Abstract
Ageing and genetic traits can only explain the increasing dementia incidence partially. Advanced healthcare services allow dementia patients to survive natural selection and pass their genes onto the next generation. Country-specific estimates of dementia incidence rates (all ages and 15–49 years old), Biological State Index expressing reduced natural selection (Is), ageing indexed by life expectancy e(65), GDP PPP and urbanization were obtained for analysing the global and regional correlations between reduced natural selection and dementia incidence with SPSS v. 27. Worldwide, Is significantly, but inversely, correlates with dementia incidence rates for both all ages and 15–49 years old in bivariate correlations. These relationships remain inversely correlated regardless of the competing contributing effects from ageing, GDP and urbanization in partial correlation model. Results of multiple linear regression (enter) have shown that Is is the significant predictor of dementia incidence among all ages and 15–49 years old. Subsequently, Is was selected as the variable having the greatest influence on dementia incidence in stepwise multiple linear regression. The Is correlated with dementia incidence more strongly in developed population groupings. Worldwide, reduced natural selection may be yet another significant contributor to dementia incidence with special regard to developed populations.
Subject terms: Evolutionary genetics, Evolutionary theory
Introduction
Dementia is not one specific disease, but a syndrome that affects patient’s ability to perform everyday activities due to a chronic or progressive deterioration of memory, thinking and behaviour1. Worldwide, around 50 million people are being affected by Alzheimer's (60–70%), vascular diseases (20–30%), Lewy bodies and frontotemporal dementia1,2. Worldwide, dementia not only affects individual patients whose human rights and freedom are unnecessarily restricted and subject to social stigma, but also has tremendous impact on families, caregivers, and society1,3.
Although extensive studies have been conducted to explore the etiological factors, only ageing and genetic susceptibility are constantly postulated as the risk factors4,5. Fox6 has hypothesized that, from evolutionary medicine perspective, humans have been living in a mismatched environment, which may be a risk factor for the increase of dementia onsets. Nevertheless, increasing evidence has shown that chronic health conditions (e.g. diabetes, obesity, hypertension, hearing impairment and cerebrovascular lesions), poor lifestyles (e.g. tobacco smoking, lack of exercise, gluten and meat eating) and psychosocial factors, such as less social engagement and depression are potential risk factors of dementia7–10. Recently, it has been shown that excessive alcohol consumption, traumatic brain injury, and air pollution could be responsible for dementia initiation11.
The World Health Organization (WHO) has estimated that, in 2021, worldwide, there were more than 55 million people living with dementia. With nearly 10 million new cases every year and the increasing proportion of older people in the population, the number of people living with dementia is expected to rise to 78 million in 2030 and 139 million in 20501,12. Similar trends were also reported in a number of individual countries, such as United Kingdom, China, Australia and United States. Population ageing has been the only risk factor used to explain this increase of annual incidence at a population level1. Almost all the studies have indicated that the increase of a population segment aged 65+ is contributing to the increasing presence of dementia.
M. Henneberg and J. Piontek advanced the Biological State Index (Ibs) based on the calculation of a population specific fertility and mortality data13,14. The Ibs measures probability for an average person in a population to have an opportunity to pass their genes to the next generation. A number of publications co-authored by M. Henneberg have revealed that this probability in different modern populations varies between 63.5% (Burkina Faso) and 99.4% (Iceland and Cyprus), and the average was 92.8%15–18.
It is a common sense that advanced healthcare services have allowed more and more people to survive to the age of reproduction and continue through their reproductive age span successfully. From the perspective of Darwinism, the underlying reason is that advanced healthcare services have relaxed natural selection leading to low mortality and low fertility19. With the greater portion of a population participating in the reproduction, a concern has been raised that the chance for deleterious genes/mutations being passed onto their next generations has been and will be increasing20. The consequence of this accumulating process is that a human population is subject to increasing incidence of non-communicable diseases. Dementia is an umbrella term for a number of non-communicable diseases, some with genetic traits, which can be passed onto the next generation.
The genetic causes of dementia have two forms: (1) directly causing dementia, including Alzheimer's, vascular, Lewy bodies and frontotemporal diseases; (2) causing comorbidities (chronic diseases: diabetes17,21, obesity15,16, hypertension, mental health disorders). Modern healthcare services have made it possible that people carrying genes/mutations contributing to dementia diseases or its comorbidities are free of dementia presence for all their life, or suffer from mild dementia. These people can reproduce and pass their dementia related genes/mutations to next generations. The portion of a population able to participate in reproduction determines the level of the deleterious genes to be inherited by the next generation. Inspired by these concepts we obtained population specific empirical and macro-level data to examine, from a worldwide perspective, whether populations with higher level of relaxed natural selection have greater dementia incidence.
Material and method
Data sources
The dependent variable are population specific incidence rates of “Alzheimer's disease and other dementias (dementia hereafter)” published in the 2020 by the Institute for Health Metrics and Evaluation22,23. The estimate of dementia incidence is expressed as the number of people per 100,000 who were newly diagnosed with dementia in the 2019.
In this cross-sectional study, natural selection is the essential part of the study method, and it is primarily related to reproduction. Therefore, for data analysis the dementia incidence rates (1) for all ages and (2) only for 15–49 years old were extracted.
The predicting variable is a current population specific level of natural selection measured by the Biological State Index, (Ibs) and its derivative, the index of opportunity for selection (Is) extracted from previous publications15,16,18.
The level of reduced natural selection is measured with the Biological State Index (Ibs) which was calculated with the population specific fertility and mortality data published by United Nations (2008) and WHO (2012) respectively24,25. The Ibs presents the probability that an average individual born into a population is able to fully participate in the reproduction of the next generation giving them an opportunity to pass their genes/mutations to their offspring13–15. Considering that the prime driver of Darwinian fitness (adaptive success) is the variance of reproductive success20,26, the opportunity for selection (Is) was calculated with the formula, Is = (1 − Ibs)/Ibs. This was considered as the independent variable15,18,20.
Except for ageing, the two other variables (GDP and urbanization) which have been associated with dementia were also included into data analyses as potential competing variables. Considering the delayed effects of these predicting factors on the dementia onset in 2019, they were backdated for 4–5 years.
Ageing is expressed with life expectancy at 65 years old (Life e(65), 2010–2015). These data are regularly published by the United Nations. This study does not take life expectancy at birth because dementia is more common in people over the age of 65, although it can also affect younger people. Another consideration for us to include Life e(65) is that the biopsychosocial functioning of people starts to decline from that age.
GDP PPP is expressed in per capita purchasing power parity in 2015 US dollars published by the World Bank27. GDP PPP has been associated with prevalence of dementia1,12, and it also influences the level of healthcare services that includes screening for dementia and their treatments.
Urbanization is expressed by the percentage of population living in urban areas in 2015 as published by the World Bank27. Urbanization entails a high level of education, but a poor lifestyle, for example, lack of exercise and social engagement, consumption of food with few nutritional benefits, more gluten and meat eating, air pollution, and consuming more salt, fat, sugar and alcohol. Urban lifestyle has been postulated as a complex risk factor for chronic diseases28.
We extracted a list of 204 populations with dementia incidence in 2019, and then we downloaded population specific Is life expectancy e(65), GDP PPP and urbanization before matching them with the dementia incidence list. A set of the population specific data for 204 countries was obtained and stored in the Microsoft Excel® for data analyses. For some populations, the estimate of one or the other variable was missing, thus specific analyses have sample sizes varying from 182 to 204. Each population was treated as an individual study subject and all of their available information was analysed.
In order to demonstrate the universal predicting effect of reduced natural selection on dementia incidence, the populations were grouped for further correlation analyses based on: (1) the WHO geographic regions29; (2) the World Bank income classifications30; (3) the United Nations gross national income (GNI) classifications31; (4) the strong contrast in terms of geographic distributions, per capita GDP PPP levels and cultural backgrounds to get seven population groupings: Asia Cooperation Dialogue (ACD)32, the Asia–Pacific Economic Cooperation (APEC)33, the Arab World33, Population with English as the official language (extracted from personal knowledge and experience), Latin America and the Caribbean (LAC)34, Organization for Economic Co-operation and Development (OECD)33, and Southern African Development Community (SADC)35. In these analyses, we only included those populations for which we could access their data for the specific groupings. Except for the population with English as the official language, all the other population listings were sourced from their respective official websites before matching them with the list of populations with dementia incidence.
Data analysis
To examine the relationships between the relaxation of natural selection and dementia incidence in different data analysis models, the analysis proceeded in four (4) steps36–39:
-
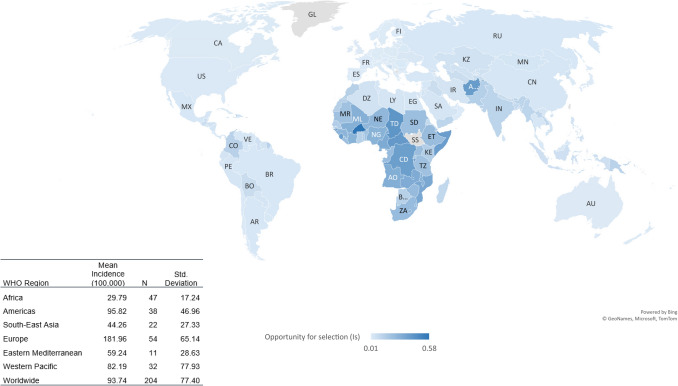
GeoNames, MS, TomTom (©, powered by Bing) was applied to integrate populations into the geographic map in the Excel (®MicroSoft 2016) depending on their locations. The darkness of colour for their areas in the map varies with their level of Is, the opportunity for natural selection. For mapping clarity and larger number of populations to be included in the map, the population label on the map is indicated as the ISO code of the population instead of the full name.
Scatter plots were also prepared for exploring and visualizing the correlation between the opportunity for natural selection and dementia incidence at a population level. Data quality and variable distributions can be examined in scatter plots as well. Mapping selection opportunity, calculations of mean Is, sample size and standard deviation, and producing scatter plots Excel (Microsoft® 2016) have been done using raw data (not log-transformed).
The 204 countries were also grouped as per the WHO geographic classifications for comparing mean Is in different regions.
Before running correlation analyses all data were logarithmed (ln), which reduced possible curvilinearity of regressions and data non-homoscedasticity due to their distributions.
Bivariate correlations (Pearson’s and nonparametric, Spearman’s “rho”) were conducted to examine the strength and direction of the correlations between all variables. Bivariate correlations were also performed for each data set of population groups to further explore and compare the correlations between Is and dementia incidence.
-
Partial correlation of Pearson’s moment-product correlation was performed to examine the correlation between selection opportunity and dementia incidence while the competing variables (ageing, GDP PPP and urbanization) were kept statistically constant.
We alternated the four variables (Is, ageing, GDP PPP and urbanization) as the predicting variable to explore its relationship with dementia incidence while controlling for all the other three variables. Thus, we could analyse and compare the levels of the independent correlations between dementia incidence and each of four potential risk factors40,41.
Standard multiple linear regression (enter model) was conducted to analyze the correlations between dementia incidence and each of the four predicting variables. Subsequently, stepwise linear regression was performed to select the predictor(s) having the best influencing effects on dementia incidence.
In the above Steps 2–4, dementia incidence rates were alternated as the dependent variables for data analyses, and results were reported in parallel.
Bivariate correlations, Pearson’s moment-product partial correlation and multiple linear regressions were performed in SPSS v. 27 (Chicago Il USA). The significance was reported when p was below 0.05, but the stronger significance levels, such as p < 0.01 and p < 0.001 were also indicated in this study. Regression analysis criteria were set at probability of F to enter ≤ 0.05 and probability of F to remove ≥ 0.10.
All the above data analysis methods were performed in accordance with the relevant guidelines and regulations.
Ethics approval and informed consent
All the data supporting our findings in this paper were freely downloaded from the United Nation agencies’ websites. No ethical approval or written informed consent for participation was required.
Consent for publication
Not applicable.
Results
Figure 1 shows that, currently every population has very low magnitude of Is because natural selection in each population has been reduced significantly by the advanced healthcare services. Worldwide, the Is ranges between 0.01 (Iceland and Cyprus) and 0.58 (Burkina Faso), and the arithmetic mean is 0.09, which has been approximately 2.5 times reduced from 100 to 150 years ago (Is = 0.22)20. In other words, worldwide, humans’ opportunity for natural selection with the advent of modern medicine has decreased approximately 2.5 times in the past 100–150 years. Among the six WHO Regions, the highest and lowest Is lied in Africa (Is = 0.23) and Europe (Is = 0.02).
Figure 1.
The magnitudes of the opportunity for natural selection Is in different countries.
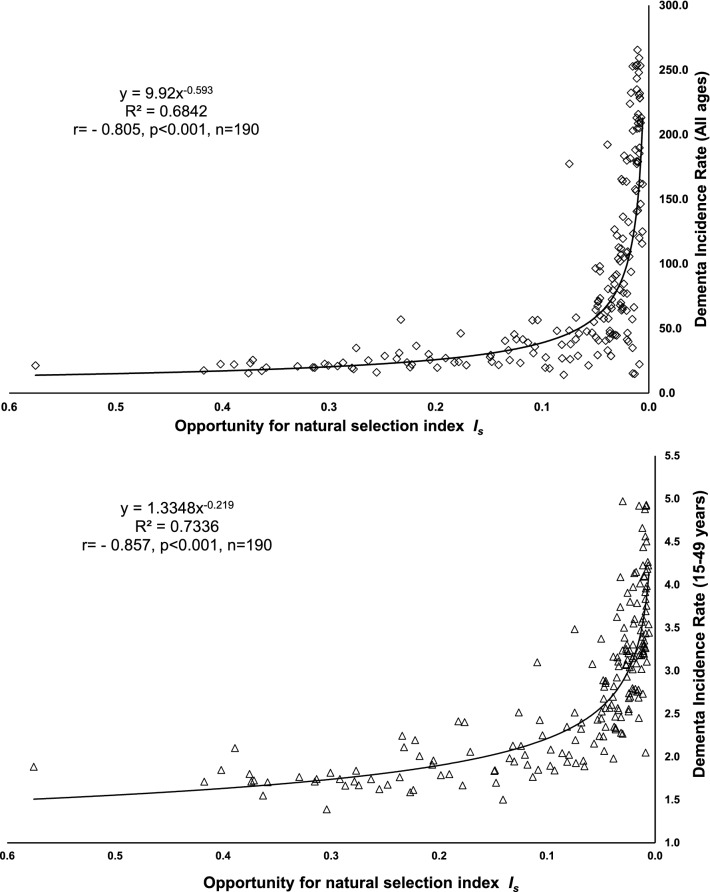
Figure 2 shows that, inversely, Is was in power correlation with dementia incidence in populations of all ages (r = − 0.805, p < 0.001, n = 190) and in 15–49 years old (r = − 0.857, p < 0.001, n = 190).
Figure 2.
The relationships between opportunity for selection Is and dementia incidence in populations of full age range and in 15–49 years old population segments.
Table 1 shows that Is is a significant predicting variable of dementia incidence in bivariate analyses. Worldwide, dementia incidence has significant and strong, but inverse, associations with Is in both Pearson’s r (r = − 0.827, p < 0.001) and Spearman’s rho (r = − 0.857, p < 0.001). Additionally, in both data analysis models, dementia incidence is in significant and moderate, but positive, associations with ageing, GDP PPP and Urbanization respectively (r > 0.500, p < 0.001). Table 1 also presents correlations between all variables.
Table 1.
Pearson’s r (above the diagonal) and Spearman’s rho (below the diagonal) between the opportunity for selection (Is) and dementia incidence.
| All ages | 15–49 years old | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dementia incidence | Is | Ageing | GDP PPP | Urban | Dementia incidence | Is | Ageing | GDP PPP | Urban | ||
| Dementia incidence | 1 | − 0.827*** | 0.773*** | 0.724*** | 0.518*** | Dementia incidence | 1 | − 0.857*** | 0.752*** | 0.793*** | 0.555*** |
| Is | − 0.830*** | 1 | − 0.827*** | − 0.866*** | − 0.594*** | Is | − 0.861*** | 1 | − 0.827*** | − 0.866*** | − 0.594*** |
| Ageing | 0.776*** | − 0.835*** | 1 | 0.754*** | 0.560*** | Ageing | 0.752*** | − 0.827*** | 1 | 0.754*** | 0.560*** |
| GDP PPP | 0.732*** | − 0.877*** | 0.767*** | 1 | 0.698*** | GDP PPP | 0.793*** | − 0.866*** | 0.754*** | 1 | 0.698*** |
| Urbanization | 0.522*** | − 0.647*** | 0.645*** | 0.738*** | 1 | Urbanization | 0.555*** | − 0.594*** | 0.560*** | 0.698*** | 1 |
Significance level: *p < 0.05; **p < 0.01; ***p < 0.001; Sample size range: 175–204.
Data sources: Dementia Incidence expressed as the number of new onsets of Alzheimer's disease and other dementias published by the Institute for Health Metrics and Evaluation (IHME) in 2020; Is measuring the magnitude of the opportunity for natural selection, extracted from the previous publication by You et al. https://doi.org/10.1111/eva.12523; Ageing expressed with life expectancy at 65 years old (2010–2015) published by the United Nations; GDP PPP expressed in purchasing power parity in 2015 US dollars published by the World Bank; Urbanization expressed in percent of population living in urban areas in 2015 published by the World Bank.
Table 2 suggests that Is is a significant risk factor for dementia incidence regardless of the competing effects of ageing, GDP PPP and urbanization. This is evidenced by examining the relationship between dementia incidence and Is, ageing, GDP PPP and urbanization respectively which were alternated as the predicting variable while the other three variables were kept statistically constant. In populations of all ages, dementia incidence significantly correlates with Is (r = − 0.429, p < 0.001) and ageing (r = 0.278, p < 0.001). However, dementia incidence correlates with Is stronger than it does with ageing where it is on the edge of significant level (z = 1.58, p = 0.0571). In population segments of 15–49 years old, dementia incidence is in significant correlation with Is (r = − 0.452, p < 0.001), ageing (r = 0.126, p < 0.001) and GDP PPP (r = 0.160, p < 0.05). Dementia incidence, however, is in significantly stronger correlation with Is than with ageing (z = 1.58, p < 0.001) and GDP PPP (z = 2.98, p < 0.001).
Table 2.
Comparison of partial correlation coefficients between dementia incidence and each variable when the other three variables were kept statistically constant.
| Variables | r | p | r | p | r | p | r | p |
|---|---|---|---|---|---|---|---|---|
| Dementia incidence rate in populations of all ages | ||||||||
| Is | − 0.429 | < 0.001 | – | – | – | – | – | – |
| Ageing | – | – | 0.278 | < 0.001 | – | – | – | – |
| GDP PPP | – | – | – | – | − 0.018 | 0.800 | – | – |
| Urban | – | – | – | – | – | – | < 0.05 | 0.763 |
| Dementia incidence rate in population segments of 15–49 years old | ||||||||
| Is | − 0.452 | < 0.001 | – | – | – | – | – | – |
| Ageing* | NA | NA | NA | NA | NA | NA | NA | NA |
| GDP PPP | – | – | – | – | − 0.160 | < 0.05 | – | – |
| Urban | – | – | – | – | – | – | < 0.01 | 0.912 |
Significance level: *p < 0.05; **p < 0.01; ***p < 0.001; sample size range: 170 for all analyses.
Data sources: Dementia Incidence expressed as the number of new onsets of Alzheimer's disease and other dementias published by the Institute for Health Metrics and Evaluation (IHME) in 2020; Is measuring the magnitude of the opportunity for natural selection, extracted from the previous publication by You et al. https://doi.org/10.1111/eva.12523; Ageing expressed with life expectancy at 65 years old (2010–2015) published by the United Nations; GDP PPP expressed in purchasing power parity in 2015 US dollars published by the World Bank; Urbanization expressed in percent of population living in urban areas in 2015 published by the World Bank.
Table 3 shows that Is is the only variable which has significant influence on dementia incidence in the linear regression model (enter) for the populations with full age ranges (β = − 0.620, p < 0.001) and segments of 15–49 years old (β = − 0.679, p < 0.001). In the subsequent stepwise multiple linear regression, Is has been selected as the variable which has the significant predicting effect on dementia incidence in populations with full age ranges (R2 = 0.685) and in population segments of 15–49 years old (R2 = 0.738). For the segments of 15–49 years old, GDP PPP significantly correlates with dementia incidence (r = 0.227, p < 0.01) in the enter linear model and is placed second to have the greatest influence on the dementia incidence (increasing R2 from 0.728 to 0.738) in the stepwise linear model. Thus, the GDP PPP seems to have less powerful predicting effect on dementia incidence in both enter and stepwise linear models.
Table 3.
Multiple linear regression analyses to examine predictors of dementia incidence.
| Variable | All ages | 15–49 years old | |||
|---|---|---|---|---|---|
| Beta | Sig | Beta | Sig | ||
| Enter | |||||
| Is | -0.620 | < 0.001 | -0.610 | < 0.001 | |
| Ageing | 0.257 | < 0.001 | 0.101 | 0.150 | |
| GDP | -0.010 | 0.921 | 0.213 | < 0.05 | |
| Urbanization | 0.008 | 0.891 | -0.037 | 0.504 | |
| Stepwise rank | All ages | Rank | 15–49 years old | ||
|---|---|---|---|---|---|
| Variable | Adjusted R2 | Variable | Adjusted R2 | ||
| 1 | Is | 0.685 | 1 | Is | 0.730 |
| 2 | Ageing | 0.704 | 2 | GDP PPP | 0.738 |
Significance level: p < 0.05, p < 0.01, p < 0.001.
Data sources: Dementia Incidence expressed as the number of new onsets of Alzheimer's disease and other dementias published by the Institute for Health Metrics and Evaluation (IHME) in 2020; Is measuring the magnitude of the opportunity for natural selection, extracted from the previous publication by You et al. https://doi.org/10.1111/eva.12523; Ageing expressed with life expectancy at 65 years old (2010–2015) published by the United Nations; GDP PPP expressed in purchasing power parity in 2015 US dollars published by the World Bank; Urbanization expressed in percent of population living in urban areas in 2015 published by the World Bank.
Table 4 indicates that, regardless of population grouping criterion, Is negatively correlates with dementia incidence, although the strength of the correlation and significance levels vary. One of the highlights of the findings is that in developed populations Is is in the stronger correlation with dementia incidence than it is in less developed population groups. This is supported by the bivariate correlation between Is and GDP PPP (r = 0.724 and 0.732 in Pearson’s r and Spearman’s rho respectively, Table 1).
Table 4.
Correlations between Is and dementia incidence in various country groupings.
| All ages | 15–49 years old | |||
|---|---|---|---|---|
| Pearson's r | Non-parametric | Pearson's r | Non-parametric | |
| WHO region | ||||
| Africa, n = 46 | − 0.756*** | − 0.556*** | − 0.811*** | − 0.603*** |
| Americas, n = 35 | − 0.759*** | − 0.721*** | − 0.769*** | − 0.736*** |
| Eastern mediterranean, n = 21 | − 0.266 | − 0.132 | − 0.638** | − 0.639** |
| Europe, n = 50 | − 0.639*** | − 0.411** | − 0.715** | − 0.691** |
| South East Asia, n = 11 | − 0.502 | − 0.373 | − 0.682* | − 0.709* |
| West Pacific, n = 27 | − 0.754*** | − 0.756*** | − 0.763*** | − 0.819*** |
| The World Bank income | ||||
| High, n = 61 | − 0.554*** | − 0.553*** | − 0.414*** | − 0.414*** |
| Upper middle, n = 53 | − 0.685*** | − 0.660*** | − 0.726*** | − 0.646*** |
| Low middle, n = 48 | − 0.788*** | − 0.789*** | − 0.711*** | − 0.752*** |
| Low, n = 28 | − 0.712*** | − 0.477** | − 0.640*** | − 0.386* |
| The United Nations Gross National Income (GNI) | ||||
| High, n = 50 | − 0.461*** | − 0.403** | − 0.351* | − 0.430** |
| Upper middle, n = 49 | − 0.652*** | − 0.622*** | − 0.695*** | − 0.624*** |
| Low middle, n = 44 | − 0.760*** | − 0.771*** | − 0.686*** | − 0.732*** |
| Low, n = 31 | − 0.710*** | − 0.523** | − 0.661*** | − 0.467** |
| Other country groupings | ||||
| ACD, n = 30 | − 0.299 | − 0.238 | − 0.609*** | − 0.638*** |
| APEC, n = 21 | − 0.724*** | − 0.681*** | − 0.532* | − 0.486* |
| Arab world, n = 22 | − 0.142 | − 0.026 | − 0.630** | − 0.624** |
| English, official language, n = 49 | − 0.839*** | − 0.820*** | − 0.883*** | − 0.872*** |
| LAC, n = 33 | − 0.749*** | − 0.665*** | − 0.768*** | − 0.694*** |
| OECD, n = 37 | − 0.602*** | − 0.327* | − 0.409* | − 0.404* |
| SADC, n = 16 | − 0.770*** | − 0.629** | − 0.933*** | − 0.779*** |
Significance level: *p < 0.05; **p < 0.01; *** p < 0.001.
Data sources: Dementia Incidence expressed as the number of new onsets of Alzheimer's disease and other dementias published by the Institute for Health Metrics and Evaluation (IHME) in 2020; Opportunity for selection expressed as Is which was extracted from the previous publication by You et al. https://doi.org/10.1111/eva.12523.
ACD Asia cooperation dialogue, APEC Asia–Pacific economic cooperation, LAC Latin America and the Caribbean, OECD organisation for economic co-operation and development, SADC Southern African Development Community.
Discussion
Dementia is a growing public health concern owing to multiple aetiologies including population ageing. By assessing the dementia incidence data for 204 populations, this study suggests that less opportunity for natural selection (Is) is another major risk factor for dementia. It is also suggested that people in developed populations have higher risk to develop dementia. In our analyses we used data on the incidence of dementia (new cases per year), however, the results based on these data can also be used, with due caution, to discuss dementia’s prevalence that is another epidemiological variable.
Dementia has strong genetic background which consists of heritable factors directly predisposing to dementia and those causing comorbidities. This has been supported by the studies which concluded that Alzheimer22 and frontotemporal42–45 dementia are familial diseases. Slooter et al. reported that about ¼ of the people aged 55+ may have genetic predisposition to develop dementia due to their family history of dementia involving their first-degree relatives46. Loy et al. conducted a systematic review listing multiple genes involved in the onset of Alzheimer's disease, up to 70% of which may be inherited from a patient's parents22. Another review and meta-analysis revealed that the onset of dementia among young people has been on the rise47. This may suggest that, due to the reduced natural selection, genetic background of dementia has become more common.
A number of studies have shown that about 20–40% of frontotemporal dementia patients have a positive family history of frontotemporal diseases42–45. The strong genetic background of vascular dementia may be attributable to a group of heterogeneous cerebrovascular disorders leading to cognitive impairment48–50. For instance, vascular dementia is sometime considered as post-stroke dementia because of the significant association between the nature of vascular dementia and stroke51,52, in particular ischemic stroke53. Dementia patients usually have a number of comorbidities which are non-communicable diseases. On average, the number of comorbidities of a patient aged 65+ is four, but people without dementia have only two54. Most of the comorbidities have genetic predispositions which may be potential triggers for dementia, such as hypertension55, depressive disorders56, obesity57 and diabetes58. Therefore, genetic traits of these non-communicable diseases may play a role in increasing predisposition to dementia.
Apparently, numerous genes predispose to dementia, but their unbalanced interactions may also increase dementia incidence in multiple ways because of the pleiotropic effects of those genes. While research into genetic basis for dementia onset is in progress, the identification of specific genes predisposing to specific kinds of dementia remains intriguing and intricate. Dementia genes and their variants are mildly deleterious, and they have not created large potential for damage to human survival59. Therefore, they will not be quickly eliminated by natural selection60,61. In this study, the inverse correlation of Is with dementia incidence suggests that relaxed selection has been caused by high level of healthcare services and public health measures in modern society. The actual prevalence of dementias in modern societies will, of course, depend on how well these diseases can be treated by healthcare services.
Globally, high level of healthcare services has relaxed the operation of natural selection in all populations in the past 100–150 years20. This was supported by the studies showing that the prevalence rates (total number of people with the condition in the given population) of nasal septa and lacrimal bone defects increased because of the decreasing operation of natural selection62. This was also evidenced by the noticeable prevalence of phenylketonuria in a population after this condition had been accumulating for several generations with about 2% increase20,63. Recently, the accumulating effects of genetic traits of some diseases have been tested on diabetes17,21, obesity15,16 and cancers18. The inverse correlation of Is with dementia incidence is compatible with alteration of mutation-selection balance.
Without being reduced by modern healthcare services, natural selection could have had an ample opportunity to eliminate the dementia associated defective genetic background introduced by mutations13,20,64–69. However, each human life deserves to be protected20. Mathematically, heritable dementia incidence could simply be doubled by generation if natural selection were reduced to zero. However, dementia incidence rates are somewhat less than doubled among populations, varying between 421.6 (per 100,000) and 14.020,22,23. Reduced natural selection has led to the greater presence of dementia associated comorbidities with genetic traits, such as diabetes17,21, obesity15,16 and cancers18.
Furthermore, worldwide, in particular in the developed countries, total fertility rate has been decreasing quickly, which has reduced biological variation in fertility70. The detrimental effects of decreasing fertility rate have been postulated as the leading risk factor for the increase in breast41 and ovarian40 cancers. These observations may be applicable to further explore dementia incidence, however, this could form a separate study.
Currently, the clinical treatment for dementias and their comorbidities only focuses on the control of symptoms71, but not gene therapy that intends to control the dementia genes/mutation. Clinically, when dementia and its comorbidities are “cured”, it only means that their symptoms, not their genetic background (genes/mutation), are under control or reversed. A recent study has advanced that larger family and household size may be a potential prevention of the dementia mortality9. However, to implement this approach the increase in human fertility rate is required which, most certainly, will cause the Earth over-population. Obviously, this is not a practical solution. Currently or for the near future, healthcare services are impotent in removing the genetic traits of dementia and of its comorbidities. For ethical reasons it is impossible to argue that natural selection should be allowed to act on modern human populations. Both, improvements in prevention and cure of dementia symptoms and future developments in gene therapies will hopefully limit detrimental effects of dementias on human life.
Strengths and limitations
This is an ecological study. Ecological data are based on aggregated quantitative data which can zoom in the rare dementia presence 1,000,000 times, thus dementia presence becomes noticeable for correlating and identifying the potential contributing effects of dementia risk factors at the population level. This necessity has been evidenced in other epidemiological studies of rare non-communicable diseases (cancer18,40,41 and Type 1 diabetes17).
The intrinsic limitations of ecological studies should be considered when we are examining the public health implications of our results. (1) The results of this study only show the correlation, not causation, of reduced natural selection with dementia incidence. (2) The relationship based on the ecological approach in this work is subject to ecological fallacy. Therefore, the protective role of less reduced natural selection may not always hold true for each individual, but it is possibly true at a population level. (3) The population level data extracted for this study might be fairly crude. The IHME, WHO, United Nations and World Bank may have made some random errors when collecting and aggregating data. (4) The opportunity for natural selection is only measured with respect to postnatal mortality, while gametic selection and intrauterine mortality are not included72.
Regardless of the limitations of the ecological data, we have consistently showed that populations with more reduced natural selection had higher dementia incidence. The findings of this study may shed light on further longitudinal studies of human evolution at a population level.
Conclusion
Worldwide, reduced natural selection may be a significant contributor to the incidence of dementia with special regard to developed populations.
Acknowledgements
The authors express their appreciation to Ms Emma Nichols from the Institute for Health Metrics and Evaluation of the University of Washington for locating and calculating the data.
Abbreviations
- GDP PPP
Gross domestic product (at purchasing power parity)
- Ibs
Biological State Index
- Is
A measure of the magnitude of natural selection which was calculated with the formula, Is = (1 − Ibs)/Ibs, it is a variance of fitness
- Life e(65)
Life expectancy at 65 years old
- UN
United Nations
- WHO
World Health Organization
- IHME
Institute for Health Metrics and Evaluation
Author contributions
W.Y. and M.H. conceived the hypothesis and discussed with M.H. and R.H. for consolidation. W.Y. extracted the data, and conducted analyses together with M.H. and R.H. before all the authors interpreted the analysis results. W.Y. drafted the text with contributions from M.H. and R.H.; and all the authors reviewed, edited and approved the final manuscript for publishing.
Data availability
The data sources have been described in the section of “Material and method”. All data for this study are freely available from the international organizations’ official websites. The formal permission to use the data for non-commercial purpose is not necessary as it is compliant with the agency’s public permission in their terms and conditions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization, Dementia fact sheet. https://www.who.int/news-room/fact-sheets/detail/dementia. 2020, WHO: WHO Official Website.
- 2.Lindau M, et al. First symptoms–frontotemporal dementia versus Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2000;11(5):286–293. doi: 10.1159/000017251. [DOI] [PubMed] [Google Scholar]
- 3.Burns, A. & Iliffe, S. Dementia. BMJ Br. Med. J. (Online) 338 (7691) (2009).
- 4.Cao Q, et al. The prevalence of dementia: A systematic review and meta-analysis. J. Alzheimers Dis. 2020;73(3):1157–1166. doi: 10.3233/JAD-191092. [DOI] [PubMed] [Google Scholar]
- 5.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009;11(2):111. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox M. Evolutionary medicine’ perspectives on Alzheimer’s disease: Review and new directions. Ageing Res. Rev. 2018;47:140–148. doi: 10.1016/j.arr.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Lee SJ, et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement. 2018;14(6):707–722. doi: 10.1016/j.jalz.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Chieffi S, et al. Exercise influence on hippocampal function: Possible involvement of orexin-A. Front. Physiol. 2017;8:85. doi: 10.3389/fphys.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You W, Henneberg M. Large household reduces dementia mortality: A cross-sectional data analysis of 183 populations. PLoS ONE. 2022;17(3):e0263309. doi: 10.1371/journal.pone.0263309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vos T, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston G, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaborators GDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henneberg M. Reproductive possibilities and estimations of the biological dynamics of earlier human populations. J. Hum. Evol. 1976;5:41–48. doi: 10.1016/0047-2484(76)90098-1. [DOI] [Google Scholar]
- 14.Henneberg M, Piontek J. Biological state index of human groups. Przeglad Anthropologiczny. 1975;XLI:191–201. [Google Scholar]
- 15.Budnik A, Henneberg M. Worldwide increase of obesity is related to the reduced opportunity for natural selection. PLoS ONE. 2017;12(1):e0170098. doi: 10.1371/journal.pone.0170098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You W, Henneberg M. Relaxed natural selection contributes to global obesity increase more in males than in females due to more environmental modifications in female body mass. PLoS ONE. 2018;13(7):e0199594. doi: 10.1371/journal.pone.0199594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You W-P, Henneberg M. Type 1 diabetes prevalence increasing globally and regionally: The role of natural selection and life expectancy at birth. BMJ Open Diabetes Res. Care. 2016;4(1):e000161. doi: 10.1136/bmjdrc-2015-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You W, Henneberg M. Cancer incidence increasing globally: The role of relaxed natural selection. Evol. Appl. 2017;00:1–13. doi: 10.1111/eva.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darwin C. The Descent of Man, and Selection in Relation to Sex. New. John Murray; 1901. [Google Scholar]
- 20.Stephan CN, Henneberg M. Medicine may be reducing the human capacity to survive. Med. Hypotheses. 2001;57(5):633–637. doi: 10.1054/mehy.2001.1431. [DOI] [PubMed] [Google Scholar]
- 21.Rühli F, van Schaik K, Henneberg M. Evolutionary medicine: The ongoing evolution of human physiology and metabolism. Physiology. 2016;31(6):392–397. doi: 10.1152/physiol.00013.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols E, et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IHME, Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. 2020, Seattle, United States: Institute for Health Metrics and Evaluation (IHME). http://ghdx.healthdata.org/gbd-results-tool.
- 24.The United Nations. World Fertility Data 2008. 2012 29.07.2015]. http://www.un.org.
- 25.WHO, World Health Statistics 2012. Life tables for WHO Member States. 2012. Geneva: World Health Organization.
- 26.Fisher, R.A.S., The genetical theory of natural selection: a complete variorum edition/by R.A. Fisher; edited with a foreword and notes by J.H. Bennett, ed. J.H. Bennett. 1999. Oxford: Oxford: Oxford University Press.
- 27.The World Bank. World Bank Open Data. 2016 12.07.2016]. http://data.worldbank.org/.
- 28.WHO, Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation (WHO technical report series; 916). 2003: Geneva. [PubMed]
- 29.WHO. WHO regional offices. [11.26.2015]. http://www.who.int.
- 30.The World Bank. Country and Lending Groups | Data. 2015. http://data.worldbank.org/about/country-and-lending-groups.
- 31.United Nations . World Economic Situation and Prospects (ISBN: 978-92-1-109180-9) United Nations; 2019. [Google Scholar]
- 32.Asia Cooperation Dialogue. Member Countries. http://www.acddialogue.com.
- 33.Asia-Pacific Economic Cooperation. Member Economies-Asia-Pacific Economic Cooperation. [11.26.2015]. http://www.apec.org.
- 34.The United Nations Educational Scientific and Cultural Organization. UNESCO Regions-Latin America and the Caribbean. 2014 [11.26.2015]. http://www.unesco.org.
- 35.South Africa Development Community. Southern African Development Community: Member States. [18.06.2015]. http://www.sadc.int.
- 36.You W, et al. Gluten consumption may contribute to worldwide obesity prevalence. Anthropol. Rev. 2020;83(3):327–348. doi: 10.2478/anre-2020-0023. [DOI] [Google Scholar]
- 37.You W, Henneberg M. Cereal crops are not created equal: Wheat consumption associated with obesity prevalence globally and regionally. AIMS Public Health. 2016;3(2):313. doi: 10.3934/publichealth.2016.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You W, et al. Total meat intake is associated with life expectancy: A cross-sectional data analysis of 175 contemporary populations. Int. J. Gen. Med. 2022;15:1833. doi: 10.2147/IJGM.S333004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You W, Henneberg M. Prostate cancer incidence is correlated to total meat intake–a cross-national ecologic analysis of 172 countries. Asian Pac. J. Cancer Prev. APJCP. 2018;19(8):2229. doi: 10.22034/APJCP.2018.19.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You W, Symonds I, Henneberg M. Low fertility may be a significant determinant of ovarian cancer worldwide: An ecological analysis of cross-sectional data from 182 countries. J. Ovarian Res. 2018;11(1):68. doi: 10.1186/s13048-018-0441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You W, et al. Decreasing birth rate determining worldwide incidence and regional variation of female breast Cancer. Adv. Breast Cancer Res. 2018;7(01):1–14. doi: 10.4236/abcr.2018.71001. [DOI] [Google Scholar]
- 42.Chow TW, et al. Inheritance of frontotemporal dementia. Arch. Neurol. 1999;56(7):817–822. doi: 10.1001/archneur.56.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratnavalli E, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615–1621. doi: 10.1212/WNL.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 44.Seelaar H, et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008;71(16):1220–1226. doi: 10.1212/01.wnl.0000319702.37497.72. [DOI] [PubMed] [Google Scholar]
- 45.Rohrer J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73(18):1451–1456. doi: 10.1212/WNL.0b013e3181bf997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slooter AJ, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: The Rotterdam Study. Arch. Neurol. 1998;55(7):964–968. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 47.Hendriks S, et al. Global prevalence of young-onset dementia: A systematic review and meta-analysis. JAMA Neurol. 2021;78(9):1080–1090. doi: 10.1001/jamaneurol.2021.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manso-Calderón R. Genetics in vascular dementia. Future Neurol. 2019;14(1):FNL5. doi: 10.2217/fnl-2018-0027. [DOI] [Google Scholar]
- 49.O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 50.Sun J-H, et al. Genetics of vascular dementia: Systematic review and meta-analysis. J. Alzheimers Dis. 2015;46(3):611–629. doi: 10.3233/JAD-143102. [DOI] [PubMed] [Google Scholar]
- 51.Chen A, et al. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-stroke dementia. Brain. 2016;139(1):242–258. doi: 10.1093/brain/awv328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mijajlović MD, et al. Post-stroke dementia–a comprehensive review. BMC Med. 2017;15(1):1–12. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vijayan M, Reddy PH. Stroke, vascular dementia, and Alzheimer’s disease: Molecular links. J. Alzheimers Dis. 2016;54(2):427–443. doi: 10.3233/JAD-160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poblador-Plou B, et al. Comorbidity of dementia: A cross-sectional study of primary care older patients. BMC Psychiatry. 2014;14(1):84. doi: 10.1186/1471-244X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sierra C. Hypertension and the risk of dementia. Front. Cardiovasc. Med. 2020;7:5. doi: 10.3389/fcvm.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011;7(6):323. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Y, et al. Higher risk of dementia in English older individuals who are overweight or obese. Int. J. Epidemiol. 2020;49(4):1353–1365. doi: 10.1093/ije/dyaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beeri MS, Bendlin BB. The link between type 2 diabetes and dementia: From biomarkers to treatment. Lancet Diabetes Endocrinol. 2020;8(9):736–738. doi: 10.1016/S2213-8587(20)30267-9. [DOI] [PubMed] [Google Scholar]
- 59.Henn BM, et al. Estimating the mutation load in human genomes. Nat. Rev. Genet. 2015;16(6):333–343. doi: 10.1038/nrg3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohta T, Gillespie JH. Development of neutral and nearly neutral theories. Theor. Popul. Biol. 1996;49(2):128–142. doi: 10.1006/tpbi.1996.0007. [DOI] [PubMed] [Google Scholar]
- 61.Marth GT, et al. The allele frequency spectrum in genome-wide human variation data reveals signals of differential demographic history in three large world populations. Genetics. 2004;166(1):351–372. doi: 10.1534/genetics.166.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Post RH. Deformed nasal septa and relaxed selection. Eugen. Q. 1966;13:101–112. doi: 10.1080/19485565.1966.9987653. [DOI] [PubMed] [Google Scholar]
- 63.Medawar, P.B. (1971) Do advances in medicine lead to genetic deterioration?. In: Bajema C. J. (Ed) Natural Selection in Human Populations. Robert E. Krieger Publishing Co., New York, pp 300–08.
- 64.Budnik A, Liczbińska G, Gumna I. Demographic trends and biological status of historic populations from Central Poland: The Ostrów Lednicki microregion. Am. J. Phys. Anthropol. 2004;125(4):369–381. doi: 10.1002/ajpa.10272. [DOI] [PubMed] [Google Scholar]
- 65.Henneberg, M. & R. Henneberg. Reconstructing medical knowledge in ancient Pompeii from the hard evidence of bones and teeth. 2002.
- 66.Henneberg, M. & R. Henneberg, Biological characteristics of the population based on analysis of skeletal remains. 1998.
- 67.Saniotis A, Henneberg M. Medicine could be constructing human bodies in the future. Med. Hypotheses. 2011;77(4):560–564. doi: 10.1016/j.mehy.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 68.Saniotis A, Henneberg M. Examining genetic load: An Islamic perspective. Med. J. Islam. World Acad. Sci. 2012;20(3):73–80. [Google Scholar]
- 69.Rühli F, Henneberg M. Biological future of humankind—Ongoing evolution and the impact of recognition of human biological variation. In: Tibayrenc M, Ayala FJ, editors. On Human Nature. Biology, Psychology, Ethics, Politics, and Religion 2016. Cambridge: Academic Press; 2016. pp. 263–275. [Google Scholar]
- 70.Henneberg M. Natural selection through differential fertility in human populations: Quantitative evaluation of selection intensity. Przeglad Antropologiczny. 1980;46:21–60. [Google Scholar]
- 71.Rossor MN, et al. The diagnosis of young-onset dementia. Lancet Neurol. 2010;9(8):793–806. doi: 10.1016/S1474-4422(10)70159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rühli F, Henneberg M. Biological future of humankind—Ongoing evolution and the impact of recognition of human biological variation. In: Tibayrenc M, Ayala FJ, editors. On Human Nature. Biology, Psychology, Ethics, Politics, and Religion. Cambridge: Elsevier; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sources have been described in the section of “Material and method”. All data for this study are freely available from the international organizations’ official websites. The formal permission to use the data for non-commercial purpose is not necessary as it is compliant with the agency’s public permission in their terms and conditions.