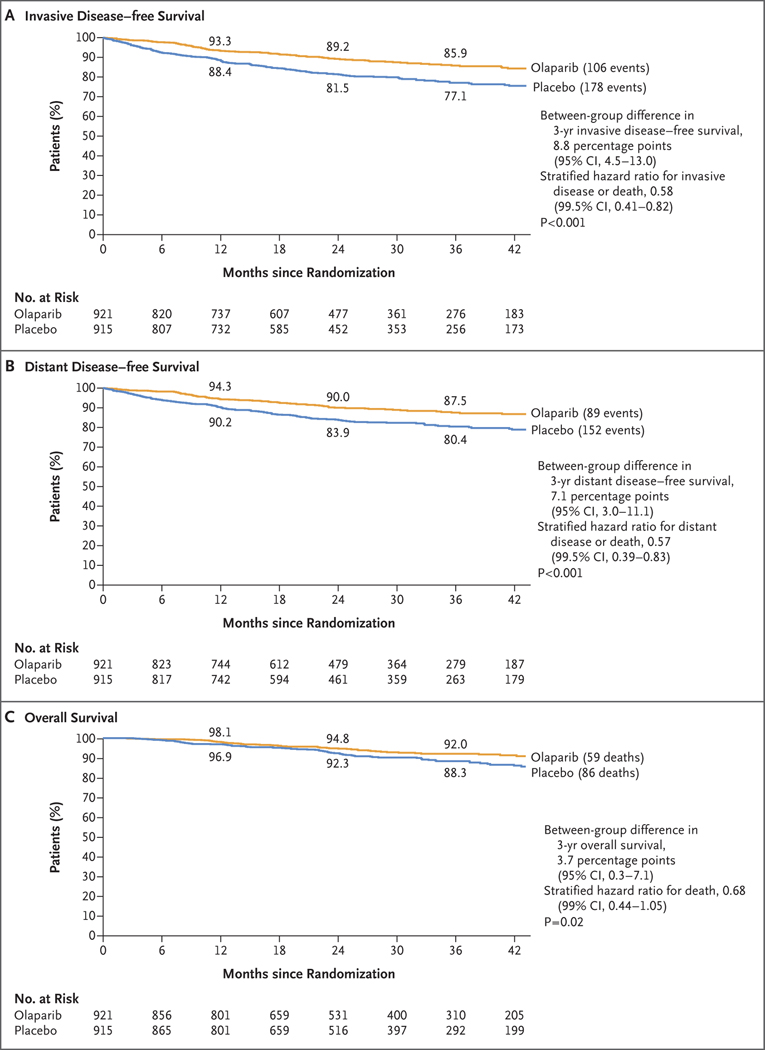
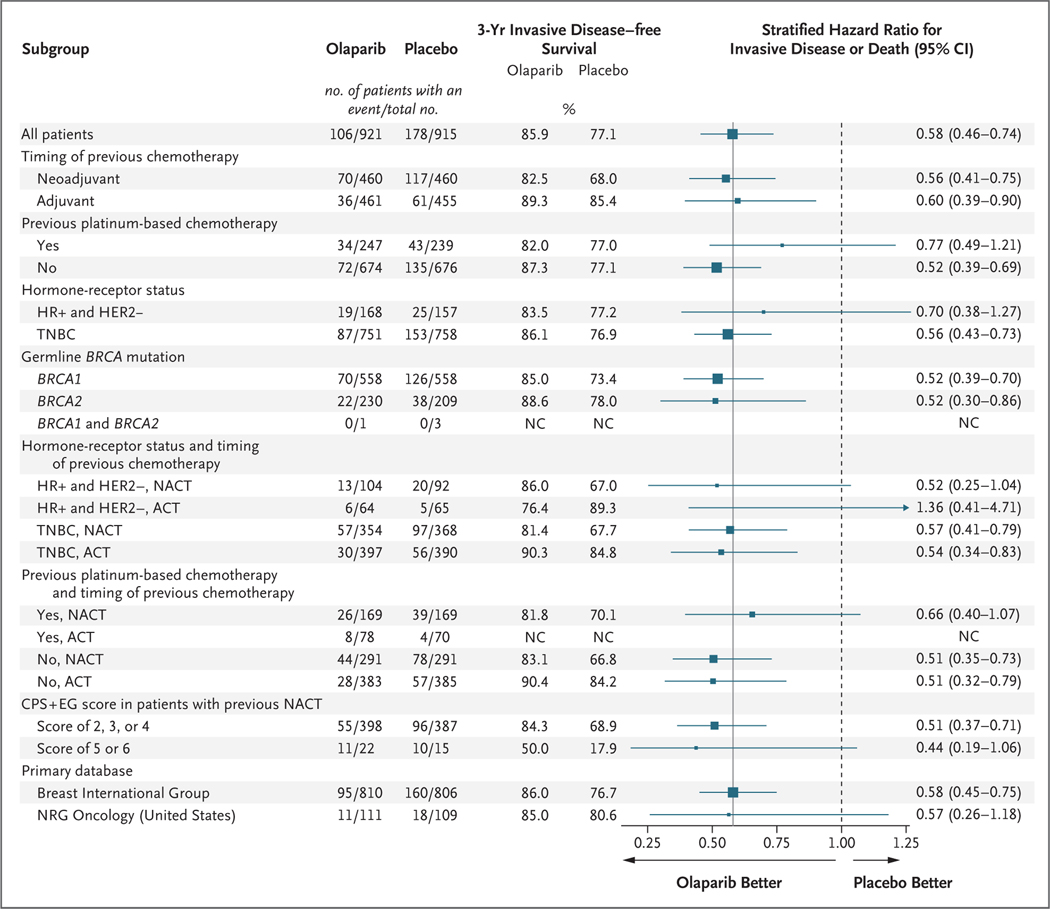
ANJ Tutt
ANJ Tutt
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
JE Garber
JE Garber
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
B Kaufman
B Kaufman
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
G Viale
G Viale
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
D Fumagalli
D Fumagalli
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
P Rastogi
P Rastogi
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
RD Gelber
RD Gelber
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
E de Azambuja
E de Azambuja
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
A Fielding
A Fielding
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
J Balmaña
J Balmaña
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
SM Domchek
SM Domchek
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
KA Gelmon
KA Gelmon
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
SJ Hollingsworth
SJ Hollingsworth
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
LA Korde
LA Korde
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
B Linderholm
B Linderholm
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
H Bandos
H Bandos
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
E Senkus
E Senkus
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
JM Suga
JM Suga
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
Z Shao
Z Shao
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
AW Pippas
AW Pippas
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
Z Nowecki
Z Nowecki
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
T Huzarski
T Huzarski
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
PA Ganz
PA Ganz
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
PC Lucas
PC Lucas
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
N Baker
N Baker
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
S Loibl
S Loibl
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
R McConnell
R McConnell
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
M Piccart
M Piccart
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
R Schmutzler
R Schmutzler
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
GG Steger
GG Steger
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
JP Costantino
JP Costantino
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
A Arahmani
A Arahmani
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
N Wolmark
N Wolmark
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
E McFadden
E McFadden
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
V Karantza
V Karantza
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
SR Lakhani
SR Lakhani
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
G Yothers
G Yothers
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
C Campbell
C Campbell
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1,
CE Geyer Jr
CE Geyer Jr
1Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research (A.N.J.T.), and the Breast Cancer Now Unit, Guy’s Hospital Cancer Centre, King’s College London (A.N.J.T.), London, AstraZeneca, Cambridge (S.J.H., N.B.), and Frontier Science (Scotland), Kincraig (R.M.C., E.M.F., C.C.) — all in the United Kingdom; Dana–Farber Cancer Institute, Harvard Medical School (J.E.G., R.D.G.), Frontier Science Foundation (R.D.G.), and Harvard T.H. Chan School of Public Health (R.D.G.) — all in Boston; the Breast Oncology Institute, Chaim Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel (B.K.); the University of Milan, European Institute of Oncology IRCCS, Milan (G.V.); Breast International Group (D.F., A.A.) and Institut Jules Bordet, l’Université Libre de Bruxelles (E.A., M.P.), Brussels; NRG Oncology (P.R., H.B., P.C.L., N.W., G.Y., C.E.G.) and the Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania (S.M.D.), Philadelphia, and the UPMC Hillman Cancer Center (P.R., P.C.L., N.W.) and the Department of Biostatistics (H.B., J.P.C., G.Y.), University of Pittsburgh, and the NSABP Foundation (N.W.), Pittsburgh — all in Pennsylvania; AstraZeneca, Gaithersburg (A.F.), and the National Cancer Institute, Rockville (L.A.K.) — both in Maryland; Vall d’Hebron Institute of Oncology and Vall d’Hebron University Hospital (J.B.) — both in Barcelona; BC Cancer, Vancouver, BC, Canada (K.A.G.); Sahlgrenska University Hospital (B.L.) and the Institute of Clinical Sciences, Department of Oncology, Sahlgrenska Academy, Gothenburg University (B.L.) — both in Gothenburg, Sweden; the Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk (E.S.), the Maria Skłodowska-Curie National Research Institute of Oncology, Warsaw (Z.N.), the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin (T.H.), and Read-Gene, Grzepnica (T.H.) — all in Poland; Kaiser Permanente Vallejo Medical Center, Vallejo (J.M.S.), and the UCLA Fielding School of Public Health, David Geffen School of Medicine at UCLA (P.A.G.), and the UCLA Jonsson Comprehensive Cancer Center (P.A.G.), Los Angeles — all in California; Fudan University Shanghai Cancer Center, Shanghai, China, (Z.S.); Georgia NCORP, Northside Hospital Cancer Institute (A.W.P.), and Piedmont Healthcare (A.W.P.) — both in Atlanta; German Breast Group, Neu-Isenburg (S.L.), the Center for Hematology and Oncology Bethanien and Goethe University, Frankfurt (S.L.), and the Center for Familial Breast and Ovarian Cancer and the Center for Integrated Oncology, Faculty of Medicine, University Hospital Cologne, Cologne (R.S.) — all in Germany; the Department of Internal Medicine I and Gaston H. Glock Research Center, Medical University of Vienna, Vienna (G.G.S.); Merck, Kenilworth, NJ (V.K.); the University of Queensland Centre for Clinical Research and Pathology Queensland (S.R.L.) — both in Brisbane, QLD, Australia; and Houston Methodist Cancer Center (C.E.G.) and Weill Cornell Medical College (C.E.G.) — both in Houston.
1;
OlympiA Clinical Trial Steering Committee Investigators1,*