Abstract
Background
Since vaccines against COVID-19 became available, rare breakthrough infections have been reported despite their high efficacies.
Purpose
To evaluate the clinical and imaging characteristics of patients with COVID-19 breakthrough infections and compare them with those of unvaccinated patients with COVID-19.
Materials and Methods
In this retrospective multicenter cohort study, the authors analyzed patient (aged ≥18 years) data from three centers that were registered in an open data repository for COVID-19 between June and August 2021. Hospitalized patients with baseline chest radiographs were divided into three groups according to their vaccination status. Differences between clinical and imaging features were analyzed using the Pearson χ2 test, Fisher exact test, and analysis of variance. Univariable and multivariable logistic regression analyses were used to evaluate associations between clinical factors, including vaccination status and clinical outcomes.
Results
Of the 761 hospitalized patients with COVID-19, the mean age was 47 years and 385 (51%) were women; 47 patients (6%) were fully vaccinated (breakthrough infection), 127 (17%) were partially vaccinated, and 587 (77%) were unvaccinated. Of the 761 patients, 412 (54%) underwent chest CT during hospitalization. Among the patients who underwent CT, the proportions without pneumonia were 22% of unvaccinated patients (71 of 326), 30% of partially vaccinated patients (19 of 64), and 59% of fully vaccinated patients (13 of 22) (P < .001). Fully vaccinated status was associated with a lower risk of requiring supplemental oxygen (odds ratio [OR], 0.24 [95% CI: 0.09, 0.64; P = .005]) and lower risk of intensive care unit admission (OR, 0.08 [95% CI: 0.09, 0.78; P = .02]) compared with unvaccinated status.
Conclusion
Patients with COVID-19 breakthrough infections had a significantly higher proportion of CT scans without pneumonia compared with unvaccinated patients. Vaccinated patients with breakthrough infections had a lower likelihood of requiring supplemental oxygen and intensive care unit admission.
© RSNA, 2022
Online supplemental material is available for this article.
See also the editorial by Schiebler and Bluemke in this issue.
Summary
Vaccinated patients with COVID-19 breakthrough infections showed fewer chest CT findings of pneumonia compared with unvaccinated patients.
Key Results
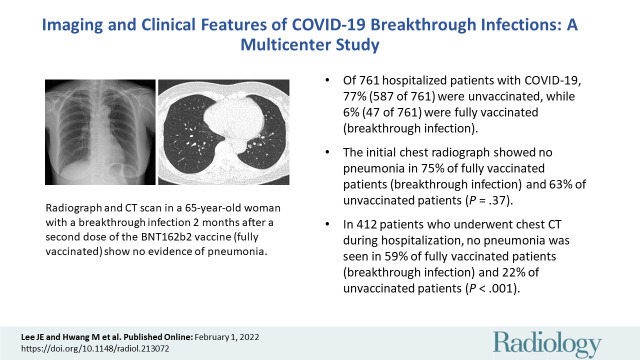
■ Of 761 hospitalized patients with COVID-19, 77% (587 of 761) were unvaccinated, while 6% (47 of 761) were fully vaccinated (breakthrough infection).
■ The initial chest radiograph showed no pneumonia in 75% of fully vaccinated patients (breakthrough infection) and 63% of unvaccinated patients (P = .37).
■ In 412 patients who underwent chest CT examination during hospitalization, no pneumonia was seen in 59% of fully vaccinated patients (breakthrough infection) and 22% of unvaccinated patients (P < .001).
Introduction
The first patient with COVID-19 disease was reported at the end of 2019. The number of confirmed cases worldwide now exceeds 270 million, with an overall mortality rate of approximately 2.0% (1). COVID-19 vaccines are effective and critical tools for bringing the pandemic under control. To date, 56% of the world’s population has received at least one dose of a COVID-19 vaccine (2). However, vaccines are not 100% effective at preventing illness. Breakthrough infections are defined as the detection of SARS-CoV-2 RNA or antigen in a respiratory specimen collected from a person at least 14 days after receiving all recommended doses of COVID-19 vaccines (3).
Although the risk of infection is much lower among vaccinated individuals and vaccination reduces the severity of illness (4–6), to our knowledge, clinical and imaging data of COVID-19 breakthrough infections have not been reported in detail. The purpose of this study was to document the clinical and imaging features of patients with COVID-19 breakthrough infections and compare them with those of infections in unvaccinated patients. In addition, we analyzed the initial imaging and clinical findings of fully, partially, and unvaccinated patients to determine the relationship of vaccination status with clinical severity.
Materials and Methods
Study Design and Population
The first COVID-19 vaccination was administered in the Republic of Korea on February 26, 2021, and the large-scale vaccination of high-risk groups, which included individuals who were 65 years or older, those with disability, or health care workers, began in May (7). As most records of in-patient vaccination history were from June 2021, this study included patients with breakthrough infections registered from June 1 to August 31, 2021.
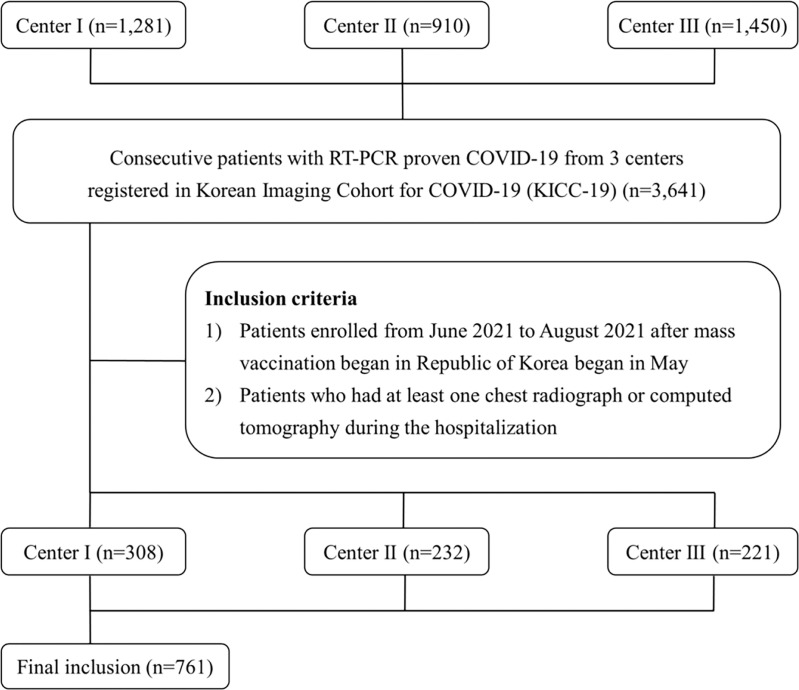
This multicenter study was composed of three centers (center 1, n = 308; center 2, n = 232; and center 3, n = 221) registered in the Korean Imaging Cohort for COVID-19 database, a nationwide open data repository (8). Patients with asymptomatic or mild symptoms were hospitalized in center 1, whereas centers 2 and 3 were main referral centers for patients with COVID-19 with severe symptoms. Consecutive adult patients aged 18 years or older who had been hospitalized for COVID-19 as confirmed by means of real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing per the guidelines for COVID-19 treatment in our country were included (Fig 1). According to these guidelines, all confirmed patients were isolated even if they had no symptoms and hospitalized at a community treatment center or hospital depending on the presence or severity of symptoms. If the patient was asymptomatic 10 days after the initial diagnosis, or if the RT-PCR tests performed at 24-hour intervals were negative at least twice, the patient could be discharged. To be included in this study, patients were required to have undergone at least one chest radiographic (posteroanterior or anteroposterior view) or CT examination during hospitalization. The study was approved by the institutional review board of the participating institution (2111–027–109), which waived the requirement for informed consent due to the retrospective nature of the study.
Figure 1:
Study flow diagram. KICC-19 = Korean Imaging Cohort for COVID-19, RT-PCR = reverse transcriptase polymerase chain reaction.
Study Definitions
Patients were categorized as unvaccinated, partially vaccinated, or fully vaccinated. Unvaccinated individuals were defined as having a positive COVID-19 RT-PCR test result with no record of vaccination against COVID-19, or as being diagnosed with COVID-19 less than 14 days after receipt of the first vaccine dose. Partially vaccinated individuals were defined as having a positive COVID-19 RT-PCR test result at least 14 days after receipt of the first vaccine dose and before receipt of the second dose with the ChAdOx1 nCoV-19 vaccine (AstraZeneca), BNT162b2 vaccine (Pfizer–BioNTech), or mRNA-1273 vaccine (Moderna). Fully vaccinated individuals were defined as having a positive COVID-19 RT-PCR test result at least 14 days after receipt of the second vaccine dose or at least 14 days after receipt of the first dose of the Ad26.COV2.S vaccine (Johnson & Johnson–Janssen).
Data Collection
Demographic characteristics (age and sex), comorbidities (hypertension, diabetes, cardiovascular disease, and cancer), clinical symptoms (fever, cough, sputum, dyspnea, myalgia, sore throat, and sensory loss), initial laboratory findings, and clinical outcomes were evaluated using the cloud-based data storage platform of the Korean Imaging Cohort for COVID-19. Initial laboratory findings included white blood cell, lymphocyte, and platelet counts as well as lactate dehydrogenase (LDH) and C-reactive protein (CRP) levels. Leukocytosis was defined as a white blood cell count of greater than 10 000/μL. Lymphocytopenia was defined as a lymphocyte count of less than 1500/μL. Thrombocytopenia was defined as a platelet count of less than 150 000/μL. The predefined clinical thresholds for LDH and CRP elevation were 50 mg/L and 250 U/L, respectively. Clinical outcomes were receipt of supplemental oxygen and mechanical ventilation, intensive care unit (ICU) admission, and in-hospital death.
Chest radiographs and CT scans of each patient obtained during hospitalization were acquired from the cloud-based data storage platform. The initial chest radiograph and CT scan were defined as those obtained at admission or within a week of symptom onset. Follow-up chest radiographs were obtained every 2 or 3 days until discharge. An initial chest radiograph was obtained in all patients for all three cohorts. An initial chest CT scan was obtained in all patients at center 1 (n = 308) and some patients at center 2 (n = 101) and center 3 (n = 3).
Image Analysis
Two radiologists (J.E.L. and Y.J.J., with 7 and 19 years of experience, respectively), unaware of patient clinical information, reviewed all images and reached conclusions by consensus in cases of interreader discrepancies. Pneumonia extent on initial and all follow-up chest radiographs, and pneumonia extent and patterns on initial and follow-up CT scans, were analyzed. Pneumonia extent in entire lung zones on chest radiographs and CT scans was scored from 0 to 2 (score 0: no evidence of pneumonia, score 1: 1%–25% involvement, and score 2: >25% involvement), which were based on a study predicting the severity of COVID-19 (9) (Fig 2). Pneumonia patterns on CT images were categorized as typical, indeterminate, atypical, or negative based on the RSNA Expert Consensus Statement (10). Peripheral bilateral ground-glass opacities (GGOs) or multifocal round GGOs, with or without consolidation or intralobular lines, or a reverse halo sign were considered as a typical appearance. An indeterminate appearance was defined as presence of GGOs with or without consolidation, but absence of typical features. An atypical appearance was defined as absence of typical or indeterminate features with presence of lobar and/or segmental consolidation without GGOs, discrete centrilobular nodules, lung cavitation, or smooth interlobular septal thickening with pleural effusion.
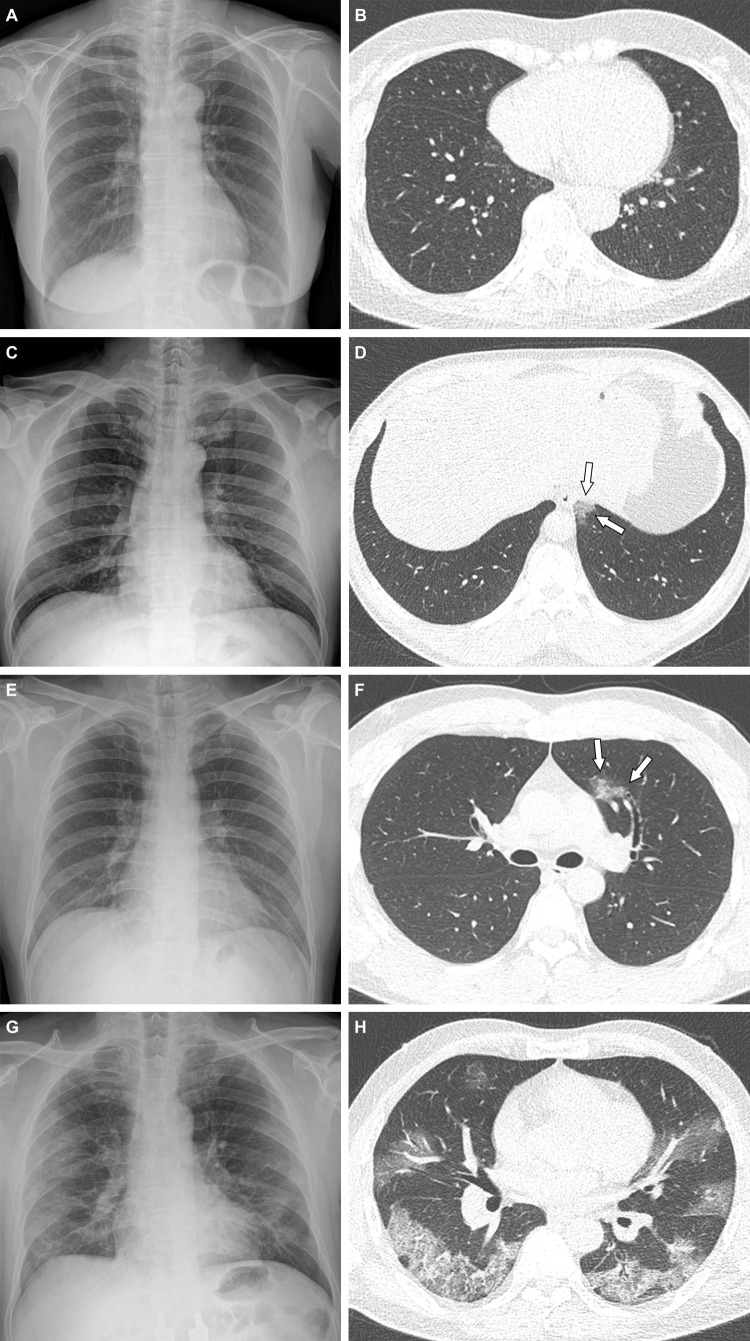
Figure 2:
Representative cases showing pneumonia extents and patterns on chest radiographs and CT images. (A, B) Images in a 65-year-old woman with a breakthrough infection 2 months after a second dose of the BNT162b2 vaccine (fully vaccinated). The patient had a history of hypertension. (A) Chest radiograph obtained at admission shows no abnormal opacification in either lung. The chest radiograph extent of pneumonia was scored as 0 (no evidence of pneumonia). (B) Axial chest CT image at the lower lobe level (obtained on the same day) is negative for pneumonia; the extent of pneumonia at CT was scored as 0 (no evidence of pneumonia). (C, D) Images in a 48-year-old man 1 month after a first dose of the ChAdOx1 nCoV-19 vaccine (partially vaccinated). The patient had no history of comorbidity. (C) Chest radiograph obtained at admission shows no abnormal opacification in either lung. The chest radiograph extent of pneumonia was scored as 0 (no evidence of pneumonia). (D) Axial chest CT image obtained on the same day shows unilateral ground-glass opacity with a nonrounded morphologic feature in the left lower lobe (arrows). The extent of pneumonia at CT was scored as 1 (1%–25% involvement) and this case was classified as an indeterminate appearance of COVID-19 according to the RSNA chest CT classification system. (E, F) Images in a 36-year-old man with no history of vaccination for COVID-19 and no history of comorbidity. (E) Chest radiograph obtained at admission shows no abnormal opacification in either lung. The chest radiograph extent of pneumonia was scored as 0 (no evidence of pneumonia). (F) Axial chest CT image obtained on the same day shows unilateral ground-glass opacity with a nonrounded morphologic feature and nonperipheral distribution in the left upper lobe (arrows). The extent of pneumonia at CT was scored as 1 (1%–25% involvement), and this case was classified as an indeterminate appearance of COVID-19 according to the RSNA chest CT classification system. (G, H) Images in a 58-year-old man with no history of COVID-19 vaccination and a history of hypertension and diabetes. He required supplemental oxygen on admission and was admitted to the intensive care unit 1 day later. (G) Chest radiograph at admission shows patchy ground-glass opacities in the middle to lower zones of both lungs. The chest radiograph extent of pneumonia was scored as 2 (>25% involvement). (H) Axial chest CT image obtained on the same day shows multifocal ground-glass opacities with a crazy-paving appearance in bilateral lungs. The extent of pneumonia at CT was scored as 2 (>25% involvement) and was classified as a typical appearance of COVID-19 according to the RSNA chest CT classification system.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics version 23.0 (IBM). Categorical variables are presented as numbers and percentages and continuous variables as means and SDs. Statistical assessment of differences between groups was determined using the Pearson χ2 test or Fisher exact test for categorical variables (sex, smoking history, comorbidities, symptoms, initial laboratory findings, three-point scales of chest radiograph and CT scores, and clinical outcomes) and with analysis of variance for continuous variables (age and hospital length of stay). Post hoc analysis was performed using the Bonferroni method. Bonferroni-adjusted P values were determined by multiplying the raw P values by the number of comparisons. Univariable and multivariable logistic regression were used to evaluate associations between clinical factors (including vaccination status) and clinical outcomes. P < .05 was considered indicative of a statistically significant difference.
Results
Demographic and Clinical Characteristics of the Patients
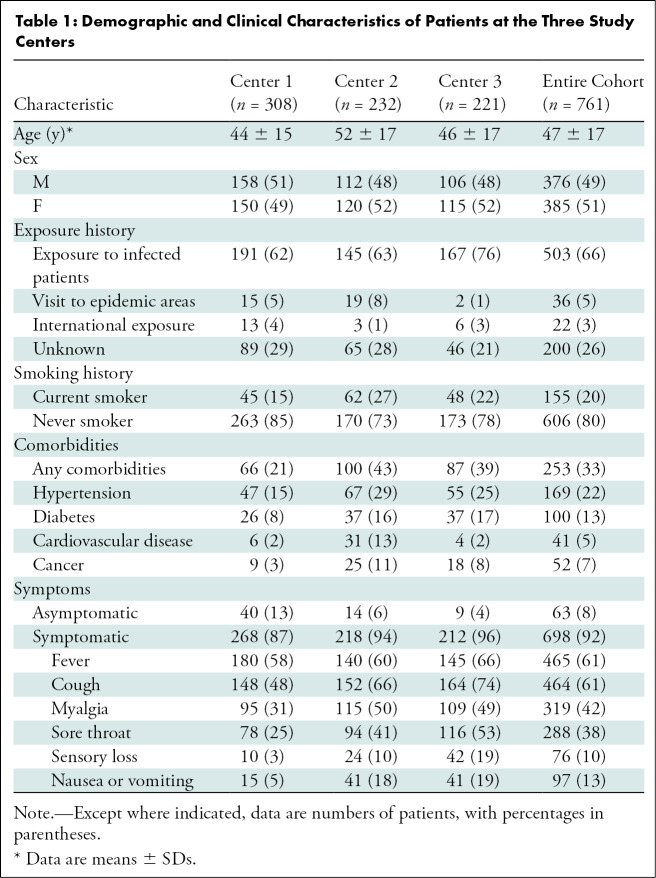
The demographic and baseline clinical characteristics of the 761 patients who met the inclusion criteria are presented in Table 1. The mean patient age was 47 years (IQR, 33–59 years); 385 of the 761 patients (51%) were women and 376 (49%) were men. Of all 761 patients, 503 (66%) had a history of contact with infected patients, 36 (5%) had visited an epidemic area, and 22 (3%) had international exposure. In 200 of the 761 patients (26%), the exposure history was unknown. Regarding smoking status, 155 of the 761 patients (20%) had a smoking history and the remaining 606 (80%) were never smokers. Moreover, 253 patients (33%) had at least one of the following comorbidities: hypertension, diabetes, cardiovascular disease, or cancer. Regarding symptoms, 698 patients (92%) had at least one of the following: fever, cough, sputum, dyspnea, myalgia, sore throat, sensory loss, nausea, or vomiting. The remaining 63 patients (8%) were asymptomatic.
Table 1:
Demographic and Clinical Characteristics of Patients at the Three Study Centers
Vaccination Status and Breakthrough Infections
Of the 761 patients, 587 (77%) were unvaccinated at the time of diagnosis, 127 (17%) were partially vaccinated, and 47 (6%) were fully vaccinated (had a breakthrough infection). The mean time between final vaccination and diagnosis was 46 days ± 26 (SD). Among the 174 vaccinated patients, 120 (69%) received the ChAdOx1 nCoV-19 vaccine, 42 (24%) received the BNT162b2 vaccine, seven (4%) received the Ad26.COV2.S vaccine, and five (3%) received the mRNA-1273 vaccine. Of the 47 patients with breakthrough infections, 22 (47%) received the ChAdOx1 nCoV-19 vaccine, 18 (38%) received the BNT162b2 vaccine, and seven (15%) received the Ad26.COV2.S vaccine. Details of patient characteristics and vaccine efficacy according to each vaccine type are summarized in Tables E1 and E2 (online).
Clinical Characteristics and Outcomes according to Vaccination Status
Baseline clinical characteristics and outcomes according to vaccination status are presented in Table 2. The mean age was higher in the fully vaccinated (65 years ± 18) and partially vaccinated (59 years ± 14) groups than in the unvaccinated group (43 years ± 15) (P < .001). The percentages of patients with at least one comorbidity were higher in the fully (55%, 26 of 47 patients) and partially (58%, 73 of 127 patients) vaccinated groups than in the unvaccinated group (26%, 154 of 587 patients) (P < .001). The percentage of asymptomatic patients at admission was higher in the fully vaccinated group (21%, 10 of 47 patients) than in the unvaccinated group (7%, 42 of 587 patients) (P = .003). The percentages of patients with leukocytosis, lymphocytopenia, thrombocytopenia, an elevated LDH level, and an elevated CRP level were not significantly different between the three groups. The mean hospital length of stay was similar in the three groups. The most common clinical outcome among patients was the need for supplemental oxygen, and the proportions of patients who required supplemental oxygen were not significantly different among the three groups. Only patients in the unvaccinated group received mechanical ventilation or died in the hospital, but differences were not significant.
Table 2:
Clinical Characteristics and Outcomes of Patients with COVID-19 according to Vaccination Status
Proportions of Chest Radiograph Scores according to Vaccination Status
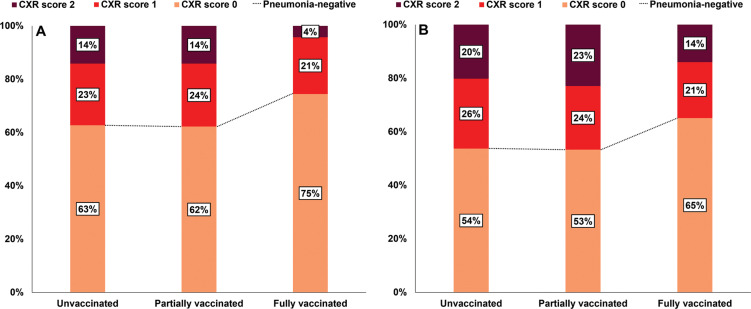
All 761 patients underwent initial chest radiography at admission, and 653 patients (86%) underwent at least one follow-up chest radiographic examination during hospitalization. At initial chest radiography assessments, 63% (368 of 587) of unvaccinated patients, 62% (79 of 127) of partially vaccinated patients, and 75% (35 of 47) of fully vaccinated patients had negative chest radiographs (score of 0) (Fig 3A). The proportion of patients with an initially negative chest radiograph was higher in the fully vaccinated group, but this was not statistically significant (Fig 3A). Additionally, 23% (136 of 587) of unvaccinated patients, 24% (30 of 127) of partially vaccinated patients, and 21% (10 of 47) of fully vaccinated patients had a chest radiograph score of 1, while 14% (83 of 587) of unvaccinated patients, 14% (18 of 127) of partially vaccinated patients, and 4% (two of 47) of fully vaccinated patients had a chest radiograph score of 2.
Figure 3:
Bar graphs show (A) initial and (B) follow-up chest radiograph scores in the 761 patients according to vaccination status. (A) The proportion of patients with an initial chest radiograph score of 0 was greater in the fully vaccinated group than in the partially vaccinated or unvaccinated groups, but not significantly so (P = .37). (B) The proportion of patients with a chest radiograph score of 0 during follow-up was also greatest in the fully vaccinated group, but not significantly so (P = .78). CXR = chest radiograph.
During hospitalization, 54% (271 of 505) of unvaccinated patients, 53% (56 of 105) of partially vaccinated patients, and 65% (28 of 43) of fully vaccinated patients had negative chest radiographs (Fig 3B). The proportion of patients with normal chest radiograph findings during follow-up was similar to that of the fully vaccinated group (Fig 3B). On follow-up images, 26% (132 of 505) of unvaccinated patients, 24% (25 of 105) of partially vaccinated patients, and 21% (nine of 43) of fully vaccinated patients had a chest radiograph score of 1 and 20% (102 of 505) of unvaccinated patients, 23% (24 of 105) of partially vaccinated patients, and 14% (six of 43) of fully vaccinated patients had a chest radiograph score of 2. The subgroup comparison of chest radiograph scores for patients with pneumonia is summarized in Table E3 (online). The factors associated with severe pneumonia (initial chest radiograph score of 2) are summarized in Table E4 (online).
Proportions of Chest CT Scores and Patterns according to Vaccination Status
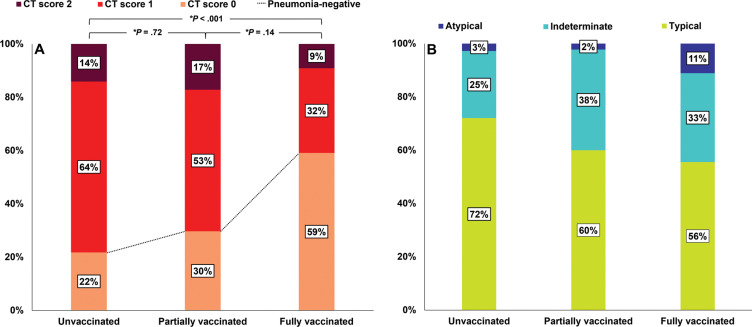
Overall, 412 of the 761 patients (54%) underwent chest CT during hospitalization; of these, 22% (71 of 326) of unvaccinated patients, 30% (19 of 64) of partially vaccinated patients, and 59% (13 of 22) of fully vaccinated patients had negative CT scans (Fig 4A). The proportion of negative CT scans was higher in the fully vaccinated group than in the unvaccinated group (Bonferroni-adjusted P < .001) (Fig 4A). Additionally, 64% (209 of 326) of unvaccinated patients, 53% (34 of 64) of partially vaccinated patients, and 32% (seven of 22) of fully vaccinated patients had a CT score of 1 and 14% (46 of 326) of unvaccinated patients, 17% (11 of 64) of partially vaccinated patients, and 9% (two of 22) of fully vaccinated patients had a CT score of 2. Of the 412 patients who underwent chest CT, 309 (75%) were positive for pneumonia, and the most common CT patterns observed in the three groups were typical (72% [184 of 255] of unvaccinated, 60% [27 of 45] of partially vaccinated, and 56% [five of nine] of fully vaccinated patients). Proportions of CT patterns in the three study groups were not significantly different (P = .22) (Fig 4B). The subgroup comparison of CT scores for pneumonia-positive cases is summarized in Table E3 (online). The factors associated with severe pneumonia (CT score of 2) are summarized in Table E5 (online).
Figure 4:
Bar graphs show (A) CT scores and (B) patterns for the 412 patients who underwent chest CT during hospitalization according to vaccination status. (A) The proportion of patients with a CT score of 0 was significantly greater in the fully vaccinated group than in the unvaccinated group (P < .001). * = Bonferroni-adjusted P value, which was determined by multiplying the raw P value by 3. (B) Among patients with pneumonia, CT patterns were not significantly different between the groups (P = .22).
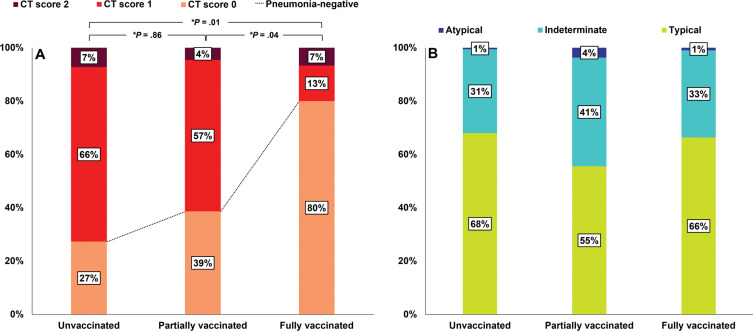
At center 1 (n = 308), all patients underwent chest CT during hospitalization; 27% (68 of 249) of unvaccinated patients, 39% (17 of 44) of partially vaccinated patients, and 80% (12 of 15) of fully vaccinated patients had negative CT scans during hospitalization. The proportion of negative CT scans was higher for fully vaccinated patients than for partially vaccinated or unvaccinated patients (Bonferroni-adjusted P = .04 and .01, respectively) (Fig 5A). Additionally, 66% (163 of 249) of unvaccinated patients, 57% (25 of 44) of partially vaccinated patients, and 13% (two of 15) of fully vaccinated patients had a CT score of 1 and 7% (18 of 249) of unvaccinated patients, 4% (two of 44) of partially vaccinated patients, and 7% (one of 15) of fully vaccinated patients had a CT score of 2. Of the 308 patients, 211 (69%) were positive for pneumonia. The most common CT patterns observed in the fully, partially, and unvaccinated groups were typical, and no intergroup difference was observed (P = .46) (Fig 5B).
Figure 5:
Bar graphs show CT scores and patterns of the 308 patients at center 1 according to vaccination status. At center 1, patients with asymptomatic or mild symptoms were hospitalized and initial chest CT scans were obtained in all patients. (A) The proportion of patients with a CT score of 0 was significantly greater in the fully vaccinated group than in the partially or unvaccinated groups (P = .04 and .01, respectively). * = Bonferroni-adjusted P value, which was determined by multiplying the raw P value by 3. (B) Among patients with pneumonia, CT patterns were not significantly different between the groups (P = .46).
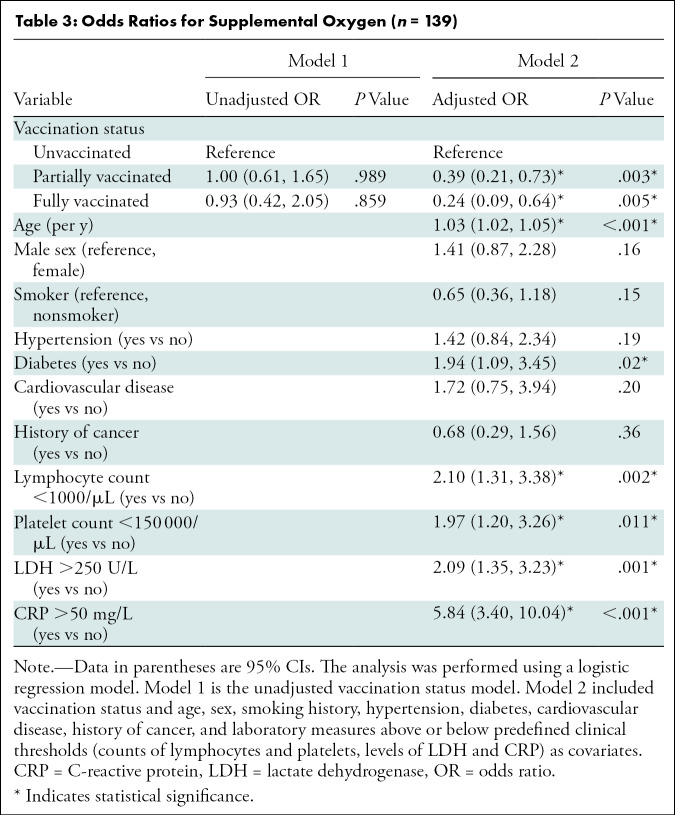
Factors Associated with the Need for Supplemental Oxygen
The unadjusted and adjusted odds ratios (ORs) for treatment with supplemental oxygen are summarized in Table 3. Adjusted multivariable analysis showed that fully vaccinated patients and partially vaccinated patients had lower ORs for requiring supplemental oxygen than unvaccinated patients (OR, 0.24 [95% CI: 0.09, 0.64; P = .005] and 0.39 [95% CI: 0.21, 0.73; P = .003], respectively). Older age, a history of diabetes, lymphocytopenia, thrombocytopenia, and elevated LDH and CRP levels were also associated with the risk of requiring supplemental oxygen.
Table 3:
Odds Ratios for Supplemental Oxygen (n = 139)
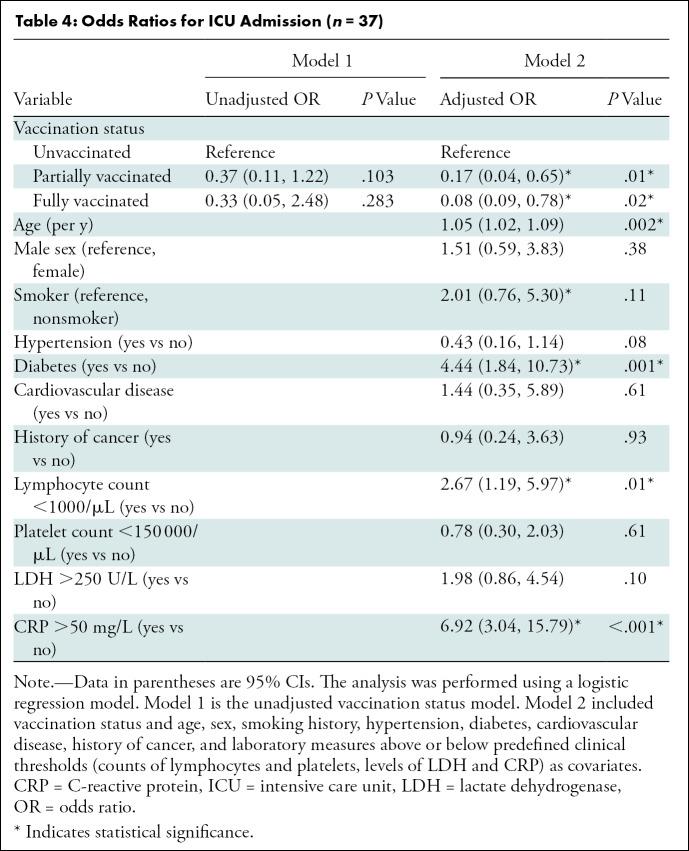
Factors Associated with ICU Admission
Unadjusted and adjusted ORs for ICU admission are summarized in Table 4. Adjusted multivariable analysis showed fully and partially vaccinated patients had significantly lower ORs for ICU admission than unvaccinated patients (OR, 0.08 [95% CI: 0.09, 0.78; P = .02] and 0.17 [95% CI: 0.04, 0.65; P = .01], respectively). In addition, older age, a history of diabetes, lymphocytopenia, and an elevated CRP level were associated with an increased risk of ICU admission.
Table 4:
Odds Ratios for ICU Admission (n = 37)
Discussion
We examined the clinical characteristics, imaging features, and clinical outcomes of patients hospitalized for COVID-19 who had been fully, partially, or not vaccinated in a multicenter cohort. Of the 761 patients, COVID-19 breakthrough infection was observed in 47 (6%) and the percentage of asymptomatic patients during admission was significantly higher in the fully vaccinated group (21%, 10 of 47) than in the unvaccinated group (7%, 42 of 587). Additionally, compared with unvaccinated patients (22%, 71 of 326), a higher percentage of fully vaccinated patients (59%, 13 of 22) had no pneumonia evidenced on CT scans (P < .001). Multivariable analysis adjusted for demographic and clinical characteristics at admission showed fully and partially vaccinated patients were at significantly lower risk of requiring supplemental oxygen or intensive care unit admission.
Reported incidences of COVID-19 breakthrough infections range from 0.4% to 9.5% and depend on the vaccine type, time elapsed after vaccination, percentage of vaccinated people, and viral variants (11–17). According to a report issued by the Washington State Department of Health, the number of breakthrough infections increased up to 30% of overall newly infected patients as the total number of infected and vaccinated numbers increased (18). In the Republic of Korea, on August 31, 2021, the prevalence of COVID-19 was 0.1%, the percentages of the Korean population partially or fully vaccinated were 62% and 33%, respectively, and breakthrough infections accounted for 1.6% of all patients who tested positive for COVID-19 (19). As of December 12, 2021, the prevalence of COVID-19 in Ontario, Canada, was approximately 0.5%, the fully vaccinated rate was 80%, and breakthrough infections were reported in approximately 6.1% (20). Further research is needed to better understand the incidence of COVID-19 breakthrough infections and to identify and understand the contributing factors.
Observed differences in clinical characteristics may reflect differences in vaccination priorities according to underlying comorbidities. During the study period from June to August 2021, high-risk groups, such as individuals 65 years and older, those with disabilities, and health care workers, were priority targets for COVID-19 vaccination in the Republic of Korea (21). Therefore, older patients and patients with at least one comorbidity were more common in the vaccinated group than in the unvaccinated group in our study. Despite these differences, mechanical ventilation and in-hospital death only occurred in the unvaccinated group. Furthermore, after adjusting for baseline clinical characteristics, multivariable analysis showed that fully vaccinated patients were at significantly lower risk of requiring supplemental oxygen and ICU admission than unvaccinated patients. Our findings concur with those of recent studies on the relationship between vaccination and disease severity in patients with COVID-19 (21–24). In addition, we analyzed imaging and clinical findings of fully, partially, and unvaccinated patients to determine the relationship of vaccination status with clinical severity, which was not investigated in previous studies (21–24).
The differences in the frequency of COVID-19–related pneumonia observed on CT scans in the three study groups may explain disease severity differences during hospitalization. In a study that examined clinical outcomes according to presence or absence of pneumonia in symptomatic patients with COVID-19, Leonard-Lorant et al (25) found that the clinical outcomes of patients with COVID-19 who had an initially pneumonia-negative CT finding were better than that of those with a positive finding. In addition, several previous studies have reported that patients with extensive pneumonia on CT scans had a poorer prognosis (26–28). In our study, the proportion of pneumonia-negative CT scans during hospital stays was significantly greater for fully vaccinated patients than unvaccinated patients. However, the proportion of pneumonia-negative chest radiograph assessments were not significantly different in the three study groups, which we attribute to the low negative predictive value of chest radiographs in patients with COVID-19–related pneumonia (29). Our findings may suggest that vaccination is negatively associated with the development of pneumonia in patients with COVID-19. Given the steady increase in vaccination rates, the role of diagnostic imaging in patients with suspected COVID-19 may need to be redefined.
After adjusting demographic and clinical characteristics at admission, as well as several laboratory biomarkers, fully or partially vaccinated patients were found to be independently associated with lower risks of requiring supplemental oxygen or ICU admission. However, hospital length of stay did not differ among the three groups as most patients had mild symptoms and were discharged from the hospital approximately 10 days after the initial diagnosis, per the aforementioned treatment guidelines of our country. We also observed associations between the risk of severe disease and clinical characteristics such as older age, history of diabetes, lymphocytopenia, thrombocytopenia, elevated LDH level, and elevated CRP level, which is in agreement with previous studies (30,31). Notably, age is an important predictor of severe disease in patients with COVID-19, even in those with a breakthrough infection (32).
Our study has several limitations that warrant mention. First, the number of patients in each group was quite different, which may have introduced bias comparing group characteristics. In addition, the sample size for the fully vaccinated group was small. Second, all asymptomatic infections may have been underestimated because we included only patients who presented at each center. Third, data on SARS-CoV-2 variants were not available and this may have confounded comparisons of clinical features. Fourth, chest radiograph and CT pneumonia scores were obtained using a three-point scale. Finally, our study cohort had a few factors that may make it difficult to extrapolate to cohorts worldwide (the majority were immunized with a non-mRNA vaccine, all patients were hospitalized). Notwithstanding these limitations, our study provides comparative clinical and imaging characteristics of COVID-19 breakthrough infections, which have not been clearly described in the literature.
In summary, patients with COVID-19 breakthrough infections had a higher proportion of CT scans without pneumonia compared with unvaccinated patients, and vaccination status was significantly associated with the need for supplemental oxygen and intensive care unit admission. This study sheds light on the clinical effectiveness of COVID-19 vaccination in the context of breakthrough infections.
J.E.L. and M.H. contributed equally to this work.
Disclosures of conflicts of interest: J.E.L No relevant relationships. M.H. No relevant relationships. Y.H.K. No relevant relationships. M.J.C. No relevant relationships. B.H.S. No relevant relationships. K.J.C. No relevant relationships. J.Y.Y. No relevant relationships. Y.J.J. No relevant relationships.
Abbreviations:
- CRP
- C-reactive protein
- ICU
- intensive care unit
- LDH
- lactate dehydrogenase
- OR
- odds ratio
- RT-PCR
- reverse transcriptase polymerase chain reaction
References
- 1. World Health Organization . Weekly epidemiological update on COVID-19 . https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---28-december-2021. Published December 28, 2021. Accessed December 29, 2021.
- 2. World Health Organization . Coronavirus (COVID-19) dashboard . https://covid19.who.int/. Accessed December 29, 2021.
- 3. Centers for Disease Control and Prevention . The possibility of COVID-19 after vaccination: breakthrough infections . https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html. Published September 7, 2021. Accessed November 3, 2021 .
- 4. Pilishvili T , Gierke R , Fleming-Dutra KE , et al . Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel . N Engl J Med 2021. ; 385 ( 25 ): e90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falsey AR , Sobieszczyk ME , Hirsch I , et al . Phase 3 safety and efficacy of AZD1222. (ChAdOx1 nCoV-19)Covid-19 vaccine . N Engl J Med 2021. ; 385 ( 25 ): 2348 – 2360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021;384(23):2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ritchie H , Beltekian D , Mathieu E , et al . Statistics and Research: Coronavirus (COVID-19) Vaccinations . https://ourworldindata.org/covid-vaccinations. Published 2021. Accessed November 3, 2021 . [Google Scholar]
- 8. Yoon SH , Ham SY , Nam BD , et al . Establishment of a nationwide Korean imaging cohort of coronavirus disease 2019 . J Korean Med Sci 2020. ; 35 ( 46 ): e413 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Au-Yong I , Higashi Y , Giannotti E , et al . Chest Radiograph Scoring Alone or Combined with Other Risk Scores for Predicting Outcomes in COVID-19 . Radiology 2021. . 10.1148/radiol.2021210986. Published online September 14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson S , Kay FU , Abbara S , et al . Radiological Society of North America Expert Consensus Document on Reporting Chest CT Findings Related to COVID-19: Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA . Radiol Cardiothorac Imaging 2020. ; 2 ( 2 ): e200152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergwerk M , Gonen T , Lustig Y , et al . Covid-19 breakthrough infections in vaccinated health care workers . N Engl J Med 2021. ; 385 ( 16 ): 1474 – 1484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brinkley-Rubinstein L , Peterson M , Martin R , Chan P , Berk J . Breakthrough SARS-CoV-2 infections in prison after vaccination . N Engl J Med 2021. ; 385 ( 11 ): 1051 – 1052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butt AA , Khan T , Yan P , Shaikh OS , Omer SB , Mayr F . Rate and risk factors for breakthrough SARS-CoV-2 infection after vaccination . J Infect 2021. ; 83 ( 2 ): 237 – 279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hacisuleyman E , Hale C , Saito Y , et al . Vaccine breakthrough infections with SARS-CoV-2 variants . N Engl J Med 2021. ; 384 ( 23 ): 2212 – 2218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uschner D , Bott M , Santacatterina M , et al . Breakthrough SARS-CoV-2 Infections after Vaccination in North Carolina . medRxiv 2021:2021.2010.2010.21264812. Posted October 13, 2021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson MG , Grant L , Meece J ; HEROES-RECOVER Network . Prevention of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. Reply . N Engl J Med 2021. ; 385 ( 19 ): 1819 – 1821 . [DOI] [PubMed] [Google Scholar]
- 17. Nixon DF , Ndhlovu LC . Vaccine Breakthrough Infections with SARS-CoV-2 Variants . N Engl J Med 2021. ; 385 ( 2 ): e7 . [DOI] [PubMed] [Google Scholar]
- 18. Washington State Department of Health . SARS-CoV-2 Vaccine Breakthrough Surveillance and Case Information Resource . https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/data-tables/420-339-VaccineBreakthroughReport.pdf. Published November 3, 2021. Accessed November 5, 2021.
- 19. Korea Disease Control and Prevention Agency . COVID-19 dashboard, Republic of Korea . https://www.kdca.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=716731&tag=&nPage=7. Published August 31, 2021. Accessed November 3, 2021 .
- 20. Ontario Agency for Health Protection and Promotion (Public Health Ontario) . Confirmed cases of COVID19 following vaccination in Ontario: December 14, 2020 to December 12, 2021 . Toronto, ON: : Queen’s Printer for Ontario; 202; . https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-epi-confirmed-cases-post-vaccination.pdf?sc_lang=en. Published December 20, 2021, Accessed December 31, 2021. [Google Scholar]
- 21. Jung J . Preparing for the coronavirus disease (COVID-19) vaccination: evidence, plans, and implications . J Korean Med Sci 2021. ; 36 ( 7 ): e59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson MG , Stenehjem E , Grannis S , et al . Effectiveness of COVID-19 vaccines in ambulatory and inpatient care settings . N Engl J Med 2021. ; 385 ( 15 ): 1355 – 1371 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olliaro P , Torreele E , Vaillant M . COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room . Lancet Microbe 2021. ; 2 ( 7 ): e279 – e280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brosh-Nissimov T , Orenbuch-Harroch E , Chowers M , et al . BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel . Clin Microbiol Infect 2021. ; 27 ( 11 ): 1652 – 1657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonard-Lorant I , Severac F , Bilbault P , et al . Normal chest CT in 1091 symptomatic patients with confirmed Covid-19: frequency, characteristics and outcome . Eur Radiol 2021. ; 31 ( 7 ): 5172 – 5177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y , Yang Z , Ai T , Wu S , Xia L . Association of “initial CT” findings with mortality in older patients with coronavirus disease 2019 (COVID-19) . Eur Radiol 2020. ; 30 ( 11 ): 6186 – 6193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Z , Jin C , Wu CC , et al . Association between initial chest CT or clinical features and clinical course in patients with coronavirus disease 2019 pneumonia . Korean J Radiol 2020. ; 21 ( 6 ): 736 – 74 . [Published correction appears in Eur Radiol 2021;31(1):567-568.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Francone M , Iafrate F , Masci GM , et al . Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis . Eur Radiol 2020. ; 30 ( 12 ): 6808 – 6817 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sverzellati N , Ryerson CJ , Milanese G , et al . Chest radiography or computed tomography for COVID-19 pneumonia? Comparative study in a simulated triage setting . Eur Respir J 2021. ; 58 ( 3 ): 2004188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gandhi RT , Lynch JB , Del Rio C . Mild or moderate Covid-19 . N Engl J Med 2020. ; 383 ( 18 ): 1757 – 1766 . [DOI] [PubMed] [Google Scholar]
- 31. Wu C , Chen X , Cai Y , et al . Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China . JAMA Intern Med 2020. ; 180 ( 7 ): 934 – 943 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butt AA , Nafady-Hego H , Chemaitelly H , et al . Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination . Int J Infect Dis 2021. ; 110 ( 353 ): 358 . [DOI] [PMC free article] [PubMed] [Google Scholar]