Abstract
Vascular cognitive impairment (VCI) is predominately caused by vascular risk factors and cerebrovascular disease. VCI includes a broad spectrum of cognitive disorders, from mild cognitive impairment to vascular dementia caused by ischemic or hemorrhagic stroke, and vascular factors alone or in a combination with neurodegeneration including Alzheimer’s disease (AD) and AD-related dementia. VCI accounts for at least 20–40% of all dementia diagnosis. Growing evidence indicates that cerebrovascular pathology is the most important contributor to dementia, with additive or synergistic interactions with neurodegenerative pathology. The most common underlying mechanism of VCI is chronic age-related dysregulation of CBF, although other factors such as inflammation and cardiovascular dysfunction play a role. Vascular risk factors are prevalent in VCI and if measured in midlife they predict cognitive impairment and dementia in later life. Particularly, hypertension, high cholesterol, diabetes, and smoking at midlife are each associated with a 20 to 40% increased risk of dementia. Control of these risk factors including multimodality strategies with an inclusion of lifestyle modification is the most promising strategy for treatment and prevention of VCI. In this review, we present recent developments in age-related VCI, its mechanisms, diagnostic criteria, neuroimaging correlates, vascular risk determinants, and current intervention strategies for prevention and treatment of VCI. We have also summarized the most recent and relevant literature in the field of VCI.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01170-y.
Keywords: Vascular cognitive impairment, Vascular risk factors, Small vessel disease, Cognition, Neuroimaging, Biomarkers
Vascular cognitive impairment (VCI) is a recently recognized entity caused predominately by cerebrovascular disease [1, 2]. VCI includes an entire spectrum of cognitive disorders, from mild cognitive impairment (MCI) to vascular dementia caused by vascular ischemic or hemorrhagic etiology and vascular factors alone or in a combination with neurodegeneration and Alzheimer’s disease (AD) [3]. In this review, we discuss recent developments in age-related VCI, including its mechanisms, diagnostic criteria, neuroimaging correlates, and vascular risk determinants. We also present current intervention strategies for prevention and treatment of VCI. Recently, several review articles have described VCI in the context of cerebral small vessel disease [4], asymptomatic carotid stenosis [5], stroke [6–8], heart disease [9, 10], and AD [11]. Here, we summarize these developments and the most recent literature in the field of VCI.
Epidemiology of VCI and the Aging Populations
Current projections suggest that 72 million people in the USA will be older than 65 years of age by 2030, which is a greater than tenfold increase in a century [12]. Age-related cognitive impairment is one of the major public health challenges of our time. The number of affected individuals in 2018 was estimated at 50 million worldwide and expected to triple by 2050 at a cost approaching $4 trillion [13]. The prevalence of VCI may be lower in low-to-middle-income countries that are early in the process of demographic transition [14]. However, these countries now see the fastest increases in the prevalence of VCI.
Vascular risk factors are prevalent in the growing older population. For example, in the community-based Framingham Heart Study, the lifetime risk for development of hypertension is more than 90% [15]. Similarly, age-related neurological diseases have increased with prolonged life expectancy. One in three people over age 65 would experience stroke, dementia, or both of these conditions during their lifetimes [16]. VCI accounts for at least 20–40% of all dementia diagnosis. Growing evidence indicates that cerebrovascular pathology is the most important contributor to dementia, with additive or synergistic interactions with neurodegenerative pathology. In the clinical-pathological analysis from the Religious Orders Study and Memory and Aging Project, only 9% of autopsy sample had isolated AD, 40% had AD plus prominent vascular pathology (macroscopic infarcts, cerebral amyloid angiopathy, atherosclerosis or arteriolosclerosis), and 44% had AD plus vascular as well as another neurodegenerative pathology [17]. This is further supported by the contribution of vascular risk factors to dementia. Vascular risk factors measured in midlife predict cognitive impairment and dementia in later life [18]. Hypertension, high cholesterol, diabetes, and smoking at midlife are each associated with a 20 to 40% increased risk of dementia and, furthermore, in dose-dependent manner such that the risk for dementia increases from 1.3 for having one risk factor to 2.4 for having four risk factors [18].
Disparities in cerebrovascular disease also translate in race-ethnic disparities in VCI and dementia. Non-Hispanic Black and Hispanics/Latino individuals have a greater burden of VCI and dementia compared to non-Hispanic white individuals [19–21]. In the Northern Manhattan Study (NOMAS), Hispanic and Black participants had greater likelihood of MCI (20%) and dementia (5%) than white participants after accounting for age and education differences [22]. Further research on the understanding of vascular risk factors, particularly in midlife as opposed to late life in the development of VCI in diverse cohorts, is needed, especially in those where participants live in the same community for comparison across groups without confounding introduced by heterogeneity in environmental and other local factors.
The most emphasis now is on identifying individuals with early cognitive impairment due to vascular risk factors and vascular pathology as these individuals are at greatest risk for developing VCI and dementia and would considerably benefit from preventive measures. However, more research is needed to understand epidemiology of a complete spectrum of VCI. Best way is to prospectively follow epidemiologic, aging, and clinical cohorts worldwide for vascular risk factors burden, structural and functional brain changes, and the intermediate vascular and cognitive phenotypes to determine their risk of transforming to VCI and clinical disease. This approach is feasible as many ongoing cohorts and aging studies are nationally or government funded (Table 1), and they are already focused on longitudinal cognitive outcomes and their prevention [23]. In this effort, major challenge remains in the harmonization of data collection, cognitive testing, and neuroimaging and other biomarkers. Based on the world pandemic of cognitive conditions, the World Stroke Organization has issued a proclamation that calls for joint prevention of stroke and dementia, data harmonization, and translation into action and is now endorsed by all major international organizations focused on brain and vascular health [24].
Table 1.
Selected list of major longitudinal epidemiology and aging cohorts studying VCI in the USA and worldwide
| • Framingham Heart Study (FHS) |
| • Atherosclerosis Risk in Communities (ARIC) |
| • Multi-Ethnic Study of Atherosclerosis (MESA) |
| • Northern Manhattan Study (NOMAS) |
| • Hispanic Community Health Study-Study of Latinos (HCHS-SOL) |
| • Reasons for Geographic and Racial Differences in Stroke (REGARDS) |
| • Religious Orders Study |
| • Rush Memory and Aging Project |
| • Einstein Aging Study (EAS) |
| • Health and Retirement Study (HRS) |
| • Age, Genes/Susceptibility study Reykjavik (AGES-RS) |
| • Three Cities study (3C) |
| • Rotterdam Study (RS) |
| • Austrian Study of Stroke Prevention (ASPS) |
| • Study of Health in Pomerania (SHIP) |
| • Singapore Longitudinal Aging Studies (SLAS) |
| • English Longitudinal Study of Ageing (ELSA) |
| • The Norwegian Life Course, Ageing and Generation Study (NorLAG) |
| • The German Ageing Survey (DEAS) |
| • Australian Longitudinal Study of Ageing (ALSA) |
| • The Japanese Study of Aging and Retirement (JSTAR) |
| • Taiwan Longitudinal Study on Aging (TLSA) |
| • China Health and Retirement Longitudinal Study (CHARLS) |
| • Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) |
Mechanism of VCI
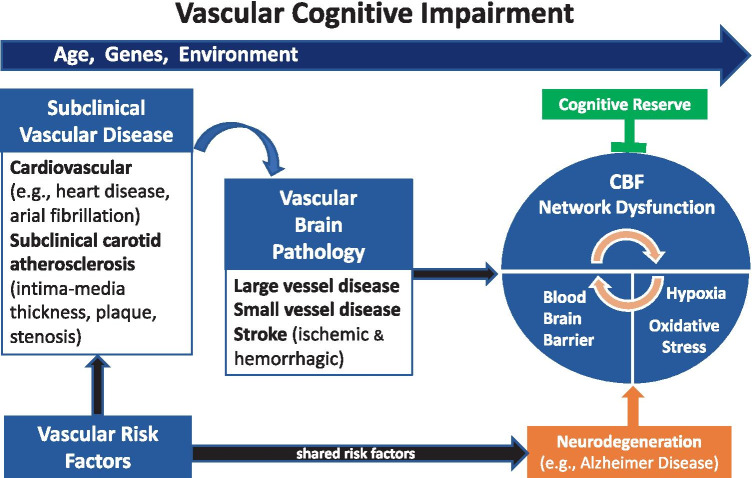
Multiple cerebrovascular etiologies can cause VCI. They include cerebral small vessel disease (SVD), large-artery atherosclerosis, brain hemorrhages, cardioembolism, and other less common etiologies of stroke [25, 26]. Age, genetic, and environmental and lifestyle factors lead to the development of vascular risk factors, subclinical arterial and brain diseases, and ultimately cause cerebral blood flow (CBF) and network dysfunction, which are hallmarks of VCI (Fig. 1). Underlying neurodegeneration through shared genetic and environmental risk factors may accelerate VCI. This process is counteracted by the individual cognitive and functional reserve and resilience. The mechanisms by which vascular pathologies contribute to VCI are not well understood. The most common underlying mechanism of these etiologies is chronic age-related dysregulation of CBF, but hypoxia, increased permeability of blood–brain barrier (BBB), endothelial dysfunction, systemic inflammation, and inflammatory clock of aging (iAge), which are tracked with multimorbidity, immunosenescence, frailty, and cardiovascular aging, are additional mechanisms among others and have been recently reviewed [27, 28]. Also, vascular pathology commonly found in autopsy studies of clinical AD patients [29] seems to occur early in the AD continuum and biomarker trajectories [30, 31].
Fig. 1.
Mechanism of Vascular Cognitive Impairment
Regulation of CBF is complex. It must ensure adequate delivery of oxygen and nutrients and rapid adjustment to CBF fluctuations. While the brain only represents about 2% of the total body mass, it consumes about 20% of oxygen and about 25% of glucose in the human body [32]. While the brain has a high metabolic demand for oxygen and glucose relative to other organs, it only contains minute energy reserves and is thus highly dependent on constant CBF to supply energy substrates. CBF regulation has to maintain constant metabolic supply and normal blood flow, volume and intracranial pressure, and prevent injury from penetration of high pressure flow from large vessels to the distal microvasculature [33]. Evidence from animal models has shown that chronic reduction in CBF can cause brain atrophy, white matter injury, lacunar infarcts, hemorrhages, memory impairment, and potentially AD [34, 35]. Vascular risk factors, particularly hypertension, have profound impact on cerebral vessel wall structure and CBF regulation. Recent studies using BOLD and arterial spin labeling MRI have documented the alteration in cerebrovascular reactivity in patients with SVD [36, 37]. However, whether the CBF dysfunction is a cause of VCI or a consequence of reduced brain metabolic demands in aging and neurodegeneration is unclear and remains to be proven [38].
The tightly controlled interaction between brain cells and the cerebral blood vessels is a central function in the mechanism of VCI, and it is conceptualized through the neurovascular unit [39, 40]. The neurovascular unit (NVU) is a complex functional and anatomical structure composed of specialized endothelial cells of the BBB surrounded by a basal lamina and the interacting neurons, astrocytes, microglia, pericytes, and an extracellular matrix. The key function of the NVU is coupling of neural activity and CBF. Growing evidence indicates that NVU dysfunction critically contributes to brain pathologies, including VCI and neurodegenerative diseases [41, 42].
There is significant heterogeneity in the interactions between neurons and cerebral vessels as well as the vasculature of the collateral circulation pathways across the brain. The circle of Willis is an anastomotic system of arteries that forms a network of collateral CBF circulation to supply nutrients to the internal brain parenchyma as well as the surface within the subarachnoid space via pial arteries and arterioles. Pial arteries dive into the substance of the brain surrounded by an extension of the subarachnoid space forming the perivascular spaces (PVS) or Virchow-Robin spaces [43]. Although the precise function of PVS is not completely understood, a perivascular pathway has long been proposed as a drainage system through retrograde travel with drainage into cervical lymph nodes [44]. This system is now known as the glymphatic system, a brain-wide network through which cerebrospinal fluid is exchanged with the interstitial fluid as a waste clearance mechanism within the brain parenchyma. The critical role of PVS is in the exchange of energy substrates, maintaining the brain immune system, and clearing of interstitial ß-amyloid [45, 46]. PVS have little to no resistance to flow [47] and have been proposed in the mechanisms of neurodegenerative disorders through a common pathway of vascular hemodynamic dysregulation and failure of the glymphatic system [48].
Multiple large and small infarcts were proposed as causes of dementia in early 1970s [49] and have been associated with high risk of dementia or worsening of cognitive function if large in size, across multiple territories or in greater number [25, 50], and particularly for those infarcts in supratentorial regions and in anterior circulation [51, 52]. There is a large interindividual variation in cognitive response to multiple infarcts and potential underlying neurodegenerative pathology; therefore, no clear infarct volume threshold has been proposed. Single infarcts may cause cognitive decline if located in strategic regions (called strategic infarcts) such as the thalamus, angular gyrus, and basal ganglia [53, 54]. Specific white matter tracks that are integrated into cortical-subcortical cognitive networks are likely playing a key role in cognitive impairments associated with these lesions [55].
Subclinical cerebral white matter lesions and microinfarcts are most common causes of VCI [56–58]. Prevalence of white matter lesions is as high as 50% in those aged 45 to 95% in those aged 80 [59, 60]. Subclinical, silent cerebral infarcts (SBIs) are prevalent up to 40%, depending on age and the burden of vascular risk factors [61, 62]. In the Rotterdam Study and the Framingham Offspring Study, SBIs doubled the risk of dementia [63, 64]. In the NOMAS, greater burden of white matter lesions and SBIs was associated with worse global cognitive performance and psychomotor speed [65]. Furthermore, those 70 years or older with greater burden of white matter performed worse in episodic and semantic memory, which is likely driven by the cumulative effects of vascular risk factors and subclinical age-related neurodegenerative pathology on cerebrovascular integrity. In Religious Orders Study, cerebral microinfarcts were associated with disturbances in episodic memory, semantic memory, and perceptual speed [66]. In support of these observations, a meta-analysis of data from different cohorts worldwide has shown prevalence of cerebral microinfarcts to be twice as high in people who died with diagnosis of dementia [67].
PVS are highly associated with other markers of SVD, including white matter hyperintensities and cerebral microinfarcts, and by many are considered a hallmark of SVD [68]. In NOMAS, PVS were associated with increased age, hypertension, presence of atherosclerotic carotid plaque, and of risk of vascular events [69, 70], most likely through the mechanism of arterial stiffness and pulse-wave reflection and propagation to the aging brain. Other proposed mechanisms for enlargement of PVS include brain atrophy, inflammation, and dysfunction of perivascular flow [45, 71]. The evidence for the associations between PVS and cognitive impairment and dementia has been conflicting; however, recent meta-analysis supports the role of PVS in cognitive impairment [72]. In a recent study, PVS were associated with greater decline in global cognition over 4 years independent of other markers of SVD and with a 2.9-fold increased risk of dementia across 8 years of follow-up [73]. The presence of PVS visible on MRI is not specific for SVD, but they are also frequently found in patients with AD, Parkinson’s disease, and multiple sclerosis [71]. Nevertheless, the mechanism behind VCI in the presence of various imaging markers of SVD (PVS, white matter lesions, SBI) remains difficult to elucidate because of shared and multiple disease processes, diffuse location, the presence of yet unrecognized pathology, and individual cognitive reserve and resilience [25].
Brain hemorrhages, intracerebral hemorrhages (ICH), and cerebral microbleeds are also associated with cognitive impairment and dementia [74, 75]. Hypertensive small vessel disease is the most common cause of deep ICH and cerebral amyloid angiopathy of lobar ICH [76]. Cerebral microbleeds (CMBs) are well-defined small and round black structures seeing on MRI gradient echo T2*-weighted imaging. The prevalence of CMBs is reported to be 3–27% in elderly individuals [77–80]. In NOMAS, the prevalence of CMBs was 5%; and 37% participants had only deep CMBs, 48% had only lobar CMBs, and 15% had CMBs in both locations [81]. The underlying mechanisms of CMBs are heterogeneous and associated with recent or old hemorrhages, vasculopathies, and various degrees of chronic ischemic injury [82]. CMBs affect cognition and risk of dementia independent of vascular risk factors and other markers of SVD [78, 79]. The mechanisms by which CMBs affect cognition are unclear, but evidence suggests that it is mediated by reduced structural brain network efficiency and disrupted connectivity [83, 84].
Cerebral amyloid angiopathies (CAA) include heterogeneous sporadic and genetic conditions characterized by amyloid deposition in the walls of cerebral arteries and arterioles. Sporadic CAA is the most common in the elderly and is characterized by vascular deposition of amyloid-beta (Aβ) [85]. The prevalence of CAA is about 2–20%, depending on age, and is present in over 80% of patients with AD on autopsy [85–87]. CAA is associated with greater number of neurofibrillary tangles, neuritic plaques, and ApoE4 presence [88]. CAA manifests with or without intracranial hemorrhage and has been associated with cognitive decline, perceptual speed, episodic memory, and semantic memory [89]. One of the key features of CAA is cortical superficial siderosis (cSS), which represents deposits of blood-breakdown products within the subarachnoid space, the leptomeninges, and the superficial cortical layers [84]. cSS is associated with transient focal neurological episodes and a high risk of future intracerebral hemorrhage. It requires appropriate blood-sensitive MR sequences that are implemented in routine scanning of patients with suspected cerebrovascular events and VCI [90]. In the Framingham and Rotterdam studies of community-dwelling older adults, 6.6% of individuals had deep microbleeds, 12.8% had strictly lobar microbleeds without cortical superficial siderosis, and 0.43% had cSS [91]. Participants with cSS were older, had the APOE ɛ4 allele more frequently, and had greater prevalence of intracerebral hemorrhage. During a mean follow-up of 5.6 years, 42% participants with cSS developed a stroke, 19% transient neurological deficits, and 4% incident dementia. Besides cSS and cerebral micro- and macro-bleeds, the mechanisms of cognitive decline in CAA also include ischemic injury to the white matter and disruption of structural and functional network integrity [92]. The CCA pathophysiology, treatment, and role of the fibrinolytic system have been recently extensively reviewed elsewhere [93].
Cardiac disease such as heart failure, ischemic heart disease, and atrial fibrillation has been associated with VCI. The most common cause of cognitive decline in heart failure is cardiac systolic dysfunction that leads to reduced cerebral perfusion [10]. Neurohormonal activation, oxidative stress, inflammation, glial activation, dendritic spine loss, and brain programmed cell death have also been proposed contributors of cognitive impairment in heart failure. A novel hypothesis has recently emerged as the misfolded protein disease is found both in the brain and the heart [94]. Elevated levels of Aβ in the heart and skeletal muscles of AD individuals indicate a possible contributor to elevated concentrations of Aβ plasma levels and potentially indirectly contributing to Aβ deposits in cerebral blood vessels and brain parenchyma [95]. Atrial fibrillation (AF) is another important cause of cognitive impairment through its thromboembolic risk, association with cerebral SVD, vascular inflammation, and genetic factors [96, 97]. New evidence shows that the use of oral anticoagulants (OACs) in AF is associated with a lower risk of cognitive impairment and dementia compared to non-OAC and antiplatelet use [98, 99].
Subclinical atherosclerosis, including carotid intima-media thickness (cIMT), atherosclerotic plaque, and carotid stenosis, has been associated with VCI. cIMT reflects thickening of the intimal and medial layers of the vessel wall, and carotid plaque represents significant atherosclerotic disease in the vessel lumen that leads to stenosis and CBF reduction [100]. Global hypoperfusion from these causes has been strongly associated with neuropathological imaging showing watershed infarcts, white matter lesions, and hippocampal sclerosis [101–103]. Although cIMT, plaque, and stenosis are validated markers of subclinical vascular disease [104–108], less is known about their ability to predict cognitive impairment and with conflicting evidence. In NOMAS, cIMT was associated with impairments in episodic and semantic memories, and processing speed, but only among APOE ε4 carriers, who represented 24% of the cohort [109], suggesting that increased cIMT may exacerbate cognitive dysfunction in those at higher risk for AD. Carotid plaque was not associated with cognitive dysfunction. In the Tromso study, however, carotid plaque but not cIMT was associated with cognition dysfunction, particularly in verbal memory [110]. The effect of aging, vascular risk factors, and their control likely affected not only the discrepancy between the studies, but also the mechanisms by which these subclinical vascular phenotypes affect cognition may differ [111]. Greater cIMT may result in increased arterial stiffness, pulsatile wave propagation to the brain, and endothelial injury, while carotid plaque may progress to significant stenosis or be a major source of cerebral emboli. Both of these mechanisms may lead to dysregulation of CBF and cerebral hypoperfusion. Carotid stenosis is a flow reduction lesion and a strong predictor of cerebrovascular events as well as cognitive impairment, although the studies with cognitive outcomes are inconsistent [5]. The mechanism by which carotid stenosis causes cognitive impairment is not fully understood. Few studies have addressed the role of SVD in the presence of carotid stenosis. Better evidence supports the role of CBF dysregulation caused by impaired cerebrovascular reserve in patients with severe carotid stenosis and who more likely have cognitive impairment and suffer further cognitive decline with time [112]. Therefore, cognitive decline can be potentially reversed by carotid revascularization and this concept is currently been tested in the CREST-H (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis — Hemodynamics) study, an ancillary study of the CREST-2 randomized clinical trial [113, 114].
Specific genetic and sporadic forms of arteriopathy are associated with VCI. The most frequent monogenic cause is cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) caused by NOTCH3 mutations and less common cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CAASIL) condition caused by HTRA1 mutations [115–117]. Although ApoE4 is a strong risk factor for AD, it seems less important for VCI [118]. Further discussion on these conditions is outside the scope of this review and is summarized elsewhere [119–121].
In some instances, there is a reversibility of cognition in VCI. Transient cognitive impairment can return to normal in about 20% patients shortly after stroke [122], depression [123], and heart failure [124]. Transient cognitive impairment in VCI does not include post-stroke delirium that is observed in up to 25% of hospitalized stroke patients [125], or post-stroke depression, found in up to 50% of stroke patients [126]. Post-stroke cognitive impairment is reversible to normal in both of these conditions.
Despite the established relationships between clinical stoke and subclinical infarcts and dementia, these relations are understudied in a systematic way in a large and diverse populations. To fill the gap in our understanding of pathophysiology of VCI, the NIH has recently established the DISCOVERY study (Determinants of Incident Stroke Cognitive Outcomes and Vascular Effects on Recovery), a large consortium to study cognitive trajectories post-ischemic and hemorrhagic stroke [6], as well as the MarkVCID to validate biomarkers of VCI due to SVD [127, 128]. Similarly, other large-scale international collaborations such as STROKOG (Stroke and Cognition Consortium), SVDs@target (Small Vessel Diseases-At-Target), and the HBC (Heart-Brain Connection) are established to investigate the mechanisms of VCI [129], with STROKOG being the largest consortium with 18,000 individuals from 32 studies and representing 18 countries [130]. Thus, understanding and targeting the mechanism of VCI are a high priority for reducing the overall burden of cognitive impairment and dementia.
Diagnostic Criteria of Vascular Cognitive Impairment and Vascular Dementia
The concept of VCI was first outlined in 2006 by the NINDS in collaboration with the Canadian Stroke Network in a statement on harmonization of minimum, common, clinical, and research standards for the description, data collection, and study of VCI [131]. In the 2011, AHA-ASA had issued a scientific statement on vascular contributions to cognitive impairment and dementia to further capture the entire VCI spectrum associated with all forms of vascular brain injury, ranging from MCI to fully developed dementia [132], and proposed the term VCI for all forms of cognitive disorder associated with cerebrovascular disease regardless of the etiology (e.g., atherosclerotic, ischemic, hemorrhagic, cardioembolic, or genetic). Thus, VCI could range from the mild cognitive deficits, through the multifocal cognitive deficits to clinical vascular dementia that is severe enough to affect social or occupational function.
The 2011 AHA/ASA scientific statement defines VCI as “a syndrome with evidence of clinical stroke or subclinical vascular brain injury and cognitive impairment affecting at least one cognitive domain.” Memory impairment is not a requirement for diagnosis of VCI as memory deficits like seen in AD are not suitable for VCI, in which memory-related structures (hippocampus, thalamus) may be intact and not causing memory impairment [133]. The need for continued development and refinement of cognitive batteries for VCI is emphasized as well as identification of imaging and soluble biomarkers of VCI.
Since the 1970s, there are a variety of vascular dementia (VaD) criteria, ranging from the clinical Hachinski Ischemic Score (HIS) and the National Institute for Neurological Diseases and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria mostly used in research [134] to the AHA-ASA vascular cognitive impairment criteria in 2011, Diagnostic and Statistical Manual (DSM-III, IIIR, IV), the International Classification of Disease 10th and 11th revision (ICD-10, ICD-11), the California Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC), and the International Society for Vascular Behavioral and Cognitive Disorders (VASCOG) criteria [135–139]. There is a considerable variability in the sensitivity of these different criteria when using pathology as a “gold standard” (ranging from 0.2 to 0.7). Specificity, however, ranges from 0.78 to 0.93; thus, most of these criteria emphasize specificity over sensitivity. Nevertheless, less than 50% of all individuals with moderately severe vascular pathology at autopsy are diagnosed during life as having VaD [140].
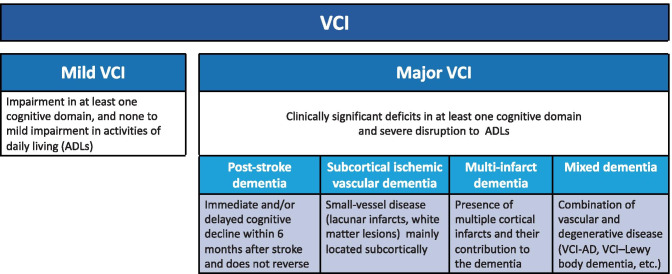
More recently, the international Vascular Impairment of Cognition Classification Consensus Study (VICCCS-1 and 2) has synthesized the conceptual framework, built the consensus, and harmonized diagnostic criteria for VCI and VaD into mild or major [141, 142]. In VICCCS-1 major VCI category, four VCI sub-types are defined: post-stroke dementia, subcortical ischemic vascular dementia, multi-infarct (cortical) dementia, and mixed dementias (Fig. 2). VICCCS-2 further discusses VCI neuroimaging markers with MRI recommended as a gold standard requirement for a diagnosis of VCI.
Fig. 2.
Vascular cognitive impairment (VCI) diagnostic criteria
Neuroimaging Correlates of Vascular Cognitive Impairment
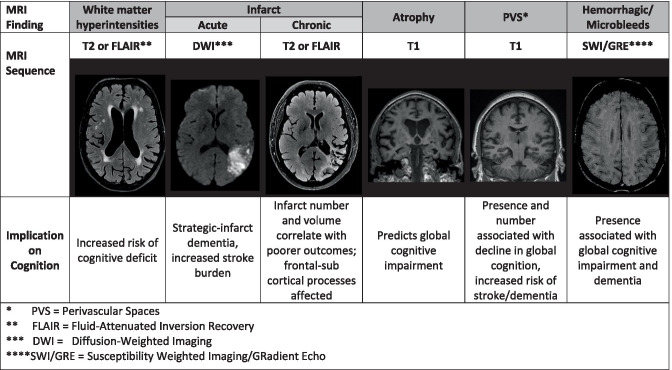
Figure 3 briefly outlines MRI sequences with most typical imaging findings and their implication on cognition. The VICCCS-2 diagnostic guidelines [142] recommend the use of imaging in the diagnosis of VCI based on the MRI measures, including the number, size and location of infarcts, and hemorrhages, extent on a quantitative or validated semiqualitative scale of WMH volume, and measures of total brain (or ventricular) and hippocampal volumes. For research purposes, the NINDS-CSC VCI harmonization guidelines [131] recommend a minimal imaging dataset with MRI 3D T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and gradient echo (GRE) sequences. Diffusion-weighted images (for acute stroke), diffusion tensor imaging (DTI) for assessing the state of the white matter tracts where abnormal DTI (lower fractional anisotropy) in normal-appearing white matter as it has been associated with vascular risk factors and poorer executive function [143], PET for β-amyloid, and non-invasive assessment of the cerebral vasculature (carotid ultrasound preferably or MR angiography) are also suggested.
Fig. 3.
Vascular MR imaging phenotypes associated with vascular cognitive impairment
White matter hyperintensities (WMHs) are often seen on FLAIR or T2 MRI sequences, increasingly with age and in the setting of vascular risk factors. They are associated with cerebral SVD, though they can be seen in other neurological conditions and in the appropriate clinical setting (e.g., multiple sclerosis). While often heterogenous in size, distribution, and quantity, their presence is noteworthy when considering vascular etiology of cognitive impairment in clinical practice. The presence of WMHs is associated with global cognitive dysfunction [144, 145], both associated with brain atrophy [146–148] and independent of atrophy [65]. Increased WMH burden has also been associated with functional decline [149]. Still, many questions remain unanswered about the cognitive implications of specific characteristics of WMH, such as asymmetry of distribution [150], impact of the underlying histopathology [151], or location (e.g., peri-ventricular vs. sub-cortical) [152]. WMHs may also be implicated in AD and AD-related dementia, though the extent of this association remains to be established [153]. Additional work demonstrates an association of WMHs with amyloid deposition, but not with tau [154]. Some suggest the increased risk from WMHs may be independent of the vascular contribution [155]. In fact, WMHs have been associated with processing speed dysfunction via direct and indirect effects on AD-specific radiographic signatures [156]. Ischemic strokes are likewise associated with VCI. The clinical cognitive outcome associated with prior infarction is impairment of frontal-subcortical functions, such as perceptual speed [55]. In addition to the cumulative effect of prior infarcts, one or more strategic infarcts in select anatomic areas associated with cognitive processes may trigger direct cognitive consequences — these are known as strategic infarcts, or single-stroke dementia. Some studies have correlated certain white matter tract lacunar infarcts to specific cognitive domain dysfunction, but concede that making cognitive outcome predictions based on location of stroke is not yet possible [54, 55]. In fact, most studies report a diversity of anatomic locations that have been associated with this VCI phenotype. The presence of more than one infarction was most strongly associated with perceptual speed and other frontal-subcortical functions [157]. Brain atrophy is measured by employing volumetric analysis of CSF (e.g., sulcal and ventricular CSF volumes) and cortical thickness (e.g., medial temporal lobe thickness). Such atrophy denotes disease progression in the context of cerebrovascular disease. Perivascular spaces on CT or MRI are visible as parenchymal hyperintensities on MRI T2-weighted images (or hypointesities on T1/FLAIR) as either linear, if run along the image plane, or round, if they are perpendicular to the image plane [158]. They are common in elderly and visible on MRI in 50–100% of individuals, depending on the imaging methods, scanner resolution, and criteria used for PVS assessment. Most commonly, PVS are seen in the basal ganglia and the centrum semiovale, and less in the hippocampus, the midbrain, the pons, and very rarely in the cerebellum [159]. The exact anatomy, structure, and the function of PVS and their role in cognitive dysfunction and risk of dementia are still unclear. Novel imaging technology to measure cerebrovascular injury is emerging and will inform future VCI research and definitions, particularly imaging of blood–brain barrier (BBB) integrity using gadolinium-enhanced MRI [160]. It measures contrast agent leakage from the blood plasma to the brain interstitial space over time and allows the detection of subtle leakage values in aging, SVD, MCI, AD, and VaD [161]. However, whether BBB leakage is associated with the variation in age-related cognitive decline remains to be investigated. The additional imaging of vascular dysfunction and BBB disruption has been recently suggested to the AD “ATN” (amyloid, tau, neurodegeneration) biomarker research framework [162, 163].
The harmonization of neuroimaging markers of cerebrovascular injury for the diagnosis of VCI is underway. The Harmonizing Brain Imaging Methods for Vascular Contributions to Neurodegeneration (HARNESS) initiative provides resources to reduce variability in measurement in MRI studies of SVD and has made available MRI protocols and analysis tools for research use [164]. This initiative complements the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria that suggests harmonized definitions of common cerebrovascular pathologies [158]. Similarly, the NIH-funded MarkVCID is established to validate both neuroimaging and serum- or fluid-based biomarkers for VCI [127, 128]. Most recently, the NIH has awarded the INDEED study (clinical significance of incidental white matter lesions on MRI among a diverse population of cognitive complaints) to investigate the role of MRI-quantified white matter lesions on cognition and health outcomes [165]. There is a great expectation that this research will soon translate the use of multiple neuroimaging biomarkers of VCI into clinical practice.
Vascular Risk Factors and Vascular Cognitive Impairment
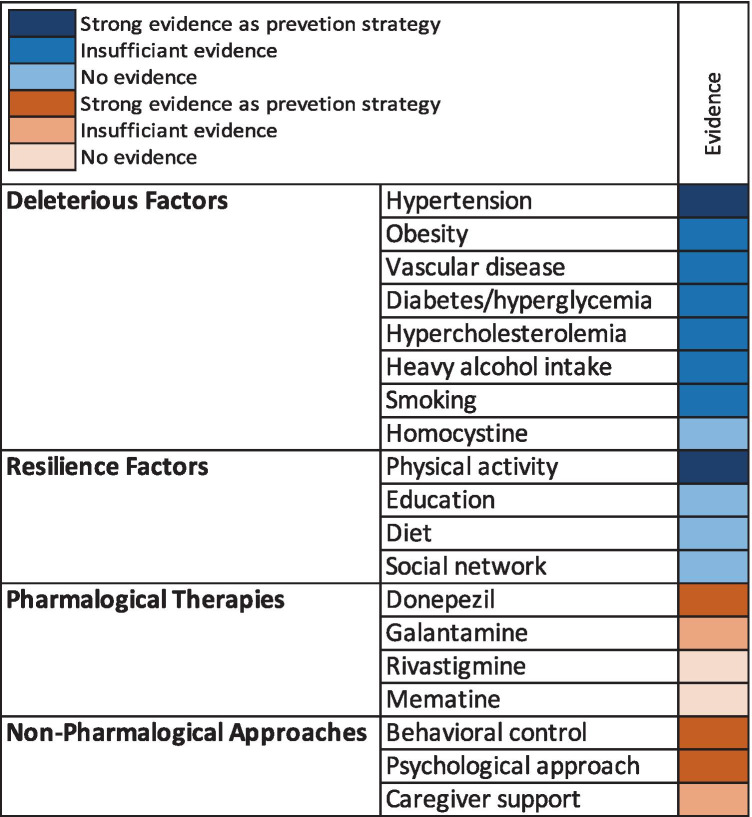
Evidence accumulated over the past several decades suggests a significant contribution of vascular risk factors to VCI [166] and AD [167, 168]. Although age remains the most significant risk factor for VCI and all-cause dementia [169], vascular risk factors come in second with strongest evidence supporting an effect of hypertension, hyperglycemia, and diabetes [25]. The evidence for the associations of deleterious and resilience factors with VCI as well as for therapeutic approaches to VCI is summarized in Fig. 4.
Fig. 4.
Evidence for VCI preventive and therapeutic strategies
Deleterious Factors
Hypertension is strongly and negatively associated with cognitive function. Hypertension has been consistently associated with poor performance in executive function [170]; greater rate of cognitive decline [171]; increased risk for MCI [171]; dementia — particularly vascular dementia (VaD) [172]; and structural and functional brain changes [173]. When uncontrolled, hypertension may lead to regional patterns of gray matter atrophy associated with white matter lesions and with worse cognitive performance [174]. In the Honolulu-Asia Aging Study, midlife systolic blood pressure (SBP) of ≥ 120 mmHg was associated with an increased risk of late-life dementia and VaD, with the latter risk being reduced with antihypertensive treatment for SBP but not DBP [175]. In NOMAS, the inverse association of SBP with processing speed/visual motor integration function was non-significant when antihypertension treatment was accounted for, in a support of the effect of hypertension control on maintenance of cognitive function [176]. The evidence of increased risk of VaD [177] is particularly strong for midlife rather than late-life hypertension [178], while for AD this relationship is less clear [179]. Furthermore, the relationship between hypertension and cognition appears to be U-shaped, with both higher and lower BP associated with worse cognitive outcomes in older adults [180]. Data pooled across 5 large cohorts suggest that racial disparities in late-life cognitive decline may be explained at least in part by higher BP levels in Black compared to white participants [181].
Obesity has been consistently linked to an increased risk of future dementia when measured in midlife, while after the age of 65, obesity appears to protect against dementia [182]. Newer evidence from a study of 39 cohorts totaling over 1.3 million dementia-free individuals suggests that the positive effect in later life may in fact be confounded by weight loss during the preclinical dementia phase, while the negative effect of midlife BMI is less likely impacted by preclinical changes and may better capture its direct effect on dementia risk [183]. Several mechanisms have been proposed to explain the link between obesity and cognitive function, including chronic low grade systemic inflammation and oxidative stress in obese individuals [184], increased permeability of the BBB [185], and increased insulin resistance leading to declines in glucose metabolism, all of which contribute to neurodegeneration and neuronal death [186]. In addition, obesity may play a significant role in the development of VCI by promoting arterial stiffness and development of atherosclerosis and SVD [187] via endothelial dysfunction [188]. Weight control is currently considered a reasonable (class IIb/level B evidence) preventive strategy for individuals at risk for VCI [132].
Diabetes and high glucose levels are consistently linked to poor cognitive performance [189], increased risk of dementia [190] and VCI [191]. Individuals < 65 years of age [192] and those with undiagnosed diabetes [189] are at particular high risk. Poor glycemic control in middle-aged patients with type 2 diabetes mellitus was found to significantly increase rate of decline in memory and reasoning in the Whitehall II cohort study [193]. These effects are supported by a strong relationship of hyperglycemia, diabetes, and insulin resistance with brain vascular changes [191, 194], alterations in cerebral flow [195], AD and non-AD pathology [196], brain infarcts [197], and changes in BBB permeability, all of which play a role in the development of VCI. Metabolic syndrome, a clustering of vascular risk factors, has been negatively linked to cognitive function across global measures [198] and cognitive domains [199] and with increased risk of MCI and its progression [200].
Lipids are the basic components of cell membranes. In the brain, long-chain polyunsaturated fatty acids (PUFAs) account for about 30% of total fatty acids including docosahexaenoic acid (DHA) and arachidonic acid [201] and are implicated in the maintenance of membrane permeability and the interaction between lipids and proteins, therefore promoting brain neurogenesis and modulating inflammation [202]. Reduction in PUFAs has been linked to lipid rafts, particularly in the frontal cortex [203], which may promote aggregation of beta amyloid and hyperphosphorylated tau [204]. Large observational studies support a link between high cholesterol particularly in midlife and cognitive impairment and development of AD and VaD in later life, independently of other vascular risk factors including hypertension and diabetes [205]. These findings are supported by a slower progression of cognitive decline in individuals taking statins, particularly among homozygous ApoE4 carriers [206]. The evidence on the effect of long-chain PUFA enriched diets on cognitive performance in older adults is conflicting, with some showing improvement in memory and executive function [207, 208] while others reporting no effects [209–211]. These conflicting results are a consequence of methodological limitations related to lack of uniform biomarkers and sufficient duration of intervention. Trials that address these limitations may help further our understanding of the effect of lipids on cognitive function in older adults and elucidate mechanisms of action [212].
Elevated homocysteine, a risk factor for vascular damage, has been linked to cognitive impairment and a greater likelihood of dementia and VaD [213], findings supported by an evidence of increased neuropathological burden in individuals with high homocysteine levels [214]. Although earlier meta-analyses did not find supporting evidence that lowering homocysteine helps prevent cognitive decline and dementia [215–217], findings from clinical trials of sufficient duration [218] and in participants already cognitively impaired [219] support a beneficial effect of lowering homocysteine with vitamin B supplementation. This positive effect was found on cognitive performance [218] and on the slower rate of brain atrophy in individuals with MCI and particularly in those with total plasma homocysteine levels > 13 μmol/L [219]. However, the benefits of lowering homocysteine on VCI prevention or progression are yet to be determined [214, 220].
Active smoking has detrimental effects on brain health. Early work had shown that nicotine, known for its short-term effects on the neuronal cholinergic system, may have potential benefits in terms of enhanced cognitive performance particularly on memory, cognitive functions that require sustained attention [221], and dementia risk [222], likely through inhibition of amyloid formation [223] and a modulation of choroid plexus function [224]. However, nicotine is just one among the thousands of compounds in tobacco smoke, with many having toxic effects on cardiovascular and pulmonary systems and the brain. The first longitudinal study to assess the impact of smoking on cognitive performance found smoking to increase the risk of cognitive impairment over a 20-year period [225], finding letter supported by subsequent studies showing greater cognitive declines in memory and executive function [226, 227] and a greater risk for dementia [228]. Smoking appears to have a greater impact on cognition in women as compared to men [229], while its relationship to VCI is unclear, although there is some evidence for a greater risk in smokers [230]. Smoking cessation is currently recommended as a reasonable (class IIa/level A) strategy for the prevention of VCI [132].
Due to a lack of homogeneity in the definition of alcohol intake, the use of reference groups, and outcomes, the impact of alcohol use on cognition is unclear [132]. However, there is reasonable evidence from large longitudinal prospective studies that drinking alcohol in moderation may have benefits in terms of a slower rate of decline in cognition [231] and reduced risk of dementia, AD, and VaD [232], potentially through a reduction in the accumulation of neuropathologic changes among individuals with a lifetime history of moderate alcohol intake [233]. In contrast, heavy drinking as well as abstinence have been linked to an increased risk for cognitive impairment [132] and dementia [232]. These reports support a modest benefit (class IIb/level B) of moderate alcohol consumption in older adults and particularly in those at risk for VCI and is therefore considered a reasonable preventative approach [132].
Resilience Risk Factors
Education is positively associated with cognitive performance. Older adults with higher education perform better on global and domain-specific measures of cognition such as working memory and reasoning [234], have a lower risk of dementia [235], and a lower risk of developing post-stroke dementia [236]. While education may not protect against vascular and other neurodegenerative pathologies [220], it may buffer the impact of the neurodegenerative pathology on clinical symptoms [237]. However, issues of confounding of the education-VCI relationship by various factors including quality of schooling, socioeconomic status, and acculturation have been raised [132], which together with a lack of its ability to prevent development of neuropathology suggest that educational interventions may not be very effective preventative approach for VCI.
Physical activity has been consistently linked to better performance on cognitive testing including global cognition [238] and executive function [239] and to a reduced risk of cognitive decline and dementia including VaD [220]. These effects may result from exercise-induced increases in expression of neurotrophic factors including brain-derived neurotrophic factor (BDNF) that promote neuro- and synaptogenesis and improve brain perfusion [132]. Other mechanisms include attenuation of age-related myelin reduction [240] and maintenance of white matter integrity [241]. The AHA/ASA guidelines gave physical activity class IIb/level B evidence as a reasonable prevention strategy for individuals at risk for VCI [132].
A healthy diet is another potential cognitively protective factor with evidence in support of the Mediterranean diet [242]. With its focus on fruits, vegetables, fish, nuts, whole grains, and monounsaturated oils, the Mediterranean diet provides adequate intake of antioxidants such as vitamins E and B12, folate, and n-3 fatty acids, the consumption of which either as part of diet or as supplements was linked to better cognitive function [220] and reduced risk of cognitive impairment [243]. These findings are however inconsistent. Several studies reported no cognitive benefit for antioxidants [244, 245] and fatty acids [246], while Mediterranean diet was not assessed specifically for VCI risk [132]. Higher circulatory levels of vitamin D have also been linked to better cognitive function and may protect against cardiovascular disease and stroke [247], although the evidence is inconsistent [248]. Finally, intake of folic acid and vitamins B6 and B12 may provide protection against cognitive decline by increasing production and metabolism of homocysteine [249]. The AHA/ASA guidelines place diet at class III/level A evidence and do not recommend diet for the prevention of cognitive impairment including VCI.
Although having an active social network and social support have been linked to better cognitive function [250] and may reduce risk of dementia [251], the quality of social support can have differential effects. While positive social support from the immediate family has been linked to reduced risk of dementia, negative social support has the opposite effect increasing the risk [252]. In addition, being married, having contact with friends, engagement in paid work, and participation in community groups may reduce the risk of incident dementia [253]. Testing these relationships in randomized clinical trials and specifically in relation to VCI is needed before a recommendation for social support as a VCI preventative strategy can be made [132].
The Impact of Combinations of Risk Factors on VCI
While individual risk factors are important to consider when assessing risk of dementia and VCI, the combined effect of risk factors within an individual is a stronger predictor of cognitive decline than independent risk factors. A higher number of vascular risk factors within an individual were associated with greater impairment in executive function and processing speed [254]. In contrast, an increasing number of ideal cardiovascular health factors may protect against decline in processing speed, executive function, and memory [255]. The Cardiovascular Risk Factors, Aging and Dementia (CAIDE), a midlife composite vascular risk score that does not require labs and better reflects the age and education distribution of the study population, was associated with greater cognitive decline [256] and found to predict dementia risk 20 years [257] and even 40 years later [258]. In addition, a higher CAIDE risk score was linked to lower performance on global and domain-specific cognitive tests and helped discriminate cognitive impairment from normal cognition, MCI and dementia cases, and particularly VCI cases from controls [259]. Other vascular risk scores such as the NOMAS GVRS (Global Vascular Risk Score) were found to be inversely associated with level of global cognition and shown to better predict declines in processing speed and memory compared to CAIDE, likely because of an additional inclusion of smoking and glucose levels [260], further supporting the idea that a higher number of vascular risk factors within an individual better predicts cognitive decline.
Strategies for VCI Prevention
One strategy for the prevention of dementia and VCI is to address modifiable vascular risk factors, with particular interest in multimodal interventions.
Several large clinical trials have assessed the impact of antihypertensive medications on cognitive outcomes including risk of dementia and VCI in patient populations with various risk conditions. One of the first and most compelling studies is the Dementia Study of the Systolic Hypertension Europe (Syst-Eur), which randomized 2418 dementia-free 60 + years adults with seated SBP between 160 and 219 mmHg and DBP < 95 mmHg to either an active treatment receiving nitrendipine (a dihydropyridine) combined or replaced by enalapril (an ACE inhibitor) and/or hydrochlorothiazide (a diuretic) or a control group [261]. After an average of 2 years of follow-up, a 50% reduction in the incidence of dementia was reported for the active treatment group. In an open-label follow-up study, a significant reduction in both AD and VaD was reported for the active treatment group [262]. The Dementia Study of the Systolic Blood Pressure Intervention Trial (SPRINT–MIND) randomized 9361 50 + years patients with SBP between 130 and 180 mmHg and increased cardiovascular risk to an intensive BP lowering regimen (< 120 mmHg) or the standard regimen of < 140 mmHg [263]. A significant 20% reduction in the risk of MCI and 15% in a composite outcome of MCI or probable dementia was reported, as well as a smaller increase in cerebral white matter lesion volume in the intensive treatment group was observed [264]. In the Dementia study of the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS), another large trial comparing 2 antiplatelet treatments (ASA + dipyridamole vs. clopidogrel and telmisartan) in 20,332 patients with prior stroke, there was no difference in the rates of cognitive decline between the 2 treatment groups, which also did not differ in risk of recurring stroke or major vascular events that were the primary outcomes [265, 266]. Additional support for a benefit of lowering BP on cognition comes from the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), in which 6105 older adults with a history of cerebrovascular events were randomized to either perindopril (ACE inhibitor) and indapamide (diuretic) vs. placebo [267]. Receiving the BP lowering treatment was associated with significant reductions in the risk of dementia and cognitive decline in patients with recurrent stroke and delayed progression of WMHs [268] in addition to substantial reductions in stroke risk [269]. The AHA/ASA guidelines gave class I/level A evidence to BP lowering as a preventative strategy in people at risk for VCI [132].
Existing evidence points to a negative contribution of midlife hypercholesterolemia to cognitive function and increased risk of dementia and VCI. Early statin trials, however, failed to demonstrate an effect on cognitive decline. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) reported no differences between a pravastatin and a control group in rates of decline in cognitive tests during the 3-year follow-up [270]. This was in line with data from the Heart Protection Study, which found no benefit of simvastatin on cognition [245]. Some evidence for a benefit for atorvastatin in improving cognitive function in older adults with dementia was reported in a small trial [271]; however, other statin clinical trials in patients with mild to moderate AD did not show cognitive benefits [272]. A benefit of simvastatin in preserving white matter microstructure was reported in cognitively normal middle-aged adults, suggesting a potential for VCI prevention [273]. Larger trials are however needed to establish a clinical benefit for statins in preventing or treating VCI. Nevertheless, maintenance of normal plasma cholesterol levels remains an important health promotion strategy. Treatment for hypercholesterolemia is currently recommended as a reasonable preventative modality for individuals at risk for VCI (class IIb/level B evidence) [132].
There was some initial evidence of an effect of diabetes treatments on cognition in small trials [274]; larger trials have been largely negative. Intensive glucose lowering in the ACCORD study in participants with type 2 diabetes mellitus (T2DM) did not significantly reduce major cardiovascular events over a period of 3.5 years and was found to increase mortality [275]. In the ACCORD MIND sub-study, intensive lowering of lipid and BP levels did not affect cognitive decline and was associated with greater decline in brain volume compared to standard therapy [276]. The latter finding could be explained by compromised brain perfusion and impaired autoregulation during intensive BP lowering, suggesting that intensive lowering of BP in older adults with T2DM may not be safe or beneficial [277]. In the largest study to date testing the efficacy of low-dose pioglitazone in delaying onset of MCI, there was no effect on delaying onset of MCI due to AD [278]. A recent meta-analysis of T2DM randomized clinical trials also found no robust evidence that T2DM treatment prevents or delays cognitive impairment [279]. Trials to assess impact of diabetes control on cognitive decline are needed in middle-aged individuals at risk for VCI as they may be able to assess benefits in preventing development of VCI. Treatment of hyperglycemia is currently considered a reasonable preventative strategy for individuals at risk for VCI (class IIb/level C of evidence) [132].
Multimodal interventions such as in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a 2-year multicenter RCT that enrolled 1260 adults 60–77 years of age into an intervention including diet, exercise, cognitive training, and vascular risk monitoring or a control group, provided strong evidence for positive cognitive change and potential for prevention of AD and VCI [280]. The FINGER model with multiple vascular risk factors control seems to be most promising brain health strategy and currently is being tested in diverse populations worldwide.
Strategies for Symptomatic Treatment
Symptomatic treatment for VCI requires a multifaceted approach that involves pharmacological therapies to directly address cognitive and behavioral symptoms as well as non-pharmacological modalities that focus on optimizing quality of life for both patients and caregivers [281].
Several clinical trials had tested the effect of cholinesterase inhibitors and NMDA receptor antagonists on cognition, global and physical functions with modest support for their efficacy in VCI [132]. The first 2 large trials of donepezil reported benefits on cognition and to a lesser extent on global function and activities of daily living outcomes, with similar side effects as observed in AD [282, 283]. A meta-analysis of 12 studies provided support for the efficacy of donepezil in improving cognitive function [284], although the improvements failed to reach clinical significance [285]. Galantamine in comparison to placebo did not show clinical significance, but showed some benefits on cognition, improved functionality measures, and reduced behavioral symptoms in mixed AD/VaD, not in pure VaD [286]. A trial that included only patients with pure VaD reported improvement in cognition, with 40% of participants in the galantamine group having a clinically significant change in ADAS-Cog score of ≤ − 4 points versus 27% in the placebo group [287]. Evidence for rivastigmine and memantine is even less robust, with two studies showing a slight improvement in executive function and behavior for rivastigmine in patients with subcortical VaD [288], in executive function in VCI patients [289], and in cognition for memantine in mild to moderate VaD patients [290, 291], although these improvements were not clinically significant. A recent meta-analysis found moderate to high evidence that donepezil has the greatest effect on cognition followed by galantamine, although effects were not of clinical significance [292]. Despite its slight effects and in the absence of other treatments, this meta-analysis supports the use of donepezil in people with VCI, which is in line with the AHA/ASA recommendations regarding the use of donepezil for cognitive enhancement in VCI [132].
Caregivers need a support system to address the challenges posed by a dementia diagnosis and clinicians are well posited to guide them as they learn to navigate the formal care system, identify community resources to address challenges in various aspects of health, assess transportation needs, plan for future care needs including placement and palliative care, and help in the management of psychological symptoms and neurobehavioral complications [132]. Another important goal is to reduce caregiving-related stress, burden, and strain, and improve their quality of life. As caregiver and patient experiences with dementia care and their impact on health are closely related and often reinforce each other, reducing caregiver stress, addressing their support needs, and improving their coping skills have the potential to elicit positive effects in VCI patient outcomes [293] and delays in nursing home placement [294].
Concluding Thoughts
Vascular cognitive impairment (VCI) includes the whole spectrum of cognitive impairment ranging from clinical mild cognitive difficulties that are evident only on cognitive testing to MCI and clinical dementia. The neuropathology of cognitive impairment in later life is often a mixture of vascular, AD, and other neurodegenerative pathology, which overlap and increase risk of cognitive impairment. Determining the contribution of vascular disease to VCI is greatly facilitated by neuroimaging, particularly by novel MRI techniques and advancements in magnetic field strength. Cerebrovascular risk factors are common among older adults and are major contributors to VCI. Currently, no specific treatments for VCI exist, but standard stroke preventive measures are recommended. Multimodality interventions that include the modifications of vascular risk factors and lifestyle are currently most promising VCI treatment and prevention strategy. VCI has been increasingly recognized as most prominent concept of vascular and mixed dementias and has received major attention worldwide with the opportunities for collaborative actions. VCI clinical and scientific framework that accounts for complexity of vascular factors and overlaying diagnoses will help drive translational research for improved understanding and ultimately lead to effective prevention and treatment of VCI in clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, et al. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;15(4):18003. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 2.Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36(2):281–288. doi: 10.1007/s10571-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Su J, Gao C, Ni W, Gao X, Li Y, et al. Progression in vascular cognitive impairment: pathogenesis, neuroimaging evaluation, and treatment. Cell Transplant. 2019;28(1):18–25. doi: 10.1177/0963689718815820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanon Zotin MC, Sveikata L, Viswanathan A, Yilmaz P. Cerebral small vessel disease and vascular cognitive impairment: from diagnosis to management. Curr Opin Neurol. 2021;34(2):246–257. doi: 10.1097/WCO.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paraskevas KI, Faggioli G, Ancetti S, Naylor AR. Editor’s choice - asymptomatic carotid stenosis and cognitive impairment: a systematic review. Eur J Vasc Endovasc Surg. 2021;61(6):888–899. doi: 10.1016/j.ejvs.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Rost NS, Meschia JF, Gottesman R, Wruck L, Helmer K, Greenberg SM, et al. Cognitive impairment and dementia after stroke: design and rationale for the DISCOVERY Study. Stroke. 2021;52(8):e499–516. doi: 10.1161/STROKEAHA.120.031611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avan A, Hachinski V. Stroke and dementia, leading causes of neurological disability and death, potential for prevention. Alzheimers Dement. 2021;17(6):1072–1076. doi: 10.1002/alz.12340. [DOI] [PubMed] [Google Scholar]
- 8.Verdelho A, Wardlaw J, Pavlovic A, Pantoni L, Godefroy O, Duering M, et al. Cognitive impairment in patients with cerebrovascular disease: a white paper from the links between stroke ESO Dementia Committee. Eur Stroke J. 2021;6(1):5–17. doi: 10.1177/23969873211000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KL, Pedelty L, Testai FD. The relationship between heart disease and cognitive impairment. Handb Clin Neurol. 2021;177:377–391. doi: 10.1016/B978-0-12-819814-8.00023-8. [DOI] [PubMed] [Google Scholar]
- 10.Jinawong K, Apaijai N, Chattipakorn N, Chattipakorn SC. Cognitive impairment in myocardial infarction and heart failure. Acta Physiol (Oxf). 2021;232(1):e13642. [DOI] [PubMed]
- 11.Loeffler DA. Modifiable, non-modifiable, and clinical factors associated with progression of Alzheimer’s disease. J Alzheimers Dis. 2021;80(1):1–27. doi: 10.3233/JAD-201182. [DOI] [PubMed] [Google Scholar]
- 12.Vespa J, Medina L, Armstrong DM. Population estimates and projections. US Census Bureau. 2020; 15.
- 13.International AD, Patterson C. World Alzheimer report 2018: the state of the art of dementia research: new frontiers. 2018 [cited 2021 Aug 20]. Available from: https://www.alzint.org/resource/world-alzheimer-report-2018/
- 14.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JCS, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 16.Wolf PA. Contributions of the Framingham Heart Study to stroke and dementia epidemiologic research at 60 years. Arch Neurol. 2012;69(5):567–571. doi: 10.1001/archneurol.2011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74–83. doi: 10.1002/ana.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 19.Noble JM, Schupf N, Manly JJ, Andrews H, Tang M-X, Mayeux R. Secular trends in the incidence of dementia in a multi-ethnic community. J Alzheimers Dis. 2017;60(3):1065–1075. doi: 10.3233/JAD-170300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimers Dement (Amst) 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed]
- 22.Wright CB, DeRosa JT, Moon MP, Strobino K, DeCarli C, Cheung YK, et al. Race/ethnic disparities in mild cognitive impairment and dementia: the Northern Manhattan Study. J Alzheimers Dis. 2021;80(3):1129–1138. doi: 10.3233/JAD-201370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshadri S, Caunca MR, Rundek T. 18 - Vascular dementia and cognitive impairment. In: Grotta JC, Albers GW, Broderick JP, Day AL, Kasner SE, Lo EH, et al. editors. Stroke (seventh edition) [Internet]. Philadelphia: Elsevier. 2022 [cited 2021 Aug 20]; 221–236.e8. Available from: https://www.sciencedirect.com/science/article/pii/B9780323694247000181
- 24.Hachinski V, Einhäupl K, Ganten D, Alladi S, Brayne C, Stephan BCM, et al. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimer’s & Dementia. 2019;15(7):961–984. doi: 10.1016/j.jalz.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120(3):573–591. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- 26.Caruso P, Signori R, Moretti R. Small vessel disease to subcortical dementia: a dynamic model, which interfaces aging, cholinergic dysregulation and the neurovascular unit. Vasc Health Risk Manag. 2019;7(15):259–281. doi: 10.2147/VHRM.S190470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlokovic BV, Gottesman RF, Bernstein KE, Seshadri S, McKee A, Snyder H, et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimer’s & Dementia. 2020;16(12):1714–1733. doi: 10.1002/alz.12157. [DOI] [PubMed] [Google Scholar]
- 28.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayed N, Huang Y, Nguyen K, Krejciova-Rajaniemi Z, Grawe AP, Gao T, et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging. 2021;1(7):598–615. doi: 10.1038/s43587-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312(1):H1–20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci (Lond). 2017;131(19):2451–68. [DOI] [PubMed]
- 35.Park J-H, Hong J-H, Lee S-W, Ji HD, Jung J-A, Yoon K-W, et al. The effect of chronic cerebral hypoperfusion on the pathology of Alzheimer’s disease: a positron emission tomography study in rats. Sci Rep. 2019;9(1):14102. doi: 10.1038/s41598-019-50681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol. 2018;31(1):36–43. doi: 10.1097/WCO.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 37.Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia. J Am Coll Cardiol. 2019;73(25):3326–3344. doi: 10.1016/j.jacc.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joutel A, Chabriat H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci (Lond) 2017;131(8):635–651. doi: 10.1042/CS20160380. [DOI] [PubMed] [Google Scholar]
- 39.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naranjo O, Osborne O, Torices S, Toborek M. In vivo targeting of the neurovascular unit: challenges and advancements. Cell Mol Neurobiol. 2021. [DOI] [PMC free article] [PubMed]
- 41.Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40(3 Suppl):S2–3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120(3):287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones EG. On the mode of entry of blood vessels into the cerebral cortex. J Anat. 1970;106(Pt 3):507–520. [PMC free article] [PubMed] [Google Scholar]
- 44.Morris AWJ, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016;131(5):725–736. doi: 10.1007/s00401-016-1555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed]
- 46.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16(3):137–153. doi: 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 47.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallina P, Nicoletti C, Scollato A, Lolli F. The, glymphatic-lymphatic system pathology and a new categorization of neurodegenerative disorders. Front Neurosci. 2021;15:527. doi: 10.3389/fnins.2021.669681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet. 1974;2(7874):207–10. [DOI] [PubMed]
- 50.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62(4):406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 51.Jaillard A, Grand S, Le Bas JF, Hommel M. Predicting cognitive dysfunctioning in nondemented patients early after stroke. Cerebrovasc Dis. 2010;29(5):415–423. doi: 10.1159/000289344. [DOI] [PubMed] [Google Scholar]
- 52.Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85(5):927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatemichi TK, Desmond DW, Prohovnik I. Strategic infarcts in vascular dementia. A clinical and brain imaging experience. Arzneimittelforschung. 1995;45(3A):371–85. [PubMed]
- 54.Biesbroek JM, Kuijf HJ, van der Graaf Y, Vincken KL, Postma A, Mali WPTM, et al. Association between subcortical vascular lesion location and cognition: a voxel-based and tract-based lesion-symptom mapping study. The SMART-MR study. PLoS One. 2013;8(4):e60541. [DOI] [PMC free article] [PubMed]
- 55.Duering M, Gesierich B, Seiler S, Pirpamer L, Gonik M, Hofer E, et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology. 2014;82(22):1946–1950. doi: 10.1212/WNL.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed]
- 57.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 58.Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39(3):800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp. 2009;30(4):1155–1167. doi: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. [DOI] [PMC free article] [PubMed]
- 61.Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 62.Dhamoon MS, Cheung Y-K, DeRosa JT, Gutierrez J, Moon YP, Sacco RL, et al. Association between subclinical brain infarcts and functional decline trajectories. J Am Geriatr Soc. 2018;66(11):2144–2150. doi: 10.1111/jgs.15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 64.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MSV, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 2015;85(5):441–449. doi: 10.1212/WNL.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42(3):722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao L, Tan L, Wang H-F, Jiang T, Zhu X-C, Yu J-T. Cerebral microinfarcts and dementia: a systematic review and metaanalysis. Curr Alzheimer Res. 2017;14(7):802–808. doi: 10.2174/1567205013666161201200429. [DOI] [PubMed] [Google Scholar]
- 68.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10(3):376–381. doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gutierrez J, Rundek T, Ekind MSV, Sacco RL, Wright CB. Perivascular spaces are associated with atherosclerosis: an insight from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2013;34(9):1711–1716. doi: 10.3174/ajnr.A3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gutierrez J, Elkind MSV, Dong C, Di Tullio M, Rundek T, Sacco RL, et al. Brain perivascular spaces as biomarkers of vascular risk: results from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2017;38(5):862–867. doi: 10.3174/ajnr.A5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams HHH, Hilal S, Schwingenschuh P, Wittfeld K, van der Lee SJ, DeCarli C, et al. A priori collaboration in population imaging: the Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement consortium. Alzheimers Dement (Amst) 2015;1(4):513–520. doi: 10.1016/j.dadm.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jie W, Lin G, Liu Z, Zhou H, Lin L, Liang G, et al. The relationship between enlarged perivascular spaces and cognitive function: a meta-analysis of observational studies. Front Pharmacol. 2020;11:715. doi: 10.3389/fphar.2020.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paradise M, Crawford JD, Lam BCP, Wen W, Kochan NA, Makkar S, et al. Association of dilated perivascular spaces with cognitive decline and incident dementia. Neurology. 2021;96(11):e1501–e1511. doi: 10.1212/WNL.0000000000011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benedictus MR, Hochart A, Rossi C, Boulouis G, Hénon H, van der Flier WM, et al. Prognostic factors for cognitive decline after intracerebral hemorrhage. Stroke. 2015;46(10):2773–2778. doi: 10.1161/STROKEAHA.115.010200. [DOI] [PubMed] [Google Scholar]
- 75.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: Systematic review, subgroup analyses and standards for study design and reporting. Brain J Neurol. 2007;1(130):1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 76.Cordonnier C, Leys D, Dumont F, Deramecourt V, Bordet R, Pasquier F, et al. What are the causes of pre-existing dementia in patients with intracerebral haemorrhages? Brain. 2010;133(11):3281–3289. doi: 10.1093/brain/awq246. [DOI] [PubMed] [Google Scholar]
- 77.Wiegman AF, Meier IB, Schupf N, Manly JJ, Guzman VA, Narkhede A, et al. Cerebral microbleeds in a multiethnic elderly community: demographic and clinical correlates. J Neurol Sci. 2014;345(1–2):125–130. doi: 10.1016/j.jns.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, et al. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75(24):2221–2228. doi: 10.1212/WNL.0b013e3182020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poels MMF, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78(5):326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 80.Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, et al. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45(5):1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caunca MR, Del Brutto V, Gardener H, Shah N, Dequatre-Ponchelle N, Cheung YK, et al. Cerebral microbleeds, vascular risk factors, and magnetic resonance imaging markers: the Northern Manhattan Study. J Am Heart Assoc. 2016;5(9):e003477. [DOI] [PMC free article] [PubMed]
- 82.van Veluw SJ, Biessels GJ, Klijn CJM, Rozemuller AJM. Heterogeneous histopathology of cortical microbleeds in cerebral amyloid angiopathy. Neurology. 2016;86(9):867–871. doi: 10.1212/WNL.0000000000002419. [DOI] [PubMed] [Google Scholar]
- 83.Tuladhar AM, van Dijk E, Zwiers MP, van Norden AGW, de Laat KF, Shumskaya E, et al. Structural network connectivity and cognition in cerebral small vessel disease. Hum Brain Mapp. 2016;37(1):300–310. doi: 10.1002/hbm.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reijmer YD, Fotiadis P, Martinez-Ramirez S, Salat DH, Schultz A, Shoamanesh A, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138(Pt 1):179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7(1):1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28(7):1418–22. [DOI] [PubMed]
- 87.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm (Vienna) 2002;109(5–6):813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 88.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58(11):1629–1634. doi: 10.1212/WNL.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 89.Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930–1936. doi: 10.1212/WNL.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron J-C, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138(Pt 8):2126–2139. doi: 10.1093/brain/awv162. [DOI] [PubMed] [Google Scholar]
- 91.Shoamanesh A, Akoudad S, Himali JJ, Beiser AS, DeCarli C, Seshadri S, et al. Cortical superficial siderosis in the general population: the Framingham Heart and Rotterdam studies. Int J Stroke. 2021;21:1747493020984559. doi: 10.1177/1747493020984559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63(9):1606–1612. doi: 10.1212/01.WNL.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 93.Mutimer CA, Keragala CB, Markus HS, Werring DJ, Cloud GC, Medcalf RL. Cerebral amyloid angiopathy and the fibrinolytic system: is plasmin a therapeutic target? Stroke. 2021;52(8):2707–2714. doi: 10.1161/STROKEAHA.120.033107. [DOI] [PubMed] [Google Scholar]
- 94.Tublin JM, Adelstein JM, Del Monte F, Combs CK, Wold LE. Getting to the heart of Alzheimer disease. Circ Res. 2019;124(1):142–149. doi: 10.1161/CIRCRESAHA.118.313563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Troncone L, Luciani M, Coggins M, Wilker EH, Ho C-Y, Codispoti KE, et al. Aβ amyloid pathology affects the hearts of patients with Alzheimer’s disease: mind the heart. J Am Coll Cardiol. 2016;68(22):2395–2407. doi: 10.1016/j.jacc.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manolis TA, Manolis AA, Apostolopoulos EJ, Melita H, Manolis AS. Atrial fibrillation and cognitive impairment: an associated burden or burden by association? Angiology. 2020;71(6):498–519. doi: 10.1177/0003319720910669. [DOI] [PubMed] [Google Scholar]
- 97.Sepehri Shamloo A, Dagres N, Müssigbrodt A, Stauber A, Kircher S, Richter S, et al. Atrial fibrillation and cognitive impairment: new insights and future directions. Heart Lung Circ. 2020;29(1):69–85. doi: 10.1016/j.hlc.2019.05.185. [DOI] [PubMed] [Google Scholar]
- 98.Mongkhon P, Fanning L, Lau WCY, Tse G, Lau KK, Wei L, et al. Oral anticoagulant and reduced risk of dementia in patients with atrial fibrillation: a population-based cohort study. 2020 [cited 2021 Aug 20]. Available from: http://hub.hku.hk/handle/10722/284984 [DOI] [PubMed]
- 99.Mongkhon P, Naser AY, Fanning L, Tse G, Lau WCY, Wong ICK, et al. Oral anticoagulants and risk of dementia: a systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev. 2019;96:1–9. doi: 10.1016/j.neubiorev.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 100.Rundek T. Beyond percent stenosis: carotid plaque surface irregularity and risk of stroke. Int J Stroke. 2007;2(3):169–171. doi: 10.1111/j.1747-4949.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- 101.Suter O-C, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33(8):1986–1992. doi: 10.1161/01.STR.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 102.George AE, de Leon MJ, Ferris SH, Kricheff II. Parenchymal CT correlates of senile dementia (Alzheimer disease): loss of gray-white matter discriminability. AJNR Am J Neuroradiol. 1981;2(3):205–213. [PMC free article] [PubMed] [Google Scholar]
- 103.Zarow C, Weiner MW, Ellis WG, Chui HC. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav. 2012;2(4):435–442. doi: 10.1002/brb3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Polak JF, O’Leary DH. Carotid intima-media thickness as surrogate for and predictor of CVD. Glob Heart. 2016;11(3):295–312.e3. doi: 10.1016/j.gheart.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 105.Touboul P-J, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18(4):346–349. doi: 10.1159/000081812. [DOI] [PubMed] [Google Scholar]
- 106.Eikendal ALM, Groenewegen KA, Anderson TJ, Britton AR, Engström G, Evans GW, et al. Common carotid intima-media thickness relates to cardiovascular events in adults aged. hypertension (Dallas, Tex : 1979) [Internet]. 2015 [cited 2021 Aug 20]; 65(4). Available from: https://portal.research.lu.se/portal/en/publications/common-carotid-intimamedia-thickness-relates-to-cardiovascular-events-in-adults-aged(0face793-571e-48df-989a-fb4a12491af2)/export.html [DOI] [PubMed]
- 107.Suzuki T, Wang W, Wilsdon A, Butler K, Adabag S, Griswold M, et al. Carotid intima-media thickness and the risk of sudden cardiac death: the ARIC Study and the CHS. J Am Heart Assoc. 2020;9(19):e016981. [DOI] [PMC free article] [PubMed]
- 108.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circulation: Cardiovascular Imaging. 2015;8(1):e002262. [DOI] [PMC free article] [PubMed]
- 109.Gardener H, Caunca MR, Dong C, Cheung YK, Elkind MSV, Sacco RL, et al. Ultrasound markers of carotid atherosclerosis and cognition. Stroke. 2017;48(7):1855–1861. doi: 10.1161/STROKEAHA.117.016921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects - the Tromsø study. Cerebrovasc Dis. 2012;33(2):159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 111.Rundek T, Della-Morte D, Gardener H, Dong C, Markert MS, Gutierrez J, et al. Relationship between carotid arterial properties and cerebral white matter hyperintensities. Neurology. 2017;88(21):2036–2042. doi: 10.1212/WNL.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamtchum-Tatuene J, Noubiap JJ, Wilman AH, Saqqur M, Shuaib A, Jickling GC. Prevalence of high-risk plaques and risk of stroke in patients with asymptomatic carotid stenosis: a meta-analysis. JAMA Neurol. 2020;77(12):1524–1535. doi: 10.1001/jamaneurol.2020.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marshall RS, Lazar RM, Liebeskind DS, Connolly ES, Howard G, Lal BK, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis – hemodynamics (CREST-H): study design and rationale. Int J Stroke. 2018;13(9):985–991. doi: 10.1177/1747493018790088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the CREST-2 clinical trials. Int J Stroke. 2017;12(7):770–778. doi: 10.1177/1747493017706238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 116.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35(11 Suppl 1):2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- 117.Hara K, Shiga A, Fukutake T, Nozaki H, Miyashita A, Yokoseki A, et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360(17):1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- 118.Markus HS, Schmidt R. Genetics of vascular cognitive impairment. Stroke. 2019;50(3):765–772. doi: 10.1161/STROKEAHA.118.020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69(2):320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beaufort N, Scharrer E, Kremmer E, Lux V, Ehrmann M, Huber R, et al. Cerebral small vessel disease-related protease HtrA1 processes latent TGF-β binding protein 1 and facilitates TGF-β signaling. PNAS. 2014;111(46):16496–16501. doi: 10.1073/pnas.1418087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haffner C, Malik R, Dichgans M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J Cereb Blood Flow Metab. 2016;36(1):158–171. doi: 10.1038/jcbfm.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rasquin SMC, Lodder J, Verhey FRJ. Predictors of reversible mild cognitive impairment after stroke: a 2-year follow-up study. J Neurol Sci. 2005;15(229–230):21–25. doi: 10.1016/j.jns.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 123.Steffens DC, Otey E, Alexopoulos GS, Butters MA, Cuthbert B, Ganguli M, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63(2):130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- 124.Heckman GA, Patterson CJ, Demers C, St Onge J, Turpie ID, McKelvie RS. Heart failure and cognitive impairment: challenges and opportunities. Clin Interv Aging. 2007;2(2):209–218. [PMC free article] [PubMed] [Google Scholar]
- 125.Oldenbeuving AW, de Kort PLM, Jansen BPW, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011;76(11):993–999. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 126.Ayerbe L, Ayis S, Wolfe CDA, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 127.Wilcock D, Jicha G, Blacker D, Albert MS, D’Orazio LM, Elahi FM, et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimers Dement. 2021;17(4):704–15. [DOI] [PMC free article] [PubMed]
- 128.Lu H, Kashani AH, Arfanakis K, Caprihan A, DeCarli C, Gold BT, et al. MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols Alzheimers Dement. 2021;17(4):716–725. doi: 10.1002/alz.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gladman JT, Corriveau RA, Debette S, Dichgans M, Greenberg SM, Sachdev PS, et al. Vascular contributions to cognitive impairment and dementia: research consortia that focus on etiology and treatable targets to lessen the burden of dementia worldwide. Alzheimers Dement (N Y) 2019;19(5):789–796. doi: 10.1016/j.trci.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sachdev PS, Lo JW, Crawford JD, Mellon L, Hickey A, Williams D, et al. STROKOG (stroke and cognition consortium): an international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring. 2017;7:11–23. doi: 10.1016/j.dadm.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 132.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lopez OL, Kuller LH, Becker JT, Jagust WJ, DeKosky ST, Fitzpatrick A, et al. Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology. 2005;64(9):1539–1547. doi: 10.1212/01.WNL.0000159860.19413.C4. [DOI] [PubMed] [Google Scholar]
- 134.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–60. [DOI] [PubMed]
- 135.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42(3 Pt 1):473–480. doi: 10.1212/WNL.42.3.473. [DOI] [PubMed] [Google Scholar]
- 136.Wetterling T, Kanitz RD, Borgis KJ. The ICD-10 criteria for vascular dementia. Dementia. 1994;5(3–4):185–188. doi: 10.1159/000106720. [DOI] [PubMed] [Google Scholar]
- 137.American Psychatric Association DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. JAMA. 1994;272(10):828–829. doi: 10.1001/jama.1994.03520100096046. [DOI] [Google Scholar]
- 138.Pohjasvaara T, Mäntylä R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences. Stroke. 2000;31(12):2952–7. [DOI] [PubMed]
- 139.Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gold G, Bouras C, Canuto A, Bergallo MF, Herrmann FR, Hof PR, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2002;159(1):82–87. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- 141.Skrobot OA, O’Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2017;13(6):624–633. doi: 10.1016/j.jalz.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 142.Skrobot OA, Black SE, Chen C, DeCarli C, Erkinjuntti T, Ford GA, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2018;14(3):280–292. doi: 10.1016/j.jalz.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 143.Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66(5):545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 144.Kloppenborg RP, Nederkoorn PJ, Geerlings MI, van den Berg E. Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology. 2014;82(23):2127–2138. doi: 10.1212/WNL.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 145.Wright CB, Dong C, Caunca MR, DeRosa J, Kuen Cheng Y, Rundek T, et al. MRI Markers predict cognitive decline assessed by telephone interview: the Northern Manhattan Study. Alzheimer Dis Assoc Disord. 2017;31(1):34–40. doi: 10.1097/WAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46(2):433–440. doi: 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thong JYJ, Hilal S, Wang Y, Soon HW, Dong Y, Collinson SL, et al. Association of silent lacunar infarct with brain atrophy and cognitive impairment. J Neurol Neurosurg Psychiatry. 2013;84(11):1219–1225. doi: 10.1136/jnnp-2013-305310. [DOI] [PubMed] [Google Scholar]
- 148.Jang J-W, Kim S, Na HY, Ahn S, Lee SJ, Kwak K-H, et al. Effect of white matter hyperintensity on medial temporal lobe atrophy in Alzheimer’s disease. Eur Neurol. 2013;69(4):229–235. doi: 10.1159/000345999. [DOI] [PubMed] [Google Scholar]
- 149.Dhamoon MS, Cheung Y-K, Moon Y, DeRosa J, Sacco R, Elkind MSV, et al. Cerebral white matter disease and functional decline in older adults from the Northern Manhattan Study: A longitudinal cohort study. PLoS Med. 2018;15(3):e1002529. [DOI] [PMC free article] [PubMed]
- 150.Dhamoon MS, Cheung Y-K, Bagci A, Alperin N, Sacco RL, Elkind MSV, et al. Differential effect of left vs. right white matter hyperintensity burden on functional decline: the Northern Manhattan Study. Frontiers in Aging Neuroscience. 2017;9:305. [DOI] [PMC free article] [PubMed]
- 151.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82(2):126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 152.Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimer’s and Dementia: Translational Research and Clinical Interventions. 2019;5:107–117. doi: 10.1016/j.trci.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TLS, et al. White matter hyperintensities are a core feature of Alzheimer’s disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol. 2016;79(6):929–939. doi: 10.1002/ana.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Jr, Rabinstein AA, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain. 2019;142(8):2483–2491. doi: 10.1093/brain/awz162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roseborough A, Ramirez J, Black SE, Edwards JD. Associations between amyloid β and white matter hyperintensities: a systematic review. Alzheimer’s & Dementia. 2017;13(10):1154–1167. doi: 10.1016/j.jalz.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 156.Caunca MR, Siedlecki K, Cheung YK, Alperin N, Lee SH, Elkind MSV, et al. Cholinergic white matter lesions, AD-signature cortical thickness, and change in cognition: the Northern Manhattan Study. J Gerontol A Biol Sci Med Sci. 2020;75(8):1508–1515. doi: 10.1093/gerona/glz279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Swartz RH, Stuss DT, Gao F, Black SE. Independent cognitive effects of atrophy and diffuse subcortical and thalamico-cortical cerebrovascular disease in dementia. Stroke. 2008;39(3):822–830. doi: 10.1161/STROKEAHA.107.491936. [DOI] [PubMed] [Google Scholar]
- 158.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39(3–4):224–231. doi: 10.1159/000375153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Montagne A, Toga AW, Zlokovic BV. Blood-brain barrier permeability and gadolinium: benefits and potential pitfalls in research. JAMA Neurol. 2016;73(1):13–14. doi: 10.1001/jamaneurol.2015.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Verheggen ICM, de Jong JJA, van Boxtel MPJ, Postma AA, Jansen JFA, Verhey FRJ, et al. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience. 2020;42(6):1751–1764. doi: 10.1007/s11357-020-00282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smith EE, Biessels GJ, De Guio F, de Leeuw FE, Duchesne S, Düring M, et al. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2019;1(11):191–204. doi: 10.1016/j.dadm.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.RePORT ⟩ RePORTER [Internet]. 2021 [cited 2021 Aug 22]. Available from: https://reporter.nih.gov/search/Qn7GwpkuREOTCQh2zP6TQQ/project-details/10144032
- 166.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 167.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pendlebury ST, Rothwell PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. The Lancet Neurology. 2019;18(3):248–258. doi: 10.1016/S1474-4422(18)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vicario A, Martinez CD, Baretto D, Diaz Casale A, Nicolosi L. Hypertension and cognitive decline: impact on executive function. J Clin Hypertens (Greenwich) 2005;7(10):598–604. doi: 10.1111/j.1524-6175.2005.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reitz C, Tang M-X, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64(12):1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rönnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40-year follow-up of a population-based cohort. Dement Geriatr Cogn Disord. 2011;31(6):460–466. doi: 10.1159/000330020. [DOI] [PubMed] [Google Scholar]
- 173.Muller M, Sigurdsson S, Kjartansson O, Aspelund T, Lopez OL, Jonnson PV, et al. Joint effect of mid- and late-life blood pressure on the brain: the AGES-Reykjavik study. Neurology. 2014;82(24):2187–2195. doi: 10.1212/WNL.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kern KC, Wright CB, Bergfield KL, Fitzhugh MC, Chen K, Moeller JR, et al. Blood pressure control in aging predicts cerebral atrophy related to small-vessel white matter lesions. Frontiers in Aging Neuroscience. 2017;9:132. doi: 10.3389/fnagi.2017.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stewart R, Xue Q-L, Masaki K, Petrovitch H, Ross GW, White LR, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. 2009;54(2):233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun X, Dong C, Levin BE, Caunca M, Zeki Al Hazzourie A, DeRosa JT, et al. Systolic blood pressure and cognition in the elderly: the Northern Manhattan Study. J Alzheimers Dis. 2021;82(2):689–99. [DOI] [PMC free article] [PubMed]
- 177.Sharp SI, Aarsland D, Day S, Sønnesyn H, Alzheimer’s Society Vascular Dementia Systematic Review Group, Ballard C. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26(7):661–9. [DOI] [PubMed]
- 178.Feldstein CA. Effects of blood pressure changes on Alzheimer’s disease. Neuroepidemiology. 2010;35(3):202–212. doi: 10.1159/000316872. [DOI] [PubMed] [Google Scholar]
- 179.Guan J-W, Huang C-Q, Li Y-H, Wan C-M, You C, Wang Z-R, et al. No association between hypertension and risk for Alzheimer’s disease: a meta-analysis of longitudinal studies. J Alzheimers Dis. 2011;27(4):799–807. doi: 10.3233/JAD-2011-111160. [DOI] [PubMed] [Google Scholar]
- 180.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45(3):374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- 181.Levine DA, Gross AL, Briceño EM, Tilton N, Kabeto MU, Hingtgen SM, et al. Association between blood pressure and later-life cognition among black and white individuals. JAMA Neurol. 2020;77(7):810–819. doi: 10.1001/jamaneurol.2020.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 183.Kivimäki M, Luukkonen R, Batty GD, Ferrie JE, Pentti J, Nyberg ST, et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement. 2018;14(5):601–9. [DOI] [PMC free article] [PubMed]
- 184.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:289645. [DOI] [PMC free article] [PubMed]
- 185.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 186.Berger AL. Insulin resistance and reduced brain glucose metabolism in the aetiology of Alzheimer’s disease. Journal of Insulin Resistance. 2016;1(1):7. doi: 10.4102/jir.v1i1.15. [DOI] [Google Scholar]
- 187.Nguyen JCD, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;19(8):375. doi: 10.3389/fnins.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Buie JJ, Watson LS, Smith CJ, Sims-Robinson C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol Dis. 2019;132:104580. [DOI] [PMC free article] [PubMed]
- 189.Saczynski JS, Jónsdóttir MK, Garcia ME, Jonsson PV, Peila R, Eiriksdottir G, et al. Cognitive impairment: an increasingly important complication of type 2 diabetes: the age, gene/environment susceptibility–Reykjavik study. Am J Epidemiol. 2008;168(10):1132–1139. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369(6):540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51(4):1256–62. [DOI] [PubMed]
- 192.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58(1):71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tuligenga RH, Dugravot A, Tabák AG, Elbaz A, Brunner EJ, Kivimäki M, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. 2014;2(3):228–235. doi: 10.1016/S2213-8587(13)70192-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cosentino F, Battista R, Scuteri A, De Sensi F, De Siati L, Di Russo C, et al. Impact of fasting glycemia and regional cerebral perfusion in diabetic subjects. Stroke. 2009;40(1):306–308. doi: 10.1161/STROKEAHA.108.520627. [DOI] [PubMed] [Google Scholar]
- 196.Roberts RO, Knopman DS, Cha RH, Mielke MM, Pankratz VS, Boeve BF, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med. 2014;55(5):759–764. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 198.Vieira JR, Elkind MSV, Moon YP, Rundek T, Boden-Albala B, Paik MC, et al. The metabolic syndrome and cognitive performance: the Northern Manhattan Study. NED. 2011;37(3–4):153–159. doi: 10.1159/000332208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Levin BE, Llabre MM, Dong C, Elkind MSV, Stern Y, Rundek T, et al. Modeling metabolic syndrome and its association with cognition: the Northern Manhattan Study. J Int Neuropsychol Soc. 2014;20(10):951–960. doi: 10.1017/S1355617714000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ng TP, Feng L, Nyunt MSZ, Feng L, Gao Q, Lim ML, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurol. 2016;73(4):456–463. doi: 10.1001/jamaneurol.2015.4899. [DOI] [PubMed] [Google Scholar]
- 201.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24(2):69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 202.Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15(12):771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 203.Díaz M, Fabelo N, Martín V, Ferrer I, Gómez T, Marín R. Biophysical alterations in lipid rafts from human cerebral cortex associate with increased BACE1/AβPP interaction in early stages of Alzheimer’s disease. J Alzheimers Dis. 2015;43(4):1185–1198. doi: 10.3233/JAD-141146. [DOI] [PubMed] [Google Scholar]
- 204.Kawarabayashi T, Shoji M, Younkin LH, Wen-Lang L, Dickson DW, Murakami T, et al. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2004;24(15):3801–3809. doi: 10.1523/JNEUROSCI.5543-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Geifman N, Brinton RD, Kennedy RE, Schneider LS, Butte AJ. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):10. doi: 10.1186/s13195-017-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6(6):456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 208.Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014;24(11):3059–3068. doi: 10.1093/cercor/bht163. [DOI] [PubMed] [Google Scholar]
- 209.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MGM, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 210.Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2010;91(6):1725–1732. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 211.Stough C, Downey L, Silber B, Lloyd J, Kure C, Wesnes K, et al. The effects of 90-day supplementation with the omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiol Aging. 2012;33(4):824.e1–3. doi: 10.1016/j.neurobiolaging.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 212.Stonehouse W. Does consumption of LC omega-3 PUFA enhance cognitive performance in healthy school-aged children and throughout adulthood? Evidence from clinical trials Nutrients. 2014;6(7):2730–2758. doi: 10.3390/nu6072730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nalder L, Zheng B, Chiandet G, Middleton LT, de Jager CA. Vitamin B12 and folate status in cognitively healthy older adults and associations with cognitive performance. J Nutr Health Aging. 2021;25(3):287–294. doi: 10.1007/s12603-020-1489-y. [DOI] [PubMed] [Google Scholar]
- 214.Smith AD, Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr. 2016;17(36):211–239. doi: 10.1146/annurev-nutr-071715-050947. [DOI] [PubMed] [Google Scholar]
- 215.Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJPM, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr. 2014;100(2):657–666. doi: 10.3945/ajcn.113.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ford AH, Almeida OP. Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J Alzheimers Dis. 2012;29(1):133–149. doi: 10.3233/JAD-2012-111739. [DOI] [PubMed] [Google Scholar]
- 217.Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. Am J Med. 2010;123(6):522–527.e2. doi: 10.1016/j.amjmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 218.Durga J, van Boxtel MPJ, Schouten EG, Kok FJ, Jolles J, Katan MB, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 219.Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010;5(9):e12244. [DOI] [PMC free article] [PubMed]
- 220.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43(11):3137–3146. doi: 10.1161/STROKEAHA.112.651778. [DOI] [PubMed] [Google Scholar]
- 221.Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007;17(3):259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 222.van Duijn CM, Hofman A. Relation between nicotine intake and Alzheimer’s disease. BMJ. 1991;302(6791):1491–1494. doi: 10.1136/bmj.302.6791.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Salomon AR, Marcinowski KJ, Friedland RP, Zagorski MG. Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry. 1996;35(42):13568–13578. doi: 10.1021/bi9617264. [DOI] [PubMed] [Google Scholar]
- 224.Lallai V, Grimes N, Fowler JP, Sequeira PA, Cartagena P, Limon A, et al. Nicotine acts on cholinergic signaling mechanisms to directly modulate choroid plexus function. eNeuro. 2019;6(2):ENEURO.0051–19.2019. [DOI] [PMC free article] [PubMed]
- 225.Galanis DJ, Petrovitch H, Launer LJ, Harris TB, Foley DJ, White LR. Smoking history in middle age and subsequent cognitive performance in elderly Japanese-American men. The Honolulu-Asia Aging Study. Am J Epidemiol. 1997;145(6):507–15. [DOI] [PubMed]
- 226.Reitz C, Luchsinger J, Tang M-X, Mayeux R. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65(6):870–875. doi: 10.1212/01.wnl.0000176057.22827.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Starr JM, Deary IJ, Fox HC, Whalley LJ. Smoking and cognitive change from age 11 to 66 years: a confirmatory investigation. Addict Behav. 2007;32(1):63–68. doi: 10.1016/j.addbeh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 228.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, et al. Associations Between Midlife Vascular Risk Factors And 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246–1254. doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lewis CR, Talboom JS, De Both MD, Schmidt AM, Naymik MA, Håberg AK, et al. Smoking is associated with impaired verbal learning and memory performance in women more than men. Sci Rep. 2021;13(11):10248. doi: 10.1038/s41598-021-88923-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stella F, Banzato CEM, Gasparetto Sé EV, Scudeler JL, Pacheco JL, Kajita RT. Risk factors for vascular dementia in elderly psychiatric outpatients with preserved cognitive functions. J Neurol Sci. 2007;257(1–2):247–249. doi: 10.1016/j.jns.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 231.Ganguli M, Vander Bilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65(8):1210–1217. doi: 10.1212/01.wnl.0000180520.35181.24. [DOI] [PubMed] [Google Scholar]
- 232.Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927. [DOI] [PMC free article] [PubMed]
- 233.Kim JW, Byun MS, Yi D, Lee JH, Ko K, Jeon SY, et al. Association of moderate alcohol intake with in vivo amyloid-beta deposition in human brain: a cross-sectional study. PLOS Medicine. 2020;17(2):e1003022. [DOI] [PMC free article] [PubMed]
- 234.Ritchie SJ, Bates TC, Deary IJ. Is education associated with improvements in general cognitive ability, or in specific skills? Dev Psychol. 2015;51(5):573–582. doi: 10.1037/a0038981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54(11):1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 236.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 237.EClipSE Collaborative Members, Brayne C, Ince PG, Keage HAD, McKeith IG, Matthews FE, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133(Pt 8):2210–6. [DOI] [PubMed]
- 238.Willey JZ, Moon YP, Ruder R, Cheung YK, Sacco RL, Elkind MSV, et al. Physical activity and cognition in the Northern Manhattan Study. NED. 2014;42(2):100–106. doi: 10.1159/000355975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tian Q, Simonsick EM, Erickson KI, Aizenstein HJ, Glynn NW, Boudreau RM, et al. Cardiorespiratory fitness and brain diffusion tensor imaging in adults over 80 years of age. Brain Res. 2014;7(1588):63–72. doi: 10.1016/j.brainres.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the Women’s Antioxidant and Cardiovascular Study. Circulation. 2009;119(21):2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 246.van de Rest O, Spiro A, Krall-Kaye E, Geleijnse JM, de Groot LCPGM, Tucker KL. Intakes of (n-3) fatty acids and fatty fish are not associated with cognitive performance and 6-year cognitive change in men participating in the Veterans Affairs Normative Aging Study. J Nutr. 2009;139(12):2329–36. [DOI] [PMC free article] [PubMed]
- 247.Buell JS, Scott TM, Dawson-Hughes B, Dallal GE, Rosenberg IH, Folstein MF, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci. 2009;64(8):888–895. doi: 10.1093/gerona/glp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Slinin Y, Paudel ML, Taylor BC, Fink HA, Ishani A, Canales MT, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74(1):33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 250.Yeh S-CJ, Liu Y-Y. Influence of social support on cognitive function in the elderly. BMC Health Serv Res. 2003;3:9. [DOI] [PMC free article] [PubMed]
- 251.Crooks VC, Lubben J, Petitti DB, Little D, Chiu V. Social network, cognitive function, and dementia incidence among elderly women. Am J Public Health. 2008;98(7):1221–1227. doi: 10.2105/AJPH.2007.115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Khondoker M, Rafnsson SB, Morris S, Orrell M, Steptoe A. Positive and negative experiences of social support and risk of dementia in later life: an investigation using the English Longitudinal Study of Ageing. J Alzheimers Dis. 2017;58(1):99–108. doi: 10.3233/JAD-161160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Saito T, Murata C, Saito M, Takeda T, Kondo K. Influence of social relationship domains and their combinations on incident dementia: a prospective cohort study. J Epidemiol Community Health. 2018;72(1):7–12. doi: 10.1136/jech-2017-209811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wiederkehr S, Laurin D, Simard M, Verreault R, Lindsay J. Vascular risk factors and cognitive functions in nondemented elderly individuals. J Geriatr Psychiatry Neurol. 2009;22(3):196–206. doi: 10.1177/0891988709335797. [DOI] [PubMed] [Google Scholar]
- 255.Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, et al. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc. 2016;5(3):e002731. [DOI] [PMC free article] [PubMed]
- 256.Kaffashian S, Dugravot A, Elbaz A, Shipley MJ, Sabia S, Kivimäki M, et al. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology. 2013;80(14):1300–6. [DOI] [PMC free article] [PubMed]
- 257.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 258.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10(5):562–570. doi: 10.1016/j.jalz.2013.05.1772. [DOI] [PubMed] [Google Scholar]
- 259.Tolea MI, Heo J, Chrisphonte S, Galvin JE. A Modified CAIDE risk score as a screening tool for cognitive impairment in older adults. J Alzheimers Dis. 2021. [DOI] [PMC free article] [PubMed]
- 260.Rundek T, Gardener H, Dias Saporta AS, Loewenstein DA, Duara R, Wright CB, et al. Global vascular risk score and CAIDE dementia risk score predict cognitive function in the Northern Manhattan Study. J Alzheimers Dis. 2020;73(3):1221–1231. doi: 10.3233/JAD-190925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Forette F, Seux ML, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene MR, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352(9137):1347–1351. doi: 10.1016/S0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 262.Forette F, Seux M-L, Staessen JA, Thijs L, Babarskiene M-R, Babeanu S, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162(18):2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- 263.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553–561. doi: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322(6):524–534. doi: 10.1001/jama.2019.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Diener H-C, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7(10):875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sacco RL, Diener H-C, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163(9):1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 268.Dufouil C, Chalmers J, Coskun O, Besançon V, Bousser M-G, Guillon P, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112(11):1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 269.Chapman N, Huxley R, Anderson C, Bousser MG, Chalmers J, Colman S, et al. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke. 2004;35(1):116–121. doi: 10.1161/01.STR.0000106480.76217.6F. [DOI] [PubMed] [Google Scholar]
- 270.Trompet S, van Vliet P, de Craen AJM, Jolles J, Buckley BM, Murphy MB, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257(1):85–90. [DOI] [PubMed]
- 271.Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Browne P, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol. 2005;62(5):753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 272.Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 273.Vogt NM, Hunt JFV, Ma Y, Van Hulle CA, Adluru N, Chappell RJ, et al. Effects of simvastatin on white matter integrity in healthy middle-aged adults. Ann Clin Transl Neurol. 2021;8(8):1656–1667. doi: 10.1002/acn3.51421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77(6):556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Williamson JD, Launer LJ, Bryan RN, Coker LH, Lazar RM, Gerstein HC, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174(3):324–333. doi: 10.1001/jamainternmed.2013.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zhang R, Vongpatanasin W, Levine BD. Faster brain shrinkage in the ACCORD MIND Study: an unexpected result? JAMA Intern Med. 2015;175(1):144. doi: 10.1001/jamainternmed.2014.6971. [DOI] [PubMed] [Google Scholar]
- 278.Budur K, Welsh-Bohmer K, Burns D, Chiang C, O’Neil J, Runyan G, et al. A Pharmacogenetics-supported clinical trialto delay onset of mild cognitive impairment due to Alzheimer’s disease using low-dose pioglitazone: an update on the TOMORROW study. Alzheimer’s & Dementia. 2014;1(10):P809–P810. [Google Scholar]
- 279.Areosa Sastre A, Vernooij RW, González-Colaço Harmand M, Martínez G. Effect of the treatment of type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:CD003804. [DOI] [PMC free article] [PubMed]
- 280.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 281.Patterson CJ, Gauthier S, Bergman H, Cohen CA, Feightner JW, Feldman H, et al. The recognition, assessment and management of dementing disorders: conclusions from the Canadian Consensus Conference on Dementia. CMAJ. 1999;160(12 Suppl):S1–15. [PMC free article] [PubMed]
- 282.Black S, Román GC, Geldmacher DS, Salloway S, Hecker J, Burns A, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke. 2003;34(10):2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1. [DOI] [PubMed] [Google Scholar]
- 283.Wilkinson D, Doody R, Helme R, Taubman K, Mintzer J, Kertesz A, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology. 2003;61(4):479–486. doi: 10.1212/01.WNL.0000078943.50032.FC. [DOI] [PubMed] [Google Scholar]
- 284.Chen Y, Zhang J, Wang Y, Yuan J, Hu W. Efficacy of cholinesterase inhibitors in vascular dementia: an updated meta-analysis. Eur Neurol. 2016;75(3–4):132–141. doi: 10.1159/000444253. [DOI] [PubMed] [Google Scholar]
- 285.Mufti M, Stabile MJ, Amico J. What is the efficacy of donepezil in patients with vascular dementia. 2019.
- 286.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359(9314):1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 287.Auchus AP, Brashear HR, Salloway S, Korczyn AD, De Deyn PP, Gassmann-Mayer C, et al. Galantamine treatment of vascular dementia: a randomized trial. Neurology. 2007;69(5):448–458. doi: 10.1212/01.wnl.0000266625.31615.f6. [DOI] [PubMed] [Google Scholar]
- 288.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Rivastigmine in subcortical vascular dementia: a randomized, controlled, open 12-month study in 208 patients. Am J Alzheimers Dis Other Demen. 2003;18(5):265–272. doi: 10.1177/153331750301800508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Narasimhalu K, Effendy S, Sim CH, Lee JM, Chen I, Hia SB, et al. A randomized controlled trial of rivastigmine in patients with cognitive impairment no dementia because of cerebrovascular disease. Acta Neurol Scand. 2010;121(4):217–224. doi: 10.1111/j.1600-0404.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 290.Orgogozo J-M, Rigaud A-S, Stöffler A, Möbius H-J, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002;33(7):1834–1839. doi: 10.1161/01.STR.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 291.Wilcock G, Möbius HJ, Stöffler A, MMM 500 group. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharmacol. 2002;17(6):297–305. [DOI] [PubMed]
- 292.Battle CE, Abdul-Rahim AH, Shenkin SD, Hewitt J, Quinn TJ. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database Syst Rev. 2021;2:CD013306. [DOI] [PMC free article] [PubMed]
- 293.Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry. 2008;16(3):229–239. doi: 10.1097/01.JGP.0000300629.35408.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial JAMA. 1996;276(21):1725–1731. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.