Abstract
Peptide- and protein-based therapeutics have drawn significant attention over the past few decades for the treatment of infectious diseases, genetic disorders, oncology, and many other clinical needs. Yet, protecting peptide- and protein-based drugs from degradation and denaturation during processing, storage and delivery remain significant challenges. In this review, we introduce the properties of peptide- and protein-based drugs and the challenges associated with their stability and delivery. Then, we discuss delivery strategies using synthetic polymers and their advantages and limitations. This is followed by a focus on silk protein-based materials for peptide/protein drug processing, storage, and delivery, as a path to overcome stability and delivery challenges with current systems.
Keywords: peptide/protein drugs, drug delivery, drug release, silk, biomaterials
Graphical Abstract
1. Introduction
Peptide- and protein-based drugs are defined as oligomeric or polymeric molecules with peptide backbones that have a therapeutic effect and are used for treatments associated with diseases involving immunology(1), infections(2), endocrinology(3), and oncology, among others(4). Rapid advances in the field of genetic engineering technology facilitated the design and synthesis of therapeutic proteins in large scale to expand protein-based pharmaceutical applications(5). Therapeutic proteins (not genetically modified) comprise 199 entities since 2018 including USA, Europe and Canada commercial proteins drugs(6), and the market value of protein drugs is estimated to reach $217 billion by 2023 according to Allied Market Research(7). At present, approximately 80 peptide drugs are on the market globally, with more than 150 peptides in clinical development, and another 400–600 peptides in preclinical studies(8). The rise in popularity for peptide- and protein-based drugs derives from their distinct properties in comparison to conventional small molecule drugs. Certain peptides and proteins play active regulatory and highly specific roles in different biological process in vivo, such as enzymatic reactions, oxygen transport, molecular recognition, biochemical cascades, antibody interactions, among many others, in the form of enzymes, antibodies, cytokines, and hormones. Peptide- and protein-based drugs can be highly efficient and selective, with high potency, low toxicity and immunogenicity, relatively low off-target-induced side-effects, and reduced risk of drug-drug interactions(9). In addition to the above advantages, peptides and proteins can be synthesized via biological processes, with fine-tuned target affinity and specificity, enhancing applicability for a range of diseases with reduced potential side effects (10).
Although peptide/protein drugs have huge potential for use as next generation therapeutics, challenges remain: 1) many proteins with intracellular targets have low cell membrane permeability because of the high molecular weight (MW) and charged amino acid residues(11), 2) susceptibility to degradation and denaturation in the presence of proteolytic enzymes and low pH, thereby diminishing bioavailability(12), 3) rapid metabolism and dissociation of subunits, resulting in decreased bioavailability(9). Significant progress has been made leveraging polymeric materials to protect peptides and proteins from loss of bioactivity due to exposure to harsh conditions during processing, storage, and after administration. Commonly used polymeric systems include biodegradable synthetic polymers such as polyesters, polyorthoesters, and polyphosphoesters(13, 14). However, using these polymeric systems has potential drawbacks, such as the involvement of organic solvents, water penetration during delivery, mechanical damage during mixing, and the generation of inflammatory byproducts during degradation. These factors can negatively affect therapeutic outcomes with peptide and protein-based drugs.
In this review, we highlight the challenges in delivering therapeutic peptides and proteins, and how polymeric materials have been developed to partially counter these challenges. We then provide a specific example on how silk-fibroin (silk)-based materials can provide new options for stabilizing and delivering peptide/protein-based drugs. Finally, we compare the advantages and disadvantages of synthetic polymers and naturally-derived silk protein in terms of peptide/protein encapsulation, delivery, and release. Our goal is to present the scientific foundation for silk as a useful polymeric matrix for the delivery of peptide and protein-based therapeutics with improved bioactivity.
2. Chemical and physical properties of peptide and protein-based drugs and their delivery challenges in biological environments
2.1. The physicochemical properties of peptides and proteins
Protein-based drugs can be divided into different categories, such as antibody-based drugs, Fc fusion proteins, anticoagulants, blood factors, hormones, interferons, interleukins, and thrombolytics(15). Peptides represent a special group of biological compounds, with the combined advantages of proteins (in terms of specificity and selectivity) and small molecule drugs (in terms of ease of synthesis, cost, and stability). Compared with many small molecule drugs, peptide/protein-based drugs are electrically charged due to the presence of anionic (carboxyl) and cationic (amine and guanidine) groups on the amino acid residues, thus, tend to be more hydrophilic and are relatively less stable physically and chemically; They also have relatively short lifetimes and limited stability and can be more sensitive to heat, ionic strength (acid, base, metal ions), organic solvents, and other external factors due to potential alterations in higher order (secondary, tertiary, and quaternary) structures(16).
2.2. Challenges in delivering peptide/protein drugs through different administration routes
Different administration routes have been utilized for protein/peptide-based drugs. Figure 1 summarizes the administration routes utilized for therapeutic peptides and proteins that are currently approved by the US Food and Drug Administration (FDA). More than 90% of peptide/protein-based drugs are administered through parenteral routes including intravenous (44%), subcutaneous (33%), and intramuscular (14%) injections as delivering peptide/protein drugs through parenteral routes can avoid most biological barriers (Figure 2E). However, several limitations are associated with parenteral administration such as pain, risk of infection, high cost, and poor patient compliance(17, 18).
Figure 1.
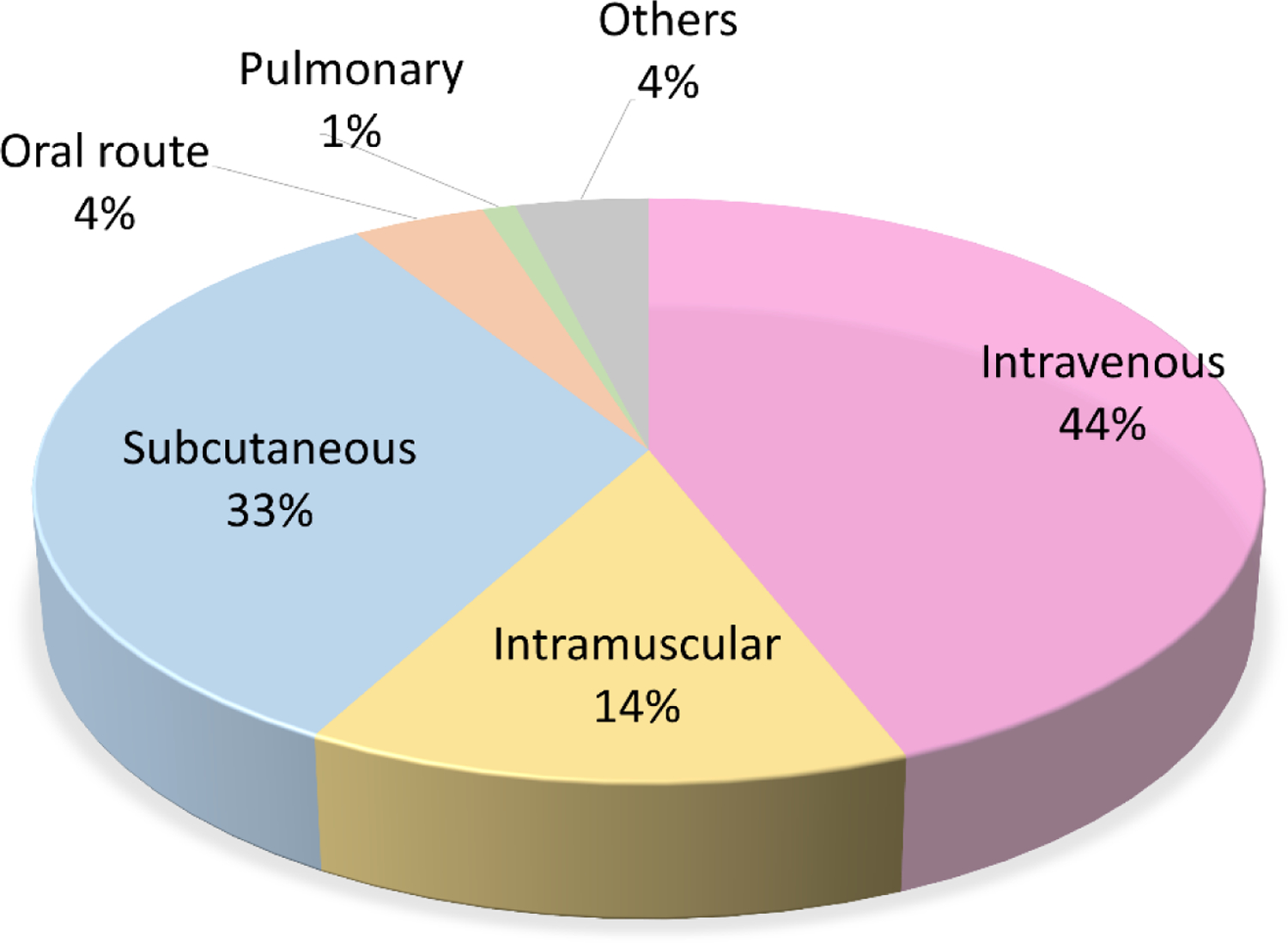
Current percentage distribution of routes of administration for therapeutic peptides and proteins. Adapted from THPdb. (https://webs.iiitd.edu.in/raghava/thpdb/index.html)
Figure 2.
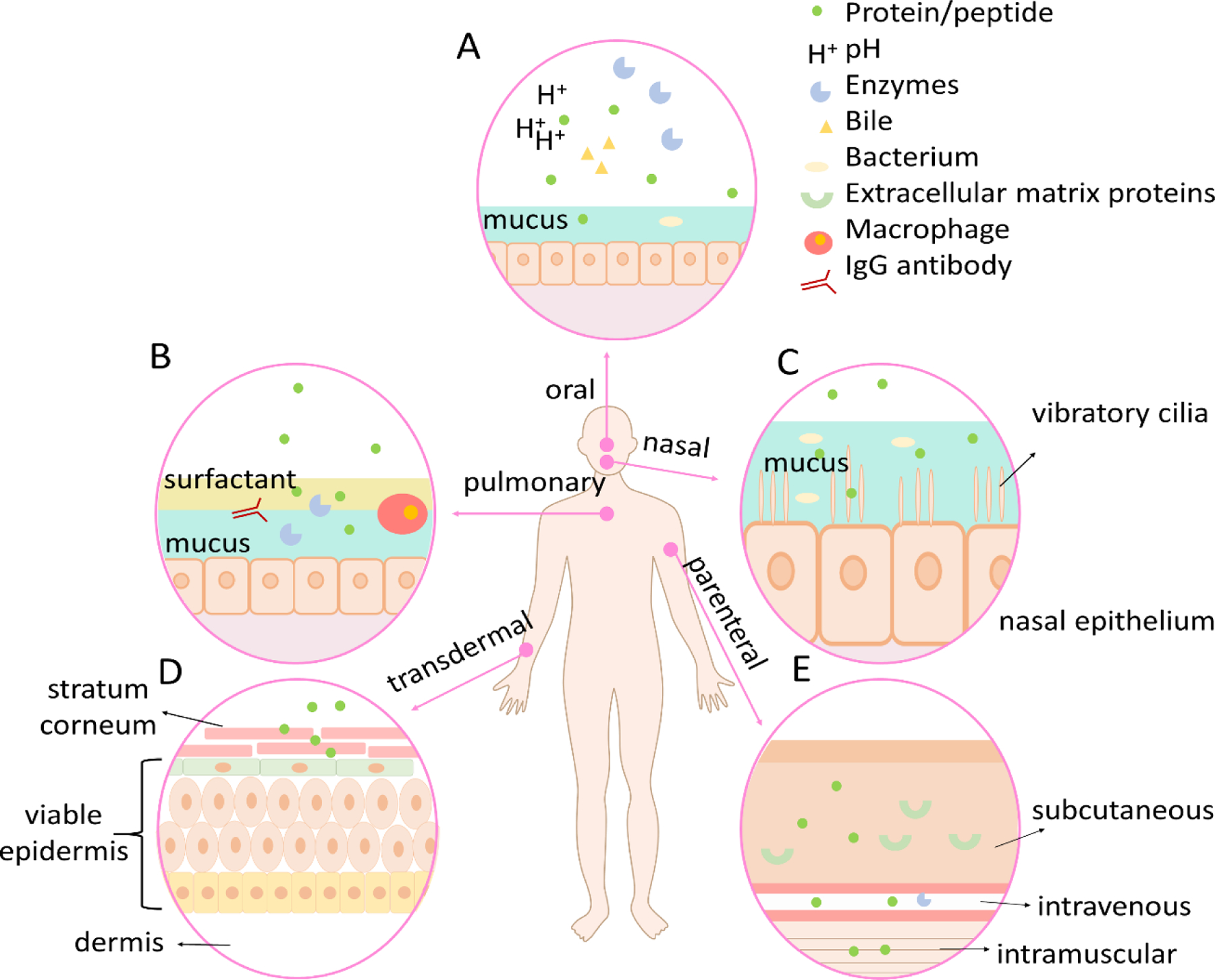
The delivery challenge of peptide/protein-based drugs in various routes. A. Mucus barrier, biochemical barrier, and cellular barrier in oral drug delivery. B. Mucus barrier, biochemical barrier, immunological barrier, and cellular barrier in pulmonary drug delivery. C. Mucus barrier, mechanical barrier, and cellular barrier in nasal drug delivery. D. Cellular barrier in transdermal drug delivery. E. three types of parenteral drug delivery.
Only 4% of the FDA approved protein/peptide drugs exploit oral administration routes although it is non-invasive, can be self-administered, and provides high patient acceptability and long-term compliance. This is because delivering peptides and proteins through oral routes remains a major challenge because of the harsh (pH, mechanical, enzymatic) gastrointestinal (GI) environment and the mucus and cellular barrier for drug uptake for absorption (Figure 2A) (19). Although there are many advantages to pulmonary drug administration (non-invasiveness, high adoption rate, overcoming hepatic first-pass metabolism) (20, 21), only 1% of FDA approved peptide/protein drugs adopt this administration route. As shown in Figure 2B, drug transport through the upper airway is limited by the relatively low surface area and low reginal blood flow. The upper airways are narrow angled passages where inertial impact occurs which prevents the entry of the particles to the lungs. The lungs consist of sophisticated networks of branching airways, which further prevents access of drug molecules to epithelial target sites (22). Drug molecules also need to overcome the lung mucus barrier, which works as a natural defense to remove particles from the airway to deliver them to the oropharynx, where the particulates are swallowed or expectorated (23). The presence of ciliated cells also prevents foreign particles from further reaching the lung epithelium (24). Cell penetration, the blood-gas barrier, immunogenicity through macrophage and IgG antibody factors, and tight junctions of alveolar and endothelial cells are all additional challenges to peptide/protein-based drug delivery in the lung (25, 26). Nasal administration offers advantages to systemic and local drug delivery, including non-invasiveness and being readily accessibility through the thin and porous epithelial barrier that is also highly vascularized with particularly advantageous for delivering drugs to brain tissues or the cerebrospinal fluid by bypassing the blood-brain barrier (BBB) (27, 28). Delivering drugs through the nasal route may, however, cause irritation to the cells and nerves in the nasal cavity (29). Epithelium adsorption efficiency is relatively low due to drug degradation by the nasal mucosa, the small surface area for absorption, and the relatively long distance transport or diffusion to reach the target region (local delivery). Vibratory cilia can also exclude foreign particles through mechanical movement, creating a mechanical barrier for drug molecules to navigate (Figure 2C). Transdermal drug administration refers to drug delivery through the skin, which consists of three stratiform tissues (Figure 2D): stratum corneum, viable epidermis, and dermis (30). This method offers several advantages including bypassing hepatic first-pass metabolism, cost-effective, and high patient compliance (18, 31). However, low absorption of macromolecules by the stratum corneum remains a significant challenge, as does adhesive membrane proteins that form tight junctions in the viable epidermis to create an extra barrier (32). We summarize and compare the key characteristics of the different administration routes for therapeutic peptides and proteins in Table 1.
Table 1.
Characteristics of different administration routes for peptide/protein-based drugs
| delivery route | challenges | benefits |
|---|---|---|
| parenteral |
|
|
| oral |
|
|
| pulmonary |
|
|
| transdermal |
|
|
| nasal |
|
|
3. Commonly used drug delivery systems and challenges to deliver protein/peptide-based drugs
To improve the efficiency of drug delivery and prolong protein/peptide drug bioavailability when administrated through various routes, polymeric delivery carriers are commonly used. Polymer cargos provide unique advantages for drug delivery because of their stability in biological fluids, versatility of formulations, support for sustained release, and protection of protein/peptide drugs from enzymatic degradation as well as the biocompatibility (48).
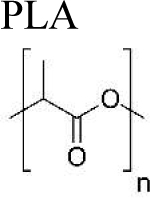
The most commonly used synthetic polymers for drug delivery include polyesters (49), poly(ortho esters) (50), polyphosphoesters (51), polyanhydrides (52), and polyelectrolytes (53). Among these, poly(D, L-lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), and poly(vinyl alcohol) (PVA) represent the most commonly used polymeric drug delivery systems due to their biodegradability, biocompatibility, and nontoxicity (54, 55). In the following section, we introduce current drug encapsulation methods, delivery routes, and drug release mechanisms using PLA, PLGA and PVA, with a focus on recurring challenges in further development. The molecular information, benefits, and limitations of these three polymeric delivery methods are also summarized in Table 2.
Table 2.
Benefits and limitations of commonly accepted drug delivery systems
| Benefits | Limitations | ||
|---|---|---|---|
|
Encapsulation | 1. flexible to tune physical properties (size, shape) |
|
| Delivery |
|
|
|
| Release |
|
|
|
|
Encapsulation |
|
|
| Delivery |
|
|
|
| Release |
|
|
|
|
Encapsulation |
|
|
| Delivery |
|
|
|
| Release |
|
|
3.1. Encapsulation challenges
PLA/PLGA are aliphatic polyesters which are relatively hydrophobic with a high static water contact angle (65). However, synthesis is usually organic solvent based, a disadvantage for peptide- and protein-based drug encapsulation (Figure 3A). PVA, as a hydrophilic polymer, is insoluble in many organic solvents and is soluble in water when heated. Protein/peptide drug encapsulation in PVA is usually low efficiency because of heat-induced denaturation issues (67, 68). Many protein- and peptide-based drugs are hydrophilic and soluble in aqueous solution. This makes encapsulation of these types of drugs using hydrophobic polymers, such as PLGA or PLA, more challenging; Therefore, PLA and PLGA-based copolymers with hydrophilic domains are often used to increase the encapsulation of peptide/protein drugs, but this adds extra steps during synthesis (69).
Figure 3.
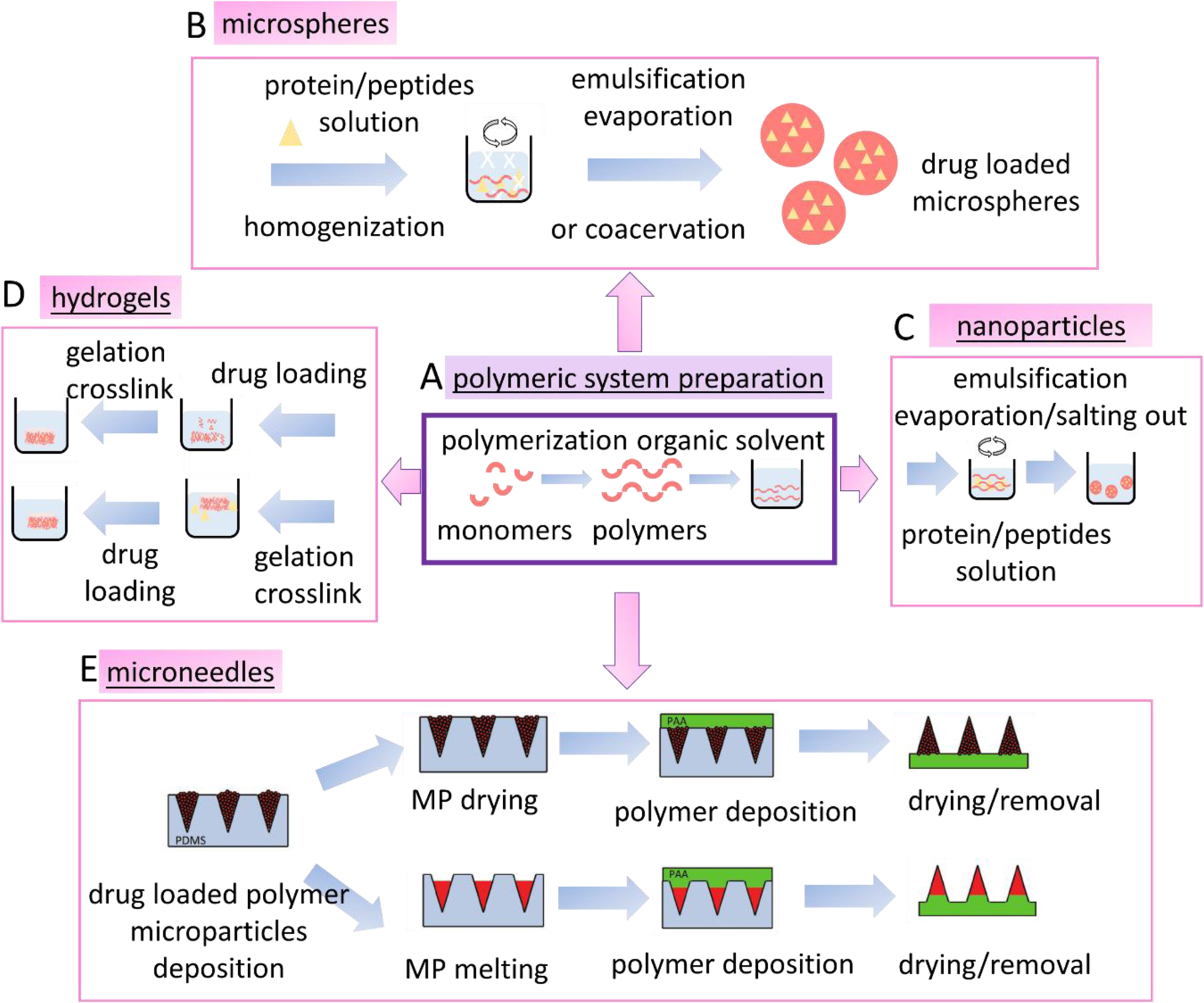
Peptide/protein-based drug encapsulation methods using synthetic polymers. A. preparation of synthetic polymers in organic solvent that are used as drug delivery carriers. B. methods to prepare polymeric microsphere to encapsulate peptide/protein-based drugs. C. methods to make polymeric nanoparticles- for encapsulating peptide/protein drugs. D. methods to prepare drug loaded polymeric hydrogels. E. methods to prepare polymeric microneedles. (adapted from DeMuth et al. (76), reproduced with permission, Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim )
Multiple material formats, including microspheres, nanoparticles, hydrogels, and microneedles, have been developed for encapsulating protein and peptide molecules in polymeric systems (Figure 3). Encapsulating peptide/protein drugs using synthetic polymers is often through microsphere/nanoparticle formation by emulsification evaporation, coacervation, or by salting out (for nanoparticles) (Figure 3B and 3C). In these processes, mechanical stirring is required to form the emulsion, and the shear forces can cause protein molecular distortions that lead to inactivation or aggregation, thus, loss of bioactivity (70–72) (56). Emulsification also requires the use of organic solvent and additives, which can lead to the denaturation or decreased activity of protein and peptide drugs.
Hydrogels have been used to encapsulate and deliver peptide- and protein-based drugs due to tissue like physical properties, good biocompatibility, and ability to efficiently encapsulate hydrophilic drugs (73). Figure 3D shows the methods to incorporate drug molecules in hydrogels, where the drugs can be loaded either before or after gelation. Crosslinking strategies are often used, including photo-initiated dimerization or polymerization, along with other chemical crosslinking methods to improve drug encapsulation efficiency. The removal of toxic reagents using dialysis could result in leaching of the loaded drugs from the hydrogel, thereby decreasing loading efficiency (74).
Microneedles have emerged as a popular method for peptide/protein drug delivery due to their ability to penetrate outer tissue layers via physical penetration to administer the drugs to the underlying vasculature (75). Figure 3E shows one approach to fabricate polymeric microneedles using micromolding (76). Drug loaded polymeric microparticles are deposited in a polydimethylsiloxane (PDMS) mold. After drying, the microparticles are coated with a layer of polyacrylic acid (PAA) polymer. The microneedles patch is obtained by removing the cast from the PDMS mold. Alternatively, microneedles can be fabricated by melting microparticles in the molds followed by polymer deposition and cast removal from the mold. This method takes advantage of the thermoplastic properties of PLA; however, the thermal molding process can be detrimental to temperature-sensitive drugs and is generally not suitable for peptide- and protein-based drugs. PVA-based microneedles can be fabricated using micromolding techniques, where thermal methods can destabilize the drug molecules (77–79). The hydrophilic nature of peptide- and protein-based drugs also decreases the encapsulation efficiency when using synthetic polymers, as they tend to be more hydrophobic. The bulk degradation mechanism of PLA/PLGA based microneedles means there is less control for peptide- and protein-based drug release.
3.2. Sterilization challenges
Sterilization is required for sustained drug delivery systems (80). High temperatures like autoclaving and dry heat sterilization can result in deformation, melting, and degradation of polymeric delivery systems, while also causing the denaturalization of the encapsulated peptide- and protein-based drugs. Ethylene oxide gas method is not applicable due to the toxic residues (81). Physical sterilization like gamma or beta irradiation(82) is more commonly used for biologic drug encapsulated systems, but can result in deterioration of polymeric systems like PLGA (83, 84). Several studies also showed that gamma irradiation can alter the release profiles of polymeric delivery systems (81, 85, 86), and can form hydroxyl radicals in the presence of water and oxygen, leading to the decrease or loss of protein biological functions (87).
3.3. Immunogenic challenges
Synthetic polymers like PLA and PLGA degrade through bulk hydrolysis of ester bonds into lactic and/or glycolic acid monomers in vivo. These low MW acids cause inflammation (96), where the severity of the inflammatory response depends on the local accumulation of the acidic products. Furthermore, these acidic products have an autocatalytic effect towards the implant or particles, causing increased rates of polymer degradation, leading to further inflammation (83). In some cases, the failure to eliminate these degraded by-products, and thus, the accumulation of acidic products, can alter biological responses of tissues (90) (91, 92). Further, during polymer erosion, the release of small fragments and particles can also cause unexpected immune response by macrophages (93). PLGA implants can result in fibrous encapsulation during implantation, which decreases vascular ingrowth and can lead to necrosis (97–99). PVA microcapsule implantation can also activate a chronic inflammatory response (100). Micro- and nanoparticles of PLA and PLGA systems also have toxicity concerns, since these micro- and nano- sized particles can enhance cellular uptake, potentially resulting in negative effects on cell growth and viability (94, 95).
3.4. Controlled drug release challenges
Synthetic polymer matrices can release encapsulated drugs through mechanisms like polymer swelling/solvent penetration (101, 102), chemical hydrolysis (89), and diffusion (Figure 6A) (103). Synthetic polymer tends to degrade via bulk erosion, meaning the degradation-controlled drug release is more challenging to control in comparison to surface erosion (such as with silk protein delivery systems) (106), (107, 108). Diffusion-based drug release is widely used to control delivery. Drug release kinetics usually depend on the concentration of the dissolved drug based on Fick’s second law (109). Non-degradable polymer matrices usually adopt a diffusion-based drug release mechanism, such as polyurethanes used for drug delivery devices (110), cardiac pacing leads (111), tissue adhesives (112) and other applications. The diffusion-controlled mechanism results in a near linear release profile which is favorable for most applications (109). However, the non-biodegradability of these matrices requires a second intervention for device removal after the treatment (113). Polymer swelling/solvent penetration controlled drug delivery involves two processes: solvent diffusion and chain disentanglement (113). This mechanism usually follows non-zero order kinetics, and the delivery rate usually depends on the permeability of the polymers and is more difficult to tune without changing the polymer compositions (114).
Figure 6.
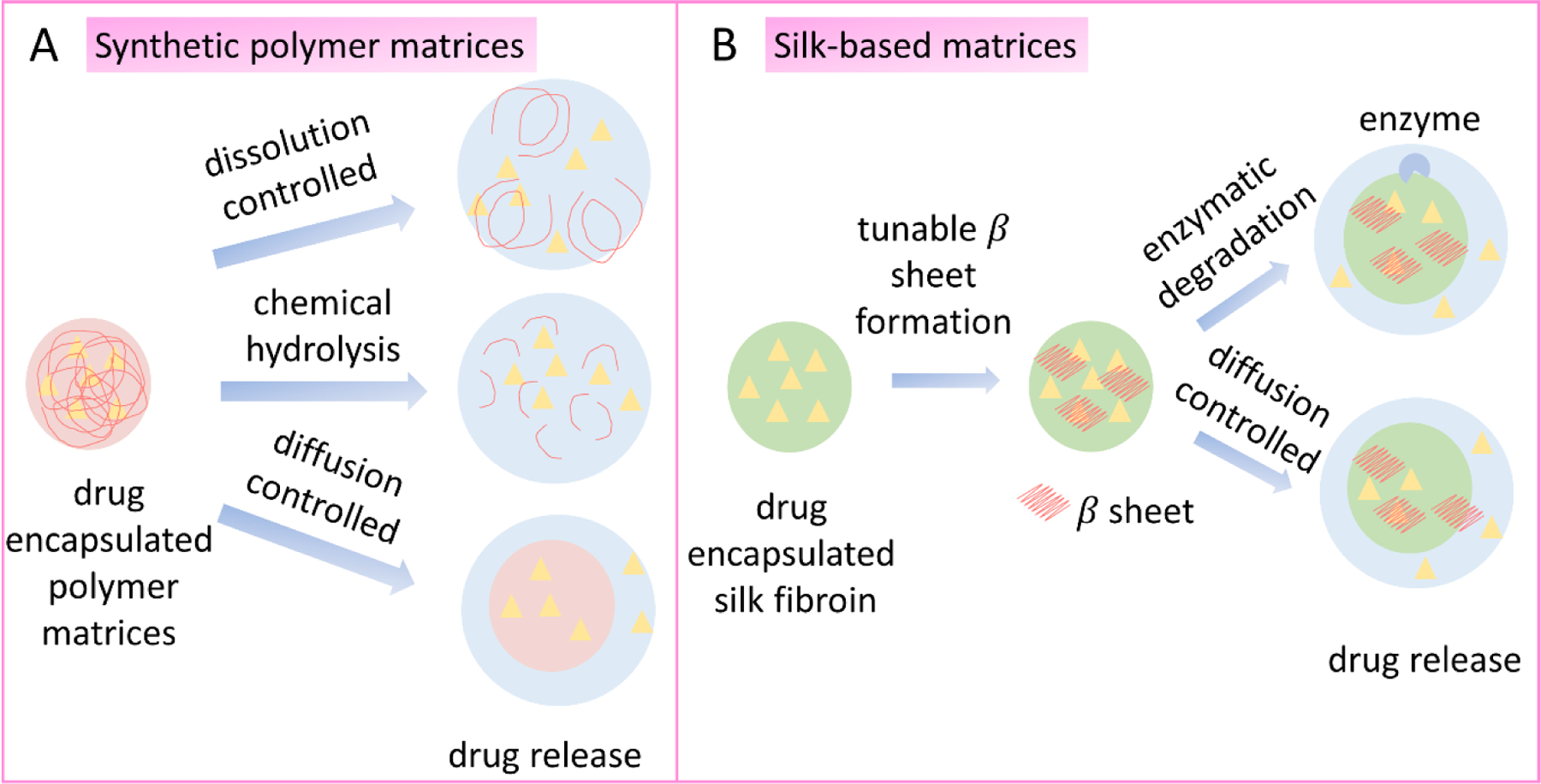
Mechanisms of drug release. A. Drug release from polymeric matrices through dissolution controlled, chemical hydrolysis and diffusion-controlled mechanisms. B. Drug release from silk encapsulation through enzymatic degradation and diffusion-controlled mechanisms.
3.5. Drug stabilization challenges
There is limited evidence of stabilization of peptide and protein based drugs by PLA, PLGA or PVA delivery systems, and the incomplete release of biologics from polymeric systems (e.g. PLGA) suggests instability of the drug molecules in the carrier (104). Efforts have been made to stabilize peptide- and protein-based drugs by adding excipients (115), chemically modifying proteins (e.g. PEGylation) (116), and using reversible protein aggregations (117). These methods add extra efforts during fabrication, and covalent modification can be highly specific but also potentially decrease the bioactivity of the protein drug (118).
4. Silk-based drug delivery strategies
Silk, a biopolymer derived from the cocoons of domesticated Bombyx mori silkworms, has been used as biomedical sutures for decades and has obtained FDA approval for application in a variety of biomaterial devices. The controlled biodegradability, biocompatibility, non-toxicity, aqueous processability, and excellent mechanical properties make silk a suitable candidate in drug delivery systems (119–124). In details, the advantages of delivering proteins and peptides-based drugs using silk-based delivery systems are summarized and listed in Table 3 in the order of encapsulation, delivery, and release. In this section, we first discuss advantageous features of silk that make it a suitable candidate for peptide/protein-based drug delivery and how it addresses the challenges faced for biologics delivery. We then introduce fabrication methods and recent advances for aqueous processing silk into different functional material formats such as micro particles, nanoparticles, hydrogels, and microneedles, which can be potentially used for therapeutic peptides and proteins delivery.
Table 3.
Advantages of peptide/protein-based drug delivery using silk-based systems.
| Aspects | Silk |
|---|---|
| Encapsulation |
|
| Delivery |
|
| Release |
|
4.1. Why silk
Water-based processing method
Regenerated silk fibroin (SF) is commonly used to encapsulate drug molecules with extra advantages for encapsulating bioactive molecules due to its water-based processing method (125–129). Silk cocoons are degummed and then dissolved using lithium bromide (LiBr) (Figure 4A) (129). Since many peptide/protein-based drugs are water soluble, they can be encapsulated in regenerated silk aqueous solution by simple mixing, and then processed into different material formats such as microspheres, nanoparticles, hydrogels, and microneedles with suitable shaping and drying methods (127, 129, 130). Water based processing enables the use of spray drying, or spray freeze drying, where aqueous solutions are directly fed into the spray nozzle to generate micro/nano particles, without the need to introduce an organic solvent phase. Harsh mechanical stirring is also avoided for emulsion methods, to help maintain the stability of protein and peptide-based drug molecules. In the preparation of microneedles, drug mixed silk aqueous solutions are directly infused into molds, avoiding a reduction in bioavailability due to heating or the use of organic solvents, unlike synthetic polymeric microneedle-based methods.
Figure 4.
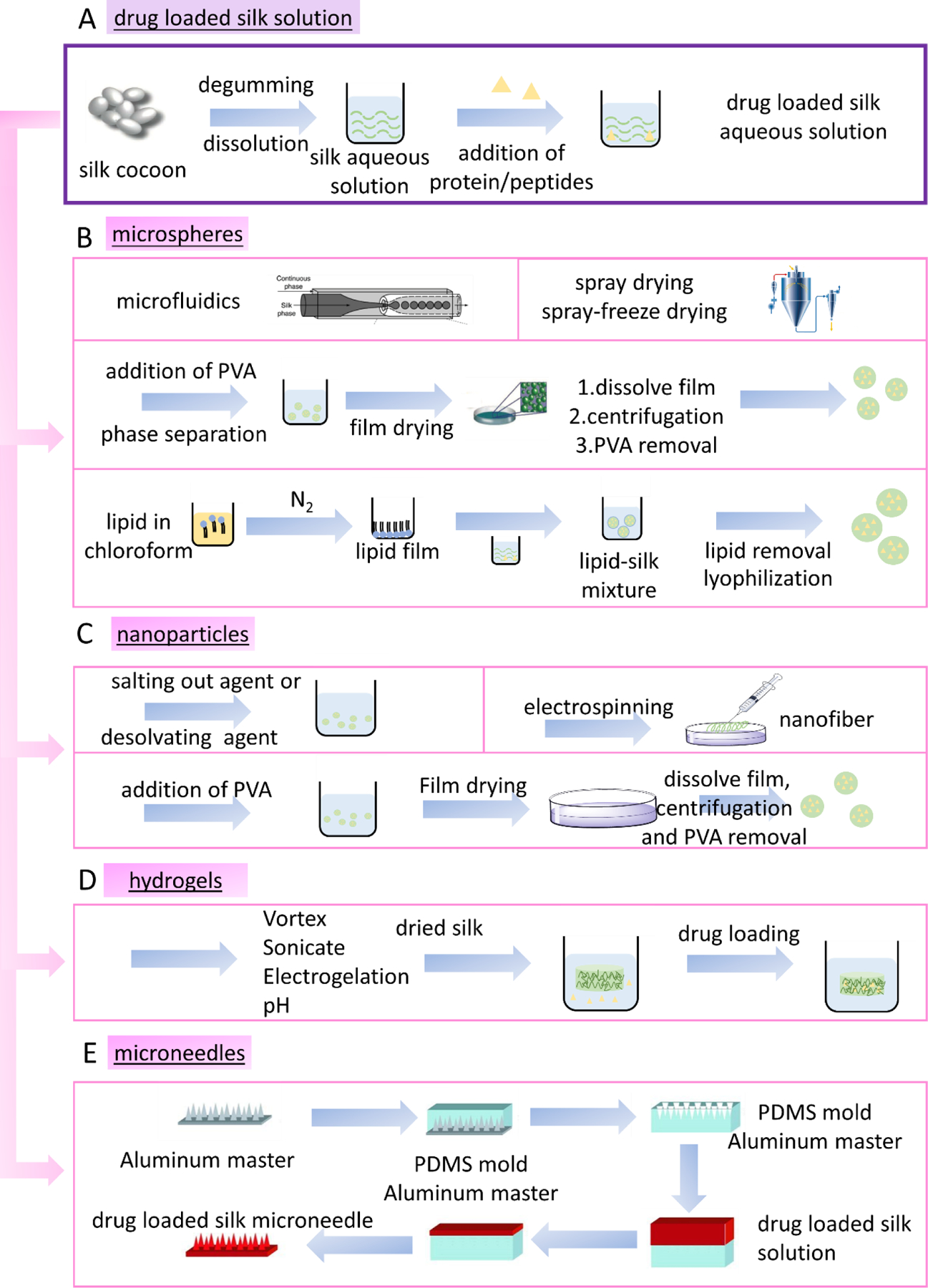
Peptide/protein-based drug encapsulation methods using silk. A. Preparation of silk aqueous solutions for peptide/protein-based drug loading. B. Methods to prepare drug encapsulated silk microspheres. C. List of methods to prepare drug loaded silk nanoparticles. D. Methods for using silk hydrogels to encapsulate peptide/protein drugs. E. Method to prepare silk microneedles for encapsulating peptide/protein drugs. (adapted from Tsioris et al. (182), reproduced with permission, copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim))
Protein stabilization
Silk plays a unique and important role in stabilizing protein-based drugs in both aqueous solution and in the solid phase (131). Silk fibroin aqueous solution is prepared by using LiBr solution to disrupt the crystalline beta-sheet structures. The polymorphic structure of silk in solution can be affected by physicochemical parameters like shear force, pH, concentration, ionic strength, and storage time. Figure 5 shows the mechanisms of silk-based stabilization. In water, peptide/protein-based drugs can be stabilized in silk solution due to preferred interactions between protein/peptide drugs and silk proteins, and hydrophobic/electrostatic shielding effect as silk is an amphiphilic protein (131). Different silk processing methods affect silk structure in the solid state. In general, regenerated silk formed into materials is regarded as a semi-crystalline polymer, where the crystallinity can be tuned by post treatment conditions with water annealing or solvents (132). In the solid state, when silk is processed into a variety of formats, the drug molecules encapsulated are stabilized due to glass dynamic supressed mobility via β-relaxation, preventing protein unfolding and mass transport of the peptide/protein molecules (133). Interactions between the silk and the protein drug lead to the stabilization of complex proteins, as demonstrated with a wide range of bioactive compounds (e.g., enzyme horseradish peroxidase (HRP) in silk films and sponges (131), protease XIV in thermoplastic molded silk (134), and glucose oxidase in silk films (135). Similarly, during drug delivery in vivo, the β-sheet crystallization in silk-based materials can prevent protein unfolding and molecule diffusion, which means silk-based materials maintain bioavailability better than with synthetic polymers due to the protection mechanisms achieved both in solution and in the solid state due to the avoidance of organic solvents.
Figure 5.
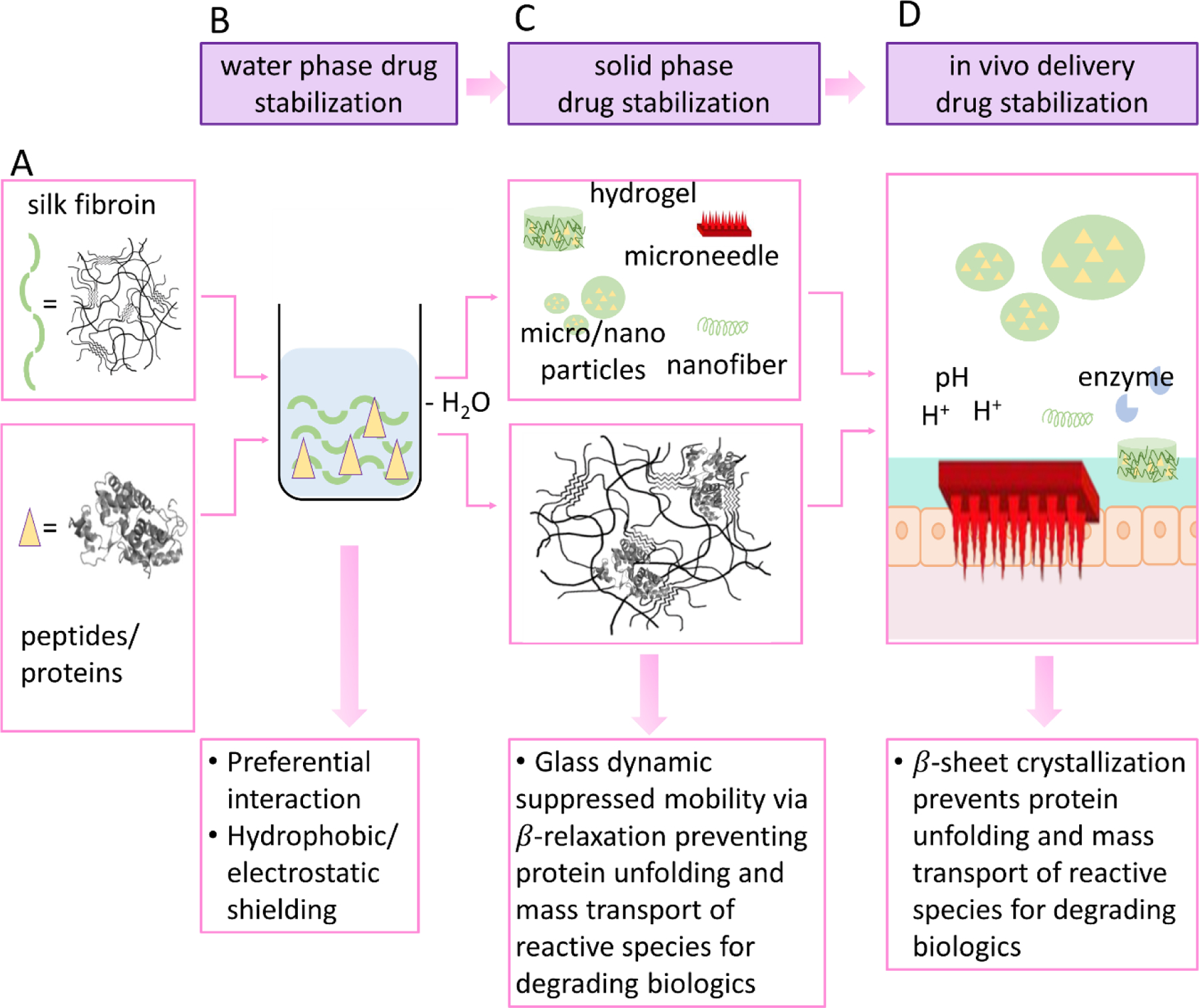
Silk-based stabilization materials and stabilization mechanisms. (adapted from Li et al.(187), reproduced with permission, copyright © 2015 Elsevier B.V. All rights reserved.) A. Silk and peptide/protein drug molecules. B. Silk-based materials stabilize drug molecules in the water phase. C. Silk-based materials stabilize drug molecules in the solid phase. D. Silk-based materials stabilize drug molecules in vivo from the harsh GI environment.
Compatible with sterilization methods
Encapsulating protein and peptide-based drugs using silk-based materials can be achieved through pre drug loading (drug molecules are premixed with the silk polymer solution and shaped into different material formats), or post drug loading (polymer-based materials are immersed in drug solutions to absorb drug molecules into the carrier). It is more straightforward to sterilize the materials with post drug loading method because only the stability of the drug carrier needs to be considered. In this scenario, silk-based materials offer advantages compared with synthetic polymeric systems such as PLA, PLGA, and PVA, because silk-based biomaterials are compatible with common sterilization methods due to the high thermal stability of the silk beta-sheet secondary structures (80). Silk has a glass transition temperature of ~190–200°C, a decomposition temperature of ~220–300°C for side chains, and >300°C for the peptide backbone (80). Thus, thermal sterilization methods (e.g., autoclaving) can be utilized, as well as physical (gamma irradiation) and chemical (ethylene oxide, peroxide) sterilization methods. For pre drug loaded materials, chemical sterilization methods are commonly used, where silk-based medical devices are advantageous due to the stabilization mechanism silk provides to protein and peptide molecules sequestered in the silk (136).
Low immunogenicity
To achieve clinical success, a key assessment for biomaterials is the in vivo response to the material, as well as to any degradation products. Silk materials induce a low if any immune response and a mild inflammatory response that decreases within the first few weeks of implantation (137). The responses involve activation of macrophages and may include a mild foreign body response depending on material format (138, 139). The number of immune cells decreases with time and granulation tissue is replaced by endogenous tissue. The low immunogenicity of silk materials supports its clinical translation, such as the FDA-approval of silk-based medical devices (140).
Robust mechanical properties
Silk has exceptionally robust mechanical properties towards shear forces. The stiffness of silk can vary from 200 Pa to 10 kPa by tuning MW and processing conditions (141). Crosslinking(142) and hybridization with other components(143) can further enhance mechanical properties. The robust mechanical property of silk provides increased stability during drug encapsulation when mechanical stirring is applied, or when injection through syringes or local implantation delivery is utilized. The high toughness of silk materials also indicates more controlled and slower degradation of the materials in vivo, resulting in longer protection of the protein and peptide drugs in the silk materials. In addition, the toughness of silk materials provides a shielding effect for protein or peptide molecules sequestered in the silk during structural distortion.
Mucoadhesive properties
Mucus membranes exist in the various delivery routes including oral, nasal, and pulmonary delivery. The mucus lubricates the interface between the internal and external environment of the body and also protects the internal environment from pathogens and toxins (38). Silk based drug delivery systems can promote drug delivery through the mucus layer by increasing particle residence time and, thus, enhancing drug efficacy (144). For example, coating liposomes with silk significantly improved mucoadhesive properties of the particles, thus increased the attachment of silk coated liposomes to cell surfaces, increasing ocular drug therapeutic efficacy (145). This interaction was due to the binding affinity of silk to glycoproteins and proteoglycans, the major components of mucus (145). The mucoadhesive properties of silk-based materials provides a significant advantage when compared with synthetic polymeric systems.
Controlled and tunable drug release mechanisms
Silk-based drug delivery systems can adopt either an enzymatic degradation-controlled mechanism or a diffusion-controlled mechanism (Figure 6B). Compared to synthetic polymeric systems, the silk release mechanisms offer an expanded set of options for tunable control of drug release.
Enzymatic degradation-controlled drug release
Silk degradation relies on proteolytic enzymes such as protease XIV, α-chymotrypsin, proteinase K, papain, and collagenase, among others (119). Enzymatic degradation of silk generates polypeptides and amino acids with the advantage that the degradation products are absorbed or metabolized in vivo, without inflammation (119). Controlling the degradation kinetics of silk hydrogels affects drug release kinetics and the degradation of silk-based hydrogels depends on many factors including MW (146), crystallinity, porosity, material format, and the local in vivo environment (147). Silk degradation by protease XIV was through surface erosion, in contrast to the bulk chemical hydrolysis of synthetic polyesters. Thus, degradation occurs from the exposed surface area (148), resulting in improved control of drug release features and more predictable outcomes. The tunability of the physiochemical properties of silk materials can also be used to tailor drug interactions with the silk matrix to control drug binding, whether for degradation-driven release or zero-order release of bound drugs (149).
Diffusion controlled drug release
Diffusion-based drug release from silk can be controlled by carrier morphology (geometry, porosity, coating thickness), crystallinity (β-sheet content) (150), and for peptide/protein drugs, molecular weight. The release of FITC-labeled dextrans with various molecular weights (4 to 40 kDa) from silk films demonstrated that increased MW of the dextran cargo led to decreased diffusion, highlighting the importance of MW for control of drug release kinetics (150). Tailoring the release kinetics can also be achieved through modifications of silk-drug interactions and by altering hydration or the surface charge of the silk matrices (151).
4.2. Silk-based delivery systems
4.2.1. Microspheres
Microspheres are a common material format for drug delivery with many advantageous features such as size (smaller than the inner diameter of most of the needles), to foster injectability. The smaller size also leads to higher surface area for bioconjugation, facile natural clearance, and enhanced penetration through tissue barriers. For example, the clearance of particles between 0.5 to 10 µm occurs by phagocytosis (152). Silk microsphere-based drug delivery systems can be fabricated by several methods that lead to different particle morphologies and polydispersities, resulting in different drug delivery efficiencies. Hence, silk-based materials provide options for tuning peptide and protein-based drug delivery properties. Figure 4B shows various routes for preparing silk-based microsphere delivery systems for peptide/protein molecules. Microfluidic devices can be used to generate silk-based microparticles with controlled size, including monodispersed microspheres (153). Microspheres with different diameters were synthesized that were tunable based on system flow rate. Silk micrococoons were also fabricated using microfluidics, with the ability to encapsulate, stabilize, and control the release of proteins including antibodies (154). Spray drying and spray-freeze drying have also been used for encapsulating peptides and proteins to form silk microspheres (155, 156). An aqueous-based preparation method based on silk/PVA films has also been reported(157) where silk and PVA were mixed and phase separated during film formation, with the silk stabilized by beta sheet crosslinking. Silk microspheres were obtained after dissolving the films in water to remove the PVA. The microspheres were used to encapsulate bovine serum albumin with a loading efficiency of 51% and slow release was achieved. Protein-based drug-loaded silk microspheres were also prepared using lipid vesicles as templates (Figure 4B). Lipid in chloroform was first prepared and dried to generate films. Drug loaded silk solution was then added to the lipid film to prepare lipid-silk mixtures. Lyophilization followed by lipid removal was used to obtain the silk microspheres. The method maintained bioactivity of protein drugs and a continuous release up to 10–15 days were observed (156).
As with synthetic polymers, silk microspheres can be prepared using emulsification-condensation by mixing silk with high MW polyethylene glycol (PEG) in solution. The delivery of a peptide drug, octreotide was demonstrated with more than 100 days of sustained release in vitro, significantly longer than Sandostatin LAR depots (octreotide acetate), a PLA based octreotide drug delivery system (158). Basic fibroblast growth factor (bFGF) was also encapsulated in porous SF microspheres using a high-voltage electrostatic method. The bioavailability of bFGF was maintained and released over 13 days (159). Recombinant human bone morphogenetic protein-2(rhBMP-2) and insulin-like growth factor I (rhIGF-I) were delivered from silk porous scaffolds incorporated as silk microspheres with concentration gradients of the protein factors. This work offers a method to deliver multiple growth factors with spatial control in a 3D culture environment (160).
4.2.2. Nanoparticles
Silk-based nanoparticles can be prepared by adding isopropyl alcohol to participate nanoparticles,(161) salting out agents (161), PVA/silk blend films (157), and electrospinning (Figure 4 C) (162). These methods have been used for encapsulating peptide/protein drugs, such as insulin-silk nanoparticle conjugates in the 40–120 nm size range (163). Silk nanoparticles (150–170 nm) were used to encapsulate vascular endothelial growth factor (VEGF) with sustained release of over 3 weeks (164). A transscleral ultrasound assisted drug delivery method was designed using silk nanoparticles with fluorescein isothiocyanate (FITC) labeled bovine serum albumin (BSA) as a model protein drug. The FITC-BSA-Silk nanoparticles provided bioadhesive (with silk as carrier material) and co-permeation properties (with assist from ultrasound). A transscleral route is a path to deliver therapeutic drugs to the posterior segment of the eye (165). Silk nanoparticle based delivery systems have also shown promising results for bone morphogenetic proteins (BMP-2) delivery (166). Three methods were used to prepare drug-loaded silk nanoparticles including self-assembly, desolvation (coacervation or precipitation of nanoparticles by adding a desolvating agent), and an oil emulsion method. Improved drug loading efficiency was reported using a fiber/growth factor dual-gradient along electrospun silk nanofibers for nerve regeneration; nerve growth factor (NGF) was encapsulated in the silk nanofibers with a concentration gradient and resulted in the increased growth and orientation of rat dorsal root ganglion (DRG) neurons (167).
4.2.3. Hydrogels
Silk-based hydrogels can be prepared by vortex (168), sonication (169), electrogelation (170), enzymatic crosslinks (171), and pH changes (Figure 4D) (146, 172–175). Encapsulating drugs into these hydrogel matrices can be achieved either by dissolving the drug in the silk solution or by loading the drug into preformed hydrogels. Pre-loading through the silk solution is not always the best option since the gelation method used can decrease the activity of the peptide or protein drug due to mechanical forces, pH changes, sonication, and electrostatic forces, depending on the method used. The methods used to prepare silk hydrogels do not require the use of chemical or photochemical crosslinking agents that are needed for some synthetic polymeric systems, avoiding major factors that can lead to the decreased activity of peptide/protein-based drugs. The successful delivery of peptide/protein-based drugs has been demonstrated using silk hydrogels, such as to deliver anti-VEGF drugs such as bevacizumab, where the daily release rate was maintained within a therapeutic range for longer than the current market products (>1–2 months) (176). BMP-2 was delivered from a silk hydrogel and promoted bone formation (177), and silk hydrogels delivered neorotrophin-2 (NT-2) for neural tissue engineering and maintained bioavailability with sustained release over 25 days (178). Albumin was delivered using a core-shell structured silk hydrogel, formed by soaking the hydrogel in methanol to form 200–850 µm thick shell structures to slow the release of albumin (179). Monoclonal antibody drugs were combined with silk gels and the in vitro release rates were tuned based on processing (180).
4.2.4. Microneedles
Microneedle-based devices are commonly prepared from synthetic polymers (section 3.1), but with limitations with peptides and protein drugs, such as the inability to precisely control drug release kinetics and the harsh processing conditions with loss of bioactivity of cargo peptides and proteins. For example, PLA-based microneedles require harsh conditions with processing temperatures >135°C, which can be detrimental to peptide and protein drug stability (181). Silk-based microneedle technology is a useful solution due to the biocompatibility, biodegradability, and strong mechanical property of the silk material. Peptide/protein-based drugs can be incorporated into the silk matrix (Figure 4) and materials microfabrication into microneedles can be carried out at ambient temperature with aqueous solutions (Figure 4E). Drug loaded silk microneedles can be processed as follows: 1) A master mold is fabricated as the microneedle shaped by high-speed milling and chemical wet etching, 2) PDMS mold is cast on the master to produce a negative mold, 3) the PDMS mold is then removed from the master mold after drying, 4) drug-loaded silk solution is cast into PDMS mold, 5) silk is dried, 6) microneedle casting is removed from PDMS mold. This method was used to encapsulate horseradish peroxidase (HRP) and enzymatic activity was maintained after microneedle fabrication along with sustained release (182). Using the same method, the sustained release of levonorgestrel, a contraceptive, was demonstrated for at least 100 days from silk-based microneedles (183). The release continued for at least a year when drug loading was through microparticles cast inside the silk microneedle patch. Insulin-loaded silk-based microneedle systems were prepared and sustained release for 60 h was achieved (184). In this system, blending proline in the system induced the transformation of the silk from random coil to β-sheet, leading to improved stability of the microneedles. Incorporating proline also helps to reduce the release rate of the insulin drugs in a controlled manner (184). Vaccine delivery was achieved using silk-based microneedle technology, with Evtrimer released over 2 weeks in the skin, correlating with increased germinal center B cell response (185). The successful delivery of a protein subunit vaccine bolus using a silk/poly(acrylic acid) (PAA) composite microneedle device demonstrated a >10-fold increase in antigen-specific T-cell and humoral immune response when compared with traditional parenteral needle-based immunization (186).
5. Conclusions and future perspectives
Peptide/protein drug delivery remains a challenge when administered through different routes, and in particular non-parenteral administration routes. The major obstacles to overcome with delivery include the GI environment due to low pH, enzymes, macrophages, antibodies, mechanical stress, mucus, transport, and cellular barriers. Encapsulation within synthetic polymers offers a strategy to protect biological drugs from loss of activity. However, encapsulation can also be detrimental due to the use of organic solvents, mechanical impacts, sterilization, the use of crosslinking agents, and chemical modifications. Silk protein offers a unique amphiphilic biopolymeric material for encapsulating and delivering peptides and proteins. During drug encapsulation, water-based silk device fabrication methods are compatible with peptide/protein drugs to maintain activity. Access to various fabrication methods enables the selection of silk material formats to encapsulate drugs. Silk can also help to stabilize protein drugs, due to the chemistry and secondary structures, providing further advantages. The robust and stable properties of silk-based materials provide mechanical durability. The biocompatibility of the silk ensures a minimum immune response and other adverse effects. The drug release mechanisms of silk-based materials following either a degradation-based or diffusion-based mechanism can be fine-tuned, while enzyme-mediated surface degradation offers a predictable outcome for sustained drug release.
While silk-based delivery systems offer to address multiple challenges with delivering peptide/protein drugs, more research is needed to understand the feasibility of silk-based materials in different drug administration routes. There remains a gap between encapsulation of drug molecules into various silk formats and the feasibility of using each format for different administration methods. For example, the role of silk-based materials to protect protein drugs from low pH and enzymatic environments in oral delivery systems would benefit from further insight, as well as related cellular interactions and penetration mechanisms.
Highlights.
The challenges of delivering peptide and protein-based drugs are discussed
The advantages and limitations of synthetic polymer delivery system is introduced for protein and peptide-based drugs
Silk based drug delivery system is highlighted for overcoming the stability and delivery challenges of protein and peptide-based drugs
Acknowledgements:
We thank the NIH (P41EB027062, R01NS094218, R01AR070975) for support of this work, and Yu-Ting L. Dingle, Ph.D. for her assistance with the illustration in Figure 7.
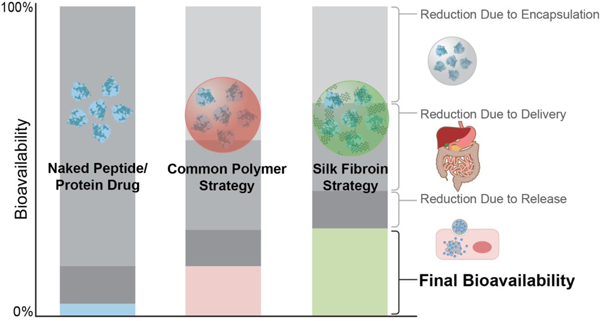
Figure 7.
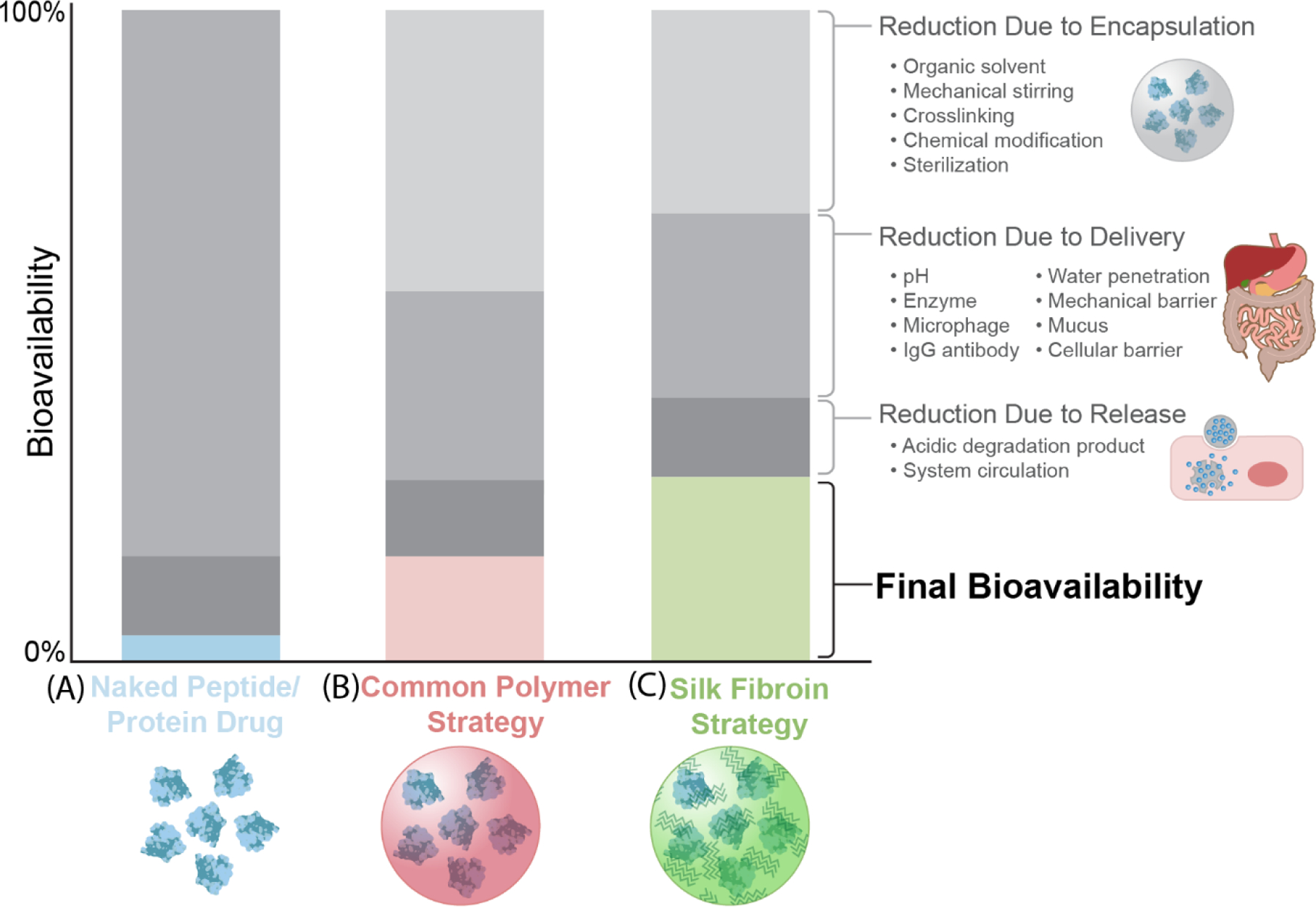
Comparison of bioavailability of the peptides/protein drugs without protection, with common synthetic polymer strategies, and with a silk protection strategy. A. The bioavailability of naked peptide/protein drugs affected by factors during encapsulation, delivery, and release. B. The bioavailability of peptide/protein drugs protected using common polymer strategies affected by factors during encapsulation, delivery, and release. C. The bioavailability of peptide/protein drugs protected using silk fibroin strategy affected by factors during encapsulation, delivery, and release. The decrease of the bioavailability in the figure is a qualitative analysis, to provide a visual comparison of the differences between the polymeric and silk strategies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gokhale AS, Satyanarayanajois S. Peptides and peptidomimetics as immunomodulators. Immunotherapy 2014;6(6):755–74. doi: 10.2217/imt.14.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oyston PCF, Fox MA, Richards SJ, Clark GC. Novel peptide therapeutics for treatment of infections. J Med Microbiol 2009;58(Pt 8):977–87. Epub 2009/06/17. doi: 10.1099/jmm.0.011122-0. [DOI] [PubMed] [Google Scholar]
- 3.Lau JL, Dunn MK. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic & Medicinal Chemistry 2018;26(10):2700–7. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 4.Fisher E, Pavlenko K, Vlasov A, Ramenskaya G. Peptide-Based Therapeutics for Oncology. Pharmaceut Med 2019;33(1):9–20. Epub 2020/01/15. doi: 10.1007/s40290-018-0261-7. [DOI] [PubMed] [Google Scholar]
- 5.Lagassé HAD, Alexaki A, Simhadri VL, Katagiri NH, Jankowski W, Sauna ZE, Kimchi-Sarfaty C. Recent advances in (therapeutic protein) drug development. F1000Res 2017;6:113-. doi: 10.12688/f1000research.9970.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geraldes DC, Beraldo-de-Araújo VL, Pardo BOP, Pessoa Junior A, Stephano MA, de Oliveira-Nascimento L. Protein drug delivery: current dosage form profile and formulation strategies. J Drug Target 2020;28(4):339–55. Epub 2019/10/03. doi: 10.1080/1061186x.2019.1669043. [DOI] [PubMed] [Google Scholar]
- 7.Research AM. Protein Therapeutics Market by Product (Monoclonal Antibodies, Insulin, Fusion Protein, Erythropoietin, Interferon, Human Growth Hormone, and Follicle Stimulating Hormone) and Application (Metabolic Disorders, Immunologic Disorders, Hematological Disorders, Cancer, Hormonal Disorders, Genetic Disorders, and Others) - Global Opportunity Analysis and Industry Forecast, 2017–20232017
- 8.Muttenthaler M, King GF, Adams DJ, Alewood PF. Trends in peptide drug discovery. Nature Reviews Drug Discovery 2021;20(4):309–25. doi: 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- 9.Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv 2013;4(11):1443–67. doi: 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des 2013;81(1):136–47. Epub 2012/12/21. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 11.Muheem A, Shakeel F, Jahangir MA, Anwar M, Mallick N, Jain GK, Warsi MH, Ahmad FJ. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharmaceutical Journal 2016;24(4):413–28. doi: 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaji J, Patole V. Protein and Peptide drug delivery: oral approaches. Indian J Pharm Sci 2008;70(3):269–77. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annu Rev Chem Biomol Eng 2010;1:149–73. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, Lin ZY, Yildirimer L, Dhinakar A, Zhao X, Wu J. Polymer-based nanoparticles for protein delivery: design, strategies and applications. Journal of Materials Chemistry B 2016;4(23):4060–71. doi: 10.1039/C6TB00308G. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrov DS. Therapeutic proteins. Methods Mol Biol 2012;899:1–26. doi: 10.1007/978-1-61779-921-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R, Singh S, Lillard JW Jr. Past, present, and future technologies for oral delivery of therapeutic proteins. J Pharm Sci 2008;97(7):2497–523. doi: 10.1002/jps.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, Cholkar K, Mitra AK. Recent developments in protein and peptide parenteral delivery approaches. Ther Deliv 2014;5(3):337–65. doi: 10.4155/tde.14.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajracharya R, Song JG, Back SY, Han H-K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput Struct Biotechnol J 2019;17:1290–308. doi: 10.1016/j.csbj.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muheem A, Shakeel F, Jahangir MA, Anwar M, Mallick N, Jain GK, Warsi MH, Ahmad FJ. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J 2016;24(4):413–28. Epub 2014/06/16. doi: 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali M CHAPTER 9 - Pulmonary Drug Delivery. In: Kulkarni VS, editor. Handbook of Non-Invasive Drug Delivery Systems Boston: William Andrew Publishing; 2010. p. 209–46. [Google Scholar]
- 21.Pond SM, Tozer TN. First-pass elimination. Basic concepts and clinical consequences. Clin Pharmacokinet 1984;9(1):1–25. Epub 1984/01/01. doi: 10.2165/00003088-198409010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Newman SP. Drug delivery to the lungs: challenges and opportunities. Ther Deliv 2017;8(8):647–61. doi: 10.4155/tde-2017-0037. [DOI] [PubMed] [Google Scholar]
- 23.Borgström L, Olsson B, Thorsson L. Degree of throat deposition can explain the variability in lung deposition of inhaled drugs. J Aerosol Med 2006;19(4):473–83. Epub 2007/01/02. doi: 10.1089/jam.2006.19.473. [DOI] [PubMed] [Google Scholar]
- 24.Bustamante-Marin XM, Ostrowski LE. Cilia and Mucociliary Clearance. Cold Spring Harb Perspect Biol 2017;9(4):a028241. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West JB. Thoughts on the pulmonary blood-gas barrier. American Journal of Physiology-Lung Cellular and Molecular Physiology 2003;285(3):L501–L13. doi: 10.1152/ajplung.00117.2003. [DOI] [PubMed] [Google Scholar]
- 26.Patil JS, Sarasija S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012;29(1):44–9. doi: 10.4103/0970-2113.92361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meredith ME, Salameh TS, Banks WA. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J 2015;17(4):780–7. Epub 2015/03/24. doi: 10.1208/s12248-015-9719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meredith ME, Salameh TS, Banks WA. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. Aaps j 2015;17(4):780–7. Epub 2015/03/25. doi: 10.1208/s12248-015-9719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dombu CY, Betbeder D. Airway delivery of peptides and proteins using nanoparticles. Biomaterials 2013;34(2):516–25. doi: 10.1016/j.biomaterials.2012.08.070. [DOI] [PubMed] [Google Scholar]
- 30.Alkilani AZ, McCrudden MTC, Donnelly RF. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015;7(4):438–70. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv 2010;1(1):109–31. doi: 10.4155/tde.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol 2008;26(11):1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner MR, Balu-Iyer SV. Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins. J Pharm Sci 2018;107(5):1247–60. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soliman E, Ranjan S, Xu T, Gee C, Harker A, Barrera A, Geddes J. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Bio-Design and Manufacturing 2018;1(3):161–70. doi: 10.1007/s42242-018-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boegh M, Nielsen HM. Mucus as a barrier to drug delivery – understanding and mimicking the barrier properties. Basic Clin Pharmacol Toxicol 2015;116(3):179–86. Epub 2014/10/29. doi: 10.1111/bcpt.12342. [DOI] [PubMed] [Google Scholar]
- 36.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev 2012;64(6):557–70. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinzón Martín S, Seeberger PH, Varón Silva D. Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators During Infection and Targets for Diagnostics and Vaccines. Frontiers in Chemistry 2019;7(710). doi: 10.3389/fchem.2019.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boegh M, Nielsen HM. Mucus as a Barrier to Drug Delivery – Understanding and Mimicking the Barrier Properties. Basic & Clinical Pharmacology & Toxicology 2015;116(3):179–86. doi: 10.1111/bcpt.12342. [DOI] [PubMed] [Google Scholar]
- 39.Yang T, Han H, Chen Y, Yang L, Parker R, Li Y, Kaplan DL, Xu Q. Study the lipidoid nanoparticle mediated genome editing protein delivery using 3D intestinal tissue model. Bioactive Materials 2021;6(11):3671–7. doi: 10.1016/j.bioactmat.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibaldi M Biopharmaceutics and clinical pharmacokinetics: Lea & Febiger; 1991. [Google Scholar]
- 41.Brown TD, Whitehead KA, Mitragotri S. Materials for oral delivery of proteins and peptides. Nature Reviews Materials 2020;5(2):127–48. doi: 10.1038/s41578-019-0156-6. [DOI] [Google Scholar]
- 42.Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. International Journal of Pharmaceutics 2013;447(1):75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siekmeier R, Scheuch G. Systemic treatment by inhalation of macromolecules–principles, problems, and examples. J Physiol Pharmacol 2008;59(Suppl 6):53–79. [PubMed] [Google Scholar]
- 44.Mastrandrea LD, Quattrin T. Clinical evaluation of inhaled insulin. Adv Drug Deliv Rev 2006;58(9–10):1061–75. Epub 2006/10/31. doi: 10.1016/j.addr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Codrons V, Vanderbist F, Verbeeck RK, Arras M, Lison D, Préat V, Vanbever R. Systemic delivery of parathyroid hormone (1–34) using inhalation dry powders in rats. J Pharm Sci 2003;92(5):938–50. Epub 2003/04/25. doi: 10.1002/jps.10346. [DOI] [PubMed] [Google Scholar]
- 46.Gessler T, Seeger W, Schmehl T. Inhaled prostanoids in the therapy of pulmonary hypertension. J Aerosol Med Pulm Drug Deliv 2008;21(1):1–12. Epub 2008/06/04. doi: 10.1089/jamp.2007.0657. [DOI] [PubMed] [Google Scholar]
- 47.H.R R, Dhamecha D, Jagwani S, Rao M, Jadhav K, Shaikh S, Puzhankara L, Jalalpure S. Local drug delivery systems in the management of periodontitis: A scientific review. Journal of Controlled Release 2019;307:393–409. doi: 10.1016/j.jconrel.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 48.Patel A, Patel M, Yang X, Mitra AK. Recent advances in protein and Peptide drug delivery: a special emphasis on polymeric nanoparticles. Protein Pept Lett 2014;21(11):1102–20. Epub 2014/08/12. doi: 10.2174/0929866521666140807114240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bialik M, Kuras M, Sobczak M, Oledzka E. Biodegradable synthetic polyesters in the technology of controlled dosage forms of antihypertensive drugs – the overview. Expert Opinion on Drug Delivery 2019;16(9):953–67. doi: 10.1080/17425247.2019.1651716. [DOI] [PubMed] [Google Scholar]
- 50.Heller J Controlled drug release from poly(ortho esters) — A surface eroding polymer. Journal of Controlled Release 1985;2:167–77. doi: 10.1016/0168-3659(85)90042-2. [DOI] [Google Scholar]
- 51.Zhao Z, Wang J, Mao H-Q, Leong KW. Polyphosphoesters in drug and gene delivery. Advanced Drug Delivery Reviews 2003;55(4):483–99. doi: 10.1016/S0169-409X(03)00040-1. [DOI] [PubMed] [Google Scholar]
- 52.Jain JP, Chitkara D, Kumar N. Polyanhydrides as localized drug delivery carrier: an update. Expert Opinion on Drug Delivery 2008;5(8):889–907. doi: 10.1517/17425247.5.8.889. [DOI] [PubMed] [Google Scholar]
- 53.Lankalapalli S, Kolapalli VRM. Polyelectrolyte Complexes: A Review of their Applicability in Drug Delivery Technology. Indian J Pharm Sci 2009;71(5):481–7. doi: 10.4103/0250-474X.58165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sung YK, Kim SW. Recent advances in polymeric drug delivery systems. Biomaterials Research 2020;24(1):12. doi: 10.1186/s40824-020-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gajra B, Pandya S, Vidyasagar G, Rabari H, Dedania R, Rao S. Poly vinyl alcohol Hydrogel and its Pharmaceutical and Biomedical Applications: A Review. International Journal of Pharmaceutical Research 2011;4:20–6. [Google Scholar]
- 56.Lee BK, Yun Y, Park K. PLA micro- and nano-particles. Adv Drug Deliv Rev 2016;107:176–91. Epub 2016/06/01. doi: 10.1016/j.addr.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kita M, Ogura Y, Honda Y, Hyon SH, Cha W 2nd, Ikada Y. Evaluation of polyvinyl alcohol hydrogel as a soft contact lens material. Graefes Arch Clin Exp Ophthalmol 1990;228(6):533–7. Epub 1990/01/01. doi: 10.1007/bf00918486. [DOI] [PubMed] [Google Scholar]
- 58.Young TH, Yao NK, Chang RF, Chen LW. Evaluation of asymmetric poly(vinyl alcohol) membranes for use in artificial islets. Biomaterials 1996;17(22):2139–45. Epub 1996/11/01. doi: 10.1016/0142-9612(96)00043-9. [DOI] [PubMed] [Google Scholar]
- 59.Paul W, Sharma CP. Acetylsalicylic acid loaded poly(vinyl alcohol) hemodialysis membranes: effect of drug release on blood compatibility and permeability. J Biomater Sci Polym Ed 1997;8(10):755–64. Epub 1997/01/01. doi: 10.1163/156856297x00290. [DOI] [PubMed] [Google Scholar]
- 60.Baker MI, Walsh SP, Schwartz Z, Boyan BD. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res B Appl Biomater 2012;100(5):1451–7. Epub 2012/04/20. doi: 10.1002/jbm.b.32694. [DOI] [PubMed] [Google Scholar]
- 61.Abdullah O, Usman Minhas M, Ahmad M, Ahmad S, Barkat K, Ahmad A. Synthesis, optimization, and evaluation of polyvinyl alcohol-based hydrogels as controlled combinatorial drug delivery system for colon cancer. Advances in Polymer Technology 2018;37(8):3348–63. doi: 10.1002/adv.22119. [DOI] [Google Scholar]
- 62.Mandal TK, Bostanian LA, Graves RA, Chapman SR. Poly(D,L-lactide-co-glycolide) encapsulated poly(vinyl alcohol) hydrogel as a drug delivery system. Pharm Res 2002;19(11):1713–9. Epub 2002/12/03. doi: 10.1023/a:1020765615379. [DOI] [PubMed] [Google Scholar]
- 63.Kim C-J, Lee PI. Composite Poly(vinyl alcohol) Beads for Controlled Drug Delivery. Pharmaceutical Research 1992;9(1):10–6. doi: 10.1023/A:1018963223484. [DOI] [PubMed] [Google Scholar]
- 64.Tamizi E, Azizi M, Seyed Dorraji MS, Dusti Z, Panahi-Azar V. Stabilized core/shell PVA/SA nanofibers as an efficient drug delivery system for dexpanthenol. Polymer Bulletin 2018;75(2):547–60. doi: 10.1007/s00289-017-2049-4. [DOI] [Google Scholar]
- 65.Baran EH, Erbil HY. Surface modification of 3D printed PLA objects by fused deposition modeling: a review. Colloids and Interfaces 2019;3(2):43. [Google Scholar]
- 66.Swider E, Koshkina O, Tel J, Cruz LJ, de Vries IJM, Srinivas M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomaterialia 2018;73:38–51. doi: 10.1016/j.actbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Barui A 3 - Synthetic polymeric gel. In: Pal K, Banerjee I, editors. Polymeric Gels: Woodhead Publishing; 2018. p. 55–90. [Google Scholar]
- 68.Yamamoto S, Kaneo Y, Maitani Y. Hydrophobized poly(vinyl alcohol) for encapsulation of amphotericin B in nanoparticles. Journal of Drug Delivery Science and Technology 2013;23(2):129–35. doi: 10.1016/S1773-2247(13)50020-7. [DOI] [Google Scholar]
- 69.Lassalle V, Ferreira ML. PLA Nano- and Microparticles for Drug Delivery: An Overview of the Methods of Preparation. Macromolecular Bioscience 2007;7(6):767–83. doi: 10.1002/mabi.200700022. [DOI] [PubMed] [Google Scholar]
- 70.Charm SE, Wong BL. Shear effects on enzymes. Enzyme and Microbial Technology 1981;3(2):111–8. doi: 10.1016/0141-0229(81)90068-5. [DOI] [Google Scholar]
- 71.Biddlecombe JG, Craig AV, Zhang H, Uddin S, Mulot S, Fish BC, Bracewell DG. Determining antibody stability: creation of solid-liquid interfacial effects within a high shear environment. Biotechnol Prog 2007;23(5):1218–22. Epub 2007/08/25. doi: 10.1021/bp0701261. [DOI] [PubMed] [Google Scholar]
- 72.Colombié S, Gaunand A, Lindet B. Lysozyme inactivation under mechanical stirring: effect of physical and molecular interfaces. Enzyme Microb Technol 2001;28(9–10):820–6. Epub 2001/06/09. doi: 10.1016/s0141-0229(01)00340-4. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater 2016;1(12):16071. Epub 2016/10/18. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer 2008;49(8):1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- 75.Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 2012;64(14):1547–68. Epub 2012/05/01. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeMuth PC, Garcia-Beltran WF, Ai-Ling ML, Hammond PT, Irvine DJ. Composite Dissolving Microneedles for Coordinated Control of Antigen and Adjuvant Delivery Kinetics in Transcutaneous Vaccination. Advanced Functional Materials 2013;23(2):161–72. doi: 10.1002/adfm.201201512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen HX, Bozorg BD, Kim Y, Wieber A, Birk G, Lubda D, Banga AK. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. European Journal of Pharmaceutics and Biopharmaceutics 2018;129:88–103. doi: 10.1016/j.ejpb.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Lee IC, He J-S, Tsai M-T, Lin K-C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. Journal of Materials Chemistry B 2015;3(2):276–85. doi: 10.1039/C4TB01555J. [DOI] [PubMed] [Google Scholar]
- 79.Liu T, Luo G, Xing M. Biomedical Applications of Polymeric Microneedles for Transdermal Therapeutic Delivery and Diagnosis: Current Status and Future Perspectives. Advanced Therapeutics 2020;3(9):1900140. doi: 10.1002/adtp.201900140. [DOI] [Google Scholar]
- 80.Yucel T, Lovett ML, Kaplan DL. Silk-based biomaterials for sustained drug delivery. J Control Release 2014;190:381–97. Epub 2014/06/10. doi: 10.1016/j.jconrel.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Igartua M, Hernández RM, Rosas JE, Patarroyo ME, Pedraz JL. γ-Irradiation effects on biopharmaceutical properties of PLGA microspheres loaded with SPf66 synthetic vaccine. European Journal of Pharmaceutics and Biopharmaceutics 2008;69(2):519–26. doi: 10.1016/j.ejpb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 82.Abuhanoğlu G, Ozer AY. Radiation sterilization of new drug delivery systems. Interv Med Appl Sci 2014;6(2):51–60. Epub 2014/06/04. doi: 10.1556/IMAS.6.2014.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ polyglycolic acid copolymers. Biomaterials 1996;17(2):93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 84.Montanari L, Costantini M, Signoretti EC, Valvo L, Santucci M, Bartolomei M, Fattibene P, Onori S, Faucitano A, Conti B, Genta I. Gamma irradiation effects on poly(dl-lactictide-co-glycolide) microspheres. Journal of Controlled Release 1998;56(1):219–29. doi: 10.1016/S0168-3659(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 85.Faisant N, Siepmann J, Richard J, Benoit J. Mathematical modeling of drug release from bioerodible microparticles: effect of gamma-irradiation. European Journal of Pharmaceutics and Biopharmaceutics 2003;56(2):271–9. [DOI] [PubMed] [Google Scholar]
- 86.Lalla J, Sapna K. Biodegradable microspheres of poly (DL-lactic acid) containing piroxicam as a model drug for controlled release via the parenteral route. Journal of microencapsulation 1993;10(4):449–60. [DOI] [PubMed] [Google Scholar]
- 87.Zbikowska HM, Nowak P, Wachowicz B. Protein modification caused by a high dose of gamma irradiation in cryo-sterilized plasma: Protective effects of ascorbate. Free Radical Biology and Medicine 2006;40(3):536–42. doi: 10.1016/j.freeradbiomed.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Tyler B, Gullotti D, Mangraviti A, Utsuki T, Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Deliv Rev 2016;107:163–75. doi: 10.1016/j.addr.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 89.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev 2016;116(4):2602–63. Epub 2016/02/08. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramot Y, Haim-Zada M, Domb AJ, Nyska A. Biocompatibility and safety of PLA and its copolymers. Adv Drug Deliv Rev 2016;107:153–62. Epub 2016/04/09. doi: 10.1016/j.addr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 91.Kohane D, Langer R, Kinney R, Lipp M, Anthony D, Louis D. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. Journal of Biomedical Materials Research 2002;59(3):450–9. doi: 10.1002/jbm.1261. [DOI] [PubMed] [Google Scholar]
- 92.Pappalardo D, Mathisen T, Finne-Wistrand A. Biocompatibility of Resorbable Polymers: A Historical Perspective and Framework for the Future. Biomacromolecules 2019;20(4):1465–77. doi: 10.1021/acs.biomac.9b00159. [DOI] [PubMed] [Google Scholar]
- 93.Lyu S, Untereker D. Degradability of polymers for implantable biomedical devices. Int J Mol Sci 2009;10(9):4033–65. doi: 10.3390/ijms10094033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elmowafy EM, Tiboni M, Soliman ME. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. Journal of Pharmaceutical Investigation 2019;49(4):347–80. doi: 10.1007/s40005-019-00439-x. [DOI] [Google Scholar]
- 95.da Luz CM, Boyles MSP, Falagan-Lotsch P, Pereira MR, Tutumi HR, de Oliveira Santos E, Martins NB, Himly M, Sommer A, Foissner I, Duschl A, Granjeiro JM, Leite PEC. Poly-lactic acid nanoparticles (PLA-NP) promote physiological modifications in lung epithelial cells and are internalized by clathrin-coated pits and lipid rafts. Journal of Nanobiotechnology 2017;15(1):11. doi: 10.1186/s12951-016-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramot Y, Haim-Zada M, Domb AJ, Nyska A. Biocompatibility and safety of PLA and its copolymers. Adv Drug Deliv Rev 2016;107:153–62. doi: 10.1016/j.addr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Cao Y, Mitchell G, Messina A, Price L, Thompson E, Penington A, Morrison W, O’Connor A, Stevens G, Cooper-White J. The influence of architecture on degradation and tissue ingrowth into three-dimensional poly(lactic-co-glycolic acid) scaffolds in vitro and in vivo. Biomaterials 2006;27(14):2854–64. Epub 2006/01/24. doi: 10.1016/j.biomaterials.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 98.Jones KS. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin Immunol 2008;20(2):130–6. Epub 2008/01/15. doi: 10.1016/j.smim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 99.Thurber AE, Omenetto FG, Kaplan DL. In vivo bioresponses to silk proteins. Biomaterials 2015;71:145–57. doi: 10.1016/j.biomaterials.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burczak K, Gamian E, Kochman A. Long-term in vivo performance and biocompatibility of poly(vinyl alcohol) hydrogel macrocapsules for hybrid-type artificial pancreas. Biomaterials 1996;17(24):2351–6. doi: 10.1016/S0142-9612(96)00076-2. [DOI] [PubMed] [Google Scholar]
- 101.Khare AR, Peppas NA. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995;16(7):559–67. doi: 10.1016/0142-9612(95)91130-Q. [DOI] [PubMed] [Google Scholar]
- 102.Carbinatto FM, de Castro AD, Evangelista RC, Cury BSF. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian Journal of Pharmaceutical Sciences 2014;9(1):27–34. doi: 10.1016/j.ajps.2013.12.002. [DOI] [Google Scholar]
- 103.Fan L-t, Singh SK. Diffusion-Controlled Release. In: Fan L-t, Singh SK, editors. Controlled Release: A Quantitative Treatment Berlin, Heidelberg: Springer Berlin Heidelberg; 1989. p. 9–88. [Google Scholar]
- 104.Hines DJ, Kaplan DL. Poly(lactic-co-glycolic) acid-controlled-release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst 2013;30(3):257–76. doi: 10.1615/critrevtherdrugcarriersyst.2013006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3(3):1377–97. Epub 2011/08/26. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ceonzo K, Gaynor A, Shaffer L, Kojima K, Vacanti CA, Stahl GL. Polyglycolic acid-induced inflammation: role of hydrolysis and resulting complement activation. Tissue Eng 2006;12(2):301–8. doi: 10.1089/ten.2006.12.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996;17(2):93–102. Epub 1996/01/01. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 108.Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials 2000;21(24):2615–21. Epub 2000/11/09. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 109.Lowinger MB, Barrett SE, Zhang F, Williams RO 3rd. Sustained Release Drug Delivery Applications of Polyurethanes. Pharmaceutics 2018;10(2):55. doi: 10.3390/pharmaceutics10020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Castel N, Soon-Sutton T, Deptula P, Flaherty A, Parsa FD. Polyurethane-coated breast implants revisited: a 30-year follow-up. Arch Plast Surg 2015;42(2):186–93. Epub 2015/03/16. doi: 10.5999/aps.2015.42.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pande GS. Thermoplastic polyurethanes as insulating materials for long-life cardiac pacing leads. Pacing Clin Electrophysiol 1983;6(5 Pt 1):858–67. Epub 1983/09/01. doi: 10.1111/j.1540-8159.1983.tb04406.x. [DOI] [PubMed] [Google Scholar]
- 112.Spring MA. Use of a Lysine-Derived Urethane Surgical Adhesive as an Alternative to Progressive Tension Sutures in Abdominoplasty Patients: A Cohort Study. Aesthet Surg J 2018;38(12):1318–29. Epub 2018/04/11. doi: 10.1093/asj/sjy094. [DOI] [PubMed] [Google Scholar]
- 113.Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv 2010;7(4):429–44. doi: 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tallury P, Alimohammadi N, Kalachandra S. Poly (ethylene-co-vinyl acetate) copolymer matrix for delivery of chlorhexidine and acyclovir drugs for use in the oral environment: effect of drug combination, copolymer composition and coating on the drug release rate. dental materials 2007;23(4):404–9. [DOI] [PubMed] [Google Scholar]
- 115.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst 2002;19(1):73–98. Epub 2002/06/06. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 116.Taluja A, Bae YH. Role of a novel multifunctional excipient poly(ethylene glycol)-block-oligo(vinyl sulfadimethoxine) in controlled release of lysozyme from PLGA microspheres. Int J Pharm 2008;358(1–2):50–9. Epub 2008/04/09. doi: 10.1016/j.ijpharm.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 117.Giteau A, Venier-Julienne M-C, Aubert-Pouëssel A, Benoit J-P. How to achieve sustained and complete protein release from PLGA-based microparticles? 2008. [DOI] [PubMed]
- 118.Buckley ST, Hubálek F, Rahbek UL. Chemically modified peptides and proteins - critical considerations for oral delivery. Tissue Barriers 2016;4(2):e1156805-e. doi: 10.1080/21688370.2016.1156805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo C, Li C, Kaplan DL. Enzymatic Degradation of Bombyx mori Silk Materials: A Review. Biomacromolecules 2020;21(5):1678–86. doi: 10.1021/acs.biomac.0c00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vepari C, Kaplan DL. Silk as a Biomaterial. Prog Polym Sci 2007;32(8–9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci. 2009;10(4):1514–24. doi: 10.3390/ijms10041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koh L-D, Cheng Y, Teng C-P, Khin Y-W, Loh X-J, Tee S-Y, Low M, Ye E, Yu H-D, Zhang Y-W, Han M-Y. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci 2015;46:86–110. doi: 10.1016/j.progpolymsci.2015.02.001. [DOI] [Google Scholar]
- 123.Numata K, Kaplan DL. Silk-based delivery systems of bioactive molecules. Adv Drug Deliv Rev 2010;62(15):1497–508. Epub 2010/03/20. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li C, Wu J, Shi H, Xia Z, Sahoo JK, Yeo J, Kaplan DL. Fiber-Based Biopolymer Processing as a Route toward Sustainability. Advanced Materials 2021;n/a(n/a):2105196. doi: 10.1002/adma.202105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chambre L, Martín-Moldes Z, Parker RN, Kaplan DL. Bioengineered elastin- and silk-biomaterials for drug and gene delivery. Adv Drug Deliv Rev 2020;160:186–98. doi: 10.1016/j.addr.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yucel T, Lovett ML, Kaplan DL. Silk-based biomaterials for sustained drug delivery. Journal of Controlled Release 2014;190:381–97. doi: 10.1016/j.jconrel.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Numata K, Kaplan DL. Silk-based delivery systems of bioactive molecules. Adv Drug Deliv Rev 2010;62(15):1497–508. Epub 2010/03/16. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seib FP, Kaplan DL. Silk for drug delivery applications: opportunities and challenges. Israel Journal of Chemistry 2013;53(9-10):756–66. [Google Scholar]
- 129.Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols 2011;6(10):1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wongpinyochit T, Johnston BF, Seib FP. Manufacture and Drug Delivery Applications of Silk Nanoparticles. J Vis Exp 2016(116):54669. doi: 10.3791/54669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li AB, Kluge JA, Guziewicz NA, Omenetto FG, Kaplan DL. Silk-based stabilization of biomacromolecules. J Control Release 2015;219:416–30. Epub 2015/09/25. doi: 10.1016/j.jconrel.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu X, Shmelev K, Sun L, Gil E-S, Park S-H, Cebe P, Kaplan DL. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011;12(5):1686–96. Epub 2011/03/22. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Franks F, editor. Material Science and the Production of Shelf Stable Biologicals 1991.
- 134.Guo C, Li C, Vu HV, Hanna P, Lechtig A, Qiu Y, Mu X, Ling S, Nazarian A, Lin SJ, Kaplan DL. Thermoplastic moulding of regenerated silk. Nature Materials 2020;19(1):102–8. doi: 10.1038/s41563-019-0560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, Kaplan DL. Stabilization of Enzymes in Silk Films. Biomacromolecules 2009;10(5):1032–42. doi: 10.1021/bm800956n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rnjak-Kovacina J, DesRochers TM, Burke KA, Kaplan DL. The effect of sterilization on silk fibroin biomaterial properties. Macromolecular bioscience 2015;15(6):861–74. Epub 2015/03/11. doi: 10.1002/mabi.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thurber AE, Omenetto FG, Kaplan DL. In vivo bioresponses to silk proteins. Biomaterials 2015;71:145–57. doi: 10.1016/j.biomaterials.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005;26(2):147–55. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 139.Navone SE, Pascucci L, Dossena M, Ferri A, Invernici G, Acerbi F, Cristini S, Bedini G, Tosetti V, Ceserani V, Bonomi A, Pessina A, Freddi G, Alessandrino A, Ceccarelli P, Campanella R, Marfia G, Alessandri G, Parati EA. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Research & Therapy 2014;5(1):7. doi: 10.1186/scrt396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Holland C, Numata K, Rnjak-Kovacina J, Seib FP. The Biomedical Use of Silk: Past, Present, Future. Advanced Healthcare Materials 2019;8(1):1800465. doi: 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- 141.Silva SS, Motta A, Rodrigues MT, Pinheiro AF, Gomes ME, Mano JF, Reis RL, Migliaresi C. Novel genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering strategies. Biomacromolecules 2008;9(10):2764–74. [DOI] [PubMed] [Google Scholar]
- 142.Wang X, Ding Z, Wang C, Chen X, Xu H, Lu Q, Kaplan DL. Bioactive silk hydrogels with tunable mechanical properties. Journal of Materials Chemistry B 2018;6(18):2739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu J, Yang F, He F, Tian X, Tang S, Chen X. A tubular gelatin scaffold capable of the time-dependent controlled release of epidermal growth factor and mitomycin C. Colloids and Surfaces B: Biointerfaces 2015;135:416–24. [DOI] [PubMed] [Google Scholar]
- 144.Brooks AE. The Potential of Silk and Silk-Like Proteins as Natural Mucoadhesive Biopolymers for Controlled Drug Delivery. Frontiers in chemistry 2015;3:65-. doi: 10.3389/fchem.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dong Y, Dong P, Huang D, Mei L, Xia Y, Wang Z, Pan X, Li G, Wu C. Fabrication and characterization of silk fibroin-coated liposomes for ocular drug delivery. Eur J Pharm Biopharm 2015;91:82–90. Epub 2015/02/04. doi: 10.1016/j.ejpb.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 146.Sahoo JK, Choi J, Hasturk O, Laubach I, Descoteaux ML, Mosurkal S, Wang B, Zhang N, Kaplan DL. Silk degumming time controls horseradish peroxidase-catalyzed hydrogel properties. Biomaterials Science 2020;8(15):4176–85. doi: 10.1039/D0BM00512F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pritchard EM, Kaplan DL. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv 2011;8(6):797–811. doi: 10.1517/17425247.2011.568936. [DOI] [PubMed] [Google Scholar]
- 148.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. In vitro degradation of silk fibroin. Biomaterials 2005;26(17):3385–93. doi: [DOI] [PubMed] [Google Scholar]
- 149.Peppas N, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. European journal of pharmaceutics and biopharmaceutics 2000;50(1):27–46. [DOI] [PubMed] [Google Scholar]
- 150.Hines DJ, Kaplan DL. Mechanisms of Controlled Release from Silk Fibroin Films. Biomacromolecules 2011;12(3):804–12. doi: 10.1021/bm101421r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yucel T, Lovett ML, Kaplan DL. Silk-based biomaterials for sustained drug delivery. J Control Release 2014;190:381–97. Epub 2014/06/05. doi: 10.1016/j.jconrel.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosales C, Uribe-Querol E. Phagocytosis: A Fundamental Process in Immunity. Biomed Res Int 2017;2017:9042851-. Epub 2017/06/12. doi: 10.1155/2017/9042851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Breslauer DN, Muller SJ, Lee LP. Generation of Monodisperse Silk Microspheres Prepared with Microfluidics. Biomacromolecules 2010;11(3):643–7. doi: 10.1021/bm901209u. [DOI] [PubMed] [Google Scholar]
- 154.Shimanovich U, Ruggeri FS, De Genst E, Adamcik J, Barros TP, Porter D, Müller T, Mezzenga R, Dobson CM, Vollrath F, Holland C, Knowles TPJ. Silk micrococoons for protein stabilisation and molecular encapsulation. Nature Communications 2017;8(1):15902. doi: 10.1038/ncomms15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hino T, Shimabayashi S, Nakai A. Silk Microspheres Prepared by Spray-drying of an Aqueous System. Pharmacy and Pharmacology Communications 2000;6(8):335–9. doi: 10.1211/146080800128736169. [DOI] [Google Scholar]
- 156.Wang X, Wenk E, Matsumoto A, Meinel L, Li C, Kaplan DL. Silk microspheres for encapsulation and controlled release. Journal of Controlled Release 2007;117(3):360–70. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 157.Wang X, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010;31(6):1025–35. Epub 2009/11/27. doi: 10.1016/j.biomaterials.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gong H, Wang J, Zhang J, Wu J, Zheng Z, Xie X, Kaplan DL, Li G, Wang X. Control of octreotide release from silk fibroin microspheres. Materials Science and Engineering: C 2019;102:820–8. doi: 10.1016/j.msec.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 159.Qu J, Wang L, Niu L, Lin J, Huang Q, Jiang X, Li M. Porous silk fibroin microspheres sustainably releasing bioactive basic fibroblast growth factor. Materials 2018;11(8):1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev 2012;64(12):1292–309. Epub 2012/02/04. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lammel AS, Hu X, Park SH, Kaplan DL, Scheibel TR. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010;31(16):4583–91. Epub 2010/03/12. doi: 10.1016/j.biomaterials.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li H, Zhu J, Chen S, Jia L, Ma Y. Fabrication of aqueous-based dual drug loaded silk fibroin electrospun nanofibers embedded with curcumin-loaded RSF nanospheres for drugs controlled release. RSC Advances 2017;7(89):56550–8. doi: 10.1039/C7RA12394A. [DOI] [Google Scholar]
- 163.Yan H-B, Zhang Y-Q, Ma Y-L, Zhou L-X. Biosynthesis of insulin-silk fibroin nanoparticles conjugates and in vitro evaluation of a drug delivery system. Journal of Nanoparticle Research 2008;11(8):1937. doi: 10.1007/s11051-008-9549-y. [DOI] [Google Scholar]
- 164.Kundu J, Chung Y-I, Kim YH, Tae G, Kundu SC. Silk fibroin nanoparticles for cellular uptake and control release. International Journal of Pharmaceutics 2010;388(1):242–50. doi: 10.1016/j.ijpharm.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 165.Huang D, Wang L, Dong Y, Pan X, Li G, Wu C. A novel technology using transscleral ultrasound to deliver protein loaded nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics 2014;88(1):104–15. doi: 10.1016/j.ejpb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 166.Shi P, Abbah SA, Saran K, Zhang Y, Li J, Wong H-K, Goh JCH. Silk Fibroin-Based Complex Particles with Bioactive Encrustation for Bone Morphogenetic Protein 2 Delivery. Biomacromolecules 2013;14(12):4465–74. doi: 10.1021/bm401381s. [DOI] [PubMed] [Google Scholar]
- 167.Dinis TM, Elia R, Vidal G, Auffret A, Kaplan DL, Egles C. Method to Form a Fiber/Growth Factor Dual-Gradient along Electrospun Silk for Nerve Regeneration. ACS Applied Materials & Interfaces 2014;6(19):16817–26. doi: 10.1021/am504159j. [DOI] [PubMed] [Google Scholar]
- 168.Yucel T, Cebe P, Kaplan DL. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009;97(7):2044–50. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008;29(8):1054–64. Epub 2007/11/26. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leisk GG, Lo TJ, Yucel T, Lu Q, Kaplan DL. Electrogelation for Protein Adhesives. Advanced Materials 2010;22(6):711–5. doi: 10.1002/adma.200902643. [DOI] [PubMed] [Google Scholar]
- 171.Hasturk O, Jordan KE, Choi J, Kaplan DL. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020;232:119720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA, Marelli B, Mitropoulos AN, Omenetto FG, Kaplan DL. Highly Tunable Elastomeric Silk Biomaterials. Advanced Functional Materials 2014;24(29):4615–24. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choi J, McGill M, Raia NR, Hasturk O, Kaplan DL. Silk Hydrogels Crosslinked by the Fenton Reaction. Advanced Healthcare Materials 2019;8(17):1900644. doi: 10.1002/adhm.201900644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Choi J, Hasturk O, Mu X, Sahoo JK, Kaplan DL. Silk Hydrogels with Controllable Formation of Dityrosine, 3,4-Dihydroxyphenylalanine, and 3,4-Dihydroxyphenylalanine–Fe3+ Complexes through Chitosan Particle-Assisted Fenton Reactions. Biomacromolecules 2021. doi: 10.1021/acs.biomac.0c01539. [DOI] [PubMed] [Google Scholar]
- 175.Yucel T, Kojic N, Leisk GG, Lo TJ, Kaplan DL. Non-equilibrium silk fibroin adhesives. Journal of Structural Biology 2010;170(2):406–12. doi: 10.1016/j.jsb.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lovett ML, Wang X, Yucel T, York L, Keirstead M, Haggerty L, Kaplan DL. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. European Journal of Pharmaceutics and Biopharmaceutics 2015;95:271–8. doi: 10.1016/j.ejpb.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 177.Diab T, Pritchard EM, Uhrig BA, Boerckel JD, Kaplan DL, Guldberg RE. A silk hydrogel-based delivery system of bone morphogenetic protein for the treatment of large bone defects. Journal of the mechanical behavior of biomedical materials 2012;11:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hopkins AM, De Laporte L, Tortelli F, Spedden E, Staii C, Atherton TJ, Hubbell JA, Kaplan DL. Silk Hydrogels as Soft Substrates for Neural Tissue Engineering. Advanced Functional Materials 2013;23(41):5140–9. doi: 10.1002/adfm.201300435. [DOI] [Google Scholar]
- 179.Yan L-P, Oliveira JM, Oliveira AL, Reis RL. Core-shell silk hydrogels with spatially tuned conformations as drug-delivery system. Journal of Tissue Engineering and Regenerative Medicine 2017;11(11):3168–77. doi: 10.1002/term.2226. [DOI] [PubMed] [Google Scholar]
- 180.Guziewicz NA, Massetti AJ, Perez-Ramirez BJ, Kaplan DL. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials 2013;34(31):7766–75. Epub 2013/07/19. doi: 10.1016/j.biomaterials.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim M, Kim T, Kim DS, Chung WK. Curved Microneedle Array-Based sEMG Electrode for Robust Long-Term Measurements and High Selectivity. Sensors 2015;15(7). doi: 10.3390/s150716265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tsioris K, Raja WK, Pritchard EM, Panilaitis B, Kaplan DL, Omenetto FG. Fabrication of Silk Microneedles for Controlled-Release Drug Delivery. Advanced Functional Materials 2012;22(2):330–5. doi: 10.1002/adfm.201102012. [DOI] [Google Scholar]
- 183.Yavuz B, Chambre L, Harrington K, Kluge J, Valenti L, Kaplan DL. Silk Fibroin Microneedle Patches for the Sustained Release of Levonorgestrel. ACS Applied Bio Materials 2020;3(8):5375–82. doi: 10.1021/acsabm.0c00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang S, Zhu M, Zhao L, Kuang D, Kundu SC, Lu S. Insulin-Loaded Silk Fibroin Microneedles as Sustained Release System. ACS Biomaterials Science & Engineering 2019;5(4):1887–94. doi: 10.1021/acsbiomaterials.9b00229. [DOI] [PubMed] [Google Scholar]
- 185.Boopathy AV, Mandal A, Kulp DW, Menis S, Bennett NR, Watkins HC, Wang W, Martin JT, Thai NT, He Y, Schief WR, Hammond PT, Irvine DJ. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proceedings of the National Academy of Sciences 2019;116(33):16473–8. doi: 10.1073/pnas.1902179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.DeMuth PC, Min Y, Irvine DJ, Hammond PT. Implantable Silk Composite Microneedles for Programmable Vaccine Release Kinetics and Enhanced Immunogenicity in Transcutaneous Immunization. Advanced Healthcare Materials 2014;3(1):47–58. doi: 10.1002/adhm.201300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li AB, Kluge JA, Guziewicz NA, Omenetto FG, Kaplan DL. Silk-based stabilization of biomacromolecules. Journal of Controlled Release 2015;219:416–30. doi: 10.1016/j.jconrel.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


