Abstract
Objective
To identify the autoantigen in 2 individuals with possible seronegative paraneoplastic neuropathy.
Methods
Serum and CSF were screened by tissue-based assay and panned for candidate autoantibodies by phage display immunoprecipitation sequencing (PhIP-Seq). The candidate antigen was validated by immunostaining knockout tissue and HEK 293T cell-based assay.
Results
Case 1 presented with gait instability, distal lower extremity numbness, and paresthesias after a recent diagnosis of serous uterine and fallopian carcinoma. Case 2 had a remote history of breast adenocarcinoma and presented with gait instability, distal lower extremity numbness, and paresthesias that progressed to generalized weakness. CSF and serum from both patients immunostained the axon initial segment (AIS) and node of Ranvier (NoR) of mice and enriched βIV-spectrin by PhIP-Seq. Patient CSF and serum failed to immunostain NoRs in dorsal root sensory neurons from βI/βIV-deficient mice. βIV-spectrin autoantibodies were confirmed by overexpression of AIS and nodal βIV-spectrin isoforms Σ1 and Σ6 by a cell-based assay. βIV-spectrin was not enriched in a combined 4,815 PhIP-Seq screens of healthy and other neurologic disease patients.
Discussion
Therefore, βIV-spectrin autoantibodies may be a marker of paraneoplastic neuropathy.
Classification of Evidence
This study provides Class IV evidence that βIV-spectrin antibodies are specific autoantibody biomarkers for paraneoplastic neuropathy.
βIV-spectrin autoantibodies have been reported in a patient with single breast cancer who developed motor neuropathy.1 However, whether βIV-spectrin antibodies are a diagnostic marker of paraneoplastic neuropathy is unknown. During a study of anti-TRIM46 neurologic syndromes,2 we identified 2 TRIM46-negative cases whose antibodies putatively localized to the axon initial segment (AIS) in the cortex (Figure 1A) and node of Ranvier (NoR) in cerebellar white matter and optic tract (not shown). Case 1, a woman in her 70s, developed gait instability, distal lower extremity numbness, and paresthesias 6 months after being diagnosed with high-grade uterine and fallopian serous carcinoma. Case 2, a woman in her 80s, was diagnosed with breast adenocarcinoma 10 years before presenting with dizziness and diplopia that rapidly progressed to dysarthria, dysphagia, and generalized weakness—a deterioration similar to the previous report of βIV-spectrin antibodies.1 Both cases tested negative for classified paraneoplastic autoantibodies (eAppendix 1 and eTable 1, links.lww.com/NXI/A722).
Figure 1. Patient Antibodies Localize to the AIS and NoR.
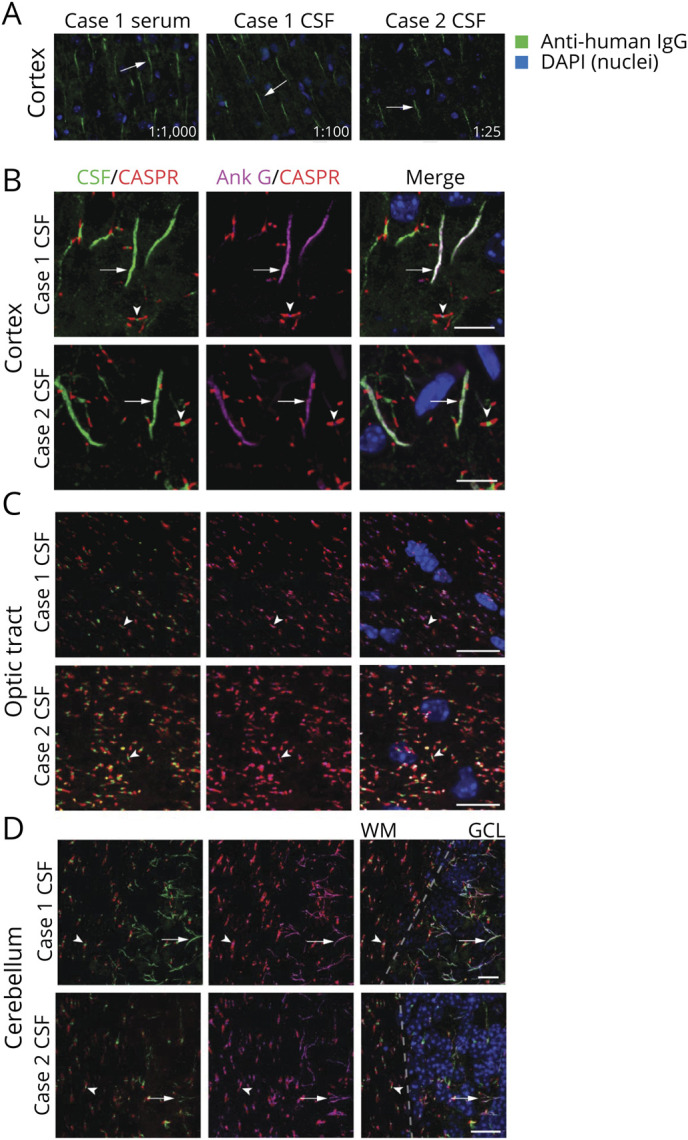
(A) Mouse brain sections were immunostained with case 1 serum (1:1,000 dilution), case 1 CSF (1:100 dilution), and case 2 CSF (1:25 dilution). In all cases, patient IgG (green) immunostained structures consistent with the AIS (arrows). Images were captured at 20× on an epifluorescent microscope. (B–D) Anatomic coimmunostaining of case 1 and 2 CSF IgG (green) with the AIS and nodal marker AnkG (magenta) in the (B) cortex, (C) optic tract, and (D) cerebellum. In D, the dotted line indicates the boundary between the cerebellar WM and GCL. Arrows indicate axon initial segments while arrowheads indicate nodes of Ranvier which are demarcated by CASPR in red. All scale bars = 10 µm. Images were captured by confocal microscopy at 60 or 100×. AIS = axon initial segment; AnkG = ankyrin G; GCL = granule cell layer; IgG = immunoglobulin G; NoR = node of Ranvier; WM = white matter.
We confirmed AIS and NoR (AIS·NoR) immunoreactivity by costaining with commercial antibodies to ankyrin G (AnkG, an AIS and NoR marker) and CASPR (a paranodal marker). In the cortex, optic tract, and cerebellar white matter, CSF immunoglobulin G (IgG) from both cases colocalized with, but was not identical to, AnkG (Figure 1, B–D). Case 1 serum IgG also colocalized with AnkG in the cortex, optic tract, and cerebellum (not shown). Case 2 serum was not available.
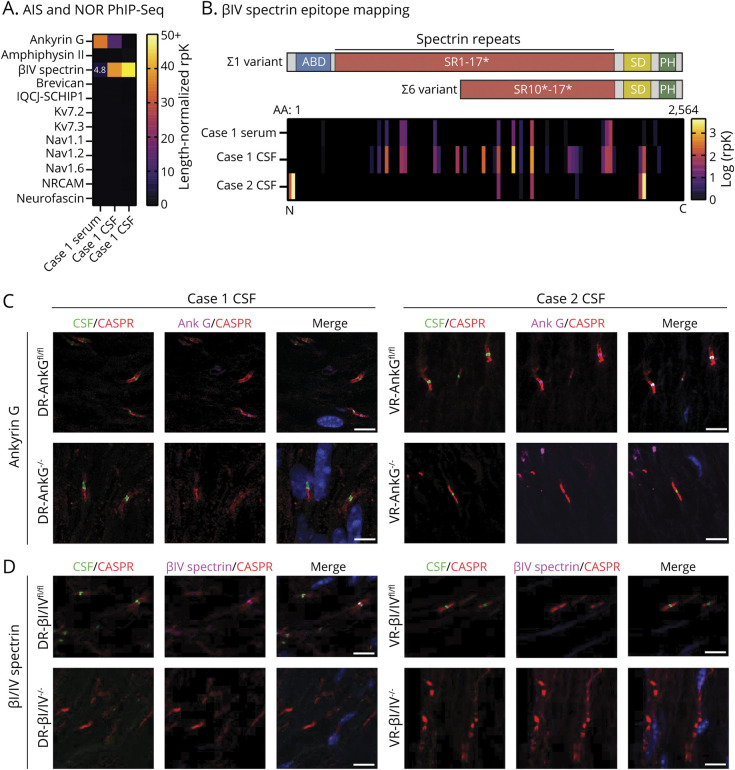
We next panned patient CSF and sera for autoantibodies by phage immunoprecipitation sequencing (PhIP-Seq) and restricted our initial analysis to proteins expressed in both AIS and NoR, as annotated by EMBL-EBI (eMethods, links.lww.com/NXI/A722). AnkG and βIV-spectrin were enriched by case 1 serum. However, case 1 CSF enriched βIV-spectrin threefold more than AnkG. Case 2 CSF strongly enriched βIV-spectrin alone (Figure 2A). Epitope mapping revealed that all 3 biospecimens enriched peptides that mapped to the major AIS and NoR βIV-spectrin isoforms, Σ1 and Σ6 (Figure 2B).3,4
Figure 2. Identification and Validation of Anti–BIV-Spectrin Antibodies.
(A) Heatmap of PhIP-Seq enrichments of proteins expressed in the both AIS and NoR. Enrichments are represented as length-normalized rpK (total rpK for the given protein/number of peptides that map to that protein, see supplemental methods). βIV-spectrin was detected in case 1 CSF with a length-normalized rpK = 4.8. All dark and unmarked cells had a length-normalized rpK <1. (B) Graphical representation of the approximate and relative locations of actin-binding domain (ABD, blue), spectrin repeats (SR, red, * = partial SR), specific domain (SD), and pleckstrin homology domain (PH, green) relative to PhIP-Seq peptide enrichments in the heatmap below spanning the full length of βIV-spectrin (amino acids 1–2564, overlapping peptides are laid end-to-end). Heatmap of βIV-spectrin peptide enrichments regarding AIS and nodal βIV-spectrin isoforms Σ1 and Σ6. Each peptide corresponds to a single peptide. N and C refer to the amino and carboxy termini of βIV-spectrin. (C) Left, case 1 CSF immunostaining of AdvillinCre/+ DR-AnkGfl/fl and DR-AnkG−/− shows nodal staining in the absence of AnkG, suggesting that AnkG is not the autoantigen. Right, case 2 CSF immunostaining of ChatCre/+ VR-AnkGfl/fl and VR-AnkG−/− tissue indicates that case 2 does not harbor AnkG antibodies. (D) Case 1 and 2 CSF immunostaining of AdvillinCre/+ DR-βI/βIVfl/fl and DR-βI/βIV−/− shows the disappearance of nodal staining in the absence of βI/V-spectrin, suggesting that βIV-spectrin is the autoantigen in both cases. For C and D, CSF was immunostained at 1:4 dilution. All scale bars = 5 µm. AIS = axon initial segment; AnkG = ankyrin G; IgG = immunoglobulin G; NoR = node of Ranvier; PhIP-Seq = phage display immunoprecipitation sequencing.
Consistent with the patients' negative paraneoplastic testing, classified paraneoplastic autoantigens were not enriched by PhIP-Seq. Notably, CSF and serum from case 1 enriched peptides to SAP25, a candidate autoantigen previously observed in anti-Yo paraneoplastic syndromes (eFigure 1, links.lww.com/NXI/A722).5
We next immunostained peripheral nerves of conditional Cre knockout mice to determine whether patient antibodies targeted AnkG or βIV-spectrin.6,7 Owing to the availability of tissue, case 1 was screened against dorsal root (DR) sensory neurons and case 2 against ventral root (VR) motor neurons. Unexpectedly, case 1 CSF and sera immunostained both AnkG-expressing (DR-AnkGfl/fl) and AnkG-deficient (DR-AnkG−/−) NoR despite enriching AnkG by PhIP-Seq (Figure 2C, serum not shown). Consistent with the PhIP-Seq data, case 2 CSF also immunostained both VR-AnkGfl/fl and VR-AnkG−/− NoR (Figure 2C).
Next, we used βI/βIV-spectrin double conditional mice to test for βIV autoantibodies because βI-spectrin is a paralog of βIV-spectrin that localizes to βIV-deficient NoR and therefore could confound the interpretation of immunostaining due to cross-reactivity.6 Case 1 serum (data not shown) and case 1 and 2 CSF immunostained DR-βI/βIVfl/lfl, but not DR-βI/βIV−/− (Figure 2D). Moreover, case 1 serum failed to immunostain DR-βI/βIV−/l− NoR that still expressed AnkG indicating that the nodal staining was solely due to anti-spectrin antibodies (eFigure 2, links.lww.com/NXI/A722).
Together, these data suggested that both patients harbored a polyclonal IgG response to βIV-spectrin, but not other classified paraneoplastic antigens. Therefore, we tested for direct binding of patient autoantibodies to βIV-spectrin by HEK 293T overexpression cell-based assay. As predicted by PhIP-Seq (Figure 2B), both cases immunostained Myc-βIV-Σ1 and Myc-βIV-Σ6 overexpressing cells, but not untransfected cells in the same well (Figure 3A). Consistent with AnkG knockout tissue staining, case 1 CSF and serum failed to bind to AnkG-mCherry in a cell-based assay (Figure 3B).
Figure 3. Direct Validation of Anti-BIV Antibodies by HEK 293T Overexpression Cell-Based Assay.
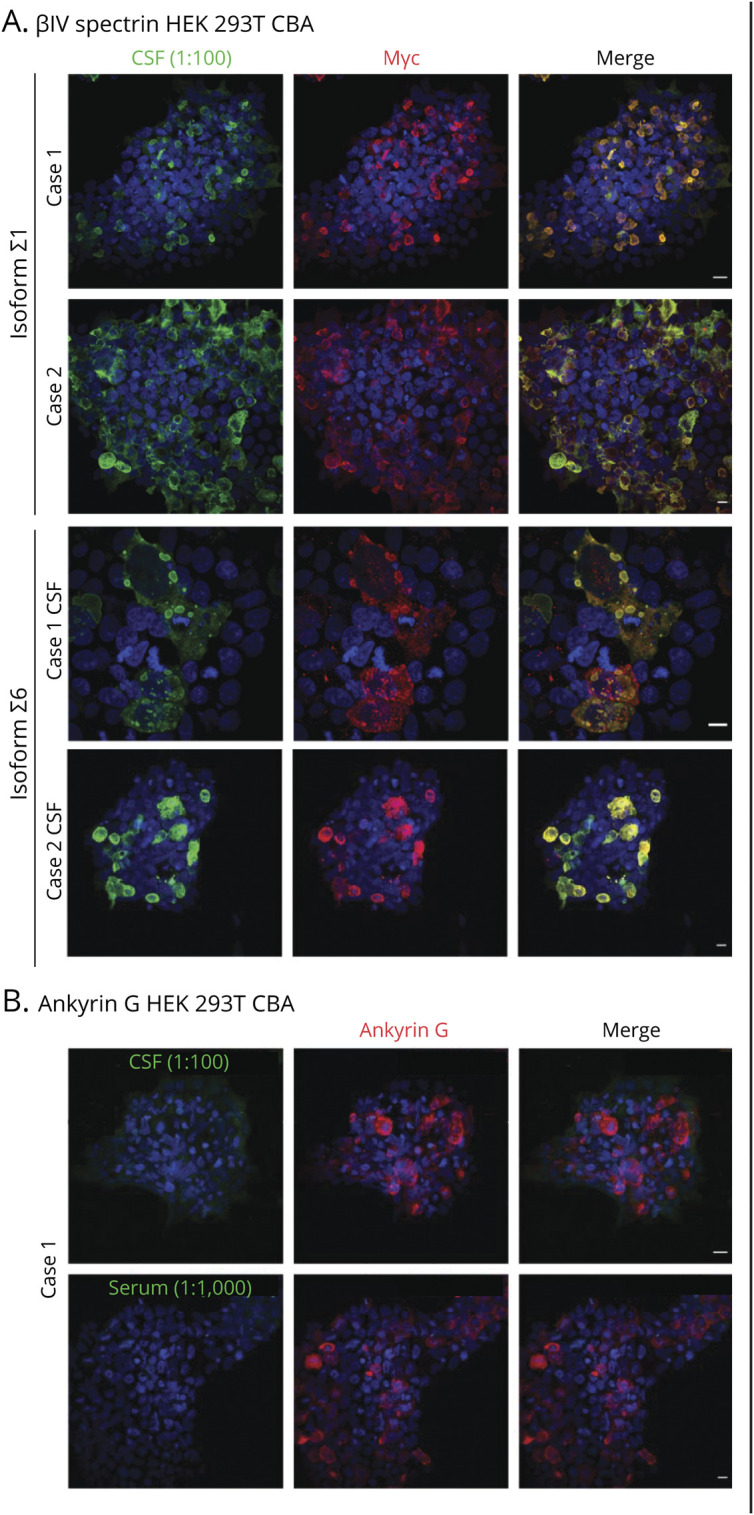
(A) HEK 293T cells were transfected with Myc-βIV-spectrin Σ1 and Σ6, fixed, permeabilized, and immunostained with case 1 or case 2 CSF at 1:100 dilution. In each case, CSF IgG (green) substantially overlapped with Myc immunostaining (red). (B) HEK 293T cells were transfected with rat RFP-AnkG, fixed, permeabilized and immunostained with case 1 (1:100) or serum (1:1,000). In both instances, case 1 IgG (green) failed to immunostain or colocalize with commercial AnkG immunostaining (red). AnkG = ankyrin G; IgG = immunoglobulin G.
In our experience, AIS·NoR-restricted CSF immunostaining is uncommon, suggesting that βIV-spectrin antibodies are rare. We found that by PhIP-Seq, case 1 and 2 biospecimens enriched βIV-spectrin moreso than 3,408 control samples (comprised beads only and healthy CSF, serum, and plasma, including technical replicates) and 1,407 samples from other neurologic disorders (comprised CSF, serum, and plasma samples, including technical replicates, from patients with other neurologic or neuroinflammatory syndromes including 15 patients with breast or ovarian cancer pathology and 3 with prominent peripheral neuropathy) (eFigure 3, links.lww.com/NXI/A722). Furthermore, βIV-spectrin was not enriched in publicly available PhIP-Seq data from 36 anti-Yo paraneoplastic patients (eMethods).
Although this study is limited by small case numbers, our evaluation of hundreds of biospecimens by tissue-based assay and thousands of biospecimens by PhIP-Seq indicate that anti–βIV-spectrin antibodies are rare and preferentially occur in patients with cancer and peripheral neuropathy with or without additional neurologic symptoms. This supports consideration of βIV-spectrin antibodies as class IV biomarkers of cancer-associated peripheral neuropathy according to Neurology's Criteria for Rating Diagnostic Accuracy Studies (eMethods, links.lww.com/NXI/A722).
Acknowledgment
The authors thank Delaine Larsen, PhD; Kari Herrington, PhD; and SoYeon Kim, PhD, of the University of California San Francisco Nikon Imaging Center for their imaging support. They also thank the patients and families for their participation in the research.
Appendix. Authors
Contributor Information
Christopher M. Bartley, Email: christopher.bartley@ucsf.edu.
Thomas T. Ngo, Email: thomas.ngo@ucsf.edu.
Bonny D. Alvarenga, Email: bonny.alvarenga@ucsf.edu.
Andrew F. Kung, Email: andrew.kung@ucsf.edu.
Lindsay H. Teliska, Email: lindsay.teliska@bcm.edu.
Michael Sy, Email: msy@hs.uci.edu.
Joseph L. DeRisi, Email: joseph.derisi@ucsf.edu.
Matthew N. Rasband, Email: rasband@bcm.edu.
Sean J. Pittock, Email: pittock.sean@mayo.edu.
Divyanshu Dubey, Email: dubey.divyanshu@mayo.edu.
Michael R. Wilson, Email: michael.wilson@ucsf.edu.
Study Funding
Confocal microscopy with the CSU-W1 spinning disk was supported by the S10 Shared Instrumentation grant (1S10OD017993-01A1). This work was further supported by NIMH R01MH122471 (S.J.P., M.R.W., and J.L.D.), R25MH060482 (C.M.B.), NINDS K08NS096117 (M.R.W.), Brain Research Foundation (S.J.P.), and Chan Zuckerberg Biohub (J.L.D.). C.M. Bartley was supported by a Hanna H. Gray Fellowship, Howard Hughes Medical Institute; President's Postdoctoral Fellowship Program, the University of California; the John A. Watson Scholar Program, the University of California, San Francisco; and the Latinx Center of Excellence (Grant No. D34HP3178).
Disclosure
C.M. Bartley has received an honorarium for speaking to the Commonwealth Club. J.L. DeRisi has received grants from the Chan Zuckerberg Biohub, personal fees from the Public Health Company, and personal fees from Allen & Company and has a patent pending for kelch-like protein 11 as a marker of neurological autoimmunity. M.N. Rasband was supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. S.J. Pittock reports grants, personal fees, and nonfinancial support from Alexion Pharmaceuticals, Inc.; grants, personal fees, nonfinancial support, and other support from MedImmune, Inc/Viela Bio.; personal fees for consulting from Genentech/Roche, UCB, and Astellas, outside the submitted work. S.J. Pittock has a patent, Patent# 8,889,102 (Application#12-678350, Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia)—issued; a patent, Patent# 9,891,219B2 (Application#12-573942, Methods for Treating Neuromyelitis Optica [NMO] by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive)—issued. S.J. Pittock also has patents pending for the following IgGs as biomarkers of autoimmune neurological disorders (septin-5, kelch-like protein 11, GFAP, PDE10A, and MAP1B). D. Dubey has attended UCB Advisory Board Meeting in Lyon, France, on September 23, 2019. D. Dubey has also consulted for UCB, Immunovant, and Astellas pharmaceuticals. All of D. Dubey's compensation for consulting activities is paid directly to Mayo Clinic. D. Dubey is on the editorial board of Journal of Clinical Medicine. D. Dubey has patents pending for kelch-like protein 11 and Leucine zipper 4 as a marker of neurological autoimmunity and germ cell tumors. M.R. Wilson has received grants from Roche/Genentech as well as personal fees from Novartis, Takeda, and Genentech and has a patent pending for kelch-like protein 11 as a marker of neurological autoimmunity. No other disclosures were reported. Go to Neurology.org/NN for full disclosures.
References
- 1.Berghs S, Ferracci F, Maksimova E, et al. Autoimmunity to beta IV spectrin in paraneoplastic lower motor neuron syndrome. Proc Natl Acad Sci USA. 2001;98(12):6945-6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valencia-Sanchez C, Knight AM, Hammami MB, et al. Characterisation of TRIM46 autoantibody-associated paraneoplastic neurological syndrome. J Neurol Neurosurg Psychiatry. 2022;93(2):196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacas-Gervais S, Guo J, Strenzke N, et al. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166(7):983-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komada M, Soriano P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156(2):337-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Donovan BD, Mandel-Brehm C, Vazquez SE, et al. Exploration of Anti-Yo and Anti-Hu paraneoplastic neurological disorders by PhIP-Seq reveals a highly restricted pattern of antibody epitopes. bioRXiv. 2018;2018:502187. [Google Scholar]
- 6.Ho TS, Zollinger DR, Chang KJ, et al. A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat Neurosc. 2014;17(12):1664-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CH, Stevens SR, Teliska LH, et al. Nodal beta spectrins are required to maintain Na(+) channel clustering and axon integrity. Elife. 2020;9:e52378. [DOI] [PMC free article] [PubMed] [Google Scholar]