Keywords: exocrine secretion, gene therapy, progenitor cell, salivary gland, xerostomia
Abstract
Salivary glands produce and secrete saliva, which is essential for maintaining oral health and overall health. Understanding both the unique structure and physiological function of salivary glands, as well as how they are affected by disease and injury, will direct the development of therapy to repair and regenerate them. Significant recent advances, particularly in the OMICS field, increase our understanding of how salivary glands develop at the cellular, molecular, and genetic levels: the signaling pathways involved, the dynamics of progenitor cell lineages in development, homeostasis, and regeneration, and the role of the extracellular matrix microenvironment. These provide a template for cell and gene therapies as well as bioengineering approaches to repair or regenerate salivary function.
CLINICAL HIGHLIGHTS
Salivary gland function and saliva composition are a window into the state of health of an organism. Saliva-based testing is a commonly used noninvasive tool for the diagnosis of viral infections such as SARS-Coronavirus-2 (SARS-CoV-2). Other viruses such as Epstein–Barr virus (EBV), hepatitis B virus, Ebola, rabies, and HIV can also be detected in oral fluids.
Salivary glands are targets for autoimmune diseases such as Sjögren’s syndrome, graft-versus-host disease, and the recently described antitumor immune checkpoint inhibitor sicca. Important molecular players have been identified in some cases; however, improving diagnostic criteria, understanding causative etiologies, increasing biomarker discovery, and identifying genetic risk signatures remain a high priority.
Salivary hypofunction remains a common side effect of radiation therapy for head and neck cancer, and research has focused on molecular mechanisms of damage and understanding the multifactorial responses preventing regeneration.
Advances in understanding the physiology of salivary secretion and the central role of neuronal input, calcium signaling, and transepithelial water transport highlight why so many drugs have side effects resulting in xerostomia.
Understanding stem/progenitor cell biology and how the extracellular microenvironment and niche signals influence lineage relationships during development and in response to specific types of damage will provide targets for novel therapeutic regeneration.
The last decade of research has provided proof of concept for functional bioengineered salivary glands, while clinical trials using gene therapy and cell-based transplantation to restore function are ongoing and promising.
1. INTRODUCTION
The primary function of salivary glands (SGs) is to produce and secrete saliva, which is critical for our oral and overall health. The physiology of saliva production, with both basal and stimulated levels of secretion, is designed so that we barely notice its presence in our lives. We notice and appreciate our saliva secretion when we see and smell “mouthwatering” food we plan to eat and salivate in anticipation similar to Pavlov’s dogs, conditioned by the sound of a bell before food. More recently, we appreciate saliva for its diagnostic value when we spit in a tube for a SARS-Coronavirus-2 (SARS-CoV-2) test rather than using a long nasopharyngeal swab to obtain a clinical sample. The importance of saliva is not appreciated until it is diminished, such as for patients undergoing radiation therapy (RT) for head and neck cancer or with autoimmune disease such as Sjögren’s syndrome (SS). Hyposalivation has detrimental effects on oral health, with loss of taste, difficulty in eating, swallowing, and talking, dental deterioration, loss of oral microbial homeostasis, increased oral lesions and oral infections, gum disease, and periodontitis, and it has a major impact on our overall well-being and can lead to malnutrition (1–5). Together, these sequelae of hypofunction often have negative psychological effects and loss of quality of life. In humans, >90% of the ∼1.5 L of saliva produced each day is secreted by the three major pairs of SGs, the parotids glands (PGs), submandibular glands (SMGs), and sublingual glands (SLGs), whereas the remaining ∼10% of saliva is secreted by minor SGs distributed throughout the oral cavity (6) (FIGURE 1).
FIGURE 1.
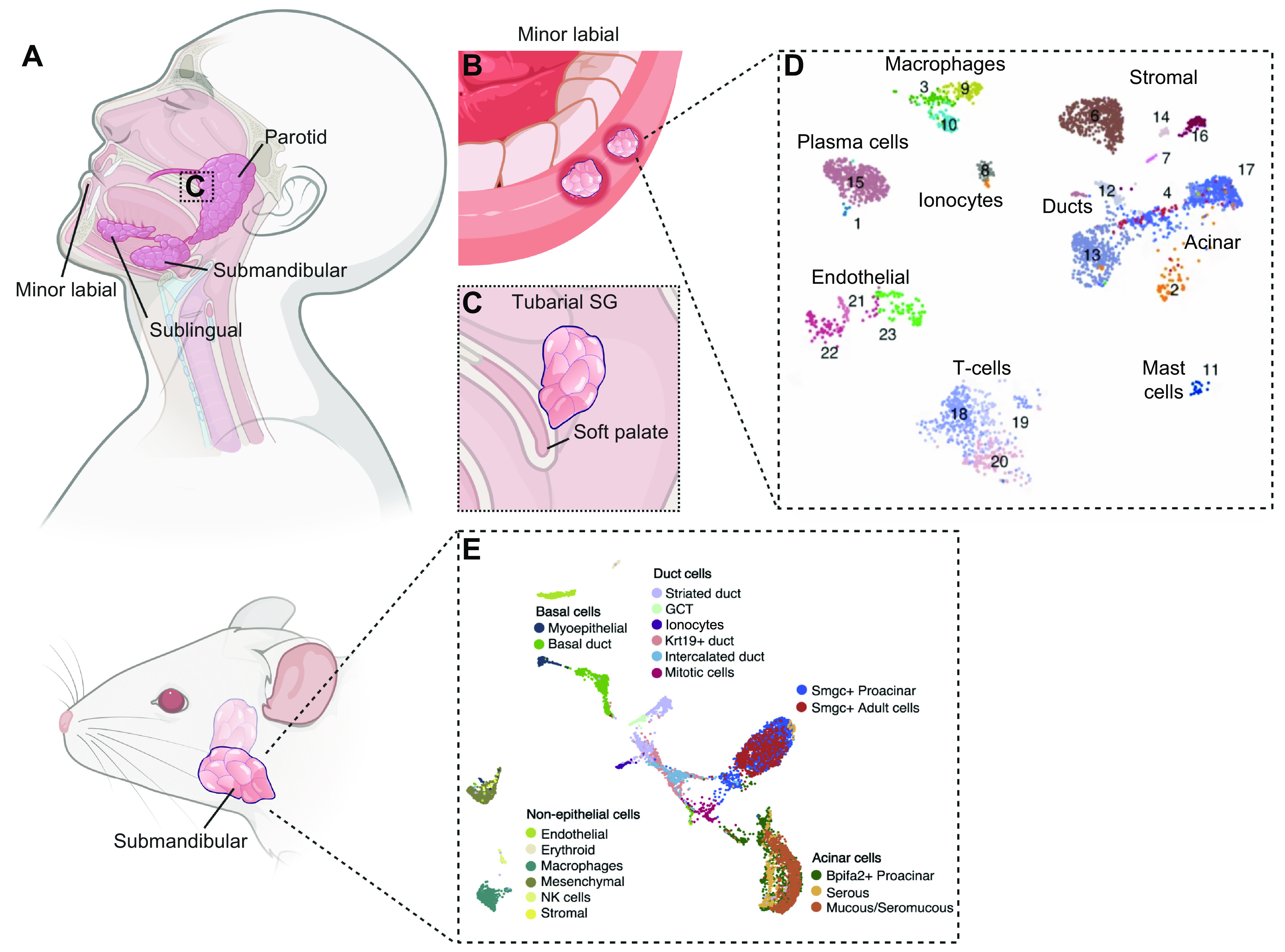
Salivary glands in human and mouse. A: the major pairs of SGs in human are the parotid, submandibular, and sublingual glands. B: hundreds of minor SGs are distributed throughout the oral cavity and include labial glands. C: an additional pair of SGs recently described are tubarial SGs localized near the torus tubarius. D and E: cellular heterogeneity of human and mouse SGs is evidenced by single-cell RNAseq. See glossary for abbreviations. Created with BioRender.com, with permission.
A major function of saliva is to work as a lubricant for the oral cavity, a first step in digestion of food, with antimicrobial properties and an ionic composition that promotes tooth mineralization. Furthermore, saliva contains numerous signaling molecules such as nerve growth factor (NGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and histatins, which are essential for wound healing of the oral mucosa and esophageal tract (5, 7–18) and also help heal dermal wounds. “Licking one’s wounds” is an instinctive response in many animals and reflects the functions of saliva in promoting clotting, enhancing epithelial repair, and having antimicrobial activity. In addition to these important functions, the molecular composition of saliva also reflects the physiological or pathological state of an organism, as it works as a solvent for secreted peptides, ions, and metabolites that are secreted or result from the breakdown of drugs and endogenous chemicals, which can serve as biomarkers for disease diagnosis. Saliva is also used in the diagnosis of many viral infections. Notably, recent studies show that SARS-CoV-2 and certain strains of enteroviruses can replicate in SG epithelium and are detected in saliva, suggesting that SGs function as a reservoir for the transmission of viral disease (19–21).
Many systemic diseases and SG disorders compromise the glandular tissue integrity and result in salivary hypofunction, characterized by alterations in both the volume and composition of saliva. These include iatrogenic diseases, such as RT for head and neck cancer, which can result in permanent gland damage (3, 22–25). There are currently no curative therapies, and treatments remain mostly palliative (4, 22). Therefore, much of the biomedical scientific effort in the field over the last decade has focused on understanding the physiology of secretion and researching potential regenerative strategies to repair SGs and restore saliva production. In the last decade, technological advances like single-cell OMICs have made it possible to evaluate the contribution of specific cell types to development of the gland and to identify factors involved in the generation of secretory cell types, which are lost during injury or disease (26–30).
In this review, we briefly cover SG anatomy and histology and focus on the last decade’s research findings on the mechanisms regulating the physiology of secretion and how this influences functions of saliva and the diagnostic implications. Given that the oral cavity is a window to the body, and that SGs are a reservoir for viral replication and saliva a transmission vector for pathogens, we review the prognostic and diagnostic applications of saliva. We then review the major disorders and diseases that affect SGs and identify areas of therapeutic opportunity informed by the recent technological advances in multi-omics and preclinical models. We discuss how these technologies have advanced our understanding of the cellular composition, lineage, and diversity of SG cell types, as well as molecular pathways and underlying genetic landscape involved in SG organogenesis, the stem cell dynamics, and the potential contributions to disease and dysfunction. This new information provides a template to develop effective regenerative strategies (31). These include the use of cell-based therapies (32–34), gene transfer (35–37), and the development of scaffolds and biomaterials (34, 38–40) that complement transplantation approaches to repair (34, 41). Finally, we conclude with some speculations for the exciting future of the field.
2. PHYSIOLOGY
The major mammalian SGs are exocrine organs that produce and secrete saliva, and to review their physiology it is important to understand their function, anatomy, and histology. In humans, saliva is ∼99% water and 1% protein, with other minor components that include ions and electrolytes (42). The PG is the largest SG and produces the majority of stimulated salivary flow, which is watery serous saliva containing amylase, whereas the SMG produces the basal salivary flow during the resting state, which is a mixture of serous and mucous saliva (43–45). The SLG produces entirely mucous saliva rich in salivary mucins (46). Hundreds of minor SGs are distributed throughout the oral cavity, as well as a recently identified pair of SGs termed tubarial glands, which in combination are responsible for the remaining saliva production (47). These differences in salivary secretions are reflected in distinct anatomy, innervation, and histology of the glands.
Although many SG functions are relatively conserved across species, some distinct histological features become evident in different glands and different species. Since mice are commonly used as a model to study SGs, it is important to note that hormone-driven sexual dimorphism in the murine SMG results in the development of specialized granular convoluted tubules (GCTs) that appear larger and more numerous in males compared with females (48). Human SGs are not reported to be sexually dimorphic and do not have GCTs. Furthermore, differences in size and contribution to overall saliva production are also notable between species with evolutionarily distinct feeding behaviors. Dogs and cats have additional pairs of major SGs called zygomatic glands, which are larger than the SLG (49). Similarly, the inferior molar, buccal, and labial glands in sheep are also larger than the SLG and considered major SGs (50). Interestingly, similar organs that produce secretions to facilitate feeding are also present across the animal kingdom, from Caenorhabditis elegans to more complex species including insects and reptiles. These include modified versions of the SGs that have evolved to produce specialized secretions, such as venom or silk in the case of snakes and spiders, respectively (51–53). However, unless otherwise specified, this review focuses primarily on studies performed with human, rodent, and porcine SGs, which have been some of the most widely used species to investigate SG biology, injury, and regeneration.
2.1. Anatomy of Human Salivary Glands
The human PG is located close to the ear at the junction of the mastoid process and sternocleidomastoid muscle, behind the lower jaw and base of the cranium. It is connected to the oral cavity via Stensen’s duct, which is localized near the upper second molar in humans and serves as a conduit to deposit saliva into the mouth (54, 55) (FIGURE 1). The gland is wrapped around the mandibular ramus and comprises two distinct lobes, the superficial lobe and the deep lobe. Between the two lobes is the facial nerve, separated from the gland by the digastric, stylohyoid, and pterygoid muscles and the styloid process. In the deep lobe of the PG, the SG tissue lies on top of cranial nerves IX to XII, the internal jugular vein, and the internal carotid artery, which serves as blood supply to the head and neck region. The PG is the largest SG in humans, with an estimated weight of 15–30 g. Approximately 20% of individuals have an accessory PG lying anteriorly over the masseter muscle, which contains both serous and mucous acinar cells (56).
The SMG is the second-largest gland in humans, weighing 7–16 g, and is found inferior to the mandible, between the anterior and posterior bellies of the digastric muscle. Similar to the PG, the SMG comprises two lobes connected by the posterior edge of the mylohyoid muscle. In this case, the deep lobe is connected to the main duct known as Wharton’s duct, which runs parallel to the hypoglossal nerve and enters the oral cavity along the lateral side of the frenulum linguae at the sublingual caruncle (54, 57). Also lateral to Wharton’s duct, the SLG lies submucosally in the floor of the mouth near the anterior lobe of the SMG (58). The SLG contains a series of smaller ducts that connect with the floor of the mouth and into Wharton’s duct. The human SLG weighs ∼3 g and represents the smallest of the major salivary glands (59).
More recently, an additional set of SGs named tubarial glands were identified in the nasopharynx near the torus tubarius. The glands extend caudally to the pharyngeal wall and cranially to Rosenmüller’s fossa. Histologically, tubarial glands are comprised primarily of mucous cells similar to SLG with no amylase expression (47). Finally, minor SGs are microscopic and can be found across oral tissues such as the lips, buccal mucosa, palate, and tongue, as well as in the aerodigestive tract. Their secretion is primarily mucous, albeit they contain both serous and secretory acinar cells. Importantly, minor SGs in the lower lip are easily biopsied and are used clinically to diagnose SS.
2.2. Salivary Gland Histology
The histology of SGs has been extensively reviewed elsewhere (6, 31, 60). In brief, the mammalian SGs are branched organs formed by a complex ductal tree that culminates in secretory units called acini, which are formed by a combination of serous and mucous acinar cells. Acinar cells are the main secretory component of the SG parenchyma, as they produce and secrete saliva that is then modified and transported by a sequential ductal system of three anatomically distinct compartments: intercalated duct (ID), striated duct (SD), and excretory duct (ED) cells that connect the gland to the oral cavity (FIGURES 2 and 3). Myoepithelial cells (MECs), another component of the SG parenchyma, wrap around acinar and duct cells and contract in response to neuronal stimuli to facilitate the expulsion of secretion. The SG microenvironment comprises the surrounding basement membrane and extracellular matrix (ECM), autonomic innervation from both sympathetic and parasympathetic nerves, a variety of immune cell populations, adipose and muscle tissue, and an intricate vasculature (59).
FIGURE 2.
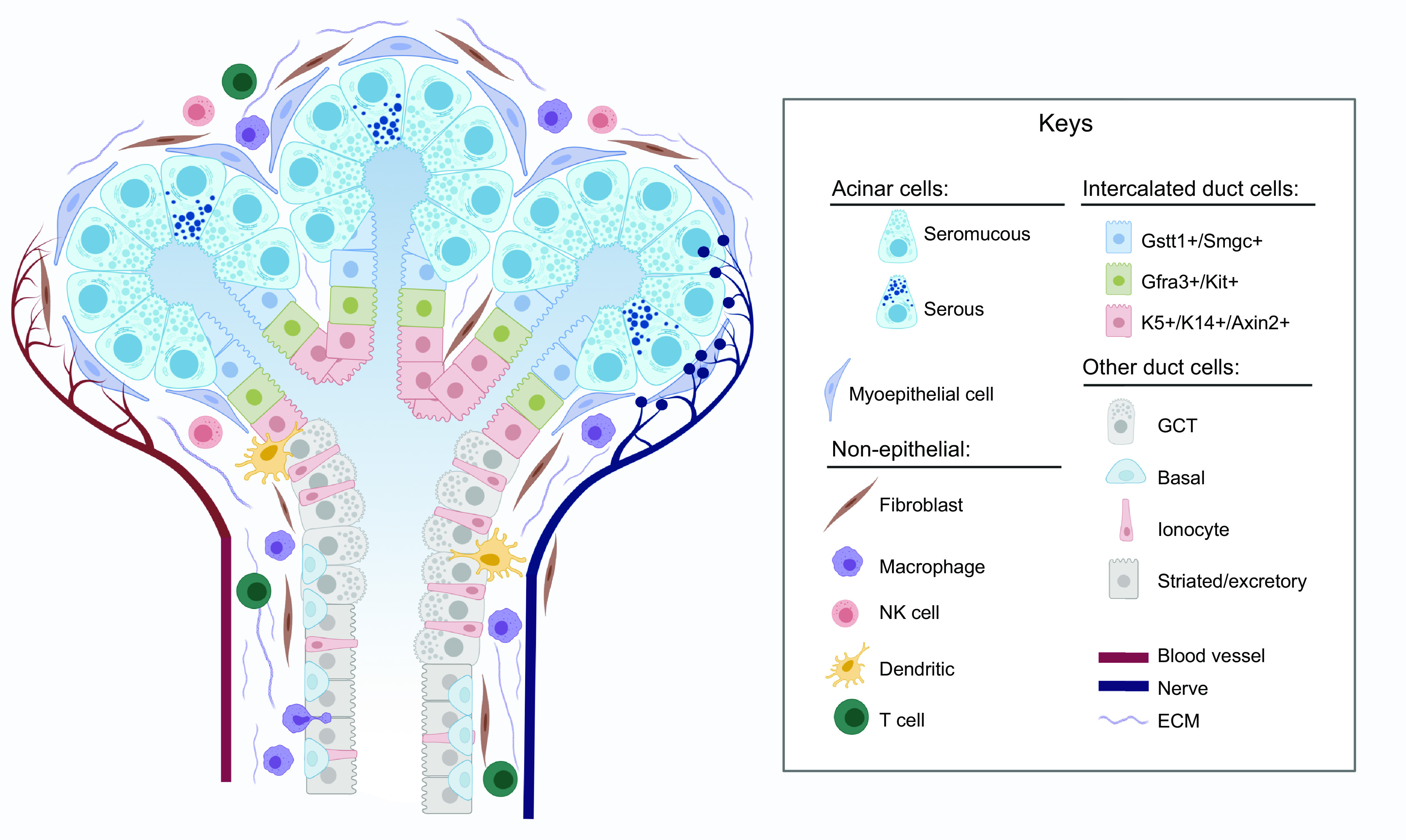
Model of major cell types of the mouse submandibular gland (SMG). SMG epithelium is divided into secretory acini and ducts. Serous and seromucous acinar cells are surrounded by MECs. Ducts are divided into sections containing specific duct cell types. Non-epithelial cell types in the surrounding ECM include fibroblasts, immune cells, blood vessels, and nerves. See glossary for abbreviations. Created with BioRender.com, with permission.
FIGURE 3.
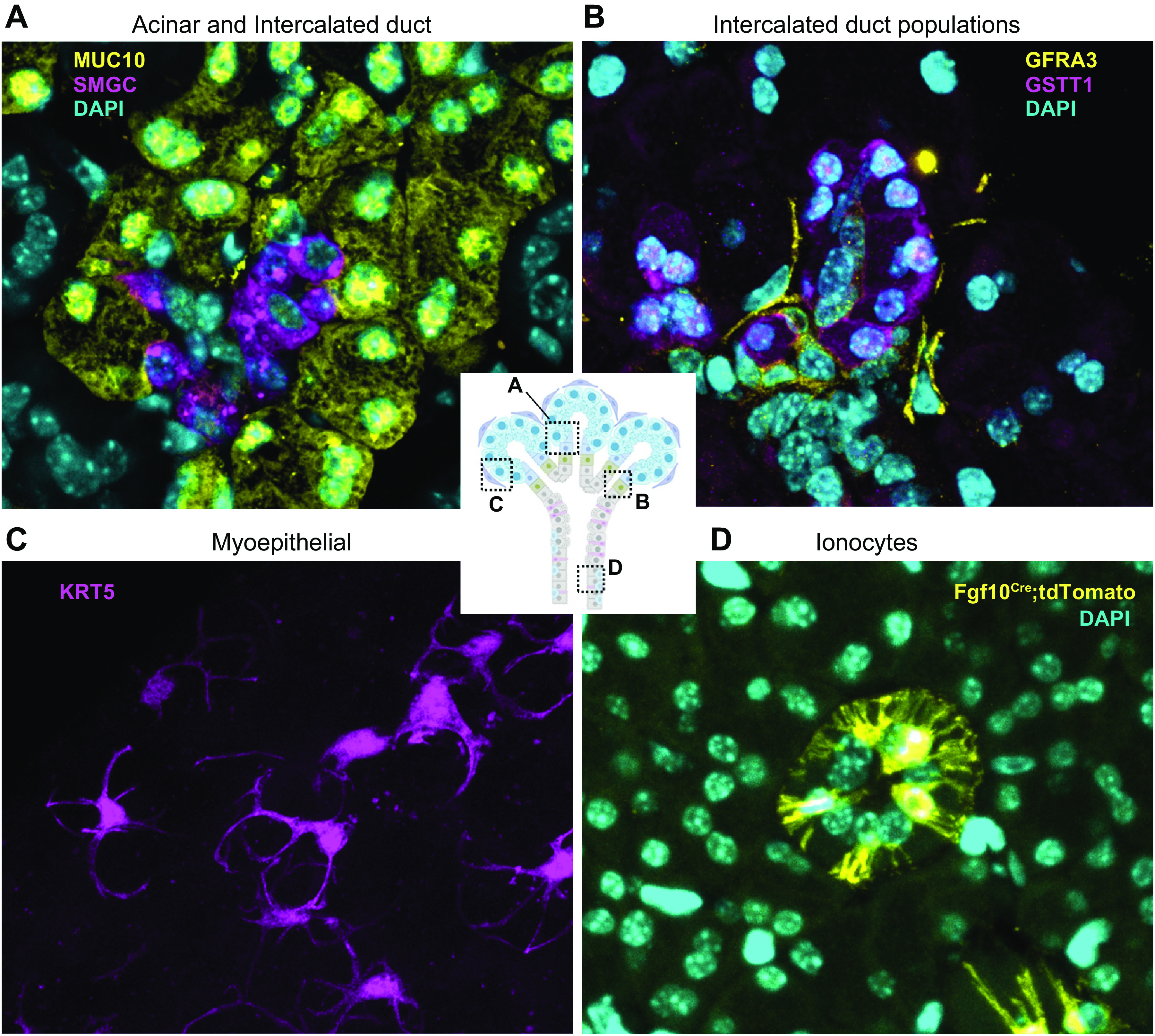
Cell types of the mouse SMG. A: secretory acinar cells (MUC10, yellow) secrete saliva into the intercalated duct (ID) (SMGC, magenta) and nuclei (DAPI, cyan). B: intercalated duct subpopulations include Gstt1+/Smgc+ (GSTT1, magenta) and Gfra3/Kit+ (GFRA3, yellow) cells and nuclei (DAPI, cyan). C: myoepithelial cells (KRT5, magenta), imaged in a thick section to highlight their stellate morphology, surround the acini and duct with long cellular processes. D: ionocytes (Fgf10Cre:TdTomatofl, yellow) with long cellular processes are located within the duct and nuclei (DAPI, cyan). See glossary for abbreviations.
2.2.1. Acinar cells.
Acini are formed by clusters of 8–12 pyramidal acinar cells connected by their lateral membranes through adherent and tight junctions. These lateral junctions serve to maintain apical-basal polarity and to prevent the free lateral transport of ions between acinar cells, which instead require specialized water channels and ion pumps to regulate secretion and ion concentration of saliva (61). Acinar cells are identified through expression of canonical markers such as aquaporin 5 (AQP5) and Mist1 (Bhlha15) (62–65). Acinar cells can be serous or mucous depending on the nature of their secretion. Serous acinar cells are characterized by abundant eosinophilic zymogen granules and a spherical nucleus, whereas mucous acinar cells contain a clear cytoplasm rich in mucins with nuclei polarized to the basal membrane of the cell (46, 66). In paraffin-embedded sections, these mucous cells appear to be capped by serous demilunes aligned to the basal membrane with a crescent shape, although it has been suggested that this could be an artifact of tissue fixation (67–69). Acini may be serous, mucous, or seromucous, depending on their composition of acinar cells, and their relative proportions vary between glands as well as species (66, 70, 71). In humans, the PG is primarily comprised of serous acini that secrete a watery solution containing amylase, proline-rich proteins, secretory immunoglobulin A, and immunoglobulins G and M. Conversely, the SLG is comprised predominantly of mucous acinar cells that secrete a viscous solution rich in mucins. Finally, SMG produces a seromucous secretion and contains both serous and mucous acinar cells (70). Recently, transcriptional profiles of human major salivary glands have provided further insight into acinar heterogeneity, which indicates subsets specialized to synthesize specific salivary proteins (30). Other secretory cells specific to murine SMGs are the GCTs, which are considered part of the ductal system although they produce an exocrine serous-like secretion. This is based on positive staining of the secretory granules with toluidine blue, electron microscopic observations of secretory granules, and the abundant production of growth factors such as NGF and EGF that are secreted into saliva (72, 73).
2.2.2. Ductal cells.
The ductal system of the SGs modifies the electrolyte content of saliva and serves as a conduit to transport secretion to the oral cavity. In general, acinar cells secrete isotonic saliva that facilitates osmotic water secretion and is deposited into the acinar lumen (70, 74, 75). As it passes through the ductal system, saliva is progressively modified into a hypotonic solution by selective reabsorption of specific ions (74, 76).
IDs are formed by a single layer of cuboidal cells with central nuclei and small secretory granules containing lysozyme and lactoferrin. In addition to their role in modifying saliva, ID cells appear to have specialized functions that vary between species. In mice, IDs are characterized by two distinct cell populations defined by expression of either Gfra3 and Kit or Gstt1 (27). Kit+ cells are also present in the IDs of human glands (77). These are thought to function as a reservoir of salivary progenitors with limited potential to regenerate the gland under specific injury conditions (77–79). Gstt1+ cells are sexually dimorphic in mice, and it is unclear whether a similar population exists in the human glands. Gstt1+ cells in the mouse ID are also defined by expression of the mucin gene Smgc in females and Serpinb11 in males (27). Notably, the mouse SMG also has sexually dimorphic GCTs at the intersection between IDs and SDs. GCT development is regulated by Runx1 in mice, but these structures are not present in the human glands (72, 80, 81). GCTs are bigger and more abundant in males compared with females, and single-cell RNA sequencing (scRNAseq) analysis of murine SMG showed that female GCTs have significantly higher expression of the mucin gene Smgc compared with male GCTs but no other transcriptional differences were detected (27). There is evidence suggesting that Smgc is secreted into the saliva of female mice and acts as a pheromone for litter recognition to prevent male mice from cannibalizing their litters (82). Therefore, it is likely that both IDs and GCTs evolved in rodents to serve a specific function that is not required in humans.
SDs participate in the bidirectional transport and reabsorption of electrolytes. They are also known as intralobular ducts and comprise the majority of the ductal system in the major SGs (83, 84). SDs are lined by a tall columnar epithelium with central nuclei and are characterized by the presence of abundant mitochondria that form cytoplasmic folds or striations in the basolateral membrane (81, 85). In addition to maintaining electrolyte balance, SDs in mammalian SGs are also involved in the secretion of organic products. Small secretory granules containing kallikreins and glycoproteins are apically localized within the cytoplasm of SD cells, albeit the specific composition of these granules may be affected by diet (84, 86, 87).
The final segment of the ductal system is the ED, which is formed by tall columnar pseudostratified epithelium with more apically located nuclei and prominent cytoplasmic striations. These ducts are found within the connective tissue in the glandular septa that separates glandular lobes and thus are also referred to as interlobular ducts (88, 89). EDs are also responsible for sodium reabsorption and potassium secretion to produce the final hypotonic saliva that is then directed to the oral cavity (75, 90). Different cell types are found within the ED based on their ultrastructural features, including light (electron lucent) and dark (electron dense) cells (89), ionocyte cells that express FGF10 and ASCL3 (91, 596), and basal progenitor cells that express P63, keratin 5 (KRT5) and keratin 14 (KRT14) (93, 94). More details on the heterogeneity and specific cell populations of the ductal system are reviewed in sect. 5.2.
2.2.3. Myoepithelial cells.
The last major component of the SG parenchyma is made up of MECs, which surround acinar cells and are occasionally found surrounding IDs (95). MECs are a type of smooth muscle epithelial cell characterized by their expression of contractile proteins such as smooth muscle actin, calponin, and myosin as well as basal cytokeratins (96). MECs are essential to the secretory process, working as a contractile force around acinar cells to facilitate the expulsion of saliva in response to neuronal stimuli (97–99). Additionally, MECs are thought to protect acinar cells from negative pressure that results from the secretory process (98, 100). Interestingly, MECs appear more prominently in SMGs than PGs (101), which may relate to the fact that SMGs produce a low basal secretion rate that may be dependent on MEC function, although this remains to be explored.
MECs are also associated with relaying neuronal stimuli and in the production of basement membrane, which is thought to contribute to the establishment and maintenance of proper tissue architecture by promoting apical-basal polarity in epithelial cells (99, 102–105). Despite these observations, MECs in SGs have remained largely understudied, and many of their functions and the regulatory mechanisms that control them are unclear. More recently, MECs in the mouse SMG were shown to exhibit injury-induced plasticity to regenerate up to 80% of acinar cells in a duct-ligation model (106), and single-cell RNAseq analysis suggests that MECs may both work as a niche source of growth factors including neurotrophins and function as a signaling hub to relay signals between multiple cell types in the gland (Chibly et al., unpublished observations). MECs of the mouse SMG differentially express neurotrophins, FGF genes, and Neuregulin 3 (Nrg3) compared with all other cell types, and they were also identified as the main source of the neurotrophic factor neurturin (NRTN) (27), which has demonstrated therapeutic potential when administered to injured SGs via gene therapy in both mice and pigs (36, 37).
2.2.4. Resident immune cells.
The presence of increased numbers of immune cells in the SGs is often associated with pathological states such as autoimmune disease, viral infections, and radiation damage. The SGs are intimately associated with salivary lymph nodes, which can be located within the PG and close to SLGs and SMGs (108). Not surprisingly, immune cells in the SGs confer immediate protection from viral infections and may even prevent spread of the disease. For instance, resident CD8+ T cells protect against cytomegalovirus infection of the SGs (109). Immune cell populations also play important roles during homeostasis and normal function of the gland, albeit these may be less understood. Plasma B cells in the gland produce immunoglobulin A, which is secreted into saliva and works as a protective agent in the oral cavity to fend off infections (110, 111). Recent scRNAseq analyses of mouse and human SGs during homeostatic and pathological states have shed light on the diversity of immune populations in the SGs and suggest that cell-cell interactions between immune cells and the epithelium are involved in both tissue homeostasis and response to injury (27, 28, 112). Numerous immune populations were identified in the healthy mouse PG, including B cells, five subtypes of T cells (CD4+, CD8+, CD4+CD8+, FoxP3+, Cxcr6+), macrophages, dendritic cells, and natural killer cells (Chibly et al., unpublished observations). Similar populations were found in a single-cell RNAseq atlas of the human minor SGs, gingiva, and oral mucosa (26). The specific role of individual immune populations in the maintenance of homeostasis or in saliva secretion are not completely understood, but OMICs resources offer new areas of opportunity for future mechanistic studies.
2.2.5. Innervation of salivary glands.
The innervation of SGs has been recently reviewed in detail (114). However, it is important to highlight the major role of innervation in both development and the physiological function of SGs. Saliva secretion is dependent on autonomic nerve signals, which are initiated by mechanosensory stimuli provided by the smell, taste, and chewing of food (115, 116). The major SGs receive innervation from both sympathetic and parasympathetic nerves, which form bundled fibers surrounded by Schwann cells (117). In the PG, parasympathetic innervation occurs via the glossopharyngeal nerve (cranial nerve IX), which carries preganglionic fibers from the inferior salivatory nucleus to the otic ganglion and postganglionic fibers from the otic ganglion back to the PG via the auriculotemporal nerve of cranial nerve V (114, 117, 118). On the other hand, sympathetic nerve fibers reach the PG from the superior cervical ganglion (115). In the SMG, innervation occurs via the lingual, hypoglossal, and facial nerves. Here, preganglionic parasympathetic fibers from the superior salivatory nucleus travel via the chorda tympani branch of the facial nerve (CNVII), which merges with the lingual branch of the mandibular nerve (CNVIII) to synapse in the submandibular ganglion (115, 119). Postganglionic secretory fibers enter both the SMG and SLG, where both sympathetic and parasympathetic nerves coexist.
Even during resting states, unstimulated saliva production requires intact autonomic nerve supply (99, 120). The SMG and SLG are responsible for the majority of unstimulated saliva production, whereas most of the secretion from the PG occurs in response to stimuli. Parasympathetic secretomotor innervation promotes vasodilation and increased blood supply to stimulate secretion of serous saliva via acetylcholine and substance P (SP), whereas sympathetic nerves respond to norepinephrine to regulate mucous secretion, peripheral blood flow, and inflammation (116, 119, 121). Neurotransmitter release may occur anywhere along the nerve fibers, and nerve terminals are identified in close proximity to most SG cell types including acinar cell, duct cell, and MEC and may be naked or surrounded by Schwann cells. Both types of innervation can act directly on secretory cells, resulting in increased levels of intracellular [Ca2+]i, changes in membrane permeability, and the corresponding secretion of organic molecules, electrolytes, water, and mucus in the form of saliva (114). Nerve stimuli also act on MECs, which express both adrenergic and cholinergic neurotransmitter receptors (122), to stimulate contraction around the secretory acini, thus facilitating degranulation and expulsion of fluid into the lumen. Inhibition of adrenoreceptors, which are expressed in MECs, slows down fluid movement but does not prevent secretion (88, 123).
In addition to neuronal stimulation of saliva flow, the composition of saliva is also affected not only by the type of stimulus provided to the gland but also by the dietary input. For instance, sweet-stimulated human parotid saliva has a higher protein content, and higher concentration of IgA is found in saliva after mastication (124–126). Similarly, higher concentrations of amylase and other proteins were secreted into rabbit parotid saliva evoked by carrots compared with standard pelleted chow (127). This may suggest that there is also higher central neural regulation controlling salivary secretion, which remains to be determined.
2.2.6. Vasculature of salivary glands.
The production of saliva and the exocrine functions of SGs are dependent on the vascular supply (31, 128, 129). Blood supply to the PG comes from the external carotid artery, which travels medial to the PG and branches into the superficial temporal and maxillary arteries. The superficial temporal artery then branches into the transverse facial artery, which supplies the PG itself, the parotid duct, and the masseter muscle. Venous outflow from the PG occurs via the retromandibular vein, which is formed by the convergence of the superficial temporal and maxillary veins within the PG. The SMG receives its blood supply from the submental branch of the facial artery and the sublingual branch of the lingual artery, and it is drained by the facial and sublingual veins (31, 57). The vascular supply to SGs is critical for fluid secretion, and damage to the vasculature is one of the early responses of the glands to IR damage (reviewed in sect. 4.5).
2.3. Physiology of Secretion
The function of SGs to produce saliva highlights the specific interactions among cell types that produce a primary fluid that is a plasma-like secretion and ultimately secrete saliva that is hypotonic, near neutral pH, mucous, viscous, and supersaturated with calcium (Ca2+) and phosphate (42, 88, 130). The orchestration of cell-specific physiological functions involving an ionic gradient to drive water secretion and preservation of ions by selective reabsorption in concert with mucin secretion for lubrication and production of proteins that bind Ca2+ and phosphate to supersaturate the oral cavity and preserve tooth mineral density highlights the complexity of secretory physiology. Saliva secretion is a two-stage process in which acinar cells produce an initial isotonic saliva that is then modified by the ductal system into a hypotonic fluid (FIGURE 4). The process is initiated by release of neurotransmitters such as acetylcholine, epinephrine, norepinephrine, SP, and neuropeptide Y, which all interact with receptors in epithelial and endothelial cells of the SG. These neurotransmitters activate muscarinic cholinergic and adrenergic receptors in acinar and endothelial cells to promote secretion, which is additionally orchestrated by tight regulation of intracellular Ca2+ concentration and electrolyte balance. In the first stage, parasympathetic activation of the muscarinic receptors M1 and M3 by acetylcholine and sympathetic stimulation of α1-adrenergic receptors by norepinephrine and epinephrine lead to increases in the [Ca2+]i, which in turn activates potassium (K+) and chloride (Cl−) channels, the sodium-potassium-chloride cotransporter (NKCC1), and the Na+/H+ (NHE1) and Cl−/bicarbonate () exchangers (71, 131) and triggers the recruitment of AQP5 to the apical acinar membrane (132). Increased [Ca2+]i and activation of K+ channels result in transepithelial flux of Cl− to produce a plasma-like isotonic fluid rich in NaCl that is secreted into the lumen of the acini (2).
FIGURE 4.
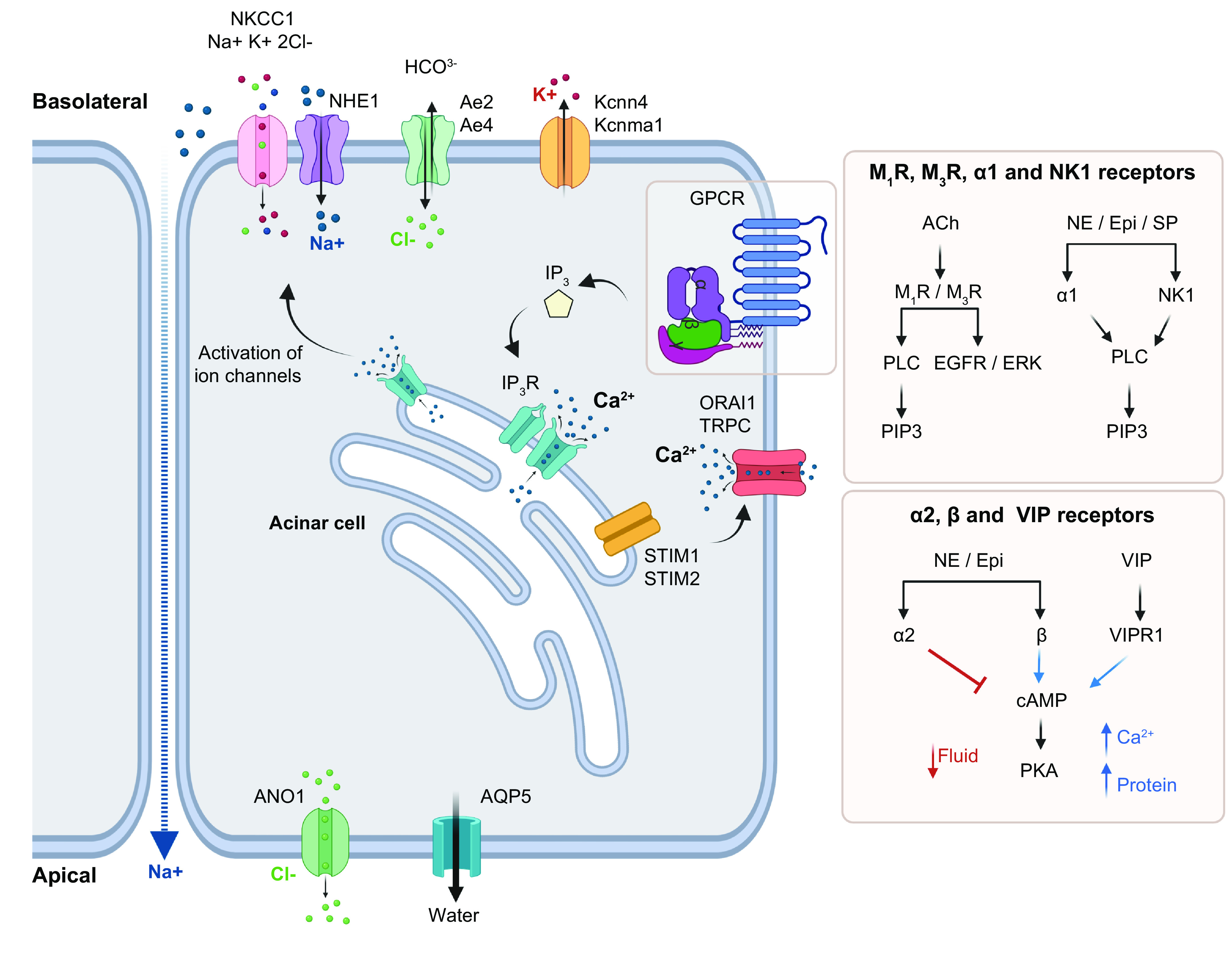
Model of stage 1 of saliva secretion. Secretion is initiated by activation of muscarinic receptors M1 or M3 by acetylcholine (ACh) or adrenergic receptors (α1) by norepinephrine (NE) and epinephrine (Epi) via specific pathways involving IP3 release or cAMP modulation. IP3 binds to its receptor in the ER to promote Ca2+ release, which is sensed by STIM1/2 in the ER membrane (EM). STIM1 recruits Orai1/TRPC to stimulate Ca2+ reentry into the cell. Ca2+ depletion from the ER recruits AQP5 to the apical membrane for water expulsion and activates NKCC1, NHE1, Ae2, Ae4, Kcnma1, and Kcnn4 in the basolateral membrane to import Na+ and Cl− into the cellular space while exporting K+ and . In turn, ANO1 is activated in the luminal membrane to secrete Cl−. Na+ also undergoes transepithelial transport toward the lumen, resulting in NaCl-rich isotonic saliva. See glossary for abbreviations. Created with BioRender.com, with permission.
In the second stage, as the initial saliva travels through the ductal system, a group of transporters expressed by duct cells are responsible for reabsorption of NaCl, effectively removing it from saliva while actively secreting K+ and (FIGURE 5). These include the apically localized epithelial Na+ channel (ENaC) and the cystic fibrosis transmembrane conductance regulator (CFTR), which are responsible for Na+ and Cl− reabsorption, respectively (133). CFTR is also involved in bicarbonate secretion, which contributes to the pH buffering of the final hypotonic saliva (74, 134–136). In pancreatic ductal cells, other transporters like NBCn1 and Slc26a6 act in concert with CFTR to secrete bicarbonate, but studies in mice indicate that these functions are not retained in SGs, where null mutations in the genes encoding the transporters did not alter content in saliva (135, 137, 138).
FIGURE 5.
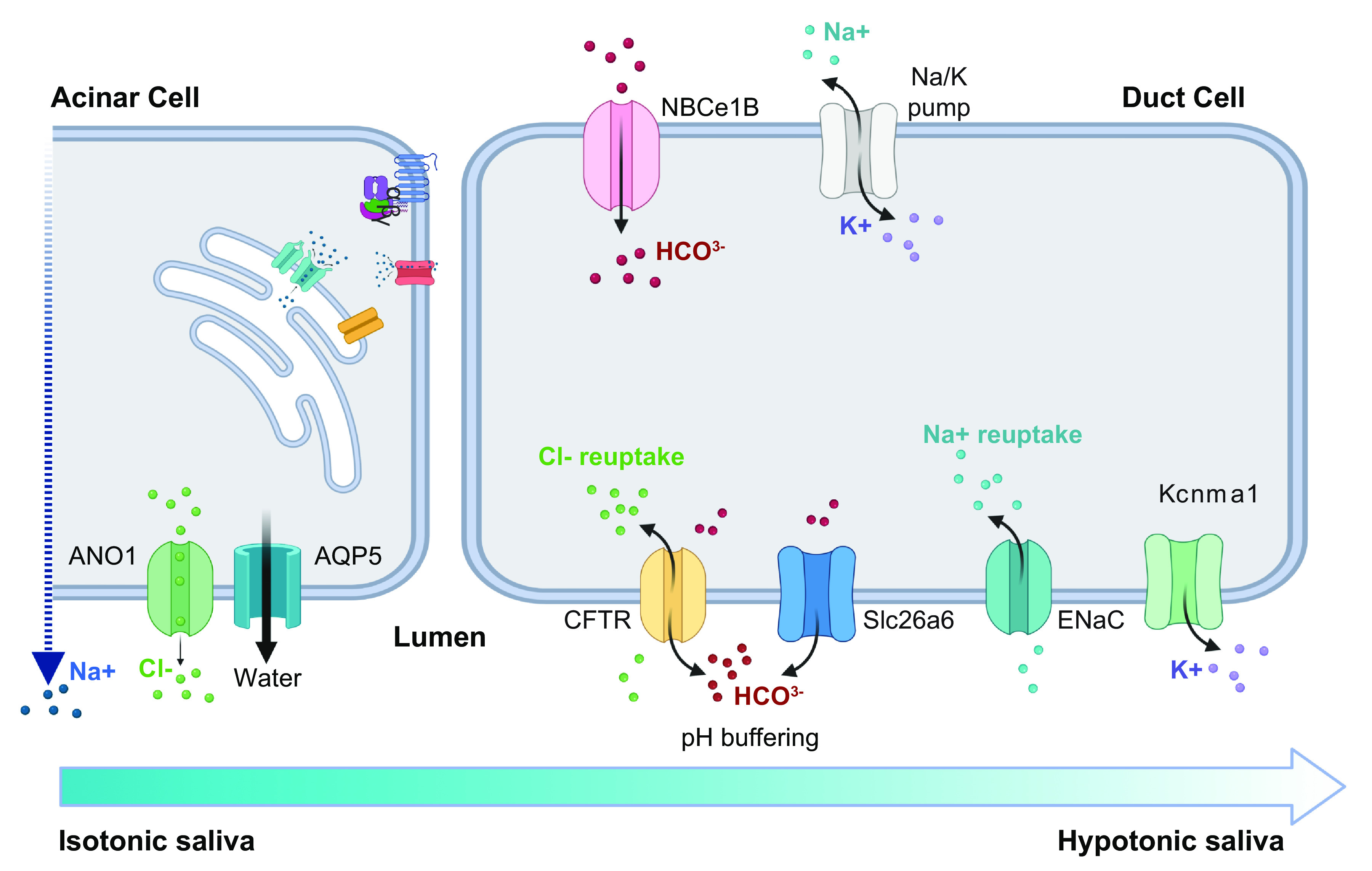
Model of stage 2 of saliva secretion. Isotonic saliva secreted by acinar cells is rich in NaCl. As it travels through the ductal system, duct cells reabsorb NaCl via CFTR and ENaC while exporting via both CFTR and Slc26a6. The resulting saliva is hypotonic and near neutral pH. See glossary for abbreviations. Created with BioRender.com, with permission.
2.3.1. Autonomic Regulation of Secretion.
Knowledge of the involvement of parasympathetic and sympathetic innervation in the process of saliva secretion dates back to the 1950s (139). It is widely accepted that parasympathetic innervation primarily drives fluid secretion by activation of muscarinic receptors in epithelial cells while sympathetic nerves are involved in the production of mucin, protein secretion, and vasoconstriction upon activation of adrenoreceptors in the epithelium and vasculature (14, 114, 115), although this is species specific and animal models suggest that saliva secretion in mucous glands, like the rat SLG, may be primarily influenced by parasympathetic innervation (140).
Parasympathetic stimulation of saliva secretion is dependent on muscarinic acetylcholine receptors (mAChRs) M1 and M3 (141, 142), which are expressed in acinar cells and MECs (143, 144). Single knockouts for either receptor show reduced secretion upon stimulation with low doses of the muscarinic agonist pilocarpine, whereas double knockouts exhibit a complete loss of pilocarpine-stimulated saliva secretion (141). M1 and M3 receptors are coupled with Gq and G11 proteins, which upon stimulation with acetylcholine activate phospholipase C (PLC) to convert phosphatidylinositol 1,4-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and release diacylglycerol (DAG). IP3 in turn binds to IP3 receptors (IP3Rs) in the endoplasmic reticulum (ER) to stimulate the release of Ca2+ from intracellular stores and increases the [Ca2+]i (145–150). Consistently, carbachol-induced increase in [Ca2+]i is impaired in M3 single knockouts and completely ablated from M1/M3 double-knockout mice (142). Both PLC and [Ca2+]i are also required for the activation of protein kinase C (PKC) for the secretion of mucin downstream of muscarinic receptors (140). Additional mechanisms downstream of M3 involve ERK activation by GPCR kinase 2 (GRK2) and arrestin-3 as well as transactivation of EGF receptor (EGFR) (147, 151).
Sympathetic regulation of saliva secretion is mediated by α- and β-adrenoreceptors expressed in epithelial and endothelial cells to promote protein exocytosis and modulate vascular resistance during saliva secretion, respectively. Norepinephrine and epinephrine primarily target α1- and α2-adrenergic receptors, whereas β-adrenoreceptors are more sensitive to isoproterenol but also respond to norepinephrine and epinephrine. In general, simultaneous stimulation of adrenergic receptors results in secretion of proteins in saliva by exocytosis, including nerve growth factor (NGF) (120), IgA (152), kallikreins (153), and amylase (154). However, each adrenergic receptor initiates distinct signaling cascades that can produce unique outcomes. Pharmacological experiments in vivo using adrenoreceptor-specific agonists and inhibitors have shown distinct functions for each receptor, where the independent activation of α1- and β-adrenergic receptors increases fluid and protein secretion (152, 154–159) and α2-adrenergic receptors inhibit fluid secretion and induce vasoconstriction (155, 160–163).
Mechanistically, α1-adrenergic receptors activate the Gq/11 family of G proteins, resulting in activation of PLC, production of IP3, and increased [Ca2+]i in a similar way to muscarinic receptors (164). In addition, the selective activation of α1-adrenergic receptors with the agonist phenylephrine leads to recruitment of AQP5 to the apical membrane of acinar cells via the Ca2+/cyclic guanosine monophosphate (cGMP)/PKG signaling pathway (132), likely contributing to fluid outflow through the apical membrane. In contrast, α2-adrenergic receptors activate the Gi/o family of G proteins and inhibit adenylate cyclase, which causes a reduction in cyclic adenosine monophosphate (cAMP) levels. Functionally, this results in vasoconstriction, reduction in fluid secretion, and decreased neurotransmitter levels (165). Finally, β-adrenergic receptors activate adenylate cyclase. resulting in the accumulation of intracellular cAMP, which in turn activates protein kinase A (PKA). It is generally accepted that cAMP-dependent PKA signaling downstream of β-adrenergic receptors does not play a significant role in the secretion of fluid, but it promotes an increase in both [Ca2+]i and protein secretion (150, 158, 166). Notably, a recent study suggested that cAMP-dependent secretion may involve distinct G proteins in a gland-specific manner, which remains to be investigated (158).
In addition to the direct stimulatory effect of autonomic innervation in SGs, centrally localized muscarinic and adrenergic receptors also modulate saliva secretion. For instance, the cholinergic agonist pilocarpine stimulates salivation through the activation of centrally localized muscarinic receptors in the brain (167, 168), and this is inhibited by centrally expressed α2-adrenoreceptors that induce vasoconstriction in the SG upon stimulation with norepinephrine (162, 169). Similarly, blockade of the norepinephrine transporter with atomoxetine leads to increases in central norepinephrine levels and induces salivary amylase secretion (154). Induction of amylase secretion is also observed in healthy individuals after receiving an infusion of norepinephrine even in the presence of the nonselective α-adrenoreceptor antagonist phentolamine, highlighting the involvement of β-adrenoreceptors (157, 170).
Saliva secretion can also be triggered by nonadrenergic, noncholinergic parasympathetic stimuli in response to neuropeptides like vasoactive intestinal peptide (VIP) and SP in a species-dependent manner. The effect of these neuropeptides in human SG function has been mostly explored in vitro. VIP stimulates protein secretion and exerts a vasodilatory effect to increase blood flow without overt changes in fluid volume across species, including humans (158, 171, 172). In contrast, SP is one of the most powerful secretagogues in rat SMGs, where it stimulates fluid output along with increased blood flow and protein secretion, but in cats and humans it causes vasodilation without fluid secretion (173, 174). Mechanistically, VIP increases cAMP levels in a similar way to β-adrenergic receptors, whereas SP binds to the neurokinin-1 receptor and leads to the activation of PLC to produce IP3 and induce Ca2+ release (14, 158).
2.3.2. Calcium regulation of secretion.
On a cellular level, an increase in cytosolic [Ca2+]i is necessary for the activation of ion channels and transporters that generate the osmotic gradient required for saliva secretion (175). The specific mechanisms driving this process in healthy and diseased SGs have been extensively reviewed elsewhere (145, 146) and are summarized in FIGURE 4. Briefly, activation of muscarinic and adrenergic receptors results in the production of IP3 and subsequent activation of IP3Rs in the ER, which triggers Ca2+ release from the ER to increase the [Ca2+]i. In SGs, IP3R2 and IP3R3 are the main isoforms expressed in the ER responsible for mediating Ca2+ release and secretion. Mice lacking either isoform show reduced saliva secretion, and double knockouts fail to secrete saliva completely and die within the first month (176). Increased [Ca2+]i activates Ca2+-dependent channels to mediate the exchange of Na+, K+, Cl−, and water across the plasma membrane (PM).
Depletion of Ca2+ concentration in the ER ([Ca2+]ER) leads to activation of the store-operated Ca2+ entry (SOCE) mechanism to promote Ca2+ entry in salivary acinar cells to maintain Ca2+ levels. In turn, Ca2+ entry is regulated by SOCE-dependent Ca2+ release-activated Ca2+ (CRAC) channels. Ca2+ influx ensures that ER stores are reloaded with Ca2+ upon termination of stimulus and allows for continuous secretion. In SGs, SOCE is initiated by Ca2+-sensing stromal interaction molecule (STIM) proteins localized to the ER membrane, which act as sensors for Ca2+ depletion and interact with Orai1 and transient receptor potential canonical (TRPC) channels in the PM to import Ca2+ into the cell.
TRPC1 and TRPC3 act as Ca2+-permeable channels in the PM. Mice lacking either TRPC1 or TRPC3 show decreased SOCE and fluid secretion, demonstrating their role in these processes (177–180). Moreover, knockdown of TRPC1 in a human SG cell line leads to a reduction of Ca2+ influx without affecting [Ca2+]ER release (179). A specific role for TRPC3 in mediating SOCE in SGs is unclear, but TRPC3−/− mice show a significant protection from pancreatitis induced by hyperactivation of SOCE, suggesting a role in mediating disease in other exocrine glands (178). Upon stimulation, TRPC1 and TRPC3 coimmunoprecipitate with STIM1, which functions as a sensor for Ca2+ depletion in the ER. This interaction is dependent on TRPC1, as the loss of TRPC1 prevents the association between STIM1 and TRPC3 at the PM-ER junction (177). Although TRPC1 mediates the formation of the complex with TRPC3 and STIM1, its function depends on both STIM1 and Orai1 (177, 181). This was demonstrated with knockdowns of Orai1 or STIM1, which completely abolished SOCE in a human SG cell line, whereas the knockdown of TRPC1 only caused a partial decrease (182–184).
The dependence of SOCE on STIM1 and Orai1 is due to their contribution to the formation of the SOCE complex in the ER-PM junctions and the formation of pores in the ER for Ca2+ entry, respectively. STIM1 has binding sites for Ca2+ in the ER and for Orai1 and TRPC1 in a cytosolic domain. Upon Ca2+ binding, STIM1 undergoes a conformational change that results in multimerization of the protein and translocation to the PM, where it works as an anchor to bring Orai1 and TRPC1 together at ER-PM junctions. Once the complex is formed, STIM1 activates Orai1 and TRPC1. Another sensor for [Ca2+]ER depletion is STIM2, which shares significant homology with STIM1. STIM2 is sensitive to small depletions of [Ca2+]ER and can promote the assembly of STIM1-Orai1 at the ER-PM junctions at low stimulus intensities (185, 186). Unlike STIM1, however, STIM2 is a poor activator of Orai1 (185, 186). Orai1 is the first channel to be activated and is the primary channel responsible for pore formation in the ER to allow for Ca2+ entry, whereas TRPC1 amplifies Orai1-mediated modulation of [Ca2+]i (184, 187). Nonetheless, both Orai1 and TRPC1 generate specific Ca2+ signals in the cytoplasm to regulate different cellular functions, which remain to be elucidated.
Targeted deletion of SOCE components and CRAC channels has corroborated their contribution to secretion. Deletion of STIM2 in SGs of STIM2fl/fl mice leads to a deficiency of saliva secretion with low stimulation (185), and TRPC1−/− mice show a 70% decrease in pilocarpine-stimulated saliva (179). SG fluid secretion has not been measured in Orai1−/− mice, but they show a 50% reduction in pilocarpine-stimulated tear secretion from lacrimal glands (188). Ubiquitous deletion of STIM1 is embryonic lethal because of cardiac defects, and thus salivary secretion cannot be measured in this model (189, 190). Conditional knockouts lacking STIM1 have been used to overcome the embryonic lethality (191). In irradiated SGs, the Ca2+-permeable nonselective cation channel transient receptor potential melastatin 2 (TRPM2) contributes to SG dysfunction by inducing caspase-3-dependent cleavage of STIM1 (180, 192), indirectly demonstrating the contribution of STIM1 to saliva secretion.
2.3.3. Ion channels and transporters involved in saliva secretion.
The opening of Cl− channels is crucial for the secretion of saliva from acinar cells and reabsorption of NaCl by duct cells to produce a hypotonic solution. Agonist-induced increases in [Ca2+]i drive saliva secretion in acinar cells via transepithelial chloride movement, which is supported by ion transporters, K+ and Cl− channels, and the Na+-K+-ATPase. Acinar cells maintain a relatively high intracellular concentration of Cl− (∼60 mM) by balancing influx/efflux of Cl− through distinct mechanisms. The main Cl− uptake mechanisms involve NKCC1, NHE1, and the Cl−/ exchangers (71, 131). NKCC1 is localized in the basolateral membrane of acinar cells, where it undergoes three cotransport cycles to facilitate entry of 3 Na+, 3 K+, and 6 Cl− ions. In mice, genetic ablation of Nkcc1 led to a dramatic decrease in Cl− uptake and ∼60% reduction in saliva secretion, which were compensated by upregulation of the Cl−/ exchanger (193). At least two Cl−/ exchangers are functionally expressed in acinar cells, namely Scl4a4 (Ae2) and Slc4a9 (Ae4). Slc4a9 has been directly implicated in saliva secretion, as Slc4a9-deficient mice show decrease in cAMP-dependent (β-adrenergic) salivation (194). Although the specific mechanism whereby Slc4a9 drives Cl− influx is not yet fully elucidated, it is postulated that Slc4a9 works as an electroneutral, nonselective cation-dependent Cl−/ exchanger that promotes Cl− influx in exchange for K+, Na+, and ions (194, 195). The export of acidifies the intracellular space, which is buffered by the activation of NHE1 in the basolateral membrane. Both NHE1 and the Cl−/ work in parallel to drive additional Cl− intake along with Na+ while exporting H+ and 2 (71, 115). NHE1-deficient mice display a 95% reduction in Na+/H+ exchange, a 34% reduction in saliva volume, and impaired NaCl reabsorption (196).
Anoctamin 1 (ANO1), also known as transmembrane member 16A (TMEM16A), is primarily found in the apical membrane of acinar cells and mediates Cl− efflux (29, 106). This is facilitated by the polarized influx of Ca2+ and subsequent opening of ion channels initiated by neurotransmitters (175). ANO1 is considered the most important Ca2+-activated Cl− channel (CaCC) involved in muscarinic-induced salivation (16). Tmem16A−/− mice die shortly after birth, and conditional deletion of Tmem16A in acinar cells completely ablates Ca2+-dependent salivation (133). ANO1 functions are regulated by TRPC1-mediated Ca2+ influx upon [Ca2+]ER depletion, and TRPC1-knockout mice show significant reduction in ANO1 currents (197, 198). In addition, activation of transient receptor potential vanilloid 4 (TRPV4) also induces ANO1-mediated Cl− currents and fluid secretion downstream of muscarinic stimulation (175, 199). Notably, stimulated saliva secretion with the β-adrenergic agonist isoproterenol is unaffected in mice with acinar deletion of Tmem16a, indicating that the channel functions independently of cAMP-dependent secretion downstream of β-adrenergic signaling (133).
The K+ imported into the cell during Cl− influx is exported via a Na+-K+-ATPase and K+ channels (14). There are two known Ca2+-activated K+ channels in SG acinar cells encoded by the KCNN4 and KCNMA1 genes (200, 201). KCNN4 is an intermediate-conductance K+ channel also known as KCa3.1, IK1, SK4, or Gardos channel, whereas KCNMA1 is a large-conductance channel referred to as KCa1.1, maxi-K, Slo1, or BK channel. These basolateral K+ channels recycle K+ into the serosal space to increase influx of Na+ via basolateral Na+-K+-ATPases. Furthermore, the opening of K+ channels hyperpolarizes the acinar cell membrane to facilitate basolateral influx of Cl− into the cellular space via NKCC1, and it maintains an optimal membrane potential for Ca2+ entry. In turn, intracellular Ca2+ activates K+ channels, creating a feedforward mechanism that contributes to concentration gradients within the cell and a wavelike pattern of secretion. Transgenic models with ablation of Kcnn4 and Kcnma1 genes in mice have demonstrated that saliva secretion is severely impaired in mice lacking both channels (200, 202). However, in the absence of one gene, the remaining channel is sufficient to maintain secretion of fluid from acinar cells of PG and SMG, albeit KCNMA1-deficient mice secreted 75% less K+ and reabsorbed significantly less Na+ than wild-type control mice (202, 203).
2.3.4. transepithelial transport.
During the second stage of saliva secretion, duct cells export and reabsorb NaCl to produce a final hypotonic fluid (FIGURE 5). This process is mediated by the Na+- cotransporters (NBCs), CFTR, and the apically localized Cl−/ exchanger Slc26a6 (17, 50, 74, 80, 129, 134–136). Cl−/ exchangers and Na+- cotransporters are encoded in distinct SLC4 genes (204), and their purpose is to regulate intracellular pH by facilitating the exchange of protons and across the membrane. The NBCe1-B channel expressed in SG is localized in the basolateral membrane of duct and acinar cells and mediates entry (75). Basolateral absorption of by NBCe1-B is balanced by the export of on the luminal membrane by Slc26a6. In addition, the NBCe1-A channel also localized at the luminal membrane is capable of absorbing , which is thought to maintain low pH and prevent salivary enzyme activation during resting states (148). IP3R binding protein released with inositol 1,4,5-triphosphate (IRBIT) is a more recently discovered regulator of transepithelial transport that becomes activated after binding of IP3 to its receptor and works as a scaffold to bind NBC channels, CFTR, and Slc26a6 at the cell membrane (205).
CFTR and ENaC are localized to the luminal membrane of ducts in rodent and human SGs, where they facilitate the reabsorption of Na+ and Cl− and secretion of K+ and . In sweat glands, the activity of ENaC is dependent on CFTR, and NaCl reabsorption by the SDs of SGs requires both CFTR and ENaC (74, 136). Although CFTR does not directly contribute to fluid secretion from epithelial cells (74), CFTR expression is markedly reduced in diseased states of SGs and pancreas, such SS and pancreatitis, respectively. In addition, transgenic overexpression of CFTR in mouse models of salivary and pancreatic injury rescued CFTR expression and localization, increased Ca2+ signaling and fluid secretion in acinar cells, and restored salivation (206). Furthermore, mutations in the Cftr gene in mice or a decrease in Cftr expression leads to inflammation, fibrosis, and tissue damage (206). However, restoration of Cftr expression reduces inflammation and SG damage (206). In a mouse model of SS, ductal Cftr was reduced and the correction of ductal CFTR function with CFTR correctors restored saliva flow, improved acinar markers, and reduced inflammation (206).
In SG ducts, Clcn2 encodes the hyperpolarization-activated chloride channel CCL-2 that mediates an inward-rectifying Cl− current; however, saliva composition, osmolality, and flow rate are all normal in Clcn2−/− mice, indicating that the channel is not required for NaCl reabsorption in the ductal system of the SGs (207). Moreover, stimulated saliva secretion with the β-adrenergic agonist isoproterenol was unaffected in mice with acinar deletion of Cftr (CftrΔF508/ΔF508) or ClC-2 (Clcn2−/−), indicating that their function is independent of β-adrenergic signaling via cAMP (133).
3. FUNCTIONS AND DIAGNOSTIC APPLICATIONS OF SALIVA
3.1. Lubricating and Protective Functions
Once saliva is secreted, modified, and delivered to the oral cavity, it plays an important role in lubrication. Lubrication enables proper swallowing of the food bolus, protects the mineralized surface of teeth, and maintains the integrity of the oral epithelial integument. This property is a result of the heavy glycosylation on many salivary proteins (208). The salivary proteome contains thousands of proteins and peptides, and major families of proteins identified in saliva include basic and acidic proline-rich proteins, salivary amylase, mucins, salivary agglutinins (gp340/DMBT1), secretory immunoglobulins, salivary cystatins, histatins, and statherins (42). The human salivary proteomes from both healthy and disease states have been generated and can be interrogated online with the Human Salivary Proteome Wiki, which is an evolving public database platform (209).
The diverse glycan landscape in the oral environment is mainly due to mucin glycoproteins in saliva, which are the most densely glycosylated salivary proteins (210). The predominant mucins in human saliva are the high-molecular-weight gel-forming mucin MUC5B and the low-molecular-weight soluble mucin MUC7 (10, 211). Other salivary mucins include MUC19, MUC1, and MUC4. In adult mice, MUC19 is the dominant gel-forming mucin, which is synthesized and secreted by the major SLGs and minor mucous glands (212). Unlike humans, rodent SMGs produce MUC10, a mouse ortholog of human MUC7 (8). Mucins are mostly decorated with O-glycans linked to serine and threonine residues in the protein backbone, conferring mucins’ bottlebrush-like structure. The O-glycans account for 80% of mucin dry molecular weight (213). In addition, the mucin backbone contains some N-glycans linked to asparagine and varying types of sialoglycans such as different types of terminal sialic acids, sulfates, modifications by O-acetylation, and the linkage to their subterminal glycan chains (214). Sialic acid and sulfate residues give mucins a net negative charge, creating an extended configuration and rendering them water retentive (215), thus playing a role mucin hydration (216). Mucins are therefore integral to rendering saliva its rheological properties such as viscosity, elasticity, and stickiness (217).
Salivary mucins along with other salivary proteins protect the oral cavity by coating the soft and hard tissues to form a thin proteinaceous film called the acquired pellicle. Both saliva and the pellicle together prevent desiccation, provide lubrication, maintain mineral homeostasis, determine the initial microbial colonizers on the tooth surface, and protect dental surface from acid attacks in the diet and those produced by oral bacteria (218). In addition, the pellicle layer protects the oral mucosa against various viruses through salivary components such as cathelicidin, lactoferrin, lysozyme, mucins, peroxidases, salivary agglutinin (gp340, DMBT1), sIgA, SLP1, and α and β defensins (219). Binding of mucins to antibacterial salivary proteins such as acidic and basic proline-rich proteins, statherins, histatin1, and sIgA increases their availability and retention time in saliva (10), whereas loss of the enzymes that glycosylate MUC10 in mice alters the composition of the oral microbiome (220). Salivary mucins protect the oral cavity by aggregating bacteria suspended in saliva via glycan-specific interactions to facilitate removal of bacteria from the oral cavity during swallowing. Statherin, a Ca2+-binding protein, prevents the precipitation of Ca2+ phosphate, thus maintaining a supersaturated Ca2+ level in saliva available for remineralization of tooth enamel and high phosphate levels for buffering of the oral environment (221).
The importance of saliva’s role in lubrication is evident in patients with dry mouth, who struggle with food mastication, swallowing, and speaking and have increased risk of tooth demineralization, dental caries, oral mucositis, and fungal infections. Analysis of the saliva of patients with dry mouth suffering from SS shows that despite having similar mucin protein concentrations, the glycoprotein structure is altered (217). Specifically, there is a decrease in the proportion of O-glycans and in sialic acid residues on mucins. Deglycosylation results in a reduction in the viscoelasticity and lubricating properties of mucins, as it affects the mucins’ structural organization (222). In addition, recent analysis by cryo-scanning electron microscopy of saliva from head and neck cancer patients after RT showed that mucins have thinner fibers and form a fragile network with larger pores, whereas saliva from healthy volunteers had thick mucin fibers that formed a strong porous network (223). The loss of negatively charged glycan residues is a proposed mechanism for oral dryness through the reduced water retention capacity of mucins, leading to reduced mucosal hydration (224). Therefore, understanding of the rheological and lubricating properties of saliva pertaining to the observed changes in mucin glycosylation provides useful insight into development of saliva substitutes for dry mouth therapy.
3.2. Wound Healing Functions
Although the mouth repeatedly experiences wounds resulting from cheek, tongue, and lip biting to tooth extraction, these wounds heal much faster and with less scarring and less frequent infections than wounds in other parts of the body (225). Saliva promotes oral wound healing in a number of ways. Saliva creates a humid environment in the oral cavity, which improves the survival and functioning of inflammatory cells that are crucial for wound healing. Saliva also contains carbohydrate complexes and glycosylated proteins, mucins, bioactive peptides such as growth factors, enzymes, and antimicrobial peptides that directly or indirectly enhance wound healing (226).
Notably, the potent nerve growth-promoting activity of snake venom and mouse saliva led to the discovery of NGF, which was first identified in snake venom and later isolated from male mouse SMGs, where it is highly expressed in the GCTs (227, 228). NGF secreted in saliva has been associated with a wide range of physiological functions including wound healing of the oral epithelia and esophageal tract, wound closure in skin, fibroblast and keratinocyte proliferation, cell migration, and, of course, peripheral nerve growth and survival. In addition to NGF, SGs are rich in EGF, FGF, VEGF, insulin-like growth factor 1 (IGF-1), transforming growth factor β (TGF-β), bone morphogenetic protein (BMP), trefoil factor 3 (TFF3), mucins, and histatins, all of which play essential roles in wound healing of the oral mucosa and esophageal tract (5, 7–18, 229).
3.3. Diagnostic Applications
The molecular composition of saliva reflects the physiological or pathological state of an organism, and most if not all of the soluble biological markers found in serum or urine can be found in saliva, including hormones, drug metabolites, and markers that are specific to both oral and systemic pathological states (230–235). Saliva is a rich reservoir of a wide spectrum of proteins/peptides, nucleic acids, electrolytes, and hormones that originate in multiple local and systemic sources. Salivary transcriptomics combined with proteomics allows the identification of the contribution of the major glands and serum to saliva (30). This is useful information as it provides a basis for using saliva as a diagnostic tool that could potentially replace blood tests. Diagnostically, a number of findings have prompted interest in the use of saliva as a source of biomarkers for a variety of conditions, including dental caries, periodontal disease, cancer, diabetes, viral infection, SS, cardiovascular disease, and many other systemic disorders (236). This potential for saliva to be used as a tool in the diagnosis of disease has been widely recognized and reviewed previously (237, 238). One caveat is that the concentration of any given compound is 10- to 100-fold lower in saliva than found in serum and thus may escape the limits of detection of specific methodologies.
Saliva harbors viral particles despite its antimicrobial properties and thus is commonly used as an inexpensive and noninvasive tool for the diagnosis of viral infections such as SARS-Coronavirus-2 (SARS-CoV-2), Epstein–Barr virus (EBV), hepatitis B virus, Ebola, rabies, and HIV. Recently, both SARS-CoV-2 and certain strains of enteroviruses, such as noroviruses, have been detected in SGs, suggesting that saliva may be a route for disease transmission (19–21). Coronaviruses (CoVs) are single-stranded RNA viruses that infect humans and other animals, including birds, livestock, and domestic animals. Seasonal variants of CoVs are responsible for mild respiratory infections, whereas severe acute respiratory syndrome coronavirus (SARS-CoV) is caused by more pathogenic strains (239). Coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 infection manifests in a broad spectrum of symptoms that range from asymptomatic disease to lethality due to sepsis and respiratory failure. Other common signs of infection include loss of taste, dry mouth, and mucosal lesions, which was recently suggested to be a consequence of an active participation of the oral cavity tissues in harboring and spreading the virus (26). Transmission of SARS-CoV-2 can occur through the propagation of saliva droplets during talking, breathing, coughing, or sneezing because of the persistence of viral particles in saliva. A study estimated that the concentration of viral particles in saliva peaked 1 wk after the onset of symptoms and could be detected up to 28 days later by RT-PCR. Interestingly, there was no difference in viral load detected in saliva of patients with mild or severe disease (240). In addition, a different study found that saliva immunoglobulin (IgG) antibodies to SARS-CoV-2 are maintained for at least 3 mo after symptom onset (241), suggesting that saliva could be used both as a diagnostic tool for the early detection of SARS-CoV-2 infections and for the development of systemic immunity against it.
Noroviruses were previously thought to only replicate in the intestines and be transmitted by the fecal-oral route through fomite contamination. Recently they have been reported to infect salivary glands of mice and primary human salivary cells, suggesting that saliva may be a new route of transmission. In addition, the oral-gut route may be involved in viral replication and infection (597). In addition, many other viruses also infect SGs, including mumps, EBV, herpes virus, parainfluenza, influenza, adenovirus, and bocavirus (243), highlighting the importance of understanding SG biology to help investigate viral pathogenesis and transmission. Furthermore, the temporal concentration of viral particles in saliva may be used as an indicator of disease progression or infectivity. This has tremendous implications for the development of saliva-based diagnostic tools and point-of-care diagnostics that may offer significant advantages over serum-based testing (233).
4. DISORDERS AND HYPOFUNCTION
4.1. Xerostomia and Hypofunction
Xerostomia is the subjective sensation of dryness felt by a patient, but it does not necessarily correlate with a reduction in salivary flow (244). In contrast, hyposalivation is the objective and measurable loss of saliva flow. The latter has debilitating consequences, as it negatively affects the ability to taste and digest food, and it predisposes patients to generalized oral dryness, cracked lips, irritation of the buccal mucosa and soft palate, loss of papillae in the tongue, tooth and enamel deterioration, increased infections, difficulty in eating and swallowing, alterations in taste and smell, halitosis, gingivitis, mouth sores, and malnutrition (245). Depending on their etiology, SG disorders can be developmental, autoimmune, physical, and oncogenic in nature, and they may be temporary, chronic, or permanent. Xerostomia is caused by alterations that directly affect SG function due to infections, duct obstruction, cancer, autoimmune and systemic disease, and cancer therapies (246). Xerostomia occurs frequently in the general population, and diagnosis is usually informed by a questionnaire that asks patients whether they feel dry mouth and with what frequency (247). In addition, the passive drool test (PDT) is the primary method to objectively measure saliva output, and new technologies continue to be developed. The BokaFlo biomedical device was recently tested to evaluate salivary flow and predict hyposalivation (saliva flow rate ≤ 0.1 mL/min) in subjects (248). Other point-of-care kits include the GC Corporation’s Salivary Check-BUFFER Kit to measure saliva consistency, pH, and flow, and the GC Saliva-Check MUTANS Kit measures the presence of Streptococcus mutans in saliva, potentially assessing the dental caries risk of patients (249).
In younger populations, xerostomia is primarily associated with medications. A 10% prevalence of xerostomia was reported in two cohorts (950 subjects) of 26- and 32-yr-old subjects; however, it was 22 times higher in those taking antidepressants (250). Iron supplements and narcotic analgesics also increased prevalence of xerostomia in this study. The nervous system plays an essential role in saliva secretion. Thus, it is no surprise that psychological and neurological factors such as stress, anxiety, Parkinson’s disease, and Alzheimer’s disease may also contribute to the development of xerostomia (251, 252). Similarly, conditions that affect nerve stimulation also impact the secretory ability of the gland. These include encephalitis, brain tumors, smoking, and a large number of medications like antihistamines, antihypertensives, opioids, antidepressants, antiepileptics, anxiolytics, anticholinergics, and antimuscarinics (251, 253). Sympathomimetic drugs also affect volume and composition of saliva by inducing the production of mucinous saliva that is more viscous (254). Obstructive injuries that cause blockade of the EDs also lead to temporary loss of function that recedes upon removal of the block, although if untreated they may cause fibrosis and permanent dysfunction. A relatively higher prevalence of up to 28% and 60% are reported in the elderly and in patients living in long-term care facilities, respectively (255, 256). However, there was the well-described association between chronic diseases and medication with the development of xerostomia, as decreases in stimulated parotid salivary flow are not related to aging (257). SGs are sensitive to both viral and bacterial infections that cause inflammation of the glands, also known as sialadenitis. Most recently, xerostomia was also reported in patients diagnosed with COVID-19 (258). Permanent loss of function is more often associated with developmental disorders that result in gland aplasia (absence of SGs), autoimmune disorders such as SS and graft-versus-host disease (GVHD), and systemic disease such as diabetes and cystic fibrosis (CF) and as a consequence of RT treatment for head and neck cancer patients (245, 259).
4.2. Developmental Disorders
Human genetic mutations in various growth factor ligands and receptors underlie diseases with SG phenotypes. For example, patients with haploinsufficiency of FGF10 suffer from one of two overlapping conditions: autosomal dominant aplasia of the lacrimal and SGs (ALSG) or autosomal dominant lacrimo-auriculo-dento-digital (LADD) syndrome (260–262). Both disorders are characterized by aplasia or hypoplasia of SGs, xerostomia, increased dental problems, and oral infections. LADD syndrome patients often display various additional features that include facial dysmorphisms, outer and inner ear anomalies and hearing loss, teeth anomalies, distal limb malformations, and, more infrequently, impairment of kidney and lung development and craniofacial, digital, and genitourinary defects (262–264). Heterozygous mutations in FGFR2 or FGFR3 can also cause LADD syndrome (265). Consistent with these human phenotypes, mouse models with deletion of either Fgf10 or Fgfr2b lack SGs and many other ectodermal organs, highlighting that these genes are critical for gland initiation (266). The heterozygous Fgf10 mouse also has hypoplastic glands with reduced saliva production (267).
Apert syndrome, which is caused by a single nucleotide substitution mutation (S252W or P253R) in FGFR2, is a congenital disorder characterized by craniosynostosis, midface hypoplasia, and bony syndactyly of the hands and feet (268, 269). The FGFR2 missense mutations result in gain of function, thus enhancing receptor occupancy by FGF ligand and/or propagating the duration of receptor signaling (269). Clinical observation of Apert syndrome patients suggests increase in saliva production, but this has not been thoroughly investigated (270). Mouse models of Apert syndrome show that SGs are larger, with hyperplasia of the parenchyma and increased branching (270, 271). Together, these mutations highlight the importance of proper FGF signaling thresholds in SG development and function.
Hypohidrotic ectodermal dysplasias (HED) is a syndrome variably characterized by absent or hypoplastic SGs, teeth, hair, sweat glands, sebaceous glands, lacrimal glands, mammary glands, and mucous glands of the bronchial, esophageal, and colonic mucosa (272, 273). HED patients suffer from dry mouth due to dramatically reduced salivary flow, and heterozygous female carriers are best identified by reduced saliva flow and altered saliva protein composition (274). Mutations in Ectodysplasia (EDA), its cognate receptor EDAR, or adaptor molecule EDARRAD or WNT10A cause HED (275, 276). Mouse mutants for Eda (Tabby), Edar (Downless), and Crinkled (Edaradd) genes also phenocopy individuals with HED (277–279). Studies using these mice models show abnormal SGs. Eda expression is found to be restricted to the SMG mesenchyme and is downstream of Wnt/β-catenin signaling (280).
CF is a multisystem developmental disease caused by mutations in CFTR that result in mislocalization and/or altered activity, which also results in altered SG function (281, 282). Patients with CF exhibit changes in salivary electrolyte and protein composition (283–285). Examination of salivary function in the homozygous mouse for the ΔF508 mutation (CftrΔF/ΔF) shows defects characteristic of CF related to fluid secretion or NaCl reabsorption. Although stimulated saliva flow was normal, the NaCl content of saliva is elevated in mice lacking CFTR (74).
Recently, the electroneutral Na+-dependent chloride transporter NKCC1 has been identified as a protein causing human disease. Clinical cases of patients with inherited mutations in NKCC1, encoded by SLC12A2 gene, show that complete absence of the protein results in severe xerostomia, deafness, mucus accumulation in lung and intestine, hypotonia, dysmorphic facial features, and severe neurodevelopmental disorder (286). Accordingly, mice lacking NKCC1 show severe impairment of salivation resulting from disrupted regulation of Cl− ions across acinar cells (193), suggesting they are a useful model to investigate disease mechanism.
4.3. Autoimmune Disease
4.3.1. Sjögren’s syndrome.
SS is a disabling autoimmune disease primarily affecting the salivary and lacrimal glands, presenting with hyposalivation and ocular dryness. SS can develop in the absence of other underlying rheumatic disorders, although it can be associated with other autoimmune diseases like rheumatoid arthritis, lupus, and psoriatic arthritis (287). The symptoms of SS are due to the loss of acinar cells and drying of mucous membranes, resulting in xerostomia, xerophthalmia, xerosis, and systemic extraglandular organ involvement including neuropathies, pulmonary manifestations, and nephritis (288, 289). SS affects 0.5–1.0% of the population, including between 400,000 and 3.1 million adults (290). It is more common in women, with a female-to-male ratio of ∼9–16:1, and mainly affects individuals between the ages of 50 and 60 yr (291, 292). SS has been extensively reviewed elsewhere, to which the reader is referred (289, 293–297).
Because of the diverse presentation of SS, a major challenge remains to improve diagnosis. The Sjögren’s International Clinical Collaborative Alliance and the American College of Rheumatology-European League Against Rheumatism have published protocols to measure focal mononuclear infiltrates of minor labial SG (LSG) biopsies and assign a focus score to aid with SS diagnosis (298–300). The most prominent histopathological finding in LSGs is the presence of well-defined foci of mostly lymphocytes surrounding ducts or small vessels. In addition, the presence of autoantibodies SS-related antigen A (SSA; also known as anti-Ro/SSA antibodies) and SSB (also known as anti-La/SSB antibodies) are key serological markers used in the diagnosis of SS (289).
Although the pathogenesis of the disease is unknown, a key hallmark is B cell hyperactivity. Hypergammaglobulinemia, autoantibody production, and alterations of B cell subpopulations are distinctive features of SS. The focal lymphocytic infiltrates within SGs consist of mainly B and T cells and a minor proportion of other mononuclear cells, including plasma cells, macrophages, myeloid and plasmacytoid dendritic cells (PDCs), and follicular dendritic cells that develop progressively in association with striated ducts or small vessels (293, 294, 300). The microenvironment of the inflamed glandular tissue is rich in chemokines and cytokines that promote B cell recruitment, homing, survival, activation, and plasma cell formation (301). In turn, the increasing long-lived plasma cells within the affected glands produce autoantigen-specific autoantibodies such as anti-SS-A/Ro and anti-SS-B/La (302). Other autoantibodies reported with SS include anti-M3 muscarinic/cholinergic receptor agonists, anti-nuclear antibodies (ANAs), and rheumatoid factor (302, 303). These autoantibodies react with the corresponding autoantigen, forming immune complexes that activate inflammatory cells via complement and Fc receptors, resulting in interferon-α (IFN-α) production by infiltrating dendritic cells (304, 305). Further activation and survival of B cells occurs and is mainly caused by B cell-activating factor (BAFF) produced by various cell types including B cells, monocytes/macrophages, dendritic cells, neutrophils, epithelial cells, and activated T cells (306). In addition, interleukins, interferon-γ (IFN-γ), and TGF-β are released by the infiltrating T cells, macrophages, and possibly the damaged glandular cells (307). This then leads to occurrence of ectopic germinal centers (GCs), which are a sign of local B cell activation. This impairs SG function and results in destruction of glandular tissue. The GCs can grow independently from the surrounding tissue and can result in non-Hodgkins and mucosa-associated lymphoid tissue (MALT) lymphomas (308–310).
During the earlier stages of SS, most of the infiltrating cells are T cells and in particular the CD4+ T cells (311). Different CD4+ T cell subsets also contribute to SS pathogenesis, including T helper 1 (Th1) and T helper 2 (Th2) cells, T follicular helper (Tfh) cells, and T helper 17 (Th17) cells (312). Tfh cells govern the ectopic germinal center formation in SG and potentiate the production of autoantibodies from B cells (313). Th17 cells, in association with Th1 and Th2 cells, are responsible for increased inflammatory cytokine production, such as IL-17, IL-21, and IL-22 (314), which are found in high concentration in the serum and LSGs of SS patients (315, 316). IL-17 promotes ectopic lymphoid tissue formation by stimulating stromal cells to produce CXCL13, which binds its receptor CXCR on B cells, which is required for homing of B cells to these sites (317). IL-6, which is also overexpressed in SS, favors Th17 and Tfh cell differentiation (318, 319). Notably, the initial activation and polarization of Th17 cells is due to dendritic cells, which secrete IL-6 and TNF in lymph nodes that drain the SGs. At later stages in SS, this Th17 cell activation may occur locally within the inflamed glandular tissue (312).
To understand the role of progenitor cells in SS, PG biopsies were cultured as organoids. Biopsies from SS patients formed fewer organoids with reduced regenerative potential that had shorter telomeres than control biopsies. This suggested that the stem cell pool had undergone an extensive replicative history and thus became senescent (320). This may be a consequence of the mitotic effect of proinflammatory cytokines (320). Generally, senescent cells have a senescence-associated secretory phenotype in which these cells secrete high levels of inflammatory cytokines, immune modulators, growth factors, and proteases (321, 322). This may enable the active spread of senescence directly to the neighboring cells, causing tissue dysfunction (322). Senescent cells express p16, which functions as an inhibitor of cell division kinase 4. Notably, the expression of p16 in basal cells of the SD (proposed progenitors) correlates with saliva production and CD45+ leukocyte cell infiltration in SS patients (323). Thus, basal cell senescence may be an early feature of SS and may contribute to reduced SG function.
An emerging role for the involvement of SG epithelial cells in SS SG dysfunction has been proposed because SG epithelial cells produce factors that attract and activate immune cells. Ductal epithelial cells from SS patients have been reported to express many cytokines and chemokines such as IL-1, IL-6, IL-7, IL-18, TNF, BAFF, CXCL10, CXCL12, and CXCL13 at higher levels than those observed in healthy individuals (293). Recently, BMP6, which is a member of the TGF-β superfamily, was reported to be overexpressed in the SGs of 54% of SS patients, which correlated with both reduced SG function and increased lymphocytic infiltration (324, 325). Accordingly, overexpression of BMP6 in murine SGs results in a SS-like phenotype (324). Treatment of either BMP6-overexpressing mice or SS mouse model (C57BL/6.NOD-Aec1Aec2) mice with pharmacological inhibitors of BMP signaling restored SG function by decreasing BMP6 signaling, downstream SMAD1/5/8 phosphorylation, and inflammatory markers (326). It remains to be determined which cells in the SS SGs are producing excess BMP6 and what stimulates its production.
Apoptosis has also been identified in SG epithelial cells in patients and mouse models of SS patients. Lysosomal proteins, which are key components involved in antigen presentation and cell survival, have been associated with apoptosis in SS (327). LAMP3 expression is increased in both infiltrating lymphocytes and SG epithelial cells in SS patients. LAMP3 is associated with the presence of serum autoantibodies, which are released via extracellular vesicles, and LAMP3 induces caspase-3 activity resulting in apoptosis (327). Mice overexpressing LAMP3 in SMGs develop SS-like phenotype with progressive SG hypofunction, immune cell infiltration, increased autoantibody production, and activation of lysosomal and immune pathways (328). LAMP3 expression, caused by increased membrane turnover and endocytic lysosomal activity of SG epithelial cells, results in degradation of membrane proteins such as NKCC1 and AQP5, both critical for saliva secretion. The role of these degradation products in autoantibody production and disease progression remains to be determined.
Interestingly, SS patients also have a higher incidence of acquired N-glycosylation sites in IgG heavy chain variable region genes that are derived from the dominant clones in PG tissue compared with controls (329). Acquisition of new N-glycans in IgG variable regions is a common feature in autoimmune diseases such as chronic Chagas heart disease and rheumatoid arthritis (330). The acquired N-glycans may generate selective advantages that allow antibody-expressing B cells to evade negative selection mechanisms in germline centers. Binding the glycosylated B cell receptor with glycan receptors such as lectins could rescue B cells from cell death. Aged mice lacking galectin-1 (Lgals1−/−), a soluble glycan-binding protein, develop a spontaneous inflammatory disorder that recapitulates SS, with increased serum autoantibodies, conspicuous SG mononuclear cell infiltrates, loss of acinar cells with an increase in ducts, increased gland weight, and a greater percentage of CD45+ infiltrating leukocytes with an increase in CD3+CD8+ T cells (331). The major ligands for galectin-1 are the N-acetyllactosamine residues present in complex N-glycans. The enzyme β1,6-N-acetylglucosaminyltransferase 5 (Mgat5) catalyzes the synthesis of β1,6-N-acetylglucosamine branched N-glycans. Aged Mgat5−/− mice also display a phenotype similar to Lgals1−/− mice. Biopsies of the minor SGs from SS patients also show reduced galectin 1 staining areas (331).
Some of the signaling pathways that contribute to SS include nuclear factor-kappaB (NF-κB), interferon (IFN), and inflammasome signaling pathways. Some patients with SS present with the type 1 IFN signature, an upregulation of IFN-stimulated genes induced by type 1 IFN. Studies have also reported the presence of activated pattern recognition receptors (PRRs) including Toll-like receptors, RNA sensor retinoic acid-inducible gene 1, and inflammasomes in infiltrating and epithelial cells of SS patient SGs (332, 333). Once NF-κB signaling is activated, it can induce gene transcription of proinflammatory cytokines, chemokines, adhesion molecules, cell cycle regulator molecules, antiapoptotic proteins, and angiogenic factors. These in turn regulate cell proliferation, apoptosis, and inflammation; however, these processes are dysregulated in SS (334). Constitutive activation of the NF-κB pathway through knockout of TNFAIP3, an inhibitor of NF-κB signaling, in KRT14-expressing epithelial cells triggers T cell-rich infiltration into the SGs, reducing saliva production (335). There is much discussion about cause and effect of specific signaling pathways, and readers are directed to recent reviews discussing these pathways (293, 332, 334).
The etiology of SS is multifunctional and may include a combination of genetic predisposition and environmental as well as epigenetic factors. Although it is not clear what factors drive SG epithelial activation, it is thought that environmental factors such as chemical compounds, viruses infecting SGs, and Toll-like receptor ligands present in saliva are all potentially involved (293, 336). To date, there is no treatment for SS, and currently standard-of-care treatments are based on the evaluation of glandular dryness and systemic symptoms. This topic has recently been extensively reviewed (295, 296). The lack of a clear etiology challenges the field to remain focused on identifying the molecular basis of the disease while still pursuing specific treatments or therapies to provide clinical relief to the SS patient’s symptoms.
4.3.2. Graft-versus-host disease.
Bone marrow transplant, also known as allogeneic hematopoietic stem cell transplant (aHSCT), is a curative treatment for hematologic cancers. However, it can cause the development of the alloimmune condition GVHD, a substantial cause of morbidity and mortality in patients receiving aHSCT. GVHD is the result of immunological attack on recipient organs or tissues by donor allogeneic T cells. The current disease model is that pretreatment conditioning, including chemotherapy and/or whole-body IR, causes loss of epithelial barrier function and tissue damage, leading to translocation of bacterial components and release of endogenous danger signals (337). These signals induce proinflammatory cytokines, activation of antigen-presenting cells, and alloreactive donor T cell differentiation (337, 338). GVHD can manifest in either acute or chronic form, each with distinct clinical presentation, timing from transplant, and pathogenesis. Acute GVHD usually presents earlier than chronic GVHD, although they can occur at the same time, called “overlap syndrome” (339). Chronic GVHD may involve multiple organs, and major targets include the oral cavity and SGs, referred to as oral GVHD (oGVHD) (340). Symptoms range from mild to severe forms and manifest as lichenoid changes, erythema, and ulcers, and in cases with SG involvement these changes include hyposalivation (341, 342). In addition, changes in saliva composition may occur, such as greater mucoid fraction and mucoid strings of saliva originating from major glands (343). Mucoceles in minor SGs often form in the lower labial and buccal mucosa (343). Histopathological patterns in minor SGs include diffuse lymphocytic infiltrate, damaged intralobular ducts, fibrosis, and a variable degree of acinar atrophy (344, 345). A small study suggested an increase in Th1 and Th2 cytokines as well as a close association between strong infiltration and Th2 cytokines, macrophage-derived chemokine, and CC chemokine receptor 4 (346). Accordingly, infiltrates are composed of CD4+ and CD8+ T cells and CD68+ macrophages, whereas CD20+ B cells are not significantly increased compared with controls (344). Still, the specific immune process in SGs is not well understood, and further work is needed to explore essential players and determine how to regenerate the damaged and fibrotic SGs.
Several murine models of GVHD provide some insight into pathophysiological mechanisms involved in fibrosis and autoantibody production (347). These models show periductal lymphocytic infiltration and parenchymal destruction and fibrosis followed by a significant decrease of saliva secretion (342). However, the molecular mechanisms and the identity of potential therapeutic targets remain to be determined. Currently there is no permanent treatment for oGVHD, and available options are focused on reduction of immune damage and symptom management (341). Early detection and concurrent intervention of oGVHD could be critical for positive outcomes. However, this relies on further work to discover novel biomarkers for early targeted therapy.
4.3.3. Immune checkpoint inhibitor sicca.
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that block tumor-derived inhibitory immune responses, resulting in a broad upregulation of the immune system that increases antitumor immunity. To date, cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death protein ligand (PD-L1) are all ICI cancer treatments (348). Although the treatment is effective against even advanced cancers, this regimen is commonly associated with so called “immune-related adverse effects” (irAEs), which are a variety of undesired autoimmune reactions occurring in any organ (349). “Sicca syndrome” refers to dryness of mucosal surfaces including the oral cavity due to a variety of etiologies, but specifically “ICI sicca” refers to the severe oral manifestation of irAE (350). ICI-induced sicca has been reported in several clinical case studies and grouped into three categories: 1) sicca with systemic symptoms, 2) sicca without systemic symptoms, and 3) sicca in the setting of preexisting autoimmune disease (summarized in Ref. 350). ICI-induced sicca is distinct from SS and is characterized as an abrupt and severe onset of dry mouth within 3 mo of ICI treatment (351). Oral manifestations of irAE also include lichenoid lesions, ulcers, and erythema multiforme (352). Histopathology suggests that an immunologic mechanism causes ICI sicca through the triggering of CD4+/CD8+ T cell activation (351, 353). Histopathological patterns in minor SGs range from mild chronic inflammation with acinar atrophy and fibrosis to severe inflammation with additional injury to the ducts, acinar atrophy, nuclear enlargement, apoptosis, fibrosis, luminal mucin inspissation, and ruptured mucin extravasation (351). Interestingly, a recent case study reported a dramatic loss of acinar cells that took on more ductal phenotype in PGs after PD-L1 treatment, indicating a potential gland-specific reaction involving a change in cell identity not observed in minor SGs (354). Further work is needed to understand the potential gland-specific effects of ICI treatments that may result in dramatic changes in epithelial cell identity.
Salivary damage or sustained autoimmunity may persist long term after completion of ICI treatment, causing persistent dry mouth and often reduced quality of life in these patients. As the use of ICI therapy is rapidly increasing, the appropriate clinical management of ICI sicca is still unclear (351, 353, 355). Future work is needed to predict the probability of developing ICI sicca, potentially by the discovery of early biomarkers of SG inflammation and possible genetic signatures of risk.
4.4. Salivary Gland Cancer
There are ∼53,000 new SG cancer cases/year worldwide (356). More than half are benign, and the majority originate from the PG. The primary cause of death is disease recurrence and distant metastasis (357), histological subtype of cancer, and lymphovascular invasion, and lymph node metastatic burden is strongly associated with recurrence or metastasis-related mortality (358, 359). Recent advances of the grading systems may identify high-risk patients (360, 361). Surgery remains the standard treatment, with RT for control of locoregional spread (362). The most common malignancies are mucoepidermoid carcinoma (MC) and adenoid cystic carcinoma (ACC). Precision diagnosis advances have been made with a classification system that improves clarity and reproducibility of cytological reports (363), definition of new tumor types, refined diagnostic criteria, and novel molecular analyses (364–366). This may facilitate more precise prognosis and allow for targeted therapy of the >20 SG malignancies now defined (367).
The use of next-generation sequencing to characterize SG cancers is increasing, and detection of genetic aberrations may serve as a powerful diagnostic tool, revealing promising prognostic biomarkers and potential therapeutic targets. For example, single-cell RNAseq of a Wnt-dependent mouse model of SG squamous cell carcinoma characterized tumor-specific cells and provided valuable insight on molecular markers to improve diagnosis and possible therapeutic targets (112). The development of a sequencing panel, the SalvGlandDx, significantly aided in classifying most SG cancer. This platform detects potential therapeutic targets, mutations, fusions, and gene expression levels of most of the known recurrent gene aberrations (368). Although clinical trials targeting overexpressed molecular markers such as HER2, ETV6-NTRK3 fusion, and AR among others have had minimal benefits, targeted therapies remain an area of active research (369, 370).
There is an increased incidence of major SG cancer in the United States, specifically of PG origin (371, 372). The increase may be due to earlier detection and improved clinical staging; however, the reason is not clear (371). Risk factors such as viral infections and IR have been implicated without a strong correlation to the pathophysiology (372). This highlights the need for further research to address risk factors, pathophysiology, and etiology of the rising trend in cases. Ultimately, it is critical to understand the molecular mechanisms of cancer initiation and progression to ensure that any regenerative approaches to improve SG function do not promote tumor formation.
4.5. Radiation Therapy-Induced Dysfunction
Head and neck cancers account for nearly 900,000 new cases annually worldwide, and RT remains the treatment of choice (373). Because of high recurrence rates, head and neck cancers are treated with relatively high doses of irradiation (IR), ranging between 70 and 76 Gy for primary tumors following a 5-day weekly regime for ∼7 wk. Intensity-modulated radiotherapy (IMRT) uses up to nine rotational beams to target the tumor while reducing damage caused to surrounding normal tissues. Descriptions of RT modalities are reviewed elsewhere (374, 375). Acute hyposalivation occurs within a week, with up to 60% loss in saliva output (376). In addition, mucositis develops in virtually all patients within the first 3 wk (377). Chronic hyposalivation may last a few weeks or months or become permanent and leads to a cascade of complications that greatly diminish quality of life (377–380), which together have negative psychological effects. Thus, IR-induced hyposalivation remains a significant problem in the clinic. Additional details can be found in a recently published review (24).
The sensitivity of SGs to IR damage and the extent of loss of function are dependent on the total dose of radiation received by the gland (381, 382). It has been estimated that ∼40% of salivary function may be retained up to 1 yr after treatment when the combined dose of radiation to the parotid gland does not exceed 50 Gy (382), and complete recovery may occur with doses under 25–30 Gy (383, 384). Furthermore, recent studies show that different regions of the gland are uniquely radiosensitive, but there is debate as to which anatomical regions are better predictors of a clinical benefit if spared from the radiation field. Some have reported that gland segmentation for dose evaluation does not improve our ability to predict the extent of remaining salivary function after treatment compared with whole gland mean dose measurements (382). Yet, there is a significant body of evidence supporting anatomically distinct regions with varying degrees of radiosensitivity. Studies from the University of Groningen have concluded that the most sensitive regions of the gland in both rodents and humans correspond to the cranial and medial aspects of the gland adjacent to the mandible, where a pool of presumptive salivary progenitors reside (385). The PARSPORT trial in the United Kingdom, which used contralateral parotid-sparing intensity-modulated radiotherapy (CLPS-IMRT), demonstrated that lower doses to the lateral and cranial components of the human parotid gland were associated with a reduction in xerostomia (386), and the most recent PARSPORT II trial using bilateral superficial lobe parotid-sparing (BSLPS)-IMRT reported a lower risk of long-term xerostomia with BSLPS-IMRT compared to CLPS-IMRT techniques and concluded that the mean dose to the superficial lobe may a better predictor of outcome (387). Finally, additional research on split delineation along the anterolateral lobe boundary has demonstrated overall reductions in the total dose received by the gland and is expected to improve retention of salivary function (388, 389). However, despite the advancement of RT technologies, estimates suggest that >80% of head and neck cancer patients exhibit salivary hypofunction after treatment and, depending on the extent of damage to the glands, 64–91% of patients suffer from chronic to permanent loss of function (384, 390, 391).
Knowledge of the histopathological features of irradiated SGs derives from clinical observations, whereas most of our understanding of the cellular and molecular mechanisms involved in the loss of function and potential regenerative mechanisms are derived from animal studies. Loss of saliva from IR damage is due to the extensive depletion of acinar cells that fail to regenerate (392, 393). Although the precise mechanisms that prevent acinar regeneration are unclear, three possible explanations are that IR 1) damages the acinar progenitor pool (394), 2) inhibits the process of acinar self-duplication that usually replaces acinar cells during homeostasis (395), or 3) creates a damaged microenvironment that prevents acinar regeneration. IR damage also disrupts gland innervation, increases vascular permeability causing interstitial edema, and promotes immune infiltration (24, 244, 396). Therefore, it is also likely that the changes in the gland microenvironment disrupt cell-cell communication signals responsible for instructing the gland to secrete or regenerate. Research in the last few years has focused on molecular mechanisms triggered by IR-induced injury in the whole gland, and work is underway to evaluate the transcriptional landscape of irradiated mouse and human SGs at a single-cell level. Furthermore, the development of a single-cell RNAseq atlas of SGs and the latest advances in lineage tracing of specific cell populations point to a previously underappreciated degree of plasticity in multiple epithelial populations that may play a role during the response of SGs to IR injury.
4.5.1. Acute mechanisms of RT-induced salivary hypofunction.
Animal models have consistently shown that acute hyposalivation results from a combination of DNA damage, acinar cell death, increased reactive oxygen species (ROS) production, and disruption of Ca2+ signaling (145, 192, 244, 397–399). DNA damage in the form of double strand breaks occurs within minutes following RT (397), and increased phosphorylation of H2A histone family member X (γH2AX) can be measured for a few hours afterwards. After DNA damage, extensive apoptosis of acinar cells, measured by the presence of cleaved caspase 3, occurs between 8 and 72 h after a single dose of IR (400) and progressively decreases to nearly normal levels. Interestingly, persistent DNA damage measured up to 2 mo after injury by elevated levels of γH2AX and 53BP1 was associated with radiation-induced senescence of SG cells without significant levels of apoptosis involved (401). Within the first few hours following damage, radiation increases phosphorylation of p53 and leads to elevated levels of Δp63 and decreased p21, inhibiting the ability of SG cells to undergo cell cycle arrest to repair DNA damage (398). Notably, preservation of function was achieved in mice via administration of IGF-1, which decreased DNA damage in a SIRT1-dependent mechanism, and the cyclin-dependent kinase inhibitor roscovitine, which increased G2/M phase arrest (397, 398, 402).
Both Akt and p53 play an essential role in regulating both the apoptotic and arrest responses of the gland through distinct yet likely interconnected mechanisms. Expression of a constitutively active form of Akt (myr-Akt1) in the SGs inhibited apoptosis via reduced p53 phosphorylation and increased MDM2 phosphorylation in vivo (403). Consistently, mice lacking p53 showed significantly lower levels of apoptosis after IR compared with wild types, and salivary function was preserved (392). Presumably upstream of p53, FGF signaling via FGF10 binding to FGFR2 has been shown to activate the AKT/PI3K pathway, leading to increased activation of antiapoptotic molecules ultimately inhibiting apoptosis (404). An alternative mechanism of radiation-induced acinar cell apoptosis occurs via protein kinase C delta (PKCδ), since both inhibition and knockdown of its kinase in both PGs and SMGs inhibits apoptosis and restores saliva production (405, 406).
ROS production occurs immediately after damage with radiation and, combined with reduction in the activity of the free radical scavenging enzymes superoxide dismutase, glutathione peroxidase, and glutathione S-transferase, leads to the accumulation of ROS and elevation of oxidative stress markers (407). ROS accumulation is detrimental to cells, as it mediates DNA damage (408). Furthermore, ROS activates TRPM2 in SGs after IR, which results in caspase-dependent cleavage of the Ca2+ sensor STIM1, ultimately disrupting SOCE and secretion (192, 399). Thus, it is not surprising that targeting ROS with antioxidant therapies has a protective effect to preserve function. Recent findings show that administration of the mitochondrially targeted antioxidant MitoTEMPO allows for almost complete preservation of saliva output in irradiated mice (409). Other antioxidant molecules like resveratrol, TEMPOL, rosmarinic acid, and α-lipoic acid also improve saliva production when administered immediately before head and neck IR in rodents (399, 410–413). ROS production may also be mediated by sustained purinergic signaling via P2X and P2Y receptors (414). Indeed, a role for purinergic signaling in mediating acute responses to radiation was described, potentially via a bystander effect in which nonirradiated cells are indirectly affected by their adjacent damaged neighbors via release of extracellular ATP (eATP) that binds to the P2X7 receptor (P2X7R). Irradiated mice lacking P2X7R have stimulated salivary flow similar to nonirradiated mice, indicating that the loss of the receptor protects against the loss of function caused by IR (400). Notably, ROS production also induces a compensatory proliferation response associated with reparative processes (415).
Apoptosis-induced compensatory proliferation has been widely reported during regenerative responses after tissue damage (416, 417). In these models, compensatory proliferation is tightly regulated and culminates in recovery of function. In SGs, a similar proliferative response occurs within days after IR injury, primarily in the acinar compartment, and remains elevated chronically without restoring secretion (398, 418, 419). Interestingly, despite the observed increase in proliferation activity, amylase-positive area is ultimately reduced after IR damage, which may explain the lack of secretion in irradiated PGs (420, 421). These observations combined with more recent lineage tracing studies show altered plasticity in epithelial cells from irradiated SGs (described in detail in sect. 5.2.3) suggest that aberrant differentiation may at least partly explain why function is not restored (77, 106, 422). Compensatory proliferation in irradiated SGs is partly regulated by the atypical protein kinase C zeta (aPKCζ), which in this context seems to act as an inhibitor of proliferation (419). Genetic ablation of aPKCζ in mice results in increased Jun kinase (JNK) signaling, translocation of the transcriptional coactivator Yap to the nucleus, and hyperproliferation of the epithelial compartment of the PG similar to that of irradiated glands (418, 419). Furthermore, pharmacological intervention with IGF-1 inhibited compensatory proliferation and restored salivary function in wild-type mice after IR (421), but it failed to do so in mice lacking aPKCζ, in which proliferation remained elevated and salivary function was reduced (419).
4.5.2. Chronic mechanisms of RT-induced salivary hypofunction.
Whereas the acute loss of function can be attributed to the loss of acinar cells, disruption of Ca2+ signaling, and inefficient compensatory proliferation, it is unclear why SGs fail to regenerate after IR, particularly given the extensive regenerative potential of the gland in other injury models (245, 422, 423). Part of the complexity in understanding the mechanisms that lead to chronic dysfunction derives from inconsistencies between clinical observations and animal models of IR-induced hyposalivation. Acute responses like apoptosis and cell proliferation are readily recapitulated by most animal models of IR-induced hyposalivation, whereas chronic associated conditions like fibrosis and extensive immune infiltration do not consistently develop in animals, particularly rodents. Nonetheless, the current state of research suggests a multifactorial response driven by cellular senescence, inflammation, fibrosis, vascular and neuronal defects, chronic impairments in cell metabolism and Ca2+ signaling, and disruption of salivary progenitors (24). Recent research in the field also suggests that cellular plasticity of surviving cells may play a crucial role in the response of the epithelial compartment of the gland to IR injury.
Chronic DNA damage after IR has been demonstrated by the sustained expression of γH2AX and 53BP1 up to 2 mo after injury, and it is associated with the expression of senescence markers like senescence-associated β-galactosidase (SA-β-gal), p19ARF, and DcR2 (401, 424). Interestingly, senescence appears to be specific to the ductal compartment of the gland given the selective expression of SA-β-gal in striated ducts and IDs after IR (401). The selective removal of senescent cells based on expression of p16Ink4a or pharmacologically with ganciclovir or the senolytic drug ABT263 increased organoid formation efficiency, and pretreatment with IL-16 prevented both senescence and SG hypofunction by promoting DNA repair (401, 425). Finally, DNA repair and avoidance of senescence was also achieved via gene delivery of sonic hedgehog (Shh) in mice, but the study did not evaluate whether salivary output was restored (424). Senescence alone is unlikely to explain both the loss of function and the lack of regeneration after IR given that it is reportedly restricted to the ductal compartment of the gland.
Damage to nonepithelial cells surrounding the salivary epithelium such as nerves and vasculature also contributes to the loss of function. Biopsies from IR human SGs show hyperinnervation characterized by an increase of tyrosine hydroxylase (TH)-positive sympathetic neurons and a lower abundance of GFRa2-positive parasympathetic nerves (426). Consistently, the neurotrophic factors brain-derived neurotrophic factor (BDNF) and NTRN and the NRTN receptor GRFα2 are reduced in irradiated SGs from rats and minipigs (427, 428). In SMG explants, IR leads to neuronal apoptosis within 24–72 h and treatment with NRTN has a protective effect that allows for neuronal survival and gland regeneration (426). Similarly, gene delivery of human NRTN to the SGs of mice and minipigs before IR results in preservation of secretory function (36, 37). Alternatively, alterations in neurotrophic factor expression in irradiated rats were prevented by pretreatment with alpha-lipoic acid and rescued via gene delivery of Shh at 4 wk after IR in minipigs (427, 428). In addition, endothelial cell death occurs as early as 4 h after IR in mice (429), and in minipigs microvascular damage is measurable by decreased blood flow and levels of VEGF up to 20 wk after damage (428). Similar to the administration of nerve-protecting agents, treatment of irradiated glands with FMS-like tyrosine kinase-3 ligand (Flt-3L), stem cell factor (SCF), and granulocyte colony-stimulating factor (G-CSF) promoted proliferation of endothelial cells and led to increased acinar cell number and salivary output (430).
In humans, the most dramatic change in the SG architecture after IR damage is the replacement of glandular tissue by excess extracellular matrix (ECM) deposition resulting in fibrosis (431, 432) that develops weeks to months after treatment. Patients treated with a regular regime of fractionated RT show glandular atrophy and periductal fibrosis along with increased immune infiltration (433). Although it is evident that fibrosis is directly associated with dysfunction, it remains unclear whether it is a cause or a consequence of it. Part of the difficulty in addressing this question is that animal models of IR damage do not consistently develop fibrosis, particularly rodents. Nonetheless, C3H mice develop fibrosis between 6 and 10 mo when treated with either a single 15-Gy dose of IR or a fractionated regime (5 × 6-Gy doses) (37, 434). Minipigs respond similarly to humans, and their glands show loss of acinar cells replaced by extensive collagen deposition even after exposure to a single 15-Gy dose of IR (37, 393). However, because of cost constraints, minipigs are an expensive model to investigate SG fibrosis, although ex vivo assays of adult pig SG explant culture may be useful to investigate the short-term effects of IR damage and define early markers of fibrosis.
Alterations in the gland microenvironment such as the nerves, vasculature, and extracellular matrix may disrupt communication signals that would otherwise instruct the gland to regenerate. Multiple lineage tracing models have consistently shown that reversible injury to the SGs results in lineage-restricted acinar cell and duct replacement (77, 422, 435, 436). However, an injury model using a 10-Gy dose of IR damage showed partial acinar cell regeneration from both acinar (MIST1+) and Keratin5 (KRT5+)-expressing cells, and MECs regenerated 80% of acinar cells in a more severe damage model in which ligation of the duct also included the nearby blood supply and nerves (79, 106, 422). The interplay among these components remains to be determined, but future studies must leverage single-cell RNAseq data from developmental and injury models with clinical data to make predictions about the cell-specific mechanisms and the cell-cell communication networks involved in post-IR SG dysfunction (Ref. 27; Chibly et al., unpublished observations).
5. DEVELOPMENT AND PROGENITOR DYNAMICS
SG development in mice has been mainly studied in the SMG and follows a stagewise process, and landmark events that characterize this process are gland initiation, branching morphogenesis, cytodifferentiation, and postnatal maturation (27, 437). SMG initiation begins with the migration of neural crest-derived cells to form a mesenchymal condensate besides the oral epithelium (FIGURE 6). At embryonic day (E)11 in mice, interactions between the mesenchyme and developing epithelium result in the thickening of the oral epithelium to form the initial placode. This process requires FGF receptor signaling, as mice lacking FGF10 or FGFR2b fail to initiate SGs. Knockout models of Pitx1 and p63 also result in absence of salivary epithelium. Invagination of the epithelial placode into the surrounding mesenchyme allows for formation of an end bud, which grows into the mesenchyme, forming a primordial stalk that will become the major ED. At this stage, neuronal precursors condense to form the parasympathetic ganglion (PSG) around the primary duct. Branching morphogenesis takes place at ∼E13 and continues throughout fetal development and involves coordinated cell proliferation, clefting, differentiation, migration, and apoptosis as well as complex cellular interactions among the epithelium, surrounding vasculature, nerves, stroma, and ECM (6, 438). Differentiation of secretory cells and MECs is evident at E16 (27), although recent findings indicate that their specification may occur as early as E14 (Murphy et al., unpublished observations). Further specialization of the epithelium continues postnatally to give rise to fully differentiated acinar cells, striated ducts and IDs, and ionocytes, and postnatally in mice granular convoluted tubules develop.
FIGURE 6.
Embryonic development of mouse SMGs. Gland initiation starts around E11.5, with an epithelial thickening growing into a condensing neural crest-derived mesenchyme. Epithelial cells are pluripotent and undergo branching morphogenesis from ∼E13. Lineage restriction occurs once differentiation starts between E15 and E16, although branching morphogenesis continues and secretory and MEC differentiation begin. See glossary for abbreviations.
The advent of single-cell OMICS and advances in lineage tracing methodologies have generated excellent new resources to answer questions about the molecular mechanisms that regulate developmental transitions, including the transcription factors and signaling pathways that determine cell fate. For instance, single-cell RNAseq studies of injury and developmental models of SGs from landmark events corresponding to gland initiation, branching morphogenesis, cytodifferentiation, and postnatal maturation (Refs. 27, 28, 112, 440, 441; Chibly et al., unpublished observations) have already proven instrumental in identifying previously uncharacterized cell populations of IDs defined by their expression of Smgc and Gstt1 in the adult SG (27). In addition, these approaches enable the characterization of distinct subsets of Sox10+ progenitor cells during development that give rise to multiple epithelial populations in the gland (Murphy et al., unpublished observations). The combination of these tools with chromatin immunoprecipitation-sequencing (ChIP-seq) analysis also allows for the identification of cell-specific epigenetic regulation (442). In addition, the Salivary Gland Molecular Anatomy Project (sgmap.nidcr.nih.gov), a searchable gene expression database for mouse SG development, healthy and IR human salivary glands, and differential gene expression of selected transgenic mouse models, contains recent sequencing efforts from distinct developmental and injury models in mouse and human SGs.
5.1. Signaling Pathways
Initiation of SGs involves epithelial-mesenchymal interactions, a coordinated developmental mechanism triggered by multiple reciprocal interactions between the epithelium and its surrounding mesenchyme. The mesenchyme produces growth factor ligands that bind corresponding receptor tyrosine kinase (RTK) receptors expressed mainly in the epithelium. The growth factor receptors transduce extracellular signals through activation of intracellular messengers or directly through receptor translocation to the nucleus. A variety of signaling pathways orchestrate the establishment of the SG structure. The regulation and cross talk of these pathways have been reviewed extensively in previous reviews (78, 443, 444). Here, we limit our review to key signaling pathways that have provided insight into the mechanisms involved in SG development (FIGURE 7).
FIGURE 7.
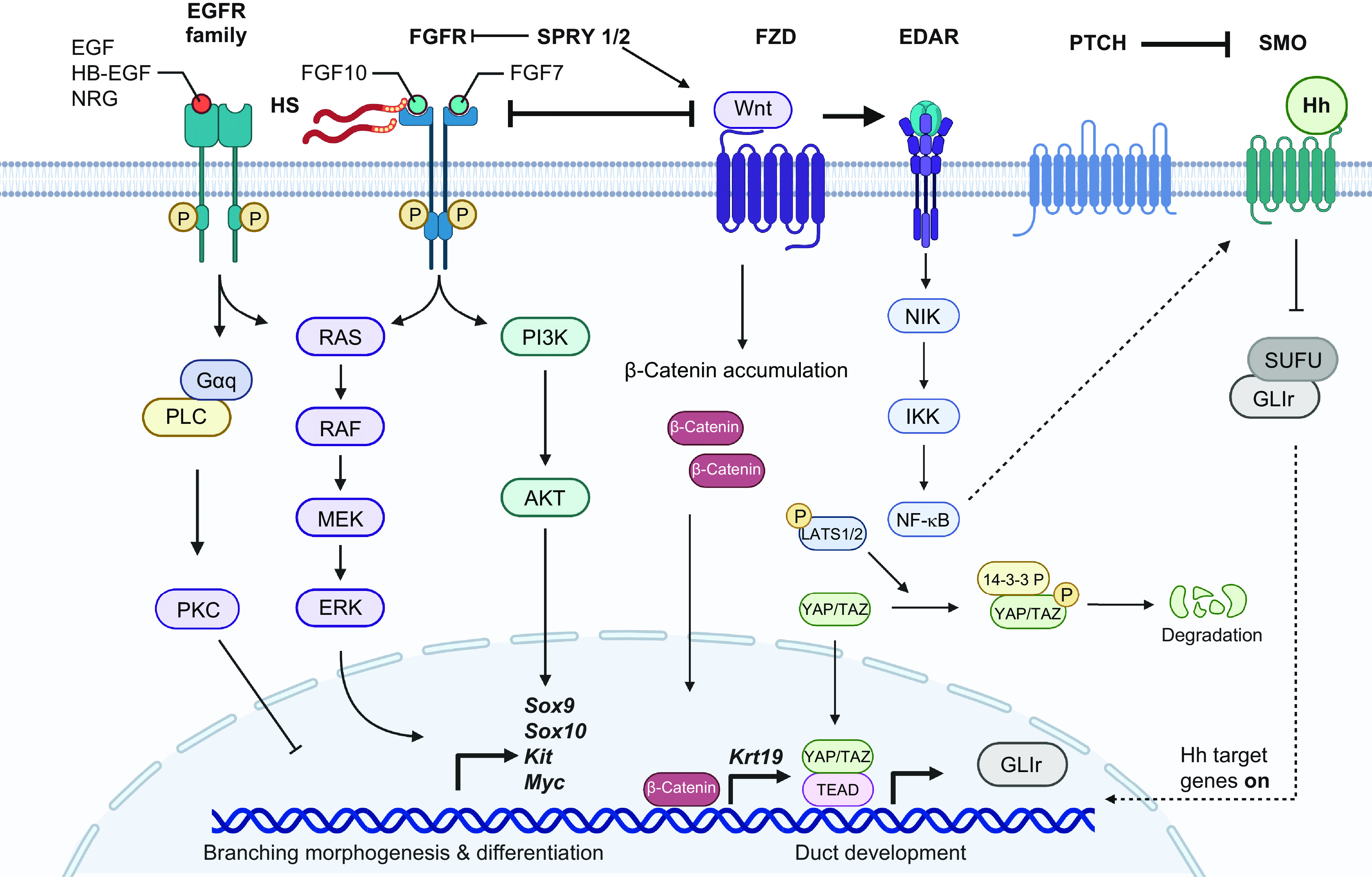
Developmental signaling pathways in mouse SMG. FGF and EGF receptor signaling are major drivers of branching morphogenesis and epithelial differentiation, particularly through induction of Sox9, Sox10, Kit, and Myc expression, which delineate embryonic progenitors in the end bud. Wnt, EDAR, Shh, and Hippo signaling effector Yap are involved in duct development and lumen formation. Wnt and FGF receptor signaling have a mutual inhibitory relationship to balance branching morphogenesis and differentiation with lumen formation and duct maturation. See glossary for abbreviations. Created with BioRender.com, with permission.
5.1.1. Retinoic acid signaling.
Retinoic acid (RA), a metabolite of retinol (vitamin A), functions as a ligand for nuclear RA receptors (RARs) that regulate SMG development. Vitamin A, an all-trans retinol, is first converted into the metabolic intermediate all-trans-retinal (atRAL) via retinol dehydrogenase 10 (RDH10). The intermediate atRAL is subsequently converted into the active product RA via enzymes of the aldehyde dehydrogenase 1A (ALDH1A) family (445). Retinoic acid signaling occurs in mandibular tissues before and during SMG initiation and continues throughout development. At E11.5, both Rhd10 and Aldh1a2 are expressed exclusively in the mesenchyme, whereas RA signaling extends into overlying epithelium (446). RAR signaling activity is required to direct the oral epithelium to initiate thickening and invagination into the underlying mesenchyme and to activate expression of SOX9, the earliest known marker of SMG fate (447). This requirement for RA signaling in gland initiation involves canonical signaling through retinoic acid receptors, mediated by retinoic acid receptor RARα (446). SMG development is impaired in Rhd10−/− mutant embryos (448). Mice with compound double or triple Rar mutations have SG developmental defects (449, 450). Additionally, in humans, exposure to excessive RA in utero has been associated with SG aplasia (451). Taken together, properly regulated RA signaling is important, as insufficient or excessive RA signaling disrupts SG development.
5.1.2. Fibroblast growth factor signaling.
Fibroblast growth factor (FGF) signaling involves 23 secreted FGFs and 5 tyrosine kinase FGFRs. FGF signaling is critical for the development of SGs, as deletion of murine Fgfr2b or its ligand Fgf10 causes gland aplasia (452, 453). However, Fgf10 heterozygous mice exhibit delayed, hypoplastic SMG development that results in reduced saliva secretion (267). Not surprising, mice with a cranial neural crest cell-specific deletion of Fgf10 (Wnt1-Cre; Fgf10F/F conditional knockout) exhibit agenesis of the SGs. A mesenchyme capsule still forms despite the lack of any branching epithelium (454). Alternatively, a mouse model for Apert syndrome caused by a single activating nucleotide substitution mutation (S252W or P253R) in FGFR2 results in a gain of function with SMGs that display enhanced branching morphogenesis with hyperplasia in the parenchyma (270).
FGF signaling promotes end bud development via ERK1/2 and/or PI3K signaling pathways when exogenous FGF7 or FGF10, both produced by the mesenchyme, is added to SMG explant cultures (455) (FIGURE 7). These two ligands for FGFR2b can also expand putative progenitor cells marked by MYC, SOX9, and KIT in the end buds (456). Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion by upregulating expression of FGFR2b-dependent transcription factors in KIT+ progenitors in the end buds (456). In addition, mesenchymal expression of FGF2 also mediates branching and differentiation, with expression of proacinar marker AQP5 in E16 SMG organoids (457).
FGFs are tightly bound to heparan sulfate proteoglycans (HSPGs) located in the basement membrane or at the cell surface and serve as cofactors to regulate specificity and affinity for signaling FGFRs (458). FGFR2b signaling requires HS to increase the affinity of FGF10 for FGFR2b, stabilizing the ternary signaling complex. The length and sulfation patterns of HS regulate FGF signaling and influence both ductal elongation and end bud expansion during SMG development (459). FGFR2b signaling directly regulates the expression of two of the HS sulfotransferases (Hs3st3a1 and Hs3st3b1) that generate a specific type of 3-O-sulfated HS epitope, and both sulfotransferase enzymes are enriched in KIT+ progenitors. The Hs3st3-modified HS in turn rapidly increases FGFR2b-mediated signaling and proliferation of the KIT+ progenitors in ex vivo SMG epithelial cultures (460). Mice lacking either Hs3st3a1 or Hs3st3b1 have reduced FGF10-dependent epithelial growth (461).
Key modulators of FGF signaling are Sprouty (Spry) proteins, which act as negative feedback antagonists (462). Excessive FGF signaling occurs in mice deficient in both Spry1 and Spry2 (Spry1/2 DKO), which prevents gangliogenesis, innervation, and KRT5+ epithelial progenitors in the developing SMG (463). Excess FGF signals in Spry1/2 DKO SMGs disrupt gangliogenesis through loss of Wnt expression by KRT5+ progenitors and in turn cause abnormal ductal differentiation, depletion of progenitors, and aberrant morphogenesis. Activation of Wnt signaling and reduction of FGF gene dosage rescued gangliogenesis and innervation in Spry1/2 DKO SMGs (463). Therefore, Sprys play important role in restricting FGF signaling to allow proper development of the SGs.
The role of FGF signaling during early development of SGs is well studied. These studies highlight that the level of FGF signaling is critical in gland development. Its role during later stages of development when acinar and MEC differentiation occurs and when postnatal maturation occurs remains to be determined.
5.1.3. Epidermal growth factor receptor family signaling.
The EGF system plays a crucial role during organ development, morphogenesis, repair, and epithelial regeneration. The EGF family includes amphiregulin (AREG), EGF, betacullulin (BTC), epiregulins, epigen, neuregulins, TGF-α, and heparin-binding EGF (HB-EGF). These ligands can bind to four receptors, EGFR/ErbB1, ErbB2, ErbB3, and ErbB4, to initiate signaling transduction via mitogen-activated protein kinase (MAPK), PI3K, and PLC/PKC pathways (464, 465) (FIGURE 7). Activation of these signaling pathways controls proliferation, differentiation, and survival (466). EGFR regulates epithelial morphogenesis during development and is important for proper branching. EGFR mediates SG branching through ERK-1/2 and PI3K pathways but also inhibits branching via activation of PKC signaling. EGFR regulates the expression of α6-integrin in the fetal SGs of mice (467). The EGFR-null SMG has impaired growth, branching, and maturation of the epithelium (468). Gefitinib, a small-molecule EGFR inhibitor, increases apoptosis of mesenchymal cells, suggesting that EGFR regulates their survival (468). In addition, EGFR transactivation is required for parasympathetic nerve-mediated proliferation of KRT5+ epithelial progenitor cells (469).
Other EGF family members have also been implicated in SG development. EGF, HB-EGF, neuregulin-1, and TGF-α induce branching of end buds (467, 470–475). HB-EGF activation of EGFR modulates ductal morphogenesis by controlling progenitor cell differentiation and expansion of the differentiated Keratin 19 (KRT19+) ductal lineage (469). Fetal SGs derived from neuregulin-1 type III-deficient mice are devoid of functional nerves and exhibit aberrant ductal morphogenesis, reduced epithelial branching, and disrupted lumen formation and expansion (476). ErbB1−/− mice exhibit smaller SMGs with impaired terminal end bud branching and reduced cell proliferation (468, 477). Furthermore, ErbB signaling regulates ductal cells by inducing Wnt expression in SMG explant epithelia, and excessive FGF signaling upsets the balance of FGF and ErbB3 signaling (463). Thus, the interaction of multiple EGF family members interacts with other major signaling pathways to regulate gland development. However, their role in epithelial regeneration has not been explored as much, requiring inducible genetic mouse models to investigate their role in regeneration after damage in adult SGs.
5.1.4. Wnt, Hippo, and Notch signaling.
Wnts are a family of secreted, cysteine-rich glycoproteins that function as short-range signaling factors and are important for SMG organogenesis. Analysis of Wnt reporter mice shows that Wnt signaling initially localizes to the mesenchyme surrounding the E12 SMG epithelium and the distal cap mesenchyme (463, 478). Inhibition of Wnt signaling early in development, when signaling is restricted to the mesenchyme, impairs branching morphogenesis (280). Mesenchymal Wnt signaling is associated with and is essential for the formation of the PSG (463). At later stages (>E13.5) Wnt localizes to the ductal epithelium and is important for duct differentiation. In contrast, inhibition of FGF signaling, which increases Wnt activity, drives duct differentiation and leads to premature ductal lumen formation (463, 478). Furthermore, mice with constitutively activated canonical Wnt signaling show defects in differentiation of acinar cells and luminal formation as a result of KIT suppression (479). In adult SGs, expression of the Wnt signaling is maintained in the duct epithelium and its activity is promoted during regeneration after injury or expansion of progenitor cells (480). Excretory basal EpCAM+ duct cells coexpressing nuclear β-catenin, a Wnt signaling intracellular signal transducer, from adult SMG can form organoids after stimulation of WNT3A and R-Spondin-1. These organoids are capable of regenerating both acinar and ductal tissues in irradiated SMGs, improving saliva secretion (481).
Hippo signaling is also involved in SG growth, and its inhibition abolishes branching morphogenesis ex vivo (482). Ablation of the Hippo pathway effector Yap in salivary epithelium leads to reduced expression of epiregulin and severely affects the epithelial patterning, with a loss of duct specification. Furthermore, restricting nuclear Yap by ablating the Lats1/2 genes results in an expansion of ductal/proximal progenitors and loss of luminal duct cells. This indicates that the Hippo pathway promotes the identity of proximal progenitors and proper expression of Yap is critical for duct maturation (483). In irradiated SGs, YAP is translocated to the nucleus in acinar and ID cells, including a population of putative label-retaining progenitors, and the process was reversed during functional restoration of the gland via systemic administration of IGF-1 (419, 484). Further research will determine whether Hippo signaling can be targeted for regenerative purposes.
Notch signaling occurs via cell-cell communication where transmembrane ligands on one cell activate transmembrane receptors on a juxtaposed cell. Notch signaling is negatively regulated by delta-like proteins 1 and 2 (DLK1, 2), which are notch receptor ligands containing epidermal growth factor-like repeats and are expressed at all stages of SMG morphogenesis. Culture of fetal SMG explants with soluble DLK1 or a pharmacological inhibitor of Notch signaling reduces branching morphogenesis as a result of reduced innervation of end buds and in turn decreases KRT5+ progenitors (485).
5.2. Progenitors and Plasticity
Defining cells harboring regenerative potential is an important step toward developing therapeutic strategies. To this end, understanding lineage relationships and cellular cues between putative progenitors and differentiated cell types both pre- and postnatally is essential. In SGs, the long-standing dogma was that differentiated cells were mostly postmitotic and that tissue turnover and regeneration were stem cell-dependent (395, 486). However, our evolving understanding of stemness and plasticity in somatic cells has resulted in a reevaluation of this dogma, and our view has become more nuanced because of major advances in the last decade (60, 79). Most of this knowledge is based on genetic mouse models defining proliferative cells and following cell lineages. This approach comes with its own caveats, such as its being dependent on specific markers and the possibility of “leaky” Cre drivers eliciting ectopic activation. However, experiments performed by independent laboratories using various models have in part mitigated these caveats. Taken together, these studies have provided some answers to lineage relationships and raised new questions about the regenerative potential of cells within the glands. Furthermore, identifying gene regulatory networks and cellular cues instructive for cell lineages is an important step toward manipulating regeneration. Here we give an updated view of SG progenitors, highlighting the plasticity of epithelial cells.
5.2.1. During development.
SGs arise from the oral epithelium with the initial placode expressing Krt14, Sox2, Sox9, Sox10, and Trp63 (441, 447, 456, 487). These intrinsic factors are important for development to progress, and their expression patterns change throughout development, which allows precise fate mapping of specific populations. This has shed light on embryonic progenitors, which are populations that give rise to more than one cell type in vivo. Once the initial bud is formed, the end bud epithelium is divided into distal and proximal progenitors identified by position and the cell-specific markers Krt14+Kit+ and Krt5+Kit+, respectively (456, 469). Distal progenitors in the end bud express Sox10, and they give rise to the entire parenchyma (487). At this point, Sox2 is not expressed in the end bud but rather restricted to the oral epithelium, and this lineage only gives rise to a subset of cells in the SMG main duct (487, 488). In the SLG, however, Sox2+ progenitors are major contributors to both duct and acini throughout development. This highlights the variations in dependence on intrinsic factors and progenitors important for development and potentially regeneration of different glands (487, 488). As cells begin to differentiate, lineages become restricted and there is no longer a clear progenitor giving rise to all different cell types in the gland. Specifically, Krt14+ distal progenitors give rise to ducts, MECs, and acinar cells up until E15, when a shift occurs, and then after E15 only contribute to ducts and MECs (77). Furthermore, once MECs are identified through specific expression of Acta2, they proliferate and are self-maintained (441). Ionocytes labeled by the transcription factor Ascl3 also proliferate and self-maintain in addition to producing some acinar progeny (91). Interestingly, genetic deletion of these cells did not negatively affect acinar cell development, which highlights cell plasticity during development (489). Still, it is not clear how ionocytes are lineage related to other progenitors in the developing gland. Also, whether there are progenitor subpopulations harboring higher potential within each compartment is not well understood and remains to be investigated further. In addition, human SGs display similar temporospatial localization patterns of intrinsic factors such as Sox2 and Sox10 during development (30, 487); however, more work is needed to understand whether in vivo lineage relationships are conserved between mouse and human.
5.2.2. During adult homeostasis.
Homeostasis in the adult SG is the stable healthy state that is maintained and regulated as needed for an organ to function properly. During homeostasis, proliferating cells maintain the integrity of the epithelium by contributing to tissue turnover (FIGURE 8A). Much of what we have learned in this area is due to mouse genetics and lineage tracing genetic models. In mouse SGs, both actively proliferating and slow-cycling cells are found, and defining such cells and their in vivo progeny is key to understanding regenerative potential. Experiments to investigate cell population dynamics using Cre drivers in conjunction with the histone 2B-green fluorescent protein (H2BGFP) mouse model indicated that actively proliferating rather than slow-cycling duct cells contribute to tissue turnover (490). This has been confirmed by several reports using genetic mouse models all concluding with lineage-restricted contribution by duct subpopulations. Specifically, ID cells labeled by Kit give rise to IDs, whereas Krt14, Krt5, and Axin2 cells in the IDs and EDs give rise to granular and luminal duct cells, respectively (77, 422, 435, 490, 491). Similar to what was discovered during late embryonic development, Acta2+ MECs are lineage restricted and self-renewing during homeostasis (77, 441). Likewise, lineage tracing of differentiated acinar cells using either Mist1- or Pip-Cre drivers have shown that they too proliferate and maintain themselves postnatally in all major SGs (395, 492). In addition, the SLG contains Sox2+ serous cells that act as progenitors for mucous cells and are important for acinar cell maintenance (436). Further work is needed to identify potential subpopulations similar to this in the SMG and PG. Taken together, recent advances point to the main mode of maintenance being through contribution by lineage-restricted progenitors and differentiated cells rather than dependence on bona fide stem cells. Even so, some data indicate the potential for multipotency or plasticity across the major compartments. For example, ID cells in the PG are reported to occasionally give rise to both ID and acinar cells, indicating that they may act as progenitors (492). Additionally, lineage tracing of basal cells and MECs reported rare events where they give rise to acinar cells (441). The significance of these findings, although not common, is the indication of multipotency or plasticity of usually lineage-restricted cells during homeostasis, which in turn may have implications for regenerative purposes after specific types of damage. In human SGs, the ducts, MECs and acini all have a baseline proliferative activity suggesting a similar mode for maintenance (493, 494); however, further work is needed to confirm this.
FIGURE 8.
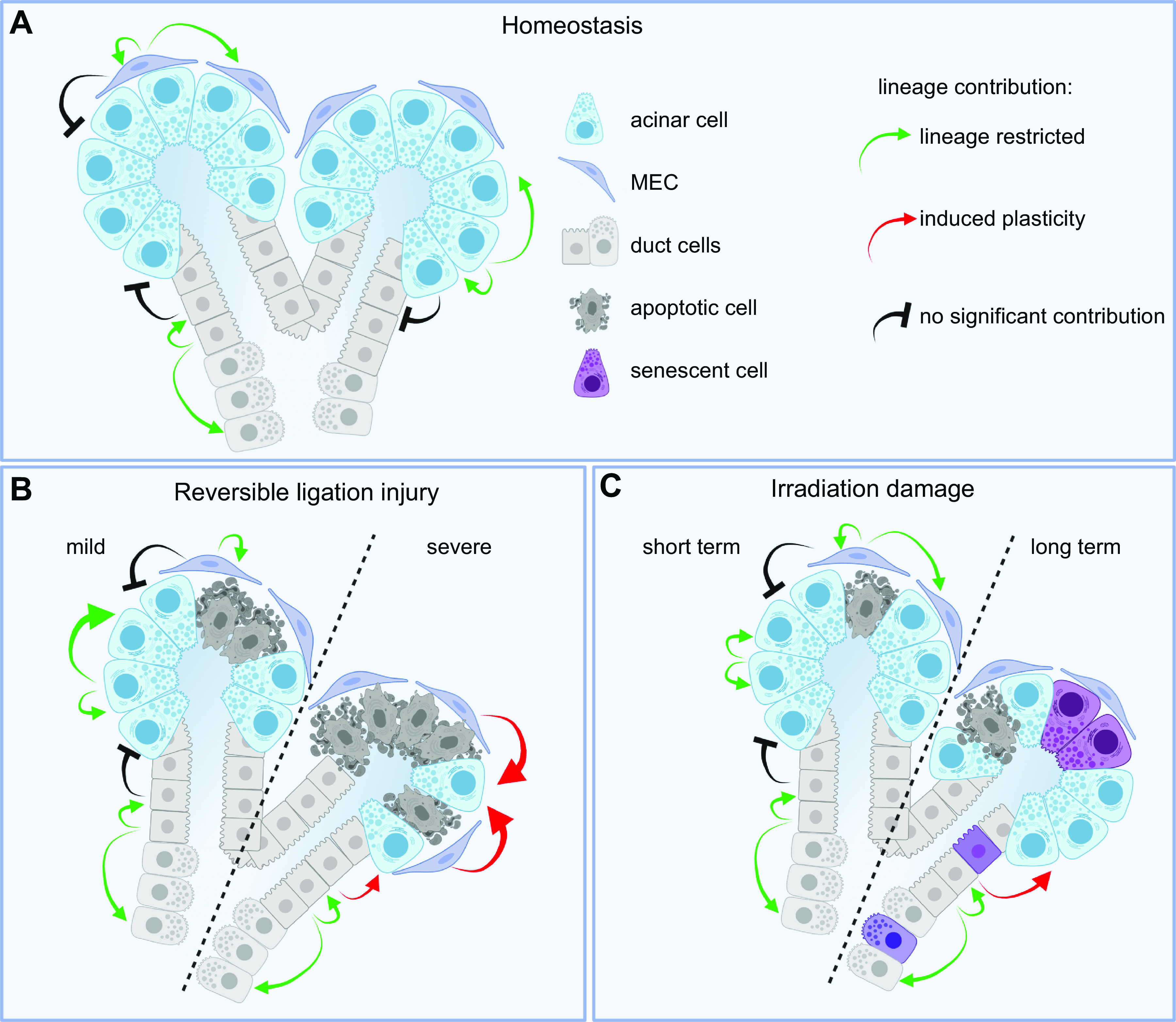
Models of epithelial lineage relationships during homeostasis and in models of damage. A and B: epithelial lineages are self-restricted, and cell types regenerate themselves during homeostasis (A) and mild reversible ligation injury (B). During severe ligation injury, cell plasticity of multiple populations becomes evident, particularly MECs and duct cells, which can regenerate all cell types, including acinar cells. C: after IR damage, duct cells can partially differentiate into acinar cells. See glossary for abbreviations. Created with BioRender.com, with permission.
5.2.3. During injury and regeneration.
Several animal models ranging from mice to monkeys have been developed to study SG injury, regenerative potential, underlying mechanisms, and therapeutic approaches. Models include duct ligation, mechanical injury, systemic diseases, inflammation, radioisotope, and IR models (recently reviewed in Ref. 495). Among these, duct ligation and IR are commonly used, and most of our knowledge on progenitors and plasticity following injury is based on these models. Duct ligation is a reversible injury that involves mechanically closing off the main duct with a ligature, which leads to gland atrophy and, upon ligation removal, gland regeneration. The severity of atrophy and apoptosis depends on whether the ligation includes vasculature and nerves in addition to the main duct (85). Mild ligation injury, which does not include ligating the vasculature and nerves, leads to atrophy of both duct and acinar cells. Regeneration following mild ligation is lineage restricted similar to homeostasis, and Krt5, Krt14, Axin2 duct cells regenerate ducts whereas KIT+ cells regenerate themselves (106, 422). However, acinar regeneration following mild ligation is independent of other cell types and has been attributed to a high number of surviving acinar cells that undergo acinar-to-ductal metaplasia, transiently expressing ductal markers (496). Indeed, upon deligation, most of them revert to an acinar phenotype, indicating their regenerative potential (395, 496), whereas also ligating the vasculature in this model leads to a more severe injury and a higher degree of acinar apoptosis (85). Under these conditions duct populations expressing Krt14, Axin2, and Kit as well as MECs are not bound to their lineage restrictions but rather step in and regenerate acini as well (106). This points to a remarkable plasticity and great regenerative potential dependent on specific types of damage, the microenvironmental cues, and a variety of epithelial cell types (FIGURE 8B). Similar to duct ligation, innate immune-mediated injury by administration of synthetic double-stranded (ds) RNA polyinosinic-polycytidylic acid [poly(I:C)] results in temporary depletion of acinar cells with subsequent replenishment. In this model, regeneration involved aurora kinase B, which was essential for cell proliferation (497).
IR damage can result in nonreversible dysfunction in a dose-dependent manner (as described in sect. 4.5). Combining IR damage with genetic mouse models has provided information on the proliferative potential and lineage relationships during both the acute and chronic phases of IR damage. After IR, acinar cells proliferate and self-duplicate in both the acute and chronic phases (FIGURE 8C) (422, 436), similar to what occurs during homeostasis and after mild injury. In the acute phase, duct cells (Krt5, Axin2) remain lineage restricted, whereas in the chronic phase they do give rise to de novo acini, indicating a change in lineage restriction, similar to that observed after severe injury (77, 422). However, after IR with high doses there is only limited regeneration, as the gland does not regain function. Nevertheless, cells with regenerative potential do remain in vivo and may be potentially directed to differentiate with specific niche signals. Combining genetic models with putative niche signals identified through transcriptional profiling of both healthy and diseased/damaged glands could identify essential pathways in the process of lineage restriction and/or gland regeneration, which could then be tested in vivo. This would be most informative in developing novel therapeutic targets for cell-based regeneration.
5.3. Extracellular Matrix Microenvironment
ECM is the noncellular component present within all tissues and organs and plays a critical role in the progenitor cell microenvironment or niche. It provides physical scaffolding for the cellular constituents and initiates crucial biochemical and biomechanical cues required for tissue morphogenesis, growth, migration, differentiation, and homeostasis (498, 499). During SG development, ECM remodeling is required to allow repetitive formation of epithelial clefts and end buds to invade the surrounding embryonic ECM. The ECM changes in composition and distribution over time. ECM exists in two main types, the interstitial matrix and the basement membrane, which differ in location and composition. The interstitial connective tissue matrix surrounds tissues to provide structural scaffolding, whereas the basement membrane, which is a specialized form of thin, flat, and laminar ECM, separates the epithelium from the surrounding stroma or mesenchyme (500). This interstitial ECM is mainly composed of collagens and various noncollagenous proteins that include elastins, fibronectins, tenascin, and laminins (498). The basement membrane is predominantly composed of ECM molecules organized into sheet-like networks of interconnected proteins including independent networks of collagen type IV and laminins, linked by nidogens, and proteoglycans including perlecan, collagen type XV, collagen type XVIII, and agrin. (501).
Collagens are the most abundant fibrous protein within the interstitial ECM and provide tensile strength, regulate cell adhesion, support chemotaxis and migration, and direct tissue development (502). Collagen type I and type III accumulate at SMG cleft sites during branching, and the initiation of cleft formation is abolished with collagenase treatment (503, 504). Collagen IV provides structural integrity to the basement membrane but, however, is not required for deposition or assembly of other basement membrane components. Proteolysis of collagen IV by membrane-type matrix metalloproteinase-2 (MT2-MMP) mediates SMG cell proliferation, morphogenesis, and collagen IV metabolism through the release of NC1 domains that signal via the PI3K-AKT pathway (505). Thus, degradation of basement membrane components through proteolysis can generate cleavage products with potential signaling functions and locally release growth factors stored in the basement membrane (506).
Fibronectin is another fibrous ECM protein intimately involved in directing the organization of the interstitial ECM and also has a crucial role in mediating cell attachment and function. However, during branching morphogenesis fibronectin is essential for stochastic cleft formation (507). The local accumulation of fibronectin rapidly induces the expression of BTBD7, a transcriptional regulator that in turn induces local expression of the epithelial-mesenchymal transition-promoting factor SNAIL2 and suppresses E-cadherin levels, thus altering cell morphology and reducing cell-cell adhesion to promote cleft progression (508).
Laminins are a family of 15 isoforms of heterotrimeric proteins that contain an α-, a β- and a γ-chain. Laminins are essential for the function of the basement membrane, as most null mutations are early embryonic lethal. Laminins play a central role in organizing complex interactions of the basement membranes, partnering with other proteins such as syndecan, nidogen, collagen, integrins, and dystroglycan and with heparan sulfates (HSs) of proteoglycans (509). Laminin α5, found in laminin-511 and -521, is one of the most widely distributed laminins that plays a key role in maintenance of basement membrane integrity as well as SG branching morphogenesis. Both Lama5−/− SMGs and wild-type ex vivo SMG organ cultures treated with Lama5 siRNA show delayed cleft formation and reduced branching morphogenesis. Laminin α5 controls SMG epithelial branching via β1-integrin signaling by regulating FGFR expression, which in turn provides positive feedback for FGFR and Lama5 expression, growth factor signaling, and further gland development (510).
Proteoglycans provide unique buffering, hydration, binding, and force resistance properties that are key for the structural integrity of the basement membrane. Proteoglycans are core proteins with attached glycosaminoglycan (GAG) side chains and include the chondroitin sulfates, dermatan sulfate, keratan sulfate, hyaluronan, and HS. Most GAGs, with the exception of hyaluronan, are covalently attached to a protein core to form a proteoglycan and mediate the majority of the ligand-binding functions of the proteoglycans (511). Selective proteoglycan synthesis inhibitors inhibit fetal SMG branching morphogenesis due to decreased deposition of sulfated GAGs in the basement membrane causing deficiency in anionic sites in the basal lamina and thus altering its structural integrity and signaling (512).
Among the ECM proteoglycans, HSPGs are critical determinants of ECM assembly and remodeling. Secreted HSPGs (perlecan, agrin, and collagen type XVIII) are directly secreted in the ECM, whereas cell surface-tethered HSPGs (syndecans, betaglycan, and glypicans) can undergo proteolytic cleavage of their ectodomains or cleavage of HS chains by heparanases, and their truncated forms can be distributed in the ECM (513). During SG branching morphogenesis, heparanase, an endoglucorunidase, is colocalized with perlecan in the basement membrane and in the clefts. Exogenous recombinant heparanase releases FGF10 bound to perlecan in the basement membrane, resulting in increased mitogen-activated protein kinase (MAPK) signaling, clefting, and branching morphogenesis (514). Thus, in the ECM, because of their negative charge, HSPGs act as a reservoir of growth factors and supply them to target cells when required. Otherwise, they may act as a barrier for growth factors, by preventing their passive diffusion over longer distances instead of confining them to the vicinity of producing cells (515, 516). The affinity of HSPGs for various growth factors varies and can regulate their concentration gradients within the microenvironment to mediate different effects on SMG development. For example, FGF10 has high affinity for HS and therefore generates a restricted diffusion range resulting in binding of FGF10 only to the proliferating tips of the elongating end bud, whereas FGF7 has low affinity for HS, which results in a broader gradient leading to expansion of end buds in multiple directions (517).
Components of the ECM and basement membrane are also rich in O-glycans, another sugar moiety present on secreted proteins, including salivary mucins, which were discussed above. O-glycosylation is controlled by a large family of enzymes called the UDP-GalNac:polypeptide N-acetylgalactosaminyltransferases (Galnts). The importance of these O-glycan structures is highlighted by loss of ppGalNAcT-1 (Galnt1), the most abundant Galnt during early SMG development. Loss of Galnt1 reduces cell proliferation and growth by specifically disrupting the secretion of basement membrane components, laminin α1 and collagen IV, resulting in decreased integrin and FGF signaling (518).
Degradation of the basement membrane proteins by proteases is important to allow remodeling of the progenitor microenvironment and results in thinning of basement membrane to allow local expansion of the branching end buds. The basement membrane surrounding these end buds is remarkably dynamic and flexible. It becomes perforated by numerous well-defined microscopic holes to facilitate pliability (519). The basement membranes as a whole constantly move toward the rear, away from the tip. Both the formation of the microscopic holes and the rearward movement require proteolytic activity and actomyosin contractility to support branching morphogenesis (520, 521).
Basement membrane organization is important for SG architecture and cell polarity. It needs to be restricted to the basal surface of epithelial tissues to mediate signals that regulate coordinated tissue organization (522). Rho-associated coiled-coil containing kinase (ROCK) promotes basement membrane remodeling during branching morphogenesis to mediate changes in tissue shape through myosin II-dependent contraction (523). ROCK1 also regulates PAR1b function and the positioning of the basement membrane at the epithelial periphery to control tissue polarity in developing SGs (522). Overall, the ECM microenvironment directs essential morphological organization and physiological function by binding growth factors and interacting with cell surface receptors to elicit signal transduction and regulate biological functions during SG development.
6. THERAPIES TO REGENERATE SALIVARY FUNCTION
6.1. Current Palliative Care and Pharmacological Therapies
Oral lubricants, saliva substitutes, and saliva stimulants continue to be the primary palliative care for patients with xerostomia, and an extensive literature on these has been previously reviewed (22). The main purpose of oral lubricants is to protect the oral mucosa, and these include mouthwashes, gels, and toothpastes, whereas the objective of saliva substitutes is to replace the normal functions of saliva beyond lubrication. However, the benefits are short-lived, with inconsistent ability to relieve xerostomia symptoms. Only 2 of 16 evaluated substitutes performed better than water in symptom relief (524), and only those substitutes that contained carrageenan, carboxymethylcellulose, pig gastric mucin, xanthan gum, and carbomer enhanced oral lubrication. Nonetheless, promising new results from a small cohort of SS patients show that the mucoadhesive chitosan-catechol (Chi-C) extended the “relief period” over sevenfold by increasing mucin recruitment and reducing friction (525).
The efficacy of saliva stimulants, i.e., secretagogues, is dependent on residual glandular function, and thus they are ineffective in cases of severe hypofunction. Pilocarpine is the most commonly prescribed; it is a cholinergic parasympathomimetic agonist that temporarily increases salivation and other exocrine secretions through M3 muscarinic receptors (526, 527). Muscarinic agonists (pilocarpine hydrochloride and cevimeline hydrochloride) all stimulate secretion, with the adverse effect of sweating that can be minimized by gradual dose escalation (527, 528). Hyperbaric oxygen therapy (HBOT), acupuncture, and electrostimulation have also been proposed to cause relief from xerostomia and in limited cases have shown to stimulate secretion. In particular, HBOT has shown short-term improvement in salivary flow and a reduction in xerostomia (529), while it promotes angiogenesis and stimulates collagen synthesis (530). The lack of an ideal control group for studies on acupuncture and transcutaneous electrostimulation make it difficult to definitively assess their effectiveness against hyposalivation. Overall, there is insufficient evidence that acupuncture alleviates xerostomia (531), but promising results from a study of patients using an intraoral device to electrostimulate the lingual nerve showed improvement in salivary flow (532).
Pharmacological approaches to treat salivary hypofunction have been studied in the context of IR damage and SS and are used either to protect the gland from IR damage (radioprotectives) or to restore function after chronic loss of function. Amifostine is the only FDA-approved radioprotective therapy for salivary hypofunction (533, 534), but its suboptimal toxicity profile limits its use in the clinic. Therefore, there is an unmet need to identify new target candidates for therapeutic intervention (see TABLE 1). In preclinical models, ROS scavengers like TEMPOL show improvements in saliva secretion after IR by limiting DNA and microvascular damage (429, 535). Similarly, the tyrosine kinase inhibitor dasatanib blocks a number of kinases including DDR1, ABL2, SIK2, RIPK2, EPHA2, and EPHB2 (536) and prevents the IR-dependent loss of secretion via a mechanism involving PKCδ (537). Other factors with potential radioprotective effects include IGF-1, FGF7, EGF, and NRTN (36, 37, 421, 538, 539).
Table 1.
Treatments and preclinical drugs aimed to improve/prevent irradiation damage in salivary glands
| Treatment/Drug | Target | Effects on Function | Effects on Histology | Species | Ref. | |
|---|---|---|---|---|---|---|
| Secretagogue treatment | Pilocarpine*/cevimeline* | Muscarinic receptors | Increased flow | n/a | Human | (527) |
| Hyperbaric oxygen therapy (HBOT) | n/a | Pilot study showed increased flow | n/a | Human | (529) | |
| Acupuncture | n/a | Insufficient evidence | n/a | Human | (531) | |
| Electrostimulation | Nerve signaling | Increased flow | n/a | Human | (532) | |
| Radioprotective therapy | TEMPOL | ROS scavenger | Partial reversed saliva flow | Microvessel protection | Mouse | (535) |
| Dasatinib | Tyrosine kinase inhibitor | Prevention of hyposalivation | Protection of AQP5+ acinar cells | Mouse | (537) | |
| IGF-1 | Cell proliferation | Prevention of hyposalivation | Protection of acinar cells | Mouse | (421) | |
| FGF7 | Blockade of p53-mediated apoptosis | Prevention of hyposalivation | Preserved cell-specific markers of acinar, duct, and MEC | Mouse | (538) | |
| NTRN | Parasympathetic nerves | Prevention of hyposalivation | Increased parasympathetic nerve markers | Mouse | (36) | |
| Rescue of long-term hyposalivation | Prevention of irradiation damage | Minipig | (37) |
Autonomic pharmacological drugs. n/a, Not applicable. See glossary for other abbreviations.
Systemic administration of IGF-1 after IR in mice also restores salivary function (419). The potential translatability of using IGF-1 in head and neck cancer patients treated with IR is very limited because of its tumor-stimulating effects. However, mouse models have been instrumental to identifying downstream mechanisms that may offer more suitable therapeutic targets. When administered immediately before IR damage, IGF-1 facilitates cell cycle arrest and promotes DNA repair in a SIRT-1-dependent manner (397). It also inhibits the nuclear translocation of Yap downstream of aPKCζ and ROCK and ultimately restores saliva production (484). However, IGF-1 fails to restore saliva secretion in irradiated mice lacking aPKCζ, indicating that the kinase is indispensable for restoring secretion downstream of IGF-1 (419). In addition to IGF receptor signaling, other growth factors, cell surface receptors, and their downstream effectors are promising candidates to restore saliva production based on results in animal models. Transient activation of EDAR with agonist monoclonal antibodies restores secretion between 30 and 90 days after IR damage (540), and inhibition of mTOR signaling with a rapamycin analog improved secretion in irradiated mice and minipigs (541, 542).
With regard to SS, there are 58 active clinical trials testing a wide arrange of compounds to ameliorate symptoms or treat disease (ClinicalTrials.gov). Currently, there is growing interest in using small-molecule inhibitors that target receptor kinases or inflammatory pathways such as NF-κB, JAK-STAT, and Toll-like receptor (TLR) signaling given the activation status of these pathways during autoimmune disease (295, 543). Specific antibodies for BAFF (belimumab) show limited relief (544), and combinations with other immune therapies like the use of monoclonal antibody anti-CD20 (rituximab), which inhibits NF-κB signaling, may improve treatment (545). Promising approaches using rituximab or anti-CD22 (epratuzumab) are also being evaluated (545–547). In addition, preclinical models have shown that inhibition of BMP6 or the ATP-gated P2X7 receptor preserves stimulated saliva production and reduces lymphocytic infiltration in a mouse model of SS.
6.2. Cell- and Tissue-Based Regenerative Therapies
Cell-based therapy falls within the interdisciplinary science of tissue engineering, combining biology with engineering approaches. It is aimed toward the use of live cells to restore, enhance, or replace organ function. As our knowledge of developmental lineage relationships and plasticity is changing, so is the approach for epithelial cell-based regeneration (79). This is reflected in the move toward non-marker-based cell isolation focusing on niche signals and microenvironments. This is in turn providing essential knowledge for the development of bioengineered glands. In line with a focus on niches, the use of nonepithelial cells is providing encouraging results. Cell-based SG therapies have made major steps forward in the last decade, and here we summarize and give an overview of recent advances and future challenges in the field.
6.2.1. Cell-based transplantation.
Cell-based transplantation in SGs aims to promote the repair response of diseased, dysfunctional, or injured tissue by transplanting isolated cells as medication. The isolation of cells exhibiting in vitro potential and expressing stem/progenitor markers known from other tissues led to the idea that these cells could be useful for transplantation. Transplant approaches involving SG stem/progenitor cells include an in vitro expansion step through spheres, organoids, or both. Dissociated cells clumping together and proliferating in floating cultures are referred to as “spheres,” whereas “organoids” describes Matrigel-grown cell clusters originating from dissociated cells isolated from either spheres or tissue. Salivary cells from both mouse and human glands harbor the potential to grow in vitro as both spheres and organoids (29, 457, 548–551). Proof of concept that transplantation of epithelial progenitors alleviates IR dysfunction was shown in a mouse model by transplanting cultured cells enriched for Kit (552). After transplant of as few as 100–300 cells, salivary function recovered ∼40% compared with nontransplanted glands. Further confirmation was given when subpopulations of Kit+ cells were found to alleviate IR dysfunction even further (553, 554). This demonstrates the viability of this approach and the regenerative potential within the epithelium. A similar Kit+ population has also been isolated and cultured from human SGs (551). Upon transplant into irradiated mouse glands human Kit+ cells improve saliva flow, indicating that they are a potential source for therapeutic use (550, 551). As our understanding of stem/progenitors in SGs has evolved, it is becoming clear that several cell populations may have regenerative potential (106, 395, 422, 436). In line with this, discovering niche signals stimulating cells based on regenerative ability rather than phenotypic identity has been shown to be a superior approach. Unselected cells preconditioned by Wnt signaling resulted in vast improvement of in vitro expansion and 80% recovery of saliva flow in a post-IR mouse model (481). Furthermore, cells undergoing intraglandular transplantation engraft and survive long term within the host gland, indicating the potential for long-term improvement of gland function (481). Although preclinical studies indicate stem cell transplant as a viable approach, it has yet to be used in clinical trials. However, the advances made in developing salivary organoids have contributed greatly to our understanding of how to manipulate cell identity, marker expression, proliferation, and niche signals, which are important for using SG-derived cells (555). These advances, in turn, enhance our understanding of salivary pathology and regeneration, which are essential for cell-based therapy (29, 320).
An alternate and attractive cell source for transplantation that has made progress toward clinical use is what are termed postnatal mesenchymal stem cells (MSCs), a broad description of which is likely tissue-specific stem/progenitors (556). MSCs are often confused with bone marrow stem cells (BMSCs), and MSCs are used widely in many fields of regenerative medicine, although whether they are the regenerative stem cell or a niche cell that produces bioactive factors often remains to be determined in each regenerative context (557). Putative MSCs have been isolated from a variety of sources including bone marrow, adipose, and dental tissues as well as CD34+ MSCs from SGs (558, 559). Although the exact mechanism of what defines them and how MSCs provide therapeutic effects is unclear, one hypothesis is that they act in a “hit- and-run” fashion by secreting soluble factors such as cytokines or through release of extracellular vesicles containing peptides, proteins, microRNA and mitochondria (558, 560). Importantly, MSCs have successfully been used in animal models as well as human trials aimed toward a wide range of organs and diseases (561). In SGs, several preclinical and clinical studies for both autoimmune disease and IR damage have shown the ability of MSCs to alleviate disease and improve salivary function (562).
After global injection of MSCs in SS mouse models, the cells migrate to diseased SGs because of changes in the microenvironment (563). Several preclinical studies indicate that transplanted MSCs modulate the immune response and thus improve gland function (564). Importantly, in SS patients, intravenous transplant of MSCs isolated from umbilical cords led to increased salivary flow rate after 2 wk as well as alleviating SS symptoms in other tissues (563). This first-in-human study provides promising results for a novel treatment of salivary dysfunction due to SS and may be useful for other autoimmune diseases as well. It will be important to perform randomized controlled trials to confirm the therapeutic use of MSCs in SS.
Recently, the use of MSCs to rescue IR damage has also shown promising results. In a randomized placebo-controlled trial, adipose-derived MSCs underwent in vitro expansion/purification for 14 days before intraglandular injection in patients suffering post-IR xerostomia (565, 566). Unstimulated saliva flow improved, and the treatment group showed decrease in fibrosis and increase of acinar and ductal area after 4 mo. Interestingly, the study found a more evident improvement in inorganic saliva composition than in flow rate. Since saliva modification is duct dependent, this indicates that both ductal and acinar function are improved after this treatment. This trial successfully shows that this strategy is safe and should encourage confirmatory trials. In an effort to circumvent in vitro purification steps, a pilot study was performed using fat grafting to introduce MSCs to SGs. This also showed postoperative improvement in patients with long-standing xerostomia and provides the groundwork for further investigation (567). Same-day fat transplant with minimal manipulation falls within already approved FDA guidelines; however, a larger randomized study would be needed to confirm efficacy of the protocol.
Another potential nonepithelial source is peripheral blood mononuclear cells (PBMCs). In vitro manipulation using five vasculogenic proteins (SCF, Flt-ligand, TPO, VEGF, IL-6) in serum-free culture media enriched the population for definite CD11b/CD206-positive (M2 macrophage-like) effectively conditioned mononuclear cells (E-MNCs) (568). Intraglandular transplantation into irradiated mouse SGs leads to a gradual recovery of salivary function through reduction of inflammatory genes, increased proliferation, blood vessel formation, and eventually increase in acinar and ductal area and reduction in fibrosis. This is now being followed up with a phase 1 first-in-human clinical trial using E-MNCs in patients experiencing IR-induced xerostomia (569). This initial study aims to verify the safety and efficacy of E-MNC transplant, and the final results are still pending. Even so, the promising use of E-MNCs comes with the advantage of being less invasive to isolate than other sources. Taken together, cell transplantation is a viable regenerative route and various cell sources are providing encouraging results. Future work is needed to confirm the safety and efficacy of these approaches; however, proof of principle is provided, and viable treatments are being investigated.
6.2.2. Organ replacement by bioengineered SGs.
The regeneration of SGs with bioengineered organ transplants aims to fully replace and restore function. An instrumental first step toward successful reconstitution of bioengineered organs is the development of the “organ germ method” (570). This approach builds on the ability of embryonic germ cells to self-organize and grow in vitro. Single cells from epithelium and mesenchyme reorganize and recapitulate development to form organ rudiments that are transplanted and grown into functional organs in vivo. This method has shown great promise in a wide range of complex organs including SGs (571). A groundbreaking study using this method with fetal mouse cells showed the potential for bioengineering and transplanting all major SGs (572). Furthermore, transplanted bioengineered glands engraft to the existing main duct, are histologically similar to existing glands, and secrete saliva upon either parasympathetic or gustatory stimulation (572, 573). Following up on this, the essential transcription factors Sox9 and Foxc1 were necessary to form salivary rudiments from embryonic stem cells, which then were able to generate functional glands after orthotopic transplantation (574). Combining the organ germ method with sphere culture enabled generation of functional human SG tissue. Long-term cultures of human SMG cells, with a stable genome, were grown into spheres and combined with E12 mouse salivary mesenchyme. The human cells responded to the mouse embryonic niche signals and developed into salivary tissue upon kidney capsule transplantation (34). This indicates that human SMG cells may be a viable source for bioengineering purposes, although this requires an understanding of critical cues that regulate cell fate. Taken together, the use of the organ germ method and human embryonic stem cells delivers a proof of principle; however, bioengineering SGs for the clinic may require alternative approaches that are not dependent on human embryonic stem cells but may use a patient-derived cell source such as induced pluripotent stem cells.
In general, the trend of bioengineering SGs has moved from the simple “blind end tube” model (575) toward the more complex three-dimensional (3-D) “branched design” that aims to mimic native tissue (576). Even so, a fully reconstructed SG made through tissue engineering techniques has not yet been achieved. Using development as a template, and considering the importance of the extracellular matrix, a major focus is identifying the optimal physical and chemical microenvironment that promotes polarization, branching, and differentiation. Ideally for transplantation, scaffolds should be well controlled, easily modified to include instructive components, and highly biocompatible and should not trigger immune responses. Several matrixes are used to grow SG cells including Matrigel, collagen type I, fibrin, silk, poly(ethylene)glycol (PEG) hydrogels, poly lactic-co-glycolic acid (PLGA), and hyaluronic acid (HA) (577, 578). Although Matrigel is successfully used in many research applications including culture of salivary organoids and embryonic stem cell manipulation as described above, its applicability for human transplantation is limited because it is a murine tumor-derived matrix (579). This has led to the increasing interest in scaffolds such as HA, PEG, and PLGA, which all can be modified to include crucial ECM motifs, derived from larger ECM proteins, that are instructive for cells (580). Generating salivary functional units for transplantation requires the 3-D reconstruction of polarized cells that are envisioned to connect to ductal structures in vivo once implanted (40). However, acinar cells undergoing cellular stress or injury often downregulate canonical acinar markers both in vitro and in vivo (496), and they readily lose their polarity and phenotype in in vitro long-term culture (581). Thus, generating or maintaining various salivary cell types and specifically acinar cells is a major challenge in the field (576).
PEG hydrogels with MMP-cleavable sequences allow for the encapsulation of primary cells that polarize with the correct localization of acinar markers NKCC1, ZO-1, and AQP5 in short-term culture (581, 582). Based on this initial work, the combination of PEG hydrogels with microbubble array technology has led to the recent development of a functional SG tissue chip with potential for high-content drug screening (583).
The use of HA-based hydrogels containing a peptide from perlecan domain IV to seed human primary cells resulted in cellular organization with lumen formation of acinar-like structures expressing AQP5 (584, 585). Furthermore, fine-tuning of this HA hydrogel culture system highlighted the potential for human salivary cells to undergo microenvironment-driven acinar differentiation (586). For transplantation purposes, 3-D spheroids grown in HA hydrogels could be transplanted into resected PGs. The cells are maintained in vivo, suggesting viability following transplantation, although additional functional regeneration was not achieved (587, 588).
Another approach used to generate 3-D organoids is bioprinting, which combines cells and biocompatible materials to mimic natural organs (589). Using primary pig SG cells or human dental pulp stem cells, a novel scaffold-free culture system called magnetic 3-D levitation allowed magnetized cells to organize and generate their own ECM over a short time period (590, 591). These organoids are organized and show SG-specific secretory activity upon cholinergic stimulation, suggesting this as an interesting and promising tool for future gland regeneration.
Recent advances in tissue engineering hold promise for building artificial SGs; however, a major challenge for the future is to fully identify appropriate niche signals instructive for organ formation, differentiation, and maintenance. This relies heavily on the continued work to understand niche factors during development, disease, and regeneration as well as optimizing tissue engineering approaches. Nevertheless, proof-of-concept studies have shown that organ replacement with bioengineered glands may be possible.
6.3. Gene Therapy
Gene therapy using viral vectors that are delivered by retro-ductal cannulation directly into the duct of the gland to deliver a gene to reduce salivary hypofunction or increase salivary flow provides a model for future regenerative therapy (FIGURE 9). First-in-human gene therapy trials as treatment for patients suffering from radiation-induced salivary hypofunction have been pioneered at NIH over the past 10 yr. Initially, adenoviral delivery of the aquaporin-1 (AQP1) water channel into surviving salivary epithelium of patients previously treated with IR for head and neck cancer resulted in short- and long-term improvements of parotid salivary flow for up to 3 yr (592, 593). Gene delivery vectors were then changed to reduce potential immune side effects of adenovirus, and adeno-associated viral vector (AAV2) delivery of AQP1 is currently being investigated in two active clinical trials to treat IR-induced salivary hypofunction (ClinicalTrials.gov NCT02446249 and NCT04043104). These trials follow from preclinical studies in irradiated minipigs where AAV2 delivery of human AQP1 recovered up to 35% of baseline secretion after AAV2-hAQP1 administration (594). Another promising target of gene therapy is NRTN. Preclinical studies in mice and minipigs show that AAV2-NRTN therapy before IR prevented subsequent hyposalivation (37). In addition, NGF was also used in AAV-mediated gene therapy in mice to reduce epithelial IR-induced apoptosis, which was dependent on dephosphorylating JNK kinase, but there was no report on salivary flow (595). Finally, AAV-Shh gene transfer has also been used in mice to repress cellular senescence and promote DNA repair in irradiated SGs (424). In summary, the path forward with gene therapy is clear, and future research is needed to identify other potential targets to prevent damage, repair function, overcome fibrosis, and regenerate functional secretory tissue.
FIGURE 9.
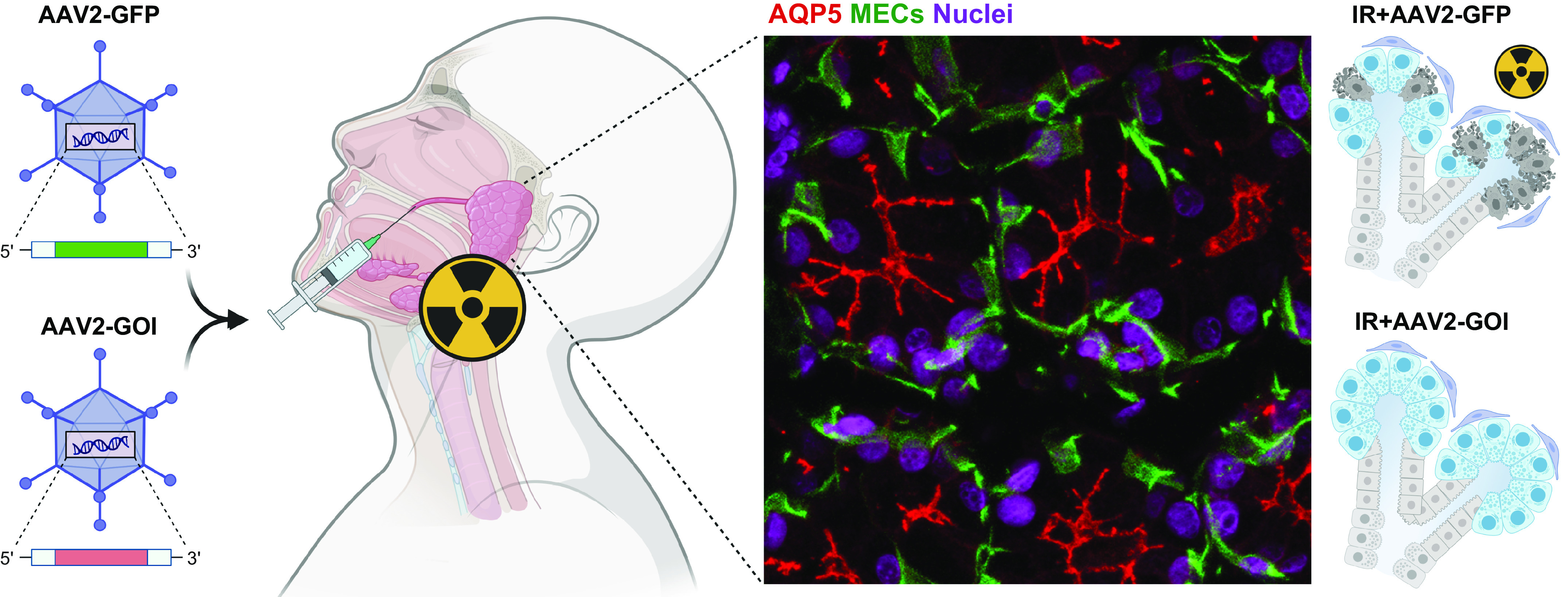
Model of gene therapy to restore salivary function. Intraductal delivery of an AAV2-based gene of interest (GOI) or a control vector. Immunostaining highlights IR-induced alterations in acinar morphology. The immunostaining shows the aquaporin water channel (AQP5, red) on the luminal surface of acinar cells and IDs that are surrounded by MECs (SMA, green) and nuclei (DAPI, magenta). Immunofluorescence image is a maximum-intensity projection of confocal sections. In the model the AAV2-GOI is expressed in the gland and prevents or repairs IR-induced epithelial damage. See glossary for abbreviations. Created with BioRender.com, with permission.
7. CONCLUSIONS
Saliva is a major contributor to oral and overall health and well-being. Thus, understanding the mechanisms that lead to loss of secretion and those involved in functional organ regeneration remains a major focus of current and future research. There has been great progress in understanding the biology of SG development and hypofunction thanks to the advent of OMICS technologies combined with more advanced animal models that mimic different aspects of disease in humans. Nonetheless, a cure for chronic salivary hypofunction has not yet been developed. Further advances in OMICS and single-cell technologies including transcriptomics, proteomics, epigenomics, and exposomics and the application of these will be instrumental in better understanding the cellular and molecular landscape in homeostasis and disease and to devise new therapies. There are a number of ongoing clinical trials to evaluate the efficacy of small-molecule inhibitors in SS, and more are expected in coming years. In addition, AAV2-AQP1 gene therapy trials in humans show promise to treat IR-induced hyposalivation. In addition, SGs are a reservoir for viral infections, which has implications for the potential development of SG-specific vectors for salivary gene therapy and the development of saliva as a diagnostic tool to evaluate disease progression or transmission.
A major paradigm shift in understanding preclinical animal models that elucidate regenerative mechanisms is a new perspective on the role of cell plasticity and salivary progenitors. Historically, the dogma was that a population of bona fide stem cells in the gland behaved similar to the stem cells of the hematopoietic system to heal the tissue. However, most lineage tracing models agree on a different model in which several cell types within the SG epithelium can serve as progenitor-like cells in an injury- and disease-dependent manner. This has tremendous implications in the development of new therapeutic approaches to regenerate the SGs and opens a new area of research to understand the microenvironmental signals that are responsible for regulating plasticity. These could be used to direct other cells from an autologous source to repair or regenerate the gland, whether directly in vivo or in the context of a cell transplant with a bioscaffold. An outstanding unanswered question is, does the degree of plasticity observed in animal models occur in human SGs and is it regulated by similar mechanisms? Single cell-based approaches will be instrumental in answering these questions, particularly when combined with injury and regeneration models. Moreover, understanding the interactions between cells and their microenvironment will be crucial to fine-tune cell-based approaches to achieve regeneration.
To conclude, we have covered the impact of using saliva as a diagnostic tool for viral disease, which proved tremendously useful amid a global pandemic to provide easy sampling for viral testing. The identification of new biomarkers associated with both disease and regeneration, as well as improved methods of detection, offer an opportunity for transdisciplinary research using the unique physiological mechanisms that drive salivary secretion to understand and diagnose not only physical disease but also behavioral, psychological, and physiological disorders.
8. GLOSSARY
- [Ca2+]ER
Calcium concentration in the ER
- [Ca2+]i
Intracellular cytosolic calcium concentration
- 53BP1
p53-binding protein 1
- AAV2
Adeno-associated viral vector 2
- AAV2-GOI
Adeno-associated viral vector 2-gene of interest
- ACC
Adenoid cystic carcinoma
- aHSCT
Allogeneic hematopoietic stem cell transplant
- ALDH1A
Aldehyde dehydrogenase 1A
- ALSG
Aplasia of the lacrimal and salivary gland
- ANA
Anti-nuclear antibodies
- ANO1
Anoctamin 1
- aPKCζ
Atypical protein kinase C zeta
- AQP1
Aquaporin 1
- AQP5
Aquaporin 5
- AREG
Amphiregulin
- atRAL
All-trans-retinal
- BAFF
B cell-activating factor
- BDNF
Brain-derived neurotrophic factor
- BMP
Bone morphogenetic protein
- BSLPS-IMRT
Bilateral superficial lobe parotid-sparing IMRT
- BTC
Betacullulin
- Ca2+
Calcium
- CaCC
Ca2+-activated Cl− channel
- cAMP
Cyclic adenosine monophosphate
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- cGMP
Calcium/cyclic guanosine monophosphate
- Chi-C
Chitosan-catechol
- Cl−
Chloride
- CLPS-IMRT
Contralateral parotid-sparing IMRT
- COVID-19
Coronavirus disease 2019 caused by SARS-CoV-2
- CoV
Coronavirus
- CRAC
Ca2+ release-activated Ca2+
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- DAG
Diacylglycerol
- DLK1, 2
Delta-like proteins 1 and 2
- E
Embryonic day
- E-MNC
Effectively conditioned PBMC
- eATP
Extracellular ATP
- EBV
Epstein–Barr virus
- ECM
Extracellular matrix
- ED
Excretory duct
- EDA
Ectodysplasin A
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- ENaC
Epithelial sodium channel
- ER
Endoplasmic reticulum
- FGF
Fibroblast growth factor
- FGFR
Fibroblast growth factor receptor
- Flt-3L
FMS-like tyrosine kinase-3 ligand
- G-CSF
Granulocyte colony-stimulating factor
- GAG
Glycosaminoglycan
- Galnt
GalNac:polypeptide N-acetylgalactosaminyltransferase
- GC
Germinal center
- GCT
Granular convoluted tubule
- GRK2
GPCR kinase 2
- GVHD
Graft-versus-host disease
- H2BGFP
Histone 2B-green fluorescent protein
- HA
Hyaluronic acid
- HB-EGF
Heparin-binding epidermal growth factor
- HBOT
Hyperbaric oxygen therapy
Bicarbonate
- HED
Hypohidrotic ectodermal dysplasia
- HS
Heparan sulfate
- HSPG
Heparan sulfate proteoglycan
- ICI
Immune checkpoint inhibitor
- ID
Intercalated duct
- IFN
Interferon
- IFN-α
Interferon-alpha
- IFN-γ
Interferon-gamma
- IGF-1
Insulin-like growth factor 1
- IgG
Immunoglobulin
- IMRT
Intensity-modulated radiotherapy
- IP3
Inositol 1,4,5-trisphosphate
- IP3R
Inositol 1,4,5-trisphosphate receptor
- IR
Irradiation
- irAE
Immune-related adverse effect
- IRBIT
IP3R binding protein released with inositol 1,4,5-trisphosphate
- JNK
Jun kinase
- K+
Potassium
- KRT5
Keratin 5
- LADD
Lacrimo-auriculo-dento-digital syndrome
- LAMP3
Lysosomal associated membrane protein 3
- LSG
Labial minor SG
- mAChR
Muscarinic acetylcholine receptor
- MALT
Mucosa-associated lymphoid tissue
- MAPK
Mitogen-activated protein kinase
- MC
Mucoepidermoid carcinoma
- MEC
Myoepithelial cell
- Mgat5
β1,6-N-acetylglucosaminyltransferase 5
- MSC
Mesenchymal stem cell
- MT2-MMP
Membrane type 2-matrix metalloproteinase
- Muc10
Mucin 10
- NBC
Sodium bicarbonate cotransporter
- NF-κB
Nuclear factor-kappaB
- NGF
Nerve growth factor
- NHE1
Na+/H+ exchanger-1
- NK-1
Neurokinin-1
- NKCC1
Sodium-potassium-chloride cotransporter 1
- Nrg3
Neuregulin 3
- NRTN
Neurturin
- oGVHD
Oral graft-versus-host disease
- P2X7R
Purinergic P2X7 receptor
- PBMC
Peripheral blood mononuclear cell
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death protein ligand 1
- PDC
Plasmacytoid dendritic cell
- PDT
Passive drool test
- PEG
Poly(ethylene)glycol
- PG
Parotid gland
- PIP2
Phosphatidylinositol 1,4-bisphosphate
- PKA
Protein kinase A
- PKC
Protein kinase C
- PKCδ
Protein kinase C delta
- PLC
Phospholipase C
- PLGA
Poly lactic-co-glycolic acid
- PM
Plasma membrane
- PRR
Pattern recognition receptor
- PSG
Parasympathetic ganglion
- RA
Retinoic acid
- RAR
Retinoic acid receptor
- RDH10
Retinol dehydrogenase 10
- ROCK
Rho-associated coiled-coil containing kinase
- ROS
Reactive oxygen species
- RT
Radiation therapy
- RTK
Receptor tyrosine kinase
- SA-β-gal
Senescence-associated beta-galactosidase
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SCF
Stem cell factor
- SD
Striated duct
- SG
Salivary gland
- Shh
Sonic hedgehog
- SLG
Sublingual gland
- SMA
Smooth muscle actin
- SMG
Submandibular gland
- SOCE
Store-operated calcium entry
- SP
Substance P
- Spry
Sprouty
- STIM
Stromal interaction molecule
- TFF3
Trefoil factor 3
- Tfh
T follicular helper
- TGF-β
Transforming growth factor beta
- TH
Tyrosine hydroxylase
- Th1
T helper 1
- Th2
T helper 2
- Th17
T helper 17
- TLR
Toll-like receptor
- TMEM16A
Transmembrane member 16A
- TRPC
Transient receptor potential canonical
- TRPM2
Transient receptor potential melastatin 2
- TRPV4
Transient receptor potential vanilloid 4
- VEGF
Vascular endothelial growth factor
- VIP
Vasoactive intestinal peptide
- γH2AX
Phos+A1:D78phorylated H2A histone family member X
GRANTS
The authors were all supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health (ZIA DE-000707 and ZIA DE-000722).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.C., M.H.A., V.N.P., and M.P.H. conceived and designed research; analyzed data; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Azuma N, Katada Y, Sano H. Deterioration in saliva quality in patients with Sjogren’s syndrome: impact of decrease in salivary epidermal growth factor on the severity of intraoral manifestations. Inflamm Regen 38: 6, 2018. doi: 10.1186/s41232-018-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res 79: 1652–1658, 2000. doi: 10.1177/00220345000790090301. [DOI] [PubMed] [Google Scholar]
- 3.Lan X, Chan JY, Pu JJ, Qiao W, Pang S, Yang WF, Wong KC, Kwong DL, Su YX. Saliva electrolyte analysis and xerostomia-related quality of life in nasopharyngeal carcinoma patients following intensity-modulated radiation therapy. Radiother Oncol 150: 97–103, 2020. doi: 10.1016/j.radonc.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Turner MD. Hyposalivation and xerostomia: etiology, complications, and medical management. Dent Clin North Am 60: 435–443, 2016. doi: 10.1016/j.cden.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Xu F, Laguna L, Sarkar A. Aging-related changes in quantity and quality of saliva: where do we stand in our understanding? J Texture Stud 50: 27–35, 2019. doi: 10.1111/jtxs.12356. [DOI] [PubMed] [Google Scholar]
- 6.Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol 25-26: 52–60, 2014. doi: 10.1016/j.semcdb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodner L, Dayan D. Epithelium and connective tissue regeneration during palatal wound healing in desalivated rats—a comparative study. Comp Biochem Physiol A Physiol 111: 415–419, 1995. doi: 10.1016/0300-9629(95)00036-7. [DOI] [PubMed] [Google Scholar]
- 8.Culp DJ, Robinson B, Cash MN, Bhattacharyya I, Stewart C, Cuadra-Saenz G. Salivary mucin 19 glycoproteins: innate immune functions in Streptococcus mutans-induced caries in mice and evidence for expression in human saliva. J Biol Chem 290: 2993–3008, 2015. doi: 10.1074/jbc.M114.597906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawes C, Pedersen AM, Villa A, Ekström J, Proctor GB, Vissink A, Aframian D, McGowan R, Aliko A, Narayana N, Sia YW, Joshi RK, Jensen SB, Kerr AR, Wolff A. The functions of human saliva: a review sponsored by the World Workshop on Oral Medicine VI. Arch Oral Biol 60: 863–874, 2015. doi: 10.1016/j.archoralbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Frenkel ES, Ribbeck K. Salivary mucins in host defense and disease prevention. J Oral Microbiol 7: 29759, 2015. doi: 10.3402/jom.v7.29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keswani SG, Balaji S, Le LD, Leung A, Parvadia JK, Frischer J, Yamano S, Taichman N, Crombleholme TM. Role of salivary vascular endothelial growth factor (VEGF) in palatal mucosal wound healing. Wound Repair Regen 21: 554–562, 2013. doi: 10.1111/wrr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med 11: 216–229, 2000. doi: 10.1177/10454411000110020501. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi S, Ohba Y, Oka T. Effect of salivary epidermal growth factor on wound healing of tongue in mice. Am J Physiol Endocrinol Metab 260: E620–E625, 1991. doi: 10.1152/ajpendo.1991.260.4.E620. [DOI] [PubMed] [Google Scholar]
- 14.Proctor GB, Shaalan AK. Salivary gland secretion. In: Physiology of the Gastrointestinal Tract, edited by Said HM. London: Academic Press, 2018, p. 813–830. [Google Scholar]
- 15.Shah D, Son KN, Kalmodia S, Lee BS, Ali M, Balasubramaniam A, Shukla D, Aakalu VK. Wound healing properties of histatin-5 and identification of a functional domain required for histatin-5-induced cell migration. Mol Ther Methods Clin Dev 17: 709–716, 2020. doi: 10.1016/j.omtm.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabak LA, Levine MJ, Mandel ID, Ellison SA. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol 11: 1–17, 1982. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 17.Torres P, Díaz J, Arce M, Silva P, Mendoza P, Lois P, Molina-Berríos A, Owen GI, Palma V, Torres VA. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. FASEB J 31: 4946–4958, 2017. doi: 10.1096/fj.201700085R. [DOI] [PubMed] [Google Scholar]
- 18.Vila T, Rizk AM, Sultan AS, Jabra-Rizk MA. The power of saliva: antimicrobial and beyond. PLoS Pathog 15: e1008058, 2019. doi: 10.1371/journal.ppat.1008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baghizadeh Fini M. Oral saliva and COVID-19. Oral Oncol 108: 104821, 2020. doi: 10.1016/j.oraloncology.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, Shen Z, Guo F, Zhang Q, Jin Y, Wang L, Wang S. Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 Patients. Cell Prolif 53: e12923, 2020. doi: 10.1111/cpr.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han P, Ivanovski S. Saliva—friend and foe in the COVID-19 outbreak. Diagnostics (Basel) 10: 290, 2020. doi: 10.3390/diagnostics10050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chibly AM, Nguyen T, Limesand KH. Palliative care for salivary gland dysfunction highlights the need for regenerative therapies: a review on radiation and salivary gland stem cells. J Palliat Care Med 4: 1000180, 2014. doi: 10.4172/2165-7386.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 76: S58–S63, 2010. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasmer KJ, Gilman KE, Munoz Forti K, Weisman GA, Limesand KH. Radiation-induced salivary gland dysfunction: mechanisms, therapeutics and future directions. J Clin Med 9: 4095, 2020. doi: 10.3390/jcm9124095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashhour K, Kamaleldin M, Hashem W. RapidArc vs conventional IMRT for head and neck cancer irradiation: is faster necessary better? Asian Pac J Cancer Prev 19: 207–211, 2018. doi: 10.22034/APJCP.2018.19.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. . SARS-CoV-2 infection of the oral cavity and saliva. Nat Med 27: 892–903, 2021. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauser BR, Aure MH, Kelly MC, Hoffman MP, Chibly AM; Genomics and Computational Biology Core. Generation of a single-cell RNAseq atlas of murine salivary gland development. iScience 23: 101838, 2020. doi: 10.1016/j.isci.2020.101838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekiguchi R, Martin D, Yamada KM; Genomics and Computational Biology Core. Single-cell RNA-seq identifies cell diversity in embryonic salivary glands. J Dent Res 99: 69–78, 2020. doi: 10.1177/0022034519883888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrano Martinez P, Cinat D, van Luijk P, Baanstra M, de Haan G, Pringle S, Coppes RP. Mouse parotid salivary gland organoids for the in vitro study of stem cell radiation response. Oral Dis 27: 52–63, 2021. doi: 10.1111/odi.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitou M, Gaylord EA, Xu E, May AJ, Neznanova L, Nathan S, Grawe A, Chang J, Ryan W, Ruhl S, Knox SM, Gokcumen O. Functional specialization of human salivary glands and origins of proteins intrinsic to human saliva. Cell Rep 33: 108402, 2020. doi: 10.1016/j.celrep.2020.108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmberg KV, Hoffman MP. Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci 24: 1–13, 2014. doi: 10.1159/000358776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalili S, Liu Y, Sumita Y, Maria OM, Blank D, Key S, Mezey E, Tran SD. Bone marrow cells are a source of undifferentiated cells to prevent Sjogren’s syndrome and to preserve salivary glands function in the non-obese diabetic mice. Int J Biochem Cell Biol 42: 1893–1899, 2010. doi: 10.1016/j.biocel.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CY, Chang FH, Chen CY, Huang CY, Hu FC, Huang WK, Ju SS, Chen MH. Cell therapy for salivary gland regeneration. J Dent Res 90: 341–346, 2011. doi: 10.1177/0022034510386374. [DOI] [PubMed] [Google Scholar]
- 34.Sui Y, Zhang S, Li Y, Zhang X, Hu W, Feng Y, Xiong J, Zhang Y, Wei S. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res Ther 11: 127, 2020. doi: 10.1186/s13287-020-01628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Pasquale G, Perez Riveros P, Tora M, Sheikh T, Son A, Teos L, Grewe B, Swaim WD, Afione S, Zheng C, Jang SI, Shitara A, Alevizos I, Weigert R, Chiorini JA. Transduction of salivary gland acinar cells with a novel AAV vector 44.9. Mol Ther Methods Clin Dev 19: 459–466, 2020. doi: 10.1016/j.omtm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira JN, Zheng C, Lombaert IMA, Goldsmith CM, Cotrim AP, Symonds JM, Patel VN, Hoffman MP. Neurturin gene therapy protects parasympathetic function to prevent irradiation-induced murine salivary gland hypofunction. Mol Ther Methods Clin Dev 9: 172–180, 2018. doi: 10.1016/j.omtm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lombaert IM, Patel VN, Jones CE, Villier DC, Canada AE, Moore MR, Berenstein E, Zheng C, Goldsmith CM, Chorini JA, Martin D, Zourelias L, Trombetta MG, Edwards PC, Meyer K, Ando D, Passineau MJ, Hoffman MP. CERE-120 prevents irradiation-induced hypofunction and restores immune homeostasis in porcine salivary glands. Mol Ther Methods Clin Dev 18: 839–855, 2020. doi: 10.1016/j.omtm.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chansaenroj A, Yodmuang S, Ferreira J. Trends in salivary gland tissue engineering: from stem cells to secretome and organoid bioprinting. Tissue Eng Part B Rev 27: 155–165, 2021. doi: 10.1089/ten.teb.2020.0149. [DOI] [PubMed] [Google Scholar]
- 39.Khan E, Farooq I, Khabeer A, Ali S, Zafar MS, Khurshid Z. Salivary gland tissue engineering to attain clinical benefits: a special report. Regen Med 15: 1455–1461, 2020. doi: 10.2217/rme-2019-0079. [DOI] [PubMed] [Google Scholar]
- 40.Ozdemir T, Fowler EW, Hao Y, Ravikrishnan A, Harrington DA, Witt RL, Farach-Carson MC, Pradhan-Bhatt S, Jia X. Biomaterials-based strategies for salivary gland tissue regeneration. Biomater Sci 4: 592–604, 2016. doi: 10.1039/c5bm00358j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim JY, Ra JC, Shin IS, Jang YH, An HY, Choi JS, Kim WC, Kim YM. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS One 8: e71167, 2013. doi: 10.1371/journal.pone.0071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carpenter GH. The secretion, components, and properties of saliva. Annu Rev Food Sci Technol 4: 267–276, 2013. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- 43.Garrett JR, Thulin A. Changes in parotid acinar cells accompanying salivary secretion in rats on sympathetic or parasympathetic nerve stimulation. Cell Tissue Res 159: 179–193, 1975. doi: 10.1007/BF00219154. [DOI] [PubMed] [Google Scholar]
- 44.Vreugdenhil AP, Nieuw Amerongen AV, de Lange GL, Roukema PA. Localization of amylase and mucins in the major salivary glands of the mouse. Histochem J 14: 767–780, 1982. doi: 10.1007/BF01033626. [DOI] [PubMed] [Google Scholar]
- 45.Roukema PA, Oderkerk CH, Salkinoga-Salonen MS. The murine sublingual and submandibular mucins. Their isolation and characterization. Biochim Biophys Acta 428: 432–440, 1976. doi: 10.1016/0304-4165(76)90051-9. [DOI] [PubMed] [Google Scholar]
- 46.Kim SK, Nasjleti CE, Han SS. The secretion processes in mucous and serous secretory cells of the rat sublingual gland. J Ultrastruct Res 38: 371–389, 1972. doi: 10.1016/S0022-5320(72)90012-3. [DOI] [PubMed] [Google Scholar]
- 47.Valstar MH, de Bakker BS, Steenbakkers RJ, de Jong KH, Smit LA, Klein Nulent TJ, van Es RJ, Hofland I, de Keizer B, Jasperse B, Balm AJ, van der Schaaf A, Langendijk JA, Smeele LE, Vogel WV. The tubarial salivary glands: a potential new organ at risk for radiotherapy. Radiother Oncol 154: 292–298, 2021. doi: 10.1016/j.radonc.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 48.Mukaibo T, Gao X, Yang NY, Oei MS, Nakamoto T, Melvin JE. Sexual dimorphisms in the transcriptomes of murine salivary glands. FEBS Open Bio 9: 947–958, 2019. doi: 10.1002/2211-5463.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Gailani M, Asking B, Emmelin N, Garrett JR. Functional and structural studies concerning the control of activity in zygomatic glands of cats. J Auton Nerv Syst 3: 71–86, 1981. doi: 10.1016/0165-1838(81)90031-X. [DOI] [PubMed] [Google Scholar]
- 50.Kay RN. The rate of flow and composition of various salivary secretions in sheep and calves. J Physiol 150: 515–537, 1960. doi: 10.1113/jphysiol.1960.sp006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva-Zacarin EC, Silva De Moraes RL, Taboga SR. Silk formation mechanisms in the larval salivary glands of Apis mellifera (Hymenoptera: Apidae). J Biosci 28: 753–764, 2003. doi: 10.1007/BF02708436. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg HI. Histology, histochemistry, and emptying mechanism of the venom glands of some elapid snakes. J Morphol 123: 133–155, 1967. doi: 10.1002/jmor.1051230204. [DOI] [PubMed] [Google Scholar]
- 53.Garb JE, Haney RA, Schwager EE, Gregorič M, Kuntner M, Agnarsson I, Blackledge TA. The transcriptome of Darwin’s bark spider silk glands predicts proteins contributing to dragline silk toughness. Commun Biol 2: 275, 2019. doi: 10.1038/s42003-019-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kessler AT, Bhatt AA. Review of the major and minor salivary glands, part 1: anatomy, infectious, and inflammatory processes. J Clin Imaging Sci 8: 47, 2018. doi: 10.4103/jcis.JCIS_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yakirevich E, Sabo E, Klorin G, Alos L, Cardesa A, Ellis GL, Shumway BS, Gnepp DR. Primary mucin-producing tumours of the salivary glands: a clinicopathological and morphometric study. Histopathology 57: 395–409, 2010. doi: 10.1111/j.1365-2559.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 56.Frommer J. The human accessory parotid gland: its incidence, nature, and significance. Oral Surg Oral Med Oral Pathol 43: 671–676, 1977. doi: 10.1016/0030-4220(77)90049-4. [DOI] [PubMed] [Google Scholar]
- 57.Ellis H. Anatomy of the salivary glands. Surgery (Oxford) 30: 569–572, 2012. doi: 10.1016/j.mpsur.2012.09.008. [DOI] [Google Scholar]
- 58.Otonari-Yamamoto M, Nakajima K, Tsuji Y, Otonari T, Curtin HD, Okano T, Sano T. Imaging of the mylohyoid muscle: separation of submandibular and sublingual spaces. AJR Am J Roentgenol 194: W431–W438, 2010. doi: 10.2214/AJR.09.3516. [DOI] [PubMed] [Google Scholar]
- 59.de Paula F, Teshima TH, Hsieh R, Souza MM, Nico MM, Lourenco SV. Overview of human salivary glands: highlights of morphology and developing processes. Anat Rec (Hoboken) 300: 1180–1188, 2017. doi: 10.1002/ar.23569. [DOI] [PubMed] [Google Scholar]
- 60.Aure MH, Symonds JM, Mays JW, Hoffman MP. Epithelial cell lineage and signaling in murine salivary glands. J Dent Res 98: 1186–1194, 2019. doi: 10.1177/0022034519864592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker OJ. Current trends in salivary gland tight junctions. Tissue Barriers 4: e1162348, 2016. doi: 10.1080/21688370.2016.1162348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Paula F, Teshima TH, Hsieh R, Souza MM, Coutinho-Camillo CM, Nico MM, Lourenco SV. The expression of water channel proteins during human salivary gland development: a topographic study of aquaporins 1, 3 and 5. J Mol Histol 48: 329–336, 2017. doi: 10.1007/s10735-017-9731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larsen HS, Aure MH, Peters SB, Larsen M, Messelt EB, Kanli Galtung H. Localization of AQP5 during development of the mouse submandibular salivary gland. J Mol Histol 42: 71–81, 2011. doi: 10.1007/s10735-010-9308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsieh MS, Jeng YM, Lee YH. Mist1: a novel nuclear marker for acinic cell carcinoma of the salivary gland. Virchows Arch 475: 617–624, 2019. doi: 10.1007/s00428-019-02600-1. [DOI] [PubMed] [Google Scholar]
- 65.Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec 259: 157–167, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 66.Punj A. Secretions of human salivary gland. In: Salivary Glands: New Approaches in Diagnostics and Treatment, edited by Güvenç IA. London: InTechOpen, 2019. [Google Scholar]
- 67.Yamashina S, Tamaki H, Katsumata O. The serous demilune of rat sublingual gland is an artificial structure produced by conventional fixation. Arch Histol Cytol 62: 347–354, 1999. doi: 10.1679/aohc.62.347. [DOI] [PubMed] [Google Scholar]
- 68.Yamashina S, Tamaki H, Katsumata O. Fine structure of the exocrine cells of rat sublingual gland revealed by rapid freezing and freeze substitution method. Eur J Morphol 38: 213–218, 2000. doi: 10.1076/0924-3860(200010)38:4;1-o;ft213. [DOI] [PubMed] [Google Scholar]
- 69.Tandler B. Are demilunes in mixed salivary glands real or fixation artifacts? A critique. J Cytol Histol 05: 218, 2014. doi: 10.4172/2157-7099.1000218. [DOI] [Google Scholar]
- 70.Kondo Y, Nakamoto T, Jaramillo Y, Choi S, Catalan MA, Melvin JE. Functional differences in the acinar cells of the murine major salivary glands. J Dent Res 94: 715–721, 2015. doi: 10.1177/0022034515570943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamoto T, Srivastava A, Romanenko VG, Ovitt CE, Perez-Cornejo P, Arreola J, Begenisich T, Melvin JE. Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am J Physiol Regul Integr Comp Physiol 292: R2380–R2390, 2007. doi: 10.1152/ajpregu.00591.2006. [DOI] [PubMed] [Google Scholar]
- 72.Gresik EW. The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc Res Tech 27: 1–24, 1994. doi: 10.1002/jemt.1070270102. [DOI] [PubMed] [Google Scholar]
- 73.Hosoi K, Nakamura T, Ueha T. Effect of testosterone on the amount of serous-like granules in convoluted tubular cells of mouse submandibular glands. J Biochem 81: 739–748, 1977. doi: 10.1093/oxfordjournals.jbchem.a131512. [DOI] [PubMed] [Google Scholar]
- 74.Catalán MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, Clarke LL, Melvin JE. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol 588: 713–724, 2010. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev 92: 39–74, 2012. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang NY, Mukaibo T, Gao X, Kurtz I, Melvin JE. Slc4a11 disruption causes duct cell loss and impairs NaCl reabsorption in female mouse submandibular glands. Physiol Rep 7: e14232, 2019. doi: 10.14814/phy2.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.May AJ, Cruz-Pacheco N, Emmerson E, Gaylord EA, Seidel K, Nathan S, Muench MO, Klein OD, Knox SM. Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development 145: dev166363, 2018. doi: 10.1242/dev.166363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emmerson E, Knox SM. Salivary gland stem cells: A review of development, regeneration and cancer. Genesis 56: e23211, 2018. doi: 10.1002/dvg.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rocchi C, Barazzuol L, Coppes RP. The evolving definition of salivary gland stem cells. NPJ Regen Med 6: 4, 2021. doi: 10.1038/s41536-020-00115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ono Minagi H, Sarper SE, Kurosaka H, Kuremoto KI, Taniuchi I, Sakai T, Yamashiro T. Runx1 mediates the development of the granular convoluted tubules in the submandibular glands. PLoS One 12: e0184395, 2017. doi: 10.1371/journal.pone.0184395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amano O, Mizobe K, Bando Y, Sakiyama K. Anatomy and histology of rodent and human major salivary glands: -overview of the Japan salivary gland society-sponsored workshop. Acta Histochem Cytochem 45: 241–250, 2012. doi: 10.1267/ahc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Isogai Y, Wu Z, Love MI, Ahn MH, Bambah-Mukku D, Hua V, Farrell K, Dulac C. Multisensory logic of infant-directed aggression by males. Cell 175: 1827–1841.e17, 2018. doi: 10.1016/j.cell.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans RL, Lau KR, Case RM. Structural and functional characterization of striated ducts isolated from the rabbit mandibular salivary gland. Exp Physiol 78: 49–64, 1993. doi: 10.1113/expphysiol.1993.sp003670. [DOI] [PubMed] [Google Scholar]
- 84.Tandler B, Gresik EW, Nagato T, Phillips CJ. Secretion by striated ducts of mammalian major salivary glands: review from an ultrastructural, functional, and evolutionary perspective. Anat Rec 264: 121–145, 2001. doi: 10.1002/ar.1108. [DOI] [PubMed] [Google Scholar]
- 85.Denny PC, Ball WD, Redman RS. Salivary glands: a paradigm for diversity of gland development. Crit Rev Oral Biol Med 8: 51–75, 1997. doi: 10.1177/10454411970080010301. [DOI] [PubMed] [Google Scholar]
- 86.Tandler B, Phillips CJ. Organic secretion by striated ducts. Eur J Morphol 38: 233–236, 2000. doi: 10.1076/0924-3860(200010)38:4;1-o;ft233. [DOI] [PubMed] [Google Scholar]
- 87.Isola M, Cabras T, Inzitari R, Lantini MS, Proto E, Cossu M, Riva A. Electron microscopic detection of statherin in secretory granules of human major salivary glands. J Anat 212: 664–668, 2008. doi: 10.1111/j.1469-7580.2008.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Proctor GB. The physiology of salivary secretion. Periodontol 2000 70: 11–25, 2016. doi: 10.1111/prd.12116. [DOI] [PubMed] [Google Scholar]
- 89.Tandler B, Pinkstaff CA, Phillips CJ. Interlobular excretory ducts of mammalian salivary glands: structural and histochemical review. Anat Rec A Discov Mol Cell Evol Biol 288: 498–526, 2006. doi: 10.1002/ar.a.20319. [DOI] [PubMed] [Google Scholar]
- 90.Catalán MA, Nakamoto T, Melvin JE. The salivary gland fluid secretion mechanism. J Med Invest 56, Suppl: 192–196, 2009. doi: 10.2152/jmi.56.192. [DOI] [PubMed] [Google Scholar]
- 91.Bullard T, Koek L, Roztocil E, Kingsley PD, Mirels L, Ovitt CE. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev Biol 320: 72–78, 2008. doi: 10.1016/j.ydbio.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nelson DA, Manhardt C, Kamath V, Sui Y, Santamaria-Pang A, Can A, Bello M, Corwin A, Dinn SR, Lazare M, Gervais EM, Sequeira SJ, Peters SB, Ginty F, Gerdes MJ, Larsen M. Quantitative single cell analysis of cell population dynamics during submandibular salivary gland development and differentiation. Biol Open 2: 439–447, 2013. doi: 10.1242/bio.20134309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Porcheri C, Mitsiadis TA. Physiology, pathology and regeneration of salivary glands. Cells 8: 976, 2019. doi: 10.3390/cells8090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Redman RS. Myoepithelium of salivary glands. Microsc Res Tech 27: 25–45, 1994. doi: 10.1002/jemt.1070270103. [DOI] [PubMed] [Google Scholar]
- 96.Makarenkova HP, Dartt DA. Myoepithelial cells: their origin and function in lacrimal gland morphogenesis, homeostasis, and repair. Curr Mol Biol Rep 1: 115–123, 2015. doi: 10.1007/s40610-015-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Emmelin N, Gjörstrup P. On the function of myoepithelial cells in salivary glands. J Physiol 230: 185–198, 1973. doi: 10.1113/jphysiol.1973.sp010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hawley D, Tang X, Zyrianova T, Shah M, Janga S, Letourneau A, Schicht M, Paulsen F, Hamm-Alvarez S, Makarenkova HP, Zoukhri D. Myoepithelial cell-driven acini contraction in response to oxytocin receptor stimulation is impaired in lacrimal glands of Sjogren’s syndrome animal models. Sci Rep 8: 9919, 2018. doi: 10.1038/s41598-018-28227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lung MA. Autonomic nervous control of myoepithelial cells and secretion in submandibular gland of anaesthetized dogs. J Physiol 546: 837–850, 2003. doi: 10.1113/jphysiol.2002.029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deugnier MA, Teulière J, Faraldo MM, Thiery JP, Glukhova MA. The importance of being a myoepithelial cell. Breast Cancer Res 4: 224–230, 2002. doi: 10.1186/bcr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardy G, Kramer B. The myoepithelium of human major salivary glands revisited. SADJ 53: 371–375, 1998. [PubMed] [Google Scholar]
- 102.Gudjonsson T, Ronnøv-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115: 39–50, 2002. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adriance MC, Inman JL, Petersen OW, Bissell MJ. Myoepithelial cells: good fences make good neighbors. Breast Cancer Res 7: 190–197, 2005. doi: 10.1186/bcr1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emmelin N, Garrett JR, Ohlin P. Neural control of salivary myoepithelial cells. J Physiol 196: 381–396, 1968. doi: 10.1113/jphysiol.1968.sp008513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shah AA, Mulla AF, Mayank M. Pathophysiology of myoepithelial cells in salivary glands. J Oral Maxillofac Pathol 20: 480–490, 2016. doi: 10.4103/0973-029X.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ninche N, Kwak M, Ghazizadeh S. Diverse epithelial cell populations contribute to the regeneration of secretory units in injured salivary glands. Development 147: dev192807, 2020. doi: 10.1242/dev.192807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abou-Foul AK. Surgical anatomy of the lymphatic drainage of the salivary glands: a systematic review. J Laryngol Otol 2020: 1–7, 2020. doi: 10.1017/S0022215120002054. [DOI] [PubMed] [Google Scholar]
- 109.Thom JT, Weber TC, Walton SM, Torti N, Oxenius A. The salivary gland acts as a sink for tissue-resident memory CD8+ T cells, facilitating protection from local cytomegalovirus infection. Cell Rep 13: 1125–1136, 2015. doi: 10.1016/j.celrep.2015.09.082. [DOI] [PubMed] [Google Scholar]
- 110.Brandtzaeg P. Secretory immunity with special reference to the oral cavity. J Oral Microbiol 2013: 5, 2013. doi: 10.3402/jom.v5i0.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aase A, Sommerfelt H, Petersen LB, Bolstad M, Cox RJ, Langeland N, Guttormsen AB, Steinsland H, Skrede S, Brandtzaeg P. Salivary IgA from the sublingual compartment as a novel noninvasive proxy for intestinal immune induction. Mucosal Immunol 9: 884–893, 2016. doi: 10.1038/mi.2015.107. [DOI] [PubMed] [Google Scholar]
- 112.Praktiknjo SD, Obermayer B, Zhu Q, Fang L, Liu H, Quinn H, Stoeckius M, Kocks C, Birchmeier W, Rajewsky N. Tracing tumorigenesis in a solid tumor model at single-cell resolution. Nat Commun 11 991, 2020. doi: 10.1038/s41467-020-14777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferreira JN, Hoffman MP. Interactions between developing nerves and salivary glands. Organogenesis 9: 199–205, 2013. doi: 10.4161/org.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci 133: 3–18, 2007. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 116.Ekström J. Autonomic control of salivary secretion. Proc Finn Dent Soc 85: 323–331, 1989. [PubMed] [Google Scholar]
- 117.Garrett JR, Kidd A. The innervation of salivary glands as revealed by morphological methods. Microsc Res Tech 26: 75–91, 1993. doi: 10.1002/jemt.1070260108. [DOI] [PubMed] [Google Scholar]
- 118.Borle RM, Jadhav A, Bhola N, Hingnikar P, Gaikwad P. Borle’s triangle: a reliable anatomical landmark for ease of identification of facial nerve trunk during parotidectomy. J Oral Biol Craniofac Res 9: 33–36, 2019. doi: 10.1016/j.jobcr.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Segal K, Lisnyansky I, Nageris B, Feinmesser R. Parasympathetic innervation of the salivary glands. Oper Tech Otolaryngol Head Neck Surg 7: 333–338, 1996. doi: 10.1016/S1043-1810(96)80005-4. [DOI] [Google Scholar]
- 120.Emmelin N. Nervous control of mammalian salivary glands. Philos Trans R Soc Lond B Biol Sci 296: 27–35, 1981. doi: 10.1098/rstb.1981.0168. [DOI] [PubMed] [Google Scholar]
- 121.Sato T, Mito K, Ishii H. Relationship between impaired parasympathetic vasodilation and hyposalivation in parotid glands associated with type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 318: R940–R949, 2020. doi: 10.1152/ajpregu.00016.2019. [DOI] [PubMed] [Google Scholar]
- 122.Ozdemir T, Srinivasan PP, Zakheim DR, Harrington DA, Witt RL, Farach-Carson MC, Jia X, Pradhan-Bhatt S. Bottom-up assembly of salivary gland microtissues for assessing myoepithelial cell function. Biomaterials 142: 124–135, 2017. doi: 10.1016/j.biomaterials.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schneyer CA, Humphreys-Beher M. Inhibitory effects of atropine and adrenergic antagonists on the changes in autonomic receptors and cyclic nucleotides of rat parotid and submandibular glands caused by sympathetic nerve stimulation. J Auton Nerv Syst 22: 23–30, 1988. doi: 10.1016/0165-1838(88)90150-6. [DOI] [PubMed] [Google Scholar]
- 124.Neyraud EH, C I, Bult J, Mesmin C, Dransfield E. Effects of different tastants on parotid saliva flow and composition. Chem Percept 2: 108–116, 2009. doi: 10.1007/s12078-009-9041-9. [DOI] [Google Scholar]
- 125.Pushpass RG, Daly B, Kelly C, Proctor G, Carpenter GH. Altered salivary flow, protein composition, and rheology following taste and TRP stimulation in older adults. Front Physiol 10: 652, 2019. doi: 10.3389/fphys.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Al-Manei K, Almotairy N, Bostanci N, Kumar A, Grigoriadis A. Effect of chewing on the expression of salivary protein composition: a systematic review. Proteomics Clin Appl 14: e1900039, 2020. doi: 10.1002/prca.201900039. [DOI] [PubMed] [Google Scholar]
- 127.Gjörstrup P. Amylase secretion in the rabbit parotid gland when stimulating the sympathetic nerves during parasympathetic activity. J Physiol 296: 443–451, 1979. doi: 10.1113/jphysiol.1979.sp013015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther 15: 2101–2106, 2007. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- 129.Kwon HR, Nelson DA, DeSantis KA, Morrissey JM, Larsen M. Endothelial cell regulation of salivary gland epithelial patterning. Development 144: 211–220, 2017. doi: 10.1242/dev.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Culp DJ, Zhang Z, Evans RL. Role of calcium and PKC in salivary mucous cell exocrine secretion. J Dent Res 90: 1469–1476, 2011. doi: 10.1177/0022034511422817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao H, Xu X, Diaz J, Muallem S. Na+, K+, and H+/HCO3− transport in submandibular salivary ducts. J Biol Chem 270: 19599–19605, 1995. doi: 10.1074/jbc.270.33.19599. [DOI] [PubMed] [Google Scholar]
- 132.Bragiel AM, Wang D, Pieczonka TD, Shono M, Ishikawa Y. Mechanisms underlying activation of alpha1-adrenergic receptor-induced trafficking of AQP5 in rat parotid acinar cells under isotonic or hypotonic conditions. Int J Mol Sci 17: 1022, 2016. doi: 10.3390/ijms17071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Catalán MA, Kondo Y, Peña-Munzenmayer G, Jaramillo Y, Liu F, Choi S, Crandall E, Borok Z, Flodby P, Shull GE, Melvin JE. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc Natl Acad Sci USA 112: 2263–2268, 2015. doi: 10.1073/pnas.1415739112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ishibashi K, Yamazaki J, Okamura K, Teng Y, Kitamura K, Abe K. Roles of CLCA and CFTR in electrolyte re-absorption from rat saliva. J Dent Res 85: 1101–1105, 2006. doi: 10.1177/154405910608501207. [DOI] [PubMed] [Google Scholar]
- 135.Mukaibo T, Munemasa T, George AT, Tran DT, Gao X, Herche JL, Masaki C, Shull GE, Soleimani M, Melvin JE. The apical anion exchanger Slc26a6 promotes oxalate secretion by murine submandibular gland acinar cells. J Biol Chem 293: 6259–6268, 2018. doi: 10.1074/jbc.RA118.002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zinn VZ, Khatri A, Mednieks MI, Hand AR. Localization of cystic fibrosis transmembrane conductance regulator signaling complexes in human salivary gland striated duct cells. Eur J Oral Sci 123: 140–148, 2015. doi: 10.1111/eos.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Munemasa T, Mukaibo T, Melvin JE. Slc26a6 is an apical membrane anion exchanger that drives HCO3(-)-dependent fluid secretion in murine pancreatic acinar cells. Am J Physiol Cell Physiol 317: C1153–C1160, 2019. doi: 10.1152/ajpcell.00257.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang NY, Mukaibo T, Kurtz I, Melvin JE. The apical Na+-HCO3- cotransporter Slc4a7 (NBCn1) does not contribute to bicarbonate transport by mouse salivary gland ducts. J Cell Physiol 9: 16376–16388, 2019. doi: 10.1002/jcp.28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Richins CA, Kuntz A. Role of sympathetic nerves in the regulation of salivary secretion. Am J Physiol 173: 470–473, 1953. doi: 10.1152/ajplegacy.1953.173.3.471. [DOI] [PubMed] [Google Scholar]
- 140.Culp DJ, Graham LA, Latchney LR, Hand AR. Rat sublingual gland as a model to study glandular mucous cell secretion. Am J Physiol Cell Physiol 260: C1233–C1244, 1991. doi: 10.1152/ajpcell.1991.260.6.C1233. [DOI] [PubMed] [Google Scholar]
- 141.Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol 66: 260–267, 2004. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K. M3 muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol 558: 561–575, 2004. doi: 10.1113/jphysiol.2004.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ryberg AT, Warfvinge G, Axelsson L, Soukup O, Götrick B, Tobin G. Expression of muscarinic receptor subtypes in salivary glands of rats, sheep and man. Arch Oral Biol 53: 66–74, 2008. doi: 10.1016/j.archoralbio.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 144.Beroukas D, Goodfellow R, Hiscock J, Jonsson R, Gordon TP, Waterman SA. Up-regulation of M3-muscarinic receptors in labial salivary gland acini in primary Sjogren’s syndrome. Lab Invest 82: 203–210, 2002. doi: 10.1038/labinvest.3780412. [DOI] [PubMed] [Google Scholar]
- 145.Ambudkar I. Calcium signaling defects underlying salivary gland dysfunction. Biochim Biophys Acta Mol Cell Res 1865: 1771–1777, 2018. doi: 10.1016/j.bbamcr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 146.Ambudkar IS. Calcium signalling in salivary gland physiology and dysfunction. J Physiol 594: 2813–2824, 2016. doi: 10.1113/JP271143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo J, Busillo JM, Benovic JL. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol Pharmacol 74: 338–347, 2008. doi: 10.1124/mol.107.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luo W, Latchney LR, Culp DJ. G protein coupling to M1 and M3 muscarinic receptors in sublingual glands. Am J Physiol Cell Physiol 280: C884–C896, 2001. doi: 10.1152/ajpcell.2001.280.4.C884. [DOI] [PubMed] [Google Scholar]
- 149.Seagrave JC, Barker S, Martinez AM, Martinez JR. Muscarinic signaling pathway in submandibular cells of adult and early postnatal rats. Proc Soc Exp Biol Med 203: 490–500, 1993. doi: 10.3181/00379727-203-43628. [DOI] [PubMed] [Google Scholar]
- 150.Tovey SC, Taylor CW. Cyclic AMP directs inositol (1,4,5)-trisphosphate-evoked Ca2+ signalling to different intracellular Ca2+ stores. J Cell Sci 126: 2305–2313, 2013. doi: 10.1242/jcs.126144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin AL, Zhu B, Zhang W, Dang H, Zhang BX, Katz MS, Yeh CK. Distinct pathways of ERK activation by the muscarinic agonists pilocarpine and carbachol in a human salivary cell line. Am J Physiol Cell Physiol 294: C1454–C1464, 2008. doi: 10.1152/ajpcell.00151.2007. [DOI] [PubMed] [Google Scholar]
- 152.Carpenter GH, Proctor GB, Ebersole LE, Garrett JR. Secretion of IgA by rat parotid and submandibular cells in response to autonomimetic stimulation in vitro. Int Immunopharmacol 4: 1005–1014, 2004. doi: 10.1016/j.intimp.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 153.Anderson LC, Garrett JR, Zhang X, Proctor GB, Shori DK. Differential secretion of proteins by rat submandibular acini and granular ducts on graded autonomic nerve stimulations. J Physiol 485: 503–511, 1995. doi: 10.1113/jphysiol.1995.sp020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Warren CM, van den Brink RL, Nieuwenhuis S, Bosch JA. Norepinephrine transporter blocker atomoxetine increases salivary alpha amylase. Psychoneuroendocrinology 78: 233–236, 2017. doi: 10.1016/j.psyneuen.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 155.Abe K, Yoneda K, Fujita R, Yokota Y, Dawes C. The effects of epinephrine, norepinephrine, and phenylephrine on the types of proteins secreted by rat salivary glands. J Dent Res 59: 1627–1634, 1980. doi: 10.1177/00220345800590101201. [DOI] [PubMed] [Google Scholar]
- 156.Elverdin JC, Chiarenza AP, Luchelli MA, Vatta M, Bianciotti LG, Boyer P, Vacas MI. Protein free diet feeding: effects on sympathetic activity and salivary evoked secretion in the submandibular gland of the rat. Arch Oral Biol 51: 621–628, 2006. doi: 10.1016/j.archoralbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 157.Kuebler U, von Känel R, Heimgartner N, Zuccarella-Hackl C, Stirnimann G, Ehlert U, Wirtz PH. Norepinephrine infusion with and without alpha-adrenergic blockade by phentolamine increases salivary alpha amylase in healthy men. Psychoneuroendocrinology 49: 290–298, 2014. doi: 10.1016/j.psyneuen.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 158.Kondo Y, Melvin JE, Catalan MA. Physiological cAMP-elevating secretagogues differentially regulate fluid and protein secretions in mouse submandibular and sublingual glands. Am J Physiol Cell Physiol 316: C690–C697, 2019. doi: 10.1152/ajpcell.00421.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wallace LJ, Partlow LM. alpha-Adrenergic regulation of secretion of mouse saliva rich in nerve growth factor. Proc Natl Acad Sci USA 73: 4210–4214, 1976. doi: 10.1073/pnas.73.11.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Edwards AV, Titchen DA. Autonomic control of protein production by the parotid gland of the sheep. Auton Neurosci 103: 38–49, 2003. doi: 10.1016/S1566-0702(02)00147-9. [DOI] [PubMed] [Google Scholar]
- 161.Kaniucki MD, Stefano FJ, Perec CJ. Clonidine inhibits salivary secretion by activation of postsynaptic alpha 2-receptors. Naunyn Schmiedebergs Arch Pharmacol 326: 313–316, 1984. doi: 10.1007/BF00501435. [DOI] [PubMed] [Google Scholar]
- 162.dos Santos Moreira T, Takakura AC, De Luca LA Jr, Renzi A, Menani JV. Inhibition of pilocarpine-induced salivation in rats by central noradrenaline. Arch Oral Biol 47: 429–434, 2002. doi: 10.1016/S0003-9969(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 163.Takakura AC, dos Santos Moreira TA, De Luca LA Jr, Renzi A, Menani JV. Central α2 adrenergic receptors and cholinergic-induced salivation in rats. Brain Res Bull 59: 383–386, 2003. doi: 10.1016/S0361-9230(02)00929-2. [DOI] [PubMed] [Google Scholar]
- 164.Hein P, Michel MC. Signal transduction and regulation: are all alpha1-adrenergic receptor subtypes created equal? Biochem Pharmacol 73: 1097–1106, 2007. doi: 10.1016/j.bcp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 165.Gyires K, Zádori ZS, Török T, Matyus P. alpha2-Adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochem Int 55: 447–453, 2009. doi: 10.1016/j.neuint.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 166.Imbery JF, Bhattacharya S, Khuder S, Weiss A, Goswamee P, Iqbal AK, Giovannucci DR. cAMP-dependent recruitment of acidic organelles for Ca2+ signaling in the salivary gland. Am J Physiol Cell Physiol 311: C697–C709, 2016. doi: 10.1152/ajpcell.00010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Renzi A, Colombari E, Mattos Filho TR, Silveira JE, Saad WA, Camargo LA, de Luca LA Jr, Deróbio JG, Menani JV. Involvement of the central nervous system in the salivary secretion induced by pilocarpine in rats. J Dent Res 72: 1481–1484, 1993. doi: 10.1177/00220345930720110401. [DOI] [PubMed] [Google Scholar]
- 168.Borella TL, De Luca LA Jr, Colombari DS, Menani JV. Central muscarinic receptor subtypes involved in pilocarpine-induced salivation, hypertension and water intake. Br J Pharmacol 155: 1256–1263, 2008. doi: 10.1038/bjp.2008.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moreira TS, Takakura AC, Menani JV, Colombari E. Activation of central alpha2-adrenoceptors mediates salivary gland vasoconstriction. Arch Oral Biol 58: 167–173, 2013. doi: 10.1016/j.archoralbio.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 170.McPherson MA, Hales CN. Control of amylase biosynthesis and release in the parotid gland of the rat. Biochem J 176: 855–863, 1978. doi: 10.1042/bj1760855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Del Fiacco M, Quartu M, Ekström J, Melis T, Boi M, Isola M, Loy F, Serra MP. Effect of the neuropeptides vasoactive intestinal peptide, peptide histidine methionine and substance P on human major salivary gland secretion. Oral Dis 21: 216–223, 2015. doi: 10.1111/odi.12249. [DOI] [PubMed] [Google Scholar]
- 172.Ekström J, Godoy T, Loy F, Riva A. Parasympathetic vasoactive intestinal peptide (VIP): a likely contributor to clozapine-induced sialorrhoea. Oral Dis 20: e90–e96, 2014. doi: 10.1111/odi.12139. [DOI] [PubMed] [Google Scholar]
- 173.Larsson O, Dunér-Engström M, Lundberg JM, Fredholm BB, Änggård A. Effects of VIP, PHM and substance P on blood vessels and secretory elements of the human submandibular gland. Regul Pept 13: 319–326, 1986. doi: 10.1016/0167-0115(86)90049-2. [DOI] [PubMed] [Google Scholar]
- 174.Bobyock E, Chernick WS. Vasoactive intestinal peptide interacts with alpha-adrenergic-, cholinergic-, and substance-P-mediated responses in rat parotid and submandibular glands. J Dent Res 68: 1489–1494, 1989. doi: 10.1177/00220345890680110401. [DOI] [PubMed] [Google Scholar]
- 175.Kasai H, Augustine GJ. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature 348: 735–738, 1990. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- 176.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309: 2232–2234, 2005. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 177.Lee KP, Choi S, Hong JH, Ahuja M, Graham S, Ma R, So I, Shin DM, Muallem S, Yuan JP. Molecular determinants mediating gating of transient receptor potential canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). J Biol Chem 289: 6372–6382, 2014. doi: 10.1074/jbc.M113.546556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology 137: 1509–1517, 2009. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1-/- mice. Proc Natl Acad Sci USA 104: 17542–17547, 2007. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu X, Ong HL, Ambudkar I. TRP channel involvement in salivary glands—some good, some bad. Cells 7: 74, 2018. doi: 10.3390/cells7070074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell 32: 439–448, 2008. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem 283: 12935–12940, 2008. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol 9: e1001025, 2011. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ong HL, de Souza LB, Zheng C, Cheng KT, Liu X, Goldsmith CM, Feske S, Ambudkar IS. STIM2 enhances receptor-stimulated Ca2+ signaling by promoting recruitment of STIM1 to the endoplasmic reticulum-plasma membrane junctions. Sci Signal 8: ra3, 2015. doi: 10.1126/scisignal.2005748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Son GY, Subedi KP, Ong HL, Noyer L, Saadi H, Zheng C, Bhardwaj R, Feske S, Ambudkar IS. STIM2 targets Orai1/STIM1 to the AKAP79 signaling complex and confers coupling of Ca2+ entry with NFAT1 activation. Proc Natl Acad Sci USA 117: 16638–16648, 2020. doi: 10.1073/pnas.1915386117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233, 2006. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 188.Xing J, Petranka JG, Davis FM, Desai PN, Putney JW, Bird GS. Role of Orai1 and store-operated calcium entry in mouse lacrimal gland signalling and function. J Physiol 592: 927–939, 2014. doi: 10.1113/jphysiol.2013.267740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohba T, Watanabe H, Murakami M, Iino K, Adachi T, Baba Y, Kurosaki T, Ono K, Ito H. Stromal interaction molecule 1 haploinsufficiency causes maladaptive response to pressure overload. PLoS One 12: e0187950, 2017. doi: 10.1371/journal.pone.0187950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mancarella S, Potireddy S, Wang Y, Gao H, Gandhirajan RK, Autieri M, Scalia R, Cheng Z, Wang H, Madesh M, Houser SR, Gill DL. Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J 27: 893–906, 2013. doi: 10.1096/fj.12-215293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Oh-Hora M, Lu X. Function of Orai/Stim Proteins Studied in Transgenic Animal Models. In: Calcium Entry Channels in Non-Excitable Cells, edited by Kozak JA, Putney JW Jr. Boca Raton, FL: CRC Press, 2018, p. 107–126. [Google Scholar]
- 192.Liu X, Gong B, de Souza LB, Ong HL, Subedi KP, Cheng KT, Swaim W, Zheng C, Mori Y, Ambudkar IS. Radiation inhibits salivary gland function by promoting STIM1 cleavage by caspase-3 and loss of SOCE through a TRPM2-dependent pathway. Sci Signal 10: eaal4064, 2017. doi: 10.1126/scisignal.aal4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl- cotransporter (NKCC1)-deficient mice. J Biol Chem 275: 26720–26726, 2000. doi: 10.1016/S0021-9258(19)61435-3. [DOI] [PubMed] [Google Scholar]
- 194.Peña-Münzenmayer G, George AT, Shull GE, Melvin JE, Catalan MA. Ae4 (Slc4a9) is an electroneutral monovalent cation-dependent Cl-/HCO3- exchanger. J Gen Physiol 147: 423–436, 2016. doi: 10.1085/jgp.201611571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peña-Münzenmayer G, Catalán MA, Kondo Y, Jaramillo Y, Liu F, Shull GE, Melvin JE. Ae4 (Slc4a9) anion exchanger drives Cl- uptake-dependent fluid secretion by mouse submandibular gland acinar cells. J Biol Chem 290: 10677–10688, 2015. doi: 10.1074/jbc.M114.612895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Park K, Evans RL, Watson GE, Nehrke K, Richardson L, Bell SM, Schultheis PJ, Hand AR, Shull GE, Melvin JE. Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J Biol Chem 276: 27042–27050, 2001. doi: 10.1074/jbc.M102901200. [DOI] [PubMed] [Google Scholar]
- 197.Jin X, Shah S, Du X, Zhang H, Gamper N. Activation of Ca2+-activated Cl- channel ANO1 by localized Ca2+ signals. J Physiol 594: 19–30, 2016. doi: 10.1113/jphysiol.2014.275107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sun Y, Birnbaumer L, Singh BB. TRPC1 regulates calcium-activated chloride channels in salivary gland cells. J Cell Physiol 230: 2848–2856, 2015. doi: 10.1002/jcp.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Derouiche S, Takayama Y, Murakami M, Tominaga M. TRPV4 heats up ANO1-dependent exocrine gland fluid secretion. FASEB J 32: 1841–1854, 2018. doi: 10.1096/fj.201700954R. [DOI] [PubMed] [Google Scholar]
- 200.Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol 284: C535–C546, 2003. doi: 10.1152/ajpcell.00044.2002. [DOI] [PubMed] [Google Scholar]
- 201.Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T. Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem 281: 27964–27972, 2006. doi: 10.1074/jbc.M603871200. [DOI] [PubMed] [Google Scholar]
- 202.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem 279: 47681–47687, 2004. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 203.Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol 294: C810–C819, 2008. doi: 10.1152/ajpcell.00511.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ohana E. Transepithelial ion transport across duct cells of the salivary gland. Oral Dis 21: 826–835, 2015. doi: 10.1111/odi.12201. [DOI] [PubMed] [Google Scholar]
- 205.Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121: 956–965, 2011. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zeng M, Szymczak M, Ahuja M, Zheng C, Yin H, Swaim W, Chiorini JA, Bridges RJ, Muallem S. Restoration of CFTR activity in ducts rescues acinar cell function and reduces inflammation in pancreatic and salivary glands of mice. Gastroenterology 153: 1148–1159, 2017. doi: 10.1053/j.gastro.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Romanenko VG, Nakamoto T, Catalán MA, Gonzalez-Begne M, Schwartz GJ, Jaramillo Y, Sepúlveda FV, Figueroa CD, Melvin JE. Clcn2 encodes the hyperpolarization-activated chloride channel in the ducts of mouse salivary glands. Am J Physiol Gastrointest Liver Physiol 295: G1058–G1067, 2008. doi: 10.1152/ajpgi.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Helmerhorst EJ, Oppenheim FG. Saliva: a dynamic proteome. J Dent Res 86: 680–693, 2007. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 209.Lau WW, Hardt M, Zhang YH, Freire M, Ruhl S. The Human Salivary Proteome Wiki: a community-driven research platform. J Dent Res 100: 1510–1519, 2021. doi: 10.1177/00220345211014432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tabak LA. The role of mucin-type O-glycans in eukaryotic development. Semin Cell Dev Biol 21: 616–621, 2010. doi: 10.1016/j.semcdb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tabak LA. Structure and function of human salivary mucins. Crit Rev Oral Biol Med 1: 229–234, 1990. doi: 10.1177/10454411900010040201. [DOI] [PubMed] [Google Scholar]
- 212.Das B, Cash MN, Hand AR, Shivazad A, Grieshaber SS, Robinson B, Culp DJ. Tissue distribution of murine Muc19/smgc gene products. J Histochem Cytochem 58: 141–156, 2010. doi: 10.1369/jhc.2009.954891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zalewska A, Zwierz K, Zółkowski K, Gindzieński A. Structure and biosynthesis of human salivary mucins. Acta Biochim Pol 47: 1067–1079, 2000. doi: 10.18388/abp.2000_3960. [DOI] [PubMed] [Google Scholar]
- 214.Cross BW, Ruhl S. Glycan recognition at the saliva-oral microbiome interface. Cell Immunol 333: 19–33, 2018. doi: 10.1016/j.cellimm.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Castro I, Sepúlveda D, Cortés J, Quest AF, Barrera MJ, Bahamondes V, Aguilera S, Urzúa U, Alliende C, Molina C, González S, Hermoso MA, Leyton C, González MJ. Oral dryness in Sjogren’s syndrome patients. Not just a question of water. Autoimmun Rev 12: 567–574, 2013. doi: 10.1016/j.autrev.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 216.Coles JM, Zhang L, Blum JJ, Warman ML, Jay GD, Guilak F, Zauscher S. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum 62: 1666–1674, 2010. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chaudhury NM, Shirlaw P, Pramanik R, Carpenter GH, Proctor GB. Changes in saliva rheological properties and mucin glycosylation in dry mouth. J Dent Res 94: 1660–1667, 2015. doi: 10.1177/0022034515609070. [DOI] [PubMed] [Google Scholar]
- 218.Chawhuaveang DD, Yu OY, Yin IX, Lam WY, Mei ML, Chu CH. Acquired salivary pellicle and oral diseases: a literature review. J Dent Sci 16: 523–529, 2021. doi: 10.1016/j.jds.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Malamud D, Abrams WR, Barber CA, Weissman D, Rehtanz M, Golub E. Antiviral activities in human saliva. Adv Dent Res 23: 34–37, 2011. doi: 10.1177/0022034511399282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Peluso G, Tian E, Abusleme L, Munemasa T, Mukaibo T, Ten Hagen KG. Loss of the disease-associated glycosyltransferase Galnt3 alters Muc10 glycosylation and the composition of the oral microbiome. J Biol Chem 295: 1411–1425, 2020. doi: 10.1074/jbc.RA119.009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hay DI, Smith DJ, Schluckebier SK, Moreno EC. Relationship between concentration of human salivary statherin and inhibition of calcium phosphate precipitation in stimulated human parotid saliva. J Dent Res 63: 857–863, 1984. doi: 10.1177/00220345840630060901. [DOI] [PubMed] [Google Scholar]
- 222.Hong Z, Chasan B, Bansil R, Turner BS, Bhaskar KR, Afdhal NH. Atomic force microscopy reveals aggregation of gastric mucin at low pH. Biomacromolecules 6: 3458–3466, 2005. doi: 10.1021/bm0505843. [DOI] [PubMed] [Google Scholar]
- 223.Winter C, Keimel R, Gugatschka M, Kolb D, Leitinger G, Roblegg E. Investigation of changes in saliva in radiotherapy-induced head neck cancer patients. Int J Environ Res Public Health 18: 1629, 2021. doi: 10.3390/ijerph18041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Alliende C, Kwon YJ, Brito M, Molina C, Aguilera S, Pérez P, Leyton L, Quest AF, Mandel U, Veerman E, Espinosa M, Clausen H, Leyton C, Romo R, González MJ. Reduced sulfation of muc5b is linked to xerostomia in patients with Sjogren syndrome. Ann Rheum Dis 67: 1480–1487, 2008. doi: 10.1136/ard.2007.078246. [DOI] [PubMed] [Google Scholar]
- 225.Iglesias-Bartolome R, Uchiyama A, Molinolo AA, Abusleme L, Brooks SR, Callejas-Valera JL, Edwards D, Doci C, Asselin-Labat ML, Onaitis MW, Moutsopoulos NM, Gutkind JS, Morasso MI. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med 10: eaap8798, 2018. doi: 10.1126/scitranslmed.aap8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brand HS, Veerman EC. Saliva and wound healing. Chin J Dent Res 16: 7–12, 2013. [PubMed] [Google Scholar]
- 227.Cohen S, Levi-Montalcini R. A nerve growth-stimulating factor isolated from snake venom. Proc Natl Acad Sci USA 42: 571–574, 1956. doi: 10.1073/pnas.42.9.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev 48: 534–569, 1968. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- 229.Oudhoff MJ, Bolscher JG, Nazmi K, Kalay H, van ’t Hof W, Amerongen AV, Veerman EC. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB j 22: 3805–3812, 2008. doi: 10.1096/fj.08-112003. [DOI] [PubMed] [Google Scholar]
- 230.Gardner A, Carpenter G, So PW. salivary metabolomics: from diagnostic biomarker discovery to investigating biological function. Metabolites 10: 47, 2020. doi: 10.3390/metabo10020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ishikawa S, Sugimoto M, Kitabatake K, Sugano A, Nakamura M, Kaneko M, Ota S, Hiwatari K, Enomoto A, Soga T, Tomita M, Iino M. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci Rep 6: 31520, 2016. doi: 10.1038/srep31520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang A, Sun H, Wang X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl Biochem Biotechnol 168: 1718–1727, 2012. doi: 10.1007/s12010-012-9891-5. [DOI] [PubMed] [Google Scholar]
- 233.Tabak LA. Point-of-care diagnostics enter the mouth. Ann NY Acad Sci 1098: 7–14, 2007. doi: 10.1196/annals.1384.043. [DOI] [PubMed] [Google Scholar]
- 234.Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DT. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev 26: 781–791, 2013. doi: 10.1128/CMR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jasim H, Carlsson A, Hedenberg-Magnusson B, Ghafouri B, Ernberg M. Saliva as a medium to detect and measure biomarkers related to pain. Sci Rep 8: 3220, 2018. doi: 10.1038/s41598-018-21131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P, Xu X, Zhou XD. Saliva in the diagnosis of diseases. Int J Oral Sci 8: 133–137, 2016. doi: 10.1038/ijos.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. Crit Rev Oral Biol Med 13: 197–212, 2002. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 238.Pereira JA, Porto-Figueira P, Taware R, Sukul P, Rapole S, Camara JS. Unravelling the potential of salivary volatile metabolites in oral diseases. A review. Molecules 25: 3098, 2020. doi: 10.3390/molecules25133098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19: 155–170, 2021. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhu J, Guo J, Xu Y, Chen X. Viral dynamics of SARS-CoV-2 in saliva from infected patients. J Infect 81: e48–e50, 2020. doi: 10.1016/j.jinf.2020.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, et al. . Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol 5: eabe5511, 2020. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Barskey AE, Juieng P, Whitaker BL, Erdman DD, Oberste MS, Chern SW, Schmid DS, Radford KW, McNall RJ, Rota PA, Hickman CJ, Bellini WJ, Wallace GS. Viruses detected among sporadic cases of parotitis, United States, 2009-2011. J Infect Dis 208: 1979–1986, 2013. doi: 10.1093/infdis/jit408. [DOI] [PubMed] [Google Scholar]
- 244.Jensen SB, Vissink A, Limesand KH, Reyland ME. Salivary gland hypofunction and xerostomia in head and neck radiation patients. J Natl Cancer Inst Monogr 2019: lgz016, 2019. doi: 10.1093/jncimonographs/lgz016. [DOI] [PubMed] [Google Scholar]
- 245.Rocchi C, Emmerson E. Mouth-watering results: clinical need, current approaches, and future directions for salivary gland regeneration. Trends Mol Med 26: 649–669, 2020. doi: 10.1016/j.molmed.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 246.Mortazavi H, Baharvand M, Movahhedian A, Mohammadi M, Khodadoustan A. Xerostomia due to systemic disease: a review of 20 conditions and mechanisms. Ann Med Health Sci Res 4: 503–510, 2014. doi: 10.4103/2141-9248.139284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.de Carvalho HN, Dos Santos YL, Bernardino IM, de Lima KC, Granville-Garcia AF, Melo de Brito Costa EM. Accuracy of a questionnaire on xerostomia as a screening tool for hyposalivation. Int Dent J 70: 427–434, 2020. doi: 10.1111/idj.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fallon BS, Chase TJ, Cooke EM, Ghazitabatabaei A, Naylor NO, Cutshall JJ, Trump BG, Weller ML. The use of BokaFlo instrument to measure salivary flow. BMC Oral Health 21: 191, 2021. doi: 10.1186/s12903-021-01477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Babu V, Hegde S, Bhat S, Sargod S. Evaluation of efficacy of three different commercially available kit for chairside cariogenic bacteria test—caries risk test, Saliva-check Mutans and CariScreen. Cureus 11: e6504, 2019. doi: 10.7759/cureus.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Murray Thomson W, Poulton R, Mark Broadbent J, Al-Kubaisy S. Xerostomia and medications among 32-year-olds. Acta Odontol Scand 64: 249–254, 2006. doi: 10.1080/00016350600633243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wolff A, Joshi RK, Ekström J, Aframian D, Pedersen AM, Proctor G, Narayana N, Villa A, Sia YW, Aliko A, McGowan R, Kerr AR, Jensen SB, Vissink A, Dawes CA. Guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: a systematic review sponsored by the World Workshop on Oral Medicine VI. Drugs R D 17: 1–28, 2017. doi: 10.1007/s40268-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zlotnik Y, Balash Y, Korczyn AD, Giladi N, Gurevich T. Disorders of the oral cavity in Parkinson's disease and parkinsonian syndromes. Parkinsons Dis 2015: 379482, 2015. doi: 10.1155/2015/379482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shetty SR, Bhowmick S, Castelino R, Babu S. Drug induced xerostomia in elderly individuals: an institutional study. Contemp Clin Dent 3: 173–175, 2012. doi: 10.4103/0976-237X.96821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Barbe AG. Medication-induced xerostomia and hyposalivation in the elderly: culprits, complications, and management. Drugs Aging 35: 877–885, 2018. doi: 10.1007/s40266-018-0588-5. [DOI] [PubMed] [Google Scholar]
- 255.Bossola M, Tazza L. Xerostomia in patients on chronic hemodialysis. Nat Rev Nephrol 8: 176–182, 2012. doi: 10.1038/nrneph.2011.218. [DOI] [PubMed] [Google Scholar]
- 256.Tanasiewicz M, Hildebrandt T, Obersztyn I. Xerostomia of various etiologies: a review of the literature. Adv Clin Exp Med 25: 199–206, 2016. doi: 10.17219/acem/29375. [DOI] [PubMed] [Google Scholar]
- 257.Wu AJ, Baum BJ, Ship JA. Extended stimulated parotid and submandibular secretion in a healthy young and old population. J Gerontol A Biol Sci Med Sci 50A: M45–M48, 1995. doi: 10.1093/gerona/50A.1.M45. [DOI] [PubMed] [Google Scholar]
- 258.Fathi H, Hoseini EG, Atoof F, Mottaghi R. Xerostomia (dry mouth) in patients with COVID-19: a case series. Future Virol 2021: 10.2217/fvl-2020-0334, 2021. doi: 10.2217/fvl-2020-0334. [DOI] [Google Scholar]
- 259.Kaae JK, Johnsen L, Hansen CR, Kristensen MH, Brink C, Eriksen JG. Relationship between patient and physician-rated xerostomia and dose distribution to the oral cavity and salivary glands for head and neck cancer patients after radiotherapy. Acta Oncol 58: 1366–1372, 2019. doi: 10.1080/0284186X.2019.1627413. [DOI] [PubMed] [Google Scholar]
- 260.Entesarian M, Dahlqvist J, Shashi V, Stanley CS, Falahat B, Reardon W, Dahl N. FGF10 missense mutations in aplasia of lacrimal and salivary glands (ALSG). Eur J Hum Genet 15: 379–382, 2007. doi: 10.1038/sj.ejhg.5201762. [DOI] [PubMed] [Google Scholar]
- 261.Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI, Jonsson R, Wahren-Herlenius M, Dahl N. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet 37: 125–127, 2005. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- 262.Milunsky JM, Zhao G, Maher TA, Colby R, Everman DB. LADD syndrome is caused by FGF10 mutations. Clin Genet 69: 349–354, 2006. doi: 10.1111/j.1399-0004.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 263.Bamforth JS, Kaurah P. Lacrimo-auriculo-dento-digital syndrome: evidence for lower limb involvement and severe congenital renal anomalies. Am J Med Genet 43: 932–937, 1992. doi: 10.1002/ajmg.1320430605. [DOI] [PubMed] [Google Scholar]
- 264.Shams I, Rohmann E, Eswarakumar VP, Lew ED, Yuzawa S, Wollnik B, Schlessinger J, Lax I. Lacrimo-auriculo-dento-digital syndrome is caused by reduced activity of the fibroblast growth factor 10 (FGF10)-FGF receptor 2 signaling pathway. Mol Cell Biol 27: 6903–6912, 2007. doi: 10.1128/MCB.00544-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rohmann E, Brunner HG, Kayserili H, Uyguner O, Nürnberg G, Lew ED, Dobbie A, Eswarakumar VP, Uzumcu A, Ulubil-Emeroglu M, Leroy JG, Li Y, Becker C, Lehnerdt K, Cremers CW, Yüksel-Apak M, Nürnberg P, Kubisch C, Kubisch C, Schlessinger J, van Bokhoven H, Wollnik B. Mutations in different components of FGF signaling in LADD syndrome. Nat Genet 38: 414–417, 2006. doi: 10.1038/ng1757. [DOI] [PubMed] [Google Scholar]
- 266.Jaskoll T, Abichaker G, Witcher D, Sala FG, Bellusci S, Hajihosseini MK, Melnick M. FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC Dev Biol 5: 11, 2005. doi: 10.1186/1471-213X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.May AJ, Chatzeli L, Proctor GB, Tucker AS. Salivary gland dysplasia in Fgf10 heterozygous mice: a new mouse model of xerostomia. Curr Mol Med 15: 674–682, 2015. doi: 10.2174/1566524015666150831141307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wilkie AO, Slaney SF, Oldridge M, Poole MD, Ashworth GJ, Hockley AD, Hayward RD, David DJ, Pulleyn LJ, Rutland P. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat Genet 9: 165–172, 1995. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]
- 269.Anderson J, Burns HD, Enriquez-Harris P, Wilkie AO, Heath JK. Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Hum Mol Genet 7: 1475–1483, 1998. doi: 10.1093/hmg/7.9.1475. [DOI] [PubMed] [Google Scholar]
- 270.Yamaji K, Morita J, Watanabe T, Gunjigake K, Nakatomi M, Shiga M, Ono K, Moriyama K, Kawamoto T. Maldevelopment of the submandibular gland in a mouse model of apert syndrome. Dev Dyn 247: 1175–1185, 2018. doi: 10.1002/dvdy.24673. [DOI] [PubMed] [Google Scholar]
- 271.Watanabe T, Kometani-Gunjigake K, Nakao-Kuroishi K, Ito-Sago M, Mizuhara M, Iwata D, Moriyama K, Ono K, Kawamoto T. A Ser252Trp substitution in mouse FGFR2 results in hyperplasia of embryonic salivary gland parenchyma. J Oral Biosci 63: 184–191, 2021. doi: 10.1016/j.job.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 272.Nordgarden H, Johannessen S, Storhaug K, Jensen JL. Salivary gland involvement in hypohidrotic ectodermal dysplasia. Oral Dis 4: 152–154, 1998. doi: 10.1111/j.1601-0825.1998.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 273.Mikkola ML. Molecular aspects of hypohidrotic ectodermal dysplasia. Am J Med Genet A 149A: 2031–2036, 2009. doi: 10.1002/ajmg.a.32855. [DOI] [PubMed] [Google Scholar]
- 274.Lexner MO, Bardow A, Hertz JM, Almer L, Nauntofte B, Kreiborg S. Whole saliva in X-linked hypohidrotic ectodermal dysplasia. Int J Paediatr Dent 17: 155–162, 2007. doi: 10.1111/j.1365-263X.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 275.Cluzeau C, Hadj-Rabia S, Jambou M, Mansour S, Guigue P, Masmoudi S, Bal E, Chassaing N, Vincent MC, Viot G, Clauss F, Manière MC, Toupenay S, Le Merrer M, Lyonnet S, Cormier-Daire V, Amiel J, Faivre L, de Prost Y, Munnich A, Bonnefont JP, Bodemer C, Smahi A. Only four genes (EDA1, EDAR, EDARADD, and WNT10A) account for 90% of hypohidrotic/anhidrotic ectodermal dysplasia cases. Hum Mutat 32: 70–72, 2011. doi: 10.1002/humu.21384. [DOI] [PubMed] [Google Scholar]
- 276.Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: GeneReviews, edited by Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mirzaa G, Amemiya A.. Seattle, WA: University of Washington, 1993. [PubMed] [Google Scholar]
- 277.Srivastava AK, Pispa J, Hartung AJ, Du Y, Ezer S, Jenks T, Shimada T, Pekkanen M, Mikkola ML, Ko MS, Thesleff I, Kere J, Schlessinger D. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci USA 94: 13069–13074, 1997. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet 22: 370–374, 1999. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- 279.Headon DJ, Emmal SA, Ferguson BM, Tucker AS, Justice MJ, Sharpe PT, Zonana J, Overbeek PA. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature 414: 913–916, 2001. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- 280.Häärä O, Fujimori S, Schmidt-Ullrich R, Hartmann C, Thesleff I, Mikkola ML. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development 138: 2681–2691, 2011. doi: 10.1242/dev.057711. [DOI] [PubMed] [Google Scholar]
- 281.Davies H, Bagg J, Goodchild MC, McPherson MA. Examination of submandibular fluid in cystic fibrosis. Acta Univ Carol Med (Praha) 36: 84–85, 1990. [PubMed] [Google Scholar]
- 282.Davies H, Bagg J, Goodchild MC, McPherson MA. Defective regulation of electrolyte and protein secretion in submandibular saliva of cystic fibrosis patients. Acta Paediatr Scand 80: 1094–1095, 1991. doi: 10.1111/j.1651-2227.1991.tb11789.x. [DOI] [PubMed] [Google Scholar]
- 283.Gonçalves AC, Marson FA, Mendonça RM, Ribeiro JD, Ribeiro AF, Paschoal IA, Levy CE. Saliva as a potential tool for cystic fibrosis diagnosis. Diagn Pathol 8: 46, 2013. doi: 10.1186/1746-1596-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Livnat G, Bentur L, Kuzmisnsky E, Nagler RM. Salivary profile and oxidative stress in children and adolescents with cystic fibrosis. J Oral Pathol Med 39: 16–21, 2010. doi: 10.1111/j.1600-0714.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 285.Goncalves AC, Marson FA, Mendonca RM, Bertuzzo CS, Paschoal IA, Ribeiro JD, Ribeiro AF, Levy CE. Chloride and sodium ion concentrations in saliva and sweat as a method to diagnose cystic fibrosis. J Pediatr (Rio J) 95: 443–450, 2019. doi: 10.1016/j.jped.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 286.Koumangoye R, Bastarache L, Delpire E. NKCC1: newly found as a human disease-causing ion transporter. Function (Oxf) 2: zqaa028, 2021. doi: 10.1093/function/zqaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mavragani CP, Moutsopoulos HM. Primary versus secondary Sjögren syndrome: is it time to reconsider these terms? J Rheumatol 46: 665–666, 2019. doi: 10.3899/jrheum.180392. [DOI] [PubMed] [Google Scholar]
- 288.Sene D, Jallouli M, Lefaucheur JP, Saadoun D, Costedoat-Chalumeau N, Maisonobe T, Diemert MC, Musset L, Haroche J, Piette JC, Amoura Z, Cacoub P. Peripheral neuropathies associated with primary Sjogren syndrome: immunologic profiles of nonataxic sensory neuropathy and sensorimotor neuropathy. Medicine (Baltimore) 90: 133–138, 2011. doi: 10.1097/MD.0b013e31820fd2d1. [DOI] [PubMed] [Google Scholar]
- 289.Brito-Zerón P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, Sivils K, Theander E, Tzioufas A, Ramos-Casals M. Sjögren syndrome. Nat Rev Dis Primers 2: 16047, 2016. doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 290.Carsons SE, Patel BC. Sjogren syndrome. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 291.Brandt JE, Priori R, Valesini G, Fairweather D. Sex differences in Sjogren’s syndrome: a comprehensive review of immune mechanisms. Biol Sex Differ 6: 19, 2015. doi: 10.1186/s13293-015-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Fox RI. Sjogren’s syndrome. Lancet 366: 321–331, 2005. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 293.Verstappen GM, Pringle S, Bootsma H, Kroese FG. Epithelial-immune cell interplay in primary Sjogren syndrome salivary gland pathogenesis. Nat Rev Rheumatol 17: 333–348, 2021. doi: 10.1038/s41584-021-00605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kroese FG, Abdulahad WH, Haacke E, Bos NA, Vissink A, Bootsma H. B-cell hyperactivity in primary Sjogren’s syndrome. Expert Rev Clin Immunol 10: 483–499, 2014. doi: 10.1586/1744666X.2014.891439. [DOI] [PubMed] [Google Scholar]
- 295.Wang X, Bootsma H, de Koning J, Kroese FG, Pringle S. Novel approaches for rescuing function of the salivary gland epithelium in primary Sjogren’s syndrome. Clin Exp Rheumatol 38, Suppl 126: 261–270, 2020. [PubMed] [Google Scholar]
- 296.Srivastava A, Makarenkova HP. Innate immunity and biological therapies for the treatment of Sjogren’s syndrome. Int J Mol Sci 21: 9172, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chivasso C, Sarrand J, Perret J, Delporte C, Soyfoo MS. The involvement of innate and adaptive immunity in the initiation and perpetuation of Sjogren’s syndrome. Int J Mol Sci 22: 658, 2021. doi: 10.3390/ijms22020658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Fisher BA, Jonsson R, Daniels T, Bombardieri M, Brown RM, Morgan P, Bombardieri S, Ng WF, Tzioufas AG, Vitali C, Shirlaw P, Haacke E, Costa S, Bootsma H, Devauchelle-Pensec V, Radstake TR, Mariette X, Richards A, Stack R, Bowman SJ, Barone F; Sjögren’s histopathology workshop group from ESSENTIAL. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjogren's syndrome. Ann Rheum Dis 76: 1161–1168, 2017. doi: 10.1136/annrheumdis-2016-210448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. . American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res 64: 475–487, 2012. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jonsson R, Brokstad KA, Jonsson MV, Delaleu N, Skarstein K. Current concepts on Sjogren’s syndrome—classification criteria and biomarkers. Eur J Oral Sci 126, Suppl: 37–48, 2018. doi: 10.1111/eos.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ibrahem HM. B cell dysregulation in primary Sjogren’s syndrome: a review. Jpn Dent Sci Rev 55: 139–144, 2019. doi: 10.1016/j.jdsr.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjogren’s syndrome. Rheum Dis Clin North Am 42: 419–434, 2016. doi: 10.1016/j.rdc.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shen L, Suresh L. Autoantibodies, detection methods and panels for diagnosis of Sjogren’s syndrome. Clin Immunol 182: 24–29, 2017. doi: 10.1016/j.clim.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 304.Båve U, Nordmark G, Lövgren T, Rönnelid J, Cajander S, Eloranta ML, Alm GV, Rönnblom L. Activation of the type I interferon system in primary Sjogren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum 52: 1185–1195, 2005. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 305.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc Natl Acad Sci USA 103: 2770–2775, 2006. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 9: 491–502, 2009. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 307.Moriyama M, Hayashida JN, Toyoshima T, Ohyama Y, Shinozaki S, Tanaka A, Maehara T, Nakamura S. Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjogren’s syndrome. Clin Exp Immunol 169: 17–26, 2012. doi: 10.1111/j.1365-2249.2012.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sene D, Ismael S, Forien M, Charlotte F, Kaci R, Cacoub P, Diallo A, Dieudé P, Lioté F. Ectopic germinal center-like structures in minor salivary gland biopsy tissue predict lymphoma occurrence in patients with primary Sjogren’s syndrome. Arthritis Rheumatol 70: 1481–1488, 2018. doi: 10.1002/art.40528. [DOI] [PubMed] [Google Scholar]
- 309.Alunno A, Leone MC, Bartoloni E, Gerli R, Carubbi F. Novel insights on lymphoma and lymphomagenesis in primary Sjogren’s syndrome. Panminerva Med 63: 491–498, 2021. doi: 10.23736/S0031-0808.20.04079-3. [DOI] [PubMed] [Google Scholar]
- 310.Alunno A, Leone MC, Giacomelli R, Gerli R, Carubbi F. Lymphoma and lymphomagenesis in primary Sjogren’s syndrome. Front Med (Lausanne) 5: 102, 2018. doi: 10.3389/fmed.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren’s syndrome. J Autoimmun 34: 400–407, 2010. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 312.Verstappen GM, Corneth OB, Bootsma H, Kroese FG. Th17 cells in primary Sjogren’s syndrome: pathogenicity and plasticity. J Autoimmun 87: 16–25, 2018. doi: 10.1016/j.jaut.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 313.Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci 20: 3394, 2019. doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ciccia F, Guggino G, Rizzo A, Ferrante A, Raimondo S, Giardina A, Dieli F, Campisi G, Alessandro R, Triolo G. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren’s syndrome. Ann Rheum Dis 71: 295–301, 2012. doi: 10.1136/ard.2011.154013. [DOI] [PubMed] [Google Scholar]
- 315.Jin JO, Yu Q. T Cell-Associated Cytokines in the Pathogenesis of Sjogren’s Syndrome. J Clin Cell Immunol S1: 11742, 2013. doi: 10.4172/2155-9899.S1-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Matsui K, Sano H. T helper 17 Cells in primary Sjogren’s syndrome. J Clin Med 6: 65, 2017. doi: 10.3390/jcm6070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 12: 639–646, 2011. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chen Z, O’Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res 41: 87–102, 2008. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- 319.Jones BE, Maerz MD, Buckner JH. IL-6: a cytokine at the crossroads of autoimmunity. Curr Opin Immunol 55: 9–14, 2018. doi: 10.1016/j.coi.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Pringle S, Wang X, Verstappen GM, Terpstra JH, Zhang CK, He A, Patel V, Jones RE, Baird DM, Spijkervet FK, Vissink A, Bootsma H, Coppes RP, Kroese FG. Salivary gland stem cells age prematurely in primary Sjogren’s syndrome. Arthritis Rheumatol 71: 133–142, 2019. doi: 10.1002/art.40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol 28: 436–453, 2018. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 322.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular senescence: defining a path forward. Cell 179: 813–827, 2019. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 323.Wang X, Bootsma H, Terpstra J, Vissink A, van der Vegt B, Spijkervet FK, Kroese FG, Pringle S. Progenitor cell niche senescence reflects pathology of the parotid salivary gland in primary Sjogren’s syndrome. Rheumatology (Oxford) 59: 3003–3013, 2020. doi: 10.1093/rheumatology/keaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yin H, Cabrera-Perez J, Lai Z, Michael D, Weller M, Swaim WD, Liu X, Catalán MA, Rocha EM, Ismail N, Afione S, Rana NA, Di Pasquale G, Alevizos I, Ambudkar I, Illei GG, Chiorini JA. Association of bone morphogenetic protein 6 with exocrine gland dysfunction in patients with Sjogren's syndrome and in mice. Arthritis Rheum 65: 3228–3238, 2013. doi: 10.1002/art.38123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lai Z, Yin H, Cabrera-Pérez J, Guimaro MC, Afione S, Michael DG, Glenton P, Patel A, Swaim WD, Zheng C, Nguyen CQ, Nyberg F, Chiorini JA. Aquaporin gene therapy corrects Sjogren's syndrome phenotype in mice. Proc Natl Acad Sci USA 113: 5694–5699, 2016. doi: 10.1073/pnas.1601992113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yin H, Kalra L, Lai Z, Guimaro MC, Aber L, Warner BM, Michael D, Zhang N, Cabrera-Perez J, Karim A, Swaim WD, Afione S, Voigt A, Nguyen CQ, Yu PB, Bloch DB, Chiorini JA. Inhibition of bone morphogenetic protein 6 receptors ameliorates Sjogren’s syndrome in mice. Sci Rep 10: 2967, 2020. doi: 10.1038/s41598-020-59443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tanaka T, Warner BM, Odani T, Ji Y, Mo YQ, Nakamura H, Jang SI, Yin H, Michael DG, Hirata N, Suizu F, Ishigaki S, Oliveira FR, Motta AC, Ribeiro-Silva A, Rocha EM, Atsumi T, Noguchi M, Chiorini JA. LAMP3 induces apoptosis and autoantigen release in Sjogren’s syndrome patients. Sci Rep 10: 15169, 2020. doi: 10.1038/s41598-020-71669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Nakamura H, Tanaka T, Pranzatelli T, Ji Y, Yin H, Perez P, Afione SA, Jang SI, Goldsmith C, Zheng CY, Swaim WD, Warner BM, Hirata N, Noguchi M, Atsumi T, Chiorini JA. Lysosome-associated membrane protein 3 misexpression in salivary glands induces a Sjogren’s syndrome-like phenotype in mice. Ann Rheum Dis 80: 1031–1039, 2021. doi: 10.1136/annrheumdis-2020-219649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Visser A, Doorenspleet ME, de Vries N, Spijkervet FK, Vissink A, Bende RJ, Bootsma H, Kroese FG, Bos NA. Acquisition of N-glycosylation sites in immunoglobulin heavy chain genes during local expansion in parotid salivary glands of primary Sjögren patients. Front Immunol 9: 491, 2018. doi: 10.3389/fimmu.2018.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Visser A, Hamza N, Kroese FG, Bos NA. Acquiring new N-glycosylation sites in variable regions of immunoglobulin genes by somatic hypermutation is a common feature of autoimmune diseases. Ann Rheum Dis 77: e69, 2018. doi: 10.1136/annrheumdis-2017-212568. [DOI] [PubMed] [Google Scholar]
- 331.Martínez Allo VC, Hauk V, Sarbia N, Pinto NA, Croci DO, Dalotto-Moreno T, Morales RM, Gatto SG, Manselle Cocco MN, Stupirski JC, Deladoey A, Maronna E, Marcaida P, Durigan V, Secco A, Mamani M, Dos Santos A, Catalán Pellet A, Pérez Leiros C, Rabinovich GA, Toscano MA. Suppression of age-related salivary gland autoimmunity by glycosylation-dependent galectin-1-driven immune inhibitory circuits. Proc Natl Acad Sci USA 117: 6630–6639, 2020. doi: 10.1073/pnas.1922778117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Shimizu T, Nakamura H, Kawakami A. Role of the innate immunity signaling pathway in the pathogenesis of Sjogren’s syndrome. Int J Mol Sci 22: 3090, 2021. doi: 10.3390/ijms22063090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Kiripolsky J, Kramer JM. Current and emerging evidence for Toll-like receptor activation in Sjogren’s syndrome. J Immunol Res 2018: 1246818, 2018. doi: 10.1155/2018/1246818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sisto M, Ribatti D, Lisi S. Understanding the complexity of Sjogren’s syndrome: remarkable progress in elucidating NF-kappaB mechanisms. J Clin Med 9: 2821, 2020. doi: 10.3390/jcm9092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wang X, Shaalan A, Liefers S, Coudenys J, Elewaut D, Proctor GB, Bootsma H, Kroese FG, Pringle S. Dysregulation of NF-kB in glandular epithelial cells results in Sjogren’s-like features. PLoS One 13: e0200212, 2018. doi: 10.1371/journal.pone.0200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hillen MR, Ververs FA, Kruize AA, Van Roon JA. Dendritic cells, T-cells and epithelial cells: a crucial interplay in immunopathology of primary Sjogren’s syndrome. Expert Rev Clin Immunol 10: 521–531, 2014. doi: 10.1586/1744666X.2014.878650. [DOI] [PubMed] [Google Scholar]
- 337.Heidegger S, van den Brink MR, Haas T, Poeck H. The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation. Front Immunol 5: 337, 2014. doi: 10.3389/fimmu.2014.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 12: 443–458, 2012. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood 129: 30–37, 2017. doi: 10.1182/blood-2016-07-686642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Margaix-Muñoz M, Bagán JV, Jiménez Y, Sarrión MG, Poveda-Roda R. Graft-versus-host disease affecting oral cavity. A review. J Clin Exp Dent 7: e138–e145, 2015. doi: 10.4317/jced.51975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Fall-Dickson JM, Pavletic SZ, Mays JW, Schubert MM. Oral complications of chronic graft-versus-host disease. J Natl Cancer Inst Monogr 2019: lgz007, 2019. doi: 10.1093/jncimonographs/lgz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nagler RM, Nagler A. Salivary gland involvement in graft-versus-host disease: the underlying mechanism and implicated treatment. Isr Med Assoc J 6: 167–172, 2004. [PubMed] [Google Scholar]
- 343.Elad S, Aljitawi O, Zadik Y. Oral graft-versus-host disease: a pictorial review and a guide for dental practitioners. Int Dent J 71: 9–20, 2021. doi: 10.1111/idj.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Soares AB, Faria PR, Magna LA, Correa ME, de Sousa CA, Almeida OP, Cintra ML. Chronic GVHD in minor salivary glands and oral mucosa: histopathological and immunohistochemical evaluation of 25 patients. J Oral Pathol Med 34: 368–373, 2005. doi: 10.1111/j.1600-0714.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 345.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, Moresi JM, Greenson J, Janin A, Martin PJ, McDonald G, Flowers ME, Turner M, Atkinson J, Lefkowitch J, Washington MK, Prieto VG, Kim SK, Argenyi Z, Diwan AH, Rashid A, Hiatt K, Couriel D, Schultz K, Hymes S, Vogelsang GB. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report . Biol Blood Marrow Transplant 12: 31–47, 2006. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 346.Hayashida JN, Nakamura S, Toyoshima T, Moriyama M, Sasaki M, Kawamura E, Ohyama Y, Kumamaru W, Shirasuna K. Possible involvement of cytokines, chemokines and chemokine receptors in the initiation and progression of chronic GVHD. Bone Marrow Transplant 48: 115–123, 2013. doi: 10.1038/bmt.2012.100. [DOI] [PubMed] [Google Scholar]
- 347.Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant 14: 365–378, 2008. doi: 10.1016/j.bbmt.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nurieva R, Divenko M, Kim S. Cancer immunology and the evolution of immunotherapy. In: Rheumatic Diseases and Syndromes Induced by Cancer Immunotherapy, edited by Suarez-Almazor ME. Cham, Switzerland: Springer, 2021, p. 3–29. [Google Scholar]
- 349.Chan KK, Bass AR. Autoimmune complications of immunotherapy: pathophysiology and management. BMJ 369: m736, 2020. doi: 10.1136/bmj.m736. [DOI] [PubMed] [Google Scholar]
- 350.Warner BM, Baer AN. Sicca Syndromes. In: Rheumatic Diseases and Syndromes Induced by Cancer Immunotherapy. Cham, Switzerland: Springer, 2021, p. 109–142. [Google Scholar]
- 351.Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, Pelayo E, Beach M, Gulley JL, Madan RA, Feliciano J, Grisius M, Long L, Powers A, Kleiner DE, Cappelli L, Alevizos I. Sicca Syndrome associated with immune checkpoint inhibitor therapy. Oncologist 24: 1259–1269, 2019. doi: 10.1634/theoncologist.2018-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Shazib MA, Woo SB, Sroussi H, Carvo I, Treister N, Farag A, Schoenfeld J, Haddad R, LeBoeuf N, Villa A. Oral immune-related adverse events associated with PD-1 inhibitor therapy: a case series. Oral Dis 26: 325–333, 2020. doi: 10.1111/odi.13218. [DOI] [PubMed] [Google Scholar]
- 353.Ortiz Brugués A, Sibaud V, Herbault-Barres B, Betrian S, Korakis I, De Bataille C, Gomez-Roca C, Epstein J, Vigarios E. Sicca syndrome induced by immune checkpoint inhibitor therapy: optimal management still pending. Oncologist 25: e391–e395, 2020. doi: 10.1634/theoncologist.2019-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Pringle S, van der Vegt B, Wang X, van Bakelen N, Hiltermann TJ, Spijkervet FK, Vissink A, Kroese FG, Bootsma H. Lack of conventional acinar cells in parotid salivary gland of patient taking an anti-PD-L1 immune checkpoint inhibitor. Front Oncol 10: 420, 2020. doi: 10.3389/fonc.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Warner B, Baer A. In reply. Oncologist 25: e396–e397, 2020. doi: 10.1634/theoncologist.2019-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71: 209–249, 2021. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 357.Hosni A, Huang SH, Goldstein D, Xu W, Chan B, Hansen A, Weinreb I, Bratman SV, Cho J, Giuliani M, Hope A, Kim J, O’Sullivan B, Waldron J, Ringash J. Outcomes and prognostic factors for major salivary gland carcinoma following postoperative radiotherapy. Oral Oncol 54: 75–80, 2016. doi: 10.1016/j.oraloncology.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 358.Park YM, Yoon SO, Koh YW, Kim SH, Lim JY, Choi EC. Clinical-pathological prognostic factors and treatment failure patterns in T1-2 high-grade parotid gland cancer. Oral Oncol 110: 104884, 2020. doi: 10.1016/j.oraloncology.2020.104884. [DOI] [PubMed] [Google Scholar]
- 359.Nam SJ, Roh JL, Cho KJ, Choi SH, Nam SY, Kim SY. Risk factors and survival associated with distant metastasis in patients with carcinoma of the salivary gland. Ann Surg Oncol 23: 4376–4383, 2016. doi: 10.1245/s10434-016-5356-3. [DOI] [PubMed] [Google Scholar]
- 360.Aro K, Ho AS, Luu M, Kim S, Tighiouart M, Clair JM, Yoshida EJ, Shiao SL, Leivo I, Zumsteg ZS. Development of a novel salivary gland cancer lymph node staging system. Cancer 124: 3171–3180, 2018. doi: 10.1002/cncr.31535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lombardi D, Tomasoni M, Paderno A, Mattavelli D, Ferrari M, Battocchio S, Missale F, Mazzola F, Peretti G, Mocellin D, Borsetto D, Fussey JM, Nankivell P, Skalidi N, Bussi M, Giordano L, Galli A, Arrigoni G, Raffetti E, Pracy P, Vander Poorten V, Nicolai P. The impact of nodal status in major salivary gland carcinoma: a multicenter experience and proposal of a novel N-classification. Oral Oncol 112: 105076, 2021. doi: 10.1016/j.oraloncology.2020.105076. [DOI] [PubMed] [Google Scholar]
- 362.Joshi NP, Broughman JR. Postoperative management of salivary gland tumors. Curr Treat Options Oncol 22: 23, 2021. doi: 10.1007/s11864-021-00820-9. [DOI] [PubMed] [Google Scholar]
- 363.Rossi ED, Baloch Z, Pusztaszeri M, Faquin WC. The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): an ASC-IAC-sponsored system for reporting salivary gland fine-needle aspiration. J Am Soc Cytopathol 7: 111–118, 2018. doi: 10.1016/j.jasc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 364.Gatta G, Guzzo M, Locati LD, McGurk M, Prott FJ. Major and minor salivary gland tumours. Crit Rev Oncol Hematol 152: 102959, 2020. doi: 10.1016/j.critrevonc.2020.102959. [DOI] [PubMed] [Google Scholar]
- 365.Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: tumors of the salivary gland. Head Neck Pathol 11: 55–67, 2017. doi: 10.1007/s12105-017-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Griffith CC, Schmitt AC, Little JL, Magliocca KR. New developments in salivary gland pathology: clinically useful ancillary testing and new potentially targetable molecular alterations. Arch Pathol Lab Med 141: 381–395, 2017. doi: 10.5858/arpa.2016-0259-SA. [DOI] [PubMed] [Google Scholar]
- 367.Rooper LM, Mansour M, Yonescu R, Oliai BR, Bishop JA, Westra WH. The decline of salivary adenocarcinoma not otherwise specified as a tumor entity: reclassification using contemporary immunohistochemical profiling and diagnostic criteria. Am J Surg Pathol 45: 753–764, 2021. doi: 10.1097/PAS.0000000000001636. [DOI] [PubMed] [Google Scholar]
- 368.Freiberger SN, Brada M, Fritz C, Höller S, Vogetseder A, Horcic M, Bihl M, Michal M, Lanzer M, Wartenberg M, Borner U, Bode PK, Broglie MA, Rordorf T, Morand GB, Rupp NJ. SalvGlandDx—a comprehensive salivary gland neoplasm specific next generation sequencing panel to facilitate diagnosis and identify therapeutic targets. Neoplasia 23: 473–487, 2021. doi: 10.1016/j.neo.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wai KC, Kang H, Ha PK. Molecular markers that matter in salivary malignancy. Otolaryngol Clin North Am 54: 613–627, 2021. doi: 10.1016/j.otc.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 370.Palsgrove D, Allahabadi S, Khan SA. Genomic analysis of salivary gland cancer and treatment of salivary gland cancers. Surg Pathol Clin 14: 151–163, 2021. doi: 10.1016/j.path.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 371.Del Signore AG, Megwalu UC. The rising incidence of major salivary gland cancer in the United States. Ear Nose Throat J 96: E13–E16, 2017. doi: 10.1177/014556131709600319. [DOI] [PubMed] [Google Scholar]
- 372.Gupta A, Koochakzadeh S, Neskey DM, Nguyen SA, Lentsch EJ. Incidence and survival trends of parotid malignancies over 42 years. Head Neck 42: 2308–2315, 2020. doi: 10.1002/hed.26172. [DOI] [PubMed] [Google Scholar]
- 373.Chow LQ. Head and neck cancer. N Engl J Med 382: 60–72, 2020. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 374.Palm A, Johansson KA. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field; implications for the long-term morbidity of cancer survivors. Acta Oncol 46: 462–473, 2007. doi: 10.1080/02841860701218626. [DOI] [PubMed] [Google Scholar]
- 375.Ratko AT, Douglas GW, de Souza AJ, Belinson EB, Aronson M. Radiotherapy Treatments for Head and Neck Cancer Update. Rockville, MD: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
- 376.Dirix P, Nuyts S, Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer 107: 2525–2534, 2006. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 377.Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol 7: 89, 2017. doi: 10.3389/fonc.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Bogaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer 16: 171–179, 2008. doi: 10.1007/s00520-007-0300-5. [DOI] [PubMed] [Google Scholar]
- 379.Gupta N, Pal M, Rawat S, Grewal MS, Garg H, Chauhan D, Ahlawat P, Tandon S, Khurana R, Pahuja AK, Mayank M, Devnani B. Radiation-induced dental caries, prevention and treatment: a systematic review. Natl J Maxillofac Surg 6: 160–166, 2015. doi: 10.4103/0975-5950.183870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Sroussi HY, Epstein JB, Bensadoun RJ, Saunders DP, Lalla RV, Migliorati CA, Heaivilin N, Zumsteg ZS. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med 6: 2918–2931, 2017. doi: 10.1002/cam4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Han P, Lakshminarayanan P, Jiang W, Shpitser I, Hui X, Lee SH, Cheng Z, Guo Y, Taylor RH, Siddiqui SA, Bowers M, Sheikh K, Kiess A, Page BR, Lee J, Quon H, McNutt TR. Dose/volume histogram patterns in salivary gland subvolumes influence xerostomia injury and recovery. Sci Rep 9: 3616, 2019. doi: 10.1038/s41598-019-40228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Clark H, Hovan A, Moiseenko V, Thomas S, Wu J, Reinsberg S. Regional radiation dose susceptibility within the parotid gland: effects on salivary loss and recovery. Med Phys 42: 2064–2071, 2015. doi: 10.1118/1.4915077. [DOI] [PubMed] [Google Scholar]
- 383.Braam PM, Roesink JM, Raaijmakers CP, Busschers WB, Terhaard CH. Quality of life and salivary output in patients with head-and-neck cancer five years after radiotherapy. Radiat Oncol 2: 3, 2007. doi: 10.1186/1748-717X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 67: 660–669, 2007. doi: 10.1016/j.ijrobp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Konings AW, Cotteleer F, Faber H, van Luijk P, Meertens H, Coppes RP. Volume effects and region-dependent radiosensitivity of the parotid gland. Int J Radiat Oncol Biol Phys 62: 1090–1095, 2005. doi: 10.1016/j.ijrobp.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 386.Buettner F, Miah AB, Gulliford SL, Hall E, Harrington KJ, Webb S, Partridge M, Nutting CM. Novel approaches to improve the therapeutic index of head and neck radiotherapy: an analysis of data from the PARSPORT randomised phase III trial. Radiother Oncol 103: 82–87, 2012. doi: 10.1016/j.radonc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 387.Miah AB, Gulliford SL, Morden J, Newbold KL, Bhide SA, Zaidi SH, Hall E, Harrington KJ, Nutting CM. Recovery of salivary function: contralateral parotid-sparing intensity-modulated radiotherapy versus bilateral superficial lobe parotid-sparing intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 28: e69–e76, 2016. doi: 10.1016/j.clon.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Xiao W, Lin Z, Zhang W, Li M, Wu VW. A split-parotid delineation approach for dose optimization in volumetric modulated arc therapy for nasopharyngeal carcinoma patients with parapharyngeal space invasion and level IIa cervical lymph node involvements. Br J Radiol 89: 20150635, 2016. doi: 10.1259/bjr.20150635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Zhang HB, Lu X, Huang SM, Wang L, Zhao C, Xia WX, Li SW, Wang FL, Zhu YL, Guo X, Xiang YQ. Superficial parotid lobe-sparing delineation approach: a better method of dose optimization to protect the parotid gland in intensity-modulated radiotherapy for nasopharyngeal carcinoma. Curr Oncol 20: e577–e584, 2013. doi: 10.3747/co.20.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L, Lopes NN, Mello AL, Muniz LV, Murdoch-Kinch CA, Nair RG, Napeñas JJ, Nogueira-Rodrigues A, Saunders D, Stirling B, von Bültzingslöwen I, Weikel DS, Elting LS, Spijkervet FK, Brennan MT; Salivary Gland Hypofunction/Xerostomia Section, Oral Care Study Group, MASCC/ISOO. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer 18: 1039–1060, 2010. doi: 10.1007/s00520-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 391.Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, Schmitz PI. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck 24: 737–747, 2002. doi: 10.1002/hed.10129. [DOI] [PubMed] [Google Scholar]
- 392.Avila JL, Grundmann O, Burd R, Limesand KH. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys 73: 523–529, 2009. doi: 10.1016/j.ijrobp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Radfar L, Sirois DA. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96: 267–274, 2003. doi: 10.1016/S1079-2104(03)00369-X. [DOI] [PubMed] [Google Scholar]
- 394.van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, Baanstra M, van der Laan HP, Kierkels RG, van der Schaaf A, Witjes MJ, Schippers JM, Brandenburg S, Langendijk JA, Wu J, Coppes RP. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med 7: 305ra147, 2015. doi: 10.1126/scitranslmed.aac4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Aure MH, Konieczny SF, Ovitt CE. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 33: 231–237, 2015. doi: 10.1016/j.devcel.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Redman RS. Histologic changes in the salivary glands following radiation therapy. In: Salivary Gland Development and Regeneration, edited by Cha S. Cham, Switzerland: Springer, 2017, p. 75–91. [Google Scholar]
- 397.Meyer S, Chibly AM, Burd R, Limesand KH. Insulin-like growth factor-1-mediated DNA repair in irradiated salivary glands is sirtuin-1 dependent. J Dent Res 96: 225–232, 2017. doi: 10.1177/0022034516677529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Mitchell GC, Fillinger JL, Sittadjody S, Avila JL, Burd R, Limesand KH. IGF1 activates cell cycle arrest following irradiation by reducing binding of DeltaNp63 to the p21 promoter. Cell Death Dis 1: e50, 2010. doi: 10.1038/cddis.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Liu X, Cotrim A, Teos L, Zheng C, Swaim W, Mitchell J, Mori Y, Ambudkar I. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat Commun 4: 1515, 2013. doi: 10.1038/ncomms2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Gilman KE, Camden JM, Klein RR, Zhang Q, Weisman GA, Limesand KH. P2X7 receptor deletion suppresses gamma-radiation-induced hyposalivation. Am J Physiol Regul Integr Comp Physiol 316: R687–R696, 2019. doi: 10.1152/ajpregu.00192.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, Cohen J, Eliashar R, Mizrachi L, Orfaig-Geva C, Baum BJ, Rose-John S, Galun E, Axelrod JH. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res 76: 1170–1180, 2016. doi: 10.1158/0008-5472.CAN-15-1671. [DOI] [PubMed] [Google Scholar]
- 402.Martin KL, Hill GA, Klein RR, Arnett DG, Burd R, Limesand KH. Prevention of radiation-induced salivary gland dysfunction utilizing a CDK inhibitor in a mouse model. PLoS One 7: e51363, 2012. doi: 10.1371/journal.pone.0051363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Limesand KH, Schwertfeger KL, Anderson SM. MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol 26: 8840–8856, 2006. doi: 10.1128/MCB.01846-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Shin HS, Lee S, Kim YM, Lim JY. Hypoxia-activated adipose mesenchymal stem cells prevents irradiation-induced salivary hypofunction by enhanced paracrine effect through fibroblast growth factor 10. Stem Cells 36: 1020–1032, 2018. doi: 10.1002/stem.2818. [DOI] [PubMed] [Google Scholar]
- 405.Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J Biol Chem 281: 9728–9737, 2006. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- 406.Arany S, Benoit DS, Dewhurst S, Ovitt CE. Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol Ther 21: 1182–1194, 2013. doi: 10.1038/mt.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Akyuz M, Taysi S, Baysal E, Demir E, Alkis H, Akan M, Binici H, Karatas ZA. Radioprotective effect of thymoquinone on salivary gland of rats exposed to total cranial irradiation. Head Neck 39: 2027–2035, 2017. doi: 10.1002/hed.24861. [DOI] [PubMed] [Google Scholar]
- 408.Srinivas US, Tan BW, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol 25: 101084, 2019. doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Liu X, Subedi KP, Zheng C, Ambudkar I. Mitochondria-targeted antioxidant protects against irradiation-induced salivary gland hypofunction. Sci Rep 11: 7690, 2021. doi: 10.1038/s41598-021-86927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Şimşek G, Gürocak Ş, Karadaǧ N, Karabulut AB, Demirtaş E, Karataş E, Pepele E. Protective effects of resveratrol on salivary gland damage induced by total body irradiation in rats. Laryngoscope 122: 2743–2748, 2012. doi: 10.1002/lary.23609. [DOI] [PubMed] [Google Scholar]
- 411.Xu L, Yang X, Cai J, Ma J, Cheng H, Zhao K, Yang L, Cao Y, Qin Q, Zhang C, Zhang Q, Sun X. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope 123: E23–E29, 2013. doi: 10.1002/lary.24276. [DOI] [PubMed] [Google Scholar]
- 412.Kim JH, Kim KM, Jung MH, Jung JH, Kang KM, Jeong BK, Kim JP, Park JJ, Woo SH. Protective effects of alpha lipoic acid on radiation-induced salivary gland injury in rats. Oncotarget 7: 29143–29153, 2016. doi: 10.18632/oncotarget.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Zhang T, Liu C, Ma S, Gao Y, Wang R. Protective effect and mechanism of action of rosmarinic acid on radiation-induced parotid gland injury in rats. Dose Response 18: 1559325820907782, 2020. doi: 10.1177/1559325820907782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Khalafalla MG, Woods LT, Jasmer KJ, Forti KM, Camden JM, Jensen JL, Limesand KH, Galtung HK, Weisman GA. P2 receptors as therapeutic targets in the salivary gland: from physiology to dysfunction. Front Pharmacol 11: 222, 2020. doi: 10.3389/fphar.2020.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vriz S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep 3: 2084, 2013. doi: 10.1038/srep02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the cell! Trends Cell Biol 18: 467–473, 2008. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Diwanji N, Bergmann A. An unexpected friend—ROS in apoptosis-induced compensatory proliferation: Implications for regeneration and cancer. Semin Cell Dev Biol 80: 74–82, 2018. doi: 10.1016/j.semcdb.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wong WY, Allie S, Limesand KH. PKCzeta and JNK signaling regulate radiation-induced compensatory proliferation in parotid salivary glands. PLoS One 14: e0219572, 2019. doi: 10.1371/journal.pone.0219572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Chibly AM, Wong WY, Pier M, Cheng H, Mu Y, Chen J, Ghosh S, Limesand KH. aPKCzeta-dependent repression of Yap is necessary for functional restoration of irradiated salivary glands with IGF-1. Sci Rep 8: 6347, 2018. doi: 10.1038/s41598-018-24678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Morgan-Bathke M, Hill GA, Harris ZI, Lin HH, Chibly AM, Klein RR, Burd R, Ann DK, Limesand KH. Autophagy correlates with maintenance of salivary gland function following radiation. Sci Rep 4: 5206, 2014. doi: 10.1038/srep05206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Grundmann O, Fillinger JL, Victory KR, Burd R, Limesand KH. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer 10: 417, 2010. doi: 10.1186/1471-2407-10-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Weng PL, Aure MH, Maruyama T, Ovitt CE. Limited regeneration of adult salivary glands after severe injury involves cellular plasticity. Cell Rep 24: 1464–1470.e3, 2018. doi: 10.1016/j.celrep.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ikai K, Sakai M, Minagi HO, Gojo N, Sakai T. DeltaNp63 is upregulated during salivary gland regeneration following duct ligation and irradiation in mice. FEBS Lett 594: 3216–3226, 2020. doi: 10.1002/1873-3468.13896. [DOI] [PubMed] [Google Scholar]
- 424.Hai B, Zhao Q, Deveau MA, Liu F. Delivery of sonic hedgehog gene repressed irradiation-induced cellular senescence in salivary glands by promoting DNA repair and reducing oxidative stress. Theranostics 8: 1159–1167, 2018. doi: 10.7150/thno.23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Peng X, Wu Y, Brouwer U, van Vliet T, Wang B, Demaria M, Barazzuol L, Coppes RP. Cellular senescence contributes to radiation-induced hyposalivation by affecting the stem/progenitor cell niche. Cell Death Dis 11: 854, 2020. doi: 10.1038/s41419-020-03074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, Hoffman MP. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun 4: 1494, 2013. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Kim JH, Jeong BK, Jang SJ, Yun JW, Jung MH, Kang KM, Kim TG, Woo SH. Alpha-lipoic acid ameliorates radiation-induced salivary gland injury by preserving parasympathetic innervation in rats. Int J Mol Sci 21: 2260, 2020. doi: 10.3390/ijms21072260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Hu L, Zhu Z, Hai B, Chang S, Ma L, Xu Y, Li X, Feng X, Wu X, Zhao Q, Qin L, Wang J, Zhang C, Liu F, Wang S. Intragland Shh gene delivery mitigated irradiation-induced hyposalivation in a miniature pig model. Theranostics 8: 4321–4331, 2018. doi: 10.7150/thno.26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Mizrachi A, Cotrim AP, Katabi N, Mitchell JB, Verheij M, Haimovitz-Friedman A. Radiation-induced microvascular injury as a mechanism of salivary gland hypofunction and potential target for radioprotectors. Radiat Res 186: 189–195, 2016. doi: 10.1667/RR14431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res 14: 7741–7750, 2008. doi: 10.1158/1078-0432.CCR-08-1449. [DOI] [PubMed] [Google Scholar]
- 431.Cheng SC, Wu VW, Kwong DL, Ying MT. Assessment of post-radiotherapy salivary glands. Br J Radiol 84: 393–402, 2011. doi: 10.1259/bjr/66754762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Luitje ME, Israel AK, Cummings MA, Giampoli EJ, Allen PD, Newlands SD, Ovitt CE. Long-term maintenance of acinar cells in human submandibular glands after radiation therapy. Int J Radiat Oncol Biol Phys 109: 1028–1039, 2021. doi: 10.1016/j.ijrobp.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Teymoortash A, Simolka N, Schrader C, Tiemann M, Werner JA. Lymphocyte subsets in irradiation-induced sialadenitis of the submandibular gland. Histopathology 47: 493–500, 2005. doi: 10.1111/j.1365-2559.2005.02256.x. [DOI] [PubMed] [Google Scholar]
- 434.Teos LY, Zheng CY, Liu X, Swaim WD, Goldsmith CM, Cotrim AP, Baum BJ, Ambudkar IS. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther 23: 572–579, 2016. doi: 10.1038/gt.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Kwak M, Ninche N, Klein S, Saur D, Ghazizadeh S. c-Kit+ cells in adult salivary glands do not function as tissue stem cells. Sci Rep 8: 14193, 2018. doi: 10.1038/s41598-018-32557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Emmerson E, May AJ, Berthoin L, Cruz‐Pacheco N, Nathan S, Mattingly AJ, Chang JL, Ryan WR, Tward AD, Knox SM. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol Med 10: e8051, 2018. doi: 10.15252/emmm.201708051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Hauser BR, Hoffman MP. Regulatory mechanisms driving salivary gland organogenesis. Curr Top Dev Biol 115: 111–130, 2015. doi: 10.1016/bs.ctdb.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Tucker AS. Salivary gland development. Semin Cell Dev Biol 18: 237–244, 2007. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 440.Gluck C, Min S, Oyelakin A, Smalley K, Sinha S, Romano RA. RNA-seq based transcriptomic map reveals new insights into mouse salivary gland development and maturation. BMC Genomics 17: 923, 2016. doi: 10.1186/s12864-016-3228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Song EC, Min S, Oyelakin A, Smalley K, Bard JE, Liao L, Xu J, Romano RA. Genetic and scRNA-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci Rep 8: 14043, 2018. doi: 10.1038/s41598-018-32343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Min S, Oyelakin A, Gluck C, Bard JE, Song EC, Smalley K, Che M, Flores E, Sinha S, Romano RA. p63 and its target follistatin maintain salivary gland stem/progenitor cell function through TGF-beta/activin signaling. iScience 23: 101524, 2020. doi: 10.1016/j.isci.2020.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Suzuki A, Ogata K, Iwata J. Cell signaling regulation in salivary gland development. Cell Mol Life Sci 78: 3299–3315, 2021. doi: 10.1007/s00018-020-03741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Mattingly A, Finley JK, Knox SM. Salivary gland development and disease. Wiley Interdiscip Rev Dev Biol 4: 573–590, 2015. doi: 10.1002/wdev.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev 21: 1113–1124, 2007. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Metzler MA, Raja S, Elliott KH, Friedl RM, Tran NQ, Brugmann SA, Larsen M, Sandell LL. RDH10-mediated retinol metabolism and RARalpha-mediated retinoic acid signaling are required for submandibular salivary gland initiation. Development 145: dev164822, 2018. doi: 10.1242/dev.164822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Chatzeli L, Gaete M, Tucker AS. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144: 2294–2305, 2017. doi: 10.1242/dev.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wright DM, Buenger DE, Abashev TM, Lindeman RP, Ding J, Sandell LL. Retinoic acid regulates embryonic development of mammalian submandibular salivary glands. Dev Biol 407: 57–67, 2015. doi: 10.1016/j.ydbio.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Lohnes D, Mark M, Mendelsohn C, Dollé P, Decimo D, LeMeur M, Dierich A, Gorry P, Chambon P. Developmental roles of the retinoic acid receptors. J Steroid Biochem Mol Biol 53: 475–486, 1995. doi: 10.1016/0960-0760(95)00094-g. [DOI] [PubMed] [Google Scholar]
- 450.Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 120: 2723–2748, 1994. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 451.Adam MP, Abramowsky CR, Brady AN, Coleman K, Todd NW. Rhabdomyomatous hamartomata of the pharyngeal region with bilateral microtia and aural atresia: a new association? Birth Defects Res A Clin Mol Teratol 79: 242–248, 2007. doi: 10.1002/bdra.20338. [DOI] [PubMed] [Google Scholar]
- 452.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277: 643–649, 2000. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- 453.De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127: 483–492, 2000. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 454.Teshima TH, Lourenco SV, Tucker AS. Multiple cranial organ defects after conditionally knocking out Fgf10 in the neural crest. Front Physiol 7: 488, 2016. doi: 10.3389/fphys.2016.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, Larsen M, Hoffman MP. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 132: 1223–1234, 2005. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- 456.Lombaert IM, Abrams SR, Li L, Eswarakumar VP, Sethi AJ, Witt RL, Hoffman MP. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Reports 1: 604–619, 2013. doi: 10.1016/j.stemcr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Hosseini ZF, Nelson DA, Moskwa N, Sfakis LM, Castracane J, Larsen M. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J Cell Sci 131: jcs208728, 2018. doi: 10.1242/jcs.208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol 4: 215–266, 2015. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, Yates EA, Turnbull JE, Ron D, Hoffman MP. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J Biol Chem 283: 9308–9317, 2008. doi: 10.1074/jbc.M709995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Patel VN, Lombaert IM, Cowherd SN, Shworak NW, Xu Y, Liu J, Hoffman MP. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev Cell 29: 662–673, 2014. doi: 10.1016/j.devcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Patel VN, Pineda DL, Berenstein E, Hauser B, Choi S, Prochazkova M, Zheng C, Goldsmith CM, Kuppevelt TV, Kulkarni A, Song Y, Linhardt RJ, Chibly AM, Hoffman MP. Loss of Hs3st3a1 or Hs3st3b1 enzymes alters heparan sulfate to reduce epithelial morphogenesis and adult salivary gland function. Matrix Biol 103-104: 37–57, 2021. doi: 10.1016/j.matbio.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Minowada G, Jarvis LA, Chi CL, Neubüser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126: 4465–4475, 1999. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 463.Knosp WM, Knox SM, Lombaert IM, Haddox CL, Patel VN, Hoffman MP. Submandibular parasympathetic gangliogenesis requires sprouty-dependent Wnt signals from epithelial progenitors. Dev Cell 32: 667–677, 2015. doi: 10.1016/j.devcel.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 44: 195–217, 2004. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- 465.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 9: 52, 2017. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Sisto M, Lorusso L, Ingravallo G, Lisi S. Exocrine gland morphogenesis: insights into the role of amphiregulin from development to disease. Arch Immunol Ther Exp (Warsz) 65: 477–499, 2017. doi: 10.1007/s00005-017-0478-2. [DOI] [PubMed] [Google Scholar]
- 467.Kashimata M, Gresik EW. Epidermal growth factor system is a physiological regulator of development of the mouse fetal submandibular gland and regulates expression of the alpha6-integrin subunit. Dev Dyn 208: 149–161, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 468.Häärä O, Koivisto T, Miettinen PJ. EGF-receptor regulates salivary gland branching morphogenesis by supporting proliferation and maturation of epithelial cells and survival of mesenchymal cells. Differentiation 77: 298–306, 2009. doi: 10.1016/j.diff.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 469.Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329: 1645–1647, 2010. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Nogawa H, Takahashi Y. Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development 112: 855–861, 1991. doi: 10.1242/dev.112.3.855. [DOI] [PubMed] [Google Scholar]
- 471.Koyama N, Kashimata M, Sakashita H, Sakagami H, Gresik EW. EGF-stimulated signaling by means of PI3K, PLCgamma1, and PKC isozymes regulates branching morphogenesis of the fetal mouse submandibular gland. Dev Dyn 227: 216–226, 2003. doi: 10.1002/dvdy.10309. [DOI] [PubMed] [Google Scholar]
- 472.Koyama N, Hayashi T, Mizukoshi K, Matsumoto T, Gresik EW, Kashimata M. Extracellular regulated kinase5 is expressed in fetal mouse submandibular glands and is phosphorylated in response to epidermal growth factor and other ligands of the ErbB family of receptors. Dev Growth Differ 54: 801–808, 2012. doi: 10.1111/dgd.12008. [DOI] [PubMed] [Google Scholar]
- 473.Kashimata M, Sayeed S, Ka A, Onetti-Muda A, Sakagami H, Faraggiana T, Gresik EW. The ERK-1/2 signaling pathway is involved in the stimulation of branching morphogenesis of fetal mouse submandibular glands by EGF. Dev Biol 220: 183–196, 2000. doi: 10.1006/dbio.2000.9639. [DOI] [PubMed] [Google Scholar]
- 474.Umeda Y, Miyazaki Y, Shiinoki H, Higashiyama S, Nakanishi Y, Hieda Y. Involvement of heparin-binding EGF-like growth factor and its processing by metalloproteinases in early epithelial morphogenesis of the submandibular gland. Dev Biol 237: 202–211, 2001. doi: 10.1006/dbio.2001.0351. [DOI] [PubMed] [Google Scholar]
- 475.Miyazaki Y, Nakanishi Y, Hieda Y. Tissue interaction mediated by neuregulin-1 and ErbB receptors regulates epithelial morphogenesis of mouse embryonic submandibular gland. Dev Dyn 230: 591–596, 2004. doi: 10.1002/dvdy.20078. [DOI] [PubMed] [Google Scholar]
- 476.Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pacheco N, Prochazka J, Haddox CL, Northrup E, Hodges C, Mostov KE, Hoffman MP, Knox SM. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev Cell 30: 449–462, 2014. doi: 10.1016/j.devcel.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Jaskoll T, Melnick M. Submandibular gland morphogenesis: stage-specific expression of TGF-alpha/EGF, IGF, TGF-beta, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TGF-beta2, TGF-beta3, and EGF-r null mutations. Anat Rec 256: 252–268, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 478.Patel N, Sharpe PT, Miletich I. Coordination of epithelial branching and salivary gland lumen formation by Wnt and FGF signals. Dev Biol 358: 156–167, 2011. doi: 10.1016/j.ydbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 479.Matsumoto S, Kurimoto T, Taketo MM, Fujii S, Kikuchi A. The WNT/MYB pathway suppresses KIT expression to control the timing of salivary proacinar differentiation and duct formation. Development 143: 2311–2324, 2016. doi: 10.1242/dev.134486. [DOI] [PubMed] [Google Scholar]
- 480.Hai B, Qin L, Yang Z, Zhao Q, Shangguan L, Ti X, Zhao Y, Kim S, Rangaraj D, Liu F. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin Cancer Res 20: 140–150, 2014. doi: 10.1158/1078-0432.CCR-13-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BN, Vries RG, Clevers H, de Haan G, van Os R, Coppes RP. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Reports 6: 150–162, 2016. doi: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Enger TB, Samad-Zadeh A, Bouchie MP, Skarstein K, Galtung HK, Mera T, Walker J, Menko AS, Varelas X, Faustman DL, Jensen JL, Kukuruzinska MA. The Hippo signaling pathway is required for salivary gland development and its dysregulation is associated with Sjogren’s syndrome. Lab Invest 93: 1203–1218, 2013. doi: 10.1038/labinvest.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Szymaniak AD, Mi R, McCarthy SE, Gower AC, Reynolds TL, Mingueneau M, Kukuruzinska M, Varelas X. The Hippo pathway effector YAP is an essential regulator of ductal progenitor patterning in the mouse submandibular gland. Elife 6: e23499, 2017. doi: 10.7554/eLife.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Wong WY, Gilman K, Limesand KH. Yap activation in irradiated parotid salivary glands is regulated by ROCK activity. PLoS One 15: e0232921, 2020. doi: 10.1371/journal.pone.0232921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Garcia-Gallastegui P, Ibarretxe G, Garcia-Ramírez JJ, Baladrón V, Aurrekoetxea M, Nueda ML, Naranjo AI, Santaolalla F, Sánchez-del Rey A, Laborda J, Unda F. DLK1 regulates branching morphogenesis and parasympathetic innervation of salivary glands through inhibition of NOTCH signalling. Biol Cell 106: 237–253, 2014. doi: 10.1111/boc.201300086. [DOI] [PubMed] [Google Scholar]
- 486.Pringle S, Van Os R, Coppes RP. Concise review: adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 31: 613–619, 2013. doi: 10.1002/stem.1327. [DOI] [PubMed] [Google Scholar]
- 487.Athwal HK, Murphy G 3rd, Tibbs E, Cornett A, Hill E, Yeoh K, Berenstein E, Hoffman MP, Lombaert IMA. Sox10 regulates plasticity of epithelial progenitors toward secretory units of exocrine glands. Stem Cell Reports 12: 366–380, 2019. doi: 10.1016/j.stemcr.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Emmerson E, May AJ, Nathan S, Cruz-Pacheco N, Lizama CO, Maliskova L, Zovein AC, Shen Y, Muench MO, Knox SM. SOX2 regulates acinar cell development in the salivary gland. Elife 6: e26620, 2017. doi: 10.7554/eLife.26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Arany S, Catalán MA, Roztocil E, Ovitt CE. Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration. Dev Biol 353: 186–193, 2011. doi: 10.1016/j.ydbio.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Kwak M, Ghazizadeh S. Analysis of histone H2BGFP retention in mouse submandibular gland reveals actively dividing stem cell populations. Stem Cells Dev 24: 565–574, 2015. doi: 10.1089/scd.2014.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Kwak M, Alston N, Ghazizadeh S. Identification of stem cells in the secretory complex of salivary glands. J Dent Res 95: 776–783, 2016. doi: 10.1177/0022034516634664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Maruyama EO, Aure MH, Xie X, Myal Y, Gan L, Ovitt CE. Cell-specific Cre strains for genetic manipulation in salivary glands. PLoS One 11: e0146711, 2016. doi: 10.1371/journal.pone.0146711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Ihrler S, Zietz C, Sendelhofert A, Lang S, Blasenbreu-Vogt S, Löhrs U. A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Arch 440: 519–526, 2002. doi: 10.1007/s004280100537. [DOI] [PubMed] [Google Scholar]
- 494.Ingalls MH, Hollomon AJ, Newlands SD, McDavid AN, Ovitt CE. Intrinsic mitotic activity supports the human salivary gland acinar cell population. FEBS Lett 594: 376–382, 2020. doi: 10.1002/1873-3468.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Kim JY, An CH, Kim JY, JK J. Experimental animal model systems for understanding salivary secretory disorders. Int J Mol Sci 21: 8423, 2020. doi: 10.3390/ijms21228423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Shubin AD, Sharipol A, Felong TJ, Weng PL, Schutrum BE, Joe DS, Aure MH, Benoit DS, Ovitt CE. Stress or injury induces cellular plasticity in salivary gland acinar cells. Cell Tissue Res 380: 487–497, 2020. doi: 10.1007/s00441-019-03157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Shaalan A, Proctor G. Salivary glands require Aurora Kinase B for regeneration after transient innate immune-mediated injury. Sci Rep 9: 11339, 2019. doi: 10.1038/s41598-019-47762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 123: 4195–4200, 2010. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev 97: 4–27, 2016. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 500.Walma DA, Yamada KM. The extracellular matrix in development. Development 147: dev175596, 2020. doi: 10.1242/dev.175596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol 57-58: 1–11, 2017. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 341: 126–140, 2010. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Grobstein C, Cohen J. Collagenase: effect on the morphogenesis of embryonic salivary epithelium in vitro. Science 150: 626–628, 1965. doi: 10.1126/science.150.3696.626. [DOI] [PubMed] [Google Scholar]
- 504.Nakanishi Y, Sugiura F, Kishi J, Hayakawa T. Collagenase inhibitor stimulates cleft formation during early morphogenesis of mouse salivary gland. Dev Biol 113: 201–206, 1986. doi: 10.1016/0012-1606(86)90122-3. [DOI] [PubMed] [Google Scholar]
- 505.Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev Cell 17: 482–493, 2009. doi: 10.1016/j.devcel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786–801, 2014. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature 423: 876–881, 2003. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 508.Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science 329: 562–565, 2010. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Guldager Kring Rasmussen D, Karsdal MA. Laminins. In: Biochemistry of Collagens, Laminins and Elastin, edited by Karsdal MA, Leeming DJ, Henriksen K, Bay-Jensen AC.. London: Academic Press, 2016, p. 163–196 [Google Scholar]
- 510.Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, Hoffman MP. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev Biol 308: 15–29, 2007. doi: 10.1016/j.ydbio.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11–55, 2015. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Thompson HA, Spooner BS. Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: effects of beta-D-xyloside, an inhibitor of branching morphogenesis. J Cell Biol 96: 1443–1450, 1983. doi: 10.1083/jcb.96.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.De Pasquale V, Pavone LM. heparan sulfate proteoglycan signaling in tumor microenvironment. Int J Mol Sci 21: 6588, 2020. doi: 10.3390/ijms21186588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, Whitelock JM, Elkin M, Vlodavsky I, Hoffman MP. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development 134: 4177–4186, 2007. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 515.De Pasquale V, Pavone LM. Heparan sulfate proteoglycans: the sweet side of development turns sour in mucopolysaccharidoses. Biochim Biophys Acta Mol Basis Dis 1865: 165539, 2019. doi: 10.1016/j.bbadis.2019.165539. [DOI] [PubMed] [Google Scholar]
- 516.Patel VN, Pineda DL, Hoffman MP. The function of heparan sulfate during branching morphogenesis. Matrix Biol 57-58: 311–323, 2017. doi: 10.1016/j.matbio.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci Signal 2: ra55, 2009. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Tian E, Hoffman MP, Ten Hagen KG. O-glycosylation modulates integrin and FGF signalling by influencing the secretion of basement membrane components. Nat Commun 3: 869, 2012. doi: 10.1038/ncomms1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Harunaga JS, Doyle AD, Yamada KM. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev Biol 394: 197–205, 2014. doi: 10.1016/j.ydbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Wang S, Sekiguchi R, Daley WP, Yamada KM. Patterned cell and matrix dynamics in branching morphogenesis. J Cell Biol 216: 559–570, 2017. doi: 10.1083/jcb.201610048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Yamada KM, Collins JW, Cruz Walma DA, Doyle AD, Morales SG, Lu J, Matsumoto K, Nazari SS, Sekiguchi R, Shinsato Y, Wang S. Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. Int J Exp Pathol 100: 144–152, 2019. doi: 10.1111/iep.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Daley WP, Gervais EM, Centanni SW, Gulfo KM, Nelson DA, Larsen M. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 139: 411–422, 2012. doi: 10.1242/dev.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev Biol 336: 169–182, 2009. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Vinke J, Kaper HJ, Vissink A, Sharma PK. Dry mouth: saliva substitutes which adsorb and modify existing salivary condition films improve oral lubrication. Clin Oral Investig 24: 4019–4030, 2020. doi: 10.1007/s00784-020-03272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wan H, Vissink A, Sharma PK. Enhancement in xerostomia patient salivary lubrication using a mucoadhesive. J Dent Res 99: 914–921, 2020. doi: 10.1177/0022034520917675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Narita T, Qi B, Murakami M, Sugiya H. Pilocarpine induces the residual secretion of salivary fluid in perfused submandibular glands of rats. PLoS One 14: e0221832, 2019. doi: 10.1371/journal.pone.0221832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Ha YJ, Lee YJ. Pharmacological management of Sjögren’s syndrome. In: Sjögren’s Syndrome and Oral Health, edited by Cha S. Cham, Switzerland: Springer, 2021, p. 197–215. [Google Scholar]
- 528.Yasuda H, Niki H. Review of the pharmacological properties and clinical usefulness of muscarinic agonists for xerostomia in patients with Sjogren’s syndrome. Clin Drug Investig 22: 67–73, 2002. doi: 10.2165/00044011-200222020-00001. [DOI] [PubMed] [Google Scholar]
- 529.Forner L, Hyldegaard O, von Brockdorff AS, Specht L, Andersen E, Jansen EC, Hillerup S, Nauntofte B, Jensen SB. Does hyperbaric oxygen treatment have the potential to increase salivary flow rate and reduce xerostomia in previously irradiated head and neck cancer patients? A pilot study. Oral Oncol 47: 546–551, 2011. doi: 10.1016/j.oraloncology.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 530.Hadley T, Song C, Wells L, Lehnhardt J, Rogers MW, Anderson J, Terry M, Novy B, Lo T. Does hyperbaric oxygen therapy have the potential to improve salivary gland function in irradiated head and neck cancer patients? Med Gas Res 3: 15, 2013. doi: 10.1186/2045-9912-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Furness S, Bryan G, McMillan R, Worthington HV. Interventions for the management of dry mouth: non-pharmacological interventions. Cochrane Database Syst Rev 8: CD009603, 2013. doi: 10.1002/14651858.CD009603.pub2. [DOI] [PubMed] [Google Scholar]
- 532.Wolff A, Koray M, Campisi G, Strietzel FP, Lafaurie GI, Beiski BZ, Ekström J. Electrostimulation of the lingual nerve by an intraoral device may lead to salivary gland regeneration: a case series study. Med Oral Patol Oral Cir Bucal 23: e552–e559, 2018. doi: 10.4317/medoral.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.King M, Joseph S, Albert A, Thomas TV, Nittala MR, Woods WC, Vijayakumar S, Packianathan S. Use of amifostine for cytoprotection during radiation therapy: a review. Oncology 98: 61–80, 2020. doi: 10.1159/000502979. [DOI] [PubMed] [Google Scholar]
- 534.Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W, Sauer R. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 18: 3339–3345, 2000. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 535.Vitolo JM, Cotrim AP, Sowers AL, Russo A, Wellner RB, Pillemer SR, Mitchell JB, Baum BJ. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res 10: 1807–1812, 2004. doi: 10.1158/1078-0432.CCR-03-0194. [DOI] [PubMed] [Google Scholar]
- 536.Montenegro RC, Howarth A, Ceroni A, Fedele V, Farran B, Mesquita FP, Frejno M, Berger BT, Heinzlmeir S, Sailem HZ, Tesch R, Ebner D, Knapp S, Burbano R, Kuster B, Müller S. Identification of molecular targets for the targeted treatment of gastric cancer using dasatinib. Oncotarget 11: 535–549, 2020. doi: 10.18632/oncotarget.27462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Wie SM, Wellberg E, Karam SD, Reyland ME. Tyrosine kinase inhibitors protect the salivary gland from radiation damage by inhibiting activation of protein kinase C-delta. Mol Cancer Ther 16: 1989–1998, 2017. doi: 10.1158/1535-7163.MCT-17-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Choi JS, Shin HS, An HY, Kim YM, Lim JY. Radioprotective effects of keratinocyte growth factor-1 against irradiation-induced salivary gland hypofunction. Oncotarget 8: 13496–13508, 2017. doi: 10.18632/oncotarget.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Cho JM, Yoon YJ, Lee S, Kim D, Choi D, Kim J, Lim JY. Retroductal delivery of epidermal growth factor protects salivary progenitors after irradiation. J Dent Res 100: 883–890, 2021. doi: 10.1177/0022034521999298. [DOI] [PubMed] [Google Scholar]
- 540.Hill G, Headon D, Harris ZI, Huttner K, Limesand KH. Pharmacological activation of the EDA/EDAR signaling pathway restores salivary gland function following radiation-induced damage. PLoS One 9: e112840, 2014. doi: 10.1371/journal.pone.0112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Zhu Z, Pang B, Iglesias-Bartolome R, Wu X, Hu L, Zhang C, Wang J, Gutkind JS, Wang S. Prevention of irradiation-induced salivary hypofunction by rapamycin in swine parotid glands. Oncotarget 7: 20271–20281, 2016. doi: 10.18632/oncotarget.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Morgan-Bathke M, Harris ZI, Arnett DG, Klein RR, Burd R, Ann DK, Limesand KH. The rapalogue, CCI-779, improves salivary gland function following radiation. PLoS One 9: e113183, 2014. doi: 10.1371/journal.pone.0113183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Pringle S, Wang X, Bootsma H, Spijkervet FK, Vissink A, Kroese FG. Small-molecule inhibitors and the salivary gland epithelium in Sjogren’s syndrome. Expert Opin Investig Drugs 28: 605–616, 2019. doi: 10.1080/13543784.2019.1631796. [DOI] [PubMed] [Google Scholar]
- 544.Mariette X, Seror R, Quartuccio L, Baron G, Salvin S, Fabris M, Desmoulins F, Nocturne G, Ravaud P, De Vita S. Efficacy and safety of belimumab in primary Sjogren’s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis 74: 526–531, 2015. doi: 10.1136/annrheumdis-2013-203991. [DOI] [PubMed] [Google Scholar]
- 545.De Vita S, Quartuccio L, Salvin S, Picco L, Scott CA, Rupolo M, Fabris M. Sequential therapy with belimumab followed by rituximab in Sjogren’s syndrome associated with B-cell lymphoproliferation and overexpression of BAFF: evidence for long-term efficacy. Clin Exp Rheumatol 32: 490–494, 2014. [PubMed] [Google Scholar]
- 546.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot JM, Perdriger A, Puéchal X, Le Guern V, Sibilia J, Gottenberg JE, Chiche L, Hachulla E, Hatron PY, Goeb V, Hayem G, Morel J, Zarnitsky C, Dubost JJ, Pers JO, Nowak E, Saraux A. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med 160: 233–242, 2014. doi: 10.7326/M13-1085. [DOI] [PubMed] [Google Scholar]
- 547.Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, Elting LS, Langendijk JA, Coppes RP, Reyland ME. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys 78: 983–991, 2010. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Nanduri LS, Baanstra M, Faber H, Rocchi C, Zwart E, de Haan G, van Os R, Coppes RP. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Reports 3: 957–964, 2014. doi: 10.1016/j.stemcr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Lee HW, Hsiao YC, Chen YC, Young TH, Yang TL. Salispheres from different major salivary glands for glandular regeneration. J Dent Res 98: 786–794, 2019. doi: 10.1177/0022034519847122. [DOI] [PubMed] [Google Scholar]
- 550.Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol 92: 466–471, 2009. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 551.Pringle S, Maimets M, van der Zwaag M, Stokman MA, van Gosliga D, Zwart E, Witjes MJ, de Haan G, van Os R, Coppes RP. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34: 640–652, 2016. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- 552.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3: e2063, 2008. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP, Coppes RP. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol 108: 458–463, 2013. doi: 10.1016/j.radonc.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 554.Xiao N, Lin Y, Cao H, Sirjani D, Giaccia AJ, Koong AC, Kong CS, Diehn M, Le QT. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest 124: 3364–3377, 2014. doi: 10.1172/JCI74096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Gao X, Wu Y, Liao L, Tian W. Oral organoids: progress and challenges. J Dent Res 100: 454–463, 2021. doi: 10.1177/0022034520983808. [DOI] [PubMed] [Google Scholar]
- 556.Robey P. “Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Res 6: 524, 2017. doi: 10.12688/f1000research.10955.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19: 35–42, 2013. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells—current trends and future prospective. Biosci Rep 35: e00191, 2015. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Togarrati PP, Sasaki RT, Abdel-Mohsen M, Dinglasan N, Deng X, Desai S, Emmerson E, Yee E, Ryan WR, da Silva MC, Knox SM, Pillai SK, Muench MO. Identification and characterization of a rich population of CD34+ mesenchymal stem/stromal cells in human parotid, sublingual and submandibular glands. Sci Rep 7: 3484, 2017. doi: 10.1038/s41598-017-03681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Prockop DJ. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy 19: 1–8, 2017. doi: 10.1016/j.jcyt.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Aldahmash A, Zaher W, Al-Nbaheen M, Kassem M. Human stromal (mesenchymal) stem cells: basic biology and current clinical use for tissue regeneration. Ann Saudi Med 32: 68–77, 2012. doi: 10.5144/0256-4947.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Jensen DH, Oliveri RS, Trojahn Kølle SF, Fischer-Nielsen A, Specht L, Bardow A, Buchwald C. Mesenchymal stem cell therapy for salivary gland dysfunction and xerostomia: a systematic review of preclinical studies. Oral Surg Oral Med Oral Pathol Oral Radiol 117: 335–342.e1, 2014. doi: 10.1016/j.oooo.2013.11.496. [DOI] [PubMed] [Google Scholar]
- 563.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, Bromberg JS, Chen W, Sun L, Wang S. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood 120: 3142–3151, 2012. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Chen W, Yu Y, Ma J, Olsen N, Lin J. Mesenchymal stem cells in primary Sjögren’s syndrome: prospective and challenges. Stem Cells Int 2018: 4357865, 2018. doi: 10.1155/2018/4357865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Grønhøj C, Jensen DH, Glovinski PV, Jensen SB, Bardow A, Oliveri RS, Specht L, Thomsen C, Darkner S, Kiss K, Fischer-Nielsen A, von Buchwald C. First-in-man mesenchymal stem cells for radiation-induced xerostomia (MESRIX): study protocol for a randomized controlled trial. Trials 18: 108, 2017. doi: 10.1186/s13063-017-1856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Grønhøj C, Jensen DH, Vester-Glowinski P, Jensen SB, Bardow A, Oliveri RS, Fog LM, Specht L, Thomsen C, Darkner S, Jensen M, Müller V, Kiss K, Agander T, Andersen E, Fischer-Nielsen A, von Buchwald C. Safety and efficacy of mesenchymal stem cells for radiation-induced xerostomia: a randomized, placebo-controlled Phase 1/2 trial (MESRIX). Int J Radiat Oncol Biol Phys 101: 581–592, 2018. doi: 10.1016/j.ijrobp.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 567.Bamba R, Shadfar S, Van Natta BW. Fat grafting as a novel treatment for xerostomia. J Craniofac Surg 32: e211–e215, 2021. doi: 10.1097/SCS.0000000000006894. [DOI] [PubMed] [Google Scholar]
- 568.I T, Sumita Y, Yoshida T, Honma R, Iwatake M, Raudales JL, Shizuno T, Kuroshima S, Masuda H, Seki M, Tran SD, Asahara T, Asahina I. Anti-inflammatory and vasculogenic conditioning of peripheral blood mononuclear cells reinforces their therapeutic potential for radiation-injured salivary glands. Stem Cell Res Ther 10: 304, 2019. doi: 10.1186/s13287-019-1414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Sumita Y, Iwamoto N, Seki M, Yoshida T, Honma R, Iwatake M, Ohba S, Takashi I, Hotokezaka Y, Harada H, Kuroshima S, Nagai K, Asahara T, Atsushi Kawakam I, Asahina I. Phase 1 clinical study of cell therapy with effective-mononuclear cells (E-MNC) for radiogenic xerostomia (first-in-human study) (FIH study on E-MNC therapy for radiogenic xerostomia). Medicine (Baltimore) 99: e20788, 2020. doi: 10.1097/MD.0000000000020788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods 4: 227–230, 2007. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 571.Ogawa M, Tsuji T. Functional salivary gland regeneration. Methods Mol Biol 1597: 135–151, 2017. doi: 10.1007/978-1-4939-6949-4_10. [DOI] [PubMed] [Google Scholar]
- 572.Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T, Tsuji T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun 4: 2498, 2013. doi: 10.1038/ncomms3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Ogawa M, Yamashita K, Niikura M, Nakajima K, Toyoshima KE, Oshima M, Tsuji T. Saliva secretion in engrafted mouse bioengineered salivary glands using taste stimulation. J Prosthodont Res 58: 17–25, 2014. doi: 10.1016/j.jpor.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 574.Tanaka J, Ogawa M, Hojo H, Kawashima Y, Mabuchi Y, Hata K, Nakamura S, Yasuhara R, Takamatsu K, Irié T, Fukada T, Sakai T, Inoue T, Nishimura R, Ohara O, Saito I, Ohba S, Tsuji T, Mishima K. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat Commun 9: 4216, 2018. doi: 10.1038/s41467-018-06469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Baum BJ, Wang S, Cukierman E, Delporte C, Kagami H, Marmary Y, Fox PC, Mooney DJ, Yamada KM. Re-engineering the functions of a terminally differentiated epithelial cell in vivo. Ann NY Acad Sci 875: 294–300, 1999. doi: 10.1111/j.1749-6632.1999.tb08512.x. [DOI] [PubMed] [Google Scholar]
- 576.Barrows CM, Wu D, Farach-Carson MC, Young S. Building a functional salivary gland for cell-based therapy: more than secretory epithelial acini. Tissue Eng Part A 26: 1332–1348, 2020. doi: 10.1089/ten.tea.2020.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Martinez M, Wu D, Farach-Carson MC, Harrington DA. Matrix biology of the salivary gland: a guide for tissue engineering. In: Salivary Gland Development and Regeneration, edited by Cha S. Cham, Switzerland: Springer, 2017. [Google Scholar]
- 578.Lee SW, Ryu JH, Do MJ, Namkoong E, Lee H, Park K. NiCHE platform: nature-inspired catechol-conjugated hyaluronic acid environment platform for salivary gland tissue engineering. ACS Appl Mater Interfaces 12: 4285–4294, 2020. doi: 10.1021/acsami.9b20546. [DOI] [PubMed] [Google Scholar]
- 579.Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater 5: 539–551, 2020. doi: 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Pradhan S, Farach-Carson MC. Mining the extracellular matrix for tissue engineering applications. Regen Med 5: 961–970, 2010. doi: 10.2217/rme.10.61. [DOI] [PubMed] [Google Scholar]
- 581.Shubin AD, Felong TJ, Graunke D, Ovitt CE, Benoit DS. Development of poly(ethylene glycol) hydrogels for salivary gland tissue engineering applications. Tissue Eng Part A 21: 1733–1751, 2015. doi: 10.1089/ten.TEA.2014.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Shubin AD, Felong TJ, Schutrum BE, Joe DS, Ovitt CE, Benoit DS. Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater 50: 437–449, 2017. doi: 10.1016/j.actbio.2016.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Song Y, Uchida H, Sharipol A, Piraino L, Mereness JA, Ingalls MH, Rebhahn J, Newlands SD, DeLouise LA, Ovitt CE, Benoit DS. Development of a functional salivary gland tissue chip with potential for high-content drug screening. Commun Biol 4: 361, 2021. doi: 10.1038/s42003-021-01876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Pradhan S, Zhang C, Jia X, Carson DD, Witt R, Farach-Carson MC. Perlecan domain IV peptide stimulates salivary gland cell assembly in vitro. Tissue Eng Part A 15: 3309–3320, 2009. doi: 10.1089/ten.TEA.2008.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Pradhan S, Liu C, Zhang C, Jia X, Farach-Carson MC, Witt RL. Lumen formation in three-dimensional cultures of salivary acinar cells. Otolaryngol Head Neck Surg 142: 191–195, 2010. doi: 10.1016/j.otohns.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 586.Srinivasan PP, Patel VN, Liu S, Harrington DA, Hoffman MP, Jia X, Witt RL, Farach-Carson MC, Pradhan-Bhatt S. Primary salivary human stem/progenitor cells undergo microenvironment-driven acinar-like differentiation in hyaluronate hydrogel culture. Stem Cells Transl Med 6: 110–120, 2017. doi: 10.5966/sctm.2016-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Pradhan-Bhatt S, Harrington DA, Duncan RL, Farach-Carson MC, Jia X, Witt RL. A novel in vivo model for evaluating functional restoration of a tissue-engineered salivary gland. Laryngoscope 124: 456–461, 2014. doi: 10.1002/lary.24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Pradhan-Bhatt S, Harrington DA, Duncan RL, Jia X, Witt RL, Farach-Carson MC. Implantable three-dimensional salivary spheroid assemblies demonstrate fluid and protein secretory responses to neurotransmitters. Tissue Eng Part A 19: 1610–1620, 2013. doi: 10.1089/ten.tea.2012.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Mota C, Camarero-Espinosa S, Baker MB, Wieringa P, Moroni L. Bioprinting: from tissue and organ development to in vitro models. Chem Rev 120: 10547–10607, 2020. doi: 10.1021/acs.chemrev.9b00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Adine C, Ng KK, Rungarunlert S, Souza GR, Ferreira JN. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials 180: 52–66, 2018. doi: 10.1016/j.biomaterials.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 591.Ferreira JN, Hasan R, Urkasemsin G, Ng KK, Adine C, Muthumariappan S, Souza GR. A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids. J Tissue Eng Regen Med 13: 495–508, 2019. doi: 10.1002/term.2809. [DOI] [PubMed] [Google Scholar]
- 592.Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Billings ME, Goldsmith CM, Tandon M, Helmerhorst EJ, Catalán MA, Danielides SJ, Perez P, Nikolov NP, Chiorini JA, Melvin JE, Oppenheim FG, Illei GG, Baum BJ. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther 24: 176–186, 2017. doi: 10.1038/gt.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, Nottingham LK, Rudy SF, Van Waes C, Whatley MA, Brahim JS, Chiorini JA, Danielides S, Turner RJ, Patronas NJ, Chen CC, Nikolov NP, Illei GG. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA 109: 19403–19407, 2012. doi: 10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Gao R, Yan X, Zheng C, Goldsmith CM, Afione S, Hai B, Xu J, Zhou J, Zhang C, Chiorini JA, Baum BJ, Wang S. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther 18: 38–42, 2011. doi: 10.1038/gt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Li SS, Wu CZ, Zhang BW, Qiu L, Chen W, Yuan YH, Liu XC, Li CJ, Li LJ. Nerve growth factor protects salivary glands from irradiation-induced damage. Life Sci 265: 118748, 2021. doi: 10.1016/j.lfs.2020.118748. [DOI] [PubMed] [Google Scholar]
- 596.Mauduit O, Aure MH, Delcroix V, Basova L, Srivastava A, Umazume T, Mays JW, Bellusci S, Tucker AS, Hajihosseini MK, Hoffman MP, Makarenkova HP. A mesenchymal to epithelial switch in Fgf10 expression specifies an evolutionary-conserved population of ionocytes in salivary glands. Cell Rep 39: 110663, 2022. doi: 10.1016/j.celrep.2022.110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Ghosh S, Kumar M, Santiana M, Mishra A, Zhang M, Labayo H, Chibly AM, Nakamura H, Tanaka T, Henderson W, Lewis E, Voss O, Su Y, Belkaid Y, Chiorini JA, Hoffman PM, Altan-Bonnet N. Enteric viruses replicate in salivary glands and infect through saliva. Nature. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


