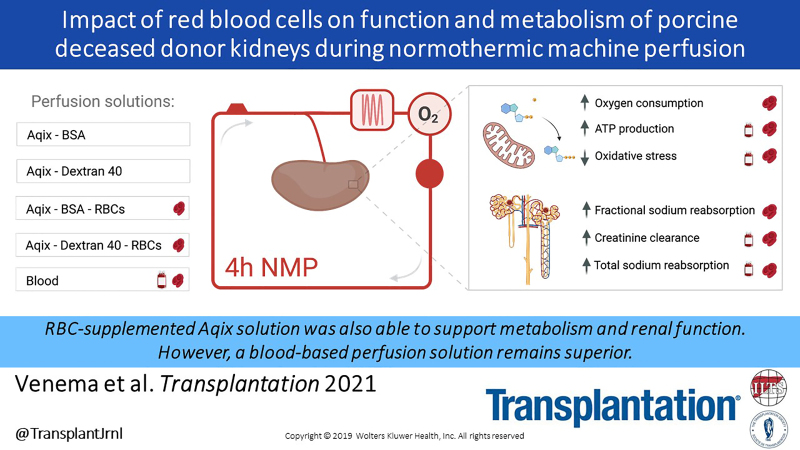
Background.
Normothermic machine perfusion (NMP) protocols using blood-based solutions are commonly used in the assessment of kidneys before transplantation. This procedure is, nevertheless, limited by blood availability and warrants the search for alternatives. We compared a blood-based solution with a serum-like preservation solution (Aqix) enriched with colloids with and without red blood cells (RBCs).
Methods.
Porcine kidneys retrieved from an abattoir were subjected to 30 min of warm ischemia, followed by 3 h of hypothermic oxygenated machine perfusion at 4 °C. Subsequently, kidneys (n = 6 per group) were evaluated with NMP for 4 h with 5 different solutions: diluted blood, Aqix with BSA ± RBCs, or Aqix with dextran 40 ± RBCs.
Results.
Throughout NMP, markers of renal function and tubular metabolism were favorable in groups with RBCs. The addition of RBCs resulted in 4- to 6-fold higher oxygen consumption rates. Controls had significantly higher ATP levels post-NMP, exhibited decreased production of oxidative stress markers, and had the highest creatinine clearance. In conclusion, this study shows that the addition of RBCs during NMP reduced renal injury, improved function, and was associated with increased renal metabolism.
Conclusions.
Although the RBC-BSA–supplemented Aqix solution was also able to support metabolism and renal function, a blood-based perfusion solution remains superior.
INTRODUCTION
Kidney transplantation is currently the standard treatment for patients with end-stage renal disease.1 Unfortunately, renal transplantation is drastically limited due to the shortage of available and suitable donor kidneys.2 To overcome this persistent shortage, most transplant centers nowadays accept older and higher-risk donor kidneys from donation after circulatory death (DCD) donors and expanded criteria donors.3,4 However, this often comes with a consequence for graft function and survival because many of these kidneys are of inferior quality when compared with standard criteria donor kidneys.5,6
Ex vivo or ex situ normothermic machine perfusion (NMP) is a technique to perfuse kidneys at 37 °C with an oxygenated blood-based perfusion solution.7 NMP can be used to evaluate organ quality by assessing kidney function and other parameters before transplantation. NMP appears especially relevant in donor kidneys with a questionable quality, such as kidneys from DCD and expanded criteria donors. In the past years, the NMP technique has been further developed, and the first clinical results have been published, giving evidence that a brief period of NMP after static cold storage preservation allows successful transplantation of kidneys that were initially declined.7-10
Currently, in the majority of perfusion strategies, blood-based solutions with red blood cells (RBCs) as oxygen carriers are used for clinical NMP of donor organs.10-14 RBC-based solutions provide a more “physiological” environment as compared to artificial (or “purely synthetic”) solutions.15 However, the usage of blood products for NMP also comes with some serious drawbacks. Among these, hemolysis is associated with the development of oxidative stress and acute kidney injury,16 endothelial and platelet activation, and adverse immune responses.15,17 In addition, blood products are precious, expensive, and have a limited shelf life.17,18 Therefore, the identification of alternatives to RBC-containing perfusion solutions for NMP is desirable, particularly if NMP is to become the new standard of care for assessing the viability of higher-risk donor organs.
AQIX RS-I (Aqix) is a nonphosphate buffered solution that aims to resemble the human interstitial fluid.19 It has been used to store biological tissue samples but also could serve as an alternative to blood-based perfusate. The osmolarity (286 mOsmol/L) is comparable with human serum, and its ionic conductivity (12.6 mS/cm) results in an iso-osmotic solution. Under both hypothermic and normothermic conditions, it has been shown that static storage of porcine kidneys in Aqix maintains the acid–base homeostasis and leads to improved recovery of cellular function.20 According to the Starling principle, the colloid osmotic pressure (COP) is essential for maintaining the fluid balance between the intravascular and extravascular compartments. Furthermore, the Starling principle states that fluid movements between blood and tissues are determined by differences in hydrostatic and colloid osmotic (oncotic) pressures between microvessels and surrounding interstitial fluid. Reduced COP leads to interstitial fluid overload and edema formation, which increases the diffusion distance for oxygen and nutrients, potentially compromising cellular metabolism.21,22 Therefore, we decided to supplement the AQIX RS-I solution with either BSA or dextran 40 (Dex). Albumin was chosen because it is the most abundant plasma protein and Dex because it has been shown to be beneficial during the hypothermic machine perfusion (HMP) of porcine kidneys.23,24 As a simple aqueous solution, Aqix will only carry oxygen in a dissolved state.19 Thus, the question remains whether dissolved oxygen will allow for an oxidative metabolic state during NMP conditions.
To determine whether AQIX RS-I combined with a colloid and RBC could serve as a preferred substitute to a blood-based perfusion solution during ex situ NMP of porcine kidneys, we have studied the effect of different perfusion solutions on renal metabolism, renal function, and injury.
MATERIALS AND METHODS
Animal Model
Porcine kidneys were retrieved from the local abattoir after a highly standardized slaughtering process simulating DCD conditions, as previously described.25 Briefly, pigs were sedated by an electrical shock and immediately exsanguinated. The blood was collected in a beaker containing 25 000 IU of heparin (LEO Pharma A/S, Ballerup, Denmark). No approval from the animal ethics committee was required because the organs of animals slaughtered for meat consumption were used and were thus considered as “waste material.”
Experimental Design
To induce ischemic injury, kidneys were subjected to 30 min of warm ischemic time, starting after exsanguination and ending when the cold flush had started. All kidneys were preserved with oxygenated HMP for 3 h. Afterward, the kidneys were perfused in the ex vivo NMP setup for 4 h with AQIX RS-I supplemented with a colloid (BSA or DEX) supplemented with or without the addition of RBCs. Kidneys that were perfused with an autologous blood-based perfusion solution served as controls. Six kidneys were included in each experimental group. For histology assessment, additional kidney tissue samples were taken from kidneys (n = 5) that were derived immediately after procurement (warm ischemic time of approximately 15 min) and that did not undergo HMP and subsequent NMP.
Kidney Preservation
After warm ischemia, all kidneys were flushed with 180 mL of saline solution at 4 °C (Baxter BV, Utrecht, The Netherlands) without the addition of heparin. Immediately after the initial flush-out, a cortical needle biopsy (Invivo, Best, The Netherlands) was taken from the upper pole of the kidney and was stored in a solution containing 0.372 g EDTA (0.744 g/L) in 130 mL H2O and NaOH (pH 10.9) + 370 mL 96% ethanol. Thereafter, kidneys were prepared for HMP by cannulating the renal artery and were connected to a Kidney Assist Transporter device (Organ Assist, Groningen, The Netherlands). HMP was performed for 3 h at a mean pressure set at 25 mm Hg at 4 °C using 100% oxygenated (100 mL/min) University of Wisconsin machine perfusion solution (Belzer MPS, Bridge to Life, London, United Kingdom).
Ex Situ NMP
After the preservation period, organ quality was assessed during 240 min of ex situ NMP with the different perfusion solutions. Control kidneys were perfused with an autologous blood-based solution (Table 1).25 The experimental groups were perfused with Aqix (AQIX RS-I, AQIX, London, United Kingdom) in combination with a colloid, that is, either BSA (Sigma-Aldrich, St Louis, MO) or Dex (Sigma-Aldrich, St Louis, MO), supplemented with or without autologous porcine RBCs that had been washed in PBS (Table 1). Before starting NMP, a second biopsy was taken, and the kidney was weighed. The kidneys were placed in an organ chamber and perfused for 4 h at 37 C using a pressure-controlled pulsatile pump set at a mean pressure of 75 mm Hg (Kidney Assist Transport, Organ Assist, Groningen, The Netherlands). A carbogen mixture (95% O2; 5% CO2) with a fixed flow of 500 mL/min was used to oxygenate the perfusate. After 30 min of NMP, another biopsy was taken and snap frozen. At the end of perfusion, the kidneys were weighed, and a cortical biopsy was snap frozen in liquid nitrogen. Perfusate and urine samples were collected 15, 60, 120, 180, and 240 min after the start of NMP. Arterial and venous blood gas analyses were performed at the same time points using an ABL90 FLEX (Radiometer, Brønshøj, Denmark).
TABLE 1.
Perfusion solution composition
| Group | Priming | Additives | Infusion (20 mL/h) |
|---|---|---|---|
| ControlHb: 5.0 ± 1.0 mmol/L | 310 mL Lactated Ringer500 mL Leukocyte-depleted blood | - 90 mg Creatinine- 1000 mg/200 mg Amoxicillin/clavulanic acid- 6 mg Dexamethasone- 6 mg Mannitol- 2 mg Sodium nitroprusside- 10 mL 8.4% Sodium bicarbonate- 10 mL 5% Glucose | - 90 mL Aminosol- 2.75 mL 8.4% Bicarbonate- 18.6 IU Insulin |
| Aqix-BSA | 800 mL AQIX RS-I17.6 g BSA (2.2%) | - 90 mg Creatinine- 1000 mg/200 mg Amoxicillin/clavulanic acid- 6 mg Dexamethasone- 6 mg Mannitol- 2 mg Sodium nitroprusside- 17 IU Insulin | No infusion |
| Aqix-Dex | 800 mL AQIX RS-I28 g Dex 40 (3.5%) | The same additives as the Aqix-BSA group | No infusion |
| Aqix-BSA-RBCHb: 5.4 ± 1.0 mmol/L | 580 mL AQIX RS-I220 mL RBC12.8 g BSA (2.2%) | The same additives as the Aqix-BSA group | No infusion |
| Aqix-Dex-RBCHb: 5.5 ± 0.8 mmol/L | 580 mL AQIX RS-I220 mL RBC20.3 g Dex (3.5%) | The same additives as the Aqix-BSA group | No infusion |
| Composition of AQIX RS-I | |||
| components | Concentration (mmol/L) | Classification | |
| NaCl | 110.00 | Salts | |
| KCl | 5.00 | ||
| CaCl2 | 1.25 | ||
| MgCl2 | 0.45 | ||
| NaHCO3 | 25.00 | pH buffer | |
| BES | 5.00 | ||
| d-Glucose | 10.00 | Metabolic substrates | |
| Glycerol | 0.11 | ||
| l-Glutamate | 0.30 | ||
| l-Glutamine | 0.40 | ||
| l-Aspartate | 0.02 | ||
| l-Carnitine | 0.05 | ||
| Choline chloride | 0.01 | ||
| Thiamin pyrophosphate | 40 | ||
| Human insulin | 28 mIU | ||
BES, N,N-bis (2-hydroxyethyl)-2-amino-ethanesulfonic acid; Dex, dextran 40; Hb, hemoglobin; RBC, red blood cells.
Evaluation of Renal Metabolism
To assess transport-related renal metabolism, renal oxygen consumption, fractional sodium excretion, and total sodium reabsorption (TNa) were calculated with the equations presented in Table 2. Creatinine and sodium concentrations were measured in both perfusate and urine samples according to routine clinical procedures at the clinical chemistry laboratory of the University Medical Center Groningen. ATP levels were measured in the biopsies collected after warm ischemia, after HMP, after 30 min NMP, and at the end of NMP (NMP240). ATP was analyzed as previously described and was normalized to the amount of protein being present in that biopsy.26
TABLE 2.
Equations for calculating renal metabolic and functional parameters
| Outcome | Unit | Equation | Abbreviations |
|---|---|---|---|
| Arterial oxygen content | mLO2/L | Hb, hemoglobin content (mmol/L).2.4794 = arterial oxygen content in mLO2/dL and is calculated by 1.54 (mLO2/g Hb at 37 °C × (Hb [mmol/L] × 1.61) × 100 (= SO2 arterial) × 0.01.po2, partial oxygen pressure kPa).K, SO2, hemoglobin saturation (%). | |
| Venous oxygen content | mLO2/L | 0.024794 = venous oxygen content in mLO2/dL and is calculated by 1.54 (mLO2/g Hb at 37 °C × (Hb [mmol/L] × 1.61) × SO2 venous × 0.01.Hb, hemoglobin content (mmol/L).po2, partial oxygen pressure (kPa).K, solubility constant of oxygen in water at 37 °C (0.0225 mLO2 per kPa).SO2, hemoglobin saturation (%).Bold part of the formula is canceled when no RBCs were used. | |
| Oxygen consumption | mLO2/min/100 g | Q, renal blood flow (L/min).g, kidney weight (g). | |
| Fractional sodium excretion | % | UNa, urine sodium concentration (mmol/L).PNa, perfusate sodium concentration (mmol/L).PCr, perfusate creatinine concentration (mmol/L).UCr, urine creatinine concentration (mmol/L). | |
| Total sodium reabsorption | mmol/min/ 100 g | CrCl, creatinine clearance (mL/min).PNa, perfusate sodium concentration (mmol/L).UNa, urine sodium concentration (mmol/L).U, urine production rate (mL/min).g, kidney weight (g). | |
| Creatinine clearance | mL/min/100 g | UCr, urine creatinine concentration (mmol/L).U, urine production rate (mL/min).PCr, perfusate creatinine concentration (mmol/L).g, kidney weight (g). | |
| TBARS production | U/L | TBARSurine, concentration of TBARS in urine (µM).U, urine production rate (mL/min).TBARSperfusate, concentration of TBARS in the perfusate (µM).P, priming volume of the NMP setup (L).I, volume of infusion during NMP (L). |
NMP, normothermic machine perfusion; TBARS, thiobarbituric acid-reactive substances.
Evaluation of Renal Function, Urine Production, and Edema Formation
Flow rate during HMP was recorded every 5 min up to 30 min and, thereafter, every 30 min. Flow rate during NMP was recorded every 15 min. Creatinine clearance was calculated according to the equation given in Table 2. Urine production was measured every 15 min during perfusion. All parameters were normalized to kidney weight measured before NMP. The variation in renal weight was calculated by subtracting the weight measured before NMP from the weight measured after NMP and was expressed as relative difference (%).
Kidney Injury, Oxidative Stress Markers, and Histological Assessment
Lactate dehydrogenase (LDH) and aspartate aminotransferase (ASAT) were analyzed by the clinical chemistry laboratory of the University Medical Center Groningen according to standard procedures. Urinary N-acetyl-beta-d-glucosaminidase (uNAG) was determined as previously described.26,27 Reactive oxygen species (ROS)–induced injury was assessed by measuring thiobarbituric acid–reactive substances (TBARS) in both urine and perfusate.28 TBARS production was calculated at every sampling time point (Table 2).
Kidney biopsies taken after NMP were fixed by immersion in 4% buffered paraformaldehyde, embedded in paraffin, and sections were cut at 4 µm. These were subsequently stained with periodic acid–Schiff and were blindly scored by a pathologist. Glomerular and vascular damage were absent in all groups. In the tubular interstitium, varying degrees of tubular damage were observed, ranging from loss of the tubular brush border integrity to loss of adherence of tubular cells to the tubular basement membrane. Damage was scored when excess cell or tubular cell debris was present within the luminal tubular area because this indicates damage to that particular segment. For that purpose, percentages of tubulointerstitial damage were given to each section: 0%, <1%, 1% to 10%, 10% to 25%, 25% to 50%, 50% to 75%, or 75% to 100%.
Statistical Analysis
All data were expressed as a mean with SEM, except histology, data which were reported as a median with an interquartile range. The area under the curve was calculated using the trapezoid rule for all parameters with multiple measurements over time. For TNa rate, the function subtracts negative areas. The area under the curve of LDH in the control group was corrected for baseline LDH level. Differences across all experimental groups were assessed by ANOVA. Subsequently, the Dunnett post hoc test was used to compare the blood group (reference) with the other experimental groups. As an exploratory analysis, all other pairwise comparisons were made with the unpaired Student t tests of which the P values were corrected using the Benjamini-Yekutieli procedure to control the false discovery rate (set at 5%).29 All statistical tests were 2-tailed, and a P ≤ 0.05 was considered statistically significant. Data were analyzed with R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Energy-demanding Processes During NMP
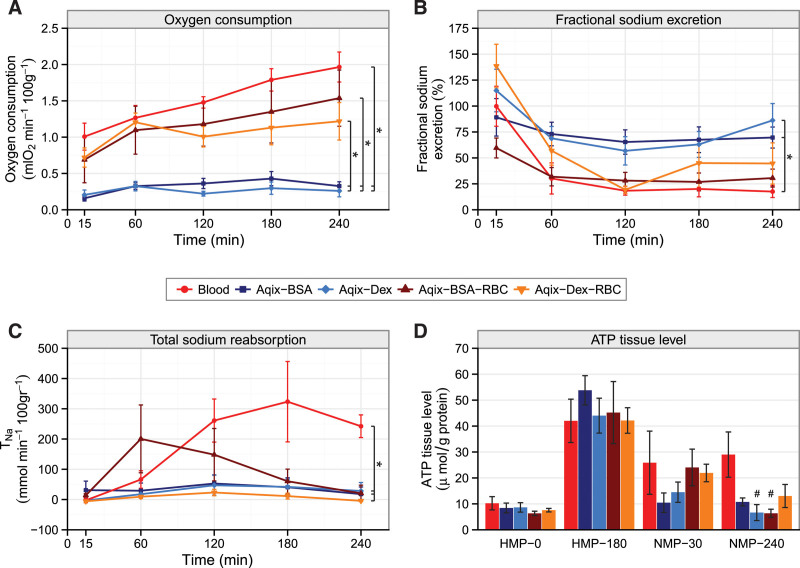
Compared with acellular perfusion solution groups (Aqix-BSA and Aqix-Dex), total oxygen consumption during NMP was significantly higher in all perfusion solutions containing RBCs (P < 0.05) (Figure 1A). The arterial oxygen content was significantly higher in the control group compared with both acellular groups (Aqix-BSA, P = 0.045; Aqix-Dex, P < 0.01) and also between Aqix-BSA-RBC and Aqix-Dex (P < 0.01) (Figure S1A, SDC, http://links.lww.com/TP/C279).
FIGURE 1.
Energy-demanding processes measured during NMP. Porcine kidneys were perfused for 4 h with blood (control) with Aqix supplemented with a colloid (2.2% BSA or 3.5% Dex) or Aqix with a colloid (BSA or Dex) and RBC. A, Oxygen consumption rates during 4 h normothermic perfusion, *P < 0.05 AUCs of all RBC-containing groups compared with acellular groups. B, Fractional sodium excretion levels during 4 h perfusion, *P < 0.02 Aqix-BSA, Aqix-Dex vs controls (Dunnett test). C, TNa during 4 h perfusion, *P < 0.001 control vs Aqix-BSA/Dex and control vs Aqix-Dex-RBC group. D, ATP content in kidney cortex tissue after 30 min warm ischemia and before preservation (HMP0), 3 h preservation (HMP180), 30 min of NMP (NMP30), and at the end of 240 min of NMP (NMP240), #P < 0.03 vs controls. Every group contains 6 kidneys. Data are presented as mean ± SEM. Except for ATP, areas under the curves were compared to assess differences between experimental groups. ATP, adenosine triphosphate; BSA, bovine serum albumin; Dex, dextran 40; HMP, hypothermic machine perfusion; NMP, normothermic machine perfusion; RBC, red blood cell; TNa, total sodium reabsorption.
Fractional sodium excretion (FENa) was calculated to estimate tubular function with a lower level corresponding to an improved tubular function. FENa (Figure 1B) levels ranged between 55% and 165% at the start of NMP. The acellular groups showed higher FENa levels and kept relatively stable at around 75% during NMP. The FENa among cellular groups tended to remain lower and decreased during NMP toward 25%. There was, however, only a significant difference between Aqix-BSA, Aqix-Dex, and the control group (P = 0.02 for each pairwise comparison). The Aqix-BSA-RBC group tended to have decreased FENa compared with the acellular groups (P = 0.059).
TNa was higher in controls than in kidneys perfused with Aqix supplemented with BSA, Dex, and Dex-RBC, respectively (P = 0.02 for each pairwise comparison). The Aqix-BSA-RBC group showed a peak in sodium reabsorption after 1 h of NMP but decreased over time.
At the start of HMP, tissue ATP levels were not different between the experimental groups (Figure 1D; HMP0). Thereafter, ATP levels remained similar among experimental groups until at the end of NMP (NMP240). At this time point, ATP levels were lower in the Aqix-Dex (P = 0.02) and Aqix-BSA-RBC (P = 0.03) groups, respectively, when compared with controls.
Lactate levels are presented in Figure S1B (SDC, http://links.lww.com/TP/C279), which shows high levels of lactate at the beginning of NMP due to the use of lactated Ringer’s solution. Compared with the control group, the increase in lactate during 4 h of NMP was significantly higher in the Aqix-Dex-RBC group (P = 0.002) and tended to be higher in the Aqix-BSA-RBC group (P = 0.068).
Renal Function and the Effects of Colloids on the Kidney
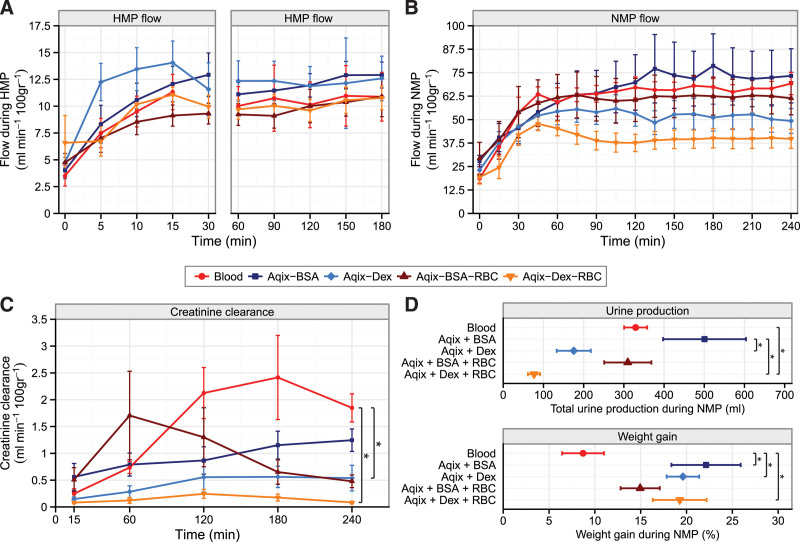
During HMP (Figure 2A, left and right panels, P = 0.61) and NMP (Figure 2B; P = 0.27), the flow first increased at the start of both machine perfusion modalities, then remained stable afterward, but did not differ between experimental groups. Irrespective of treatment group, NMP flow (40–75 mL/min/100 g) was 4- to 7-fold higher than HMP flow (10–13 mL/min/100 g). Creatinine clearance was the highest in the control group (Figure 2C) and was significantly better in comparison with the Aqix-Dex-RBC group (P = 0.01) and the Aqix-Dex group (P = 0.02), respectively. Urine production was significantly lower in the Aqix-Dex-RBC group compared with the control group (Figure 2D; Figure S2, SDC, http://links.lww.com/TP/C279; P = 0.02). The Aqix-BSA group showed significantly higher urine production compared with Aqix-Dex and Aqix-Dex-RBC (P < 0.05). Compared with controls, weight gain during NMP was increased in the Aqix-BSA, Aqix-Dex, and Aqix-Dex-RBC groups, respectively (Figure 2D; P < 0.05).
FIGURE 2.
Renal function and the effects of colloids on the kidney. Porcine kidneys were perfused for 4 h with blood (control) with Aqix supplemented with a colloid (2.2% BSA or 3.5% Dex) or Aqix with a colloid (BSA or Dex) and RBC. A, Renal flow rates during 3 h of HMP and (B) during 4 h NMP; (C) creatinine clearance during 4 h NMP, *P = 0.02 control vs Aqix-Dex-RBC group; (D) total urine production and weight gain, respectively, during 4 h NMP, *P ≤ 0.05. Every group contains 6 kidneys. Data are presented as mean ± SEM. Except for urine production and weight gain, areas under the curves were compared to assess differences between experimental groups. BSA, bovine serum albumin; Dex, dextran 40; HMP, hypothermic machine perfusion; NMP, normothermic machine perfusion; RBC, red blood cell.
Renal Injury and Oxidative Stress During NMP
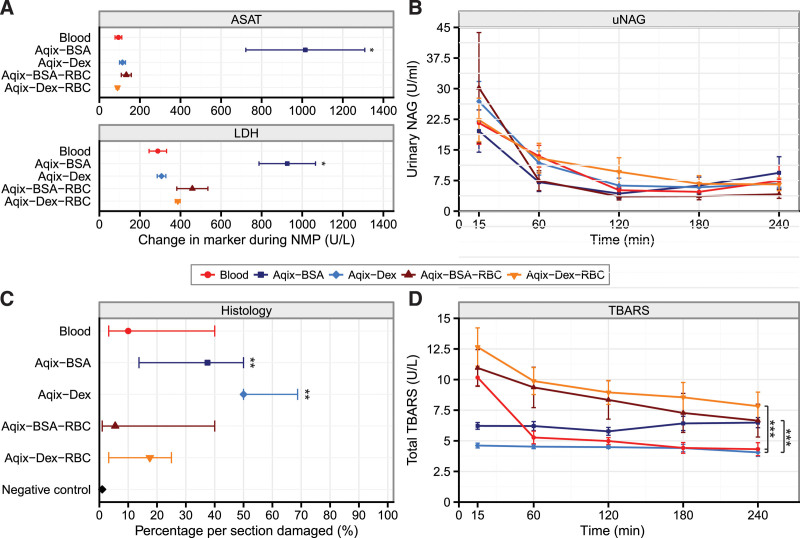
Compared with all other experimental groups, kidneys perfused with Aqix including BSA exhibited significantly increased ASAT and LDH production during NMP (Figure 3A; Figure S3, SDC http://links.lww.com/TP/C279; P < 0.001). Despite increased release of injury markers, no differences in the specific groups were found in regard to uNAG or total TBARS production, the latter being a marker for oxidative stress (Figure 3B and D, respectively). Notably, kidneys perfused with Aqix-BSA-RBC and Aqix-Dex-RBC had increased TBARS production compared with the Aqix-Dex group. Also, kidneys perfused with Aqix-Dex-RBC had higher TBARS production compared with controls (Figure 3D; P = 0.013). Histology examination revealed that kidneys perfused with Aqix-BSA and Aqix-Dex had a significantly higher level of damage per section compared with the negative control samples, indicating that most renal damage occurred in kidneys perfused with acellular solutions (Figure 3C; P < 0.05). No differences between groups were found; however, the median percentage of damage was lower in the cellular groups compared with the acellular groups. Histology sections can be found in Figure S4 (SDC, http://links.lww.com/TP/C279).
FIGURE 3.
Kidney injury and oxidative stress markers during NMP. Porcine kidneys were perfused for 4 h with blood (control) with Aqix supplemented with a colloid (2.2% BSA or 3.5% Dex) or with a colloid (BSA or Dex) and RBC. A, ASAT and LDH levels during 4 h NMP, *P < 0.001 Aqix + BSA vs all other experimental groups. B, uNAG levels during 4 h NMP. C, Histology damage scores from sections taken after 4 h NMP. Negative control samples are additional kidney tissue samples taken directly after death of the pig, **P < 0.05. D, Total TBARS production during 4 h perfusion, **P < 0.04. Every group contains 6 kidneys. Data are presented as mean ± SEM, except in Figure 3C, data are presented as median ± interquartile range. ASAT, aspartate aminotransferase; BSA, bovine serum albumin; Dex, dextran 40; LDH, lactate dehydrogenase; NMP, normothermic machine perfusion; RBC, red blood cell; TBARS, thiobarbituric acid-reactive substances; uNAG, urinary N-acetyl-beta-d-glucosaminidase.
DISCUSSION
The objective of our study was to determine whether acellular AQIX RS-I (Aqix) in combination with a colloid and RBC as an oxygen carrier could be a viable substitute for blood-based perfusion solution during ex situ NMP of porcine kidneys. The control group consisted of perfusion with leukocyte-depleted autologous blood diluted with Ringers’ lactate, supplemented with antibiotics, a vasodilator, a corticosteroid, mannitol, and nutrients; this solution has been successfully used as a standard preclinical perfusion solution by our group.25 The main finding in this study was that the oxygen consumption rates were 4- to 6-fold lower in the acellular solutions when compared with the RBC-containing groups with a significantly higher sodium transport in the control group. In addition, a trend toward improved fractional sodium excretion in both the control and Aqix-BSA-RBC groups was observed. Interestingly, sodium transport and reabsorption were impaired when dextran was added as a colloid.
Comparison With Other Studies
NMP is an upcoming technique that has only been introduced in clinical practice on a limited scale for kidney transplantation. It has been previously demonstrated that 1 h of NMP is feasible and safe using a blood-based electrolyte perfusion solution without the addition of a colloid.7-10 Subsequently, it became evident that longer perfusions than 1 h are needed for better graft assessment and possible organ repair.30,31 Several studies have shown that NMP with acellular perfusion solutions of donor liver and lungs can be successful. However, there is only limited experience with acellular perfusion solutions for kidney NMP.17,18,32-34 Similar outcomes were found when comparing a synthetic acellular hemoglobin-based oxygen carrier with packed RBCs during an NMP of discarded human kidneys.35 The comparison between a cellular and an acellular solution has not been made yet. There are some preclinical data on acellular (re)perfusions in which renal function of porcine kidneys was evaluated after preservation with various techniques using an ex vivo normothermic reperfusion protocol without the supplementation of oxygen carriers. During 90 min of perfusion, differences in renal function could be observed, providing evidence for the suitability of oxygen carrier-free solutions to assess organ function.23,36-40 Our data do not support this finding because renal function, based on fractional sodium excretion and creatinine clearance, was very low in the acellular groups. Relatively low quality of the kidneys in our study due to the standardized slaughtering process and exposure to 30 min of warm ischemia could explain these findings. However, it was our aim to evaluate the different solutions used for NMP in donor kidneys that have been injured and need to be properly assessed. A limitation of this study, but also the studies that used acellular (re)perfusion techniques to assess renal function, is that the donor kidneys were not transplanted. Therefore, it is unknown whether perfusion with oxygen carrier-free solutions may affect safety and long-term organ function. As NMP of donor kidneys is still in its infancy, the exact needs of the organ during NMP are not yet known, making the prediction of posttransplant outcomes based on kidney function parameters measured during NMP impossible. In this regard, we cannot say which level of renal clearance, tubular function, or oxygen consumption during NMP might be predictive of posttransplant adequate renal function. During normothermia, it is known that cellular metabolism is fully active and the demand of oxygen is high.41 Therefore, the question remains whether sufficient oxygen is present to support cell metabolism during NMP when perfused with acellular solutions.
Renal Metabolism
Although representing <0.5% of the body weight, kidneys use approximately 10% of the whole-body oxygen consumption.42 Such an oxygen requirement results from active sodium reabsorption by tubular cells.43 In that process, both oxygen and metabolic substrates are necessary to fuel oxidative phosphorylation.44,45 All RBC-containing groups showed higher levels of oxygen consumption compared with the acellular groups by a 4- to 6-fold factor. Nevertheless, the only significant improvement in TNa and ATP levels was seen in the control group. Although AQIX RS-I appears to have essential components to fuel oxidative phosphorylation, the solution did not support ATP production and subsequent sodium reabsorption at the same level when compared with the control group. The Aqix-BSA-RBC group showed an almost significant decrease in ATP production when comparing 30 and 240 min of NMP (P = 0.06). Furthermore, this group showed a peak in sodium reabsorption after 1 h of NMP but decreased over time. This indicates that Aqix-BSA-RBC is not favorable to fuel oxidative phosphorylation during NMP for longer perfusion periods. We have previously reported similar results when comparing static cold-stored kidneys with hypothermic machine perfused kidneys using different oxygen concentrations,25 and although cold-stored kidneys consumed oxygen (with a comparable consumption as found in this study), the handling of sodium was disrupted (FENa, 70%–100%) because oxygen consumption did not result in ATP production but in ROS formation, as measured by TBARS levels during perfusion. In the present study, we also found higher TBARS levels in the Aqix-Dex-RBC and Aqix-BSA-RBC group compared with controls, suggesting increased oxidative injury in this group.46,47 Our data possibly suggest mitochondrial malfunction or uncoupling, whereby oxygen is partially reduced, producing ROS. Higher lactate production during NMP suggests increased anaerobic metabolism in both acellular groups but also in the Aqix-BSA/Dex-RBC groups, indicating that insufficient oxygen is delivered to the renal cells to perform oxidative phosphorylation. Metabolic tracer studies, such as isotopic labeling of central metabolites, could provide additional insight into renal metabolism during NMP.48
In the absence of transport-related processes, the kidney exhibits a low, basal oxygen consumption rate of approximately 15% of total renal oxygen consumption of a filtering/reabsorbing kidney.43,49 In contrast, oxygen consumption has been shown to increase linearly with glomerular filtration rate, which in turn is associated with TNa through tubuloglomerular feedback.42,43 Because sodium reabsorption appeared to be almost absent in the acellular groups, we hypothesize this is the reason that the tubular injury marker uNAG was not increased among these groups despite insufficient oxygen supply.
Effect of Different Colloids During NMP
A significant increase in weight gain compared with control was observed in the Aqix-BSA, Aqix-Dex, and Aqix-Dex-RBC groups, reflecting tissue swelling and possible edema formation. Edema increases the diffusion distance for oxygen and could therefore compromise cellular metabolism, as observed in the Aqix-BSA, Aqix-Dex, and Aqix-Dex-RBC groups.21,22 A higher concentration of colloids and so a higher COP could lead to less edema formation and so improved cellular metabolism; however, we believe that an oxygen carrier is still of essence for optimal support of the kidney its metabolic needs.
Dex was tested as a potential artificial colloid during NMP.50 Based on pilot experiments, we determined that 3.5% Dex was most beneficial for minimalizing edema formation when looking at the wet/dry ratio; however, weight gain during NMP was significantly higher in both Dex groups compared with the control. Furthermore, sodium transport and reabsorption were impaired in both Dex groups. We did not observe severe renal injury based on injury markers measured in the perfusate (ASAT, LDH) or urine (uNAG) in dextran perfused kidneys. However, histology findings showed significantly increased cell damage compared with our negative controls and kidneys that were perfused with dextran that, without RBC, showed the highest median percentage of cell damage per section scored by the pathologist. This finding is in line with other studies that have shown that dextran can cause tubular damage.51 Little is known about using Dex during machine perfusion,23,24 and additional studies should be conducted to determine the pharmacokinetics and toxicity of Dex during ex vivo NMP.
Limitations of the Study
Due to the use porcine kidneys obtained from a slaughterhouse, we were not able to provide posttransplant outcomes. Furthermore, these pigs were sedated followed by exsanguination and were not exposed to the agonal phase that is commonly seen in DCD donation.52 Therefore, functional warm ischemia is not considered in this model. Nonetheless, our model has shown to be a validated technique to assess donor kidney quality after reperfusion.25,41,53-56 Slaughterhouse kidneys provide the opportunity to test early preclinical hypotheses in organs of large animals without the need of laboratory animals, thereby reducing both costs and animals for research. Ultimately, a transplant model with laboratory DCD porcine donors will be required as a next step toward translation to clinical practice with human kidneys. Another limitation is that we did not include the addition of an acellular synthetic oxygen carrier, such as hemoglobin-based oxygen carrier or other alternatives. We cannot answer the questions on whether RBCs are necessary or whether another oxygen carrier could also perform the task of transporting oxygen toward the kidney during NMP.
In conclusion, this study shows that the addition of RBCs to the perfusion solution significantly enhances oxygen consumption of ex vivo perfused kidneys under normothermic conditions. We have demonstrated that RBCs are necessary to support sodium reabsorption during NMP, indicating that to be able to perform this energy-consuming process, sufficient oxygen transportation is mandatory to support basal renal function. The Aqix supplemented with BSA and RBC is partly able to support renal metabolism and function, but the control blood-based perfusion solution was found to be superior. To fully apprehend these new findings, further evaluation in the transplant setting is required.
Moreover, such studies are essential for the development of emerging concepts of organ-tailored preservation, and potential repair protocols suggesting that the role of NMP will provide a window for organ assessment before transplantation.
ACKNOWLEDGMENTS
The authors are very grateful to Kroon Vlees and butchery Kuipers for their support in providing kidneys for this research. Their special thanks go to Gert Maring and Henk Luinge for making the logistics to perform scientific research possible in a commercial slaughterhouse. Furthermore, they would like to thank Petra Ottens, Janneke Wiersema-Buist, and Jacco Zwaagstra for performing analyses.
Supplementary Material
Footnotes
This project has received funding from the European Union’s 7th Framework Programme for Research, Technological Development, and Demonstration under grant agreement 305934.
The authors declare no conflicts of interest.
L.H.V. participated in research design, performed experiments, wrote the article, and performed data analysis. L.L.v.L. participated in research design, performed experiments, and participated in writing of the article. R.A.P. performed statistical analysis, made figures, and participated in writing the article. H.v.G. performed histological analysis and participated in writing the article. R.J.P. did supervision and participated in writing the article. P.H. participated in research design and data analysis. T.H. and T.M. participated in writing the article. H.G.D.L. participated in research design, supervision, and the writing article.
Supplemental Visual Abstract; http://links.lww.com/TP/C280.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall IE, Schröppel B, Doshi MD, et al. Discard and function after transplantation. Am J Transplant. 2015;15:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. Am J Transplant. 2007;7:2769–2774. [DOI] [PubMed] [Google Scholar]
- 4.Peters-Sengers H, Homan van der Heide JJ, Heemskerk MBA, et al. Similar 5-year estimated glomerular filtration rate between kidney transplants from uncontrolled and controlled donors after circulatory death-a dutch cohort study. Transplantation. 2017;101:1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 6.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 annual data report: kidney. Am J Transplant. 2018;18(suppl 1):18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosgood SA, Thompson E, Moore T, et al. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br J Surg. 2018;105:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 2013;13:1246–1252. [DOI] [PubMed] [Google Scholar]
- 9.Hosgood SA, Barlow AD, Hunter JP, et al. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg. 2015;102:1433–1440. [DOI] [PubMed] [Google Scholar]
- 10.Hosgood SA, Saeb-Parsy K, Wilson C, et al. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017;7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–1787. [DOI] [PubMed] [Google Scholar]
- 12.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327–1335. [DOI] [PubMed] [Google Scholar]
- 13.Karangwa SA, Burlage LC, Adelmeijer J, et al. Activation of fibrinolysis, but not coagulation, during end-ischemic ex situ normothermic machine perfusion of human donor livers. Transplantation. 2017;101:e42–e48. [DOI] [PubMed] [Google Scholar]
- 14.Watson CJ, Kosmoliaptsis V, Randle LV, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant. 2016;16:353–357. [DOI] [PubMed] [Google Scholar]
- 15.Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int. 2015;28:657–664. [DOI] [PubMed] [Google Scholar]
- 16.Mamikonian LS, Mamo LB, Brian Smith P, et al. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants and children. 2015;15:e111–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matton APM, Burlage LC, van Rijn R, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl. 2018;24:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries Y, Matton APM, Nijsten MWN, et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am J Transplant. 2019;19:1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay MD, Hosgood SA, Harper SJ, et al. Normothermic versus hypothermic ex vivo flush using a novel phosphate-free preservation solution (AQIX) in porcine kidneys. J Surg Res. 2011;171:275–282. [DOI] [PubMed] [Google Scholar]
- 20.Kay MD, Hosgood SA, Harper SJ, et al. Static normothermic preservation of renal allografts using a novel nonphosphate buffered preservation solution. Transpl Int. 2007;20:88–92. [DOI] [PubMed] [Google Scholar]
- 21.Groeneveld AB. Albumin and artificial colloids in fluid management: where does the clinical evidence of their utility stand? Crit Care. 2000;4(suppl 2):S16–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scallan J, Huxley VH, Korthuis RJ. Capillary Fluid Exchange: Regulation, Functions, and Pathology. Morgan and Claypool Publishers; 2010. [PubMed] [Google Scholar]
- 23.Gallinat A, Fox M, Lüer B, et al. Role of pulsatility in hypothermic reconditioning of porcine kidney grafts by machine perfusion after cold storage. Transplantation. 2013;96:538–542. [DOI] [PubMed] [Google Scholar]
- 24.Gallinat A, Lüer B, Swoboda S, et al. Use of the new preservation solution custodiol-N supplemented with dextran for hypothermic machine perfusion of the kidney. Cryobiology. 2013;66:131–135. [DOI] [PubMed] [Google Scholar]
- 25.Venema LH, Brat A, Moers C, et al. ; COPE Consortium. Effects of oxygen during long-term hypothermic machine perfusion in a porcine model of kidney donation after circulatory death. Transplantation. 2019;103:2057–2064. [DOI] [PubMed] [Google Scholar]
- 26.Mahboub P, Ottens P, Seelen M, et al. Gradual rewarming with gradual increase in pressure during machine perfusion after cold static preservation reduces kidney ischemia reperfusion injury. PLoS One. 2015;10:e0143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogland ER, de Vries EE, Christiaans MH, et al. The value of machine perfusion biomarker concentration in DCD kidney transplantations. Transplantation. 2013;95:603–610. [DOI] [PubMed] [Google Scholar]
- 28.Hoeksma D, Rebolledo RA, Hottenrott M, et al. Inadequate antioxidative responses in kidneys of brain-dead rats. Transplantation. 2017;101:746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–857. [DOI] [PubMed] [Google Scholar]
- 30.Kaths JM, Echeverri J, Goldaracena N, et al. Eight-hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment, and repair of kidney grafts. Transplantation. 2016;100:1862–1870. [DOI] [PubMed] [Google Scholar]
- 31.Kaths JM, Cen JY, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant. 2017;17:957–969. [DOI] [PubMed] [Google Scholar]
- 32.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. [DOI] [PubMed] [Google Scholar]
- 33.Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant. 2015;15:993–1002. [DOI] [PubMed] [Google Scholar]
- 34.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144:1200–1206. [DOI] [PubMed] [Google Scholar]
- 35.Aburawi MM, Fontan FM, Karimian N, et al. Synthetic hemoglobin-based oxygen carriers are an acceptable alternative for packed red blood cells in normothermic kidney perfusion. Am J Transplant. 2019;19:2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Horn C, Minor T. Improved approach for normothermic machine perfusion of cold stored kidney grafts. Am J Transl Res. 2018;10:1921–1929. [PMC free article] [PubMed] [Google Scholar]
- 37.Minor T, Sutschet K, Witzke O, et al. Prediction of renal function upon reperfusion by ex situ controlled oxygenated rewarming. Eur J Clin Invest. 2016;46:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minor T, Efferz P, Lüer B. Hypothermic reconditioning by gaseous oxygen persufflation after cold storage of porcine kidneys. Cryobiology. 2012;65:41–44. [DOI] [PubMed] [Google Scholar]
- 39.Gallinat A, Efferz P, Paul A, et al. One or 4 h of “in-house” reconditioning by machine perfusion after cold storage improve reperfusion parameters in porcine kidneys. Transpl Int. 2014;27:1214–1219. [DOI] [PubMed] [Google Scholar]
- 40.Minor T, von Horn C, Paul A. Role of erythrocytes in short-term rewarming kidney perfusion after cold storage. Artif Organs. 2019;43:584–592. [DOI] [PubMed] [Google Scholar]
- 41.Hendriks KDW, Brüggenwirth IMA, Maassen H, et al. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J Transl Med. 2019;17:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen JJ. Relationship between energy requirements for Na+ reabsorption and other renal functions. Kidney Int. 1986;29:32–40. [DOI] [PubMed] [Google Scholar]
- 43.Lassen NA, Lassen U, Munck O, et al. Oxygen consumption and sodium reabsorption by the kidney. Acta Physiol Scand. 1961;51:371–84. [DOI] [PubMed] [Google Scholar]
- 44.Singh P, Ricksten SE, Bragadottir G, et al. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin Exp Pharmacol Physiol. 2013;40:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990;258:C377–C389. [DOI] [PubMed] [Google Scholar]
- 46.Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. 2018;1859:940–950. [DOI] [PubMed] [Google Scholar]
- 47.Jastroch M, Divakaruni AS, Mookerjee S, et al. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel K, Smith TB, Neil DAH, et al. The effects of oxygenation on ex vivo kidneys undergoing hypothermic machine perfusion. Transplantation. 2019;103:314–322. [DOI] [PubMed] [Google Scholar]
- 49.Evans RG, Harrop GK, Ngo JP, et al. Basal renal O2 consumption and the efficiency of O2 utilization for Na+ reabsorption. Am J Physiol Renal Physiol. 2014;306:F551–F560. [DOI] [PubMed] [Google Scholar]
- 50.Mitra S, Khandelwal P. Are all colloids same? How to select the right colloid? Indian J Anaesth. 2009;53:592–607. [PMC free article] [PubMed] [Google Scholar]
- 51.Feest TG. Low molecular weight dextran: a continuing cause of acute renal failure. Br Med J. 1976;2:1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters-Sengers H, Houtzager JHE, Heemskerk MBA, et al. DCD donor hemodynamics as predictor of outcome after kidney transplantation. Am J Transplant. 2018;18:1966–1976. [DOI] [PubMed] [Google Scholar]
- 53.Maassen H, Hendriks KDW, Venema LH, et al. Hydrogen sulphide-induced hypometabolism in human-sized porcine kidneys. PLoS One. 2019;14:e0225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pool M, Eertman T, Parrage JS, et al. Infusing mesenchymal stromal cells into porcine kidneys during normothermic machine perfusion: intact MSCs can be traced and localised to glomeruli. Int J Mol Sci. 2019;20:3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huijink TM, Venema LH, Posma RA, et al. Metformin preconditioning and postconditioning to reduce ischemia reperfusion injury in an isolated ex vivo rat and porcine kidney normothermic machine perfusion model. Clin Transl Sci. 2021;14:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Posma RA, Venema LH, Huijink TM, et al. Increasing metformin concentrations and its excretion in both rat and porcine ex vivo normothermic kidney perfusion model. BMJ Open Diabetes Res Care. 2020;8:e000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.