Abstract
The prolonged exposure to obesogenic diets disrupts the mesocortical dopaminergic input to the prefrontal cortex (PFC). This leads to suboptimal dopamine levels in this brain region, which affects cognition and control of food intake. Treatments that restore mesocortical dopaminergic neurotransmission may improve obesity‐associated cognitive dysfunction and modulate food intake to induce weight loss. Given the complexity and multifactorial nature of obesity, combination treatments would likely achieve sizeable and sustained body weight loss and improve obesity‐linked outcomes, such as cognitive dysfunction. Given this background, we hypothesize that concomitant activation of serotonin 5‐HT2C and histamine H1 receptors, coupled with antagonism of histamine H3 receptors, synergistically modulates mesocortical dopamine neurotransmission and ameliorates obesity‐induced cognitive dysfunction. We propose to test the hypothesis in a diet‐induced obesity (DIO) rat model by treating animals with the 5‐HT2C agonist lorcaserin and the H1 agonist and H3 antagonist betahistine. Consistent with our hypothesis, both lorcaserin and betahistine have been shown to reduce body weight in humans with obesity and animals. Both drugs have been demonstrated to improve cognitive functions by influencing dopaminergic signaling in the PFC. The proposed combination treatment addresses the paucity of studies on obesity treatments that improve cognitive function. This research may also help identify a potential targetable mechanism connecting obesity and neurocognitive outcomes.
Keywords: betahistine, cognition, combination treatment, lorcaserin, obesity
Hypothesis: Amelioration of obesity‐induced cognitive dysfunction via a lorcaserin‐betahistine combination treatment.
Abbreviations
- 5‐HT2C
serotonin 2C
- BMI
body mass index
- D2
dopamine 2
- DIO
diet‐induced obesity
- H1
histamine 1
- H3
histamine 5
- PFC
prefrontal cortex
- VTA
ventral tegmental area
1. INTRODUCTION
Obesity is considered as “the epidemic of the 21st century,” 1 with worldwide rates having more than doubled since 1980. The health consequences of obesity are far‐reaching—it increases the risk of not only diabetes, cardiovascular disease, and certain cancers, but also dementia such as Alzheimer's disease. 2 , 3 Notably, obesity diminishes cognitive function throughout life, even in the absence of metabolic syndrome or diabetes. 3 Considering the rising global prevalence of obesity, research that delves into the mental health consequences of obesity is extremely important. Furthermore, drug development in this area merits priority in the absence of specific treatments that directly address obesity‐induced cognitive outcomes.
The prefrontal cortex (PFC), one of the critical brain areas subserving cognition, learning, and memory functions, is significantly impacted by diet‐evoked obesity. 3 , 4 Moreover, the prolonged exposure to obesogenic diets disrupts the mesocortical dopaminergic input to the PFC, resulting in circuit dysregulation and subsequent alterations in cognition and eating behavior. 4 Of note, marked reductions in PFC dopamine have been observed in both obese humans and animals, 2 , 4 , 5 implying that dopamine plays a vital role in obesity and associated cognitive deficits and dysfunctional eating behavior. Thus, treatments that restore mesocortical dopaminergic neurotransmission may improve obesity‐associated cognitive dysfunction and modulate food intake to facilitate weight loss. Psychostimulants (e.g., amphetamine and phentermine), which increase brain dopamine levels, 6 are widely known to produce cognitive enhancement and weight loss. 7 , 8 However, the abuse liability, coupled with other detrimental side effects of these drugs, warrant identification of non‐psychostimulant anti‐obesity treatments.
Aside from dopamine, the monoamine neurotransmitters serotonin and histamine also regulate cognition and appetite. 9 , 10 , 11 Moreover, serotonin 5‐HT2C and histamine (H1) and (H3) receptors have been demonstrated to affect dopaminergic signaling and may thus modulate cognitive functions and food intake directly and indirectly through dopamine neurotransmission. 9 , 10 , 11 For example, the selective 5‐HT2C receptor agonist lorcaserin increased the firing rate of dopamine neurons. 12 Blockade of H3 receptors by thioperamide also increased the firing activity of dopaminergic cells. 11 Lorcaserin also improved the cognitive functions of obese animals. 13 Betahistine, an H1 receptor agonist and H3 receptor antagonist, enhanced cognitive function in schizophrenic patients. 14 Lorcaserin and betahistine have also been reported to decrease food intake and promote weight loss in animals and humans. 9 , 12 , 15 , 16 Notably, H3 receptor antagonists have been shown to increase extracellular dopamine concentrations in the prefrontal cortex. 17 , 18 , 19 There is some evidence suggesting that H3 receptor blockade does not increase dopamine levels in the striatum, 17 which may indicate lack of abuse liability of H3 antagonists. Similarly, lorcaserin altered firing rate and pattern of the ventral tegmental area dopamine neurons but did not affect dopamine release in the nucleus accumbens. 20
The complexity and multifactorial nature of obesity indicate that combination treatment strategies would achieve the greatest likelihood of success in facilitating weight loss and in treating obesity‐linked outcomes. 21 Combination treatments of anti‐obesity drugs with different pharmacodynamic actions and produce effects on multiple pathways may also overcome the natural compensatory mechanisms involved in energy homeostasis which could reduce their efficacy. 22 In the same vein, combination treatments of drugs that work synergistically or in parallel could also produce the most significant benefit in treating obesity‐related cognitive dysfunction.
1.1. Hypothesis
We hypothesize that concomitant treatment with lorcaserin and betahistine synergistically modulates mesocortical dopamine neurotransmission and ameliorates obesity‐induced cognitive deficits in a diet‐induced obesity (DIO) rat model. The two drugs also synergistically enhance weight loss in obese subjects. The rationale for this hypothesis lies in the reported improved outcomes (e.g., enhanced weight loss) of combination treatment vs. monotherapy in obesity. 21 The scientific premise supporting the selection of the two drugs is based on 1) the reported efficacy of these non‐psychostimulant agents to target receptors that influence dopamine signaling in the PFC and 2) their ability to reduce body weight in obesity clinical trials. 9 , 10 Notably, high doses of betahistine enhanced working memory of healthy human subjects. 23 No changes in cognition was observed with up to 1 month of lorcaserin treatment in obese humans, 24 although animal studies showed cognitive‐enhancing effects of lorcaserin in obese mice. 13 In view of these findings, we hypothesize that lorcaserin and betahistine, given individually, do not robustly improve cognitive functions, or higher doses of each drug are required to achieve that effect. When co‐administered, these drugs would synergize to enhance mesocortical dopamine release (Figure 1). By restoring mesocortical dopaminergic signaling, the combination treatment may not only ameliorate obesity‐induced cognitive dysfunction but also strengthen the cognitive control of food intake. These effects may occur at low doses of each drug that produce minimum side effects.
FIGURE 1.
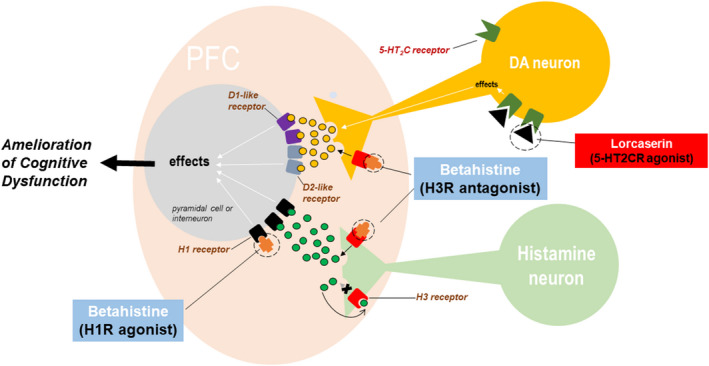
Hypothesis: Lorcaserin plus betahistine synergistically enhances mesocortical dopamine and ameliorates cognitive dysfunction in obese animals. Both lorcaserin and betahistine increase dopamine release in the PFC, but they produce the effect via different mechanisms. Lorcaserine activates serotonine 5‐HT2C receptors on mesocortical dopaminergic neurons to increase their firing rate. Betahistine blocks the inhibitory histamine H3 receptors on dopaminergic neurons. When co‐administered, the two drugs would act synergistically to restore the mesocortical dopamine deficit in obese animals and ameliorate cognitive dysfunction caused by the reduced activation of both D1‐ and D2‐like receptors on PFC pyramidal cells and interneurons. Betahistine may exert additional pro‐cognitive effects by stimulating H1 receptors and increasing the release of histamine and other transmitters such as acetylcholine and norepinephrine in the PFC 25
2. STRATEGIES TO EVALUATE THE HYPOTHESIS
2.1. Determine the efficacy of combination lorcaserin and betahistine to ameliorate cognitive dysfunction in a diet‐induced animal model of obesity
Obesity in Western countries is driven primarily by overconsumption of obesogenic diets. 2 Therefore, the DIO model would appropriately reproduce human overweight and obesity, and obesity‐induced alterations in PFC‐dependent cognitive functions, as described previously. 4 DIO can be achieved by giving adult (12 weeks old) Wistar rats an obesogenic diet (41% kcal from fat) for 8 weeks. The control group could be assigned standard chow (13% kcal from fat). In our hands, an 8‐week exposure to an obesogenic diet is enough to induce obesity in rats and to alter PFC‐dependent functions. 26 , 27 Successful induction of obesity in rats can be verified through anthropometric measurements performed weekly, including measuring the percentage of body weight gain, abdominal circumference (AC) and Body Mass Index (BMI). 28 Of note, the BMI positively correlated with fat mass in rats, indicating a reliable estimate of body fat. To measure the effects of DIO on cognitive functions, rodent behavioral tests for cognition can be used, such as the novel object recognition test, Y‐maze tasks, and object‐in‐place test. Notably, the Y‐maze and object‐in‐place tasks are PFC‐dependent cognitive assays. 3 Ideally, the behavioral tests should not require too much locomotion to avoid the potential confounds of obesity‐related decreases in physical performance. DIO results in persistent cognitive impairments in our model even when obese rats are placed on a control diet (unpublished finding).
After verifying obesity‐induced cognitive dysfunction, rats can be randomized to receive either saline or drugs while being maintained on their respective diets. Control rats can also be given either saline or drugs. To measure the effects of long‐term drug treatment on cognition, rats could be implanted with osmotic minipumps to facilitate continuous drug delivery for several weeks. The use of osmotic minipumps would also allow chronic drug delivery without introducing additional stressors. The lorcaserin and betahistine doses used in the combination treatment should ideally be minimum effective doses or lower than those that produce robust weight loss in obese rats when the two drugs are given individually. This strategy could help reduce dose‐related adverse effects of and improve tolerability to the combination treatment. 22 To verify additive or synergistic effects of the combination treatment, an isobologram analysis may be employed by comparing theoretical versus experimental ED50s of the drug combination, as described in previous studies. 29 , 30
It is important to ensure that induction of obesity and cognitive behavioral testing are conducted in adult rats, considering the reported increased risk of late‐life cognitive impairment with being obese during the midlife. 2 Aside from the above‐described behavioral tests, locomotor activity assays may also be employed to ensure responses in cognitive tests are not affected by obesity‐related decreases in physical performance.
Although changes in cognitive functions would be the primary outcome of this study, it would also be interesting to examine whether a relationship exists between the outcomes related to cognitive function and weight loss effects through correlation analysis. A positive correlation would lead to another interesting question of whether the improvement of cognitive function results from weight loss or vice‐versa. However, it is possible that obese rats given lorcaserin plus betahistine show either enhanced cognitive functions or more significant weight loss compared with monotherapy, but not both. This finding may suggest a dissociation between cognition and food intake and a specific PFC‐associated function (cognition or food intake) influenced explicitly by the combination treatment. On the other hand, there might be within‐group variations in cognitive and weight loss improvements in lorcaserin plus betahistine‐treated rats. This may reflect the clinical case of the heterogeneous response of obese individuals to drug treatment. Future studies will determine whether metabolic outcomes (e.g., blood glucose and insulin and leptin resistance) play a role in obesity‐induced cognitive dysfunction in DIO, and if the combined treatment also improves those outcomes.
2.2. Demonstrate the efficacy of the combination treatment to restore mesocortical dopamine signaling in obese rats
The mesocortical dopamine pathway plays a pivotal role in regulating cognition and food intake. 4 Obese patients and animal models of obesity show a significant reduction in PFC dopamine levels, 2 , 4 , 31 suggesting dysfunction in this pathway, which may contribute to cognitive dysfunction, poor inhibitory control, and compulsive overeating. 4 Therefore, restoring mesocortical dopamine signaling may improve cognitive functions and strengthen cognitive control of food intake.
Our preliminary in vivo electrophysiology studies showed decreased dopamine neuron activity in the ventral tegmental area (VTA) of DIO rats (unpublished finding), consistent with the reported decreased dopamine release and reduced biochemical activity of VTA‐dopamine neurons in obese rats and humans. 2 , 31 We also found that injection of lorcaserin dose‐dependently increased the firing rate of mesocortical dopamine neurons of non‐obese rats. Lorcaserin, therefore, may help restore dysregulated dopaminergic signaling in obese animals. Moreover, the addition of betahistine may amplify this effect of lorcaserin, and together, these drugs may synergistically modulate mesocortical dopamine neurotransmission and ameliorate obesity‐induced cognitive deficits in animals (Figure 1).
To demonstrate the efficacy of combination lorcaserin and betahistine to restore mesocortical dopaminergic signaling in DIO rats, in vivo electrophysiology may be employed. In vivo electrophysiology allows simultaneous recordings of the PFC local field potentials (LFPs) and dopamine neuron single unit activities as described in our previous studies. 32 , 33 Antagonists selective for 5‐HT2C and H1 receptors may then be used to confirm that the effects of lorcaserin and betahistine are mediated by 5‐HT2C and H1 receptors, respectively. H3 antagonists (e.g. thioperamide) could also be employed to determine whether the drug partially mimics the effects of the combination treatment. At the end of in vivo recordings, the cell's response to low doses of the dopamine D2 receptor agonist apomorphine (1–8 μg/kg) could then be examined. Unlike mesolimbic dopamine neurons, mesocortical dopamine neurons do not possess somatodendritic D2 autoreceptors 34 and, therefore, should not be inhibited by low doses of apomorphine. The above‐described experiment will demonstrate that restoring mesocortical dopamine signaling is responsible for improving cognitive functions in obese rats.
One possible finding of this experiment is that lorcaserin plus betahistine produces different effects on mesocortical dopamine neurons in obese rats, which could be expected given the heterogeneity of dopamine neurons in the VTA. 34 This response heterogeneity must be noted during the data analysis. To verify synergistic effects of the combination treatment, an isobologram analysis may also be conducted as described above. Moreover, it would be logical to compare the acute and chronic effects of the combination treatment on the above parameters. Other experiments, e.g., brain microdialysis or fiber photometry, should be employed to establish that the combination treatment, indeed, increases PFC dopamine levels.
3. SIGNIFICANCE OF THE HYPOTHESIS
This study is significant because it addresses the paucity of studies on obesity treatments that improve cognition. Studies that explore the mental consequences of obesity are few in number. Because animal models have excellent predictive validity for the clinical efficacy of anti‐obesity drugs, the findings of this study are likely to reach clinical testing in the near future. Additionally, this research may help identify a potential targetable mechanism connecting obesity and mental health. Dopamine is a potent mediator underpinning the individual effects of obesity on the brain and behavior. This study has the potential to show that the targetable mesocortical dopamine pathway is involved in DIO and cognitive dysfunction.
Furthermore, this study is essential because it utilizes drug repurposing to discover pharmacological treatments that may ameliorate obesity‐induced cognitive dysfunction. Few studies have evaluated evidence‐based pharmacotherapy to reduce neurocognitive consequences of obesity. Finding new uses for approved drugs will provide the quickest possible transition from bench to bedside. Finally, pharmacological treatments that restore cognitive dysfunction in obesity will demand practical interventions for long‐term weight management in individuals with obesity. They could potentially enhance dietary self‐regulatory abilities resulting in better food and lifestyle choices in obese individuals.
4. NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 35 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 36
DISCLOSURE
None of the authors have any relevant actual or potential conflicts of interest.
AUTHOR CONTRIBUTION
ICD conceived the original idea. ICD, WSX, and JDF contributed equally to the design of the research. ICD wrote the manuscript with input from all authors.
ACKNOWLEDGMENT
This study was funded by the American Association of Colleges of Pharmacy (AACP) New Investigator Grant awarded to ICD. It was also partly supported by the National Institutes of Health NIH grants (DK124727, GM060507, MD006988) and the Loma Linda University School of Medicine GRASP Seed Funds to JDF and ICD.
dela Peña IC, Figueroa JD, Shi W‐X. Hypothesis: Amelioration of obesity‐induced cognitive dysfunction via a lorcaserin–betahistine combination treatment. Pharmacol Res Perspect. 2022;10:e00947. doi: 10.1002/prp2.947
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Rossner S. Obesity: the disease of the twenty‐first century. Int J Obes. 2002;26(Suppl 4):S2‐4. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen JCD, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8(375). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bocarsly ME, Fasolino M, Kane GA, et al. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci. 2015;112(51):15731‐15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowe CJ, Reichelt AC, Hall PA. The prefrontal cortex and obesity: a health neuroscience perspective. Trends Cogn Sci. 2019;23(4):349‐361. [DOI] [PubMed] [Google Scholar]
- 5. Geiger BM, Behr GG, Frank LE, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity‐prone rats. FASEB J. 2008;22(8):2740‐2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. dela Peña I, Gevorkiana R, Shi W‐X. Psychostimulants affect dopamine transmission through both dopamine transporter‐dependent and independent mechanisms. Eur J Pharmacol. 2015;764:562‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mariotti CK, Rossato GL, Fröehlich EP, Limberger PR. Amphetamine‐type medicines: a review of pharmacokinetics, pharmacodynamics, and toxicological aspects. Current Clinical Pharmacology. 2013;8(4):350‐357. [DOI] [PubMed] [Google Scholar]
- 8. Repantis D, Bovy L, Ohla K, Kühn S, Dresler M. Cognitive enhancement effects of stimulants: a randomized controlled trial testing methylphenidate, modafinil, and caffeine. Psychopharmacology. 2021;238(2):441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barak N, Greenway FL, Fujioka K, Aronne LJ, Kushner RF. Effect of histaminergic manipulation on weight in obese adults: a randomized placebo controlled trial. Int J Obes (2005), 2008;32(10):1559‐1565. [DOI] [PubMed] [Google Scholar]
- 10. Greenway FL, Shanahan W, Fain R, Ma T, Rubino D. Safety and tolerability review of lorcaserin in clinical trials. Clin. Obes. 2016;6(5):285‐295. [DOI] [PubMed] [Google Scholar]
- 11. Flik G, Folgering JH, Cremers TI, Westerink BH, Dremencov E. Interaction between brain histamine and serotonin, norepinephrine, and dopamine systems. In vivo microdialysis and electrophysiology study. J. Mol. Neurosci: MN. 2015;56(2):320‐328. [DOI] [PubMed] [Google Scholar]
- 12. Xu P, He Y, Cao X, et al. Activation of serotonin 2C receptors in dopamine neurons inhibits binge‐like eating in mice. Biol Psychiat. 2017;81(9):737‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang H, Huang F, Ni M, et al. Cognitive function is impaired by obesity and alleviated by Lorcaserin treatment in mice. CNS Neurosci Ther. 2015;21(5):472‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Huang X, Fan H, et al. High‐dose Betahistine improves cognitive function in patients with schizophrenia: a randomized double‐blind placebo‐controlled trial. Front Psychiatry. 2021;12:762656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hurren KM, Berlie HD. Lorcaserin: an investigational serotonin 2C agonist for weight loss. Am J Health‐Syst Pharm. 2011;68(21):2029‐2037. [DOI] [PubMed] [Google Scholar]
- 16. Lian J, Huang XF, Pai N, Deng C. Preventing olanzapine‐induced weight gain using betahistine: a study in a rat model with chronic olanzapine treatment. PLoS One. 2014;9(8):e104160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fox GB, Esbenshade TA, Pan JB, et al. Pharmacological properties of ABT‐239 [4‐(2‐{2‐[(2R)‐2‐Methylpyrrolidinyl]ethyl}‐benzofuran‐5‐yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther. 2005;313(1):176‐190. [DOI] [PubMed] [Google Scholar]
- 18. Ligneau X, Perrin D, Landais L, et al. BF2.649 [1‐{3‐[3‐(4‐Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J Pharmacol Exp Ther. 2007;320(1):365‐375. [DOI] [PubMed] [Google Scholar]
- 19. Medhurst AD, Atkins AR, Beresford IJ, et al. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer's disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther. 2007;321(3):1032‐1045. [DOI] [PubMed] [Google Scholar]
- 20. De Deurwaerdère P, Ramos M, Bharatiya R, et al. Lorcaserin bidirectionally regulates dopaminergic function site‐dependently and disrupts dopamine brain area correlations in rats. Neuropharmacology. 2020;166: 107915. [DOI] [PubMed] [Google Scholar]
- 21. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342‐362. [DOI] [PubMed] [Google Scholar]
- 22. Gadde KM, Allison DB. Combination therapy for obesity and metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(5):353‐358. [DOI] [PubMed] [Google Scholar]
- 23. van Ruitenbeek P, Mehta MA. Potential enhancing effects of histamine H1 agonism/H3 antagonism on working memory assessed by performance and bold response in healthy volunteers. Br J Pharmacol. 2013;170(1):144‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farr OM, Upadhyay J, Gavrieli A, et al. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion‐ and salience‐related changes correlate with weight loss effects: a 4‐week‐long randomized, placebo‐controlled double‐blind clinical trial.. Diabetes. 2016;65(10):2943‐2953. doi 10.2337/db16-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol. 2008;154(6):1166‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalyan‐Masih P, Vega‐Torres JD, Miles C, et al. Western high‐fat diet consumption during adolescence increases susceptibility to traumatic stress while selectively disrupting hippocampal and ventricular volumes. Eneuro. 2016;3(5): 10.1523/ENEURO.0125-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vega‐Torres JD, Haddad E, Lee JB, et al. Exposure to an obesogenic diet during adolescence leads to abnormal maturation of neural and behavioral substrates underpinning fear and anxiety. Brain Behav Immun. 2018;70:96‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novelli ELB, Diniz YS, Galhardi CM, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41(1):111‐119. [DOI] [PubMed] [Google Scholar]
- 29. Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Pers. 2015;3(3):e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nasehi M, Hajikhani M, Ebrahimi‐Ghiri M, Zarrindast MR. Interaction between NMDA and CB2 function in the dorsal hippocampus on memory consolidation impairment: an isobologram analysis. Psychopharmacology. 2017;234(3):507‐514. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen JCD, Ali SF, Kosari S, et al. Western diet chow consumption in rats induces striatal neuronal activation while reducing dopamine levels without affecting spatial memory in the radial arm maze. Fron Behav Neurosci. 2017;11(22): 10.3389/fnbeh.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. dela Peña IC, Shen G, Shi W‐X. Methylphenidate significantly alters the functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. Neuropharmacology. 2018;131:431‐439. [DOI] [PubMed] [Google Scholar]
- 33. Gao M, Liu CL, Yang S, Jin GZ, Bunney BS, Shi WX. Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci. 2007;27(20):5414‐5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760‐773. [DOI] [PubMed] [Google Scholar]
- 35. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Res. 2017;46(D1):D1091‐D1106. doi: 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alexander SPH, Fabbro D, Kelly E, et al. The concise guide to pharmacology 2019/20: enzymes. Br J Pharmacol. 2019;176(S1):S297‐S396. doi: 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


