Abstract
Background:
Despite reductions in exposure for workers and the general public, radon remains a leading cause of lung cancer. Prior studies of underground miners depended heavily upon information on deaths among miners employed in the early years of mine operations when exposures were high and tended to be poorly estimated.
Objectives:
To strengthen the basis for radiation protection, we report on the follow-up of workers employed in the later periods of mine operations for whom we have more accurate exposure information and for whom exposures tended to be accrued at intensities that are more comparable to contemporary settings.
Methods:
We conducted a pooled analysis of cohort studies of lung cancer mortality among 57,873 male uranium miners in Canada, Czech Republic, France, Germany, and the United States, who were first employed in 1960 or later (thereby excluding miners employed during the periods of highest exposure and focusing on miners who tend to have higher quality assessments of radon progeny exposures). We derived estimates of excess relative rate per 100 working level months (ERR/100 WLM) for mortality from lung cancer.
Results:
The analysis included person-years of observation and 1,217 deaths due to lung cancer. The relative rate of lung cancer increased in a linear fashion with cumulative exposure to radon progeny (ERR/100 ; 95% CI: 0.89, 1.88). The association was modified by attained age, age at exposure, and annual exposure rate; for attained ages , the ERR/100 WLM was 8.38 (95% CI: 3.30, 18.99) among miners who were exposed at of age and at annual exposure rates of working levels. This association decreased with older attained ages, younger ages at exposure, and higher exposure rates.
Discussion:
Estimates of association between radon progeny exposure and lung cancer mortality among relatively contemporary miners are coherent with estimates used to inform current protection guidelines. https://doi.org/10.1289/EHP10669
Introduction
Following World War II, the development of technologies for nuclear weapons, power generation, and propulsion led to a surge in demand for uranium.1 As a result, hundreds of thousands of miners have been engaged in uranium mining over the last 75 y.2 Exposure to radon and its progeny is an occupational hazard of underground mining and an established cause of lung cancer.3 Exposure to radon is also a problem for the population generally because it is ubiquitous in indoor environments, sometimes reaching concentrations as high as those encountered in mines.4 To guide policies and regulations to protect both the public and workers, the epidemiological studies of underground miners have been used to derive risk models, beginning in the 1980s.
A major update to these models was reported in the Biological Effects of Ionizing Radiation (BEIR) VI report released in 1999.5 The report’s models were based on analyses of an international pooled study of lung cancer among underground miners that included cohorts of uranium, as well as tin, iron, and fluorspar, miners from Australia, Canada, China, Czech Republic, France, Sweden, and the United States.6 These BEIR VI risk models have been influential for setting residential and occupational radiation protection standards and guidelines for radon and its progeny.5,7–11 In the decades since that pooled study, many of the uranium miner cohorts have been updated,12–15 and a new large German cohort study of uranium miners was established.16–18 The resulting data from this expanded set of uranium miner cohorts provides an opportunity to update radon risk models and address critical sources of uncertainty regarding the risks of contemporary occupational and residential radon exposures.
The Pooled Uranium Miners Analysis (PUMA) brings together data from cohorts of uranium miners from Canada, Czech Republic, France, Germany, and the United States. These are among the largest and most informative cohorts of uranium miners in the world.19 A first article analyzed causes of death in regard to national mortality rates,20 and analyses of the association between cumulative exposure to radon progeny and mortality are ongoing. Here, we report on the results of an analysis of miners first employed in 1960 or later, thereby excluding miners employed during the periods of highest exposure and focusing on those miners who tend to have higher-quality assessments of radon progeny exposures that occurred at lower exposure rates. This is important because residential, as well as most contemporary occupational, exposures to radon progeny occur at low rates [annual exposure rates working level (WL)],4,21 and the availability of results derived from studies of individuals with low exposure rates is of substantial interest for radiation protection.11 Therefore, our focus in the present analysis is on estimates of the risk of lung cancer after exposure to low-level radon progeny, which is the setting of primary concern in contemporary radiation protection.
Materials and Methods
The protocol for pooling the data from participating cohorts has been previously described.19 The PUMA project was established to undertake combined analyses of cohorts of uranium miners, including open pit, underground, and surface workers.19 People who were only ever employed as millers are not included in the study. The PUMA is restricted to cohorts of uranium miners; consequently, the PUMA project does not include the three non-uranium miner cohorts that were included in the report from the U.S. National Academies of Sciences BEIR VI committee,6,22 namely tin miners from China,23 fluorspar miners from Canada,24 and iron miners from Sweden.25,26 The PUMA project only includes cohorts of uranium miners for which there are quantitative estimates of exposure to radon progeny and for which there are peer-reviewed published results, as well as an ongoing active research program.19 Thus, the Radium Hill cohort in Australia was excluded, although it was part of the earlier pooled analysis.5 Only a few cohorts included women and their total number in the PUMA study was small ().19 We therefore restricted this study to male miners in the PUMA project. The PUMA project pools information from seven cohorts of uranium miners (Table 1).
Table 1.
Description of individual study cohorts in the present analysis. Pooled Uranium Miners Analysis (PUMA) of uranium miners in Canada, Czech Republic, France, Germany, and the United States, male miners hired in 1960 or later.
| Study | Location | Period of follow-up | Miners | Average age at first exposure (y) | Average duration of employment (y) | Average duration of follow-up (y) | Mean cumulative exposure [WLM (max)] | Mean annual exposure rate [WL, (max)] |
|---|---|---|---|---|---|---|---|---|
| Eldorado27 | Canada | 1960–1999 | 6,593 | 27.2 | 2.0 | 25.0 | 6.6 (768.6) | 0.2 (26.1) |
| Ontario28 | Canada | 1960–2007 | 15,810 | 27.5 | 5.8 | 29.5 | 6.2 (232.3) | 0.4 (2.4) |
| Czech Republic14 | Czech Republic | 1960–2014 | 5,532 | 24.3 | 6.4 | 35.3 | 6.6 (91.9) | 0.2 (0.8) |
| France13 | France | 1960–2007 | 2,159 | 27.5 | 16.9 | 28.9 | 12.1 (118.3) | 0.1 (0.6) |
| Wismut16 | Germany | 1960–2013 | 25,067 | 21.2 | 10.0 | 36.2 | 18.4 (334.8) | 0.3 (4.6) |
| New Mexico29 | USA | 1960–2012 | 2,537 | 26.8 | 9.2 | 37.6 | 38.5 (461.7) | 4.7 (156.6) |
| Colorado Plateau12 | USA | 1960–2005 | 175 | 28.6 | 2.2 | 35.1 | 192.8 (1,849.9) | 7.5 (135.1) |
Note: max, maximum; WL, working level; WLM, working level months.
Data were obtained for miners employed by the Eldorado Mining and Refining Company at the Port Radium mine in the Northwest Territories, Canada, at the Beaverlodge uranium mine in Saskatchewan, Canada, and miners from Ontario, Canada;27,28 Western and Central Bohemia, Czech Republic;14 miners employed by the Commissariat à l’Energie Atomique–Compagnie Générale des Matières Nucléaires (CEA-COGEMA) primarily working in the regions of Limousin, Vendée, Forez, and Hérault, France;13 miners employed by the Wismut company in the regions of Saxony and Thuringia, Germany;16 and miners in New Mexico and in the Colorado Plateau region of the United States.12,29 Worker information was taken from existing records, with no direct contact with any cohort member; because there is minimal risk to cohort members, the associated institutional review boards waived requirements for informed consent. All aspects of the study protocol were approved by the institutional review boards of the University of North Carolina at Chapel Hill, North Carolina.
Vital status was ascertained through linkages with either registration offices, local health offices, death registries, employer and tax records, and Social Security Administration records, depending on the cohort. The underlying cause of death was coded according to the International Classification of Diseases.30 The outcome of interest in these analyses is death due to lung cancer (International Classification of Diseases, Ninth Revision code 162).
Cumulative exposure to radon decay products, expressed as working level months (WLMs), was calculated as the product of time in a workplace (in units of working months) and concentration of radon decay products in the workplace air (in units of working levels), where 1 WL denotes the total potential alpha energy contained in of air upon complete decay of the short-lived radon progeny. Individual annual estimates of WLM are available for all miners and are the estimates of WLM used in prior analyses of each of the participating cohort studies. Individual estimates of radon exposure were primarily based on ambient measurements of radon decay products in each mine and, in some cases, personal portable monitoring, supplemented in some of the earlier cohorts by estimation.19
For the present analysis, we restricted the study to miners first employed in 1960 or later. These are miners who typically encountered low concentrations of radon progeny in the workplace and for whom estimates of the concentration of radon decay products in the workplace air are based primarily on contemporaneous area monitoring of radon decay products and individual measurements. A major factor contributing to the relatively low-level exposures to radon progeny experienced by uranium miners employed in 1960 or later was the introduction of mechanical ventilation of uranium mines for air exchange that occurred around 1955 in the Czech,31 German,16 and French mines13 and around 1960 in the Canadian and U.S. mining industry; these interventions were often accompanied by improvements in exposure monitoring for radiation protection purposes.5,28,32 More than 90% of the employed working years in 1960 or later involved exposures to radon progeny at annual exposure rates of WL.
Statistical Methods
Person-years and lung cancer deaths were tabulated in categories defined by the cross-classification of cumulative exposure to radon progeny, attained age, calendar year, study cohort, and employment duration. Attained age and calendar year were categorized in 5-y intervals, seven categories of study cohort were defined, and employment duration was defined in three categories (, , and years). Cumulative radon progeny exposure was treated in a time-dependent fashion and was calculated as the sum of annual estimates of WLM that were recorded on a calendar year basis. In all analyses, cumulative exposures were tabulated under a minimal 5-y lag assumption to allow for the development of death from cancer and to facilitate comparison with other studies of lung cancer among underground miners.5,18 Age at exposure was assigned to annual exposure estimates based on a miner’s age at the midpoint of each calendar year. Annual exposure rate (in units of WL) was assigned to annual exposure estimates based on the estimated number of working months that a miner was employed in a calendar year. Cumulative exposure groups were defined as 0, , , , , , , , , , , , , , , , , , , , , , , and WLM. Cumulative exposures were calculated in two age-at-exposure windows ( or ), three time-since-exposure windows (5–14, 15–24, or y), and three annual exposure-rate windows (, , or WL). In each cell of the table (defined by the cross-classification of categories of covariates and cumulative exposure) the person-time weighted mean value of the cumulative exposures was calculated and used for estimation of exposure–response coefficients.33 Methods were applied for the analysis of grouped cohort data based on maximum likelihood estimation of Poisson regression models for mortality rates.5,6,34,35 Adjustment for potential confounding by attained age, calendar year, study cohort, and employment duration was obtained by background stratification.36 Cumulative exposure–lung cancer mortality associations were quantified using a model under which the relative rate of lung cancer mortality was a function of , where represents the excess relative rate per 100 working level months (ERR/100 WLM), and D represents the cumulative exposure to radon progeny. Departure from linearity in the effect of cumulative exposure was evaluated by fitting a model that included a higher-order polynomial function of that exposure. Heterogeneity of cumulative exposure–lung cancer mortality associations across study cohorts was assessed by inclusion of product terms between study cohort and cumulative radon exposure. We also classified cohorts as either North American or European and assessed evidence of heterogeneity between these groups.
Prior analyses suggest modification of the ERR/100 WLM by temporal factors.5,6,34,35 To allow for modification of the ERR/100 WLM with attained age, we fitted a model of the form , where parameters describe modification of the ERR/100 WLM, index categories defined by attained age (, 55–64, 65–74, y), and by definition for attained age . To allow for modification of the ERR/100 WLM across the two age-at-exposure windows, we fitted a model of the form , where , where the parameter describes modification by age at exposure, and D is partitioned into temporal exposure windows with and defining the cumulative exposures accrued at ages and . To further allow for modification of the ERR/100 WLM with annual exposure rate, that model was extended such that where and describe modification by annual exposure rate, and D is partitioned into exposure windows , , , , , and defining the cumulative exposures accrued at ages at annual exposure rates of , , and WL, and cumulative exposures accrued at ages at annual exposure rates of , , and WL, respectively.
To allow for modification of the ERR/100 WLM across the three time-since-exposure windows, we fitted a model of the form , where D is partitioned into temporal exposure windows with , , and defining the cumulative exposures accrued 5–14, 15–24, or prior to the current attained age, such that , where the parameters and describe modification by time since exposure. To further allow for modification of the ERR/100 WLM with annual exposure rate, that model was extended such that where index cumulative exposure in windows defined by time since exposure tse and annual exposure rate er.
Effect measure modification by temporal factors was assessed with regard to the change in deviance upon inclusion of the additional terms in the regression model. Noting the reduced power of such tests to detect a statistical interaction (relative to the power to detect main effects), we allowed for a type I error probability of 0.2, favoring an increase in model complexity if additional terms were unnecessarily included in the model over elimination of modifiers that could undermine validity and obscure important patterns of modification.37
We undertook sensitivity analyses in which we restricted analyses to the person-time and events at lower cumulative exposures ( WLM), and lower annual exposure rates ( WL). Sensitivity analyses were undertaken in which older miners (i.e., attained age ) were excluded owing to concerns about the accuracy of death certification at older ages. Sensitivity analyses were undertaken in which person-time and events contributed by miners with short terms of employment were excluded (i.e., those with employment durations of or ).
Estimates of excess relative rate of lung cancer per 100 WLM were derived using the SAS statistical software package (version 9.2) (SAS Institute Inc.) and the Epicure software package (Risk Sciences International).38,39 We present 95% likelihood-based confidence intervals (CIs) for estimated ERR/100 WLM coefficients.
Results
Table 1 describes the cohort-specific distribution of miners, exposures, duration of employment, and follow-up. The study cohorts included 57,873 male uranium miners who provided person-years of observation and 1,217 deaths due to lung cancer (Table 2). The Wismut and Ontario cohorts contributed the most information to the combined analysis; the French and Colorado cohorts contributed the least information. Few lung cancer deaths were observed at of age. Approximately 40% of the lung cancer deaths and 30% of person-years were contributed by people who were employed as uranium miners for .
Table 2.
Distribution of lung cancer deaths and person-year by categories of baseline covariates. Pooled Uranium Miners Analysis (PUMA) of uranium miners in Canada, Czech Republic, France, Germany, and the United States, male miners hired in 1960 or later.
| Categories | Lung cancer deaths | Person-years |
|---|---|---|
| Study cohort | ||
| Eldorado, Canada | 91 | 164,487 |
| Ontario, Canada | 299 | 466,968 |
| Czech Republic | 228 | 195,348 |
| France | 19 | 62,447 |
| Wismut, Germany | 470 | 894,313 |
| New Mexico, USA | 94 | 95,291 |
| Colorado Plateau, USA | 16 | 8,238 |
| Attained age (y) | ||
| 0 | 389,602 | |
| 30–34 | 4 | 242,794 |
| 35–39 | 15 | 257,214 |
| 40–44 | 43 | 256,061 |
| 45–49 | 99 | 232,433 |
| 50–54 | 141 | 189,375 |
| 55–59 | 218 | 142,030 |
| 60–64 | 272 | 92,862 |
| 65–69 | 217 | 50,056 |
| 70–74 | 134 | 22,655 |
| 75–79 | 56 | 8,151 |
| 80–84 | 15 | 2,783 |
| 3 | 1,076 | |
| Calendar year period | ||
| 1960–1964 | 0 | 16,330 |
| 1965–1969 | 4 | 55,353 |
| 1970–1974 | 12 | 101,027 |
| 1975–1979 | 32 | 172,896 |
| 1980–1984 | 51 | 241,895 |
| 1985–1989 | 89 | 267,255 |
| 1990–1994 | 146 | 270,309 |
| 1995–1999 | 191 | 262,268 |
| 2000–2004 | 256 | 223,778 |
| 2005–2009 | 275 | 181,722 |
| 2010–2014 | 161 | 94,259 |
| Duration of employment (y) | ||
| 529 | 979,454 | |
| 5– | 174 | 367,064 |
| 514 | 540,574 | |
| Total | 1,217 | 1,887,092 |
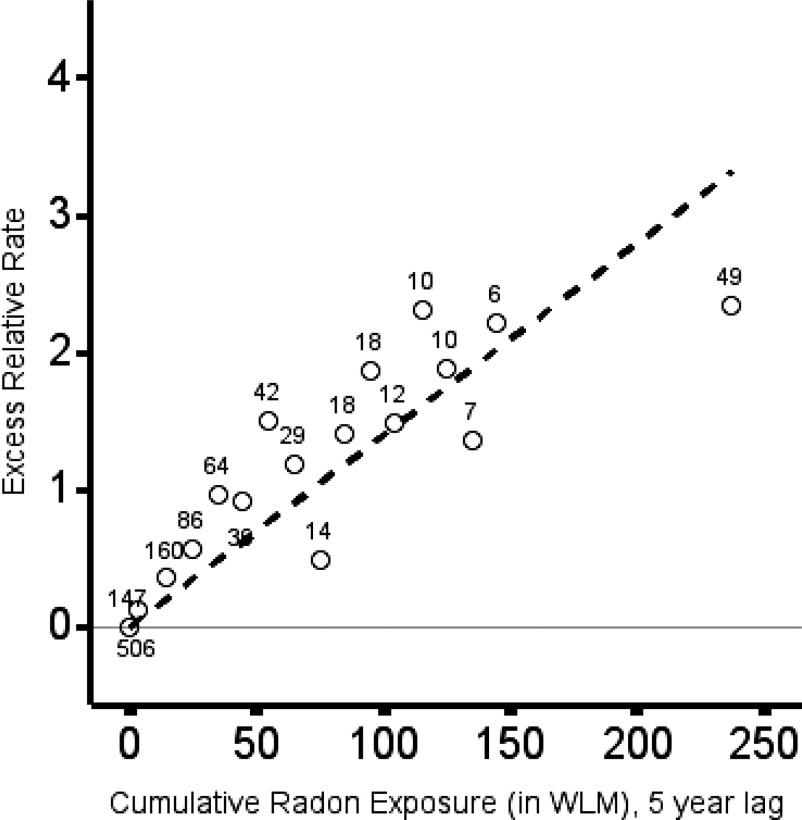
The estimate from a simple linear model for lung cancer mortality per cumulative exposure to radon progeny, lagged 5 y, was ERR/100 (95% CI: 0.89, 1.88). The distribution of lung cancer deaths by categories of cumulative exposure is indicated in Figure 1 (category-specific estimates are reported in Table S1). A linear model describing the association fitted the data well [Figure 1; linear fitted line displays ERR/100 (95% CI: 0.89, 1.88)]; and, a linear-quadratic model for the association led to negligible improvement in model goodness of fit compared with a simple linear model [, 1 degree of freedom (df), ; Table S2]. We investigated heterogeneity between study cohorts in the association between cumulative exposure to radon progeny and lung cancer mortality; a formal test for heterogeneity in a simple linear model by study cohort was rejected (, 6 df, ; Table S2). We also investigated heterogeneity in the association between cumulative exposure to radon progeny and lung cancer mortality between North American and European cohorts (, 1 df, ).
Figure 1.
Excess relative rate of lung cancer mortality (circles) and observed number of lung cancer deaths (numbers), by categories of cumulative exposure to radon progeny (category-specific estimates of excess relative rate of lung cancer mortality are reported in Table S1). Simple linear model for the association between cumulative exposure, lagged 5 y, and lung cancer mortality [dashed line: ERR/100 (95% CI: 0.89, 1.88)]. Background stratified by study cohort, attained age, calendar period, and duration of employment as a uranium miner. Pooled Uranium Miners Analysis (PUMA) of uranium miners in Canada, Czech Republic, France, Germany, and the United States, male miners hired in 1960 or later. Note: CI, confidence interval; ERR, excess relative rate; WLM, working level months.
A model that allowed for modification of the association by attained age fitted the data better than a simple linear model for the association between cumulative exposure and lung cancer mortality (, 3 df, ; Table S2). At attained ages , the ERR/100 WLM was 3.24 (95% CI: 1.58, 5.91), and this association decreased monotonically with increasing attained age. Allowing modification by attained age and age at exposure provided additional improvement in fit compared with a model that allowed for modification of the association by attained age (, 1 df, ; Table S2); the estimated magnitude of association diminished with increasing attained ages and diminished when radon progeny exposures occurred at younger ages (). Further allowing for effect modification by annual exposure rate provided a substantial additional improvement in fit compared with a model that allowed for modification of the association by attained age and age at exposure (, 2 df, ; Table S2). For attained ages , the ERR/100 WLM was 8.38 (95% CI: 3.30, 18.99) among miners who were exposed at of age and at annual exposure rates WL. This estimated association decreased with older attained ages, younger ages at exposure, and when the annual exposure rate was WL (Table 3).
Table 3.
Regression estimates of the excess relative rate (ERR) of lung cancer mortality per 100 working level months (WLMs) with effect modification by attained age, age at exposure, and exposure rate. Pooled Uranium Miners Analysis (PUMA) of uranium miners in Canada, Czech Republic, France, Germany, and the United States, male miners hired in 1960 or later.
| Parameters | Estimate | 95% CI |
|---|---|---|
| ERR/100 WLM | 8.38 | (3.30, 18.99) |
| Age at exposure (y) | ||
| 1 | — | |
| 0.59 | (0.30, 1.20) | |
| Attained age (y) | ||
| 1 | — | |
| 55–64 | 0.55 | (0.24, 1.30) |
| 65–74 | 0.20 | (0.06, 0.53) |
| 0.14 | (n.d., 0.64) | |
| Exposure rate (WL) | ||
| 1 | — | |
| 0.5– | 1.23 | (0.49, 2.77) |
| 0.33 | (0.13, 0.75) | |
Note: Background stratified by attained age, calendar period, study cohort, and duration of employment as a uranium miner. —, not applicable; CI, confidence interval; n.d., lower bound not determined; WL, working level.
In a sensitivity analysis of person-time and events restricted to WLM, WL, or attained age , the estimated ERR/100 WLM, as well as estimated coefficients describing effect measure modification, were comparable to the estimate obtained over the full range of cumulative exposure (Table 4). Similar results were obtained in a sensitivity analysis restricted to person-time and events observed among miners with a duration of employment of or (Table S3). In a sensitivity analysis, we excluded from analysis the person-time and events accrued among miners employed for a duration of and obtained estimates of ERR/100 WLM similar to (albeit slightly smaller than) those obtained using information for the full study base with adjustment for categories of employment duration (Table S3).
Table 4.
Sensitivity of regression estimates of the excess relative rate (ERR) of lung cancer mortality per 100 working level months (WLMs) to restrictions based on attained age, cumulative exposure, or exposure rate. Pooled Uranium Miners Analysis (PUMA) of uranium miners in Canada, Czech Republic, France, Germany, and the United States, male miners hired in 1960 or later.
| Person-time and events restricted to: | Attained age ya | WLMb | WLc | |||
|---|---|---|---|---|---|---|
| Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | |
| ERR/100 WLM | 7.82 | (2.95, 17.99) | 7.97 | (2.43, 16.25) | 7.56 | (2.58, 22.62) |
| Age at exposure (y) | ||||||
| 1 | — | 1 | — | 1 | — | |
| 0.61 | (0.31, 1.29) | 0.59 | (0.26, 1.40) | 0.62 | (0.24, 1.66) | |
| Attained age (y) | ||||||
| 1 | — | 1 | — | 1 | — | |
| 55–64 | 0.55 | (0.24, 1.29) | 0.83 | (0.30, 2.72) | 0.75 | (0.24, 2.64) |
| 65–74 | 0.20 | (0.06, 0.54) | 0.19 | (0.04, 0.86) | 0.12 | (0.01, 0.63) |
| — | 0.12 | (0.00, 1.22) | 0.43 | (0.06, 2.44) | ||
| Exposure rate (WL) | ||||||
| 1 | — | 1 | — | 1 | — | |
| 0.5– | 1.26 | (0.49, 2.88) | 0.97 | (0.17, 2.59) | 0.79 | (0.04, 2.37) |
| 0.37 | (0.15, 0.85) | 0.30 | (, 1.14) | — | — | |
| Lung cancer deaths () | 1,143 | — | 1,000 | — | 962 | — |
| Person-years () | 18.75 | — | 17.93 | — | 17.43 | — |
Note: Adjusted for attained age, calendar period, study cohort, and duration of employment as a uranium miner. —, not applicable; WL, working level.
Restricted to person-time and events for which attained age .
Restricted to person-time and events for which cumulative exposure, lagged 5 y, WLM.
Restricted to person-time and events for which annual exposure rate WL.
Finally, we fitted an alternative model for the cumulative radon exposure–lung cancer mortality association that allowed for modification by time since exposure (Table S4). In the period 5–14 y after exposure, the ERR/100 WLM was 2.86 (95% CI: 0.47, 6.71) and the magnitude of the estimate of association decreased with increasing time since exposure. However, a model that allowed for modification of the association by time since exposure did not fit as well as a model that allowed for modification of the association by attained age (Table S2, model 7). A model that allowed for modification by attained age, time since exposure, and exposure rate describes a set of effect measure modifiers that is similar to the BEIR VI model5; the estimated ERR/100 WLM was highest in the earliest window of time since exposure (5–14 y after exposure), youngest attained age category (attained age ), and at lower annual exposure rates (annual exposure rates WL). The estimated magnitude of association between exposure to radon progeny and lung cancer mortality diminished at older attained ages, diminished when radon progeny annual exposure rates were WL, and diminished with increasing time since exposure (Table 5). However, that model did not fit the data better than a model that allows for effect modification by attained age, age at exposure, and annual exposure rate, and required a larger number of estimated model parameters (Table S2).
Table 5.
Comparison of reported estimates for the exposure–age–concentration model shown in the U.S. National Academy of Science’s BEIR VI committee report (without restriction by calendar period of hire) with those estimates obtained fitting a similar model to the Pooled Uranium Miners Analysis (PUMA) of uranium miners in Canada, Czech Republic, France, Germany, and the United States, male miners hired in 1960 or later.
| BEIR VI reporta | PUMAb | ||
|---|---|---|---|
| Estimate (SE)c | Estimate | (95% CI) | |
| ERR/100 WLM | 7.68 (1.94) | 6.98 | (1.97, 16.15) |
| Time since exposure (y) | |||
| 5–14 | 1. | 1. | — |
| 15–24 | 0.78 | 0.64 | (0.17, 2.43) |
| 0.51 | 0.89 | (0.34, 3.01) | |
| Attained age (y) | |||
| 1.00 | 1.00 | — | |
| 55–64 | 0.57 | 0.64 | (0.25, 1.68) |
| 65–74 | 0.29 | 0.22 | (0.06, 0.67) |
| 0.09 | 0.17 | (n.d., 0.85) | |
| Mean exposure rate (WL) | |||
| 1. | — | — | |
| 0.5–1.0 | 0.49 | — | — |
| 1.0–3.0 | 0.37 | — | — |
| 3.0–5.0 | 0.32 | — | — |
| 5.0–15.0 | 0.17 | — | — |
| 0.11 | — | — | |
| Annual exposure rate (WL) | |||
| — | 1. | — | |
| 0.5–1.0 | — | 1.00 | (0.38, 2.36) |
| — | 0.29 | (0.11, 0.68) | |
Note: Cohorts of uranium miners in Canada, Czech Republic, France, and the United States are included in both the BEIR VI report and the PUMA analysis. —, not applicable; BEIR VI, Biological Effects of Ionizing Radiation VI; ERR/100 WLM, excess relative rate per 100 working level months; n.d., not determined; SE, standard error; WL, working level.
Adjusted for attained age, calendar period and study cohort.
Adjusted for attained age, calendar period, study cohort, and duration of employment as a uranium miner.
Approximate standard error; in the BEIR VI report this is reported as exp[sqrt(var[])].
Discussion
Despite reductions in exposure for workers and the general population, radon remains a leading cause of lung cancer.5 The potential for relatively high occupational exposure still occurs in metal and nonmetal underground mines, particularly phosphate, fluorspar, talc, and slate mines, where concentrations of airborne radon progeny can reach or exceed the radon levels typically encountered in uranium mines.40,41 Internationally, there are almost workers employed in underground metal and nonmetal mines.42 Aside from underground mining, there are other workplaces where radon can pose a significant hazard, including workplaces below ground, such as subways, tunnels, utility service ducts, underground parking, tourist caves, and waste repositories. In addition, there are many above-ground workplaces where high levels of radon progeny may occur, including groundwater treatment facilities and workplaces where large quantities of materials with elevated radium concentrations are stored or processed, such as phosphate fertilizer plants and monazite sands mining, oil refineries, and natural gas and oil piping facilities.43 Exposure to radon progeny also occurs in residential settings, where concentrations are typically low but may vary markedly depending upon geology, building construction, ventilation, and heating.43 Policies to reduce indoor radon have been controversial, in part because of uncertainties concerning risks at typical indoor radon levels. The PUMA project provides a foundation for addressing these uncertainties in a new generation of risk models.
Since the report by the BEIR IV Committee in 1988, which pooled four miner studies,22 one of the primary quantitative sources of information regarding the association between exposure to radon progeny and lung cancer has been the epidemiological studies of miners.5,44,45 Many of those studies drew heavily on deaths among miners employed in the early years of mine operations when exposures were highest.5,6,22 Recently, however, new cohorts of uranium miners have been enumerated, existing cohorts have been expanded, and follow-up has been extended substantially.11 We now have long-term follow-up of workers employed in more contemporary periods of mine operation for whom we have better exposure information and for whom exposures tended to be accrued at lower intensities more relevant to contemporary settings.
The PUMA study provides clear evidence of positive associations between protracted low-level radon progeny exposure and lung cancer mortality. The association appears linear in the low exposure range (Figure 1), consistent with theoretical and experimental work that suggests a linear exposure–response pattern for radon exposure and lung cancer at low annual exposure rates.5 It has been posited that exposures to low concentrations of radon progeny result in a higher excess risk of lung cancer per unit exposure than exposures to higher concentrations of radon progeny (a so-called inverse dose rate effect).5 Findings from our analyses of contemporary miners, who primarily experienced exposures to low concentrations of radon progeny, support current risk estimates that were derived from earlier epidemiological studies of underground miners; our findings directly address questions regarding effects of low-level exposures that are of primary concern today.10,46,47 Our subcohort analyses restricted to WL annual exposure rate further focus the analysis on effects observed at low radon exposure rates.
The reduced measurement error in radon exposure assessments, as a consequence of our restriction to more recent employment periods, is a strength of our analysis. In the early years of operations, working conditions in uranium mines were poor and exposures were high and often poorly characterized.5,6,22,44 For example, prior to 1955 in French and German uranium mines and prior to 1950 in the Colorado Plateau mines, there was no systematic exposure assessment. Annual radon exposure estimates for these early periods were reconstructed by expert ratings. Subsequently, ambient measurements were taken, and in France, for example, personal dosimetry was introduced in 1983. The size of measurement errors decreased over time and became relatively small in the 1960s and later when compared with the early years of operation when contemporaneous measurements often were entirely lacking.32,48,49 The impact of exposure measurement error, particularly associated with the early years of operation, has been a major concern in prior studies of radon exposure and lung cancer among underground miners. There also have been concerns that trends in data quality could influence not only an estimate of the overall radon-associated lung cancer risk coefficient but also estimates of parameters that describe the relationship of excess risk with time-related variables, such as exposure rate and time since exposure.5,22
Of course, restriction of the PUMA cohort to miners hired in 1960 or later reduces person-years when compared with analyses of the full PUMA cohort, with small contributions made by cohorts that are predominately constituted by miners employed in earlier periods. Restriction to the relatively shorter follow-up of more contemporary miners also may limit characterization of temporal modifiers of the association. However, in the current analysis, we have information derived from decades of follow-up for those employed since 1960, and this restriction to more contemporary workers may strengthen inference about temporal modifiers because it reduces concern about the influence of uncertainties in exposure estimates on parameters that describe time-related modifiers of the association of interest.
Characterization of temporal modifiers of radon-associated lung cancer risks is important for risk assessments for both workers and the general population. Models that imply that effects diminish with attained age and age at exposure, for example, imply markedly different projections of population attributable risk than models that do not. For miners hired in 1960 or later, a model that allows for effect measure modification by attained age, age at exposure, and annual exposure rate fitted better, and required fewer estimated model parameters than a model that allowed effect measure modification by time since exposure, attained age, and exposure rate (such as employed in the report of the BEIR VI committee, a prior pooled analysis of Czech and French miners,31 and an analysis of German miners17) (Table S2). Although prior work has suggested that a model that allows for effect modification by age at exposure should be adjusted for time since exposure,14,31,50 (a related, albeit slightly different, timescale to attained age), we note that age at exposure plus attained age defines time since exposure. With continued follow-up of these cohorts, the information available to understand temporal modifiers of the association between radon and lung cancer among miners hired in 1960 or later is expected to increase substantially.
We lack individual-level information for many miners in the PUMA project on smoking and occupational risk factors, such as silica, arsenic, and diesel exhaust. These exposures might confound or modify the association between radon progeny and lung cancer. Prior analyses of individual cohorts in the PUMA project have assessed confounding by smoking based on smoking history information collected for miners hired in 1960 or later in the Wismut, Colorado Plateau, and New Mexico cohorts, as well as information collected for nested case–control subsamples of the Czech, Canadian, and French cohorts. Those prior analyses indicate minimal evidence of confounding of the radon progeny–lung cancer association by smoking in the Wismut,51,52 Colorado Plateau,12 New Mexico,29 Czech,53 Canadian Beaverlodge,54 and French55,56 cohorts. Prior case–control analyses of European uranium miners suggested that the ERR/100 WLM was larger for nonsmokers than smokers, and a marginal estimate was weighted toward the association among smokers, reflecting the high prevalence of smoking among miners.55,56 We could not assess effect measure modification by tobacco smoking in the PUMA project; consequently, our results reflect a weighted average of associations for smokers and nonsmokers, and given the high prevalence of smokers in the PUMA project,19 will tend to be weighted toward the radon–lung cancer association among smokers. We therefore suggest caution when transporting estimates between populations with differing smoking distributions. Prior work suggests that neither arsenic nor diesel exhaust are strong confounders,57 in part due to their relatively weak associations with lung cancer and in part due to weak associations with cumulative radon exposure. For example, diesel engine–powered equipment was used infrequently in the Beaverlodge mines in Canada because the vehicles there were primarily powered by electricity, and diesel engine–powered equipment was not used in the Czech mines because most ore transport was done manually in the Jachymov mines, and electric locomotives were used in the Pribram mines. Our previous report on standardized mortality analyses of the PUMA project compared cause-specific mortality in the cohort with that of the general population, which further contextualizes our results: Relative excesses of mortality due to cancers of the lung, liver and gallbladder, stomach, and pleura were observed, as well as deaths due to external causes and silicosis. Although excess silicosis mortality was observed, prior assessments of silica exposure suggest minimal positive confounding (i.e., away from the null) of the cumulative radon–lung cancer association.12,57,58
Our study outcomes were assessed using information on underlying cause of death as recorded on death certificates. One limitation of relying upon death certificates is that we do not have the ability to describe associations by histological subtypes. Moreover, death certificates are known to have imperfect sensitivity and specificity as a tool for ascertainment of cancers, including lung cancer,59,60 and the accuracy of cause of death coding may be particularly poor for deaths that occur at the oldest attained ages. To indirectly address the latter concern, we undertook sensitivity analyses that excluded person-time and events in the oldest attained age group and found similar results to that obtained without such exclusion. In addition, given trends in the diagnosis of lung cancer, we anticipated more accurate identification of deaths from lung cancer during the more recent follow-up time.
The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) has regularly reviewed the literature on effects of exposure to radon.7–10 The most recent UNSCEAR report included a meta-analysis of published estimates of the radon–lung cancer association (without effect measure modifiers) in analyses restricted to miners employed in recent periods (or miners having low exposure to radon). The corresponding estimate of ERR/100 (95% CI: 1.11, 1.94) is consistent with our linear estimate of the ERR/100 (95% CI: 0.89, 1.88) under a 5-y lag assumption.8 That UNSCEAR report also included a meta-analysis of published estimates of ERR/WLM derived from the full miner cohorts (i.e., with no restrictions based on period of employment or exposure).8 The ratio between the two meta-estimates was about a factor of two, with an estimated ERR/WLM that was higher when derived from analyses restricted to recent periods of employment/low radon exposure. Given that these meta-analyses did not include effect modifiers such as time since exposure, attained age, and exposure rate, the interpretation was considered limited and preference was therefore given to models that include effect modifiers for better comparability across studies and for improved risk transfer to specified combinations of modifying factors.8 This underscores the importance of results from the PUMA project, which allow examination of effect modification in pooled analyses based on common criteria applied to all constituent cohorts (Tables 3 and 4).
Characterization of the association between exposure to radon progeny and lung cancer is a foundation for risk assessments that inform public health decision-making and cancer prevention strategies.61 Improved knowledge of the relationship between lung cancer and radon at low exposure rates is needed for lifetime risk calculation and conversion of radon concentration into effective dose for contemporary occupational settings.62,63 The PUMA project substantially strengthens the quantitative estimates needed for radon risk assessments, and the present analysis, with its focus on relatively contemporary miners, notably allows us to directly address questions about the low-level exposures to radon that typically occur in contemporary workplaces and homes. The coherence of our findings with the models based on the earlier analyses that currently inform radiation protection guidelines for radon exposure reduces uncertainty and strengthens conclusions regarding the adequacy of those standards.
Supplementary Material
Acknowledgments
D.B.R. wrote the original draft. D.B.R., E.R., P.A.D., M.T.D., N.F., V.D., M.K., J.S., S.B., K.K.-R., M.S.B., L.T., L.B.Z., C.W., and D.L. contributed to writing, reviewing, and editing the paper.
This work was supported by the Centers for Disease Control and Prevention (CDC; R03 OH010946). The construction of the French cohort was partially supported by the Institute for Radiological Protection and Nuclear Safety (IRSN). IRSN thanks ORANO for its cooperation in the elaboration of the French cohort. For the U.S. contribution, funding was provided by the National Institute for Occupational Safety and Health. L.B.Z.’s work was funded and supported by the CDC in association with the National Institute for Occupational Safety and Health (NIOSH; R21OH011452). For the Czech cohort, funding was provided by the National Radiation Protection Institute (SURO; MV-25972-2/OBV). Work on the Ontario cohort was funded by the Canadian Nuclear Safety Commission, the Ontario Ministry of Labor, and the Canadian Cancer Society. The findings and conclusions of this report are those of the authors and do not necessarily reflect those of the NIOSH or the International Agency for Research on Cancer.
The funding sources had no role in the study design, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper for publication.
References
- 1.Rhodes R. 1986. The Making of the Atomic Bomb. New York, NY: Simon & Schuster, Inc. [Google Scholar]
- 2.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). 2017. Annex D: biological effects of selected internal emitters—uranium. In: Sources and Effects of Ionizing Radiation. UNSCEAR 2016 Report to the General Assembly. Scientific Annexes A, B, C and D. New York, NY: United Nations. [Google Scholar]
- 3.International Agency for Research on Cancer. 2012. A Review of Human Carcinogens. Part D: Radiation. Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 4.National Research Council Panel on Dosimetric Assumptions Affecting the Application of Radon Risk Estimates. 1991. Comparative Dosimetry of Radon in Mines and Homes. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 5.National Research Council Committee on Health Risks of Exposure to Radon. 1999. Health Effects of Exposure to Radon: BEIR VI. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 6.Lubin JH, Boice JD Jr, Edling C, Hornung RW, Howe G, Kunz E, et al. . 1994. Radon and Lung Cancer Risk: A Joint Analysis of 11 Underground Miners Studies. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- 7.United Nations Scientific Committee on the Effects of Atomic Radiation. 1988. Sources, Effects, and Risks of Ionizing Radiation. New York, NY: United Nations. [Google Scholar]
- 8.United Nations Scientific Committee on the Effects of Atomic Radiation. 2000. Sources, Effects, and Risks of Ionizing Radiation. New York, NY: United Nations. [Google Scholar]
- 9.United Nations Scientific Committee on the Effects of Atomic Radiation. 2006. Effects of Ionizing Radiation. New York, NY: United Nations. https://www.unscear.org/unscear/en/publications/2006_1.html. [Google Scholar]
- 10.United Nations Scientific Committee on the Effects of Atomic Radiation. 2019. Sources, Effects, and Risks of Ionizing Radiation. New York, NY: United Nations. [Google Scholar]
- 11.Tirmarche M, Harrison JD, Laurier D, Paquet F, Blanchardon E, Marsh JW, et al. . 2010. ICRP publication 115. Lung cancer risk from radon and progeny and statement on radon. Ann ICRP 40(1):1–64, PMID: , 10.1016/j.icrp.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Schubauer-Berigan MK, Daniels RD, Pinkerton LE. 2009. Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado Plateau cohort. Am J Epidemiol 169(6):718–730, PMID: , 10.1093/aje/kwn406. [DOI] [PubMed] [Google Scholar]
- 13.Rage E, Caër-Lorho S, Drubay D, Ancelet S, Laroche P, Laurier D. 2015. Mortality analyses in the updated French cohort of uranium miners (1946–2007). Int Arch Occup Environ Health 88(6):717–730, PMID: , 10.1007/s00420-014-0998-6. [DOI] [PubMed] [Google Scholar]
- 14.Tomasek L. 2012. Lung cancer mortality among Czech uranium miners—60 years since exposure. J Radiol Prot 32(3):301–314, PMID: , 10.1088/0952-4746/32/3/301. [DOI] [PubMed] [Google Scholar]
- 15.Navaranjan G, Berriault C, Do M, Villeneuve PJ, Demers PA. 2016. Cancer incidence and mortality from exposure to radon progeny among Ontario uranium miners. Occup Environ Med 73(12):838–845, PMID: , 10.1136/oemed-2016-103836. [DOI] [PubMed] [Google Scholar]
- 16.Kreuzer M, Schnelzer M, Tschense A, Walsh L, Grosche B. 2010. Cohort profile: the German uranium miners cohort study (WISMUT cohort), 1946–2003. Int J Epidemiol 39(4):980–987, PMID: , 10.1093/ije/dyp216. [DOI] [PubMed] [Google Scholar]
- 17.Kreuzer M, Sobotzki C, Schnelzer M, Fenske N. 2018. Factors modifying the radon-related lung cancer risk at low exposures and exposure rates among German uranium miners. Radiat Res 189(2):165–176, PMID: , 10.1667/RR14889.1. [DOI] [PubMed] [Google Scholar]
- 18.Walsh L, Tschense A, Schnelzer M, Dufey F, Grosche B, Kreuzer M. 2010. The influence of radon exposures on lung cancer mortality in German uranium miners, 1946–2003. Radiat Res 173(1):79–90, PMID: , 10.1667/RR1803.1. [DOI] [PubMed] [Google Scholar]
- 19.Rage E, Richardson DB, Demers PA, Do M, Fenske N, Kreuzer M, et al. . 2020. PUMA—Pooled Uranium Miners Analysis: cohort profile. Occup Environ Med 77(3):194–200, PMID: , 10.1136/oemed-2019-105981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson DB, Rage E, Demers PA, Do MT, DeBono N, Fenske N, et al. . 2021. Mortality among uranium miners in North America and Europe: the Pooled Uranium Miners Analysis (PUMA). Int J Epidemiol 50(2):633–643, PMID: , 10.1093/ije/dyaa195. [DOI] [PubMed] [Google Scholar]
- 21.Field RW, Smith BJ, Steck DJ, Lynch CF. 2002. Residential radon exposure and lung cancer: variation in risk estimates using alternative exposure scenarios. J Expo Anal Environ Epidemiol 12(3):197–203, PMID: , 10.1038/sj.jea.7500215. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council Committee on the Biological Effects of Ionizing Radiation (BEIR IV). 1988. Health Risks of Radon and other Internally Deposited Alpha-Emitters. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 23.Xuan XZ, Lubin JH, Li JY, Yang LF, Luo AS, Lan Y, et al. . 1993. A cohort study in southern China of tin miners exposed to radon and radon decay products. Health Phys 64(2):120–131, PMID: , 10.1097/00004032-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Villeneuve PJ, Morrison HI, Lane R. 2007. Radon and lung cancer risk: an extension of the mortality follow-up of the Newfoundland fluorspar cohort. Health Phys 92(2):157–169, PMID: , 10.1097/01.HP.0000239127.43136.89. [DOI] [PubMed] [Google Scholar]
- 25.Björ O, Damber L, Jonsson H, Nilsson T. 2015. A comparison between standard methods and structural nested modelling when bias from a healthy worker survivor effect is suspected: an iron-ore mining cohort study. Occup Environ Med 72(7):536–542, PMID: , 10.1136/oemed-2014-102251. [DOI] [PubMed] [Google Scholar]
- 26.Björ O, Jonsson H, Damber L, Wahlström J, Nilsson T. 2013. Reduced mortality rates in a cohort of long-term underground iron-ore miners. Am J Ind Med 56(5):531–540, PMID: , 10.1002/ajim.22168. [DOI] [PubMed] [Google Scholar]
- 27.Lane RSD, Frost SE, Howe GR, Zablotska LB. 2010. Mortality (1950–1999) and cancer incidence (1969–1999) in the cohort of Eldorado uranium workers. Radiat Res 174(6):773–785, PMID: , 10.1667/RR2237.1. [DOI] [PubMed] [Google Scholar]
- 28.Navaranjan G, Berriault C, Do M, Villeneuve PJ, Demers PA. 2015. Ontario Uranium Miners Cohort Study Report. Final report prepared for the Canadian Nuclear Safety Commission. Toronto, ON, Canada: University of Toronto. [Google Scholar]
- 29.Samet JM, Pathak DR, Morgan MV, Key CR, Valdivia AA, Lubin JH. 1991. Lung cancer mortality and exposure to radon progeny in a cohort of New Mexico underground uranium miners. Health Phys 61(6):745–752, PMID: , 10.1097/00004032-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization (WHO). 1998. International Classification of Diseases, Ninth Revision (ICD-9).
- 31.Tomasek L, Rogel A, Tirmarche M, Mitton N, Laurier D. 2008. Lung cancer in French and Czech uranium miners: radon-associated risk at low exposure rates and modifying effects of time since exposure and age at exposure. Radiat Res 169(2):125–137, PMID: , 10.1667/RR0848.1. [DOI] [PubMed] [Google Scholar]
- 32.Navaranjan G, Chambers D, Thompson PA, Do M, Berriault C, Villeneuve PJ, et al. . 2019. Uncertainties associated with assessing Ontario uranium miners’ exposure to radon daughters. J Radiol Prot 39(1):136–149, PMID: , 10.1088/1361-6498/aaf1eb. [DOI] [PubMed] [Google Scholar]
- 33.Richardson D, Loomis D. 2004. The impact of exposure categorization for grouped analyses of cohort data. Occup Environ Med 61(11):930–935, PMID: , 10.1136/oem.2004.014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubin JH. 1988. Models for the analysis of radon-exposed populations. Yale J Biol Med 61(3):195–214, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 35.Breslow NE, Day NE. 1987. Statistical Methods in Cancer Research. Vol II, The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer. [PubMed] [Google Scholar]
- 36.Richardson DB, Langholz B. 2012. Background stratified Poisson regression analysis of cohort data. Radiat Environ Biophys 51(1):15–22, PMID: , 10.1007/s00411-011-0394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvin S. 1996. Statistical Analysis of Epidemiologic Data. 2nd ed. New York, NY: Oxford University Press. [Google Scholar]
- 38.SAS Institute Inc. SAS OnlineDoc® 9.2. PDF Files. 2nd ed. Cary, NC: SAS Institute Inc. [Google Scholar]
- 39.Preston DL, Lubin JH, Pierce DA, McConney ME, Shilnikova NS. 2008. Epicure Risk Regression and Person-Year Computation Software: Command Summary and User Guide. Ottawa, ON, Canada: Risk Sciences International. [Google Scholar]
- 40.National Institute for Occupational Safety and Health. 1987. A Recommended Standard for Occupational Exposure to Radon Progeny in Underground Mines. Cincinnati, OH: National Institute for Occupational Safety and Health. [Google Scholar]
- 41.Daniels RD, Schubauer-Berigan MK. 2017. Radon in US workplaces: a review. Radiat Prot Dosimetry 176(3):278–286, PMID: , 10.1093/rpd/ncx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.International Atomic Energy Agency. 2004. Radiation, People and the Environment. Vienna, Austria: IAEA Publications. [Google Scholar]
- 43.Field RW. 1999. Radon occurrence and health risk. https://cheec.uiowa.edu/sites/cheec.uiowa.edu/files/radon_occ.pdf [accessed 16 May 2022].
- 44.Archer VE, Coons T, Saccomanno G, Hong DY. 2004. Latency and the lung cancer epidemic among United States uranium miners. Health Phys 87(5):480–489, PMID: , 10.1097/01.hp.0000133216.72557.ab. [DOI] [PubMed] [Google Scholar]
- 45.Sevc J, Kunz E, Placek V. 1977. Lung cancer in uranium miners and long-term exposure to radon daughter products. Health Phys 30:433–437, PMID: , 10.1097/00004032-197606000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Tomasek L. 2020. Lung cancer lifetime risks in cohort studies of uranium miners. Radiat Prot Dosimetry 191(2):171–175, PMID: , 10.1093/rpd/ncaa143. [DOI] [PubMed] [Google Scholar]
- 47.Tomasek L, Rogel A, Laurier D, Tirmarche M. 2008. Dose conversion of radon exposure according to new epidemiological findings. Radiat Prot Dosimetry 130(1):98–100, PMID: , 10.1093/rpd/ncn117. [DOI] [PubMed] [Google Scholar]
- 48.Allodji RS, Leuraud K, Bernhard S, Henry S, Bénichou J, Laurier D. 2012. Assessment of uncertainty associated with measuring exposure to radon and decay products in the French uranium miners cohort. J Radiol Prot 32(1):85–100, PMID: , 10.1088/0952-4746/32/1/85. [DOI] [PubMed] [Google Scholar]
- 49.Laurier D, Marsh JW, Rage E, Tomasek L. 2020. Miner studies and radiological protection against radon. Ann ICRP 49(1 suppl):57–67, PMID: , 10.1177/0146645320931984. [DOI] [PubMed] [Google Scholar]
- 50.Tomasek L. 2014. Effect of age at exposure in 11 underground miners studies. Radiat Prot Dosimetry 160(1–3):124–127, PMID: , 10.1093/rpd/ncu068. [DOI] [PubMed] [Google Scholar]
- 51.Kreuzer M, Fenske N, Schnelzer M, Walsh L. 2015. Lung cancer risk at low radon exposure rates in German uranium miners. Br J Cancer 113(9):1367–1369, PMID: , 10.1038/bjc.2015.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnelzer M, Hammer GP, Kreuzer M, Tschense A, Grosche B. 2010. Accounting for smoking in the radon-related lung cancer risk among German uranium miners: results of a nested case-control study. Health Phys 98(1):20–28, PMID: , 10.1097/HP.0b013e3181b8ce81. [DOI] [PubMed] [Google Scholar]
- 53.Tomasek L. 2013. Lung cancer risk from occupational and environmental radon and role of smoking in two Czech nested case-control studies. Int J Environ Res Public Health 10(3):963–979, PMID: , 10.3390/ijerph10030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.L’Abbé KA, Howe GR, Burch JD, Miller AB, Abbatt J, Band P, et al. . 1991. Radon exposure, cigarette smoking, and other mining experience in the Beaverlodge Uranium Miners Cohort. Health Phys 60(4):489–495, PMID: , 10.1097/00004032-199104000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Leuraud K, Billon S, Bergot D, Tirmarche M, Caër S, Quesne B, et al. . 2007. Lung cancer risk associated to exposure to radon and smoking in a case-control study of French uranium miners. Health Phys 92(4):371–378, PMID: , 10.1097/01.HP.0000252259.72683.2a. [DOI] [PubMed] [Google Scholar]
- 56.Leuraud K, Schnelzer M, Tomasek L, Hunter N, Timarche M, Grosche B, et al. . 2011. Radon, smoking and lung cancer risk: results of a joint analysis of three European case-control studies among uranium miners. Radiat Res 176(3):375–387, PMID: , 10.1667/rr2377.1. [DOI] [PubMed] [Google Scholar]
- 57.Sogl M, Taeger D, Pallapies D, Brüning T, Dufey F, Schnelzer M, et al. . 2012. Quantitative relationship between silica exposure and lung cancer mortality in German uranium miners, 1946–2003. Br J Cancer 107(7):1188–1194, PMID: , 10.1038/bjc.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samet JM, Pathak DR, Morgan MV, Coultas DB, James DS, Hunt WC. 1994. Silicosis and lung cancer risk in underground uranium miners. Health Phys 66(4):450–453, PMID: , 10.1097/00004032-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Percy C, Stanek E III, Gloeckler L. 1981. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health 71(3):242–250, PMID: , 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demers PA, Vaughan TL, Checkoway H, Weiss NS, Heyer NJ, Rosenstock L. 1992. Cancer identification using a tumor registry versus death certificates in occupational cohort studies in the United States. Am J Epidemiol 136(10):1232–1240, PMID: , 10.1093/oxfordjournals.aje.a116431. [DOI] [PubMed] [Google Scholar]
- 61.Rushton L, Hutchings SJ, Fortunato L, Young C, Evans GS, Brown T, et al. . 2012. Occupational cancer burden in Great Britain. Br J Cancer 107(suppl 1):S3–S7, PMID: , 10.1038/bjc.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsh JW, Tomášek L, Laurier D, Harrison JD. 2021. Effective dose coefficients for radon and progeny: a review of ICRP and UNSCEAR values. Radiat Prot Dosimetry 195(1):1–20, PMID: , 10.1093/rpd/ncab106. [DOI] [PubMed] [Google Scholar]
- 63.Harrison JD. 2021. Lung cancer risk and effective dose coefficients for radon: UNSCEAR review and ICRP conclusions. J Radiol Prot 41(2):433, PMID: , 10.1088/1361-6498/abf547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.