Abstract
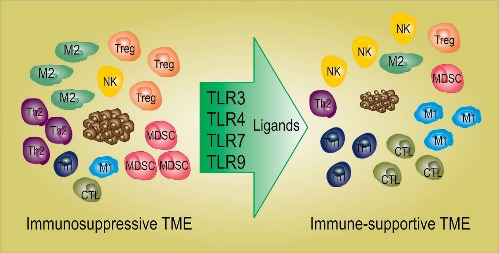
Immunotherapy is considered a promising approach for cancer treatment. An important strategy for cancer immunotherapy is the use of cancer vaccines, which have been widely used for cancer treatment. Despite the great potential of cancer vaccines for cancer treatment, their therapeutic effects in clinical settings have been limited. The main reason behind the lack of significant therapeutic outcomes for cancer vaccines is believed to be the immunosuppressive tumor microenvironment (TME). The TME counteracts the therapeutic effects of immunotherapy and provides a favorable environment for tumor growth and progression. Therefore, overcoming the immunosuppressive TME can potentially augment the therapeutic effects of cancer immunotherapy in general and therapeutic cancer vaccines in particular. Among the strategies developed for overcoming immunosuppression in TME, the use of toll-like receptor (TLR) agonists has been suggested as a promising approach to reverse immunosuppression. In this paper, we will review the application of the four most widely studied TLR agonists including agonists of TLR3, 4, 7, and 9 in cancer immunotherapy.
Keywords: Immunotherapy, Cancer, TLRs, Microenvironment, Agonist, Vaccine
Introduction
During the past two decades, there has been great success in developing and clinical application of immunotherapeutic approaches in cancer treatment. 1 The purpose of cancer immunotherapy is to stimulate and boost the patient's immune system towards the eradication of malignant cells. 2-4 Cancer immunotherapy started with Coley's toxin in 1891. Coley showed that injecting the bacterial toxin into a patient with inoperable cancer results in the shrinking of the malignant tumor. Coley’s study was one of the first examples of immunotherapy and set many scientists on a path leading to the development of many immunotherapy drugs. 4 The main advantage of cancer immunotherapy over other conventional cancer therapies such as chemotherapy (CT), is the generation of cancer-specific and long-lasting effects against cancer. 5-7 Despite great promise, cancer immune-therapeutics in general and cancer vaccines particular, have not lived to their potential and did not achieve the expected therapeutic endpoints in the clinic. 8,9 Immunosuppression in the tumor microenvironment (TME) has been recognized as one of the main barriers to the success of cancer immunotherapy. 7,10
The therapeutic outcome of cancer immunotherapy much depends on TME, where cancer cells interact with different types of stroma cells including immune cells. 6 Several clinical and preclinical studies have shown that TME is immunosuppressive at the time of diagnosis in many cancers and it plays a vital role in resistance to therapy. 7 Activation of immunosuppressive immune cells such as tolerogenic dendritic cells (DCs) and regulatory T (Treg) cells is found to be crucial in the establishment of immunosuppression in TME. The application of antibodies that suppress the function of Treg cells, thereby improving effector T cells (Teff) function is one of the first strategies used to increase the therapeutic efficacy of cancer vaccines by modulation of immunosuppression in TME. 6 In normal conditions, PD-1/PDL-1 (Programmed death-1/Programmed death-ligand-1) and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) act as the brakes of the immune system. PD-1/PDL-1 and CTLA-4 are upregulated in human malignancies and display an essential role in suppressing anti-cancer immune responses in TME. PD-L1 expressed on tumor cells negatively regulates T cell-mediated immune responses. PD-L1 binds to PD-1 receptors expressed on T cells leading to the induction of T cell exhaustion, which in turn make a significant contribution to the formation of immunosuppressive TME. 6,11,12 Moreover, binding of CTLA-4 on Treg cells to B7 ligands on APCs results in blocking of essential activation signal for T cells actuation leading to anergy in T cells or generation of more Treg cells in TME. 13 Blocking these inhibitory receptors through antibodies, have been shown to reduce immunosuppression in TME and enhance the therapeutic effects of anti-cancer immune responses induced by cancer vaccines. 10,14 PD-1/PD-L1 and CTLA-4 blockers have also been shown to improve the activation and tumor infiltration of cancer-specific CTLs (cytotoxic T lymphocytes). 6
Another critical strategy to reverse the immunosuppression in TME is to target tolerogenic DCs that play a vital role in activating immunosuppressive immune cells including Treg cells. Previous findings show that the function of tolerogenic DCs can be restored using adjuvants, which bind to pattern recognition receptors (PRRs) on DCs and induce their activation and functional maturation. Toll-like receptors (TLRs) are the most well-known type of PRRs found on antigen-presenting cells (APCs) such as DCs and macrophages. TLR ligands have been extensively studied for the modulation of anti-tumor immune responses. 6,10 Here, we review the evidential studies, investigating the effect of TLR in overcoming immunosuppression in TME, as a significant obstacle to successful cancer immunotherapy.
TME as a barrier to the success of cancer immunotherapy
Oncogenic signaling and TME are two significant phenomena responsible for escaping cancer from immune surveillance, leading to tumor progression. 1,8,15 The heterogeneous nature and immunogenic status of the TME (cold or hot) can affect the outcome of cancer immunotherapy. 9 Various studies have demonstrated that hot TME, which is associated with inflamed T lymphocytes (T cells), high rates of mutation, and neoantigens rather than cold TME (non-T cells inflamed, lower mutation, and neoantigen) exerts effective response rates to immunotherapy. Thus, turning cold tumors into hot ones through modulation of the TME may induce a better response to immunotherapy.
TLR ligands can activate APCs and induce Th1 (T helper) type inflammatory response in TME leading to better infiltration and function of anti-cancer immune effector cells. 16 The activation of TLR signaling on APCs enhances their antigen presentation capacity, induces the expression of co-stimulatory factors and Th1 inflammatory cytokines such as IL-12. Th1 inflammatory cytokines promote the switching of CD4+ T cells from Th2 to Th1, enhance CD8+ T cell responses, and inhibit Treg cell activity. 17 Manipulation of TME by intratumoral TLR agonists (acting as in situ vaccination) can induce local immune response upon generation or expanding pre-existing anti-tumor T cells. Combination of TLR agonists with other therapies such as checkpoint inhibitors (CPIs) can trigger both local and systemic immune responses leading to significant clinical response to CPIs in the treatment of metastasis lesions that show resistance to CPIs. 9,18 Recent studies also show that combining TLR agonists with some other immunotherapy approaches such as cell-based immunotherapy can improve the effectiveness of immunotherapy regimens in cancer patients. 18 In this paper, we have provided a comprehensive review of preclinical and clinical data showing that TLR agonists can improve the efficacy of various cancer immunotherapy approaches in particular cancer vaccines.
Immunosuppression in TME is induced by various types of cells including immunosuppressive macrophages, tolerogenic DCs, innate lymphocytes, myeloid-derived suppressor cells, (MDSCs), and Treg cells (Fig. 1). 19 Among the immunosuppressive cells, tolerogenic DCs play the main role in the formation of an immunosuppressive TME. Tolerogenic DCs suppress immune responses against cancer and induce tolerance to cancer. Most human cancers are shown to produce immunosuppressive factors, promoting the activation of tolerogenic DCs, which leads to the activation of Treg cells, known to play a major role in the suppression of anti-cancer immune cells. 20 Tolerogenic DCs and Treg cells also produce a high level of immunosuppressive factors, including IL-10, TGF-β, and IL-35, which not only suppress the anti-cancer function of immune cells such as Teff, NK (natural killer) cells, and DCs but also induce the activation of more immunosuppressive cells such as MDSCs in TME. This vicious cycle of immunosuppression continues and paralyzes every aspect of anti-cancer immune responses. 21,22 Targeting tolerogenic DCs is thought to be a good way to break the cycle of immunosuppression in the TME.
Fig. 1.
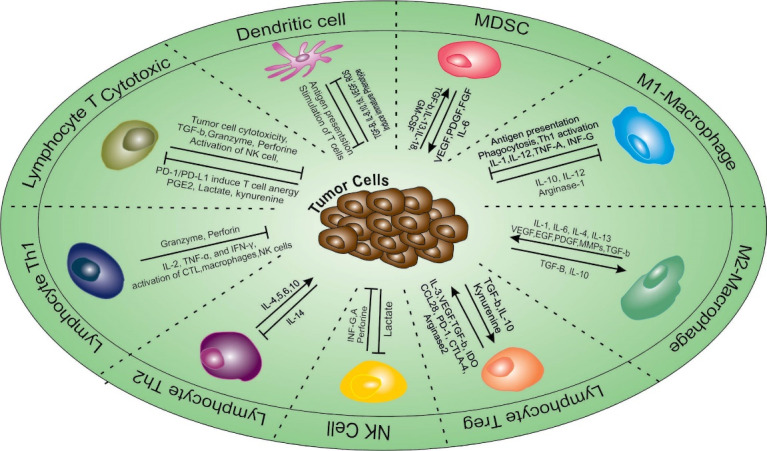
Tumor microenvironment. There is reciprocal interaction between several types of immune cells and tumor cells. Pro-tumor immune cells induce tumor cells growth through expression of many factors; in contrast, anti-tumor immune cells inhibit tumor cells growth and development.
Treg: regulatory T; TLRs: Toll like receptors; DC: Dendritic cell; Th-1: T helper cells; CTL: Cytotoxic T lymphocytes; PD-L1: Programmed death-ligand-1; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN: Interferon-α; TNF-α: Tumor necrosis factor; NKC: NK cytotoxicity; FGF: fibroblast growth factor.
TLR signaling pathway and agonists
TLRs are the sensors of danger imposed by pathogens or cellular damage. TLRs-expressed on APCs recognize highly conserved molecules on invading pathogens known as pathogen-associated molecular patterns (PAMPs). Viral double-stranded RNA (dsRNA) and lipopolysaccharide (LPS) are examples of PAMPs. TLRs also sense internal danger signals known as damage-associated molecular patterns (DAMPs), which are produced from damaged and stressed cells. Examples of DAMPs include high mobility group protein B1 (HMGB), heat shock proteins (HSPs), micro RNAs (miRNAs), 23 and deoxynucleic acid (DNA)- nucleoprotein. Both types of danger signals activate DCs and induce their functional maturation, which is required for the activation of the adaptive immune responses. 24,25 Under normal physiological circumstances, potential threats such as pathogens or cellular stress arouse the innate immune system as a frontlineof protection in which DCs recognize PAMPs and DAMPs via PPRs. Engagement of PPRs by PAMPs and DAMPs results in the maturation of DCs which in turn activates T cells leading to the initiation of the adaptive immune response. 10 TLRs are the most well-known type of PRRs and TLR ligands have been widely studied as an adjuvant in cancer therapy. So far, 13 members of TLR have been identified, 10 of which exist in humans. TLRs are expressed either on the endosomes or on the cell surface of APCs. TLR3, 7, 8, and 9 are expressed on the endosomes, and TLR1, 2, 4, 5, 6, and 10 are found on the cell membrane. 24,26 TLR2, 4, and 5 are expressed on the surface of APCs and their activation results in antibody-mediated as well as cell-mediated immunity. 27 On the other hand, TLR7 and TLR9 are expressed in the endosome and their activation mainly induces potent cell-mediated immune responses. 3 Redecke et al compared immune responses in mice immunized with a TLR2 agonist (Pam3Cys) or TLR9 agonist (ODN). Activation of TLR2, in contrast to activation of TLR9, induced a great Th2- mediated immune response. TLR2 increased serum level of Th2-related effector molecules (IL-13, IL-1-β, and granulocyte-macrophage colony-stimulating factor [GM-CSF]) and decreased Th1-associated cytokines (IL-12, interferon-gamma [IFN-γ]), IL-18, IL-27). 28 In another study, Didierlaurent et al demonstrated that TLR5 agonist (flagellin) in mice drives Th2-type immunity via the up-regulation of IL-4 and IL-13 by CD4+ T cells and downregulation of the Th1-promoting cytokine (IL-12). 29 Dillon et al showed that administration of LPS (TLR4 agonist) to mice results in the maturation of DCs, which mainly secret IL-12 and promotes Th1 responses. In contrast, Pam-3-cys (TLR2 agonist) elicits abundant IL-10 and less IL-12, which favors Th2 responses. 30
Upon TLRs stimulation, the downstream signaling pathways are activated leading to the initiation of adaptive immune responses. TLRs are found on the immune cells including DCs, macrophages, granulocytes, T and B cells, NK cells, and mast cells as well as endothelial, epithelial, and cancer cells. 31,32 Stimulation of TLRs on immune cells results in the activation of nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway (Fig. 2). 3,26 The stimulation of these signaling pathways leads to an up-regulation of genes encoding IFN-I, a variety of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1, IL-6, and IL-12, co-stimulatory molecules (CD40, CD80, and CD86), chemokines (C-C motif chemokine ligand 2 (CCL2), CCL3, and CCL4) as well as anti-inflammatory factors such as IL-10, prostaglandin E2 (PGE2), and cyclooxygenase-2 (COX-2). These released factors trigger maturation and activation of innate immune cells (DCs, phagocytes, and NK cells) and adaptive immune cells (Teff and antigen-specific B-cells) leading to an inflammatory environment. Each TLR has a distinctive pattern of cellular expression and cytokine induction based on its role in recognizing various types of pathogens. 25,33 Indeed, the specific functions of each TLR vary according to the kind of ligand and cell. 24 Depending on the type of involving TLR ligand, the response can have anti-or pro-tumor effects. 3,32,34 Besides, recent researches have reported that expression and regulation patterns of TLRs, as well as TLRs genetic polymorphisms, are involved in the outcomes of TLR treatment. Therefore, personalized TLRs therapy has been investigated in many cancers such as colorectal cancer (CRC). 23 On the other hand, several studies have shown that TLRs are expressed on different cancer cells, such as lung cancer, colorectal cancer, ovarian cancer, bladder cancer, melanomas, and renal cell cancer cells. Stimulation of TLRs on tumor cells can directly induce cancer cell death through apoptosis, autophagy, and necroptosis. 35
Fig. 2.
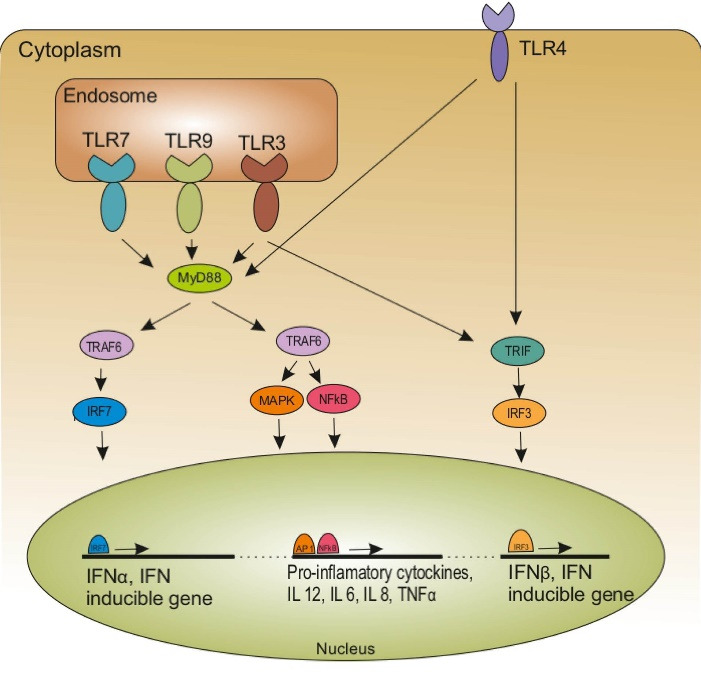
TLR activation and its downstream signaling pathways. TLR ligation results in the activation of NF-Κb, MAPK, and IRF signaling pathway leading to an up-regulation of genes encoding IFN-I, a variety of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-12.
TLR: toll‐like receptor; NF-κB: nuclear factor-kappa B; IRF: IFN regulatory factor; IFN: interferon; TNF-α tumor necrosis factor-α; TRIF: TIR-domain-containing adapter-inducing IFN-β; TRAF: TNF receptor-associated factor; MAPK: mitogen-activated protein kinase
Among the immune cells expressing TLRs, DCs are the well-studied cells in the context of TLRs signaling and the effects of TLRs stimulation on the activation/polarization of adaptive immune responses. DCs are central in the orchestration of various forms of immunity and tolerance in TME. 36 The mode of DCs maturation depends on the type of DAMPs or PAMPs detected by TLRs, which in turn determine the types of cytokines released by DCS. The cytokine secreted has polarizing effects on the activation of naïve Th cells and their differentiation to Th1 or Th2 cells. For instance, while signals mediated by endosomal TLRs such as TLR3, 7, and 9 result in the expression of IL-12 and induction of Th1 cells, activation of TLR2 and TLR5 induces the release of IL-4 leading to activation Th2 responses. TLR2-driven Th2 responses result in up-regulation of IL-10 and IL-6, inhibition of IFN-γ and IL-12 leading to proliferation and survival of Treg Cells. 37-39 Stimulation of TLR7 on DCs by guanosine analogs induces both cellular and humoral immune responses with antiviral ability via secretion of IL-12 and IFN-I. 36,39
Growing evidence demonstrated that maturation and differentiation of myeloid DCs (mDCs) are suppressed in the TME 40 Numerous studies have shown that TLRs signaling can restore the function of immunosuppressed DCs and might be useful for reversing immunosuppression in TME. 41,42 TLR ligands have been widely utilized as adjuvants for the development of anti-cancer vaccines, some of which have shown great success in the clinical setting. Vo et al have demonstrated that TLR4 agonists, such as Rv2299c (antigen derived from mycobacteria), can be used as a potential adjuvant in DC-based vaccines, which can generate a potent cellular immune response and effective anti-tumor immunity in the TME. They showed that administration of Rv2299c in combination with tumor antigen (TA)-loaded-DC vaccine in CRC mouse models leads to a remarkable reduction in the frequency of suppressor cells such as MDSC and Treg cells as well as an increase in the function of CTL and NK cells. These effects were correlated with greater inhibition of tumor progression in the mice treated with combination therapy as compared with those treated with monotherapy alone. 43 To date, Food and Drug Administration (FDA) has approved three TLR agonists such as imiquimod (IMQ; TLR7 agonist), bacillus Calmette-guérin (BCG; TLR4 agonist), and monophosphoryl lipid A (MPLA; TLR2/4 agonist) for treating skin carcinoma, bladder cancer, and cervical cancer, respectively. 24 In this review, we will present and discuss several lines of evidence, which show that TLR agonists can restore the function of tolerogenic DCs and reverse the immunosuppression in TME leading to an improved therapeutic outcome in cancer.
TLR ligands and cancer therapy
During the last two decades, stimulation and activation of DCs using TLR ligands have shown promising results in cancer immunotherapy. 44 Each TLR has a specific potential to modify T cell responses, which provide opportunities for controlling and directing the adaptive immunity during vaccination strategies and immunotherapies. 39,45 The timely introduction of TLR ligands to TME during an immunotherapy procedure can maximize the functional maturation of DCs and activation of anti-cancer immune responses. 44 It has been shown that introducing TLR agonists to APCs in TME prior to antigen encounter may not be useful due to defective cross-presentation of antigens by APCs and suppression of cross-priming in premature APCs. 46 In this manner, the utilization of TLR ligands may be most effective at the appropriate time of, or closely following the intratumoral release of cancer antigens, which usually results from the killing of cancer cells by CT or radiotherapy (RT).
Multiple lines of evidence show that TLR agonists are a promising class of anti-cancer agents that induce effective anti-cancer immune responses in TME. Modulation of TME by TLR agonists has been found to enhance the infiltration and functional activity of anti-tumor lymphocytes in the TME. 24 Increasing studies have shown that the administration of TLR agonists alone or in combination with other therapies can successfully eliminate tumors due to their powerful effects on initiation of innate and adaptive immune responses in the TME. 33 TLR agonists have been applied alone as adjuvants in cancer vaccines or in combined with other conventional standard treatments such as CT, RT, and other immunotherapies (e.g. CPIs). 24 Administration of TLR ligands in combination with CT and RT has been shown to enhance the growth-inhibitory effects of them in the mouse cancer model. 44,47 Preclinical studies have shown that TLR agonists can augment anti-tumor immune responses induced by RT and diminish resistance to RT in numerous tumor types. TLR agonists improve DC-mediated T cell priming after RT, which is found to lead to the stimulation of systemic anti-tumor immune responses and long-lasting memory in some animal cancer models. 48
Research in the area of TLR targeting for cancer treatment has revealed that while TLR ligands have anti-cancer effects in most cases, they might also have tumorigenic effects in cancer settings (Fig. 3). 49 Some studies have reported that TLR agonists can induce cancer initiation and progression by cancer immune evasion and supporting cancer cell survival. 49 The tumorigenic effects of TLR stimulation are suggested to be more evident in tumor initiation and development. Some studies also show that some TLR agonists promote cancer invasion, resistance to CT, and escape from the immune system. Based on the well-known link between chronic inflammation (bad inflammation) and cancer, it seems that constitutive stimulation of TLRs and DCs activation can result in chronic inflammation paving the path for cancer initiation and development. 31,50,51 NF-κB pathway activated via any TLR stimulation leads to activation and recruitment of immune cells in the TME. Stimulation of the NF-κB pathway can also cause accumulation of pro-tumor and anti-apoptotic molecules such as vascular endothelial growth factor (VEGF) and TNF-α in TME, which can form a favorable environment for tumor development and metastasis. Moreover, TLR agonists can also induce metabolic reprogramming in DCs, which results in tumor cell survival. 52 Besides the pro-tumor or anti-tumor effects resulting from TLR targeting, might be dependent on the type of targeted TLR and the cells expressing that specific TLR. Recent studies have demonstrated that TLR signals have a dual role sword on the MDSCs activity. It has been reported that TLR2 and TLR4 signaling can induce the immunosuppressive activity of MDSCs in cancer. In contrast to this report, other studies show that activation of TLR3, 7/8, and 9 decreases the MDSC frequency thereby enhancing their antitumor effects. 53 Sautès-Fridman et al have demonstrated that stimulation of tumor cells through TLR for viral ssRNAs or bacteria (TLR7/8 or TLR4 agonists) improve cell viability and induces chemo-resistance. On the other hand, stimulation of TLR3, the receptor for dsRNA, diminishes tumor cell survival and trigger chemosensitivity in some lung cancer cell lines. 54
Fig. 3.
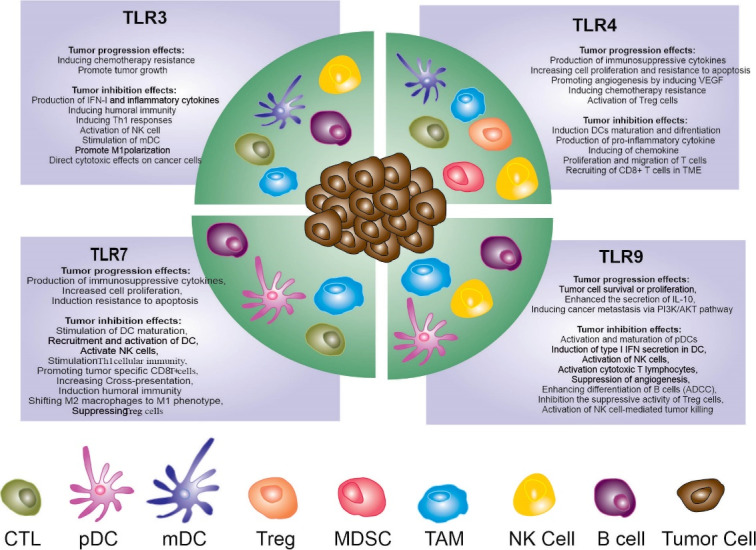
Function of TLRs-expressed immune cells in TME. Immune cells depend on expressing TLRs and engagement with certain ligand can induce tumor progression or inhibition.
mDC: myeloid dendritic cells; pDC: plasmacytoid dendritic cells; Treg: regulatory T; TLRs: Toll like receptors; DC: Dendritic cell; Th-1: T helper cells; CTL: Cytotoxic T lymphocytes; TME: Tumor microenvironment; MDSC: myeloid-derived suppressor cells; TAM: tumor-associated macrophages; NK cell: natural killer cells; ADCC: Antibody-dependent cellular cytotoxicity.
Given the reported dual effects of TLR targeting in cancer, selection of the TLR agonist for targeting in cancer greatly depends on the cancer type, stage, the type of TLR and its downstream signaling pathway, the type of cells expressing TLR, and the degree of tumor immunogenicity. 24,49 In recent years, the utilization of synthetic TLR agonists as adjuvants to favorably stimulate Th1 or Th2 immune responses has developed as truthful therapeutic agents, especially for the development of cancer vaccines against poorly immunogenic tumors. 55 Promising results from preclinical studies using TLR agonists both as monotherapy and in combination with other anti-cancer therapy have led to the clinical application of TLR agonists in patients with progressive cancers. Several ongoing clinical trials are assessing TLR agonists alone or in combination with different treatments including CT, RT, targeted therapy, and immunotherapy agents (Table 1). TLR 3, 4, 7/8, and 9 have the highest therapeutic potential in the treatment of many diseases as well as infections and cancer. The reported anti-cancer effects of TLR3, 4, 7/8, and 9 agonists have been good enough to make them used as immunotherapeutic agents with robust potential in clinical oncology. 25,32,56
Table 1. Summary of the ongoing clinical trial with TLR3/4/7/9 alone or in combination with other therapies (2020) .
| TLR | Ligand | Cancer | Combination | No. clinical trial | Phase | Institute sponsor |
|---|---|---|---|---|---|---|
| 3 | Poly-ICLC | Low-grade lymphoma | Intratumoral Flt3L with low-dose RT | NCT01976585 |
Recruiting Phase I/II |
Joshua Brody |
| 3 |
Resiquimod or polyICLC |
Brain tumors | DC vaccination and tumor lysate-pulsed DC | NCT01204684 |
Active, Phase II |
Jonsson Comprehensive Cancer Center |
| 3 | Poly-ICLC | Metastatic colon cancer | Pembrolizumab | NCT02834052 |
Recruiting Phase I/II |
Asha Nayak |
| 3 | Hiltonol | Recurrent advanced ovarian cancer | Oregovomab | NCT03162562 |
Active, Phase I |
OncoQuest Inc. |
| 3 | PolyICLC | Advanced, measurable, biopsy-accessible cancers | Tremelimumab and IV Durvalumab | NCT02643303 |
Recruiting Phase I/II |
Ludwig Institute for Cancer Research |
| 7 | MGN1703 | Advanced solid malignancies | Ipilimumab | NCT02668770 |
Active, Phase I |
M.D. Anderson Cancer Center |
| 7 | SHR2150 | Unresectable/ metastatic solid tumors | CT Plus PD-1 or CD47 antibody | NCT04588324 |
Recruiting Phase I |
Chinese PLA General Hospital |
| 7 | RO7119929 | Hepatocellular, biliary tract cancer, secondary liver cancer, liver metastases | Tocilizumab | NCT04338685 |
Recruiting Phaes1 |
Hoffmann-La Roche |
| 7 | SHR2150 | Solid tumors | Anti-cancer agent | NCT04588324 |
Recruiting Phase I/II |
Chinese PLA General Hospital |
| 7 | DSP-0509, | Advanced solid tumors | Pembrolizumab | NCT03416335 |
Recruiting Phase I/II |
Sumitomo Dainippon Pharma Oncology, Inc |
| 7 | Imiquimod | Advanced solid tumors | Standard of Care PD-1 Therap | NCT04116320 |
Recruiting Phase I |
Craig L Slingluff, Jr |
| 7 | BNT411 | Solid tumor, extensive-stage small cell lung cancers | Atezolizumab, carboplatin, etoposide | NCT04101357 |
Recruiting Phase I/II |
BioNTech Small Molecules GmbH |
| 7 | TQ-A3334 | Non-small cell lung cancer | Anlotinib | NCT04273815 |
Recruiting Phase I/II |
Chia Tai Tianqing Pharmaceutical Group |
| 7 | BDC-1001 | Advanced HER2-expressing solid tumors | Pembrolizumab | NCT04278144 |
Recruiting Phase I/II |
Bolt Biotherapeutics, Inc. |
| 7 | Imiquimod | High-grade cervical intraepithelial neoplasia | Topical fluorouracil | NCT03196180 |
Active, Phase I |
National Cancer Institute (NCI) |
| 7 | NKTR-262 | Locally advanced or metastatic solid tumors malignancies | Bempegaldesleukin, nivolumab | NCT03435640 |
Recruiting Phase I/II |
Nektar Therapeutics |
| 7 | Imiquimod | High-grade cervical intraepithelial neoplasia | Topical fluorouracil | NCT03196180 |
Active, Phase I |
National Cancer Institute (NCI) |
| 9 | CpG7909 | Esophageal cancer | URLC10-177 and TTK-567 peptide vaccine | NCT00669292 |
Unknown Phase I/II |
Wakayama Medical University |
| 9 | CMP-001 | Pancreatic cancer and other melanoma | INCAGN01949 (an activating antibody against OX40) | NCT04387071 |
Not yet Recruiting |
University of Southern California |
| 9 | Tilsotolimod | Solid Tumors (ILLUMINATE-206) | Nivolumab and Ipilimumab | NCT03865082 | Recruiting | Idera Pharmaceuticals, Inc. |
| 9 | Tilsotolimod | Patients with advanced cancers | Ipilimumab and Intravenous Nivolumab | NCT04270864 | Recruiting | Gustave Roussy, Cancer Campus, Grand Paris |
| 9 | SD-101 | CT-Refractory Metastatic Pancreatic Cancer | Nivolumab, radiation therapy | NCT04050085 |
Recruiting Phase I |
University of California, Davis |
| 9 | DUK-CPG-001 | Myeloid or lymphoid malignancies | NK cell enriched-DLI | NCT02452697 |
Recruiting Phase II |
David Rizzieri, MD |
| 9 | CpG | Metastatic pancreatic cancer | Irreversible electroporation (IRE), Nivolumab | NCT04612530 |
Recruiting Phase I |
VU University Medical Center |
| 9 | CMP-001 | Melanoma patients, lymph node cancer | Nivolumab | NCT03618641 |
Active, Phase II |
Diwakar Davar |
| 9 | SD-101 | Prostate cancer | Pembrolizumab | NCT03007732 |
Recruiting Phase II |
Lawrence Fong |
| 9 | CMP-001 | Advanced cancer |
Avelumab, Utomilumab, PF-04518600, PD 0360324 |
NCT02554812 |
Recruiting Phase II |
Pfizer |
| 9 | CMP-001 | Metastatic colorectal cancer |
Radiation therap, Nivolumab, Ipilimumab |
NCT03507699 |
Recruiting Phase I |
Sheba Medical Center |
| 9 | CMP-001 | Nivolumab | Melanoma | NCT04401995 |
Recruiting Phase II |
Diwakar Davar |
| 9 | Tilsotolimod | Malignant melanoma | Saline | NCT04126876 |
Recruiting Phase II |
A.J.M. van den Eertwegh |
| 9 | SD-101 | Low-grade B-cell non-Hodgkin lymphomas | Anti-OX40 antibody BMS 986178, radiation therapy | NCT03410901 |
Recruiting Phase I |
Ronald Levy |
| 9 | SD-101 | Relapsed or refractory grade 1-3A Follicular lymphoma | Ibrutinib, radiation therapy | NCT02927964 | Recruiting Phase II | Robert Lowsky |
| 9 | IMO-2125 | Metastatic melanoma | Ipilimumab | NCT03445533 | Active, Phase III | Idera Pharmaceuticals, Inc. |
TLR3 targeting for cancer therapy
TLR3 is expressed in different types of immune cells and TLR3 signaling induces cell-mediated immune responses. TLR3 is expressed on endosomal membranes in mDCs, as well as on both cell and endosomal surfaces in macrophages, fibroblasts, and epithelial cells. TLR3 recognizes virus-derived dsRNA, small interfering RNAs (siRNA), and self- RNAs derived from injured cells. 26 TLR3 signals through a myeloid differentiation primary response 88 (MyD88)-independent pathway resulting in the activation of NF-κB, IFN regulatory factor3 (IRF3), and activator protein-1 (AP-1). Stimulation of the MyD88-independent signaling pathway leads to the secretion of cytokines (IL-6, TNF-α, and IL-12) and IFN-I (especially IFN-β), which in turn leads to the initiation of cellular immune responses. While most of PRRs induce systemic IFN/cytokinemia upon activation, TLR3 stimulation mainly results in local inflammation. 57,58 TLR3 ligands act as inducers of cellular immune responses through activation of mDCs which in turn stimulate NK cells and CTLs. 59
TLR3 agonists
Over the last two decades, a few synthetic ligands for TLR3 have been developed. The ligands developed for TLR3 are the synthetic analog of dsRNA which include polyinosinic-polycytidylic acid (poly I:C; Ampligen), polyadenylic:polyuridylic (polyA:U), and ARNAX, which consists of DNA-capped dsRNA. The poly-ICLC (Hiltonol) is an analog of poly I:C that has been made stable with poly-l-lysine and carboxymethylcellulose and it is resistant to nucleases. Poly-ICLC stimulates the release of cytotoxic cytokines and induces IFN-γ production, which in turn can increase the tumoricidal activity of various immune cells. 60,61 Moreover, another nontoxic analog of Poly I:C is Poly I:C which boosts anti-tumor immunity through stimulation of effective NK and T cell responses. 60 Poly I:C has shown promising effects when it is administered single or in combination with other agents in both human malignancy and murine tumor models. 61,62 The poly I:C has favorable safety, and immunogenicity profiles as well as a robust record in the production of type-I IFNs/cytokines. 63 Poly I:C induces mDC-NK reciprocal activation by cell-to-cell contact. 64 Moreover, it has been shown that poly I:C engagement to TLR3 on tumor cells cause cancer cell apoptosis and provides an anti-tumor IFN-I rich environment. 61
In the context of DC-priming, targeting TLR3 is considered a promising approach for the stimulation of anti-tumor CTL responses. 58 ARNAX (TLR3-specific ligand) is a non-inflammatory DC-priming adjuvant that induces local cytokine release effectively and activates DCs for cross-priming of CD8+ T cells. Combination therapy using ARNAX combined with tumor-associated antigens (TAAs), triggers tumor-specific CTL without systemic cytokine production, modulates the TME to establish Th1-type anti-tumor immune responses, and inhibits tumor growth without systemic inflammation in mice carrying EG7 (OVA expressing T lymphoma) and MO5 (melanoma) tumors. 58,65,66 Therefore, TLR3 agonists may represent advanced adjuvants for anti-cancer therapy and serve as safe means of enhancing this strategy in the clinic. 67,68
Four main characteristics of TLR3 signaling make it a promising target for cancer therapy: (i) TLR3 is highly expressed in various types of human cancers and TLR3 agonists directly kill TLR3-expressing cancer cells, (ii) TLR3 is mainly expressed in cross-presenting DCs and triggers cross-priming of endogenous antigens leading to induce robust CD8+ T cell responses, (iii) TLR3 agonists induce the expression of chemokines that attract Th1, CD8+ and NK cells, (iv) TLR3 is expressed on NK cells and TLR3 agonists increase the anti-cancer function of NK cells. 61,69
TLR3 activation results in anti-cancer effects through various mechanisms. It has been shown that stimulation of TLRs improves the intratumoral infiltration of preexisting tumor-reactive CTLs and converts tumor-associated macrophages (TAMs) from an M2- to an M1-like phenotype. 22 Also, the endogenous human TLR3 in cancer cells exert direct pro-apoptotic activity. 70 TLR3 is also shown to contribute to the processing of antigens derived from apoptotic cells and their presentation to CD8+ T cells. 71 Besides TLR3 agonists bind to TLR3 expressed on DCs, which results in the maturation of DCs and cross-presentation of TAAs leading to the activation of cancer-specific CTL. 61 TLR3 deficiency has been reposed to result in the development of T cell leukemia and increased tumor growth in prostate mice model cancer. 57 A preclinical study has shown that TLR3 induces apoptosis in lung cancer cell lines upon caspase-3 activation. 71 Another study has shown that the targeted delivery of TLR3 agonists to tumor cells induces IFN-I expression and apoptosis in cancer cells resulting in tumor regression in the HT1376 xenografts immune-deficient mice model. 72 Clinical studies have shown high intratumoral TLR3 expression levels in hepatocellular carcinoma (HCC) patients are associated with prolonged survival. 69 On the one hand, TLR3 expression on lung cancer patients is associated with a good prognosis. 71 While TLR3 signaling predominantly stimulates anti-tumor immunity and results in anti-cancer effects, some limited research has reported that TLR3 agonists can enhance the viability of tumor cells and increase their resistance to CT. 57,73
TLR3 combination therapy
TLR3 agonists have been used alone or in combination with other anti-cancer agents in cancer treatment. 33 Chemo-immunotherapy with a TLR3 agonist (poly I:C) and low-dose cisplatin has been shown to down-regulate drug transporters (e.g., permeability glycoprotein (P-gp) and multi-drug resistance-1 (MRP-1). Poly I:C and cisplatin combination therapy were found to increase the cytoplasmic level of cisplatin and to significantly augment cisplatin-induced cell death which was dependent on the TLR3 signaling pathway. In a similar preclinical study the pretreatment of oral squamous cell carcinoma (OSCC) mice model tumor with poly I:C, sensitized them to the low dose cisplatin-based CT. The polyI-C-cisplatin regimen was found to significantly decrease the number of MDSCs, TAM, and cancer-associated fibroblast (CAF) in TME, leading to tumor regression and alleviating adverse events (AEs). 74 Application of TLR3 agonists with a TLR9 agonist (CpG: 5'-cytosine-phosphate-guanine-3') combined with adoptive T cell transfer (ACT) resulted in a significant increase in the frequency of neutrophils, macrophages, NK cells, B cells, CD4+ and CD8+ T cells in tumor-draining lymph nodes. This combination therapy augmented the eradication of murine melanoma cells, which led to the improvement of survival in tumor-bearing mice up to two-fold as compared with untreated mice (9 vs. 18 days). 67 In vitro and in vivo studies have shown that combining the poly-ICLC with sorafenib (an oral multi-kinase inhibitor) could control HCC tumor growth. This combination therapy increased apoptosis and reduced the proliferation of HCC cell lines. In HCC mouse models, poly-ICLC and sorafenib combination therapy enhanced tumor cell apoptosis and host immune responses in the TME compared with monotherapy or phosphate-buffered saline (PBS)-treated controls. Remodeling of TME as a result of poly-ICLC with sorafenib combination therapy was characterized by local activation of NK cells, T cells, macrophages, and DCs as well as decreased expression of PD-1 and PD-L1 in tumor-infiltrating CD8+ T cells and tumor cells. 75
Preclinical studies
Animal model investigations have shown that TLR3 agonists augment the therapeutic efficacy of anti-cancer drugs by modulation of immunosuppression in TME. It has been shown that poly I:C and poly-ICLC trigger powerful tumor-antigen specific CTL, NK, and natural killer T cell (NKT) immune responses leading to tumor regression and long-term survival of tumor-bearing mice. 60 Treatment of Lewis lung carcinoma (LLC) tumor-implant mice with poly I:C lead to tumor reversion not only by mDCs maturation for cross-priming and NK cells activation but also by promoting M1 macrophages polarization. 76 Recently Roselli et al reported that poly A:U delays tumor growth and improves survival in various mice cancer models. Poly A:U switches the immune-suppressive TME to anti-tumor immunity through changes in TME composition in favor of antigen-specific CD8+ granzyme B+ T cells (lower Treg/CD8+ cells ratio). 77 Damo M. and colleagues demonstrated that poly I:C is a promising TLR agonist for induction of DCs functional maturation required for induction of cellular immune responses against cancer. Intradermal administration of poly I:C-based Dexo (DC-derived exosomes) vaccine to B16F10 tumor-bearing mice was found to significantly inhibit the growth of B16F10 tumors and enhance prolonged survival of the mice as compared with the control groups. Further analysis showed that vaccination of tumor-bearing mice with Dexo, composed of DCs loaded with melanoma tumor antigens along with poly I:C, induces strong activation of tumor-specific CD8+ T cells and tumor infiltration of CTL, NK, and NK-T cells to the TME, as compared with the group treated with Dexo (B16) or Dexo (pIC) alone. 2 Other studies show that TLR3 agonists not only cause DCs maturation but also induce PD-L1 trafficking to the cell membrane and limit T cell activation through the prevention of contact between DCs and T cells. These findings suggest that combination TLR3 agonists with PD-L1 blocker can increase the efficacy of cancer immunotherapy. 61
Clinical trials
Clinical studies show that TLR3 targeting is a promising approach to treat human cancer (Table 2). Several studies have shown that administration of poly A:U and poly-ICLC in cancer patients significantly enhance NK cell cytotoxicity and increase the levels of IFN and NK cells. 78-80 Kyi et al investigated immune responses of treatment with intratumoral followed with intramuscular poly-ICLC in recurrent metastatic patients (head and neck squamous cell cancer and melanoma) with prior systemic therapy failed. The poly-ICLC was well tolerated in the patients with general AEs at the injection site. In the patients with clinical benefit, the frequency of CD4+, CD8+, PD-1, and PD-L1 in TME increased compared with patients with advanced disease. In addition, gene expression associated with chemokine activity, T cell stimulation, and antigen presentation in tumor and peripheral blood mononuclear cells (PBMC) were higher in the responded patients compared with the non-responded group. These findings show that poly-ICLC induces both local and systemic immune responses. 81 Salazar M.A. and colleagues reported that sequential intratumoral and intramuscular administration of poly-ICLC induces in situ vaccination in a patient with rhabdomyosarcoma, who failed CT and radiotherapy. This regime induced local tumor inflammation and systemic immune response that caused a significant reduction in a facial tumor. Their findings showed that the regression in tumor results from the activation of both local and systemic anti-cancer immunity induced by intratumoral and intramuscular injection of poly-ICLC. Based on their model, intratumoral injection of poly-ICLC causes the release of tumor antigens from the cells that died by the innate immune system, leading to the maturation of DCs and induction of Th1 immune responses. Their findings suggest that intramuscular poly-ICLC maintenance therapy is responsible for systemic anti-tumor immune response through induction of chemokine, co-stimulators, inflammasome formation, and an increase in the Teff/Treg cells ratio. 82 Several studies have shown that administration of poly-ICLC with DC-based immunotherapy is safe and rapidly develops significantly higher,quick, and numerous antibody and T cell responses than vaccination alone or without adjuvant. 63,83,84 Mehrotra S. and colleagues have demonstrated that intradermal injections of peptide-pulsed DC vaccines (human telomerase reverse transcriptase (hTERT), carcinoembryonic antigen (CEA), survivin (SRV.A2) followed immediately with intramuscular administration of poly-ICLC, was well tolerated in pancreatic cancer patients. This combination therapy triggers DC activation and enhances the expansion of tumor-infiltrating lymphocytes (TILs). Pre- and post-DC vaccination analysis of peripheral blood revealed that more than half of the DCs up-regulated CD80, CD86, and HLA-D (Human Leukocyte Antigen – DR isotype) without significant alteration between the overall B, NK, and T cells subsets. Half of the patients who completed the study, exhibited stable disease, while the others experienced the developing disease. Patients with stable disease showed a little increase in IFN-γ via specific antigen stimulation and effective formation of antigen-specific T cells. The median of progression-free survival (PFS) and overall survival (OS) was 3 and 7.7 months, respectively. 83 Melssen et al reported vaccination with melanoma antigen combined with TLR agonists (poly-ICLC or LPS) plus incomplete Freund’s adjuvant (IFA) is safe and trigger effective and robust CD8+ T cell immune responses in melanoma patients. The frequency of IFN-γ-secreting cells was significantly higher with the inclusion of IFA compared with the use of TLR agonists alone. IFA augmented CD8+ T cell responses to peptide vaccines when associated with TLR agonists (72% vs. 18%). All patients enrolled in the study, experienced high OS and disease-free survival (DFS). 63 Sabbatini et al evaluated the immunogenicity of overlapping long peptides (OLP) from cancer-testis antigen NY-ESO-1(New York esophageal squamous cell carcinoma-1) in combination with plus Montanide (as oil adjuvants for human vaccines) and poly-ICLC in patients with progressive ovarian cancer. The vaccine was generally well-tolerated and NY-ESO-1–specific CD4+T cells were detected in all studied patients. Administrating of OLP alone did not show CD8+T cell response. The level of NY-ESO-1–specific antibody and CD8+ T cells in OLP, Montanide, and poly-ICLC vaccinated patients, where more than that in the patients treated with OLP and Montanide (62% vs. 91%). These results suggest that poly-ICLC further augmented the initiation of specific immune responses against NY-ESO-1. 84 In a similar study, Pavlick et al have shown that vaccination of melanoma patients with a vaccine composed of NY-ESO-1 protein and poly-ICLC with or without Montanide was safe and well-tolerated. The vaccine regimens increased the serum level of NY-ESO-1-specific CD4+ T cell and antibody titers in all the vaccinated patients. NY-ESO- 1-specific CD8+ T cell levels in blood circulation were higher in patients vaccinated with NY-ESO-1, poly-ICLC, and Montanide as compared with what was observed in poly-ICLC alone. 85 The results of clinical studies using TLR3 agonists in several human cancers have suggested TLR3 ligands as promising agents for future anti-cancer therapy. 69
Table 2. Clinical applications of TLR3 agonists in cancer immunotherapy .
| TLR | Agonist | Combination | No. of samples | Cancer type | Response | Ref./The year |
| 3 | Poly(A:U) | - | 40 | Breast cancer | Enhancing level of 2-5A synthetase and NKC | 78 (1985) |
| 3 | Poly-ICLC | - |
28 10 13 |
ALL, ANLL, Neuroblastoma | Increasing the levels of IFN | 79 (1985) |
| 3 | Poly-ICLC | IL2 |
5 6 5 1 8 |
Colorectal, melanoma, renal cell, mycosis fungoides, other solid tumors | Enhancing lytic NK activity and increaseing the number of CD56+ cells | 80 (1992) |
| 3 | Poly-ICLC | NY-ESO-1 and Montanide | 28 | Ovarian cancer | Increasing antibody, CD4+ and CD8+ T-cells | 84 (2012) |
| 3 | Poly-ICLC | Autologous DC | 12 | Pancreatic cancer | Induction of tumor specific CD8+T cell population | 83 (2017) |
| 3 | Poly-ICLC | - |
7 1 |
Head and neck, squamous cell cancers, melanoma | Local and systemic immune responses through augmentation CD4+, CD8+ T cells, PD1, and PD-L1 levels | 81 (2018) |
| 3 | Poly-ICLC | Montanide and NY-ESO-1 | 4 | Melanoma | Induction of robust humoral and cellular immune responses specific for NY-ESO-1 | 85 (2020) |
| 3 or 4 | Poly-ICLC or LPS | IFA, melanoma peptides and Tet | 51 | Melanoma | Safe and effective vaccine adjuvants associated with IFA | 63 (2019) |
Abbreviation: ALL: acute lymphoblastic leukemia; ANLL: acute nonlymphocytic leukemia; DC: Dendritic cell; PD-L1: Programmed death-ligand-1; IFA: Incomplete Freund’s adjuvant; Tet: tetanus helper peptide; IFN: Interferon-α; NKC: NK cytotoxicity; Poly(A:U): Polyadenylic:polyuridylic (polyA:U); Poly-ICLC: poly-l-lysine and carboxymethylcellulose; LPS: Lipopolysaccharide; NY-ESO-1:New York esophageal squamous cell carcinoma 1.
In summary, TLR3 agonists act as safe adjutants for cancer immunotherapy with low AEs. These agonists exert direct and indirect anti-cancer effects through induction of apoptosis in tumor cells and stimulation of the effector immune system, respectively. TLR3 ligands remodel immune components of TME in favor of tumor growth suppression leading to tumor regression and survival enhancement. Finally, TLR3 agonists have been shown to improve the therapeutic efficacy of tumor chemotherapeutic and immunotherapeutic agents in preclinical and clinical studies.
TLR4 targeting for cancer therapy
TLR4 is the first known member of the TLR family that is expressed in various types of immune cells. TLR4 specifically recognizes bacterial toxin LPS and several other endogenous molecules (i.e DAMPs) which are produced during tissue damage. 55,86 TLR4 is highly expressed on immune cell membranes such as DCs, macrophages, and lymphocytes. TLR4 is poorly expressed in epithelial and endothelial cells. 56 TLR4 ligands have been shown to activate various intracellular pathways including NF-𝜅B, AP-1, PI3K/Akt, and the MAPK signaling pathways. 87,88 Among TLRs identified to date, TLR4 is the only TLR, which is found to activate two distinct signaling pathways (MyD88 and TRIF (TIR-domain-containing adapter-inducing IFN-β) dependent pathways) leading to the production of both pro-inflammatory cytokines (IL-1β and TNF-α) and IFN-I, respectively. Besides, TLR4 is the only known cell surface PRR that is capable of inducing the secretion of IFN-I. 88,89
Several studies show that DAMPs play an important role in activating TLR4 in TME. 90 TLR4 is found to promote sterile inflammation upon interaction with a diverse range of endogenous molecules (DAMPs) including HSPs, extracellular matrix degradation products, HMGB-1, and minimally modified low-density lipoprotein (LDL), which are often released or exposed to immune cells by the cells undergoing death or stress. These endogenous molecules are also extensively released by tumor cells dying as a result of RT and/or CT. 91 Fang et al found oxaliplatin (OXA) and/or 5-fluorouracil (5-Fu) treated-CRC cells release high levels of DAMPs (i.e HMGB1 and HSP70). DAMPs promote mouse and human DCs maturation through up-regulation of HLA-DR, CD80 and CD86 receptors, IL-1β, and TNF-α. Therefore, DAMPs released from chemically stressed tumor cells can remodel TME through DCs activation via TLR4 leading to the development of an anti-tumor T cell immune response. 92 Several studies have reported that the frequency of TILs correlates with the production of HMGB1 by tumor cells. 93 It has been suggested that the fundamental mechanism might be correlated to the activation of TLR4 by HMGB1 in TME. 90 These findings show that the combination of TLR4 agonists with chemotherapeutic agents can induce synergistic anti-cancer effects and reduce cancer relapse due to the induction of anti-cancer immune responses. 94
In contrast to the numerous studies reporting the anti-cancer effects of TLR4 stimulation, some evidence shows pro-tumor effects resulting from TLR4 activation. 92,95-97 Thus many consider TLR4 activation in tumor and immune cells as a "double-edged sword". 90,98 While the activation of the TLR4 on immune cells triggers anti-tumor immune responses, the stimulation of TLR4 expressed on tumor cells has shown different effects in various cancers. Stimulation of TLR4 has pro-apoptotic activity on gynecological cancers, but in lung and CRC cells, it induces immunosuppressive cytokines and apoptosis resistance. 99-101 Ou et al have hypothesized that administration of long-term low doses of TLR4 ligands (endogenous or microbial) induces tumor growth (prostate) whereas injection of a single high dose of TLR4 enhances antigen-specific anti-tumor immune responses. 102 In another study, Chen et al have reported that in bone marrow(BM) precursor cell cultures, prolonged stimulation of TLR4 with MPLA results in defective DCs differentiation, and accumulation of MDSCs. The expression of immunosuppressive cytokines such as IL-10 increased and a decrease in the level of Th1 inflammatory cytokines such as IL-6, IL-12, and TNF-α was observed. In a wild-type mice model study, prolonged stimulation of TLR4 by intravenous injection of MPLA increased the number of MDSCs while short stimulation by TLR4 down-regulated the percentage of MDSCs in the spleen. 103 Other pro-tumor effects of TLR4 are found to be through its role in the induction of angiogenesis which is suggested to be mediated by HMGB1. 90 Some tumor-secreted growth factors such as TGF, VEGF, PDGF, and EGF have been found to act as TLR4 ligands, which has been associated with remodeling of TME in favor of tumor progression. 104 Another study has reported that endogenous TLR4 ligands such as MMP2 can promote tumor growth through a change in the composition of immune cells in TME. 105 The pro-tumor effects reported for TLR4 stimulation have made the use of TLR4 ligands for cancer immunotherapy a challenge where the scientists try to find strategies to eliminate the possible tumor-promoting effects of TLR4 ligands. 106
TLR4 agonists
Over the last two decades, numerous TLR4 ligands have been developed and evaluated for the induction of immune response. The best-characterized ligands developed for TLR4, are LPS, MPLA, BCG, and glucopyranosyl lipid A (GLA). 88 Among the developed TLR4 ligands, MPLA has been approved by the FDA for use as an adjuvant in Cervarix® (prophylaxis of human papillomavirus (HPV)-associated cervical cancer), Fendrix® and SuperVax® (hepatitis B vaccines), and Pollinex® (an allergy vaccine). BCG is also approved for the treatment of bladder carcinoma. 55,107,108 TLR4 ligands induce DCs maturation as indicated by the overexpression of several cell membrane markers including CD40, CD86, HLA-DR, and CD83 45 promote cell-mediated immunity. 109 LPS is the first microbial component identified as a TLR agonist and its efficacy as a vaccine adjuvant has been shown in preclinical models. 63,109 LPS cannot be used in clinical applications due to toxicity. MPLA and GLA are nontoxic derivatives forms of LPS, which can activate TLR4 ligands and are used in clinical studies. 110,111 Several studies show that MPLA induces a robust Th1-polarized inflammatory response leading to cell-mediated immunity. 111 Hamdy et al showed that vaccination of mice bearing melanoma with TLR4 ligand (7-acyl lipid A) plus tyrosinase-related protein 2 (TRP2), melanoma antigen, encapsulated in poly (lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) induce anti-tumor responses can break immunotolerance to cancer-associated self-antigens. Vaccine-induced activation of TRP2-specific CD8+ T cells secreting IFN-γ at lymph nodes and spleens in the vaccinated mice. Immunization with TRP2/7-acyl lipid A-NP increased the level of pro-inflammatory Th1- related cytokines including IFN-α, TNF-γ, IL-6, and IL-2 and decreased the level of VEGF compared with the control group. Variation in the level of pro-inflammatory cytokines induced immunostimulatory TME and reduced growth of tumor compared with the control group. 112 In another study, they reported that vaccination of transgenic mouse model (DO11.10) with ovalbumin (OVA) and MPLA encapsulated in PLGA-NP lead to DC maturation/activation and induction of CD4 +and CD8 +T cells expressing activation/memory markers. 97 GLA is a synthetic non-toxic analog, which is shown to induce full maturation of DCs and Th1 differentiation. 113-115 Induction of cellular immunity is of great importance in active immunotherapy of cancer, therefore TLR4 ligands are considered to be a promising class of adjuvants for the development of cancer vaccines. 111
TLR4 ligands as an adjuvant for cancer vaccines
The potential of TLR4 ligands as an adjuvant for cancer vaccines has been shown in many preclinical studies. 27 Lee et al reported that a TLR4 agonist named Rv0652 (50S ribosomal protein from mycobacterium tuberculosis) can significantly improve the therapeutic efficacy of DC-based tumor immunotherapy. In vitro study showed that Rv0652 induces DCs activation, and pro-inflammatory cytokine secretion (TNF-α, IL-1β, and IL-6). Activated DCs polarize naïve T cells toward IFN-γ secreting Th cells and induces T cell-mediated-cytotoxicity. Immunization of E.G7 thymoma model mice with Rv0652-stimulated OVA-pulsed DCs induces an antigen-specific CD8+ T cell response resulting in suppression of tumor growth, and prolonged survival. 116 It has been shown that NPs loaded with lipooligosaccharide (LOS) and plant pathogen Xanthomonas campestris pv. campestris (Xcc)(as TLR4 agonist) induce antigen-specific CTL effector and memory responses against highly aggressive and poorly immunogenic murine melanomas. Importantly, anti-tumor-specific immune responses with co-treatment of LOS and TLR4 agonists are augmented when they are used in combination with PD-L1 blockers. 108 Albershardt et al showed that the TLR4 agonist, GLA (G100), prompted a general expansion in effector cells including T cells and NK cells, macrophages, DCs, and tissue-resident memory cells. Intratumoral administration of G100 with ACT in melanoma or glioblastoma (GBM) mice models caused complete tumor eradication compared with control animals. Anti-cancer effects of GLA and ACT combination therapy was associated with a significant escalation in the infiltration of Teff cells in TME. 117
Preclinical studies
Various animal model studies have shown that TLR4 ligands can turn immunosuppressive TME into an immune-supportive one and significantly improve the therapeutic outcome of cancer vaccines. 117,118 We have reported that intratumoral administration of MPLA in combination with a signal transducer and activator of transcription 3 (STAT3) inhibitory molecule significantly reduces immunosuppression in TME leading to an enhancement in intratumoral infiltration of T cells with activation/memory markers in the mouse melanoma model. 119 In line with our observations, it has been shown that the administration of DC vaccine with LPS leads to a significant up-regulation of NK cells and a remarkable decrease of Treg cells in TME in the ovarian cancer mice model. 120 This study showed no significant survival benefits in the mice treated with DC vaccine and LPS combination therapy compared with the mice of the DC mono-treated group. Davis et al also showed that the intratumoral injection of LPS formulated into GVAX (TEGVAX), induces an efficient anti-tumor response in B16, CT26, and SCCFVII/SF tumor-bearing mice. A significant expansion in the intratumoral infiltration of tumor-specific CTL, CD4+, and CD8+ T cellsas well as an enhanced level of DC activationin the local draining lymph nodes, were observed in the mice received TEGVAX as compared with the control (PBS) and the GVAX treated tumors. The tumor growth rate in the mice treated with TEXVAX was found to be significantly lower than that in the mice who received GVAX alone. Intratumoral TEGVAX caused tumor regression in 40-60% of CT26 bearing mice, which then rejected subsequent challenges with CT26, indicating the induction of long-lasting immunity to this type of cancer. In all three murine models, the reduced tumor growth rate correlated with enhanced survival of the mice treated with TEGVAX as compared with the GVAX group. 121 In another study, Rommelfanger et al have shown the combination of LPS with intratumoral VSV (oncolytic vesicular stomatitis virus) significantly improves the anti-tumor efficacy of VSV through activation of different components of innate immunity including increasing serum levels of TNF-α and IL-6. 118 Interestingly, it has been also shown that intravenous administration of MPLA, CpG, and LPS can potentiate the anti-tumor activity of ACT in melanoma mouse models. 122
Clinical trials
The potential of TLR4 agonists for cancer immunotherapy has been shown in several clinical studies (Table 3). Isambert et al showed intravenous administration of OM-174, an analog of lipid A, was well tolerated with general reaction and without limiting toxicity in patients with different metastatic solid tumors. OM-174 increased the frequency and activity of NK cells in patients receiving OM-174 at high and repeated doses. Increased serum levels of IL-8 and IL-10 and the reduced level of TNF-α and IL-6 over time after each injection of OM-174, suggest the induction drug tolerance in these patients. 123 In another clinical trial, Neidhart et al reported immunization of metastatic CRC (mCRC) patients with subcutaneous injections of the recombinant baculovirus-derived KSA (KSA; Epithelial cell adhesion molecule (EpCAM)) vaccine formulated with MPLA in the liposomal emulsion. This combination was shown to be safe and was well-tolerated without significant local reactions. This regime was found to trigger significant KSA-specific humoral and cellular immune responses associated with lymphoproliferation and IFN-γ expression. Co-administration of recombinant GM-CSF (rGM-CSF) with recombinant baculovirus-derived KSA vaccine augmented generating KSA-specific Th1 associated cellular immune responses. In this trial, no patient manifested a clinical response perhaps due to insufficient cellular immune response. 124 Intratumoral injection of G100 in patients with Merkel cell carcinoma (MCC) with/without RT was durable and increased expression of pro-inflammatory genes and activated CD8+ and CD4+ T cells in the TME which lead to local tumor regression. 125 Mahipal et al investigated immune response in patients with solid tumors expressing NY-ESO-1 who were treated with G305, a recombinant NY-ESO-1 protein vaccine, mixed with GLA in a stable emulsion (SE). This regime was safe and tolerable at all doses of GLA-SE (2–10 μg). Overall, vaccination increased antibody response to NY-ESO-1 and CD4+/CD8+ T cell immune responses in the systemic circulation of patients. 126
Table 3. Clinical applications of TLR4 agonists in cancer immunotherapy .
| TLR | Agonist | Combination | No. of samples | Cancer type | Response | Ref./The year |
| 4 | MPLA | GM-CSF | 11 | Colorectal cancers | Specific antibody and cellular immune responses | 124 (2004) |
| 4 | OM-174 | - | 17 | Metastatic solid tumor | Increasing NK cells number and activity, IL-8, IL-10, TNF-α, and IL-6 concentration | 123 (2013) |
| 4 | G100 | Radiotherapy | 10 | Merkel cell carcinoma | Increasing expression of pro-inflammatory genes and activated CD8+ and CD4+ T cells in the TME | 125 (2019) |
| 4 | GLA | Recombinant NY-ESO-1 vaccine | 12 | Solid tumors expressing NY-ESO-1 | Increasing antibody response to NY-ESO-1 and CD4+/CD8+ T-cell responses in systemic circulation | 126 (2019) |
Abbreviation: GM-CSF: granulocyte-macrophage colony-stimulating factor; TNF-α: Tumor necrosis factor; MPLA: Monophosphoryl lipid A; GLA: glucopyranosyl lipid-A; NY-ESO-1: New York esophageal squamous cell carcinoma 1.
Briefly, TLR4 agonists induce anti-tumor effects through stimulation of immune effector cells, thereby remodeling TME for better activation and function of anti-cancer immune responses. TLR4 agonists have been reported to enhance the therapeutic efficacy of other cancer treatment modalities including chemotherapy, radiotherapy, and some immunomodulatory agents (i.e anti-STAT3 drugs). While many studies have shown the anti-cancer effects induced by activation TLR4 expressed on immune cells, findings on stimulation of TLR4 agonists on cancer cells are conflicting showing both pro-tumorogenic and antitumor activity. The direction of the response may be related to the type of cancer and/or dosing of TLR4 agonist.
TLR7 targeting for cancer therapy
TLR7 is expressed on several types of immune cells and it gets activated upon binding to microbial nucleoside components. TLR7 is an endosomal receptor and recognizes purine-rich (GU-rich or poly-U sequences) ssRNA species and synthetic small molecules. 127,128 Predominantly, TLR7 is expressed in plasmacytoid DCs (pDCs) and B cells. The expression of TLR7 on T cells and macrophages is found to be context-dependent. 26,41 TLR7 is similar to another TLR family member, TLR8, in terms of phylogenetic and the ligands it binds to. 128 Recognition of a ligand by TLR7/8 is followed by the activation of MAPKs and NF-ĸB signaling pathways resulting in the up-regulation of pro-inflammatory cytokines, chemokines, IFN-α, and co-stimulatory molecules. 129 Activation of TLR7/8 has been shown to induce Th1 immune responses, suppress Th2 responses as well as regulatory mechanisms. 45,127,128 TLR7-activated cell adhesion molecules such as CD11a/CD18 integrin causes pDC cluster formation, which naturally happens in viral infections and is considered to be the major source of IFN-α. 130,131 Recent preclinical research has shown that TLR7 stimulation in tumor cells enhances CD8+ T cell cytotoxic responses and suppresses PD-1 expression on T cells that improved anti-tumor immunity. 132
Several studies have shown that TLR7 ligands are useful for cancer therapy. TLR7 agonists activate APCs and prime Th1 responses leading to the indication of cell-mediated immunity, which is the appropriate type of immune response against cancer. Besides, TLR7 agonists have been shown to activate NK cells and inhibit the function of Treg cells, which play a main role in the inhibition of immune responses against cancer. 133,134 In addition, some TLR7 ligands have been found to induce apoptosis in cancer cells and sensitize them to the killing effects of CTLs and CT. 134-136 Localized immunotherapy with TLR7/8 agonists has been found to polarize immune responses toward Th1 type immune responses, which inhibit tumor growth in mouse model tumors. 17
TLR7 agonists
Over the last two decades, several types of synthetic and natural nucleoside ligands have been developed for targeting TLR7/8 in cancer immunotherapy. The most-studied class of TLR7 agonists is imidazoquinolines derivatives including IMQ (R-837), resiquimod (R-848), and gardiqiomod. IMQ (also called Aldara) is a synthetic nucleotide-like TLR7 ligand, which can easily penetrate the epidermal barrier due to its small size and its hydrophobic nature. The topical formulation of IMQ (5% cream) has been accepted for the therapy of dermatological cancers such as basal cell carcinoma (BCC) and viral lesions such as HPV. IMQ selectively binds to TLR7 and activates downstream signaling pathways including NF-κB and MAPK. Moreover, IMQ has been shown to bind to TLR8 with less selectivity as compared with TLR7. Due to the similar structure of IMQ to adenosine nucleoside, it can bind to adenosine receptors (AR). 128 In the absence of TLR7 and TLR8 on immune cells, TLR7/8 agonists can interact with AR and stimulate the secretion of pro-inflammatory cytokines and suppress an important feedback mechanism of inflammation. 137,138 The other two TLR7 ligands, resiquimod, and gardiquimod have been shown to induce more potent immune responses as compared with IMQ. 128 Resiquimod shows anti-cancer effects and potently induce Th1 type inflammatory responses. It has been shown that resiquimod induces DCs maturation and secretion of Th1 response-related cytokines including IL-6, IFN-γ, and TNF-α, leading to initiation of antigen-specific CD8+ T cell responses. 130,139 A preclinical study has also shown resiquimod inhibits angiogenesis and induces apoptosis in 4T1 bearing mice model. Interestingly, dying cancer cells treated with resiquimod, release a high level of HMGB-1 which in turn can act as a danger signal and activate anti-cancer immune cells through binding to TLR4. 140 Gardiquimod, another synthetic ligand for TLR7, is known to be a potent anti-cancer agent. Several in vitro and in vivo studies have proven the anti-tumor effects of gardiquimod in cancer therapy. Gardiquimod induces the expression of co-stimulatory molecules and IL-12 by DCs, leading to the activation of Th1 cells and cellular immune responses against cancer. Gardiquimod has been also shown to trigger apoptosis in cancer cells and suppress cancer metastasis. 130,134
Remodeling of TME
TLR7 agonists remodel TME through activation and recruitment of immune cells. Multiple lines of evidence prove that the anti-cancer effects of TLR7 agonists are associated with their ability to alter immunosuppression in TME and make it more supportive for the activation and function of anti-cancer immune cells. The topical administration of IMQ in the melanoma mouse model significantly decreases the mRNA expression of Treg cells-related chemokines and increases the expression of cytotoxic molecules (granzyme B, perforin) in the tumor. IMQ was also found to decrease Tregs and increase CD8+ cells in the TME. 141 It has shown that the intratumoral application of resiquimod with anti-CD200R in colon carcinoma mouse model inhibits tumor growth. Resiquimod reprograms the tumor-promoting microenvironment to a tumor-suppressive one by shifting the phenotype of tumor-infiltrating macrophages from predominantly M2 to M1. 142 In another study, Chen et al developed personalized immunotherapy through in situ immunization by utilizing the antigens-derived tumor, which was co-encapsulated with resiquimod in NPs. Immunotherapy of mice bearing CT26 tumor using resiquimod-tumor lysate NPs resulted in the induction of DCs maturation, activation of a robust T cell activation, CD3+ T cell infiltration, and granzyme B secretion in TME. Analysis of excised primary tumors showed that combination therapy exhibited a significant drop in the relative density of MDSCs, but not M2 macrophages, in the TME of the tumor-bearing mice as compared with what was observed in untreated or monotherapies groups. In this study, alteration in TME was correlated with significant suppression of tumor growth and induction of apoptosis. This regime was also found to induce a long-lasting immunologic memory and prevent cancer recurrence and metastasis. Interestingly, tumor re-challenging led to tumor rejection in all of the survived mice from the initial challenge. 143 Another study has reported that the intratumoral administration of TLR7 agonists (SZU101) not only elicit a systemic anti-tumor response but it modulates the TME via increasing CD4+ and CD8+ and decreasing Treg cells in murine breast tumor model. 144
Preclinical studies
The anti-cancer effect of IMQ in preclinical studies has been shown in several types of malignancies such as prostate cancer 145 and bladder cancer mice models. A previous study has shown that IMQ induces reactive oxygen species (ROS) production, which stimulates the ATM/ATR (ataxia-telangiectasia mutated/ATM- and Rad3-Related) pathway, leading to p53-dependent apoptosis in humane BCC cell line. Inhibition of ATM/ATR signaling or silencing/mutation of p53 significantly reduces the IMQ-induced apoptosis but it enhances autophagy. 146 Suzuki et al have reported that application of topical IMQ in the skin of healthy mice induces maturation and migration of epidermal Langerhans cells to the local lymph nodes leading to the activation of specific T cell response and production of perforin and granzyme B. 147 Han et al have shown that IMQ reduces cell growth and prompts cell cycle arrest at the G2/M phase and direct apoptosis in a mouse model ofprostate cancer. In addition, preclinical research has reported an anti-angiogenic effect for IMQ via four mechanisms inducing; i. production of anti-angiogenic cytokines such as IFNs, IL- 10, IL-12, and IL18, ii. down-regulation of pro-angiogenic molecules including fibroblast growth factor-β (FGF-β) and metalloproteinase-9 (MMP-9), iii. overexpression of endogenous angiogenesis blockers (IFNs, IP10), iv. and the induction of endothelial apoptosis. 148,149 In line with these findings, Majewski et al have shown that the application of topical IMQ on the murine skin results in the reduction of tumor cell-induced angiogenesis. The anti-angiogenic effect of IMQ was found to be mediated by enhancing IL-18 and IFN-γ and downregulation of IL-10 expression. 150 Another preclinical study has shown that the application of resiquimod in mice bearing mammary carcinoma reduces the immunosuppressive activity of MDSCs on T cells, enhances the proliferation of T cells, and induces MDSCs differentiation to macrophages and DCs. 151 Systemic application of resiquimod in the colon cancer model mice has been found not only to reduce the frequency of MDSC in TME but also it stimulates MDSCs and make them functional APCs capable of inducing T cells activation compared with control PBS-treated mice. 152 Michaelis K-A and colleagues have shown that intraperitoneal administration of resiquimod in mice bearing pancreatic ductal adenocarcinoma (PDAC), can reduce Treg population in TME and increase intratumoral infiltration, cytotoxicity, and activation of CD8+ T cell. These effects cause the suppression of tumor growth and a significant improvement in survival of treated mice compared with vehicle treatment. 127 In another study, Rodell et al have demonstrated that resiquimod loaded in NP, can remodel TME via promotion of Th1 responses which in turn resulted in the induction of long-term anti-tumor memory in MC38 and B16.F10 bearing mice models. 153
TLR7 combination therapy
Several preclinical studies have reported that TLR7 ligands can enhance the therapeutic efficacy of cancer conventional treatments. The superior anti-cancer activity of combination therapy with TLR7 agonists and RT and/or CT has been reported in various types of animal cancer models such as colorectal, pancreatic cancer, 154 and lymphoma. 155 It has been shown that the systemic application of the novel TLR7 agonist, DSR-6434, in combination with RT, induces expression of IFN-1, activation of T- and B-cells, NK cells, and NK-T cells. DSR-6434 and RT combination therapy was found to enhance the therapeutic efficacy of IR and improve survival in mice bearing CT26 and KHT (murine sarcoma cell line) tumors. 156 Cho et al have demonstrated that combinational therapy with IMQ and RT induces anti-cancer and anti-metastatic effects in mouse melanoma tumors. The pretreatment of TLR7-expressed melanoma tumor with IMQ before RT has been found to increase autophagic cell death leading to a significant inhibition in tumor growth and the number of the metastatic area as compared with control groups. IMQ and IR combination therapy increased the recruitment of CD4+ and CD8+ T cells and decreased the number of Treg cells and MDSCs in TME compared with the untreated group. These results suggest that IMQ and IR combinational treatment induces systemic anti-cancer immune responses, which can lead to a reduction in the metastatic activity of circulating tumor cells. Importantly, the median survival of treated mice with IMQ combined with IR was significantly higher than that of untreated mice. 157 Karashima et al reported combination treatment with oral sorafenib (a multi-kinase inhibitor) and transcutaneous IMQ was tolerated well in mice with orthotopic kidney cancer cells (RENCA) model. IMQ and sorafenib combination therapy induced CD8+ and CD4+ cells and significantly reduced the growth of RENCA cell carcinoma in the kidney as compared with the tumors treated with the control vehicle. 158 Gao N. and colleagues have shown that application of a TLR7 agonist (SZU-101) along with tyrosine kinase inhibitors (TKIs; lapatinib), enhances tumor clearance without affecting the TLR7-NF-κB signaling in breast tumor-bearing mice models. 159 Animal studies have also shown that TLR agonists can reduce the AEs of CT due to the need for a reduced dose of chemotherapeutic agents in combinational therapy. 130
Previous studies have reported that the anti-tumor efficacy of TLR7/8 agonists is enhanced when they are used in combination with CPIs and co-stimulatory agonists. 17 Recently, Nishii et al reported that systemic monotherapy with low-dose resiquimod in two PD-L1 blockade-resistant tumor mouse models (Colon 26 and SCCVII), did not result in beneficial anti-cancer effects. In contrast, the combination of resiquimod with PD-L1 blockade was associated with a significant diminish in tumor size, rapid activation of DCs, attenuation of Treg cells, and an increased ratio of CD8+T cells/Treg cells in TME in both tumor models. 160 Systemic injection of resiquimod in combination with obinutuzumab (anti-CD20 mAb) has been reported to induce lymphoma depletion and resistance to disease recurrence in murine lymphoma models. 161 The therapeutic effects of co-treatment with resiquimod and obinutuzumab were found to be through activation of both NK cells and CD4+ T cells without effect on CD8+ T cells. The well-known anti-cancer effects of IMQ make IMQ a promising agent for cancer immunotherapy; however, the clinical application of IMQ requires more studies for developing an effective formulation for maximal immunogenicity. 46,127,128,162
Some studies have reported that IMQ may induce the production of some immunosuppressive factors including IL-10, hypoxia-inducible factor-1α (HIF-1α), indoleamine 2, 3-dioxygenase (IDO), and nitric oxide synthase (NOS) in TME. Therefore, combination therapy with IMQ and anti-cancer agents blocking the function of IDO, IL-10, and NOS or HIF-1α inhibitors, have been used to enhance the therapeutic efficacy of IMQ. 163-165 Ito et al have shown that intratumoral injection of IMQ associated with the oral L-NAME (an iNOS inhibitor) augments the anti-cancer effects of IMQ compared with IMQ monotherapy in EG7 and CT26 tumor-bearing mice. Combination therapy with IMQ and L-NAME significantly impaired tumor growth in both models. 164 In another study, Huang et al reported that targeting HIF-1α protein by 17-AAG, augments the anti-tumor effects of IMQ in B16F10 tumor-bearing mice. The anti-cancer effects of IMQ and 17-AAG combinational therapy were correlated with a significant increase in the apoptotic cell content. 166 Ghosh et al have demonstrated that in vitro stimulation of human PBMC with the combination of TLR7/8 and TLR4 agonists (3M-003 and LPS) or TLR7/8 and TCR stimulatory agents (the combination of phytohemagglutinin (PHA), anti-CD3, and anti-CD28), up-regulates of IFN-γ and IFN-α compared with the cells treated with individual agents. These combination therapies showed no effect on the levels of the pro-inflammatory cytokines including TNFα, IL-6, and IL-8 as compared with monotherapy groups. 167
Clinical trials
The anti-cancer effects of TLR7 ligands have been evaluated in several clinical trials (Table 4). In 1993, before the exploration of TLRs on immune cells, Witt et al reported that oral administration of IMQ (100-500 mg capsules) in some cancer patients results in biological and immunological effects. Although clinical anti-tumor responses were not observed, the serum level of IFN-α, β2-microglobulin, and neopterin increased without changes in IL-1β or TNF-α levels. 168 Urosevic et al investigated the mechanism by which topical IMQ induces anti-cancer effects in basal cell carcinoma (BCC) patients. They demonstrated that IMQ increases apoptosis via downregulation of Bcl-2 (B-cell lymphoma 2) expression. Topical IMQ was also found to enhance intratumoral recruitment of lymphocytes CD4+ and CD8+ secreting IFN-γ and macrophages. The expression of Bcl-2 and FasL/Fas, ICAM-1 (Intercellular adhesion molecule 1) on infiltrated cells was significantly reduced in IMQ treated samples as compared with pretreatment samples. 169 In line with this study, Berman et al have evaluated the FasR expression level on BCC after very short-term application of IMQ 5% cream or vehicle. While excised samples on IMQ-treated patients expressed FasR (3⁄4 patients) and T-lymphocytes, none of the vehicle-treated tumors expressed FasR and T-lymphocytes (0/5 patients). 170 This data suggest that the efficiency of IMQ 5% in the improvement of BCC may be due to IMQ-induced FasR-mediated apoptosis. In another clinical trial, Vidal et al compared the level of apoptosis-related genes such as Bcl-2, Ki67, and p53 in IMQ treated-BCC lesions and the control treated with Aldara-(3M Pharmaceuticals) excipient. The BCC tumors treated with IMQ showed a decrease in the Bcl-2 expression and an increase in the apoptotic profile compared with BCC lesions treated with the excipient. The expression of Ki67 and p53 remained unchanged in all of the groups. 171 Another research showed that topical application of IMQ in BCC patients induces tumor rejection. IMQ induces inflammatory TME composed of CD8+ and CD4+ T cells, granzyme B and perforin expressing mDCs, and pDCs releasing TNF-related apoptosis-inducing ligand (TRAIL). Therefore IMQ-activated DCs are not only involved in the initiation phase of the immune response but also act as effector cells and directly induce apoptosis in tumor cells. 172 Giorgi et al evaluated the immunophenotype of the TME in skin biopsies from BCC patients, pre- and post- IMQ treatment. IMQ treatment resulted in a significant increase in intratumoral infiltration of the mononuclear inflammatory cells including mostly CD4+ T cells and to a lesser extent the CTLs, B cells, Langerhans cells, DCs, neutrophils, NK cells, eosinophils, and melanophages. After IMQ therapy, all excisional specimens showed a relative increase in CTL/suppressor lymphocytes as compared with pretreatment biopsies. IMQ therapy also reduced the expression of the anti-apoptotic marker, Bcl-2 and it enhanced the expression of the pro-apoptotic marker Bax, Fas/FasL. 173 Huang et al have shown that the excised samples from squamous cell carcinoma (SCC) patients, who were treated with topical IMQ, contained a higher level of Teff cells. Cancer cell apoptosis and histologic evidence of tumor regression were found in the samples from IMQ-treated patients as compared with the untreated group. IMQ also increased the expression of IFN-γ, granzyme, and perforin by Teff cells and reduced the level of IL-10 and TGF-β as compared with the control group. 174 Another study has shown that the application of topical IMQ (5% cream) on skin metastases in breast cancer patients, stimulates local anti-tumor immune responses leading to regression of lesions. Topical IMQ also stimulates a significant expansion in the intratumoral infiltration of CD8+ and CD4+ T cells, which locally produce inflammatory cytokines such as IFN-γ. 175 Recently, Nguyen A. et al have demonstrated that topical IMQ cream is a treatment option for cutaneous metastasis of breast cancer. They have reported that treatment of breast cancer metastatic skin lesions of a woman with topical IMQ 5% cream twice per day for 5 days a week, lead to a reduction in the size, thickness, and pigmentation of the lesions. The therapeutic efficacy of topical IMQ is believed to be due to its ability to induce a Th1 inflammatory response in TME. 176
Table 4. Clinical applications of TLR7 agonists in cancer immunotherapy .
| TLR | Agonist | Combination | No. of samples | Cancer type | Response | Ref./The year |
|---|---|---|---|---|---|---|
| 7 | IMQ | - | 14 | Lung, colon, ovarian, melanoma, carcinoid, renal cell, breast, mesothelioma, chondrosarcoma, rectal, pancreatic | Induction of IFN-α, β-microglobulin and neopterin | 168 (1993) |
| 7 | IMQ | Flt3 ligand and tumor antigens | 8 | Metastatic melanoma | Developing both cutaneous and peptide-specific CD8+ T-cell responses | 182 (2004) |
| 7 | IMQ | - | 7 | BCC | Increasing mDCs and pDCs | 172 (2007) |
| 7 | IMQ | Recombinant NY-ESO-1 | 9 | Melanoma | Stimulation of humoral and cellular responses and IFNγ-secreting NY-ESO-1-specific CD4+ T cells | 46 (2008) |
| 7 | IMQ | Therapeutic HPV vaccination | 19 |
VIN associated with HPV16 infection |
Altering the balance of useful CD4+- and CD8+-directed effector cells | 185 (2010) |
| 7 | IMQ | - | 10 | Breast cancer skin metastases | Tumor regression, lymphocytic infiltrate and production of cytokines | 175 (2012) |
| 7 | IMQ | - | 8 | Breast cancer skin metastases | Upregulation of gene-related with Th1/cytotoxic immune response, migration of Th-1 and CTLs into the tumor, | 178 (2019) |
| 7 | IMQ | - | 11 | BCC | Increasing infiltration of the mononuclear inflammatory cell in the TME, decreasing the Bcl-2 | 173 (2009) |
| 7 | IMQ | - | 6 | BCC | Increasing apoptosis through downregulation of Bcl-2 expression | 169 (2003) |
| 7 | IMQ | - | 1 | Melanoma skin metastases | Upregulation of IFN-α, TIMP-1 and KiSS-1, MMP-1, and THBS1 downregulation of MMP-9, FGF2 | 177 (2004) |
| 7 | IMQ | - | 30 | BCC | Decreasing Bcl-2 expression and an increase in the apoptotic index | 171 (2004) |
| 7 | IMQ | Multipeptide cancer vaccine | 4 | Melanoma | Activation of T CD8+ and NK cells and infiltration in the TME | 184 (2016) |
| 7 | IMQ | - | 10 | BCC | Expression of FasR and increasing of T-lymphocytes | 170 (2003) |
| 7 |
Resiquimod (Topical) |
- | 12 | CTCL | Increasing T-cell clones, T-cell effector, NK cells, and circulating DCs functions | 179 (2015) |
| 7 |
Resiquimod (Topical) |
NY-ESO-1 and Montanide | 3 | Melanoma | Induction both specific humoral and CD4+ T-cell responses | 139 (2015) |
| 7 | 852A | - | 25 | Advanced cancer | Production of IFN-a, cytokine increasing, immunologic activity, and clinical symptoms | 180 (2007) |
| 7 | 852A | - | 13 | CT-refractory metastatic melanoma | Increasing of IP-10 and IFN-α and Th-1 cytokines (IFN-γ) | 186 (2008) |
| 7 | 852A | - | 15 | CLL | Increasing of TNF-α, Il-6, INF-α, and IgM | 136 (2010) |
| 7 or 3 | IMQ or poly-ICLC | Autologous tumor lysate and DC vaccine | 23 | Glioblastoma | Activation of DC, trafficking to lymph nodes, and the priming of anti-tumor antigen-specific T lymphocytes | 183 (2011) |
Abbreviations: CTCL: Cutaneous T-cell lymphoma; CLL: Chronic lymphocytic leukemia; BCC: Basal cell carcinoma; VIN: Vulval intraepithelial neoplasia; HPV: Human papillomavirus; mDC: myeloid dendritic cells; pDC: plasmacytoid dendritic cells; TLRs: Toll-like receptors; DC: Dendritic cell; Th-1: T helper cells; CTL: Cytotoxic T lymphocytes; IFN: Interferon-α; TNF-α: Tumor necrosis factor; (polyA:U); Poly-ICLC: poly-l-lysine and carboxymethylcellulose; IMQ: Imiqiumod; NY-ESO-1: New York esophageal squamous cell carcinoma 1; Flt3: fms like tyrosine kinase 3; Bcl-2: B-cell lymphoma 2; TIMP-1: Tissue inhibitor matrix metalloproteinase 1; KiSS-1: Kisspeptins; MMP: Matrix metallopeptidases; THBS1: Thrombospondin-1; FGF2: fibroblast growth factor; IP-10: Interferon gamma-induced protein 10.
Several clinical studies have reported the effects of IMQ on cancer metastasis and angiogenesis. Hesling et al have reported the expression profiles of molecules related to metastasis and angiogenesis in the biopsied lesion of a woman with skin melanoma metastases who had received topical IMQ once daily for 8 weeks. Analysis of the skin lesion before and after treatment showed that IMQ upregulated expression of IFN-α, MMP inhibitors (i.e TIMP-1 and KiSS-1), MMP-1, and THBS1 (angiogenesis inhibitor). In contrast, IMQ downregulated MMP-9 and FGF2. VEGF expression remained at a basal level. 177 Recently, Rozenblit et al have evaluated transcriptomic profiles in baseline and post-treatment tumor samples from skin metastases of breast cancer patients treated with topical IMQ. They have found that the majority of the transcripts related to chemo-attraction, antigen presentation, and cytotoxic mechanisms were upregulated in responding patients. Further analysis of the responding patients showed that IMQ triggers a strong Th1 type immune response, which is associated with upregulation of genes related to Th-1 chemokines and their receptors, migration of Th-1 and CTLs into the tumor, and markers of lymphocyte activation. In addition, they observed activation of innate immune cells including NK cells, neutrophils, functional mature DCs in tumor samples from the responding patients. In contrast, samples from the partial responder (PR) patients, displayed a permissive environment characterized by the overexpression of genes related to leukocyte adhesion and migration, antigen presentation, chemokine receptor, and T cell cytotoxic markers. 178 Altogether, these findings show that the application of topical IMQ can prompt anti-tumor immune responses through the recruitment and activation of DCs and T cells in TME.
In addition to IMQ, the therapeutic efficacy of some other TLR7 agonists has been also evaluated in clinical trials. Rook et al have reported that the application of the topical resiquimod gel (0.03% and 0.06%) in patients with cutaneous T cell lymphoma (CTCL), triggers not only local but also systemic immune responses leading to the regression of both treated and untreated lesions. Complete eradication of malignant T cells and depletion of clonal malignant T cells were found in 30% and 90% of patients treated with resiquimod, respectively. Topical resiquimod also enhanced the recruitment and expansion of effector T cell clones and NK cell functions in skin lesions. 179 Although the topical formulation of TLR7 agonist has shown promising anti-tumor immune response in skin tumors, systemic administration is required to treat tumors of internal organs. Intravenous administration of a TLR7 agonist (i.e 852A) was well tolerated in patients with refractory solid organ tumors, at doses up to 1.2 mg/m2. Induction of anti-tumor immune responses included an increase in IFN-α and the clinical symptoms of tumor regression were found in all the patients who received a dose of ≥0.6 mg/m2. 180 Intravenous injections of 852A (0.3-0.6 mg/m2) in patients with relapsed chronic lymphocytic leukemia (CLL) were also reported to be safe but showed weak clinical efficacy. In this study, an increase in the serum levels of TNF-α, IL-6, IFN-α, and IgM was not correlated with the dose of 852A. 136
Despite the proven anti-cancer effects of TLR7 agonists, their clinical use has been limited due to toxicity, AEs, and TLR tolerance that influence the efficacy of TLR7 in cancer immunotherapy. 17,180 TLR7 tolerance due to repetitive receptor stimulation not only blunts cytokines and IFN-I secretion in DCs but also enhances the secretion of the suppressive cytokine IL-10 in tolerogenic DCs. To avoid TLR7 tolerance and positive systemic immune response, careful evaluation of the induced cytokines pattern, dose schedule, and the timing of TLR stimulation is essential for effective immunotherapy. 133,181 TLR7 combination therapy may enhance the efficacy of treatment due to tolerance prevention by both enhancing the secretion of pro-inflammatory cytokines and preventing the high secretion of IL-10. 133
The administration of cancer vaccines in combination with the topical form of TLR7 agonists has shown promising results in clinical trials. Intradermal application of NY-ESO-1 antigen into pre- and post-treated topical IMQ lesions was well tolerated in malignant melanoma patients. The IMQ/NY-ESO-1 combination treatment resulted in the activation and infiltration of APCs at the vaccination site leading to the activation of both humoral and cell-mediated immunity. Intratumoral infiltration of IFN-γ-secreting NY-ESO-1-specific CD4+ T cells, monocytes, macrophages, mDCs, and NK cells were higher than what was observed in the untreated control skin of the same patients. 46 Shackleton et al have evaluated the immune responses of triple combination therapy including topical IMQ, subcutaneously Flt3 ligand (FL), and peptides vaccination (influenza (Flu), Melan-A (Mel), tyrosinase (Tyr), and NY-ESO-1) in melanoma patients. The results showed that IMQ stimulates maturation of FL-increased immature DCs leading to an increase in TAs- specific CD8+ T immune responses in vaccine sites. 182 Topical application of resiquimod combined with subcutaneous administration of NY-ESO-1 plus Montanide in melanoma patients stimulates NY-ESO-1-specific antibodies and CD4+ T cell responses without evidence of CD8+ T cell responses. 139 Another clinical study has reported that combination therapy with TLR3/TLR7 agonists (poly-ICLC, or 5% IMQ cream) and autologous DC vaccination are well tolerated in patients with GBM. Combinational therapy with DC vaccines and TLR3 or TLR7 was found to enhance tumor-infiltrating lymphocytes (TIL) in patients with a subtype of GBM characterized with mesenchymal gene expression signature. 183 Mauldin et al have reported that treatment of melanoma metastases with topical IMQ plus subcutaneously and intradermally injection of a multi-peptide cancer vaccine (12 melanoma peptides and a tetanus toxoid-derived helper peptide), were well tolerated with mild AEs and without dose-limiting toxicities. IMQ induced CD8+ T and NK cells activation and infiltration in the TME and favorable gene signatures in the patients with T cell responses. Evaluation of gene expression profiles in pre- and post-IMQ-treated tumor samples showed up-regulation of the genes related to adaptive immunity (TCR, IFNγ, and IL-2 signaling pathways), innate immunity (TLR signaling and complement activation), and T cell/NK cell recruitment (IL2RG, IL2RB, CD3G, CD2, SLAMF6, and CD38). Moreover, IMQ up-regulates the gene-related to tryptophan metabolism, fatty acid omega oxidation, apoptotic execution phase, IL-1, and TLR signaling. 184 Topical IMQ followed by therapeutic HPV vaccination in patients with valval intraepithelial neoplasia (VIN), was well tolerated and induced systemic lymphoproliferation and local inflammatory environment. IMQ treatment significantly reduced the size of lesions, cleared HPV infection, and improved symptoms in patients. Administration of IMQ prior to HPV vaccines enhanced intratumoral infiltration of CD4+ and CD8+ T cells and reduced the population of Treg cells in TME. Interestingly, the higher level of pre-existing and post-treatment Treg cells was associated with the non-responsiveness of the lesion to treatment. 185 Dummer et al have reported the intravenous infusion of 852A (0.6 to 0.9 mg/m2) in CT-refractory metastatic melanoma patients was well tolerated as the majority of patients completed the treatment period (13 out of 21) and just 2 patients have shown AEs. Co-treatment of patients with 852A with RT induced systemic immune responses through increasing dose-dependent expression of serum cytokine (i.e IFN-I) which was associated with prolonged disease stability. 186 Despite promising results of TLR7 agonists in clinical trials, more research is needed to enhance their efficacy, reduce toxicity, optimize the dose and administration route.
Altogether, the previous studies show that TLR7 agonists remodel the TME in favor of anti-cancer immune response leading to suppression of tumor progression. Reversal of immunosuppression by TLR7 agonists is related to better tumor infiltration and function of immune cells as well as a significant decrease in the level of immunosuppressive factors in the TME. TLR7 agonists have been also shown to directly induce tumor cell apoptosis by overexpression of pro-apoptotic proteins and down-regulation of anti-apoptotic factors in tumor cells. TLR7 ligands have been also reported to enhance the therapeutic efficacy of CT or RT in preclinical and clinical studies. Limited studies are showing that TLR7 agonists increase some cellular metabolites in cancer cells and exert pro-tumor effects, which can be neutralized by combination therapy. The therapeutic efficacy of TLR7 agonists can be optimized by the evolution of the effects of the administration route, dose, and timing of administration when is combined with other anti-cancer drugs.
TLR9 targeting for cancer therapy
TLR9 is an endosomal receptor, which is mainly expressed in immune cells. TLR9 interacts with nucleoside-like ligands leading to the activation of immune responses. TLR9 downstream signaling pathway is activated upon binding to exogenous (bacterial DNA and viral DNA/RNA) or endogenous (self-derived DNA; such as mitochondrial DNA (mtDNA)) danger signals such as single-strand unmethylated CpG dinucleotide motifs. It is worth noting that TLR9 signaling gets suppressed upon binding to methylated DNA in the vertebrate. 187,188 TLR9 exerts the narrowest expression pattern in humans. Thus far, pDCs and B cells are the only immune cells that constitutively express TLR9. Other immune cells such as neutrophils, monocytes, monocyte-derived cells, and CD4+ T cells only express TLR9 during activation. Stimulation of TLR9 triggers activation of MAPK and NF-ĸB signaling cascade in pDCs, which leads to the overexpression of pro-inflammatory cytokines (IFN-I) and co-stimulatory molecules such as CD80 (B7.1) and CD86 (B7.2). pDCs activation through TLR9 results in the induction of Th1 cells leading to CTL responses. 187
TLR9 stimulation has various effects on the function of immune cells. Several studies have demonstrated that TLR9 ligands directly induce the maturation and activation of pDCs. On the other hand, TLR9 agonists induce B cell differentiation into plasma cells and promote antibody-dependent cellular cytotoxicity (ADCC). 187,189 In addition, direct ligation of TLR9 agonists inhibits the suppressive function of Treg cells. 190 Several studies have indicated that DNA released by dying cancer cells after CT, acts as a TLR9 agonist and modulates the TME through activation of DCs and induction of tumor-specific CTLs. 191 TLR9 signaling can augment tumor regression either directly through upregulation of IFN-γ, or indirectly, via killing of tumor cells by activated-NK cells. 25
TLR9 agonists
Several synthetic CpG oligodeoxynucleotide (CpG ODN) have been developed as TLR9 agonists for the treatment of cancer. 187 CpG ODNs motifs are potent inducers of the innate and adaptive immune response. CpG ODNs motifs are considered promising adjuvants for vaccines in particular for anti-cancer vaccines because CpG ODNs activate cell-mediated immune responses. 192,193 CpG ODNs motifs act as a danger signal and activate DCs leading to activation of Th1 type inflammatory responses. Moreover, CpG ODNs induce NK cell activity. CpG ODNs have been also reported to reduce the number of Treg cells in the draining lymph node. 33 According to immunologic effects, CpG ODNs are divided into three different subtypes including CpG-A, CpG-B, and CpG-C. CpG-A motifs are potent stimulators of NK cells. CpG-B motifs induce antigen presentation by pDCs and production of Th1 cytokines. CpG-B motifs also promote the differentiation of anti-tumor Th17 cells, which have been shown to induce intratumoral CTL recruitment and subsequent regression of established tumors. 194 CpG-C motifs show a combination of immunologic effects induced by CpG-A and CpG-B motifs. 33
Among TLR9 ligands developed to date, PF-3512676 (also known as CPG 7909 and agatolimod), EnanDIM®, Lefitolimod (MGN1703), and IMO-2125 (tilsotolimod) are the most well-studied CpG-ODN TLR-9 agonists. While cancer immunotherapy with PF-3512676 has been found to result in poor therapeutic outcomes in cancer patients, promising results have been reported with EnanDIM® and MGN1703. Several preclinical research studies have shown that MGN1703 significantly activates both the innate and adaptive immune cells (DCs, NK cells, monocytes, CTL, and B-cells) and induces the secretion of a variety of cytokines (IFN-α, IFN-γ, IL-8, and IL-6) and chemokines (CD86, CD40, CD169, CD69, IP-10) from activated immune cells. 195,196 Recently, Schleimann et al have shown that MGN1703 induces B cell differentiation, IgG production, and a potent IFN response in lymph nodes as well as pDC, NK cells, and T cell activation in human immunodeficiency virus (HIV) patients. 197 A preclinical study in the CT26 mice model has shown that intratumoral administration of MGN1703, modulates TME and induces tumor infiltration of T cells, specifically CD8+ T cells. 198 Recently Yang et al have developed Y-shaped dsDNA nanostructures (Y-DNAs) that interact with TLR9 and could activate innate immune cells. Their results show that treatment of mouse BM-derived DCs (BMDCs) and macrophages with Y-DNA, results in increasing of co-stimulatory factors (CD80 and CD86) as well as up-regulation of Th1-type pro-inflammatory cytokines (IL-12 and TNF-α). 199
TLR9 combination therapy
Preclinical studies show that TLR9 agonists are useful for cancer therapy when they are applied in combination with other anti-cancer therapies such as RT, CT, and anti-cancer vaccines. 187 We have previously reported that the intratumoral application of CpG ODNs combined with a selective inhibitor of STAT3 (JSI-124), reverses the immunosuppression in TME leading to a significant expansion in tumor infiltration of activated CD4+ and CD8+ T cells in tumor-bearing mice. In this study, the presence of activated T cells in TME was correlated with tumor regression and long-term immune memory in melanoma-bearing mice. 200 In line with this report, intravenous injection of PF-3512676 in combination with a STAT3 inhibitory agent has been reported to upregulate the genes related to antigen processing/presentation, Th1 cell activation in mouse model lymphoma. Up-regulation of genes related to Th1 responses was correlated with cancer-specific lymphoma CD8+/CD4+ T immunity, which significantly inhibited tumor growth resulting in the long-term survival of mice receiving the combination therapy. 201
Combination of MGN1703 with CPIs was found to enhance the anti-tumor effect of the CPIs, which was correlated with further tumor regression, prolonged survival, and long-lasting anti-tumor immune memory indicated with tumor re-challenge studies. 195 Gallotta et al have reported that intranasal administration of TLR9 agonist (SD-101) significantly enhances the anti-cancer effects of immunotherapy with anti-PD-1 in mice carrying a poorly immunogenic lung cancer. Co-treatment of anti-PD-1 antibody and TLR9 agonist administered by inhalation resulted in the regression of both lung tumors and distant metastatic lesion and significantly increased the survival of tumor-bearing mice. The observed anti-cancer effects were correlated with a significant increase in trafficking and function of Teff cells in the lung. 202 Local administration of TLR9 ligand (CpG) enhances the expression of OX40 (OX40; a type of TNF receptor) on both Teff cells and in Treg cells in the TME. Sagiv-Barfi I et al have shown that in situ vaccination of TLR9 or TLR7 ligands (CpG or IMQ) with agonistic anti-OX40 antibody into one site of mice tumor models (B cell lymphoma tumors (A20), breast carcinoma (4T1), colon cancer (CT26) and melanoma (B16F10) induce a tumor-specific immune response. This regime led to the regression of treated and untreated syngeneic tumors throughout the body without effect on non-homologous distant tumors. Agonistic anti-OX40 augment CpG efficacy and induces long-lasting immunity that leads to the cure of most of the mice tumors with a major effect on the survival of these mice. 203
Preclinical studies
Several preclinical studies have been evaluated anti-cancer effects of TLR9 agonists. Wang et al have shown that the intratumoral administration of IMO-2125 in the CRC mice model stimulates anti-tumor immune responses resulting in the regression of the tumor. IMO-2125 was found to induce both local and systemic Th1 immune response and long-lasting tumor-specific CTL response, resulting in growth inhibition of primary tumors as well as metastatic lesions. In addition, IMO-2125 was found to modulate TME resulting in the recruitment of cancer-specific T cells into tumors. 16 Kapp et al have reported that subcutaneous injection of EnanDIM® activates innate and adaptive immune response without toxicity in multiple mice cancer models. EnanDIM® was found to remodel TME leading to significant infiltration of CD8+ T cells within the CT26 tumor mice model, which was associated with tumor regression. Treatment of A20 lymphoma and EMT-6 (breast carcinoma model) mice model with EnanDIM have been also found to result in the complete destruction of the established tumor in more than half of the mice in the test group, which showed a significantly improved rate of survival as compared with the control group. All surviving mice rejected re-challenging with A20 and EMT-6 cells suggesting the development of long-lasting immunity. 195 Kapp et al have reported intratumoral administration of MGN1703 in CT26 tumor-bearing mice, increases intratumoral infiltration and anti-cancer activity of tumor-specific CD8+ T cells as well as the ratio of M1/M2 macrophages within TME, which was correlated with tumor regression. They also showed that intratumoral MGN1703 reduces tumor growth and enhances survival in B16 melanoma and EMT-6 breast cancer mice model. These preclinical data suggest that the application of TLR9 agonists in human malignancies can be beneficial. 189
Clinical trials
Over the last decade, many clinical studies have reported that TLR9 agonists are well tolerated in cancer patients and they show therapeutic effects in some types of human malignancies (Table 5). 187 PF-3512676, one of the first developed TLR9 agonists, has been evaluated for its ability to induce anti-cancer effects in several clinical studies. It has been shown that subcutaneous vaccinations of melanoma patients with low-dose PF-3512676 along with melanoma antigen and IFA, results in the rapid and strong expansion of antigen-specific T cell responses. 204 Another study has reported that intradermal injections of PF-3512676 in melanoma patients lead to an increase in the frequency of pDC, mDC, the release of a variety of inflammatory cytokines, and a significant decrease in the frequency of Treg cells in the sentinel lymph node (SLN). 205 Pashenkov et al have shown that the subcutaneous administration of PF-3512676 (6 mg weekly) in melanoma patients induces the regression of metastatic lesions in patients without notable toxicity. 206 In line with these findings, it has been demonstrated that the intratumoral application of PF-3512676 (up to 10 mg) in patients with BCC or melanoma was well tolerated and caused the regression of primary local tumors. The observed anti-cancer effects with PF-3512676 were correlated with an increase in serum levels of IL-6, IP-10, IL-12p40 as well as infiltration of lymphocytes CD4+ and CD8+ T cells, CD14+ monocytes, and CD68+ macrophages into the lesions. 189 Another study has shown that subcutaneous administration of PF-3512676 (0.08-0.81mg/kg; weekly) in renal cell carcinoma (RCC) patients was tolerable and resulted in a significant reduction in tumor size. 4 The therapeutic efficacy and safety of PF-3512676 have been assessed in lymphoma patients. Link et al have shown that intravenous administration of PF-3512676 (0.01 to 0.64 mg/kg) in patients with Hodgkin lymphoma can be safe. PF-3512676 treatment increased the number and activity of NK-cells but did not affect the level of serum cytokines (IL-12, IL-18, TNF-a), chemokines (IP-10, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1b (MIP-1b), or immune activation markers (IgM, IgG, C-reactive protein (CRP)). 207 Another study has shown that subcutaneous injection of PF-3512676 (0.08-0.36 mg/kg), was well tolerated in CTCL patients with a clinical response rate being 32%. 208 Another clinical trial has demonstrated that administration of a single intravenous and subcutaneous dose of PF-03512676 in CLL patients was well tolerated up to 1.05 mg/kg and 0.45 mg/kg respectively without significant clinical response. 209 Due to AEs and lack of therapeutic efficacy, clinical studies with PF-03512676 have been discontinued.
Table 5. Clinical applications of TLR9 agonists in cancer immunotherapy .
| TLR | Agonist | Combination | No. of samples | Cancer type | Response | Ref./The year |
| 9 | CpG-28 | - | 24 | Recurrent glioblastoma | Well tolerated at doses up to 20mg | 193 (2006) |
| 9 | CpG-28 | RT and temozolomide | 81 | Glioblastoma | Without effect on OS and PFS | 210 (2017) |
| 9 | CpG-B | GM-CSF | 30 | Melanoma | Improve RFS | 211 (2017) |
| 9 | SD-101 | RT | 29 | Lymphoma | Tumor regression, increasing IFN responsive genes, infiltration of CD8+ and CD4+ effector T-cells, decreasing number of Th and Tregs | 220 (2018) |
| 9 | SD-101 | RT | 13 | B-cell lymphoma | Well tolerated and exert anti-tumor activity with induction of CD8+ T cells and INF-α | 221 (2016) |
| 9 | PF-3512676 | RT | B-cell lymphoma | Induction tumor-reactive memory CD8+ T cells and systemic antilymphoma clinical responses | 218 (2010) | |
| 9 | PF-3512676 | Rituximab | 50 | Relapsed B cell non-Hodgkin’s lymphoma | Safe in combination with rituximab | 217 (2007) |
| 9 | PF-3512676 | - | 10 | BCC or melanoma metastases | Local tumor regressions, increasing of IL-6 cellular infiltrates of lymphocytes | 189 (2008) |
| 9 | PF-3512676 | Gemcitabine and cisplatin | 839 | NSCLC | Without effect on OS or PFS | 215 (2012) |
| 9 | PF-3512676 | - | 41 | CLL | Increasing serum level of NK and T cells and the expression of CD20, CD86, and TRAIL | 209 (2012) |
| 9 | PF-3512676 | - | 20 | Melanoma | Activation of blood pDCs, increasing of NKC and NK cell recruitment into tissues | 206 (2006) |
| 9 | PF-3512676 | - | 39 | Renal cell carcinoma | Increasing serum level of the IP-10, MCP-1, and IFN-α and reduction tumor | 4 (2009) |
| 9 | PF-3512676 | - | 23 | Non-Hodgkin lymphoma | Increasing concentrations and activation of NK-cell and ADCC activity | 207 (2006) |
| 9 | PF-3512676 | Paclitaxel/carboplatin | 828 | NSCLC | Did not improve OS or PFS and represented serious AEs | 216 (2010) |
| 9 | PF-3512676 | - | 28 | CTCL | Well tolerated | 208 (2011) |
| 9 | PF-3512676 | Melanoma antigen and IFA | 8 | Melanoma | Induction of strong antigen-specific T cell responses | 204 (2005) |
| 9 | PF-3512676 | - | 23 | Melanoma | Increasing frequency of pDC, mDC, releasing of inflammatory cytokines, and lower frequencies of Treg cells in the SLN | 205 (2007) |
| 9 | PF-3512676 | RT | 7 | B-cell lymphoma | Safe and well tolerated | 219 (2006) |
| 9 | MGN1703 | - | 28 | Metastatic solid tumor | Expression of cytokines and chemokines | 213 (2015) |
| 9 | MGN1703 | Platinum-based CT | 103 | ES-SCLC | Safe and induction of monocytes activation and expression of IP-10 | 225 (2018) |
| 9 | MGN1703 | - | 59 | mCRC | Induction of durable and prolonged PFS and disease control | 212 (2014) |
Abbreviation: CTCL: Cutaneous T-cell lymphoma; CLL: Chronic lymphocytic leukemia; NSCLC: Non-small-cell lung carcinoma; ES-SCLC: Extensive-stage small-cell lung cancer; BCC: Basal cell carcinoma; mCRC: metastatic colorectal carcinoma; mDC: myeloid dendritic cells; pDC: plasmacytoid dendritic cells; Treg: regulatory T; TLRs: Toll-like receptors; DC: Dendritic cell; Th-1: T helper cells; CTL: Cytotoxic T lymphocytes; IFA: Incomplete Freund’s adjuvant; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN: Interferon-α; NKC: NK cytotoxicity; IP-10: Interferon gamma-induced protein 10; MCP-1: monocyte chemoattractant protein-1; ADCC: Antibody-dependent cellular cytotoxicity; AEs: Adverse events; SLN: sentinel lymph node; TME: Tumor microenvironment; PFS: progression-free survival; OS: Overall survival; RT: Radiotherapy; CT: chemotherapy; CpG: RFS:recurrence-free survival.
Anti-tumor effects of other TLR9 agonists have been studied in several clinical trials. Carpentier et al have shown that intratumoral injection of CpG-280 (5–20 mg) in patients with recurrent GBM is well tolerated at doses up to 20 mg, but the clinical response was poor. 193 Another study has reported that local immunotherapy with CpG-280 (10 mg) injected into the surgical cavity in patients with GBM after tumor removal and followed by standard of care (SOC; RT and temozolomide), was well tolerated. However, this treatment did not show a significant effect on OS and recurrence-free survival (RFS) in this trial. 210 Koster et al have shown that intradermal administration of low-dose CpG-B (1-8 mg) at the primary tumor excision site in melanoma patients induces local and systemic immune responses that protect patients against disease relapse and improve RFS up to 26 months compared with untreated patients (22 months). 211 MGN1703, a novel TLR9 agonist, has shown promising effects in clinical studies. Subcutaneous administration of MGN1703 (60mg) as maintenance treatment in mCRC has been found to significantly induce durable and prolonged PFS versus placebo (2.8 to 2.6 months). 212 Another clinical trial has reported that the use of MGN1703 in patients with metastatic solid tumors is safe and well-tolerated even in doses of up to 60 mg. 213
Several clinical studies have assessed the therapeutic efficacy of combination therapy with TLR9 agonists and other conventional anti-cancer drugs. Goldinger et al have reported that combination therapy with subcutaneous injection of CpG-ODN G10, topical IMQ, and melanoma peptide antigen with or without Montanide, stimulated specific T cell responses, effector-memory-phenotype cells, and humoral immune responses in melanoma patients. However, this regimen was not found to have a significant clinical beneficiary response in patients. 214 A phase III clinical trial using PF-3512676 along with gemcitabine and cisplatin in non-small cell lung cancer (NSCLC) patients, did not show clinical response and was without effect on OS or PFS. This clinical trial was discontinued due to increased toxicity and insufficient therapeutic response. 215 In a similar study, Hirsh V. and coworkers evaluated clinical benefits of subcutaneous administration of PF-3512676 (0.2 mg/kg) as monotherapy and in combination with paclitaxel/carboplatin CT in advanced NSCLC patients. They reported that this combination therapy did not improve OS or PFS and resulted in serious AEs in most patients. 216 Another study has shown that co-treatment of PF-3512676 with rituximab in lymphoma patients, induces Th1 cytokines and chemokines. 217 The intratumoral injection of PF-3512676 following low-dose RT in patients with low-grade B-cell lymphoma has been reported to be well tolerated without treatment-limiting AEs. This combination therapy resulted in the activation of tumor-reactive memory CD8+ T cells and significant clinical responses indicated with regression of large disseminated tumors. 218 A phase I/II study has reported that the intratumoral application of PF-3512676 in combination with low-dose RT was well tolerated in low-grade lymphoma patients. Five out of 7 patients who completed the treatment showed a partial response with tumor regression observed at all un-irradiated sites and 2 remaining patients had stable disease. 219 Combination therapy with intratumoral SD-101 and low-dose RT in lymphoma patients resulted in tumor regression in injected and non-injected tumors. SD-101 was found to modulate TME through an increased expression of IFN responsive genes and infiltration of CD8+ and CD4+ effector T cells. Also, SD-101 treatment resulted in a decrease in the number of T follicular helper and Treg cells into tumors. 220 In a similar study, Levy et al conducted a phase I/II trial for escalating intratumoral doses of SD-101 in untreated B-cell lymphoma patients and found no dose-limiting toxicities up to 8 mg/dose and the majority of related AEs were mild. Intratumoral SD-101 following RT, exerts anti-tumor activity with the induction of CD8+ T cells and IFN-α. A reduction in tumor sizes was observed in the period of study (46.1% on day 90 to 68.6% on day 540). 221
Combination therapy with TLR9 agonists and CPIs has also shown promising effects in clinical studies. Ribas et al have evaluated the safety and anti-tumor activity of co-treatment of intratumoral SD-101 and pembrolizumab in patients with melanoma. This combination therapy was well tolerated with mild-to-moderate AEs. In this study, the expected 12-month PFS, overall response rate (ORR), and OS rate were 88%, 78%, and 89% respectively. The improved survival of the patients treated with SD-101 and pembrolizumab was associated with a significant expansion of the number of CTL, NK cells, DCs, and B cells in TME. These results demonstrated that combining SD-101 administration with PD-1 blockade potentially increases clinical efficiency and decreases PD-1 blockade-related toxicity. 222 Several clinical trials are ongoing evaluating the safety and effectiveness of IMO-2125 in combination with different immune CPIs including ipilimumab (anti-CTLA4), pembrolizumab, and nivolumab in melanoma patients and refractory solid tumors. Co-treatment of IMO-2125 with ipilimumab is well tolerated and shows promising therapeutic efficacy in patients with anti-PD-1 resistant metastatic melanoma. Intratumoral administration of IMO-2125 in patients with refractory solid tumors was generally well tolerated and it induced TME reprogramming through immune checkpoint upregulation, activation of DCs, and stimulation of IFN-I signaling. 223 Moreover, it has been shown that combination therapy with IMO-2125 and Ipilimumab triggers DCs maturation, IFN-γ upregulation, and activation of T cell in distant lesions in anti-PD-1 refractory metastatic melanoma patients. 224 The results of phase II clinical trial showed that the administration of MGN1703 following platinum-based CT led to a significant increase in the rate of monocyte activation and IP-10 expression in extensive-stage small-cell lung cancer (ES-SCLC). While MGN1703 had no relevant effect on PFS and OS in patients with ES-SCLC, it significantly enhanced the OS of the patients with chronic obstructive pulmonary disease (COPD) as compared with the control group that only received platinum-based CT. 225
Overall, the results of immunotherapy with TLR9 ligands are very promising. The findings summarized in this section, clearly show that TLR9 agonist can reverse immunosuppression in TME for better tumor infiltration and function of anti-cancer effector cells. Preclinical and clinical studies show that reversal of immunosuppression in TME by TLR9 agonists results in therapeutic outcomes in most cases. The induction of long-lasting immunity by TLR9 agonists reported in several studies is very encouraging for more work on the development of new TLR9 agonists for cancer immunotherapy. The findings presented in this section, also suggest that the most clinical benefits from TLR9 agonists therapy are obtained in combination regimens included with RT or CT or other therapy. Some studies have reported the termination of clinical studies with some TLR9 agonists due to the intolerability of patients to the potential side effects. These reports suggest that further studies are needed to evaluate the newly developed TLR9 ligands for dosing and the route and timing of administration, to achieve an optimized therapeutic outcome with minimum side effects.
Review Highlights
What is the current knowledge?
√ Immunosuppressive TME is believed to be the main barrier to the effectiveness of cancer immunotherapies in particular cancer vaccines.
√ The TME counteracts the therapeutic effects of cancer vaccines and provides a favorable environment for tumor growth and progression.
What is new here?
√ TLR agonists reverse the immunosuppressive TME and potentially augment the therapeutic effects of cancer therapies in particular cancer vaccines in clinical settings.
√ Several clinical studies provide proof of principle that TLR3, 4, 7, and 9 agonists can improve the clinical outcome of cancer patients.
Concluding Remarks
Several preclinical and clinical findings provide proof of principles for how immunotherapy with TLRs agonists, improves the clinical outcome of cancer patients. Given the significant impact of TME on the therapeutic effects of cancer treatments, modulation of TME by TLR ligands seems to be the main mechanism by which these immunomodulators induce anticancer effects and enhance the therapeutic effects of other cancer treatments in particular cancer vaccines.
The development of cancer vaccines is considered to be a promising approach for cancer treatment due to its specificity and long-lasting effects. Despite promising results in preclinical studies, the effectiveness of cancer vaccines in the induction of effective anti-cancer immune responses and the therapeutic outcome of this method remains poor in many clinical trials. Over the last two decades, many studies have found that the main reason behind the poor therapeutic efficacy of cancer vaccines is related to immunosuppressive TME, which inhibits the infiltration and function of anti-cancer immune cells. Therefore, reversal of immunosuppression in TME is suggested to be the main strategy for increasing the therapeutic efficacy of cancer vaccines. One of the effective methods to modulate immunosuppression in TME is the use of TLR agonists, which have a successful track record of use as adjuvants in cancer immunotherapy. Based on the findings presented in this paper, TLR3, 4, 7, and 9 agonists, reverse the immunosuppression in TME by activating different types of immune cells, thereby positively influencing the therapeutic efficacy of cancer vaccines and other conventional cancer therapies. Altogether, these findings show that TLR ligands can be beneficial in the treatment of cancer.
Funding sources
No funding.
Ethical statement
Not applicable.
Competing interests
None to declare.
Authors’ contribution
OM devised the main conceptual idea, designed the structure of the article, and made a major contribution to writing, editing, and critical revision of the paper. LR did the major contributions to the literature search, data collection, drafting the article, and writing the manuscript. MR helped in the literature search and drafting of the article. FR, BB, RL, and AL contributed to the editing and critical revision of the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damo M, Wilson DS, Simeoni E, Hubbell JAJSr. TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci Rep. 2015;5:1–15. doi: 10.1038/srep17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobold S, Wiedemann G, Rothenfußer S, Endres S. Modes of action of TLR7 agonists in cancer therapy. Immunotherapy. 2014;6:1085–95. doi: 10.2217/imt.14.75. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JA, Kuzel T, Drucker BJ, Urba WJ, Bukowski RM. Safety and efficacy of PF-3512676 for the treatment of stage IV renal cell carcinoma: an open-label, multicenter phase I/II study. Clin Genitourin Cancer. 2009;7:E58–65. doi: 10.3816/CGC.2009.n.025. [DOI] [PubMed] [Google Scholar]
- 5. Why Cancer Immunotherapy? https://www.cancerresearch.org/immunotherapy/why-immunotherapy.
- 6.Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Guo F. Recent updates on cancer immunotherapy. Precis Clin Med. 2018;1:65–74. doi: 10.1093/pcmedi/pby011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Guo J, Huang L. Modulation of tumor microenvironment for immunotherapy: focus on nanomaterial-based strategies. Theranostics. 2020;10:3099–117. doi: 10.7150/thno.42998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal S, Kandimalla ER. Intratumoural immunotherapy: activation of nucleic acid sensing pattern recognition receptors. Immuno-Oncology Technology. 2019;3:15–23. doi: 10.1016/j.iotech.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–92. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 11.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure) Ann Oncol. 2016;27:1492–504. doi: 10.1093/annonc/mdw217. [DOI] [PubMed] [Google Scholar]
- 13.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38:255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma N, Vacher J, Allison JP. TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral Treg depletion. Proc Natl Acad Sci U S A. 2019;116:10453–62. doi: 10.1073/pnas.1819004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Q, Zhang H, Zheng J, Zhang L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer. 2020;6:605–18. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Jiang W, Zhu F, Mao X, Agrawal S. Modulation of the tumor microenvironment by intratumoral administration of IMO-2125, a novel TLR9 agonist, for cancer immunotherapy. Int J Oncol. 2018;53:1193–203. doi: 10.3892/ijo.2018.4456. [DOI] [PubMed] [Google Scholar]
- 17.Mullins SR, Vasilakos JP, Deschler K, Grigsby I, Gillis P, John J. et al. Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies. J Immunother Cancer. 2019;7:244. doi: 10.1186/s40425-019-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pahlavanneshan S, Sayadmanesh A, Ebrahimiyan H, Basiri M. Toll-Like Receptor-Based Strategies for Cancer Immunotherapy. J Immunol Res. 2021;2021:9912188. doi: 10.1155/2021/9912188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J. et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallois A, Bhardwaj N. Dendritic cell-targeted approaches to modulate immune dysfunction in the tumor microenvironment. Front Immunol. 2013;4:436. doi: 10.3389/fimmu.2013.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Molecular Cancer. 2020;19:116. doi: 10.1186/s12943-020-01234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hossam N, Matboli M, Shehata HH, Aboelhussein MM, Hassan MK, Eissa S. Toll-like receptor immune modulatory role in personalized management of colorectal cancer, review of literature. Expert Review of Precision Medicine and Drug Development. 2020;5:455–68. doi: 10.1080/23808993.2020.1816136. [DOI] [Google Scholar]
- 24.Braunstein MJ, Kucharczyk J, Adams S. Targeting Toll-Like Receptors for Cancer Therapy. Target Oncol. 2018;13:583–98. doi: 10.1007/s11523-018-0589-7. [DOI] [PubMed] [Google Scholar]
- 25.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/jci31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Sunagar R, Gosselin E. Bacterial Protein Toll-Like-Receptor Agonists: A Novel Perspective on Vaccine Adjuvants. Front Immunol. 2019;10:1144. doi: 10.3389/fimmu.2019.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redecke V, Häcker H, Datta SK, Fermin A, Pitha PM, Broide DH. et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–43. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 29.Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H. et al. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172:6922–30. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 30.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A. et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–43. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Alvero AB, Silasi DA, Mor G. Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. Am J Reprod Immunol. 2007;57:93–107. doi: 10.1111/j.1600-0897.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 32.Holldack J. Toll-like receptors as therapeutic targets for cancer. Drug Discov Today. 2014;19:379–82. doi: 10.1016/j.drudis.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949–64. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban-Wojciuk Z, Khan MM, Oyler BL, Fahraeus R, Marek-Trzonkowska N, Nita-Lazar A. et al. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front Immunol. 2019;10:2388. doi: 10.3389/fimmu.2019.02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Feng R, Wang YZ, Sun HW, Zou QM, Li HB. Toll-like receptors: Triggers of regulated cell death and promising targets for cancer therapy. Immunol Lett. 2020;223:1–9. doi: 10.1016/j.imlet.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Kapsenberg ML. Dendritic-cell control of pathogen-driven T cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira-Nascimento L, Massari P, Wetzler LM. The Role of TLR2 in Infection and Immunity. Front Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Netea MG, Van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob Agents Chemother. 2005;49:3991–6. doi: 10.1128/AAC.49.10.3991-3996.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong H, Gutkin DW, Han B, Ma Y, Keskinov AA, Shurin MR. et al. Origin and pharmacological modulation of tumor-associated regulatory dendritic cells. Int J Cancer. 2014;134:2633–45. doi: 10.1002/ijc.28590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaelis KA, Norgard MA, Zhu X, Levasseur PR, Sivagnanam S, Liudahl SM. et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat Commun. 2019;10:4682. doi: 10.1038/s41467-019-12657-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mende I, Engleman EG. Breaking self-tolerance to tumor-associated antigens by in vivo manipulation of dendritic cells. Methods Mol Biol. 2007;380:457–68. doi: 10.1007/978-1-59745-395-0_29. [DOI] [PubMed] [Google Scholar]
- 43.Vo MC, Lee HJ, Kim JS, Hoang MD, Choi NR, Rhee JH. et al. Dendritic cell vaccination with a toll-like receptor agonist derived from mycobacteria enhances anti-tumor immunity. Oncotarget. 2015;6:33781–90. doi: 10.18632/oncotarget.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li K, Qu S, Chen X, Wu Q, Shi M. Promising Targets for Cancer Immunotherapy: TLRs, RLRs, and STING-Mediated Innate Immune Pathways. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Re F, Strominger JL. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J Immunol. 2004;173:7548–55. doi: 10.4049/jimmunol.173.12.7548. [DOI] [PubMed] [Google Scholar]
- 46.Adams S, O'Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K. et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. Journal of immunology (Baltimore, Md : 1950) 2008;181:776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewan MZ, Vanpouille-Box C, Kawashima N, DiNapoli S, Babb JS, Formenti SC. et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res. 2012;18:6668–78. doi: 10.1158/1078-0432.Ccr-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walshaw RC, Honeychurch J, Choudhury A, Illidge TM. Toll-Like Receptor Agonists and Radiation Therapy Combinations: An Untapped Opportunity to Induce Anticancer Immunity and Improve Tumor control. Int J Radiat Oncol Biol Phys. 2020;108:27–37. doi: 10.1016/j.ijrobp.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Huang L, Xu H, Peng G. TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell Mol Immunol. 2018;15:428–37. doi: 10.1038/cmi.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300–1. doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu J, Liu Y, Xie B, Ye P, Huang J, Lu Z. Roles of toll-like receptors: From inflammation to lung cancer progression. Biomed Rep. 2018;8:126–32. doi: 10.3892/br.2017.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J COMCO, Lu Y. Toll-Like Receptor Agonists in Cancer Therapy. Nov Appro in Can Study. 2020;4 doi: 10.31031/NACS.2020.04.000597. [DOI] [Google Scholar]
- 53.Zhou H, Jiang M, Yuan H, Ni W, Tai G. Dual roles of myeloid-derived suppressor cells induced by Toll-like receptor signaling in cancer. Oncol Lett. 2021;21:149. doi: 10.3892/ol.2020.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sautès-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I. et al. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011;30:13–25. doi: 10.1007/s10555-011-9279-y. [DOI] [PubMed] [Google Scholar]
- 55.Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi: 10.3389/fimmu.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shetab Boushehri MA, Lamprecht A. TLR4-Based Immunotherapeutics in Cancer: A Review of the Achievements and Shortcomings. Mol Pharm. 2018;15:4777–800. doi: 10.1021/acs.molpharmaceut.8b00691. [DOI] [PubMed] [Google Scholar]
- 57.Pradere JP, Dapito DH, Schwabe RF. The Yin and Yang of Toll-like receptors in cancer. Oncogene. 2014;33:3485–95. doi: 10.1038/onc.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsumoto M, Takeda Y, Tatematsu M, Seya T. Toll-Like Receptor 3 Signal in Dendritic Cells Benefits Cancer Immunotherapy. Front Immunol. 2017;8:1897. doi: 10.3389/fimmu.2017.01897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–12. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol. 2016;28:329–38. doi: 10.1093/intimm/dxw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varthaman A, Moreau HD, Maurin M, Benaroch P. TLR3-Induced Maturation of Murine Dendritic Cells Regulates CTL Responses by Modulating PD-L1 Trafficking. PLOS ONE. 2016;11:e0167057. doi: 10.1371/journal.pone.0167057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seya T, Takeda Y, Matsumoto M. A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy. Adv Drug Deliv Rev. 2019;147:37–43. doi: 10.1016/j.addr.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Melssen MM, Petroni GR, Chianese-Bullock KA, Wages NA, Grosh WW, Varhegyi N. et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund's adjuvant in melanoma patients. J Immunother Cancer. 2019;7:163. doi: 10.1186/s40425-019-0625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K. et al. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207:2675–87. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takeda Y, Kataoka K, Yamagishi J, Ogawa S, Seya T, Matsumoto M. A TLR3-Specific Adjuvant Relieves Innate Resistance to PD-L1 Blockade without Cytokine Toxicity in Tumor Vaccine Immunotherapy. Cell Rep. 2017;19:1874–87. doi: 10.1016/j.celrep.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 66.Takeda Y, Yoshida S, Takashima K, Ishii-Mugikura N, Shime H, Seya T. et al. Vaccine immunotherapy with ARNAX induces tumor-specific memory T cells and durable anti-tumor immunity in mouse models. Cancer Sci. 2018;109:2119–29. doi: 10.1111/cas.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amos SM, Pegram HJ, Westwood JA, John LB, Devaud C, Clarke CJ. et al. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol Immunother. 2011;60:671–83. doi: 10.1007/s00262-011-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pearson FE, Chang K, Minoda Y, Rojas IML, Haigh OL, Daraj G. et al. Activation of human CD141(+) and CD1c(+) dendritic cells in vivo with combined TLR3 and TLR7/8 ligation. Immunol Cell Biol. 2018;96:390–400. doi: 10.1111/imcb.12009. [DOI] [PubMed] [Google Scholar]
- 69.Chew V, Abastado JP. Immunomodulation of the tumor microenvironment by Toll-like receptor-3 (TLR3) ligands. Oncoimmunology. 2013;2:e23493. doi: 10.4161/onci.23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 71.Bianchi F, Alexiadis S, Camisaschi C, Truini M, Centonze G, Milione M. et al. TLR3 Expression Induces Apoptosis in Human Non-Small-Cell Lung Cancer. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21041440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schau I, Michen S, Hagstotz A, Janke A, Schackert G, Appelhans D. et al. Targeted delivery of TLR3 agonist to tumor cells with single chain antibody fragment-conjugated nanoparticles induces type I-interferon response and apoptosis. Sci Rep. 2019;9:3299. doi: 10.1038/s41598-019-40032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shcheblyakov DV, Logunov DY, Tukhvatulin AI, Shmarov MM, Naroditsky BS, Gintsburg AL. Toll-Like Receptors (TLRs): The Role in Tumor Progression. Acta Naturae. 2010;2:21–9. [PMC free article] [PubMed] [Google Scholar]
- 74.Ding L, Ren J, Zhang D, Li Y, Huang X, Ji J. et al. The TLR3 Agonist Inhibit Drug Efflux and Sequentially Consolidates Low-Dose Cisplatin-Based Chemoimmunotherapy while Reducing Side Effects. Mol Cancer Ther. 2017;16:1068–79. doi: 10.1158/1535-7163.MCT-16-0454. [DOI] [PubMed] [Google Scholar]
- 75.Ho V, Lim TS, Lee J, Steinberg J, Szmyd R, Tham M. et al. TLR3 agonist and Sorafenib combinatorial therapy promotes immune activation and controls hepatocellular carcinoma progression. Oncotarget. 2015;6:27252–66. doi: 10.18632/oncotarget.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shime H, Matsumoto M, Oshiumi H, Tanaka S, Nakane A, Iwakura Y. et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci U S A. 2012;109:2066–71. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roselli E, Araya P, Nunez NG, Gatti G, Graziano F, Sedlik C. et al. TLR3 Activation of Intratumoral CD103(+) Dendritic Cells Modifies the Tumor Infiltrate Conferring Anti-tumor Immunity. Front Immunol. 2019;10:503. doi: 10.3389/fimmu.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hovanessian AG, Youn JK, Buffet-Janvresse C, Riviere Y, Michelson M, Lacour J. et al. Enhancement of natural killer cell activity and 2-5A synthetase in operable breast cancer patients treated with polyadenylic; polyuridylic acid. Cancer. 1985;55:357–62. doi: 10.1002/1097-0142(19850115)55:2<357::aidcncr2820550210>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 79.Lampkin BC, Levine AS, Levy H, Krivit W, Hammond D. Phase II trial of a complex polyriboinosinic-polyribocytidylic acid with poly-L-lysine and carboxymethyl cellulose in the treatment of children with acute leukemia and neuroblastoma: a report from the Children's Cancer Study Group. Cancer Res. 1985;45:5904–9. [PubMed] [Google Scholar]
- 80.Ewel CH, Urba WJ, Kopp WC, Smith JW, Steis RG, Rossio JL. et al. Polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose in combination with interleukin 2 in patients with cancer: clinical and immunological effects. Cancer Res. 1992;52:3005–10. [PubMed] [Google Scholar]
- 81.Kyi C, Roudko V, Sabado R, Saenger Y, Loging W, Mandeli J. et al. Therapeutic Immune Modulation against Solid Cancers with Intratumoral Poly-ICLC: A Pilot Trial. Clin Cancer Res. 2018;24:4937–48. doi: 10.1158/1078-0432.CCR-17-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res. 2014;2:720–4. doi: 10.1158/2326-6066.Cir-14-0024. [DOI] [PubMed] [Google Scholar]
- 83.Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M. et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol. 2017;10:82. doi: 10.1186/s13045-017-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K. et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18:6497–508. doi: 10.1158/1078-0432.Ccr-12-2189. [DOI] [PubMed] [Google Scholar]
- 85.Pavlick A, Blazquez AB, Meseck M, Lattanzi M, Ott PA, Marron TU. et al. Combined Vaccination with NY-ESO-1 Protein, Poly-ICLC, and Montanide Improves Humoral and Cellular Immune Responses in Patients with High-Risk Melanoma. Cancer Immunol Res. 2020;8:70–80. doi: 10.1158/2326-6066.CIR-19-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–97. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fang W, Bi D, Zheng R, Cai N, Xu H, Zhou R. et al. Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264.7 macrophages. Sci Rep. 2017;7:1663. doi: 10.1038/s41598-017-01868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molteni M, Bosi A, Rossetti C. Natural Products with Toll-Like Receptor 4 Antagonist Activity. Int J Inflam. 2018;2018:2859135. doi: 10.1155/2018/2859135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li J, Yang F, Wei F, Ren X. The role of toll-like receptor 4 in tumor microenvironment. Oncotarget. 2017;8:66656–67. doi: 10.18632/oncotarget.19105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peri FP, M M. Peri FP, MTherapeutic Targeting of Innate Immunity with Toll-like Receptor 4 (TLR4) Antagonists. Biotechnol Adv. 2012;30(1):251–60. doi: 10.1016/j.biotechadv.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 92.Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y. et al. TLR4 is essential for dendritic cell activation and anti-tumor T cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol. 2014;11:150–9. doi: 10.1038/cmi.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park IA, Heo SH, Song IH, Kim YA, Park HS, Bang WS. et al. Endoplasmic reticulum stress induces secretion of high-mobility group proteins and is associated with tumor-infiltrating lymphocytes in triple-negative breast cancer. Oncotarget. 2016;7:59957–64. doi: 10.18632/oncotarget.11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Garay RPV, P.; Bauer, J.; Normier, G.; Bardou, M.; Jeannin, J.-F.; Chiavaroli, C.. Eur. J. Cancer Relapse under Chemotherapy: Why TLR2/4 Receptor Agonists Can Help. Pharmacol 2007; 563 (1-3). [DOI] [PubMed]
- 95.Awasthi S. Toll-like receptor-4 modulation for cancer immunotherapy. Front Immunol. 2014;5:328. doi: 10.3389/fimmu.2014.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X, Li X, Zhang X, Zang L, Yang H, Zhao W. et al. Toll-like receptor 4-induced inflammatory responses contribute to the tumor-associated macrophages formation and infiltration in patients with diffuse large B-cell lymphoma. Ann Diagn Pathol. 2015;19:232–8. doi: 10.1016/j.anndiagpath.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Hamdy S, Elamanchili P, Alshamsan A, Molavi O, Satou T, Samuel J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly(D,L-lactic-co-glycolic acid) nanoparticles. J Biomed Mater Res A. 2007;81:652–62. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- 98. Ran S BN, Patel R, Volk-Draper L. LR4-Induced Inflammation Is a Key Promoter of Tumor Growth, Vascularization, and Metastasis. Translational Studies on Inflammation: IntechOpen; 2019.
- 99. Won JL, Y.; Chang, K.; Hong, Y. Evidence for the Involvement of Toll-Like Receptor 4 (TLR4)-Mediated Apoptosis in Gynecological Cancers. Preprints 2016; 2016120107.
- 100.He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850–9. doi: 10.1016/j.molimm.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 101.Tang X, Zhu Y. TLR4 signaling promotes immune escape of human colon cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Oncol Res. 2012;20:15–24. doi: 10.3727/096504012x13425470196092. [DOI] [PubMed] [Google Scholar]
- 102.Ou T, Lilly M, Jiang W. The Pathologic Role of Toll-Like Receptor 4 in Prostate Cancer. Front Immunol. 2018;9:1188. doi: 10.3389/fimmu.2018.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen J, Sun B, Zhao X, Liang D, Liu J, Huang Y. et al. Monophosphoryl lipid A induces bone marrow precursor cells to differentiate into myeloid-derived suppressor cells. Mol Med Rep. 2013;8:1074–8. doi: 10.3892/mmr.2013.1653. [DOI] [PubMed] [Google Scholar]
- 104.Kashani B, Zandi Z, Pourbagheri-Sigaroodi A, Bashash D, Ghaffari SH. The role of toll-like receptor 4 (TLR4) in cancer progression: A possible therapeutic target? Journal of Cellular Physiology. 2021;236:4121–37. doi: 10.1002/jcp.30166. [DOI] [PubMed] [Google Scholar]
- 105.Muniz-Bongers LR, McClain CB, Saxena M, Bongers G, Merad M, Bhardwaj N. MMP2 and TLRs modulate immune responses in the tumor microenvironment. JCI Insight. 2021;6 doi: 10.1172/jci.insight.144913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang X, Quinn PJ. Endotoxins: structure, function and recognition: Springer Science & Business Media; 2010.
- 108.Traini G, Ruiz-de-Angulo A, Blanco-Canosa JB, Zamacola Bascarán K, Molinaro A, Silipo A. et al. Cancer Immunotherapy of TLR4 Agonist-Antigen Constructs Enhanced with Pathogen-Mimicking Magnetite Nanoparticles and Checkpoint Blockade of PD-L1. Small. 2019;15:e1803993. doi: 10.1002/smll.201803993. [DOI] [PubMed] [Google Scholar]
- 109.Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev Vaccines. 2003;2:219–29. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- 110.Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982;257:11808–15. [PubMed] [Google Scholar]
- 111.Vermaelen K. Vaccine Strategies to Improve Anti-cancer Cellular Immune Responses. Front Immunol. 2019;10:8. doi: 10.3389/fimmu.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z. et al. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–57. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 113.Pantel A, Cheong C, Dandamudi D, Shrestha E, Mehandru S, Brane L. et al. A new synthetic TLR4 agonist, GLA, allows dendritic cells targeted with antigen to elicit Th1 T cell immunity in vivo. Eur J Immunol. 2012;42:101–9. doi: 10.1002/eji.201141855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dubois Cauwelaert N, Desbien AL, Hudson TE, Pine SO, Reed SG, Coler RN. et al. The TLR4 Agonist Vaccine Adjuvant, GLA-SE, Requires Canonical and Atypical Mechanisms of Action for TH1 Induction. PLoS One. 2016;11:e0146372. doi: 10.1371/journal.pone.0146372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Orr MT, Desbien AL, Cauwelaert ND, Reed SG. Mechanisms of activity of the combination TLR4 agonist and emulsion adjuvant GLA-SE. The Journal of Immunology 2016; 196: 75.2.
- 116.Lee SJ, Shin SJ, Lee MH, Lee M-G, Kang TH, Park WS. et al. A potential protein adjuvant derived from Mycobacterium tuberculosis Rv0652 enhances dendritic cells-based tumor immunotherapy. PloS one. 2014;9:e104351. doi: 10.1371/journal.pone.0104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Albershardt TC, Leleux J, Parsons AJ, Krull JE, Berglund P, Ter Meulen J. Intratumoral immune activation with TLR4 agonist synergizes with effector T cells to eradicate established murine tumors. NPJ Vaccines. 2020;5:50. doi: 10.1038/s41541-020-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rommelfanger DM, Compte M, Diaz RM, Ilett E, Alvarez-Vallina L, Thompson JM. et al. The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice. Mol Ther. 2013;21:348–57. doi: 10.1038/mt.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Molavi O, Ma Z, Hamdy S, Lavasanifar A, Samuel J. Immunomodulatory and anticancer effects of intra-tumoral co-delivery of synthetic lipid A adjuvant and STAT3 inhibitor, JSI-124. Immunopharmacol Immunotoxicol. 2009;31:214–21. doi: 10.1080/08923970802380452. [DOI] [PubMed] [Google Scholar]
- 120.Vindevogel E, Baert T, A VANH, Verbist G, Vande Velde G, Garg AD. et al. The Use of Toll-like Receptor 4 Agonist to Reshape the Immune Signature in Ovarian Cancer. Anticancer Res. 2016;36:5781–92. doi: 10.21873/anticanres.11162. [DOI] [PubMed] [Google Scholar]
- 121.Davis MB, Vasquez-Dunddel D, Fu J, Albesiano E, Pardoll D, Kim YJ. Intratumoral administration of TLR4 agonist absorbed into a cellular vector improves antitumor responses. Clin Cancer Res. 2011;17:3984–92. doi: 10.1158/1078-0432.CCR-10-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nelson MH, Bowers JS, Bailey SR, Diven MA, Fugle CW, Kaiser ADM. et al. Toll-like receptor agonist therapy can profoundly augment the antitumor activity of adoptively transferred CD8+ T cells without host preconditioning. Journal for ImmunoTherapy of Cancer. 2016;4:6. doi: 10.1186/s40425-016-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Isambert N, Fumoleau P, Paul C, Ferrand C, Zanetta S, Bauer J. et al. Phase I study of OM-174, a lipid A analogue, with assessment of immunological response, in patients with refractory solid tumors. BMC Cancer. 2013;13:172. doi: 10.1186/1471-2407-13-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Neidhart J, Allen KO, Barlow DL, Carpenter M, Shaw DR, Triozzi PL. et al. Immunization of colorectal cancer patients with recombinant baculovirus-derived KSA (Ep-CAM) formulated with monophosphoryl lipid A in liposomal emulsion, with and without granulocyte-macrophage colony-stimulating factor. Vaccine. 2004;22:773–80. doi: 10.1016/j.vaccine.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 125.Bhatia S, Miller NJ, Lu H, Longino NV, Ibrani D, Shinohara MM. et al. Intratumoral G100, a TLR4 Agonist, Induces Antitumor Immune Responses and Tumor Regression in Patients with Merkel Cell Carcinoma. Clin Cancer Res. 2019;25:1185–95. doi: 10.1158/1078-0432.Ccr-18-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahipal A, Ejadi S, Gnjatic S, Kim-Schulze S, Lu H, ter Meulen JH. et al. First-in-human phase 1 dose-escalating trial of G305 in patients with advanced solid tumors expressing NY-ESO-1. Cancer Immunology, Immunotherapy. 2019;68:1211–22. doi: 10.1007/s00262-019-02331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michaelis KA, Norgard MA, Zhu X, Levasseur PR, Sivagnanam S, Liudahl SM. et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nature Communications. 2019;10 doi: 10.1038/s41467-019-12657-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–9. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 129.Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist. 2008;13:859–75. doi: 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- 130.Chi H, Li C, Zhao FS, Zhang L, Ng TB, Jin G. et al. Anti-tumor Activity of Toll-Like Receptor 7 Agonists. Front Pharmacol. 2017;8:304. doi: 10.3389/fphar.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saitoh SI, Abe F, Kanno A, Tanimura N, Mori Saitoh Y, Fukui R. et al. TLR7 mediated viral recognition results in focal type I interferon secretion by dendritic cells. Nat Commun. 2017;8:1592. doi: 10.1038/s41467-017-01687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zahm CD, Colluru VT, McIlwain SJ, Ong IM, McNeel DG. TLR Stimulation during T cell Activation Lowers PD-1 Expression on CD8+ T Cells. Cancer Immunology Research. 2018;6:1364–74. doi: 10.1158/2326-6066.Cir-18-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hotz C, Bourquin C. Systemic cancer immunotherapy with Toll-like receptor 7 agonists: Timing is everything. Oncoimmunology. 2012;1:227–8. doi: 10.4161/onci.1.2.18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma F, Zhang J, Zhang J, Zhang C. The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cell Mol Immunol. 2010;7:381–8. doi: 10.1038/cmi.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schon M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J. et al. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003;95:1138–49. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- 136.Spaner DE, Shi Y, White D, Shaha S, He L, Masellis A. et al. A phase I/II trial of TLR-7 agonist immunotherapy in chronic lymphocytic leukemia. Leukemia. 2010;24:222–6. doi: 10.1038/leu.2009.195. [DOI] [PubMed] [Google Scholar]
- 137.Power Coombs MR, Belderbos ME, Gallington LC, Bont L, Levy O. Adenosine modulates Toll-like receptor function: basic mechanisms and translational opportunities. Expert Rev Anti Infect Ther. 2011;9:261–9. doi: 10.1586/eri.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schön MP, Schön M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–47. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 139.Sabado RL, Pavlick A, Gnjatic S, Cruz CM, Vengco I, Hasan F. et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol Res. 2015;3:278–87. doi: 10.1158/2326-6066.CIR-14-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin T, He S, Wang Y. Toll-like receptor 7/8 agonist, R848, exhibits antitumoral effects in a breast cancer model. Mol Med Rep. 2015;12:3515–20. doi: 10.3892/mmr.2015.3885. [DOI] [PubMed] [Google Scholar]
- 141.Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Aiba S. Immunomodulatory effects of imiquimod cream on tumor-infiltrating leukocytes in B16F10 melanomas. Journal of Dermatological Science. 2016;84:e176. [Google Scholar]
- 142.Pilch Z, Tonecka K, Braniewska A, Sas Z, Skorzynski M, Boon L. et al. Antitumor Activity of TLR7 Is Potentiated by CD200R Antibody Leading to Changes in the Tumor Microenvironment. Cancer Immunol Res. 2018;6:930–40. doi: 10.1158/2326-6066.Cir-17-0454. [DOI] [PubMed] [Google Scholar]
- 143.Chen PM, Pan WY, Wu CY, Yeh CY, Korupalli C, Luo PK. et al. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials. 2020;230:119629. doi: 10.1016/j.biomaterials.2019.119629. [DOI] [PubMed] [Google Scholar]
- 144.Diao Y, Wang X, Wan Y, Zhong J, Gao D, Liu Y. et al. Antitumor activity of a novel small molecule TLR7 agonist via immune response induction and tumor microenvironment modulation. Oncol Rep. 2016;35:793–800. doi: 10.3892/or.2015.4436. [DOI] [PubMed] [Google Scholar]
- 145.Han JH, Lee J, Jeon SJ, Choi ES, Cho SD, Kim BY. et al. In vitro and in vivo growth inhibition of prostate cancer by the small molecule imiquimod. Int J Oncol. 2013;42:2087–93. doi: 10.3892/ijo.2013.1898. [DOI] [PubMed] [Google Scholar]
- 146.Huang SW, Chang SH, Mu SW, Jiang HY, Wang ST, Kao JK. et al. Imiquimod activates p53-dependent apoptosis in a human basal cell carcinoma cell line. J Dermatol Sci. 2016;81:182–91. doi: 10.1016/j.jdermsci.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 147.Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA. et al. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114:135–41. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 148.Li VW, Li WW, Talcott KE, Zhai AW. Imiquimod as an antiangiogenic agent. J Drugs Dermatol. 2005;4:708–17. [PubMed] [Google Scholar]
- 149.Sato Y, Goto Y, Narita N, Hoon DS. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer Microenviron. 2009;2 Suppl 1:205–14. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Majewski S, Marczak M, Mlynarczyk B, Benninghoff B, Jablonska S. Imiquimod is a strong inhibitor of tumor cell‐induced angiogenesis. International journal of dermatology. 2005;44:14–9. doi: 10.1111/j.1365-4632.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 151.Lee M, Park CS, Lee YR, Im SA, Song S, Lee CK. Resiquimod, a TLR7/8 agonist, promotes differentiation of myeloid-derived suppressor cells into macrophages and dendritic cells. Arch Pharm Res. 2014;37:1234–40. doi: 10.1007/s12272-014-0379-4. [DOI] [PubMed] [Google Scholar]
- 152.Spinetti T, Spagnuolo L, Mottas I, Secondini C, Treinies M, Ruegg C. et al. TLR7-based cancer immunotherapy decreases intratumoral myeloid-derived suppressor cells and blocks their immunosuppressive function. Oncoimmunology. 2016;5:e1230578. doi: 10.1080/2162402X.2016.1230578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–88. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schölch S, Rauber C, Tietz A, Rahbari NN, Bork U, Schmidt T. et al. Radiotherapy combined with TLR7/8 activation induces strong immune responses against gastrointestinal tumors. Oncotarget. 2014;6:4663–76. doi: 10.18632/oncotarget.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao D, Li W, Wang W, Cai Y, Wang Y, Luo X. et al. Synergy of purine-scaffold TLR7 agonist with doxorubicin on systemic inhibition of lymphoma in mouse model. Journal of Cancer. 2017;8:3183–9. doi: 10.7150/jca.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adlard AL, Dovedi SJ, Telfer BA, Koga-Yamakawa E, Pollard C, Honeychurch J. et al. A novel systemically administered Toll-like receptor 7 agonist potentiates the effect of ionizing radiation in murine solid tumor models. Int J Cancer. 2014;135:820–9. doi: 10.1002/ijc.28711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cho JH, Lee HJ, Ko HJ, Yoon BI, Choe J, Kim KC. et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget. 2017;8:24932–48. doi: 10.18632/oncotarget.15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karashima T, Udaka K, Niimura M, Suzuki K, Osakabe H, Shimamoto T. et al. Therapy with transcutaneous administration of imiquimod combined with oral administration of sorafenib suppresses renal cell carcinoma growing in an orthotopic mouse model. Oncol Lett. 2017;14:1162–6. doi: 10.3892/ol.2017.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gao N, Zhong J, Wang X, Jin Z, Li W, Liu Y. et al. Immunomodulatory and Antitumor Effects of a Novel TLR7 Agonist Combined with Lapatinib. Scientific Reports. 2016;6 doi: 10.1038/srep39598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nishii N, Tachinami H, Kondo Y, Xia Y, Kashima Y, Ohno T. et al. Systemic administration of a TLR7 agonist attenuates regulatory T cells by dendritic cell modification and overcomes resistance to PD-L1 blockade therapy. Oncotarget. 2018;9:13301–12. doi: 10.18632/oncotarget.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheadle EJ, Lipowska-Bhalla G, Dovedi SJ, Fagnano E, Klein C, Honeychurch J. et al. A TLR7 agonist enhances the antitumor efficacy of obinutuzumab in murine lymphoma models via NK cells and CD4 T cells. Leukemia. 2017;31:1611–21. doi: 10.1038/leu.2016.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 163.Ito H, Ando T, Arioka Y, Saito K, Seishima M. Inhibition of indoleamine 2,3-dioxygenase activity enhances the anti-tumour effects of a Toll-like receptor 7 agonist in an established cancer model. Immunology. 2015;144:621–30. doi: 10.1111/imm.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ito H, Ando T, Ogiso H, Arioka Y, Seishima M. Inhibition of induced nitric oxide synthase enhances the anti-tumor effects on cancer immunotherapy using TLR7 agonist in mice. Cancer Immunol Immunother. 2015;64:429–36. doi: 10.1007/s00262-014-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spirig R, Djafarzadeh S, Regueira T, Shaw SG, von Garnier C, Takala J. et al. Effects of TLR agonists on the hypoxia-regulated transcription factor HIF-1alpha and dendritic cell maturation under normoxic conditions. PLoS One. 2010;5:e0010983. doi: 10.1371/journal.pone.0010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Huang S-W, Kao J-K, Wu C-Y, Wang S-T, Lee H-C, Liang S-M. et al. Targeting aerobic glycolysis and HIF-1α expression enhance imiquimod-induced apoptosis in cancer cells. Oncotarget. 2014;5:1363. doi: 10.18632/oncotarget.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ghosh TK, Mickelson DJ, Lipson KE, Alkan SS. Inhibition of in vitro tumor cell proliferation by cytokines induced by combinations of TLR or TLR and TCR agonists. Int Immunopharmacol. 2007;7:1471–82. doi: 10.1016/j.intimp.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 168.Witt PL, Ritch PS, Reding D, McAuliffe TL, Westrick L, Grossberg SE. et al. Phase I trial of an oral immunomodulator and interferon inducer in cancer patients. Cancer Res. 1993;53:5176–80. [PubMed] [Google Scholar]
- 169.Urosevic M, Maier T, Benninghoff B, Slade H, Burg G, Dummer R. Mechanisms underlying imiquimod-induced regression of basal cell carcinoma in vivo. Arch Dermatol. 2003;139:1325–32. doi: 10.1001/archderm.139.10.1325. [DOI] [PubMed] [Google Scholar]
- 170.Berman B, Sullivan T, De Araujo T, Nadji M. Expression of Fas-receptor on basal cell carcinomas after treatment with imiquimod 5% cream or vehicle. Br J Dermatol. 2003;149 Suppl 66:59–61. doi: 10.1046/j.0366-077x.2003.05634.x. [DOI] [PubMed] [Google Scholar]
- 171.Vidal D, Matías-Guiu X, Alomar A. Efficacy of imiquimod for the expression of Bcl-2, Ki67, p53 and basal cell carcinoma apoptosis. Br J Dermatol. 2004;151:656–62. doi: 10.1111/j.1365-2133.2004.06094.x. [DOI] [PubMed] [Google Scholar]
- 172.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–51. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De Giorgi V, Salvini C, Chiarugi A, Paglierani M, Maio V, Nicoletti P. et al. In vivo characterization of the inflammatory infiltrate and apoptotic status in imiquimod-treated basal cell carcinoma. Int J Dermatol. 2009;48:312–21. doi: 10.1111/j.1365-4632.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 174.Huang SJ, Hijnen D, Murphy GF, Kupper TS, Calarese AW, Mollet IG. et al. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol. 2009;129:2676–85. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Adams S, Kozhaya L, Martiniuk F, Meng TC, Chiriboga L, Liebes L. et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748–57. doi: 10.1158/1078-0432.Ccr-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chong E. Treating Cutaneous Metastasis of Breast Cancer with Topical Imiquimod. Loma Linda University Student Journal. 2019;3:4. [Google Scholar]
- 177.Hesling C, D'Incan M, Mansard S, Franck F, Corbin-Duval A, Chèvenet C. et al. In vivo and in situ modulation of the expression of genes involved in metastasis and angiogenesis in a patient treated with topical imiquimod for melanoma skin metastases. Br J Dermatol. 2004;150:761–7. doi: 10.1111/j.0007-0963.2004.05898.x. [DOI] [PubMed] [Google Scholar]
- 178.Rozenblit M, Hendrickx W, Heguy A, Chiriboga L, Loomis C, Ray K. et al. Transcriptomic profiles conducive to immune-mediated tumor rejection in human breast cancer skin metastases treated with Imiquimod. Sci Rep. 2019;9:8572. doi: 10.1038/s41598-019-42784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rook AH, Gelfand JM, Wysocka M, Troxel AB, Benoit B, Surber C. et al. Topical resiquimod can induce disease regression and enhance T cell effector functions in cutaneous T cell lymphoma. Blood. 2015;126:1452–61. doi: 10.1182/blood-2015-02-630335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dudek AZ, Yunis C, Harrison LI, Kumar S, Hawkinson R, Cooley S. et al. First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clin Cancer Res. 2007;13:7119–25. doi: 10.1158/1078-0432.CCR-07-1443. [DOI] [PubMed] [Google Scholar]
- 181.Koga-Yamakawa E, Murata M, Dovedi SJ, Wilkinson RW, Ota Y, Umehara H. et al. TLR7 tolerance is independent of the type I IFN pathway and leads to loss of anti-tumor efficacy in mice. Cancer Immunol Immunother. 2015;64:1229–39. doi: 10.1007/s00262-015-1730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T. et al. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 183.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH. et al. Gene expression profile correlates with T cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.Ccr-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mauldin IS, Wages NA, Stowman AM, Wang E, Olson WC, Deacon DH. et al. Topical treatment of melanoma metastases with imiquimod, plus administration of a cancer vaccine, promotes immune signatures in the metastases. Cancer Immunol Immunother. 2016;65:1201–12. doi: 10.1007/s00262-016-1880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL. et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102:1129–36. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dummer R, Hauschild A, Becker JC, Grob JJ, Schadendorf D, Tebbs V. et al. An exploratory study of systemic administration of the toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma. Clin Cancer Res. 2008;14:856–64. doi: 10.1158/1078-0432.Ccr-07-1938. [DOI] [PubMed] [Google Scholar]
- 187.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–7. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- 188.Williamson RD, McCarthy FP, Kenny LC, McCarthy CM. Activation of a TLR9 mediated innate immune response in preeclampsia. Scientific Reports. 2019;9 doi: 10.1038/s41598-019-42551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–7. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 190.Sharma RK, Sharma J, Khan ZK, Pattekar A, Gupta V, Bansal R. et al. Diminished TLR2-TLR9 mediated CD4+ T cell responses are associated with increased inflammation in intraocular tuberculosis. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-32234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kang TH, Mao CP, Kim YS, Kim TW, Yang A, Lam B. et al. TLR9 acts as a sensor for tumor-released DNA to modulate anti-tumor immunity after chemotherapy. J Immunother Cancer. 2019;7:260. doi: 10.1186/s40425-019-0738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Krieg AMJTJoci. Development of TLR9 agonists for cancer therapy. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A. et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. 2006;8:60–6. doi: 10.1215/s1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guéry L, Dubrot J, Lippens C, Brighouse D, Malinge P, Irla M. et al. Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression. Cancer Res. 2014;74:6430–40. doi: 10.1158/0008-5472.Can-14-1149. [DOI] [PubMed] [Google Scholar]
- 195.Kapp K, Volz B, Oswald D, Wittig B, Baumann M, Schmidt M. Beneficial modulation of the tumor microenvironment and generation of anti-tumor responses by TLR9 agonist lefitolimod alone and in combination with checkpoint inhibitors. Oncoimmunology. 2019;8:e1659096. doi: 10.1080/2162402x.2019.1659096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wittig B, Schmidt M, Scheithauer W, Schmoll HJ. MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol. 2015;94:31–44. doi: 10.1016/j.critrevonc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 197.Schleimann MH, Kobberø ML, Vibholm LK, Kjær K, Giron LB, Busman-Sahay K. et al. TLR9 agonist MGN1703 enhances B cell differentiation and function in lymph nodes. EBioMedicine. 2019;45:328–40. doi: 10.1016/j.ebiom.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. K. Kapp1 BV, D. Oswald1, B. Wittig2, M. Schmidt. The TLR9 agonist lefitolimod modulates tumor microenvironment and improves anti-tumor effect of checkpoint inhibitors in vivo. Annals of Oncology 2017.
- 199.Yang G, Koo JE, Lee HE, Shin SW, Um SH, Lee JY. Immunostimulatory activity of Y-shaped DNA nanostructures mediated through the activation of TLR9. Biomed Pharmacother. 2019;112:108657. doi: 10.1016/j.biopha.2019.108657. [DOI] [PubMed] [Google Scholar]
- 200.Molavi O, Ma Z, Hamdy S, Lai R, Lavasanifar A, Samuel J. Synergistic antitumor effects of CpG oligodeoxynucleotide and STAT3 inhibitory agent JSI-124 in a mouse melanoma tumor model. Immunol Cell Biol. 2008;86:506–14. doi: 10.1038/icb.2008.27. [DOI] [PubMed] [Google Scholar]
- 201.Zhao X, Zhang Z, Moreira D, Su YL, Won H, Adamus T. et al. B Cell Lymphoma Immunotherapy Using TLR9-Targeted Oligonucleotide STAT3 Inhibitors. Mol Ther. 2018;26:695–707. doi: 10.1016/j.ymthe.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gallotta M, Assi H, Degagne E, Kannan SK, Coffman RL, Guiducci C. Inhaled TLR9 Agonist Renders Lung Tumors Permissive to PD-1 Blockade by Promoting Optimal CD4(+) and CD8(+) T cell Interplay. Cancer Res. 2018;78:4943–56. doi: 10.1158/0008-5472.CAN-18-0729. [DOI] [PubMed] [Google Scholar]
- 203.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS. et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F. et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–46. doi: 10.1172/jci23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Molenkamp BG, van Leeuwen PA, Meijer S, Sluijter BJ, Wijnands PG, Baars A. et al. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin Cancer Res. 2007;13:2961–9. doi: 10.1158/1078-0432.Ccr-07-0050. [DOI] [PubMed] [Google Scholar]
- 206.Pashenkov M, Goëss G, Wagner C, Hörmann M, Jandl T, Moser A. et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–24. doi: 10.1200/jco.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 207.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M. et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–68. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 208.Kim YH, Girardi M, Duvic M, Kuzel T, Link BK, Pinter-Brown L. et al. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T cell lymphoma. J Am Acad Dermatol. 2010;63:975–83. doi: 10.1016/j.jaad.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 209.Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG. et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:211–7. doi: 10.3109/10428194.2011.608451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ursu R, Carpentier A, Metellus P, Lubrano V, Laigle-Donadey F, Capelle L. et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric, randomised study. Eur J Cancer. 2017;73:30–7. doi: 10.1016/j.ejca.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 211.Koster BD, van den Hout M, Sluijter BJR, Molenkamp BG, Vuylsteke R, Baars A. et al. Local Adjuvant Treatment with Low-Dose CpG-B Offers Durable Protection against Disease Recurrence in Clinical Stage I-II Melanoma: Data from Two Randomized Phase II Trials. Clin Cancer Res. 2017;23:5679–86. doi: 10.1158/1078-0432.CCR-17-0944. [DOI] [PubMed] [Google Scholar]
- 212.Schmoll HJ, Wittig B, Arnold D, Riera-Knorrenschild J, Nitsche D, Kroening H. et al. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol. 2014;140:1615–24. doi: 10.1007/s00432-014-1682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weihrauch MR, Richly H, von Bergwelt-Baildon MS, Becker HJ, Schmidt M, Hacker UT. et al. Phase I clinical study of the toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours. Eur J Cancer. 2015;51:146–56. doi: 10.1016/j.ejca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 214.Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A. et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8⁺ T cell responses in melanoma patients. Eur J Immunol. 2012;42:3049–61. doi: 10.1002/eji.201142361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JSK, Blasinska-Morawiec M. et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. 2012;23:72–7. doi: 10.1093/annonc/mdr030. [DOI] [PubMed] [Google Scholar]
- 216.Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE. et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2667–74. doi: 10.1200/jco.2010.32.8971. [DOI] [PubMed] [Google Scholar]
- 217.Leonard JP, Link BK, Emmanouilides C, Gregory SA, Weisdorf D, Andrey J. et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin's lymphoma. Clin Cancer Res. 2007;13:6168–74. doi: 10.1158/1078-0432.Ccr-07-0815. [DOI] [PubMed] [Google Scholar]
- 218.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH. et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/jco.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ai WYZ, Kim Y, Hoppe RT, Shah S, Horning SJ, Tibshirani R. et al. Preliminary Report on a Phase I/II Study of Intratumoral Injection of PF-3512676 (CpG 7909), a TLR9 Agonist, Combined with Radiation in Recurrent Low-Grade Lymphomas. Blood. 2006;108:2716. [Google Scholar]
- 220.Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK. et al. In Situ Vaccination with a TLR9 Agonist and Local Low-Dose Radiation Induces Systemic Responses in Untreated Indolent Lymphoma. Cancer Discov. 2018;8:1258–69. doi: 10.1158/2159-8290.Cd-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Levy R, Reagan PM, Friedberg JW, Bartlett NL, Gordon LI, Leung A. et al. SD-101, a Novel Class C CpG-Oligodeoxynucleotide (ODN) Toll-like Receptor 9 (TLR9) Agonist, Given with Low Dose Radiation for Untreated Low Grade B-Cell Lymphoma: Interim Results of a Phase 1/2 Trial. Blood. 2016;128:2974. doi: 10.1182/blood.V128.22.2974.2974. [DOI] [Google Scholar]
- 222.Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ. et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discovery. 2018;8:1250–7. doi: 10.1158/2159-8290.Cd-18-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Focused on providing solutions for patients with significant unmet medical needs. https://www.iderapharma.com/pipeline-programs/.
- 224. Haymaker C. Reactivating the anti-tumor response by targeting innate and adaptive immunity in a trial of intratumoral IMO 2125. Oral Presentation 2016.
- 225.Thomas M, Ponce-Aix S, Navarro A, Riera-Knorrenschild J, Schmidt M, Wiegert E. et al. Immunotherapeutic maintenance treatment with toll-like receptor 9 agonist lefitolimod in patients with extensive-stage small-cell lung cancer: results from the exploratory, controlled, randomized, international phase II IMPULSE study. Ann Oncol. 2018;29:2076–84. doi: 10.1093/annonc/mdy326. [DOI] [PMC free article] [PubMed] [Google Scholar]


