Abstract
Tedizolid has activity against Gram-positive pathogens as well as Mycobacterium spp and Nocardia spp. Real-world evidence supporting long-term tolerability and clinical success of tedizolid is lacking. Prolonged tedizolid therapy (median, 188 days; interquartile range, 62–493 days) appeared to be well tolerated in 37 patients (8.1% experienced adverse effect leading to discontinuation). Clinical success was 81.3% in those evaluated.
Keywords: Gram-positive infections, long-term tolerability, tedizolid, thrombocytopenia
Real-world evidence supporting long-term tolerability and clinical success of tedizolid is lacking. Prolonged tedizolid therapy (median, 188 days; interquartile range, 62–493 days) in 37 patients was well tolerated with few serious adverse effects and good clinical success (81.3%).
Tedizolid is an oxazolidinone antibiotic approved for treatment of acute bacterial skin and skin structure infections with a 6-day course of therapy [1, 2]. Tedizolid is an appealing option for facilitating transitions-of-care or outpatient management of complicated infections due to its high bioavailability, oral formulation, and in vitro potency against commonly encountered Gram-positive pathogens. Specifically, it is one of few orally available agents that provide potent activity against resistant Gram-positive organisms including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus (MRSA) [3, 4].
The 2 commercially available oxazolidinones, tedizolid and linezolid, offer similar spectra of antimicrobial activity. However, long-term use of linezolid is limited by hematological adverse effects (thrombocytopenia and anemia in 10%–48% and 10%–44%, respectively), peripheral neuropathy (2%–13%), optic neuritis (2%–4%), and serotonergic drug interactions [5–9]. Prospective trials of short-course tedizolid have proven both efficacy and tolerability [1, 2]. Reports of prolonged tedizolid use suggest a low frequency of adverse events, including among patients with prior toxicity related to linezolid; however, these reports are limited in patient numbers included and leave questions about prolonged durations of therapy [10–15]. Differences in safety may be due to reduced drug exposures to tedizolid related to lower total daily doses, higher plasma protein binding, and decreased central nervous system penetration [16–18]. Despite these potential advantages, long-term use of tedizolid is often avoided based on the presumption that adverse effects seen with linezolid represent a class effect. Given the paucity of tolerability data when used beyond typical durations, the primary objective of this report was to describe real-world clinical experience with tedizolid with an emphasis on long-term tolerability and clinical success.
MATERIALS AND METHODS
This retrospective case series evaluated adult patients receiving tedizolid, based on prescriber/clinician discretion, from January 2015 through November 2020 who were identified through electronic medical records. Patients were eligible for inclusion if they were ≥18 years old, received inpatient/outpatient tedizolid for ≥28 consecutive days, had a baseline complete blood count (CBC) analysis obtained ≤6 months before tedizolid initiation, and had ≥1 additional CBC analysis obtained ≥14 days after tedizolid initiation. Patients with incomplete medical records were excluded.
The primary objective was to describe the incidence of any adverse effects (hematological, central nervous system, or other) potentially associated with tedizolid when used for long (≥28 consecutive days) durations with or without concomitant serotonergic agents. We also aimed to describe clinical success of tedizolid when used for clinical indications requiring longer durations of therapy.
Thrombocytopenia at baseline and/or during therapy was differentiated as mild (100–150 × 109/L), moderate (50–100 × 109/L), or severe (<50 × 109/L). Clinical success was defined based on whether tedizolid was used for treatment of active infection or long-term infection suppression. Long-term infection suppression was defined as prescriber’s use of long-term or indefinite tedizolid administration for chronic infection without curative intent [14]. Clinical success of treatment was defined as lack of hospital admission/readmission, recurrence of infection with index pathogen, or infection-related mortality, all during therapy or within 60 days after tedizolid discontinuation. Clinical success during suppressive therapy was defined as lack of relapse of infection with the index pathogen and/or death during tedizolid use. Mycobacterium spp and Nocardia spp were excluded from clinical success evaluations due to the 60-day follow-up period not being appropriate for these infections. Baseline renal insufficiency was defined as any staging of chronic kidney disease per KDIGO criteria [19]. To assess risk and/or occurrence of serotonin syndrome, a list of serotonergic medications was developed using primary and tertiary resources that included selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, atypical antipsychotics, and other medications with serotonergic activity [20].
Descriptive statistics and paired t test or Wilcoxon matched-pair signed rank test were used for analysis using SPSS software, version 26.0 (IBM Corp., Armonk, NY) and R, version 4.02 (R Core Team). Continuous descriptive data were reported as mean (±standard deviation [SD]) or median (interquartile range [IQR]) for parametric and non-parametric data, respectively. Before initiation, the protocol was submitted to the Colorado Multiple Institutional Review Board and determined to be exempt from full board review.
RESULTS
A total of 37 patients met inclusion criteria. The mean age and weight were 61 (SD ±14) years and 85 (SD ±28) kg, respectively; patients were predominantly female (21 of 37; 56.8%) and white (30 of 37; 81.1%). Common comorbid diseases at baseline included diabetes mellitus (15 of 37; 40.5%), peripheral neuropathy (14 of 37; 37.8%), and renal insufficiency (12 of 37; 32.4%), with median serum creatinine of 0.96 (IQR, 0.66–1.1) mg/dL among those not receiving chronic hemodialysis (35 of 37; 94.6%). At least 1 concomitant serotonergic medication was received by 20 of 37 (54.1%) patients, with 10 of 37 (27.0%) receiving ≥2 serotonergic medications.
Tedizolid was most often used for suppression of chronic infections (24 of 37; 64.9%). Prosthetic joint infections involving the knee or hip were most common (11 of 37; 29.7%), followed by hardware-associated vertebral infections (7 of 37; 18.9%) and osteomyelitis without associated hardware (5 of 37; 13.5%). It should be noted that, in this case series, tedizolid was primarily used for US Food and Drug Administration (FDA) nonlabeled indications. Although sometimes difficult to determine from retrospective review, tedizolid was selected over other possible agents primarily based on factors such as need for long-term therapy, desirability of oral treatment options, and risk of long-term adverse effects or proven intolerability of other agents, or desire to avoid potential drug-drug interactions. Of the 56 isolated pathogens, Enterococcus spp was most common (16 of 56; 28.6%), 68.8% of which were vancomycin-resistant Enterococcus faecium and 25.0% were Enterococcus faecalis. Additional organisms included methicillin-susceptible S aureus (9 of 56; 16.1%), MRSA (6 of 56; 10.7%), Mycobacterium spp (3 of 56; 5.4%), and Nocardia spp (2 of 56; 3.6%). Polymicrobial infections were present in 14 of 37 (37.8%) cases, and 21 of 37 (56.8%) patients had hardware or prostheses while on suppression/treatment. The most common tedizolid regimen was 200 mg daily (97.3%), whereas the median duration of tedizolid use was 188 (IQR, 62–493) days. Incremental durations of tedizolid use were as follows: 28–60 days, 9 of 37 (24.3%); 61–120 days, 5 of 37 (13.5%); 121–180 days, 4 of 37 (10.8%); 181–300 days, 6 of 37 (16.2%); 301–500 days, 4 of 37 (10.8%); 501–700 days, 5 of 37 (13.5%); and ≥701 days, 4 of 37 (10.8%). Within 1 month of tedizolid initiation, 8 of 37 (21.6%) patients had also previously received linezolid. Concomitant antibiotics were used in 19 of 37 (51.4%) cases, most commonly being fluoroquinolones (7 of 19; 36.8%), beta-lactams (6 of 19; 31.6%), and metronidazole (3 of 19; 15.8%). Clinical success was observed in 26 of 32 (81.3%) patients using tedizolid for treatment or suppression (6 of 8 [75.0%] and 20 of 24 [83.3%] patients for treatment and suppression, respectively).
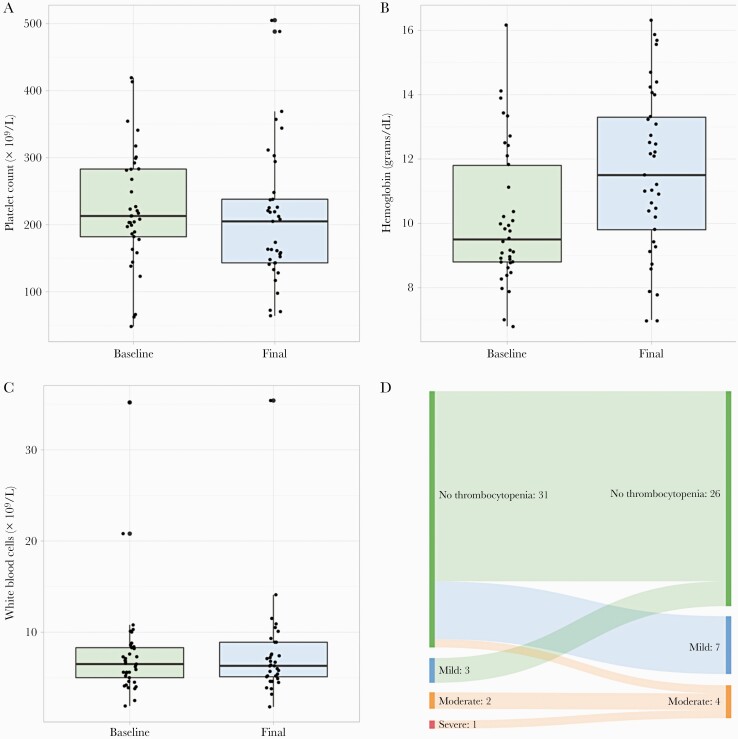
There were no significant differences in median CBC indices when comparing baseline to last laboratory values obtained while on tedizolid, with the exception of a statistically/clinically significant increase in hemoglobin values from baseline to last measurement: 9.5 (IQR, 8.8–11.9) vs 11.5 (IQR, 9.8–13.3) g/dL, respectively; P < .001 (Figure 1A–C). The mean (±SD) change in CBC indices from baseline to last laboratory measurement were as follows: platelets −14 [SD ±81] × 109/L; hemoglobin +1.5 [SD ±1.7] g/dL; and white blood cells −0.1 [SD±2.3] × 109/L. Before tedizolid initiation, a total of 6 patients were documented with mild (3 of 37; 8.1%), moderate (2 of 37; 5.4%), or severe (1 of 37; 2.7%) thrombocytopenia. The last platelet count obtained on therapy revealed 7 of 37 (18.9%) and 4 of 37 (10.8%) patients with mild or moderate thrombocytopenia, respectively, whereas no patients had documented severe thrombocytopenia (Figure 1D). Baseline CBC analyses were obtained a median of 2 (IQR, 1–15) days before tedizolid initiation, whereas the last CBC analyses obtained while on therapy were a median of 114 (IQR, 45–297) days after tedizolid initiation. A median of 5 (IQR, 3–22) CBC analyses were drawn throughout tedizolid use. After the last CBC analysis while on therapy, patients continued tedizolid for a median of 16 (IQR, 1–77) additional days.
Figure 1.
Comparison of baseline and final laboratory draws while on tedizolid for platelets (A), hemoglobin (B), and white blood cells (C). Schematic of degree of thrombocytopenia between baseline and last platelet draw while on tedizolid for each patient included (D).
No patients were documented to have new/worsening peripheral neuropathy, optic neuritis or visual changes, or serotonin syndrome while receiving tedizolid. However, 1 patient each (3 of 37; 8.1%) experienced dizziness, lactic acidosis, and macrocytic anemia, which led to tedizolid discontinuation; the durations of tedizolid therapy in these patients before drug discontinuation were 675, 745, and 123 days, respectively. The patient experiencing dizziness also received amitriptyline, bupropion, tramadol, venlafaxine, ziprasidone, and antihypertensive medications while on tedizolid; the dizziness was ascribed to antihypertensive medications, and the patient was successfully restarted on tedizolid for continued suppressive therapy. The patient experiencing lactic acidosis was receiving escitalopram and several other medications while on tedizolid; the association with tedizolid was unclear because the lactic acidosis did not resolve after tedizolid discontinuation.
DISCUSSION
During our approximately 6-year observational period, long-term use of tedizolid appeared to be well tolerated during treatment or chronic suppression of a variety of complicated Gram-positive infections. It should be noted that some patients did experience reductions in their overall hematological indices (Figure 1A–D), but most of these reductions were mild and did not result in tedizolid discontinuation. Because tedizolid myelosuppression appears to be dose-dependent, the minor decreases observed in our cohort may have been due to enhanced exposures among these individuals; however, this was not confirmed due to a lack of available therapeutic drug monitoring [21].
Given that many patients are not candidates for surgical removal of infected hardware, safe and efficacious oral options for suppressive therapy are often needed. Other commonly used oral options for Gram-positive infections include doxycycline and sulfamethoxazole/trimethoprim (SXT); however, these agents have variable activity against Enterococcus spp and require multiple daily dosing. Further issues with SXT include hypersensitivity reactions, potential drug-drug interactions, and other adverse effects that can limit long-term use (eg, anemia, renal insufficiency).
The findings of this case series demonstrate that tedizolid may be an attractive option, particularly with regard to tolerability, for patients requiring long-term therapy/suppression due to poor source control, retained hardware, or nonsurgical candidacy. Particularly given that patients with infections caused by nontuberculous Mycobacteria spp and Nocardia spp typically require ≥6 months of therapy, tedizolid could represent a potentially safe option if proven to be clinically efficacious [22, 23]. As with all antimicrobial regimens used for prolonged periods of time, the clinician and patient should evaluate factors such as costs, potential adverse effects, and threat of the emergence of antimicrobial resistance.
In addition, although not directly compared with linezolid, the low rate of adverse events observed in this study contrasts with previous studies evaluating long-term linezolid use [7–9]. It has been shown that tedizolid has lower steady-state tissue/plasma ratios in rat bone and brain in comparison to linezolid, which may be a mechanistic explanation of the observed low incidence of hematological adverse effects and central nervous system interactions [3]. With a median duration of 188 (IQR, 62–493) days, the tedizolid duration in this report far surpasses both the time period when adverse effects associated with linezolid would be expected to be observed as well as other published reports on long-term tedizolid use [6, 10, 11, 13]. Two recent studies that evaluated the safety profile of tedizolid exhibited similar rates of adverse effects (11.1% and 5.9%, overall; 8.6% and 0%, hematologic-related); however, the durations of tedizolid exposure in these investigations were shorter compared with those described in this report (median of 28 [IQR, 14–59] and 29 [IQR, 15–44] days) [10, 11].
This case series remains relatively limited in sample size, and the heterogeneity of patient and infection characteristics further limits the external validity. The possibility of selection bias is apparent, and the lack of control group limits our ability to rigorously evaluate the effectiveness and safety of long-term tedizolid use. Furthermore, most patients received the majority of their tedizolid regimen in the outpatient setting, and it is therefore not possible to determine actual adherence to therapy. The FDA labeling for tedizolid includes a warning/precaution for use in patients with neutrophil counts <1000 cells/mm3 [24]. Baseline neutrophil counts were not obtained as part of data collection (only total white blood cell counts); therefore, tolerability of tedizolid in this patient population specifically in respect to neutrophil counts cannot be determined from this sample. However, as described in results and shown in Figure 1, no patients experienced notable changes in total white blood cell counts during the period of tedizolid exposure. Finally, susceptibility data for tedizolid was unavailable. However, this is likely due to the delay of validated antimicrobial susceptibility testing methods after novel antimicrobial approvals and low rates of linezolid resistance.
CONCLUSIONS
Despite these potential limitations, long-term use of tedizolid seems to be a reasonable alternative to linezolid in regard to tolerability and safety during prolonged courses for complicated infections.
Acknowledgments
We thank our team at University of Colorado Health, specifically the inpatient and outpatient Divisions of Infectious Diseases.
Disclaimer. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
Financial support. This work was funded in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp.
Potential conflicts of interest. T. M., S. W. M., M. A. M., and D. N. F. have received research funding from Merck & Co., Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Taylor Morrisette, Department of Pharmacy, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, Colorado, USA; Department of Pharmacy-Infectious Diseases, University of Colorado Hospital, Aurora, Colorado, USA; Present Affiliation: Department of Clinical Pharmacy and Outcomes Sciences, Medical University of South Carolina, College of Pharmacy, Charleston, South Carolina, USA; Present Affiliation: Department of Pharmacy Services, Medical University of South Carolina Shawn Jenkins Children’s Hospital, Charleston, South Carolina, USA.
Kyle C Molina, Department of Pharmacy-Infectious Diseases, University of Colorado Hospital, Aurora, Colorado, USA.
Beatriz Da Silva, Department of Pharmacy, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, Colorado, USA.
Scott W Mueller, Department of Pharmacy, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, Colorado, USA.
Laura Damioli, Department of Medicine, University of Colorado Hospital, Aurora, Colorado, USA; Division of Infectious Diseases, University of Colorado School of Medicine, Aurora, Colorado, USA.
Martin Krsak, Department of Medicine, University of Colorado Hospital, Aurora, Colorado, USA; Division of Infectious Diseases, University of Colorado School of Medicine, Aurora, Colorado, USA.
Matthew A Miller, Department of Pharmacy-Infectious Diseases, University of Colorado Hospital, Aurora, Colorado, USA.
Douglas N Fish, Department of Pharmacy, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, Colorado, USA; Department of Pharmacy-Infectious Diseases, University of Colorado Hospital, Aurora, Colorado, USA.
References
- 1. Prokocimer P, De Anda C, Fang E, et al. . Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 2013; 309:559–69. [DOI] [PubMed] [Google Scholar]
- 2. Moran GJ, Fang E, Corey GR, et al. . Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2014; 14:696–705. [DOI] [PubMed] [Google Scholar]
- 3. Ong V, Flanagan S, Fang E, et al. . Absorption, distribution, metabolism, and excretion of the novel antibacterial prodrug tedizolid phosphate. Drug Metab Dispos 2014; 42:1275–84. [DOI] [PubMed] [Google Scholar]
- 4. Locke JB, Zurenko GE, Shaw KJ, Bartizal K.. Tedizolid for the management of human infections: in vitro characteristics. Clin Infect Dis 2014; 58(Suppl 1):S35–42. [DOI] [PubMed] [Google Scholar]
- 5. French G. Safety and tolerability of linezolid. J Antimicrob Chemother 2003; 51:ii45–53. [DOI] [PubMed] [Google Scholar]
- 6. Gerson SL, Kaplan SL, Bruss JB, et al. . Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 2002; 46:2723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rao N, Hamilton CW.. Efficacy and safety of linezolid for Gram-positive orthopedic infections: a prospective case series. Diagn Microbiol Infect Dis 2007; 59:173–9. [DOI] [PubMed] [Google Scholar]
- 8. Legout L, Valette M, Dezeque H, et al. . Tolerability of prolonged linezolid therapy in bone and joint infection: protective effect of rifampicin on the occurrence of anaemia? J Antimicrob Chemother 2010; 65:2224–30. [DOI] [PubMed] [Google Scholar]
- 9. Vazquez JA, Arnold AC, Swanson RN, et al. . Safety of long-term use of linezolid: results of an open-label study. Ther Clin Risk Manag 2016; 12:1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vendrell MM, Pitarch MT, Lletí MS, et al. Safety and tolerability of more than 6 days of tedizolid treatment. Antimicrob Agents Chemother 2021; doi: 10.1128/AAC.00356-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benavent E, Morata L, Escrihuela-Vidal F, et al. . Long-term use of tedizolid in osteoarticular infections: benefits among oxazolidinone drugs. Antibiotics (Basel) 2021; 10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nigo M, Luce AM, Arias CA.. Long-term use of tedizolid as suppressive therapy for recurrent methicillin-resistant Staphylococcus aureus graft infection. Clin Infect Dis 2018; 66:1975–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poon YK, La Hoz RM, Hynan LS, et al. . Tedizolid vs linezolid for the treatment of nontuberculous Mycobacteria infections in solid organ transplant recipients. Open Forum Infect Dis 2021; 8:ofab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferry T, Conrad A, Senneville E, et al. . Safety of tedizolid as suppressive antimicrobial therapy for patients with complex implant-associated bone and joint infection due to multidrug-resistant Gram-positive pathogens: results from the TediSAT Cohort Study. Open Forum Infect Dis 2021; 8:ofab351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Senneville E, Dinh A, Ferry T, Beltrand E, Blondiaux N, Robineau O.. Tolerance of prolonged oral tedizolid for prosthetic joint infections: results of a multicentere prospective study. Antibiotics (Basel) 2021; 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Vriese AS, Coster RV, Smet J, et al. . Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis 2006; 42:1111–7. [DOI] [PubMed] [Google Scholar]
- 17. Flanagan S, Bartizal K, Minassian SL, et al. . In vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. Antimicrob Agents Chemother 2013; 57:3060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flanagan S, McKee EE, Das D, et al. . Nonclinical and pharmacokinetic assessments to evaluate the potential of tedizolid and linezolid to affect mitochondrial function. Antimicrob Agents Chemother 2015; 59:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney inter, Suppl 2013; 3:1–150. [Google Scholar]
- 20. Volpi-Abadie J, Kaye AM, Kaye AD.. Serotonin syndrome. Ochsner J 2013; 13:533–40. [PMC free article] [PubMed] [Google Scholar]
- 21. Lodise TP, Bidell MR, Flanagan SD, et al. . Characterization of the haematological profile of 21 days of tedizolid in healthy subjects. J Antimicrob Chemother 2016; 71:2553–8. [DOI] [PubMed] [Google Scholar]
- 22. Brown-Elliott BA, Wallace RJ Jr. In vitro susceptibility testing of tedizolid against nontuberculous mycobacteria. J Clin Microbiol 2017; 55:1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown-Elliott BA, Wallace RJ Jr. In vitro susceptibility testing of tedizolid against isolates of Nocardia. Antimicrob Agents Chemother 2017; 61:e01537–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tedizolid [package insert]. Whitehouse Station, NJ: Merck and Co., Inc.; 2021. [Google Scholar]