Highlight
-
•
Prior MA is associated with an earlier onset of cardiovascular onset which is striking in young adults.
-
•
Both the races displayed the prior MA use associated earlier age of CVD onset with marked differences in the latency to cardiovascular onset between the Blacks and Whites.
-
•
Hypertension emerged as the most frequently observed CVD in the MA group.
Keywords: Substance use, Cardiovascular, Methamphetamine
Abstract
Introduction
The illicit use of methamphetamine (MA), a dangerous psychostimulant has become a global epidemic. Studies have demonstrated a link between illicit substance use and cardiovascular consequences. The objective of this study was to assess whether MA use is associated with an early onset of cardiovascular diseases (CVD).
Methods
Retrospective analysis was conducted using data collected from 1376 individuals at Louisiana State University Health Sciences Center - Shreveport between 2011 and 2020. Cardiovascular patients with and without a history of MA use were divided into the MA and Control groups. The age of CVD onset was assessed. Descriptive statistics for patient characteristics, Two Samples T-Test for continuous and Pearson's χ^2- tests for categorical variables were calculated. Hazard ratios (HR) and time ratios (TR) were calculated.
Results
The age of CVD onset in patients with prior MA use occurred on average 8 year earlier than the age of CVD onset (mean age ± SD = 44 ± 12.04) in controls (mean age ± SD = 52 ± 10.70) (unpaired t-test, p < 0.0001). The findings were noted in both the races (Time Ratio = 0.93, 95% CI = 0.89 to 0.97, p-value < 0.001), with a striking difference in the latency to CVD onset between Black and White subjects. A 12-fold increase in subjects who showed a premature onset of CVD (<30 years of age) in the MA group was observed. Our data analysis revealed that hypertension was the most frequently observed CVD.
Conclusions
MA use likely accelerates early onset of CVD and contributes to CVD complications in young adults.
1. Introduction
The rate of stimulant misuse has reached an epidemic level in the United States. Approximately 1.9 million people aged 12 years or older have reported past year methamphetamine use; 1.05 million reported methamphetamine use disorder (SAMHSA, 2019). The prevalence of amphetamine-type stimulants is on the rise with upwards of 51 million users worldwide. Among these, the illicit use of methamphetamine (MA) has escalated, trailing cannabis and opioid misuse, and emerged as a global health crisis (Abbruscato & Trippier, 2018). The precipitous increase in MA use nationwide led the US congress to introduce the Methamphetamine Response Act to forestall or prevent the development of this crisis in its early stages. The psychosocial burden caused by the COVID-19 pandemic may have worsened the surge of addiction (both new and relapse) across the globe (Dubey et al., 2020).
MA is a powerful amphetamine derivative, with a rapid onset of action due to its fast brain distribution and a prolonged activity post administration (Kirkpatrick et al., 2012). In general, the central nervous system mediated effects of MA include increased wakefulness, physical activity, euphoria, psychosis, and other psychiatric and cognitive sequelae (Pavese, Rimoldi, Gerhard, Brooks, & Piccini, 2004). These effects are largely attributed to the ability of MA to increase levels of brain catecholamines, mainly via promoting cytosolic vesicular release of dopamine and norepinephrine in presynaptic nerve terminals. Other mechanisms potentially involved in mediating the effect of MA, which increases brain monoamine levels, include inhibition of presynaptic reuptake of catecholamines and blocking breakdown of monoamines by inhibition of monoamine oxidase (Panenka et al., 2013).
It has been well documented that MA elicits a wide range of other adverse effects in addition to the detrimental neurological consequences. Moreover, previous clinical studies have reported that the predominant health concern in MA users is death resulting from cardiovascular disease (CVD) following accidental overdoses (Darke, Duflou, & Kaye, 2017). MA is a sympathomimetic that acts indirectly to stimulate alpha- and beta-adrenergic receptors, thereby elevating heart rate and blood pressure, especially during excessive and uncontrolled MA use (Kevil et al., 2019). The primary cardiotoxic mechanism for MA entails increased catecholamine levels in the peripheral nervous system. This in turn leads to cardiovascular complications of vasoconstriction, vasospasm, tachycardia, atherosclerotic plaque formation likely due to enhanced oxidative stress (Rezvan, Ni, Alberts-Grill, & Jo, 2011) (Bauch, Grunwald, Vischer, Gerlach, & Hauss, 1987), cardiac arrhythmias, and cardiomyopathy (Kaye, S & McKetin, R. 2005). Although the mechanisms surrounding these altered pathophysiological responses remain elusive, some of the key players implicated in mediating MA-related cardiovascular dysfunction consist of reduced mitochondrial function and enhanced reactive oxygen stress (Lord et al., 2010).
Previous reports have indicated that chronic drug use is associated with premature mortality resulting from the interaction between genetic, environmental, behavioral factors and disease. For instance, the median life expectancy of smokers was found to be decreased by at least 10 years due to the toxic multi-system effects of cancer, cardiovascular, cerebrovascular and pulmonary dysfunction (Nakama et al., 2011). Similarly, MA use also leads to cardiovascular complications (Kevil et al., 2019) and has been previously reported to promote rapid decline in mental function (Dean, Groman, Morales, & London, 2013). However, few studies have evaluated the association between prior MA use and early onset of CVD. Thus, the goal of our study was to test the hypothesis that individuals with a history of MA use or misuse develop CVD at younger ages than individuals with CVD who do not have a recorded history of MA use or misuse.
2. Materials and methods
2.1. Study design
A retrospective chart review was conducted on patients between the ages of 20 and 80 years who presented to the hospital at Louisiana State University Health Sciences Center - Shreveport between 2011and 2020. The study was carried out following the approved IRB protocol. The study design consisted of 2 groups: cardiovascular patients only (Control group) and cardiovascular patients with a history of methamphetamine use (MA group).
2.2. Inclusion and exclusion criteria
Electronic Health Record (EHR) database was queried to identify subjects who met the study criteria: patients who experienced a cardiovascular disease identified using the diagnosis code ICD-10:1–10 through I-152. This query resulted in 57, 711 records. Inclusion criteria for the control group included a diagnosis of CVD only. Of the 57, 711 records, n = 783 patient records were randomly selected for analysis in this study (Control group). Similarly, for the MA group, EHR was queried to identify subjects who had documented MA abuse, using the International Classification of Diseases-10th Revision-Clinical Modification (ICD-10-CM) code F15 (any diagnosis related to methamphetamines), a positive urine toxicology screen for amphetamines. Of that group, we identified patients who experienced a cardiovascular event as described by the diagnosis code ICD-10:1–10 through I-152. We excluded patients identified as positive for prescription of amphetamines and a diagnosis of Attention-deficit/Hyperactivity Disorder (ADHD). This query resulted in 2274 records. A subset of electronic health records (n = 593) were randomly selected for analysis in this study (Case group). Patients with documented MA abuse and a recorded age of first CVD diagnosis were included in the MA group, and patients identified with a CVD diagnosis were included in the control group. The cohort selection flow chart is schematically represented in Supplemental Fig. 1.
2.3. Data collection
Following random selection of 1376 charts, clinical and demographic data were collected for the two groups. These data included date of birth, age at time of first registered CVD diagnosis, date of first diagnosis of amphetamine or MA use, sex, ethnicity, urine toxicology, use of tobacco products, misuse of alcohol, and other drugs as well as other medical comorbidities. Patient medication history was also recorded. Of the 1376 patients selected, 783 were in the control group and 593 in the MA group. Based on a power analysis, a two-sided log-rank test with a sample size of 226 (113 patients each in case and control group) is necessary to achieve 80% power at α = 0.05 to detect a difference in the onset of CVD between methamphetamine users and non-methamphetamine users.
2.4. Statistical analysis
An unpaired t-test comparing the means ± SEM of the two independent groups (control and MA) was performed to determine whether there were differences in the age of CVD onset between the two groups. Descriptive statistics were used to report patient characteristics and prevalence of tobacco and cocaine use. Patient characteristics were expressed as n (%) for categorical variables and mean (standard deviation, SD) for continuous variables, as appropriate. P-values for continuous variables were calculated using a two Samples t-test and for categorical variables using a Pearson's χ^2- tests. Time-to-CVD onset was estimated based on the age when the patient received the diagnosis. Kaplan-Meier (KM) curves were used to visualize differences in the time-to-CVD onset, comparing prior tobacco use, prior cocaine use, Non-Hispanic Black and Non-Hispanic White patients, and female and male patients. Cox proportional hazard models estimated the magnitude of risk for each group described for the KM curves. Parametric regression (accelerated-failure-time models) with Weibull distributions were used to estimate Time Ratios (TRs) that mainly investigates the latency to severity. Our study consisting of different time unit (in this case age of onset) utilized Time Ratios to better reflect this aspect of analysis. The mathematical properties of TRs have been described in previous publications (Yang, Flores, Katz, Nathan, & Mehta, 2017). TRs are an alternative and effective measure of Hazard ratios (HR). TRs > 1 correspond to a prolonged latency-to-CVD onset, while TRs < 1 correspond to a shortened time-to-CVD onset. For all analyses, the Type-I error rate to evaluate statistical significance was set at 0.05. Data management and analyses were performed with STATA/IC statistical software, version 16 (StataCorp, LLC).
3. Results
3.1. Patient characteristics
Table 1 provides the patient characteristics identified for this retrospective cross-sectional design comparing the control group to the MA group. The control group comprised predominantly of women (79%) compared to 44% in the MA group (p < 0.001).
Table 1.
A schematic representation of the patient characteristics is represented for the two groups: Control and MA respectively. Values are mean ± SD or n (%).
| Control N = 783 |
MA N = 593 |
P | |
|---|---|---|---|
| Sex | <0.001 | ||
| Female | 623 (79.6%) | 262 (44.2%) | |
| Male | 160 (20.4%) | 331 (55.8%) | |
| Race | <0.001 | ||
| Blacks | 698 (89.1%) | 253 (42.7%) | |
| Whites | 85 (10.9%) | 340 (57.3%) | |
| Tobacco use | <0.001 | ||
| No | 600 (76.6%) | 114 (19.2%) | |
| Yes | 183 (23.4%) | 479 (80.8%) | |
| Cocaine use | <0.001 | ||
| No | 781 (99.7%) | 403 (68.0%) | |
| Yes | 2 (0.3%) | 190 (32.0%) |
The racial distribution varied in the two groups with a majority of Black patients in the control group (89%) compared to 43% in the MA group (p < 0.001). There were few patients from other racial groups in either of the study groups. The prevalence of tobacco or cocaine use was substantially higher in the MA group compared to controls.
3.1.1. Methamphetamine use is associated with an early onset of CVD observed in the MA group
As shown in Fig. 1, individuals in the methamphetamine group (N = 593) were significantly younger at first diagnosis of CVD compared to those in the control group (N = 783) (p < 0.001). There was a marked difference of about 8 years between the two groups.
Fig. 1.
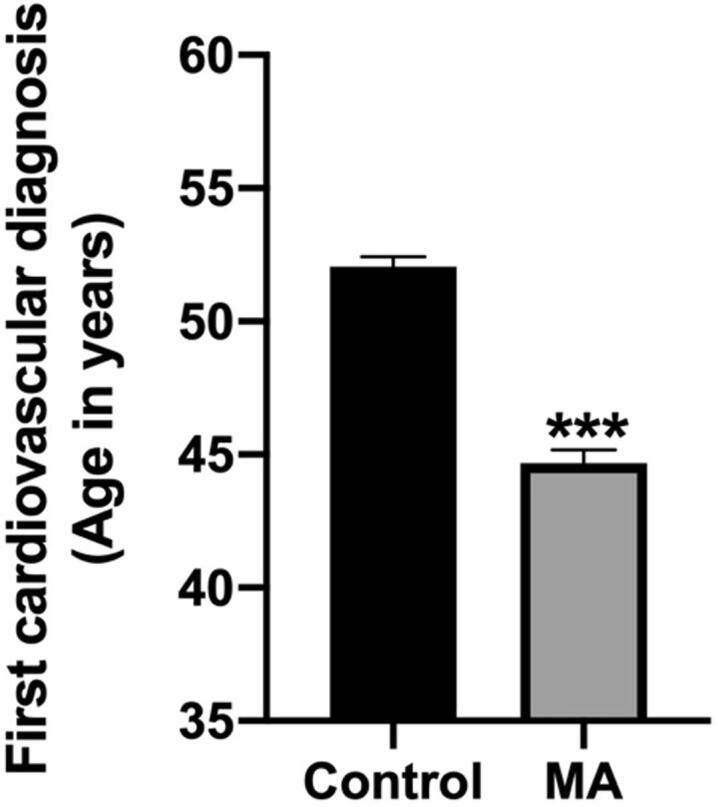
MA use was associated with an earlier age at first diagnosis of CVD compared to matched control group. All values represent the mean + SEM, *** = p < 0.001 as assessed by unpaired t-test.
3.1.2. MA use is associated with an earlier onset of CVD across both the races
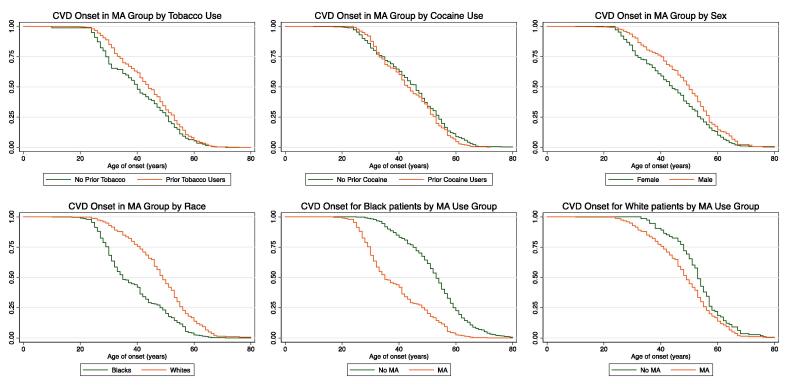
After observing the remarkable differences in the age of CVD onset between the MA and control groups, we next investigated whether risk factors such as prior tobacco or cocaine use, sex or race influenced the age of first recorded CVD diagnosis. Parametric regression analysis was used to estimate Time Ratios (TRs) for within and between group analysis and Hazard Ratios were calculated to estimate the risk for each group. The Kaplan-Meier graphs in Fig. 2A and 2B show that prior tobacco or cocaine use did not affect the latency to CVD onset in the MA group (HRTobacco = 1.18, 95% CI = 0.96 to 1.46, p = 0.12; HRCocaine = 1.08, 95% CI = 0.91 to 1.28, p = 0.38) (TRTobacco = 0.96, 95% CI = 0.91 to 1.00, p = 0.081; TRCocaine = 0.98, 95% CI = 0.94 to 1.02, p = 0.25). These data indicated that the MA use associated with early CVD onset was not influenced by a history of tobacco or cocaine use. The results remained unchanged after adjusting for prior tobacco use and sex as outlined in Supplementary Tables 1 and 2 (Tables S1 and S2).
Fig. 2.
A-F: Kaplan Meier curves, adjusted for sex and tobacco use, showing the influence of predictor variables on MA use associated early CVD onset. A) Tobacco use in MA group did not affect the latency to CVD onset. B) Sex differences were not observed in the latency to CVD onset. C) Prior cocaine use did not affect the latency to CVD onset. D) MA use was associated with racial differences in the latency to CVD onset. E) Black patients with prior MA use exhibited a shorter latency to CVD onset compared to compared to the control group. F) White patients with prior MA use demonstrated an earlier latency to CVD onset compared to the control group.
We next sought to determine whether sex differences were observed in MA use – associated early onset of CVD in our retrospective analysis. As shown in Supplementary Tables 1 and 2 (Table S1 and S2), sex differences were not observed in MA use – associated early onset of CVD (HR = 1.09, 95% CI = 0.92 to 1.28, p-value = 0.34; TR = 0.98, 95% CI = 0.94 to 1.02, p-value = 0.43). Group differences with respect to sex are graphically presented in Fig. 2C.
Taken together, these results suggest that risk factors such as prior tobacco or cocaine use and biological characteristics such as sex had no impact on the early CVD onset phenotype associated with MA use.
In contrast to the above results, MA use- associated early onset of CVD was notably observed in both the races. As shown in Fig. 2D, there was a striking difference in the latency-to-CVD onset between Black and White patients, with Blacks exhibiting an earlier onset of CVD compared to Whites. The cox proportional hazards regression model and Weibull distribution models for TR adjusted for prior tobacco use and sex are shown in Supplementary Tables 1 and 2 (Table S1 and S2) (HR = 1.32, 95% CI = 1.12 to 1.56, p-value < 0.01; TR = 0.93, 95% CI = 0.90 to 0.97, p-value < 0.001). The data in Fig. 2E and 2F further demonstrat that use of MA was associated with an early onset of CVD in both the races. The HR and TR for these analyses are presented in Supplementaty Tables 1 and 2 (Table S1 and S2) (HRBlacks = 1.95, 95% CI = 1.69 to 2.26, p-value < 0.01; TRBlacks = 0.86, 95% CI = 0.84 to 0.89, p-value < 0.001 and HRWhites = 1.76, 95% CI = 1.39 to 2.27, p-value < 0.01; TRWhites = 0.89, 95% CI = 0.84 to 0.93, p-value < 0.001) when between group comparisons were performed.
Models were adjusted for sex and tobacco use without changes in statistical significance as depicted in Supplementary Tables 1 and 2 (Table S1 and S2).
3.1.3. MA use - associated early diagnosis of CVD is predominant in the young cohort
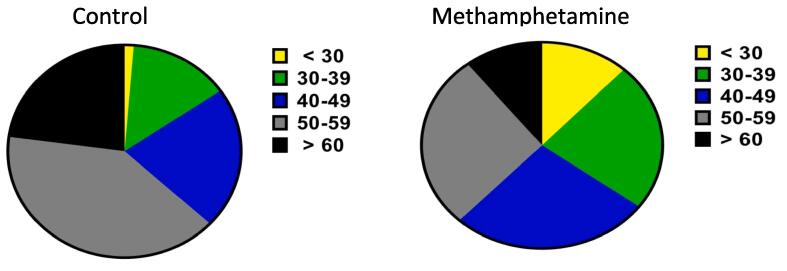
The dramatic difference in the age of CVD onset between the control and MA groups prompted us to evaluate which age group within the MA group was more prone to the cardiotoxic effect. Data for the age distribution for the first diagnosis of CVD in the control and methamphetamine groups is presented in Fig. 3. The pie charts depict a clear link between early onset of CVD and MA use with an almost 10-fold increase in the risk of early onset of CVD. A distinct shift is observed in the young cohort; compared to the control group (1%), a greater percentage (12%) of the individuals in the MA group received a diagnosis of CVD prior to the age of 30.
Fig. 3.
A comparative shift in the age distribution for early onset of CVD is observed between the two groups. A pie chart showing frequency of age distribution is presented.
3.1.4. Prevalence and nature of the CVD disease burden in the MA group
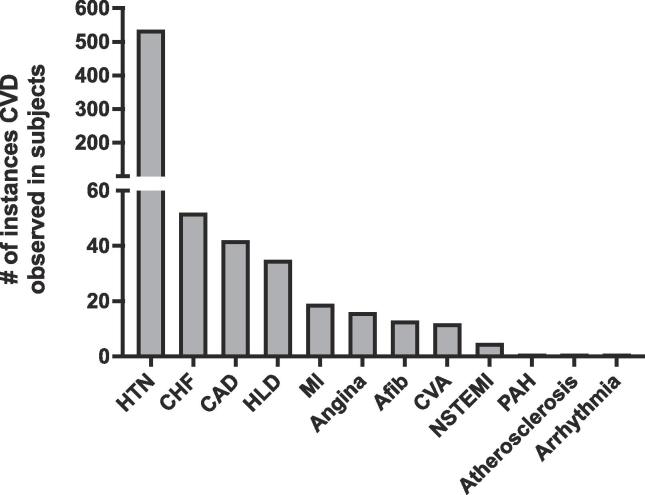
We also wanted to investigate the prevalence and nature of the different cardiovascular complications that together constitute the CVD disease burden associated with MA use. As shown in Fig. 4, hypertension was the most frequently observed cardiovascular event. We performed sensitivity analysis to evaluate differences in the previously described results. There were no differences in the statistical significance of the results as presented in Supplementary Tables 3 and 4 (Table S3 and S4).
Fig. 4.
Prevalence and nature of the different cardiovascular events in the MA group that together constitute the CVD burden collapsed across gender and age is presented. High levels of CVD are observed amongst cases of MA use with hypertension being a major contributing factor compared to the other CVDs.
Hypertension was followed by congestive heart failure (CHF), coronary artery disease (CAD), hyperlipidemia (HLD), myocardial infarction (MI), angina, atrial fibrillation (Afib), cerebrovascular accident / stroke (CVA), non-ST segment elevation myocardial infarction (NSTEMI), pulmonary arterial hypertension (PAH), atherosclerosis, and arrhythmia.
4. Discussion
This retrospective study set out to compare whether early onset of CVD is more prevalent in patients with a history of MA use compared to controls. Our findings indicate that patients with a history of MA use had a significantly shorter latency to the onset of CVD compared to controls. MA – use associated earlier onset of CVD persisted in both the racial cohorts compared to the control group with the effect being much stronger in Black than White patients. In addition, MA use was associated with a staggering increase in very early onset CVD in the cohort under 30 years of age. Methamphetamine is a sympathomimetic with a range of adverse effects that act on multiple organs systems. Of these, cardiovascular complications such as hypertension, cardiomyopathy (Paratz, Cunningham, & MacIsaac, 2016) and arrhythmias (Haning & Goebert, 2007) are prevalent amongst MA users, which contributes to an earlier age of CVD onset.
The remarkable 8 year difference in onset between the two groups was found in our retrospective cross-sectional study. Similar observations in earlier onset of neurocognitive decline and cellular alterations following psychostimulant use has been previously reported (Astarita et al., 2015, Bartzokis et al., 2000, Verdejo-Garcia and Perez-Garcia, 2007).
In addition to the above findings, we also present data showing that the young cohort (aged < 30 years) was more susceptible to the early onset of CVD associated with MA use. A distinct 12-fold increase in the risk of early onset of CVD was observed in this young cohort (aged < 30 years) in the MA group compared to controls. This upward shift in age at onset is in agreement with those of other studies of posthumous patient data from the literature that have reported CVD and ischemic heart disease to be more prevalent in young patients with a history of cocaine abuse (Bachi et al., 2017). Another recent case study reported a 22-year-old male patient with illicit MA abuse - associated cardiomyopathy (Schwab, Katus, & Raake, 2019). This key finding suggests that if it remains undetected, early onset of CVD observed with MA use may result in increased rates of clinical CVD encounters, culminating eventually in an immense economic, personal, and social burden (Zhao et al., 2021).
Although CVD is prevalent in individuals of all races/ethnicities, epidemiological data have indicated disproportionate rates of CVD prevalence at the population level. Black patients are at an elevated risk for cardiovascular complications compared to Whites (Graham, 2015). The exact pathophysiology underlying these racial differences is unclear but could be related to some unexplained factors such as different drug use patterns or accentuation of the pathological processes that inherently predispose Black patients to an earlier CVD onset.
To further explore which cardiac complication is associated with the early onset of CVD in MA users, we investigated the prevalence and nature of the different cardiac complications with respect to the age of early onset of CVD in both groups. Hypertension emerged as the most frequently reported cardiac event in MA users followed by CHF and others. This observation in our retrospective study is consistent with previously reported studies that have indicated hypertension and a range of other CVD complications to be associated with MA use (Darke et al., 2017).
While the findings presented here reveal a premature CVD phenotype observed in the MA group, some important limitations of our study include the weaknesses associated with a retrospective design. Foremost, although minimized by adjusting for sex and prior tobacco use, the inhomogeneity in our sample size may have confounded some of the results. Additionally, information regarding the duration and route of MA use seems to play a vital role in determining the nature of the CVD event associated with MA use; however, this was incomplete in our cohort. Furthermore, majority of MA users are polydrug users, but inadequate information concerning the duration and amount of tobacco or cocaine use limited risk factor analysis of how these factors contributed towards the earlier age of CVD onset. Finally, these retrospective data were acquired from a single center and may not generalize to other populations.
5. Conclusions
Overall, our key findings suggest that the rising prevalence of MA abuse is likely to promote an earlier onset of CVD and therefore the burden of cardiovascular complications in young adults. Risk factors such as prior tobacco or cocaine use, sex difference and racial disparity are implicated in early onset of CVD. It is important to note that prior MA use was as an independent risk factor for early CVD onset in our retrospective analysis. Nevertheless, the risk factors may be differentially present in individuals with a history of MA use and may interact with MA use to promote the early CVD onset. For example, tobacco use was four times more common in our MA group than in the control group. Although more research is needed to dissociate this complexity, our report and others highlight cardiovascular complications that are likely driven in key ways by MA use (Neeki et al., 2016). This poses an argument that assessment of drug use self-reports along with a positive urine drug screens for drugs of abuse could be useful in the clinical evaluation of CVD complications in young patients.
6. Contributors
VB: Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft, review and editing. JP and KM: Conceptualization, Methodology, Supervision. YG, LN, MP, AE, SK, ST, CC and SJ: Data collection. NB: Formal data analysis. SM and PD: Clinical expertise and manuscript review. BK: Study design. All authors contributed to the manuscript and have approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the department of Psychiatry and Behavioral Medicine for providing access to the database and the Louisiana Addiction Research Center for providing the resources needed for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.abrep.2022.100435.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abbruscato T.J., Trippier P.C. DARK Classics in Chemical Neuroscience: Methamphetamine. ACS Chemical Neuroscience. 2018;9(10):2373–2378. doi: 10.1021/acschemneuro.8b00123. [DOI] [PubMed] [Google Scholar]
- Astarita G., Avanesian A., Grimaldi B., Realini N., Justinova Z., Panlilio L.V.…Piomelli D. Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PLoS ONE. 2015;10(2) doi: 10.1371/journal.pone.0116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachi K., Mani V., Jeyachandran D., Fayad Z.A., Goldstein R.Z., Alia-Klein N. Vascular disease in cocaine addiction. Atherosclerosis. 2017;262:154–162. doi: 10.1016/j.atherosclerosis.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G., Beckson M., Lu P.H., Edwards N., Rapoport R., Wiseman E., Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: Implications for addiction research. Psychiatry Research. 2000;98(2):93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Bauch H.J., Grunwald J., Vischer P., Gerlach U., Hauss W.H. A possible role of catecholamines in atherogenesis and subsequent complications of atherosclerosis. Experimental Pathology. 1987;31(4):193–204. doi: 10.1016/s0232-1513(87)80001-4. [DOI] [PubMed] [Google Scholar]
- Darke S., Duflou J., Kaye S. Prevalence and nature of cardiovascular disease in methamphetamine-related death: A national study. Drug and Alcohol Dependence. 2017;179:174–179. doi: 10.1016/j.drugalcdep.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Dean A.C., Groman S.M., Morales A.M., London E.D. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38(2):259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey M.J., Ghosh R., Chatterjee S., Biswas P., Chatterjee S., Dubey S. COVID-19 and addiction. Diabetes Metab Syndr. 2020;14(5):817–823. doi: 10.1016/j.dsx.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–245. doi: 10.2174/1573403x11666141122220003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haning W., Goebert D. Electrocardiographic abnormalities in methamphetamine abusers. Addiction. 2007;102(Suppl 1):70–75. doi: 10.1111/j.1360-0443.2006.01776.x. [DOI] [PubMed] [Google Scholar]
- Kevil C.G., Goeders N.E., Woolard M.D., Bhuiyan M.S., Dominic P., Kolluru G.K.…Orr A.W. Methamphetamine Use and Cardiovascular Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(9):1739–1746. doi: 10.1161/ATVBAHA.119.312461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M.G., Gunderson E.W., Johanson C.E., Levin F.R., Foltin R.W., Hart C.L. Comparison of intranasal methamphetamine and d-amphetamine self-administration by humans. Addiction. 2012;107(4):783–791. doi: 10.1111/j.1360-0443.2011.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K.C., Shenouda S.K., McIlwain E., Charalampidis D., Lucchesi P.A., Varner K.J. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovascular Research. 2010;87(1):111–118. doi: 10.1093/cvr/cvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama H., Chang L., Fein G., Shimotsu R., Jiang C.S., Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106(8):1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeki M.M., Kulczycki M., Toy J., Dong F., Lee C., Borger R., Adigopula S. Frequency of Methamphetamine Use as a Major Contributor Toward the Severity of Cardiomyopathy in Adults </=50 Years. American Journal of Cardiology. 2016;118(4):585–589. doi: 10.1016/j.amjcard.2016.05.057. [DOI] [PubMed] [Google Scholar]
- Panenka W.J., Procyshyn R.M., Lecomte T., MacEwan G.W., Flynn S.W., Honer W.G., Barr A.M. Methamphetamine use: A comprehensive review of molecular, preclinical and clinical findings. Drug and Alcohol Dependence. 2013;129(3):167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Paratz E.D., Cunningham N.J., MacIsaac A.I. The Cardiac Complications of Methamphetamines. Heart Lung Circ. 2016;25(4):325–332. doi: 10.1016/j.hlc.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Pavese N., Rimoldi O., Gerhard A., Brooks D.J., Piccini P. Cardiovascular effects of methamphetamine in Parkinson's disease patients. Movement Disorders. 2004;19(3):298–303. doi: 10.1002/mds.10651. [DOI] [PubMed] [Google Scholar]
- Rezvan A., Ni C.W., Alberts-Grill N., Jo H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: Role of oxidative stress. Antioxidants & Redox Signaling. 2011;15(5):1433–1448. doi: 10.1089/ars.2010.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab D.M., Katus H.A., Raake P.W. Cardiomyopathy in a 22-year-old man with a long history of methamphetamine abuse. Internist (Berl) 2019;60(3):304–308. doi: 10.1007/s00108-019-0559-x. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A., Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: Common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190(4):517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Yang Z., Flores J., Katz S., Nathan C.A., Mehta V. Comparison of Survival Outcomes Following Postsurgical Radioactive Iodine Versus External Beam Radiation in Stage IV Differentiated Thyroid Carcinoma. Thyroid. 2017;27(7):944–952. doi: 10.1089/thy.2016.0650. [DOI] [PubMed] [Google Scholar]
- Zhao S.X., Deluna A., Kelsey K., Wang C., Swaminathan A., Staniec A., Crawford M.H. Socioeconomic Burden of Rising Methamphetamine-Associated Heart Failure Hospitalizations in California From 2008 to 2018. Circ Cardiovasc Qual Outcomes. 2021;14(7) doi: 10.1161/CIRCOUTCOMES.120.007638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.