Emerging evidence has linked cell mechanics to functional behaviors 1. The biophysical traits of a single cell are inextricably linked to the cytoskeleton. It has become increasingly evident that intrinsic and extrinsic mechanical properties, which describe the resistance to deformation (elasticity) or flow (viscosity) in response to an applied force, regulate cellular activities, such as cell morphology, adhesion, migration, and trafficking. Recent studies have demonstrated that solid tumor cells with higher migratory and invasive potential are softer than cells with lower migration and invasion potential 2-5. However, how cell intrinsic mechanical properties might affect liquid tumor (leukemia) development and progression remains unclear.
Ptpn21 (protein tyrosine phosphatase, non-receptor type 21), a poorly studied tyrosine phosphatase 6, binds to actin filaments and regulates cytoskeleton-associated cellular processes 7. We have recently shown that Ptpn21 plays an important role in maintaining cell mechanical properties 8, and that it helps retain hematopoietic stem cells (HSCs) in the bone marrow (BM) niche through a biomechanical mechanism 8. Knock-out of Ptpn21 results in impaired retention of HSCs within BM niches. Ptpn21 knock-out stem cells exhibit enhanced mobility and spontaneous egress into the peripheral blood. These phenotypes were attributable to the decrease in cellular mechanical stiffness and the increase in cell deformability 8. Mechanistically, Ptpn21 functions by dephosphorylating Spetin1 (Tyr246) 8, a rarely described component of the cytoskeleton. Importantly, missense mutations and frameshift truncating mutations in PTPN21 have been identified in chronic lymphocytic leukemia (IntOGen - mutational cancer drivers database) and colon cancer 9-11, respectively. However, the pathogenic effects of PTPN21 loss of function mutations remain to be determined.
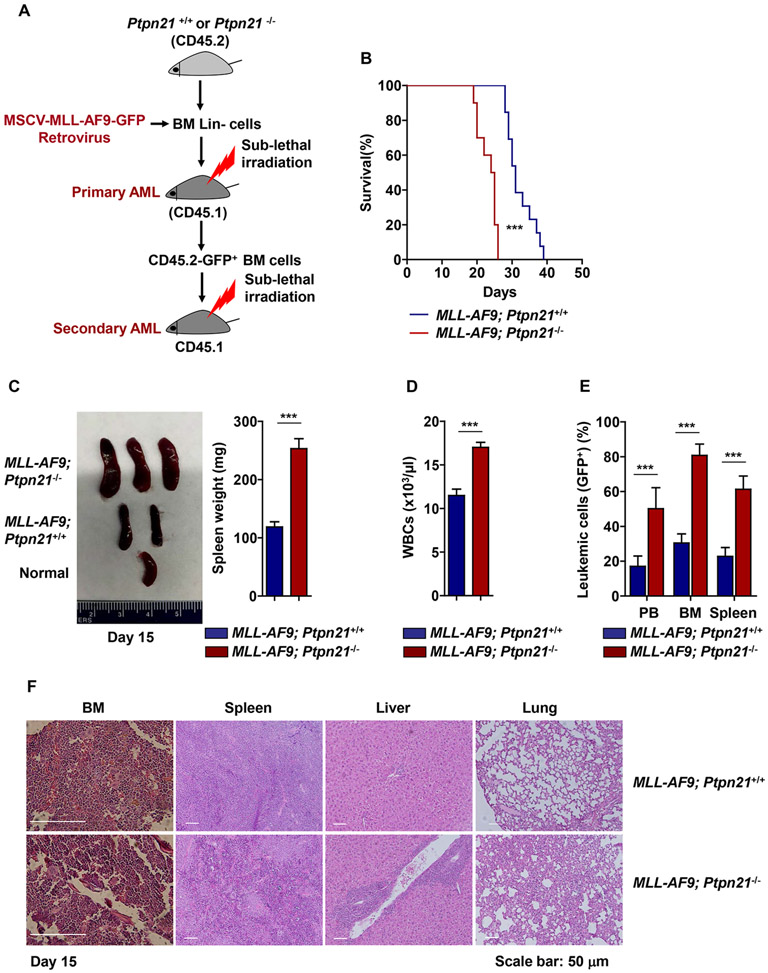
Given the decreased cell mechanical tension and increased deformability displayed by Ptpn21 knock-out hematopoietic cells 8, we utilized Ptpn21 knock-out (Ptpn21−/−) mice as a model to examine the biomechanical regulation of leukemic development and progression. BM lineage negative (Lin−) cells isolated from Ptpn21−/− or Ptpn21+/+ mice (CD45.2+) were transduced with acute myeloid leukemia (AML)-associated oncogene MLL-AF9 and transplanted into sublethally irradiated congenic BoyJ mice (CD45.1+). Following in vivo expansion (to obtain sufficient MLL-AF9-transduced cells), leukemic cells were harvested and inoculated into BoyJ mice again (Figure 1A). Surprisingly, although Ptpn21 was reported to play a positive role in cell signaling (c-Src activation) 12, 13, the recipient mice inoculated with MLL-AF9-Ptpn21−/− leukemic cells developed AML more quickly than the mice receiving MLL-AF9-Ptpn21+/+ control leukemic cells. The survival of MLL-AF9-Ptpn21−/− cell recipients was shortened (Figure 1B). Leukemic burden, as determined by white blood cell counts, spleen weights, and leukemic cells (GFP+) in the peripheral blood, BM, and spleen, was markedly increased in the transplants inoculated with MLL-AF9-Ptpn21−/− cells 15 (Figure 1C-1E) and 25 days (Supplementary Figure 1A-1C) after the inoculation. Moreover, pathological examination of the BM, spleen, liver, and lung revealed more severe leukemic cell infiltration in MLL-AF9-Ptpn21−/− compared to MLL-AF9-Ptpn21+/+ cell recipients (Figure 1F). As MLL-AF9-transduced leukemic cells were expanded in vivo initially, to rule out the possibility that the observed effects of Ptpn21 deficiency on leukemia was caused by the expansion process, we scaled up retroviral gene transduction and transplanted freshly isolated GFP+ MLL-AF9 transduced cells into congenic mice. The homing ability of MLL-AF9-Ptpn21−/− leukemic cells was similar to that of MLL-AF9-Ptpn21+/+ leukemic cells (Supplementary Figure 2A). However, MLL-AF9-Ptpn21−/− leukemic cells proliferated much faster than MLL-AF9-Ptpn21+/+ leukemic cells in the transplants, consistent with the data shown in Figure 1. White blood cell counts, and leukemic cells (GFP+) in the peripheral blood, BM and spleen were significantly increased in MLL-AF9-Ptpn21−/− cell recipients (Supplementary Figure 2A-2C). Indeed, the cell cycling of MLL-AF9-Ptpn21−/− leukemic cells in the transplants was faster compared to that of MLL-AF9-Ptpn21+/+ leukemic cells (Supplementary Figure 2D).
Figure 1. Deletion of Ptpn21 promotes development and progression of MLL-AF9-induced leukemia.
(A) Lin− cells isolated from Ptpn21−/− and Ptpn21+/+ mice were infected with MSCV-MLL-AF9-IRES-GFP retrovirus. Entire cell populations (5×105 cells/mouse) were transplanted into sublethally irradiated (700 rad; with a cesium irradiator) BoyJ mice for in vivo expansion of leukemic cells. GFP+ leukemic cells sorted from the recipient mice were transplanted into sublethally irradiated BoyJ mice. (B) Kaplan-Meier survival curve of the secondary mice receiving 1×105 GFP+ MLL-AF9-tranduced Ptpn21−/− (n=10 mice) or MLL-AF9-tranduced Ptpn21+/+ (n=13 mice) cells. (C-E) Fifteen days after leukemic cell inoculation, recipient mice (n=5 mice per genotype) were sacrificed. Spleens were weighted (C), white blood cell counts in the peripheral blood were determined by a hematology analyzer (D), and leukemic cells (GFP+) in the peripheral blood, BM, and spleen (E) were assessed by FACS analyses. (F) Histopathological examination of the BM, spleen, liver, and lung dissected from the recipient mice transplanted with MLL-AF9-Ptpn21−/− or MLL-AF9-Ptpn21+/+ cells. Representative images from 5 mice per genotype are shown. Data shown in (C) – (E) are mean ± standard deviation of all mice examined. *** P < .001.
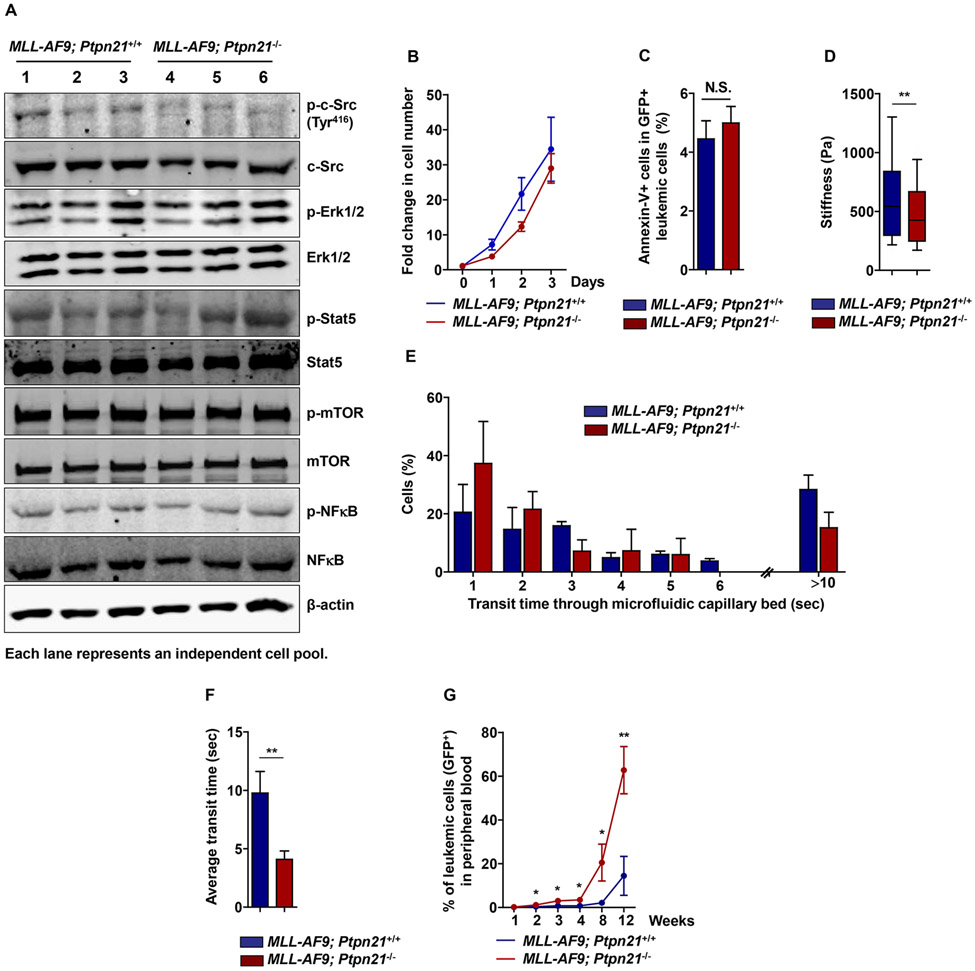
Ptpn21 has been shown to be a positive regulator of c-Src signaling12, 13. We therefore compared cell signaling activities in leukemic cells with and without Ptpn21. c-Src activity in MLL-AF9-Ptpn21−/− leukemic cells was marginally decreased relative to that in MLL-AF9-Ptpn21+/+ counterparts. The impact of Ptpn21 deficiency on other signaling pathways, such as Erk, Stat5, mTOR, and NF-κB activities, was undetectable (Figure 2A). In agreement with these results, MLL-AF9-Ptpn21−/− leukemic cells did not show any growth advantages in culture dishes (Figure 2B). Apoptosis in these cells was also comparable to that in MLL-AF9-Ptpn21+/+ control cells (Figure 2C). These data apparently did not account for the enhanced leukemia development and progression in MLL-AF9-Ptpn21−/− cell transplants. Atomic force microscopy measurements of cell stiffness showed that Ptpn21-deleted leukemic cells were softer than control leukemic cells (Figure 2D), similar to “normal” Ptpn21−/− hematopoietic cells. A consequence of decreased cell stiffness can be enhanced cell deformability 1. To further examine the relative deformability of Ptpn21–deleted leukemic cells, we utilized a microfluidic device, an established bioengineering instrument with multiple microchannels (5.9 ± 0.8 μm in width) mimicking capillary beds, to determine relative cell deformability based on transit time 14, 15. Compared to control cells, Ptpn21-deleted leukemic cells traversed the microfluidic channels faster and a lower proportion of the cells obstructed the device (>10 sec transit time) (Figure 2E, 2F), consistent with the important role of Ptpn21 in cell mechanics and motility 8. Importantly, in the transplants, Ptpn21-deleted leukemic cells proliferated much faster than control cells (Figure 2G). Taken together, these data suggest that the cell mechanical changes (decreased tension and increased deformability) rather than the impact on cell signaling, are likely responsible for the accelerated leukemia progression in MLL-AF9-Ptpn21−/− cell transplants.
Figure 2. Cellular mechanical properties but not cell signaling activities are impacted in Ptpn21 deleted MLL leukemic cells that proliferate faster than control cells in vivo.
(A) Lin− cells isolated from Ptpn21−/− and Ptpn21+/+ mice were infected with MSCV-MLL-AF9-IRES-GFP retrovirus. GFP+ MLL-AF9-transduced cells were sorted by FACS. Sorted cells were lysed and subjected to immunoblotting analyses with the indicated antibodies (n= 3 mice per genotype). (B, C) Sorted GFP+ MLL-AF9-transduced cells were cultured in IMDM medium containing 10%FBS, 50 ng/mL SCF, 50 ng/mL Flt3L, 20 ng/mL IL-3, and 20 ng/mL IL-6, for the indicated periods of time. Cell growth rates were determined (n=4-6 mice per genotype) (B). Apoptosis in the cells cultured for 24 hours were assayed by FACS (n=4-6 mice per genotype) (C). (D) Leukemic cells isolated were measured for mechanical stiffness by atomic force microscopy (n= 3 mice per genotype; 108 cells examined per mouse sample). (E, F) Leukemic cells isolated were assayed by the microfluidic system (n=3 mice per genotype, 30 cells examined per mouse sample). Cells in transit were recorded using a bright-field microscopy, transit times were manually calculated (E) to determine average transit time through the device (F). Data in (B) – (F) are shown as mean ± standard deviation. (G) Sorted GFP+ MLL-AF9-tranduced cells (1×105 cells/mouse) were transplanted into sublethally irradiated (200 rad with an X-ray irradiator) BoyJ mice (n=6-7 mice per group). GFP+ leukemic cells in the peripheral blood were assessed at the indicated time points before all recipient animals receiving MLL-AF9-Ptpn21−/− leukemic cells died. * P < .05, ** P < .01; N.S., not significant.
This work raises an intriguing possibility, that is, cell mechanical rigidity is a tumor suppressing mechanism. The biomechanical regulation by Ptpn21 appears to have an anti-leukemic effect. Consistent with this notion, AML patients with lower PTPN21 expression levels in leukemic cells had poorer prognosis compared to those with higher PTPN21 levels (Supplementary Figure 3). Nevertheless, it remains unclear how Ptpn21-deleted leukemic cells proliferated faster than control cells in the in vivo setting. Since the in vitro growth rate of Ptpn21 deleted leukemic cells was comparable to that of control leukemic cells, conceivably many factors in the in vivo microenvironment that the leukemic cells were associated with might have contributed to the overall elevated growth of the softer leukemic cells lacking Ptpn21 in mice, such as the viscosity of the plasma, the shearing forces, and/or potential immune evasion. However, the possibility that other unknown effects caused by Ptpn21 deficiency on signaling or cellular physiology might promote increased proliferation cannot be fully excluded. Further studies are required to address these possibilities. Finally, given the leukemia-suppressing role of Ptpn21 identified in this study, we would predict that the loss-of-function mutations of PTPN21 found in leukemia (IntOGen - mutational cancer drivers database) and colon cancer 9-11 might promote tumor development and/or progression through impacting biomechanics. A deeper understanding of the relationship between the cell mechanical alterations and tumor cell activities might ultimately lead to rational design of more effective therapies to inhibit PTPN21-mutated malignancies.
Supplementary Material
Acknowledgments.
This work was supported by the National Institutes of Health grants HL130995 and DK092722 (to C.K.Q.).
Footnotes
Competing financial interests. The authors declare no competing financial interests.
References
- 1.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature 2010. Jan 28; 463(7280): 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tse HT, Gossett DR, Moon YS, Masaeli M, Sohsman M, Ying Y, et al. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci Transl Med 2013. Nov 20; 5(212): 212ra163. [DOI] [PubMed] [Google Scholar]
- 3.Byun S, Son S, Amodei D, Cermak N, Shaw J, Kang JH, et al. Characterizing deformability and surface friction of cancer cells. Proc Natl Acad Sci U S A 2013. May 07; 110(19): 7580–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol 2007. Dec; 2(12): 780–783. [DOI] [PubMed] [Google Scholar]
- 5.Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, et al. The nanomechanical signature of breast cancer. Nat Nanotechnol 2012. Nov; 7(11): 757–765. [DOI] [PubMed] [Google Scholar]
- 6.Moller NP, Moller KB, Lammers R, Kharitonenkov A, Sures I, Ullrich A. Src kinase associates with a member of a distinct subfamily of protein-tyrosine phosphatases containing an ezrin-like domain. Proc Natl Acad Sci U S A 1994. Aug 02; 91(16): 7477–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlucci A, Gedressi C, Lignitto L, Nezi L, Villa-Moruzzi E, Avvedimento EV, et al. Protein-tyrosine phosphatase PTPD1 regulates focal adhesion kinase autophosphorylation and cell migration. J Biol Chem 2008. Apr 18; 283(16): 10919–10929. [DOI] [PubMed] [Google Scholar]
- 8.Ni F, Yu WM, Wang X, Fay ME, Young KM, Qiu Y, et al. Ptpn21 controls hematopoietic stem cell homeostasis and biomechanics. Cell Stem Cell 2019. April 4; 24(4):608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannakis M, Hodis E, Jasmine Mu X, Yamauchi M, Rosenbluh J, Cibulskis K, et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet 2014. Dec; 46(12): 1264–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korff S, Woerner SM, Yuan YP, Bork P, von Knebel Doeberitz M, Gebert J. Frameshift mutations in coding repeats of protein tyrosine phosphatase genes in colorectal tumors with microsatellite instability. BMC Cancer 2008. Nov 10; 8: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, et al. Recurrent R-spondin fusions in colon cancer. Nature 2012. Aug 30; 488(7413): 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardone L, Carlucci A, Affaitati A, Livigni A, DeCristofaro T, Garbi C, et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol 2004. Jun; 24(11): 4613–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlucci A, Porpora M, Garbi C, Galgani M, Santoriello M, Mascolo M, et al. PTPD1 supports receptor stability and mitogenic signaling in bladder cancer cells. J Biol Chem 2010. Dec 10; 285(50): 39260–39270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbluth MJ, Lam WA, Fletcher DA. Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab Chip 2008. Jul; 8(7): 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fay ME, Myers DR, Kumar A, Turbyfield CT, Byler R, Crawford K, et al. Cellular softening mediates leukocyte demargination and trafficking, thereby increasing clinical blood counts. Proc Natl Acad Sci U S A 2016. Feb 23; 113(8): 1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.