Abstract
Background
Solid tumour perfusion can be unstable, creating transiently hypoxic cells that can contribute to radiation resistance. We investigated the in vivo lifetime of transiently hypoxic tumour cells and chronically hypoxic tumour cells during tumour growth and following irradiation.
Methods
Hypoxic cells in SiHa and WiDr human tumour xenografts were labelled using pimonidazole and EF5, and turnover was quantified as the loss of labelled cells over time. The perfusion-modifying drug pentoxifylline was used to reoxygenate transiently hypoxic cells prior to hypoxia marker administration or irradiation.
Results
Chronically hypoxic cells constantly turnover in SiHa and WiDr tumours, with half-lives ranging from 42–82 h and significant numbers surviving >96 h. Transiently hypoxic cells constitute 26% of the total hypoxic cells in WiDr tumours. These transiently hypoxic cells survive at least 24 h, but then rapidly turnover with a half-life of 34 h and are undetectable 72 h after labelling. Transiently hypoxic cells are radiation-resistant, although vascular dysfunction induced by 10 Gy of ionising radiation preferentially kills transiently hypoxic cells.
Conclusions
Transiently hypoxic tumour cells survive up to 72 h in WiDr tumours and are radiation-resistant, although transiently hypoxic cells are sensitive to vascular dysfunction induced by high doses of ionising radiation.
Subject terms: Cancer microenvironment, Tumour heterogeneity, Radiotherapy
Introduction
Solid tumours often develop regions with low oxygen content (i.e. hypoxia), and patients with tumours that contain high proportions of hypoxic cells have the worse therapeutic outcome. Hypoxic tumour cells are resistant to treatment with ionising radiation, and the presence of hypoxic cells in solid tumours is correlated with reduced local control after radiotherapy and with decreased disease-free and overall survival [1–7]. Patients with hypoxic primary tumours also have increased metastases [2, 8–10] and worse outcome after surgical resection of the primary tumour [2], indicating that hypoxia promotes a more aggressively metastatic tumour phenotype [11, 12].
Tumour hypoxia develops due to vascular and microenvironmental deficiencies in oxygen delivery relative to the high oxygen demand by metabolically active tumour cells. “Chronic” or “diffusion-limited” hypoxia occurs due to the proliferation of cells near tumour blood vessels forcing adjacent tumour cells further away from the blood supply. Oxygen consumption by proliferating tumour cells reduces the amount of oxygen available for diffusion deeper into tumour tissue [13, 14], with chronically hypoxic cells being located 70–150 µm away from functional tumour blood vessels (beyond the diffusion distance of oxygen). Chronically hypoxic cells are often adjacent to areas of necrosis, where oxygen and nutrient availability limit tumour cell survival [15–19]. While chronically hypoxic cells exhibit reduced or halted proliferation [16, 20], division of better-oxygenated cells pushes daughter cells radially outwards from perfused blood vessels, leading to downstream cells being pushed into regions of chronic hypoxia and pushing chronically hypoxic cells into areas of necrosis [16, 21–23]. New chronically hypoxic cells are therefore generated as existing chronically hypoxic cells die, with the majority of chronically hypoxic cells surviving for 24–48 h [21, 22].
Tumour blood vessels can also be poorly functional and prone to compression by high interstitial fluid pressure [24, 25] and/or collagen-rich extracellular matrix in the tumour [26–29]. Vascular compression combined with aggregation of erythrocytes and leukocytes can severely restrict blood flow and oxygen delivery to portions of the tumour [30–32]. Blood flow through tumour vasculature can be unstable [33], with periods of temporary flow reversal or stasis [34, 35] changing oxygen delivery over time and creating regions of “transient”, “acute” or “perfusion-limited” hypoxia [36]. Unstable tumour perfusion has been observed using fluorescent markers of vessel function in murine tumours [37, 38] or laser doppler flowmetry to monitor erythrocyte flux combined with oxygen probes in rat tumours [39], canine tumours [40] and human tumours [41]. Non-invasive imaging modalities including intravital imaging [42, 43], magnetic resonance imaging [44–46] and optoacoustic imaging [47] also demonstrate fluctuations in tumour perfusion and oxygenation over time in several tumour types. These studies illustrate remarkable heterogeneity in tumour perfusion and oxygenation fluctuations over time, with transient hypoxic periods lasting anywhere from a few minutes to over an hour, and indicate that tumour regions can be exposed to repeated cycles of hypoxia and reoxygenation over time [48]. Transiently hypoxic tumour cells are important contributors to radiation resistance [49–54] with ramifications for local-regional control of solid tumours. Cells exposed to cycles of hypoxia and reoxygenation in vitro and in vivo have increased expression of metastasis-associated genes [55] and increased metastatic growth compared to chronically hypoxic cells [56, 57]. These data suggest that transiently hypoxic tumour cells are important contributors to the known association between tumour hypoxia and poor patient outcome [50], although the lifetime of transiently hypoxic tumour cells in vivo is currently unknown.
Hypoxia markers, such as pimonidazole [58] and EF5 [59], have been used for decades to quantify hypoxia across a wide range of pre-clinical tumour models and in the clinic. Rather than providing an instantaneous snapshot of tumour hypoxia, the circulation half-lives of pimonidazole and EF5 (30 and 40 m, respectively, in mice [60, 61]) produce time-integrated cumulative measurements of hypoxic cells, including chronically hypoxic cells and cells that are transiently hypoxic during the circulation and labeling time of the drug. Distinguishing between chronically and transiently hypoxic tumour cells, therefore, requires sequential administration of different hypoxia markers [62] and/or the use of perfusion-modifying drugs such as pentoxifylline to temporarily stabilise oxygen delivery and reduce hypoxia marker labeling in transiently hypoxic cells [49, 63]. Pentoxifylline is a hemorheological drug that increases erythrocyte deformability [64], reduces blood viscosity and increases blood flow through tortuous and constricted vasculature that can otherwise cause transient hypoxia. Pentoxifylline does not increase blood oxygen content [65, 66] suggesting the drug has minimal influence on oxygen delivery to chronically hypoxic cells. Indeed, we have previously shown that pentoxifylline stabilises microregional tumour perfusion and temporarily reduces transient hypoxia [49, 63] without affecting chronic hypoxia. Pentoxifylline is therefore a useful tool to distinguish between the lifetime of chronically hypoxic and transiently hypoxic tumour cells in vivo.
We, and others, have found that transiently hypoxic cells exist more proximal to tumour blood vessels than chronically hypoxic cells [20, 49, 62]. We, therefore, hypothesised that transiently hypoxic cells may live longer than chronically hypoxic cells, and that transiently hypoxic cells may be sensitive to vascular destruction induced by high doses of radiation [67, 68]. Herein we show that chronically hypoxic cells in our xenograft models constantly turnover, with significant numbers dying within 24 h while some chronically hypoxic cells can survive >96 h. Transiently hypoxic cells survive for at least 24 h after labeling, followed by complete turnover over the next 48 h. We also show that transiently hypoxic cells survive ionising radiation similarly to chronically hypoxic cells, although are susceptible to large single doses of radiation that induce vascular dysfunction. Overall, our data highlight the importance of identifying transient hypoxia in the clinic and developing therapeutic strategies to target transiently hypoxic tumour cells to improve patient outcome.
Methods
Cells and mice
Ten-week-old male NOD/SCID-gamma mice were housed under specific pathogen-free conditions. Mice were acclimatised to housing rooms for at least 2 weeks prior to initiating experimental procedures. WiDr [69] human colon adenocarcinoma and SiHa [70] human cervical squamous cell carcinoma cell lines were maintained in minimal essential medium + 10% fetal bovine serum and used within 25 passages. Cells were received from ATCC and tested negative for mycoplasma by PCR test prior to initiating in vivo and in vitro experiments. Flank tumours were produced by subcutaneous injection of 106 cells in 100 μl of filter-sterilised phosphate-buffered saline. All experiments were performed in accordance with Institutional and Canadian Council on Animal Care guidelines. For irradiation, mice were fixed in a lead jig so only the tumour was subjected to x-irradiation (250 keV; 3 Gy/min; X-RAD320, Precision X-ray). Excised tumours were cut in half, with half embedded in optimal cutting temperature medium (Tissue Tek) and frozen for sectioning and microscopy, and the other half processed into single-cell suspension for flow cytometry and/or clonogenic assays as previously described [49, 62, 63].
For in vitro experiments, cells were cultured at 37 °C, 5% CO2 in either standard cell culture incubators (ThermoFisher Scientific), a hypoxia chamber (Coy Lab Products) maintained at 37 °C, 5% CO2, 1% O2, or in a 37 °C benchtop incubator for cycling hypoxia experiments. For the latter, cells were plated in T25 flasks (Corning) and individually gassed with variable O2/5% CO2/balance N2 (Praxair). To ensure rapid oxygen equilibration, culture flasks were on a table-top rocker within the incubator. O2 concentrations were measured in parallel flasks with contactless optical oxygen sensor spots and a FireStingO2 (FSO2–2) meter (Pyroscience); O2 tensions were within 5% of the target O2 tension within 5 m. Trypsinised cells were counted using a Z2 Cell and Particle Counter (Beckman). Growth rates of cultured tumour cells were calculated as:
where gr is the growth rate, Ni and Nf are the initial and final numbers of cells, respectively, and t is the time elapsed (hours). Doubling times were calculated as ln(2)/gr.
Chemicals
Pentoxifylline (50 mg/kg; Millipore Sigma), pimonidazole (100 mg/kg; Hypoxyprobe) and/or EF5 (60 mg/kg; Millipore Sigma) were administered by intraperitoneal injection. Intravenous injection of 1 mg Hoechst 33342 10 m prior to mouse sacrifice was used to label cells surrounding perfused blood vessels [37, 71]. For in vitro experiments, cells were labelled with 200 μM pimonidazole in media for three hours at 1% O2. The cyclin-dependent kinase (CDK) 4/6 inhibitors abemaciclib (10 μM; MedChemExpress) and palbociclib (50 μM; MedChemExpress) were used to induce G1 cell cycle arrest in vitro.
Immunofluorescent microscopy and flow cytometry
For microscopy experiments, three 10 μm thick sections were cut from OCT-embedded frozen tumours, with each section being >200 μm apart to ensure representative sampling of the tumour. Pimonidazole was detected using a monoclonal antibody (Hypoxyprobe FITC-Mab) conjugated to fluorescein isothiocyanate (FITC). EF5 was detected using a Cy5-conjugated ELK3–51 antibody (Millipore Sigma EF5011). Slides were stained for CD31 (BD Pharmingen 553370) or carbonic anhydrase IX (CAIX, Novus Biologicals NB100–417) with Alexa 594 secondary antibody (Life Technologies A-11012), and with 2 μM 4,6-diamidino-2-phenyl-indole (DAPI; Sigma) to label cell nuclei. Tumour sections were imaged as previously described [63]. Briefly, immunofluorescent images were captured using a robotic Zeiss Axiomager Z1 fluorescence microscope with a cooled monochrome Retiga 4000 R CCD camera and a motorised slide loader and x-y stage. Regions of tumour tissue that did not retain integrity during the sectioning process (whether due to necrosis or technical issues) were excluded prior to quantitative analyses using ImageJ macros developed in-house.
For flow cytometry-based cell cycle analyses, permeabilised cells were labelled with 100 μM propidium iodide to measure DNA content. To track cell divisions, cell cultures were exposed to 10 μM CellTrace Yellow (CTY; ThermoFisher Scientific) in phosphate-buffered saline for 20 m. Flow cytometry analyses were conducted using a Fortessa (BD Biosciences) flow cytometer with FlowJo 7.6 software (Tree Star).
Statistical analysis
Statistics were performed using Graphpad Prism 8 software with p < 0.05 considered significant. Specific statistical tests are listed in figure legends. Data were assessed for normality using the Shapiro-Wilk test, which determined whether groups would be compared using parametric or non-parametric statistical tests. Data are presented as mean with variation as defined in figure legends. Data were tested for an equal variance before the selection of statistical tests. Replicates for in vitro experiments represent data from independent experimental repeats run on different days; replicates for in vivo experiments represent individual tumours grown in separate mice. Mice were randomly assigned to parallel experimental treatment groups at the time of tumour implant, equally dividing groups among cage mates. No tumour-bearing animals were excluded from our analyses, and researchers were not blinded to group allocations during the experiments. Sample sizes were selected based on previous work using these tumour models for similar experimental procedures [49, 63].
Results
We have previously found that WiDr tumour xenografts exhibit microregional perfusion fluctuations and transient hypoxia, while microregional perfusion and tumour oxygenation are comparatively stable in SiHa tumours [49, 62, 63, 72]. We, therefore, used these two tumour lines for our study. The hypoxic fraction of solid tumours can increase during the early stages of tumour growth, so we first assessed EF5 labelling of hypoxic cells in WiDr and SiHa tumours in order to identify tumour volumes that contained consistent fractions of hypoxic cells. For SiHa tumours, we found that the fraction of tumour labelled by EF5 was consistent between 21 and 29 days of tumour growth (Fig. 1a, b), while the EF5-positive hypoxic fraction of WiDr tumours was consistent between 26 and 35 days of tumour growth (Fig. 1c, d). Subsequent experiments were therefore conducted on SiHa and WiDr tumours harvested 29 and 32 days post-implant, respectively.
Fig. 1. Pimonidazole positive tumour area decreases with time.
a SiHa EF5+ tumour area and b mass versus time post-implant. c WiDr EF5+ tumour area and d mass versus time post-implant. Data points are individual tumours with n = 11–29, mean ± 95% CI, Tukey multiple comparisons test with p < 0.01 for a vs b in c; no significant differences in a. Retrospective data were analyzed for these panels. e Timeline of in vivo experiments. f Representative images of WiDr and SiHa tumours for indicated times between pimonidazole injection and tumour harvest. DAPI (nuclei) is blue, pimonidazole (Pimo) is green, EF5 is red, overlap of Pimo/EF5 is yellow. Sample regions singly labelled by EF5 are indicated by ‘>’, regions of poor tissue integrity (due to tumour necrosis or sectioning issues) are outlined with dashed lines and indicated by ‘#’, scale bars = 500 μm. g Total EF5+ area for SiHa and h WiDr tumours. i SiHa tumour area that was Pimo−EF5+ or j Pimo+EF5− over time. k WiDr tumour area that was Pimo−EF5+ or l Pimo+EF5− over time. m Total Pimo+ area for SiHa and n WiDr tumours. N = 9–12, mean ± 95% CI, Tukey multiple comparisons test with p < 0.05 for a vs b, p < 0.001 for a vs c, p < 0.01 for b vs c. o Decrease in Pimo+ area relative to EF5+ area over time. Total EF5+ hypoxic fractions for SiHa (dashed line) and WiDr (dotted line) shown for reference, n = 9–12, mean ± 95% CI. Data are combined from 3 independent experiments.
To investigate hypoxic cell turnover in vivo, tumour-bearing mice were administered pimonidazole (Pimo) between 96 h and 3 h prior to tumour harvest with EF5 given 3 h prior to harvest (Fig. 1e). Representative images are shown in Fig. 1f, with tumour areas labelled with Pimo in green, with EF5 in red, both Pimo and EF5 in yellow, and DAPI in blue. As expected, the EF5+ hypoxic fraction was consistent within tumour types irrespective of the time between Pimo injection and tumour harvest (Fig. 1f–h). We observed strong agreement between co-administered Pimo and EF5 (Fig. 1f), with only 4–6% of viable tumour area singly labelled for Pimo or EF5 in either WiDr or SiHa tumours (Fig. 1i–l). We found that 30–40% of WiDr and SiHa tumour cells label with Pimo or EF5 after a 3 h labeling period.
The proportion of SiHa and WiDr tumour area labeled with Pimo decreased with time after Pimo injection (Fig. 1f, m, n). The proportion of tumour area singly labelled by Pimo remained unchanged or decreased with time in SiHa and WiDr tumours (Fig. 1j, l), indicating that surviving Pimo-labelled tumour cells were likely to remain hypoxic (and therefore label with subsequent EF5). Consistently, singly labelled EF5+ tumour cells increased with time between Pimo and EF5 administration in both SiHa and WiDr tumours (Fig. 1i, k) despite no change in the total EF5+ hypoxic fraction within tumour types (Fig. 1g, h). Collectively, these data indicate that during a 96-hour period of tumour growth with a stable total hypoxic fraction, tumour cells labelled by a single injection of Pimo decrease over time.
Using EF5 as a baseline hypoxic fraction, we quantified the amount of Pimo+ tumour area that was lost over time (Fig. 1o). Our data indicate the half-life of Pimo-labelled hypoxic cells was approximately 42 ± 3 h in SiHa tumours and 54 ± 9 h (mean ± SEM) in WiDr tumours. Notably, the decrease in Pimo area over time was not equivalent to the total EF5+ hypoxic fraction in WiDr or SiHa tumours (Fig. 1o; dashed/dotted lines), indicating that some proportion of Pimo-labeled hypoxic cells was detectable 96 h later in both tumour types.
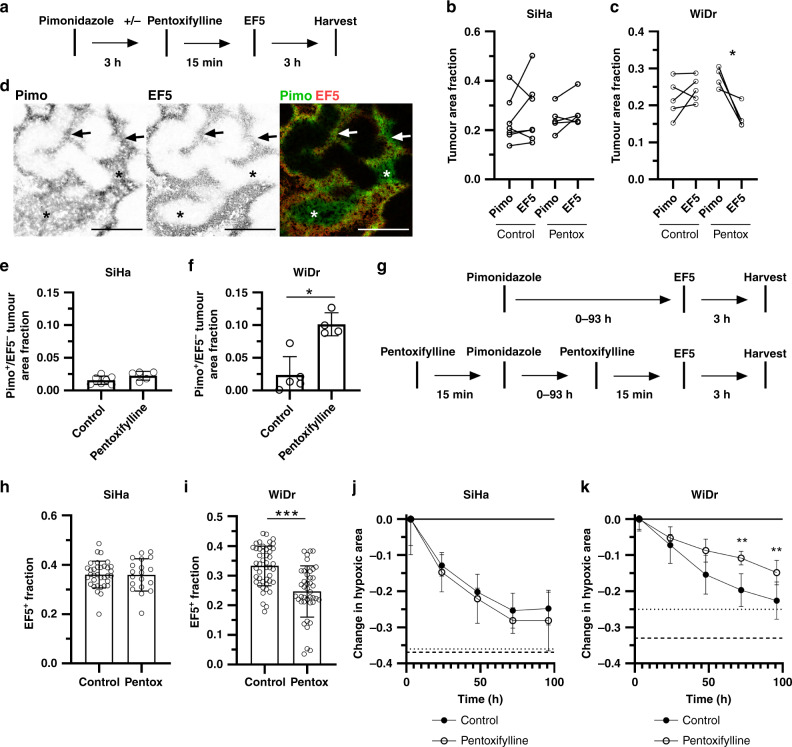
We were next interested in distinguishing between the loss of chronically hypoxic and transiently hypoxic cells in these models. We have previously shown that pentoxifylline temporarily stabilises microregional tumour perfusion and reoxygenates transiently hypoxic cells in WiDr tumours within 15 m of injection [49, 63]. To validate that pentoxifylline reoxygenates transiently hypoxic tumour cells, we administered Pimo to label both chronically and transiently hypoxic tumour cells followed by pentoxifylline to temporarily reoxygenate transiently hypoxic tumour cells prior to EF5 labeling (Fig. 2a). Pentoxifylline significantly reduced the EF5+ hypoxic fraction of WiDr tumours, but not SiHa tumours (Fig. 2b, c), consistent with our previous work [25, 26]. EF5 labeling indicative of chronic hypoxia was not affected by pentoxifylline (Fig. 2d, arrows). Accordingly, pentoxifylline increased the fraction of Pimo+EF5- tumour area in WiDr tumours, but not in SiHa tumours (Fig. 2e, f). In WiDr tumours, we observed distinct Pimo+ hypoxic tumour regions that were EF5- after pentoxifylline treatment (Fig. 2d, stars), indicating that pentoxifylline-mediated stabilisation of microregional perfusion reoxygenated cells in these regions. Taken together, these data confirm that a single hypoxia marker injection can label tumour cells that are chronically and transiently hypoxic, and that tumour cells labeled with hypoxia markers after pentoxifylline treatment represent chronically hypoxic cells. Differences in hypoxia marker labeling between control and pentoxifylline-treated tumours, therefore, reflect the amount of transiently hypoxic cells present in the tumour.
Fig. 2. Pentoxifylline induces 2-nitroimidazole mismatch and reveals chronically and transiently hypoxic cell turnover.
a Experimental timeline. b Paired analysis of Pimo+ and EF5+ tumour area in control and pentoxifylline-treated mice with SiHa tumours or c WiDr tumours. N = 4–5, paired Student’s t-test, *p < 0.05. d Representative images of a WiDr tumour treated with pentoxifylline prior to EF5 injection. Pimo is green, EF5 is red, overlap of Pimo/EF5 is yellow, scale bars = 500 μm. Arrows indicate regions of chronic hypoxia; stars indicate transiently hypoxic regions reoxygenated by pentoxifylline prior to EF5 injection. e Pimo+EF5- area in control or pentoxifylline-treated mice with SiHa tumours or (F) WiDr tumours. N = 4–5, mean ± SD, Mann–Whitney test, *p < 0.05. g Experimental timelines for control and pentoxifylline treatment groups. h EF5+ area in control or pentoxifylline-treated mice with SiHa tumours or i WiDr tumours. N = 19–46, mean ± 95% CI, Mann–Whitney test, ***p < 0.001. j Decrease in Pimo+ area relative to EF5+ area over time in control or pentoxifylline-treated mice with SiHa tumours or k WiDr tumours. Total EF5+ hypoxic fractions for control (dashed line) and pentoxifylline-treated mice (dotted line) shown for reference. N = 9–12, mean ± 95% CI, Sidak multiple comparisons test between control and pentoxifylline time points, **p < 0.01. Data are combined from 3 independent experiments.
The data in Fig. 1o indicate the turnover of total hypoxic tumour cells, and we, therefore, incorporated pentoxifylline prior to Pimo and EF5 injections (Fig. 2g) in order to quantify the specific turnover of chronically hypoxic tumour cells. Pentoxifylline had no effect on the EF5+ hypoxic fraction of SiHa tumours (Fig. 2h), but we observed a 26% decrease in EF5+ area of WiDr tumours (Fig. 2i). These data indicate that transiently hypoxic tumour cells are not present in SiHa tumours and constitute approximately 26% of the hypoxic fraction in WiDr tumours, consistent with our previous work [49, 62, 63, 72]. Pentoxifylline did not affect the loss of Pimo+ tumour area over time in SiHa tumours (Fig. 2j), indicating the curves in Fig. 1o and 2j represent turnover of chronically hypoxic cells in SiHa tumours (with a half-life of 42 ± 3 hr). Interestingly, when pentoxifylline was used to prevent Pimo and EF5 from labeling transiently hypoxic cells in WiDr tumours, we found significant changes in turnover between chronically hypoxic cells (Fig. 2k; open circles) and total hypoxic cells (black circles; chronically and transiently hypoxic cells). Firstly, the turnover of Pimo+ cells within the first 24 h of labeling was unaffected by pentoxifylline, indicating chronically hypoxic tumour cells are primarily turning over within the first 24 h. Chronically hypoxic cells continue to turnover while the separation between the curves from 48–96 h indicates loss of transiently hypoxic cells over time. Indeed, since transiently hypoxic cells make up 26% of the total hypoxic fraction in WiDr tumours, the difference between the two curves in Fig. 2k suggests that transiently hypoxic tumour cells are lost within 72 h of Pimo labelling. Based on the rates of cell loss over time, we calculated the half-life of chronically hypoxic WiDr tumour cells as 82 ± 6 h and the half-life of transiently hypoxic WiDr tumour cells as 34 ± 9 h. Overall, these data suggest that transiently hypoxic cells in WiDr tumours survive for 24 h after Pimo labelling, but are no longer detectable by 72 h after labelling.
Our conclusions regarding hypoxic cell turnover are dependent upon loss of Pimo+ cells occurring primarily due to cell death rather than loss or dilution of Pimo adducts over time in viable cells. We, therefore, investigated the rate of Pimo loss over time in vitro under different oxygenation conditions. To model proliferation-associated dilution of Pimo staining intensity, synchronised cells were labeled with Pimo at 1% O2 for 3 h, followed by labelling with CTY to track cell divisions over time at 21% O2, 1% O2 or 0.5% O2. WiDr and SiHa CTY mean fluorescence intensity (MFI) decreased over time in both 21% O2 and 1% O2 (Fig. 3a, b), indicating ongoing cellular proliferation. WiDr and SiHa CTY MFI did not significantly change from 24–72 h of culture in 0.5% O2 (Fig. 3a, b), consistent with severe hypoxia inducing cell cycle arrest and in vivo data indicating that hypoxic cells are poorly proliferative [16, 19, 23]. Pimo MFI decreased over time in WiDr and SiHa cells cultured in either 21% O2 or 1% O2, although fluorescence intensity remained significantly greater than control cells (antibody-only; no Pimo) for up to 72 h at 21% O2 and 96 h at both 1% O2 and 0.5% O2 (Fig. 3c, d).
Fig. 3. Hypoxia reduces tumour cell proliferation and loss of pimonidazole staining intensity.
WiDr or SiHa cells were labelled with Pimo at 1% O2 for 3 h and CTY for 20 m before culture in 21% O2, 1% O2 or 0.5% O2. a CTY mean fluorescent intensity (MFI) for WiDr cells and b SiHa cells over time. CTY data are log2-transformed so that a 50% reduction in CTY MFI as cells divide can be more readily observed. N = 3 independent repeats, mean ± SD, Sidak’s multiple comparisons test, *p < 0.05, **p < 0.01, ***p < 0.001. c Pimo MFI for WiDr cells and d SiHa cells over time. N = 3 independent repeats, mean ± SD, Dunnett’s multiple comparison test comparing each time point to the antibody-only (no Pimo) control, *p < 0.05, **p < 0.01, ***p < 0.001. e WiDr cells were labeled with Pimo and CTY prior to treatment with either 10 μM abemaciclib or 50 μM palbociclib at 21% O2 or f 1% O2. Linear regression lines are shown. N = 3 independent repeats, mean ± SEM, Dunnett’s multiple comparison test comparing each group to DMSO control, ***p < 0.001, *p < 0.05. g WiDr and h SiHa cells were cultured in 5% O2, 1% O2, 0.5% O2 or cycles of 25 m at 5% O2 and 25 m at 0.5% O2 for 48 h. Growth rates (exponential change in cell number per hour) were calculated based on cell numbers quantified by automated cell counting. N = 6, mean ± SD, Tukey multiple comparisons test, *p < 0.05, **p < 0.01, ***p < 0.001. Data are from 1 experimental day.
To confirm that proliferation was the primary mechanism for Pimo MFI loss over time in cells cultured in 21% O2 and 1% O2, we treated Pimo and CTY labelled cells with the CDK inhibitors abemaciclib or palbociclib. Neither drug affected cell viability compared to dimethyl sulfoxide controls (Supplemental Fig. 1), and both abemaciclib and palbociclib reduced the proportions of cells in S/G2/M phase for 24–96 h in both 21% O2 and 1% O2 (Supplemental Fig. 2). Both abemaciclib and palbociclib significantly reduced the rate of Pimo MFI loss over time in cells at 21% O2 and 1% O2 (Fig. 3e, f). These data indicate that proliferation is the major mechanism of Pimo MFI loss over time in WiDr cells and that Pimo MFI is comparably stable in non-proliferating cells. While these data show that tumour cell proliferation reduces Pimo MFI over time, it is important to note that Pimo fluorescence intensity remains greater than background levels for 72–96 h (~3–4 cell divisions) whether or not cells are cultured in hypoxic conditions or are allowed to fully reoxygenate. Since Pimo labelling intensity in vivo is highest in cells below 0.5% oxygen [73, 74], and tumour cells are rarely dual labelled by simultaneously administered Pimo and the proliferation marker iodo-deoxyuridine [20], it is unlikely that chronically hypoxic tumour cells will undergo enough cell divisions within 96 h of Pimo labelling in vivo to dilute Pimo fluorescence below detection levels.
We next tested the effect of transient hypoxia on tumour cell proliferation. Cell cultures were cycled between 25 m of 5% O2 and 25 m of 0.5% O2 for a total of 48 h, consistent with timeframes of microregional perfusion fluctuations observed in vivo [37–39, 41, 75–78]. We found that the growth rate of cells (i.e., exponential growth of the cell population per hour) exposed to cycles of hypoxia and reoxygenation were intermediate between the growth rates of cells cultured in 5% and 0.5% O2 alone (Fig. 3g, h). Based on the growth rates, the population doubling times were 23–26 h for WiDr and SiHa tumour cells at 21% or 1% O2, 86 h for SiHa cells at 0.5% O2 and 75 h for cells cycling between 5% and 0.5% O2. These data indicate that WiDr tumour cells are only proliferative at oxygen tensions above 0.5% O2, and that cells cycling between 0.5% O2 and 5% O2 have significantly reduced proliferative rates. Taken together, the data in Fig. 3 indicate that Pimo labeling intensity can be reduced by cellular proliferation in vitro, but our data also indicate that even in the presence of continued proliferation, Pimo staining intensity in formerly hypoxic cells remains above background levels for 72–96 h (3–4 cell divisions). Since both chronically and transiently hypoxic cells have significantly reduced proliferation, and proliferation of oxygenated tumour cells in vivo pushes downstream hypoxic cells further from functional blood vessels over time into regions of more severe hypoxia and therefore progressively less supportive of proliferation [21, 22, 79, 80], we conclude that proliferation-associated dilution of Pimo adducts does not interfere with our ability to detect Pimo+ tumour cells in vivo within 96 h of labelling. The data in Figs. 1 and 2, therefore, reflect the lifetime of chronically and transiently hypoxic tumour cells in our xenograft models.
A major therapeutic implication of tumour hypoxia is the survival of hypoxic tumour cells following irradiation, and transiently hypoxic cells limit the radiation response of WiDr tumours [49, 63]. In addition to directly killing tumour cells in vivo, ionising radiation doses greater than ~8 Gy may also cause endothelial cell apoptosis and loss of vascular function that can indirectly kill additional tumour cells over time [67, 68]. We were interested in quantifying the response of chronically hypoxic and transiently hypoxic tumour cells to direct and indirect cell killing following irradiation. WiDr tumours were irradiated with a single dose of either 5 or 10 Gy, or with two doses of 5 Gy administered 6 h apart to allow repair of DNA damage induced by the first dose. We harvested tumours 3 h after irradiation to quantify direct radiation-induced cell kill, and 27 h after irradiation as an indication of direct and indirect (i.e. vascular damage-associated) cell kill.
To investigate how the WiDr tumour microenvironment responds to each irradiation protocol, we quantified the density of CD31+ tumour blood vessels that were labelled with intravenously injected Hoechst 33342 as an indicator of microregional vessel function. Neither 5 Gy nor 2×5 Gy affected the CD31+ vascular density (Fig. 4a), the density of functional perfused tumour vasculature (Fig. 4b), or the EF5+ hypoxic areas (Fig. 4c) in tumours 3 or 27 h after irradiation. However, we observed significant reductions in vascular density and Hoechst 33342+ functional blood vessels (Fig. 4a, b, d), and a concomitant increase in EF5+ hypoxic areas (Fig. 4c, d), in tumours harvested 27 h following 10 Gy irradiation compared to tumours harvested 3 h after 10 Gy irradiation. These data support previously published work indicating that larger doses of x-irradiation can impair vascular function [67, 68]. Taken together, these data indicate that a single dose of 10 Gy x-irradiation impairs vascular function and increases hypoxia in WiDr tumours 27 h after treatment, while 5 Gy and 2 × 5 Gy doses do not.
Fig. 4. Hypoxic cell response to direct and indirect effects of ionising radiation.
a Density of CD31+ blood vessels, b density of perfused Hoechst 33342+ CD31+ blood vessels and c fraction of tumour area positive for EF5 in WiDr tumours 3 or 27 h after irradiation with 5, 2 × 5 or 10 Gy. d Representative images of WiDr tumours 3 or 27 h after 10 Gy. CD31 is red, Hoechst 33342 is blue, EF5 is green, scale bars = 500 μm. Arrow heads are Hoechst negative, EF5+ blood vessels. e Clonogenic survival of cells from WiDr tumours 3 or 27 h after irradiation with 5 Gy, f 2 × 5 Gy or g 10 Gy. Mice were treated with PBS (control; black circles) or pentoxifylline (white circles) 15 m prior to irradiation. Clonogenic data are corrected for plating efficiency from 5 non-irradiated control or pentoxifylline-treated tumours. N = 5, mean ± SD, Tukey multiple comparisons test with a vs b or b vs c p < 0.05, a vs c p < 0.001. Data are from 1 experimental day.
We next quantified clonogenic survival of tumour cells isolated from irradiated tumours. For WiDr tumours administered 5 Gy or 2 × 5 Gy, clonogenic survival was similar whether tumours were harvested 3 h or 27 h post-irradiation (Fig. 4e-f; black circles). Tumours irradiated with 10 Gy had lower surviving fractions than either 5 Gy or 2 × 5 Gy, and the surviving fraction dropped from 0.101 ± 0.004 to 0.050 ± 0.010 (mean ± SEM) from 3 to 27 h after 10 Gy irradiation (Fig. 4g; black circles). These data indicate increased tumour cell death with time after 10 Gy irradiation, consistent with vascular impairment 27 h following 10 Gy (Fig. 4b–d).
We also used pentoxifylline 15 m prior to irradiation [49, 63] to reoxygenate transiently hypoxic cells during the radiation dose(s). Increasing microregional tumour perfusion with pentoxifylline prior to irradiation did not affect the vascular function or hypoxia assessed 3 or 27 h after any radiation treatment (data not shown). We found that pentoxifylline reduced tumour cell survival 3 h after irradiation by ~28%, 35% and 30% for tumours irradiated with 5, 2 × 5 and 10 Gy, respectively (Fig. 4e–g; white circles), validating that transiently hypoxic cells are resistant to direct radiation cell killing [49, 63]. Interestingly, WiDr tumour cell survival 27 h after 10 Gy was not further decreased by treating the mice with pentoxifylline prior to irradiation (Fig. 4g), indicating no difference in survival between chronically hypoxic cells (white circles) and a mixed population of chronically and transiently hypoxic cells (black circles). These data suggest that transiently hypoxic cells did not survive the vascular damage induced by 10 Gy irradiation. Taken together, our data indicate that transiently hypoxic WiDr tumour cells are resistant to direct cell killing induced by 5, 2 × 5 and 10 Gy irradiation, that 10 Gy induces vascular dysfunction in WiDr tumours that increases tumour cell death over time, and that transiently hypoxic tumour cells are sensitive to radiation-induced vascular damage.
Discussion
Transient (or acute) hypoxia was first described decades ago [36], and since that time several studies have expanded our understanding of the heterogeneity of transient tumour perfusion and hypoxia. Transient changes in tumour blood flow and oxygenation can occur in the order of minutes, to hours, or even days in vivo [48]. Changes in microregional perfusion and erythrocyte flux can occur over minutes to hours, and are exacerbated by blood vessel tortuosity and compression of vasculature that restricts blood flow. Vascular remodeling can also cause blood flow and oxygenation changes over longer timeframes. The propensity of transient hypoxia seems to depend on the tumour type, with some tumour models exhibiting transient perfusion and hypoxia while other models have relatively stable perfusion and hypoxia over time. For example, previous studies have shown that microregional perfusion in WiDr tumours can change over 20 m intervals, while SiHa tumours have stable perfusion over similar time periods [72]. Hypoxia markers such as pimonidazole or EF5 continually label hypoxic cells during the circulation and binding times of the drugs, thereby providing time-integrated measurements of tumour hypoxia. We have previously shown that 1–2 injections of pimonidazole are sufficient to label virtually all chronically and transiently hypoxic cells in WiDr tumours [62], indicating that hypoxia markers can be used to provide cumulative measurements of transient hypoxia induced by relatively frequent changes in perfusion. While hypoxia markers are not able to capture real-time changes in hypoxia over time, hypoxia markers enable tracking of labeled hypoxic cells to assess survival and response to therapy.
In the current study, we found that the half-life of pimonidazole-labelled hypoxic cells was 42 h in SiHa tumours and 54 h in WiDr tumours. Chronically hypoxic cells were constantly turning over in both tumour types, with significant turnover in the first 24 h after labeling and yet a significant proportion of hypoxic cells surviving 96 h. Using pentoxifylline to discriminate between chronically and transiently hypoxic cells, we found that 26% of the hypoxic cells in WiDr tumours were transiently hypoxic, and that chronically hypoxic cells have a longer half-life than transiently hypoxic cells in WiDr tumours. Transiently hypoxic cells survived the first 24 h after labelling with pimonidazole, but were reduced to undetectable levels 72 h after labelling, with a half-life of 34 h. These data indicate relatively rapid loss of transiently hypoxic cells within a 48 h period, while chronically hypoxic cells died with comparably slower kinetics. Transiently hypoxic cells are located closer to vasculature than chronically hypoxic cells in WiDr tumours [62], although it is unclear from our data whether transiently hypoxic cells die in place or whether these cells are pushed into regions of chronic hypoxia and necrosis within 72 h. The development of methodologies or markers to specifically label transiently hypoxic tumour cells would help to further improve our understanding of transiently hypoxic cell turnover in vivo.
We interpreted the loss of pimonidazole-labelled tumour cells over time in vivo to indicate cell death, although intracellular macromolecule-bound pimonidazole adducts can be diluted during the proliferation of viable cells. It was therefore important to model proliferation-associated dilution of pimonidazole adducts under optimal growth conditions (21% O2), hypoxic conditions, and transiently hypoxic conditions to determine whether pimonidazole labeling intensity could be diluted below detection levels. We found that pimonidazole is indeed lost over time in WiDr and SiHa cells cultured in either 21% O2 or 1% O2 in vitro, but that labeling intensity remained above background levels for 72–96 h. Treatment with cytostatic agents significantly reduced the rate of pimonidazole loss, indicating that dilution of pimonidazole is significantly inhibited in cells with reduced proliferation. Consistently, pimonidazole adduct dilution was significantly reduced in WiDr and SiHa cells cultured in 0.5% O2, which is sufficiently hypoxic to inhibit the proliferation of these cells. Whether dilution of pimonidazole adducts is adversely affecting our ability to detect hypoxic cells over time, therefore, depends on the pimonidazole labeling intensity of hypoxic cells, the prevalence of subsequent proliferation of hypoxic cells and the pimonidazole detection sensitivity at the end of the experiment. Firstly, pimonidazole significantly binds in tumour cells that are below 10 mmHg O2 (1.3% O2), and pimonidazole labeling intensity further increases in cells below 0.5% O2 [73, 74]. Our in vitro data indicate that significant pimonidazole intensity remains in cells even after 3–4 cell divisions, and that hypoxic cells have reduced proliferation whether the cells are exposed to 0.5% O2 or cycles of hypoxia and reoxygenation. Chronically hypoxic cells in vivo transit down the oxygen diffusion gradient and into regions less supportive of proliferation, and pimonidazole-labeled hypoxic WiDr tumour cells in vivo are largely non-proliferative [20]. Further, the in vivo cell cycle time of WiDr tumour cells is 24 h [23]. Taken together, our data and previously published work indicate that proliferation-associated dilution of pimonidazole adducts would not significantly impair our ability to quantify the lifetime of hypoxic tumour cells in vivo, particularly when the overall timecourse of the experiment is limited to 96 h.
We also found that transiently hypoxic tumour cells survive 5, 2×5 and 10 Gy irradiation in similar proportions to their estimated abundance in WiDr tumours. This further supports the existing evidence that transiently hypoxic tumour cells are important considerations for tumour radiation resistance [49–54, 63]. We found that 10 Gy induced a decrease in the density of Hoechst 33342 perfused CD31-positive blood vessels 27 h post-irradiation, leading to an increase in tumour hypoxia and also decreased tumour cell survival that was not observed with 5 Gy or 2×5 Gy. These observations are in agreement with past reports that vascular dysfunction can be induced by single doses of ionising radiation greater than ~8 Gy [67, 68]. Importantly, our data suggest that transiently hypoxic tumour cells were disproportionately affected by radiation-induced vascular dysfunction compared to chronically hypoxic cells, suggesting that hypofractionated radiation regimens designed to disrupt tumour blood vessel function may be useful to target transiently hypoxic cells. However, the significant induction in tumour hypoxia we observed 27 h following 10 Gy could limit the overall efficacy of subsequent radiation doses. Indeed, past studies suggest that interventions against tumour hypoxia are required for each dose of hypofractionated radiotherapy delivered within a 1-week period in order to optimise tumour cell kill [81]. Consistent with the sensitivity of transiently hypoxic tumour cells to radiation-induced vascular dysfunction, transiently hypoxic cells may be particularly susceptible to vascular disrupting agents [82–84]. We have also recently shown that the angiotensin II receptor blocker telmisartan decreases collagen deposition, improves microregional tumour perfusion and reduces transient hypoxia in WiDr tumours [63]. Our understanding of transient tumour hypoxia continues to improve and non-invasive, real-time measurements of tumour perfusion and hypoxia will facilitate testing additional therapeutic strategies to target transiently hypoxic tumour cells.
Overall, our data support that chronically and transiently hypoxic cells in solid tumours die with different kinetics. We found that transiently hypoxic tumour cells survive in untreated WiDr tumours for 24 h after labelling with pimonidazole, followed by rapid turnover and complete loss by 72 h after labelling. Chronically hypoxic cells have a constant turnover in WiDr and SiHa tumours, with significant numbers dying within 24 h while some chronically hypoxic cells can survive >96 h. We found that transiently hypoxic tumour cells are resistant to radiation, confirming that these cells are important targets to improve the overall radiation response of some tumours. We also found that transiently hypoxic tumour cells are killed by large single doses of radiation that can induce vascular dysfunction. Overall, our data provide parameters to model transient hypoxia in vitro, demonstrate the relative lifetime of transiently hypoxic cells in vivo and support additional studies to develop new therapeutic strategies to target this radiation-resistant population of tumour cells.
Supplementary information
Acknowledgements
We would like to thank Drs. Andrew Minchinton and Alastair Kyle for use of their fluorescent microscope slide scanner and associated custom image processing programs.
Author contributions
BJW contributed to project conceptualisation and experimental design, data collection, data analysis and interpretation and writing of the manuscript. CML contributed to data collection and analysis. KLB contributed to project conceptualisation, experimental design, data interpretation and writing of the manuscript.
Funding information
This work was supported by the Canadian Institutes of Health Research (CIHR; MOP-126138 and PJT-159513). BJW was supported by a CIHR Doctoral Research Award. BJW and CML were scholars in the Strategic Training in Transdisciplinary Radiation Science for the 21st Century (STARS21) program. KLB was a Michael Smith Foundation of Health Research Biomedical Research Scholar.
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were approved by the University of British Columbia Animal Care Committee in accordance with Canadian Council on Animal Care guidelines.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01691-5.
References
- 1.Höckel M, Knoop C, Schlenger K, Vorndran B, Baußnann E, Mitze M, et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 2.Höckel M, Schlenger K, Aral B, Mitze M, Schäffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. [PubMed] [Google Scholar]
- 3.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–9. doi: 10.1016/S0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 4.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/S0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 5.Nordsmark M, Alsner J, Keller J, Nielsen OS, Jensen OM, Horsman MR, et al. Hypoxia in human soft tissue sarcomas: adverse impact on survival and no association with p53 mutations. Br J Cancer. 2001;84:1070–5. doi: 10.1054/bjoc.2001.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Sun A, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–56. doi: 10.1016/S0167-8140(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 7.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–7. doi: 10.1016/S0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 8.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–3. [PubMed] [Google Scholar]
- 9.Pitson G, Fyles A, Milosevic M, Wylie J, Pintilie M, Hill R. Tumor size and oxygenation are independent predictors of nodal diseases in patients with cervix cancer. Int J Radiat Oncol Biol Phys. 2001;51:699–703. doi: 10.1016/S0360-3016(01)01662-5. [DOI] [PubMed] [Google Scholar]
- 10.Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20:680–7. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- 11.Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer. 2011;11:504. doi: 10.1186/1471-2407-11-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–80. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewhirst MW, Secomb TW, Ong ET, Hsu R, Gross JF. Determination of local oxygen consumption rates in tumors. Cancer Res. 1994;54:3333–6. [PubMed] [Google Scholar]
- 14.Secomb TW, Hsu R, Ong ET, Gross JF, Dewhirst MW. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995;34:313–6. doi: 10.3109/02841869509093981. [DOI] [PubMed] [Google Scholar]
- 15.Tannock IF. Oxgen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972;45:515–24. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- 16.Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968;22:258–73. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–49. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirst DG, Hirst VK, Joiner B, Prise V, Shaffi KM. Changes in tumour morphology with alterations in oxygen availability: further evidence for oxygen as a limiting substrate. Br J Cancer. 1991;64:54–58. doi: 10.1038/bjc.1991.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirst DG, Denekamp J, Hobson B. Proliferation kinetics of endothelial and tumour cells in three mouse mammary carcinomas. Cell Prolif. 1982;15:251–61. doi: 10.1111/j.1365-2184.1982.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 20.Durand RE, Aquino-Parsons C. Non-constant tumour blood flow: Implications for therapy. Acta Oncol. 2001;40:862–9. doi: 10.1080/02841860152703508. [DOI] [PubMed] [Google Scholar]
- 21.Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K, et al. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat Commun. 2012;3:710–83. doi: 10.1038/ncomms1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ljungkvist ASE, Bussink J, Kaanders JHAM, Rijken PFJW, Begg AC, Raleigh JA, et al. Hypoxic cell turnover in different solid tumor lines. Int J Radiat Oncol Biol Phys. 2005;62:1157–68. doi: 10.1016/j.ijrobp.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Durand RE, Sham E. The lifetime of hypoxic human tumor cells. Int J Radiat Oncol Biol Phys. 1998;42:711–5. doi: 10.1016/S0360-3016(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 24.Lunt SJ, Kalliomaki TMK, Brown A, Yang VX, Milosevic M, Hill RP. Interstitial fluid pressure, vascularity and metastasis in ectopic, orthotopic and spontaneous tumours. BMC Cancer. 2008;8:1–14.. doi: 10.1186/1471-2407-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011;71:3872–80. doi: 10.1158/0008-5472.CAN-10-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108:2909–14. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V, Boucher Y, Liu H, Ferreira D, Hooker J, Catana C, et al. Noninvasive assessment of losartan-induced increase in functional microvasculature and drug delivery in pancreatic ductal adenocarcinoma. Transl Oncol. 2016;9:431–7. doi: 10.1016/j.tranon.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stylianopoulos T, Munn LL, Jain RK. Reengineering the tumor vasculature: improving drug delivery and efficacy. Trends Cancer. 2018;4:258–9. doi: 10.1016/j.trecan.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Secomb TW, Hsu R, Dewhirst MW, Klitzman B, Gross JF. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys. 1993;25:481–9. doi: 10.1016/0360-3016(93)90070-C. [DOI] [PubMed] [Google Scholar]
- 31.Baish JW, Gazit Y, Berk DA, Nozue M, Baxter LT, Jain RK. Role of tumor vascular architecture in nutrient and drug delivery: an invasion percolation-based network model. Microvasc Res. 1996;51:327–46. doi: 10.1006/mvre.1996.0031. [DOI] [PubMed] [Google Scholar]
- 32.Pogue BW, O’Hara JA, Wilmot CM, Paulsen KD, Swartz HM. Estimation of oxygen distribution in RIF-1 tumors by diffusion model-based interpretation of pimonidazole hypoxia and eppendorf measurements. Radiat Res. 2001;155:15–25. doi: 10.1667/0033-7587(2001)155[0015:EOODIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–37. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–58. [PubMed] [Google Scholar]
- 35.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- 36.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–6. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 37.Trotter MJ, Chaplin DJ, Durand RE, Olive PL. The use of fluorescent probes to identify regions of transient perfusion in murine tumors. Int J Radiat Oncol Biol Phys. 1989;16:931–4. doi: 10.1016/0360-3016(89)90889-4. [DOI] [PubMed] [Google Scholar]
- 38.Durand RE, Lepard NE. Contribution of transient blood flow to tumour hypoxia in mice. Acta Oncol. 1995;34:317–23. doi: 10.3109/02841869509093982. [DOI] [PubMed] [Google Scholar]
- 39.Braun RD, Lanzen JL, Dewhirst MW. Fourier analysis of fluctuations of oxygen tension and blood flow in R3230Ac tumors and muscle in rats. Am J Physiol Heart Circ Physiol. 1999;277:H551–H568. doi: 10.1152/ajpheart.1999.277.2.H551. [DOI] [PubMed] [Google Scholar]
- 40.Brurberg KG, Skogmo HK, Graff BA, Olsen DR, Rofstad EK. Fluctuations in pO2 in poorly and well-oxygenated spontaneous canine tumors before and during fractionated radiation therapy. Radiother Oncol. 2005;77:220–6. doi: 10.1016/j.radonc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Pigott KH, Hill SA, Chaplin DJ, Saunders MI. Microregional fluctuations in perfusion within human tumours detected using laser Doppler flowmetry. Radiother Oncol. 1996;40:45–50. doi: 10.1016/0167-8140(96)01730-6. [DOI] [PubMed] [Google Scholar]
- 42.Simonsen TG, Gaustad JV, Leinaas MN, Rofstad EK. Vascular abnormalities associated with acute hypoxia in human melanoma xenografts. Radiother Oncol. 2012;105:72–78. doi: 10.1016/j.radonc.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Gaustad JV, Simonsen TG, Hansem LMK, Rofstad EK. Intravital microscopy of tumor vessel morphology and function using a standard fluorescence microscope. Eur J Nucl Med Mol Imaging. 2021;48:3089–3100. doi: 10.1007/s00259-021-05243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishna MC, Matsumoto S, Yasui H, Saito K, Devasahayam N, Subramanian S, et al. Electron paramagnetic resonance imaging of tumor pO2. Radiat Res. 2012;177:376–86. doi: 10.1667/RR2622.1. [DOI] [PubMed] [Google Scholar]
- 45.Baudelet C, Ansiaux R, Jordan BF, Havaux X, Macq B, Gallez B. Physiological noise in murine solid tumours using T2*-weighted gradient-echo imaging: a marker of tumour acute hypoxia? Phys Med Biol. 2004;49:3389–411. doi: 10.1088/0031-9155/49/15/006. [DOI] [PubMed] [Google Scholar]
- 46.Magat J, Jordan BF, Cron GO, Gallez B. Noninvasive mapping of spontaneous fluctuations in tumor oxygenation using 19F MRI. Med Phys. 2010;37:5434–41. doi: 10.1118/1.3484056. [DOI] [PubMed] [Google Scholar]
- 47.Ron A, Deán-Ben XL, Gottschalk S, Razansky D. Volumetric optoacoustic imaging unveils high-resolution patterns of acute and cyclic hypoxia in a murine model of breast cancer. Cancer Res. 2019;79:4767–75. doi: 10.1158/0008-5472.CAN-18-3769. [DOI] [PubMed] [Google Scholar]
- 48.Bader SB, Dewhirst MW, Hammond EM. Review cyclic hypoxia: an update on its characteristics, methods to measure it and biological implications in cancer. Cancers. 2021;13:1–20. doi: 10.3390/cancers13010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennewith KL, Durand RE. Drug-induced alterations in tumour perfusion yield increases in tumour cell radiosensitivity. Br J Cancer. 2001;85:1577–84. doi: 10.1054/bjoc.2001.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vordermark D, Horsman M. Hypoxia as a biomarker and for personalized radiation oncology. Recent Results Cancer Res. 2016;198:123–42. doi: 10.1007/978-3-662-49651-0_6. [DOI] [PubMed] [Google Scholar]
- 51.Siemann DW, Horsman MR. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther. 2015;153:107–24. doi: 10.1016/j.pharmthera.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: Results of a phase III randomized trial. J Clin Oncol. 2012;30:1777–83. doi: 10.1200/JCO.2011.35.9315. [DOI] [PubMed] [Google Scholar]
- 53.Kaanders JHAM, Wijffels KIEM, Marres HAM, Ljungkvist ASE, Pop L, van den Hoogen F, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–74. [PubMed] [Google Scholar]
- 54.Lee I, Kim JH, Levitt SH, Song CW. Increases in tumor response by pentoxifylline alone or in combination with nicotinamide. Int J Radiat Oncol Biol Phys. 1992;22:425–9. doi: 10.1016/0360-3016(92)90846-A. [DOI] [PubMed] [Google Scholar]
- 55.Chaudary N, Hill RP. Increased expression of metastasis-related genes in hypoxic cells sorted from cervical and lymph nodal xenograft tumors. Lab Invest. 2009;89:587–96. doi: 10.1038/labinvest.2009.16. [DOI] [PubMed] [Google Scholar]
- 56.Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61:8903–8. [PubMed] [Google Scholar]
- 57.Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res. 2004;64:2054–61. doi: 10.1158/0008-5472.CAN-03-3196. [DOI] [PubMed] [Google Scholar]
- 58.Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: Pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72:889–95. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2, 2, 3, 3, 3-pentafluoropropyl)acetamide]: analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer. 1995;72:869–74. [DOI] [PMC free article] [PubMed]
- 60.Walton MI, Bleehen NM, Workman P. Effects of localised tumour hyperthermia on pimonidazole (Ro 03-8799) pharmacokinetics in mice. Br J Cancer. 1989;59:667–73. doi: 10.1038/bjc.1989.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laughlin KM, Tracy M, Chan CY, Lord EM. Biodistribution of the Nitroimidazole EF5 (2-[2-nitro-1H-imidazol-1-yl]-N-(2,2,3,3,3-pentafluoropropyl) acetamide) in Mice Bearing Subcutaneous. J Pharmacol Exp Ther. 1996;277:1049–57. [PubMed]
- 62.Bennewith KL, Durand RE. Quantifying transient hypoxia in human tumor xenografts by flow cytometry. Cancer Res. 2004;64:6183–9. doi: 10.1158/0008-5472.CAN-04-0289. [DOI] [PubMed] [Google Scholar]
- 63.Wadsworth BJ, Cederberg RA, Lee C-M, Firmino NS, Franks SE, Pan J, et al. Angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and improves tumour response to radiation. Cancer Lett. 2020;493:31–40. doi: 10.1016/j.canlet.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 64.Ehrly AM. The effect of pentoxifylline on the flow properties of human blood. Curr Med Res Opin. 1978;5:608–13. doi: 10.1185/03007997809110195. [DOI] [PubMed] [Google Scholar]
- 65.Price MJ, Li LT, Tward JD, Bublik I, Mcbride WH, Lavey RS. Effect of nicotinamide and pentoxifylline on normal tissue and fsa tumor oxygenation. Acta Oncol. 1995;34:391–5. doi: 10.3109/02841869509093995. [DOI] [PubMed] [Google Scholar]
- 66.Zywietz F, Böhm L, Sagowski C, Kehrl W. Pentoxifylline enhances tumor oxygenation and radiosensitivity in rat rhabdomyosarcomas during continuous hyperfractionated irradiation. Strahlenther Onkol. 2004;180:306–14. doi: 10.1007/s00066-004-1198-1. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 68.Song CW, Griffin RJ, Lee Y-J, Cho H, Seo J, Park I, et al. Reoxygenation and repopulation of tumor cells after ablative hypofractionated radiotherapy (SBRT and SRS) in murine tumors. Radiat Res. 2019;192:159–68. doi: 10.1667/RR15346.1. [DOI] [PubMed] [Google Scholar]
- 69.Noguchi P, Wallace R, Johnson J, Earley EM, O’Brien S, Ferrone S, et al. Characterization of WiDr: a human colon carcinoma cell line. In Vitro. 1979;15:401–8. doi: 10.1007/BF02618407. [DOI] [PubMed] [Google Scholar]
- 70.Friedl F, Kimura I, Osato T, Ito Y. Studies on a new human cell line (SiHa) derived from carcinoma of uterus. I. Its establishment and morphology. Proc Soc Exp Biol Med. 1970;135:543–5. doi: 10.3181/00379727-135-35091a. [DOI] [PubMed] [Google Scholar]
- 71.Trotter MJ, Chaplin DJ, Olive PL. Use of a carbocyanine dye as a marker of functional vasculature in murine tumours. Br J Cancer. 1989;59:706–9. doi: 10.1038/bjc.1989.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durand RE, Aquino-Parsons C. Clinical relevance of intermittent tumour blood flow. Acta Oncol. 2001;40:929–36. doi: 10.1080/02841860152708206. [DOI] [PubMed] [Google Scholar]
- 73.Mahy P, De Bast M, Gallez B, Gueulette J, Koch CJ, Scalliet P, et al. In vivo colocalization of 2-nitroimidazole EF5 fluorescence intensity and electron paramagnetic resonance oximetry in mouse tumors. Radiother Oncol. 2003;67:53–61. doi: 10.1016/S0167-8140(03)00028-8. [DOI] [PubMed] [Google Scholar]
- 74.Koch CJ. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002;352:3–31. doi: 10.1016/S0076-6879(02)52003-6. [DOI] [PubMed] [Google Scholar]
- 75.Chaplin DJ, Hill SA. Temporal heterogeneity in microregional erythrocyte flux in experimental solid tumours. Br J Cancer. 1995;71:1210–3. doi: 10.1038/bjc.1995.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kimura H, Braun RD, Ong ET, Hsu R, Secomb TW, Papahadjopoulos D, et al. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996;56:5522–8. [PubMed] [Google Scholar]
- 77.Chaplin DJ, Horsman MR, Trotter MJ. Effect of nicotinamide on the microregional heterogeneity of oxygen delivery within a murine tumor. J Natl Cancer Inst. 1990;82:672–6. doi: 10.1093/jnci/82.8.672. [DOI] [PubMed] [Google Scholar]
- 78.Minchinton AI, Durand RE, Chaplin DJ. Intermittent blood flow in the KHT sarcoma-flow cytometry studies using hoechst 33342. Br J Cancer. 1990;62:195–200. doi: 10.1038/bjc.1990.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moore JV, Hasleton PS, Buckley CH. Tumour cords in 52 human bronchial and cervical squamous cell carcinomas: Inferences for their cellular kinetics and radiobiology. Br J Cancer. 1985;51:407–13. doi: 10.1038/bjc.1985.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ljungkvist ASE, Bussink J, Kaanders JHAM, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167:127–45. doi: 10.1667/RR0719.1. [DOI] [PubMed] [Google Scholar]
- 81.Wittenborn TR, Horsman MR. Targeting tumour hypoxia to improve outcome of stereotactic radiotherapy. Acta Oncol. 2015;54:1385–92. doi: 10.3109/0284186X.2015.1064162. [DOI] [PubMed] [Google Scholar]
- 82.Zhou H, Hallac RR, Lopez R, Denney R, MacDonough MT, Li L, et al. Evaluation of tumor ischemia in response to an indole-based vascular disrupting agent using BLI and (19)F MRI. Am J Nucl Med Mol Imaging. 2015;5:143–53. [PMC free article] [PubMed] [Google Scholar]
- 83.Liu L, O’Kelly D, Schuetze R, Carlson G, Zhou H, Trawick ML, et al. Non-invasive evaluation of acute effects of tubulin binding agents: a review of imaging vascular disruption in tumors. Molecules. 2021;26:2551. doi: 10.3390/molecules26092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iversen AB, Busk M, Bertelsen LB, Laustsen C, Munk OL, Nielsen T, et al. The potential of hyperpolarized 13-C magnetic resonance spectroscopy to monitor the effect of combretastatin based vascular disrupting agents. Acta Oncol. 2017;56:1626–33. doi: 10.1080/0284186X.2017.1351622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.