Abstract
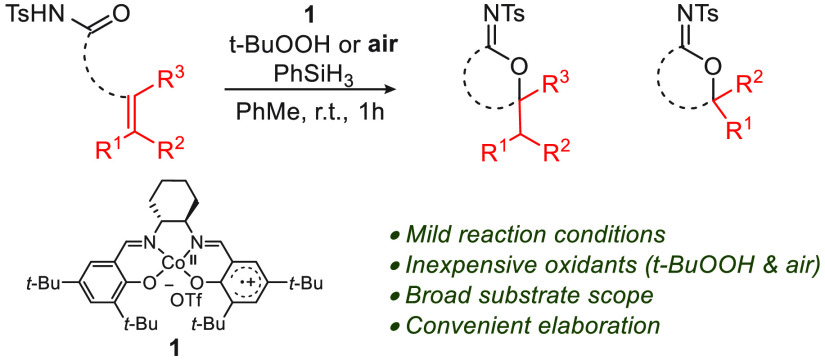
The cycloisomerization of β-, γ-, and δ-unsaturated N-acyl sulfonamides to N-sulfonyl lactams and imidates is reported. This transformation is effected in the presence of a CoIII(salen) catalyst using t-BuOOH or air as the oxidant. The method shows good functional group tolerance (alkyl, aryl, heteroaryl, ether, N-Boc) and furnishes an underexplored class of cyclic building blocks. The strong solvent dependence of the transformation is investigated, and the synthetic versatility of the N-sulfonyl imidate product class is highlighted.
Keywords: cycloisomerization, N-acyl sulfonamides, N-sulfonyl imidates, cobalt catalysis, diverted Mukaiyama hydration
Hydrofunctionalization of unactivated olefins using inexpensive first-row transition metals, such as Fe, Mn, Co, and Ni, has received considerable attention.1,2 Early examples of their application include earth-abundant metal-mediated olefin hydration3 and hydroperoxidation,4 pioneered by Drago5 and Mukaiyama,6 respectively. Since then there has been considerable evolution of olefin hydrofunctionalization to include oximation,7 cyanation,8 hydrazination,9 azidation,8b,10 amination,11 and halogenation.8b,12 More recently, Shigehisa and others have demonstrated intermolecular olefin hydroalkoxylation,13 hydrofluorination,13b intramolecular hydroarylation,14 hydrothioetherification,15 and hydroamination.13e,16 Herein we report the Co-catalyzed cycloisomerization of unsaturated N-acyl sulfonamides, which provides ready access to a wide range of cyclic N-sulfonyl imidates, an underexplored functional group (Scheme 1). In addition to examination of conditions with t-BuOOH, a salient feature of the process is our discovery that the reaction can be performed with air as a convenient and safe oxidant. Additionally, over the course of this study we observed a notable solvent effect that governs the product distribution, which we attribute to solvent donor ability and the attendant electronic character of the cobalt metal complex.
Scheme 1. Cyclization of Alkenyl N-Acyl Sulfonamides.
Imidates are a synthetically versatile class of compounds that act as activated amide equivalents through their dual behavior as both electrophiles and nucleophiles. Thus, they provide access to a wide range of structural motifs, including oxazolines, indazoles, and isoquinolines.17 Acyclic N-sulfonyl imidates have been investigated as surrogates for azahetarenes in steroidal antiproliferative agents.18 They have also been examined in drug discovery as prodrugs for esters and sulfonamides, addressing challenges associated with bioavailability and metabolism.19 While probenazole, a member of the class, has found applications as an antifungal agent against rice blast fungus and leaf blight, erythromycin derivatives modified through the incorporation of a cyclic imidate showed 4-fold increased activity against Streptococcus pneumoniae.20 Cyclic N-sulfonyl imidates are less well studied, despite the fact that they could be useful in a prodrug approach as precursors toward lactones.
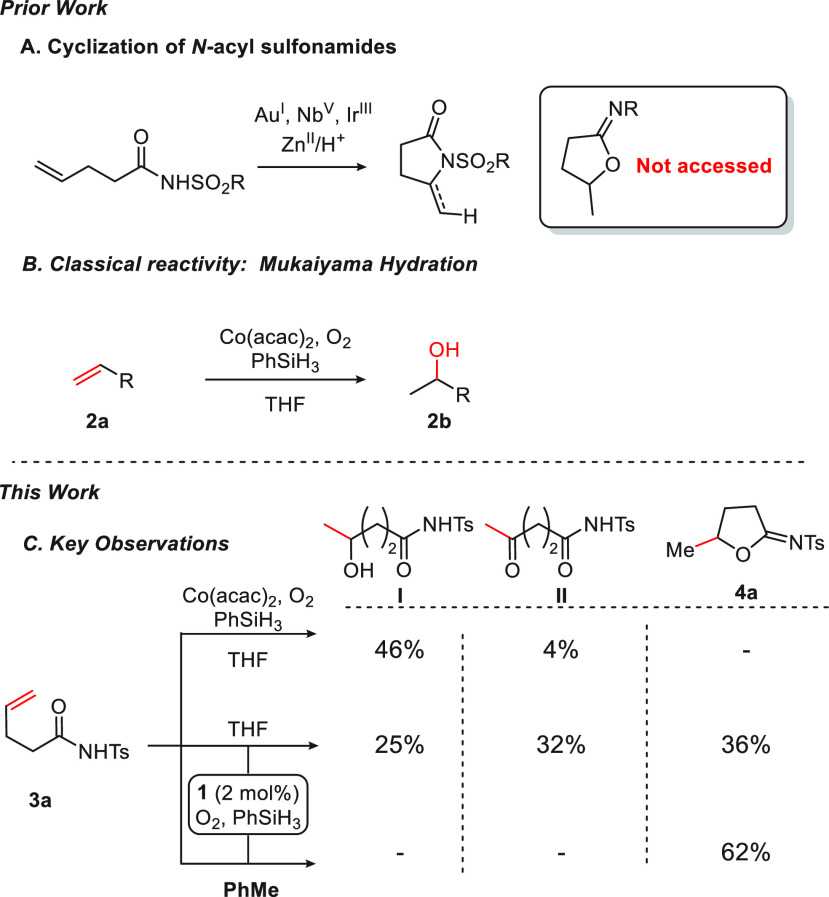
Cycloisomerizations of alkenyl N-acyl sulfonamides to N-sulfonyl lactams have been described, mediated by Ph3PAuCl,21 Ir catalysts,22 NbCl5,23 and Zn(OTf)2/TfOH24 (Figure 1A). Cyclic N-sulfonyl imidates have generally been accessed through electrophilic cyclization of N-acyl sulfonyl alkenes mediated by iodine,25m-CPBA,26 Ph2Se2,27 or TsN3.28,29,26 The direct cycloisomerization of alkenyl N-acyl sulfonamides to furnish cyclic N-sulfonyl imidates is unknown.13e,16a
Figure 1.
Background and initial observations.
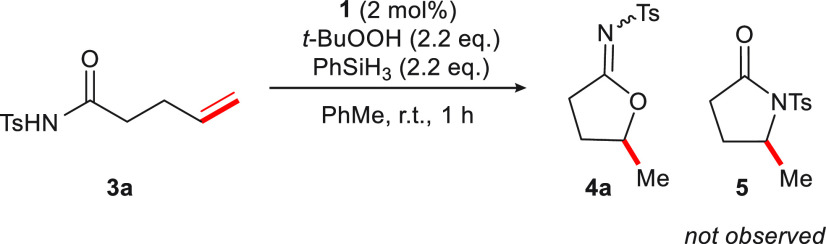
We have been interested in identifying novel reactivity of olefins mediated by base metal–salen complexes.9a,9c,10,30 In the presence of a CoII catalyst, silane, and oxidant, olefins classically proceed through Mukaiyama hydration pathways (Figure 1B). When we subjected N-acyl sulfonamide 3a to Mukaiyama hydration conditions, a mixture of products I and II was formed in 46% and 4% yield, respectively, in line with early observations by Mukaiyama.6a−6c,6f Interestingly, when Co(acac)2 was replaced by CoIII(salen)OTf complex 1 under otherwise identical conditions, the formation of N-sulfonyl imidate 4a was observed (36%). Importantly, resubjecting I to Mukaiyama hydration conditions in the presence of catalyst 1 did not lead to 4a (see the Supporting Information (SI)). When THF was replaced with toluene, 4a was formed in 62% yield. Neither alcohol I nor ketone II was isolated from the reaction mixture. Collectively, these results compelled us to examine further the reaction in which Mukaiyama hydration of olefinic N-acyl sulfonamides is derailed to form cyclic imidates.
Although the cycloisomerization is isohypsic,31 it has been noted that for Co-mediated olefin functionalization reactions, the addition of hydroperoxide can be beneficial.6f,10a Examination of the reaction conditions led to the identification of a procedure in toluene with 2 mol%1, t-BuOOH (2.2 equiv), and PhSiH3 (2.2. equiv) that effects the cycloisomerization reaction 3a → 4a in 85% yield (Table 1, entry 1; see the SI). Control experiments revealed interesting details. No reactivity was observed in the absence of silane or catalyst 1 (entries 2 and 3). The use of a CoII(salen-tBu,tBu) catalyst under otherwise identical conditions gave the product in merely 17% yield (entry 4; see the SI). Under an inert atmosphere, identical product yields were obtained (entry 5). Importantly, we found that the reaction could be conducted with air as the oxidant, leading to 4a in 56% yield after 48 h (entry 6). Under conditions in which the catalyst loading was increased to 10 mol% with 4 equiv of silane and air, product 4a was formed in 82% yield (entry 7). The fact that reaction conditions prescribe air and avoid the use of expensive or toxic oxidants makes this transformation attractive.32 Moreover, the use of air instead of oxygen renders the process safer.
Table 1. Optimization of the Reaction Conditions.
| entry | deviation from the standard conditions | yield of 4a (%) |
|---|---|---|
| 1 | none | 85 |
| 2 | without PhSiH3 | 0 |
| 3 | without 1 | 0 |
| 4 | CoII(salen-tBu,tBu) instead of 1 | 17 |
| 5 | under N2 | 84 |
| 6 | air instead of t-BuOOH, 48 h | 56 |
| 7 | air, 10 mol% 1, and 4 equiv of PhSiH3 | 82 |
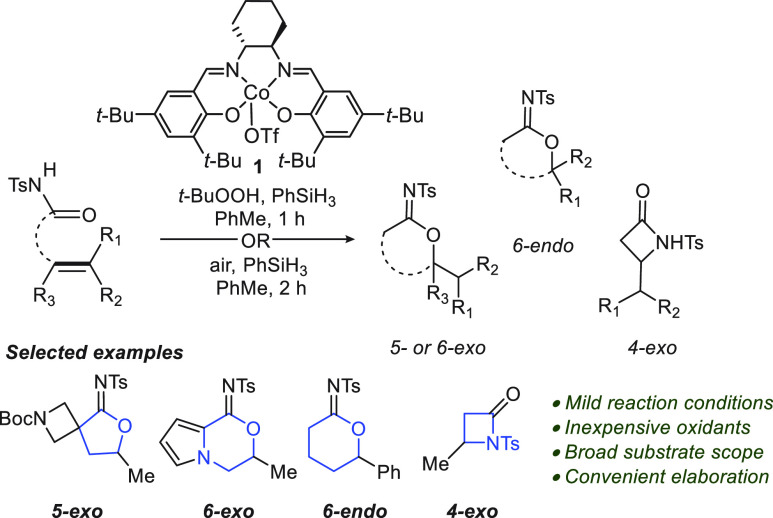
With the optimized reaction conditions in hand, the scope and functional group tolerance of this transformation was investigated (Figure 2). Substrates incorporating a wide range of alkyl or aryl substituents, including rings at Cα, were well-tolerated in the transformation, leading to products 4b–g (62–83%). Ether-containing and N-Boc-amine-substituted alkenyl N-acyl sulfonamides readily underwent cycloisomerization and gave rise to products 4h–j in 63–97% yield. β-Substituted olefins also underwent cyclization to give products 4k–m. The reaction tolerated disubstituted olefins, allowing access to N-sulfonyl imidate 4n in 55% yield.
Figure 2.
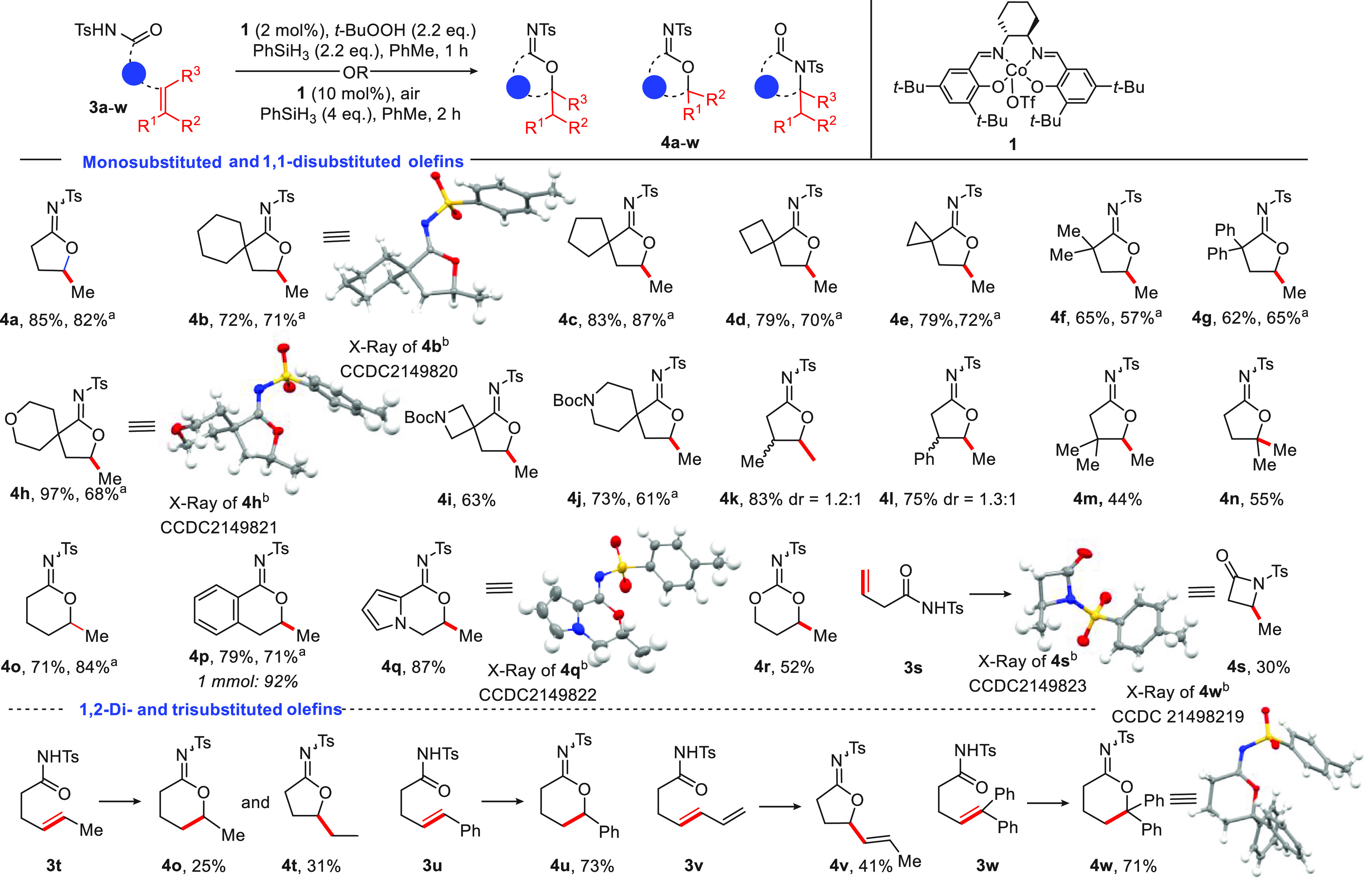
Substrate scope of the Co-catalyzed cycloisomerization of alkenyl N-acyl sulfonamides. aReaction yield obtained employing 10 mol%1, 4 equiv of PhSiH3, and air. bThermal ellipsoids are shown at the 50% probability level.
For selected examples, namely, 4a–h, 4j, 4p, and 4o, the cycloisomerization reaction was repeated to showcase the broad applicability of air as the oxidant. The products were generally obtained in yields comparable to those under conditions employing t-BuOOH. The structures of the imidate products 4b, 4h, 4q, and 4w formed in this study were assigned by X-ray crystallography. For characterization of the remaining products, IR spectroscopy proved to be useful. N-Sulfonyl imidates display distinctive IR C=N absorbances at 1620 cm–1, in stark contrast to νC=O = 1720 cm–1 for N-tosyl lactams.
We next evaluated the formation of six-membered N-sulfonyl imidates. Gratifyingly, 3o–q provided access to 4o–q in 71–87% yield. Imidocarbonate 4r was prepared in 52% yield from homoallylic N-sulfonyl carbamate 3r. When 3s was subjected to the reaction conditions, β-lactam 4s was formed in 30% yield. The transformation was also amenable to 1,2-disubstituted olefins, with alkenoyl N-acyl sulfonamide 3t giving a mixture of five- and six-membered-ring products. In contrast, styrene derivative 4u and diene 4v were formed as single products. Finally, we established the feasibility of performing the reaction in a system with a trisubstituted olefin (3w), affording 4w in 71% yield.
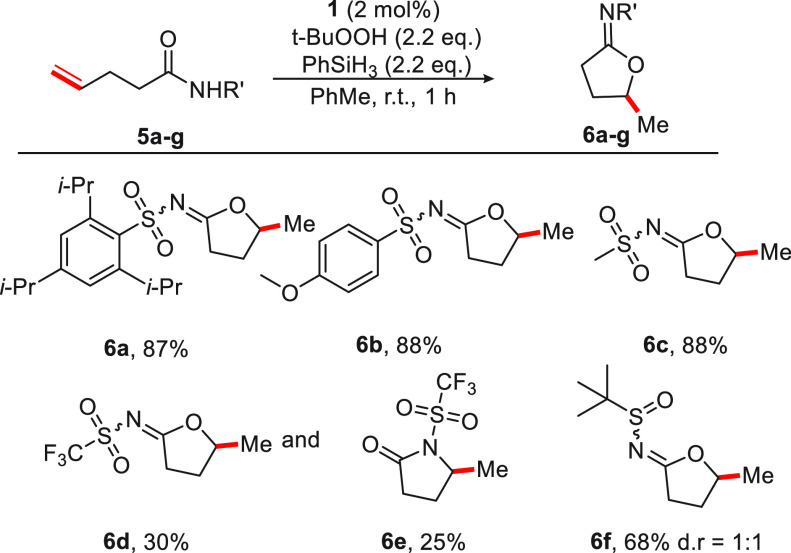
The effect of different sulfonamides on the transformation was investigated next (Figure 3). High yields were maintained for aryl sulfonamide substrates bearing arene groups substituted with sterically demanding and electron-donating substituents (6a and 6b). Methanesulfonamide derivative 5c also produced the corresponding cyclic N-sulfonyl imidate 6c in 88% yield. The use of olefinic N-acyl trifluoromethane sulfonamide 5d yielded cycloisomerization products, albeit as a mixture of imidate 6d and lactam 6e in 30% and 25% yield, respectively. N-Sulfinyl imidate 6f was formed in 68% yield, expanding the reaction to N-acyl sulfinamides.
Figure 3.
Co-catalyzed cycloisomerization of a variety of sulfonamide and sulfinamide derivatives.
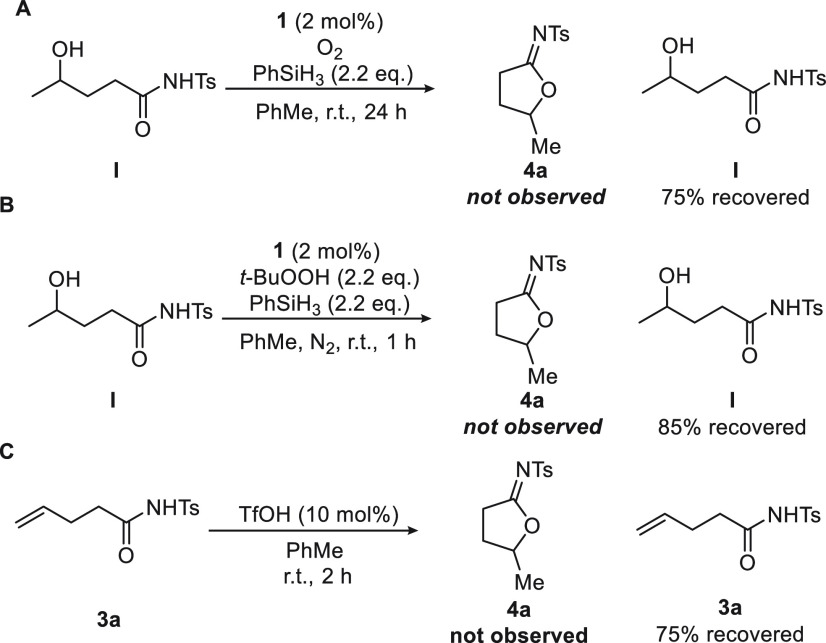
The observation that the reaction conditions resemble those typically employed for Mukaiyama hydration6b of olefins compelled us to conduct key control experiments (Figure 4A,B). In this respect, when I was subjected to either Mukaiyama hydration conditions or the reaction conditions, imidate 4a was not formed, and the starting secondary alcohol was reisolated in 75 or 85% yield, respectively. This result precludes a mechanistic pathway for the overall transformation 3a → 4a involving Mukaiyama hydration of the olefin followed by ring closure. To rule out the possibility that the reaction is mediated by triflic acid formed from CoIIIOTf catalyst 1, starting material 3a was exposed to a catalytic amount of TfOH (10 mol%) (Figure 4C). No product formation was observed after 2 h, and 3a was recovered in 75% yield.
Figure 4.
Control experiments for the Co-catalyzed cycloisomerization reaction.
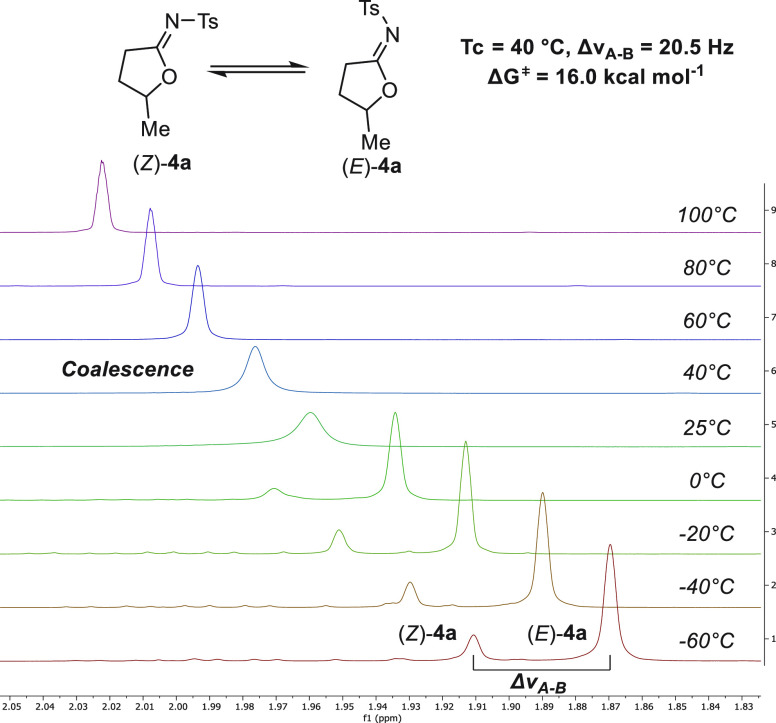
Interestingly, there is paucity of data on the behavior of cyclic N-sulfonyl imidates. Accordingly, we conducted a brief study on 4a. It displayed fast E/Z isomerization at room temperature as determined by HSQC and NOE NMR spectroscopy. The isomer (E)-4a prevails in toluene-d8 (see the SI) on the basis of a cross-peak between the ring H2Cα and o-CHaryl. Cyclic imidates bearing Cα substituents crystallized solely as the Z isomer (see 4b, 4h, and 4q). The barrier for E/Z isomerization of parent compound 4a was determined to be ΔG⧧ = 16.0 kcal mol–1 (Figure 5).33
Figure 5.
Determination of the barrier to E/Z isomerization in N-sulfonyl imidate 4a. 1H NMR spectra were recorded at 500 MHz in toluene-d8 at −60 to 100 °C.
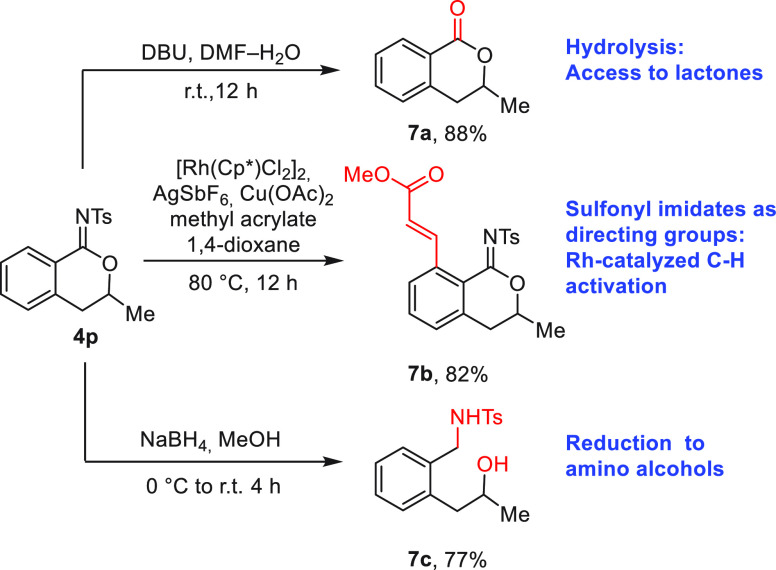
We then investigated derivatization of this underexplored product class (Figure 6). Compound 4p was hydrolyzed to lactone 7a in 88% yield (DMF–H2O, 0.2 equiv of DBU, r.t., 12 h).29b,34 We wondered whether N-sulfonyl imidates could be employed as directing groups for arene C–H functionalization. The closest analogy we could find was in the work with Rh-catalyzed functionalization of cyclic N-sulfonyl ketimines.35 Subjecting 4p to [(Cp*)RhCl2]2, AgSbF6, Cu(OAc)2, and methyl acrylate in 1,4-dioxane led to formation of o-C–H-alkenylated imidate 7b (82% yield). Finally, reduction of 4p with NaBH4 gave amino alcohol 7c in 77% yield.
Figure 6.
Selected functionalization of sulfonyl imidates.
The reactivity reported herein is intriguing and unexpected given previous studies involving cobalt complexes and simple olefins under similar conditions.9a,9c,10,30b A number of spectroscopic and computational investigations have suggested that canonical CoIII(salen) complexes such as 1 may be in equilibrium with species described as CoII(salen•+).36 Various independent studies have separately indicated that equilibria involving Co species are sensitive to factors such as temperature, counterions, donor ligands, and solvent (CD2Cl2 vs DMSO-d6 vs pyridine). Combined NMR and quantum-chemical studies revealed that Co(salen)Cl in THF-d8 is present in diamagnetic and paramagnetic forms.36
Our leading results outlined in Figure 1C hinted at a solvent effect (THF vs toluene), with the latter proving optimal for cyclization vis-à-vis the formation of Mukaiyama hydration/oxidation products I and II. Accordingly, we conducted a study of the cyclization reaction of 3a with air as the oxidant over a range of solvents (Figure 7A). In proceeding from toluene to DMF, the relative amount of cyclization product decreases while the Mukaiyama products increase. A plot of the yield of cyclization product 4a against solvent donor number37 reveals a linear relationship (Figure 7; see the SI for details), with the yield of 4a decreasing as a function of solvent coordination ability.38 A recent report on olefin hydroamination reactions suggests a dependence of the product distribution S (cyclization vs Mukaiyama products) on solvent viscosity.16c However, in the cyclization reactions of 3a we did not observe such a dependence on the viscosity (R2 = 0.02; see the SI); instead, once again a strong correlation (R2 = 0.94) involving S and solvent donor ability was observed (Figure 7C).
Figure 7.
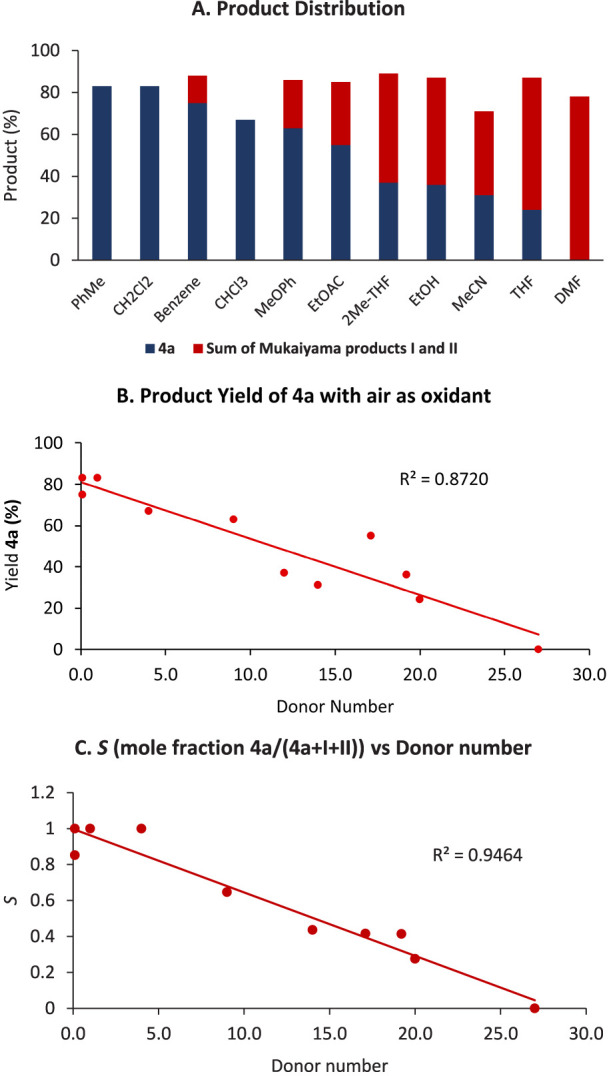
Solvent trends in the cycloisomerization of N-acyl sulfonamides. (A) Product distribution in various solvents. (B) Relationship between solvent donor number (Gutmann37) and yield of 4a. (C) Correlation between S (=4a/(4a + I + II)) and solvent donor number.
For reactions involving Co complexes, silane, and an oxidant, it has been suggested in the literature that hydrometalation of the starting olefin affords an organocobalt species.9a,9c,10,13a−13d,14,16a,30b Our results are consistent with this putative intermediate partitioning itself between two possible pathways as a function of the solvent, namely, reaction with oxygen to form Mukaiyama hydration products I and II or, alternatively, a diverted course to give 4a. It remains unclear whether the cyclization process we describe subsequently proceeds via cationic or radical intermediates.39 In mechanistic inorganic studies of Co(salen) complexes,36a Fuji has discussed the importance of the redox-active salen ligand in understanding the electronic structure of these complexes. This raises the question of whether the ability of canonical 1 to access its CoII(salen•+) form may be crucial to diverting the reaction from classical Mukaiyama reactivity to cycloisomerization. The design of complexes incorporating redox-noninnocent ligands for olefin functionalization reactions may provide new avenues for the identification of preparatively useful transformations.
In conclusion, we have reported the Co-catalyzed cycloisomerization of olefinic N-acyl sulfonamides employing t-BuOOH or air as the oxidant. The transformation was successful for a broad spectrum of olefin substrates and tolerated a variety of functional groups.40 The barrier to E/Z isomerization of the imidates was determined by NMR coalescence experiments. We elaborated the imidate products in further transformations, including cleavage of the sulfonamide, N-tosyl imidate reduction, and CH functionalization. Finally, we investigated the product distribution as a function of solvent, confirming that cycloisomerization is preferred over the traditional Mukaiyama hydration pathways in noncoordinating solvents. The access to cyclic N-sulfonyl imidate products provided by this method opens new possibilities for these structures as potentially useful building blocks in small-molecule discovery endeavors.
Acknowledgments
This work was funded by the European Research Council (OLECAT, Grant ID 833540). We are grateful to Dr. N. Trapp and M. Solar for X-ray crystallographic analysis, Dr. M.-O. Ebert and R. Falkenstein for low- and high-temperature NMR experiments, and T. Horak for starting material synthesis (all ETH Zurich). M.B. thanks the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00186.
The authors declare no competing financial interest.
Supplementary Material
References
- a Crossley S. W. M.; Obradors C.; Martinez R. M.; Shenvi R. A. Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev. 2016, 116, 8912–9000. 10.1021/acs.chemrev.6b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kyne S. H.; Lefevre G.; Ollivier C.; Petit M.; Ramis Cladera V. A.; Fensterbank L. Iron and cobalt catalysis: new perspectives in synthetic radical chemistry. Chem. Soc. Rev. 2020, 49, 8501–8542. 10.1039/D0CS00969E. [DOI] [PubMed] [Google Scholar]
- Ludwig J. R.; Schindler C. S. Catalyst: Sustainable Catalysis. Chem. 2017, 2, 313–316. 10.1016/j.chempr.2017.02.014. [DOI] [Google Scholar]
- Lee N.-H.; Byun J.-C.; Baik J.-S.; Han C.-H.; Han S.-b. Development of Mn(III)(Schiff Base) Complexes for the Catalyst of Olefin Oxygenation to Alcohols in the Presence of NaBH4. Bull. Korean Chem. Soc. 2002, 23, 1365–1366. 10.5012/bkcs.2002.23.10.1365. [DOI] [Google Scholar]
- a Matsushita Y.-I.; Sugamoto K.; Nakama T.; Matsui T. Synthesis of γ-hydroperoxy-α,β-unsaturated carbonyl compounds from α,β,γ,δ-unsaturated carbonyl compounds by cobalt(II) porphyrin-catalysed hydroperoxygenation. J. Chem. Soc., Chem. Commun. 1995, 567–568. 10.1039/C39950000567. [DOI] [Google Scholar]; b Sugamoto K.; Matsushita Y.-I.; Matsui T. Direct hydroperoxygenation of conjugated olefins catalyzed by cobalt(II) porphyrin. J. Chem. Soc., Perkin Trans. 1 1998, 3989–3998. 10.1039/a805888a. [DOI] [Google Scholar]; c Tokuyasu T.; Kunikawa S.; Masuyama A.; Nojima M. Co(III)–Alkyl Complex- and Co(III)–Alkylperoxo Complex-Catalyzed Triethylsilylperoxidation of Alkenes with Molecular Oxygen and Triethylsilane. Org. Lett. 2002, 4, 3595–3598. 10.1021/ol0201299. [DOI] [PubMed] [Google Scholar]; d O’Neill P. M.; Hindley S.; Pugh M. D.; Davies J.; Bray P. G.; Park B. K.; Kapu D. S.; Ward S. A.; Stocks P. A. Co(thd)2: a superior catalyst for aerobic epoxidation and hydroperoxysilylation of unactivated alkenes: application to the synthesis of spiro-1,2,4-trioxanes. Tetrahedron Lett. 2003, 44, 8135–8138. 10.1016/j.tetlet.2003.09.033. [DOI] [Google Scholar]; e Tokuyasu T.; Kunikawa S.; McCullough K. J.; Masuyama A.; Nojima M. Synthesis of Cyclic Peroxides by Chemo- and Regioselective Peroxidation of Dienes with Co(II)/O2/Et3SiH. J. Org. Chem. 2005, 70, 251–260. 10.1021/jo048359j. [DOI] [PubMed] [Google Scholar]; f Hurlocker B.; Miner M. R.; Woerpel K. A. Synthesis of silyl monoperoxyketals by regioselective cobalt-catalyzed peroxidation of silyl enol ethers: application to the synthesis of 1,2-dioxolanes. Org. Lett. 2014, 16, 4280–4283. 10.1021/ol5020015. [DOI] [PubMed] [Google Scholar]
- Zombeck A.; Hamilton D. E.; Drago R. S. Novel catalytic oxidations of terminal olefins by cobalt(II)-Schiff base complexes. J. Am. Chem. Soc. 1982, 104, 6782–6784. 10.1021/ja00388a051. [DOI] [Google Scholar]
- a Isayama S.; Mukaiyama T. Hydration of olefins with molecular oxygen and triethylsilane catalyzed by bis(trifluoroacetylacetonato)cobalt(II). Chem. Lett. 1989, 18, 569–572. 10.1246/cl.1989.569. [DOI] [Google Scholar]; b Inoki S.; Kato K.; Takai T.; Isayama S.; Yamada T.; Mukaiyama T. Bis(trifluoroacetylacetonato)cobalt(II) catalyzed oxidation-reduction hydration of olefins selective formation of alcohols from olefins. Chem. Lett. 1989, 18, 515–518. 10.1246/cl.1989.515. [DOI] [Google Scholar]; c Isayama S.; Mukaiyama T. A New Method for Preparation of Alcohols from Olefins with Molecular Oxygen and Phenylsilane by the Use of Bis(acetylacetonato)cobalt(II). Chem. Lett. 1989, 18, 1071–1074. 10.1246/cl.1989.1071. [DOI] [Google Scholar]; d Inoki S.; Kato K.; Isayama S.; Mukaiyama T. A new and facile method for the direct preparation of α-hydroxycarboxylic acid esters from α,β-unsaturated carboxylic acid esters with molecular oxygen and phenylsilane catalyzed by Bis(dipivaloylmethanato)manganese(II) complex. Chem. Lett. 1990, 19, 1869–1872. 10.1246/cl.1990.1869. [DOI] [Google Scholar]; e Mukaiyama T.; Yamada T. Recent advances in aerobic oxygenation. Bull. Chem. Soc. Jpn. 1995, 68, 17–35. 10.1246/bcsj.68.17. [DOI] [Google Scholar]; f Isayama S.; Mukaiyama T. Novel method for the preparation of triethylsilyl peroxides from olefins by the reaction with molecular oxygen and triethylsilane catalyzed by bis(1,3-diketonato)cobalt(II). Chem. Lett. 1989, 18, 573–576. 10.1246/cl.1989.573. [DOI] [Google Scholar]
- a Okamoto T.; Kobayashi K.; Oka S.; Tanimoto S. Cobalt-catalyzed reaction of nitric oxide with aryl-substituted olefins in the presence of tetrahydroborate ion. J. Org. Chem. 1987, 52, 5089–5092. 10.1021/jo00232a005. [DOI] [Google Scholar]; b Okamoto T.; Kobayashi K.; Oka S.; Tanimoto S. A high-yield regiospecific synthesis of keto oximes from aryl-conjugated ethylenes and ethyl nitrite in the presence of cobalt complex and BH4- ion. J. Org. Chem. 1988, 53, 4897–4901. 10.1021/jo00256a001. [DOI] [Google Scholar]; c Kato K.; Mukaiyama T. A novel method for the preparation of 2-hydroxyiminocarboxylic acid esters. Cobalt (II) catalyzed α-oximation of α, β-unsaturated esters with butyl nitrite and phenylsilane. Chem. Lett. 1990, 19, 1917–1920. 10.1246/cl.1990.1917. [DOI] [Google Scholar]; d Kato K.; Mukaiyama T. Iron (III) complex catalyzed nitrosation of terminal and 1,2-disubstituted olefins with butyl nitrite and phenylsilane. Chem. Lett. 1992, 21, 1137–1140. 10.1246/cl.1992.1137. [DOI] [Google Scholar]; e Sugamoto K. A Synthesis of Oximes from Olefins by Cobalt(II) Porphyrin-Catalyzed Reduction-Nitrosation. Synlett 1998, 1998, 1270–1272. 10.1055/s-1998-1933. [DOI] [Google Scholar]; f Prateeptongkum S.; Jovel I.; Jackstell R.; Vogl N.; Weckbecker C.; Beller M. First iron-catalyzed synthesis of oximes from styrenes. Chem. Commun. 2009, 1990–1992. 10.1039/b900326f. [DOI] [PubMed] [Google Scholar]
- a Gaspar B.; Carreira E. M. Mild Cobalt-Catalyzed Hydrocyanation of Olefins with Tosyl Cyanide. Angew. Chem. 2007, 119, 4603–4606. 10.1002/ange.200700575. [DOI] [PubMed] [Google Scholar]; b Leggans E. K.; Barker T. J.; Duncan K. K.; Boger D. L. Iron(III)/NaBH4-Mediated Additions to Unactivated Alkenes: Synthesis of Novel 20′-Vinblastine Analogues. Org. Lett. 2012, 14, 1428–1431. 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Waser J.; Carreira E. M. Convenient Synthesis of Alkylhydrazides by the Cobalt-Catalyzed Hydrohydrazination Reaction of Olefins and Azodicarboxylates. J. Am. Chem. Soc. 2004, 126, 5676–5677. 10.1021/ja048698u. [DOI] [PubMed] [Google Scholar]; b Sato M.; Gunji Y.; Ikeno T.; Yamada T. Stereoselective α-Hydrazination of α,β-Unsaturated Carboxylates Catalyzed by Manganese(III) Complex with Dialkylazodicarboxylate and Phenylsilane. Chem. Lett. 2005, 34, 316–317. 10.1246/cl.2005.316. [DOI] [Google Scholar]; c Waser J.; González-Gómez J. C.; Nambu H.; Huber P.; Carreira E. M. Cobalt-Catalyzed Hydrohydrazination of Dienes and Enynes: Access to Allylic and Propargylic Hydrazides. Org. Lett. 2005, 7, 4249–4252. 10.1021/ol0517473. [DOI] [PubMed] [Google Scholar]; d Zheng J.; Qi J.; Cui S. Fe-Catalyzed Olefin Hydroamination with Diazo Compounds for Hydrazone Synthesis. Org. Lett. 2016, 18, 128–131. 10.1021/acs.orglett.5b03317. [DOI] [PubMed] [Google Scholar]
- a Waser J.; Nambu H.; Carreira E. M. Cobalt-Catalyzed Hydroazidation of Olefins: Convenient Access to Alkyl Azides. J. Am. Chem. Soc. 2005, 127, 8294–8295. 10.1021/ja052164r. [DOI] [PubMed] [Google Scholar]; b Carreira E.; Gaspar B.; Waser J. Cobalt-Catalyzed Synthesis of Tertiary Azides from α,α-Disubstituted Olefins under Mild Conditions Using Commercially Available Reagents. Synthesis 2007, 2007, 3839–3845. 10.1055/s-2007-1000817. [DOI] [Google Scholar]
- a Gui J.; Pan C.-M.; Jin Y.; Qin T.; Lo J. C.; Lee B. J.; Spergel S. H.; Mertzman M. E.; Pitts W. J.; La Cruz T. E.; et al. Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886–891. 10.1126/science.aab0245. [DOI] [PubMed] [Google Scholar]; b Obradors C.; Martinez R. M.; Shenvi R. A. Ph(i-PrO)SiH2: An Exceptional Reductant for Metal-Catalyzed Hydrogen Atom Transfers. J. Am. Chem. Soc. 2016, 138, 4962–71. 10.1021/jacs.6b02032. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhu K.; Shaver M. P.; Thomas S. P. Amine-bis(phenolate) Iron(III)-Catalyzed Formal Hydroamination of Olefins. Chem. - Asian J. 2016, 11, 977–80. 10.1002/asia.201501098. [DOI] [PubMed] [Google Scholar]; d Ma W.; Zhang X.; Fan J.; Liu Y.; Tang W.; Xue D.; Li C.; Xiao J.; Wang C. Iron-Catalyzed Anti-Markovnikov Hydroamination and Hydroamidation of Allylic Alcohols. J. Am. Chem. Soc. 2019, 141, 13506–13515. 10.1021/jacs.9b05221. [DOI] [PubMed] [Google Scholar]
- a Gaspar B.; Carreira E. M. Catalytic hydrochlorination of unactivated olefins with para-toluenesulfonyl chloride. Angew. Chem., Int. Ed. 2008, 47, 5758–60. 10.1002/anie.200801760. [DOI] [PubMed] [Google Scholar]; b Taniguchi T.; Goto N.; Nishibata A.; Ishibashi H. Iron-Catalyzed Redox Radical Cyclizations of 1,6-Dienes and Enynes. Org. Lett. 2010, 12, 112–115. 10.1021/ol902562j. [DOI] [PubMed] [Google Scholar]; c Ma X.; Herzon S. B. Non-classical selectivities in the reduction of alkenes by cobalt-mediated hydrogen atom transfer. Chem. Sci. 2015, 6, 6250–6255. 10.1039/C5SC02476E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shigehisa H.; Aoki T.; Yamaguchi S.; Shimizu N.; Hiroya K. Hydroalkoxylation of unactivated olefins with carbon radicals and carbocation species as key intermediates. J. Am. Chem. Soc. 2013, 135, 10306–9. 10.1021/ja405219f. [DOI] [PubMed] [Google Scholar]; b Shigehisa H. Functional Group Tolerant Markovnikov-Selective Hydrofunctionalization of Unactivated Olefins Using a Cobalt Complex as Catalyst. Synlett 2015, 26, 2479–2484. 10.1055/s-0035-1560171. [DOI] [Google Scholar]; c Shigehisa H.; Hayashi M.; Ohkawa H.; Suzuki T.; Okayasu H.; Mukai M.; Yamazaki A.; Kawai R.; Kikuchi H.; Satoh Y.; Fukuyama A.; Hiroya K. Catalytic Synthesis of Saturated Oxygen Heterocycles by Hydrofunctionalization of Unactivated Olefins: Unprotected and Protected Strategies. J. Am. Chem. Soc. 2016, 138, 10597–604. 10.1021/jacs.6b05720. [DOI] [PubMed] [Google Scholar]; d Shigehisa H.; Kikuchi H.; Hiroya K. Markovnikov-Selective Addition of Fluorous Solvents to Unactivated Olefins Using a Co Catalyst. Chem. Pharm. Bull. 2016, 64, 371–374. 10.1248/cpb.c15-01024. [DOI] [PubMed] [Google Scholar]; e For a singular example of an N-phenyl-N-tosylurea undergoing cyclization via oxygen, see:Nagai T.; Mimata N.; Terada Y.; Sebe C.; Shigehisa H. Catalytic Dealkylative Synthesis of Cyclic Carbamates and Ureas via Hydrogen Atom Transfer and Radical-Polar Crossover. Org. Lett. 2020, 22, 5522–5527. 10.1021/acs.orglett.0c01872. [DOI] [PubMed] [Google Scholar]; f Ebisawa K.; Izumi K.; Ooka Y.; Kato H.; Kanazawa S.; Komatsu S.; Nishi E.; Shigehisa H. Catalyst- and Silane-Controlled Enantioselective Hydrofunctionalization of Alkenes by Cobalt-Catalyzed Hydrogen Atom Transfer and Radical-Polar Crossover. J. Am. Chem. Soc. 2020, 142, 13481–13490. 10.1021/jacs.0c05017. [DOI] [PubMed] [Google Scholar]
- Shigehisa H.; Ano T.; Honma H.; Ebisawa K.; Hiroya K. Co-Catalyzed Hydroarylation of Unactivated Olefins. Org. Lett. 2016, 18, 3622–3625. 10.1021/acs.orglett.6b01662. [DOI] [PubMed] [Google Scholar]
- Date S.; Hamasaki K.; Sunagawa K.; Koyama H.; Sebe C.; Hiroya K.; Shigehisa H. Catalytic Direct Cyclization of Alkenyl Thioester. ACS Catal. 2020, 10, 2039–2045. 10.1021/acscatal.9b05045. [DOI] [Google Scholar]
- a Shigehisa H.; Koseki N.; Shimizu N.; Fujisawa M.; Niitsu M.; Hiroya K. Catalytic hydroamination of unactivated olefins using a Co catalyst for complex molecule synthesis. J. Am. Chem. Soc. 2014, 136, 13534–13537. 10.1021/ja507295u. [DOI] [PubMed] [Google Scholar]; The cyclization of two singular N-phenyl amides, 2-allyl-N-phenylbenzamide and 2-vinyl-N-phenylbenzamide, under HAT conditions to yield the corresponding cyclic N-phenyl imidates was reported herein.; b Ohuchi S.; Koyama H.; Shigehisa H. Catalytic Synthesis of Cyclic Guanidines via Hydrogen Atom Transfer and Radical-Polar Crossover. ACS Catal. 2021, 11, 900–906. 10.1021/acscatal.0c05359. [DOI] [Google Scholar]; c Ye W.-T.; Zhu R. Dioxygen-promoted cobalt-catalyzed oxidative hydroamination using unactivated alkenes and free amines. Chem. Catal. 2022, 2, 345–357. 10.1016/j.checat.2021.12.003. [DOI] [Google Scholar]
- Thakur R.; Jaiswal Y.; Kumar A. Imidates: an emerging synthon for N-heterocycles. Org. Biomol Chem. 2019, 17, 9829–9843. 10.1039/C9OB01899A. [DOI] [PubMed] [Google Scholar]; Traditionally, acyclic N-sulfonyl imidates can be accessed through acid-catalyzed condensation of nitriles with alcohols (Pinner synthesis) followed by sulfonylation of the resultant imidate. Alternatively, they may be generated through dipolar cycloaddition of N-tosyl azide with alkynes in the presence of oxygen nucleophiles.
- Volkova Y. A.; Kozlov A. S.; Kolokolova M. K.; Uvarov D. Y.; Gorbatov S. A.; Andreeva O. E.; Scherbakov A. M.; Zavarzin I. V. Steroidal N-Sulfonylimidates: Synthesis and biological evaluation in breast cancer cells. Eur. J. Med. Chem. 2019, 179, 694–706. 10.1016/j.ejmech.2019.06.048. [DOI] [PubMed] [Google Scholar]
- Bundgaard H.; Larsen J. D. N-Sulfonyl imidates as a novel prodrug form for an ester function or a sulfonamide group. J. Med. Chem. 1988, 31, 2066–2069. 10.1021/jm00119a002. [DOI] [PubMed] [Google Scholar]
- a Iwata M. Probenazole—a plant defence activator. Pestic. Outlook 2001, 12, 28–31. 10.1039/b100805f. [DOI] [Google Scholar]; b Zhang L.; Jiao B.; Yang X.; Liu L.; Ma S. Synthesis and antibacterial activity of new 4″-O-carbamates of 11,12-cyclic carbonate erythromycin A 6,9-imino ether. J. Antibiot. 2011, 64, 243–247. 10.1038/ja.2010.166. [DOI] [PubMed] [Google Scholar]
- Liu X.-Y.; Li C.-H.; Che C.-M. Phosphine Gold(I)-Catalyzed Hydroamination of Alkenes under Thermal and Microwave-Assisted Conditions. Org. Lett. 2006, 8, 2707–2710. 10.1021/ol060719x. [DOI] [PubMed] [Google Scholar]
- a Nagamoto M.; Yanagi T.; Nishimura T.; Yorimitsu H. Asymmetric Cyclization of N-Sulfonyl Alkenyl Amides Catalyzed by Iridium/Chiral Diene Complexes. Org. Lett. 2016, 18, 4474–4477. 10.1021/acs.orglett.6b01954. [DOI] [PubMed] [Google Scholar]; b Nishimura T.; Nagamoto M.; Yorimitsu H. Iridium-Catalyzed Intramolecular Oxidative Cyclization of Alkenyl Amides and Alkenoic Acids. Synthesis 2017, 49, 4272–4282. 10.1055/s-0036-1588435. [DOI] [Google Scholar]
- Ferrand L.; Tang Y.; Aubert C.; Fensterbank L.; Mouriès-Mansuy V.; Petit M.; Amatore M. Niobium-Catalyzed Intramolecular Addition of O–H and N–H Bonds to Alkenes: A Tool for Hydrofunctionalization. Org. Lett. 2017, 19, 2062–2065. 10.1021/acs.orglett.7b00657. [DOI] [PubMed] [Google Scholar]
- Chou T.-H.; Yu B.-H.; Chein R.-J. ZnI2/Zn(OTf)2-TsOH: a versatile combined-acid system for catalytic intramolecular hydrofunctionalization and polyene cyclization. Chem. Commun. 2019, 55, 13522–13525. 10.1039/C9CC07242J. [DOI] [PubMed] [Google Scholar]
- Arai T.; Watanabe O.; Yabe S.; Yamanaka M. Catalytic Asymmetric Iodocyclization of N-Tosyl Alkenamides using Aminoiminophenoxy Copper Carboxylate: A Concise Synthesis of Chiral 8-Oxa-6-azabicyclo[3.2.1]octanes. Angew. Chem., Int. Ed. 2015, 54, 12767–12771. 10.1002/anie.201505748. [DOI] [PubMed] [Google Scholar]
- Takesue T.; Fujita M.; Sugimura T.; Akutsu H. A series of two oxidation reactions of ortho-alkenylbenzamide with hypervalent iodine(III): a concise entry into (3R,4R)-4-hydroxymellein and (3R,4R)-4-hydroxy-6-methoxymellein. Org. Lett. 2014, 16, 4634–4637. 10.1021/ol502225p. [DOI] [PubMed] [Google Scholar]
- Cheng X.; Hasimujiang B.; Xu Z.; Cai H.; Chen G.; Mo G.; Ruan Z. Direct Electrochemical Selenylation/Cyclization of Alkenes: Access to Functionalized Benzheterocycles. J. Org. Chem. 2021, 86, 16045–16058. 10.1021/acs.joc.1c01267. [DOI] [PubMed] [Google Scholar]
- Franz J. E.; Dietrich M. W.; Henshall A.; Osuch C. Reactions of Sulfonyl Azides and Sulfonamides with Vinyl Ethers. J. Org. Chem. 1966, 31, 2847–2853. 10.1021/jo01347a026. [DOI] [PubMed] [Google Scholar]
- a Neilson D. G.Imidates including cyclic imidates. In Amidines and Imidates; John Wiley & Sons, 1991; pp 425–483. [Google Scholar]; b Matsubara R.; Berthiol F.; Kobayashi S. Sulfonylimidates as Nucleophiles in Catalytic Addition Reactions. J. Am. Chem. Soc. 2008, 130, 1804–1805. 10.1021/ja077054u. [DOI] [PubMed] [Google Scholar]
- a Du Bois J.; Hong J.; Carreira E. M.; Day M. W. Nitrogen Transfer from a Nitridomanganese(V) Complex: Amination of Silyl Enol Ethers. J. Am. Chem. Soc. 1996, 118, 915–916. 10.1021/ja953659r. [DOI] [Google Scholar]; b Waser J.; Carreira E. M. Catalytic hydrohydrazination of a wide range of alkenes with a simple mn complex. Angew. Chem., Int. Ed. 2004, 43, 4099–102. 10.1002/anie.200460811. [DOI] [PubMed] [Google Scholar]; c Balkenhohl M.; Kölbl S.; Georgiev T.; Carreira E. M. Mn- and Co-Catalyzed Aminocyclizations of Unsaturated Hydrazones Providing a Broad Range of Functionalized Pyrazolines. JACS Au 2021, 1, 919–924. 10.1021/jacsau.1c00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson J. B. Systematic characterization of structures and reactions for use in organic synthesis. J. Am. Chem. Soc. 1971, 93, 6847–6854. 10.1021/ja00754a026. [DOI] [Google Scholar]
- This is in contrast to previous methods employing N-fluorocollidinium salts as oxidants, which either require the reaction to be carried out under an inert atmosphere (hydrothioetherifcation, acetoxylation) or even prescribe degassing with argon to avoid the formation of Mukayiama hydration (hydrofluorination, hydrohydroxylation, and hydroamination) products.
- a Gunther H.NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry, 3rd ed.; John Wiley & Sons, 2013. [Google Scholar]; b Huggins M. T.; Kesharwani T.; Buttrick J.; Nicholson C. Variable Temperature NMR Experiment Studying Restricted Bond Rotation. J. Chem. Educ. 2020, 97, 1425–1429. 10.1021/acs.jchemed.0c00057. [DOI] [Google Scholar]
- Gesmundo N. J.; Grandjean J. M.; Nicewicz D. A. Amide and amine nucleophiles in polar radical crossover cycloadditions: synthesis of gamma-lactams and pyrrolidines. Org. Lett. 2015, 17, 1316–9. 10.1021/acs.orglett.5b00316. [DOI] [PubMed] [Google Scholar]
- Wang N. J.; Mei S. T.; Shuai L.; Yuan Y.; Wei Y. Aerobic oxidative C-H olefination of cyclic N-sulfonyl ketimines catalyzed by a rhodium catalyst. Org. Lett. 2014, 16, 3040–3043. 10.1021/ol501152a. [DOI] [PubMed] [Google Scholar]
- a Kurahashi T.; Fujii H. Unique Ligand-Radical Character of an Activated Cobalt Salen Catalyst That Is Generated by Aerobic Oxidation of a Cobalt(II) Salen Complex. Inorg. Chem. 2013, 52, 3908–3919. 10.1021/ic302677f. [DOI] [PubMed] [Google Scholar]; b Kemper S.; Hrobárik P.; Kaupp M.; Schlörer N. E. Jacobsen’s Catalyst for Hydrolytic Kinetic Resolution: Structure Elucidation of Paramagnetic Co(III) Salen Complexes in Solution via Combined NMR and Quantum Chemical Studies. J. Am. Chem. Soc. 2009, 131, 4172–4173. 10.1021/ja806151g. [DOI] [PubMed] [Google Scholar]; c Kochem A.; Kanso H.; Baptiste B.; Arora H.; Philouze C.; Jarjayes O.; Vezin H.; Luneau D.; Orio M.; Thomas F. Ligand Contributions to the Electronic Structures of the Oxidized Cobalt(II) salen Complexes. Inorg. Chem. 2012, 51, 10557–10571. 10.1021/ic300763t. [DOI] [PubMed] [Google Scholar]
- Gutmann V.The Donor-Acceptor Approach to Molecular Interactions; Plenum Press: New York, 1978. [Google Scholar]
- A similar trend was observed for the transformation with t-BuOOH as the oxidant (see the SI).
- A more detailed discussion of feasible mechanistic pathways for the transformation described herein can be found in the SI.
- When the reaction was attempted in an intermolecular fashion employing phenylbut-4-ene and an excess of N-acetyl sulfonamide, no product was isolated.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.