Abstract
Nicotinamide adenine dinucleotide (NAD+) is an essential and pleiotropic coenzyme involved not only in cellular energy metabolism, but also in cell signaling, epigenetic regulation, and post-translational protein modifications. Vascular disease risk factors are associated with aberrant NAD+ metabolism. Conversely, the therapeutic increase of NAD+ levels through the administration of NAD+ precursors or inhibitors of NAD+-consuming enzymes reduces chronic low-grade inflammation, reactivates autophagy and mitochondrial biogenesis, and enhances oxidative metabolism in vascular cells of humans and rodents with vascular pathologies. As such, NAD+ has emerged as a potential target for combatting age-related cardiovascular and cerebrovascular disorders. This review discusses NAD+-regulated mechanisms critical for vascular health and summarizes new advances in NAD+ research directly related to vascular aging and disease, including hypertension, atherosclerosis, coronary artery disease, and aortic aneurysms. Finally, we enumerate challenges and opportunities for NAD+ repletion therapy while anticipating the future of this exciting research field, which will have a major impact on vascular medicine.
Keywords: Vascular disease, Hypertension, Nicotinamide adenine dinucleotide, Inflammation, Mitochondria, Autophagy, Aging
INTRODUCTION
Cardiovascular and cerebrovascular diseases are the leading causes of morbidity in the elderly and are responsible for at least one in every 3 deaths globally.1,2 Hence, identifying the pathophysiological mechanisms contributing to age-related vascular decline is key to the prevention and treatment of these disorders and has the potential to exert a major impact on human health.3 In this regard, emerging experimental and epidemiological evidence indicates that aging is associated with a systemic decline in nicotinamide adenine dinucleotide (NAD+), which is an essential coenzyme in cellular metabolism.4,5,6 Accordingly, dysregulated NAD+ metabolism has been implicated in the age-related functional decline of various tissues and organs, including those composing the circulatory system.7,8,9 Restoring NAD+ homeostasis through supplementation of its precursors (also known as vitamin B3 derivatives), including nicotinamide riboside (NR), nicotinamide (NAM, also named niacinamide) and nicotinic acid (NA), in addition to the NAD+ intermediate nicotinamide mononucleotide (NMN), mitigates age-associated diseases in many clinically relevant animal models.4,10,11 These findings have transformed views on NAD+ metabolism and shaped further research activities with the aim to gain a deeper understanding of why NAD+ levels decline during aging, and how that decline affects body functions in health and disease.
In the context of vascular disease, NAD+ metabolism is increasingly recognized as an attractive actionable target. Replenishment of NAD+ in vascular cells—either by the stimulation of NAD+ synthesis or the inhibition of its degradation—protects against age-related arterial stiffening and endothelial dysfunction8 and improves conditions characterized by abnormal blood flow, such as ischemia/reperfusion injury.12 This is particularly important as these vascular pathologies often co-exist. Indeed, hypertension is a risk factor for atherosclerosis, and coronary artery disease often occurs via thrombotic complications of atherosclerotic plaques. Although intense research in recent years has revolutionized our view on NAD+ biology and generated new and evolving concepts related to the biosynthesis, transport, catabolism, and bioactivity of NAD+ in health and disease,13 we are only beginning to understand the pathophysiological implications of dysregulated vascular NAD+ metabolism. In this review, we summarize how disruption of NAD+ metabolism affects vascular function, which vasoprotective mechanisms are regulated by NAD+, and how the restoration of NAD+ homeostasis mitigates common vascular diseases, including hypertension, atherosclerosis, coronary artery disease, and aortic aneurysm. For a comprehensive overview of NAD+ biochemistry and metabolism, as well as of its role in other organs, including the heart, we refer readers to other relevant in-depth reviews.5,14,15,16
VASOPROTECTIVE MECHANISMS OF NAD+
1. NAD+ suppresses vascular inflammation
Epidemiological and experimental studies suggest that old age and chronic systemic low-grade inflammation (i.e., inflammaging) are the principal drivers of cardiovascular and cerebrovascular diseases.17,18 Very recently, Covarrubias and colleagues demonstrated a causal link between age-dependent decrease in NAD+ and persistent low-grade inflammation.19 The authors found that senescent cells promote the proliferation of M1-like mouse macrophages expressing high levels of CD38, which is a major NAD+-consuming enzyme in mammals.20 Accordingly, high CD38 levels contribute to the age-dependent decline of NAD+, at least in metabolically active tissues, such as the liver and adipose tissue. Of note, CD38 is strongly expressed in endothelial cells,21 as well as in human macrophages and monocytes in inflammatory conditions22 and in blood samples from aged individuals.23 Therefore, reduced NAD+ consumption, increased NAD+ synthesis, or a combination of both have been proposed as plausible strategies to attenuate age-induced inflammatory processes (Fig. 1). In support of this idea, chronic supplementation of the NAD+ precursor NAM reduced inflammation and improved many aspects of healthspan in aged mice fed a high-fat diet,10 likely by promoting the differentiation of monocytes to macrophages with reduced pro-inflammatory phenotype.24 Interestingly, similar anti-inflammatory actions have also been reported for niacin, which stimulated M2 polarization of peripheral monocytes in vitro, both in mice and humans.25 In another study, NAM reduced renal mRNA levels of inflammatory markers, which was associated with lowered arterial blood pressure in hypertensive mice with genetically or pharmacologically induced dysfunction of endothelial nitric oxide synthase (eNOS).26 Since NAM administration is safe in humans,27 this observation merits further evaluation in the subgroup of patients with hypertension who have an impaired eNOS system, in whom the inhibition of inflammation might be particularly efficient.
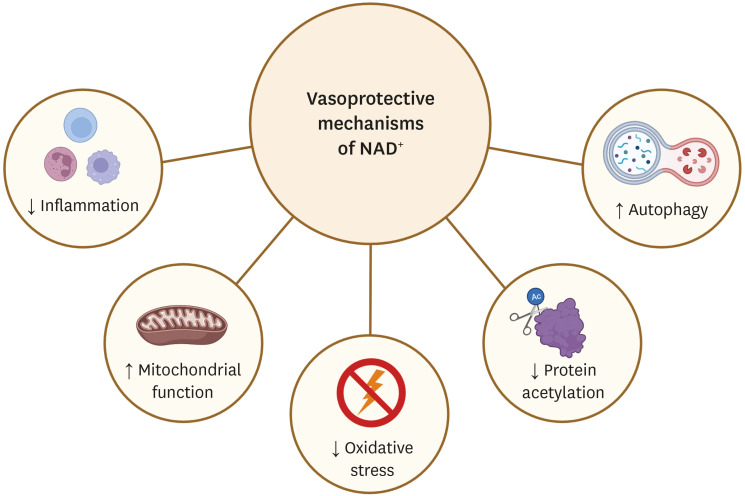
Fig. 1. Elevating cellular NAD+ activates various vasoprotective mechanisms. Pharmacological modulation of NAD+ levels via NAD+ precursors or inhibitors of NAD+-consuming enzymes reduces chronic low-grade inflammation and protein acetylation, reactivates autophagy and mitochondrial biogenesis, and enhances oxidative metabolism in vascular cells. Up-arrows indicate increases, down-arrows indicate decreases. The clip art included in this figure was created with BioRender.com.
NAD+, nicotinamide adenine dinucleotide.
Similar anti-inflammatory effects were reported for alternative NAD+ precursors, such as NR and NMN, which inhibit interleukin-1β and tumor necrosis factor-α (TNF-α)-induced inflammation in cultured endothelial cells and improve endothelial dysfunction in aortic rings ex vivo.28 Interestingly, NMN reversed endothelial dysfunction and inflammation by extracellular conversion to NR via CD73, an ecto-5′-nucleotidase localized on the luminal surface of endothelial cells, whereas the NR-induced vasoprotective effects were CD73-independent.28 Although the precise mechanisms underlying the vasoprotective effects of NR remain elusive, endothelial SIRT1 (an NAD+-dependent lysine deacetylase) appears to be involved,28 likely through the modulation of eNOS activity.29 In another study, the activation of SIRT1 with SRT1720 was shown to ameliorate vascular endothelial dysfunction in aged mice by reducing arterial inflammation and oxidative stress, but these effects were linked to elevated COX-2 signaling rather than increased nitric oxide (NO) production.30 Regardless, NR also inhibits TNF-α signaling, thereby lowering systolic blood pressure and, at least in part, reducing multimorbidity and premature aging in mice with dysfunctional mitochondria owing to mitochondrial transcription factor A (TFAM) deficiency in T cells.31 In sum, considering that inflammation renders the vasculature prone to dysfunction, pharmacological strategies to increase vascular NAD+ concentrations might constitute a promising approach to prevent inflammatory-mediated endothelial dysfunction and consequent vascular disease.
2. NAD+ attenuates vascular oxidative stress and mitochondrial dysfunction
The depletion of intracellular NAD+ levels impedes mitochondrial fatty acid β-oxidation and oxidative phosphorylation, underscoring the critical role of NAD+ in maintaining mitochondrial function in the vasculature and beyond. Preclinical studies have demonstrated that supplementation of different NAD+ precursors reduces mitochondrial oxidative stress and reverses related vascular dysfunction (Fig. 1).32,33 For example, chronic NMN supplementation improved NO-related endothelial dysfunction and decreased aortic pulse wave propagating velocity (a proxy of arterial stiffness) by attenuating collagen accumulation and increasing elastin content in aged mouse arteries.32 These NMN-induced vasoprotective effects correlated with reduced oxidative stress and increased SIRT1 activity. Similarly, NMN administration normalized mitochondrial production of reactive oxygen species (ROS) and improved mitochondrial bioenergetics in primary cerebrovascular endothelial cells of old mice.34 Additionally, the neurovascular protective effects of NMN were accompanied by the transactivation of genes involved in mitochondrial rejuvenation, anti-inflammatory, and anti-apoptotic pathways.35 NMN, especially in combination with exogenous hydrogen sulfide, also improved skeletal muscle blood flow by attenuating the age-associated reduction in capillary density (i.e., microvascular rarefaction) through the activation of vascular endothelial growth factor signaling in a SIRT1-dependent manner.8 In the same vein, many health benefits of SIRT1 activation are related to improved mitochondrial function. Indeed, similar to NAD+ precursors, SIRT1-activating compounds such as resveratrol and SRT1720 induced mitochondrial biogenesis,36 attenuated mitochondrial oxidative stress,37,38 activated the antioxidant defense response39 and inhibited apoptosis in endothelial and vascular smooth muscle cells from old mice and rats.40 NMN also activates SIRT3,41 which deacetylates numerous mitochondrial proteins (e.g., superoxide dismutase 2, SOD2), thereby reducing vascular oxidative stress.42,43 In the aged mouse aorta, NMN reverted changes in the microRNA expression profile, which correlated with enhanced mitochondrial biogenesis.44 However, future studies are required to explain the relationship between microRNAs and age-related vascular diseases, and to delineate the mechanistic role of microRNA gene expression regulatory networks in the vasoprotective effects of NMN.
Another strategy for raising intracellular NAD+ levels is to inhibit its degradation by blocking NAD+-consuming enzymes,5 such as the cyclic ADP-ribose synthase CD38, which is considered the principal NADase in mammalian tissues.20 CD38 is highly expressed in the endothelium,21 where it is strongly activated by hypoxia-reoxygenation, leading to loss of eNOS-mediated NO generation and exaggerated eNOS uncoupling. Of note, CD38 is inhibited by the naturally occurring flavonoid apigenin, resulting in elevated NAD+ and decreased global acetylation in cell cultures.45 In old mice, apigenin rescued endothelial dysfunction, which was associated with increased NO bioavailability, normalized arterial ROS, and reduced oxidative stress.46 Additionally, in vitro, apigenin prevented the formation and accumulation of foam cells, which are known to propagate the development of atherosclerotic lesions, and alleviated age-associated aortic stiffening, reducing adverse remodeling of the extracellular matrix and suppressing vascular inflammation. Considering that apigenin is a Food and Drug Administration-approved dietary supplement, these preclinical findings provide an experimental basis for future translational studies testing the potential of this CD38 inhibitor to improve arterial dysfunction and reduce vascular disease risk in the elderly.
In sum, considering the key role of mitochondrial homeostasis in maintaining vascular health,47 age- and disease-related depletion of NAD+ might have severe consequences on mitochondrial redox balance with implications for vascular disease risk.
3. NAD+ activates vascular autophagy
In recent years, substantial progress has been made towards better understanding the connection between cardiovascular dysfunction and autophagy.48,49 As is also true for several other tissues, autophagic flux is reduced in the vasculature of aged mice and humans.50 Consistent with this finding, genetic manipulation studies have demonstrated that reducing or completely blocking autophagy by inactivating essential autophagy genes in vascular endothelial or smooth muscle cells markedly deteriorates vascular physiology.51 For instance, mice harboring a vascular smooth muscle cell-specific Atg7 deficiency showed premature defects in calcium homeostasis, as well as abnormal vascular reactivity and smooth muscle cell contractility.52 Along similar lines, mice with endothelial cell-specific deletion of Prkaa—an α catalytic subunit of AMP-activated protein kinase that regulates mitochondrial biogenesis, function, and turnover—displayed reduced autophagy, which was sufficient to cause aortic endothelial dysfunction and mitochondrial fragmentation.53 These findings indicate that manipulation of autophagy may impair vascular functions and that intact autophagic responses are required for vascular homeostasis. Increasing evidence suggests that enhancing NAD+ availability stimulates autophagic flux to protect from ischemic vascular diseases, including in the heart and brain.54,55 For example, NAD+ treatment preserved coronary microvascular density, reduced infarct size, and improved postischemic vascular repair by rescuing coronary microvascular endothelial cells upon ischemia/reperfusion damage in the rat heart.12 This microvascular protection was mediated, at least in part, through TFEB-induced lysosomal autophagy, which was also reported to stimulate postischemic angiogenesis in a mouse hindlimb ischemia model.56
Mechanistically, recent studies have identified that sirtuins play an important role in mediating NAD+-induced autophagy.57 In fact, SIRT1 can induce autophagy by epigenetic mechanisms, namely through histone modifications that influence autophagy-related gene expression and by post-translational mechanisms through the action of forkhead box transcription factors.58 Moreover, SIRT1 directly deacetylates several essential proteins of the autophagy machinery, including the products of the autophagy genes Atg5, Atg7, and Atg8.59,60 Since NAD+ might affect multiple other downstream targets relevant for autophagy, further research is warranted to obtain a full understanding of the mechanisms underlying NAD+-dependent autophagy activation. Of note, SIRT6 has been recently implicated in autophagy activation and reduced macrophage foam cell formation, suggesting that yet another NAD+-responsive sirtuin might protect against atherosclerosis progression.61
Taken together, emerging evidence underscores that autophagic flux plays a major role in the NAD+-induced maintenance of vascular homeostasis (Fig. 1). Conversely, defective autophagy appears to be a common cause of vascular aging and the development of associated pathologies. However, further studies testing causality and dose–response relationships are required to confirm whether and to which extent autophagy underlies NAD+-induced vasoprotection. Future research is required to delineate the role of autophagy subroutines, such as mitophagy (i.e., mitochondrial autophagy). This is particularly important because age-dependent impairment of mitophagy might cause endothelial dysfunction, metabolic imbalance, inflammation, and senescence, which may collectively contribute to atherosclerosis.62 Hence, future studies focusing on the mitochondrial axis are needed to elucidate the role of NAD+ in decelerating the manifestation of age-related diseases.63
TARGETING NAD+ METABOLISM IN VASCULAR DISEASE
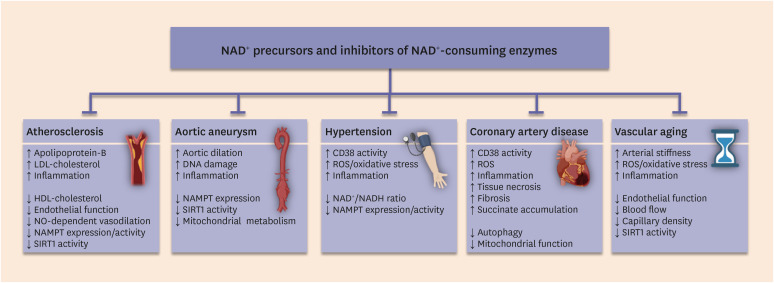
A decrease in NAD+ is centrally involved in cardiovascular diseases, such as cardiac ischemia in the context of coronary artery disease.64,65,66 Disrupted NAD+ homeostasis also accompanies other human vascular pathologies, including hypertension,67 atherosclerosis,68 and aortic aneurysm,69 and restoration of NAD+ content via different NAD+ precursors has yielded promising results in animal models (Fig. 2 and Table 1).
Fig. 2. Targeting NAD+ metabolism to treat vascular diseases. Restoration of NAD+ content through different NAD+ precursors and inhibitors of NAD+-depleting enzymes is an emerging therapeutic strategy to improve hallmarks of various vascular disorders. Up-arrows indicate increases, down-arrows indicate decreases. The clip art included in this figure was created with BioRender.com.
NAD+, nicotinamide adenine dinucleotide; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NO, nitric oxide; NAMPT, nicotinamide phosphoribosyltransferase; ROS, reactive oxygen species.
Table 1. Vascular disorders, against which NAD+ precursors show beneficial effects.
| Vascular disease | NAD+ precursor | Experimental setting | Effects | Ref. |
|---|---|---|---|---|
| Hypertension | NAM | Dahl salt-sensitive rats | Reduced high blood pressure | 26 73 |
| eNOS−/− mice | Improved endothelial function | |||
| L-NAME-treated mice | Reduced inflammation | |||
| NA | Nephrectomized rats | Reduced high blood pressure | 76 | |
| Reduced inflammation | ||||
| Reduced oxidative stress | ||||
| NR | Middle-aged and old humans | A trend towards reduced blood pressure and aortic stiffness | 72 | |
| Atherosclerosis | NA | Humans and (APOE) mice with atherosclerosis | Decreased LDL cholesterol | 83 132 |
| Increased HDL cholesterol | ||||
| NAM | APOE mice | Improved protection against ApoB-containing lipoprotein oxidation | 79 | |
| Reduced inflammation and atherogenesis | ||||
| Coronary artery disease | NAD+ | A swine model of myocardial ischemia-reperfusion | Decreased necrosis, fibrosis and stiffness | 103 |
| Improved recovery of cardiac function | ||||
| Reduced inflammation | ||||
| NAM | Ischemia-reperfusion injury in rats | Decreased myocardial infarction size | 104 133 | |
| Reduced oxidative stress | ||||
| NR | Ischemia-reperfusion injury in mice | Improved ejection fraction and reduced infarct size | 105 | |
| NMN | Ischemia-reperfusion injury in mice and aged rats | Smaller infarct size | 106 107 | |
| Ameliorated cardiac function | ||||
| Improved ROS and mitochondrial membrane potential | ||||
| Aortic aneurysm | NA and NAM | Calcium chloride- and angiotensin II-treated mice | Decreased formation of abdominal aortic aneurysms | 112 |
| Reduced inflammation and immune cell infiltration | ||||
| Lower matrix degradation | ||||
| Vascular aging | NMN | Naturally aged mice | Reduced arterial stiffness | 32 34 |
| Cerebromicrovascular protection | ||||
| Improved neurovascular coupling | ||||
| Improved endothelial function | ||||
| Lower oxidative stress |
NAD+, nicotinamide adenine dinucleotide; NAM, nicotinamide; eNOS, endothelial nitric oxide synthase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; L-NAME, N[ω]-nitro-l-arginine methyl ester; ROS, reactive oxygen species; NA, nicotinic acid; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide.
1. Hypertension
Currently, one in 3 adults worldwide has hypertension,70 and the global epidemic of hypertension is expected to rise owing to the demographic shift towards ever more aged populations. Although hypertension is a modifiable risk factor for cardiovascular disorders, including ischemic and hemorrhagic stroke, coronary and valvular heart diseases, as well as heart or renal failure,71 hypertension management has remained a major public health challenge. This has been largely attributed to the multifactorial nature of hypertension and its complex pathogenesis, which remains incompletely understood.
In this respect, NAD+ metabolism has emerged as a potential therapeutic target for hypertension and associated vascular dysfunction.72 The expression of the rate-limiting enzyme in NAD+ biosynthesis, nicotinamide phosphoribosyltransferase (NAMPT), has been recently found downregulated both in mice and humans with hypertension,67 implying that restoring NAD+ homeostasis might have an anti-hypertensive effect. In support of this possibility, systemic overexpression of NAMPT was found to protect against angiotensin II-induced hypertension in a SIRT1-dependent manner by reducing ROS production in aortic endothelial cells and vascular smooth muscle cells.67 By contrast, mice with systemic Nampt haploinsufficiency displayed elevated blood pressure and ROS levels in response to angiotensin II infusion, and the administration of recombinant human NAMPT reversed this effect.67 Along the same lines, increased NAMPT-mediated NAD+ biosynthesis upon NAM supplementation prevented the increase in systolic blood pressure induced by the non-selective NOS inhibitor, L-NAME (N[ω]-nitro-l-arginine methyl ester). Consistently, NAM lowered the elevated systolic blood pressure in Dahl salt-sensitive rats as well as in eNOS−/− mice, likely through reduced inflammation.26,73 In pregnant mice with pre-eclampsia, NAM lowered arterial blood pressure through the reduction of cADPR,74 a product of CD38-mediated NAD+ consumption that regulates calcium signaling.75 Similarly, NA attenuated high blood pressure, inflammation, and oxidative stress in rats with chronic kidney disease,76 whereas NR treatment lowered systolic blood pressure in mice with T cell-specific TFAM deficiency while restoring the NAD+/NADH ratio.31 These findings were corroborated in a recent human phase I study showing that NR supplementation led to a mild reduction in blood pressure and aortic stiffness in middle-aged and old, otherwise healthy, individuals.72
Pharmacological and genetic CD38 inhibition, which increases cellular NAD+, significantly attenuated angiotensin II-induced hypertension and vascular remodeling in mice.77 CD38−/− mice and WT mice treated with NMN or the CD38-specific inhibitor 78c displayed lower blood pressures, reduced vascular media thickness, media-to-lumen ratio, and collagen deposition, as well as normalized elastin expression. Moreover, NMN supplementation and CD38 inhibition alleviated the senescence of vascular smooth muscle cells.77
In aggregate, restoring NAD+ levels by supplementation of NAD+ precursors or CD38 inhibitors is being explored as an adjuvant therapy for hypertension. However, large clinical trials are warranted to provide conclusive evidence as to whether incrementation of NAD+ levels provides tangible benefits.
2. Atherosclerosis
Atherosclerosis is associated with endothelial dysfunction, the recruitment of pro-inflammatory M1-like macrophages, and the degeneration of smooth muscle cells in the vasculature. During early atherosclerosis, macrophages differentiate into foam cells by ingesting modified low-density lipoprotein cholesterol, which in turn promotes the formation of atherosclerotic plaques. NAD+-dependent activation of SIRT1 has been shown to have beneficial effects on all these cell types and to protect against atherosclerosis.78 For instance, dietary NAM supplementation in ApoE-deficient mice prevented atherogenesis and improved protection against ApoB-containing lipoprotein oxidation and aortic inflammation.79 Of note, the protective effects of NAM might also be achieved by increasing the plasma concentration of N-methyl-nicotinamide (methyl-NAM, a metabolic product of NAM). In fact, epidemiological studies have demonstrated that methyl-NAM may exert anti-thrombotic and anti-inflammatory effects on the endothelium by promoting NO-dependent vasodilation, thereby improving endothelial function.80 In the same vein, methyl-NAM was found to be atheroprotective in ApoE−/−/Ldlr−/− mice, which displayed improved endothelial dysfunction associated with reduced atherosclerotic plaque area, plaque inflammation, and cholesterol content in the brachiocephalic artery.81 Similarly, the aortas of ApoE−/− mice fed methyl-NAM and a high-fat, high-cholesterol diet exhibited improved endothelium-dependent vasorelaxation.82 Mechanistically, this effect was attributed, at least in part, to decreased asymmetric dimethylarginine concentrations due to the induction of dimethylarginine dimethylaminohydrolase 2.82
Niacin (nicotinic acid, NA) is a well-known lipid-lowering compound that reduces apolipoprotein-B-containing lipoproteins while raising the levels of atheroprotective high-density lipoproteins.83 Notably, the anti-dyslipidemia effects of niacin were known long before the discovery of statins and the link between NAD+ and sirtuins.84 Although niacin has a potent anti-atherogenic effect, it failed to reduce the residual cardiovascular risk in patients receiving statins.85,86,87 Furthermore, a recent meta-analysis revealed that the combinatory administration of niacin and standard lipid-lowering therapy using statins might be associated with adverse effects on survival.88,89 It is important to note, however, that niacin monotherapy was previously shown to reduce mortality.90 Regardless, niacin-treated patients exhibit poor compliance due to an unpleasant flushing side effect; thus, niacin is no longer recommended or only prescribed to statin-intolerant patients.91,92 Although more tolerable formulations of NA have been developed,93 available preclinical evidence on the role of NAD+, and especially NAMPT—the rate-limiting enzyme of NAD+ salvage biosynthesis94—in atherosclerosis is rather scarce and contradictory. On the one hand, leukocyte-specific overexpression of NAMPT attenuated atherosclerotic plaques in low-density lipoprotein receptor-deficient (Ldlr−/− ) mice.95 Additionally, a reduced number of atherosclerotic plaques in Ldlr−/− mice coincided with increased macrophage resistance to apoptosis and skewed polarization towards a more anti-inflammatory M2 phenotype.95 On the other hand, systemic NAMPT inhibition has been reported to exert an atheroprotective effect,96 while global NAMPT overexpression aggravated atherosclerosis in ApoE−/− mice.97
In light of the inconsistent findings regarding the impact of NAD+ on atherosclerosis, more studies geared towards targeted and cell type-specific interventions must examine the general importance of maintaining cellular NAD+ levels in atherosclerosis and delineate the specific role of NAMPT in atherogenesis. Furthermore, it is important to mention that, despite the initial discouraging clinical effects of niacin on patients with cardiometabolic risk, current research is shifting towards other NAD+ precursors, which do not necessarily reduce lipid levels, but arguably possess higher NAD+ repletion capacity than niacin.5 In view of the pleiotropic actions of NAD+, it would not be surprising if the possible (cardio)vascular benefits of NAD+ precursors might be uncoupled from the correction of hyperlipidemia. In fact, as we discuss in the next sections, various studies have consistently shown that, both in the presence or absence of adiposity, NAD+ repletion counteracts life-threatening vascular disorders.
3. Coronary artery disease
Experimental models of coronary artery disease induced by transient coronary artery ligation have clearly demonstrated that myocardial ischemia is associated with NAD+ depletion.64,65,66 One possible explanation for the NAD+ decline in postischemic hearts is the over 50-fold higher CD38 activity in endothelial cells than in cardiomyocytes.21 This CD38 overactivation appears to be an important cause of postischemic endothelial dysfunction, suggesting that CD38 is an actionable target to prevent this dysfunction in unstable coronary syndrome.98 In this regard, both genetic deletion and pharmacological inhibition of CD38 by luteolinidin and the thiazoloquin(az)olin(on)e 78c protected against ischemia/reperfusion injury, preserved contractile function, enhanced coronary flow, and decreased infarct size.99,100,101 Similarly, NAD+ administration (10 mg/kg body weight [BW] per day intraperitoneally) lowered the ischemic accumulation of succinate and ROS, which were both associated with reduced cardiac injury in isolated rat hearts.102 Furthermore, intravenous NAD+ administration (20 mg/kg BW before reperfusion) attenuated ischemic cardiac tissue necrosis, fibrosis, and inflammation upon reperfusion of the transiently occluded left anterior descending coronary artery in pigs.103 NAD+ precursors, including NAM, NR, and NMN exert similar protective effects. For instance, dietary administration of NAM (0.5 g/kg diet) reduced infarction size in an ex vivo model of myocardial ischemia-reperfusion.104 Mice treated with the alternative precursor NR (100 mg/kg BW) also exhibited improved cardiac function and smaller infarcts.105 The NAD+ intermediate NMN consistently normalized alterations in the mitochondrial membrane potential and ROS levels associated with ischemic myocardial injury in aged rats.106 NMN not only protected against ischemic injury, but also had beneficial effects against coronary reperfusion injuries.107 Of note, the NAD+-induced protective effects in coronary artery disease models coincide with the reactivation of autophagy flux.12,54 However, more studies are required to elucidate whether autophagy is protective or detrimental in this setting.108
Accumulating evidence implicates NAD+ deficiency in coronary artery disease and associated cardiac events. Like several CD38 inhibitors, various NAD+ precursors have been shown to improve postischemic endothelial dysfunction and, thus, protect against experimental ischemia/reperfusion injury of the myocardium. Therefore, future clinical trials should examine whether treatment with NAD+ precursors may exert beneficial effects in patients with acute coronary syndrome. Furthermore, the harmful effects of CD38 overactivation in the postischemic heart highlight the need for further research to delineate the mechanisms involved.98 In this respect, future studies should focus on the mechanisms of CD38 activation in response to hypoxia-reoxygenation in endothelial cells, which display the highest CD38 expression among all major cardiac cell types.19
4. Aortic aneurysm
Apart from lowering lipid levels, NA mediates potent anti-inflammatory effects on human endothelial and immune cells.109,110 In this regard, persistent adventitial and medial infiltration of immune cells contributes to the pathogenesis of abdominal aortic aneurysms. Consistently, NA (0.3% w/v in the drinking water)111 reduced immune cell infiltration and matrix degradation, thereby protecting against abdominal aortic aneurysm formation in mice subjected to calcium chloride or angiotensin II infusion.112 Interestingly, NAM (0.4% w/v), which ostensibly does not exert significant lipid-lowering effects,113 also protected against abdominal aortic aneurysms.112 Notably, NAM-treated mice exhibited increased SIRT1 activity, and co-administration of the SIRT1 inhibitor EX-527 effectively abolished the vasoprotective effects of NAM.112 Similarly, the alternative NAD+ precursor NR has been recently shown to improve mitochondrial metabolism, aortic function, and aortic diameter, thereby reversing Marfan syndrome-associated aortic aneurysms in a relevant mouse model.114 In support of a causal role of NAD+ in the development of aortic dilation and aneurysms, mice with smooth muscle cell-specific knockout of Nampt exhibited increased susceptibility to angiotensin II-induced aortic aneurysms, as denoted by exaggerated medial hemorrhage and dissection.69 In human subjects with thoracic aortic aneurysms, unrepaired DNA strand breakages were detected in smooth muscle cells, and this damage was particularly enriched in smooth muscle cells with the lowest NAMPT expression.69
In sum, supplementation of NAD+ precursors improves aortic wall structure and function as it protects or even reverses aortic aneurysms in mice. Various mechanisms, including reduced pro-inflammatory signals, enhanced mitochondrial metabolism, and sirtuin activation may mediate these vasoprotective effects. Taking into account that the aortic diameter in patients with aortic aneurysm inversely correlates with NAMPT expression in smooth muscle cells,69 future clinical studies should explore whether NAD+ precursors may improve the course of this disease in humans.
5. Aging and related vascular decline
The integrity of most organs and tissues relies on an ample and functional microcapillary network that provides transport routes for the circulation of cells, oxygen, nutrients, and metabolic waste products.115 Recent observations suggest that the organ-specific loss of vascular abundance is an important characteristic of aging tissues in mice and humans.116 On the one hand, impaired vascular function and structure comprises stiffening of the large elastic arteries, intimal thickening, and media calcification, which are mediated by increases in oxidative stress, inflammation, and vascular smooth muscle tone. On the other hand, vascular dysfunction also encompasses a decrease in the number and function of endothelial cells at the interface between circulating blood and tissues. Endothelial dysfunction, which is characterized by reduced NO production and bioavailability, as well as an imbalance between the vasoconstrictors and vasodilators derived from the endothelium, leads to local dysregulation of the vascular tone and, thus, to reduced blood flow to tissues, culminating in end-organ damage.117,118
A decline in the cellular NAD+ pool is closely related to cellular aging, whereas an increase in NAD+ synthesis or a decrease in its degradation delays aging in various organ systems.15 Similar geroprotective effects have been reported for the vascular system. For example, aged mice treated with NMN, although not suffering from hyperlipidemia or hypertension, exhibited several vascular benefits, including restored endothelial-dependent vascular relaxation, reduced arterial stiffness, and reduced oxidative stress.32 In another study, NMN administration to naturally aged mice conferred cerebromicrovascular protective effects, which led to improved neurovascular coupling and cognitive function.34 In addition, NMN supplementation improved blood flow and increased treadmill endurance in old mice by promoting a SIRT1-dependent increase in capillary density in the skeletal muscle.8 Since adequate blood flow is vital to every tissue and organ, not only skeletal muscle, it will be important to test whether increased endothelial NAD+ availability stimulates angiogenesis and improves blood flow in the aging brain and heart.
NAD+ METABOLISM AS A TARGET TO IMPROVE VASCULAR HEALTH IN HUMANS
Despite extensive preclinical evidence on the benefits of NAD+, clinical studies still lag behind. In fact, only a handful of trials have been concluded, and these trials mainly focused on safety, as well as on the ability of NAD+ precursors to increase NAD+ bioavailability. Besides NA, which has been historically tested for its lipid-lowering impact, NR is the most common precursor evaluated in ongoing trials with vascular endpoints (Table 2). NR supplementation appears to be safe, well-tolerated, has no apparent side effects, and is effective in increasing whole-blood NAD+ levels.72 Importantly, in healthy middle-aged and older adults, NR tended to lower blood pressure and reduce aortic stiffness.72 In addition, NR improved mitochondrial fitness and dampened activation of the NLRP3 inflammasome in circulating leukocytes isolated from healthy volunteers.119 However, not all studies support the therapeutic potential of NR supplementation. For example, NR administration failed to ameliorate endothelial dysfunction, as determined by brachial artery flow-mediated dilation in middle-aged and older adults.72 Similarly, oral NR failed to improve blood flow, mitochondrial bioenergetics, and metabolism of skeletal muscle, although it did succeed in reducing the levels of circulating inflammatory cytokines in 70- to 80-year-old men.120 Future large-scale trials are needed to provide conclusive evidence on the putative health benefits provided by NR.
Table 2. Ongoing NAD+ clinical trials with vascular endpoints.
| NAD+ precursor | Dose | Condition (demographics) | Trial design and phase | No. of recruited participants | Vascular endpoint(s) | Expected completion | Identifier |
|---|---|---|---|---|---|---|---|
| NAM | 2,500 mg/day | Early-onset pre-eclampsia (age: 18–55 years; gender: women) | Single group, open-label (phase 2) | 25 | Changes in mean blood pressure | July 2020 | NCT03419364 |
| NA | Up to 2,000 mg/day | Healthy volunteers (age: 18–99 years; gender: men and women) | Single group, open-label (phase 2) | 24 | Changes in lipoprotein composition and function as well as vascular compliance | July 2020 | NCT02322203 |
| NR | 1,000 mg/day | Hypertension (SBP >130 mmHg; age: 65–105 years; gender: men and women) | Randomized, placebo-controlled, double-blind (phase 1) | 74 | Changes in systolic blood pressure and arterial stiffness | May 2021 | NCT04112043 |
| 1,000 mg/day | Moderate to severe chronic kidney disease (age: 35–80 years; gender: men and women) | Randomized, placebo-controlled, double-blind (phase 2) | 118 | Changes in aortic stiffness and arterial blood pressure | September 2024 | NCT04040959 | |
| 1,000 mg/day | (Pre)hypertension (SBP: 120–139 mmHg; age: 50–79 years; gender: men and women) | Randomized, placebo-controlled, double-blind (phase 2) | 118 | Changes in systolic blood pressure and arterial stiffness | December 2023 | NCT03821623 | |
| 1,000 mg/day | Peripheral artery disease (age: >18 years; gender: men and women) | Randomized, placebo-controlled, double-blind (phase 3) | 90 | Effects on walking performance, physical activity, quality of life, and skeletal muscle phenotype | April 2022 | NCT03743636 | |
| NMN | 300 mg/day | Middle-aged and old healthy volunteers (age: 40–65 years; gender: men and women) | Randomized, placebo-controlled, double-blind (phase: N/A) | 66 | Safety and efficacy in reducing systolic and diastolic blood pressures | March 2021 | NCT04228640 |
| 400 mg/day | Healthy volunteers (age: 30–60 years; gender: men and women) | Single group, open-label (phase: N/A) | 20 | Tolerability, pharmacodynamics and cardiovascular effects, including arterial blood pressure; heart rate, blood lipids | October 2021 | NCT04862338 | |
| 800 mg/day | Hypertension (SBP: 140–159 mmHg and DBP: 90-99 mmHg; age: 18–65 years; gender: men and women) | Randomized, single (assessor)-blind (phase 4) | 20 | Changes in flow-mediated dilation, pulse wave velocity, as well as systolic and diastolic blood pressures | July 2022 | NCT04903210 |
We searched the US clinical trial registry (https://www.clinicaltrials.gov/) using terms “nicotinamide” and “vascular disease” for recently completed or ongoing clinical trials of NAD+ supplementation that have yet to publish results (from database inception to January 2022).
NAD+, nicotinamide adenine dinucleotide; NAM, nicotinamide; NA, nicotinic acid; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide; DBP, diastolic blood pressure; SBP, systolic blood pressure; N/A, not available.
Observational findings indicate that a diet rich in NAM (and NA) is linked to lower blood pressure and a reduced risk of cardiac mortality in humans.73 In view of the good tolerability of NAM in relatively high doses over months or even years,121 it is conceivable to examine the therapeutic utility of NAM as an adjuvant therapy for hypertension. Although 2 recent clinical studies have shown that NMN supplementation is safe and can increase NAD+ bioavailability in blood,122,123 the impact of NMN supplementation on vascular health has not yet been reported. Results from ongoing studies examining the effects of NMN on vascular function and arterial blood pressure in older adults and individuals with hypertension (Table 2) are awaited to determine whether NMN has the potential to improve vascular health.
Despite the promising vasoprotective effects of CD38 inhibitors, which have vasorelaxant and antioxidant properties in experimental models of cardiac ischemia/reperfusion injury,99 only a few clinical studies have so far been completed. The flavonoids epicatechin and quercetin (both abundant in tea) were tested in middle-aged and old men and women with increased systolic blood pressure.124 Supplementation with both CD38 inhibitors failed to improve flow-mediated dilation, arterial stiffness, NO bioavailability, or blood lipid profiles. Similarly, the polyphenol and CD38 inhibitor quercetin failed to increase endothelial function in healthy men with the APOE genotype.125 However, quercetin lowered postprandial systolic blood pressure, which was associated with decreased postprandial triacylglycerol concentrations in parallel with increased high-density lipoprotein cholesterol concentrations. In light of the recent finding that CD38 hyperactivation might drive NAD+ depletion in aged mice,19 further research efforts are necessary to determine whether specific CD38 inhibition may alleviate age-related vascular remodeling. In support of this idea, new preclinical evidence suggests that suppressing vascular smooth muscle cell senescence by means of CD38 inhibitors delays vascular aging.77
In aggregate, the available human studies suggest that oral administration of various NAD+ precursors is safe and modestly increases levels of NAD+ or its metabolites, albeit to varying degrees and in a tissue-specific fashion.126 Hence, well-powered and carefully designed clinical trials should determine whether chronic supplementation of NAD+ precursors, especially those with high NAD+-increasing capacity, may improve vascular health, perhaps independently from lipid-lowering effects.126
CONCLUSION AND FUTURE PERSPECTIVES
Although NAD+ studies focusing on the vasculature have been largely overshadowed by cardiac-centric studies, the significant benefits of restoring NAD+ homeostasis in animal models of vascular disease have spurred interest in the therapeutic potential of NAD+ at the clinical level. However, many difficulties and challenges related to the administration of NAD+-regenerative therapeutics must be resolved to translate the experimental findings to medical practice. For this, future large-scale clinical studies with long-term follow-up that extends beyond treatment discontinuation are needed. These trials should consider adapting drug doses from rodent studies to human studies while considering major differences in metabolic rate and body surface area between mice and humans, but rather similar cellular NAD+ turnover rates in both species.127 Another important question is how NAD+ precursors are best administered (i.e., at which dose, formulation, and route of administration, and at what time of day, considering chronobiological variations in NAD+ levels).128 On the one hand, utilizing the human-equivalent dosage of NAD+ precursors could have a more favorable and consistent effect on vascular-related endpoints. On the other hand, high doses of NAD+ precursors can cause hepatotoxicity and other adverse effects in patients,16,129 emphasizing the necessity to rigorously measure therapeutic and toxicological endpoints in healthy and diseased states. Another important consideration is the standardization and development of reliable biomarkers of NAD+ metabolism, including the quantitation of NAD+ precursors and metabolites in the circulatory system as well as the proxies of their bioactivity, which can encompass specific patterns of protein acetylation, autophagy, and mitophagy. Solving these current limitations will be critical for designing future NAD+-centered therapeutic interventions in patients.
Based on current evidence, both NR and NMN seem promising candidates for boosting NAD+ levels in vascular cells. In addition to bypassing the rate-limiting step in NAD+ synthesis, another advantage of administering NR or the NAD+ intermediate NMN might reside in the fact that both precursors avoid the negative feedback exerted by NAM on sirtuin deacetylases (which typically produce NAM as an end product). However, recent in vivo data challenge this long-held view, as all 3 precursors (i.e., NAM, NMN, and NR) exerted ambiguous effects on global protein acetylation in various tissues including the heart41,73,130,131 and liver.10 Furthermore, a recent study has demonstrated that almost all NAD+ precursors are metabolized to NAM before reaching peripheral tissues,127 implying that the inhibitory feedback exerted by NAM might occur irrespective of the chemical nature of the NAD+ precursor that has been administered. Regardless, this is an emerging area of investigation, and future head-to-head comparisons must elucidate the exact (and perhaps subtle) effects of different NAD+-increasing therapeutics on protein acetylation, which might depend on the precise (vascular) cell types and subcellular compartments where target proteins reside. Other open questions involve the cell type-specific mechanisms underlying the vasoprotective benefits of NAD+ repletion. Given the central role of NAD+ in mitochondrial metabolism and bioenergetics, future studies should examine mitochondrion-initiated stress pathways, with a particular focus on the mitochondrial unfolded protein response in mammalian models in vivo to identify key signaling molecules involved in mitochondrioprotection. We anticipate that this type of knowledge will advance our understanding of vascular diseases associated with mitochondrial dysfunction, and will accelerate the discovery of novel targets to modulate this proteotoxic stress-sensing pathway.
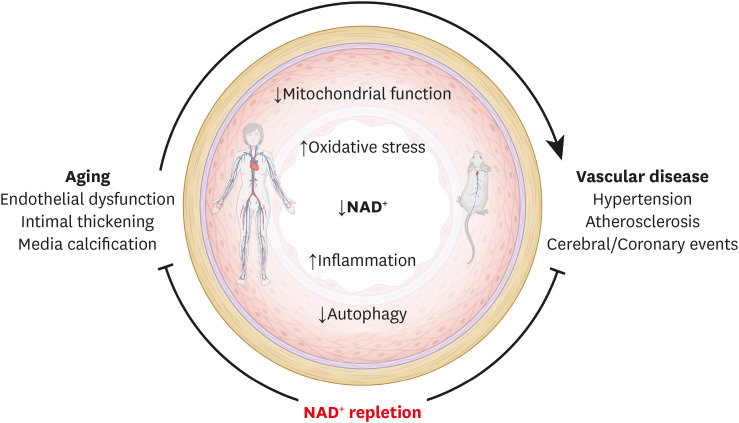
In summary, targeting vascular NAD+ metabolism holds significant therapeutic potential for the clinical management of age-related cardiovascular and cerebrovascular disorders. Although much remains to be done, based on ever-accumulating evidence, the pharmacological modulation of NAD+ levels via NAD+ precursors or inhibitors of NAD+-consuming enzymes appears to be an attractive strategy for reducing chronic low-grade inflammation, reactivating autophagy and mitochondrial biogenesis, and enhancing oxidative metabolism in vascular cells (Fig. 3). These approaches represent an exciting avenue to improve vascular health.
Fig. 3. NAD+ repletion therapy delays vascular aging and improves vascular health. Aging and related vascular disorders are associated with a decline in cellular NAD+ content, which coincides with reduced mitochondrial function and autophagy, as well as increased oxidative stress and pro-inflammatory signaling. Collectively, these contribute significantly to vascular pathologies, including hypertension, atherosclerosis, coronary artery disease, and aortic aneurysm. Hence, emerging NAD+-regenerative therapies are increasingly recognized as a potential strategy to protect vascular health during aging and disease both in animals and humans. Up-arrows indicate increases, down-arrows indicate decreases. The clip art included in this figure was created with BioRender.com.
NAD+, nicotinamide adenine dinucleotide.
Footnotes
Funding: This work was supported by the European Research Area Network on Cardiovascular Diseases through the MINOTAUR consortium to S.S. (Austrian Science Fund—FWF, I3301) and G.K. (Agence National de la Recherche). M.A. acknowledges funding received from the European Commission (H2020-MSCA-IF-2020, SPeR-ToNE), the Austrian Society of Cardiology (Präsidentenstipendium der ÖKG), and the Medical University of Graz (Start Fund). G.K. is supported by the Ligue contre le cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. H.B. acknowledges funding from the Austrian Science Fund-FWF, P-33874-B.
Conflict of Interest: Drs. Abdellatif and Sedej are involved in a patent application related to the cardiometabolic effects of caloric restriction mimetics, including nicotinamide. G.K. has held research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Samsara, Sanofi, Sotio, Vascage, and Vasculox/Tioma. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. G.K. is a scientific co-founder of everImmune, Samsara Therapeutics, and Therafast Bio. G.K. is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. H.B. reports no conflicts.
H.B. is an editor of Journal of Lipid and Atherosclerosis; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
- Conceptualization: Sedej S.
- Supervision: Sedej S.
- Writing - original draft: Sedej S, Abdellatif M.
- Writing - review & editing: Sedej S, Abdellatif M, Bugger H, Kroemer G.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Otín C, Kroemer G. Hallmarks of health. Cell. 2021;184:33–63. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 5.Abdellatif M, Sedej S, Kroemer G. NAD+ metabolism in cardiac health, aging, and disease. Circulation. 2021;144:1795–1817. doi: 10.1161/CIRCULATIONAHA.121.056589. [DOI] [PubMed] [Google Scholar]
- 6.Yoshino J, Baur JA, Imai SI. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27:513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, et al. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;316:H1253–H1266. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89.e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane AE, Sinclair DA. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res. 2018;123:868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 2018;27:667–676.e4. doi: 10.1016/j.cmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buqué A, Bloy N, Perez-Lanzón M, Iribarren K, Humeau J, Pol JG, et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun. 2020;11:3819. doi: 10.1038/s41467-020-17644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YJ, Zhang M, Zhao X, Shi K, Ye M, Tian J, et al. NAD+ administration decreases microvascular damage following cardiac ischemia/reperfusion by restoring autophagic flux. Basic Res Cardiol. 2020;115:57. doi: 10.1007/s00395-020-0817-z. [DOI] [PubMed] [Google Scholar]
- 13.Chini CC, Zeidler JD, Kashyap S, Warner G, Chini EN. Evolving concepts in NAD+ metabolism. Cell Metab. 2021;33:1076–1087. doi: 10.1016/j.cmet.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat Metab. 2020;2:9–31. doi: 10.1038/s42255-019-0161-5. [DOI] [PubMed] [Google Scholar]
- 16.Reiten OK, Wilvang MA, Mitchell SJ, Hu Z, Fang EF. Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mech Ageing Dev. 2021;199:111567. doi: 10.1016/j.mad.2021.111567. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covarrubias AJ, Kale A, Perrone R, Lopez-Dominguez JA, Pisco AO, Kasler HG, et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat Metab. 2020;2:1265–1283. doi: 10.1038/s42255-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Boslett J, Hemann C, Christofi FL, Zweier JL. Characterization of CD38 in the major cell types of the heart: endothelial cells highly express CD38 with activation by hypoxia-reoxygenation triggering NAD(P)H depletion. Am J Physiol Cell Physiol. 2018;314:C297–C309. doi: 10.1152/ajpcell.00139.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amici SA, Young NA, Narvaez-Miranda J, Jablonski KA, Arcos J, Rosas L, et al. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front Immunol. 2018;9:1593. doi: 10.3389/fimmu.2018.01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polzonetti V, Carpi FM, Micozzi D, Pucciarelli S, Vincenzetti S, Napolioni V. Population variability in CD38 activity: correlation with age and significant effect of TNF-α -308G>A and CD38 184C>G SNPs. Mol Genet Metab. 2012;105:502–507. doi: 10.1016/j.ymgme.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Weiss R, Schilling E, Grahnert A, Kölling V, Dorow J, Ceglarek U, et al. Nicotinamide: a vitamin able to shift macrophage differentiation toward macrophages with restricted inflammatory features. Innate Immun. 2015;21:813–826. doi: 10.1177/1753425915602545. [DOI] [PubMed] [Google Scholar]
- 25.Kong D, Li J, Shen Y, Liu G, Zuo S, Tao B, et al. Niacin promotes cardiac healing after myocardial infarction through activation of the myeloid prostaglandin D2 receptor subtype 1. J Pharmacol Exp Ther. 2017;360:435–444. doi: 10.1124/jpet.116.238261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huynh PK, Wilder J, Hiller S, Hagaman J, Takahashi N, Maeda-Smithies N, et al. Beneficial effects of nicotinamide on hypertensive mice with impaired endothelial nitric oxide function. J Exp Nephrol. 2020;1:1–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med. 2018;24:1351–1359. doi: 10.1038/s41591-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateuszuk Ł, Campagna R, Kutryb-Zając B, Kuś K, Słominska EM, Smolenski RT, et al. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem Pharmacol. 2020;178:114019. doi: 10.1016/j.bcp.2020.114019. [DOI] [PubMed] [Google Scholar]
- 29.D’Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28:711–732. doi: 10.1089/ars.2017.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desdín-Micó G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabandé-Rodríguez E, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368:1371–1376. doi: 10.1126/science.aax0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong G, Zheng D, Zhang L, Ni R, Wang G, Fan GC, et al. Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic Biol Med. 2018;123:125–137. doi: 10.1016/j.freeradbiomed.2018.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiss T, Nyúl-Tóth Á, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020;42:527–546. doi: 10.1007/s11357-020-00165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-κB activation in the nonhuman primate Macaca mulatta . J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csiszár A, Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, et al. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci. 2015;70:303–313. doi: 10.1093/gerona/glu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2:93885. doi: 10.1172/jci.insight.93885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Flynn CR, Uzhachenko R, et al. Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ Res. 2017;121:564–574. doi: 10.1161/CIRCRESAHA.117.310933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, et al. Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ Res. 2020;126:439–452. doi: 10.1161/CIRCRESAHA.119.315767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41:419–439. doi: 10.1007/s11357-019-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, et al. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clayton ZS, Hutton DA, Brunt VE, VanDongen NS, Ziemba BP, Casso AG, et al. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am J Physiol Heart Circ Physiol. 2021;321:H185–H196. doi: 10.1152/ajpheart.00118.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkman DL, Robinson AT, Rossman MJ, Seals DR, Edwards DG. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2021;320:H2080–H2100. doi: 10.1152/ajpheart.00917.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdellatif M, Ljubojevic-Holzer S, Madeo F, Sedej S. Autophagy in cardiovascular health and disease. Prog Mol Biol Transl Sci. 2020;172:87–106. doi: 10.1016/bs.pmbts.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Ljubojević-Holzer S, Kraler S, Djalinac N, Abdellatif M, Voglhuber J, Schipke J, et al. Loss of autophagy protein ATG5 impairs cardiac capacity in mice and humans through diminishing mitochondrial abundance and disrupting Ca2+ cycling. Cardiovasc Res. 2021:cvab112. doi: 10.1093/cvr/cvab112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G. Autophagy in cardiovascular aging. Circ Res. 2018;123:803–824. doi: 10.1161/CIRCRESAHA.118.312208. [DOI] [PubMed] [Google Scholar]
- 52.Michiels CF, Fransen P, De Munck DG, De Meyer GR, Martinet W. Defective autophagy in vascular smooth muscle cells alters contractility and Ca2+ homeostasis in mice. Am J Physiol Heart Circ Physiol. 2015;308:H557–H567. doi: 10.1152/ajpheart.00659.2014. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, Wu S, Zhu H, Ding Y, Dai X, Ouyang C, et al. Deletion of PRKAA triggers mitochondrial fission by inhibiting the autophagy-dependent degradation of DNM1L. Autophagy. 2017;13:404–422. doi: 10.1080/15548627.2016.1263776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- 56.Fan Y, Lu H, Liang W, Garcia-Barrio MT, Guo Y, Zhang J, et al. Endothelial TFEB (transcription factor EB) positively regulates postischemic angiogenesis. Circ Res. 2018;122:945–957. doi: 10.1161/CIRCRESAHA.118.312672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang HN, Dai Y, Zhang CH, Omondi AM, Ghosh A, Khanra I, et al. Sirtuins family as a target in endothelial cell dysfunction: implications for vascular ageing. Biogerontology. 2020;21:495–516. doi: 10.1007/s10522-020-09873-z. [DOI] [PubMed] [Google Scholar]
- 58.Lapierre LR, Kumsta C, Sandri M, Ballabio A, Hansen M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy. 2015;11:867–880. doi: 10.1080/15548627.2015.1034410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang R, Xu Y, Wan W, Shou X, Qian J, You Z, et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell. 2015;57:456–466. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 61.He J, Zhang G, Pang Q, Yu C, Xiong J, Zhu J, et al. SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J. 2017;284:1324–1337. doi: 10.1111/febs.14055. [DOI] [PubMed] [Google Scholar]
- 62.Fang EF, Tao J. Targeting on the NAD+-mitophagy axis to treat cardiovascular disease. Aging Med (Milton) 2020;3:151–152. doi: 10.1002/agm2.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmermann A, Madreiter-Sokolowski C, Stryeck S, Abdellatif M. Targeting the mitochondria-proteostasis axis to delay aging. Front Cell Dev Biol. 2021;9:656201. doi: 10.3389/fcell.2021.656201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sukoyan GV, Kavadze IK. Effect of nadcin on energy supply system and apoptosis in ischemia-reperfusion injury to the myocardium. Bull Exp Biol Med. 2008;146:321–324. doi: 10.1007/s10517-008-0268-2. [DOI] [PubMed] [Google Scholar]
- 65.Sukoyan GV, Andriadze NA, Guchua EI, Karsanov NV. Effect of NAD on recovery of adenine nucleotide pool, phosphorylation potential, and stimulation of apoptosis during late period of reperfusion damage to myocardium. Bull Exp Biol Med. 2005;139:46–49. doi: 10.1007/s10517-005-0208-3. [DOI] [PubMed] [Google Scholar]
- 66.Liaudet L, Yang Z, Al-Affar EB, Szabó C. Myocardial ischemic preconditioning in rodents is dependent on poly (ADP-ribose) synthetase. Mol Med. 2001;7:406–417. [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou L, Zhang S, Bolor-Erdene E, Wang L, Tian D, Mei Y. NAMPT/SIRT1 attenuate Ang II-induced vascular remodeling and vulnerability to hypertension by inhibiting the ROS/MAPK pathway. Oxid Med Cell Longev. 2020;2020:1974265. doi: 10.1155/2020/1974265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, et al. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127:386–396. doi: 10.1161/CIRCULATIONAHA.112.124404. [DOI] [PubMed] [Google Scholar]
- 69.Watson A, Nong Z, Yin H, O’Neil C, Fox S, Balint B, et al. Nicotinamide phosphoribosyltransferase in smooth muscle cells maintains genome integrity, resists aortic medial degeneration, and is suppressed in human thoracic aortic aneurysm disease. Circ Res. 2017;120:1889–1902. doi: 10.1161/CIRCRESAHA.116.310022. [DOI] [PubMed] [Google Scholar]
- 70.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021;13:eabd7064. doi: 10.1126/scitranslmed.abd7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li F, Fushima T, Oyanagi G, Townley-Tilson HW, Sato E, Nakada H, et al. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc Natl Acad Sci U S A. 2016;113:13450–13455. doi: 10.1073/pnas.1614947113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guse AH. Regulation of calcium signaling by the second messenger cyclic adenosine diphosphoribose (cADPR) Curr Mol Med. 2004;4:239–248. doi: 10.2174/1566524043360771. [DOI] [PubMed] [Google Scholar]
- 76.Cho KH, Kim HJ, Rodriguez-Iturbe B, Vaziri ND. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F106–F113. doi: 10.1152/ajprenal.00126.2009. [DOI] [PubMed] [Google Scholar]
- 77.Gan L, Liu D, Liu J, Chen E, Chen C, Liu L, et al. CD38 deficiency alleviates Ang II-induced vascular remodeling by inhibiting small extracellular vesicle-mediated vascular smooth muscle cell senescence in mice. Signal Transduct Target Ther. 2021;6:223. doi: 10.1038/s41392-021-00625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Méndez-Lara KA, Letelier N, Farré N, Diarte-Añazco EM, Nieto-Nicolau N, Rodríguez-Millán E, et al. Nicotinamide prevents apolipoprotein B-containing lipoprotein oxidation, inflammation and atherosclerosis in apolipoprotein E-deficient mice. Antioxidants. 2020;9:E1162. doi: 10.3390/antiox9111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Domagala TB, Szeffler A, Dobrucki LW, Dropinski J, Polanski S, Leszczynska-Wiloch M, et al. Nitric oxide production and endothelium-dependent vasorelaxation ameliorated by N1-methylnicotinamide in human blood vessels. Hypertension. 2012;59:825–832. doi: 10.1161/HYPERTENSIONAHA.111.183210. [DOI] [PubMed] [Google Scholar]
- 81.Mateuszuk L, Jasztal A, Maslak E, Gasior-Glogowska M, Baranska M, Sitek B, et al. Antiatherosclerotic effects of 1-methylnicotinamide in apolipoprotein E/low-density lipoprotein receptor-deficient mice: a comparison with nicotinic acid. J Pharmacol Exp Ther. 2016;356:514–524. doi: 10.1124/jpet.115.228643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang N, Wang M, Song J, Liu Y, Chen H, Mu D, et al. N-methylnicotinamide protects against endothelial dysfunction and attenuates atherogenesis in apolipoprotein E-deficient mice. Mol Nutr Food Res. 2016;60:1625–1636. doi: 10.1002/mnfr.201501019. [DOI] [PubMed] [Google Scholar]
- 83.Creider JC, Hegele RA, Joy TR. Niacin: another look at an underutilized lipid-lowering medication. Nat Rev Endocrinol. 2012;8:517–528. doi: 10.1038/nrendo.2012.22. [DOI] [PubMed] [Google Scholar]
- 84.Romani M, Hofer DC, Katsyuba E, Auwerx J. Niacin: an old lipid drug in a new NAD+ dress. J Lipid Res. 2019;60:741–746. doi: 10.1194/jlr.S092007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 86.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 87.Philpott AC, Hubacek J, Sun YC, Hillard D, Anderson TJ. Niacin improves lipid profile but not endothelial function in patients with coronary artery disease on high dose statin therapy. Atherosclerosis. 2013;226:453–458. doi: 10.1016/j.atherosclerosis.2012.10.067. [DOI] [PubMed] [Google Scholar]
- 88.Jenkins DJ, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71:2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 89.Jenkins DJ, Spence JD, Giovannucci EL, Kim YI, Josse RG, Vieth R, et al. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment: JACC focus seminar. J Am Coll Cardiol. 2021;77:423–436. doi: 10.1016/j.jacc.2020.09.619. [DOI] [PubMed] [Google Scholar]
- 90.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 91.Zeman M, Vecka M, Perlík F, Hromádka R, Staňková B, Tvrzická E, et al. Niacin in the treatment of hyperlipidemias in light of new clinical trials: has niacin lost its place? Med Sci Monit. 2015;21:2156–2162. doi: 10.12659/MSM.893619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D’Andrea E, Hey SP, Ramirez CL, Kesselheim AS. Assessment of the role of niacin in managing cardiovascular disease outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e192224. doi: 10.1001/jamanetworkopen.2019.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang Y, Jin M, Chen J, Yan J, Liu P, Yao M, et al. Discovery of a novel niacin-lipoic acid dimer N2L attenuating atherosclerosis and dyslipidemia with non-flushing effects. Eur J Pharmacol. 2020;868:172871. doi: 10.1016/j.ejphar.2019.172871. [DOI] [PubMed] [Google Scholar]
- 94.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 95.Bermudez B, Dahl TB, Medina I, Groeneweg M, Holm S, Montserrat-de la Paz S, et al. Leukocyte overexpression of intracellular NAMPT attenuates atherosclerosis by regulating PPARγ-dependent monocyte differentiation and function. Arterioscler Thromb Vasc Biol. 2017;37:1157–1167. doi: 10.1161/ATVBAHA.116.308187. [DOI] [PubMed] [Google Scholar]
- 96.Nencioni A, da Silva RF, Fraga-Silva RA, Steffens S, Fabre M, Bauer I, et al. Nicotinamide phosphoribosyltransferase inhibition reduces intraplaque CXCL1 production and associated neutrophil infiltration in atherosclerotic mice. Thromb Haemost. 2014;111:308–322. doi: 10.1160/TH13-07-0531. [DOI] [PubMed] [Google Scholar]
- 97.Kong YY, Li GQ, Zhang WJ, Hua X, Zhou CC, Xu TY, et al. Nicotinamide phosphoribosyltransferase aggravates inflammation and promotes atherosclerosis in ApoE knockout mice. Acta Pharmacol Sin. 2019;40:1184–1192. doi: 10.1038/s41401-018-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reyes LA, Boslett J, Varadharaj S, De Pascali F, Hemann C, Druhan LJ, et al. Depletion of NADP(H) due to CD38 activation triggers endothelial dysfunction in the postischemic heart. Proc Natl Acad Sci U S A. 2015;112:11648–11653. doi: 10.1073/pnas.1505556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boslett J, Hemann C, Zhao YJ, Lee HC, Zweier JL. Luteolinidin protects the postischemic heart through CD38 inhibition with preservation of NAD(P)(H) J Pharmacol Exp Ther. 2017;361:99–108. doi: 10.1124/jpet.116.239459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boslett J, Helal M, Chini E, Zweier JL. Genetic deletion of CD38 confers post-ischemic myocardial protection through preserved pyridine nucleotides. J Mol Cell Cardiol. 2018;118:81–94. doi: 10.1016/j.yjmcc.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boslett J, Reddy N, Alzarie YA, Zweier JL. Inhibition of CD38 with the thiazoloquin(az)olin(on)e 78c protects the heart against postischemic injury. J Pharmacol Exp Ther. 2019;369:55–64. doi: 10.1124/jpet.118.254557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu L, Wang Q, Zhao B, Wu Q, Wang P. Exogenous nicotinamide adenine dinucleotide administration alleviates ischemia/reperfusion-induced oxidative injury in isolated rat hearts via Sirt5-SDH-succinate pathway. Eur J Pharmacol. 2019;858:172520. doi: 10.1016/j.ejphar.2019.172520. [DOI] [PubMed] [Google Scholar]
- 103.Zhai X, Han W, Wang M, Guan S, Qu X. Exogenous supplemental NAD+ protect myocardium against myocardial ischemic/reperfusion injury in swine model. Am J Transl Res. 2019;11:6066–6074. [PMC free article] [PubMed] [Google Scholar]
- 104.Sukhodub A, Du Q, Jovanović S, Jovanović A. Nicotinamide-rich diet protects the heart against ischaemia-reperfusion in mice: a crucial role for cardiac SUR2A. Pharmacol Res. 2010;61:564–570. doi: 10.1016/j.phrs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nie H, Zhang Y, Yu H, Xiao H, Li T, Yang Q. Oral delivery of carrier-free dual-drug nanocrystal self-assembled microspheres improved NAD+ bioavailability and attenuated cardiac ischemia/reperfusion injury in mice. Drug Deliv. 2021;28:433–444. doi: 10.1080/10717544.2021.1886198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hosseini L, Vafaee MS, Badalzadeh R. Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats. J Cardiovasc Pharmacol Ther. 2020;25:240–250. doi: 10.1177/1074248419882002. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdellatif M. Autophagy promotes longevity-except in the presence of ‘leaky’ mitochondria. Cardiovasc Res. 2019;115:e118–e120. doi: 10.1093/cvr/cvz224. [DOI] [PubMed] [Google Scholar]
- 109.Digby JE, Martinez F, Jefferson A, Ruparelia N, Chai J, Wamil M, et al. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler Thromb Vasc Biol. 2012;32:669–676. doi: 10.1161/ATVBAHA.111.241836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu BJ, Chen K, Barter PJ, Rye KA. Niacin inhibits vascular inflammation via the induction of heme oxygenase-1. Circulation. 2012;125:150–158. doi: 10.1161/CIRCULATIONAHA.111.053108. [DOI] [PubMed] [Google Scholar]
- 111.van der Hoorn JW, de Haan W, Berbée JF, Havekes LM, Jukema JW, Rensen PC, et al. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler Thromb Vasc Biol. 2008;28:2016–2022. doi: 10.1161/ATVBAHA.108.171363. [DOI] [PubMed] [Google Scholar]
- 112.Horimatsu T, Blomkalns AL, Ogbi M, Moses M, Kim D, Patel S, et al. Niacin protects against abdominal aortic aneurysm formation via GPR109A independent mechanisms: role of NAD+/nicotinamide. Cardiovasc Res. 2020;116:2226–2238. doi: 10.1093/cvr/cvz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 114.Oller J, Gabandé-Rodríguez E, Ruiz-Rodríguez MJ, Desdín-Micó G, Aranda JF, Rodrigues-Diez R, et al. Extracellular tuning of mitochondrial respiration leads to aortic aneurysm. Circulation. 2021;143:2091–2109. doi: 10.1161/CIRCULATIONAHA.120.051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T, et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021;373:eabc8479. doi: 10.1126/science.abc8479. [DOI] [PubMed] [Google Scholar]
- 116.Chen J, Sivan U, Tan SL, Lippo L, De Angelis J, Labella R, et al. High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci Adv. 2021;7:eabd7819. doi: 10.1126/sciadv.abd7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shabir O, Berwick J, Francis SE. Neurovascular dysfunction in vascular dementia, Alzheimer’s and atherosclerosis. BMC Neurosci. 2018;19:62. doi: 10.1186/s12868-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Traba J, Kwarteng-Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest. 2015;125:4592–4600. doi: 10.1172/JCI83260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Reports. 2019;28:1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 122.Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372:1224–1229. doi: 10.1126/science.abe9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J. 2020;67:153–160. doi: 10.1507/endocrj.EJ19-0313. [DOI] [PubMed] [Google Scholar]
- 124.Dower JI, Geleijnse JM, Gijsbers L, Zock PL, Kromhout D, Hollman PC. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am J Clin Nutr. 2015;101:914–921. doi: 10.3945/ajcn.114.098590. [DOI] [PubMed] [Google Scholar]
- 125.Pfeuffer M, Auinger A, Bley U, Kraus-Stojanowic I, Laue C, Winkler P, et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr Metab Cardiovasc Dis. 2013;23:403–409. doi: 10.1016/j.numecd.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 126.Abdellatif M, Baur JA. NAD+ metabolism and cardiometabolic health: the human evidence. Cardiovasc Res. 2021;117:e106–e109. doi: 10.1093/cvr/cvab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu L, Su X, Quinn WJ, 3rd, Hui S, Krukenberg K, Frederick DW, et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018;27:1067–1080.e5. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Winter SL, Boyer JL. Hepatic toxicity from large doses of vitamin B3 (nicotinamide) N Engl J Med. 1973;289:1180–1182. doi: 10.1056/NEJM197311292892208. [DOI] [PubMed] [Google Scholar]
- 130.Diguet N, Trammell SA, Tannous C, Deloux R, Piquereau J, Mougenot N, et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137:2256–2273. doi: 10.1161/CIRCULATIONAHA.116.026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tong D, Schiattarella GG, Jiang N, Altamirano F, Szweda PA, Elnwasany A, et al. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ Res. 2021;128:1629–1641. doi: 10.1161/CIRCRESAHA.120.317046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kühnast S, Louwe MC, Heemskerk MM, Pieterman EJ, van Klinken JB, van den Berg SA, et al. Niacin reduces atherosclerosis development in APOE*3Leiden.CETP mice mainly by reducing nonHDL-cholesterol. PLoS One. 2013;8:e66467. doi: 10.1371/journal.pone.0066467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tong DL, Zhang DX, Xiang F, Teng M, Jiang XP, Hou JM, et al. Nicotinamide pretreatment protects cardiomyocytes against hypoxia-induced cell death by improving mitochondrial stress. Pharmacology. 2012;90:11–18. doi: 10.1159/000338628. [DOI] [PubMed] [Google Scholar]