Abstract
Opioids continue to be of use for the treatment of pain. Most clinically used analgesics target the mu opioid receptor (MOR) whose activation results in adverse effects like respiratory depression, addiction, and abuse liability. Various approaches have been used by the field to separate receptor mediated analgesic actions from its adverse effects. These include biased agonism, opioids targeting multiple receptors, allosteric modulators, heteromers, and splice variants of the MOR. This review will focus on the current status of the field and some upcoming targets of interest which may lead to a safer next generation of analgesics.
Introduction
Chronic pain is defined as pain that persists beyond the normal healing time (>3 months), and affects over 100 million Americans. The total economic and social cost of chronic pain is estimated to be at least $600 billion. There are currently no universally effective treatments for chronic pain, particularly neuropathic pain, which is associated with poor health outcomes including anxiety, depression, poor sleep, inability to work, and increased rates of suicide. Neuropathic pain is caused by dysfunction or damage to the somatosensory nervous system that often results from stroke, diabetes, multiple sclerosis, spinal cord injury, or from peripheral nerve damage or dysfunction, and has a worldwide prevalence of 7–10% and is associated with a poor quality of life. The majority of chronic pain sufferers do not experience complete pain relief with first line treatments such as calcium channel inhibitors (e.g. gabapentin, pregabalin), antidepressants, and/or anticonvulsants, and patients are often unable to tolerate effective doses because of serious adverse effects. Second-line treatments include opioids that target the mu-opioidreceptor(MOR) such as morphine, tramadol, and fentanyl. However, they rapidly cause tolerance and have side-effects such as respiratory depression and addiction. Moreover, misuse of prescription opioids has led to an opioid abuse crisis with opioid overdoses killing over 93,000 people in the US in 2020 according to recent CDC data. This highlights the urgent unmet need for identifying new therapeutic targets to not only treat chronic pain effectively, but to do so without causing side effects such as addiction.
One promising approach over the last 10 to 15 years has been biased signaling at the MOR which was proposed to differentiate analgesic signaling (e.g. GαI/O) from side-effect signaling (e.g. βarrestin2). Combined with other molecular discoveries, including negative feedback systems upregulated during opioid treatment, receptor heterodimerization, and allosteric modulators, these findings suggest new drug discovery approaches that are fundamentally different from previous target-based strategies, since they simultaneously engage multiple targets or activate their receptor targets in new ways. These novel approaches hold the potential to develop opioids without adverse effects that we have been pursuing for more than a century. The approaches profiled in this review are summarized in Figure 1, and exemplary novel ligands created to exploit these new approaches are shown in Figure 2, Figure 3 and Figure 4. Please note, receptor nomenclature has been adjusted to conform to BJP’s Concise Guide to Pharmacology (Alexander et al., 2019).
Figure 1: Summary of Novel Approaches to Improve Opioid Therapy.
A summary scheme for each novel approach profiled in this review is shown. Each includes exemplary novel ligands created to exploit these strategies, all of which result in enhanced analgesia with reduced or maintained side effects. MOR = mu opioid receptor; NOP = nociceptin orphanin/FQ receptor; KOR = kappa opioid receptor; DOR = delta opioid receptor; HSP90 = heat shock protein 90; βarr2 = βarrestin2; MDOR = mu/delta opioid receptor heterodimer; 6TM-MOR = 6 transmembrane segment MOR splice variant. Figure created with BioRender.com.
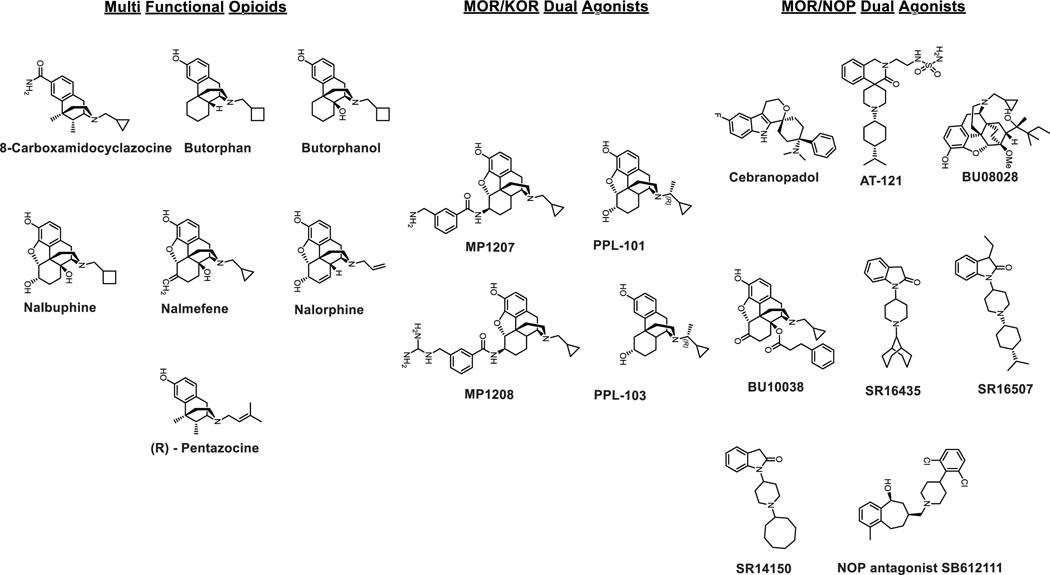
Figure 2.
Chemical structures of multifunctional opioids and dual agonists at MOR-KOR and MOR-NOP.
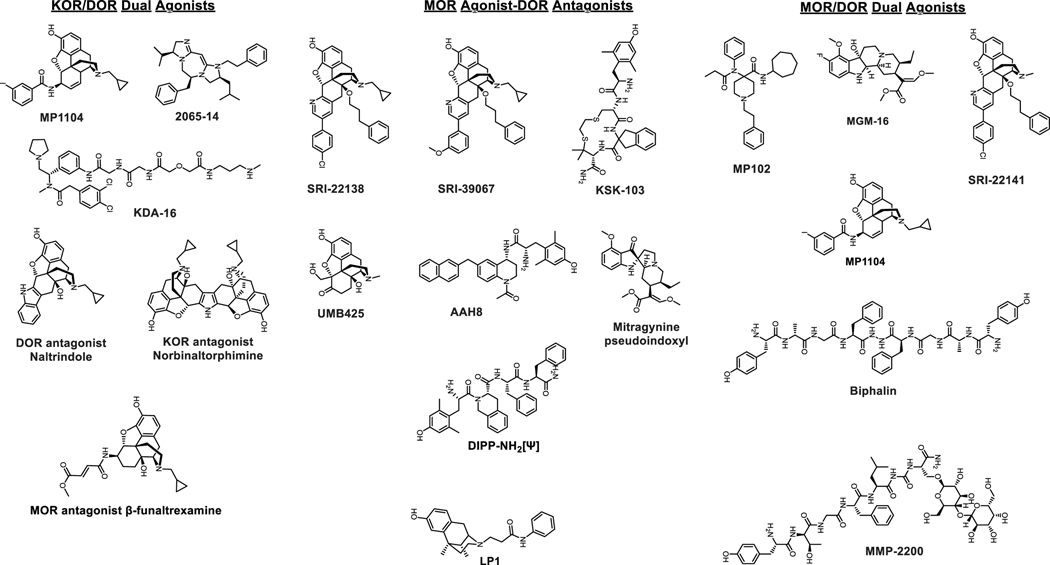
Figure 3.
Chemical structures of dual agonists at KOR-DOR, MOR agonist-DOR antagonists and dual agonists at MOR-DOR.
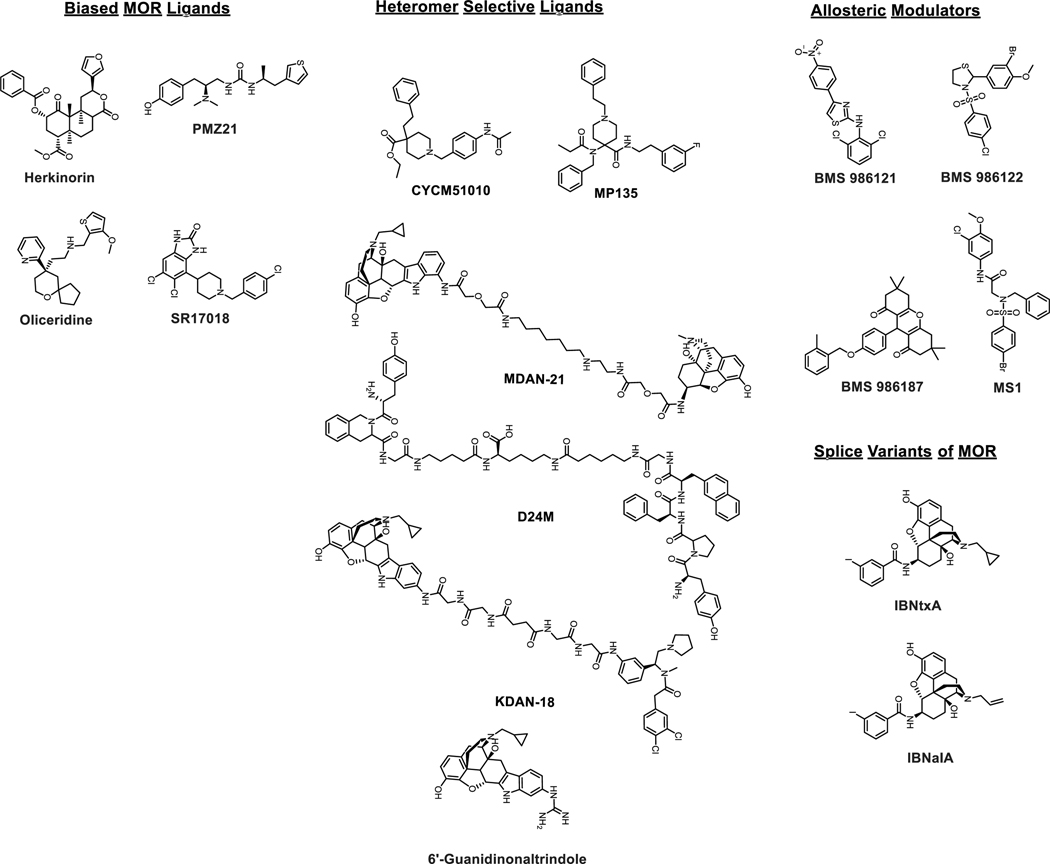
Figure 4.
Chemical structures of biased MOR agonists, heteromer selective ligands, allosteric modulators and ligands targeting truncated splice variants of MOR.
Multi-Functional Opioids
One strategy utilized to overcome the undesirable effects of receptor mediated on target side effects is to target multiple opioid receptors simultaneously. The four members of the opioid receptor family are the mu opioid receptor (MOR), kappaopioidreceptor(KOR), deltaopioidreceptor(DOR), and nociceptinopioidreceptors(NOP); all 4 of these receptors have been shown to modulate pain. Mixed opioid receptor agonists and mixed agonist-antagonists may be a viable strategy to develop analgesics with reduced side-effects. Mixed agonists could maintain antinociceptive effects, associated with activation of an individual subtype, while the antagonism and/or agonism of the other subtype may be used to dial out the adverse effects. This approach was first suggested by the observation that when the MOR system is chronically stimulated, other negative feedback systems are also upregulated to compensate, which can drive side effects. Several of these feedback systems have been identified, and when targeted, have been shown to alleviate opioid side effects.
In this section we will review promising newer mixed target profiles like MOR-KOR agonism, MOR-NOP agonism, MOR-DOR dual agonism, MOR agonism-DOR antagonism, and KOR-DOR agonism. While some of these profiles have been explored for years, their results have not yet been translated to human testing.
MOR/KOR Dual Agonists
As opposed to MOR activation, which besides antinociception causes respiratory depression, inhibition of gut motility, and induces reinforcing effects, KOR activation induces antinociception without respiratory depression and inhibition of gut motility. Because KOR agonists often induce dysphoria they are generally considered not to have abuse liability. Prior evidence from preclinical studies suggest that dual activation of MOR and KOR may produce synergistic analgesia, while offsetting respective liabilities associated with individual subtypes. For example, U50,488h shows no respiratory depression on its own and has been reported to reduce the respiratory depression mediated by DAMGO administered supraspinally (Haji & Takeda, 2001). Another dual agonist, nalbuphine, with moderate efficacy MOR partial agonism and high efficacy KOR partial agonism in animals shows negligible respiratory depression (Schmidt, Tam, Shotzberger, Smith, Clark & Vernier, 1985). Dezocine is a MOR/KOR partial agonist that shows antinociception with reduced side effects, and is in clinical use in China (Wang et al., 2018b). Older mixed agents include 8-Carboxamidocyclazocine (8-CAC) which is a full agonist at both KOR and MOR, butorphan which is a partial agonist at both MOR and KOR, and agents like nalbuphine, pentazocine, nalmefene, nalorphine, and butorphanol, which are KOR agonist-MOR partial agonist/antagonists. These older ligands and their literature are reviewed in (Paton, Atigari, Kaska, Prisinzano & Kivell, 2020). More recent examples in this category include PPL-101/103 and MP1207/08.
PPL-101 and PPL-103 (Khroyan et al., 2017) are based on the morphine template and contain a chiral alpha methyl N-substituent leading to a unique combination of high binding affinities at both MOR and KOR and partial agonist activities at all three opioid receptors (MOR, KOR and DOR) with lowest efficacy at MOR and highest efficacy at KOR. They did not produce conditioned place aversion or preference, increased locomotor activity, or cause locomotor sensitization in mice.
MP1207 and MP1208 are based on the 6β-amidomorphinan template (Uprety et al., 2021), both of which displayed MOR/KOR Gi - partial agonism with diminished arrestin signaling (bias factors of 8 and 22, respectively at KOR and no arrestin signaling at MOR), while retaining efficacious analgesia when administered icv without inducing respiratory depression, hyperlocomotion (as compared to morphine at equianalgesic doses), or conditioned place preference/aversion in mice. One caveat: given that both classes of ligands are partial agonists, it is possible that partial agonism as opposed to bias plays a critical role in the improved in vivo profile observed for these compounds.
MOR/NOP Dual Agonists
Agonists targeting the fourth opioid receptor subtype, the nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor, have been shown to have antinociceptive properties on their own. The roles of NOP in antinociception are complicated depending on the routes of administration in rodents (Kiguchi & Ko, 2019; Toll, Bruchas, Calo, Cox & Zaveri, 2016). However, in non-human primates (NHPs) NOP agonists exert antinociception supraspinally, spinally, as well as systemically (Kiguchi & Ko, 2019). More importantly NOP agonists do not show respiratory depression, itch, physical dependence, or addiction and in turn beneficially modulate the pain-relieving and addictive properties of MOR agonists (Toll, Bruchas, Calo, Cox & Zaveri, 2016) at spinal and systemic levels (Cremeans, Gruley, Kyle & Ko, 2012; Hu, Calo, Guerrini & Ko, 2010; Kiguchi, Ding & Ko, 2016). Taken together this has led to the development of bifunctional NOP/MOR agonists as a viable approach to develop non-addictive antinociceptive agents. Several molecules based on this hypothesis are now known like Cebranopadol (Lambert, Bird & Rowbotham, 2015), AT121 (Ding et al., 2018), BU08028 (Ding et al., 2016) and BU10038 (Kiguchi et al., 2019).
Cebranopadol was the first molecule of the non-morphinan template in this series and was discovered in 2014 (Linz et al., 2014). The compound showed high binding affinity at MOR, KOR, and NOP, and was found to be a full agonist at both MOR and NOP in [35S]GTPγS functional assays (Linz et al., 2014; Tzschentke, Linz, Koch & Christoph, 2019). In cell lines cebranopadol was found to have 12-fold selectivity for MOR over NOP with comparable maximal stimulation of 89% and 103% at NOP and MOR compared to appropriate control agonists at each subtype. In rodents, cebranopadol showed anti-nociceptive effects in acute, chronic, and neuropathic pain models. These in vivo actions were consistent with combined treatment of NOP and MOR agonists when administered systemically. In rodents, cebranopadol exerted potent anti-allodynic effects irrespective of route of administration (spinal, supraspinal, or systemic). In conditioned place preference (CPP) and in drug discrimination assays in rodents it behaved similarly to morphine, which may be a consequence of its full agonist actions at MOR (de Guglielmo, Matzeu, Kononoff, Mattioni, Martin-Fardon & George, 2017; Tzschentke & Rutten, 2018). In NHPs it showed antinociceptive actions spinally as well as systemically without causing itch, which is commonly associated with MOR-based antinociceptive agents (Kiguchi & Ko, 2019).
In clinical studies, cebranopadol administered orally showed antinociception as well as typical opioid side-effects like respiratory depression and miosis, though these side-effects were present for shorter intervals compared to the drug’s antinociceptive actions. These findings suggest that cebranopadol exhibits a safer pharmacological profile over other clinically used MOR analgesics (Tzschentke, Linz, Koch & Christoph, 2019) like morphine though a head to head comparison with morphine or fentanyl was not made in the same trial. However, in the past Dahan and colleagues reported respiratory depression with MOR agonists like morphine and in comparison cebranopadol fared favorably (Dahan et al., 2017; Dahan, Romberg, Teppema, Sarton, Bijl & Olofsen, 2004). Cebranopadol has also been evaluated for lower back pain, diabetic neuropathy, and in cancer pain patients (Eerdekens et al., 2019). In all cases the drug was found to have analgesic actions with constipation, nausea, and dizziness in patients suffering from low back pain (Christoph, Eerdekens, Kok, Volkers & Freynhagen, 2017) seen in <10% cases. The compound is expected to be in phase III clinical trials soon. Whether the rewarding properties seen in rodents translates to humans still remains to be seen.
AT121 (Ding et al., 2018) is a partial agonist of NOP and MOP in comparison to cebranopadol which is a full agonist at both receptors. AT121 activated both MOR and NOP equipotently. Maximal efficacy in a [35S]GTPγS assay was found to be 41% and 14% for NOP and MOR respectively. In NHPs, AT-121 showed antinociceptive effects comparable with morphine while showing no respiratory depression, reinforcing behavior, itching, opioid-induced hyperalgesia, or physical dependence. In addition, this NOP/MOR partial agonist attenuated the reinforcing effects of oxycodone without disrupting food intake. Its antinociception was antagonized by the NOP antagonist J-13397 and MOR antagonist naltrexone.
The Husbands group reported an analog of buprenorphine with a similar NOP/MOR partial agonism profile to AT121. BU08028 (Ding et al., 2016) had 13 fold higher selectivity for MOR over NOP. Its maximal efficacy at NOP was 48% compared to 21% at MOR. BU08028 showed antinociception in NHPs mediated in vivo by NOP as well as MOR. Similar to AT121 this drug showed no reinforcing behavior, respiratory depression, itching, or physical dependence. Another naltrexone based analog, BU10038 (Kiguchi et al., 2019), was recently characterized. BU10038 had a 14 fold MOR to NOP selectivity in binding assays, similar to BU 08028. Efficacy at the receptors was also similar, with 18% at MOR and 34% at NOP. Spinal administration of BU10038 showed antinociception without itching or analgesic tolerance when compared to morphine, while systemically it showed antinociception from both NOP and MOR while not showing respiratory depression, abuse liability, or physical dependence.
Taken together pharmacological evaluation of cebranopadol, AT121, BU08028, and BU10038 suggest that balanced partial agonist activation of NOP and MOR may lead to safer pain relievers. Challenges towards this hypothesis remain, e.g. the ratio of NOP:MOR efficacy and potency that leads to analgesia but not CPP is less clear. This is best illustrated by compounds from the Zaveri group (Khroyan, Polgar, Jiang, Zaveri & Toll, 2009; Khroyan et al., 2007). SR16435 (Khroyan et al., 2007) with a nearly equipotent/equi-efficacious ratio between MOR (EC50=29 nM, EMax=30%) and NOP (EC50=28 nM, EMax=45%) as well as partial agonism at both subtypes did show antinociception mediated by MOR but also showed CPP in spite of an in vitro profile similar to AT121 described above. SR14150 (NOP: EC50=20nM, EMax=54%; MOR: EC50=99 nM, EMax=23%) mediated analgesia in rodents through MOR and showed no CPP. Another compound, SR16507, had equipotency at both subtypes and was a full agonist at NOP and partial agonist at MOR (EMax=47%) and showed MOR mediated antinociception while also showing CPP. In a chronic pain model however, both SR14150 and SR16435 showed potent anti-allodynic activity and in contrast to thermal nociception, this effect was completely blocked by the NOP receptor antagonist SB 612111 (Khroyan et al., 2009) but was not altered by naloxone. This suggests that NOP activation plays a bigger role in rodents in chronic pain models while MOR activation mediates in vivo actions in acute pain models.
Some of this lack of agreement between compounds could simply be in vitro results not translating to in vivo outcomes and/or differences in translatability between rodents and non-human primates (Cremeans, Gruley, Kyle & Ko, 2012). Further possibilities include meaningful differences in compound pharmacokinetics driving different outcomes, or aspects of pharmacodynamics that are not measured could impact compound performance, such as intrinsic efficacy. The antagonism of MOR mediated antinoception by NOP activation seen in rodents as well as antinociception by NOP activation is route dependent (Kiguchi, Ding, Kishioka & Ko, 2020; Kiguchi & Ko, 2019). It is hoped that with newer NOP/MOR ligands which are characterized in NHPs may lead to a better understanding of how NOP co-activation modulates MOR analgesia and reinforcing behavior and lead to a safer next generation of pain-relieving agents.
KOR/DOR Dual Agonists
Both KOR and DOR agonists are known to have antinociceptive actions. Complicating this anti-nociceptive activity, activation of DOR by some agonists is known to cause convulsions (Jutkiewicz, Baladi, Folk, Rice & Woods, 2006), although not all DOR agonists cause convulsions (Saitoh et al., 2018), leading to some confusion and controversy as to the role of the DOR in epileptic activity. On the other hand, activation of KOR is known to have anti-convulsant activity (Zangrandi, Burtscher, MacKay, Colmers & Schwarzer, 2016) in addition to blocking the addiction potential of drugs of abuse like cocaine and morphine (e.g. (Mello & Negus, 2000)). In addition, intrathecally administered KOR agonist U50,488H and DOR agonist DPDPE have been shown to have a synergistic anti-nociceptive effect (Miaskowski, Taiwo & Levine, 1990), suggesting that co-activation of DOR along with KOR in a balanced fashion may lead to fewer side-effects. This hypothesis has led to the synthesis of a few dual KOR-DOR agonists.
A chemical entity named MP1104 (Che et al., 2018) had dual KOR/DOR agonist actions in mice (Varadi et al., 2015a). MP1104 had antinociceptive actions in a radiant heat tail flick assay as well as the formalin assay (Ulker, Toma, White, Uprety, Majumdar & Damaj, 2020). The antinociceptive actions of MP1104 were intact in MOR KO mice and not blocked by the selective MOR antagonist β-funaltrexamine. The antinociception was blocked partially in KOR KO mice and by selective KOR and DOR antagonists, norBinaltorphimine(norBNI) and naltrindole (NTI) respectively. A combination of norBNI and NTI or KOR KO in the presence of NTI completely blocked the antinociception mediated by MP1104. Consistent with dual KOR/DOR agonist actions, MP1104 did not show typical KOR-like conditioned place aversion (CPA) in mice or rats (Atigari, Uprety, Pasternak, Majumdar & Kivell, 2019; Varadi et al., 2015a) while also not showing seizures in mice. Blockage of DOR receptors with NTI resulted in MP1104 showing CPA, suggestive that activation of DOR may blunt the aversive phenotype associated with KOR agonists. MP1104 also blocked cocaine CPP in mice and cocaine self-administration in rats while showing no anxiogenic, pro-depressive phenotypes in rats.
Two additional compounds which share this mixed DOR/KOR dual agonism include KDA 16 (Tang, Yang, Lunzer, Powers & Portoghese, 2011) and a diimidazodiazepine peptidomimetic, 2065–14 (Eans et al., 2015). When administered supraspinally, diimidazodiazepine, similar to MP1104, showed neither CPP nor CPA, supporting the hypothesis that DOR co-activation blunts KOR-mediated dysphoria. Taken together these probes demonstrate the promise of a dual-action DOR and KOR agonist to achieve pain relief with low side effects in vivo, suggesting that additional study of this topic is warranted in the near future.
MOR Agonist/DOR Antagonist Compounds
Chemical entities with MOR agonism and DOR antagonism have been heavily investigated with numerous probes now available. These ligands rest on the hypothesis that the DOR mediates at least some of the negative side effects of MOR activation, particularly tolerance and dependence/withdrawal. Support for this hypothesis came from early studies in rodents co-administered with the MOR agonist morphine with the DOR antagonist NTI, which demonstrated attenuated analgesic tolerance and physical dependence when compared to morphine alone (Abdelhamid, Sultana, Portoghese & Takemori, 1991). Furthermore, antisense knock-down of DOR produces a similar phenotype, suggesting a role for DOR in the development of morphine tolerance and physical dependence (Kest, Lee, McLemore & Inturrisi, 1996). Similarly, DOR antagonists are known to block morphine-induced reinforcing behavior (e.g. (Moron, Gullapalli, Taylor, Gupta, Gomes & Devi, 2010)). Together these studies suggested that a chemotype with MOR agonist and DOR antagonist activity would produce pain relief with reduced side effects.
Overall bifunctional peptides/peptidomimetics/small molecules with mixed MOR agonist/DOR antagonist activity displayed reduced tolerance and physical dependence, which was attributed to DOR blockade. Results from these molecules thus broadly and consistently support the hypothesis. Examples in this class include DIPP-NH2[Ψ] (a peptide) (Schiller et al., 1999); KSK103 (peptidomimetic) (Mosberg et al., 2014); and SRI-22138 (Ananthan et al., 2012), SRI-39067 (Vekariya et al., 2020), UMB425 (Healy et al., 2013), AAH8 (Anand et al., 2018), and mitragynine pseudoindoxyl (Chakraborty & Majumdar, 2021; Varadi et al., 2016) (non-peptide small molecules). While compounds with this activity remain moderately studied, mitragynine pseudoindoxyl, AAH8 and SRI-39067 notably demonstrated no conditioned place preference and less tolerance and withdrawal than morphine. Together, these studies validate a rational approach to target DOR with antagonists in order to attenuate side effects associated with MOR agonists.
These studies have generated a number of compounds from different labs which all show the same general benefits, providing strong support to the hypothesis. Outside of LP1 which has been characterized in carrageenan and chronic constriction injury chronic pain models (Parenti et al., 2013), however, the compounds were all studied using acute pain assays, mostly tail flick, with the side effects measured in naïve mice. The performance of this ligand class is thus understudied in chronic pain models, especially neuropathic pain, including the side effect profile of these ligands in chronic pain models. This limitation provides a clear path for future investigation.
MOR/DOR Dual Agonists
As discussed above, multifunctional MOR agonist/DOR antagonist compounds have been suggested to produce anti-nociception with reduced side effects by multiple groups. It is thus surprising and seemingly paradoxical that MOR/DOR dual agonists have also been described as beneficial, by producing anti-nociception with some of the same side effects also reduced. The data supporting this finding is more limited than that for the agonist/antagonist compounds, but still clear.
Perhaps the first dual agonist compound described was the peptide biphalin, first created by Lipkowsky (Lipkowski, Konecka & Sroczynska, 1982). This peptide was shown to produce potent anti-nociception, with reduced dependence liability (Shen & Crain, 1995). This peptide then served as a scaffold for the creation of numerous cyclized and linear derivatives, mainly headed by Adriano Mollica’s research group. These daughter compounds also showed potent anti-nociception, and in some cases reduced side effect liability (e.g. (Stefanucci et al., 2019)).
Another peptide ligand with a similar profile was MMP-2200 (a.k.a. lactomorphin) created by the Robin Polt research group. This highly potent dual agonist showed highly efficacious anti-nociception with reduced tolerance, dependence, and reward liability (Lowery, Raymond, Giuvelis, Bidlack, Polt & Bilsky, 2011; Mabrouk, Falk, Sherman, Kennedy & Polt, 2012; Stevenson et al., 2015). Notably, this peptide was glycosylated, which the Polt group has shown permits systemic administration with stable pharmacokinetics and blood-brain barrier penetration (Mabrouk, Falk, Sherman, Kennedy & Polt, 2012). Beyond peptides, small molecule dual agonists also showed similar benefits. A series of fentanyl-based analogs with dual agonist activity showed potent anti-nociception in multiple pain models with reduced motor liability (Podolsky et al., 2013). Our lab developed 6β-aminomorphinan compounds and a carfentanyl amide (MP102; (Gutridge et al., 2020; Varadi et al., 2015b)) with dual agonist activity, potent anti-nociception, and reduced respiratory depression (Varadi et al., 2013). A mitragynine derivative (MGM-16) showed potent anti-nociception in neuropathic pain (Matsumoto et al., 2014). Together these findings provide a firm foundation for the hypothesis that dual mu/delta agonism produces anti-nociception with reduced side effects. One caveat however is that abuse liability for these compounds has not been studied, with the exception of MMP-2200 above; this liability must thus be examined during further development.
What then accounts for the seeming paradox of agonist/antagonists and dual agonists producing some of the same benefits? One thing to keep in mind is that most of the studies above as well as the agonist/antagonist studies tested antinociception in the same way. The acute tail flick test was used for most anti-nociceptive testing, and chronic compound was only given to naïve mice for tolerance/dependence testing. Chronic dosing in chronic pain models was not often used, and the roles of the MOR vs. DOR were not teased out. We thus recently reported the results of our comprehensive study of the small molecule dual agonist SRI-22141 (Lei, Vekariya, Ananthan & Streicher, 2020). Like many of the studies above, we found this compound produced potent acute anti-nociception in the tail flick test; we also showed potent anti-nociception in 2 different models of chronic neuropathic pain. However, we also gave the drug chronically to naïve as well as chronic pain mice, showing reduced tolerance and dependence in the context of those pain states. When we used a selective delta opioid antagonist, we found that the delta receptor contributed strongly to neuropathic pain relief, but did not contribute to tail flick pain relief. Importantly, we also found that the dual agonist produced an anti-inflammatory effect in the spinal cords of mice suffering from neuropathic pain, which was reversed by delta antagonist.
We thus hypothesize that the benefits produced by dual agonism are acting via a different mechanism. The delta activity is probably not contributing to acute pain relief in naïve mice in no pain or that are uninjured via tail flick test or similar, while it does contribute strongly to pain states with an inflammatory component like neuropathic pain. The benefits to tolerance and dependence are thus mediated by the anti-inflammatory effect of delta opioid activation. In support of this hypothesis, it has been long established that the delta receptor is made competent for pain relief by inflammatory stimulation (Brackley, Gomez, Akopian, Henry & Jeske, 2016). Stimulation of the delta receptor has also been shown to produce anti-inflammatory effects in several models, including our study above (Shrivastava et al., 2017). Lastly, neuroinflammation has been shown to play a key role in side effects like opioid tolerance and dependence (Li, Csakai, Jin, Zhang & Yin, 2016). Testing this hypothesis fully may help resolve this basic science paradox, and determine the mechanisms by which dual agonists and agonist/antagonists both produce effective pain relief with reduced side effects. These studies may also indicate which pain types both ligand classes could be most effective in treating; this knowledge will be needed as these compounds advance to clinical testing.
G-Protein Biased Agonism
The development of opioid agonists biased against βarrestin2 recruitment has been a major area of focus in the opioid field. The first piece of the story for this approach was published by Laura Bohn in the lab of Marc Caron in 1999 when she discovered that βarrestin2 knockout in mice enhanced the anti-nociceptive efficacy of morphine (Bohn, Lefkowitz, Gainetdinov, Peppel, Caron & Lin, 1999). This finding was consistent with the known role of βarrestin2 in desensitizing/internalizing G Protein Coupled Receptors (GPCR). While interesting, this finding would not have been translationally meaningful if side effect efficacy was similarly boosted. Soon after the same group published a study showing that morphine tolerance was also blocked, consistent with a desensitizing role of βarrestin2, but not physical dependence (Bohn, Gainetdinov, Lin, Lefkowitz & Caron, 2000). Bohn and colleagues published another study showing that morphine reward learning was actually enhanced by βarrestin2 knockout; this finding raises safety concerns about biased agonists and reward that have never been fully addressed (Bohn et al., 2003).
True excitement for βarrestin2 knockout was generated when Laura Bohn’s group published more comprehensive studies of opioid side effects in her own independent lab. A 2005 study showed that morphine constipation and respiratory depression were all blocked by βarrestin2 knockout (Raehal, Walker & Bohn, 2005). A more comprehensive study in 2011 further found that tolerance and dependence/withdrawal were blocked by βarrestin2 knockout (Raehal & Bohn, 2011). One very important caveat to this study, however, is that high doses of morphine were able to produce equivalent side effects, and βarrestin2 knockout had no impact on side effects caused by fentanyl, methadone, and oxycodone. While collectively these studies did suggest that βarrestin2 knockout could improve the therapeutic index of opioids, these caveats and limitations, especially the enhanced reward seen with βarrestin2 knockout, must be kept in mind.
These studies suggested that stimulation of the opioid receptor without recruitment of βarrestin2 could result in improved analgesia with reduced side effects. A way to accomplish this goal came from the parallel field of biased agonism, also called functional selectivity. These studies, performed in other receptor systems apart from the opioid receptors, suggested that different ligands could activate the same receptor to produce different signal transduction cascades and thus different functional outcomes (reviewed in (Kenakin, 2007)). This concept was applied to the opioid field to screen for novel opioid agonists which could activate the mu opioid receptor (MOR) without recruiting βarrestin2.
Early reports identified biased compounds such as herkinorin, providing proof-of-concept, even if those particular drugs had poor drug-like properties (Groer et al., 2007; Tidgewell et al., 2008; Xu et al., 2007). Notably, to our knowledge, all identified biased agonists have been characterized for arrestin recruitment using one of several in vitro assays, which have poor sensitivity, and may not reflect arrestin recruitment in vivo. Perhaps due to this limitation, a recent study has found herkinorin to be unbiased at MOR (Manglik et al., 2016). PZM21 was identified fortuitously as a biased agonist as part of a drug design project with other aims; this unusual compound displayed decreased opioid side effects, but was also active in some pain assays but not others (Manglik et al., 2016). TRV130/Oliceridine was discovered by the pharmaceutical company Trevena; early in vivo reports showed decreased side effects in rodent models (Chen et al., 2013; DeWire et al., 2013). Based on these results TRV130/Oliceridine was advanced to clinical trials, which demonstrated rapid onset analgesia with potentially reduced respiratory depression (Soergel et al., 2014a; Soergel et al., 2014b; Viscusi et al., 2016). Although initially denied, Oliceridine was recently approved by the FDA for human use as a therapeutic with potentially improved safety, providing clinical validation for the biased agonist approach. It must be noted that TRV130 in preclinical studies has been reported to be addictive and recent literature argues it is a partial agonist in vivo as well (Faouzi, Varga & Majumdar, 2020; Singleton, Baptista-Hon, Edelsten, McCaughey, Camplisson & Hales, 2021). Laura Bohn’s group also published a recent paper with an entire new series of opioid biased agonists, and further demonstrated a linear relationship between bias factor and therapeutic index (Schmid et al., 2017).
These basic science, translational, and clinical studies do provide support for the use of biased agonists as improved pain therapeutics. However, recent studies have cast doubt on these findings (Cuitavi, Hipolito & Canals, 2021; Faouzi, Varga & Majumdar, 2020). A MOR knock-in mutant mouse with no C-terminal phosphorylation sites, and thus no desensitization or arrestin recruitment, was recently created (Kliewer et al., 2019). This mouse did have enhanced opioid anti-nociception and reduced tolerance, as expected from the known roles of βarrestin2, and in agreement with earlier studies. However, this mouse also had unchanged or worsened respiratory depression, constipation, and withdrawal, in direct contradiction to the side effect studies above. A replication study was also performed using a newly derived βarrestin2 knockout line on a backcrossed and clean genetic background, performed by 3 different labs in different countries (Kliewer et al., 2020). This βarrestin2 knockout mouse had morphine-induced constipation and respiratory depression that was indistinguishable from wild type. A similar negative replication study has also recently been published (Bachmutsky, Wei, Durand & Yackle, 2021). These findings, along with other recent studies, have cast doubt on the basic science foundation of this approach.
At the same time, recent studies have cast doubt on the biased agonist findings. Using PZM21 newly synthesized as well as obtained from the original reporting lab, a recent study found that this ligand recruited βarrestin2 in vitro and caused respiratory depression (40 mg/kg i.p. PZM21 showed equivalent respiratory depression to 10 mg/kg i.p. morphine) and tolerance in vivo (Hill et al., 2018). These findings are in direct contradiction to the results reported earlier (Manglik et al., 2016). A recent study (Gillis et al., 2020) also found that low intrinsic efficacy, instead of arrestin recruitment, could explain the observed benefits of biased agonists; this study directly tested TRV130/Oliceridine, PZM21, and SR-17018, the lead compound reported in (Schmid et al., 2017). The same study also found that a higher dose of PZM21 (100 mg/kg i.p.) displayed about half the respiratory depression in comparison to morphine at 10 mg/kg i.p., suggesting some separation of analgesia from respiratory depression. A recent preprint from Laura Bohn’s group also contests the findings that benefits seen with PZM21, SR17018 and TRV130 are due to low intrinsic efficacy and not biased signaling (Stahl & Bohn, 2020).
These contradictory findings assault both the basic science foundation and translational application of biased agonists for improved pain therapy. It still remains unclear if biased agonism can lead to less addictive drugs. While PZM21 has not shown CPP by multiple labs (Kudla et al., 2019; Manglik et al., 2016), it was found to produce reinforcing behavior in non-human primates (Ding et al., 2020). Clearly more study will be required to determine the true role of βarrestin2 in promoting analgesia vs. side effects, and whether G-protein biased agonists with intrinsic efficacy similar to the prototypic ligand DAMGO (in less amplified systems) are good clinical candidates. New studies should utilize refined methods to transcend the limitations of tools like germline knockout; the MOR phosphorylation mutant cited above is a good example of such an approach (Kliewer et al., 2019). New approaches should also be developed to assess ligand bias; as mentioned above, current in vitro assays of arrestin recruitment are very insensitive and may poorly reflect arrestin recruitment in neurons. Such studies are strongly needed to resolve this controversy and chart a new course forward for the field.
Opioid Receptor Heterodimers
The vast majority of studies that have been used to assign a particular role to a receptor or to determine the pharmacodynamic profile of a particular ligand use simple approaches that treat the receptor as an isolated unit. Studies on ligand selectivity and functional profile overwhelmingly use in vitro systems with a single overexpressed receptor, without other relevant receptors present. When those ligands are then used to assess the role of a particular receptor in vivo, the assumptions and limitations of those in vitro models are carried over to interpreting the in vivo results. However, it’s now clear that receptors do not function as isolated units; they engage in higher levels of organization within the cell, including receptor homo- and heterodimerization. A particular receptor in vivo could engage in multiple signaling types, including as an isolated receptor, as a homodimer pair, a heterodimer pair, or even larger units of organization that are still being described. A ligand targeting that receptor could thus evoke different clusters of signaling outputs, depending on how that receptor is organized and how that ligand interacts with these different organizational configurations. However, that in vivo complexity is missed when the assumptions from simple in vitro studies are used to interpret in vivo ligand results. Even other approaches like genetic knockdown/knockout do not avoid this problem, since a knocked out protein will be knocked out in all configurations, from single unit receptors to higher order heterodimer clusters. Appreciation for and investigation into these higher orders of complexity in receptor signaling is now being actively investigated in the opioid and pain field, with implications for drug discovery and development.
Perhaps the best described opioid heterodimer is the mu-delta opioid receptor heterodimer (MDOR). The MDOR has been unambiguously shown to exist in multiple in vitro cell culture models co-expressing both receptors (e.g. (Ananthan et al., 2012)). Using these models, the MDOR was suggested to engage in preferential signaling vs. the monomers, including βarrestin2 recruitment and ERK MAPK signaling (Rozenfeld & Devi, 2007). The chaperone protein RTP4 was also suggested to mediate the process of heterodimerization, and was further suggested to increase this activity in response to chronic morphine (Decaillot, Rozenfeld, Gupta & Devi, 2008; Fujita, Yokote, Gomes, Gupta, Ueda & Devi, 2019).
While useful, these models all involve overexpression in simplified cell lines, and could thus be an artifact of the culture model. There is some evidence of the presence and function of the MDOR in vivo, but the data is less clear, and has generated some controversy, in part due to the lack of MDOR-selective tools. Lakshmi Devi and colleagues used an MDOR-selective antibody generated by a screening approach to demonstrate broad expression of the MDOR in brain, which further increased in expression in response to chronic morphine (Gupta et al., 2010). This increase in response to morphine links with the RTP4 results above (Fujita, Yokote, Gomes, Gupta, Ueda & Devi, 2019), suggesting a mechanism by which MDOR could increase with morphine treatment. Jennifer Whistler and colleagues used a cocktail of drugs to stabilize MDOR expression, which led to a decrease in anti-nociceptive potency of opioid drugs (Milan-Lobo, Enquist, van Rijn & Whistler, 2013). Combined with the above findings, this suggests the MDOR could act as an anti-opioid negative feedback loop, stimulated by chronic opioid, which would then act to dampen the opioid system. In support of this hypothesis, another study found that the MDOR promoted opioid anti-nociceptive tolerance (Beaudry, Gendron & Moron, 2015). Further studies suggested specific signaling evoked by the MDOR in the CNS (Kabli, Fan, O’Dowd & George, 2014; Schuster, Metcalf, Kitto, Messing, Fairbanks & Wilcox, 2015), while another suggested that MDOR activation could have anti-depressive and anti-anxiety effects (Kabli, Nguyen, Balboni, O’Dowd & George, 2014). However, these approaches in general used indirect methods with unclear MDOR selectivity, which has generated some controversy as to the presence and role of the MDOR in vivo, abetted by other studies suggesting no functional MDOR interaction in vivo (Wang et al., 2018a). These limitations point out the need to develop specific tools to investigate the MDOR.
Along these lines, some pharmacological tools have been developed. The small molecule agonist CYM51010 was found to be MDOR-selective, and evoked anti-nociception with reduced tolerance (Gomes et al., 2013). However, this ligand was only 5–6 fold selective for the MDOR over MOR and DOR, and thus could cause a mix of heterodimer and monomer activity. We recently developed the compound MP135, with a higher MDOR agonist selectivity; we showed that this compound produced anti-nociception, but also significant opioid-like side effects including respiratory depression and abuse liability (Faouzi et al., 2020). MDAN21 was developed by the Portoghese group, which is a bivalent linked ligand with mu agonist and delta antagonist pharmacophores. This ligand caused highly potent anti-nociception in mice, with reduced tolerance, dependence, and reward (Daniels, Lenard, Etienne, Law, Roerig & Portoghese, 2005; Lenard, Daniels, Portoghese & Roerig, 2007). While this ligand did have a U-shaped linker length to potency relationship, suggesting heterodimer activity, the molecular pharmacology of this compound at the MDOR was not defined. In addition, it is not clear what pharmacodynamic effect this ligand would have at the MDOR with a mix of agonist and antagonist pharmacophores; it could even potentially cause MDOR antagonism or disruption. The results of these studies thus do not definitively suggest a MDOR role in vivo, although they do suggest this activity profile could be a promising therapeutic approach if a drug-like molecule could be developed. Lastly, we developed a bivalent linked compound with dual antagonist pharmacophores, deemed D24M. We showed this compound was ~90 fold selective for the MDOR over either monomer, and reduced morphine withdrawal in mice (Olson, Keresztes, Tashiro, Daconta, Hruby & Streicher, 2018). We further showed that D24M suppressed opioid anti-nociception via suppression of Src and CaMKII signaling in the brain, also supporting this hypothesis (Keresztes et al., 2021). These results are more clear for the role of the MDOR, since they use a ligand with a high selectivity and defined molecular pharmacology. These results further support the hypothesis that the MDOR acts as an anti-opioid negative feedback loop. Taken together, these studies all suggest that the MDOR could be targeted as a therapeutic candidate, most likely using a selective antagonist, to reverse the anti-opioid negative feedback loop caused by chronic morphine treatment. This should enhance anti-nociception while reducing side effects like tolerance and withdrawal. However, studies on the MDOR are still in the early stages, and much more work remains to be done on the basic science and translational potential of this target. This is especially true for abuse liability, which has not been definitively tested for the MDOR.
Other opioid heterodimers have also been found in the literature, and some could be exploited for improved pain therapy. Kelly Berg and colleagues have described a kappa-delta opioid heterodimer (KDOR) in peripheral nociceptors that can produce anti-nociception without the typical side effects of central mu or kappa ligands (Berg et al., 2012; Jacobs, Pando, Jennings, Chavera, Clarke & Berg, 2018; Jacobs et al., 2019). Some ligands have also been developed which may engage this target. A bivalent linked ligand KDAN18 has been developed and suggested to be KDOR-selective, and was also shown to produce anti-nociception (Daniels, Kulkarni, Xie, Bhushan & Portoghese, 2005). One caveat to this ligand though is that it has mixed kappa agonist and delta antagonist pharmacophores, and thus like MDAN21, the molecular pharmacology of this ligand at the KDOR is not clear. The small molecule 6’-guanidinonaltrindole (6’-GNTI) was also suggested to be KDOR-selective by the Berg and Whistler groups (Jacobs, Pando, Jennings, Chavera, Clarke & Berg, 2018; Waldhoer et al., 2005), and does produce anti-nociception. However other groups have reported that this ligand is a potent G-protein biased agonist at kappa opioid monomers, calling this selectivity into question (Rives, Rossillo, Liu-Chen & Javitch, 2012; Schmid, Streicher, Groer, Munro, Zhou & Bohn, 2013). Further work will thus be needed to develop proof-of-concept selective KDOR agonists and evaluate their therapeutic profile.
Among the opioid heterodimers observed, the MDOR and KDOR above are the best established. Other pairs have been described however, and in some cases linked to antinociception, including mu opioid-vasopressin1b, mu opioid-kappa opioid, mu opioid-GPR139, and more. These newly observed heterodimers have been recently reviewed by Zhang and colleagues (Zhang, Zhang, Hang & Liu, 2020). In general, these pairs are described by only one or a few studies, and some have not been validated in vivo. There are also no selective ligands for these targets, meaning that future basic science and translational work must be done to establish these heterodimers as valid therapeutic targets and to create new selective ligands that have the potential to modulate these targets to improve pain therapy. Much more work also has to be performed to study the abuse liability interactions of these heterodimer pairs, including the MDOR and KDOR, which in general has not been studied.
Opioid Receptor Allosteric Activation
Most MOR, KOR and DOR ligands described to date target the orthosteric binding site. In contrast, allosteric ligands bind to sites distinct from the orthosteric binding pocket and may have some advantages in terms of spatial-temporal control of receptor activation. Allosteric modulators, in particular positive allosteric modulators (PAMs), can enhance affinity, potency, and efficacy of exogenous drugs or endogenous opioid peptides. Crucially, a pure PAM will only potentiate receptors which already have an agonist bound, such as an endogenous peptide. This means that unlike an exogenous orthosteric agonist, not all receptors in the body will be activated, only those currently activated by endogenous systems. Negative allosteric modulators (NAMs) on the other hand can inhibit binding of orthosteric ligand agonists and/or decrease ligand functional activity. Silent allosteric modulators (SAMs) do not affect binding or activity of the orthosteric site agonists directly but instead block the activity of NAMs or PAMs. An ideal allosteric modulator will enhance the in vivo agonist effect of the endogenous peptides while at the same time reducing the negative adverse effects that accompany opioid receptor activation, since receptors in those circuits may not be activated by endogenous systems.
Two promising MOR PAMs are now known, BMS-986121 and BMS-986122, both of which enhance G-protein and βarrestin-2 signaling of orthosteric site MOR ligands (Burford et al., 2013) though G-protein signaling is enhanced more (discussed later). In addition, BMS986122 increased the potency but not efficacy of MOR full agonists and only enhanced the efficacy of partial agonists like morphine without effecting potency. As expected it did nothing to antagonists (Livingston & Traynor, 2014). Another probe, MS1, showed an increase of MOR βarrestin-2 over G-protein signaling, suggestive that this ligand can be used in conjunction with orthosteric ligands to induce biased agonism as a pharmacological tool, albeit not as a clinical candidate (Bisignano et al., 2015). Other PAMs of interest include BMS 986187, a biased allosteric modulator of DOR. BMS 986187 showed reduced receptor phosphorylation and low receptor internalization, compared to the full agonist SNC80 (Burford et al., 2015; Stanczyk, Livingston, Chang, Weinberg, Puthenveedu & Traynor, 2019).
Recent studies from the Traynor group have also suggested that BMS-986122 allosterically disrupts the Na+ binding site of MOR (Livingston & Traynor, 2014). Studies dating back to the 1970s showed that incubation of MOR with NaCl inhibits agonist binding and function while not affecting antagonist binding. The Na+ site is located in the middle of the 7-TM bundle and involves coordination with D1142.50, N1503.35, N3287.45, S3297.46, and S1543.39, along with a number of highly organized water molecules across TM domains II, III, VI and VII (Katritch, Fenalti, Abola, Roth, Cherezov & Stevens, 2014). Importantly, the Na+ ion is absent in the structures of active state GPCRs like MOR (Huang et al., 2015). It is believed that expulsion of the Na+ from the pocket pushes TM6 outwards, along with an inward shift of TM7 at the level of the conserved NPxxY motif, thus facilitating G-protein binding and activation.
BMS-986122 and Na+ ions oppose each other’s action, and BMS-986122 binds at a distinct site from Na+ to allosterically disrupt the binding of Na+. While Na+ favors the inactive state of the receptor, the PAM BMS-986122 stabilizes the active state of the receptor, explaining the decreased potency of Na+ ions to inhibit binding of the agonist DAMGO in the presence of BMS-986122. A molecular dynamics simulation study proposes that BMS986122 stabilizes TM7 by engaging with Trp7.35 and prevents Na+ from disrupting agonist actions (Bartuzi, Kaczor & Matosiuk, 2016). Confirmation for this proposed model will require validation by other biophysical methods like NMR, and possibly atomic structures using X-ray or cryoEM of allosteric modulators bound to opioid receptors.
BMS-986122 was recently evaluated in vitro, ex vivo, and in vivo as well. In cell lines it increased G-protein activation of the endogenous opioid peptide Met-enkephalin to a greater extent than for β-arrestin2. This was confirmed in mouse brain homogenates as well as in electrophysiological assays for GABA-release in the periaqueductal gray, where it also increased G-protein activity of Met-enkephalin. In mice it had antinociceptive actions mediated through the MOR while showing reduced constipation, conditioned place preference, and respiratory depression. Taken together the allosteric modulation approach represents a highly innovative strategy to enhance the body’s ability to fight pain while not recruiting adverse effects associated with orthosteric site binders, potentially including abuse liability (Kandasamy et al., 2021).
Targeting Opioid Receptor Splice Variants
The mu opioid receptor gene (OPRM1) undergoes extensive alternative splicing to produce multiple splice variants that are conserved in rodents and humans. These splice variants can be classified under three categories: a) full length seven transmembrane (7TM) domain C-terminal variants, b) truncated six transmembrane domain variants (6TM) missing the first transmembrane of MOR, and finally c) truncated single TM variants that only have the first TM.
The significance of 7TM C-terminal variants have been demonstrated by seminal work from Pasternak and Pan. These include differences in G-protein signaling, phosphorylation, and internalization, as well as differences in the pharmacological actions of morphine in mice (reviewed in (Pasternak, Childers & Pan, 2020)). Recent evidence also suggests that these C-terminal variants may lead to differential bias for a variety of MOR ligands (Narayan, Hunkele, Xu, Bassoni, Pasternak & Pan, 2021). Given the complexity involved with these variants it is unlikely these variants can be individually targeted for drug development. The single TM variants do not bind any opioids; instead they are believed to act as chaperones increasing the receptor expression of MOR at the cell surface and aid in increasing the analgesic potency of morphine in mice (Xu et al., 2013). This also makes the single TM variants a poor choice for drug development.
In contrast, the 6TM-MOR variants are of high interest for drug development based on the actions of the 6TM-selective agonist 3’-iodobenzoyl-6β-naltrexamide (IBNtxA) and its analog IBNalA (Grinnell et al., 2014; Majumdar et al., 2011b; Majumdar et al., 2012). In mice as well as rats, IBNtxA was found be an analgesic in the radiant heat tail flick and hot plate assays. In both species, IBNtxA showed no respiratory depression, physical dependence, or cross-tolerance with morphine. Furthermore in mice IBNtxA showed no reward behavior (Islam et al., 2020; Majumdar et al., 2011b) and was found to be a potent analgesic in neuropathic as well as inflammatory pain assays (Wieskopf et al., 2014).
The mechanism of action of IBNtxA is unique. In mice lacking the full length MOR gene (Oprm1 exon 1 KO mice) IBNtxA retained its analgesic actions, while morphine’s analgesic actions were totally lost. In a 6TM variant KO mice (Oprm1 exon 11–KO mice) which retains full length 7TM MOR variants, the analgesic actions of IBNtxA were lost while the analgesic actions of morphine remained intact. IBNtxA analgesia was independent of 7TM MOR, KOR and DOR, as mice lacking genes for 7TM MOR, KOR, and DOR (triple opioid KO mice preserving 6TM variants, (Majumdar et al., 2011b)) retained the analgesic actions of IBNtxA while the actions of morphine, U50,488h, and DPDPE was lost in these mice. The 6TM-dependent mechanism of action was further confirmed using lentivirus-mediated delivery of 6TM variant MOR-1G in mice lacking both 6TM as well as 7TM MOR variants (exon 1 and exon 11 Oprm1 KO mouse). While both morphine as well as IBNtxA were inactive in the parent mutant animal, the analgesic action of only IBNtxA and not morphine was rescued when 6TM variant MOR 1G was expressed in mice again (Lu, Xu, Rossi, Majumdar, Pasternak & Pan, 2015; Lu et al., 2018).
While these results are exciting and interesting, several unanswered questions still remain about the mechanism of 6TM action in vivo. Besides the actions of the synthetic drug IBNtxA, the 6TM variants also mediate the analgesic actions of KOR, DOR and α2 adrenergic agents like clonidine; however, none of these agents have any binding or functional activity at the 6TM receptor expressed alone in HEK cells (Marrone et al., 2016a). Morphine in Exon 11 MOR KO mice lacking 6TM variants also shows reduced tolerance, hyperalgesia, and locomotor effects while retaining its analgesic actions (Marrone et al., 2017). Other MOR agonists like buprenorphine, endomorphins, and DAPP (Dmt-D-Ala-Phe-Phe-NH2) (Marrone et al., 2016b) in the same mice show attenuated actions in mice lacking 6TM (Oprm1 exon 11–KO mice) as well as the classical 7TM KO mice (Oprm1 exon 1–KO mice). Taken together it appears 6TM MOR agonists can be classified under three categories: 1) 6TM MOR dependent/7TM MOR independent (IBNtxA), 2) 6TM dependent-7TM MOR dependent- (buprenorphine, peptides like DAPP and endomorphin), and 3) 6TM dependent/non MOR-KOR-DOR and α2 agents.
One hypothesis proposed to explain the complexity of 6TM actions is dimerization with a GPCR or non-GPCR protein. [125I]IBNtxA binding was seen in triple opioid KO mice (mice lacking 7TM MOR, KOR and DOR) similar to wild type membranes, while lost in membranes from 6TM KO mice (Oprm1 exon 11–KO mice) mice. Binding of [125I]IBNtxA was seen in membranes co-expressing 6TM variant MOR 1G along with ORL1 and not in MOR 1G alone (Majumdar et al., 2011a); this suggests that a 6TM variant may dimerize or physically associate with another non-opioid GPCR to initiate its actions, although the exact identity of the binding site has not yet been determined (Majumdar et al., 2011a). A recent manuscript reported co-expression of MOR 1G along with full length 7TM MOR led to enhancement of MOR expression at the cell surface, leading to functional 7TM MOR at the protein level. MOR-1G physically interacts with 7TM MOR starting at the endoplasmic reticulum, enhancing MOR expression at the plasma membrane through a chaperone like function (Zhang, Xu & Pan, 2020). Heteromerization of 6TM with β2AR has also been reported by Diatchenko and co-workers as a mechanism for synergistic analgesia when the 6TM and β2-adrenoreceptor drugs are co-administered (Samoshkin et al., 2015).
It remains to be seen if the analgesic actions of IBNtxA and other 6TM dependent drugs can be linked to either ORL1 or other GPCRs in vivo. Selectivity of drug action at 6TM targets also remains a challenge. IBNtxA is a very nonselective drug with high affinity and activity at MOR, KOR and DOR (Grinnell et al., 2021; Islam et al., 2020; Majumdar et al., 2011a). Structure-activity relationships (SAR) on the IBNtxA template did lead to partial selectivity for 6TM over DOR, and showed that the phenyl ring at the 6-position of the epoxymorphinan moiety is required for 6TM affinity (Majumdar et al., 2012). SAR studies have been hindered by the lack of a functional assay due to the unknown identity of the partner protein or proteins with which the 6TM variant MOR1G physically associates. Characterization of IBNtxA in ORL1 and 6TM/ORL1 double KO mice would aid in revealing the composition of the site labelled by IBNtxA. Identification of a partner receptor will permit development of an assay and in turn lead to selective 6TM drugs which retain the behavioral profile seen with the parent drug IBNtxA while also elucidating 6TM opioid function in vivo.
Inhibition of Heat Shock Protein 90
One recent target that has been uncovered for novel pain therapy is the central chaperone protein Heat shock protein 90 (Hsp90). Hsp90 is a highly conserved and highly expressed protein in all cells, where it occupies a central role in cell biology. Hsp90 carries out numerous roles, including acting as a chaperone to assist in protein folding and stabilization, regulating target protein location within the cell, forming specific protein complexes, and regulating the specificity and onset of signal transduction. Despite this central importance, Hsp90 has barely been studied in the context of neuroscience, pain, or opioids.
Some studies have suggested that Hsp90 inhibition is anti-inflammatory, suggesting the use of this protein for managing inflammatory pain. Non-selective Hsp90 inhibitors were shown to prevent the onset and duration of neuropathic pain via this anti-inflammatory activity (Lewis et al., 2010). While this suggests potential direct use in the management of inflammatory-related pain modalities, these studies do not show an impact on opioid anti-nociception or signaling. A few studies did show that Hsp90 was upregulated in the brain in response to chronic morphine, and that non-selective Hsp90 inhibition reduced the severity of opioid withdrawal (Abul-Husn et al., 2011). A supportive study found that Hsp90 inhibition reduced cellular markers of dependence and withdrawal in an in vitro model (Koshimizu et al., 2010). These studies are suggestive, but do not establish the role of Hsp90 in regulating opioid signaling and whether this target can be used to enhance opioid therapy.
To this end, we’ve engaged in a long-term investigation into the role of Hsp90 in regulating opioid anti-nociception and signal transduction in the central nervous system. Our studies to date have shown a regional difference in Hsp90 activity. We found that in the brain, Hsp90 promoted ERK MAPK activation and thus promoted opioid anti-nociception; thus inhibition of Hsp90 in the brain blocked ERK MAPK and opioid anti-nociception (Lei et al., 2017). We found this effect consistent across many acute and chronic pain states, including post-surgical paw incision, HIV peripheral neuropathy, and chemotherapy-induced peripheral neuropathy (Lei et al., 2017; Stine et al., 2020). In contrast, we found that in the spinal cord, Hsp90 blocked ERK MAPK activation and thus blocked opioid anti-nociception, with Hsp90 inhibition in the spinal cord thus promoting opioid anti-nociception (Duron et al., 2020). This enhancement of opioid anti-nociception in the spinal cord suggested we could use this activity to enhance opioid therapy. We found this hypothesis was correct, in that spinal Hsp90 inhibition consistently improved morphine potency in acute and chronic pain models by 2–4 fold, while improving tolerance, rescuing established tolerance, and not changing the potency of constipation and reward (unpublished data in review).
These results suggest a translational path to target Hsp90 to improve opioid therapy. Spinal cord inhibition improves the therapeutic index of morphine, enabling a dose-reduction strategy. This means that a lower dose of opioid could be given to patients, which due to enhanced anti-nociceptive potency, would retain analgesic efficacy. However, since side effects are either improved or not changed, the lower opioid dose would result in fewer side effects, especially tolerance. While high efficacy agonists like fentanyl produce less tolerance, they do not produce less reward and similar side effects, suggesting the potential superiority of a dose-reduction approach. One roadblock to this approach however was our finding that non-selective inhibitors given systemically always result in a brain-like phenotype, with blocked opioid anti-nociception (Duron et al., 2020). One way around this roadblock could be to target specific Hsp90 isoforms only active in the spinal cord. In our initial investigations, we found that the Hsp90α isoform and the p23 and Cdc37 co-chaperones were responsible for regulating opioid anti-nociception in the brain (Lei, Duron, Stine, Mishra, Blagg & Streicher, 2019). In contrast, our in-progress studies suggest that additional isoforms and co-chaperones regulate opioid anti-nociception in the spinal cord (Hsp90β, Grp94, Aha1). By using systemic isoform-selective inhibitors targeting these spinal-only isoforms, we were able to boost anti-nociception and reduce tolerance while giving drug by a translationally relevant route (unpublished data).
Together these findings do suggest a way forward to target Hsp90 to improve opioid therapy. General Hsp90 inhibitors can be given to block inflammation, preventing and easing inflammatory and neuropathic pain. More specifically, isoform-selective Hsp90 inhibitors can be given to selectively boost the anti-nociceptive efficacy of opioids to manage multiple pain types, while enabling opioid dose-reduction and reduced side effects. While current studies do suggest a benefit in managing opioid reward as above, this topic must be explored in further detail.
Future Directions
As reviewed above, many promising pre-clinical approaches are in development which may result in the next generation of efficacious but non-addictive painkillers. However, from past experience in the field, it’s clear there is no one “silver bullet” approach. Any of these approaches may fail to bridge the “valley of death” between preclinical development and clinical testing and approval. Many such approaches have failed in the past, even with very promising animal model data. General translational challenges include species differences between rodents/dogs/primates typically used during development and human patients, and business challenges like raising capital for development and clinical testing. Beyond the molecular pharmacodynamic approaches reviewed here, there is a robust debate in the field over translational methods like the animal models used to assess pain and side effects. Future exploration of the basic behavioral science of pain may result in more translational animal models which will improve our ability to predict ligand performance in humans. We must explore every avenue in full and expand our basic science knowledge at all levels of pain and analgesia if we are to be successful in creating that next generation of analgesic drugs. Past experience has shown there are no easy fixes, we must fully understand this system if we are to solve the twin crises of chronic pain and opioid abuse and overdose.
Acknowledgements
S.M. is supported by funds from NIH grants DA045884 and DA046487, and start-up funds from the Center for Clinical Pharmacology, Washington University and Faculty Research Incentive Funds (FRIF) from the University of Health Sciences & Pharmacy. J.M.S. is supported by NIH grants R01NS091238, R01DA038635 (and DA038635-S1), R21DA044509 and UG3DA047717; an Arizona Biomedical Research Commission New Investigator Award #ADHS18–198875; and institutional funds from the University of Arizona.
Abbreviations:
- Aha1
Activator of 90 kDa heat shock protein ATPase homolog 1
- CPA
conditioned place aversion
- CPP
conditioned place preference
- cryoEM
Cryogenic electron microscopy
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- DOR
delta opioid receptor
- ERK
extracellular signal-regulated kinase
- FDA
food and drug administration
- 7TM
full length seven transmembrane
- GPCR
G protein coupled receptors
- GABA
gamma aminobutyric acid
- GPR139
G grotein coupled receptor 139
- Grp94
glucose-regulated protein 94
- GαI/O
guanine nucleotide-binding protein alpha family member i/o isoform
- Hsp90
heat shock protein 90
- Hsp90β
heat shock protein 90 β isoform
- KDOR
kappa-delta opioid receptor heterodimer
- KOR
kappa opioid receptor
- MAPK
mitogen-activated protein kinase
- MOR
mu opioid receptor
- MDOR
mu-delta opioid receptor heterodimer
- N/OFQ
nociceptin/orphanin FQ
- NAMs
negative allosteric modulators
- NHP
nonhuman primates
- NMR
nuclear magnetic resonance
- NOP
nociceptin opioid peptide receptor
- OPRM1
opioid receptor mu 1
- ORL1
opioid-receptor-like 1
- PAMs
positive allosteric modulators
- RTP4
receptor transporter protein 4
- SAMs
silent allosteric modulators
- TM7
seventh transmembrane domain of GPCRs
- 6TM
truncated six transmembrane domain variants
Footnotes
Conflict of Interest
The authors declare the following competing financial interest(s): SM is the co-founder of Sparian Biosciences. JMS is a co-founder and equity holder in Teleport Pharmaceuticals, LLC, and an equity holder in Botanical Results, LLC.
References
- Abdelhamid EE, Sultana M, Portoghese PS, & Takemori AE (1991). Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther 258: 299–303. [PubMed] [Google Scholar]
- Abul-Husn NS, Annangudi SP, Ma’ayan A, Ramos-Ortolaza DL, Stockton SD Jr., Gomes I, et al. (2011). Chronic morphine alters the presynaptic protein profile: identification of novel molecular targets using proteomics and network analysis. PloS one 6: e25535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, et al. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. Br J Pharmacol 176 Suppl 1: S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand JP, Kochan KE, Nastase AF, Montgomery D, Griggs NW, Traynor JR, et al. (2018). In vivo effects of mu-opioid receptor agonist/delta-opioid receptor antagonist peptidomimetics following acute and repeated administration. Br J Pharmacol 175: 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthan S, Saini SK, Dersch CM, Xu H, McGlinchey N, Giuvelis D, et al. (2012). 14-Alkoxy- and 14-acyloxypyridomorphinans: mu agonist/delta antagonist opioid analgesics with diminished tolerance and dependence side effects. J Med Chem 55: 8350–8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atigari DV, Uprety R, Pasternak GW, Majumdar S, & Kivell BM (2019). MP1104, a mixed kappa-delta opioid receptor agonist has anti-cocaine properties with reduced side-effects in rats. Neuropharmacology 150: 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmutsky I, Wei XP, Durand A, & Yackle K (2021). ss-arrestin 2 germline knockout does not attenuate opioid respiratory depression. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartuzi D, Kaczor AA, & Matosiuk D (2016). Interplay between Two Allosteric Sites and Their Influence on Agonist Binding in Human mu Opioid Receptor. J Chem Inf Model 56: 563–570. [DOI] [PubMed] [Google Scholar]
- Beaudry H, Gendron L, & Moron JA (2015). Implication of delta opioid receptor subtype 2 but not delta opioid receptor subtype 1 in the development of morphine analgesic tolerance in a rat model of chronic inflammatory pain. Eur J Neurosci 41: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, et al. (2012). Allosteric interactions between delta and kappa opioid receptors in peripheral sensory neurons. Mol Pharmacol 81: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisignano P, Burford NT, Shang Y, Marlow B, Livingston KE, Fenton AM, et al. (2015). Ligand-Based Discovery of a New Scaffold for Allosteric Modulation of the mu-Opioid Receptor. J Chem Inf Model 55: 1836–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, & Caron MG (2000). Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408: 720–723. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, et al. (2003). Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci 23: 10265–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, & Lin FT (1999). Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495–2498. [DOI] [PubMed] [Google Scholar]
- Brackley AD, Gomez R, Akopian AN, Henry MA, & Jeske NA (2016). GRK2 Constitutively Governs Peripheral Delta Opioid Receptor Activity. Cell Rep 16: 2686–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford NT, Clark MJ, Wehrman TS, Gerritz SW, Banks M, O’Connell J, et al. (2013). Discovery of positive allosteric modulators and silent allosteric modulators of the mu-opioid receptor. Proc Natl Acad Sci U S A 110: 10830–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford NT, Livingston KE, Canals M, Ryan MR, Budenholzer LM, Han Y, et al. (2015). Discovery, synthesis, and molecular pharmacology of selective positive allosteric modulators of the delta-opioid receptor. J Med Chem 58: 4220–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, & Majumdar S (2021). Natural Products for the Treatment of Pain: Chemistry and Pharmacology of Salvinorin A, Mitragynine, and Collybolide. Biochemistry 60: 1381–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che T, Majumdar S, Zaidi SA, Ondachi P, McCorvy JD, Wang S, et al. (2018). Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 172: 55–67 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XT, Pitis P, Liu G, Yuan C, Gotchev D, Cowan CL, et al. (2013). Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem 56: 8019–8031. [DOI] [PubMed] [Google Scholar]
- Christoph A, Eerdekens MH, Kok M, Volkers G, & Freynhagen R (2017). Cebranopadol, a novel first-in-class analgesic drug candidate: first experience in patients with chronic low back pain in a randomized clinical trial. Pain 158: 1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, & Ko MC (2012). Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuitavi J, Hipolito L, & Canals M (2021). The Life Cycle of the Mu-Opioid Receptor. Trends Biochem Sci 46: 315–328. [DOI] [PubMed] [Google Scholar]
- Dahan A, Boom M, Sarton E, Hay J, Groeneveld GJ, Neukirchen M, et al. (2017). Respiratory Effects of the Nociceptin/Orphanin FQ Peptide and Opioid Receptor Agonist, Cebranopadol, in Healthy Human Volunteers. Anesthesiology 126: 697–707. [DOI] [PubMed] [Google Scholar]
- Dahan A, Romberg R, Teppema L, Sarton E, Bijl H, & Olofsen E (2004). Simultaneous measurement and integrated analysis of analgesia and respiration after an intravenous morphine infusion. Anesthesiology 101: 1201–1209. [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, & Portoghese PS (2005). A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J Med Chem 48: 1713–1716. [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, & Portoghese PS (2005). Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A 102: 19208–19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Matzeu A, Kononoff J, Mattioni J, Martin-Fardon R, & George O (2017). Cebranopadol Blocks the Escalation of Cocaine Intake and Conditioned Reinstatement of Cocaine Seeking in Rats. J Pharmacol Exp Ther 362: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaillot FM, Rozenfeld R, Gupta A, & Devi LA (2008). Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A 105: 16045–16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, et al. (2013). A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344: 708–717. [DOI] [PubMed] [Google Scholar]
- Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, et al. (2016). A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A 113: E5511–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Perrey DA, Nguyen T, Czoty PW, Hsu FC, et al. (2020). Antinociceptive, reinforcing, and pruritic effects of the G-protein signalling-biased mu opioid receptor agonist PZM21 in non-human primates. British journal of anaesthesia 125: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, et al. (2018). A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Science Translational Medicine 10: 3483–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron DI, Lei W, Barker NK, Stine C, Mishra S, Blagg BSJ, et al. (2020). Inhibition of Hsp90 in the spinal cord enhances the antinociceptive effects of morphine by activating an ERK-RSK pathway. Sci Signal 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eans SO, Ganno ML, Mizrachi E, Houghten RA, Dooley CT, McLaughlin JP, et al. (2015). Parallel Synthesis of Hexahydrodiimidazodiazepines Heterocyclic Peptidomimetics and Their in Vitro and in Vivo Activities at mu (MOR), delta (DOR), and kappa (KOR) Opioid Receptors. J Med Chem 58: 4905–4917. [DOI] [PubMed] [Google Scholar]
- Eerdekens MH, Kapanadze S, Koch ED, Kralidis G, Volkers G, Ahmedzai SH, et al. (2019). Cancer-related chronic pain: Investigation of the novel analgesic drug candidate cebranopadol in a randomized, double-blind, noninferiority trial. Eur J Pain 23: 577–588. [DOI] [PubMed] [Google Scholar]
- Faouzi A, Uprety R, Gomes I, Massaly N, Keresztes AI, Le Rouzic V, et al. (2020). Synthesis and Pharmacology of a Novel mu-delta Opioid Receptor Heteromer-Selective Agonist Based on the Carfentanyl Template. J Med Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faouzi A, Varga BR, & Majumdar S (2020). Biased Opioid Ligands. Molecules (Basel, Switzerland) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita W, Yokote M, Gomes I, Gupta A, Ueda H, & Devi LA (2019). Regulation of an Opioid Receptor Chaperone Protein, RTP4, by Morphine. Mol Pharmacol 95: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, et al. (2020). Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal 13. [DOI] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Gupta A, Saldanha SA, Negri A, Pinello CE, et al. (2013). Identification of a mu-delta opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci U S A 110: 12072–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell SG, Majumdar S, Narayan A, Le Rouzic V, Ansonoff M, Pintar JE, et al. (2014). Pharmacologic characterization in the rat of a potent analgesic lacking respiratory depression, IBNtxA. J Pharmacol Exp Ther 350: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell SG, Uprety R, Varadi A, Subrath J, Hunkele A, Pan YX, et al. (2021). Synthesis and Characterization of Azido Aryl Analogs of IBNtxA for Radio-Photoaffinity Labeling Opioid Receptors in Cell Lines and in Mouse Brain. Cell Mol Neurobiol 41: 977–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, et al. (2007). An opioid agonist that does not induce micro-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol 71: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. (2010). Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal 3: ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutridge AM, Robins MT, Cassell RJ, Uprety R, Mores KL, Ko MJ, et al. (2020). G protein-biased kratom-alkaloids and synthetic carfentanil-amide opioids as potential treatments for alcohol use disorder. Br J Pharmacol 177: 1497–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, & Takeda R (2001). Effects of a kappa-receptor agonist U-50488 on bulbar respiratory neurons and its antagonistic action against the mu receptor-induced respiratory depression in decerebrate cats. Jpn J Pharmacol 87: 333–337. [DOI] [PubMed] [Google Scholar]
- Healy JR, Bezawada P, Shim J, Jones JW, Kane MA, MacKerell AD, Jr., et al. (2013). Synthesis, modeling, and pharmacological evaluation of UMB 425, a mixed mu agonist/delta antagonist opioid analgesic with reduced tolerance liabilities. ACS Chem Neurosci 4: 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, et al. (2018). The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175: 2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Calo G, Guerrini R, & Ko MC (2010). Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 148: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, et al. (2015). Structural insights into micro-opioid receptor activation. Nature 524: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A, Rahman MA, Brenner MB, Moore A, Kellmyer A, Buechler HM, et al. (2020). Abuse Liability, Anti-Nociceptive, and Discriminative Stimulus Properties of IBNtxA. ACS Pharmacol Transl Sci 3: 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BA, Pando MM, Jennings E, Chavera TA, Clarke WP, & Berg KA (2018). Allosterism within delta Opioid-kappa Opioid Receptor Heteromers in Peripheral Sensory Neurons: Regulation of kappa Opioid Agonist Efficacy. Mol Pharmacol 93: 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BA, Pando MM, Jennings EM, Jamshidi RJ, Zamora JC, Chavera TS, et al. (2019). Signaling characteristics and functional regulation of delta opioid-kappa opioid receptor (DOP-KOP) heteromers in peripheral sensory neurons. Neuropharmacology 151: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, & Woods JH (2006). The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmacol Exp Ther 317: 1337–1348. [DOI] [PubMed] [Google Scholar]
- Kabli N, Fan T, O’Dowd BF, & George SR (2014). mu-delta opioid receptor heteromer-specific signaling in the striatum and hippocampus. Biochemical and biophysical research communications 450: 906–911. [DOI] [PubMed] [Google Scholar]
- Kabli N, Nguyen T, Balboni G, O’Dowd BF, & George SR (2014). Antidepressant-like and anxiolytic-like effects following activation of the mu-delta opioid receptor heteromer in the nucleus accumbens. Mol Psychiatry 19: 986–994. [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Hillhouse TM, Livingston KE, Kochan KE, Meurice C, Eans SO, et al. (2021). Positive allosteric modulation of the mu-opioid receptor produces analgesia with reduced side effects. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, & Stevens RC (2014). Allosteric sodium in class A GPCR signaling. Trends Biochem Sci 39: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T (2007). Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol 72: 1393–1401. [DOI] [PubMed] [Google Scholar]
- Keresztes A, Olson K, Nguyen P, Lopez-Pier MA, Hecksel R, Barker NK, et al. (2021). Antagonism of the mu-delta opioid receptor heterodimer enhances opioid antinociception by activating Src and calcium/calmodulin-dependent protein kinase II signaling. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Lee CE, McLemore GL, & Inturrisi CE (1996). An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain research bulletin 39: 185–188. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Cippitelli A, Toll N, Lawson JA, Crossman W, Polgar WE, et al. (2017). In Vitro and In Vivo Profile of PPL-101 and PPL-103: Mixed Opioid Partial Agonist Analgesics with Low Abuse Potential. Front Psychiatry 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Jiang F, Zaveri NT, & Toll L (2009). Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J Pharmacol Exp Ther 331: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Orduna J, Jiang F, Olsen C, Toll L, et al. (2009). Activity of new NOP receptor ligands in a rat peripheral mononeuropathy model: potentiation of morphine anti-allodynic activity by NOP receptor antagonists. Eur J Pharmacol 610: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, et al. (2007). SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther 320: 934–943. [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, et al. (2019). BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. British journal of anaesthesia 122: e146–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Kishioka S, & Ko MC (2020). Nociceptin/Orphanin FQ Peptide Receptor-Related Ligands as Novel Analgesics. Curr Top Med Chem 20: 2878–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, & Ko MC (2016). Central N/OFQ-NOP Receptor System in Pain Modulation. Advances in pharmacology 75: 217–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, & Ko MC (2019). Effects of NOP-Related Ligands in Nonhuman Primates. Handbook of experimental pharmacology 254: 323–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, et al. (2020). Morphine-induced respiratory depression is independent of beta-arrestin2 signalling. Br J Pharmacol 177: 2923–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, et al. (2019). Phosphorylation-deficient G-protein-biased mu-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu TA, Tsuchiya H, Tsuda H, Fujiwara Y, Shibata K, Hirasawa A, et al. (2010). Inhibition of heat shock protein 90 attenuates adenylate cyclase sensitization after chronic morphine treatment. Biochemical and biophysical research communications 392: 603–607. [DOI] [PubMed] [Google Scholar]
- Kudla L, Bugno R, Skupio U, Wiktorowska L, Solecki W, Wojtas A, et al. (2019). Functional characterization of a novel opioid, PZM21, and its effects on the behavioural responses to morphine. Br J Pharmacol 176: 4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DG, Bird MF, & Rowbotham DJ (2015). Cebranopadol: a first in-class example of a nociceptin/orphanin FQ receptor and opioid receptor agonist. British journal of anaesthesia 114: 364–366. [DOI] [PubMed] [Google Scholar]
- Lei W, Duron DI, Stine C, Mishra S, Blagg BSJ, & Streicher JM (2019). The Alpha Isoform of Heat Shock Protein 90 and the Co-chaperones p23 and Cdc37 Promote Opioid Anti-nociception in the Brain. Front Mol Neurosci 12: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Mullen N, McCarthy S, Brann C, Richard P, Cormier J, et al. (2017). Heat-shock protein 90 (Hsp90) promotes opioid-induced anti-nociception by an ERK mitogen-activated protein kinase (MAPK) mechanism in mouse brain. J Biol Chem 292: 10414–10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Vekariya RH, Ananthan S, & Streicher JM (2020). A Novel Mu-Delta Opioid Agonist Demonstrates Enhanced Efficacy With Reduced Tolerance and Dependence in Mouse Neuropathic Pain Models. J Pain 21: 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard NR, Daniels DJ, Portoghese PS, & Roerig SC (2007). Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol 566: 75–82. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, et al. (2010). Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience 165: 569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Csakai A, Jin J, Zhang F, & Yin H (2016). Therapeutic Developments Targeting Toll-like Receptor-4-Mediated Neuroinflammation. ChemMedChem 11: 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, et al. (2014). Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349: 535–548. [DOI] [PubMed] [Google Scholar]
- Lipkowski AW, Konecka AM, & Sroczynska I (1982). Double-enkephalins--synthesis, activity on guinea-pig ileum, and analgesic effect. Peptides 3: 697–700. [DOI] [PubMed] [Google Scholar]
- Livingston KE, & Traynor JR (2014). Disruption of the Na+ ion binding site as a mechanism for positive allosteric modulation of the mu-opioid receptor. Proc Natl Acad Sci U S A 111: 18369–18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, & Bilsky EJ (2011). In vivo characterization of MMP-2200, a mixed delta/mu opioid agonist, in mice. J Pharmacol Exp Ther 336: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu J, Rossi GC, Majumdar S, Pasternak GW, & Pan YX (2015). Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. The Journal of clinical investigation 125: 2626–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu J, Xu M, Rossi GC, Majumdar S, Pasternak GW, et al. (2018). Truncated mu-Opioid Receptors With 6 Transmembrane Domains Are Essential for Opioid Analgesia. Anesthesia and analgesia 126: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk OS, Falk T, Sherman SJ, Kennedy RT, & Polt R (2012). CNS penetration of the opioid glycopeptide MMP-2200: a microdialysis study. Neurosci Lett 531: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Burgman M, Haselton N, Grinnell S, Ocampo J, Pasternak AR, et al. (2011a). Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett 21: 4001–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, et al. (2011b). Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci U S A 108: 19778–19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Subrath J, Le Rouzic V, Polikar L, Burgman M, Nagakura K, et al. (2012). Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated mu opioid receptor (MOR-1) splice variants. J Med Chem 55: 6352–6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. (2016). Structure-based discovery of opioid analgesics with reduced side effects. Nature 537: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone GF, Grinnell SG, Lu Z, Rossi GC, Le Rouzic V, Xu J, et al. (2016a). Truncated mu opioid GPCR variant involvement in opioid-dependent and opioid-independent pain modulatory systems within the CNS. Proc Natl Acad Sci U S A 113: 3663–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone GF, Le Rouzic V, Varadi A, Xu J, Rajadhyaksha AM, Majumdar S, et al. (2017). Genetic dissociation of morphine analgesia from hyperalgesia in mice. Psychopharmacology (Berl) 234: 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone GF, Lu Z, Rossi G, Narayan A, Hunkele A, Marx S, et al. (2016b). Tetrapeptide Endomorphin Analogs Require Both Full Length and Truncated Splice Variants of the Mu Opioid Receptor Gene Oprm1 for Analgesia. ACS Chem Neurosci 7: 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Narita M, Muramatsu N, Nakayama T, Misawa K, Kitajima M, et al. (2014). Orally active opioid mu/delta dual agonist MGM-16, a derivative of the indole alkaloid mitragynine, exhibits potent antiallodynic effect on neuropathic pain in mice. J Pharmacol Exp Ther 348: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, & Negus SS (2000). Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci 909: 104–132. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Taiwo YO, & Levine JD (1990). Kappa- and delta-opioid agonists synergize to produce potent analgesia. Brain Res 509: 165–168. [DOI] [PubMed] [Google Scholar]
- Milan-Lobo L, Enquist J, van Rijn RM, & Whistler JL (2013). Anti-analgesic effect of the mu/delta opioid receptor heteromer revealed by ligand-biased antagonism. PloS one 8: e58362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Gullapalli S, Taylor C, Gupta A, Gomes I, & Devi LA (2010). Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology 35: 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, et al. (2014). Development of a bioavailable mu opioid receptor (MOPr) agonist, delta opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J Med Chem 57: 3148–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan A, Hunkele A, Xu J, Bassoni DL, Pasternak GW, & Pan YX (2021). Mu Opioids Induce Biased Signaling at the Full-Length Seven Transmembrane C-Terminal Splice Variants of the mu Opioid Receptor Gene, Oprm1. Cell Mol Neurobiol 41: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KM, Keresztes A, Tashiro JK, Daconta LV, Hruby VJ, & Streicher JM (2018). Synthesis and Evaluation of a Novel Bivalent Selective Antagonist for the Mu-Delta Opioid Receptor Heterodimer that Reduces Morphine Withdrawal in Mice. J Med Chem 61: 6075–6086. [DOI] [PubMed] [Google Scholar]
- Parenti C, Turnaturi R, Arico G, Gramowski-Voss A, Schroeder OH, Marrazzo A, et al. (2013). The multitarget opioid ligand LP1’s effects in persistent pain and in primary cell neuronal cultures. Neuropharmacology 71: 70–82. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Childers SR, & Pan YX (2020). Emerging Insights into Mu Opioid Pharmacology. Handbook of experimental pharmacology 258: 89–125. [DOI] [PubMed] [Google Scholar]
- Paton KF, Atigari DV, Kaska S, Prisinzano T, & Kivell BM (2020). Strategies for Developing kappa Opioid Receptor Agonists for the Treatment of Pain with Fewer Side Effects. J Pharmacol Exp Ther 375: 332–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky AT, Sandweiss A, Hu J, Bilsky EJ, Cain JP, Kumirov VK, et al. (2013). Novel fentanyl-based dual mu/delta-opioid agonists for the treatment of acute and chronic pain. Life Sci 93: 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, & Bohn LM (2011). The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, & Bohn LM (2005). Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314: 1195–1201. [DOI] [PubMed] [Google Scholar]
- Rives ML, Rossillo M, Liu-Chen LY, & Javitch JA (2012). 6’-Guanidinonaltrindole (6’-GNTI) is a G protein-biased kappa-opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem 287: 27050–27054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, & Devi LA (2007). Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 21: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Tominaga H, Ogawa Y, Irukayama-Tomobe Y, Yamada M, Yanagisawa M, et al. (2018). Effects of the delta opioid receptor agonist KNT-127 on electroencephalographic activity in mice. Pharmacol Rep 70: 350–354. [DOI] [PubMed] [Google Scholar]
- Samoshkin A, Convertino M, Viet CT, Wieskopf JS, Kambur O, Marcovitz J, et al. (2015). Structural and functional interactions between six-transmembrane mu-opioid receptors and beta2-adrenoreceptors modulate opioid signaling. Scientific reports 5: 18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TM, Lemieux C, et al. (1999). The opioid mu agonist/delta antagonist DIPP-NH(2)[Psi] produces a potent analgesic effect, no physical dependence, and less tolerance than morphine in rats. J Med Chem 42: 3520–3526. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, et al. (2017). Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell 171: 1165–1175 e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, & Bohn LM (2013). Functional selectivity of 6’-guanidinonaltrindole (6’-GNTI) at kappa-opioid receptors in striatal neurons. J Biol Chem 288: 22387–22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WK, Tam SW, Shotzberger GS, Smith DH Jr., Clark R, & Vernier VG (1985). Nalbuphine. Drug Alcohol Depend 14: 339–362. [DOI] [PubMed] [Google Scholar]
- Schuster DJ, Metcalf MD, Kitto KF, Messing RO, Fairbanks CA, & Wilcox GL (2015). Ligand requirements for involvement of PKCepsilon in synergistic analgesic interactions between spinal mu and delta opioid receptors. Br J Pharmacol 172: 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KF, & Crain SM (1995). Biphalin, an enkephalin analog with unexpectedly high antinociceptive potency and low dependence liability in vivo, selectively antagonizes excitatory opioid receptor functions of sensory neurons in culture. Brain Res 701: 158–166. [DOI] [PubMed] [Google Scholar]
- Shrivastava P, Cabrera MA, Chastain LG, Boyadjieva NI, Jabbar S, Franklin T, et al. (2017). Mu-opioid receptor and delta-opioid receptor differentially regulate microglial inflammatory response to control proopiomelanocortin neuronal apoptosis in the hypothalamus: effects of neonatal alcohol. J Neuroinflammation 14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton S, Baptista-Hon DT, Edelsten E, McCaughey KS, Camplisson E, & Hales TG (2021). TRV130 partial agonism and capacity to induce anti-nociceptive tolerance revealed through reducing available mu-opioid receptor number. Br J Pharmacol 178: 1855–1868. [DOI] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, et al. (2014a). Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 155: 1829–1835. [DOI] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, et al. (2014b). First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol 54: 351–357. [DOI] [PubMed] [Google Scholar]
- Stahl EL, & Bohn LM (2020). Re-evaluating how low intrinsic efficacy and apparent bias for G protein activation relates to the improved side effect profiles of new opioid agonists. bioRxiv: 2020.2011.2019.390518. [Google Scholar]
- Stanczyk MA, Livingston KE, Chang L, Weinberg ZY, Puthenveedu MA, & Traynor JR (2019). The delta-opioid receptor positive allosteric modulator BMS 986187 is a G-protein-biased allosteric agonist. Br J Pharmacol 176: 1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanucci A, Lei W, Pieretti S, Dimmito MP, Luisi G, Novellino E, et al. (2019). Novel Cyclic Biphalin Analogues by Ruthenium-Catalyzed Ring Closing Metathesis: in Vivo and in Vitro Biological Profile. ACS Med Chem Lett 10: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Luginbuhl A, Dunbar C, LaVigne J, Dutra J, Atherton P, et al. (2015). The mixed-action delta/mu opioid agonist MMP-2200 does not produce conditioned place preference but does maintain drug self-administration in rats, and induces in vitro markers of tolerance and dependence. Pharmacol Biochem Behav 132: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine C, Coleman DL, Flohrschutz AT, Thompson AL, Mishra S, Blagg BS, et al. (2020). Heat shock protein 90 inhibitors block the antinociceptive effects of opioids in mouse chemotherapy-induced neuropathy and cancer bone pain models. Pain 161: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Yang J, Lunzer MM, Powers MD, & Portoghese PS (2011). A kappa Opioid Pharmacophore Becomes a Spinally Selective kappa-delta Agonist When Modified with a Basic Extender Arm. ACS Med Chem Lett 2: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidgewell K, Groer CE, Harding WW, Lozama A, Schmidt M, Marquam A, et al. (2008). Herkinorin analogues with differential beta-arrestin-2 interactions. J Med Chem 51: 2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo G, Cox BM, & Zaveri NT (2016). Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev 68: 419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Linz K, Koch T, & Christoph T (2019). Cebranopadol: A Novel First-in-Class Potent Analgesic Acting via NOP and Opioid Receptors. Handbook of experimental pharmacology 254: 367–398. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, & Rutten K (2018). Mu-opioid peptide (MOP) and nociceptin/orphanin FQ peptide (NOP) receptor activation both contribute to the discriminative stimulus properties of cebranopadol in the rat. Neuropharmacology 129: 100–108. [DOI] [PubMed] [Google Scholar]
- Ulker E, Toma W, White A, Uprety R, Majumdar S, & Damaj MI (2020). The antinociceptive effects of a dual kappa-delta opioid receptor agonist in the mouse formalin test. Behav Pharmacol 31: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uprety R, Che T, Zaidi SA, Grinnell SG, Varga BR, Faouzi A, et al. (2021). Controlling opioid receptor functional selectivity by targeting distinct subpockets of the orthosteric site. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, Hosztafi S, Le Rouzic V, Toth G, Urai A, Noszal B, et al. (2013). Novel 6beta-acylaminomorphinans with analgesic activity. Eur J Med Chem 69: 786–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, Marrone GF, Eans SO, Ganno ML, Subrath JJ, Le Rouzic V, et al. (2015a). Synthesis and characterization of a dual kappa-delta opioid receptor agonist analgesic blocking cocaine reward behavior. ACS Chem Neurosci 6: 1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, Marrone GF, Palmer TC, Narayan A, Szabo MR, Le Rouzic V, et al. (2016). Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit beta-Arrestin-2. J Med Chem 59: 8381–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, Palmer TC, Haselton N, Afonin D, Subrath JJ, Le Rouzic V, et al. (2015b). Synthesis of Carfentanil Amide Opioids Using the Ugi Multicomponent Reaction. ACS Chem Neurosci 6: 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekariya RH, Lei W, Ray A, Saini SK, Zhang S, Molnar G, et al. (2020). Synthesis and Structure-Activity Relationships of 5’-Aryl-14-alkoxypyridomorphinans: Identification of a mu Opioid Receptor Agonist/delta Opioid Receptor Antagonist Ligand with Systemic Antinociceptive Activity and Diminished Opioid Side Effects. J Med Chem 63: 7663–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, et al. (2016). A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain 157: 264–272. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, et al. (2005). A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci U S A 102: 9050–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Tawfik VL, Corder G, Low SA, Francois A, Basbaum AI, et al. (2018a). Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits. Neuron 98: 90–108 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Chai JR, Xu XJ, Ye RF, Zan GY, Liu GY, et al. (2018b). Pharmacological Characterization of Dezocine, a Potent Analgesic Acting as a kappa Partial Agonist and mu Partial Agonist. Scientific reports 8: 14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieskopf JS, Pan YX, Marcovitz J, Tuttle AH, Majumdar S, Pidakala J, et al. (2014). Broad-spectrum analgesic efficacy of IBNtxA is mediated by exon 11-associated splice variants of the mu-opioid receptor gene. Pain 155: 2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, et al. (2007). A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse 61: 166–175. [DOI] [PubMed] [Google Scholar]
- Xu J, Xu M, Brown T, Rossi GC, Hurd YL, Inturrisi CE, et al. (2013). Stabilization of the mu-opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J Biol Chem 288: 21211–21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangrandi L, Burtscher J, MacKay JP, Colmers WF, & Schwarzer C (2016). The G-protein biased partial kappa opioid receptor agonist 6’-GNTI blocks hippocampal paroxysmal discharges without inducing aversion. Br J Pharmacol 173: 1756–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang JT, Hang L, & Liu T (2020). Mu Opioid Receptor Heterodimers Emerge as Novel Therapeutic Targets: Recent Progress and Future Perspective. Frontiers in pharmacology 11: 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xu J, & Pan YX (2020). A Truncated Six Transmembrane Splice Variant MOR-1G Enhances Expression of the Full-Length Seven Transmembrane mu-Opioid Receptor through Heterodimerization. Mol Pharmacol 98: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]