Background.
Kidney transplantation (KT) is the optimal treatment for kidney failure and is associated with better quality of life and survival relative to dialysis. However, knowledge of the current capacity of countries to deliver KT is limited. This study reports on findings from the 2018 International Society of Nephrology Global Kidney Health Atlas survey, specifically addressing the availability, accessibility, and quality of KT across countries and regions.
Methods.
Data were collected from published online sources, and a survey was administered online to key stakeholders. All country-level data were analyzed by International Society of Nephrology region and World Bank income classification.
Results.
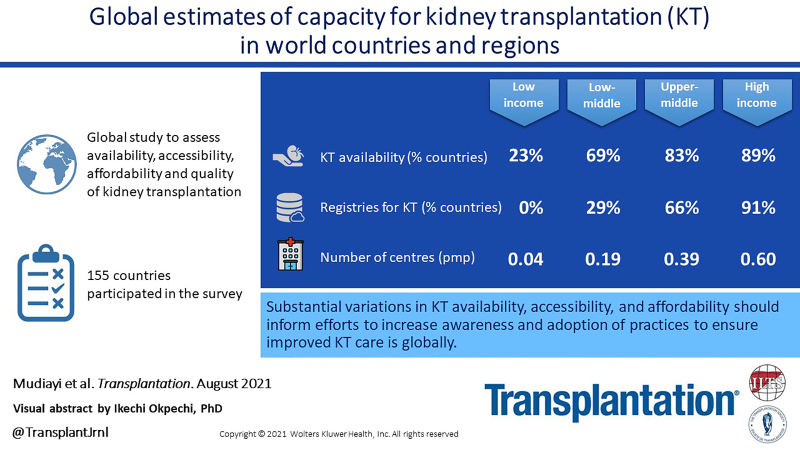
Data were collected via a survey in 182 countries, of which 155 answered questions pertaining to KT. Of these, 74% stated that KT was available, with a median incidence of 14 per million population (range: 0.04–70) and median prevalence of 255 per million population (range: 3–693). Accessibility of KT varied widely; even within high-income countries, it was disproportionately lower for ethnic minorities. Universal health coverage of all KT treatment costs was available in 31%, and 57% had a KT registry.
Conclusions.
There are substantial variations in KT incidence, prevalence, availability, accessibility, and quality worldwide, with the lowest rates evident in low- and lower-middle income countries. Understanding these disparities will inform efforts to increase awareness and the adoption of practices that will ensure high-quality KT care is provided around the world.
INTRODUCTION
Kidney failure is a major public health challenge, and its burden is projected to increase dramatically because of the aging general population and rising prevalence of diabetes and hypertension.1,2 Although dialysis is the most commonly used treatment for kidney failure worldwide, studies in both high-income countries and middle-income countries have shown that kidney transplantation (KT) is more cost effective and associated with better quality of life, increased survival, and higher economic productivity.3,4 The International Society of Nephrology’s (ISN) framework for integrated kidney care prioritizes KT above all other methods of kidney replacement therapy.5 Nevertheless, the applicability of KT is limited by numerous factors, including patient suitability, donor availability, cultural bias against organs from deceased donors, local or regional expertise, and costs of KT surgery and immunosuppressive medications.6,7 Strong demand for organs has also been linked to transplant tourism and a black market in kidneys for decades.8 Although it is likely that these constraints on access to KT are felt more acutely in low-income countries than in countries at other income levels, estimates of the availability, accessibility, and quality of KT care have not been reported.
In this article, we review the latest International Society of Nephrology Global Kidney Health Atlas (ISN-GKHA) survey data on the global quality of KT care and estimate the availability, accessibility, and quality of KT care worldwide. We also highlight barriers to improving access to high-quality KT care worldwide.
MATERIALS AND METHODS
Setting
As described elsewhere,9-11 the ISN-GKHA is a project led by the ISN targeted at monitoring and evaluating the global capacity of kidney care. The ISN-GKHA project combines an international survey of key stakeholders with desk research, including a review of published scientific literature, government reports, and other relevant data sources on various aspects of the epidemiology of kidney failure and health system characteristics corresponding to each of the World Health Organization (WHO) universal health coverage domains (ie, service delivery, health workforce, information systems, medicines and medical products, financing, and leadership).12
Data Collection
Data on the global epidemiology of KT (incidence and prevalence data) were extracted from annual reports of renal registries (Appendix 1, SDC, http://links.lww.com/TP/C285), and the global observatory on donation and transplantation (GODT).13 Prevalence of KT was defined as the reported number of patients with a functioning kidney transplant as shown for the year of reporting in the registry databases and was presented as number per million population (pmp). Incidence of KT (including preemptive, living donor and deceased donor KT) was defined as the total number of kidney transplants performed in a given year for each category and was presented as number pmp as reported in the GODT database. However, we did not separate incidence of KT performed in a country from KT performed only on residents of that country. All other data reported in this article were collected via a survey developed in alignment with the WHO framework on monitoring health systems. Details about survey development and validation have been published elsewhere.10,11 To date, 2 iterations of the survey have been conducted in 2016 and 2018.14 Here, we report on survey items from the 2018 iteration designed to assess the status of KT across world countries and regions. We invited key experts in nephrology to participate based on their knowledge of kidney care and their ability to accurately represent their countries. Countries surveyed are listed in Table S1 (SDC, http://links.lww.com/TP/C285) by ISN Regions and WHO income groups. However, we could not obtain information from 27 countries listed in Table S2 (SDC, http://links.lww.com/TP/C285) as those countries did not participate in the survey. In total, 2 to 3 representatives from 182 countries were invited to participate in the survey. Respondents (country head of nephrology organization, policymaker [eg, head of a large government hospital and director at State Ministry of Health], and lead at a patient advocacy organization [eg, country-level National Kidney Foundation]) were identified by ISN regional board members in each country in the region. We administered the survey online via REDCap Cloud (www.redcapcloud.com) from July to September 2018 and stored the data in a centralized database.
We questioned respondents about their country’s capacity to deliver KT (ie, availability, funding, essential workforce, quality, and outcomes). The survey questionnaire is available as an online supplement (Supplementary Appendix 2 http://links.lww.com/TP/C285). In addition to reporting whether KT was available in their country (yes/no/unknown), respondents were asked to rate various elements of optimal care in KT. Accessibility to KT services was defined as the proportion of patients with kidney failure who were suitable for transplant and could access KT services (low accessibility: <11%; high accessibility: >11%; this value was selected based on the median value from our results). Intensive follow-ups were conducted by email reminders (at least thrice) and telephone to ensure complete and timely responses during the study period. The University of Alberta Research Ethics Committee approved this project (Protocol number: PRO00063121) and all participants provided informed consent.
Data Analysis
Data are presented as number (%) or median [interquartile range (IQR)] as appropriate. Percentage values were calculated using the number of responding countries as the denominator. For the purposes of this study, we focused our analysis on survey items, density of global KT centers, accessibility of KT, types of KT (living versus deceased donors) including elements of preemptive KT, and availability of services for KT care. The annual incidence and prevalence of KT estimates were directly extracted from the most up to date registry reports and global databases. Data on kidney transplant prevalence and incidence of preemptive kidney transplants were obtained, as reported, from the most recent country kidney and transplant registries, which varied per country (Supplementary Appendix 1 http://links.lww.com/TP/C285). We relied primarily on the GODT database (2015–2017) as source of data for all incidence KT variables. Where such data were not available, we looked for incidence data from other sources including data from registries and published studies for such countries. All prevalence data were obtained from registries and published reports. Data from registries and published studies were sometimes dated and may not have been representative of the current status of KT in some countries (Supplementary Appendix 1 http://links.lww.com/TP/C285).
Countries without a kidney disease and transplant registry, those with no data included in GODT and those we could not identify publications that reported on the assessed KT parameters were not included in the desk research analysis for KT incidence and prevalence in each region. Before data analysis, the survey data were checked for inconsistencies from the responses obtained from each country. If significant discrepancies existed, ISN regional leaders, with knowledge on the status of kidney care in their region, were asked to clarify such discrepancies. Using STATA 15 software (Stata Corporation, 2017), we analyzed the data at the country level to produce descriptive statistics. Findings were reported as overall aggregate scores, stratified by ISN region and World Bank income group.
RESULTS
Results of this study are presented in tables and figures and broadly summarized into 2 categories: desk research (Table 1 and Figures S1–S4, SDC, http://links.lww.com/TP/C285) and survey responses (Tables 2–4, Figures 1–2 and Tables S1–S10, SDC, http://links.lww.com/TP/C285). Figure 3 combined desk and survey data.
TABLE 1.
Global epidemiology of kidney transplantation
| Category | Global incidence of kidney transplantation (pmp) | Global prevalence of kidney transplantation (pmp) | Global incidence of deceased donor kidney transplantation (pmp) | Global incidence of living donor kidney transplantation (pmp) | Global incidence of preemptive kidney transplantation (pmp) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | N | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | |
| Overall | 13.5 (5.2–37.8) | 98 | 255.0 (58.0–432.0) | 75 | 15.1 (2.9–29.4) | 75 | 5.3 (2.6–10.8) | 97 | 5.1 (1.5–6.9) | 20 |
| ISN region | ||||||||||
| Africa | 4.6 (1.5–10.8) | 9 | 25.3 (25.3–25.3) | 1 | 1.1 (0.3–2.0) | 4 | 2.1 (0.9–5.3) | 9 | – | 0 |
| Eastern & Central Europe | 26.3 (9.6–29.6) | 16 | 282.0 (88.4–302.0) | 13 | 21.9 (8.3–26.5) | 15 | 2.9 (2.1–4.5) | 16 | 0.9 (0.5–1.5) | 4 |
| Latin America & the Caribbean | 9.8 (6.0–20.2) | 18 | 68.8 (28.8–205.0) | 20 | 7.2 (2.6–16.1) | 17 | 3.8 (2.8–6.4) | 18 | – | 0 |
| Middle East | 12.9 (11.7–25.2) | 9 | 277.0 (106.0–311.0) | 7 | 4.4 (2.6–7.1) | 5 | 12.7 (9.1–14.6) | 9 | – | 0 |
| NIS & Russia | 5.4 (2.9–12.3) | 7 | 27.0 (25.0–58.0) | 3 | 4.2 (0.9–22.0) | 4 | 2.9 (1.4–5.4) | 7 | – | 0 |
| North America | 56.0 (48.4–63.6) | 2 | 6.8 (3.7–560.1) | 5 | 40.6 (35.5–45.7) | 2 | 15.4 (12.9–17.9) | 2 | 8.1 (5.3–10.8) | 2 |
| North & East Asia | 6.7 (6.5–13.1) | 3 | 141.0 (67.0–352.0) | 3 | 3.3 (1.4–5.2) | 2 | 6.7 (1.3–11.7) | 3 | – | 0 |
| Oceania & South East Asia | 4.7 (2.5–12.6) | 9 | 190.8 (58.0–380.0) | 6 | 6.1 (0.4–25.1) | 7 | 3.2 (2.0–5.6) | 9 | 4.8 (4.5–5.0) | 2 |
| South Asia | 4.8 (3.5–5.0) | 5 | – | 0 | – | 0 | 4.8 (3.5–5.0) | 5 | – | 0 |
| Western Europe | 46.5 (38.9–51.9) | 20 | 535.5 (468.0–573.7) | 17 | 35.3 (25.2–41.3) | 19 | 10.6 (6.8–15.1) | 19 | 6.3 (3.3–7.7) | 12 |
| World Bank income group | ||||||||||
| Low income | 3.5 (3.5–3.5) | 1 | – | 0 | – | 0 | 3.5 (3.5–3.5) | 1 | – | 0 |
| Lower-middle income | 4.3 (1.8–6.6) | 22 | 27.0 (5.0–33.0) | 7 | 0.2 (0.1–0.6) | 7 | 4.0 (1.6–5.5) | 22 | – | 0 |
| Upper-middle income | 9.3 (5.6–18.6) | 31 | 80.0 (50.0–114.1) | 25 | 5.2 (2.5–8.5) | 27 | 2.9 (1.7–10.4) | 31 | 0.7 (0.3–1.2) | 3 |
| High income | 38.5 (23.1–48.0) | 44 | 363.0 (269.0–535.5) | 43 | 27.1 (17.5–37.0) | 41 | 7.5 (4.8–14.5) | 43 | 5.8 (3.4–6.9) | 17 |
ISN, International Society of Nephrology; IQR, interquartile range; NIS, newly independent states; pmp, per million population.
TABLE 2.
Availability of kidney transplantation, donor type, transplant waitlist types, and kidney transplantation centers
| Category | Kidney transplantation availability | Donor type | Transplant waitlist | Kidney transplantation centers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Yes, n (%) | n | Deceased donors only, n (%) | Live donors only, n (%) | Combination, n (%) | n | National, n (%) | Regional only, n (%) | None, n (%) | n | Transplant centers pmp, median (IQR) | |
| Overall | 155 | 114 (74) | 114 | 0 (0) | 32 (28) | 82 (72) | 113 | 70 (62) | 22 (19) | 21 (19) | 113 | 0.42 (0.20–0.72) |
| ISN region | ||||||||||||
| Africa | 41 | 14 (34) | 14 | 0 (0) | 11 (79) | 3 (21) | 14 | 5 (36) | 2 (14) | 7 (50) | 14 | 0.15 (0.04–0.18) |
| Eastern & Central Europe | 19 | 18 (95) | 18 | 0 (0) | 2 (11) | 16 (89) | 18 | 16 (89) | 2 (11) | 0 (0) | 18 | 0.71 (0.47–0.80) |
| Latin America & the Caribbean | 18 | 17 (94) | 17 | 0 (0) | 2 (12) | 15 (88) | 16 | 10 (63) | 4 (25) | 2 (13) | 16 | 0.50 (0.30–0.86) |
| Middle East | 11 | 11 (100) | 11 | 0 (0) | 3 (27) | 8 (73) | 11 | 5 (45) | 4 (36) | 2 (18) | 11 | 0.41 (0.34–0.43) |
| NIS & Russia | 7 | 7 (100) | 7 | 0 (0) | 4 (57) | 3 (43) | 7 | 2 (29) | 2 (29) | 3 (43) | 7 | 0.33 (0.26–0.51) |
| North America | 9 | 5 (56) | 5 | 0 (0) | 2 (40) | 3 (60) | 5 | 2 (40) | 1 (20) | 2 (40) | 5 | 0.75 (0.59–0.82) |
| North & East Asia | 7 | 7 (100) | 7 | 0 (0) | 1 (14) | 6 (86) | 7 | 7 (100) | 0 (0) | 0 (0) | 7 | 0.55 (0.36–1.23) |
| Oceania & South East Asia | 15 | 10 (67) | 10 | 0 (0) | 3 (30) | 7 (70) | 10 | 6 (60) | 3 (30) | 1 (10) | 10 | 0.41 (0.13–0.85) |
| South Asia | 7 | 6 (86) | 6 | 0 (0) | 3 (50) | 3 (50) | 6 | 0 (0) | 2 (33) | 4 (67) | 6 | 0.10 (0.06–0.19) |
| Western Europe | 21 | 19 (90) | 19 | 0 (0) | 1 (5) | 18 (95) | 19 | 17 (89) | 2 (11) | 0 (0) | 19 | 0.52 (0.37–0.72) |
| World Bank income group | ||||||||||||
| Low income | 22 | 5 (23) | 5 | 0 (0) | 5 (100) | 0 (0) | 5 | 0 (0) | 1 (20) | 4 (80) | 5 | 0.04 (0.01–0.06) |
| Lower-middle income | 35 | 24 (69) | 24 | 0 (0) | 15 (63) | 9 (38) | 24 | 2 (8) | 9 (38) | 13 (54) | 24 | 0.19 (0.08–0.31) |
| Upper-middle income | 41 | 34 (83) | 34 | 0 (0) | 8 (24) | 26 (76) | 34 | 23 (68) | 8 (24) | 3 (9) | 34 | 0.39 (0.26–0.71) |
| High income | 57 | 51 (89) | 51 | 0 (0) | 4 (8) | 47 (92) | 50 | 45 (90) | 4 (8) | 1 (2) | 50 | 0.60 (0.42–0.85) |
ISN, International Society of Nephrology; IQR, interquartile range; NIS, newly independent states; pmp, per million population.
TABLE 4.
Accessibility (defined as proportion of patients with kidney failure receiving kidney transplant) to kidney transplantation and variation based on geography and patient characteristics
| Category | n | Access to transplantation | Variation based on geography | Variation based on patient characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 1–10% | 11%–25% | 26%–50% | >50% | Yes | No | N/A | Other | Yes | No | N/A | Other | ||
| Overall | 154 | 29 (19) | 51 (33) | 18 (12) | 10 (6) | 46 (30) | 32 (21) | 98 (64) | 23 (15) | 1 (1) | 31 (20) | 103 (67) | 19 (12) | 1 (1) |
| ISN region | ||||||||||||||
| Africa | 41 | 22 (54) | 15 (37) | 1 (2) | 1 (2) | 2 (5) | 7 (17) | 18 (44) | 16 (39) | 0 (0) | 6 (15) | 19 (46) | 15 (37) | 1 (2) |
| Eastern & Central Europe | 19 | 1 (5) | 3 (16) | 2 (11) | 2 (11) | 11 (58) | 1 (5) | 16 (84) | 1 (5) | 1 (5) | 2 (11) | 16 (84) | 1 (5) | 0 (0) |
| Latin America & the Caribbean | 18 | 0 (0) | 9 (50) | 4 (22) | 2 (11) | 3 (17) | 8 (44) | 9 (50) | 1 (6) | 0 (0) | 8 (44) | 10 (56) | 0 (0) | 0 (0) |
| Middle East | 11 | 0 (0) | 0 (0) | 2 (18) | 1 (9) | 8 (73) | 1 (9) | 10 (91) | 0 (0) | 0 (0) | 0 (0) | 11 (100) | 0 (0) | 0 (0) |
| NIS & Russia | 7 | 0 (0) | 2 (29) | 1 (14) | 1 (14) | 3 (43) | 1 (14) | 6 (86) | 0 (0) | 0 (0) | 1 (14) | 6 (86) | 0 (0) | 0 (0) |
| North America | 9 | 3 (33) | 2 (22) | 2 (22) | 0 (0) | 2 (22) | 1 (11) | 5 (56) | 3 (33) | 0 (0) | 2 (22) | 5 (56) | 2 (22) | 0 (0) |
| North & East Asia | 7 | 0 (0) | 7 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (14) | 6 (86) | 0 (0) | 0 (0) | 0 (0) | 7 (100) | 0 (0) | 0 (0) |
| Oceania & South East Asia | 15 | 2 (13) | 8 (53) | 1 (7) | 0 (0) | 4 (27) | 7 (47) | 7 (47) | 1 (7) | 0 (0) | 8 (53) | 6 (40) | 1 (7) | 0 (0) |
| South Asia | 7 | 1 (14) | 5 (71) | 1 (14) | 0 (0) | 0 (0) | 5 (71) | 1 (14) | 1 (14) | 0 (0) | 4 (57) | 3 (43) | 0 (0) | 0 (0) |
| Western Europe | 20 | 0 (0) | 0 (0) | 4 (20) | 3 (15) | 13 (65) | 0 (0) | 20 (100) | 0 (0) | 0 (0) | 0 (0) | 20 (100) | 0 (0) | 0 (0) |
| World Bank income group | ||||||||||||||
| Low income | 22 | 14 (64) | 8 (36) | 0 (0) | 0 (0) | 0 (0) | 3 (14) | 8 (36) | 11 (50) | 0 (0) | 3 (14) | 8 (36) | 10 (45) | 1 (5) |
| Lower-middle income | 35 | 9 (26) | 18 (51) | 5 (14) | 0 (0) | 3 (9) | 14 (40) | 16 (46) | 5 (14) | 0 (0) | 12 (34) | 19 (54) | 4 (11) | 0 (0) |
| Upper-middle income | 41 | 5 (12) | 15 (37) | 6 (15) | 5 (12) | 10 (24) | 13 (32) | 22 (54) | 6 (15) | 0 (0) | 10 (24) | 26 (63) | 5 (12) | 0 (0) |
| High income | 56 | 6 (11) | 8 (14) | 5 (9) | 5 (9) | 32 (57) | 2 (4) | 52 (93) | 1 (2) | 1 (2) | 6 (11) | 50 (89) | 0 (0) | 0 (0) |
Data are n (%) of countries.
ISN, International Society of Nephrology; N/A, not available; NIS, newly independent states.
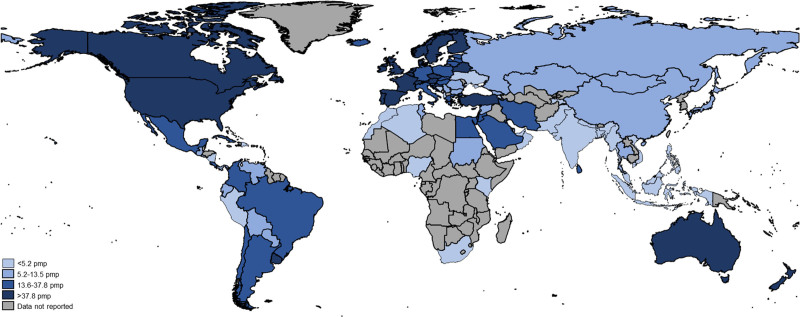
FIGURE 1.
Global incidence of kidney transplantation. pmp, per million population.
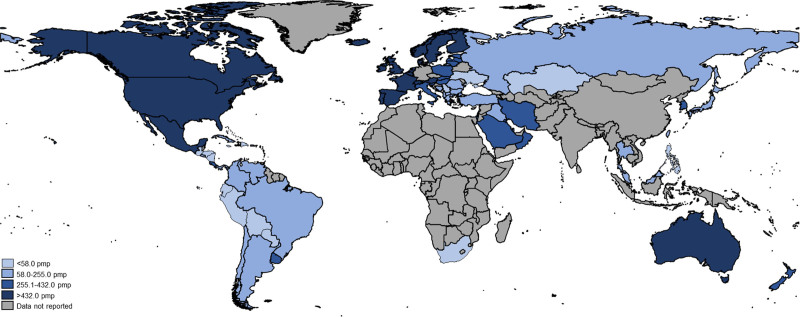
FIGURE 2.
Global prevalence of kidney transplantation. pmp, per million population.
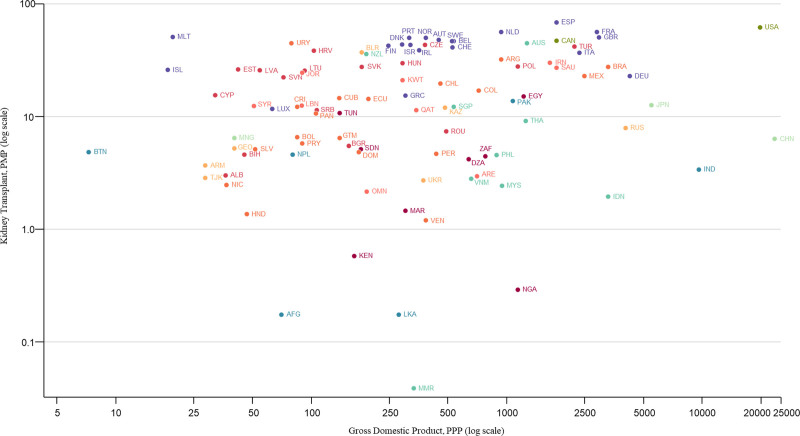
FIGURE 3.
Incidence of kidney transplantation by country gross domestic product. AFG, Afghanistan; ALB, Albania; ARE, United Arab Emirates; ARG, Argentina; ARM, Armenia; AUS, Australia; AUT, Austria; BEL, Belgium; BGR, Bulgaria; BIH, Bosnia and Herzegovina; BLR, Belarus; BOL, Bolivia; BRA, Brazil; BTN, Bhutan; CAN, Canada; CHE, Switzerland; CHL, Chile; CHN, China; COL, Colombia; CRI, Costa Rica; CUB, Cuba; CYP, Cyprus; CZE, Czech Republic; DEU, Germany; DNK, Denmark; DOM, Dominican Republic; DZA, Algeria; ECU, Ecuador; EGY, Egypt; ESP, Spain; EST, Estonia; FIN, Finland; FRA, France; GBR, United Kingdom; GEO, Georgia; GRC, Greece; GTM, Guatemala; HND, Honduras; HRV, Croatia; HUN, Hungary; IDN, Indonesia; IND, India; IRL, Ireland; IRN, Iran; ISL, Iceland; ISR, Israel; ITA, Italy; JOR, Jordan; JPN, Japan; KAZ, Kazakhstan; KEN, Kenya; KWT, Kuwait; LBN, Lebanon; LKA, Sri Lanka; LTU, Lithuania; LUX, Luxembourg; LVA, Latvia; MAR, Morocco; MEX, Mexico; MLT, Malta; MMR, Myanmar; MNG, Mongolia; MYS, Malaysia; NGA, Nigeria; NIC, Nicaragua; NLD, Netherlands; NOR, Norway; NPL, Nepal; NZL, New Zealand; OMN, Oman; PAK, Pakistan; PAN, Panama; PER, Peru; PHL, Philippines; PMP, per million population; POL, Poland; PPP, purchasing power parity; PRT, Portugal; PRY, Paraguay; QAT, Qatar; ROU, Romania; RUS, Russian Federation; SAU, Saudi Arabia; SDN, Sudan; SGP, Singapore; SLV, El Salvador; SRB, Serbia; SVK, Slovak Republic; SVN, Slovenia; SWE, Sweden; SYR, Syrian Arab Republic; THA, Thailand; TJK, Tajikistan; TUN, Tunisia; TUR, Turkey; UKR, Ukraine; URY, Uruguay; USA, United States; VEN, Venezuela; VNM, Vietnam; ZAF, South Africa.
Response Rate and Participation
Out of 182 countries contacted, individuals from 155 countries (85.2%) responded to items designed to assess various domains of access to and quality of maintenance KT for patients with kidney failure. No survey data were available for individuals from 27 countries that did not respond. Importantly, the countries in the survey represented >95% of the total world population. Participation was generally high across national income levels (high-income: n = 58, 88%; upper-middle-income: n = 41, 85%; lower-middle-income: n = 38, 90%; low-income: n = 23, 88%). Overall, 317 individuals participated in the survey, including nephrologists (n = 260, 82%); nonnephrologist physicians (n = 22, 7%); other health professionals (n = 7, 2%); administrators, policymakers, or civil servants (n = 17, 5%); and others (n = 11, 3%) (Table S3, SDC, http://links.lww.com/TP/C285).
Global Epidemiology of KT
There was significant variability in the global epidemiology of KT across world countries and regions (Table 1). Individuals from 98 (45%) countries reported data on the incidence of KT. The median global incidence of KT was 14 pmp (IQR: 5–38), ranging from 0.04 pmp to 70 pmp (Figure 1). The median global prevalence of KT was 255 pmp (IQR: 58–432) but varied over 200-fold across countries, from 3 pmp in the Bahamas to 693 pmp in Portugal (Figure 2). Data on prevalence of KT were not provided by respondents from low-income countries. The median prevalence of KT was highest in Western Europe (536 pmp) and lowest in Africa (25 pmp). The incidence of KT by gross domestic product is shown in Figure 3. The 5 countries with KT incidence lower than 1 pmp were either low-income or lower-middle-income countries while high-income countries had high KT incidence.
Respondents from 75 (34%) countries reported data on the incidence of deceased donor KT. The median global incidence of deceased donor KT was 15 pmp (IQR: 3–29), ranging from 0.5 pmp in Algeria to 63 pmp in Spain (Figure S1, SDC, http://links.lww.com/TP/C285). High variation in the incidence of deceased donor KT also was observed within each World Bank country income category, with 45-fold variation across the 41 high-income countries. Median incidence of deceased donor KT was highest in high-income countries that were members of the Organization for Economic Co-operation and Development (OECD; 29 pmp) compared with 19 pmp in non-OECD high-income countries. The incidence of deceased donor KT varied 700-fold across the 27 upper-middle income countries and 18-fold across the 7 lower-middle-income countries. Median deceased donor KT incidence was highest in North America (41 pmp) and lowest in Africa (0 pmp) (Table 1; Figure S1, SDC, http://links.lww.com/TP/C285).
Respondents from 97 (44%) countries reported data on the incidence of living donor KT. The median global incidence of living donor KT was 5 pmp (IQR: 3–11), ranging from 0.04 pmp in Myanmar to 33 pmp in Turkey (Table 1; Figure S2, SDC, http://links.lww.com/TP/C285). Only 1 country (Nepal) from the low-income group provided data on the incidence of living donor KT (3.5 pmp). Median living donor KT incidence was highest in North America (15 pmp) and lowest in Africa (2 pmp) (Table 1; Figure S2, SDC, http://links.lww.com/TP/C285).
Respondents from 20 (9%) countries reported data on the incidence of preemptive KT. The median global incidence of preemptive KT was 5 pmp (IQR: 2–7), ranging from 0.3 pmp in Bosnia and Herzegovina to 12.4 pmp in Norway (Table 1; Figure S3, SDC, http://links.lww.com/TP/C285). preemptive KT incidence varied 31-fold across the 17 OECD high-income countries and 4-fold across the 3 upper-middle-income countries which reported data. The median incidence of preemptive KT was highest in North America (8 pmp) and lowest in Eastern and Central Europe (1 pmp) (Table 1, Figure S3, SDC, http://links.lww.com/TP/C285).
Availability of KT Services
Overall, as depicted in Table 2, respondents from 155 countries replied to the question regarding availability of KT services, and respondents from 114 (74%) countries reported that KT services were generally available. The median number of centers providing KT services was 0.4 pmp (IQR: 0.2–0.7), with 1000-fold global variance, ranging from 0.01 pmp in Ethiopia to 10.4 pmp in Antigua and Barbuda (Figure S4, SDC, http://links.lww.com/TP/C285). The median number of centers providing KT were 0.6 pmp (IQR: 0.4–0.9) in high-income countries, 0.4 pmp (IQR: 0.3–0.7) in upper-middle-income countries, 0.2 pmp (IQR: 0.1–0.3) in lower-middle-income countries, and 0.04 pmp (IQR: 0.01–0.06) in low-income countries (Table 2). Furthermore, the associations between quartiles of KT centers and incidence of KT and incidence rate ratio (IRR) of KT are summarized in Table 3. Incidence of KT (pmp) was seen to increase with increasing quartiles of KT centers and although the IRR of KT was also observed to increase with increasing quartiles of KT centers, the IRR in the fourth quartile was slightly and nonsignificantly lower than the third quartile of KT centers (IRR = 0.98; 95% CI = 0.88, 1.08) (Table 3).
TABLE 3.
Association between quartiles of kidney transplant center numbers and incidence of kidney transplant
| Transplant center quartile | n | Incidence of kidney transplant (pmp), median (IQR) | IRR (95% CI) | ||
|---|---|---|---|---|---|
| First quartile | 20 | 4.45 (1.75–11.53) | 1 [Reference] | – | – |
| Second quartile | 24 | 7.42 (5.27–22.56) | 1.35 (1.14, 1.60) | 1 [Reference] | – |
| Third quartile | 26 | 28.49 (11.74–45.09) | 2.75 (2.37, 3.19) | 1.99 (1.75, 2.26) | 1 [Reference] |
| Fourth quartile | 22 | 26.80 (15.83–42.00) | 2.68 (2.31, 3.12) | 2.04 (1.80, 2.31) | 0.98 (0.88, 1.08) |
CI, confidence interval; IQR, interquartile range; IRR, incidence rate ratio; pmp, per million population.
Among the 113 countries offering KT, 70 (62%) had national transplantation waitlists, 22 (19%) only had regional waitlists, and 21 (19%) did not have any type of waitlist. Among the 5 low-income countries with KT services, 1 (Afghanistan) had a regional waitlist and the other 4 did not have any type of waitlist (Table 2). National waitlists were most common in high-income (n = 45, 90%) and upper-middle-income (n = 23, 68%) countries. Among lower-middle-income countries, only 2 (2/24, 8%) had national waitlists, and more than half did not have any type of waitlist (Table 2). The distribution of waiting list by donor type and the relative risk ratio of living donor KT is summarized in Table S4 (SDC, http://links.lww.com/TP/C285). Given that no country had a deceased donor only KT program, we compared the risk of having a KT in programs with live donor KT only to those with combined live and deceased KT. An individual in a country with a regional waiting list will have a higher chance of receiving a live donor KT than an individual in a country with a national waiting list (Table S4, SDC, http://links.lww.com/TP/C285).
Accessibility to KT Services
Respondents from 41% (n = 51) of countries where KT is available, reported <11% of patients with kidney failure who were suitable candidates for transplants were able to access KT services (Table 4). Access to existing services was lowest in North and East Asia, South Asia, Africa, and Oceania and South East Asia. Access was highest in Western Europe, Eastern and Central Europe, and the Middle East. Access to KT increased with income level (Table 4).
In 60% (n = 92) of participating countries, respondents reported “early provision of culturally appropriate information” as generally available with a trend for higher availability in high-income countries (n = 49, 88%), relative to upper-middle (n = 27, 66%), lower-middle (14, 40%), and low-income countries (n = 2, 9%) (Table S5, SDC, http://links.lww.com/TP/C285). Similar income-based trends were observed in the 69% (n = 106) of countries which reported on the availability of “effective preventive therapy to control infections” (Table S5, SDC, http://links.lww.com/TP/C285). Between 62% and 71% of countries reported on the general availability of vital infrastructures, which are critical to KT accessibility, such as “timely access to operating space,” “availability of appropriate immunosuppression and antirejection treatment,” “availability of appropriate facilities for immunosuppression drugs monitoring,” “availability of a multidisciplinary team,” and the “availability of standard organ procurement frameworks” (Tables S5–S7, SDC, http://links.lww.com/TP/C285). Overall, general availability was highest in high-income countries, followed by upper-middle, lower-middle, and low-income countries (Tables S5–S7, SDC, http://links.lww.com/TP/C285).
Quality Reporting of KT Services
Among the countries with KT services, a large proportion reported patient survival (77%, n = 86), kidney allograft function (73%, n = 82) and graft survival (72%, n = 80) in registries. Most countries also reported delayed graft function (65%, n = 73) and rejection rates (59%, n = 66) (Tables S8–S10, SDC, http://links.lww.com/TP/C285).
Health Information Systems and KT Services
Worldwide, 88 (57%) participating countries had a KT registry. KT registries were most common in high-income countries and became progressively less common with decreasing country income (Table S10, SDC, http://links.lww.com/TP/C285). No low-income countries reported KT registries. Among countries with transplant registries, 85% (n = 75) were national. Regional and local registries existed in 10% (n = 9) and 13% (n = 11) of countries, respectively. National transplant registries existed in most upper-middle (96%, n = 26) and high (84%, n = 43) income countries, and in just over half of lower-middle (60%, n = 6) income countries. Regional transplant registries existed in 7 (14%) high and 2 (20%) lower-middle income countries; local transplant registries existed in 7 (14%) high, 1 (4%) upper-middle, and 3 (30%) lower-middle income countries.
The majority (97%, n = 85) of countries with KT registries captured the donor source (living or deceased), and most collected data on patient mortality (86%, n = 76) and the cause of kidney failure (85%, n = 75). Less than half collected data on process-based measures (42%, n = 37) or hospitalizations (41%, n = 36), and few (20%, n = 18) collected PROMs. Registries were used to capture the cause of kidney failure in nearly all high-income countries (n = 48) as well as in 21 upper-middle and 7 lower-middle income countries (Table S10, SDC, http://links.lww.com/TP/C285). Donor type (living or deceased) and patient mortality were reported in all registries in high-income countries and most registries in upper-middle (96% and 67%, respectively) and lower-middle (80% for each measure) income countries. Hospitalizations were reported in fewer than half of the registries in high (47%, n = 24) and upper-middle (26%, n = 7) income countries, and half of the registries in lower-middle (50%, n = 5) income countries. Irrespective of income level, few KT registries collected PROMs: 22% (n = 11) of registries in high, 19% (n = 5) of registries in upper-middle, and 20% (n = 2) of registries in lower-middle-income countries collected these data (Table S10, SDC, http://links.lww.com/TP/C285).
DISCUSSION
This study represents a major attempt by the ISN to describe the current state of infrastructure, capacity, and services for KT across countries. Our data, although heterogenous and dated in a few instances, identifies significant variations in KT activity and availability of services around the world and suggest that increasing global access to KT will be a complex and challenging task. Lower-income countries lack the prerequisite transplant facilities, waitlists, workforce, sufficient government-backed initiatives and publicly funded healthcare systems to facilitate increased access to KT, especially deceased donor and preemptive KT.
We found that the prevalence and incidence of KT generally correlates with country income level. However, discrepancies in these trends (eg, Japan’s low incidence of KT) reveal that even in high-income countries, cultural practices and considerations regarding deceased donation can limit uptake of KT.15 Variable rates of prevalence and incidence of KT in middle-income countries appear to be greatly influenced by government funding models for KT services, availability of donors, and healthcare delivery models.16,17 In low-income regions such as Africa (excluding South Africa), no prevalence data were available; a review of incidence data and the literature shows that Nigeria, Ghana, Kenya, Sudan, Morocco, Tunisia, and Algeria have some KT capabilities, but low incidence rates.18 South Africa and Sudan are the countries in sub-Saharan Africa where substantial numbers of transplants are performed on an annual basis.6,18
Increased uptake of organs from deceased donors continues to play a strong role in partly addressing the demand for KT, particularly in upper-middle- and high-income countries where global incidence of living and deceased donor KT are strikingly similar.19 Our findings are aligned with previous studies that have shown low participation in deceased donor programs in low-income countries. This low participation is likely because of high costs of deceased donor programs, lack of infrastructure to support deceased organ donation including lack of critical care beds, lack of histocompatibility laboratory capacity, lack of legal frameworks for determining death due to the absence of neurological function, an absence of rigorous engagement with the government to formulate policy on all organ donor programs and especially deceased donor programs, and societal, cultural, or religious attitudes.6,7 Consequently, almost all kidney transplants in low-income countries involve organs from living donors, although availability of KT in these countries is greatly influenced by patients’ financial situation.6 As for living donation, our findings support past studies showing that preemptive KT is primarily limited to high-income countries.20 Our data show that low-income countries lack the prerequisite transplant facilities, waitlists, and workforces that make preemptive KT possible. Of note, in low-income countries where dialysis is costly, preemptive KT should be highly encouraged, as the literature suggests the utility of preemptive KT as a cost saving strategy in places with limited access to dialysis.21-23
Beyond increasing access to deceased and living donor KT, improving access to KT is contingent on a multitude of financial, governmental, and societal factors. First, a lack of transplantation registries and waitlists continues to limit KT access at the national and international levels. Registries and waitlists are necessary for the surveillance of KT practices and outcomes, and thus play a key role in strategies to improve access to KT, as seen even in high-income countries like the United States.24 Second, previous studies have noted that adequate financing of healthcare systems is a key determinant of high-quality and equitable healthcare delivery.25 Out of pocket costs tend to be higher in low-income countries where wealth is often unequally distributed and only a limited number of people are capable of paying. Access to KT in middle- and low-income countries is heavily influenced by a country’s gross national income per capita, as this factor has been shown to have strong downstream effects on healthcare system design, oversight, service delivery, infrastructure, and retention of multidisciplinary teams with the healthcare expertise required for the implementation of KT programs.4,25,26
The WHO Commission on Social Determinants of Health has argued for the expansion of universal health coverage and elimination of user fees as important and necessary means of increasing global healthcare access.27,28 There is a lack of consensus among experts about the best way to implement such systems, given the complex socio-cultural, political, and economic dynamics in many lower-middle- and low-income countries.25,29 Despite this complexity, previous reports have shown increased access to both healthcare services and KT care when governments of middle- and low-income countries (eg, Pakistan, Iran, Kenya, Philippines) have implemented hybrid funding models for healthcare systems or public-private partnerships.29-31
Finally, this study confirms previous findings which show that access to KT is disproportionately limited in low-income countries. Targeted approaches via strategies to obtain support from government on local initiatives developed to improve organ donation (eg, increased use of deceased kidney donors, and tissue typing services) as well as patient and healthcare provider awareness campaigns can be used to address the low availability of KT in some countries.22,31-33 Although these resource-intensive models have shown potential for addressing the disparities in KT access for vulnerable (racial, ethnic minorities, geographically remote) populations in high-income countries, their applicability is limited in middle- and low-income countries.
The strengths of our study include the rigorous methodology of the ISN-GKHA project, which is based on the WHO’s well-validated conceptual framework for assessing chronic disease.12 The survey had high-external validity, involving 182 countries with good coverage across regions and income levels. Data were collected from regional and national stakeholders familiar with local contexts. Another strength of this study is the utilization of tested and trusted international data sources (eg, GODT, European Renal Association Registry, United States Renal Data System, and Australia and New Zealand Dialysis and Transplant Registry).
The limitations of the study include the use of a questionnaire survey, which may have introduced bias based on the knowledge, expertise, and perceptions of respondents. The survey questions were designed based on the assumption that respondents would have the knowledge to fairly and accurately report on the capacity for KT care in their countries. For this reason, we selected respondents representing various capacities within the domain of KT care delivery. We are unable to draw any definitive conclusions about KT availability or other variables affecting KT access and quality in the 15% of countries from which no respondent data were collected. Some of the countries from which stakeholder responses were not obtained were island nations with small populations for whom KT was not an available service. For example, Curacao relies on a trans-Atlantic KT program with the Netherlands.34 However, the reason for nonresponse from individuals in countries with significantly larger populations, like Rwanda, was that they did not have a KT program35 while for others like Uzbekistan with a functioning KT program, the reason is unclear.36
Other limitations of our data set include the lack of data on within-country variability of KT prevalence, use of the most recent data available (eg, available data, either registry or publication, for some countries were well over a decade old) due to inconsistent or unavailable annual reporting of KT data, as well as uncertainty of KT estimates from low-income and lower-middle-income countries due to lack of rigorous data from those regions. We are aware that survey data can be subjective and can be subject to social desirability bias, as respondents might not like to portray their countries or regions in a negative way. For instance, respondents (including nephrology leaders, government officials like health minsters and Directors of Ministries of Health) may have wanted to or had to portray their country’s capacity according to an official line or they may just not have known. Therefore, the survey data on KT may have been affected by recall bias or opinions and may not have necessarily reflected granular, patient-level information in the country of respondents, and the accuracy of the survey data is therefore dependent on how correctly respondents represented the status of services in their country. While responses between stakeholders from the same country were concordant >80% of the time, there were some discordant responses. Although such discrepancies were resolved by consulting ISN leaders in the various regions with comprehensive knowledge of the status of kidney care in those regions, the presence of discordance reduces confidence in those data.
A predominant response from providers of kidney medical care as opposed to healthcare managers or other administrators could also be construed as a limitation. Finally, our survey did not collect data about comorbidities (eg, prevalence rates of diabetes mellitus, hypertension), which may have greatly contributed to inter-country variability of deceased donor, living donor, and preemptive KT. Despite these limitations, our data provides an important overview of the capacity and variations in various aspects of KT across countries and regions which will inform efforts to improve practices that will ensure improved KT care globally.
In conclusion, the growing global burden of CKD and kidney failure dictates the need for this study and for future studies that estimate the capacity for KT across countries and regions, especially given the stagnation in the supply of living organ donors, the under-utilization of deceased donor organs, and the financial constraints of dialysis therapies.37,38 Although low-income countries have many competing healthcare and economic challenges, inadequate funding for healthcare, poor state of healthcare infrastructure and healthcare system design are some significant barriers to improving access to quality KT care in low-income countries. The lack of robust government-backed local initiatives tailored toward improving organ donation and use, updated legislation on transplantation policies and access to KT clinical research and training programs need to be addressed across mostly low-income and lower-middle-income regions. In contrast, high-income countries also need to address challenges peculiar to their settings including in some cases low public awareness and education, high levels of community resistance to deceased organ donation, and lack of access among geographically remote populations to ensure equitable access to quality KT care.29-31 Understanding how different challenges are faced by countries in different income strata will inform efforts to increase awareness and the adoption of practices that will ensure high-quality KT care is provided around the world.
ACKNOWLEDGMENTS
We thank Kara Stephenson Gehman for carefully editing the English text of the ISN-GKHA and a draft of this article.
Supplementary Material
Footnotes
This work was supported by the International Society of Nephrology (Grant RES0033080 to the University of Alberta).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
D.W.J. has received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care; consultancy fees from Astra Zeneca and AWAK, speaker’s honoraria and travel sponsorships from ONO; and travel sponsorships from Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. V.J. has received grants, speaker honoraria, or consultancy fees from GlaxoSmithKline, Biocon, Baxter, Janssen, Medtronic, and NephroPlus. He has a policy of all funds being paid to his employer. A.K.B. has received consultancy fees from Janssen. The International Society of Nephrology provided administrative support for the design and implementation of the study and data collection activities. The authors were responsible for data management, analysis, and interpretation, as well as article preparation, review, and approval and the decision to submit the article for publication. The remaining authors have no conflicts of interest to declare.
A.K.B. and D.W.J. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A.K.B., A.L., and D.W.J. did study concept and design. All authors did acquisition, analysis, or interpretation of data. D.M., F.Y., I.O., and A.K.B. drafted the article. All authors did critical revision of the article for important intellectual content. I.O. did the graphical abstract. F.Y. did statistical analysis. A.K.B., D.W.J., and A.L. obtained funding. A.K.B. and D.W.J. did study supervision.
Supplemental Visual Abstract; http://links.lww.com/TP/C288.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Hamer RA, El Nahas AM. The burden of chronic kidney disease. BMJ. 2006;332:563–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. [DOI] [PubMed] [Google Scholar]
- 3.Howard K, Salkeld G, White S, et al. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology (Carlton). 2009;14:123–132. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M, Nkunu V, Varghese C, et al. Framework for establishing integrated kidney care programs in low- and middle-income countries. Kidney Int Suppl (2011). 2020;10:e19–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson B, Du Toit T, Jones ESW, et al. Outcomes and challenges of a kidney transplant programme at Groote Schuur Hospital, Cape Town: a South African perspective. PLoS One. 2019;14:e0211189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naicker S, Ashuntantang G. End stage renal disease in Sub-Saharan Africa. Garcia-Garcia G, Agodoa L, Norris K, eds. In: Chronic Kidney Disease in Disadvantaged Populations. Elsevier; 2017:125–137. [Google Scholar]
- 8.Shimazono Y. The state of the international organ trade: a provisional picture based on integration of available information. Bull World Health Organ. 2007;85:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bello AK, Johnson DW, Feehally J, et al. Global Kidney Health Atlas (GKHA): design and methods. Kidney Int Suppl (2011). 2017;7:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello AK, Levin A, Tonelli M, et al. Assessment of Global Kidney Health Care Status. JAMA. 2017;317:1864–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bello AK, Levin A, Lunney M, et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ. 2019;367:l5873. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. 2010. Accessed July 16, 2020. Available at https://www.who.int/healthinfo/systems/WHO_MBHSS_2010_full_web.pdf.
- 13.Global Observatory on Donation and Transplantation. WHO-ONT. 2018. Accessed August 16, 2020. Available at http://www.transplant-observatory.org/.
- 14.Bello AK, Levin A, Tonelli M, et al. Global Kidney Health Atlas: A Report by the International Society of Nephrology on the Current State of Organization and Structures for Kidney Care Across the Globe. International Society of Nephrology; 2017. [Google Scholar]
- 15.Aikawa A. Current status and future aspects of kidney transplantation in Japan. Ren Replace Ther. 2018;4:1–12. [Google Scholar]
- 16.Garcia-Garcia G, Chavez-Iñiguez JS. The tragedy of having ESRD in Mexico. Kidney Int Rep. 2018;3:1027–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saidi RF, Broumand B. Current challenges of kidney transplantation in Iran: moving beyond The “Iranian Model.” Transplantation. 2018;102:1195–1197. [DOI] [PubMed] [Google Scholar]
- 18.Muller E. Transplantation in Africa—an overview. Clin Nephrol. 2016;86:90–95. [DOI] [PubMed] [Google Scholar]
- 19.Wu DA, Watson CJ, Bradley JA, et al. Global trends and challenges in deceased donor kidney allocation. Kidney Int. 2017;91:1287–1299. [DOI] [PubMed] [Google Scholar]
- 20.Barsoum RS. Overview: end-stage renal disease in the developing world. Artif Organs. 2002;26:737–746. [DOI] [PubMed] [Google Scholar]
- 21.Arze Aimaretti L, Arze S. Preemptive renal transplantation—the best treatment option for terminal chronic renal failure. Transplant Proc. 2016;48:609–611. [DOI] [PubMed] [Google Scholar]
- 22.Akoh JA. Renal transplantation in developing countries. Saudi J Kidney Dis Transpl. 2011;22:637–650. [PubMed] [Google Scholar]
- 23.el-Agroudy AE, Donia AF, Bakr MA, et al. Preemptive living-donor kidney transplantation: clinical course and outcome. Transplantation. 2004;77:1366–1370. [DOI] [PubMed] [Google Scholar]
- 24.Fissell RB, Srinivas T, Fatica R, et al. Preemptive renal transplant candidate survival, access to care, and renal function at listing. Nephrol Dial Transplant. 2012;27:3321–3329. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Garcia G, Jha V. CKD in disadvantaged populations. Am J Nephrol. 2015;41:116–120. [DOI] [PubMed] [Google Scholar]
- 26.Bello AK, Alrukhaimi M, Ashuntantang GE, et al. Global overview of health systems oversight and financing for kidney care. Kidney Int Suppl (2011). 2018;8:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmot M, Allen J, Bell R, et al. WHO European review of social determinants of health and the health divide. Lancet. 2012;380:1011–1029. [DOI] [PubMed] [Google Scholar]
- 28.Evans DB, Marten R, Etienne C. Universal health coverage is a development issue. Lancet. 2012;380:864–865. [DOI] [PubMed] [Google Scholar]
- 29.Lagomarsino G, Garabrant A, Adyas A, et al. Moving towards universal health coverage: health insurance reforms in nine developing countries in Africa and Asia. Lancet. 2012;380:933–943. [DOI] [PubMed] [Google Scholar]
- 30.Guy-Frank CJ, Persaud K, Butsenko D, et al. Developing a sustainable renal transplant program in low- and middle-income countries: outcome, challenges, and solutions. World J Surg. 2019;43:2658–2665. [DOI] [PubMed] [Google Scholar]
- 31.Rizvi SAH, Naqvi SAA, Zafar MN, et al. A kidney transplantation model in a low-resource country: an experience from Pakistan. Kidney Int Suppl (2011). 2013;3:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salim A, Ley EJ, Berry C, et al. Effect of community educational interventions on rate of organ donation among Hispanic Americans. JAMA Surg. 2014;149:899–902. [DOI] [PubMed] [Google Scholar]
- 33.Wasser WG, Boner G, Koslowsky M, et al. Emergence of an Israel faith-based community organization facilitating live donor kidney transplantation. BMC Nephrol. 2018;19:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minnee RC, Lardy N, Ajubi N, et al. Ten-yr results of the trans-Atlantic kidney transplant airlift between the Dutch Caribbean and the Netherlands. Clin Transplant. 2011;25:302–307. [DOI] [PubMed] [Google Scholar]
- 35.Chironda G, Ngendahayo F, Mudasumbwa G, et al. Renal replacement therapy (RRT) in Rwanda: benefits, challenges and recommendations. RMJ. 2019;76:1–6. [Google Scholar]
- 36.Khadjibaev A, Khadjibaev F, Anvarov K, et al. Organ donation in Uzbekistan: achievements and prospects for further development. Exp Clin Transplant. 2020;18:54–57. [DOI] [PubMed] [Google Scholar]
- 37.Reese PP, Boudville N, Garg AX. Living kidney donation: outcomes, ethics, and uncertainty. Lancet. 2015;385:2003–2013. [DOI] [PubMed] [Google Scholar]
- 38.Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96:414–422C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.