Abstract
Skeletal muscle is one of the largest organs in the body and is essential for maintaining quality of life. Loss of skeletal muscle mass and function can lead to a range of adverse consequences. The gut microbiota can interact with skeletal muscle by regulating a variety of processes that affect host physiology, including inflammatory immunity, protein anabolism, energy, lipids, neuromuscular connectivity, oxidative stress, mitochondrial function, and endocrine and insulin resistance. It is proposed that the gut microbiota plays a role in the direction of skeletal muscle mass and work. Even though the notion of the gut microbiota–muscle axis (gut–muscle axis) has been postulated, its causal link is still unknown. The impact of the gut microbiota on skeletal muscle function and quality is described in detail in this review.
1. Introduction
Skeletal muscle is one of the largest organs, accounting for roughly half of the total body weight. Skeletal muscle produces heat, regulates blood sugar, storing amino acids, and alters the physiological characteristics of the body [1]. Skeletal muscle mass and function decline have been reported to affect 8%–13% of older adults [2], with clinical effects including frailty, loss of mobility, falls, fractures, disability, and increased mortality [3]. Numerous factors contribute to the loss of skeletal muscle mass and function, such as inflammatory states [4], age-related changes in the hormonal environment [5], insulin resistance [6], gut physiology [7], DNA damage, and mitochondrial dysfunction [8]. These mechanisms are enhanced in the presence of insufficient protein energy [9].
The physiological characteristics of skeletal muscle have been extensively studied in the past few decades, providing unique insights into the interconnection among organs [10]. As with the products secreted by skeletal muscle, external factors that may act on skeletal muscle can also play an important role in peripheral tissues. The gut microbiota has the potential to influence muscle function and quality [11]. The gut microbiota is increasingly being seen as a key factor in human wellbeing and disease, especially in older adults [12]. Although the gut microbiota is known for its role in nutrient absorption, it is closely associated with many other physiological processes [13]. Therefore, the interaction between the gut microbiota and human organs has become the focus of recent research [14].
Recent studies have demonstrated the existence of a gut microbiota-muscle axis, i.e., that muscle function and metabolism are largely dependent on the quantity and composition of the gut microbiota, and that the gut microbiota is expected to be a potential biological target for the prevention and treatment of muscle-related diseases such as sarcopenia and muscular dystrophy [15]. Furthermore, it is critical to clarify how the gut microbiota affects exercise load, modulates muscle function, and improves host fitness. The gut microbiota has a profound effect on skeletal muscle function and mass, and intervening in this axis may reverse the decline in skeletal muscle function and mass [13, 15–19]. This article reviews the progress of research on the effects of gut microbiota on the biological function of skeletal muscle and its mechanisms.
2. Gut Microbiota and Intestinal Barrier
2.1. Gut Microbiota
The human body consists of approximately 30 trillion cells that coexist with various microbial communities [20]. The human gut microbiome consists of 10–100 trillion microbes that are highly diverse, complex, constantly evolving, and colonize the digestive tract [21]. For host physiology, body homeostasis, and long-term health, functional interactions between gut microorganisms and hosts are critical. Although several studies have revealed how the gut microbiota impacts the liver and intestinal metabolism [22], there are few reports on how the gut microbiota regulates skeletal muscle, which is also one of the key metabolic organs [23]. The composition of the gut microbiome is influenced by a variety of factors, including genetics, age, diet, and exercise [24]. The human gut microbiota is dynamic throughout the life cycle, with the composition of gut microbes tending toward a steady state during the early years, but new research has found that the gut microbiota changes significantly in older adults (≥65 years) [25]. Antibiotics are known to cause changes in the microbiota composition, and older people are more inclined to use antibiotics more frequently [26], which may be one of the reasons for the changes in their gut microbiota composition.
To date, more than 9.9 million microbial genes have been found in human feces, with Bacteroides and Firmicutes accounting for the majority [27]. Probiotics are beneficial bacteria (e.g., Lactobacillus, Bifidobacterium, Clostridium butyricum, and Bacillus subtilis) [28]. Prebiotics are largely found in our gastrointestinal tract. Prebiotics are organic substances that the host cannot digest or absorb but which benefit the host's health. They feed beneficial bacteria and promote the growth and reproduction of beneficial bacteria [29]. The aging gut microbiota is highly characterized by a decrease in microbial diversity and beneficial bacteria, as well as a rearrangement of Bacteroides and Firmicutes, especially in older people, where individual differences in microorganisms can be greater [30, 31].
2.2. Intestinal Barrier
The intestinal tract of the organism has a relatively complete functional barrier, and intestinal barrier function refers to the function of the intestinal epithelium that can separate the intestinal lumen from the internal environment of the organism and prevent the invasion of pathogenic antigens. The normal intestinal barrier consists of mechanical barrier, chemical barrier, immune barrier, and biological barrier together [32].
The mechanical barrier is an intact intestinal mucosal epithelial structure closely connected to each other, which consists of a mucosal layer, intestinal epithelial cells, intercellular tight junctions, and submucosal lamina propria, and the intact intestinal mucosal epithelial cells and tight junctions between epithelial cells are the structural basis of the mechanical barrier [33]. Gastric acid, bile, various digestive enzymes, lysozyme, digestive juices, and antibacterial substances produced by parasitic bacteria in the intestinal lumen constitute the chemical barrier of the intestinal tract [34]. Stomach acid can destroy bacteria entering the gastrointestinal tract and inhibit bacterial adhesion and colonization of the gastrointestinal epithelium; lysozyme can destroy the cell wall of bacteria and cause bacterial lysis; digestive juices secreted by the intestine can dilute toxins and flush the intestinal lumen, making it difficult for potentially pathogenic bacteria to adhere to the intestinal epithelium [35, 36]. The immune barrier of the gut consists of immune cells, immune factors, and gut-associated lymphoid tissue. Immune cells initiate immune responses and form the intestinal mucosal immune system to protect the gut from external stimuli [36]. Immune factors enhance gut barrier function through immune rejection and bacterial clearance, in which immunoglobulin IgA plays an important role in regulating gut microbiota and maintaining immune homeostasis [37]. Gut-associated lymphoid tissue neutralizes antigenic substances by triggering local immune responses and can also secrete immunoglobulins to block the binding of bacteria to intestinal epithelial receptors, thereby effectively blocking the adhesion of harmful substances to the intestinal mucosa [38]. The normal parasitic flora in the intestine forms the biological barrier of the intestinal mucosa, and the metabolism of the gut microbiota can also regulate the mechanical, chemical, and immune barriers of the intestinal tract [39]. The biological barrier of the gut maintains the stability of the gut microbiota, and dysregulation of gut microbial homeostasis can lead to a decrease in beneficial microbes and an increase in harmful microbes, thereby compromising the health of the host [40].
Since birth, the microbiota has colonized the gastrointestinal tract and participates in many physiological processes in the host. Intestinal immune and endocrine function, energy homeostasis, and health are all influenced by the complex microbiota [41], which regulates inflammatory gene expression, innate immune effector cells (monocytes and macrophages), glucose tolerance, and gut hormone release, among other metabolic pathways [42, 43]. The gut microbiota and the gut barrier interact with each other. Intestinal cells regulate the composition of the gut microbiota by secreting antimicrobial peptides, and conversely, the gut microbiota can also affect the growth process of intestinal epithelial cells [34]. In mice, depletion of the gut microbiota compromises the intestinal epithelium, leading to altered patterns of microvillus formation and reduced cell renewal [44]. Probiotics form a biofilm to cover the intestinal mucosa, preventing the invasion of foreign bacteria, and they also produce acidic metabolites that lower the pH of the intestinal tract, thereby inhibiting the growth of harmful bacteria [45]. In addition, the accumulation of anaerobic bacteria and the invasion of exogenous pathogenic bacteria can lead to dysbiosis of the gut microbiota, damage the intestinal epithelial cells, and destroy the gut microbiota barrier [46].
2.3. Gut Microbiota Affects Skeletal Muscle Mass and Function
According to emerging evidence, the gut microbiota appears to play a role in regulating several muscle metabolic pathways [47]. Individual differences in gut microbiota relative abundance are linked to muscle mass and body weakness [48, 49], and higher gut microbiota diversity is linked to increased muscle mass [50]. In young women, the diversity of the gut microbiota is also related to skeletal muscle mass [51]. Increased numbers of Oscillospira and Ruminococcus and decreased numbers of Barnesellacae and Christensenellacea taxa are found in people with muscle wasting and physical weakness [48]. When compared to older people with low functional muscular strength, those with higher levels of Prevotella, Barnesiella, and Barnesiella intestinihominis have greater muscle strength [52]. Barnesiella and Prevotella have genes that produce short-chain fatty acids (SCFAs) [53].
Several studies from rodents have suggested that gut microbes may be related to the function and quality of skeletal muscle. The effects of gut microbiota shortage on skeletal muscle were studied in two animal investigations, which revealed that a lack of gut bacteria causes muscle mass loss [54, 55].The abundant Rikenellaceae group found in the gut microbiota of older mice is linked to a dose-dependent rise in muscular frailty index [56]. Higher Sutterella to Barnesiella ratio, altered inflammation and immune function, and decreased gastrocnemius and triceps size in rats with muscle atrophy were compared with healthy adult rats [47]. Comparison of germ-free (GF) mice lacking gut microbiota and pathogen-free (PF) mice with gut microbiota revealed skeletal muscle atrophy and decreased muscle mass in GF mice [23]. Ghrelin-deficient mice develop microbial dysbiosis at a young age and then lose muscle mass and function as they get older [57]. A decrease in gut bacteria can directly lead to muscle atrophy, according to two new studies [23, 54].
Antibiotics change the microbiota, and metronidazole has been shown to upregulate the expression of neurogenic atrophy-related proteins in skeletal muscle in earlier studies, as well as histone deacetylase 4, myostatin (MyoG), and FOXO1/FXOX3-mediated protein degradation, leading to skeletal muscle atrophy, thereby reducing muscle mass in the hind limb and muscle fiber volume in the tibialis anterior muscle of mice [58]. Similarly, antibiotic-treated mice resulted in muscle atrophy, reduced muscle mass, decreased running endurance, and increased ex vivo muscle fatigue [26, 59]. However, after inoculation with natural microbes in antibiotic-treated mice, the mice had increased muscle mass and a muscle mass/body weight ratio [59].
In vitro studies have also shown that gut microbial products can directly affect muscle mass [60]. The levels of two intestinal microbial metabolites (indoxyl sulfate and p-cresol sulfate) increase with age and play a vital part in muscle function [61]. Indoxyl sulfate, a biomarker of uremic sarcopenia, accelerates muscle atrophy by increasing inflammation levels, oxidative stress, and myasthenic gene expression and is negatively correlated with muscle strength and physical exercise [62]. Similarly, the gut microbiota that produces p-cresol sulfate, through insulin resistance and increasing muscle lipid content, ultimately contributes to poor muscle status [63]. Conversely, SCFAs are the end product of colonic protein fermentation and have many important physiological functions.
3. The Gut Microbiome Regulates Skeletal Muscle through a Variety of Mechanisms
3.1. Inflammation, Immunity, and Autophagy
One of the major mechanisms contributing to the loss of skeletal muscle mass and function is systemic chronic inflammation. As research has progressed, the importance of the gut microbiota in skeletal muscle metabolism and immunological function has become recognized. The gut microbiota promotes metabolic homeostasis and immune function by strengthening the intestinal barrier [64]. Gut microbial disorders and loss of variety, in contrast, compromise the integrity of the intestinal barrier, allowing hazardous microbial products such as lipopolysaccharide (LPS) to enter the bloodstream, and these harmful substances trigger systemic inflammation and lead to metabolic disorders and decreased muscle function and mass [15]. Elevated LPS levels activate Toll-like receptor (TLR) 4 signaling, which leads to metabolic endotoxemia [65]. Activation of the TLR4 signaling pathway causes a significant increase in nuclear factor- (NF-) κB protein levels (p50 and p65) and c-Jun N-terminal kinase phosphorylation, resulting in a decrease in human immune function [65]. Specifically, the TLR4 signaling pathway induces upregulation of proinflammatory cytokines (interleukin-6 and tumor necrosis factor-α) through a cascade response, thereby inducing a systemic inflammatory response [66] (Figure 1).
Figure 1.
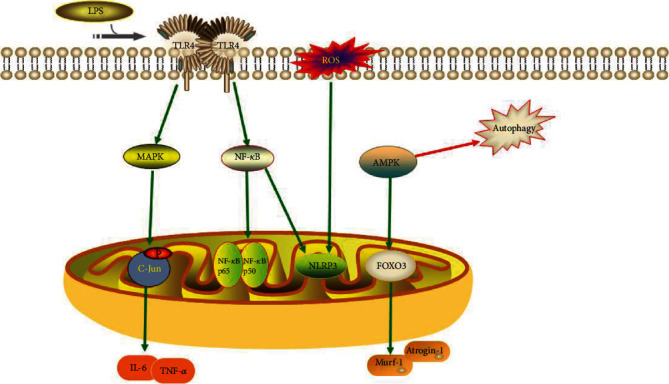
TLR4 signaling and the production of ROS induce inflammatory responses. AMPK signaling regulates autophagic activity and produces muscle atrophy factors.
In recent years, autophagy has received a lot of attention as a fundamental element in skeletal muscle mass and function regulation. Autophagy ensures skeletal muscle quality and function by removing dysfunctional organelles from senescent cells [67]. The AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-coactivator- (PGC-) 1 signaling pathways are known to regulate cellular metabolism and play essential roles in autophagy, inflammation, insulin resistance, and skeletal muscle. In addition, AMPK and PGC-1α signaling pathways are associated with the gut microbiota–muscle axis [68]. The activation of AMPK and PGC-1 decreases with age [69], and inhibition of AMPK and PGC-1α signaling pathways decreases autophagic activity, leading to a decrease in skeletal muscle mass and function [70]. Decreased autophagic activity exacerbates the inflammatory response, which in turn inhibits activation of the AMPK signaling pathway [71]. The reduced autophagic activity also clusters dysfunctional organelles in senescent cells, thereby increasing the production of reactive oxygen species (ROS). The level of the inflammasomes, including Nod-like receptor 3 (NLRP3), is stimulated by ROS [72]. The NF-κB signaling mentioned above also stimulates the production of NLRP3 inflammasomes [73]. Thus, dysregulated autophagic activity and inflammatory responses play a pivotal part in the loss of skeletal muscle mass and function, and AMPK and PGC-1α signaling pathways are closely associated with the gut microbiota–muscle axis [68]. Further research into the relationships between the AMPK and PGC-1 signaling pathways, autophagy, inflammatory responses, and the gut microbiome could aid in the treatment of disorders characterized by skeletal muscle mass and function loss (Figure 1).
Increased expression of atrophy marker genes, particularly Murf-1 and Atrogin-1, which play a critical role in muscle atrophy, is linked to the role of microbiota in the reduction of muscle mass and function [74]. FOXO transcription factors influence the production of Murf-1 and Atrogin-1 [74]. By activating the FOXO3-mediated protein breakdown pathway, AMPK modulates muscle fiber size [75]. Decreased muscle mass and strength in GF mice are associated with increased expression of FOXO, Murf-1, and Atrogin-1. The MyoG and FOXO3 pathways and their downstream target genes are regulated by the gut microbiota and their derived metabolites during protein synthesis and degradation [76]. The activation of AMPK signaling in GF mouse muscle suggests that the AMPK/FOXO3/Atrogin-1/Murf-1 signaling pathway may be implicated in the gut microbiota–muscle axis [23] (Figure 1).
3.2. Endocrine System
The endocrine system has an important role in muscle mass regulation, with insulin, insulin-like growth factor- (IGF-) 1, and growth hormone influencing muscle growth and development [77]. In general, insulin acts on skeletal muscle to promote glucose uptake and upregulates anabolic signaling, which influences the rate of muscle protein synthesis [78]. Dysregulation of the gut microbiota and disruption of epithelial regeneration can be founded in intestinal epithelial IGF-1 gene-deficient mice compared with normal mice [79]. Mechanistically, IGF-1 regulates muscle growth through the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway and inhibits the mRNA transcription and translation process of muscle protein synthesis (MPS) [80]. The PI3K/AKT signaling pathway is a well-known insulin-resistance pathway [81], and it is disrupted in diabetic patients. Insulin production and beta-cell activity may be diminished once this route is blocked, worsening insulin resistance even more [82]. Insulin resistance causes muscle cells to be unable to utilize glucose and instead rely on glycogen or fat, which can lead to a loss of muscle mass and function [83] (Figure 2).
Figure 2.
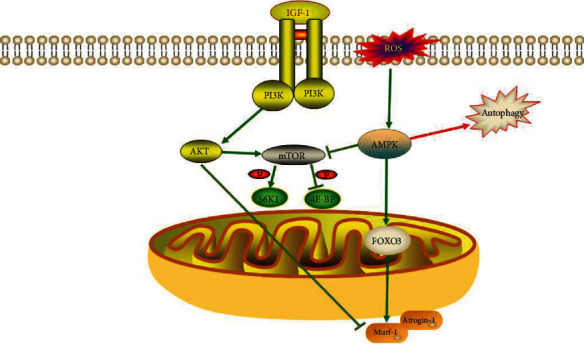
IGF-1 activates mTOR through PI3K/AKT signaling to stimulate protein synthesis. The PI3K/AKT signaling pathway inhibits the expression of myasthenic markers (Murf-1 and Atrogin-1). ROS inhibits mTOR activity by activating the AMPK signaling pathway.
Glucocorticoids can induce skeletal muscle atrophy under pathological conditions [84]. One of the target genes for glucocorticoid receptor activation is Kruppel-like factor (KLF) 15, which is implicated in metabolic activities in skeletal muscle such as overexpression of branched-chain aminotransferase2, which leads to degradation of branched-chain amino acids (BCAAs) [85]. Loss of gut microbiota also leads to the degradation of BCAAs in muscle. Increased catabolism of BCAAs in GF mice is a key factor in muscle atrophy, and increased expression of genes involved in BCAA metabolism leads to reductions in muscle mass, hindlimb grip strength, and spontaneous activity in mice [23]. Catabolism of BCAAs is linked to skeletal muscle proteolysis and has the ability to modulate protein synthesis [86] (Figure 3).
Figure 3.

Glucocorticoids inhibit protein synthesis by activating KLF15, which leads to the degradation of BCAAs.
3.3. Protein Anabolism
A balance between protein synthesis and breakdown keeps skeletal muscle mass in check. A state of negative muscle protein balance occurs when the rate of muscle protein breakdown (MPB) exceeds the rate of MPS over time, resulting in a reduction in skeletal muscle function and mass [87]. It is widely believed that the decrease in muscle function and mass is caused by diminished ability to stimulate MPS rather than by acceleration of MPB [88]; a metabolic phenomenon known as muscle anabolic resistance.
Mammalian target of rapamycin (mTOR) is a downstream target of PI3K/Akt. mTOR stimulates protein synthesis in two ways: phosphorylation and inactivation of eukaryotic initiation factor 4E-binding protein1 and phosphorylation and activation of ribosomal S6 kinase1 [89]. Many studies have demonstrated that mTOR signaling regulates MPS, and that inhibition of mTOR signaling results in decreased muscle function and muscle loss [90]. IGF-1 can activate mTOR activity by activating the PI3K/Akt signaling pathway, thereby stimulating protein synthesis [91]. Production of myasthenic markers (Murf-1 and Atrogin-1) is downregulated by the PI3K/Akt pathway [92]. However, phosphorylation and activation of AMPK can inhibit mTOR activity [93]. Decreased insulin sensitivity and inflammatory responses also reduce mTOR signaling. Reduced insulin sensitivity inhibits mTOR activity by reducing IGF-1 levels, and overproduction of inflammatory factors as well as ROS can inhibit the mTOR pathway by activating the AMPK pathway [9] (Figure 2).
An increasing number of studies have shown that the gut microbiota can produce a large number of bacterial metabolites to activate diverse receptors in host cells, thus maintaining homeostasis in the host. Bile acids (BAs) are metabolites produced by the microbiota [94]. BAs bind to cellular BA receptors, one of which is the nuclear farnesoid X receptor (FXR), to modulate host glucose and lipid metabolic signaling [95]. FXR is activated in the ileum and produces fibroblast growth factor (FGF) 19, which is called FGF15 in rodents. In previous research, BAs, BA receptors, and the FXR-FGF15/19 signaling pathway have all been linked to skeletal muscle mass and function [96]. The expression of FGF15/19 activates the protein kinase (ERK) signaling pathway and phosphorylation of ERK downstream targets p90 ribosomal S6 kinase and ribosomal protein S6 to catalyze protein synthesis [97]. In short, gut microbiota disorders inhibit the BA/FXR/FGF15/19/ERK signaling pathway, resulting in restricted protein synthesis and thus skeletal muscle atrophy [98] (Figure 4).
Figure 4.
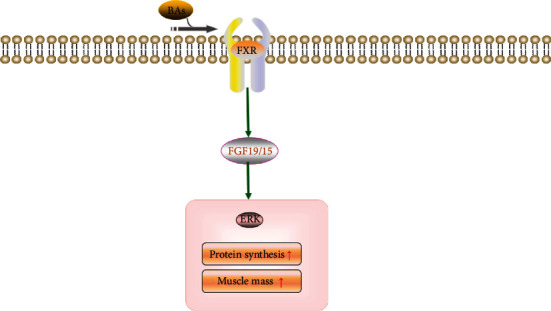
BAs promote protein synthesis and strengthen muscle mass through the FXR/FGF15/19 signaling pathway.
3.4. Peroxisome Proliferator-Activated Receptors
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor family of transcription factors that are activated by fatty acids and their derivatives. After activation by ligand binding, PPAR heterodimerizes with retinoid X receptors, forming a heterodimer that binds to a PPAR response element upstream of the target gene promoter, ultimately regulating the transcription of the target gene [99]. There are three subtypes of PPAR: PPARα, β/δ, and γ. PPARα is highly expressed not only in the liver, heart, brown adipose tissue, and kidney but also in skeletal muscle [100]. It plays an important role in fatty acid catabolism by regulating peroxisomal and mitochondrial β-oxidation and microsomal ω-oxidation of fatty acids; it is also involved in glucose metabolism and is key in controlling energy expenditure and suppressing inflammatory responses [101]. The expression of PPARβ/δ is more widespread in skeletal muscle, and it plays an important role in glucose and lipid metabolism, inflammatory response, energy expenditure, and muscle fiber type switching [102]. PPARγ is highly expressed in adipocytes and is associated with lipid deposition in muscle and other organs, affecting adipogenesis as well as triglyceride storage [103].
It has been shown that mice lacking PPARβ/δ have a reduced number of muscle satellite cells with decreased regenerative capacity, ultimately leading to muscle atrophy and decreased muscle mass and body weight, suggesting that PPARβ/δ regulates postnatal myogenesis and regeneration in mice [104]. Some mice with specific active PPARβ/δ have shown greater resistance to fatigue [105]. Abnormal energy metabolism and reduced muscle fibers have been observed in mice with PPARβ/δ knockout in muscle and adipocyte hypertrophy, and glucose intolerance with insulin resistance has also been observed [106]. PGC-1α has been shown to be a downstream target gene of PPARβ/δ [107]. The expression of PPARβ/δ also increases the level of PGC-1α, which affects fatty acid oxidation and glucose metabolism [108]. These results also show that PPAR agonists can improve the deficiency of myotonic proteins, compensate for the loss of muscle fibers, and improve myotonic dystrophy [109]. Experiments using antibiotics to treat mice with changes in muscle peripheral biological clock mechanisms and metabolic regulators (PPARγ) have suggested that disturbances in the gut microbiota are associated with the expression of genes that regulate muscle peripheral circadian mechanisms and metabolism [26].
PPAR primarily interacts with the gut microbiota in inflammation and metabolism [110]. PPARα protects the intestine from an inflammation-induced increase in intestinal permeability by preventing neutrophil infiltration, and the microbiota activates PPARα through TLR4 signaling, thereby acting to reduce inflammation [111]. Previously, it was reported that treatment of mice with type I diabetes with a PPARα agonist (bezafibrate) resulted in improved skeletal muscle insulin sensitivity through activation of PI3K/AKT signaling [112]. Similarly, PPARβ/δ and PPARγ play a role in reducing inflammation in the intestines, thereby regulating the composition of the intestinal flora [113]. PPARβ/δ suppresses the inflammatory response and enhances insulin sensitivity by activating the AMPK signaling pathway and inhibiting the extracellular regulated protein kinase ERK1/2 [114]. PPARγ in muscle promotes glucose utilization by muscle through activation of glucose transporter protein (GLUT) 1 and GLUT4 [115].
3.5. Mitochondrial Function and Neuromuscular Connectivity
Skeletal muscle mitochondrial dysfunction is also a cause of decreased muscle mass and function [116]. Skeletal muscle mitochondrial function and content decrease with age, and electron microscopy shows abnormally expanded mitochondrial segments [117]. The production of IGF-1 by the gut microbiota connects mitochondrial skeletal muscle to the gut microbiota. It was discovered that IGF-1 levels in GF mice were lower than in PF mice, and that the expression of genes encoding mitochondrial oxidative phosphorylation complexes was lower in GF mouse skeletal muscle, resulting in a loss in mitochondrial function [23].
The central nervous system controls skeletal muscle function via neurotransmission at the neuromuscular junction [118]. Acetylcholine, a key neurotransmitter for signaling between muscles and nerves, was reduced in GF mice when compared to PF mice, as was the expression of the acetylcholine receptor subunit Rapsyn and low-density lipoprotein receptor-related protein 4; both of which are important for neuromuscular junction assembly [23] (Figure 5).
Figure 5.
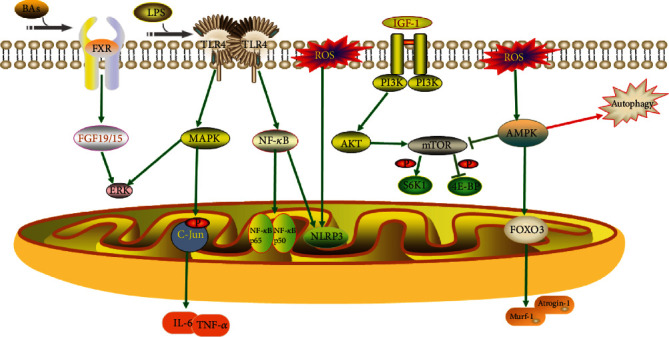
Mechanisms involved in the gut microbiota–skeletal muscle axis.
4. Interventions
To date, there have been many preclinical and human studies that have directly or indirectly demonstrated a link between gut microbiota and muscle mass/function (Table 1). Various interventions have been proposed for the gut microbiota, and probiotics and/or prebiotics, SCFAs, dietary supplementation, and exercise have all been effective in enhancing muscle mass and host function (Figure 6). Dietary habits influence the composition of the gut microbiota and can induce changes in the microbiota that are important for the function of the organism [119]. In the context of skeletal muscle aging, eating disorders cause reduced microbial diversity and increased intestinal permeability, which inhibit cytokine-mediated protein anabolism [120]. Supplementation of prebiotics and/or probiotics improves intestinal homeostasis and promotes skeletal muscle metabolism and synthesis [121]. Exercise or physical activity is also a factor in regulating the gut microbiota [122].
Table 1.
The effects of gut microbiota on skeletal muscle.
| References | Objects | Methods | Results | Remarks |
|---|---|---|---|---|
| Chen et al. [123] | Mice | Supplementation of LP10 | Forelimb grip strength and endurance swimming time were increased | LP10 reduces the inflammatory response, improves glucose utilization, and increases the number of type I muscle fibers in the gastrocnemius muscle |
| Storelli et al. [124] | Drosophila | Supplementation of Lactobacillus plantarum | Increased protein synthesis and enhanced muscle anabolism | Upregulation of mTOR pathway and enhancement of MPS |
| Chen et al. [125] | Mice | Supplementation of NCE | Forelimb grip strength and endurance swimming time were increased | NCE alters gut microbiota composition and increases tissue glycogen content |
| Okamoto et al. [126] | LMC diet mice | Inulin supplementation combined with microbial transplantation | Endurance was improved | Muscle mass improvement was not found, and it may be difficult to promote muscle growth with a single supplement of inulin |
| Katsuki et al. [127] | Mice | Supplementation of Lactobacillus curvatus CP2998 | The myotubular diameter was restored | CP2998 prevents dexamethasone-induced muscle atrophy by inhibiting glucocorticoid receptor activation |
| Hsu et al. [128] | Mice | Supplementation of kefir | Significant improvement in forelimb grip strength score, endurance swim time, and muscle mass | Altered gut microbiota composition and increased tissue glycogen content |
| Ni et al. [129] | Mice | Supplementation of Lactobacillus casei LC122 or Bifidobacterium longum BL986 | Improved muscle strength and function | Improved intestinal barrier function and reduced inflammatory response |
| Chen et al. [130] | Mice | Supplementation of Lactobacillus paracasei PS23 | Reduced risk of sarcopenia | Improved mitochondrial function and decreased secretion of proinflammatory cytokines |
| Huang et al. [131] | Mice | Colonization of Eubacterium rectale or Clostridium coccoides | Endurance swimming time was increased | / |
| Scheiman et al. [132] | Mice | Inoculation of Veillonella atypica | Treadmill running exhaustion time was increased | Veillonella atypica converts lactic acid metabolism to propionic acid |
| Fielding et al. [52] | Mice | Fecal samples from older adults | The grip strength of mice in the high-function group increased significantly | Altered gut microbiome and strengthened intestinal barrier in high-functioning mice |
| Munukka et al. [133] | Mice | Supplementation of Faecalibacterium prausnitzii | Muscle mass was increased | Enhanced mitochondrial respiration, reduced inflammatory response, altered gut microbiota composition, and improved intestinal integrity |
| Lee et al. [134] | Mice | Supplementation of SA-03 | Significant improvement in muscle strength and endurance performance | Increased liver and muscle glycogen stores, decreased levels of lactate, blood urea nitrogen, ammonia, and creatine kinase |
| Lee et al. [135] | Mice | Supplementation of OLP-01 | Increased grip strength and endurance in mice | Increased SCFA, liver, and muscle glycogen |
| Hsu et al. [54] | Mice | Supplementation of Bacteroides fragilis | Increased muscle mass and endurance swimming time | Serum superoxide dismutase activity was lower than GF mice |
| Lahiri et al. [23] | Germ-free mice | Supplementation of SCFA | Increased muscle mass and function and grip strength | SCFA reduces the expression of Atrogin-1 and Murf-1 |
| Walsh et al. [138] | Mice | Supplementation of butyrate | Prevention of hind limb muscle atrophy in mice | Increase in muscle fibers, prevention of intramuscular fat accumulation, improvement of mitochondrial function and glucose metabolism |
| Buihues et al. [139] | Elderly people (≥65 years) | Supplementation of prebiotic:inulin plus fructooligosaccharides | Improved muscle strength and endurance, less fatigue | Prebiotics promote the growth of beneficial bacteria and reduce proinflammatory cytokines |
| Huang et al. [140] | Triathletes | Supplementation of Lactobacillus plantarum PS128 | Significantly improves triathletes' endurance | Regulate gut microbiota composition and increase SCFA content |
| Huang et al. [141] | Healthy adults | Supplementation of LP10 | Increased muscle mass and fatigue resistance | LP10 improves aerobic endurance performance |
| Barger et al. [142] | Older men | High dietary fiber diet | Higher grip strength and physical performance indicators | High dietary fiber promotes butyrate production |
| Morita et al. [143] | Older women | 12 weeks of aerobic training | Increased trunk muscle strength | Increased gut microbiota diversity and fecal SCFA content |
| Shing et al. [144] | Male runners | Supplementation of probiotic capsules | Prolonged fatigue exercise at high temperatures | / |
| Salarkia et al. [145] | Female swimmers | Supplementation of probiotic yogurt | Improved aerobic performance | Improved maximum oxygen uptake |
Figure 6.
Diet, exercise, prebiotics/probiotics, and SCFA supplementation can alter the gut microbiota and improve muscle mass and function.
In a mouse model, forelimb grip strength and endurance swimming time were significantly increased after 6 weeks of supplementation with Lactobacillus plantarum TWK10 (LP10), which increased glucose utilization and reduced the inflammatory response by increasing the number of types I muscle fibers in the gastrocnemius muscle, thereby increasing endurance exercise time [123]. In a study of Drosophila, Lactobacillus plantarum can increase protein synthesis and upregulate mTOR, thereby promoting MPS and enhancing muscle anabolism [124]. Curcumin as a prebiotic can alter the composition of gut microbiota and improve endurance, swimming time, and forelimb grip strength in mice, possibly due to a significant increase in tissue glycogen content in mice after supplementation with nanobubble curcumin extract (NCE) [125]. Inulin combined with microbial transplantation improves endurance in mice on a low microbiome-accessible carbohydrate (LMC) diet, but no improvement in muscle mass was found [126]. Myotube diameter was significantly reduced after treatment of mouse skeletal muscle C2C12 myotubes with dexamethasone, whereas Lactobacillus curvatus CP2998 (CP2998) restored mouse myotube diameter by inhibiting glucocorticoid receptor activation and prevented muscle atrophy [127]. After oral administration of kefir supplementation, the forelimb grip strength scores, endurance swimming time, and muscle mass of mice were significantly higher than in controls, and the composition of the gut microbiota of mice was changed (reduced Firmicutes/Bacteroidetes ratio) and tissue glycogen content was also significantly increased after kefir supplementation [128]. After oral administration of Lactobacillus casei LC122 or Bifidobacterium longum BL986 for 12 weeks, these two probiotics improved intestinal barrier function, increased muscle strength, and reduced oxidative stress and inflammation in peripheral tissues [129]. Lactobacillus paracasei PS23 restores mitochondrial dysfunction due to aging in mice, reduces inflammatory factor activity, and has potential therapeutic implications for decreased skeletal muscle function and quality [130]. Colonization of Eubacterium rectale or Clostridium coccoides in mice increases endurance swimming fatigue time [131]. Veillonella atypica was isolated from fecal samples of marathon runners. Inoculation of this strain into mice significantly increases treadmill running exhaustion time, and Veillonella atypica improves running time by converting exercise-induced lactate metabolism to propionic acid [132]. Transferring fecal samples from older adults (high-functioning/low-functioning group) into GF mice found significantly increased grip strength in high-functioning mice compared to low-functioning mice [52]. Treatment with Faecalibacterium prausnitzii increased muscle mass in high-fat-fed mice, which may be associated with enhanced mitochondrial respiration, altered intestinal microbiota composition, reduced inflammatory response, and improved intestinal integrity [133]. Lactobacillus salivarius subspecies salicinius (SA-03) was isolated from the gut microbiota of gold medal weight lifters and then orally fed to mice for 4 weeks, resulting in a significant improvement in muscle strength and endurance performance and an increase in liver and muscle glycogen stores [134]. Similarly, Bifidobacterium longum (OLP-01), isolated from gold medal winners in weightlifting, was supplemented into mice and found that OLP-01 supplementation improved grip strength and endurance in mice and significantly increased liver and muscle glycogen levels [135]. Compared with GF mice, mice in the Bacteroides fragilis group showed increased endurance swimming time, reduced physical fatigue, and lower serum superoxide dismutase activity than GF mice [54].
Many studies have demonstrated that the gut microbiota can produce SCFA by fermenting indigestible carbohydrates [136]. SCFAs consist of three primary components: acetate, propionate, and butyrate; all of which are absorbed in the intestinal lumen and influence muscle and fat metabolism [137]. After feeding SCFA to GF mice, it was found that GF mice showed greater gastrocnemius muscle mass and strength, and the grip strength of GF mice was increased, which was consistent with the fact that SCFA increased muscle density, muscle mass, and function in GF mice by regulating the expression of Atrogin-1 and Murf-1 [23]. Butyrate prevents the loss of skeletal muscle mass and function during aging. After butyrate treatment, aged mice were found to have increased muscle fibers, prevented intramuscular fat accumulation, decreased fat mass in mice, and improved glucose metabolism and mitochondrial function in skeletal muscle [138].
After 13 weeks of oral administration of prebiotics consisting of a mixture of inulin plus fructooligosaccharides to elderly people aged 65 and over with frailty syndrome, these participants were found to have improved muscle strength and reduced fatigue, possibly because the prebiotics affected the body's immune function by promoting the growth of beneficial bacteria, inhibiting the growth of pathogens, and reducing other proinflammatory cytokines [139]. In triathletes, Lactobacillus plantarum PS128 increased endurance running performance, which was linked to changes in microbiota composition and greater levels of SCFAs [140]. Lactobacillus plantarum TWK10 has been shown in previous studies to improve exercise performance in mouse models, and LP10 has also been shown to do the same in human experiments. In healthy adults taking LP10 daily, it was found that LP10 significantly increased human exercise capacity in a dose-dependent manner, as well as improved fatigue-related performance and significantly increased muscle mass [141]. An observational study of older men found that a diet high in dietary fiber had higher physical performance indicators, higher scores on the short physical performance battery (SPPB), and higher grip strength, and that a diet high in dietary fiber may have a positive effect on the body's production of butyrate [142]. In a test of 32 sedentary older women over the age of 65, 12 weeks of aerobic training altered the participants' gut microbiota diversity and increased trunk muscle strength, and fecal SCFA level content has also been increased [143]. After supplementing 10 male runners with probiotic capsules daily for 4 weeks, it was found that probiotic supplements significantly increased runners' fatigued exercise time in the heat [144]. In a test of young adult female swimmers, it was found that after 8 weeks of supplementation with probiotic yogurt, the athletes' aerobic performance improved [145].
5. Conclusion and Future Perspectives
The role of the gut microbiota–muscle axis plays a crucial role in both humans and animals. The gut microbiota interacts with skeletal muscle through inflammatory immunity, autophagy, protein anabolism, energy, lipids, neuromuscular connectivity, oxidative stress, mitochondrial function, and endocrine and insulin resistance, thus affecting the physiological functions of the body (Figure 7). Specifically, the host's diet provides nutritional resupply to the gut microbiota, which maintains the structural integrity and the health of the gut, and participates in and mediates nutrient absorption and metabolism in the gut, which provides the material basis for muscle growth and development. Substances such as neurotransmitters, SCFAs, and bile acids produced by the metabolism of the gut microbiota regulate energy consumption and storage through the nervous and circulatory systems, providing energy for muscle development. The gut microbiota also influences the secretion of insulin, glucocorticoids, and leptin through the endocrine system, hormones that are important regulators of muscle growth and development. In addition, disturbance of the gut microbiota and invasion of exogenous harmful substances can lead to the impaired intestinal barrier and increased secretion of proinflammatory cytokines, which can negatively affect muscle growth and development.
Figure 7.
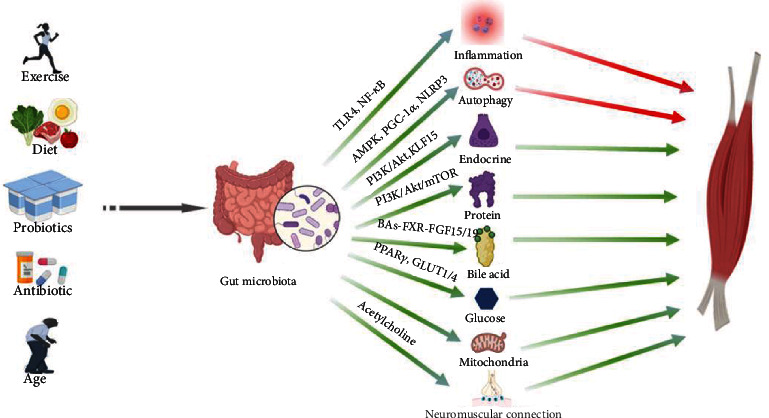
The gut–muscle axis under physiological and pathological conditions. Red arrows represent negative effects on muscles, and green arrows represent positive effects on muscles.
Dietary supplementation, probiotics and/or prebiotics, SCFAs, and exercise can influence the composition of the gut microbiota, improving skeletal muscle mass and function. Although there is now a large body of research demonstrating a strong link and communication between gut microbiota and muscle tissue, there are no clear experiments showing which type or types of probiotics and/or prebiotics, SCFA, promote muscle growth and development, and there is also a lack of research on the quantitative nature of supplements.
To validate the above influencing factors and the mechanisms involved, a large number of high-quality interventional experimental studies are needed to demonstrate how dietary supplementation, probiotics and/or prebiotics, SCFAs, and exercise affect the gut microbiota. It is believed that as research methods continue to advance, the understanding of the gut microbiota–muscle axis will become more advanced. By regulating the gut microbiota, people can improve several diseases caused by reduced skeletal muscle mass and function.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81701965) and the Natural Science Foundation of Liaoning Province (20180550116 and 2019-MS-069).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Guangyao Li and Binghui Jin contributed equally to this work.
References
- 1.Lieber R. L., Roberts T. J., Blemker S. S., Lee S. S., Herzog W. Skeletal muscle mechanics, energetics and plasticity. Journal of Neuroengineering and Rehabilitation . 2017;14(1):p. 108. doi: 10.1186/s12984-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. Journal of Diabetes & Metabolic Disorders . 2017;16(1):1–10. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A. J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing . 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer I., Mets T., Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Current Opinion in Clinical Nutrition and Metabolic Care . 2012;15(1):12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 5.Sakuma K., Yamaguchi A. Sarcopenia and age-related endocrine function. International Journal of Endocrinology . 2012;2012:10. doi: 10.1155/2012/127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleasby M. E., Jamieson P. M., Atherton P. J. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. The Journal of Endocrinology . 2016;229(2):R67–R81. doi: 10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- 7.Azzolino D., Passarelli P. C., De Angelis P., Piccirillo G. B., D’Addona A., Cesari M. Poor oral health as a determinant of malnutrition and sarcopenia. Nutrients . 2019;11(12):p. 2898. doi: 10.3390/nu11122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson M. J., McArdle A. Role of reactive oxygen species in age-related neuromuscular deficits. The Journal of Physiology . 2016;594(8):1979–1988. doi: 10.1113/JP270564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson M. D., Cross B. L., Grosicki G. J. Evidence for the contribution of gut microbiota to age-related anabolic resistance. Nutrients . 2021;13(2):p. 706. doi: 10.3390/nu13020706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henningsen J., Rigbolt K. T. G., Blagoev B., Pedersen B. K., Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Molecular & Cellular Proteomics . 2010;9(11):2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shreiner A. B., Kao J. Y., Young V. B. The gut microbiome in health and in disease. Current Opinion in Gastroenterology . 2015;31(1):69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lustgarten M. S. Classifying aging as a disease: the role of microbes. Frontiers in Genetics . 2016;7 doi: 10.3389/fgene.2016.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przewłócka K., Folwarski M., Kaźmierczak-Siedlecka K., Skonieczna-Żydecka K., Kaczor J. J. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients . 2020;12(5):p. 1451. doi: 10.3390/nu12051451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carabottia M., Sciroccoa A., Masellib M. A., Severia C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology . 2015;28(2):203–209. [PMC free article] [PubMed] [Google Scholar]
- 15.Grosicki G. J., Fielding R. A., Lustgarten M. S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut-muscle axis. Calcified Tissue International . 2018;102(4):433–442. doi: 10.1007/s00223-017-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lochlainn M. N., Bowyer R., Steves C. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients . 2018;10(7):p. 929. doi: 10.3390/nu10070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picca A., Fanelli F., Calvani R., et al. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators of Inflammation . 2018;2018:15. doi: 10.1155/2018/7026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ticinesi A., Nouvenne A., Cerundolo N., et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients . 2019;11(7):p. 1633. doi: 10.3390/nu11071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lustgarten M. S. The role of the gut microbiome on skeletal muscle mass and physical function: 2019 update. Frontiers in Physiology . 2019;10:p. 1435. doi: 10.3389/fphys.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biology . 2016;14(8, article e1002533) doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang L., Li P., Wang D., Wang T., Hao D., Qu X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Scientific Reports . 2021;11(1):p. 4628. doi: 10.1038/s41598-021-84031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montagner A., Korecka A., Polizzi A., et al. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Scientific Reports . 2016;6(1):1–15. doi: 10.1038/srep23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahiri S., Kim H., Garcia-Perez I., et al. The gut microbiota influences skeletal muscle mass and function in mice. Science Translational Medicine . 2019;11(502) doi: 10.1126/scitranslmed.aan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ticinesi A., Lauretani F., Milani C., et al. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: is there a gut-muscle axis? Nutrients . 2017;9(12):p. 1303. doi: 10.3390/nu9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heintz C., Mair W. You are what you host: microbiome modulation of the aging process. Cell . 2014;156(3):408–411. doi: 10.1016/j.cell.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manickam R., Oh H., Tan C., Paramalingam E., Wahli W. Metronidazole causes skeletal muscle atrophy and modulates muscle chronometabolism. International Journal of Molecular Sciences . 2018;19(8):p. 2418. doi: 10.3390/ijms19082418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Consortium M. H. I. T., Jia H., et al. An integrated catalog of reference genes in the human gut microbiome. Nature Biotechnology . 2014;32(8):834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 28.Liguori I., Russo G., Aran L., et al. Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clinical Interventions in Aging . 2018;Volume 13:913–927. doi: 10.2147/CIA.S149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dave M., Higgins P. D., Middha S., Rioux K. P. The human gut microbiome: current knowledge, challenges, and future directions. Translational Research . 2012;160(4):246–257. doi: 10.1016/j.trsl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 30.O’Toole P. W., Jeffery I. B. Microbiome-health interactions in older people. Cellular and Molecular Life Sciences: CMLS . 2018;75(1):119–128. doi: 10.1007/s00018-017-2673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt T. S. B., Raes J., Bork P. The human gut microbiome: from association to modulation. Cell . 2018;172(6):1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Ren Z., Guo C., Yu S., et al. Progress in mycotoxins affecting intestinal mucosal barrier function. International Journal of Molecular Sciences . 2019;20(11):p. 2777. doi: 10.3390/ijms20112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otani T., Furuse M. Tight junction structure and function revisited. Trends in Cell Biology . 2020;30(10):805–817. doi: 10.1016/j.tcb.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Hao W., Hao C., Wu C., Xu Y., Jin C. Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers. Chemosphere . 2022;288(2, article 132556) doi: 10.1016/j.chemosphere.2021.132556. [DOI] [PubMed] [Google Scholar]
- 35.Ohland C. L., MacNaughton W. K. Probiotic bacteria and intestinal epithelial barrier function. American Journal of Physiology. Gastrointestinal and Liver Physiology . 2010;298(6):G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn K. A., Pedraza I., Demoruelle M. K. Mucosal immune responses to microbiota in the development of autoimmune disease. Rheumatic Diseases Clinics of North America . 2014;40(4):711–725. doi: 10.1016/j.rdc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Rossi O., Van Baarlen P., Wells J. M. Host-recognition of pathogens and commensals in the mammalian intestine. Current Topics in Microbiology and Immunology . 2013;358:291–321. doi: 10.1007/82_2011_191. [DOI] [PubMed] [Google Scholar]
- 38.Mörbe U. M., Jørgensen P. B., Fenton T. M., et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunology . 2021;14(4):793–802. doi: 10.1038/s41385-021-00389-4. [DOI] [PubMed] [Google Scholar]
- 39.Wu J., Zhao Y., Wang X., et al. Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Critical Reviews in Food Science and Nutrition . 2022;62(3):783–797. doi: 10.1080/10408398.2020.1828813. [DOI] [PubMed] [Google Scholar]
- 40.Tsilimigras M. C., Fodor A., Jobin C. Carcinogenesis and therapeutics: the microbiota perspective. Nature Microbiology . 2017;2(3) doi: 10.1038/nmicrobiol.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delzenne N. M., Cani P. D. Interaction between obesity and the gut microbiota: relevance in nutrition. Annual Review of Nutrition . 2011;31(1):15–31. doi: 10.1146/annurev-nutr-072610-145146. [DOI] [PubMed] [Google Scholar]
- 42.Martin A. M., Sun E. W., Rogers G. B., Keating D. J. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Frontiers in Physiology . 2019;10 doi: 10.3389/fphys.2019.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Research . 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takiishi T., Fenero C. I. M., Câmara N. O. S. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers . 2017;5(4, article e1373208) doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karakula-Juchnowicz H., Rog J., Juchnowicz D., et al. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutrition Journal . 2019;18(1):p. 50. doi: 10.1186/s12937-019-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grochowska M., Wojnar M., Radkowski M. The gut microbiota in neuropsychiatric disorders. Acta Neurobiologiae Experimentalis . 2018;78(2):69–81. doi: 10.21307/ane-2018-008. [DOI] [PubMed] [Google Scholar]
- 47.Siddharth J., Chakrabarti A., Pannérec A., et al. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging . 2017;9(7):1698–1720. doi: 10.18632/aging.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picca A., Ponziani F. R., Calvani R., et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients . 2020;12(1) doi: 10.3390/nu12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro‐Mejía J. L., Khakimov B., Krych Ł., et al. Physical fitness in community-dwelling older adults is linked to dietary intake, gut microbiota, and metabolomic signatures. Aging Cell . 2020;19(3, article e13105) doi: 10.1111/acel.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellanos N., Diez G. G., Antúnez-Almagro C., et al. A critical mutualism-competition interplay underlies the loss of microbial diversity in sedentary lifestyle. Frontiers in Microbiology . 2020;10 (3142 doi: 10.3389/fmicb.2019.03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bressa C., Bailén-Andrino M., Pérez-Santiago J., et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One . 2017;12(2, article e0171352) doi: 10.1371/journal.pone.0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fielding R. A., Reeves A. R., Jasuja R., Liu C., Barrett B. B., Lustgarten M. S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Experimental Gerontology . 2019;127:p. 110722. doi: 10.1016/j.exger.2019.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Louis P., Flint H. J. Formation of propionate and butyrate by the human colonic microbiota. Environmental Microbiology . 2017;19(1):29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 54.Hsu Y. J., Chiu C. C., Li Y. P., et al. Effect of intestinal microbiota on exercise performance in mice. Journal of Strength and Conditioning Research . 2015;29(2):552–558. doi: 10.1519/JSC.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 55.Blanton L. V., Charbonneau M. R., Salih T., et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science . 2016;351(6275) doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langille M. G., Meehan C. J., Koenig J. E., et al. Microbial shifts in the aging mouse gut. Microbiome . 2014;2(1):p. 50. doi: 10.1186/s40168-014-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C. S., Wei Q., Wang H., et al. Protective effects of ghrelin on fasting-induced muscle atrophy in aging mice. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences . 2020;75(4):621–630. doi: 10.1093/gerona/gly256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suriano F., Van Hul M., Cani P. D. Gut microbiota and regulation of myokine-adipokine function. Current Opinion in Pharmacology . 2020;52:9–17. doi: 10.1016/j.coph.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Nay K., Jollet M., Goustard B., et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. American Journal of Physiology Endocrinology and Metabolism . 2019;317(1):E158–E171. doi: 10.1152/ajpendo.00521.2018. [DOI] [PubMed] [Google Scholar]
- 60.De Spiegeleer A., Elewaut D., Van Den Noortgate N., et al. Quorum sensing molecules as a novel microbial factor impacting muscle cells. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease . 2020;1866(3, article 165646) doi: 10.1016/j.bbadis.2019.165646. [DOI] [PubMed] [Google Scholar]
- 61.Viaene L., Thijs L., Jin Y., et al. Heritability and clinical determinants of serum indoxyl sulfate and p-cresyl sulfate, candidate biomarkers of the human microbiome enterotype. PLoS One . 2014;9(5, article e79682) doi: 10.1371/journal.pone.0079682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saoi M., Li A., McGlory C., et al. Metabolic perturbations from step reduction in older persons at risk for sarcopenia: plasma biomarkers of abrupt changes in physical activity. Metabolites . 2019;9(7):p. 134. doi: 10.3390/metabo9070134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thome T., Salyers Z. R., Kumar R. A., et al. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. American Journal of Physiology - Cell Physiology . 2019;317(4):C701–C713. doi: 10.1152/ajpcell.00098.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rastelli M., Cani P. D., Knauf C. The gut microbiome influences host endocrine functions. Endocrine Reviews . 2019;40(5):1271–1284. doi: 10.1210/er.2018-00280. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh S., Lertwattanarak R., Garduño J. D., et al. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences . 2015;70(2):232–246. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thevaranjan N., Puchta A., Schulz C., et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe . 2017;21(4):455–66.e4. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benton M. Sarcopenic obesity: strategies for management. The American Journal of Nursing . 2011;111(12):38–44. doi: 10.1097/01.NAJ.0000408184.21770.98. quiz 5-6. [DOI] [PubMed] [Google Scholar]
- 68.Ryu J. Y., Choi H. M., Yang H. I., Kim K. S. Dysregulated autophagy mediates sarcopenic obesity and its complications via AMPK and PGC1α signaling pathways: potential involvement of gut dysbiosis as a pathological link. International Journal of Molecular Sciences . 2020;21(18):p. 6887. doi: 10.3390/ijms21186887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salminen A., Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Research Reviews . 2012;11(2):230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Ren J., Zhang Y. Targeting autophagy in aging and aging-related cardiovascular diseases. Trends in Pharmacological Sciences . 2018;39(12):1064–1076. doi: 10.1016/j.tips.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salminen A., Kaarniranta K., Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging . 2012;4(3):166–175. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sho T., Xu J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnology and Applied Biochemistry . 2019;66(1):4–13. doi: 10.1002/bab.1700. [DOI] [PubMed] [Google Scholar]
- 73.Afonina I. S., Zhong Z., Karin M., Beyaert R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nature Immunology . 2017;18(8):861–869. doi: 10.1038/ni.3772. [DOI] [PubMed] [Google Scholar]
- 74.Schakman O., Kalista S., Barbé C., Loumaye A., Thissen J. P. Glucocorticoid-induced skeletal muscle atrophy. The International Journal of Biochemistry & Cell Biology . 2013;45(10):2163–2172. doi: 10.1016/j.biocel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 75.Park S. S., Seo Y. K., Kwon K. S. Sarcopenia targeting with autophagy mechanism by exercise. BMB Reports . 2019;52(1):64–69. doi: 10.5483/BMBRep.2019.52.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu C., Cheung W. H., Li J., et al. Understanding the gut microbiota and sarcopenia: a systematic review. Journal of Cachexia, Sarcopenia and Muscle . 2021;12(6):1393–1407. doi: 10.1002/jcsm.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez A. M., Hoffman J. R., Stout J. R., Fukuda D. H., Willoughby D. S. Intramuscular anabolic signaling and endocrine response following resistance exercise: implications for muscle hypertrophy. Sports Medicine . 2016;46(5):671–685. doi: 10.1007/s40279-015-0450-4. [DOI] [PubMed] [Google Scholar]
- 78.Deane C. S., Ely I. A., Wilkinson D. J., Smith K., Phillips B. E., Atherton P. J. Dietary protein, exercise, ageing and physical inactivity: interactive influences on skeletal muscle proteostasis. The Proceedings of the Nutrition Society . 2021;80(2):106–117. doi: 10.1017/S0029665120007879. [DOI] [PubMed] [Google Scholar]
- 79.Zheng Y., Song Y., Han Q., et al. Intestinal epithelial cell-specific IGF1 promotes the expansion of intestinal stem cells during epithelial regeneration and functions on the intestinal immune homeostasis. American Journal of Physiology Endocrinology and Metabolism . 2018;315(4):E638–E649. doi: 10.1152/ajpendo.00022.2018. [DOI] [PubMed] [Google Scholar]
- 80.Barclay R. D., Burd N. A., Tyler C., Tillin N. A., Mackenzie R. W. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Frontiers in Nutrition . 2019;6 doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z., Liu H., Liu J. Akt activation: A potential strategy to ameliorate insulin resistance. Diabetes Research and Clinical Practice . 2019;156, article 107092 doi: 10.1016/j.diabres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Huang X., Liu G., Guo J., Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. International Journal of Biological Sciences . 2018;14(11):1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merz K. E., Thurmond D. C. Role of skeletal muscle in insulin resistance and glucose uptake. Comprehensive Physiology . 2020;10(3):785–809. doi: 10.1002/cphy.c190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martín A. I., Priego T., López-Calderón A. Hormones and muscle atrophy. Advances in Experimental Medicine and Biology . 2018;1088:207–233. doi: 10.1007/978-981-13-1435-3_9. [DOI] [PubMed] [Google Scholar]
- 85.Fan L., Hsieh P. N., Sweet D. R., Jain M. K. Krüppel-like factor 15: regulator of BCAA metabolism and circadian protein rhythmicity. Pharmacological Research . 2018;130:p. 123. doi: 10.1016/j.phrs.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 86.Fan L., Hsieh P. N., Sweet D. R., Jain M. K. Regulation of skeletal muscle function by amino acids. Nutrients . 2020;12(1):p. 261. doi: 10.3390/nu12010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atherton P. J., Smith K. Muscle protein synthesis in response to nutrition and exercise. The Journal of Physiology . 2012;590(5):1049–1057. doi: 10.1113/jphysiol.2011.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tipton K. D., Hamilton D. L., Gallagher I. J. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Medicine . 2018;48(Suppl 1):53–64. doi: 10.1007/s40279-017-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duan Y., Haybaeck J., Yang Z. Therapeutic potential of PI3K/AKT/mTOR pathway in gastrointestinal stromal tumors: Rationale and Progress. Cancers . 2020;12(10):p. 2972. doi: 10.3390/cancers12102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoon M. S. mTOR as a key regulator in maintaining skeletal muscle mass. Frontiers in Physiology . 2017;8 doi: 10.3389/fphys.2017.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshida T., Delafontaine P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cell . 2020;9(9):p. 1970. doi: 10.3390/cells9091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwarzer M., Makki K., Storelli G., et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science . 2016;351(6275):854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y., Liu Z., Shu S., Cai J., Tang C., Dong Z. AMPK/mTOR signaling in autophagy regulation during cisplatin-induced acute kidney injury. Frontiers in Physiology . 2020;11 doi: 10.3389/fphys.2020.619730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wahlström A., Sayin S. I., Marschall H. U., Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabolism . 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Sun L., Xie C., Wang G., et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nature Medicine . 2018;24(12):1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo A., Li K., Xiao Q. Fibroblast growth factor 19 alleviates palmitic acid-induced mitochondrial dysfunction and oxidative stress via the AMPK/PGC-1α pathway in skeletal muscle. Biochemical and Biophysical Research Communications . 2020;526(4):1069–1076. doi: 10.1016/j.bbrc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 97.Kir S., Beddow S. A., Samuel V. T., et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science . 2011;331(6024):1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiu Y., Yu J., Li Y., et al. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Annals of Medicine . 2021;53(1):508–522. doi: 10.1080/07853890.2021.1900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manickam R., Wahli W. Roles of peroxisome proliferator-activated receptor β/δ in skeletal muscle physiology. Biochimie . 2017;136:p. 42. doi: 10.1016/j.biochi.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Bougarne N., Weyers B., Desmet S. J., et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocrine Reviews . 2018;39(5):760–802. doi: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- 101.Naiman S., Huynh F. K., Gil R., et al. SIRT6 promotes hepatic beta-oxidation via activation of PPARα. Cell Reports . 2019;29(12):4127–4143. doi: 10.1016/j.celrep.2019.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan N. S., Vázquez-Carrera M., Montagner A., Sng M. K., Guillou H., Wahli W. Transcriptional control of physiological and pathological processes by the nuclear receptor PPARβ/δ. Progress in Lipid Research . 2016;64:98–122. doi: 10.1016/j.plipres.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 103.Christofides A., Konstantinidou E., Jani C., Boussiotis V. A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism . 2021;114, article 154338 doi: 10.1016/j.metabol.2020.154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chandrashekar P., Manickam R., Ge X., et al. Inactivation of PPARβ/δ adversely affects satellite cells and reduces postnatal myogenesis. American Journal of Physiology Endocrinology and Metabolism . 2015;309(2):E122–E131. doi: 10.1152/ajpendo.00586.2014. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y. X., Zhang C. L., Yu R. T., et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biology . 2004;2(10, article e294) doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schuler M., Ali F., Chambon C., et al. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metabolism . 2006;4(5):407–414. doi: 10.1016/j.cmet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Aguilar-Recarte D., Palomer X., Wahli W., Vázquez-Carrera M. The PPARβ/δ-AMPK connection in the treatment of insulin resistance. International Journal of Molecular Sciences . 2021;22(16):p. 8555. doi: 10.3390/ijms22168555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yarmohammadi F., Hayes A. W., Karimi G. Targeting PPARs signaling pathways in cardiotoxicity by natural compounds. Cardiovascular Toxicology . 2022;22(4):281–291. doi: 10.1007/s12012-021-09715-5. [DOI] [PubMed] [Google Scholar]
- 109.Miura P., Chakkalakal J. V., Boudreault L., et al. Pharmacological activation of PPAR / stimulates utrophin A expression in skeletal muscle fibers and restores sarcolemmal integrity in mature mdx mice. Human Molecular Genetics . 2009;18(23):4640–4649. doi: 10.1093/hmg/ddp431. [DOI] [PubMed] [Google Scholar]
- 110.Duszka K., Wahli W. Enteric microbiota−gut−brain axis from the perspective of nuclear receptors. International Journal of Molecular Sciences . 2018;19(8):p. 2210. doi: 10.3390/ijms19082210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mukherji A., Kobiita A., Ye T., Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell . 2013;153(4):812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 112.Franko A., Huypens P., Neschen S., et al. Bezafibrate improves insulin sensitivity and metabolic flexibility in STZ-induced diabetic mice. Diabetes . 2016;65(9):2540–2552. doi: 10.2337/db15-1670. [DOI] [PubMed] [Google Scholar]
- 113.Salvadó L., Barroso E., Gómez-Foix A. M., et al. PPARs and microbiota in skeletal muscle health and wasting. International Journal of Molecular Sciences . 2020;21(21):p. 8056. doi: 10.3390/ijms21218056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salvadó L., Barroso E., Gómez-Foix A. M., et al. PPARβ/δ prevents endoplasmic reticulum stress-associated inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia . 2014;57(10):2126–2135. doi: 10.1007/s00125-014-3331-8. [DOI] [PubMed] [Google Scholar]
- 115.Paravamsivam P., Heng C. K., Malek S. N., Sabaratnam V., Kuppusamy U. R. Giant oyster mushroom Pleurotus giganteus (Agaricomycetes) enhances adipocyte differentiation and glucose uptake via activation of PPARγ and glucose transporters 1 and 4 in 3T3-L1 cells. International Journal of Medicinal Mushrooms . 2016;18(9):821–831. doi: 10.1615/IntJMedMushrooms.v18.i9.60. [DOI] [PubMed] [Google Scholar]
- 116.Hood D. A., Memme J. M., Oliveira A. N., Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annual Review of Physiology . 2019;81(1):19–41. doi: 10.1146/annurev-physiol-020518-114310. [DOI] [PubMed] [Google Scholar]
- 117.Kim Y., Triolo M., Hood D. A. Impact of aging and exercise on mitochondrial quality control in skeletal muscle. Oxidative Medicine and Cellular Longevity . 2017;2017:16. doi: 10.1155/2017/3165396.3165396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swenarchuk L. E. Nerve, Muscle, and Synaptogenesis. Cell . 2019;8(11):p. 1448. doi: 10.3390/cells8111448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh R. K., Chang H. W., Yan D. I., et al. Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine . 2017;15(1):p. 73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salazar N., González S., Nogacka A. M., et al. Microbiome: effects of ageing and diet. Current Issues in Molecular Biology . 2020;36:33–62. doi: 10.21775/cimb.036.033. [DOI] [PubMed] [Google Scholar]
- 121.Salazar N., Valdés-Varela L., González S., Gueimonde M., De Los Reyes-Gavilán C. G. Nutrition and the gut microbiome in the elderly. Gut Microbes . 2017;8(2):82–97. doi: 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strasser B., Wolters M., Weyh C., Krüger K., Ticinesi A. The effects of lifestyle and diet on gut microbiota composition, inflammation and muscle performance in our aging society. Nutrients . 2021;13(6):p. 2045. doi: 10.3390/nu13062045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Y. M., Wei L., Chiu Y. S., et al. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients . 2016;8(4):p. 205. doi: 10.3390/nu8040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metabolism . 2011;14(3):403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 125.Chen Y. M., Chiu W. C., Chiu Y. S., Li T., Sung H. C., Hsiao C. Y. Supplementation of nano-bubble curcumin extract improves gut microbiota composition and exercise performance in mice. Food & Function . 2020;11(4):3574–3584. doi: 10.1039/C9FO02487E. [DOI] [PubMed] [Google Scholar]
- 126.Okamoto T., Morino K., Ugi S., et al. Microbiome potentiates endurance exercise through intestinal acetate production. American Journal of Physiology Endocrinology and Metabolism . 2019;316(5):E956–E966. doi: 10.1152/ajpendo.00510.2018. [DOI] [PubMed] [Google Scholar]
- 127.Katsuki R., Sakata S., Nakao R., Oishi K., Nakamura Y. Lactobacillus curvatus CP2998 prevents dexamethasone-induced muscle atrophy in C2C12 myotubes. Journal of Nutritional Science and Vitaminology . 2019;65(5):455–458. doi: 10.3177/jnsv.65.455. [DOI] [PubMed] [Google Scholar]
- 128.Hsu Y. J., Huang W. C., Lin J. S., et al. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients . 2018;10(7):p. 862. doi: 10.3390/nu10070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ni Y., Yang X., Zheng L., et al. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Molecular Nutrition & Food Research . 2019;63(22, article e1900603) doi: 10.1002/mnfr.201900603. [DOI] [PubMed] [Google Scholar]
- 130.Chen L. H., Huang S. Y., Huang K. C., et al. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging . 2019;11(2):756–770. doi: 10.18632/aging.101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang W. C., Chen Y. H., Chuang H. L., Chiu C. C., Huang C. C. Investigation of the effects of microbiota on exercise physiological adaption, performance, and energy utilization using a gnotobiotic animal model. Frontiers in Microbiology . 2019;10:p. 10. doi: 10.3389/fmicb.2019.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scheiman J., Luber J. M., Chavkin T. A., et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nature Medicine . 2019;25(7):1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Munukka E., Rintala A., Toivonen R., et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. The ISME Journal . 2017;11(7):1667–1679. doi: 10.1038/ismej.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee M. C., Hsu Y. J., Ho H. H., et al. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms . 2020;8(4):p. 545. doi: 10.3390/microorganisms8040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee M. C., Hsu Y. J., Chuang H. L., et al. In vivo ergogenic properties of the Bifidobacterium longum OLP-01 isolated from a weightlifting gold medalist. Nutrients . 2019;11(9):p. 2003. doi: 10.3390/nu11092003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yatsunenko T., Rey F. E., Manary M. J., et al. Human gut microbiome viewed across age and geography. Nature . 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu J., Lin S., Zheng B., Cheung P. C. K. Short-chain fatty acids in control of energy metabolism. Critical Reviews in Food Science and Nutrition . 2018;58(8):1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 138.Walsh M. E., Bhattacharya A., Sataranatarajan K., et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell . 2015;14(6):957–970. doi: 10.1111/acel.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buigues C., Fernández-Garrido J., Pruimboom L., et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. International Journal of Molecular Sciences . 2016;17(6):p. 932. doi: 10.3390/ijms17060932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang W. C., Pan C. H., Wei C. C., Huang H. Y. Lactobacillus plantarum PS128 improves physiological adaptation and performance in triathletes through gut microbiota modulation. Nutrients . 2020;12(8):p. 2315. doi: 10.3390/nu12082315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang W. C., Lee M. C., Lee C. C., et al. Effect of Lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients . 2019;11(11):p. 2836. doi: 10.3390/nu11112836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barger K., Langsetmo L., Orwoll E. S., Lustgarten M. S. Investigation of the diet-gut-muscle axis in the osteoporotic fractures in men study. The Journal of Nutrition, Health & Aging . 2020;24(4):445–452. doi: 10.1007/s12603-020-1344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morita E., Yokoyama H., Imai D., et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients . 2019;11(4):p. 868. doi: 10.3390/nu11040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shing C. M., Peake J. M., Lim C. L., et al. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. European Journal of Applied Physiology . 2014;114(1):93–103. doi: 10.1007/s00421-013-2748-y. [DOI] [PubMed] [Google Scholar]
- 145.Salarkia N., Ghadamli L., Zaeri F., Sabaghian Rad L. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: a randomized controlled trial. Medical Journal of the Islamic Republic of Iran . 2013;27(3):141–146. [PMC free article] [PubMed] [Google Scholar]


