Abstract
Immunocompromised individuals are at high risk of poor coronavirus disease 2019 (COVID-19) outcomes and demonstrate a lower immune response to COVID-19 vaccines, including to the novel mRNA vaccines that have been shown to elicit high neutralizing antibody levels. This review synthesized available data on the immune response to COVID-19 and critically assessed mRNA COVID-19 vaccine immunogenicity in this vulnerable subpopulation. Patients with various immunocompromising conditions exhibit diverse responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and COVID-19 severity and mortality, and available vaccines elicit lower immune responses, particularly in solid organ transplant recipients. Strategies to improve vaccine responses in immunocompromised individuals are being implemented in vaccine recommendations, including the use of a third and fourth vaccine dose beyond the two-dose series. Additional doses may enhance vaccine effectiveness and help provide broad coverage against emerging SARS-CoV-2 variants. Continued investigation of vaccines and dosing regimens will help refine approaches to help protect this vulnerable subpopulation from COVID-19.
Keywords: COVID-19, COVID-19 vaccines, Immunocompromised individuals, Immunogenicity, SARS-CoV-2, Malignancy, Chronic inflammatory disease, Hemodialysis, Solid organ transplant recipients, Human immunodeficiency virus
Key Summary Points
| Despite prioritization of immunocompromised individuals within COVID-19 vaccine protocols, this population remains vulnerable to adverse effects of SARS-CoV-2 infection, including high morbidity and mortality. |
| Patients with various immunocompromising conditions exhibit diverse responses to SARS-CoV-2 infection, and available vaccines elicit lower immune responses, particularly in solid organ transplant recipients. |
| Additional COVID-19 vaccine doses may enhance vaccine effectiveness and protect against emerging SARS-CoV-2 variants. |
| Continued investigation of COVID-19 vaccine dosing and intervals, heterologous vaccination regimens, and breadth of coverage of emerging variants is needed to optimize vaccination recommendations to protect this vulnerable population. |
Introduction
Coronavirus disease 2019 (COVID-19) manifests in a range of outcomes, from asymptomatic infection to critical outcomes, including respiratory failure, septic shock, and death [1]. Among those who recover, long-term sequelae may occur, characterized by persistent symptoms or organ dysfunction.
Multiple COVID-19 vaccines across different platforms are under investigation; several are available via emergency use authorization (EUA), including two mRNA vaccines, BNT162b2 and mRNA-1273 (Table 1; [2–9]), which represent the first use of an mRNA vaccine platform outside of clinical trial settings [10–15]. BNT162b2 is now licensed in the United States for those ≥ 16 years old [16] and has EUA for prevention of COVID-19 in those 5–15 years old [17, 18]. mRNA-1273 recently received US licensure for those 18 years and older [19]. Both mRNA vaccines elicit strong humoral responses (i.e., neutralizing antibody production) and strong cellular responses via CD4+ and CD8+ T-cell induction and Th1 cytokine expression [20, 21]. mRNA vaccine-elicited neutralizing antibody titers (NATs) are the highest among the various vaccine platforms [22, 23]. High efficacy for both vaccines was shown in phase 3 trials, and real-world data from their pragmatic use have demonstrated high effectiveness, including against circulating variants [2, 6, 24–30].
Table 1.
Summary of mRNA COVID-19 vaccines
| Vaccine | Characteristics | Dosing | Reported populations |
|---|---|---|---|
| BNT162b2 [3–6, 8, 9] | Encodes the full length of the SARS-CoV-2 spike, modified by two proline mutations locking it in a prefusion conformation |
2 doses at a 21-day interval Third dose ≥ 28 days after dose 2 in immunocompromised individuals or solid organ transplant recipients 5 years and older Booster dose ≥ 5 months after primary series in individuals 12 years and older (18 and older for heterologous booster; same interval and dose) |
5 years and older Healthy or with stable chronic medical conditions (e.g., HIV, HBV, HCV infection)a |
| mRNA-1273 [2, 7] | LNP-encapsulated and expressing the prefusion-stabilized spike |
2 doses at a 1-month interval Third dose ≥ 1 month after dose 2 in immunocompromised individuals or solid organ transplant recipients Booster dose ≥ 5 months after primary series (same interval and dose for heterologous booster) |
18 years and older In a medically stable condition |
In those with immunocompromising conditions, immune responses to infection, treatment, and vaccination are often altered [31]. Data on COVID-19 outcomes in this population are emerging. Results from a UK study of 17 million patients from 2020 suggest that immunocompromised individuals have increased risk of COVID-19–related death [32]. Case reports also indicate that immunocompromised individuals have higher risk of prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, shedding, and viral evolution [33–36]. The severity of COVID-19 outcomes in this population suggests that cytokine storm–mediated effects are occurring in infected patients, although immunosuppression leading to a depressed immunosuppressive immunologic phenotype may be an alternative explanation for this observation [37]. Additionally, because immunocompromised individuals were excluded from initial COVID-19 vaccine trials, vaccination responses in this population are being investigated in observational studies. Therefore, this review synthesized available data on the immune response to COVID-19 and, using a targeted literature search, critically assessed mRNA COVID-19 vaccine immunogenicity in immunocompromised individuals, and considers the available tolerability and safety data from these studies. Data supporting the benefit of additional vaccine doses beyond the two-dose series used initially in this population are also reviewed.
Methods
A targeted search of PubMed and preprint servers (medRxiv and SSRN) was conducted on May 10, 2021, using the following search terms: (coronavirus OR “Severe acute respiratory syndrome” OR covid-19 OR nCoV OR COVID OR SARS OR MERS OR middle east respiratory syndrome) AND (“immune disorders” OR “immunocompromised condition” OR “autoimmune disease” OR “autoimmune disorder” OR “immunosuppressive therapy” OR “immunodeficienc*” OR “chronic inflammatory disease” OR “hematologic malignancy” OR “cancer” OR “hemodialysis” OR transplant OR HIV-1). Reviews were excluded. The following data were extracted from the identified publications: article type, study population (e.g., immunocompromised condition), country, vaccine used (if applicable; type, dosing, and interval), treatments, main immunologic findings (e.g., anti-spike protein immunoglobulin G [IgG] titers, neutralizing antibodies, and cell response), safety and tolerability profile of the vaccine, and any other relevant findings.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Outcomes in Specific Immunocompromised Conditions
Malignancy
COVID-19 Characteristics
Large studies and systematic assessments have synthesized the available literature on COVID-19 characteristics in cancer patients. In a US and European community-based survey that included 20,000 cancer patients, risk of COVID-19 test positivity or hospitalization was 60% greater in cancer patients; associations between cancer and COVID-19 increased among older individuals, men, and those receiving cancer treatment [38]. Other analyses support these findings, including reports of increased risk of severe disease, intensive care unit (ICU) admission, and mortality in cancer patients with COVID-19 [39–42]. The type of cancer may also confer higher risk of COVID-19-associated death, particularly among patients with lung or hematologic malignancies [31, 40, 42]. Worse outcomes may be associated with recent receipt of cancer therapy, patient age, and male sex.
Vaccination Response
Four studies evaluating COVID-19 vaccine immune responses in 348 patients with hematologic malignancies, including multiple myeloma (MM), chronic lymphocytic leukemia (CLL), and myeloproliferative neoplasms (MPNs), were identified (Table 2) [43–46]. Responses were evaluated after two BNT162b2, mRNA-1273, or AZD1222 (a COVID-19 adenovirus vaccine) doses (2 studies; assessed 2–3 weeks post-dose) or one BNT162b2 dose (2 studies; assessed 21 days post-dose). All studies measured IgG-anti-spike protein levels; one also evaluated NATs and T-cell responses.
Table 2.
Overview of studies assessing COVID-19 vaccine responses among immunocompromised patients
| Reference | Country | Population | Men, % | Median age, years | Therapeutic regimena | Vaccine | Timing of serology assessment | Immunoassays | Antibody response | Vaccine safety |
|---|---|---|---|---|---|---|---|---|---|---|
| Hematologic malignancies | ||||||||||
| Agha et al. [43] | USA | Patients with various hematologic malignancies (n = 67); most commonly MM (43%) | 52 | 71 |
Cancer-directed therapy within 3 months of treatment Observation: 57% Active treatment: 43% |
BNT162b2 mRNA-1273 |
PD2 (time period NR) | IgG-anti-spike protein levels | 54% (PD2) | NR |
| Bird et al. [44] | UK | Patients with MM (n = 93) | 59 | 67 |
On therapy: 71% Most common therapy Immunomodulatory drug: 47% PI: 19% Steroid: 45% Anti-CD38 mAb: 23% Other: 11% |
BNT162b2 AZ1222 |
≥ 21 days PD1 | IgG-anti-spike protein levels | 56% (PD1) | NR |
| Herishanu et al. [45] | Israel | Patients with CLL (n = 167) | 67 | 71 |
Protocol of currently treated patients BTKi: 67% Venetoclax ± anti-CD20 mAb: 29% Other: 4% |
BNT162b2 | 2–3 weeks PD2 | IgG-anti-spike protein levels | 40% (PD2) |
Mild local reactions: 31% PD1; 34% PD2 (most commonly injection site pain) Mild systemic events: 13% PD1; 23% PD2 (most commonly weakness) No significant differences in AE rates between patients actively treated vs. not actively treated at time of vaccination |
| Harrington et al. [46] | UK | Patients with myeloproliferative neoplasms (n = 21) | 33 | 58 |
Peg-IFN: 19% Surveillance: 33% Hydroxycarbamide: 19% Ruxolitinib: 29% |
BNT162b2 | 21 days PD1 |
IgG-anti-spike protein levels Neutralizing antibodies Cell response |
86% (PD1) |
Localized inflammation: 57% Systemic events: 48% |
| Solid tumor malignancies | ||||||||||
| Barrière et al. [47] | France | Patients with solid tumors (n = 122); HV (n = 29) | 53 | 69 | Chemotherapy ± targeted therapy: 86% | BNT162b2 | 3–4 weeks PD2 | IgG-anti-spike protein levels |
48% (PD1) 95% (PD2) |
No SAE reported |
| Palich et al. [48] | France | Patients with solid tumors (n = 110), which was most commonly breast (34%); HV (n = 25) | 40 | 66 |
Chemotherapy: 35% Targeted therapy: 24% Immunotherapy: 16% Hormonal therapy: 15% Radiotherapy: 6% Surveillance: 16% |
BNT162b2 | 4 weeks PD1 | IgG-anti-spike protein levels | 58% (PD1) | NR |
| Chronic inflammatory diseases | ||||||||||
| Geisen et al. [52] | Germany | Patients with CID (n = 26); HVs (n = 42) | 36 | 50.5 |
Biological DMARD: 77% Conventional DMARD: 31% Corticosteroid: 27% |
BNT162b2 mRNA-1273 |
7 days PD2 |
IgG-anti-spike protein levels Neutralizing antibodies |
87% (PD2) | Although side effects were comparable in study groups, patients with CID had marginal propensity towards mild AEs vs. HVs |
| Braun-Moscovici et al. [53] | Israel | Patients with CID (n = 264 vaccinated); most commonly RA (37%) | 24 | 57.6 |
Biological DMARD: 67% Conventional DMARD: 61% Colchicine: 0.2% Nintedanib: 0.1% Corticosteroid: 35% |
BNT162b2 | 4–6 weeks PD2 | IgG-anti-spike protein levels | 86% (PD2) |
Local reactions: 58% Fatigue: 30% Myalgia: 12% Headache: 20% Low-grade fever: 3% |
| Deepak et al. [54] | USA | Patients with CID (n = 133) compared with immunocompetent controls (n = 53); most commonly IBD (32%) and RA (29%) | 26 | 45.5b |
Biologic therapy: 59% Conventional DMARD: 63% Prednisone: 13% Janus kinase inhibitors: 8% NSAID: 20% No immunosuppression: 7% |
BNT162b2 mRNA-1273 |
Within 20 days PD2 |
IgG-anti-spike protein levels Neutralizing antibodies |
89% (PD2) | NR |
| Boyarsky et al. [55] | USA | Patients with rheumatic and musculoskeletal diseases (n = 123); most commonly inflammatory arthritis (28%) | 5 | 50 |
None: 28% Conventional DMARD: 19% Biologic DMARD: 14% Corticosteroid monotherapy: 3% Combination therapy: 37% |
BNT162b2 mRNA-1273 |
Median 22 days PD1 | IgG-anti-spike protein levels | 74% (PD1) | NR |
| Wong et al. [56] | USA | IBD (n = 48) | 48 | 35.2 (mean) |
Biologics: 85% Oral corticosteroid: 6% Non therapy: 10% |
BNT162b2 mRNA-1273 |
NR | IgG-anti-spike protein levels |
67% (PD1) 100% (PD2) |
NR |
| Hemodialysis | ||||||||||
| Schrezenmeier et al. [61] | Germany | Dialysis patients with stage 5 CKD (vaccinated, n = 36; unvaccinated n = 18); controls (n = 44) | 69 | 74 | NR | BNT162b2 | 1–10 weeks PD2 |
IgG-anti-spike protein levels Neutralizing antibodies Cell response |
56% (1 week PD2) 89% (3 week PD2) |
NR |
| Goupil et al. [62] | Canada | Hemodialysis patients (n = 154); controls (n = 40)b | 63 | 70–76 (mean) | Immunosuppressive medications: 16% | BNT162b2 | 4 weeks PD1 | IgG-anti-spike protein levels |
43% (PD1; without prior SARS-CoV-2 infection) 84% (PD1; with prior SARS-CoV-2 infection) |
NR |
| Simon et al. [63] | Austria | Hemodialysis patients (n = 81); controls (n = 80) | 55 | 67 (mean) |
RAAS inhibitor: 43% Immunosuppressants: 11% Vitamin D: 75% EPO: 91% |
BNT162b2 | 3 weeks PD2 | Neutralizing antibodies | 53% (PD2) |
No grade 4 AEs Hemodialysis patients had significantly fewer local and systemic events compared with control group |
| Grupper et al. [64] | Israel | Hemodialysis patients (n = 56); HV (n = 95) | 75 | 74 | NR | BNT162b2 | PD2 | IgG-anti-spike protein levels | 96% (PD2) | NR |
| Solid organ transplant | ||||||||||
| Boyarsky et al. [75] | USA | SOT (n = 436) | 39 | 55.9 |
Tacrolimus: 83% Corticosteroids: 54% Mycophenolate: 66% Azathioprine: 9% Sirolimus: 4% Everolimus: 2% |
BNT162b2 mRNA-1273 |
17–24 days PD1 | Anti-spike protein levels | 17% (PD1) | NR |
| Sattler et al. [76] | Germany | Kidney transplant (n = 39); HV (n = 39)c | 72 | 57.4 (mean) |
CS + TAC + MMF: 56% CS + CyA + MMF: 33% mTORi + MMF ± CS: 8% mTORi + CyA + MMF: 3% CMV seropositive pre-treatment: 67% |
BNT162b2 | 7–22 days PD2 |
IgG-anti-spike protein levels Neutralizing antibodies Cell response |
3% (PD2) | NR |
| Itzhaki Ben Zadok et al. [77] | Israel | Heart transplant (n = 42) | 83 | 61 |
Calcineurin inhibitor: 81% mTOR inhibitor: 57% Oral steroid: 69% Anti-metabolite: 55% |
BNT162b2 | 14–19 days PD2 | IgG-anti-spike protein levels |
15% (PD1) 49% (PD2) |
Injection site pain: 71% Fatigue: 14% Arthralgia: 12% Myalgia: 10% |
| Basic-Jukic and Ivo [78] | Croatia | Kidney transplant (< 50 patients received vaccination at the time of the report) | NR | NR | NR | BNT162b2 | N/A | NR | NR | NR |
| Rusk et al. [79] | USA | Case report of patient with bilateral lung transplant (n = 1) | 0 | 55 | Various post-transplant medications including prednisone, mycophenolate, tacrolimus, and alendronate | BNT162b2 | 16 days PD2 | IgG-anti-spike protein levels | 0% (PD2) | Mild injection site pain PD1 and PD2 |
| Grupper et al. [80] | Israel | Kidney transplant (n = 136); HV (n = 25) | 82 | 58.6 (mean) |
Calcineurin inhibitor backbone: 90% Prednisone: 89% MMF: 76% Combination regimen: 79% Prednisone + tacrolimus + MMF: 60% |
BNT162b2 | 10–20 days PD2 | IgG-anti-spike protein levels | 38% (PD2) |
Local pain: 52% Systemic symptoms: 19% |
| Rozen-Zvi et al. [81] | Israel | Kidney transplant (n = 308) | 64 | 57.5 |
Mycophenolic acid: 73% Tacrolimus: 93% Cyclosporine: 8% mTOR inhibitor: 8% Corticosteroid: 8% Rituximab: 2% Antithymocyte globulin: 5% |
BNT162b2 | 4 weeks PD2 | IgG-anti-spike protein levels | 36% (PD2) | NR |
| Narasimhan et al. [82] | USA | Lung transplant (n = 73) | 74 | 65 | Anti-metabolite: 99% |
BNT162b2 mRNA-1273 |
Up to ~ 50 days PD2 |
IgG-anti-spike protein levels Cell response |
25% (PD2) | NR |
| Peled et al. [83] | Israel | Heart transplant (n = 77) | 65 | 62 |
Mycophenolic acid: 75% Everolimus: 26% Prednisone: 75% |
BNT162b2 | 3 weeks PD2 |
IgG-anti-spike protein levels Neutralizing antibodies |
18% (PD2) |
Local reaction: 49–56% (most commonly pain) Systemic event: 37–49% (most commonly fatigue or headache) |
| Rincon-Arevalo et al. [84] | Germany | Kidney transplant (n = 40); HV (n = 35)d | 70 | 62 |
MMF: 98% Steroid: 93% Calcineurin inhibitor: 93% |
BNT162b2 | 7 days PD2 |
IgG-anti-spike protein levels Neutralizing antibodies Cell response |
2.5% (PD2) | NR |
| HIV | ||||||||||
| Levy et al. [94] | Israel | PLWH (n = 143); HV (n = 261) | 92 | 49.8 (mean) | ART: 100% | BNT162b2 | 2–3 weeks PD2 |
IgG-anti-spike protein levels Neutralizing antibodies |
97% (PD2) |
Local reaction: 26–41% (most commonly mild pain) Systemic event: 20–48% (most commonly fatigue and headache PD1 and fatigue and fever PD2) |
AE adverse event, ART antiretroviral therapy, BTKi Bruton’s tyrosine kinase inhibitor, CID chronic inflammatory disease, CKD chronic kidney disease, CLL chronic lymphocytic leukemia, COVID-19 coronavirus disease 2019, CS + CyA + MMF corticosteroids + cyclosporin A + mycophenolate mofetil, CS + Tac + MMF corticosteroids + tacrolimus + mycophenolate mofetil, DMARD disease-modifying antirheumatic drug, EC50 50% effective concentration, EPO erythropoietin, HV healthy volunteer, IBD inflammatory bowel disease, IgG immunoglobulin G, mTORi + CyA + MMF mTOR inhibitor + cyclosporin A + mycophenolate mofetil, mTORi + MMF ± CS mTOR inhibitor + mycophenolate mofetil ± corticosteroids, N/A not applicable, NR not reported, NSAID nonsteroidal anti-inflammatory drug, mAb monoclonal antibody, MM multiple myeloma, MMF mycophenolate mofetil or mycophenolate sodium, mTOR mammalian target of rapamycin, PD1 post-dose 1, PD2 post-dose 2, Peg-IFN pegylated interferon, PI protease inhibitor, PLWH people living with HIV, RA rheumatoid arthritis, RAAS renin–angiotensin–aldosterone system, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, SAE serious adverse event, SOT solid organ transplant, TKI tyrosine kinase inhibitor
aMay not total 100% because patients could receive ≥ 1 class of treatment
bIncluded participants with and without prior SARS-CoV-2 infection
cStudy also included 26 patients with kidney failure undergoing hemodialysis
dStudy also included patients on maintenance hemodialysis (n = 41), and peritoneal dialysis (n = 4)
After two mRNA vaccine doses, humoral responses ranged from 40% in CLL patients to 54% in patients with various hematologic malignancies [43, 45]. In a study in CLL patients that included comparison to sex- and age-matched healthy controls (HCs), antibody-mediated response to mRNA vaccination was significantly reduced in patients (52% vs. 100%; adjusted odds ratio [OR] = 0.010; P < 0.001) [45]. In the study assessing NATs and cellular responses, 86% of MPN patients had positive neutralizing antibodies and 80% memory T-cell responses, with CD4+ and CD8+ T-cell responses in 75% and 35% of patients, respectively, after one BNT162b2 dose [46]. In this study, no significant differences in humoral or T-cell responses were observed between those receiving active treatment and those undergoing active surveillance.
Two studies comprising a total of 232 patients with solid tumors were identified; both compared BNT162b2 responses to HCs (Table 2) [47, 48]. Responses were evaluated after one dose (1 study; assessed 4 weeks post-dose) or two doses (1 study; assessed 3–4 weeks post-dose); both only evaluated IgG-anti-spike protein levels. Across both studies, approximately 50% (58/122; 64/110) of patients had IgG-anti-spike protein responses post-dose 1. In the study assessing response post-dose 2, the percentage of responders among evaluable patients increased to 95% (n = 40) [47]. After adjustment for potential confounders, one study found older age (OR = 3.58; P = 0.008) and chemotherapy receipt (OR = 4.34; P = 0.003) were strongly associated with lack of seroconversion among patients [48].
Three studies in patients with malignancies reported on the safety and tolerability profile of BNT162b2 [45–47]. In a study in patients with CLL who received BNT162b2, mild local reactions were reported in 31% and 34% of patients after dose 1 and 2, respectively, and were most commonly related to injection site pain [45]. Systemic events were reported in 13% and 23% of patients after dose 1 and 2, respectively, and were most commonly related to weakness. No significant differences in adverse event (AE) frequencies were observed between patients receiving active CLL treatment compared with those who were not. Finally, in a study in patients with MPNs, localized inflammation was reported in 57% and systemic events (e.g., flu-like illness, fatigue, gastrointestinal symptoms) in 48% of patients after receipt of one BNT162b2 dose [46]. In a study in patients with various solid tumors, no serious AEs (SAEs) were reported among BNT162b2 recipients [47].
Chronic Inflammatory Diseases
COVID-19 Characteristics
Chronic inflammatory diseases (CIDs) are associated with increased infection risk, although immunosuppressive treatments might limit the cytokine storm associated with severe COVID-19 [49]. Consistent with this hypothesis, a cohort of 6792 US patients with and without CIDs found that CID patients with COVID-19 were more likely to be hospitalized, but having CID was not an independent risk factor for severe COVID-19 [49]. Patients receiving corticosteroids had increased severe disease risk, while biologic or non-biologic immunosuppressive treatments were not significantly associated with this risk. Consistent with this finding, a prospective study of 103 inflammatory arthritis (IA) patients found that COVID-19 outcomes were worse in those receiving corticosteroids compared with those receiving anti-cytokines [50]. The type of CID might also be associated with severe COVID-19. In a study of 228 hospitalized patients with IA or connective tissue diseases, the latter was associated with severe disease and poor outcomes [51].
Vaccination Response
Five studies describing immunogenicity responses in 594 CID patients receiving mRNA vaccines were identified; a range of CIDs were included, most commonly rheumatoid arthritis, IA, and inflammatory bowel disease (IBD) (Table 2) [52–56]. Immune responses were evaluated after two BNT162b2 or mRNA-1273 doses (4 studies; assessed 1–6 weeks post-dose) or one BNT162b2 or mRNA-1273 dose (1 study; assessed ~ 3 weeks post-dose). All studies evaluated IgG-anti-spike protein levels; two evaluated NATs. Cellular immune responses were not assessed.
Humoral responses in CID patients were observed after one (67–74%) or two (86–100%) doses [52–56]. In two studies that included HCs, IgG-anti-spike protein levels and NATs were lower in CID patients versus HCs, with B-cell–depleting and corticosteroid therapies associated with reduced responses [52, 54]. In patients who received B-cell–depleting therapies, the lack of vaccine response was predominantly observed in those who had recently received therapy. One study of patients with IA treated with disease-modifying antirheumatic drugs (DMARDs) found that B-cell–depleting treatments were associated with significantly lower immune responses to vaccination [53]. Two other studies also analyzed immune responses to vaccination according to CID treatment. In patients with CID who received BNT162b2 or mRNA-1273, 94% of those receiving anti-tumor necrosis factor (TNF) therapy had immune responses to vaccination [55]. In a study in patients with IBD, receipt of anti-TNF or vedolizumab therapy was associated with decreased humoral responses compared with HCs (anti-TNF: anti–receptor-binding domain [RBD] total immunoglobulin; vedolizumab: anti-RBD total immunoglobulin, anti-RBD IgG, and anti-spike protein IgG) [56].
Two studies in patients with CID described the safety and tolerability profile after vaccination [52, 53]. In one study, 58% of patients experienced a local reaction, and fatigue (30%) and headache (20%) were the most common systemic events after receipt of BNT162b2 [53]. One patient reported new-onset arthritis 2 months after vaccination, but no patients experienced a flare of inflammatory rheumatic disease at up to 2 months of follow-up. In the second study, in which patients received either BNT162b2 or mRNA-1273, patients with CID experienced generally milder AEs compared with HCs, with mild fatigue (54% vs. 43%) and myalgia (42% vs. 32%) more frequent in patients, and fever more common in HCs (0% vs. 14%) [52].
Hemodialysis
COVID-19 Characteristics
Hemodialysis recipients may be at greater COVID-19 risk because of immunosuppression and the older age, comorbidities, and frequent healthcare visits common in this population [57–59]. In a European registry study of 4298 kidney transplant and dialysis patients, COVID-19 incidence was slightly increased in dialysis patients versus the general population, which was attributed to the older age or more frequent SARS-CoV-2 testing undertaken in patients [59]. Other studies support these findings. A systematic review of 396,062 hemodialysis patients (COVID-19 cases, n = 3261) found a 15-fold increase in COVID-19 incidence and greater mortality risk in patients versus the general population [57]. Mortality risk may be lower in hemodialysis patients from Asia, which is potentially associated with their younger age and diagnostic approaches in this region allowing for timely intervention [57]. In another systematic review of > 1.1 million patients with chronic kidney disease (CKD), COVID-19 incidence was higher in patients receiving maintenance dialysis [60].
Vaccination Response
Four studies assessed responses to BNT162b2 vaccination in 325 hemodialysis recipients (Table 2) [61–64]. All included comparator groups (vaccinated vs. unvaccinated patients or HCs). Three reported immune responses post-dose 2 (assessed 1–10 weeks post-dose); the fourth assessed responses 4 weeks post-dose 1. All evaluated IgG-anti-spike protein levels. One study also assessed NATs and cellular immune responses.
Humoral responses in hemodialysis recipients were 43–84% and 53–96% after one and two BNT162b2 doses, respectively; responses were lower in patients versus HCs [61–64]. One study of 154 patients receiving hemodialysis found that humoral responses were dependent on past SARS-CoV-2 infection, with patients previously infected mounting a delayed antibody response post-dose 1 [62]. Delayed responses were also reported in some patients who received two doses [63]. In this study, men had lower median titers versus women, while increasing age was inversely associated with titer level. In a study of two doses, the neutralizing antibody capacity was 78% (28/36) and SARS-CoV-2 T cells were detected in 68% of patients (21/31) post-dose 2, supportive of vaccine-induced B- and T-cell responses [61]. Another study found older age to be significantly associated with decreased antibody levels in hemodialysis recipients [64]. One study found that receipt of immunosuppressive medication did not appear to affect the immune response to vaccination [62].
One study in hemodialysis recipients described the safety and tolerability profile of BNT162b2 [63]. No grade 4 AEs were reported, and hemodialysis recipients had significantly fewer local and systemic events compared with HCs after either vaccine dose.
Solid Organ Transplant Recipients
COVID-19 Characteristics
The weakened T-cell–mediated immune response of solid organ transplant recipients (SOTRs) makes them vulnerable to many viral infections, although increased vulnerability to Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) has not been reported [65]. Immunosuppressive treatments and comorbidities common in this population may also increase vulnerability to infection, causing substantial morbidity and mortality [66, 67]. Systematic reviews have assessed morbidity in SOTRs. One that included 22 studies found that among SOTRs with COVID-19, 81% were hospitalized, of which 29% required ICU admission and 26% mechanical ventilation [65]. These rates are consistent with those in kidney and liver transplant recipients [68–70]. Among 415 heart transplant recipients, 34% had severe and 14% critical COVID-19, while 23% required ICU admission and 16% mechanical ventilation [71]. In a systematic review of SOTRs that included 37 articles, all-cause mortality was 19%, which varied by transplant type [65]. However, mortality is generally consistent across transplantation type, including kidney and liver transplant recipients [68–70, 72, 73]. COVID-19–associated mortality of 24–26% was reported in two studies of 39 and 415 heart transplant recipients, respectively; diabetes mellitus and stage 3 or higher CKD were associated with increased mortality in one study [71, 74]. A systematic review in 5559 kidney transplant recipients found those who died from COVID-19 were older, had a shorter time since transplantation, or were living in Asia–Pacific or Europe (vs. the United States) [68].
Vaccination Response
Nine studies and one case report of vaccine responses in approximately 1200 SOTRs were identified and included kidney, heart, and lung transplantation recipients (Table 2) [75–84]. Responses were evaluated after two (8 studies; assessed 7 to ~ 50 days post-dose) or one dose (1 study; assessed 17–24 days post-dose) of BNT162b2 or mRNA-1273; one study did not specify schedule or response period. Nine evaluated IgG-anti-spike protein levels, three NATs, and three cellular responses.
Among these studies, < 50% of SOTRs had immune response to vaccination, with limited development of neutralizing antibodies and diminished cellular responses [75–77, 79, 81–84]. Two patients developed COVID-19 after the second BNT162b2 dose in a study of < 50 kidney transplant recipients [78]. Patient age, longer maintenance dialysis duration and lower estimated glomerular filtration rate (for kidney transplant recipients) pre-transplantation, and specific COVID-19 vaccine administered were factors associated with poor responses [75, 77, 80, 81, 83]. Factors associated with poor responses also included use of maintenance antimetabolite immunosuppressive therapy and type of immunosuppressant regimen received [75–77, 80, 81, 83]. In two studies, those receiving antimetabolite immunosuppressive therapy were less likely to develop an antibody response [75, 77]. In two studies in kidney transplant recipients, multivariate analyses found receipt of high-dose corticosteroids, maintenance triple immunosuppressive medications, mycophenolate mofetil (MMF)–containing regimens, and mammalian target of rapamycin (mTOR) inhibitors were associated with poor humoral responses [80, 81]. Another study in heart transplant recipients found that patients with a high antibody response to vaccination had a lower use of MMF and higher use of mTOR inhibitors compared with patients with low antibody responses [83]. However, a study in kidney transplant recipients found immune responses did not differ significantly between patients receiving tacrolimus and cyclosporin A [76].
Three studies in SOTRs described safety and tolerability after BNT162b2 administration [77, 79, 83]. In two studies in heart transplant recipients, local reactions occurred in 49–76% and systemic events in 37–49% of patients, which were most commonly injection site pain, fatigue, headache, arthralgia, and myalgia [77, 83]. In a study in kidney transplant recipients, local pain was experienced by 52% and systemic symptoms by 19% of patients after vaccination, and there was a similar reactogenicity rate between patients and HCs [80].
HIV
COVID-19 Characteristics
Because HIV infection is associated with abnormal humoral and T-cell–mediated immune responses, people living with HIV (PLWH) may be at increased risk of adverse COVID-19 outcomes [85, 86]. However, determining effects of SARS-CoV-2 infection in PLWH is complicated by the potential benefit of antiretroviral (ART) and protease inhibitor therapies, some of which have been investigated as COVID-19 treatments; PLWH may also be less susceptible to the cytokine storm associated with severe COVID-19 because of dysfunctional immune systems [87]. Systematic reviews have assessed epidemiology and effects of COVID-19 in PLWH. Two systematic reviews with approximately 850,000 PLWH reported 0.7–0.9% COVID-19 and 5.3–8.8% mortality rates; the hospitalization rate was 28%, which included 2.5% who were severe-critical and 3.5% requiring ICU care [88, 89]. Some systematic reviews found no apparent correlation with concurrent HIV and SARS-CoV-2 infection and severe disease and adverse outcomes, although conflicting findings were reported [85, 86, 89–91]. One review of > 89,000 patients with coinfection found those with advanced HIV stages had less severe COVID-19 and lower mortality versus patients with earlier stages, although some studies provided conflicting findings [92]. Other systematic reviews, which included 23–63 studies, found that men with coinfection had higher morbidity and mortality; higher morbidity and mortality from COVID-19 was also reported in patients who had multiple comorbidities, were older, or had high viral load [85, 87, 93]. In other systematic reviews, which included 14–82 studies, the effect of ART in reducing risk of infection and COVID-19–associated mortality was inconclusive [87–89, 91].
Vaccination Response
One study was identified assessing response to BNT162b2 in PLWH, which evaluated IgG-anti-spike protein and neutralizing antibody responses (Table 2) [94]. Immunocompetent healthcare workers were included as controls; responses were assessed 2–3 weeks post-dose 2. Overall, 18% (26/143) of patients had AIDS and all were receiving ART. The mean (range) of nadir CD4+ T cells was 345 (2–900)/µL, and 10% and 18% of PLWH had nadir CD4+ T cells < 100 cells/µL and < 200 cells/µL, respectively.
In this study, anti–receptor-binding domain IgG was positive and NATs developed in 97% of patients each after dose 2 [94]. In an adjusted linear regression analysis, PLWH had significantly lower IgG concentrations than HCs (P = 0.008). The population included in the study had a high CD4+ cell count at baseline; of the three patients with a CD4+ count < 200 cells/µL, all had high immune responses to BNT162b2. However, a statistically significant decrease from baseline in CD4+ counts after dose 1 and 2 (P < 0.0001) was found, which persisted up to 4 months after dose 2 and was not associated with clinical changes.
In PLWH, local reactions were more common after the first BNT162b2 dose (41% vs. 26%), while systemic events were more common after the second dose (20% vs. 48%) [94]. Injection site pain was the most common local reaction, which was mild and resolved within 24 h. Fatigue and headache were the most common systemic events after dose 1, and fatigue and fever after dose 2. No immediate or delayed hypersensitivity reactions were observed.
Additional COVID-19 Vaccine Doses for Immunocompromised Individuals
With COVID-19 vaccination programs initiated since December 2020, the need for additional doses was soon debated, including whether required for all individuals, timing post-primary series, and whether booster and primary vaccine matching is needed [95]. Differing recommendations regarding the need for a third and fourth dose have resulted, with several countries/regions issuing recommendations for additional doses in immunocompromised individuals (Table 3; [96–102]).
Table 3.
Recommendations for additional COVID-19 vaccine doses in immunocompromised individuals
| Country or region | Issuing body | Date of recommendation | Recommendation |
|---|---|---|---|
| France [98, 99] | Government of France | April 11, 2021 | Third dose 4 weeks PD2 in severely immunocompromised individuals |
| Government of France | January 28, 2022 | Administration of a fourth dose in severely immunocompromised individuals within 3 months of the third dose | |
| Israel [100] | December 30, 2021 |
Fourth dose in individuals who have undergone organ or stem cell transplants, with hematologic cancers, autoimmune diseases, and who are receiving specific immunosuppressive medications Those with breast, lung, or colon cancer do not qualify |
|
| Global [96] | WHO | October 26, 2021 |
Additional dose recommended in moderately to severely immunocompromised individuals 1–3 months after the primary series. This recommendation applies to individuals with active cancer, solid organ and stem cell transplant recipients, individuals with immunodeficiency, HIV, and those receiving immunosuppressive therapies Homologous additional doses are recommended, but heterologous platforms may be considered because of vaccine supply and access |
| USA [97] | CDC | February 17, 2022 |
Individuals 5–11 years old who are moderately or severely immunocompromised should receive a primary series of three doses of BNT162b2, with the second dose given 3 weeks after the first dose and the third dose given ≥ 4 weeks after the second dose; a fourth dose is not recommended at this time Individuals 12 years and older (for BNT162b2) and 18 years and older (for mRNA-1273) who are moderately or severely immunocompromised should receive a third dose ≥ 4 weeks after receiving their second dose; a booster dose ≥ 3 months after the third dose is recommended; mRNA COVID-19 vaccines are preferred This recommendation extends to individuals receiving cancer treatment, who have received an organ transplant and are using immunosuppressive therapies, who received a stem cell transplant in the past 2 years or are taking immunosuppressive therapies, with moderate or severe primary immunodeficiency, with advanced or untreated HIV infection, or receiving active treatment with high-dose corticosteroids or other immunosuppressive therapies |
| EU [101] | EMA | October 4, 2021 | An additional dose of BNT162b2 or mRNA-1273 may be given to individuals with severely weakened immune systems ≥ 28 days after the second dose |
| UK [102, 103] | MHRA | September 9, 2021 | One dose of BNT162b2 may be administered as a third dose ≥ 8 weeks after the second dose of an mRNA or adenovirus-vectored COVID-19 vaccine if the potential benefits are greater than any potential risks |
| UK Health Security Agency | February 11, 2022 | For those 12 years and older, a reinforcing dose 3 months after the third dose is recommended |
CDC US Centers for Disease Control and Prevention, COVID-19 coronavirus disease 2019, EMA European Medicines Agency, MHRA Medicines and Healthcare Products Regulatory Agency, PD2 post-dose 2, SOT solid organ transplant, WHO World Health Organization
Current US recommendations were based on the US Food and Drug Administration (FDA) granting EUA for mRNA vaccines allowing administration of a third dose in SOTRs and other individuals with comparable immunocompromised status on August 12, 2021 [103]. On August 16, 2021, the US Centers for Disease Control and Prevention (CDC) recommended that moderately to severely immunocompromised adults receive an additional mRNA vaccine dose [104]; since then, the recommendation has been extended to include immunocompromised children 5 years and older [105]. The initial recommendation was based on limited serologic evidence from two small, randomized controlled trials. In a study of 50 hemodialysis recipients, five of whom had prior COVID-19, three BNT162b2 doses were administered to those without prior infection and two to those previously infected [106]. In the latter, 89% (40/45) had antibody responses post-dose 2 and 93% (42/45) post-dose 3; two who did not respond to dose 2 had robust responses post-dose 3. Antibody responses after three doses in those not previously infected were comparable to those measured post-dose 2 in patients with a COVID-19 history. In a study of 120 SOTRs who received two mRNA-1273 doses, a third mRNA-1273 dose or placebo was administered 2 months later [107]. One month post-dose 3, 55% of mRNA-1273 and 18% of placebo recipients had serologic responses (P < 0.001); the median percent virus neutralization was 71% and 13%, respectively. In their recommendations, the CDC cited evidence that immunocompromised individuals (e.g., those with hematologic cancers or on hemodialysis or receiving certain immunosuppressive therapies [108]) do not always have the same degree of immune response to vaccination [105].
Additional evidence has accumulated in systematic reviews of data regarding the immune response to COVID-19 vaccination and vaccine efficacy among immunocompromised subpopulations [109]. While the majority of the data come from mRNA vaccines, there is some evidence from other platforms, such as viral vector and inactivated whole virus vaccines, showing diminished immune responses to vaccination and reduced vaccine efficacy among immunocompromised individuals, thereby making the case for administering additional doses to help protect against COVID-19 [96]. In line with this accumulating evidence, various public health agencies have updated their recommendations. For instance, the CDC recommends that moderately or severely immunocompromised adults who have received one dose of the Ad26.COV2.S viral vector vaccine should receive two subsequent doses of an mRNA vaccine (dose 2 at ≥ 4 weeks post-dose 1 and dose 3 at ≥ 2 months post-dose 2) [97]. Similarly, the UK Joint Committee on Vaccination and Immunisation (JCVI) recommends a preference for mRNA vaccines for the third vaccine dose in immunocompromised individuals, while the AZD1222 adenovirus vaccine may be an option for those 16 years and older who have received this vaccine previously and in whom the mRNA vaccines are contraindicated [102].
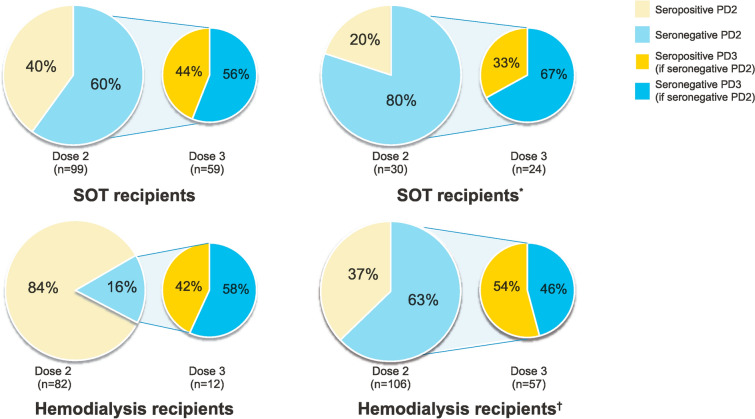
Emerging evidence for increased seropositivity rates in immunocompromised individuals following a third mRNA vaccine dose is summarized in Fig. 1. In studies that included SOTRs and hemodialysis patients, 33–50% of immunocompromised individuals who were seronegative to the initial two-dose series developed antibody responses to a third dose administered approximately 1–3 months post-dose 2 [110–113]. The MELODY (Mass Evaluation of Lateral flow immunOassays for the Detection of SARS-CoV-2 antibodY responses in immunosuppressed people) study, which opened in December 2021, aims to evaluate antibody responses to a third COVID-19 vaccine dose in SOTRs, individuals with hematologic malignancies, and individuals with autoimmune disease [114]. These data may help inform vaccination strategies and future recommendations in immunocompromised individuals.
Fig. 1.
Changes in seroresponse to an additional mRNA COVID-19 dose in immunocompromised individuals. *Recipients received primary dosing with a homologous mRNA vaccine and BNT162b2; they received mRNA-1273, BNT162b2, or Ad26.COV2.S at dose 3. †Seronegative includes individuals with low or no response. COVID-19 coronavirus disease 2019, PD2 post-dose 2, PD3 post-dose 3, SOT solid organ transplant [110–113]
Discussion
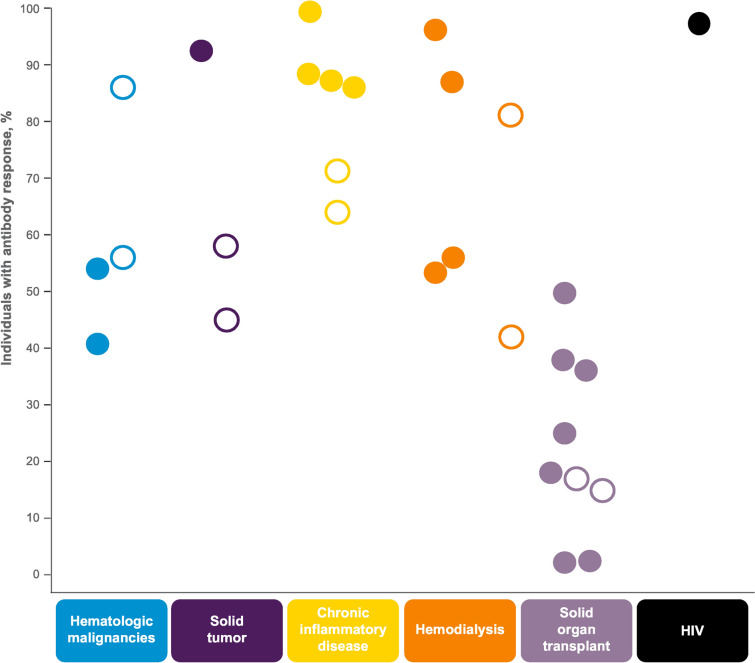
Our literature review shows reduced antibody responses to one or two mRNA vaccine doses in patients with a range of immunocompromising conditions, although this diminished response appears more pronounced in some conditions, particularly SOTRs (Fig. 2). Although responses to two doses versus one dose were higher, even two-dose regimens generally provided diminished responses. Our review also found a generally consistent safety and tolerability profile of mRNA vaccines in immunocompromised individuals compared with the profile in other adult populations.
Fig. 2.
Percentage of individuals with antibody responses after 1–2 mRNA COVID-19 vaccine doses. Open circles indicate antibody response after one vaccine dose and closed circles indicate antibody responses after two vaccine doses. The approach for assessing antibody responses differed between studies
The findings from our review suggest that despite prioritizing immunocompromised individuals within COVID-19 vaccine protocols, this population remains vulnerable to adverse effects of SARS-CoV-2 infection, including high morbidity and mortality. One approach to improve protection against COVID-19 in immunocompromised individuals is to administer additional vaccine doses. This is supported by emerging clinical data showing enhanced antibody response without detrimental safety and tolerability effects post-dose 3 in immunocompromised adults [106, 110–113]. Additional doses in this population are now recommended in some countries and regions.
Importantly, the two-dose schedule for mRNA vaccines administered approximately 3–4 weeks apart was implemented in initial clinical trials and within mass vaccination programs in response to the rapidly evolving pandemic. As we learn more regarding transmission, infection, and disease, as well as the characteristics of SARS-CoV-2 variants and the evolving epidemiology among various populations, it is anticipated that vaccine schedules and recommendations, including among immunocompromised individuals, will evolve.
We have considered transferable lessons from other vaccines used in immunocompromised individuals in the context of COVID-19 vaccination in this population, as this population is also recommended to receive immunization against several infectious diseases to reduce infection-associated morbidity and mortality [115]. Unfortunately, there is low vaccine uptake and limited data on safety, efficacy, effectiveness, and immunogenicity of vaccines in immunocompromised individuals. Accordingly, strategies to overcome these limitations have been investigated. For instance, to protect immunocompromised individuals against influenza, the timing of vaccination, administration of two doses in an influenza season, vaccine formulation modifications, and vaccination of close contacts of the patient and healthcare workers have been proposed [116].
Further investigation to optimize COVID-19 vaccination recommendations for immunocompromised individuals is required, including consideration of timing, immunosuppression protocols, dosing and intervals, heterologous primary and booster vaccination regimens, and breadth of coverage of emerging SARS-CoV-2 variants including omicron. Additionally, identification of an absolute correlate of protection for COVID-19 vaccines will be extremely helpful in the further development and implementation of these vaccines, although neutralizing antibody responses are the most promising candidate [117], and were used extensively in the studies included within our review. Given the small study sizes identified within our review effort, larger prospective trials in this population will be informative, and several such studies in both adults and children are ongoing (Table 4). With the remarkable global uptake of COVID-19 vaccinations, with > 10 billion doses administered as of February 28, 2022 [118], it is also anticipated that real-world effectiveness data in these vulnerable populations will emerge.
Table 4.
Clinical trials evaluating mRNA COVID-19 vaccines in immunocompromised individualsa
| Vaccine | Clinical trial identifier | Details | Status |
|---|---|---|---|
| BNT162b2 | NCT04952766 | Phase 4 study assessing humoral response against SARS-CoV-2 after vaccination in immunocompromised adults compared with healthy volunteers | Recruiting (estimated primary completion March 2022) |
| BNT162b2 | NCT04895982 | Phase 2 study assessing a three-dose schedule in immunocompromised children and adults | Recruiting |
| BNT162b2 | NCT04969601 | Phase 1/2 study assessing a two-dose schedule in children 1–15 years of age with acute leukemia and in their siblings (12–15 years of age) | Recruiting (estimated primary completion May 2022) |
| BNT162b2 mRNA-1273 | NCT04805125 | Phase 3 study using Swiss cohorts of immunocompromised patients to assess the comparative effectiveness and safety of BNT162b2 and mRNA-1273 | Ongoing (estimated completion July 2022) |
| BNT162b2 | NCT04844489 | Study to assess the humoral and cellular responses to vaccination against SARS-CoV-2 variants in immunocompromised individuals | Recruiting |
| mRNA-1273 | NCT04847050 | Phase 2 study assessing the safety and immunogenicity of vaccination in adults with hematologic malignancies, various regimens of immunosuppression, and in participants with solid tumors on PD1/PDL1 inhibitor therapy | Recruiting (estimated primary completion June 2023) |
COVID-19 coronavirus disease 2019, PD1 post-dose 1, PD2 post-dose 2, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
aAs of January 13, 2022
Limitations of the data set from our literature review should be noted. For instance, several immunocompromising conditions were included, and within each study, the assessed populations were generally heterogeneous, limiting assessment of immune responses to disease and vaccination between subgroups (e.g., by type of cancer or CID or by stage of disease including patients undergoing flares or in remission). A comprehensive assessment of the association between these factors and immune responses would require large data sets over time.
Conclusion
Patients with various immunocompromising conditions exhibit diverse responses to SARS-CoV-2 infection and COVID-19 severity and mortality, and available vaccines elicit lower immune responses but have a similar safety and tolerability profile to that observed in other adult populations. Additional doses may enhance vaccine effectiveness and protect against emerging variants. Continued investigation will help refine approaches to help protect this vulnerable population.
Acknowledgements
Funding
This work was supported by Pfizer Inc. Pfizer Inc also funded the journal’s Rapid Services Fee.
Medical writing, editorial, and other assistance
Editorial/medical writing support was provided by Tricia Newell, PhD, and Allison Gillies, PhD, of ICON (Blue Bell, PA, USA) and was funded by Pfizer Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors contributed to the conceptualization of this work. The literature search and data analysis were performed by Daniel Curcio, Amit Srivastava, and Tricia Newell. The first draft of the manuscript was written by Tricia Newell under direction from the authors, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Norka I. Napuri, Daniel Curcio, and Amit Srivastava are employees of Pfizer Inc and David L. Swerdlow is a retired employee of Pfizer Inc, and all authors may hold stock or stock options. David L. Swerdlow was an employee of Pfizer Inc at the initiation of the manuscript.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
This article is based on published literature and therefore does not contain any applicable data sets.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health (2021). https://www.covid19treatmentguidelines.nih.gov/. Accessed 17 May 2022. [PubMed]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). https://www.fda.gov/media/153714/download. Accessed Jan 14, 2022.
- 5.Frenck RW, Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration. Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). https://www.fda.gov/media/144637/download. Accessed Jan 12, 2022.
- 8.US Food and Drug Administration. Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). https://www.fda.gov/media/153713/download. Accessed Jan 12, 2022.
- 9.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed Aug 9, 2021.
- 11.World Health Organization. Status of COVID-19 vaccines within WHO EUL/PQ evaluation process. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_23March2021_0.pdf. Accessed March 31, 2021.
- 12.European Medicines Agency. COVID-19 vaccines: authorised. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section. Accessed March 31, 2021.
- 13.US Food and Drug Administration. COVID-19 vaccines. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines. Accessed March 31, 2021.
- 14.Teo SP. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. 2021 doi: 10.1177/08971900211009650. [DOI] [PubMed] [Google Scholar]
- 15.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. FDA approves first COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine. Accessed Sept 27, 2021.
- 18.US Food and Drug Administration. FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age. Accessed 5 Jan 2022.
- 19.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA takes key action by approving second COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine. Accessed 17 May 2022.
- 20.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 21.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 23.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for C-V Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasreen S, Chung H, He S, et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario. Canada. medRxiv. 2021 doi: 10.1101/2021.06.28.21259420. [DOI] [Google Scholar]
- 29.Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385:320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatcher SM, Endres-Dighe SM, Angulo FJ, et al. COVID-19 vaccine effectiveness: a review of the first 6 months of COVID-19 vaccine availability (1 January–30 June 2021) Vaccines. 2022;10:393. doi: 10.3390/vaccines10030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72:340–350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truong TT, Ryutov A, Pandey U, et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: a consecutive case series. EBioMedicine. 2021;67:103355. doi: 10.1016/j.ebiom.2021.103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hensley MK, Bain WG, Jacobs J, et al. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: a case study. Clin Infect Dis. 2021;73:e815–e821. doi: 10.1093/cid/ciab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baang JH, Smith C, Mirabelli C, et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2021;223:23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5:e140329. doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KA, Ma W, Sikavi DR, et al. Cancer and risk of COVID-19 through a general community survey. Oncologist. 2021;26:e182–e185. doi: 10.1634/theoncologist.2020-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian Y, Qiu X, Wang C, et al. Cancer associates with risk and severe events of COVID-19: a systematic review and meta-analysis. Int J Cancer. 2021;148:363–374. doi: 10.1002/ijc.33213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarifkar P, Kamath A, Robinson C, et al. Clinical characteristics and outcomes in patients with COVID-19 and cancer: a systematic review and meta-analysis. Clin Oncol (R Coll Radiol) 2021;33:e180–e191. doi: 10.1016/j.clon.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19-infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113:371–380. doi: 10.1093/jnci/djaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8:ofab353. doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrington P, de Lavallade H, Doores KJ, et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;35:3573–3577. doi: 10.1038/s41375-021-01300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrière J, Chamorey E, Adjtoutah Z, et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palich R, Veyri M, Marot S, et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32:1051–1053. doi: 10.1016/j.annonc.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungaro RC, Agrawal M, Park S, et al. Autoimmune and chronic inflammatory disease patients with COVID-19. ACR Open Rheumatol. 2021;3:111–115. doi: 10.1002/acr2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haberman RH, Castillo R, Chen A, et al. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheumatol. 2020;72:1981–1989. doi: 10.1002/art.41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79:1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 52.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021;80:1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 54.Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong SY, Dixon R, Martinez Pazos V, et al. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021;161:715–8 e4. doi: 10.1053/j.gastro.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen CY, Shao SC, Chen YT, et al. Incidence and clinical impacts of COVID-19 infection in patients with hemodialysis: systematic review and meta-analysis of 396,062 hemodialysis patients. Healthcare (Basel) 2021;9:47. doi: 10.3390/healthcare9010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruchfeld A. The COVID-19 pandemic: consequences for nephrology. Nat Rev Nephrol. 2021;17:81–82. doi: 10.1038/s41581-020-00381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung EYM, Palmer SC, Natale P, et al. Incidence and outcomes of COVID-19 in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2021;78:804–815. doi: 10.1053/j.ajkd.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrezenmeier E, Bergfeld L, Hillus D, et al. Immunogenicity of COVID-19 Tozinameran vaccination in patients on chronic dialysis. Front Immunol. 2021;12:690698. doi: 10.3389/fimmu.2021.690698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goupil R, Benlarbi M, Beaubien-Souligny W, et al. Short-term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ. 2021;193:E793–E800. doi: 10.1503/cmaj.210673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36:1709–1716. doi: 10.1093/ndt/gfab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grupper A, Sharon N, Finn T, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:1037–1042. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Low JM, Gu Y, Ng MSF, et al. BNT162b2 vaccination induces SARS-CoV-2 specific antibody secretion into human milk with minimal transfer of vaccine mRNA. medRxiv. 2021 doi: 10.1101/2021.04.27.21256151. [DOI] [Google Scholar]
- 66.Prabhu M, Murphy EA, Sukhu AC, et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahalingasivam V, Craik A, Tomlinson LA, et al. A systematic review of COVID-19 and kidney transplantation. Kidney Int Rep. 2021;6:24–45. doi: 10.1016/j.ekir.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kremer D, Pieters TT, Verhaar MC, et al. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: lessons to be learned. Am J Transplant. 2021;21:3936–3945. doi: 10.1111/ajt.16742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kulkarni AV, Tevethia HV, Premkumar M, et al. Impact of COVID-19 on liver transplant recipients—a systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayant K, Reccia I, Virdis F, et al. COVID-19 in hospitalized liver transplant recipients: an early systematic review and meta-analysis. Clin Transplant. 2021;35:e14246. doi: 10.1111/ctr.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diaz-Arocutipa C, Carvallo-Castaneda D, Luis-Ybanez O, et al. COVID-19 in heart transplant recipients during February–August 2020: a systematic review. Clin Transplant. 2021;35:e14390. doi: 10.1111/ctr.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phanish M, Ster IC, Ghazanfar A, et al. Systematic review and meta-analysis of COVID-19 and kidney transplant recipients, the South West London kidney transplant network experience. Kidney Int Rep. 2021;6:574–585. doi: 10.1016/j.ekir.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samidoust P, Nikoupour H, Hemmati H, Samidoust A. Clinical manifestations and characterization of COVID-19 in liver transplant recipients: a systematic review of case reports and case series. Ethiop J Health Sci. 2021;31:429–438. doi: 10.4314/ejhs.v31i2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Granger C, Guedeney P, Arnaud C, et al. Clinical manifestations and outcomes of coronavirus disease-19 in heart transplant recipients: a multicentre case series with a systematic review and meta-analysis. Transpl Int. 2021;34:721–731. doi: 10.1111/tri.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Investig. 2021;131:14. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—a prospective cohort study. Eur J Heart Fail. 2021;23:1555–1559. doi: 10.1002/ejhf.2199. [DOI] [PubMed] [Google Scholar]
- 78.Basic-Jukic N, Ivo J. SARS-CoV-2 infection after two doses of mRNA vaccine in renal transplant recipients. Transplant Infect Dis. 2021;2021:e13628. doi: 10.1111/tid.13628. [DOI] [PubMed] [Google Scholar]
- 79.Rusk DS, Strachan CC, Hunter BR. Lack of immune response after mRNA vaccination to SARS-CoV-2 in a solid organ transplant patient. J Med Virol. 2021;93:5623–5625. doi: 10.1002/jmv.27044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(1173):e1–e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narasimhan M, Mahimainathan L, Clark AE, et al. Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines. Vaccines (Basel) 2021;9:708. doi: 10.3390/vaccines9070708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rincon-Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6:eabj1031. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 85.Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. 2021;25:85–92. doi: 10.1007/s10461-020-02983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hariyanto TI, Rosalind J, Christian K, Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: a systematic review and meta-analysis. South Afr J HIV Med. 2021;22:1220. doi: 10.4102/sajhivmed.v22i1.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel RH, Acharya A, Chand HS, Mohan M, Byrareddy SN. Human immunodeficiency virus and severe acute respiratory syndrome coronavirus 2 coinfection: a systematic review of the literature and challenges. AIDS Res Hum Retroviruses. 2021;37:266–282. doi: 10.1089/aid.2020.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee KW, Yap SF, Ngeow YF, Lye MS. COVID-19 in people living with HIV: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:3554. doi: 10.3390/ijerph18073554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang M, Luo N, Chen M, et al. Prevalence and mortality due to COVID-19 in HIV co-infected population: a systematic review and meta-analysis. Infect Dis Ther. 2021;10:1267–1285. doi: 10.1007/s40121-021-00447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sarkar S, Khanna P, Singh AK. Impact of COVID-19 in patients with concurrent co-infections: a systematic review and meta-analyses. J Med Virol. 2021;93:2385–2395. doi: 10.1002/jmv.26740. [DOI] [PubMed] [Google Scholar]
- 91.Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11:6283. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.SeyedAlinaghi S, Karimi A, MohsseniPour M, et al. The clinical outcomes of COVID-19 in HIV-positive patients: a systematic review of current evidence. Immun Inflamm Dis. 2021;9:1160–1185. doi: 10.1002/iid3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Costenaro P, Minotti C, Barbieri E, Giaquinto C, Donà D. SARS-CoV-2 infection in people living with HIV: a systematic review. Rev Med Virol. 2021;31:1–12. doi: 10.1002/rmv.2155. [DOI] [PubMed] [Google Scholar]
- 94.Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect. 2021;27:1851–1855. doi: 10.1016/j.cmi.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oliver S. Overview of data to inform recommendations for booster doses of COVID-19 vaccines; 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/06-COVID-Oliver-508.pdf. Accessed 17 May 2022.
- 96.World Health Organization. Interim recommendations for an extended primary series with an additional vaccine dose for COVID-19 vaccination in immunocompromised persons. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-immunocompromised-persons. Accessed Jan 13, 2022.
- 97.US Centers for Disease Control and Prevention. COVID-19 vaccines for moderately or severely immunocompromised people. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed Feb 23, 2022.
- 98.Republique Francaise. Vaccins Contre la COVID-19: Modalites D'administration Des Rappels. https://solidarites-sante.gouv.fr/IMG/pdf/dgs-urgent_no2022-16_vaccins_personnes_immunodeprimes_pdf. Accessed Feb 28, 2022.
- 99.Israel Ministry of Health. Fourth dose of the vaccine approved for people with a weakened immune system. https://www.gov.il/en/departments/news/30122021-05. Accessed Jan 13, 2022.
- 100.European Medicines Agency. Comirnaty and Spikevax: EMA recommendations on extra doses and boosters. https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters. Accessed Jan 18, 2022.
- 101.Government of the United Kingdom. Decision: information for healthcare professionals on COVID-19 vaccine Pfizer/BioNTech (Regulation 174). GOV.UK. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/information-for-healthcare-professionals-on-pfizerbiontech-covid-19-vaccine. Accessed Jan 6, 2022.
- 102.UK Health Security Agency. COVID-19 vaccination programme. Information for healthcare practitioners. Version 4.1. February 11, 2022. https://www.gov.uk/government/publications/covid-19-vaccination-programme-guidance-for-healthcare-practitioners. Accessed 17 May 2022.
- 103.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. Accessed Sept 17, 2021.
- 104.US Centers for Disease Control and Prevention. COVID-19 vaccines for moderately to severely immunocompromised people. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html#print. Accessed Aug 18, 2021.
- 105.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed Jan 13, 2022.
- 106.Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100:702–704. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.US Centers for Disease Control and Prevention. Science brief: COVID-19 vaccines and vaccination. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html. Accessed Aug 18, 2021. [PubMed]
- 109.Lee A, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Longlune N, Nogier MB, Miedouge M, et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36:1704–1709. doi: 10.1093/ndt/gfab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Espi M, Charmetant X, Barba T, et al. Justification, safety, and efficacy of a third dose of mRNA vaccine in maintenance hemodialysis patients: a prospective observational study. medRxiv. 2021 doi: 10.1101/2021.07.02.21259913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Imperial College London. MELODY study. https://www.imperial.ac.uk/medicine/research-and-impact/groups/melody-study/. Accessed Jan 18, 2022.
- 115.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44-100. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 116.Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311–1322. doi: 10.1080/21645515.2018.1445446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27:1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 118.World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed June 23, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article is based on published literature and therefore does not contain any applicable data sets.