Key Points
Question
What is the stroke rate in patients with asymptomatic severe carotid stenosis without surgical intervention?
Findings
This retrospective cohort study included 3737 participants with 70% to 99% asymptomatic carotid stenosis identified between 2008 and 2012 and followed-up through 2019 who did not undergo surgical intervention. The estimated rate of ipsilateral carotid-related acute ischemic stroke was 4.7% over 5 years.
Meaning
These findings may inform decision-making regarding treatment for patients with asymptomatic severe carotid stenosis.
Abstract
Importance
Optimal management of patients with asymptomatic severe carotid stenosis is uncertain, due to advances in medical care and a lack of contemporary data comparing medical and surgical treatment.
Objective
To estimate stroke outcomes among patients with medically treated asymptomatic severe carotid stenosis who did not undergo surgical intervention.
Design, Setting, and Participants
Retrospective cohort study that included 3737 adult participants with asymptomatic severe (70%-99%) carotid stenosis diagnosed between 2008 and 2012 and no prior intervention or ipsilateral neurologic event in the prior 6 months. Participants received follow-up through 2019, and all were members of an integrated US regional health system serving 4.5 million members.
Exposures
Imaging diagnosis of asymptomatic carotid stenosis of 70% to 99%.
Main Outcomes and Measures
Occurrence of ipsilateral carotid-related acute ischemic stroke. Censoring occurred with death, disenrollment, or ipsilateral intervention.
Results
Among 94 822 patients with qualifying imaging studies, 4230 arteries in 3737 (mean age, 73.8 [SD 9.5 years]; 57.4% male) patients met selection criteria including 2539 arteries in 2314 patients who never received intervention. The mean follow-up in this cohort was 4.1 years (SD 3.6 years). Prior to any intervention, there were 133 ipsilateral strokes with a mean annual stroke rate of 0.9% (95% confidence interval [CI], 0.7%-1.2%). The Kaplan-Meier estimate of ipsilateral stroke by 5 years was 4.7% (95% CI, 3.9%-5.7%).
Conclusions and Relevance
In a community-based cohort of patients with asymptomatic severe carotid stenosis who did not undergo surgical intervention, the estimated rate of ipsilateral carotid-related acute ischemic stroke was 4.7% over 5 years. These findings may inform decision-making regarding surgical and medical treatment for patients with asymptomatic severe carotid artery stenosis.
This retrospective cohort study estimated stroke outcomes among 3737 adult participants with asymptomatic severe carotid stenosis who did not undergo surgical intervention.
Introduction
Since the publication of several randomized clinical trials in 1995 and 2010, optimal treatment of asymptomatic carotid disease has involved selective intervention.1,2 Since then, medical therapy has progressed, and reports have demonstrated improved outcomes with medical therapy alone, raising questions about the absolute incremental benefit of intervention to prevent stroke in asymptomatic patients.3,4,5
The stroke risk of asymptomatic carotid disease without surgical revascularization is difficult to ascertain because data sources have been limited to nonsurgical groups of clinical trials, rare prospective studies, or smaller intervention-based studies.6,7,8 Furthermore, given the age of these studies, they do not account for the recent advances in cardiovascular risk reduction in the population. The objective of this study was to describe the long-term risk of stroke among patients with asymptomatic severe carotid stenosis who do not undergo intervention in a more contemporary and racial and ethnically diverse community population.
Methods
This project was approved by the Kaiser Permanente Northern California (KPNC) institutional review board with a specified waiver of consent for a data-only retrospective study. KPNC is an integrated health care delivery system that serves over 4.5 million members across 21 medical centers and hospitals. Demographics in the health system are comparable with the broader population of insured patients in the region.9
Disease Cohort Assembly
A previously described method for the identification of carotid disease from archived imaging data within the health system was used for cohort assembly.10 Patients with severe carotid stenosis (70%-99% according to the Society of Radiologists in Ultrasound Consensus Criteria for ultrasound studies11 and the North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria for axial imaging12) were identified from 2008-2012. If 2 studies were performed within a short time period and the results were different the axial study was generally used to define the stenosis category. If 2 ultrasounds were performed in the similar time period, the second one was used if informative and distinct from the first one. If the stenosis category from the second ultrasound was less than 70%, that artery was excluded from the current study. To stratify a subcategory of high-grade stenosis both at baseline and during follow-up, a high-grade sonographic threshold was designated by an internal carotid artery peak systolic velocity of 350 cm/s or greater or a NASCET grade of 90% to 99% lesion on axial imaging. Exclusion criteria were as follows: (1) patients with no active health plan membership for 1 year prior to the index study; (2) nonatherosclerotic lesions of the carotid artery (eg, dissection, fibromuscular dysplasia, trauma); (3) ipsilateral carotid interventions (carotid endarterectomy and carotid artery stenting identified using Current Procedural Terminology-4 codes and International Statistical Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] and ICD-10-CM procedure codes) any time prior to the index study; and (4) stroke or transient ischemic attack, using ICD-9-CM and ICD-10-CM diagnosis codes within 6 months prior to the index study. Manual chart review of operative reports, clinical notes, and other relevant documentation was performed to confirm the laterality, timing, and presence of an asymptomatic lesion. Eligible arteries were followed through December 31, 2019, with censoring at death, membership disenrollment, or an ipsilateral carotid intervention.
Covariates
Baseline characteristics were collected, including demographics, relevant comorbidities, cardiovascular medication, and numerical values for blood pressure and laboratory values. The values obtained closest to the date of the index study were used as baseline. Race and ethnicity, which were reported by the patient during routine medical charting based on fixed categories, were included to aid readers in understanding the diversity of the community cohort. A previously assembled internally13 and externally14 validated comorbidity index was used as a general risk-adjustment score (comorbidity point score, version 2 [COPS2]) for patients, based on diagnosis codes and automated physiological data capture.
Baseline cardiovascular medication use (eg, statins, antihypertensives) was defined as at least one filled prescription in the 12 months prior to study entry. Adherence was assessed by the medication possession ratio (MPR) ascertained from outpatient pharmacy records. Baseline MPR was calculated by dividing the total number of days of dispensed outpatient prescriptions supplied in the prior year by 365 days. During follow-up, this calculation was amended to include days supplied among all dispensed medication up to the end of follow-up per each study year as the numerator and maximal follow-up time or study year as the denominator. Calculated MPR was a continuous percentage, and for dichotomous analytic purposes, an MPR of greater than 80% (hereafter termed MPR80) was defined as adherent.15
Outcomes
The primary outcome was defined as acute ischemic stroke ipsilateral to the carotid lesion. Each identified event was manually reviewed by a vascular surgeon or stroke neurologist; disagreements were adjudicated by consensus. Available imaging reports (computed tomography, magnetic resonance imaging), consultation reports from Neurology or Vascular Surgery, or other relevant documentation were reviewed. Strokes were confirmed and then assigned laterality, location, and etiology with the appropriate clinical evidence. Subtypes of ischemic stroke were categorized using published criteria.16 When imaging data were informative, presence of ischemic injury on diagnostic imaging was counted as a stroke even if the duration of symptoms was less than 24 hours.17 Despite a multispecialty approach to stroke care, some cases could not be definitively designated, so these ipsilateral cases were conservatively assigned to the primary outcome category. During adjudication, if there was a presumed lacunar stroke ipsilateral to the carotid stenosis, it was judged as being related to the carotid lesion. In addition, strokes of unknown etiology or multiple possible etiologies that were ipsilateral to the carotid lesion of interest were included in the primary outcome. Secondary outcomes included stenosis progression to high grade or occlusion, medication prescription, medication adherence, blood pressure, low-density lipoprotein (LDL) levels, and all-cause mortality.
Sample Size Calculation
Due to the descriptive nature of this study, sample size calculations were not computed, and the cohort size was fixed by the chosen criteria. Adequacy of sample size was assessed by the CIs around the primary point estimates.
Statistical Analysis
Baseline characteristics of the group receiving intervention are described in eTable 1 in the Supplement and intervention-related outcomes in the KPNC health system have been previously reported and are not reported here.18 Missing data, including blood pressure, creatinine, high-density lipoprotein (HDL), LDL, hematocrit, triglycerides, and body mass index (calculated as weight in kilograms divided by height in meters squared), were replaced with median values of study participants with data by sex. These characteristics were compared between the asymptomatic intervention group and the no intervention group with χ2 or Fisher exact tests for categorical variables as appropriate, and continuous variables were compared between groups using independent 2-sample t tests. COPS2 scores were compared using the Mann-Whitney U (Wilcoxon rank-sum) test.
In the case of bilateral asymptomatic severe stenosis, each artery was analyzed independent of the contralateral artery. Crude rates of outcomes of interest were calculated, and the life table method (indirect method without a comparison group) and Kaplan-Meier survival analyses were performed for ipsilateral stroke. We also examined stroke risk while accounting for the competing risks of death and intervention.19 A competing-risks model was developed using a nonparametric cumulative incidence function to determine the cumulative incidence of ipsilateral stroke. In the presence of a competing event such as mortality, the cumulative incidence function was dependent on the hazard rate of the event of interest (stroke) as well as of the hazard rate of the competing event(s). The cumulative incidence function in competing risks can be estimated using the Aalen-Johansen estimator. This model accounts for the competing events of death and intervention in determining the cumulative incidence of the primary outcome.
A Cox proportional hazards model was used to identify risk factors for the primary outcome. Covariates in the regression analyses were determined based on significant association with the outcome of interest, as well as clinical relevance. Variables included in the model included age, sex, high-grade stenosis, history of contralateral stroke, COPS2 score, diabetes, anticoagulation or statin medication use and adherence, and blood pressure, LDL, HDL, and triglycerides levels. The proportional hazards assumption for the covariates in the Cox model was assessed based on cumulative sums of Martingale residuals over follow-up20 and through scaled Schoenfeld residual plots.21 While the tests of proportional hazards were significant for age (P = .03), high-grade stenosis, (P = .02), and HDL (P = .01), indicating nonproportionality, Schoenfeld residual plots for these variables indicated only slight changes in the hazard ratios (HRs) over time. Since the deviation from the proportional hazards assumption is small for these variables, we report the estimated HRs under the proportional hazards assumption and interpret the effect estimates for these variables as mean HRs across follow-up.
All analyses were performed using SAS 9.4 (SAS Institute Inc) with the threshold of significance set at a 2-sided P value of less than .05. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Results
Figure 1 illustrates the assembly of the cohort of interest. Overall, 4230 arteries with asymptomatic severe stenosis in 3737 patients were included in the final cohort. Baseline characteristics of the group receiving intervention are described in Table 1 and in eTable 1 in the Supplement. In brief, those patients not receiving intervention tended to be older, female and with a larger overall comorbidity burden as reflected in the COPS2 score at baseline. The mean age of the cohort was 73.8 (SD 9.5) years, 58.1% were male, and 73.0% were White. Of the 3737 patients, 2314 patients with 2539 included arteries did not undergo intervention prior to the study end point. Among patients not meeting the primary end point or undergoing intervention, 1553 (36.7%) patients died, and 268 (6.3%) patients disenrolled from the health plan during the study period.
Figure 1. Identification of Carotid Arteries With Asymptomatic Severe Stenosis, 2008-2012.
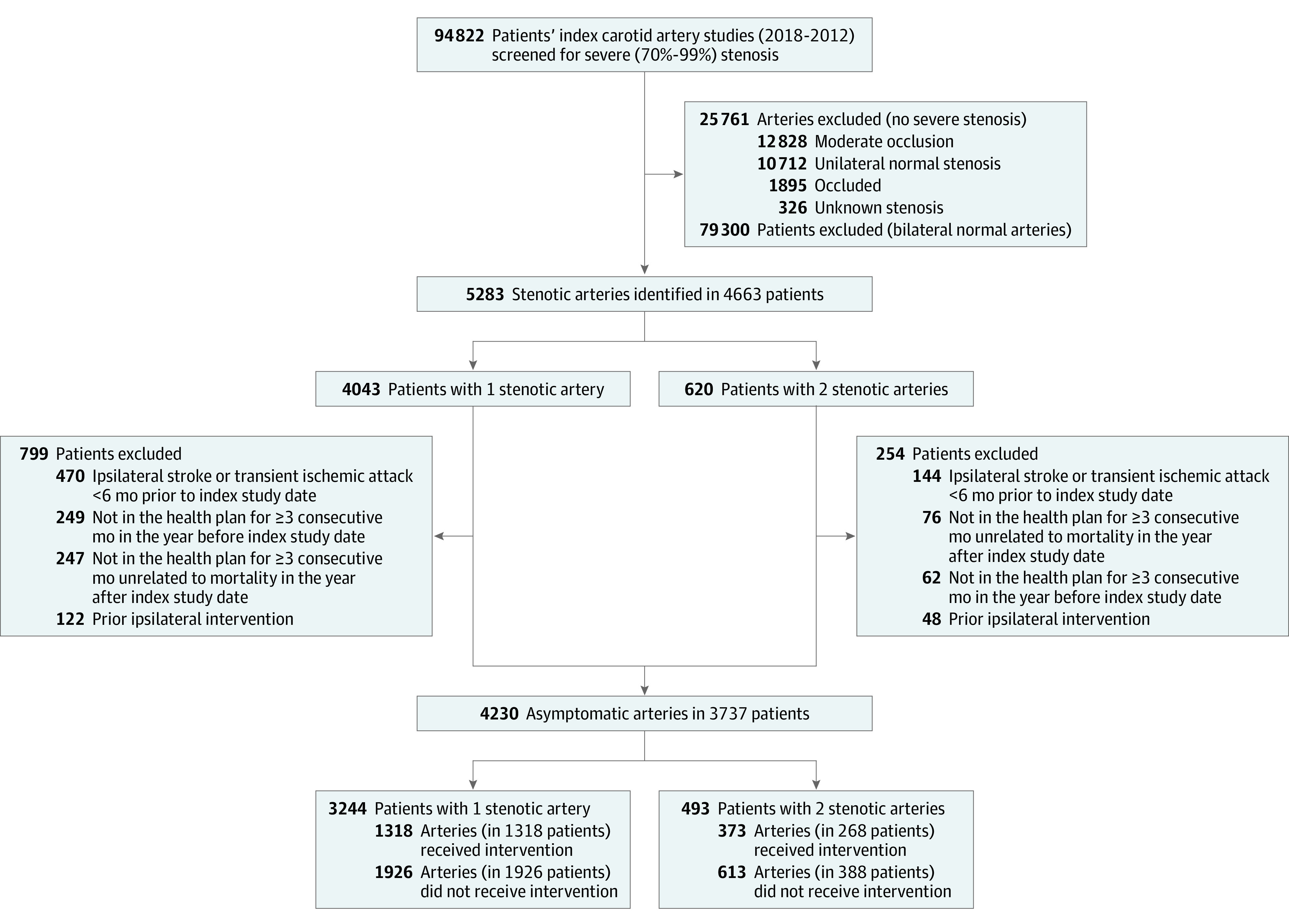
Table 1. Baseline Characteristics of Study Cohort.
| Characteristics | No. (%) | |
|---|---|---|
| Unique arteries (N=4230) | Unique patients (N=3737) | |
| Men | 2428 (57.4) | 2171 (58.1) |
| Women | 1802 (43.6) | 1566 (41.9) |
| Age, mean (SD), y | 73.8 (9.5) | 73.8 (9.5) |
| Median (IQR), y | 74 (68-81) | 74 (68-81) |
| Race and ethnicitya | ||
| Hispanic/Latinx | 406 (9.6) | 360 (9.6) |
| Non-Hispanic/Latinx Asianb | 246 (5.8) | 214 (5.7) |
| Non-Hispanic/Latinx Black | 178 (4.2) | 162 (4.3) |
| Non-Hispanic/Latinx White | 3099 (73.3) | 2727 (73.0) |
| Otherc | 297 (7.0) | 270 (7.2) |
| Unknown | 4 (0.1) | 4 (0.1) |
| Systolic blood pressure, mean (SD), mm Hgd | 130.8 (19.1) | 130.8 (19.0) |
| Median (IQR), mm Hg | 130 (120-140) | 130 (120-140) |
| Diastolic blood pressure, mean (SD), mm Hgd | 68.2 (11.2) | 68.3 (11.1) |
| Median (IQR), mm Hg | 68 (60-75.7) | 68 (60-76) |
| Blood pressure <140/90 mm Hgd | 3132 (74.0) | 2767 (74.0) |
| Creatinine, mean (SD), mg/dLd | 1.2 (0.9) | 1.2 (0.9) |
| Median (IQR), mg/dL | 1 (0.9-1.3) | 1 (0.9-1.3) |
| High-density lipoprotein, mean (SD), mg/dLd | 46.4 (12.8) | 46.4 (12.8) |
| Median (IQR), mg/dL | 44 (38-52) | 44 (38-52) |
| Low-density lipoprotein, mean (SD), mg/dLd | 93.5 (33.4) | 93.3 (33.3) |
| Median (IQR), mg/dL | 87 (72-109) | 87 (72-108) |
| Hematocrit, mean (SD), %d | 38.5 (4.9) | 38.6 (4.9) |
| Median (IQR), % | 39 (35.4-41.6) | 39.1 (35.5-41.8) |
| Triglyceride, mean (SD), mg/dLd | 147.8 (91.7) | 148.1 (92.0) |
| Median (IQR), mg/dL | 126 (100-168) | 126 (100-168) |
| High-grade carotid stenosis | 473 (11.2) | 434 (11.6) |
| Contralateral disease | ||
| Normale | 1870 (44.2) | 1870 (50.0) |
| Moderate | 1185 (28.0) | 1185 (31.7) |
| Severef | 1002 (23.7) | 509 (13.6) |
| Occluded | 173 (4.1) | 173 (4.6) |
| Remote history of strokeg | 81 (1.9) | 72 (1.9) |
| Coronary artery disease | 186 (4.4) | 161 (4.3) |
| Diabetes | 909 (21.5) | 804 (21.5) |
| Chronic obstructive pulmonary disease | 39 (0.9) | 34 (0.9) |
| Peripheral arterial disease | 208 (4.9) | 181 (4.8) |
| Atrial fibrillation | 93 (2.2) | 81 (2.2) |
| Hypertension | 697 (16.5) | 606 (16.2) |
| COPS2, mean (SD)h | 37.4 (33.9) | 37.2 (33.8) |
| Median (IQR) | 27 (10-50) | 27 (10-50) |
| Body mass index, mean (SD)i | 27.8 (5.5) | 27.8 (5.4) |
| Median (IQR) | 27.1 (24.1-30.6) | 27.1 (24.2-30.5) |
| Smoking history, No./total (%) | ||
| Never | 1103/3758 (29.4) | 988/3319 (29.8) |
| Current | 516/3758 (13.7) | 455/3319 (13.7) |
| Former | 2139/3758 (56.9) | 1876/3319 (56.5) |
Abbreviation: COPS2, comorbidity point score, version 2.
SI conversion factors: To convert creatinine from mg/dL to μmol/L, multiply by 88.4; high-density lipoprotein and low-density lipoprotein from mg/dL to mmol/L, multiply by 0.0259; and triglycerides from mg/dL to mmol/L, multiply by 0.0113.
Race and ethnicity data were self-reported and based on fixed categories.
Included individuals who identified as Pacific Islander and Native Hawaiian.
Included individuals who identified as Non-Hispanic/Latinx American Indian, Alaska Native, or more than 1 race.
Missing data replaced with median of study participants with data by sex: blood pressure = 93, creatinine = 155, high-density lipoprotein = 538, low-density lipoprotein = 465, hematocrit = 466, triglycerides = 701, and body mass index = 127.
Includes 11 carotid arteries with unknown stenosis.
Includes 493 patients with bilateral asymptomatic severe carotid arteries and 16 patients with a unilateral asymptomatic severe carotid artery with a symptomatic contralateral severe stenosis at baseline (excluded from the study cohort).
Defined as any stroke occurring more than 6 months prior to the index study found in the electronic health record.
COPS2 is a validated comorbidity index used as a predictor of 30-day mortality (score range, 0-700 with increasing risk of death as score increases). Scores between 41 and 60 predict 30-day mortality of less than 4%.
Calculated as weight in kilograms divided by height in meters squared.
Primary Outcome: Ipsilateral Carotid-Related Stroke
There were 133 ipsilateral carotid-related ischemic strokes in 129 patients, with the median time from study entry to stroke event of 24.8 (IQR, 7.9-46.5) months. Of the strokes reported as the primary outcome, 3 cases were lacunar in nature, 18 cases had multiple possible etiologies, and 13 cases had an unknown or undefined etiology. The crude (unadjusted) annual ipsilateral stroke rate was 0.9% (95% CI, 0.7%-1.2%). Among the 133 ipsilateral stroke events, 35 patients (27.1%) underwent subsequent intervention and 86 (66.7%) patients died (all causes) including 15 within 30 days and 19 additional patients within 1 year of stroke. There were 43 patients who survived through the study period after stroke. The median survival after ipsilateral stroke, including those receiving intervention, was 25.3 (IQR, 9.7-54.5) months.
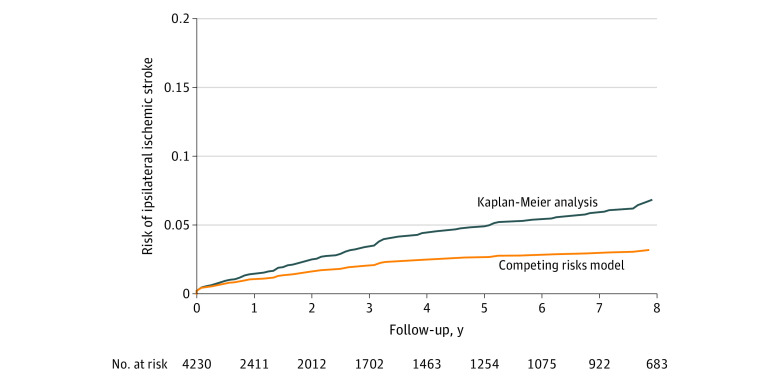
The unadjusted overall mean yearly rates and cumulative 5-year risk of stroke by type are shown in Table 2. The cumulative risk of ipsilateral carotid related stroke at 5 years in Kaplan-Meier analysis was 4.7% (95% CI, 3.9%-5.7%) (Table 2, Figure 2 A) among patients who received the intervention, and it was 4.1% (95% CI, 3.3%-5.0%) in patients who did not receive the intervention. When accounting for the competing risk of death and intervention for asymptomatic disease, the cumulative incidence of ipsilateral stroke was 1.0% (95% CI, 0.8%-1.4%) at 1 year and 2.7% (95% CI, 2.2%-3.2%) at 5 years (Table 2, Figure 2 B).
Table 2. Stroke and All-Cause Mortality (per Unique Artery).
| Outcomes | Cohort, % (95% CI) |
|---|---|
| (N=4230) | |
| Unadjusted overall crude rates, No. (%) | |
| Ipsilateral carotid-related stroke | 133 (3.1) |
| All-cause mortality | 2353 (55.6) |
| Mean yearly crude rates | |
| Ipsilateral carotid-related stroke | 0.9 (0.7-1.2) |
| All-cause mortality | 13.6 (12.6-14.7) |
| Kaplan-Meier unadjusted cumulative probabilities at 5 y | |
| Risk of ipsilateral carotid-related stroke | 4.7 (3.9-5.7) |
| All-cause mortality | 45.2 (43.4-46.9) |
| Unadjusted cumulative incidence of ipsilateral stroke (competing risk analysis), y | |
| 1 | 1.0 (0.8-1.4) |
| 2 | 1.6 (1.3-2.0) |
| 3 | 2.1 (1.7-2.5) |
| 4 | 2.5 (2.0-3.0) |
| 5 | 2.7 (2.2-3.2) |
| 6 | 2.8 (2.3-3.4) |
| 7 | 2.9 (2.5-3.5) |
| 8 | 3.2 (2.7-3.8) |
Figure 2. Cumulative Risk of Ipsilateral Ischemic Stroke per Unique Artery After Initial Diagnosis of Asymptomatic Severe Carotid Stenosis.
Competing risks model comprises death and carotid intervention. Median observation time was 1.7 (IQR, 0.2-6.1) years.
In multivariable Cox regression analysis, significant independent variables associated with ipsilateral ischemic stroke included age (for every 10-year increase: adjusted HR, 1.25 [95% CI, 1.02-1.53]; P = .03), a high-grade lesion at baseline (adjusted HR, 1.73 [95% CI, 1.06-2.84]; P = .03), and history of nonipsilateral stroke (adjusted HR, 2.81 [95% CI, 1.63-4.84]; P < .001). Use of any statin during study follow-up was associated with a reduced risk of stroke (adjusted HR, 0.38 [95% CI, 0.21-0.72]; P = .003). A comprehensive list of covariates used in the regression model is seen in eTable 2 in the Supplement.
Secondary Outcomes
Baseline Stenosis and Contralateral Artery Status
Among the 3737 patients in the overall asymptomatic cohort, 493 (13.2%) had bilateral severe carotid stenosis meeting the selection criteria. Of the 4230 arteries, 473 (11.2%) were considered high grade at baseline, 1185 (28.0%) had a contralateral moderate stenosis, 1002 (23.7%) had contralateral severe stenosis, and 173 (4.1%) had a contralateral carotid occlusion at baseline (Table 1).
Carotid Disease Progression During Follow-up
Among the 3078 arteries that underwent 1 or more additional imaging studies during follow-up, 1815 were in patients not receiving intervention. Of these severe but not high-grade stenosis arteries, 391 (21.5%) progressed to high grade during follow-up and 153 (8.4%) progressed to occlusion. Among 216 arteries with high-grade stenosis at baseline also not undergoing intervention, 61 (28.2%) progressed to occlusion.
Seventy-seven of the 133 ipsilateral strokes (57.9%) were associated with severe baseline stenosis without progression, 32 (24.1%) were associated with progression to high-grade stenosis, and 19 (14.3%) were associated with baseline high-grade stenosis. Of the 163 (3.9%) arteries that were observed to have interval carotid occlusion, 17 (12.8% of the 133 ipsilateral carotid-related strokes) arteries observed with this clinical change were associated with ipsilateral carotid-related stroke (10 with baseline severe stenosis; 7 with baseline high-grade stenosis).
All-Cause Mortality
The unadjusted rate of all-cause mortality was 51.4% (Table 2) with a mean yearly crude rate of 13.6% (95% CI, 12.6%-14.7%) during the mean (SD) follow-up time of 4.1 (3.6) years. Life-table analysis provided unadjusted cumulative probabilities of death due to any cause at 1 year (9.9% [95% CI, 9.0%-10.9%]) and at 5 years (45.2% [95% CI, 43.4%-46.9%]).
Medication Usage and Adherence
Median baseline blood pressures of the cohort (unique patients) were 130 mm Hg (IQR, 120-140 mm Hg) for systolic and 68 mm Hg (IQR, 60-76 mm Hg) for diastolic, including 71.8% who had at least 1 baseline blood pressure lower than 140/90 mm Hg. Median baseline LDL was 87 mg/dL (IQR, 72-108 mg/dL), and the active smoking prevalence among patients with known smoking history data at baseline was 13.7% (455/3319). Additional descriptive characteristics are seen in Table 1.
Seventy-four percent of the patients (n = 2768) were prescribed a statin at baseline with baseline statin adherence of 45.2% in the year prior to baseline. During follow-up, any statin use improved to 91.1% with statin adherence increasing to 70.7%. Of the 3299 patients with an antihypertensive medication prescribed in the year prior to study entry, 82.2% (n = 2711) demonstrated medication adherence at baseline. Of the 3526 patients who were prescribed antihypertensive medications during follow-up, 88.5% (n = 3120) demonstrated medication adherence. Figure 3 (panels A through D) demonstrates time-dependent observations on yearly medication use and adherence of these medications along with the associated measured blood pressures and LDL levels. As shown in these figures, both indices appeared to be well-controlled and remained below recommended target levels during the study period.
Figure 3. Per-Patient Medication Use, Adherence, and Effect Over Time.
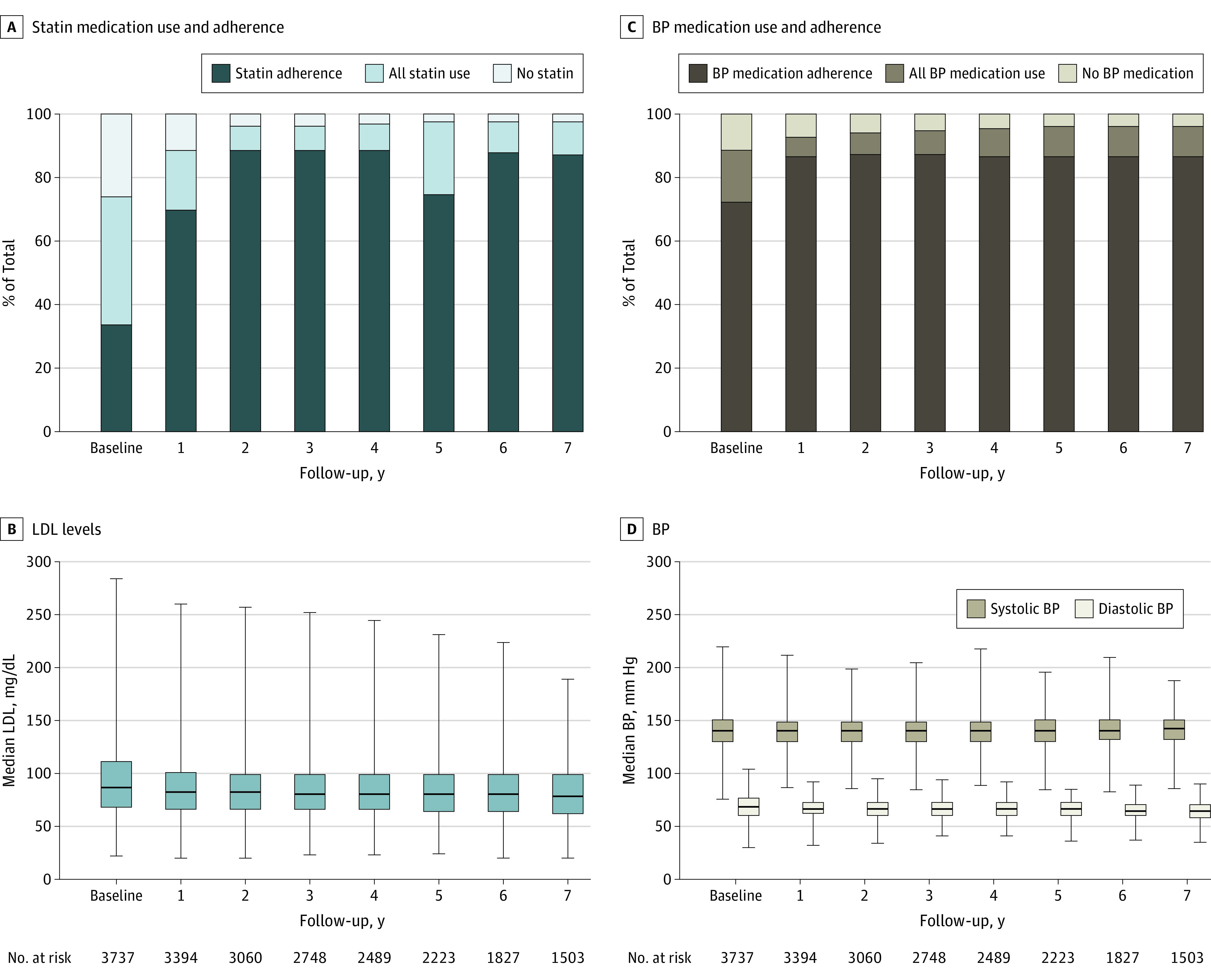
Data are reported for baseline (year 0) and for the next 7 years.
In panels B and D, the horizontal bar in each box indicates the median, box tops and bottoms indicate the interquartile range, and the whiskers indicate the minimum and maximum (full range) values.
BP indicates blood pressure, and LDL indicates low-density lipoprotein.
Discussion
In a community-based cohort of patients with asymptomatic severe carotid stenosis who did not undergo surgical intervention, the estimated rate of ipsilateral carotid-related acute ischemic stroke was lower than historical reports. To our knowledge, this study is the largest and most current assessment of long-term ipsilateral ischemic stroke risk and provides insight into the factors that are associated with stroke in the context of significant mortality in this high-risk population.
In addition, this study provides insight into the true stroke risk in this population because the competing risks model used in the outcome analysis can be more accurate than a traditional proportional hazards model, accounting for death and carotid intervention as competing events rather than censoring those patients who died or had intervention during the follow-up period. This difference is especially relevant in a patient population with many comorbidities and a correspondingly high mortality rate. The more commonly used Kaplan-Meier analysis of event rates treats death as a censoring event and incorrectly assumes that those who are censored have the same risk of stroke as those who are not, thereby overestimating the cumulative incidence of stroke.19,22
Data on outcomes of modern medical treatment for asymptomatic carotid disease are sparse. The largest prospective study in this regard was the Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) study,8 which enrolled 1121 patients with 50% to 99% stenosis from non-US centers from 1998 to 2002. This cohort was observed to have 130 ipsilateral events of which 59 were strokes. In addition, the study demonstrated an association between disease progression and risk of stroke.6 One critique of this study was the grouping of stenosis between 50% and 99% because conventional US practice and preoperative risk counseling subdivides this category as the Society of Radiologists in Ultrasound criteria delineates. In the current study, inclusion criteria specified a stenosis of 70% to 99% to qualify for study entry. Current practice is also informed by the deferred group of the Asymptomatic Carotid Surgery Trial 1 (ACST-1), which randomized patients from 1993-2003 with 60% to 90% stenosis to immediate or deferred treatment. Using survival analysis, that study observed a 10% risk of any nonperioperative stroke in the deferred group at 5 years.2
In contrast, there are more recent reports regarding the reduced risk of stroke. A 2009 meta-analysis showed a persistent decline in risk among asymptomatic patients in studies published after 1990 compared with the era of the randomized trials.4 The conclusion that the effects of contemporary medical therapy were now similar to the effects of procedural intervention for stroke risk reduction was controversial; there was concern about inclusion of moderate stenosis in the calculation of risk, which arguably diluted stroke risk among some patients who would not otherwise have been considered for intervention.23 The current study reports the stroke risk in a cohort of surgically relevant carotid stenoses using recent consensus criteria.24
In this cohort, the system-wide stroke reporting was standardized, leading to high fidelity in detecting outcome events. Stroke care in KPNC is regionalized, and all stroke cases are confirmed from both the medical record and billing perspectives as part of the core practice guidelines. In addition, the embedded nature of the electronic health records and the low disenrollment rate allowed comprehensive follow-up and granular assessment of associated variables. Liberal methods for estimating stroke rates were used, including unknown and likely lacunar strokes in the numerator when calculating ipsilateral stroke rates as there can be uncertainty about etiology. This category included patients without brain imaging or when 2 or more etiologies were clinically possible (eg, cardiac arrhythmia and carotid stenosis). Thus, the true stroke rate due to carotid disease may be lower than the presented estimates. The broad representation of the study cohort and the KPNC population in general implies that these results may be generalizable to insured patients in a similar geographic region in the US.
There were several associations between stenosis progression and arterial occlusion with the incidence of stroke. Proportional hazards modeling showed that a high-grade lesion at baseline or progression of a severe lesion to high-grade stenosis or occlusion was associated with an increased risk for stroke. Although this association has not been demonstrated in randomized clinical trials, these results echo findings from the ACSRS report.8 The implication of these findings suggests that the actual cohort of patients at higher risk for stroke and who therefore are reasonable candidates for intervention may be far smaller than current standards dictate.
The study design included time-dependent variations of risk-factor modification. During the time of Asymptomatic Carotid Artery Stenosis study and ACST-1 trial enrollments, there was no established protocol for risk factor reduction, nor was there widespread use of statins for lipid-lowering therapy and cardiovascular risk reduction. However, statin therapy is associated with reduced risk of stroke and cardiovascular outcomes in both primary and secondary prevention.25,26 KPNC has an established program to medically manage this high-risk population and has a robust tracking mechanism for medication adherence and other relevant parameters.27 Accordingly, details of antihypertensive and lipid-lowering medications, both at baseline and during follow-up, were included. Although adherence was imperfect, relevant indices (blood pressure and LDL) appeared generally well-controlled. In the multivariable model, any use of statin medication was significantly associated with a lower risk of stroke. Total mortality was high and likely reflects efforts to include all patients with carotid disease and not only patients who were deemed acceptable surgical candidates as one might see in an intervention-based study.
Limitations
This study has several limitations. First, there was not a practical method to assess raw imaging data as might be done in a clinical trial, nor was there comprehensive information on vascular laboratory accreditation or quality assurance during the study period. In addition, there was no available information regarding plaque characteristics, whether by ultrasound or magnetic resonance imaging, as is more customary in international practice.28
Second, aspirin use was not assessed since it is an over-the-counter medication and is not specifically tracked by pharmacy databases or the electronic health record.
Third, although stroke diagnosis coding in the electronic health record was found to be comprehensive, assessment of documented transient ischemic attacks was unreliable, and thus the effect of transient ischemic attack on cohort composition and outcome cannot be fully accounted for. Difficulty with transient ischemic attack diagnosis and documentation has been previously reported in this health system.29
Fourth, there is selection bias in a retrospective cohort study like this when intervention is applied for known and unknown reasons. Treatment decisions and resource utilization cannot be accounted for. This methodology allowed upstream analysis of all patients with abnormal carotid imaging and not just those referred to a specialty practice, capturing a broader group of patients. Accordingly, the differing characteristics outlined in eTable 1 (Supplement) between the group receiving intervention and those patients who did not receive intervention illustrate treatment strategy differences in the preferences of the patient and caregiver.
Fifth, although the inception of this cohort is now 12 to 13 years old, it likely remains relevant to the current era given that stroke care in the health system has not appreciably changed since the study outset.
Conclusions
In a community-based cohort of patients with asymptomatic severe carotid stenosis who did not undergo surgical intervention, the estimated rate of ipsilateral carotid-related acute ischemic stroke was 4.7% over 5 years. These findings may inform decision-making regarding surgical and medical treatment for patients with asymptomatic severe carotid artery stenosis.
eTable 1. Baseline Characteristics by Intervention Status (Per Unique Artery)
eTable 2. Predictors of Ipsilateral Acute Ischemic Stroke With Cox Regression Analysis
References:
- 1.Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273(18):1421-1428. doi: 10.1001/jama.1995.03520420037035 [DOI] [PubMed] [Google Scholar]
- 2.Halliday A, Harrison M, Hayter E, et al. ; Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group . 10-Year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376(9746):1074-1084. doi: 10.1016/S0140-6736(10)61197-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarenco P, Bogousslavsky J, Callahan A III, et al. ; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators . High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559. doi: 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 4.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40(10):e573-e583. doi: 10.1161/STROKEAHA.109.556068 [DOI] [PubMed] [Google Scholar]
- 5.den Hartog AG, Achterberg S, Moll FL, et al. ; SMART Study Group . Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke. 2013;44(4):1002-1007. doi: 10.1161/STROKEAHA.111.669267 [DOI] [PubMed] [Google Scholar]
- 6.Nicolaides AN, Kakkos SK, Kyriacou E, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52(6):1486-1496.e1-e5. doi: 10.1016/j.jvs.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 7.Conrad MF, Michalczyk MJ, Opalacz A, Patel VI, LaMuraglia GM, Cambria RP. The natural history of asymptomatic severe carotid artery stenosis. J Vasc Surg. 2014;60(5):1218-1226. doi: 10.1016/j.jvs.2014.05.047 [DOI] [PubMed] [Google Scholar]
- 8.Kakkos SK, Nicolaides AN, Charalambous I, et al. ; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group . Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg. 2014;59(4):956-967.e1. doi: 10.1016/j.jvs.2013.10.073 [DOI] [PubMed] [Google Scholar]
- 9.Gordon NP. Similarity of adult Kaiser Permanente members to the adult population in Kaiser Permanente’s Northern California service area: comparisons based on the 2017/2018 cycle of the California Health Interview Survey. November 8, 2020. Accessed December 14, 2021. https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/compare_kp_ncal_chis2017-18.pdf
- 10.Chang RW, Tucker L-Y, Rothenberg KA, et al. . Establishing a carotid artery stenosis disease cohort for comparative effectiveness research using natural language processing. J Vasc Surg. 2021;74(6):1937-1947.e3. doi: 10.1016/j.jvs.2021.05.054 [DOI] [PubMed] [Google Scholar]
- 11.Grant EG, Benson CB, Moneta GL, et al. ; Society of Radiologists in Ultrasound . Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis—Society of Radiologists in Ultrasound consensus conference. Ultrasound Q. 2003;19(4):190-198. doi: 10.1097/00013644-200312000-00005 [DOI] [PubMed] [Google Scholar]
- 12.Moneta GL, Edwards JM, Chitwood RW, et al. Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 99% internal carotid artery stenosis with duplex scanning. J Vasc Surg. 1993;17(1):152-157. doi: 10.1016/0741-5214(93)90019-I [DOI] [PubMed] [Google Scholar]
- 13.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. doi: 10.1097/MLR.0b013e3181589bb6 [DOI] [PubMed] [Google Scholar]
- 14.Wong J, Taljaard M, Forster AJ, Escobar GJ, van Walraven C. Derivation and validation of a model to predict daily risk of death in hospital. Med Care. 2011;49(8):734-743. doi: 10.1097/MLR.0b013e318215d266 [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206-213. doi: 10.1001/jamacardio.2018.4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams HP Jr, Biller J. Classification of subtypes of ischemic stroke: history of the trial of org 10172 in acute stroke treatment classification. Stroke. 2015;46(5):e114-e117. doi: 10.1161/STROKEAHA.114.007773 [DOI] [PubMed] [Google Scholar]
- 17.Easton JD, Saver JL, Albers GW, et al. ; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease . Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke. 2009;40(6):2276-2293. doi: 10.1161/STROKEAHA.108.192218 [DOI] [PubMed] [Google Scholar]
- 18.Rothenberg KA, Tucker LY, Gologorsky RC, et al. Long-term stroke risk with carotid endarterectomy in patients with severe carotid stenosis. J Vasc Surg. 2021;73(3):983-991. doi: 10.1016/j.jvs.2020.06.124 [DOI] [PubMed] [Google Scholar]
- 19.Huebner M, Wolkewitz M, Enriquez-Sarano M, Schumacher M. Competing risks need to be considered in survival analysis models for cardiovascular outcomes. J Thorac Cardiovasc Surg. 2017;153(6):1427-1431. doi: 10.1016/j.jtcvs.2016.12.039 [DOI] [PubMed] [Google Scholar]
- 20.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557-572. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 21.Schoenfield D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239-241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 22.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cambria RP. The endovascular revolution stopped at the carotid bifurcation … or did it? J Vasc Surg. 2012;56(6):1748-1760. doi: 10.1016/j.jvs.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 24.Howard VJ, Meschia JF, Lal BK, et al. ; CREST-2 study investigators . Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials. Int J Stroke. 2017;12(7):770-778. doi: 10.1177/1747493017706238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008-2024. doi: 10.1001/jama.2015.15629 [DOI] [PubMed] [Google Scholar]
- 26.Meschia JF, Bushnell C, Boden-Albala B, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension . Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754-3832. doi: 10.1161/STR.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155-2165. doi: 10.1056/NEJMoa0908610 [DOI] [PubMed] [Google Scholar]
- 28.Huibers A, de Borst GJ, Bulbulia R, Pan H, Halliday A; ACST-1 collaborative group . Plaque echolucency and the risk of ischaemic stroke in patients with asymptomatic carotid stenosis within the first Asymptomatic Carotid Surgery Trial (ACST-1). Eur J Vasc Endovasc Surg. 2016;51(5):616-621. doi: 10.1016/j.ejvs.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 29.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284(22):2901-2906. doi: 10.1001/jama.284.22.2901 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics by Intervention Status (Per Unique Artery)
eTable 2. Predictors of Ipsilateral Acute Ischemic Stroke With Cox Regression Analysis