Abstract
Background:
The objective of this study was to evaluate factors associated with intraventricular hemorrhage (IVH) expansion and its association with long-term outcomes.
Methods:
We performed a post-hoc analysis of the international, multi-center Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) trial which enrolled IVH patients between September 1, 2009 and January 31, 2015. The exposure was IVH expansion, defined as >1mL increase in volume between baseline and stability computed tomography scans, prior to treatment randomization. We assessed factors associated with IVH expansion and secondarily assessed the relationship of IVH expansion with clinical outcomes: composite of death or major disability (modified Rankin Score > 3), and mortality alone at 6 months. The relationship of IVH expansion on ventriculoperitoneal shunt (VPS) placement was additionally explored. Multivariable logistic regression was used for all analyses.
Results:
Of 500 IVH patients analyzed, the mean age was 59 (+/−11) years old, 44% were female, and 135 (27%) had IVH expansion. In multivariable regression models, factors associated with IVH expansion were baseline parenchymal intracerebral hemorrhage (ICH) volume (adjusted OR 1.04 per 1 mL increase; 95%CI: 1.01–1.08), presence of parenchymal hematoma expansion: >33% (adjusted OR 6.63; 95%CI: 3.92–11.24), time to stability head CT (adjusted OR 0.71 per 1 hour increase; 95%CI: 0.54–0.94) and thalamic hematoma location (adjusted OR 1.68; 95%CI: 1.01–2.79) while additionally adjusting for age, sex, and race. In secondary analyses, IVH expansion was associated with higher odds of poor 6-month outcomes (adjusted OR 1.84; 95% CI: 1.12–3.02), but not mortality (OR 1.40; 95%CI: 0.78–2.50) after adjusting for baseline ICH volume, thalamic ICH location, age, anticoagulant use, Glasgow Coma Scale score, any withdrawal of care order, and treatment randomization arm. However, there were no relationships of IVH expansion on subsequent VPS placement (adjusted OR 1.02; 95% CI: 0.58–1.80) after adjusting for similar covariates.
Conclusions:
In a clinical trial cohort of patients with large IVH, acute hematoma characteristics, specifically larger parenchymal volume, hematoma expansion, and thalamic ICH location were associated with IVH expansion. Given that IVH expansion resulted in poor functional outcomes, exploration of treatment approaches to optimize hemostasis and prevent IVH expansion, particularly in patients with thalamic ICH, require further study.
Keywords: Intracerebral hemorrhage, intraventricular hemorrhage, intraventricular hemorrhage expansion, hematoma expansion, outcome
Introduction
Intracerebral hemorrhage (ICH) is the most devastating type of stroke, with a high morbidity and mortality1. Parenchymal hematoma volume and intraventricular hemorrhage (IVH) presence are strong independent predictors of poor outcome, in addition to age2. Although both reflect blood in the brain, albeit in different compartments, the former is often measured as a continuous variable, while IVH is primarily defined as a dichotomous measure (the presence or absence of IVH)3. Yet, IVH, like parenchymal ICH volume, is a dynamic process and can change over time. Studies have suggested that delayed appearance of IVH, even when absent on initial presentation, is a marker for poor outcomes4. This implies that IVH expansion, like parenchymal hematoma expansion, also has an adverse effect on outcomes, and emerging studies have advocated the incorporation of IVH expansion to improve the predictive accuracy of existing prognostic scales5.
However, our understanding of IVH expansion is limited by a paucity of data. While it is increasingly recognized that small amounts of IVH expansion is harmful in patients who present initially with either no or small IVH volumes5,6, it is unknown whether these findings are generalizable to patients who present with already large volume obstructive IVH. Similarly, factors associated with IVH expansion are unclear in these types of IVH patients. Prior studies evaluating factors associated with IVH expansion were limited by single center cohorts, small sample sizes, and cohorts with small or absent baseline IVH volumes. In a secondary analysis of data from the recombinant Factor VIIa in spontaneous ICH trial, admission blood pressure, baseline ICH volume, and treatment randomization to placebo (vs. recombinant factor VII) were associated with IVH expansion6. However, administration of hemostatic therapy within the first 4 hours hampered the generalizability of the results. We therefore sought to investigate factors associated with IVH expansion and the relationships of IVH expansion with outcome in a multi-center international cohort of patients with large IVH with protocolized imaging, centrally adjudicated imaging data, and blinded endpoint assessments.
Methods
Data availability
The data used in this analysis may be obtained from the National Institute of Neurological Disorders and Stroke (NINDS) clinical trial repository, or from the CLEAR III steering committee, after a formal request with a written proposal.
Study design and Patient Population
We performed an ad hoc secondary analysis of data from the multi-center, international, randomized, placebo-controlled trial, CLEAR III (Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage). Patients with large IVH were randomized between September 1, 2009 and January 31, 2015 to receive an external ventriculostomy drain (EVD) plus alteplase versus EVD plus saline, to facilitate removal of IVH. Full study details have been published previously7. The trial protocol was approved by an ethics committee at each enrolling site, and written informed consent was obtained from each participant or the legal surrogate. The present study was approved by the Weill Cornell Medicine institutional review board. For this study, we included all patients enrolled in the CLEAR III trial and given completeness of data and there were no exclusion criteria. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies8.
Measurements
Head computed tomography (CT) was obtained on admission for IVH diagnosis and the final head CT per trial protocol was 24 hours post last dose of the study agent. Interim stability scans were obtained after clinically indicated placement of an EVD to ensure parenchymal ICH stability as well as the absence of significant tract hemorrhage (≤ 5 milliliter [mL]) prior to enrollment and treatment randomization. If there was evidence of initial hemorrhage growth (defined in the trial as >5mL for parenchymal hematoma and >2 mm width of the lateral ventricle most compromised by blood for IVH), additional screening with follow-up scans were allowed up to 72 hours from the initial hemorrhage to evaluate for stabilization of the hematoma9. Hematoma volumes (both parenchymal and intraventricular) were calculated using semi-automated planimetry and read centrally by radiologists and neurologists blinded to treatment assignment and outcomes. ICH location similarly was adjudicated centrally by radiology core7. The final stability head CT prior to drug/placebo administration was considered as the stability scan analyzed for the purposes of this study. Given potential variations in timing of the final stability head CT, times from ictus to stability CT scans were recorded.
The primary outcome in this study was IVH expansion defined as greater than 1 mL increase in the absolute IVH volume between the baseline and final stability CT scans. This cut off for IVH expansion has been validated in prior studies5. Hematoma expansion was defined as an interval increase of 33% or more in the parenchymal ICH volume, given that the majority of ICHs in the CLEAR III trial were small deep hemorrhages10,11. The secondary outcomes were a composite of major disability or death, defined as a modified Rankin Scale (mRS) score >3, as well as death only, assessed at 6 months7. Lastly, exploratory analyses evaluated the relationships of IVH expansion with ventriculoperitoneal shunt (VPS) placement, given the known association of VPS with poor ICH outcomes12.
Statistical analysis
We used Student’s t-test or the Wilcoxon rank-sum test for continuous variables depending on the normality of distribution, and the χ2 test for categorical variables. We performed two different analyses. The first analysis entailed using multivariable logistic regression to study the factors associated with IVH expansion. The covariates for the regression models were chosen based on a significance of p<0.2 in the bivariate regression. Additionally, universal confounders chosen a priori: age, sex, race (white vs non-white), baseline ICH volume (as a continuous absolute volume measurement in mL), and ictus to stability head CT timing (time in hours between ICH symptom onset to final stability head CT acquisition) were also included in the models regardless of the p value. In the second analysis, we evaluated the association between IVH expansion and functional outcomes. The covariates for the models included known predictors of poor ICH outcomes: baseline ICH volume, thalamic ICH location, age, anticoagulant use, Glasgow Coma Scale score (continuous variable), any withdrawal of care order, and treatment randomization arm2,13,14. Exploratory regression analyses were performed to assess relationships of IVH expansion on VPS placement after adjusting for appropriate covariates12. In cases with missing data, complete analyses were performed if this was <15% of the entire cohort. The threshold for statistical significance was set at α=0.05. All analyses were performed using Stata/MP version 13 (College Station, TX).
Results
Of the 500 patients with IVH enrolled in the CLEAR III trial, the mean age was 59 (standard deviation [SD]: 11) years old and 44% were female. We identified that IVH expansion occurred in 135 (27%) subjects when assessing the IVH volume change between baseline and final stability head CTs. When assessing IVH change as a continuous variable of absolute growth or reduction (in mL), IVH expanders had a median expansion of 5.3 mL (IQR: 2.3–10.2 mL) compared to non-expanders who had a median reduction of IVH volume of −4.3 mL (IQR: −8.0 to −1.5 mL). Patients with IVH expansion were older, predominantly white, and were more likely to have preceding anticoagulant medication use (Table 1). Patients with IVH expansion also had larger baseline and stability parenchymal ICH volumes and were more likely to encounter hematoma expansion (Figure 1 and Supplemental Figure I). Similarly, at the end of treatment (alteplase vs placebo), patients with IVH expansion also had larger parenchymal ICH (9.6 vs. 5.5 mL) and IVH volumes (12.8 vs. 7.2 mL) compared to those without IVH expansion on the final, end of treatment scans. No missing IVH volume outcome variables were identified.
Table 1:
Baseline demographics, comorbidities and clinical severity factors in intracerebral hemorrhage patients with concurrent intraventricular hemorrhage
| Demographic factors | IVH Expansion N=135 (27.0%) |
No IVH Expansion N= 365 (73.0%) |
P value |
|---|---|---|---|
| Mean age (SD), years | 60.5 (11.3) | 57.9 (11.2) | 0.01 |
| Male (n,%) | 64 (47.4) | 158 (43.3) | 0.41 |
| Other | 1 (0.7) | 19 (5.2) | |
| Hypertension (n,%) | 107 (79.3) | 263 (72.1) | 0.10 |
| Hyperlipidemia (n,%) | 130 (96.3) | 355 (97.3) | 0.58 |
| Prior anticoagulant use (n,%) | 20 (14.8) | 29 (7.9) | 0.02 |
| Prior antiplatelet use (n,%) | 39 (28.9) | 89 (24.4) | 0.31 |
| Baseline Clinical Factors | |||
| Median Glasgow Coma Scale (IQR) | 8 (6–10) | 10 (7–13) | <0.001 |
| Mean systolic blood pressure (SD), mm Hg | 193 (35) | 195 (39) | 0.64 |
| Imaging Factors | |||
| Primary IVH | 14 (10.4) | 32 (8.7) | |
| Thalamic location (n,%) | 87 (64.4) | 206 (56.4) | 0.11 |
| Baseline ICH volume1, mL | 8.5 (4.1–14.6) | 7.5 (2.8–13.6) | 0.39 |
| Baseline IVH volume1, mL | 21.2 (11.5–37.3) | 25.6 (15.6–40.3) | 0.08 |
| Stability ICH volume1, mL | 11.2 (5.5–19.6) | 6.6 (2.3–12.8) | <0.001 |
| Stability IVH volume1, mL | 29.7 (16.7–48.5) | 20.4 (11.5–31.1) | <0.001 |
| End of Treatment ICH volume1, mL | 9.6 (2.3–17.5) | 5.5 (1.6–11.4) | <0.001 |
| End of Treatment IVH volume1, mL | 12.8 (6.7–28.1) | 7.2 (2.6–14.1) | <0.001 |
| ICH expansion (n,%) | 64 (47.4) | 54 (14.8) | <0.001 |
| Time from ictus to stability CT scan, hours1 | 41.1 (25.2–49.6) | 45.1 (28.2–60.0) | 0.01 |
| Intraventricular alteplase use (n,%) | 63 (48.8) | 183 (50.1) | 0.84 |
| Withdrawal of care (n,%) | 24 (18.6) | 35 (9.6) | 0.01 |
Abbreviations: ICH: Intracerebral hemorrhage; IVH: Intraventricular hemorrhage; N: number; mL: milliliters; SD: Standard deviation; IQR: interquartile range; mm Hg: millimeters of mercury
values presented as median (interquartile range)
6 patients without clear ICH location
Figure 1:
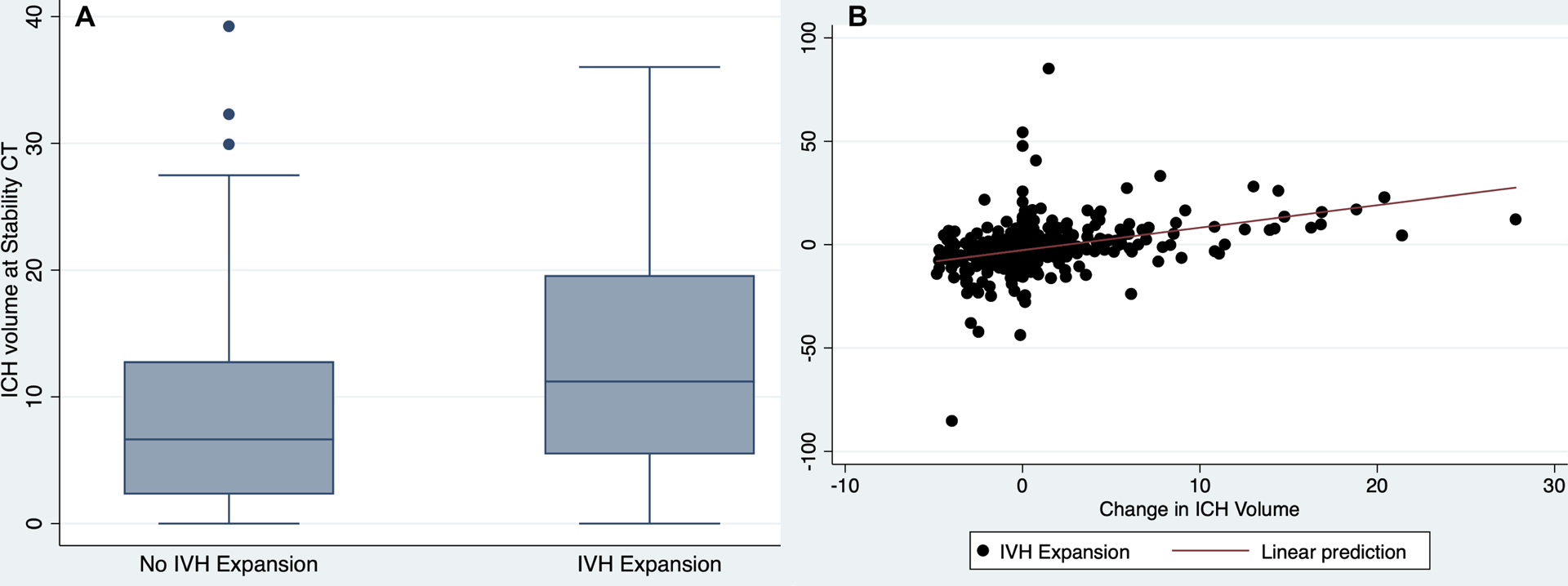
Relationships between Changes in ICH and IVH volumes from Baseline to Stability CT Scans. Box plot showing a change in ICH volume (Y-axis) plotted against the change in IVH volume (Panel A), and scatter plot of change in IVH volume (Y-axis) plotted against the change in ICH volume (Panel B).
IVH: intraventricular hemorrhage; ICH: intracerebral hemorrhage; CT: computed tomography
Results of the bivariate logistic regression assessing factors associated with IVH expansion are shown in Table 2. In the multivariable logistic regression models, baseline parenchymal ICH volume (adjusted OR 1.04 per 1 mL increase; 95%CI: 1.01–1.08), presence of parenchymal HE (adjusted OR 6.63; 95%CI: 3.92–11.24), time from ictus to stability head CT (adjusted OR 0.71 per 1 hour increase; 95%CI: 0.54–0.94) and thalamic hematoma location (adjusted OR 1.68; 95%CI: 1.01–2.79) were independently associated with higher odds of IVH expansion (Table 2). Additionally, we performed in a post hoc sensitivity analysis where we substituted time from ictus to stability CT scan with the time from baseline CT scan to stability CT scan in the multivariable logistic regression model. The results were similar to those noted in the primary analysis (Supplemental Table I).
Table 2:
Factors associated with IVH Expansion among Patients Enrolled in the CLEAR III Trial
| Covariates | ||||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, per year increase | 1.02 (1.01–1.04) | 0.02 | 1.01 (0.99–1.03) | 0.39 |
| Female | 0.85 (0.57–1.25) | 0.41 | 1.29 (0.83–2.02) | 0.26 |
| Black race | 0.68 (0.43–1.05) | 0.08 | 0.87 (0.52–1.46) | 0.60 |
| Hypertension | 1.48 (0.92–2.38) | 0.10 | 1.28 (0.74–2.23) | 0.38 |
| Hyperlipidemia | 0.73 (0.25–2.18) | 0.56 | ||
| Prior anticoagulant use | 2.01 (1.09–3.70) | 0.02 | 1.50 (0.72–3.13) | 0.28 |
| Prior antiplatelet use | 1.26 (0.81–1.96) | 0.31 | ||
| Glasgow Coma Scale at screening | 0.89 (0.83–0.93) | <0.001 | ||
| Primary IVH | 1.22 (0.51–2.92) | 0.66 | ||
| Thalamic ICH | 1.40 (0.92–2.10) | 0.11 | 1.68 (1.01–2.79) | 0.04 |
| Baseline IVH volume, per 1 mL | 0.99 (0.97–1.03) | 0.27 | ||
| Baseline ICH volume, per 1 mL | 1.01 (0.98–1.04) | 0.36 | 1.04 (1.01–1.08) | 0.01 |
| Ictus to stability CT scan time, per 1 hour increase | 0.98 (0.96–0.99) | 0.01 | 0.71 (0.54–0.94) | 0.02 |
| ICH expansion | 5.2 (3.32–8.10) | <0.001 | 6.63 (3.92–11.24) | <0.001 |
| Intraventricular alteplase use | 0.95 (0.64–1.42) | 0.80 | ||
| Admission systolic blood pressure | 0.99 (0.97–1.01) | 0.74 |
Abbreviations: CI, confidence interval; CT, computed tomography; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; OR: Odds ratio; P value <0.05 was considered significant
We then evaluated the relationship between IVH expansion and outcomes. In the adjusted regression models, IVH expansion was associated with increased odds of poor functional outcome (adjusted OR 1.84; 95% CI: 1.12–3.02), but not mortality (adjusted OR 1.40; 95%CI: 0.78–2.50) at 6 months.
We additionally explored whether initial IVH expansion impacted later IVH clearance by investigating IVH volume changes between the stability and end of treatment CT scans. While we did note that IVH expanders had slightly larger absolute IVH clearance volumes by the end of treatment (median [IQR]: 12.6 mL [6.5–23.3 mL] vs. 10.4 mL [5.5–17.8 mL]; p=0.008), given their larger IVH volumes on stability imaging these IVH expanders actually had less IVH clearance when quantified as a relative percent change compared to non-expanders (median [IQR]: 49% [28–71%] vs 60% [39–80%]; p=0.002). Furthermore, IVH expanders had less clearance compared to non-expanders in both the alteplase (median [IQR]: 67% [52–78%] vs 74% [52–90%]; p=0.03) and placebo arms (median [IQR]: 33% [23–50%] vs 48% [32–67%]; p=0.001) suggesting that acute IVH expansion results in less relative IVH clearance downstream regardless of the study intervention. Despite less IVH clearance seen in IVH expanders, we did not identify an association of IVH expansion with VPS placement after adjusting for relevant covariates (Table 3).
Table 3:
Multivariate Analyses Showing the Relationship between IVH Expansion and Outcomes
| Outcome | IVH Expansion | No IVH Expansion | Unadjusted OR (95% CI) |
P value | Adjusted OR (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Modified Rankin Score 4–6 at 6 months1 | 91 (67.4) | 182 (49.9) | 2.07 (1.37–3.14) | <0.001 | 1.84 (1.12–3.02) | 0.02 |
| Mortality at 6 months1 | 43 (31.9) | 76 (20.9) | 1.78 (1.14–2.76) | 0.01 | 1.40 (0.78–2.50) | 0.25 |
| Permanent CSF shunt2 | 24 (17.8) | 66 (18.1) | 0.97 (0.58–1.64) | 0.94 | 1.02 (0.58–1.80) | 0.93 |
Abbreviations: CI: confidence interval; IVH, intraventricular hemorrhage; OR: odds ratio
P value <0.05 was considered significant
Model was adjusted for age, sex, race, diagnostic parenchymal hematoma volume, hematoma location, withdrawal of care, and treatment randomization arm.
Model was adjusted for age, sex, race, diagnostic parenchymal hematoma volume, treatment randomization arm, thalamic location, and ventriculostomy output per day.
Discussion
In this secondary analysis of a large, multicenter clinical trial cohort of patients with large IVH, factors associated with IVH expansion were larger parenchymal hematoma volume, ICH expansion, and thalamic ICH location. Patients with IVH expansion had a nearly two-fold higher odds of poor functional outcome, compared to those without.
Our results build on the findings of prior studies where acute ICH characteristics were associated with IVH expansion5,6. Notably, we identified that thalamic location appeared to independently drive IVH expansion suggesting that the close proximity of the thalamus to the ventricular system not only increases the probability of developing acute IVH, but is also driver of IVH expansion6,15. Interestingly, the association between thalamic location and IVH expansion was independent of hematoma expansion, implying that ongoing bleeding does not necessarily translate to parenchymal hematoma expansion, but may extend primarily into the ventricular space16.
We additionally identified that IVH expansion was associated with poor outcomes. While prior studies have identified that small volumes of IVH expansion are associated with poor outcomes, these studies were limited to patients having either small or initially absent baseline IVH volumes4–6,17. Furthermore, these prior studies were appropriately limited to patients not requiring initial EVD placement to avoid confounding of these relationships via CSF diversion. In our cohort of patients with moderate to severe IVH necessitating EVD placement, we similarly identified relationships of IVH expansion with poor outcomes. These collective findings may suggest that even small amounts of IVH expansion deleteriously impact outcomes regardless of the amount of IVH present at baseline. However, it is also important to note that the impact of IVH expansion on poor outcomes may differ based on baseline presenting IVH volumes. In other words, it is plausible that small IVH expansion may not have as deleterious of an impact on outcome in patients presenting with already large baseline IVH volumes. Though our IVH expander patient cohort had a good distribution of both small and large IVH expansion, it was notable that the impact of IVH expansion on poor long term outcomes in our data was smaller compared to prior studies5. It is unclear whether this difference was from differences of the patient cohort itself, limitations of the utilized IVH thresholds in our patient cohort, or CSF/IVH clearance via an EVD. However, further work may be required to best characterize best IVH thresholds associated with outcome in patients who present with large obstructive IVH.
While the mechanism underlying the relationship of IVH expansion and poor outcomes in our cohort is unknown, we did note that patients with IVH expansion had less relative IVH volume clearance at the end of treatment, most likely reflecting the larger IVH volumes seen in this group. However, this did not appear to drive a relationship of IVH expansion with shunt dependency, a known factor associated with worse ICH outcomes12. Mechanistically, it is plausible that the additive burden of blood in the ventricular system led to increased iron burden from hemosiderin breakdown and ensuing subsequent inflammation18–20, the increased risk of intracranial hypertension and suboptimal cerebral perfusion pressure21, and an increased risk of stroke with larger IVH volumes22 leading to the poor outcomes seen in our IVH expander group. Though further work is required to clarify the mechanisms behind our findings, our findings may indicate that previously identified poor outcomes after thalamic ICH14 may not be driven merely from the thalamic injury itself, but the compounded injury with concurrent IVH and IVH expansion in these patients14. This may suggest the importance of considering whether IVH expansion prevention treatment strategies, such as ultra-early anti-hypertensive treatment or coagulation treatments23,24, can improve long term outcomes in ICH patients, and perhaps thalamic ICH patients specifically.
While our study strengths included the use of a large prospective, multicenter, trial cohort of patients with large IVH, protocolized neuroimaging, centrally adjudicated neuroimaging measurement assessments, and blinded outcome assessments, there are several limitations worth noting. First, the stringent inclusion criteria resulted in a cohort primarily comprised of patients with deep parenchymal ICH locations with smaller baseline hematoma volumes and large IVH volumes. This may have resulted in selection bias thus limiting the generalizability of our findings. Similarly, because large obstructive IVH at baseline was required for enrollment, we did not have the data or patient cohort to assess for delayed IVH in settings where patients initially presented with absent or minimal IVH volumes. Second, because an EVD was placed between baseline and stability scans, and cerebrospinal fluid diversion practices were variable between centers, we were unable to create accurate IVH expansion thresholds best associated with poor outcomes in our severe IVH cohort without the confounding effect of CSF or IVH removal. Despite these limitations, we were still able to identify that previously identified IVH expansion thresholds (>1 mL) associated with poor outcome5,6. It is also worth noting potential limitations of the use of semi-automated planimetry in IVH volumetric analyses in identifying true expansion versus “pseudo-expansion” that may occur with the re-distribution of casted IVH. Planimetric techniques would not discriminate between these two entities if hounsfield unit thresholds were still met, which could be a general limitation of such measurement approaches. Finally, we did not have data on central, peripheral inflammatory markers, iron burden, or more granular outcome measurements (including cognitive assessments) to evaluate for potential mechanistic factors that could be driving the association between IVH expansion and poor outcome.
Conclusion
In a post hoc analysis of a clinical trial cohort of patients with large IVH, we identified that patients with thalamic ICH location were more likely to encounter IVH expansion. Given that IVH expansion portends poor outcomes, further work is required to assess whether treatment efforts to prevent IVH expansion in thalamic ICH patients can improve outcomes.
Supplementary Material
Acknowledgments
Access to data: Drs. Roh and Murthy had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
This study was supported by the NIH/NHLBI K23HL151901 (Dr. Roh), NIH/NINDS 5U01 NS062851-05 (Drs. Hanley and Ziai) and NIH/NINDS K23NS105948 (Dr. Murthy). The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
DJR is supported by the NIH/NHLBI K23HL151901, NIH NHLBI R01HL148151-03, National Blood Foundation Scientific Research Grant, and the Department of Defense W81XWH-20-PRMRP-IIRA-COV. IA is supported by the NIH (1U01NS080824 and U01NS106513). DFH is supported by the NIH (U01NS080824 and U24TR001609), and reports personal fees from Op2Lysis, personal fees from BrainScope and Neurotrope, and non-financial support from Genentech outside the submitted work. WCZ is supported by the NIH (1U01NS080824, R01NS102583, and U01NS106513), and receives consulting fees from C.R. Bard, Inc. outside of the area of work commented on here. SBM is supported by the NIH (K23NS105948) and has received personal fees for medicolegal consulting on neurological disorders. All remaining authors declare no competing interests.
Non-Standard Abbreviations:
- CLEAR III
Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage
- CT
computed tomography
- EVD
external ventriculostomy drain
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- mRS
modified Rankin Scale
- VPS
ventriculoperitoneal shunt
Footnotes
Clinical Trial Registration Information: NCT00784134 (https://clinicaltrials.gov/ct2/show/NCT00784134)
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 2.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke J. Cereb. Circ 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 3.Roeder SS, Sprügel MI, Sembill JA, Giede-Jeppe A, Macha K, Madžar D, Lücking H, Hoelter P, Gerner ST, Kuramatsu JB, et al. Influence of the Extent of Intraventricular Hemorrhage on Functional Outcome and Mortality in Intracerebral Hemorrhage. Cerebrovasc. Dis. Basel Switz 2019;47:245–252. [DOI] [PubMed] [Google Scholar]
- 4.Maas MB, Nemeth AJ, Rosenberg NF, Kosteva AR, Prabhakaran S, Naidech AM. Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology. 2013;80:1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yogendrakumar V, Ramsay T, Fergusson D, Demchuk AM, Aviv RI, Rodriguez-Luna D, Molina CA, Silva Y, Dzialowski I, Kobayashi A, et al. New and expanding ventricular hemorrhage predicts poor outcome in acute intracerebral hemorrhage. Neurology. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, Skolnick BE, Davis SM. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–773; discussion 773–774. [DOI] [PubMed] [Google Scholar]
- 7.Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, Dawson J, Gandhi D, Ullman N, Mould WA, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. The Lancet. 2017;389:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 9.Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, Butcher K, Vespa P, Wright DW, Keyl PM, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Int. J. Stroke Off. J. Int. Stroke Soc 2014;9:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roh D, Boehme A, Young C, Roth W, Gutierrez J, Flaherty M, Rosand J, Testai F, Woo D, Elkind MSV. Hematoma expansion is more frequent in deep than lobar intracerebral hemorrhage. Neurology. 2020; 2020. Dec 15;95(24):e3386–e3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy SB, Awad I, Harnof S, Aldrich F, Harrigan M, Jallo J, Caron J-L, Huang J, Camarata P, Lara LR, et al. Permanent CSF shunting after intraventricular hemorrhage in the CLEAR III trial. Neurology. 2017;89:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TWK, Vashkevich A, McNamara KA, Valant V, Schwab K, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delcourt C, Sato S, Zhang S, Sandset EC, Zheng D, Chen X, Hackett ML, Arima H, Hata J, Heeley E, et al. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology. 2017;88:1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Li R, Zhao L-B, Yang X-M, Yang W-S, Deng L, Lv X-N, Wu G-F, Tang Z-P, Wei M, et al. Intraventricular Hemorrhage Growth: Definition, Prevalence and Association with Hematoma Expansion and Prognosis. Neurocrit. Care 2020; 2020. Dec;33(3):732–739. [DOI] [PubMed] [Google Scholar]
- 16.Dowlatshahi D, Deshpande A, Aviv RI, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, Kobayashi A, Boulanger J-M, Lum C, et al. Do Intracerebral Hemorrhage Nonexpanders Actually Expand Into the Ventricular Space? Stroke. 2018;49:201–203. [DOI] [PubMed] [Google Scholar]
- 17.Witsch J, Bruce E, Meyers E, Velazquez A, Schmidt JM, Suwatcharangkoon S, Agarwal S, Park S, Falo MC, Connolly ES, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q, Tang J, Tan L, Guo J, Tao Y, Li L, Chen Y, Liu X, Zhang JH, Chen Z, et al. Intracerebral Hematoma Contributes to Hydrocephalus After Intraventricular Hemorrhage via Aggravating Iron Accumulation. Stroke. 2015;46:2902–2908. [DOI] [PubMed] [Google Scholar]
- 19.Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 2003;23:629–652. [DOI] [PubMed] [Google Scholar]
- 20.Selim M, Foster LD, Moy CS, Xi G, Hill MD, Morgenstern LB, Greenberg SM, James ML, Singh V, Clark WM, et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziai WC, Thompson CB, Mayo S, McBee N, Freeman WD, Dlugash R, Ullman N, Hao Y, Lane K, Awad I, et al. Intracranial Hypertension and Cerebral Perfusion Pressure Insults in Adult Hypertensive Intraventricular Hemorrhage: Occurrence and Associations With Outcome. Crit. Care Med 2019;47:1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera-Lara L, Murthy SB, Nekoovaght-Tak S, Ali H, McBee N, Dlugash R, Ram M, Thompson R, Awad IA, Hanley DF, et al. Influence of Bleeding Pattern on Ischemic Lesions After Spontaneous Hypertensive Intracerebral Hemorrhage with Intraventricular Hemorrhage. Neurocrit. Care 2018;29:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Warren AD, Qureshi AI, Morotti A, Falcone GJ, Sheth KN, Shoamanesh A, Dowlatshahi D, Viswanathan A, Goldstein JN. Ultra-Early Blood Pressure Reduction Attenuates Hematoma Growth and Improves Outcome in Intracerebral Hemorrhage. Ann. Neurol 2020;88:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naidech AM, Grotta J, Elm J, Janis S, Dowlatshahi D, Toyoda K, Steiner T, Mayer SA, Khanolkar P, Denlinger J, et al. Recombinant factor VIIa for hemorrhagic stroke treatment at earliest possible time (FASTEST): Protocol for a phase III, double-blind, randomized, placebo-controlled trial. Int. J. Stroke Off. J. Int. Stroke Soc 2021;17474930211042700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this analysis may be obtained from the National Institute of Neurological Disorders and Stroke (NINDS) clinical trial repository, or from the CLEAR III steering committee, after a formal request with a written proposal.


