Abstract
Background
Liver graft fibrosis affects long-term graft and patient survival in liver transplant recipients. Transient elastography and magnetic resonance elastography are widely used for the assessment of liver fibrosis in routine clinical practice, but are limited in liver transplant settings. The aims of the present study were to evaluate the accuracy of magnetic resonance elastography and transient elastograph in the assessment of liver fibrosis in liver transplant recipients, and to determine the recurrence rates of post-transplant hepatic steatosis and liver fibrosis.
Methods
A total of 126 consecutive liver transplant recipients were included. Magnetic resonance elastography and transient elastography were performed for to measure liver stiffness.
Results
The most common cause of liver transplantation was hepatitis B virus-induced cirrhosis (50%). The mean liver stiffness value with transient elastography was 6.1 ± 3.0 kPa, and the mean magnetic resonance elastography value was 2.7 ± 1.0 kPa. A significant positive correlation was found between magnetic resonance elastography and transient elastography in terms of liver stiffness measurement (r = 0.61, P < .001). Obesity and the underlying etiology of liver diseases did not have any significant negative effect on magnetic resonance elastography and transient elastography measurements. During the follow-up, the post-transplant recurrence rates of hepatic steatosis and hepatic fibrosis were 26% and 37%, respectively. The recurrence rates of post-transplant hepatic steatosis and liver fibrosis were slightly higher in recipients with non-alcoholic fatty liver disease-related cirrhosis than those with viral hepatitis-related etiologies (44% vs 27%, P = .43; 44% vs 30%, P = .45, respectively).
Conclusion
Magnetic resonance elastography and transient elastography are accurate in assessing liver fibrosis in the liver transplant setting. Obesity and the underlying etiology of primary liver disease do not influence the measurements.
Keywords: Aspartate aminotransferase to platelet ratio index (APRI), fibrosis score 4 (FIB-4), liver fibrosis, magnetic resonance elastography, liver transplantation, transient elastography
Introduction
Liver graft fibrosis affects long-term graft and patient survival in liver transplant recipients.1 Liver biopsy remains the gold standard for the evaluation of liver inflammation and fibrosis. However, its utility is still subject to debate due its invasiveness, potential complications, and diagnostic variability.2,3 In fact, sequential liver biopsy is not applicable as a diagnostic method for serial monitoring of post-transplant liver fibrosis. Most liver transplantation (LT) centers no longer perform protocol liver biopsies because of low patient and physician acceptability. Since the degree of liver fibrosis predicts graft and patient survival, accurately assessing and monitoring liver graft fibrosis changes using non-invasive methods is necessary.
Several non-invasive biochemical-based biomarkers, including the aspartate aminotransferase (AST) to platelet ratio index (APRI) and fibrosis score 4 (FIB-4), and imaging methods such as transient elastography (TE) and magnetic resonance elastography (MRE) are widely used for the assessment of liver fibrosis in routine clinical practice.4-11 FIB-4 and APRI combined with clinical parameters are accurate indirect measures of liver fibrosis.5,6,10-12 Vibration-controlled (VC) TE (FibroScan; Echosens, Paris, France) measures the controlled attenuation parameter (CAP) for hepatic steatosis and liver stiffness for liver fibrosis.9,11,13-16 MR imaging (MRI) methods such as MR spectroscopy, proton density fat fraction (MRI-PDFF), and MRE have been used for the determination of hepatic steatosis and liver stiffness.17-20 Previous studies have reported that MRE is significantly more accurate in the assessment of hepatic fibrosis in patients with chronic liver disease (CLD) than other non-invasive techniques.8,11,21,22
Extensive clinical data on the utility and performance of elastography in native livers are available in the literature. However, few studies have evaluated the accuracy of MRE and TE in assessing liver fibrosis in the LT setting. The aims of the present study were to evaluate the accuracy and performance of MRI methods and TE in the assessment of liver fibrosis and hepatic steatosis in liver transplant recipients and to determine the post-transplant hepatic fibrosis and steatosis recurrence rates using MRI methods.
Materials and Methods
Patients
This was a retrospective study on prospectively collected data that included 126 consecutive recipients (M/F: 72/54) followed up in the Ankara University School of Medicine, Department of Gastroenterology, Liver Diseases Outpatient Clinic, between September 10, 2019 and March 9, 2020. All patients were followed up for at least 12 months after LT. Data were collected from outpatient visit charts. This study was approved by the local ethics committee of the Ankara University School of Medicine (I3-184-20).
MATERIALS AND METHODS
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), bilirubin, and complete blood cell counts were measured in our central laboratory. The values for APRI and FIB-4 were calculated on the day that TE was performed, as follows:
APRI: AST (U/L)/upper limit of normal AST (U/L)/platelets (109/L x 100);23
FIB-4: Age (years) x AST (U/L)/ALT (U/L)1/2 x platelets x109/L.24
The FIB-4 index gives values between 0.2 and 10. It can accurately differentiate mild to moderate liver fibrosis from advanced fibrosis and cirrhosis. An FIB-4 score of <1.45 indicates no advanced fibrosis with a negative predictive value of 90%, whereas an FIB-4 score of >3.25 indicates advanced fibrosis with 97% specificity and a positive predictive value of 65%.10,12
Hepatic Steatosis and Liver Stiffness Measurement
Hepatic steatosis measurement and liver stiffness measurement (LSM) were obtained using a FibroScan probe (Echosens, Paris, France) with an M or an XL probe to cater to patients with different body build types. All measurements were performed by one of the authors (ZME). Patients were examined after fasting overnight. The FibroScan probe was placed in the appropriate intercostal space window on the anterior axillary line. At least 10 valid measurements were obtained within 5-10 minutes. The ratio of the median of 10 successive measurements to the interquartile range (IQR) was <30%. TE was used to measure the CAP (dB/m) and liver stiffness (kPa) simultaneously. For F2-F4 fibrosis, the cut-off values ranged from 6.2 kPa to 11 kPa, with 62-90% sensitivity and 74-100% specificity; for F3-F4 fibrosis, the cut-off values ranged from 8 kPa to 12 kPa, with 84-100% sensitivity and 83-97% specificity.9 Morbidly obese patients (BMI > 40 kg/m2) and severely underweight patients (BMI < 16 kg/m2), patients with ascites, and patients with moderate and severe cholestasis were excluded.
MRI was performed with a 1.5-T MR system (Magnetom Aera, Siemens Healthcare, Erlangen, Germany). A 30-channel phased-array body coil was used for this acquisition. The patients were examined in the supine position. A 3-plane localization imaging gradient echo sequence was performed at the beginning of the examination. The MRE parameters were as follows: TR/TE 50 ms/21.41 ms, flip angle 25°, section thickness 50 mm, and field of view (FOV) 350 x 350 mm2 with a passive driver frequency of 60 Hz. Using a workstation (syngo.via VB10; Siemens Medical Solutions), regions of interest (ROIs) were drawn as geographic areas guided by the magnitude image to include liver parenchyma by excluding major vessels. Measurements were repeated on the confidence map images. The multi-echo Dixon method was used with a VIBE sequence (Siemens Healthcare) with the following parameters: repetition time 15.6 ms, 6 echo times (1.23, 2.48, 3.73, 4.98, 6.23, and 7.48 ms), flip angle 4°, readout echo bandwidth 1080 Hz/pixel, FOV 450 mm, and slice thickness 3.5 mm. Using a workstation (syngo.via VB10; Siemens Medical Solutions), 3 elliptic ROIs of approximately 2 cm2 were placed on the PDFF maps, and the average was calculated.
Immunosuppression
The immunosuppressive protocol consisted of tacrolimus or cyclosporine plus mycophenolate mofetil (MMF) and a steroid. Tacrolimus or cyclosporine was administered with a therapeutic target level. Corticosteroids were gradually tapered over 12 weeks and discontinued for 24-48 weeks after LT. Alternative immunosuppressive agents, including sirolimus or everolimus, were used in a few patients who were intolerant of calcineurin inhibitors.
Definitions
The primary end point was the assessment of liver fibrosis and hepatic steatosis by MRI methods and TE, and was a comparison of the MRE and TE results with the APRI and FIB-4 scores.
The secondary end point was the determination of post-transplant liver fibrosis and hepatic steatosis recurrence rates using MRI methods.
The detailed diagnostic values of MRE for the detection of each stage of liver fibrosis and the optimal thresholds were previously provided.8
Statistical Analysis
Means and standard deviations, medians and ranges, and frequencies and percentages were used for descriptive statistics. Comparisons between 2 groups were assessed with Student’s t-test or the Mann–Whitney U-test, depending on the distribution of the data. Categorical variables were assessed with the chi-square test or Fisher’s exact test. Correlations between continuous and/or ordinal variables were calculated using Pearson’s correlation coefficient or Spearman’s rho analysis, where applicable. Multiple linear regression analysis was performed to determine relationships between a continuous dependent variable and a set of independent variables. A P value of less than .05 was considered statistically significant.
Results
Patients
All 126 patients were Caucasian, their median age was 57 years (range: 18-74 years), and their gender was predominantly male (58%). The median body mass index (BMI) was 25.7 kg/m2 (range: 16-37.6 kg/m2): 40.2% of the patients were overweight (25-29.9 kg/m2), and 17.5% were obese (≥30 kg/m2). Among the patients, 35% had diabetes mellitus, 28% had hypertension, and 27% were active smokers. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of All Liver Transplant Recipients
| Overall, (n = 126) | |
|---|---|
| Age (years) | 53.3 ± 11.1 |
| 57 (18-74) | |
| Gender (M/F) | 58%/42% |
| BMI (kg/m2) | 26.2 ± 4.5 |
| 25.7 (16-37.6) | |
| Serum ALT (U/L) | 24.7 ± 20.9 |
| 19.0 (6-169) | |
| Serum AST (U/L) | 24.9 ± 14.6 |
| 22.0 (12-145) | |
| Serum GGT (U/L) | 49.1±67.1 |
| 28.5 (7-587) | |
| Serum ALP (U/L) | 136.0 ± 89.2 |
| 109.0 (43-634) | |
| Serum total bilirubin (mg/dL) | 0.92 ± 0.5 |
| 0.8 (0.3-3.0) | |
| INR | 1.2 ± 0.4 |
| 1.1 (0.5-4.0) | |
| Serum albumin (g/dL) | 4.3 ± 3.0 |
| 4.3 (3.3-5.0) | |
| Platelet count (103/µL). | 207.2 ± 65.8 |
| 198.0 (50.0-471.0) | |
| APRI Score | 0.29 ± 0.2 |
| 0.24 (0.08-1.53) | |
| FIB-4 Score | 1.50 ± 0.7 |
| 1.44 (0.15-3.92) | |
| TE (kPa) | 6.1 ± 3.0 |
| 5.5 (2.1-19.0) | |
| MRE (kPa) | 2.71 ± 1.0 |
| 2.43 (1.57-6.72) | |
| CAP (dB/m) | 249.5 ± 70.5 |
| 254.5 (100.0-399.0) | |
| MR-PDFF (%) | 4.9 ± 7.0 |
| 2.0 (0.1-30.0) |
Data were given as mean ± SD, Median (Min, Max).
APRI, aspartate aminotransferase (AST) to platelet ratio index; CAP, controlled attenuation parameter; FIB 4, fibrosis score 4; INR, internal normalized ratio; PDFF, proton density fat fraction.
The reason for LT was hepatitis B virus (HBV)-induced cirrhosis in 63 patients (50%), autoimmune liver diseases in 12 patients (9.5%), NAFLD cirrhosis in 11 patients (8.7%), hepatitis D co-infection-related cirrhosis in 9 patients (7.1%), cryptogenic cirrhosis in 8 patients (6.4%), hepatitis C virus (HCV)-induced cirrhosis in 7 patients (5.6%), alcoholic liver disease (ALD) in 4 patients (3.2%), and miscellaneous factors in 12 patients (10.2%). Nine patients had been diagnosed with hepatocellular carcinoma (HCC) prior to LT. Living donor LT (LDLT) was performed on 91 patients (72.2%), while cadaveric donor LT (CDLT) was performed on the remaining 35 patients (27.8%). The donors were predominantly male (65% vs 35% females). Most patients (85%) were on tacrolimus-based combination therapy (tacrolimus plus MMF with/without prednisolone), followed by 7.1% on cyclosporin-based therapy, 9.5% on everolimus-based therapy, and 4.0% on sirolimus-based therapy.
The median serum ALT level was 19 U/L (range: 6-169 U/L); the median AST level was 22 U/L (range: 12-145 U/L); the median GGT level was 28.5 U/L (range: 7-587 U/L); the median ALP level was 109 U/L (range: 43-634 U/L); the median total bilirubin level was 0.8 mg/dL (range: 0.3-3.0 mg/dL); the median albumin level was 4.3 g/L (range: 3.3-5.0 g/L) and the median platelet count was 198 × 103/µL (range: 50-471 × 103/µL). The median international normalized ratio (INR) was 1.1 (range: 0.5-4.0) (Table 1).
Hepatic Steatosis and Liver Stiffness Measurements
The mean FIB-4 and APRI scores were 1.50 ± 0.7 (median: 1.44, range: 0.15-3.92) and 0.29 ± 0.2 (median: 0.24, range: 0.08-1.53) respectively. Sixty-five patients (51.2%) had low fibrosis score (<1.45), whereas 5 patients (3.9%) presented with advanced fibrosis (FIB-4 > 3.25).
The median time interval between LT and TE was 75.5 months (range: 12-372 months). TE was performed on all recipients with a median IQR/M of 14% (range: 2-29%). An M probe was used in 71 patients, whereas an XL probe was used in 55 patients. The mean CAP and liver stiffness values were 249.5 ± 70.5 dB/m (median: 254.5 dB/m, range: 100-399 dB/m) and 6.1 ± 3.0 kPa (median: 5.5 kPa, range: 2.1-19.0 kPa), respectively (Table 1).
MRI was performed for 78 available patients. The median time interval between TE and MRE was 118 days (range: 1-179 days). The mean MRI-PDFF and MRE values were 4.9% ± 7.0% (median: 2%, range: 0.1-30%) and 2.71 ± 0.96 kPa (median: 2.43 kPa, range: 1.57-6.72 kPa), respectively (Table 1).
A comparison between the LDLT and CDLT groups revealed no significant difference in liver stiffness determined by TE and MRE. The mean TE and MRE values were 6.77 ± 3.71 kPa (median: 5.7 kPa) and 2.82 ± 1.07 kPa (median: 2.46 kPa), respectively, in the LDLT group, and 5.28 ± 2.17 kPa (median: 4.8 kPa) and 2.38 ± 0.38 kPa (median: 2.33 kPa), respectively, in the CDLT group.
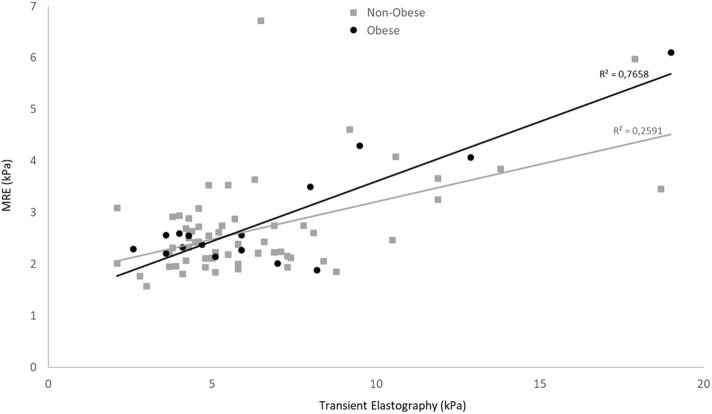
A significant positive correlation was found between MRE and TE in terms of LSMs (r = 0.613, 95% CI: 0.452-0.735, P < .001) (Figure 1). There was also a significant positive correlation between FIB-4 and the APRI (r = 0.727, 95% CI: 0.577-0.803, P < .001). In contrast, no significant correlations were found between imaging techniques and biochemical tests. Of note, MRE and TE accurately identified mild or moderate fibrosis using a FIB-4 cutoff value <1.45 (n = 37). The correlation between MRE and TE reached 0.79 (P < .001). Obesity did not have a significant negative effect on LSM, in MRE and TE. There was a significant difference in the MRE and TE correlation between obese and non-obese patients (r = 0.88 and r = 0.51, respectively; P = .008) (Figure 2). The correlation between MRE and TE was slightly stronger using an XL probe than an M probe (r = 0.68 and r = 0.48, respectively, P = .19). The underlying etiology of primary disease did not significantly affect the correlation between MRE and TE. No significant difference in the MRE and TE correlation was observed between patients with viral etiologies and those with non-viral etiologies (r = 0.70 and r = 0.54, respectively; P = .267).
Figure 1.
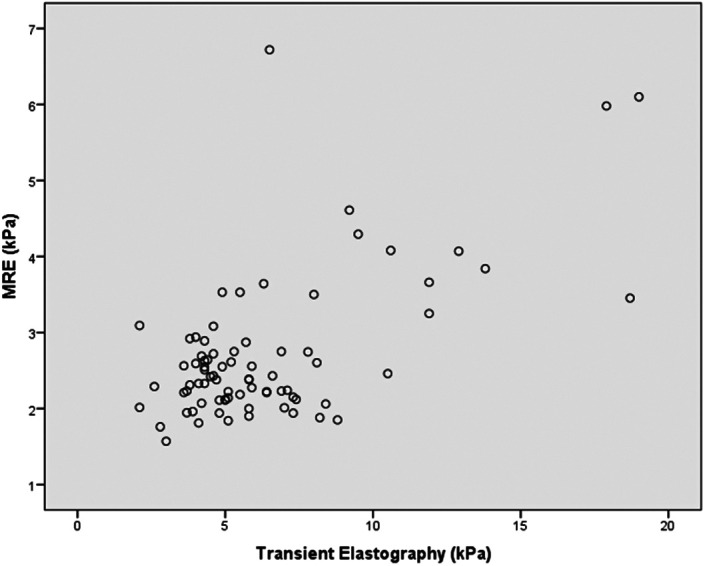
Scatterplots show a significant, moderate correlation between MRE and TE for identifying liver fibrosis. TE, transient elastography; MRE, magnetic resonance elastography.
Figure 2.
Obesity did not negatively affect the correlation between MRE and TE for identifying liver fibrosis. TE, transient elastography; MRE, magnetic resonance elastography.
A significant correlation was also observed between MRI-PDFF and FibroScan CAP in terms of hepatic steatosis quantification (r = 0.44, P < .001). Obesity did not negatively affect the correlation. There was no significant difference in terms of the correlation between MRI-PDFF and FibroScan-CAP between obese and non-obese patients (r = 0.50 vs r = 0.27; P = .374). The correlation with an XL probe was 0.42.
Recurrence of Hepatic Steatosis and Liver Fibrosis
During the follow-up at a median of 75.5 months after LT, the post-transplant hepatic fibrosis recurrence rate based on MRE (≥ 2.61 kPa) was 37.2% (29/78). Seventeen of these patients (58.6%) presented with significant fibrosis (≥ 2.97 kPa). The recurrence rate was slightly higher in recipients with NAFLD cirrhosis (44.4%, 4/9) than in those with viral hepatitis-related etiologies (29.5%, 13/44; P = .45). Significant fibrosis (≥ 2.97 kPa) was detected in 2 of the 4 recipients with NAFLD cirrhosis and in 7 of the 13 recipients with viral hepatitis-related etiologies. The rate of post-transplant hepatic steatosis recurrence based upon MRI-PDFF (≥ 5%) was 25.6% (20/78). The recurrence rate was slightly higher in recipients with NAFLD cirrhosis (44.4%, 4/9) than in those with viral hepatitis-related etiologies (27.3%, 12/44; P = .43).
Discussion
This study compared the accuracy of elastography techniques in assessing the degree of liver fibrosis in the LT setting. We found a statistically significant moderate correlation between MRE and TE (r = 0.61, P < .001). This finding is compatible with a pooled analysis of 4 studies involving a total of 141 liver transplant recipients (138 HCV-infected), which reported a high diagnostic accuracy for detection of advanced fibrosis in HCV-infected liver transplant recipients.25 In contrast to the pooled analysis, which mainly comprised HCV-infected patients, in the present study, we observed a good correlation between MRE and TE in liver transplant recipients with any etiology of CLD. This is a new finding. In addition, a significant correlation between MRI-PDFF and FibroScan-CAP in terms of hepatic steatosis quantification (r = 0.44, P < .001) was also observed in the present study. These findings indicate that MRI methods and TE accurately determine liver fibrosis and quantify hepatic steatosis in the LT setting and that their diagnostic performance is similar in any CLD etiology.
No head-to-head study comparisons between blood-based biomarkers and elastography techniques for liver fibrosis assessments in the LT settings are available in the literature. Blood-based biomarkers and elastography techniques are generally excellent at excluding advanced fibrosis and cirrhosis in native livers.6,7,11,26-33 A meta-analysis of TE studies in the LT setting reported excellent sensitivity (98%) and specificity (84%) for detecting cirrhosis and good estimates for detecting significant fibrosis in HCV-infected liver transplant recipients,28,29,32,33 which are comparable to the reported sensitivity and specificity of MRE (100% and 95%, respectively).25 Blood-based biomarkers are not affected by high BMIs; however, they may be affected by the patients’ condition, potential drug effects on aminotransferase levels, immunosuppression, and effects of underlying CLD etiology. Moreover, the reliability of biochemical tests has been questioned. In the present study, a statistically significant moderate correlation between FIB-4 and APRI in the assessment of liver fibrosis was found. However, we observed no significant correlations between the imaging techniques and biochemical tests. This finding suggests that elastography imaging techniques, both MRE and TE, could replace liver biopsy for the determination of the severity of liver allograft fibrosis.
The diagnostic performance of imaging-based techniques is now well known. Several factors, such as a high BMI, recent food intake, excess alcohol consumption, acute hepatitis, cholestasis, and hepatic steatosis, affect LSM.9,11,13-16 The failure rate of TE is significantly higher than that of MRE in obese patients, although it is reduced with the use of the XL probe. Previous studies have reported failed or unreliable TE measurements, at rates between 17% and 35% in obese patients.14,25,28,30-35 However, data on obese liver transplant recipients are lacking. In the present study, obesity did not affect the correlations between MRE and TE (P = .008), and between MRI-PDFF and CAP (P = .374). Interestingly, the correlation was stronger in obese (r = 0.88) than in non-obese patients (r = 0.51). Moreover, with an obesity-specific (XL) probe, the correlation was slightly stronger, both in the assessment of liver fibrosis and in the quantification of hepatic steatosis (r = 0.68 and r = 0.50, respectively). This result indicates that the number of failed or unreliable scans is decreased by MRE, suggesting that the TE XL probe may increase the utility of TE in the obese recipients.
Post-transplant NAFLD is a risk factor for NASH and liver fibrosis. NASH may be present in the setting of normal or near-normal serum aminotransferases levels.28,36-38 Malik et al37 reported recurrent NAFLD, NASH and significant fibrosis (≥ F2) rates of 70%, 25%, and 18%, respectively, in NAFLD patients, within a mean of 18 months after LT.37 In the present study, during a median follow-up period of 76 months after LT, the post-transplant hepatic steatosis and fibrosis recurrence rates based on MRE were 25.6% and 37.2%, respectively. Among the recipients with liver fibrosis, the majority of the patients (59%) presented with significant fibrosis (≥ 2.97 kPa). The recurrence rates were slightly higher in recipients with NAFLD cirrhosis than in those with viral hepatitis-related etiologies.
This study is the first comparative study of imaging methods, MRE and TE, and biochemical-based biomarkers for the assessment of allograft fibrosis. It is also the first to demonstrate a similar diagnostic performance of MRE and TE in liver transplant recipients with any underlying CLD etiology. MRE and TE are not available in every transplant center. This, unfortunately, reduces the clinical applications in practice. Unfortunately, since our center no longer performs protocol liver biopsies in liver transplant recipients, we were unable to directly compare liver biopsies to MRE and TE. Another limitation is the relatively small number of advanced fibrosis cases (n = 5) based on the FIB-4 score, limiting interferences from subgroup analyses.
In conclusion, MRI methods and TE are accurate in determining the degree of liver fibrosis and in quantifying hepatic steatosis in the LT setting. Obesity and the underlying etiology of primary liver disease do not negatively influence LSM. Recurrent NAFLD is relatively common following LT for NAFLD cirrhosis. Non-invasive methods can be used to eliminate or reduce the number of liver biopsies for liver allograft fibrosis assessments in such patients.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by the local ethics committee of the Ankara University School of Medicine (I3-184-20).
Informed Consent: N/A.
Peer Review: Externally peer-reviewed.
Author Contributions: Consept – R.İ.; Design – R.İ.; Supervision – H.O.; Resources – R.İ., Z.M.E., A.K., İ.S.İ., Y.B. ; Materials – R.İ., Z.M.E., A.K., İ.S.İ.; Data Collection and/or Processing –R.İ., Z.M.E., A.K, Y.B., İ.S.İ.; Analysis and/or Interpretation – A.H.E., M.K, İ.S.İ; Literature Search – R.İ., Z.M.E.; Writing Manuscript – R.İ., Z.M.E.; Critical Review – R.İ., Z.M.E., A.K., İ.S.İ., Y.B., M.K.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 annual data report: liver. Am J Transplant. 2019;19:184 283. 10.1111/ajt.15276) [DOI] [PubMed] [Google Scholar]
- 2. . Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495 500. 10.1056/NEJM200102153440706) [DOI] [PubMed] [Google Scholar]
- . . Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898 1906. 10.1053/j.gastro.2005.03.084). [DOI] [PubMed] [Google Scholar]
- 4. . Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104 1112. 10.1016/j.cgh.2009.05.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. . Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617 649. 10.3109/07853890.2010.518623) [DOI] [PubMed] [Google Scholar]
- 6. . Adams LA, George J, Bugianesi E, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26(10):1536 1543. 10.1111/j.1440-1746.2011.06774.x) [DOI] [PubMed] [Google Scholar]
- 7. . Zarski JP, Sturm N, Guechot J, et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol. 2012;56(1):55 62. 10.1016/j.jhep.2011.05.024) [DOI] [PubMed] [Google Scholar]
- 8. . Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with non-alcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17(4):630 637.e8. 10.1016/j.cgh.2018.05.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Zhang X, Wong GLH, Wong VWS. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26(2):128 141. 10.3350/cmh.2019.0001n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. . Castera L. Non-invasive tests for liver fibrosis in NAFLD: creating pathways between primary healthcare and liver clinics. Liver Int. 2020;40(S1):77 81. 10.1111/liv.14347) [DOI] [PubMed] [Google Scholar]
- 11. . Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69(7):1343 1352. 10.1136/gutjnl-2018-317593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Festi D, Schiumerini R, Marzi L, et al. Review Article: the diagnosis of non-alcoholic fatty liver disease: availability and accuracy of non-invasive methods. Aliment Pharmacol Ther. 2013;37(4):392 400. 10.1111/apt.12186) [DOI] [PubMed] [Google Scholar]
- 13. . Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH: current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461 478. 10.1038/s41575-018-0014-9) [DOI] [PubMed] [Google Scholar]
- 14. . Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156 163.e2. 10.1016/j.cgh.2018.04.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. . Vuppalanchi R, Siddiqui MS, Van Natta ML, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67(1):134 144. 10.1002/hep.29489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. . Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, Apri, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343 350. 10.1053/j.gastro.2004.11.018) [DOI] [PubMed] [Google Scholar]
- 17. . Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: Quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767 775. [DOI] [PubMed] [Google Scholar]
- 18. . Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60(6):1920 1928. 10.1002/hep.27362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. . Kennedy P, Wagner M, Castéra L, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology. 2018;286(3):738 763. 10.1148/radiol.2018170601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26(5):1431 1440. 10.1007/s00330-015-3949-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. . Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486 1501. 10.1002/hep.29302) [DOI] [PubMed] [Google Scholar]
- 22. . Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology. 2016;63(2):453 461. 10.1002/hep.28337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726 736. 10.1002/hep.24105) [DOI] [PubMed] [Google Scholar]
- 24. . Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317 1325. 10.1002/hep.21178) [DOI] [PubMed] [Google Scholar]
- 25. . Singh S, Venkatesh SK, Keaveny A, et al. Diagnostic accuracy of magnetic resonance elastography in liver transplant recipients: a pooled analysis. Ann Hepatol. 2016;15(3):363 376. 10.5604/16652681.1198808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. . Rigamonti C, Donato MF, Fraquelli M, et al. Transient elastography predicts fibrosis progression in patients with recurrent hepatitis C after liver transplantation. Gut. 2008;57(6):821 827. 10.1136/gut.2007.135046) [DOI] [PubMed] [Google Scholar]
- 27. . Vinciguerra T, Brunati A, David E, et al. Transient elastography for non-invasive evaluation of post-transplant liver graft fibrosis in children. Pediatr Transplant. 2018;22(2). 10.1111/petr.13125) [DOI] [PubMed] [Google Scholar]
- 28. . Winters AC, Mittal R, Schiano TD. A review of the use of transient elastography in the assesment of fibrosis and steatosis in the post-liver transplant patient. Clin Transplant. 2019;33(10). 10.1111/ctr.13700) [DOI] [PubMed] [Google Scholar]
- 29. . Adebajo CO, Talwalkar JA, Poterucha JJ, Kim WR, Charlton MR. Ultrasound-based transient elastography for the detection of hepatic fibrosis in patients with recurrent hepatitis C virus after liver transplantation: a systemic review and meta-analysis. Liver Transpl. 2012;18(3):323 331. 10.1002/lt.22460) [DOI] [PubMed] [Google Scholar]
- 0. . Degos F, Perez P, Roche B, et al. Diagnostic accuracy of Fibroscan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53(6):1013 1021. 10.1016/j.jhep.2010.05.035) [DOI] [PubMed] [Google Scholar]
- 1. . Sterling RK, King WC, Wahed AS, et al. Evaluating noninvasive markers to identify advanced fibrosis by liver biopsy in HBV/HIV co-infected adults. Hepatology. 2020;71(2):411 421. 10.1002/hep.30825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Siddiqui MS, Idowu MO, Stromberg K, et al. Diagnostic performance of vibration-controlled transient elastography in liver transplant recipients. Clin Gastroenterol Hepatol. 2021;19(2):367 374. 10.1016/j.cgh.2020.03.067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Chayanupatkul M, Dasani DB, Sogaard K, Schiano TD. The utility of assessing liver allograft fibrosis and steatosis post-liver transplantation using transient elastography with controlled attenuation parameter. Transpl Proc. 2020;53(1):159 165. 10.1016/j.transproceed.2020.02.160 [DOI] [PubMed] [Google Scholar]
- 4. . Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13 369 examinations. Hepatology. 2010;51(3):828 835. 10.1002/hep.23425) [DOI] [PubMed] [Google Scholar]
- 5. . Bota S, Sporea I, Sirli R, et al. Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of Acoustic Radiation Force Impulse (ARFI) elastography: analysis of a cohort of 1,031 subjects. Eur J Radiol. 2014;83(2):268 272. 10.1016/j.ejrad.2013.11.019) [DOI] [PubMed] [Google Scholar]
- 6. . Losurdo G, Castellaneta A, Rendina M, Carparelli S, Leandro G, Di Leo A. Systematic review with meta-analysis: de novo non-alcoholic fatty liver disease in liver-transplanted patients. Aliment Pharmacol Ther. 2018;47(6):704 714. 10.1111/apt.14521) [DOI] [PubMed] [Google Scholar]
- 7. . Malik SM, Devera ME, Fontes P, Shaikh O, Sasatomi E, Ahmad J. Recurrent disease following liver transplantation for non-alcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15(12):1843 1851. 10.1002/lt.21943) [DOI] [PubMed] [Google Scholar]
- 8. . Kappus M, Abdelmalek M. De novo and recurrence of nonalcoholic steatohepatitis after liver transplantation. Clin Liver Dis. 2017;21(2):321 335. 10.1016/j.cld.2016.12.006) [DOI] [PubMed] [Google Scholar]