Abstract
Congenital disorders of glycosylation (CDG) are ultra-rare, genetically, and clinically heterogenous metabolic disorders. Although the number of identified CDG is growing rapidly, there are few therapeutic options. Most treatments involve dietary supplementation with monosaccharides or other precursors. These approaches are relatively safe, but in many cases the molecular and biochemical underpinnings are incomplete. Recent studies demonstrate that yeast, worm, fly and zebrafish models of CDG are powerful tools in screening repurposed drugs, ushering a new avenue to search for novel therapeutic options. Here we present a perspective on compounds which are currently in use for CDG treatment or have a potential to be applied as therapeutics in the near future.
INTRODUCTION
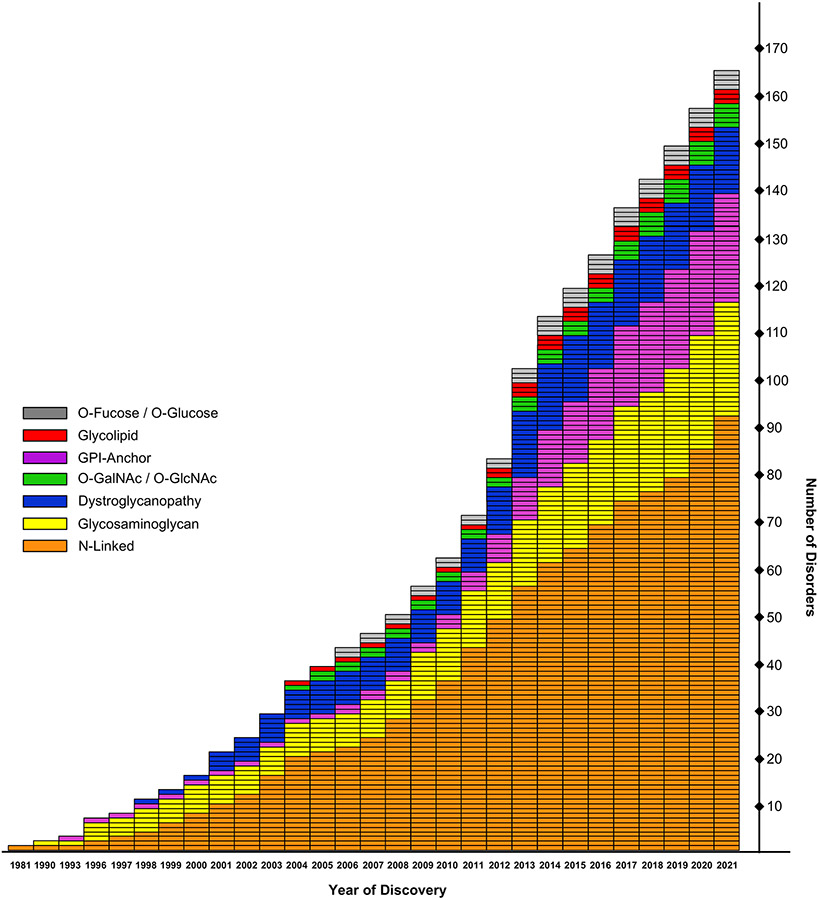
Congenital disorders of glycosylation (CDG) are ultra-rare metabolic diseases resulting from defects in various steps of glycan synthesis. 165 different CDG have currently been identified and more than half of them are due to deficiencies that affect the N-glycosylation pathway (Fig. 1) 1. For physicians, a CDG diagnosis is frequently challenging, even in an academic setting because they are often unfamiliar with glycoscience. Also, CDG often present with a broad spectrum of clinical symptoms, such as developmental delay, intellectual disability, hypotonia and seizures 2. Expanded use of efficient DNA sequencing technologies, declining cost, and coverage by some insurance plans has helped speed identification of a pathogenic variants in glycosylation-related genes and biochemical approaches can validate pathogenicity. In some cases, glycosylation of serum glycoproteins, such as transferrin (TF) or apolipoprotein C-III, can be used as a diagnostic biomarkers, however this is not ideal as not all CDG affect N- or O-glycosylation. There is no serum marker that can be used for diagnostic purposes in the dystroglycanopathies or defects in GPI-anchor biosynthesis. In addition, serum only represents a subpopulation of the cells as it mainly contains proteins produced by the liver and B-cells. To make it even more challenging, in some authentic subjects, serum glycosylation spontaneously or gradually normalizes over time hampering the diagnostic process 1.
Figure 1. Identification of congenital disorders of glycosylation.
Graph represents the distribution of CDG by the year they were identified, which were grouped according to affected glycosylation pathway. This figure is an updated version adapted from 68, 69.
A significant deficiency in the field is the lack of good cellular models to study molecular mechanisms of different CDG. Access to fibroblasts derived from affected individuals is limited, however they remain the most commonly used model in studying CDG and are often employed to confirm or validate a diagnosis. They are also used to study potential therapeutic compounds. However, their utility is finite as many disorders exhibit tissue-specific phenotypes, not recapitulated by these cells 3. Recent studies using yeast, worm, zebrafish and fly models provided promising candidates for the treatment of a few glycosylation disorders, ushering in a new avenue for repurposing approved drugs 4, 5. However, positive impact of such compounds needs to be cross validated using other model organisms. Induced pluripotent stem cells (iPSCs) provide an alternative to proposed approaches that can be used for in vitro drug screening tests, although iPSCs generation is only possible when fibroblasts are available 6.
The growing number of identified disorders underscores the paucity of current therapies, which are only available for approximately 10% of the identified CDG (Table 1) 7. However, if one considered a total number of diagnosed cases the number of individuals for whom the treatment is available is significantly less. Several therapeutic strategies rely on supplementation of the individual’s diet with the appropriate monosaccharide. These approaches are relatively safe and have been applied in a growing number of cases, even though the mechanism behind the successful treatment is often incompletely understood 8. Depending on the disorder the mode of action determining successful therapy is different, e.g., in some cases monosaccharide supplementation increases the cytosolic concentration of limiting nucleotide sugar (like in PGM1-CDG), in others it provides an alternative substrate for nucleotide sugar biosynthesis (like in MPI-CDG). It is important to remember that in the majority of therapies more efforts are required to fully understand the mechanism determining the success or failure of the dietary supplementation with monosaccharides. Other treatment options include organ transplantation and gene therapy; however, they are only feasible in a small number of disorders. Current efforts focus on developing alternative approaches, such as delivery of activated sugar compounds or pharmacological chaperones, which bind directly to the mutated protein, rescuing its function 7. It is important to underline that CDG are congenital, therefore not all the symptoms can be reversed with a therapy. The defects related to processes with a limited prenatal development window such as impaired brain development, will not improve upon the treatment. In contrast, ongoing metabolic process, such as bone growth, might benefit from a therapy. Here we focus on selected therapeutic strategies for the treatment of selected glycosylation disorders, focusing on different group of compounds and trying to address their possible mechanism of action.
Table 1. List of congenital disorders of glycosylation with available therapy.
Adapted from Sosicka et al. 3.
| Disorder | Gene | Function | Therapy |
|---|---|---|---|
| MPI-CDG | MPI | Fruc-6P ←→ Man-6P | Oral supplementation with Man |
| PGM1-CDG | PGM1 | Glc-6P ←→ Glc-1P | Oral supplementation with Gal |
| SLC35A2-CDG | SLC35A2 | Golgi localized UDP-Gal transporter | Oral supplementation with Gal |
| SLC39A8-CDG | SLC39A8 | Plasma membrane transporter of Mn, Zn, Cd, Fe | Oral supplementation with Gal or Mn |
| TMEM165-CDG | TMEM165 | Supplies Ca2+ and Mn2+ ions to the Golgi in exchange for proton | Oral supplementation with Gal or Mn |
| SLC35C1-CDG | SLC35C1 | Golgi localized GDP-Fuc transporter | Oral supplementation with Fuc |
| CAD-CDG | CAD | De novo biosynthesis of pyridine nucleotides | Oral supplementation with uridine |
| GNE myopathy | GNE | UDP-GlcNAc → ManNAc + UDP ManNAc + UTP → ManNAc-6P + PPi |
Oral supplementation with ManNAc Gene therapy |
| NANS deficiency | NANS | ManNAc-6P + PEP → Neu5Ac-9P + Pi | Experimental therapy with oral Neu5Ac supplementation |
| Fukuyama-type CMD | FKTN | Transferase adding the first Rib-5P to core M3 of O-Man glycans | Experimental therapy with oral Rib supplementation |
| DOLK-CDG | DOLK | Dol + CTP → Dol-P + PPi | Heart transplantation |
| ATP6P1-CDG | ATP6AP1 | V-ATPase subunit, Golgi pH maintainer | Liver transplantation |
| CCDC115-CDG | CCDC115 | Participates in V-ATPase assembly | Liver transplantation |
| PNH | PIGA | Catalytic subunit of GlcNAc-PI synthase | Bone marrow transplantation |
| PGM3-CDG | PGM3 | GlcNAc-6P ←→ GlcNAc-1P | Bone marrow transplantation |
Fruc – fructose; Man – mannose; Glc – glucose; Gal – galactose; GlcNAc – N-acetylglucosamine; Fuc – fucose; ManNAc – N-acetylmannosamine; Neu5Ac – N-acetylneuraminic acid; PEP – phosphoenolpyruvate; Rib – ribitol; Dol – dolichol; PNH – paroxysmal nocturnal hemoglobinuria; PI – phosphatidylinositol
THERAPEUTIC APPROACHES IN PMM2-CDG
Pathogenic variants in PMM2 cause PMM2-CDG, the most prevalent disorder of glycosylation, with an estimated 1000 cases worldwide 1. Phosphomannomutase 2 (PMM2) converts mannose-6-phosphate (mannose-6P) to mannose-1P, which supplies GDP-mannose production. This autosomal recessive CDG is characterized by multisystem phenotypes, mostly showing neurological involvement, due to N-linked hypoglycosylation of proteins. Around 20% of PMM2-CDG children die in the first four years of life.
In 2016, the first clinically relevant mouse model of PMM2-CDG was generated. These heterozygous mice, carrying the most frequent human variant in PMM2, R137H (human R141H) as well as a variant F115L (human F119L), recapitulated many disease features seen in the affected subjects. Prenatal lethality was high, and the survivors had significantly stunted growth, likely due to a deficiency in growth-related glycoproteins such as insulin-like grow factor 1 9. Similar to the fibroblasts derived from PMM2-CDG individuals 10, mouse fibroblasts exhibit reduced PMM2 activity, decreased levels of GDP-mannose and truncated lipid-linked oligosaccharide precursors as well as hypoglycosylation of total cellular N-glycoproteins 9.
The first attempts to treat PMM2-CDG relied on dietary supplementation with mannose, which was, at the time, already applied as a therapy in another CDG (described in detailed in the next section). Initial studies in PMM2-CDG fibroblasts reveled that the addition of mannose to the cell culture medium increased the GDP-mannose pool and partially corrected the impaired synthesis of lipid linked oligosaccharide 11. However, pilot clinical studies did not support a benefit of mannose supplementation in these individuals. Although the therapy did not cause any serious side effects, the level of hypoglycosylated serum TF did not change or even increased in some subjects 12, 13. In 2020, a single study on PMM2-CDG individuals supplemented with a high doses of mannose showed promising results indicating improved protein glycosylation upon the treatment 14. However, one needs to be very careful while interpreting these results, because they stand in opposition to many other clinical reports gathered over the last 20 years and, critically, the study lacked any untreated controls. Intravenous mannose treatment in a single PMM2-CDG individual was also not effective 15. Moreover, the most recent research demonstrates that glycosylation of TF spontaneously improves with time in multiple PMM2-CDG subjects 16. Recently, in a pilot trial, of oral galactose therapy in PMM-CDG was conducted, as this treatment had been successfully applied to a few other CDG (described in detailed in the next section). Overall, oral galactose therapy did not result in significant improvement, but the authors claim some milder subjects showed positive clinical changes with a trend toward improved TF glycosylation 17. However, it is not possible to determine whether these limited improvements were spontaneous or the result of galactose supplements.
Attempts have been made to deliver membrane permeable derivatives of mannose-1P with some of these compounds improving glycosylation in PMM2-CDG fibroblasts. However, most were highly toxic due to subsequent cleavage of acetoxymethyl groups covering the phosphate charge 18-20. That is why current trials focus on encapsulating mannose-1P into liposomes, even though such a compound would be quickly removed by the liver. Alternative studies concentrate on the delivery of GDP-mannose, using nanoparticles 21. This approach may be particularly useful as nanoparticles can cross the brain blood barrier when coated with a glycopeptide linked to the endothelial opioid receptor. Considering most PMM2-CDG symptoms are due to neurological impairment, these features might be beneficial for affected individuals 22, although some pathology is irreversible since it occurred during embryonic development. Currently, none of these compounds are in clinical trials.
It had been reported that 80% of all pathogenic variants in PMM2 are missense mutations 23 and most are likely to result in the increased susceptibility to degradation and/or aggregation. Therefore, functional characterization of these disease-causing variants in PMM2-CDG led to therapeutic strategies involving pharmacological chaperones, which are small molecules that bind to and stabilize PMM2 protein, facilitating its dimerization which is required for PMM2 function 24. Screening of 10,000 low-molecular-weight pharmacological chaperones identified compounds capable of stabilizing both wild-type and mutant PMM2 and in some cases increased mutant PMM2 activity by 10-30%. Although this increase is promising, PMM2 variants remain less active than a wild-type protein 24. Recently, studies focusing on pharmacological chaperones designed based on the PMM2 natural ligand, α-glucose-1,6-bisphosphate, identified β-glucose-1,6-bisphosphate as a compound efficiently stabilizing PMM2. This ligand acts as a mild non-covalent inhibitor of PMM2, that was proved to stabilize the homodimers of F119L and V129M pathogenic mutants, however its effect on their activity has not been tested 25.
Several pathogenic variants of PMM2, including L32R, D65Y, V231M and T237M, affect normal mRNA splicing, making them a potential candidate for antisense therapy that involves the use of antisense oligonucleotides to target and repair mutant mRNA 22. It is worth mentioning that none of these variants has a high prevalence. Antisense morpholino oligonucleotides targeting mutant splicing sites in PMM2-CDG fibroblasts restored a normal splicing profile and 30-40% increase of the PMM2 enzymatic activity, but it was still below the normal activity range 26. Some PMM2-CDG subjects have high residual activity of the enzyme and even a single boost may be sufficient to observe a clinical improvement. Although some antisense therapies have already been approved for other disorders, no clinical trials have been introduced for PMM2-CDG and one has to be aware that a number of such treatments failed during trials.
In 2019, acetazolamide, an FDA-approved drug that lowers plasma pH and increases intracellular calcium availability, was repurposed as a treatment for PMM2-CDG. A study on 24 affected subjects demonstrated acetazolamide improves coagulation parameters, clinical severity, ataxia, epilepsy, and lipodystrophy in examined individuals. 27. PMM2 activity depends on calcium binding and the authors claim it is probably how acetazolamide helps PMM2-CDG subjects 7. Currently, the drug is in phase II clinical trials to establish if it could be repurposed to treat cerebellar impairment in PMM2-CDG.
Recently, Iyer et al. generated a novel PMM2-CDG worm model, as part of a multispecies drug repurposing screen 5. Using PMM2-CDG fibroblasts, they tested drug candidates, as well as some other promising hits identified using a yeast model, which was performed in a different study 5, 28. Insights from structure-activity relationship revealed that epalrestat, an aldose reductase inhibitor, effectively increased enzymatic activity of all four tested PMM2 variants. How this drug could help PMM2-CDG subjects is yet unknown, but one possibility is aldose reductase inhibition diverts glucose from the polyol pathway to glucose-1,6-bisphosphate, an endogenous stabilizer, and coactivator of PMM2 homodimerization 5. Currently, a pilot clinical trial testing applicability of epalrestat in PMM2-CDG has begun.
MANNOSE SUPPLEMENTATION IN MPI-CDG
Mannose phosphate isomerase (MPI) catalyzes interconversion of fructose-6P and mannose-6P, providing a substrate for PMM2 and playing a key role in maintaining the supply of GDP-mannose required for glycosylation. MPI-CDG differs from many other glycosylation disorders as it lacks neurological involvement, manifesting with severe diarrhea and vomiting and liver abnormalities 3. MPI-CDG was the first disorder of glycosylation found to be efficiently treated by dietary supplementation with a monosaccharide, mannose 29. This therapy has been successfully applied in at least 26 affected individuals described in the literature. Recommended dose ranges vary between 150 to 170 mg/kg/dose 4-5 times daily. Mannose is well absorbed in the gut and within 1 to 2 hours after ingestion reaches peak blood concentrations. It disappears from the blood with an estimated half-time of 4 hours. High levels of mannose have been correlated with elevated hemoglobin A1C and diabetes in humans, because of its elevated activity in glycation, so dosage needs to be carefully monitored 30. Main side effects of mannose therapy include bloating and diarrhea; however, this can be controlled by appropriated dosing.
A long-term follow-up on eight MPI-CDG subjects, who started their treatment with a median age of seven months and have been on oral mannose for an average of over 12 years, proved efficacy and safety of this therapy. Oral supplementation with mannose corrected serum proteins N-glycosylation and resolved recurring diarrheas. However, liver fibrosis persisted despite treatment, even though two subjects showed an improvement in liver architecture 31.
DIETARY SUPPLEMENTATION WITH FUCOSE
SLC35C1 encodes a Golgi localized transporter of GDP-fucose, and its dysfunction leads to SLC35C1-CDG also known as leukocyte adhesion deficiency II (LADII). In this disorder hypofucosylation of glycans impairs endothelium homing of leukocytes leading to severe immunodeficiency. Dietary supplementation with fucose quickly elevates serum levels of this monosaccharide and normalizes elevated leukocytes by promoting synthesis of Sialyl-Lewis X on neutrophiles, which improves their rolling on the endothelium 32, 33. Successful application of this therapy is inconsistent with the broadly accepted dogma established in mid-1970s, demonstrating over 90% of glycan-associated fucose is supplied by the de novo pathway, where GDP-fucose originates either from mannose or glucose. Less than 10% of GDP-fucose is produced from exogenous fucose, and strongly suggests dietary supplementation with this monosaccharide should not have been effective 34, 35. One possible explanation is the study only used a single fucose concentration 0.3 μM and one cell line, HeLa. This amount of fucose was insignificantly low compared to <5 μM serum concentration of fucose and no dose response analysis was conducted. Therefore, much more effort is required to fully understand glycan-associated metabolism of fucose. If dietary fucose was a more prevalent source of fucose, it may increase a pool of GDP-fucose, making it more available to the SLC35C1 transporter, having an overall positive impact on fucosylation in LADII subjects. Our most recent research strongly indicates a superior role of exogenous fucose over that produced by the de novo pathway. Low concentrations of exogenous fucose progressively inhibit the de novo pathway, and completely by ~30-50μM 36. This observation can help explain the success of fucose therapy in some cases of LADII.
Fucosyltransferase 8 (FUT8) is a Golgi-localized α1,6-fucosyltransferase, essential for transferring the core fucose onto N-glycans. In FUT8-CDG, severe neurological and skeletal abnormalities occur due to the hypofucosylation of N-glycoproteins 37. Recently, non-identical twins with severe FUT8-CDG received oral fucose supplements over a five month period and showed moderate clinical improvement, however without proper controls or a natural history of this disorder, it is difficult to show fucose was responsible. The treatment showed only mild improvement of fucosylation. No side effects were noted during fucose supplementation, with regular monitoring showing normal liver and kidney function tests 38. It indicates dietary supplementation with fucose is relatively safe in FUT8-CDG subjects, however more studies are required to confirm the benefits of this treatment.
Fucokinase (FCSK) activates fucose to fucose-1P, which directly enters the cell or is salvaged from glycoproteins and is further converted to GDP-fucose. In 2018 our group identified two individuals with pathogenic variants in FCSK, leading to decreased enzymatic activity of fucokinase 39. Serum N-glycan analysis from one of the subjects did not reveal any changes in fucosylation. However, serum primary represents glycans produced by the liver and it is possible that this organ was not affected in this individual. If hypofucosylation was identified in FCSK-CDG individuals, oral fucose therapy may also be helpful in these cases, however no trials have been performed yet.
DIETARY SUPPLEMENTATION WITH GALACTOSE
Phosphoglucomutase 1 (PGM1) catalyzes the interconversion of glucose-1P and glucose-6P. Subsequently, glucose-1P can be converted to UDP-glucose, serving as a substrate in biosynthesis of UDP-galactose, UDP-glucuronic acid and UDP-xylose 8. PGM1 deficiency affects glycogen metabolism, glycolysis and protein glycosylation. This disorder characterizes with dual TF glycosylation pattern, with reduced site occupancy and hypogalactosylation of glycans. A pilot study on six PGM1-CDG individuals showed that dietary supplementation with galactose effectively corrected glycosylation defects and improved some clinical parameters 40. Further research on a larger cohort showed galactose therapy was safe in doses up to 1.5 g/kg/day with quick and significant clinical and metabolic improvements in PGM1-CDG subjects. However, it does not completely normalize protein glycosylation and TF profiles remain abnormal. When hypoglycosylation of TF corrects upon the treatment much more efficiently then reduced site occupancy, it is unknown how the latter phenotype improves over the galactose supplementation 41. It is hypothesized galactose therapy in PGM1-CDG bypasses the metabolic defects in UDP-galactose and UDP-glucose production by boosting depleted levels of galactose-1P. Replenishing UDP-glucose rescues ER-linked glycosylation, while increasing supplies of UDP-galactose improves Golgi-associated glycosylation 42. A single case study demonstrated that in some individuals even very high doses of galactose i.e., 2.5 g/kg/day did not result in complete normalization of protein glycosylation and did not improve a growth rate.
SLC35A2 encodes a UDP-galactose transporter, which occurs in two splice variants, one localizing to the Golgi apparatus and the other one occupying the ER. Surprisingly, although pathogenic variants in this X-linked gene cause neurological impairments and skeletal abnormalities, most SLC35A2-CDG individuals do not show abnormalities in serum TF N-glycosylation, which impedes the diagnosis. In many SLC35A2-CDG subjects, analysis of TF showed it was only abnormal in the early stages of life and this abnormality normalizes over time. One possible explanation is that liver cells, which produce TF, regenerate and over this process they may select for those carrying the normal allele. That leads to a change in wild type to mutant allele ratios and TF becoming less abnormal 43. Clinical parameters of a cohort of ten SLC35A2-CDG subjects have been evaluated on oral supplementation with galactose and 9 out of 10 significantly improved. However, it is hard to conclude how the treatment affects glycosylation, since in many affected individuals TF glycosylation spontaneously corrects over the time 44. As it was demonstrated for PGM1-CDG where galactose supplementation increased concentration of UDP-galactose 42, it is likely a similar effect in SLC35A2-CDG. However, for the individuals carrying pathogenic variants that cause a complete loss of function or that trap the transporter in the ER this therapy will not be beneficial 45.
Dietary supplementation with galactose was also implemented in two other glycosylation disorders, SLC39A8-CDG and TMEM165-CDG. The latter transports Mn2+, Zn2+, Cd2+ and Fe2+ ions across the plasma membrane and the former most likely supplies Ca2+ and Mn2+ to the Golgi in exchange for protons, maintaining a stable Golgi pH. In both disorders hypogalactosylation of N-glycans is likely due to the poor function of β1,4-galactosyltransferase, caused by insufficient access to Mn2+ ions which are required for enzyme activity 46, 47. These defects can be overcome by increasing UDP-galactose concentration inside the Golgi, which likely happens while subjects supplement their diet with galactose.
URIDINE TREATMENT IN CAD-CDG
CAD encodes a trifunctional protein associated with the enzymatic activities for the first three steps in the pyrimidine biosynthesis pathway: carbamoyl phosphate synthetase, aspartate transcarbamylase and dihydroorotase 48. The process of de novo pyrimidine nucleotide biosynthesis is essential for human cell proliferation and the rate-limiting step on this pathway is catalyzed by the carbamoyl phosphate synthetase, domain of a multifunctional CAD enzyme 49. Pathogenic variants in CAD cause a dramatic decrease in the amount of pyrimidine nucleotides and corresponding nucleotide sugars leading to neurological and hematological aberrations 50. A few studies demonstrated dietary supplementation with uridine quickly and significantly improves health of these subjects 50, 51. However, not all variants in CAD are pathogenic, therefore Caño-Ochoa et al. introduced an assay that allows assessment of pathogenicity of CAD mutants. Initially, affected individuals had been identified using an assay measuring CAD activity-dependent incorporation of 3H-labeled aspartate into nucleic acids and nucleotide sugars. Unfortunately, this method has a low resolution and narrow dynamic range 51. A novel approach to discern benign verses pathogenic variants relies on a CAD-knockout cell line whose growth is dependent on either expression of a functional CAD (carrying a benign variant) or uridine supplementation in the medium. Expression of CAD carrying a pathogenic variant will result in no growth. Testing over thirty CAD variants showed only approximately half were deleterious for CAD enzymatic activity 50. This emphasis the importance of such cell-based analysis methods, especially if possible, therapies exist, as it quickly eliminates benign variants and allows to implement the treatment to authentic cases only.
BIOMETALS IN CDG THERAPY
Biometals plays an important role in a variety of biochemical processes including cell-cell adhesion, energy supply, growth and development, protein folding, maintenance of membrane potential, metabolism of amino acids, lipids, proteins, and glycosylation. They are often used as cofactors for enzymes performing nearly all biosynthesis and redox reaction in energy production and cell metabolism. In glycosylation, glycosyltransferases utilize Mg2+ and Mn2+ as cofactors, which localize in the active sites of these enzymes, playing a role in proper substrate positioning. Ca2+ is essential for the activity of hydrolytic enzymes: glycosidases and sulfatases, which participate in biosynthesis of N-glycans and proteoglycans respectively. Golgi α-mannosidase 2A, lysosomal α-mannosidase 2B and MPI require Zn2+ as a cofactor, while cytosolic α-mannosidase 2C member 1 and lysosomal α-mannosidase 2B member 1 need Co+ to maintain proper activity 52.
As previously mentioned, TMEM165 and SLC39A8 are bivalent ions transporter localized in the Golgi apparatus and plasma membrane respectively. Their defects cause CDG with hypogalactosylation of N-glycans, that can be corrected by dietary supplementation with galactose46, 47. As an alternative, supplementation with Mn2+ was proven to work efficiently in both disorders. A dose of 15-20 mg of MnSO4/kg bodyweight/day was given to two SLC39A8-CDG individuals and showed some improvement in glycosylation and had a considerable impact on clinical parameters. However, the study only involved two subjects and did not include any controls, therefore much more work is required to prove applicability of this treatment 53. In TMEM165-CDG, manganese supplementation completely rescued N-glycosylation defects in TMEM165 knockout HEK293 cells, similar to galactose supplementation. However, in this study, only galactose therapy was tested in TMEM165-CDG subjects, where it partially improved clinical and biochemical parameters, including TF glycosylation. More extensive research is needed to confirm applicability of either galactose or manganese therapy in these subjects, as current observations is insufficient to determine usefulness of either treatment 47. The main obstacle toward successful Mn2+ supplementation is the possibility of toxic effect as chronic exposure to manganese leads to manganism, a disorder characterized by Parkinsonism as well as psychiatric symptoms 54. Therefore, during this treatment, blood Mn2+ measurements are necessary to prevent manganese toxicity.
MAGT1 is a non-catalytic subunit of the STT3B oligosaccharyltransferase complex. Pathogenic variants in MAGT1 leads to primary immunodeficiency, which characterizes with chronic Epstein-Barr virus (EBV) infections attributed to a Mg2+ homeostasis defect known as XMEN 55. In healthy subjects natural killer group 2 member D receptor (NKG2D) exhibits antiviral activity against EBV, however in XMEN individuals this N-glycoprotein is degraded due to hypoglycosylation and does not provide any protection against the virus, allowing it to proliferate 56. The other phenotypes found in MAGT1-deficient subjects include intellectual and developmental disability which are likely due to N-glycosylation defects carried out by STT3B complex 55. Initial in vitro and in vivo studies on a few XMEN individuals showed Mg+ supplementation partially restored NKG2D expression, which improved the cytotoxic function against EBV, however not all the subjects responded to this therapy. Current studies indicate this treatment does not appear effective in the clinical intervention in XMEN disorder 57.
TREATMENT OF NGLY1 DEFICIENCY
NGLY1 encodes N-glycanase-1, a cytosolic glycosidase that catalyzes deglycosylation of retrotranslocated misfolded N-glycoproteins by chopping off the glycan chain before the protein get degraded by the proteasome. It is also a component of the endoplasmic reticulum-associated degradation (ERAD) pathway. NGLY1 deficiency represents the first identified congenital disorder of deglycosylation (NGLY1-CDDG). These children are characterized with developmental delay, neurological involvement, liver disease and alacrima – reduced or absent tear formation 3. Alacrima may be explained by the down-regulation of aquaporins upon NGLY1 knock-down or caused by an enzymatic-independent function of NGLY1 in transcription regulation of multiple aquaporins through transcription factors ATF1/CREB1. Reduced aquaporins expression determines cells resistance to hypotonic-induced cell lysis 58. In the future, this phenotype can be utilized as an easy to monitor marker when assessing the pathogenicity of novel NGLY1 variants.
Although it is expected NGLY1-deficient cells should accumulate misfolded glycoprotein in the cytoplasm, much like a lysosomal storage disorder, this accumulation is not found. Instead, aspartylglycosamine (Asn-GlcNAc) levels significantly increases in the blood of seven analyzed NGLY1-CDDG subjects. Mechanistically, in an absence of NGLY1 activity, endo-β-N-acetylglucosaminidase cleaves the high mannose glycan and generates the Asn-GlcNAc fragment 59. Another characteristic biomarker of this disorder is Asn-GlcNAc-Gal-Neu5Ac oligosaccharide, present in the urine of affected individuals. It is likely derived from addition of galactose and sialic acid to the Asn-GlcNAc primer, probably originating from cleavage of high mannose glycans by cytoplasmic endo-β-N-acetylglucosaminidase 60.
Studies using Drosophila melanogaster as a model organism shed some light on possible therapeutic approaches in NGLY1-CDDG. Owings et al. used flies with NGLY1 deficiency, that resulted in developmental delay and lethality, to test if GlcNAc supplementation was a possible therapy. They observed that the treatment rescued lethality and had a positive impact on the longevity of the flies 61. In another study Rodriguez et al. used a fly model of NGLY1-CDDG to conduct a screen of over 2,500 FDA approved drugs, bioactive compounds, and natural product. They found cholesterol-derived ecdysteroid molting hormone 20-hydroxyecdysone (20E) was able to partially rescue the global developmental delay in the mutant flies. A similar phenotype was observed upon the overexpression of human NGLY1 in tissues involved in ecdysteroidogenesis, e.g., prothoracic gland. However, since 20E is an insect-specific developmental hormone it should not be considered as a drug candidate for NGLY1-CDDG in humans 62.
NGLY1 deficiency has also been shown to impair mitophagy and lead to severely fragmented mitochondria and reduced mitochondrial function 63. The transcription factor NRF1, which regulates mitophagy and gene expression during proteotoxic stress, is deglycosylated and activated by NGLY1 64. Using NGLY1-deficient worm and fly models, Iyer et al. performed a phenotypic drug screen using a library of over 20,000 compounds and identified activators of NRF2, a transcription factor responsible for activating similar pathways/genes as NRF1. Anti-inflammatory drugs as clinical approaches to address the mitochondrial defect observed in NGLY1-deficient cells were also identified 4. Defects in mitophagy results in release of genetic material from mitochondria to the cytoplasm, which activates immune signaling. Hyperactivation of this pathway has been described in several autoimmune and autoinflammatory diseases 64, which may explain the identification of anti-inflammatory drugs as a potential therapeutic hits. In addition, they found that catecholamines and catecholamine receptor activators as another group of drug candidates 4. This is in line with the clinical observation, that NGLY1-CDDG individuals have reduced levels of catecholamine precursors in the cerebral spinal fluid 65.
Recent studies on flies identified Ncc69, fly homologue of human NKCC1, as a modifier of NGLY1 deficiency 66. NKCC1 is a conserved ion co-transporter aiding in the active transport of sodium, potassium and chloride in or out of the cells 67 In cells derived from an NGLY1 −/− mouse model NKCC1 migrates at a higher average molecular weight due to N-glycosylation that does not normally occur. It results in ~50% reduction of its activity, that may explain some of the prominent features of NGLY1 deficiency, such as alacrimia, reduced saliva and sweat production. NKCC1 may be a promising target for NGLY1-CDDG therapies 66.
CONCLUDING REMARKS
Over the last decade, the number of diagnosed genetic disorders resulting from abnormal glycosylation has almost tripled (Fig. 1). However, the rapid increase of identified CDG does not correspond to a significant boost in the discovery rate for novel therapies. In the last couple years, several studies demonstrated the power of model organisms in studying CDG and searching for therapeutic compounds 4, 5, 66, but it is insufficient considering rapidly growing number of disorders, therefore more efforts are needed to understand the biology behind these successful treatments.
Diagnostics is yet another area which requires further improvement. Although next generation sequencing provides a great platform for initial identification of potentially pathogenic variants, they must be distinguished from benign ones. This indicates an urgency in searching for novel biomarkers, that will support the diagnostic process. In parallel, there will be continued necessity to development biochemical assays and cell-based models that will allow us to assess the pathogenicity of these variants. All these aspects should be the main focus of the field in the next several years.
FUNDING:
The Rocket Fund and National Institutes of Health (NIH) grant R01DK099551 supported this work. PS was supported by Frontiers in Congenital Disorders of Glycosylation Career Developmental Award U54 NS115198.
KEYWORDS
- Glycosylation
a post-translational modification which predominantly occurs in the Golgi apparatus and endoplasmic reticulum of eukaryotic cells. In this enzymatic process monosaccharides are covalently attached to macromolecules.
- Hypoglycosylation
reduced and/or insufficient glycosylation.
- Congenital disorders of glycosylation (CDG)
ultra-rare metabolic diseases resulting from defects in various steps of glycan synthesis.
- Transferrin
Serum glycoprotein carrying two N-glycosylation sites and used as a CDG diagnostic biomarker.
- PMM2-CDG
The most common CDG with an estimated 1000 cases worldwide
- MPI-CDG
First CDG for which a therapy has been proposed. It relies on dietary supplementation with mannose.
- NGLY1-CDDG
First described congenital disorder of degycosylation (CDDG).
REFERENCES
- 1.Ng BG; Freeze HH, Perspectives on Glycosylation and Its Congenital Disorders. Trends Genet 2018. 34 (6), 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeze HH; Eklund EA; Ng BG; Patterson MC, Neurological aspects of human glycosylation disorders. Anna Rev Neurosci 2015, 38, 105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosicka P; Ng BG; Freeze HH, Congenital Disorders of Glycosylation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer S; Mast JD; Tsang H; Rodriguez TP; DiPrimio N; Prangley M; Sam FS; Parton Z; Perlstein EO, Drug screens of NGLY1 deficiency in worm and fly models reveal catecholamine, NRF2 and anti-inflammatory-pathway activation as potential clinical approaches. Dis Model Mech 2019, 12 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer S; Sam FS; DiPrimio N; Preston G; Verheijen J; Murthy K; Parton Z; Tsang H; Lao J; Morava E; Perlstein EO, Repurposing the aldose reductase inhibitor and diabetic neuropathy drug epalrestat for the congenital disorder of glycosylation PMM2-CDG. Dis Model Mech 2019. 12 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiesler CT; Cajic S; Hoffmann D; Thiel C; van Diepen L; Hennig R; Sgodda M; Weiβmann R; Reichl U; Steinemann D; Diekmann U; Huber NM; Oberbeck A; Cantz T; Kuss AW; Körner C; Schambach A; Rapp E; Buettner FF, Glycomic Characterization of Induced Pluripotent Stem Cells Derived from a Patient Suffering from Phosphomannomutase 2 Congenital Disorder of Glycosylation (PMM2-CDG). Mol Cell Proteomics 2016, 15 (4), 1435–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verheijen J; Tahata S; Kozicz T; Witters P; Morava E, Therapeutic approaches in Congenital Disorders of Glycosylation (CDG) involving N-linked glycosylation: an update. Genet Med 2022. 22 (2), 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosicka P; Ng BG; Freeze HH, Therapeutic Monosaccharides: Looking Back, Moving Forward. Biochemistry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan B; Clasquin M; Smolen GA; Histen G; Powe J; Chen Y; Lin Z; Lu C; Liu Y; Cang Y; Yan Z; Xia Y; Thompson R; Singleton C; Dorsch M; Silverman L; Su SM; Freeze HH; Jin S, A mouse model of a human congenital disorder of glycosylation caused by loss of PMM2. Hum Mol Genet 2016, 25 (11), 2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panneerselvam K; Freeze HH, Mannose corrects altered N-glycosylation in carbohydrate-deficient glycoprotein syndrome fibroblasts. J Clin Invest 1996, 97 (6), 1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rush JS; Panneerselvam K; Waechter CJ; Freeze HH, Mannose supplementation corrects GDP-mannose deficiency in cultured fibroblasts from some patients with Congenital Disorders of Glycosylation (CDG). Glycobiology 2000, 10 (8), 829–35. [DOI] [PubMed] [Google Scholar]
- 12.Kjaergaard S; Kristiansson B; Stibler H; Freeze HH; Schwartz M; Martinsson T; Skovby F, Failure of short-term mannose therapy of patients with carbohydrate-deficient glycoprotein syndrome type 1A. Acta Paediatr 1998, 87 (8), 884–8. [DOI] [PubMed] [Google Scholar]
- 13.Mayatepek E; Kohlmüller D, Mannose supplementation in carbohydrate-deficient glycoprotein syndrome type I and phosphomannomutase deficiency. Eur J Pediatr 1998, 157 (7), 605–6. [DOI] [PubMed] [Google Scholar]
- 14.Taday R; Grüneberg M; DuChesne I; Reunert J; Marquardt T, Dietary mannose supplementation in phosphomannomutase 2 deficiency (PMM2-CDG). Orphanet J Rare Dis 2020, 15 (1), 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grünert SC; Marquardt T; Lausch E; Fuchs H; Thiel C; Sutter M; Schumann A; Hannibal L; Spiekerkoetter U, Unsuccessful intravenous D-mannose treatment in PMM2-CDG. Orphanet J Rare Dis 2019, 14 (1), 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witters P; Edmondson AC; Lam C; Johnsen C; Patterson MC; Raymond KM; He M; Freeze HH; Morava E, Spontaneous improvement of carbohydrate-deficient transferrin in PMM2-CDG without mannose observed in CDG natural history study. Orphanet J Rare Dis 2021, 16 (1), 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witters P; Andersson H; Jaeken J; Tseng L; van Karnebeek CDM; Lefeber DJ; Cassiman D; Morava E, D-galactose supplementation in individuals with PMM2-CDG: results of a multicenter, open label, prospective pilot clinical trial. Orphanet J Rare Dis 2021, 16 (1), 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutschow S; Thiem J; Kranz C; Marquardt T, Membrane-permeant derivatives of mannose-1-phosphate. Bioorg Med Chem 2002, 10 (12), 4043–9. [DOI] [PubMed] [Google Scholar]
- 19.Eklund EA; Merbouh N; Ichikawa M; Nishikawa A; Clima JM; Dorman JA; Norberg T; Freeze HH, Hydrophobic Man-1-P derivatives correct abnormal glycosylation in Type I congenital disorder of glycosylation fibroblasts. Glycobiology 2005, 15 (11), 1084–93. [DOI] [PubMed] [Google Scholar]
- 20.Hardré R; Khaled A; Willemetz A; Dupré T; Moore S; Gravier-Pelletier C; Le Merrer Y, Mono, di and tri-mannopyranosyl phosphates as mannose-1-phosphate prodrugs for potential CDG-Ia therapy. Bioorg Med Chem Lett 2007, 17 (1), 152–5. [DOI] [PubMed] [Google Scholar]
- 21.Bortot B; De Martino E; Tesser A; Ura B; Ruozi B; Aloisio M; Biffi S; Addobbati R; Tosi G; Dolcetta D; Severini GM, In vitro treatment of congenital disorder of glycosylation type Ia using PLGA nanoparticles loaded with GDP-Man. Int J Mol Med 2019, 44 (1), 262–272. [DOI] [PubMed] [Google Scholar]
- 22.Gámez A; Serrano M; Gallego D; Vilas A; Pérez B, New and potential strategies for the treatment of PMM2-CDG. Biochim Biophys Acta Gen Snbj 2020, 1864 (11), 129686. [DOI] [PubMed] [Google Scholar]
- 23.Stenson PD; Mort M; Ball EV; Shaw K; Phillips A; Cooper DN, The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 2014, 133 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuste-Checa P; Brasil S; Gámez A; Underhaug J; Desviat LR; Ugarte M; Pérez-Cerdá C; Martinez A; Pérez B; Pharmacological Chaperoning: A Potential Treatment for PMM2-CDG. Hum Mutation, 2017. 38 (2), 160–168. [DOI] [PubMed] [Google Scholar]
- 25.Monticelli M; Liguori L; Allocca M; Andreotti G; Cubellis MV, β-Glucose-1,6-Bisphosphate Stabilizes Pathological Phophomannomutase2 Mutants In Vitro and Represents a Lead Compound to Develop Pharmacological Chaperones for the Most Common Disorder of Glycosylation, PMM2-CDG. Int J Mol Sci 2019, 20 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vega AI; Pérez-Cerdá C; Desviat LR; Matthijs G; Ugarte M; Pérez B, Functional analysis of three splicing mutations identified in the PMM2 gene: toward a new therapy for congenital disorder of glycosylation type Ia. Hum Mutat 2009, 30 (5), 795–803. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Monseny AF; Bolasell M; Callejón-Póo L; Cuadras D; Freniche V; Itzep DC; Gassiot S; Arango P; Casas-Alba D; de la Morena E; Corral J; Montero R; Pérez-Cerdá C; Pérez B; Artuch R; Jaeken J; Serrano M, AZATAX: Acetazolamide safety and efficacy in cerebellar syndrome in PMM2 congenital disorder of glycosylation (PMM2-CDG). Ann Neurol 2019, 85 (5), 740–751. [DOI] [PubMed] [Google Scholar]
- 28.Lao JP; DiPrimio N; Prangley M; Sam FS; Mast JD; Perlstein EO, Yeast Models of Phosphomannomutase 2 Deficiency, a Congenital Disorder of Glycosylation. G3 (Bethesda) 2019, 9 (2), 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niehues R; Hasilik M; Alton G; Körner C; Schiebe-Sukumar M; Koch HG; Zimmer KP; Wu R; Harms E; Reiter K; von Figura K; Freeze HH; Harms HK; Marquardt T, Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest 1998, 101 (7), 1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Čechová A; Altassan R; Borgel D; Bruneel A; Correia J; Girard M; Harroche A; Kiec-Wilk B; Mohnike K; Pascreau T; Pawliński Ł; Radenkovic S; Vuillaumier-Barrot S; Aldamiz-Echevarria L; Couce ML; Martins EG; Quelhas D; Morava E; de Lonlay P; Witters P; Honzik T, Consensus guideline for the diagnosis and management of mannose phosphate isomerase-congenital disorder of glycosylation. J Inherit Metab Dis 2020, 43 (4), 671–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard M; Douillard C; Debray D; Lacaille F; Schiff M; Vuillaumier-Barrot S; Dupré T; Fabre M; Damaj L; Kuster A; Torre S; Mention K; McLin V; Dobbelaere D; Borgel D; Bauchard E; Seta N; Bruneel A; De Lonlay P, Long term outcome of MPI-CDG patients on D-mannose therapy. J Inherit Metab Dis 2020, 43 (6), 1360–1369. [DOI] [PubMed] [Google Scholar]
- 32.Karsan A; Cornejo CJ; Winn RK; Schwartz BR; Way W; Lannir N; Gershoni-Baruch R; Etzioni A; Ochs HD; Harlan JM, Leukocyte Adhesion Deficiency Type II is a generalized defect of de novo GDP-fucose biosynthesis. Endothelial cell fucosylation is not required for neutrophil rolling on human nonlymphoid endothelium. J Clin Invest 1998, 101 (11), 2438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquardt T; Lühn K; Srikrishna G; Freeze HH; Harms E; Vestweber D, Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 1999, 94 (12), 3976–85. [PubMed] [Google Scholar]
- 34.Yurchenco PD; Atkinson PH, Fucosyl-glycoprotein and precursor polls in HeLa cells. Biochemistry 1975, 14 (14), 3107–14. [DOI] [PubMed] [Google Scholar]
- 35.Yurchenco PD; Atkinson PH, Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry 1977, 16 (5), 944–53. [DOI] [PubMed] [Google Scholar]
- 36.Sosicka P; Ng BG; Wong M; Xia ZJ; Scott DA; Lebrilla C; Freeze HH, Novel insights into the fucose metabolism–challenging the old dogma. In Faseb j, 2020; Vol. 34(S1), pp 1–1. [Google Scholar]
- 37.Ng BG; Dastsooz H; Silawi M; Habibzadeh P; Jahan SB; Fard MAF; Halliday BJ; Raymond K; Ruzhnikov MRZ; Tabatabaei Z; Taghipour-Sheshdeh A; Brimble E; Robertson SP; Faghihi MA; Freeze HH, Expanding the molecular and clinical phenotypes of FUT8-CDG. J Inherit Metab Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JH; Reunert J; He M; Mealer RG; Noel M; Wada Y; Grüneberg M; Horváth J ; Cummings RD; Schwartz O; Marquardt T, L-Fucose treatment of FUT8-CDG. Mol Genet Metab Rep 2020, 25, 100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng BG; Rosenfeld JA; Emrick L; Jain M; Burrage LC; Lee B; Craigen WJ; Bearden DR; Graham BH; Freeze HH, Pathogenic Variants in Fucokinase Cause a Congenital Disorder of Glycosylation. Am J Hum Genet 2018, 103 (6), 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tegtmeyer LC; Rust S; van Scherpenzeel M; Ng BG; Losfeld ME; Timal S; Raymond K; He P; Ichikawa M; Veltman J; Huijben K; Shin YS; Sharma V; Adamowicz M; Lammens M; Reunert J; Witten A; Schrapers E; Matthijs G; Jaeken J; Rymen D; Stojkovic T ; Laforêt P; Petit F; Aumaître O; Czarnowska E; Piraud M; Podskarbi T; Stanley CA; Matalon R; Burda P; Seyyedi S; Debus V; Socha P; Sykut-Cegielska J; van Spronsen F; de Meirleir L; Vajro P; DeClue T; Ficicioglu C; Wada Y; Wevers RA; Vanderschaeghe D; Callewaert N; Fingerhut R; van Schaftingen E; Freeze HH; Morava E; Lefeber DJ; Marquardt T, Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med 2014, 370 (6), 533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong SY; Gadomski T; van Scherpenzeel M; Honzik T; Hansikova H; Holmefjord KSB; Mork M; Bowling F; Sykut-Cegielska J; Koch D; Hertecant J; Preston G; Jaeken J; Peeters N; Perez S; Nguyen DD; Crivelly K; Emmerzaal T; Gibson KM; Raymond K; Abu Bakar N; Foulquier F; Poschet G; Ackermann AM; He M; Lefeber DJ; Thiel C; Kozicz T; Morava E, Oral D-galactose supplementation in PGM1-CDG. Genet Med 2017, 19 (11), 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radenkovic S; Bird MJ; Emmerzaal TL; Wong SY; Felgueira C; Stiers KM; Sabbagh L; Himmelreich N; Poschet G; Windmolders P; Verheijen J; Witters P; Altassan R; Honzik T; Eminoglu TF; James PM; Edmondson AC; Hertecant J; Kozicz T; Thiel C; Vermeersch P; Cassiman D; Beamer L; Morava E; Ghesquière B, The Metabolic Map into the Pathomechanism and Treatment of PGM1-CDG. Am J Hum Genet 2019, 104 (5), 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng BG; Sosicka P; Agadi S; Almannai M; Bacino CA; Barone R; Botto LD; Burton JE; Carlston C; Chung BH; Cohen JS; Coman D; Dipple KM; Dorrani N; Dobyns WB; Elias AF; Epstein L; Gahl WA; Garozzo D; Hammer TB; Haven J; Héron D; Herzog M; Hoganson GE; Hunter JM; Jain M; Juusola J; Lakhani S; Lee H; Lee J; Lewis K ; Longo N; Lourenço CM; Mak CCY; McKnight D; Mendelsohn BA; Mignot C; Mirzaa G; Mitchell W; Muhle H; Nelson SF; Olczak M; Palmer CGS; Partikian A; Patterson MC; Pierson TM; Quinonez SC; Regan BM; Ross ME; Guillen Sacoto MJ; Scaglia F; Scheffer IE; Segal D; Singhal NS; Striano P; Sturiale L; Symonds JD; Tang S; Vilain E; Willis M; Wolfe LA; Yang H; Yano S; Powis Z; Suchy SF; Rosenfeld JA; Edmondson AC; Grunewald S; Freeze HH, SLC35A2-CDG: Functional characterization, expanded molecular, clinical, and biochemical phenotypes of 30 unreported Individuals. Hum Mutat 2019, 40 (7), 908–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witters P; Tahata S; Barone R; Õunap K; Salvarinova R; Grønborg S; Hoganson G; Scaglia F; Lewis AM; Mori M; Sykut-Cegielska J; Edmondson A; He M; Morava E, Clinical and biochemical improvement with galactose supplementation in SLC35A2-CDG. Genet Med 2020, 22 (6), 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nolting K; Park JH; Tegtmeyer LC; Zühlsdorf A; Grüneberg M; Rust S; Reunert J; Du Chesne I; Debus V; Schulze-Bahr E; Baxter RC; Wada Y; Thiel C; van Schaftingen E; Fingerhut R; Marquardt T, Limitations of galactose therapy in phosphoglucomutase 1 deficiency. Mol Genet Metab Rep 2017, 13, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JH; Hogrebe M; Grüneberg M; DuChesne I; von der Heiden AL; Reunert J; Schlingmann KP; Boycott KM; Beaulieu CL; Mhanni AA; Innes AM; Hörtnagel K; Biskup S; Gleixner EM; Kurlemann G; Fiedler B; Omran H; Rutsch F; Wada Y; Tsiakas K; Santer R; Nebert DW; Rust S; Marquardt T, SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am J Hum Genet 2015, 97 (6), 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morelle W; Potelle S; Witters P; Wong S; Climer L; Lupashin V; Matthijs G; Gadomski T; Jaeken J; Cassiman D; Morava E; Foulquier F, Galactose Supplementation in Patients With TMEM165-CDG Rescues the Glycosylation Defects. J Clin Endocrinol Metab 2017, 102 (4), 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simmer JP; Kelly RE; Rinker AG Jr.; Zimmermann BH; Scully JL; Kim H; Evans R; Mammalian dihydroorotase: nucleotide sequence, peptide sequences, and evolution of the dihydroorotase domain of the multifunctional protein CAD. Proc Natl Acad Sci U S A 1990, 87 (1), 174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graves LM; Guy HI; Kozlowski P; Huang M; Lazarowski E; Pope RM; Collins MA; Dahlstrand EN; Earp HS 3rd; Evans DR, Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature 2000, 403 (6767), 328–32. [DOI] [PubMed] [Google Scholar]
- 50.Del Caño-Ochoa F; Ng BG; Abedalthagafi M; Almannai M; Cohn RD; Costain G; Elpeleg O; Houlden H; Karimiani EG; Liu P; Manzini MC; Maroofian R; Muriello M; Al-Otaibi A; Patel H; Shimon E; Sutton VR; Toosi MB; Wolfe LA; Rosenfeld JA; Freeze HH; Ramón-Maiques S, Cell-based analysis of CAD variants identifies individuals likely to benefit from uridine therapy. Genet Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng BG; Wolfe LA; Ichikawa M; Markello T; He M; Tifft CJ; Gahl WA; Freeze HH, Biallelic mutations in CAD, impair de novo pyrimidine biosynthesis and decrease glycosylation precursors. Hum Mol Genet 2015, 24 (11), 3050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foulquier F; Legrand D, Biometals and glycosylation in humans: Congenital disorders of glycosylation shed lights into the crucial role of Golgi manganese homeostasis. Biochim Biophys Acta Gen Subj 2020, 1864 (10), 129674. [DOI] [PubMed] [Google Scholar]
- 53.Park JH; Hogrebe M; Fobker M; Brackmann R; Fiedler B; Reunert J; Rust S; Tsiakas K; Santer R; Grüneberg M; Marquardt T, SLC39A8 deficiency: biochemical correction and major clinical improvement by manganese therapy. Genet Med 2018, 20 (2), 259–268. [DOI] [PubMed] [Google Scholar]
- 54.Avila DS; Puntel RL; Aschner M, Manganese in health and disease. Met Ions Life Sci 2013, 13, 199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blommaert E; Péanne R; Cherepanova NA; Rymen D; Staels F; Jaeken J; Race V; Keldermans L; Souche E; Corveleyn A; Sparkes R; Bhattacharya K; Devalck C; Schrijvers R; Foulquier F; Gilmore R; Matthijs G, Mutations in MAGT1 lead to a glycosylation disorder with a variable phenotype. Proc Natl Acad Sci U S A 2019, 116 (20), 9865–9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravell JC; Matsuda-Lennikov M; Chauvin SD; Zou J; Biancalana M; Deeb SJ; Price S; Su HC; Notarangelo G; Jiang P; Morawski A; Kanellopoulou C; Binder K; Mukherjee R; Anibal JT; Sellers B; Zheng L; He T; George AB; Pittaluga S; Powers A; Kleiner DE; Kapuria D; Ghany M; Hunsberger S; Cohen JI; Uzel G; Bergerson J; Wolfe L; Toro C; Gahl W; Folio LR; Matthews H; Angelus P; Chinn IK; Orange JS; Trujillo-Vargas CM; Franco JL; Orrego-Arango J; Gutiérrez-Hincapié S; Patel NC; Raymond K; Patiroglu T; Unal E; Karakukcu M; Day AG; Mehta P; Masutani E; De Ravin SS; Malech HL; Altan-Bonnet G; Rao VK; Mann M; Lenardo MJ; Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease. J Clin Invest 2020, 130 (1), 507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravell JC; Chauvin SD; He T; Lenardo M, An Update on XMEN Disease. J Clin Immunol 2020, 40 (5), 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tambe MA; Ng BG; Freeze HH, N-Glycanase 1 Transcriptionally Regulates Aquaporins Independent of Its Enzymatic Activity. Cell Rep 2019, 29 (13), 4620–4631.e4. [DOI] [PubMed] [Google Scholar]
- 59.Haijes HA; de Sain-van der Velden MGM; Prinsen H; Willems AP; van der Ham M; Gerrits J; Couse MH; Friedman JM; van Karnebeek CDM; Selby KA; van Hasselt PM; Verhoeven-Duif NM; Jans JJM; Aspartylglycosamine is a biomarker for NGLY1-CDDG, a congenital disorder of deglycosylation. Mol Genet Metab 2019, 127 (4), 368–372. [DOI] [PubMed] [Google Scholar]
- 60.Hall PL; Lam C; Alexander JJ; Asif G; Berry GT; Ferreira C; Freeze HH; Gahl WA; Nickander KK; Sharer JD; Watson CM; Wolfe L; Raymond KM, Urine oligosaccharide screening by MALDI-TOF for the identification of NGLY1 deficiency. Mol Genet Metab 2018, 124 (1), 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owings KG; Lowry JB; Bi Y; Might M; Chow CY, Transcriptome and functional analysis in a Drosophila model of NGLY1 deficiency provides insight into therapeutic approaches. Hum Mol Genet 2018, 27 (6), 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez TP; Mast JD; Hartl T; Lee T; Sand P; Perlstein EO, Defects in the Neuroendocrine Axis Contribute to Global Development Delay in a Drosophila Model of NGLY1 Deficiency. G3 (Bethesda) 2018, 8 (7), 2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang K; Huang R; Fujihira H; Suzuki T; Yan N, N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J Exp Med 2018, 215 (10), 2600–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomlin FM; Gerling-Driessen UIM; Liu YC; Flynn RA; Vangala JR; Lentz CS; Clauder-Muenster S; Jakob P; Mueller WF; Ordoñez-Rueda D; Paulsen M; Matsui N; Foley D; Rafalko A; Suzuki T; Bogyo M; Steinmetz LM; Radhakrishnan SK; Bertozzi CR, Inhibition of NGLY1 Inactivates the Transcription Factor Nrf1 and Potentiates Proteasome Inhibitor Cytotoxicity. ACS Cent Sci 2017, 3 (11), 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lam C; Ferreira C; Krasnewich D; Toro C; Latham L; Zein WM; Lehky T; Brewer C; Baker EH; Thurm A; Farmer CA; Rosenzweig SD; Lyons JJ; Schreiber JM; Gropman A; Lingala S; Ghany MG; Solomon B; Macnamara E; Davids M; Stratakis CA; Kimonis V; Gahl WA; Wolfe L, Prospective phenotyping of NGLY1-CDDG, the first congenital disorder of deglycosylation. Genet Med 2017, 19 (2), 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talsness DM; Owings KG; Coelho E; Mercenne G; Pleinis JM; Partha R; Hope KA; Zuberi AR; Clark NL; Lutz CM; Rodan AR; Chow CY, A Drosophila screen identifies NKCC1 as a modifier of NGLY1 deficiency. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haas M, The Na-K-Cl cotransporters. Am J Physiol 1994, 267 (4 Pt 1), C869–85. [DOI] [PubMed] [Google Scholar]
- 68.Freeze HH; Chong JX; Bamshad MJ; Ng BG, Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet 2014, 94 (2), 161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.In Essentials of Glycobiology, Varki A; Cummings RD; Esko JD; Stanley P; Hart GW; Aebi M; Darvill AG; Kinoshita T; Packer NH; Prestegard JH; Schnaar RL; Seeberger PH, Eds. Cold Spring Harbor Laboratory Press, Copyright 2015-2017 by The Consortium of Glycobiology Editors, La Jolla, California. All rights reserved.: Cold Spring Harbor (NY), 2015. [Google Scholar]