Abstract
A bacterial strain capable of growing on cyclohexylamine (CHAM) was isolated by using enrichment and isolation techniques. The strain isolated, strain IH-35A, was classified as a member of the genus Brevibacterium. The results of growth and enzyme studies are consistent with degradation of CHAM via cyclohexanone (CHnone), 6-hexanolactone, 6-hydroxyhexanoate, and adipate. Cell extracts obtained from this strain grown on CHAM contained CHAM oxidase, and the model for CHAM oxidation by this enzyme was similar to the model for deamino oxidation of amine by amine oxidase.
Cyclohexylamine (CHAM) is widely used as an insecticide and antiseptic in various industries, and considerable attention has been devoted to possible environmental pollution by CHAM and CHAM toxicity to humans because CHAM is suspected of being a weak carcinogen (6, 10, 11).
CHAM is a fairly strong base (pKa 10.6) and therefore should be excreted mainly unchanged when it is administered to animals (16). In fact, the metabolism of CHAM has not been fully investigated. Renwick and Williams (12) reported that CHAM was excreted largely unchanged and that only 4 to 5% of CHAM was metabolized to cyclohexanol, trans-cyclohexane-1,2-diol, and trans-3-, cis-3-, trans-4-, and cis-4-aminocyclohexanols in rats, guinea pigs, and humans.
Microbial degradation of simple alicyclic compounds, such as cyclohexane, cyclohexanol, cyclohexane 1,2-diol, and cyclohexanecarboxylic acid, has been thoroughly investigated (1, 3, 4, 7, 9, 13–15). However, little is known about the degradation of CHAM by microorganisms.
In this paper, we describe the isolation and properties of a pure culture of a bacterium that grows on CHAM.
Isolation of the CHAM-utilizing bacterium.
An organism was isolated from a soil sample from Osaka Prefecture by selective enrichment by using minimal salts medium (MSM) containing yeast extract (100 mg per liter) and CHAM · HCl (1.0 g per liter). It was maintained on either liquid or solid mineral salts medium containing CHAM · HCl as the sole carbon source. MSM contained (per liter of distilled water) 1.0 g of NH4NO3, 1.5 g of KH2PO4, 1.5 g of Na2HPO4, 0.5 g of MgSO4 · 7H2O, 0.01 g of CaCl2 · 2H2O, 0.005 g of FeSO4 · 7H2O, and 0.002 g of MnSO4 · 4H2O. The isolate was a gram-positive, rod-shaped nonmotile, oxidase-negative, catalase-positive organism. This strain had the following additional characteristics: gelatin was liquified, nitrate was not reduced to nitrite, β-galactosidase was not produced, and esculin was hydrolyzed. Identification and classification of this strain as a member of Brevibacterium oxydans were confirmed by NCIMB Japan Co. Ltd. (Shizuoka, Japan); this strain was designated strain IH-35A.
Growth substrates.
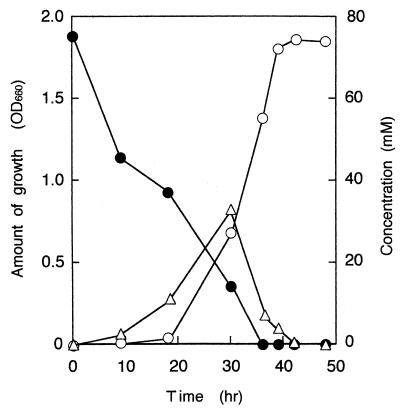
Growth of B. oxydans IH-35A on CHAM was tested in MSM (Fig. 1). This strain also grew well in nitrogen-free MSM (MSM without NH4NO3 and yeast extract) containing 1.0% (wt/vol) (73.7 mM) CHAM · HCl as the sole source of carbon, nitrogen, and energy. The ability of B. oxydans IH-35A to grow on various substrates was tested in liquid media containing the carbon sources at a concentration of 0.1% (wt/vol). It grew on N-methycyclohexylamine, cyclohexanone (CHnone), 2-chlorocyclohexanone, and 6-hexanolactone but did not grow on cyclohexanol, 4-aminocyclohexanol, or 2-, 3-, or 4-methylcyclohexanone. None of our 25 cyclohexanol-utilizing bacterial strains grew on CHAM.
FIG. 1.
Degradation of CHAM by B. oxydans IH-35. Increases in cell density (○) were determined photometrically at 660 nm. The disappearance of CHAM (●) was measured by GLC. The GLC analysis was carried out with a gas chromatograph (model GC-14A; Shimazu) equipped with a 30-m type DB-1 glass capillary column. The column was operated at 60°C, and the temperature was programmed to increase to 220°C at a rate of 10°C/min. The flow rate of the carrier gas, He, was 1.36 ml/min. The CHAM peak (retention time, 2.55 min) was detected with a flame ionization detector. Accumulation of CHnone (▵) was determined by HPLC as described in the text. OD660, optical density at 660 nm.
Transformation of CHAM by resting cell suspensions.
The amount of ammonia that accumulated in the medium during transformation of CHAM was measured by the method described by Fawcett and Scott (5). Cells of strain IH-35A grown in MSM were washed and suspended in 50 mM sodium-potassium phosphate buffer (pH 7.0) containing CHAM. Stoichiometric amounts of ammonia were released into the medium concomitant with degradation of CHAM, and then CHnone accumulated in the medium. In addition, cyclohexanol, trans-cyclohexane-1,2-diol, and 3- and 4-aminocyclohexanols were not detected in the medium (data not shown).
Enzyme activities in cell extracts of the CHAM-grown Brevibacterium strain.
To confirm the major routes of metabolism of CHAM by B. oxydans IH-35A, studies were performed with crude cell extracts. The cell extracts were prepared by ultrasonication with a Sonifire model 250 apparatus (Branson, Danbury, Conn.) by using six 20-s bursts on ice.
The enzymatic reaction product was determined by high-performance liquid chromatography (HPLC), gas-liquid chromatography (GLC), or thin-layer chromatography (TLC). HPLC was performed with a Capcell C18 column (length, 250 mm; diameter, 4.6 mm) by using methanol as the mobile phase at a flow rate of 1.0 ml/min. CHnone was detected by photometric detection at 280 nm and was identified by comparison with an authentic standard. For GLC, solutions of acidic reaction products in dry ether were methylated by using the procedure of Metcalf and Schmitz (8). GLC was performed with a column (2 m by 3 mm) that was packed with 10% (wt/wt) methylsilicone gum on Chromosorb W. The following conditions were used: carrier gas, H2 at a flow rate of 25 ml/min; oven temperature, 150°C; injection temperature, 170°C; and thermal conductivity detector temperature, 170°C. TLC of the 2,4-dinitrophenyl-hydrazine derivative was carried out on 0.25-mm-thick layers of Kieselgel F254 developed with solvent A (hexane-ethyl formate, 4/1 [vol/vol]) or with solvent B (benzene-tetrahydrofuran, 19/1 [vol/vol]). 2,4-Dinitrophenylhydrazone was detected by direct visual observation. TLC of carboxylic acids was carried out on 0.25-mm-thick layers of Kieselgel F254 developed with solvent C (benzene-ethyl acetate-formic acid, 25/25/2 [vol/vol/vol]) or with solvent D (benzene-dioxane-acetic acid, 40/8/4 [vol/vol/vol]). Carboxylic acids were detected by spraying thoroughly dried plates with 0.1% (wt/vol) bromocresol green in aqueous 95% (vol/vol) ethanol adjusted to pH 6.0 with NaOH.
Incubation of crude cell extracts (48,000-×-g supernatants) with CHAM demonstrated that CHAM-dependent stimulation of O2 consumption occurred. Incubation of a 48,000-×-g supernatant (1.26 mg of protein) with CHAM under aerobic conditions, followed by extraction and identification of the reaction product by TLC (Rf with solvent A, 0.69; Rf with solvent B, 0.58) and HPLC (retention time, 2.98 min), indicated that the product of this reaction was CHnone. When the reaction stoichiometry for CHAM was measured with a Warburg apparatus, ca. 0.5 μmol of O2 was consumed per μmol of CHAM oxidized (Table 1). Furthermore, investigation of the reaction stoichiometry demonstrated that oxidation of 1 μmol of CHAM was accompanied by the formation of ca. 1 μmol of NH3 and ca. 1 μmol of CHnone (Table 2).
TABLE 1.
Reaction stoichiometry for consumption of oxygen during deamination of CHAM by cell extracts of B. oxydans IH-35A
| Amt of CHAM added (μmol) | Amt of oxygen consumed (μmol) | CHAM added/oxygen consumed ratio |
|---|---|---|
| 2.50 | 1.28 | 0.51 |
| 5.00 | 2.48 | 0.50 |
| 10.00 | 4.48 | 0.45 |
The main compartments of Warburg flasks contained, in a volume of 2.0 ml, 450 μmol of sodium-potassium phosphate buffer (pH 7.2), 1.26 mg of 48,000-×-g supernatant, and CHAM. The center well contained 0.1 ml of 20% (wt/vol) KOH. Manometers were equilibrated at 30°C, and reactions were started by adding proteins from the side arms. The level of catalase activity in the 48,000-×-g supernatant was 3.18 U/mg. The data are averages of the data from three different experiments.
TABLE 2.
Reaction stoichiometry for production of CHnone and formation of ammonia during deamination of CHAM by cell extracts of B. oxydans IH-35Aa
| Amt of CHAM added (μmol) | Production of CHnone
|
Formation of ammonia
|
||
|---|---|---|---|---|
| Amt of CHnone produced (μmol) | CHAM added/ CHnone pro-duced ratio | Amt of NH3 formed (μmol) | CHAM added/ NH3 formed ratio | |
| 10.0 | 9.87 | 1.02 | ||
| 30.0 | 30.02 | 1.00 | ||
| 50.0 | 50.60 | 0.99 | ||
| 50.0b | 0 | |||
| 2.50 | 2.58 | 0.97 | ||
| 5.00 | 4.98 | 1.01 | ||
| 10.00 | 9.58 | 0.96 | ||
| 10.00b | 0 | |||
Each reaction mixture contained, in a volume of 1.0 ml, 450 μmol of sodium-potassium phosphate buffer (pH 7.2) and 1.26 mg of 48,000-×-g supernatant. Reactions at 30°C were started by adding limited amounts of CHAM. The CHnone concentrations were determined by HPLC. The NH3 concentrations were determined by the method of Fawcett and Scott (5). The data are averages of the data from three different experiments.
Boiled-extract control.
On the basis of these results, we presume that the model for CHAM oxidation by this enzyme is similar to the model for deamino oxidation of amine by amine oxidase, which catalyzes oxidative deamination of various amines to form the corresponding aldehydes, hydrogen peroxide, and ammonia according to the following equilibrium: R-CH2NH-R′ + H2O + O2 → R-CHO + R′-NH2 + H2O2, where R is an alkyl group or an aryl group and R′ is H or an aminoalkyl group. However, the production of H2O2 and the exact consumption of O2 are not clear because of catalase contained in the cell extracts of strain IH-35A.
Several methods for assaying activity relating to CHnone degradation were examined. When the 48,000-×-g supernatant was incubated with NADPH, CHnone-stimulated consumption of O2 occurred. No activity was detected when NADPH was replaced by NADH. An investigation of the reaction stoichiometry demonstrated that oxidation of 1 μmol of CHnone was accompanied by consumption of ca. 1 μmol of NADPH and ca. 1 μmol of O2, which is close to the stoichiometry theoretically required for a mixed-function oxygenase. The enzyme reaction product identified by TLC (Rf in solvent C, 0.37; Rf in solvent D, 0.59) and GLC (retention time, 3.50 min) was 6-hydroxyhexanoate. This observation is consistent with the formation of 6-hydroxyhexanoate from CHnone by cell extracts of cyclohexanol-grown Nocardia globerula CL1(9) or Acinetobacter sp. strain NCIMB 9871(4) or cyclohexane-grown Xanthobacter species (15).
The presence of a 6-hexanolactone hydrolase in the 48,000-×-g supernatant was demonstrated by incubation of the 48,000-×-g supernatant with 6-hexanolactone. The residual 6-hexanolactone content was measured by a procedure involving alkaline hydroxamate formation, followed by acidification conversion into the ferric hydroxamate and measurement of absorbance at 510 nm, as described by Cain (2). The expected product of the reaction, 6-hydroxyhexanoate, was identified by TLC (Rf with solvent C, 0.37; Rf with solvent D, 0.59) and GLC (retention time, 3.50 min) after diethylether extraction of a 1-h reaction mixture containing 200 μmol of 6-hexanolactone and 48,000-×-g supernatant (1.85 mg of protein).
The 48,000-×-g supernatant from CHAM-grown cells catalyzed the reduction of NADP+ in the presence of 6-hydroxyhexanoate. Addition of NAD+ to the reaction mixture did not result in any further increase in absorbance at 340 nm. The product of 6-hydroxyhexanoate oxidation cochromatographed with adipic acid in the TLC systems (Rf with solvent C, 0.47; Rf with solvent D, 0.70) described by Donoghue and Trudgill (4).
The results of our studies with cell extracts of strain IH-35A are consistent with the following main route for CHAM degradation: CHAM → CHnone → 6-hexanolactone → 6-hydroxyhexanoate →→ adipate. The dependence on monooxygenation for ring cleavage was based on the presence of a CHnone monooxygenase and a 6-hexanolactone hydrolase. Although strain IH-35A did not grow on cyclohexanol, CHnone is converted to adipate by enzymes in strain IH-35A cell extracts. When the CHnone degradative pathway is considered, this finding supports the results of Norris and Trudgill (9) and Donoghue and Trudgill (4) obtained for oxidation of cyclohexanol by N. globerula CL1 and Acinerobacter sp. strain NCIMB 9871.
Induction of the enzymes catalyzing CHAM oxidation.
A comparison of the activities of the enzymes responsible for CHAM metabolism in crude extracts of Brevibacterium cells grown on CHAM, CHnone, or succinate is shown in Table 3. The results clearly demonstrate that CHAM oxidase, CHnone monooxygenase, and 6-hexanolactone hydrolase activities are induced by growth of B. oxydans IH-35A on CHAM and are present in the cells at levels that are several dozenfold greater than the levels in an extract of succinate-grown cells. Furthermore, CHAM oxidase is not induced by growth on CHnone.
TABLE 3.
pH optima, cofactor specificities, and activities of enzymes involved in CHAM degradation by cell extracts of B. oxydans IH-35A after growth on CHAM, CHnone, and succinatea
| Enzymeb | pH opti-mumc | Cosubstrate specificity | Activity (μmol min−1 [mg of protein]−1)
|
||
|---|---|---|---|---|---|
| CHAM-grown cells | CHnone-grown cells | Succinate-grown cells | |||
| CHAM oxidase | 7.0–7.5 | None | 0.26 | <0.01 | <0.01 |
| CHnone monooxy-genase | 9.0–9.5 | NADPH | 0.75 | 0.83 | <0.01 |
| 6-Hexanolactone hydrolase | 7.2–7.4 | None | 15.63 | 14.27 | <0.01 |
| 6-Hydroxyhexanoate dehydrogenase | 7.0–7.5 | NADP | 0.02 | 0.02 | <0.01 |
The data are averages of the data from three different experiments.
CHAM oxidase activity was assayed by measuring the formation of NH3 accompanying the degradation of CHAM. CHnone monooxygenase activity was assayed by monitoring the CHnone-dependent oxidation of NADPH at 340 nm by the method of Norris and Trudgill (9). 6-Hexanolactone hydrolase activity was assayed by the method of Cain (2). 6-Hydroxyhexanoate dehydrogenase activity was assayed by measuring the increase in absorbance at 340 nm due to NAD+ reduction by using the method of Donoghue and Trudgill (4).
The effects of pH on enzyme-catalyzed reactions were determined by using five different buffer mixtures, each at a concentration of 20 mM, covering the pH range from 4 to 11. The buffers used were sodium acetate, sodium citrate, phosphate, Tris-HCl, and glycine-NaOH.
Finally, to the best of our knowledge, this is the first report of CHAM metabolism by a bacterium.
To gain a better understanding of degradation of CHAM by B. oxydans IH-35A, a biochemical and genetic characterization study of CHAM oxidase is in progress.
Acknowledgments
Financial support for this study was provided by Kansai University research grants (Grant-in-Aid for Joint Research, 1998) and by the Special Research Fund of The Institute of Industrial Technology, Kansai University.
REFERENCES
- 1.Anderson M S, Hall R A, Griffin M. Microbial metabolism of alicyclic hydrocarbons: cyclohexane catabolism by a pure strain of Pseudomonas sp. J Gen Microbiol. 1980;120:89–94. [Google Scholar]
- 2.Cain R B. The metabolism of protocatechuic acid by a Vibrio. Biochem J. 1961;79:298–312. doi: 10.1042/bj0790298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey J F, Trudgill P W. The metabolism of transcyclohexan-1,2-diol by an Acinetobacter species. Eur J Biochem. 1977;74:115–127. doi: 10.1111/j.1432-1033.1977.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 4.Donoghue N A, Trudgill P W. The metabolism of cyclohexanol by Acinetobacter NCIB 9871. Eur J Biochem. 1975;60:1–7. doi: 10.1111/j.1432-1033.1975.tb20968.x. [DOI] [PubMed] [Google Scholar]
- 5.Fawcett J K, Scott J E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroes R, Peters P W J, Berkvens J M, Verschuuren H G, De Vries T, Van Esch G J. Long term toxicity and reproduction study (including a teratogenicity study) with cyclamate, saccharin and cyclohexylamine. Toxicology. 1977;8:285–300. doi: 10.1016/0300-483x(77)90100-7. [DOI] [PubMed] [Google Scholar]
- 7.Magor A N, Warburton J, Trower M K, Griffin M. Comparative study of the ability of three Xanthobacter species to metabolize cycloalkanes. Appl Environ Microbiol. 1986;52:665–671. doi: 10.1128/aem.52.4.665-671.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metcalf L D, Schmitz A A. The rapid preparation of fatty acid esters for gas analysis. Anal Chem. 1961;33:363–364. [Google Scholar]
- 9.Norris D B, Trudgill P W. The metabolism of cyclohexanol by Nocardia globerula CL1. Biochem J. 1971;121:363–370. doi: 10.1042/bj1210363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen K W, Legator M S, Figge F H J. Dominant-lethal effects of cyclohexylamine in C57 B1/Fe mice. Mutat Res. 1972;14:126–129. doi: 10.1016/0027-5107(72)90116-9. [DOI] [PubMed] [Google Scholar]
- 11.Price J M, Biava C G, Oser B L, Vogin E E, Steinfeld J, Ley H L. Bladder tumors in rats fed cyclohexylamine or high doses of a mixture of cyclamate and saccharin. Science. 1970;167:1131–1132. doi: 10.1126/science.167.3921.1131. [DOI] [PubMed] [Google Scholar]
- 12.Renwick A G, Williams R T. The metabolites of cyclohexylamine in man and certain animals. Biochem J. 1972;129:857–867. doi: 10.1042/bj1290857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rho E M, Evans W C. The aerobic metabolism of cyclohexanecarboxylic acid by Acinetobacter anitratum. Biochem J. 1975;148:11–15. doi: 10.1042/bj1480011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stirling L A, Watkinson R J. Microbial metabolism of alicyclic hydrocarbons: isolation and properties of a cyclohexane-degrading bacterium. J Gen Microbiol. 1977;99:119–125. [Google Scholar]
- 15.Trower M K, Buckland R M, Higgings R, Griffin M. Isolation and characterization of a cyclohexane-metabolizing Xanthobacter sp. Appl Environ Microbiol. 1985;49:1282–1289. doi: 10.1128/aem.49.5.1282-1289.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams R T. The fate of cyclohexylamine in man and other species. In: Roe F J C, editor. Metabolic aspects of food safety. Oxford, United Kingdom: Blackwell Scientific Publications; 1970. pp. 230–231. [Google Scholar]