Abstract
Objective
Crystal structures activate innate immune cells, especially macrophages, and initiate inflammatory responses. We aimed to understand the role of the mechanosensitive TRPV4 channel in crystal-induced inflammation.
Methods
Real-time RT-PCR, RNAscope in situ hybridization, and Trpv4eGFP mice were used to examine TRPV4 expression and whole-cell patch-clamp recording and live-cell Ca2+ imaging were used to study TRPV4 function in mouse synovial macrophages and human peripheral blood mononuclear cells (PBMCs). Both genetic deletion and pharmacological inhibition approaches were used to investigate the role of TRPV4 in NLRP3 inflammasome activation induced by diverse crystals in vitro and in mouse models of crystal-induced pain and inflammation in vivo.
Results
TRPV4 was functionally expressed by synovial macrophages and human PBMCs and TRPV4 expression was upregulated by stimulation with monosodium urate (MSU) crystals and in human PBMCs from patients with acute gout flares. MSU crystal-induced gouty arthritis were significantly reduced by either genetic ablation or pharmacological inhibition of TRPV4 function. Mechanistically, TRPV4 mediated the activation of NLRP3 inflammasome by diverse crystalline materials but not non-crystalline NLRP3 inflammasome activators, driving the production of inflammatory cytokine IL-1β which elicited TRPV4-dependent inflammatory responses in vivo. Moreover, chemical ablation of the TRPV1-expressing nociceptors significantly attenuated the MSU crystal-induced gouty arthritis.
Conclusion
TRPV4 is a common mediator of inflammatory responses induced by diverse crystals through NLRP3 inflammasome activation in macrophages. TRPV4-expressing resident macrophages are critically involved in MSU crystal-induced gouty arthritis. A neuroimmune interaction between the TRPV1-expressing nociceptors and the TRPV4-expressing synovial macrophages contributes to the generation of acute gout flares.
Keywords: Crystalline materials, TRPV4, macrophage, NLRP3 inflammasome, IL-1β
Graphical Abstract
Introduction
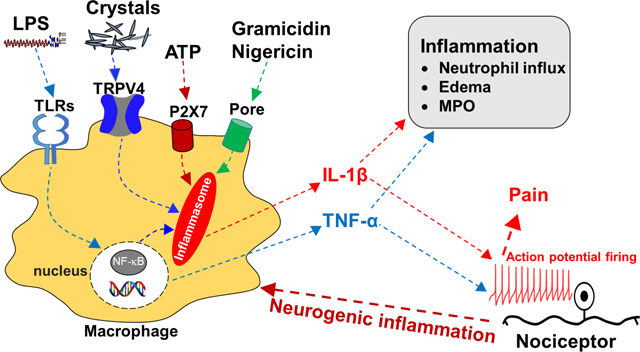
Innate immune cells recognize a variety of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) to mount a rapid inflammatory response.1, 2 One way in which cells mediate this process is through the NLRP3 inflammasome, an intracellular sensor of both exogenous and endogenous stimuli such as microbes, misfolded proteins, and ATP. Depending on the signal, this pathway activates a variety of cellular processes including inflammatory cytokine production and programmed cell death by pyroptosis to drive an innate antimicrobial response.1 However, as with many host-protective responses, this process can also become highly pathologic.
Many exogenous or endogenously formed crystal structures with different compositions can act as DAMPs and in turn trigger NLRP3 inflammasome activation.3, 4 For instance, exogenous silica crystals and asbestosis fibers are well-known to cause fibrosis and chronic airway disease. It is also recognized that innate immune cells such as macrophages (MΦs) internalize both endogenous and exogenous crystals and thereby activate the NLRP3 inflammasome, driving many inflammatory diseases such as gout, pseudogout, atherosclerosis and silicosis.5, 6 Currently, there are at least three hypotheses for MΦ recognition and NLRP3 inflammasome activation by crystals and nanoparticles: (1) The NLRP3 inflammasome senses the disturbance of cellular homeostasis rather than directly recognizing various types of crystals;7 (2) Distinct membrane-bound receptors serve as molecular sensors for distinct crystals to initiate NLRP3 inflammasome activation upon interaction;5 (3) Phagocyte membrane engagement by crystals may directly activate the NLRP3 inflammasome in a receptor-independent manner.8 However, the molecular events underlying the activation of the NLRP3 inflammasome induced by cell surface contact with crystals remain unclear.
Growing evidence shows that ion channels are critical signaling mediators in both innate and adaptive immune cells9 and their expression and function on MΦs are tightly associated with NLRP3 inflammasome activation.10 Transient receptor potential (TRP) ion channels are important signaling components in both neurosensory and inflammatory pathways, giving way to their functions as polymodal sensors of environmental cues and as key mediators in sensory transduction.11–15 Among them, the non-selective multifunctional cation channel TRPV4 is highly expressed in cartilage chondrocytes and activated by hypo-osmolality, heat, and arachidonic acid metabolites16. TRPV4 has also been functionally linked to the skeletal dysplasia.17, 18 Although studies reported that TRPV4 is expressed by primary nociceptors and contributes to acute nociception caused by hypotonic stress and neuroinflammation,19–21 emerging evidence also showed that TRPV4 expressed by non-neuronal cells including MΦs is involved in the development of acute/chronic itch and regulation of gastrointestinal motility.22, 23 However, it remains unknown whether and how TRPV4 channel is involved in crystal-induced inflammation in MΦs.
In this study, we show that MΦ-expressed TRPV4 is selectively involved in the inflammation and reflexive pain-related responses induced by both endogenous and exogenous crystals through activation of the NLRP3 inflammasome. Our results suggest that TRPV4 is a potential drug target for treating crystal-induced inflammatory disorders.
MATERIALS AND METHODS
Materials and methods are described in the online supplemental file.
Results
TRPV4 is required for MSU crystal-induced reflexive pain-related responses and inflammation in a mouse model of gout.
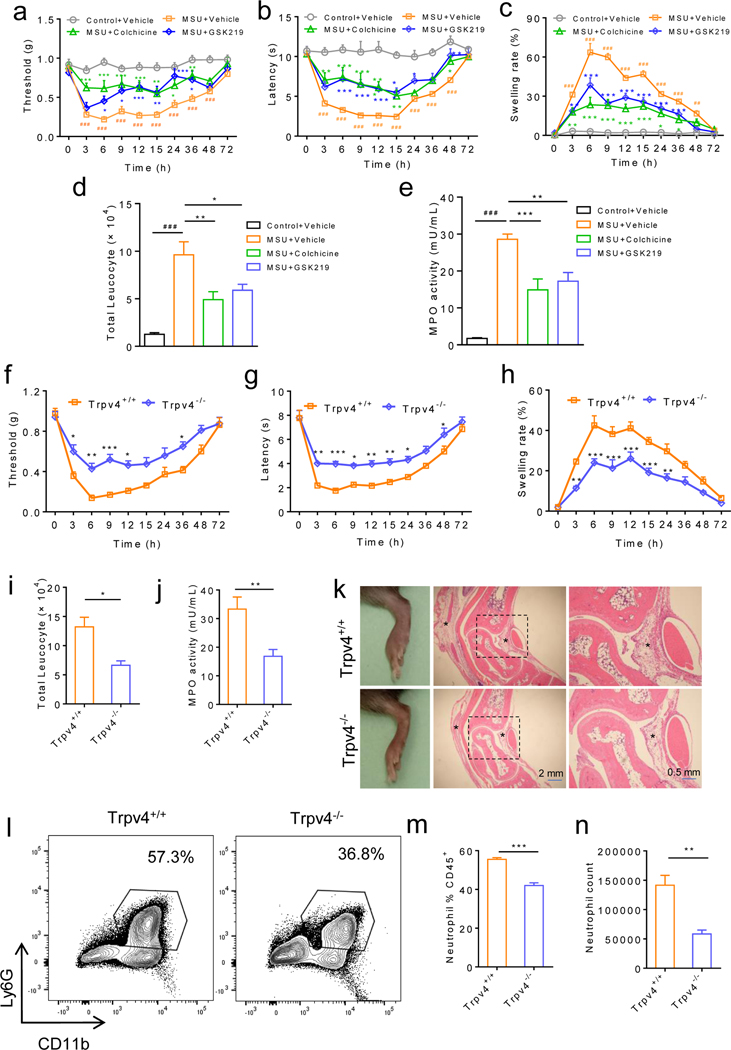
To determine the role of TRPV4 in inflammatory joint disease, we first employed the well-established mouse model of gout.24 As expected, wild-type (wt) mice treated with intra-articular (IA) injections of monosodium urate (MSU) crystals (0.8 mg per site) developed severe joint swelling and reflexive mechanical and thermal pain-related responses (figure 1a–c). Strikingly, intraperitoneal (I.P.) administration of GSK219, a potent and selective TRPV4 inhibitor,23, 25 either 3 days before injections of MSU crystals (figure 1a–e) or 5 hrs after MSU crystal injections (online supplemental figure 1a–e) significantly reduced MSU crystal-induced reflexive pain-related responses as well as inflammation-related parameters including ankle edema, leukocyte infiltration and myeloperoxidase (MPO) activity. The inhibitory effect of GSK219 was comparable to that of colchicine, a standard of care (SoC) treatment for acute gout flares (figure 1a–e). Moreover, genetic ablation of TRPV4 function significantly alleviated MSU crystal-induced reflexive pain-related responses, and joint inflammation (ankle edema, synovium lesion/inflammation, neutrophil infiltration and MPO activity) when compared with Trpv4+/+ control mice (figure 1f–n), suggest that TRPV4 function is critically required for the generation of MSU crystal-induced gouty arthritis.
Figure 1: Pharmacological inhibition or genetic ablation of TRPV4 function reduces inflammation and reflexive pain-related responses in a mouse model of gout.
(a-c) IA injections of MSU crystals (0.8 mg/site) in wt C57BL/6J mice produced reflexive mechanical (a) and heat (b) pain-related responses as well as joint swelling (c) in a time-dependent manner. Mice were treated with either GSK219 (5 mg/kg, i.p.) or vehicle 3 days before injections of MSU crystals. Colchicine (1 mg/kg, i.p.) was used as a positive control. n=6–8 for each group. (d, e) Increased leucocyte infiltration (d) and myeloperoxidase activity (e) were detected 6 hr after IA injections of MSU crystals in the presence of GSK219 (5 mg/kg, i.p.) or vehicle. Colchicine (1 mg/kg, i.p.) was used as a positive control. n=6 for the vehicle control and 7–8 for all other groups. (f-h) Time courses for reflexive mechanical (f) and heat (g) pain-related responses as well as joint swelling (h) induced by IA injections of MSU crystals in the Trpv4+/+ and Trpv4−/− mice, n=7–8 for each group. (i, j) Both total leucocyte infiltration (i) and myeloperoxidase activity (j) were measured 6 hr after IA injections of MSU crystals, n=7–8 for each group. (k) Representative HE-staining showing edema and inflammation of ankles in both Trpv4+/+ and Trpv4−/− mice 6 hr after IA injections of MSU crystals. Magnifications, ×5 (middle) and ×20 (right). Asterisks indicate the infiltrating leukocytes. n=3 independent repeats with similar results for both groups. (l) Representative FACS plots of neutrophils in the articular cavity from the Trpv4+/+ and Trpv4−/− mice subjected to IA injections of MSU crystals. (m, n) Summarized data on the right show the percentile of neutrophils within the CD45+ population (m) and the comparison of the neutrophil counts (n) between the Trpv4+/+ and Trpv4−/− mice subject to IA injections of MSU crystals. n=6 for all groups. Statistical significance was determined using Tukey post hoc tests (multiple comparisons, a-c), one-way ANOVA with Tukey’s post-test (d-e), two-way ANOVA followed by Bonferroni’s post-hoc test (f-h), and Student’s t test (i, j, m, n). *P<.05, **P<.01, ***P<.001.
TRPV4 expression and function are increased in the setting of acute gout flares.
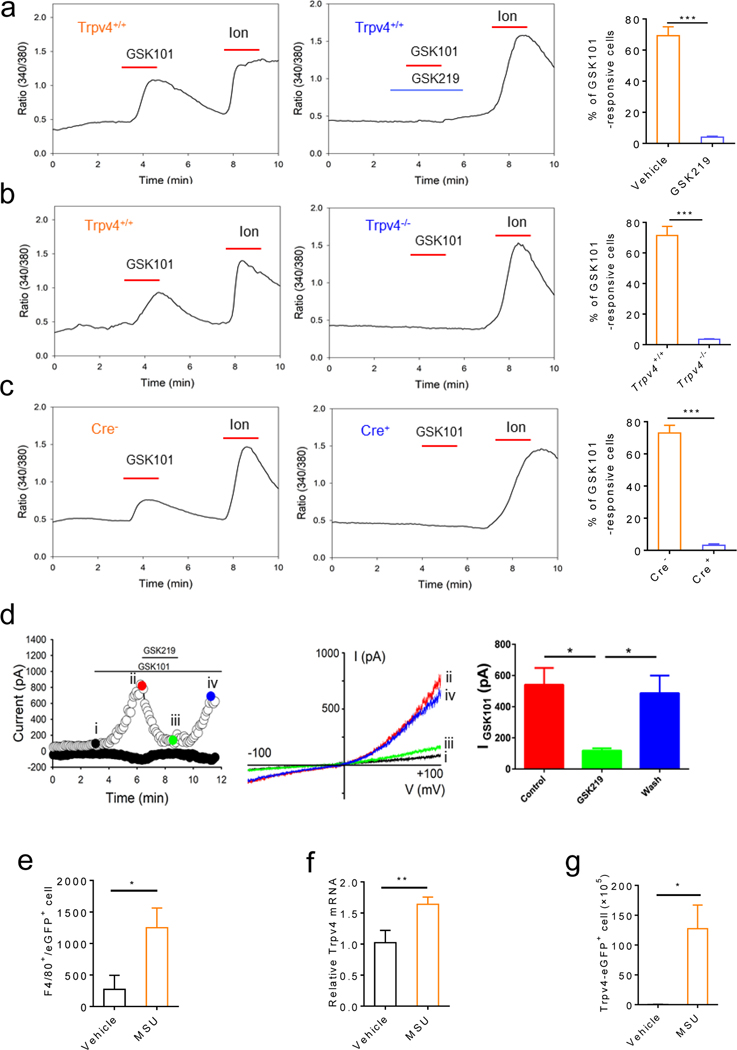
Although TRPV4 is reported to be expressed by the primary nociceptors,26, 27 surprisingly, we did not detect TRPV4 expression and function in the dorsal root ganglion (DRG) cell bodies and nerve terminals in the skin using the Trpv4eGFP mice, RNAscope in situ hybridization, and live-cell Ca2+ imaging on ex vivo DRG explants (online supplemental figure 2a–i). On the other hand, TRPV4-eGFP+ cells were found in the synovial membrane and overlapped with well-known synovial MΦ markers such as F4/80, Cx3cr1, and CD68 (online supplemental figure 3a and b). To determine the functionality of TRPV4 in the synovial MΦs, we used live-cell Ca2+ imaging on sort-purified CD45+ synovial MΦs using CD11b/MHCII (figure 2a–c). Application of a potent and selective TRPV4 activator GSK101 elicited a robust [Ca2+]i response in the synovial MΦs isolated from the Trpv4+/+ mice,28, 29 which was nearly abolished by GSK219 (figure 2a). GSK101 GSK101-induced [Ca2+]i response was absent in synovial MΦs from either the Trpv4−/− mice or the Cre+ MΦ-specific Trpv4 cKOs (Cx3cr1CreERT;Trpv4f/f) in which TRPV4 expression is selectively ablated in MΦs after tamoxifen induction22 (figure 2b–c). Moreover, GSK101 activated TRPV4-like whole-cell currents in the eGFP+ synovial MΦs isolated from the Trpv4eGFP mice, which was inhibited by GSK219 (figure 2d). Flow cytometry further revealed that most of the TRPV4-eGFP+/CD45+ cells were CD11b+/F4/80+ (figure 2e). Strikingly, both TRPV4 mRNA transcripts (figure 2f) and the number of TRPV4-eGFP+ cells were markedly increased in the joints 6 hrs after IA injections of MSU crystals (0.8 mg/site) (figure 2g and online supplemental figure 3c). Consistent with the data from mouse studies, TRPV4 expression was significantly increased in PBMCs, the most relevant cell type of resident MΦs, from patients with acute gout flares when compared with that from healthy subjects (online supplemental figure 3d). In marked contrast, the expression of TRPV1, TRPA1, or TRPM8 was not significantly different between these two groups (online supplemental figure 3d). Moreover, PBMCs from gout patients displayed a stronger [Ca2+]i response upon application of TRPV4 agonist GSK101 (100 nM) when compared with that from healthy control subjects (online supplemental figure 3e and f).
Figure 2: TRPV4 is functionally expressed by the synovial MΦ and TRPV4 expression and the number of TRPV4+ MΦs are increased by MSU crystals.
(a) Representative traces showing GSK101 (0.3 μM)-elicited [Ca2+]i response in freshly dissociated synovial MΦ single-cell suspensions from the Trpv4+/+ mice. Pre- and co-applied GSK219 (1 μM) abolished the GSK101 action. Summarized data on the right show the reduction of the percentage of GSK101-responsive synovial MΦs by GSK219. (b) Representative traces showing that GSK101 induced a [Ca2+]i response in the synovial MΦ single-cell suspensions from the Trpv4+/+ but not Trpv4−/− mice. Summarized data on the right show that genetic ablation of TRPV4 function reduces the percentage of GSK101-responsive synovial MΦs. (c) Representative traces showing the GSK101-induced [Ca2+]i response in the synovial MΦ single-cell suspensions from the Cre- but not Cre+ Cx3cr1CreERT; Trpv4f/f mice. Summarized data on the right show reduced the percentage of GSK101-responsive synovial MΦs isolated from the MΦ-specific TRPV4 cKOs. (d) Left: Representative time course of membrane currents evoked by GSK101 (0.3 μM) at +100 mV and −100 mV membrane potentials with and without co-applied GSK219 (1 μM). Horizontal bars denote the time courses for applications of GSK101 and GSK219. Middle: Representative current-voltage curves taken at time points i, ii, iii, and iv (color-coded) from the time course on the left. A ramp protocol elicited by a voltage ramp from −100 mV to +100 mV was used. Quantification of the effect of GSK219 on GSK101-activated whole-cell membrane current recorded at +100 mV is shown on the right. (e) Flow cytometry shows that the number of TRPV4-eGFP+ cells increased significantly 6 hr after IA injections of MSU crystals (0.8 mg/site). (f) TRPV4 mRNA expression in the synovial lining of the Trpv4eGFP mice treated with vehicle or MSU crystals. (g) Quantification of the number of the F4/80+/eGFP+ synovial MΦs in response to treatment with MSU crystals or vehicle. Statistical significance was determined using Student’s t test (a-g), *P<.05, **P<.01, ***P<.001. n=5–6 per group.
MSU crystal-induced inflammation and reflexive pain-related responses are attenuated in MΦ-specific Trpv4 cKOs.
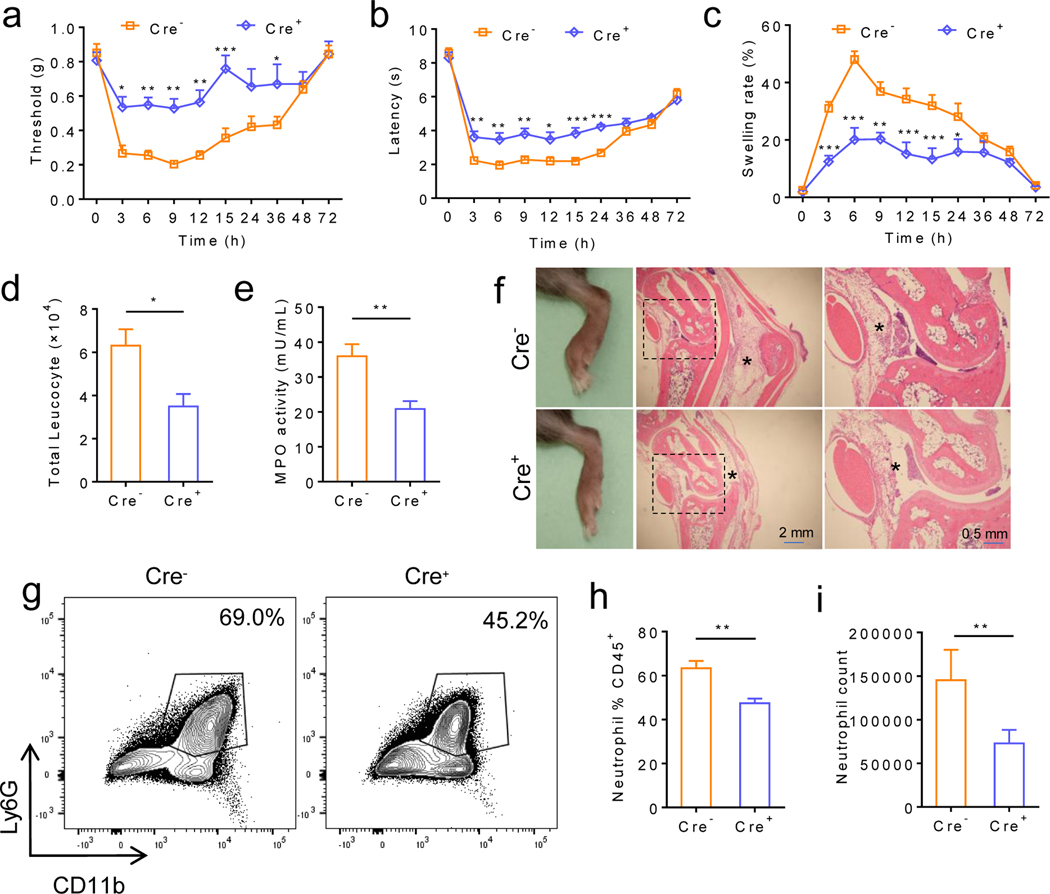
To test whether MΦ-expressed TRPV4 is required for MSU crystal-induced gouty arthritis, we used the MΦ-specific Trpv4 cKOs (Cx3cr1CreERT; Trpv4f/f).22 Indeed, the Cre+ mice displayed significantly reduced reflexive pain-related responses, joint inflammation and synovium lesion when compared with the Cre- littermates in response to IA injections of MSU crystals (figure 3).
Figure 3: MΦ-specific Trpv4 cKOs display reduced joint swelling and reflexive pain-related responses in MSU crystal-induced gouty arthritis.
(a-c) Time courses for reflexive mechanical (a) and heat (b) pain-related responses as well as joint swelling (c) induced by IA injections of MSU crystals (0.8 mg/site) in both Cre+ and Cre- Cx3cr1CreERT; Trpv4f/f mice (Experiments were performed 1 week after the last tamoxifen administration). n=6–8 for each group. (d, e) Changes of total leucocyte infiltration (d) and myeloperoxidase activity (e) in both Cre+ and Cre- Cx3cr1CreERT; Trpv4f/f mice 6 hr after IA injections of MSU crystals (0.8 mg/site), n=6–8 for each group. (f) Representative HE-staining showing edema and inflammation of ankle sections 6 hr after IA injections of MSU crystals (0.8 mg/site) in the Cre+ and Cre- Cx3cr1CreERT; Trpv4f/f mice. Magnifications, ×5 (middle) and ×20 (right). Asterisks indicate the infiltrating leukocytes. n=3 independent experiments with similar results for both groups. (g) Representative FACS plots of synovial neutrophils from both Cre- and Cre+ Cx3cr1CreERT; Trpv4f/f mice subjected to IA injections of MSU crystals. (h, i) Summarized data show the comparison of the percentile of neutrophils within the CD45+ population (h) and the neutrophil count (i) between the Cre- and Cre+ Cx3cr1CreERT; Trpv4f/f mice subjected to IA injections of MSU crystals. n=5 for each group. Statistical significance was determined using two-way ANOVA followed by Bonferroni’s post-hoc test (a-c), and Student’s t test (d, e, h, i), *P<.05, **P<.01, ***P<.001.
Since the synovial MΦs can arise from either tissue-resident or circulating monocyte-derived MΦs, we also tested the inducible MΦ-specific Trpv4 cKOs 4 weeks after tamoxifen administration when tissue-resident MΦs retain their identity but the monocyte-derived MΦs are replaced with wt MΦs produced by bone-marrow-derived progenitors as shown by fate mapping studies.30 Both reflexive pain-related responses and ankle swelling were still significantly reduced in the Cre+ Cx3cr1CreERT; Trpv4f/f mice when compared to their Cre- littermates (online supplemental figure 4). These results recapitulate the reduced reflexive pain-related responses and inflammation phenotypes displayed by the Trpv4−/− mice and further demonstrate that TRPV4 expression in the tissue-resident MΦs is the primary contributor to MSU crystal-induced gouty arthritis.
TRPV4 is also functionally expressed by neutrophils31 and articular chondrocytes that are involved in age-related and post-traumatic osteoarthritis (OA).32 Indeed, in addition to synovial MΦs, the number of eGFP+ neutrophils was also markedly increased by MSU crystals as revealed by flow cytometry and immunofluorescence (online supplemental figure 5a–c). Notably, we and others have used neutrophil infiltration as an inflammation marker for MSU crystal-induced gouty inflammation.33 To test if TRPV4 expression in these cell types also contributes to gouty inflammation and reflexive pain-related responses, we generated the neutrophil-specific (S100a8Cre; Trpv4f/f) and cartilage-specific (Col2a1CreERT+; Trpv4f/f) Trpv4 cKOs. Neither of these Cre+ cKOs displayed changes in ankle swelling and reflexive pain-related responses after IA injections of MSU crystals compared to their respective Cre- groups (online supplemental figure 5d–i). Consistent with the absence of TRPV4 expression and function in primary nociceptors, genetic ablation of TRPV4 function in the TRPV1 lineage neurons (Trpv1Cre; Trpv4f/f) had no effect on MSU crystal-induced gouty arthritis (online supplemental figure 5j–l). Together, these results suggest that neutrophil-, articular chondrocyte- or sensory nociceptor-expressed TRPV4 is dispensable for the development of MSU crystal-induced gouty arthritis.
TRPV4 function is critically involved in MSU crystal-induced NLRP3 inflammasome activation and IL-1β production.
Activation of NLRP3 inflammasome is a hallmark of MΦ-mediated inflammatory responses, which requires at least two signaling events:7, 34 The first event involves nuclear factor-κB (NF-κB)-dependent upregulation of NLRP3 along with pro-IL-1β, which is triggered by PAMPs including Toll-like receptors (TLRs), DAMPs or cytokines. Of note, NF-κB signaling also promotes the production and release of tumor necrosis factor-α (TNF-α), which can be independent of NLRP3 inflammasome activation. The second event involves the assembly of a complex of multiple proteins including NLRP3, ASC (the adaptor molecule apoptosis-associated speck-like protein containing a CARD), and pro-caspase-1, resulting in the activation of caspase-1. Subsequently, active caspase-1 processes pro-IL-1β to mature IL-1β, which is then released from dying MΦs.
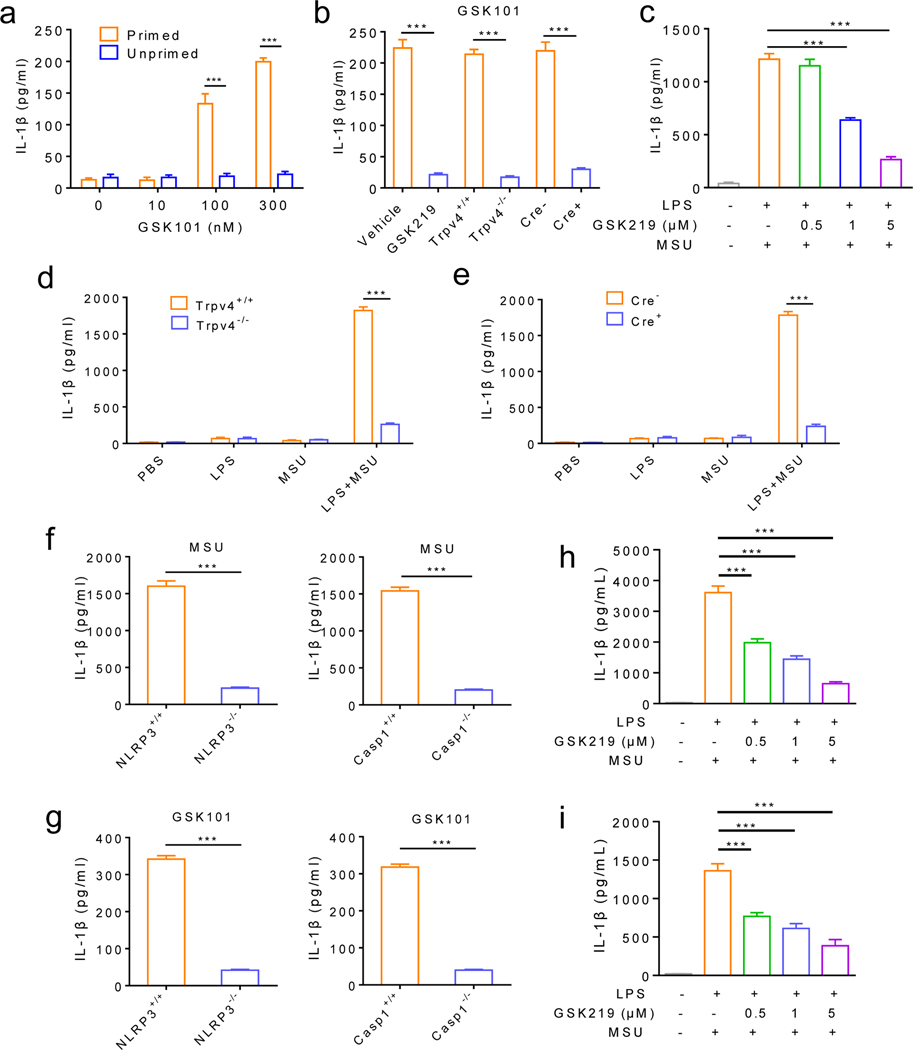
Strikingly, application of the TRPV4 activator GSK101 was sufficient to induce the production of IL-1β in a concentration-dependent manner in the Trpv4+/+ BMDMs primed with LPS but not in LPS-unprimed group, which could be blunted by either pharmacological inhibition or genetic ablation of TRPV4 function (figure 4a and b, online supplemental figure 6a), although GSK101 did not affect pro-IL-1β and pro-caspase-1 in the lysates (Input) of LPS-primed BMDMs isolated from the Trpv4+/+ and Trpv4−/− mice (online supplemental figure 6a). Similar to GSK101, application of MSU crystals also markedly increased the IL-1β production from LPS-primed wt BMDMs (figure 4c, online supplemental figure 6a) while the levels of both pro-IL-1β and pro-caspase-1 were comparable in lysates (Input) of LPS-primed and MSU crystal-treated BMDMs isolated from either Trpv4+/+ or Trpv4−/− mice (online supplemental figure 6a). GSK219 suppressed MSU crystal-induced IL-1β production from LPS-primed BMDMs in a concentration-dependent manner (figure 4c). MSU crystal-induced IL-1β production was also abolished in the LPS-primed BMDMs isolated from either the Trpv4−/− mice or Cre+ MΦ-specific Trpv4 cKOs when compared with their respective control groups (figure 4d and e, online supplemental figure 6a). Moreover, LPS-primed BMDMs from mice deficient in NLRP3 or caspase-1 were unable to release cleaved IL-1β in response to either MSU crystals (figure 4f) or GSK101 (figure 4g). These results suggest that TRPV4 is involved in the second event of NLRP3 inflammasome activation in LPS-primed BMDMs and NLRP3-caspase-1 signaling is required for TRPV4-mediated IL-1β production induced by both MSU crystals and GSK101. Consistent with results from mouse BMDMs, MSU crystals also induced robust inflammasome activation in cultured primary human MΦs (THP-1 cells) and human PBMCs isolated from healthy subjects, which was significantly reduced by GSK219 (figure 4h and i, and online supplemental figure 6b and c), suggesting that TRPV4 is a key mediator of MSU crystal-induced NLRP3 inflammasome activation in innate immune cells from both rodents and humans.
Figure 4: TRPV4 function is critical to MSU crystal-induced NLRP3 inflammasome activation and IL-1β production.
(a) GSK101 promotes IL-1β production in LPS-primed but not in LPS-unprimed BMDMs in a concentration-dependent manner. (b) MSU crystal-induced IL-1β production from LPS-primed BMDMs was severely suppressed by TRPV4 antagonism and genetic ablation of TRPV4 function in both global Trpv4 KO mice and MΦ-specific Trpv4 cKOs compared with their respective controls. (c) MSU crystal-induced of IL-1β production from LPS-primed BMDMs was inhibited by GSK219 in a concentration-dependent manner. (d) MSU crystal-induced IL-1β production was markedly reduced in LPS-primed BMDMs isolated from the Trpv4−/− mice when compared with the Trpv4+/+ mice. (e) MSU crystal-induced IL-1β production was markedly reduced in LPS-primed BMDMs isolated from the Cre+ Cx3cr1CreERT; Trpv4f/f mice when compared with their Cre- littermates. Note PBS, LPS, and MSU crystals alone did not increase IL-1β production in (d) and (e). (f) MSU crystal-induced IL-1β production in LPS-primed BMDMs from NLRP3-deficient mice and Casp-1-deficient mice and their respective control mice. (g) GSK101-induced IL-1β production in LPS-primed BMDMs from NLRP3-deficient mice and Casp-1-deficient mice and their respective control mice. (h) ELISA analysis of IL-1β in supernatants from PMA-differentiated THP-1 cells treated with various concentrations of GSK219 and then stimulated with MSU crystals (200 μg/mL). (i) ELISA analysis of IL-1β in supernatants from human PBMCs pretreated with various concentrations of GSK219 and then stimulated with MSU crystals (200 μg/mL). Statistical significance was determined using two-way ANOVA followed by Bonferroni’s post-hoc test (a), Tukey post hoc tests (multiple comparisons, c, h and i), and Student’s t test (b, d-g). ***P<.001. n=6 per group.
Interestingly, neither GSK101 nor GSK219 showed any significant effects on the release of TNF-α from the LPS-primed wt BMDMs (online supplemental figure 7a and b). Moreover, TNF-α production was not affected in the LPS-primed BMDMs isolated from either the Trpv4−/− mice or the Cre+ MΦ-specific Trpv4 cKOs when compared to their respective controls (online supplemental figure 7c and d), suggesting that TRPV4 is unlikely involved in the first event of NLRP3 inflammasome activation and TRPV4 function is not required for TNF-α production in our MSU crystal-induced gout model.
Distinct mechanisms are involved in TRPV4-mediated NLRP3 inflammasome activation induced by GSK101 and MSU Crystals.
Although the precise mechanism of MSU crystal-induced NLRP3 activation is not completely understood, the involvement of several key events including lysosomal rupture, activation of reactive oxygen species (ROS), and intracellular Ca2+ signaling has been reported.35 As TRPV4 regulates numerous cellular functions through intracellular Ca2+ signaling, we first tested BAPTA-AM, a cell-permeant Ca2+ chelator, on GSK101- and MSU crystal-induced IL-1β production from the LPS-primed wt BMDMs. Surprisingly, BAPTA-AM effectively blocked GSK101-induced IL-1β release while it only suppressed about half of MSU crystal-induced IL-1β release (figure 5a, b). In contrast, cytochalasin D (Cyt D), an actin polymerization inhibitor that blocks >90% of phagocytosis, nearly abolished MSU crystal-induced IL-1β production without a significant effect on the GSK101-induced IL-1β production (figure 5a, b). These results suggest that the effect of GSK101-induced IL-1β production is largely dependent on TRPV4-mediated intracellular Ca2+ signaling while MSU crystals can induce IL-1β production through phagocytosis without completely relying on TRPV4-dependent intracellular Ca2+ signaling.
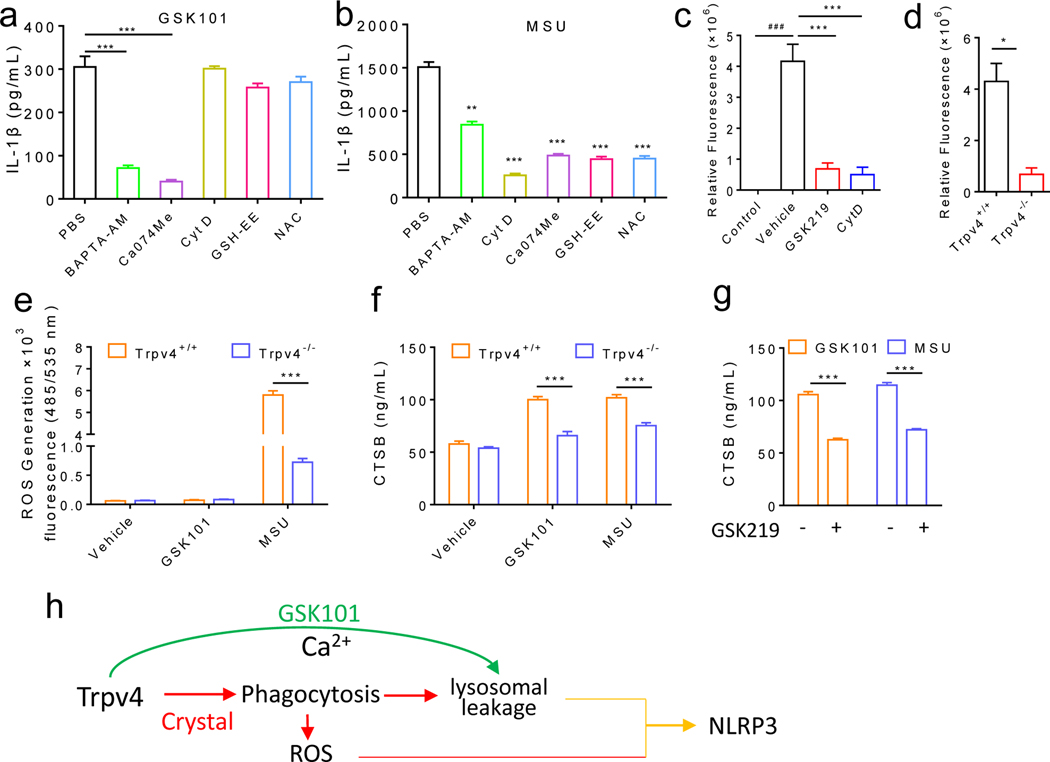
Figure 5: GSK101 and MSU crystals use distinct mechanisms to drive TRPV4-dependent NLRP3 inflammasome activation.
(a, b) GSK101- or MSU crystal- induced IL-1β production from LPS-primed BMDMs in the presence of various chemical inhibitors: Bapta-AM (a cell permeant Ca2+ chelator), cytochalasin D (Cyt D, a potent phagocytosis inhibitor), CA-074Me (a selective inhibitor of CTSB), or ROS scavengers GSH-EE (ethyl ester of glutathione) and NAC (N-acetylcysteine). PBS is the vehicle control for all chemicals. (c) Effect of GSK219, Cyt D on phagocytosis of pHrodo™ Red E. coli BioParticles® in LPS-primed BMDMs using a fluorescent microplate reader. (d) Phagocytic activity of BMDMs from the Trpv4+/+ and Trpv4−/− mice was measured using a fluorescence plate reader. (e) GSK101- and MSU crystal-induced ROS production in LPS-primed BMDMs from the Trpv4+/+ and Trpv4−/− mice. (f) GSK101- and MSU crystal-induced increase in CTSB levels in LPS-primed BMDMs isolated from the Trpv4+/+ and Trpv4−/− mice. (g) Effect of GSK219 on CTSB production induced by GSK101 or MSU crystals in LPS-primed wt BMDMs. (h) Schematic diagram of the working hypothesis. Statistical significance was determined using Student’s t test (a-g). *P<.05, **P<.01, ***P<.001. n=4–5 for each group.
Previous studies also showed that crystals can use receptor-independent membrane-based immune sensing to initiate the activation of NLRP3 inflammasome, which is blunted by a non-selective cation channel blocker ruthenium red, also a TRPV4 channel blocker.36, 37 We thus speculated that MΦ-expressed TRPV4 might be involved in the cell surface contact with various crystals. Indeed, both pharmacological blockade and genetic ablation of TRPV4 function significantly reduced MΦ phagocytosis (figure 5c, d, online supplemental figure 8a and b). Phagocytosis of crystal structures is known to produce large amounts of ROS.38 Interesting, application of MSU crystals but not GSK101 promoted ROS production in the LPS-primed BMDMs in a TRPV4-dependent manner (figure 5e). Consistent with these results, IL-1β production induced by MSU crystals but not GSK101 was markedly reduced by two ROS scavengers: ethyl ester of glutathione (GSH-EE) and N-acetylcysteine (NAC) (figure 5a, b).
Increased phagocytosis triggered by crystal structures also leads to lysosomal damage resulting in the cellular release of lysosomal contents such as cathepsin B (CTSB) that can be sensed by NLRP3 inflammasome.39, 40 Both MSU crystals and GSK101 significantly increased CTSB release from LPS-primed BMDMs, which was suppressed by either genetic or pharmacological inhibition of TRPV4 function (figure 5f, g). Further, IL-1β production induced by both GSK101 and MSU crystals was blunted by CA-074Me, a selective inhibitor of CTSB (figure 5a, b). Collectively, these findings suggest that TRPV4 is critically involved in the phagocytosis of MSU crystals by MΦs, which leads to the production of ROS and lysosomal leakage and ultimately NLRP3 inflammasome activation. On the other hand, the GSK101-induced NLRP3 inflammasome activation is accomplished by lysosomal leakage following TRPV4-dependent intracellular Ca2+ signaling (figure 5h).
TRPV4 is involved in NLRP3 inflammasome activation produced by crystalline but not non-crystalline inflammasome activators.
Besides MSU crystals, other medically relevant crystals, such as CPPD which causes pseudogout, SiO2, and alum adjuvant, as well as classic non-crystalline NLRP3 inflammasome activators such as ATP and pore-forming toxins nigericin and gramicidin also activate NLRP3 inflammasome and increase IL-1β production. We thus tested if TRPV4 is involved in the signaling pathways shared by these activators. Surprisingly, GSK219 pretreatment or genetic ablation of TRPV4 function from LPS-primed MΦs markedly reduced IL-1β production induced by CPPD, alum, or silica crystals (figure 6a), whereas IL-1β production induced by ATP, nigericin or gramicidin was unaffected (online supplemental figure 9a–c), suggesting that TRPV4 function is essential for the activation of NLRP3 inflammasome by commonly used crystalline NLRP3 activators but not the noncrystalline activators ATP and pore-forming toxins in vitro.
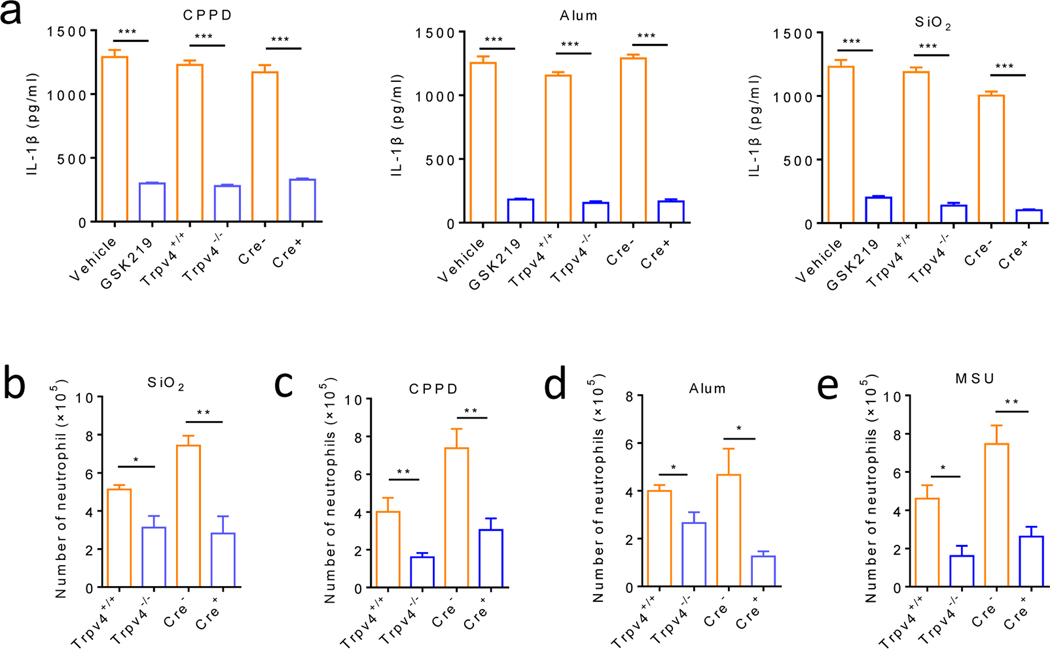
Figure 6: TRPV4 mediates both in vitro and in vivo inflammatory responses produced by crystalline but not non-crystalline NLRP3 inflammasome activators.
(a) Pharmacological inhibition (GSK219, 1 μM) and genetic ablation of TRPV4 function using both global and MΦ-specific cKOs severely reduced IL-1β release from LPS-primed BMDMs stimulated with CPPD (100 ng/mL), Alum (200 ng/mL) or SiO2 (100 ng/mL). (b) Orotracheal instilled silica crystals increased neutrophil infiltration in the bronchoalveolar lavage fluid in the Trpv4+/+ and Cre- Cx3cr1CreERT; Trpv4f/f mice, which was significantly reduced in the Trpv4−/− and Cre+ Cx3cr1CreERT; Trpv4f/f mice respectively. (c-e) Intraperitoneal injections of MSU crystals (c), CPPD (d), or alum (e) crystals markedly promoted neutrophil infiltration in the Trpv4+/+ and Cre- Cx3cr1CreERT; Trpv4f/f mice, which was significantly reduced in the Trpv4−/− and Cre+ Cx3cr1CreERT; Trpv4f/f mice, respectively. Statistical significance was determined using Student’s t test (a-e). *P<.05, **P<.01, ***P<.001. n=5–6 for each group.
To further investigate the role of TRPV4 in crystal-induced inflammation in vivo, we transorally instilled silica crystals into the Trpv4−/− mice or Cre+ Cx3cr1CreERT; Trpv4f/f Trpv4 cKOs and their respective controls. Flow cytometry detected a robust neutrophil infiltration in bronchoalveolar lavage fluids from both the Trpv4+/+ mice and Cre- Cx3cr1CreERT; Trpv4f/f Trpv4 cKOs, which was significantly reduced in the Trpv4−/− mice and Cre+ Cx3cr1CreERT; Trpv4f/f Trpv4 cKOs, respectively (figure 6b). Similar effect was also observed for MSU, CPPD, and aluminum hydroxide crystals in a mouse model of peritonitis in which the recruitment of neutrophils induced by intraperitoneal injections of diverse crystals was markedly reduced by genetic ablation of TRPV4 function (figure 6c–e), confirming that TRPV4 is required for crystal-induced inflammation in vivo.
Neuroimmune interaction between the TRPV1-expressing sensory nociceptors and TRPV4-expressing MΦs contributes to MSU crystal-induced gouty arthritis.
Prior studies have demonstrated that cytokines released from activated immune cells contribute to MSU crystal-induced pain and inflammation through sensitizing TRPV1-expressing nociceptive sensory neurons, suggesting that the TRPV1-expressing primary nociceptors are the downstream mediator of MSU crystal-induced sensory hypersensitivity.41, 42 Moreover, besides pain-related responses, MSU crystal-induced inflammation was also inhibited in mice deficient in nociceptor-expressed TRPA1 or TRPV1 channels,41–47 which prompted us to hypothesize that activation of the TRPV1-expressing primary nociceptors during MSU crystal-induced acute gout flares can further enhance joint inflammation by promoting the function of the TRPV4-expressing MΦs, forming a positive feedback loop. To test this hypothesis, we selectively ablated the TRPV1-expressing primary nociceptors with a super potent TRPV1 agonist resiniferatoxin (RTX).48 As expected, RTX treatment effectively reduced the reflexive mechanical (online supplemental figure 10a) and thermal (online supplemental figure 10b) pain-related responses. Strikingly, joint swelling (online supplemental figure 10c), IL-1β expression (online supplemental figure 10d), the number of neutrophils and MΦs (online supplemental figure 10e and f), the MPO activity (online supplemental figure 10g), and the TRPV4 mRNA expression in synovial resident MΦs (online supplemental figure 10h) in the MSU crystal-induced gout model were all markedly attenuated, supporting our hypothesis that the MSU crystal-induced MΦ-dependent inflammation requires the presence of the TRPV1-expressing primary nociceptors and a neuroimmune interaction between the TRPV1-expressing primary nociceptors and the TRPV4-expressing synovial MΦs is essential for the generation of MSU crystal-induced acute gout flares.
Discussion
In this study, we showed that MΦ-expressed TRPV4 is selectively involved in the inflammatory responses induced by diverse crystals. The conclusion was supported by multiple experimental measures. First, we showed that TRPV4 is functional expressed by MΦs and TRPV4 expression is upregulated in both MSU crystal-stimulated mouse synovial MΦs and PBMCs from human patients with acute gout flares. Second, we provided evidence that TRPV4 function is required for the activation of the NLRP3 inflammasome and subsequent IL-1β production induced by diverse crystals including MSU, CPPD, and SiO2 but not non-crystalline NLRP3 inflammasome activators ATP, nigericin or gramicidin. Third, the reflexive pain-related responses and inflammation induced by IA injections of MSU crystals were markedly attenuated by both pharmacological inhibition and genetic ablation of TRPV4 function. Last, the TRPV1-expressing nociceptors are required for MSU crystal-induced gouty arthritis through increasing TRPV4 expression, the number of synovial resident MΦs and the release of MΦ-derived cytokines. which supports the hypothesis that a neuroimmune axis of the TRPV4-expressing resident MΦs-TRPV1-expressing primary nociceptors plays a critical role in the generation of crystal-induced gouty arthritis in mice. Of note, our findings might explain why the crystals are in patients for years without causing overt gout flares. It is well known that acute gout flares are tightly associated with the levels of uric acid in patients. For example, the accumulation of high level of uric acid caused by a diet rich in red meat, seafood and beverages sweetened with fructose49 might activate TRPV4-expressing synovial resident MΦs, resulting in release of inflammatory cytokines which subsequently provoke action potential firing in nociceptors, thereby transmitting pain to the brain and driving the neurogenic inflammation to release neurotransmitters such as substance P and CGRP in the affected joints and promote joint inflammation through regulating macrophage functions,50, 51 forming a positive feedback loop and driving the production of acute gout flares.
Our findings also challenge the long-held assumption that TRPV4 is expressed by primary nociceptors mediating neurogenic inflammation and nociception52–56 because we did not find any evidence supporting a functional expression of TRPV4 in the primary nociceptors. First, we did not detect TRPV4 expression in DRG neurons using RNAscope in situ hybridization which otherwise revealed a robust expression of TRPV1 in many DRG neurons. Second, GSK101 did not elicit TRPV4-dependent Ca2+ response in DRG neurons where capsaicin induced a robust Ca2+ response. Third, the MSU crystal-induced gouty arthritis were not significantly affected in sensory neuron-specific TRPV4 cKOs using the Trpv1Cre line which covers ~80% of the nociceptors.57 These results demonstrate that TRPV4 unlikely engages in crystal-induced joint pain and inflammation through a direct neurogenic action, like that mediated by nociceptor-expressed TRPV1 and TRPA1 channels.43, 58 It should be noted that nerve injury and inflammation can induce macrophage expansion in the DRG,59, 60 leading to heightened pain responses. TRPV4 expression in these MΦs but not sensory neurons might contribute to the generation of pain-related responses. Future studies are required to test this possibility.
TRPV4 is functionally expressed in articular chondrocytes in multiple species, 61–63 and promotes distinct mechanoelectrical transduction pathways to regulate the metabolic response of chondrocytes to dynamic loading.64, 65 Interestingly, although chondrocyte-expressed TRPV4 was reported to contribute to age-related OA, 32 knockout of TRPV4 channels was shown to promote the development of OA in male mice.66 Moreover, IA injections of a TRPV4 agonist stimulated chondrocyte anabolic changes and decreased the length of the damaged area in a surgically induced rat model of OA, suggesting that TRPV4 activation protects rats from the development of OA.67 Surprisingly, our results using cartilage-specific Trpv4 cKOs showed that the chondrocyte-expressed TRPV4 does not contribute to MSU crystal-induced gouty arthritis. Therefore, chondrocyte-expressed TRPV4 plays distinct roles in the settings of different types of joint inflammation.
The classic NLRP3 inflammasome activators including ATP, nigericin, gramicidin, and diverse crystals (MSU, CPPD, alum and SiO2) use distinct mechanisms to activate the NLRP3 inflammasome.68 For instance, ATP acts on the P2X7 receptors while nigericin and gramicidin are pore forming toxins, although membrane permeability is engaged in both pathways.69–71 On the other hand, crystal signaling involves phagolysosome and lysosome rupture.7 Our data showed that TRPV4 is involved in the NLRP3 inflammasome activation induced by diverse crystals but not ATP and pore-forming toxins. Therefore, TRPV4 likely engages in the crystal-induced signaling in MΦs which is separate from these classics signaling pathways. Although the precise mechanism how TRPV4 channel is activated in the gout model remains controversial, our findings suggest that mechanosensitive TRPV4 channel might be critically involved in the cell surface contact by various crystals and be directly activated by swelling-related cell volume change (online supplemental figure 11) associated with the phagocytosis of MSU crystals, which subsequently causes lysosomal leakage and drives ROS production, leading to NLRP3 inflammasome activation in the joint MΦs.39, 72, 73 On the other hand, TRPV4-mediated NRLP3 inflammasome activation induced by GSK101 relies primarily on intracellular Ca2+ signaling and subsequent lysosomal leakage although the relationship between the TRPV4-mediated intracellular Ca2+ signaling and lysosomal leakage requires further investigation. Considering TRPV4 is activated by many forms of physical and chemical stimuli,74, 75 it might play a critical role in inflammation through converging many signaling pathways driving NLRP3 inflammasome activation.
In summary, we demonstrated that the TRPV4-expressing resident MΦs are the key mediator of MSU crystal-induced gouty arthritis in mice. Mechanistically, the mechanosensitive TRPV4 channel can be selectively activated by crystal-induced cell volume change, which leads to NLRP3 inflammasome activation and subsequent production and release of inflammatory cytokines that drive the joint inflammation and pain-related responses. We also showed that this process requires the presence of the TRPV1-expressing nociceptors and demonstrated that a neuroimmune interaction between the TRPV1-expressing primary nociceptors and the TRPV4-expressing synovial MΦs is a critical cellular mechanism underlying acute gout flares. Identification of the function of TRPV4 in crystal-induced inflammation should facilitate the development of new therapeutic interventions to treat inflammatory conditions associated with crystal disposition.
Supplementary Material
Key messages.
What is already known about this subject?
Many exogenous or endogenously formed crystal structures with different compositions can act as DAMPs and in turn trigger NLRP3 inflammasome activation.
Ion channels are critical signaling mediators in both innate and adaptive immune cells.
What does this study add?
Macrophage-expressed TRPV4 is essential to the generation of crystal-induced joint pain and inflammation and inflammatory responses evoked by diverse crystalline structures.
TRPV4 is critically involved in the activation of macrophage NLRP3 inflammasome and production of the inflammatory cytokine IL-1β induced by diverse crystalline materials but not non- crystalline NLRP3 inflammasome activators.
A neuroimmune interaction between the TRPV1-expressing primary nociceptors and the TRPV4-expressing synovial macrophages is an important cellular mechanism behind acute gout flares.
How might this impact on clinical practice or future developments?
Our findings show that macrophage-expressed TRPV4 is a common regulator of crystal-induced inflammatory responses and shed new insights into the development of new therapeutic interventions to treat inflammatory conditions associated with crystal disposition.
Funding
This work was supported by grants from the National Institutes of Health R01DK103901, R01AR077183, and R01AA027065 to H.H.
Competing interests
The authors declare no competing interests. B.S.K. has served as a consultant for AbbVie, ABRAX Japan, Almirall, Cara Therapeutics, Maruho, Menlo Therapeutics, Pfizer, and Third Rock Ventures. B.S.K. is also founder, chief scientific officer, and stockholder of Nuogen Pharma and stockholder of Locus Biosciences.
References
- 1.Liston A, Masters SL. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol 2017; 17:208–214. [DOI] [PubMed] [Google Scholar]
- 2.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 2020; 20:95–112. [DOI] [PubMed] [Google Scholar]
- 3.Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep 2011; 13:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin BS, Mangan MS, Latz E. Crystal Formation in Inflammation. Annu Rev Immunol 2016; 34:173–202. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama M. Macrophage Recognition of Crystals and Nanoparticles. Front Immunol 2018; 9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renaudin F, Orliaguet L, Castelli F et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1beta activation on macrophages. Ann Rheum Dis 2020; 79:1506–1514. [DOI] [PubMed] [Google Scholar]
- 7.He Y, Hara H, Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci 2016; 41:1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng G, Sharma K, Ward SM et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 2008; 29:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol 2015; 33:291–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong T, Yang Y, Jin T, Jiang W, Zhou R. Orchestration of NLRP3 Inflammasome Activation by Ion Fluxes. Trends Immunol 2018; 39:393–406. [DOI] [PubMed] [Google Scholar]
- 11.Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation 2015; 12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, Feng J, Liu S, Walters ET, Hu H. Molecular and cellular mechanisms that initiate pain and itch. Cell Mol Life Sci 2015; 72:3201–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aroke EN, Powell-Roach KL, Jaime-Lara RB et al. Taste the Pain: The Role of TRP Channels in Pain and Taste Perception. Int J Mol Sci 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman HA, Chen A, Kravatz NL, Chavan SS, Chang EH. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front Immunol 2020; 11:590261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly S, Chapman RJ, Woodhams S et al. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis 2015; 74:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darby WG, Grace MS, Baratchi S, McIntyre P. Modulation of TRPV4 by diverse mechanisms. Int J Biochem Cell Biol 2016; 78:217–228. [DOI] [PubMed] [Google Scholar]
- 17.Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep 2013; 14:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNulty AL, Leddy HA, Liedtke W, Guilak F. TRPV4 as a therapeutic target for joint diseases. Naunyn Schmiedebergs Arch Pharmacol 2015; 388:437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Kanju P, Fang Q et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 2014; 155:2662–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y, Wang S, Yang Z et al. Bilirubin alleviates alum-induced peritonitis through inactivation of NLRP3 inflammasome. Biomed Pharmacother 2019; 116:108973. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Fan H, Li X et al. Trpv4 regulates Nlrp3 inflammasome via SIRT1/PGC-1alpha pathway in a cuprizone-induced mouse model of demyelination. Exp Neurol 2021; 337:113593. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Qian A, Oetjen LK et al. TRPV4 Channel Signaling in Macrophages Promotes Gastrointestinal Motility via Direct Effects on Smooth Muscle Cells. Immunity 2018; 49:107–119 e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Feng J, Yu G et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J Allergy Clin Immunol 2018; 141:608–619 e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres R, Macdonald L, Croll SD et al. Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Ann Rheum Dis 2009; 68:1602–1608. [DOI] [PubMed] [Google Scholar]
- 25.Thorneloe KS, Cheung M, Bao W et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 2012; 4:159ra148. [DOI] [PubMed] [Google Scholar]
- 26.Liu TT, Bi HS, Lv SY, Wang XR, Yue SW. Inhibition of the expression and function of TRPV4 by RNA interference in dorsal root ganglion. Neurol Res 2010; 32:466–471. [DOI] [PubMed] [Google Scholar]
- 27.Vergnolle N, Cenac N, Altier C et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br J Pharmacol 2010; 159:1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pankey EA, Zsombok A, Lasker GF, Kadowitz PJ. Analysis of responses to the TRPV4 agonist GSK1016790A in the pulmonary vascular bed of the intact-chest rat. Am J Physiol Heart Circ Physiol 2014; 306:H33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorneloe KS, Sulpizio AC, Lin Z et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}−3-hydroxypropanoyl)-1 -piperazinyl]carbonyl}−3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther 2008; 326:432–442. [DOI] [PubMed] [Google Scholar]
- 30.Bajpai G, Bredemeyer A, Li W et al. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res 2019; 124:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin J, Michalick L, Tang C et al. Role of Transient Receptor Potential Vanilloid 4 in Neutrophil Activation and Acute Lung Injury. Am J Respir Cell Mol Biol 2016; 54:370–383. [DOI] [PubMed] [Google Scholar]
- 32.O’Conor CJ, Ramalingam S, Zelenski NA et al. Cartilage-Specific Knockout of the Mechanosensory Ion Channel TRPV4 Decreases Age-Related Osteoarthritis. Sci Rep 2016; 6:29053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scanu A, Luisetto R, Oliviero F et al. High-density lipoproteins inhibit urate crystal-induced inflammation in mice. Ann Rheum Dis 2015; 74:587–594. [DOI] [PubMed] [Google Scholar]
- 34.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 2016; 13:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012; 13:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hari A, Zhang Y, Tu Z et al. Activation of NLRP3 inflammasome by crystalline structures via cell surface contact. Sci Rep 2014; 4:7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voets T, Prenen J, Vriens J et al. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 2002; 277:33704–33710. [DOI] [PubMed] [Google Scholar]
- 38.Dupre-Crochet S, Erard M, Nubetae O. ROS production in phagocytes: why, when, and where? J Leukoc Biol 2013; 94:657–670. [DOI] [PubMed] [Google Scholar]
- 39.Halle A, Hornung V, Petzold GC et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 2008; 9:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hornung V, Bauernfeind F, Halle A et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008; 9:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmeister C, Silva MA, Rossato MF et al. Participation of the TRPV1 receptor in the development of acute gout attacks. Rheumatology (Oxford) 2014; 53:240–249. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmeister C, Trevisan G, Rossato MF, de Oliveira SM, Gomez MV, Ferreira J. Role of TRPV1 in nociception and edema induced by monosodium urate crystals in rats. Pain 2011; 152:1777–1788. [DOI] [PubMed] [Google Scholar]
- 43.Galindo T, Reyna J, Weyer A. Evidence for Transient Receptor Potential (TRP) Channel Contribution to Arthritis Pain and Pathogenesis. Pharmaceuticals (Basel) 2018; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moilanen LJ, Hamalainen M, Lehtimaki L, Nieminen RM, Moilanen E. Urate crystal induced inflammation and joint pain are reduced in transient receptor potential ankyrin 1 deficient mice--potential role for transient receptor potential ankyrin 1 in gout. PLoS One 2015; 10:e0117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trevisan G, Hoffmeister C, Rossato MF et al. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum 2013; 65:2984–2995. [DOI] [PubMed] [Google Scholar]
- 46.Trevisan G, Hoffmeister C, Rossato MF et al. TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic Biol Med 2014; 72:200–209. [DOI] [PubMed] [Google Scholar]
- 47.Yin C, Liu B, Li Y et al. IL-33/ST2 induces neutrophil-dependent reactive oxygen species production and mediates gout pain. Theranostics 2020; 10:12189–12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng J, Yang P, Mack MR et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat Commun 2017; 8:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakse B, Jakse B, Pajek M, Pajek J. Uric Acid and Plant-Based Nutrition. Nutrients 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol 2019; 62:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinho-Ribeiro FA, Verri WA Jr., Chiu IM. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol 2017; 38:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 2005; 118:70–79. [DOI] [PubMed] [Google Scholar]
- 53.Grant AD, Cottrell GS, Amadesi S et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 2007; 578:715–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sipe WE, Brierley SM, Martin CM et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 2008; 294:G1288–1298. [DOI] [PubMed] [Google Scholar]
- 55.Liedtke W, Choe Y, Marti-Renom MA et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000; 103:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brierley SM, Page AJ, Hughes PA et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 2008; 134:2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J 2011; 30:582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gouin O, L’Herondelle K, Lebonvallet N et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017; 8:644–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 2015; 112:E6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu X, Liu H, Hamel KA et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 2020; 11:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phan MN, Leddy HA, Votta BJ et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum 2009; 60:3028–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hdud IM, El-Shafei AA, Loughna P, Barrett-Jolley R, Mobasheri A. Expression of Transient Receptor Potential Vanilloid (TRPV) channels in different passages of articular chondrocytes. Int J Mol Sci 2012; 13:4433–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez JC, Lopez-Zapata DF, Wilkins RJ. TRPV4 channels activity in bovine articular chondrocytes: regulation by obesity-associated mediators. Cell Calcium 2014; 56:493–503. [DOI] [PubMed] [Google Scholar]
- 64.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A 2014; 111:1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum 2010; 62:2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atobe M, Nagami T, Muramatsu S et al. Discovery of Novel Transient Receptor Potential Vanilloid 4 (TRPV4) Agonists as Regulators of Chondrogenic Differentiation: Identification of Quinazolin-4(3 H)-ones and in Vivo Studies on a Surgically Induced Rat Model of Osteoarthritis. J Med Chem 2019; 62:1468–1483. [DOI] [PubMed] [Google Scholar]
- 68.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011; 469:221–225. [DOI] [PubMed] [Google Scholar]
- 69.Gulbransen BD, Bashashati M, Hirota SA et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 2012; 18:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mariathasan S, Weiss DS, Newton K et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006; 440:228–232. [DOI] [PubMed] [Google Scholar]
- 71.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 2009; 28:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruchard M, Mignot G, Derangere V et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19:57–64. [DOI] [PubMed] [Google Scholar]
- 73.Fujisawa A, Kambe N, Saito M et al. Disease-associated mutations in CIAS1 induce cathepsin B-dependent rapid cell death of human THP-1 monocytic cells. Blood 2007; 109:2903–2911. [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Elias A, Mrkonjic S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA. The TRPV4 channel. Handb Exp Pharmacol 2014; 222:293–319. [DOI] [PubMed] [Google Scholar]
- 75.Verma P, Kumar A, Goswami C. TRPV4-mediated channelopathies. Channels (Austin) 2010; 4:319–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.