This randomized clinical trial analyzes data comparing treatment regimens according to prior stroke, transient ischemic attack, or thromboembolism to determine the safety and efficacy of apixaban, vitamin K antagonists, and aspirin.
Key Points
Question
In patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention receiving a P2Y12 inhibitor, does treatment effect vary according to prior stroke, transient ischemic attack (TIA), or thromboembolism (TE)?
Findings
In this randomized clinical trial, a post hoc analysis found that patients with vs without prior stroke/TIA/TE had a higher risk of ischemic and bleeding events. Among those with prior stroke/TIA/TE, patients treated with apixaban had less bleeding, death, or hospitalization than patients treated with vitamin K antagonists; patients treated with aspirin had a higher bleeding risk than those receiving placebo.
Meaning
The safety and efficacy of apixaban compared with vitamin K antagonists were consistent with the overall trial findings, irrespective of history of stroke/TIA/TE.
Abstract
Importance
Data are limited regarding the risk of cerebrovascular ischemic events and major bleeding in patients with atrial fibrillation (AF) and recent acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI).
Objective
Determine the efficacy and safety of apixaban or vitamin K antagonists (VKA) and aspirin or placebo according to prior stroke, transient ischemic attack (TIA), or thromboembolism (TE).
Design, Setting, and Participants
In this prospective, multicenter, 2-by-2 factorial, randomized clinical trial, post hoc parallel analyses were performed to compare randomized treatment regimens according to presence or absence of prior stroke/TIA/TE using Cox proportional hazards models. Patients with AF, recent ACS or PCI, and planned use of P2Y12 inhibitors for 6 months or longer were included; 33 patients with missing data about prior stroke/TIA/TE were excluded.
Interventions
Apixaban (5 mg or 2.5 mg twice daily) or VKA and aspirin or placebo.
Main Outcomes and Measures
Major or clinically relevant nonmajor (CRNM) bleeding.
Results
Of 4581 patients included, 633 (13.8%) had prior stroke/TIA/TE. Patients with vs without prior stroke/TIA/TE were older; had higher CHA2DS2-VASC and HAS-BLED scores; and more frequently had prior bleeding, heart failure, diabetes, and prior oral anticoagulant use. Apixaban was associated with lower rates of major or CRNM bleeding and death or hospitalization than VKA in patients with (hazard ratio [HR], 0.69; 95% CI, 0.46-1.03) and without (HR, 0.68; 95% CI, 0.57-0.82) prior stroke/TIA/TE. Patients without prior stroke/TIA/TE receiving aspirin vs placebo had higher rates of bleeding; this difference appeared less substantial among patients with prior stroke/TIA/TE (P = .01 for interaction). Aspirin was associated with numerically lower rates of death or ischemic events than placebo in patients with (HR, 0.71; 95% CI, 0.42-1.20) and without (HR, 0.93; 95% CI, 0.72-1.21) prior stroke/TIA/TE (not statistically significant).
Conclusions and Relevance
The safety and efficacy of apixaban compared with VKA was consistent with the AUGUSTUS findings, irrespective of prior stroke/TIA/TE. Aspirin increased major or CRNM bleeding, particularly in patients without prior stroke/TIA/TE. Although aspirin may have some benefit in patients with prior stroke, our findings support the use of apixaban and a P2Y12 inhibitor without aspirin for the majority of patients with AF and ACS and/or PCI, regardless of prior stroke/TIA/TE status.
Trial Registration
ClinicalTrials.gov Identifier: NCT02415400
Introduction
Choosing the ideal antithrombotic therapy strategy for patients with atrial fibrillation (AF) treated with dual antiplatelet therapy for acute coronary syndrome (ACS) and/or recent percutaneous coronary intervention (PCI) can be challenging. Recent data from randomized trials and subsequent meta-analyses demonstrated the benefit of a dual-pathway strategy with a P2Y12 inhibitor coupled with oral anticoagulation, without aspirin, as the optimal strategy to balance the ischemic and bleeding risks.1 In the AUGUSTUS trial,2 apixaban resulted in less bleeding and fewer hospitalizations than vitamin K antagonist (VKA), and aspirin resulted in more bleeding than placebo, in patients with AF and ACS and/or PCI treated with a P2Y12 inhibitor.
Patients with a history of stroke may be at increased risk for both ischemic and bleeding outcomes. Prior studies have demonstrated that AF, prior stroke, transient ischemic attack (TIA), and thromboembolism (TE) are associated with an increased risk of recurrent stroke/TE and major bleeding.3,4 Prior stroke was also associated with an increased risk of subsequent stroke, particularly intracranial hemorrhage (ICH), in patients treated with elective PCI or during ACS.5,6,7 However, data are limited regarding the risk of cerebrovascular ischemic events and major bleeding in patients with AF with recent ACS/PCI. Because of its 2-by-2 factorial design, the AUGUSTUS trial2 provides a unique opportunity to further understand the clinical characteristics and ischemic and bleeding outcomes stratified by prior stroke status in patients with AF and recent ACS/PCI who were treated with apixaban or VKA and aspirin or placebo.
Methods
The design8 and results2 of the AUGUSTUS trial (NCT02415400) have been published. In brief, AUGUSTUS was a prospective, multicenter, 2-by-2 factorial, randomized clinical trial comparing apixaban with VKA and aspirin with placebo in patients with AF who had a recent ACS or underwent PCI (or both). The study was approved by ethics committees at participating sites, and all patients provided written informed consent before enrollment.
Eligible patients met the following inclusion criteria: age 18 years or older; previous, persistent, permanent, or paroxysmal AF and planned long-term use of an oral anticoagulant; recent ACS or PCI; and planned use of a P2Y12 inhibitor for at least 6 months. Patients using anticoagulation for other conditions (eg, prosthetic valves, venous thromboembolism, and mitral stenosis) were not eligible. Other key exclusion criteria were severe kidney insufficiency; a history of ICH; recent or planned coronary artery bypass graft surgery; coagulopathy or ongoing bleeding; and contraindication to a VKA, apixaban, all P2Y12 inhibitors, or aspirin. Patients’ baseline risk for stroke and bleeding was assessed using CHA2DS2-VASc and a modified HAS-BLED at time of randomization.9 For the calculation of HAS-BLED score, patients not taking a VKA were assumed to not have a labile international normalized ratio.
Randomization was stratified according to indication (ACS or PCI) at enrollment. In accordance with the apixaban label instructions for stroke prevention in patients with AF, patients randomized to receive apixaban were directed to take 5 mg twice daily or 2.5 mg twice daily if they met 2 or more of the following dose-adjustment criteria: age 80 years or older, weight 60 kg or less, and creatinine 1.5 mg/dL or greater (to convert to μmol/L, multiply by 88.4). Patients randomly assigned to receive VKA had the dose adjusted to reach a target international normalized ratio within a range of 2.0 to 3.0. For the comparison of aspirin with placebo, patients received aspirin at a dose of 81 mg or matching placebo once daily. The treatment regimen comparing apixaban with VKA was open-label; however, the regimen comparing aspirin with matching placebo was double-blind. After 6 months, patients were transitioned from their 2 trial interventions to antiplatelet and anticoagulant therapy according to the local standard of care.
The primary outcome for both factorial comparisons was major bleeding or clinically relevant nonmajor (CRNM) bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH). Bleeding was defined as ISTH major bleeding if it resulted in death, occurred in a critical organ (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, intramuscular with compartment syndrome, or pericardial), or was associated with either a decrease in the hemoglobin level of at least 2 g/dL or a transfusion of at least 2 units of packed red blood cells. Bleeding was defined as CRNM bleeding if it resulted in hospitalization, medical or surgical intervention for bleeding, an unscheduled clinic visit, or a change in physician-directed antithrombotic therapy.10
Secondary outcomes included the composite of death or hospitalization and the composite of death or ischemic events (stroke, myocardial infarction, stent thrombosis [definite or probable], or urgent revascularization). Exploratory outcomes included individual components of the secondary outcomes. All bleeding and ischemic events (except for urgent revascularization) were independently adjudicated by the Clinical Events Classification Committee at Duke Clinical Research Institute, whose members were unaware of the trial group assignments.
Statistical Analysis
In this post hoc analysis, patient characteristics at baseline are presented according to the presence of prior stroke/TIA/TE. Continuous variables were summarized as median (IQR) or mean (SD) and compared between patients with and without prior stroke/TIA/TE using the t test or Wilcoxon rank sum test. Categorical variables were summarized as counts with percentages and compared using the Pearson χ2 test or Fisher exact test. Missing values were excluded from the denominators for the percentages.
Using the Kaplan-Meier method, the cumulative incidence was estimated for safety outcomes (ISTH major or CRNM bleeding) at 6 months from the start of the intervention and for efficacy outcomes at 6 months from randomization. The log-rank test was used to compare the cumulative incidence of the outcomes between patients with and without prior stroke/TIA/TE.
The treatment effect of apixaban compared with VKA on the outcomes was assessed using Cox proportional hazards models that included the treatment group, the presence of prior stroke/TIA/TE, and the interaction between the two. Similar analyses were performed to assess the treatment effect of aspirin compared with placebo on the outcomes. Results are presented as hazard ratios (HRs) with 95% CIs and P values for interaction. The proportional hazard assumption was assessed using weighted Schoenfeld residuals. The proportional hazard assumption was met for both comparisons (anticoagulant and antiplatelet) for all end points in both subgroups (prior stroke/TIA/TE and no prior stroke/TIA/TE), except for death or ischemic events in the subgroup of patients without prior stroke/TIA/TE for the apixaban vs VKA comparison. Because the departure from the proportional hazard assumption was mild, we decided to include average HRs. For all analyses, a 2-sided P value of less than .05 was considered statistically significant, and no corrections for multiple testing were applied. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Of the 4614 patients enrolled in AUGUSTUS, 33 had no data on prior stroke/TIA/TE and were excluded; therefore, this analysis included the remaining 4581 patients. Of those, 633 (13.8%) had a history of stroke/TIA/TE. The Table presents demographic and clinical characteristics of patients with and without prior stroke/TIA/TE. Patients with prior stroke/TIA/TE were older and were more likely to have a history of bleeding, heart failure, and diabetes compared with those without prior stroke/TIA/TE. In addition, they had higher CHA2DS2-VASC and HAS-BLED scores, along with higher rates of prior oral anticoagulant use, and a longer time from ACS or PCI to randomization when compared with those without prior stroke/TIA/TE. A HAS-BLED score was calculated in 4405 of 4614 patients (95%) in AUGUSTUS. Among patients assigned to receive apixaban, a higher proportion of patients with prior stroke/TIA/TE received the adjusted dose (2.5 mg twice daily) than patients without prior stroke/TIA/TE.
Table. Patient Baseline Characteristics According to Presence of Prior Stroke, Transient Ischemic Attack, or Thromboembolism.
| No./total No. (%) | P value | |||
|---|---|---|---|---|
| Overall (n = 4581) | Prior stroke/TIA/TE (n = 633) | No prior stroke/TIA/TE (n = 3948) | ||
| Age, median (IQR), y | 71 (64-77) | 72 (66-78) | 70 (64-77) | <.001 |
| Female sex | 1329/4581 (29.0) | 183/633 (28.9) | 1146/3948 (29.0) | .95 |
| Male sex | 3252/4581 (71.0) | 450/633 (71.1) | 2802/3948 (71.0) | |
| Race and ethnicitya | .17 | |||
| American Indian and Alaska Native | 16/4524 (0.4) | 3/624 (0.5) | 13/3900 (0.3) | |
| Asian | 140/4524 (3.1) | 20/624 (3.2) | 120/3900 (3.1) | |
| Black | 59/4524 (1.3) | 14/624 (2.2) | 45/3900 (1.2) | |
| White | 4152/4524 (91.8) | 570/624 (91.3) | 3582/3900 (91.8) | |
| Other | 157/4524 (3.5) | 17/624 (2.7) | 140/3900 (3.6) | |
| Serum creatinine, mg/dL | .61 | |||
| <1.5 | 4128/4504 (91.7) | 565/620 (91.1) | 3563/3884 (91.7) | |
| ≥1.5 | 376/4504 (8.3) | 55/620 (8.9) | 321/3884 (8.3) | |
| CHA2DS2-VASc score, mean (SD) | 3.9 (1.6) | 5.9 (1.4) | 3.6 (1.3) | <.001 |
| HAS-BLED score, mean (SD) | 2.9 (0.9) | 3.7 (1.0) | 2.7 (0.9) | <.001 |
| Prior bleeding | 50/4561 (1.1) | 16/632 (2.5) | 34/3929 (0.9) | <.001 |
| Hypertension leading to medication use | 4073/4581 (88.9) | 576/633 (91.0) | 3497/3948 (88.6) | .07 |
| Heart failure | 1973/4581 (43.1) | 301/633 (47.6) | 1672/3948 (42.4) | .01 |
| Diabetes | 1678/4581 (36.6) | 264/633 (41.7) | 1414/3948 (35.8) | .004 |
| Concomitant P2Y12 inhibitor at randomization | .09 | |||
| Clopidogrel | 4142/4469 (92.7) | 557/615 (90.6) | 3585/3854 (93.0) | |
| Prasugrel | 49/4469 (1.1) | 8/615 (1.3) | 41/3854 (1.1) | |
| Ticagrelor | 278/4469 (6.2) | 50/615 (8.1) | 228/3854 (5.9) | |
| Previous use of oral anticoagulant | 2247/4581 (49.1) | 344/633 (54.3) | 1903/3948 (48.2) | .004 |
| Qualifying index event | .38 | |||
| ACS and PCI | 1705/4564 (37.4) | 222/629 (35.3) | 1483/3935 (37.7) | |
| Medically managed ACS | 1095/4564 (24.0) | 163/629 (25.9) | 932/3935 (23.7) | |
| Elective PCI | 1764/4564 (38.7) | 244/629 (38.8) | 1520/3935 (38.6) | |
| No. of days from ACS or PCI to randomization, mean (SD) | 6.6 (4.2) | 7.0 (4.2) | 6.6 (4.2) | .006 |
| Reduced-dose apixaban (2.5 mg) in patients randomized to receive apixaban | 227/2274 (10.0) | 37/324 (11.4) | 190/1950 (9.7) | .35 |
Abbreviations: ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; TE, thromboembolism; TIA, transient ischemic attack.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Race and ethnicity data for the patients were determined by the investigator and recorded on the case-report forms. The category other includes those not in the other listed groups.
Cumulative Incidence of Outcomes by the Presence of Prior Stroke/TIA/TE
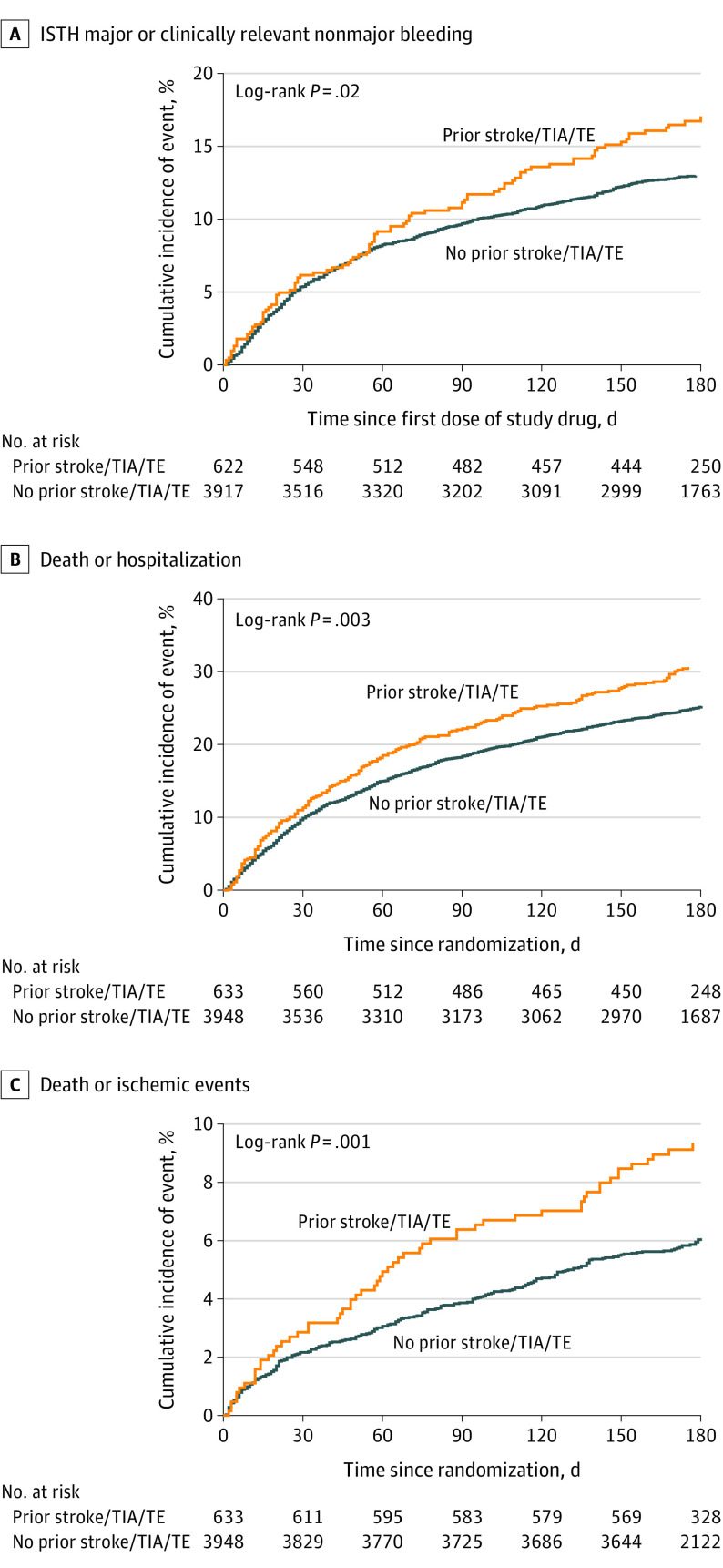
Compared with patients without prior stroke/TIA/TE, patients with prior stroke/TIA/TE had a 3-fold higher risk of ischemic stroke (1.60% vs 0.50%; P = .002) and were more likely to have ISTH major or CRNM bleeding (17.1% vs 13.0%; P = .02), death or hospitalization (30.6% vs 25.2%; P = .003), and death or ischemic events (9.4% vs 6.1%; P = .001) (Figure 1). Kaplan-Meier curves for all 4 groups for ISTH major or CRNM bleeding, death or hospitalization, and death or ischemic events are included in eFigures 1-3 in Supplement 1.
Figure 1. Kaplan-Meier Curves for Outcomes by Presence of Prior Stroke, Transient Ischemic Attack (TIA), or Thromboembolism (TE).
ISTH indicates International Society on Thrombosis and Haemostasis.
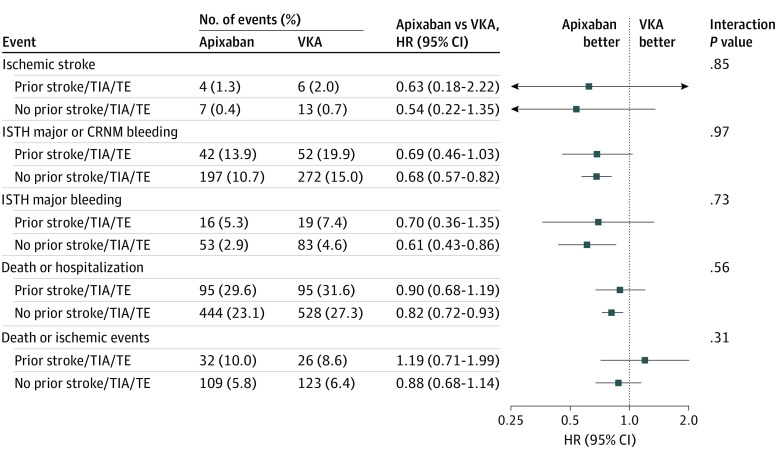
Treatment Effect of Apixaban vs VKA on Outcomes by the Presence of Prior Stroke/TIA/TE
There was no significant interaction between the anticoagulant treatment and prior stroke/TIA/TE on any of the outcomes. Patients treated with apixaban had a lower risk of ISTH major or CRNM bleeding and death or hospitalization than those receiving VKA among patients with no prior stroke/TIA/TE. Consistent with the main trial results, patients with and without prior stroke/TIA/TE who were treated with apixaban had fewer ischemic strokes, fewer major bleeding events, and fewer deaths or hospitalizations than patients treated with VKA. There were no significant differences in any other outcomes between apixaban and VKA (Figure 2).
Figure 2. Treatment Effect of Apixaban vs Vitamin K Antagonist (VKA) on Outcomes by Presence of Prior Stroke, Transient Ischemic Attack (TIA), or Thromboembolism (TE).
CRNM indicates clinically relevant nonmajor; HR, hazard ratio; ISTH, International Society on Thrombosis and Haemostasis.
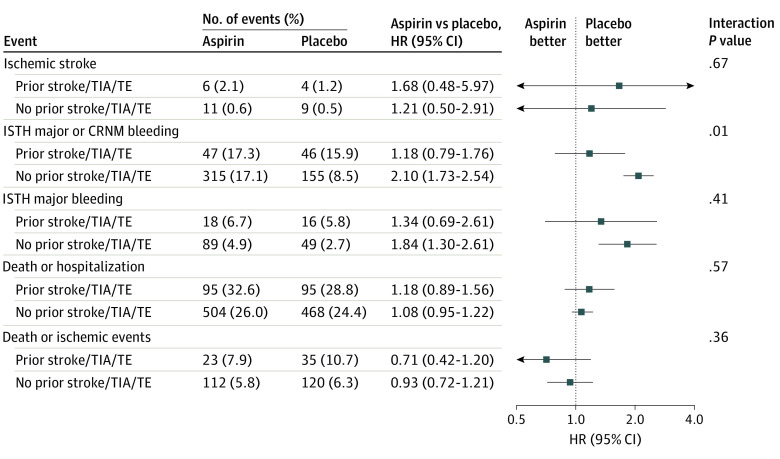
Treatment Effect of Aspirin vs Placebo on Outcomes by the Presence of Prior Stroke/TIA/TE
There was a significant interaction between the antiplatelet treatment and prior stroke/TIA/TE for ISTH major or CRNM bleeding (Figure 3). Patients treated with aspirin had a higher risk of bleeding than those in the placebo group among patients without prior stroke/TIA/TE. While there were no significant differences between aspirin and placebo observed among patients with prior stroke/TIA/TE, aspirin was associated with numerically lower rates of death or ischemic events than placebo in patients with prior stroke/TIA/TE (HR, 0.71; 95% CI, 0.42-1.20). There were no significant differences in any other outcomes between aspirin and placebo.
Figure 3. Treatment Effect of Aspirin vs Placebo on Outcomes by Presence of Prior Stroke, Transient Ischemic Attack (TIA), or Thromboembolism (TE).
CRNM indicates clinically relevant nonmajor; HR, hazard ratio; ISTH, International Society on Thrombosis and Haemostasis.
Discussion
The AUGUSTUS trial provided a unique opportunity to assess the overall risk of stroke, bleeding, and ischemic events in patients with AF presenting with ACS and/or undergoing PCI and the treatment effect of apixaban compared with warfarin and aspirin compared with placebo. In this subgroup analysis of the AUGUSTUS trial, patients with prior stroke/TIA/TE had a 3-fold increased risk of ischemic stroke and a higher risk of ISTH major or CRNM bleeding, death or hospitalization, and death or ischemic events than those without prior stroke/TIA/TE. The safety and efficacy of apixaban compared with VKA was consistent with the overall trial findings, irrespective of history of stroke/TIA/TE. Aspirin increased major or CRNM bleeding, particularly in patients without prior stroke/TIA/TE.
Until the publication of trials assessing dual antithrombotic therapy with direct oral anticoagulants,2,11,12,13 patients with AF presenting with ACS and/or PCI represented a clinical challenge because of their elevated risk of stroke, stent thrombosis, and bleeding. Three of these 4 studies (RE-DUAL PCI, AUGUSTUS, and ENTRUST-AF PCI) included patients who had a history of stroke/TIA/TE.2,12 Patients with AF and prior stroke/TIA/TE have an increased risk of recurrent stroke or embolic events3; the CHA2DS2-VASc score assigns additional weight to prior stroke/TIA/TE. Several studies with direct oral anticoagulants have also shown an increased risk of stroke or systemic embolism in patients with prior stroke/TIA.4,14,15,16
In a subgroup analysis of the ARISTOTLE study,4 patients with previous stroke or TIA had a 2- to 3-times higher risk of stroke or systemic embolism, major bleeding, ICH, and mortality than those without previous stroke or TIA. Risk of major bleeding was about one-third higher in patients with previous stroke than in those without, whereas rates of gastrointestinal bleeding were similar between groups. In AUGUSTUS, patients with and without prior stroke/TIA/TE who were treated with apixaban seemed to have fewer strokes, fewer major bleeding events (including ICH), and lower mortality than patients treated with warfarin, although these benefits were not all statistically significant. The present analysis extends these findings from the AUGUSTUS trial by showing overall better safety and efficacy with apixaban compared with VKA in patients with prior stroke/TIA/TE.
Prior studies have demonstrated that previous stroke is also associated with an increased risk of subsequent stroke, particularly ICH, in patients treated with PCI, either electively or following ACS.5,6,7 In an analysis using the British Cardiovascular Intervention Society database5 that included more than 500 000 patients, prior stroke was associated with a 1.7-times higher risk of ischemic stroke in patients undergoing PCI or ACS and a 4-fold increased risk of hemorrhagic stroke after elective PCI. In a pooled cohort of 19 475 patients from 3 Japanese studies in PCI,6 the authors assessed the influence of prior stroke on ischemic and bleeding outcomes after PCI. Prior hemorrhagic stroke and prior ischemic stroke, compared with no prior stroke, were associated with a significant 4.4- and 1.5-fold increased risk of ICH, respectively. There was also a 1.5-fold statistically significant increased risk for ischemic events, driven mainly by higher risk for ischemic stroke in patients with prior hemorrhagic stroke and prior ischemic stroke compared with no prior stroke.
To our knowledge, this is the first study to analyze the risk of stroke, bleeding, and ischemic events in a contemporary cohort of patients who had prior stroke/TIA/TE and AF who were presenting with ACS or undergoing PCI. Patients with prior stroke/TIA/TE were older and had higher CHA2DS2-VASC, even when prior stroke was excluded from the score. The higher mean HAS-BLED score of patients with prior stroke/TIA/TE compared with patients without prior stroke was primarily driven by the presence of prior stroke. This analysis found that patients with prior stroke/TIA/TE had an increased risk of ischemic and bleeding events compared with those without prior stroke/TIA/TE. The treatment effect of apixaban compared with warfarin was consistent with the overall AUGUSTUS study results regarding recurrent ischemic stroke, ISTH major or CRNM bleeding, and death or hospitalization.
Aspirin increased ISTH major or CRNM bleeding, particularly in patients without prior stroke/TIA/TE. The magnitude of harm with aspirin compared with placebo is more pronounced in patients without prior stroke/TIA/TE than in those with prior stroke/TIA/TE. Aspirin increases the risk of ISTH major or CRNM bleeding in patients with and without prior stroke/TIA/TE. This finding might be due to a differential risk of aspirin use in patients with or without prior stroke/TIA/TE. As described in the Table, patients with prior stroke/TIA/TE had significantly more prior bleeding events compared with those without stroke/TIA/TE. The use of the P2Y12 inhibitors prasugrel and ticagrelor was higher in patients with vs without prior stroke/TIA/TE; clopidogrel use was similar between the 2 groups. There is no clear benefit of aspirin compared with placebo regarding death or ischemic events in patients with or without prior stroke, although we did observe a nonsignificant decrease in death or ischemic events in patients with prior stroke/TIA/TE assigned to receive aspirin. As such, there may be a role for aspirin in patients with prior stroke/TIA/TE in appropriate clinical situations.
Limitations
This study has some limitations that warrant consideration. We captured whether study participants had a history of the aggregate of stroke/TIA/TE but not the individual components alone. It is possible that each specific event may confer a differential risk of future ischemic or bleeding events. Further, we did not use specific definitions for each of these events; thus, the true frequency of prior stroke, TIA, and TE beyond a documented history is unknown. The characteristics of the 33 excluded patients may be different from the overall study population, but they account for less than 1% of the overall study population. Individuals from racial and ethnic minority groups, and Black patients in particular, were marginally represented in this study. Further, this is a post hoc analysis subject to type I error, and subgroup analyses are prone to resulting in chance findings and are typically underpowered to show robust findings in the independent subgroups. These results should be considered provocative and a basis for future dedicated trials.
Conclusions
Among patients with AF presenting with ACS and/or PCI, those with a history of stroke/TIA/TE had a higher risk of ischemic and bleeding events than those without. Among patients with prior stroke/TIA/TE, patients treated with apixaban had less bleeding, death, and hospitalization than those treated with VKA, whereas patients treated with aspirin had a higher bleeding risk than those treated with placebo. Although it is possible that aspirin might have some benefit in patients with prior stroke, in general, our findings support the use of apixaban and a P2Y12 inhibitor without aspirin for the majority of patients with AF and ACS and/or PCI, regardless of prior stroke/TIA/TE status.
eFigure 1. Kaplan-Meier curves for ISTH major or CRNM bleeding in patients with (A) and without (B) prior stroke/TIA/TE
eFigure 2. Kaplan-Meier curves for death or hospitalization in patients with (A) and without (B) prior stroke/TIA/TE
eFigure 3. Kaplan-Meier curves for death or ischemic events in patients with (A) and without (B) prior stroke/TIA/TE
Data sharing statement
References
- 1.Lopes RD, Hong H, Harskamp RE, et al. Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention: an updated network meta-analysis. JAMA Cardiol. 2020;5(5):582-589. doi: 10.1001/jamacardio.2019.6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes RD, Heizer G, Aronson R, et al. ; AUGUSTUS Investigators . Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509-1524. doi: 10.1056/NEJMoa1817083 [DOI] [PubMed] [Google Scholar]
- 3.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 4.Easton JD, Lopes RD, Bahit MC, et al. ; ARISTOTLE Committees and Investigators . Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11(6):503-511. doi: 10.1016/S1474-4422(12)70092-3 [DOI] [PubMed] [Google Scholar]
- 5.Myint PK, Kwok CS, Roffe C, et al. ; British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research . Determinants and outcomes of stroke following percutaneous coronary intervention by indication. Stroke. 2016;47(6):1500-1507. doi: 10.1161/STROKEAHA.116.012700 [DOI] [PubMed] [Google Scholar]
- 6.Natsuaki M, Morimoto T, Watanabe H, et al. ; CREDO‐Kyoto PCI/CABG registry cohort‐2, RESET and NEXT trial investigators . Ischemic and bleeding risk after percutaneous coronary intervention in patients with prior ischemic and hemorrhagic stroke. J Am Heart Assoc. 2019;8(22):e013356. doi: 10.1161/JAHA.119.013356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 8.Lopes RD, Vora AN, Liaw D, et al. An open-label, 2 × 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: rationale and design of the AUGUSTUS trial. Am Heart J. 2018;200:17-23. doi: 10.1016/j.ahj.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Harskamp RE, Fanaroff AC, Lopes RD, et al. Antithrombotic therapy in patients with atrial fibrillation after acute coronary syndromes or percutaneous intervention. J Am Coll Cardiol. 2022;79(5):417-427. doi: 10.1016/j.jacc.2021.11.035 [DOI] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 11.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423-2434. doi: 10.1056/NEJMoa1611594 [DOI] [PubMed] [Google Scholar]
- 12.Cannon CP, Bhatt DL, Oldgren J, et al. ; RE-DUAL PCI Steering Committee and Investigators . Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513-1524. doi: 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- 13.Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394(10206):1335-1343. doi: 10.1016/S0140-6736(19)31872-0 [DOI] [PubMed] [Google Scholar]
- 14.Hankey GJ, Patel MR, Stevens SR, et al. ; ROCKET AF Steering Committee Investigators . Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11(4):315-322. doi: 10.1016/S1474-4422(12)70042-X [DOI] [PubMed] [Google Scholar]
- 15.Diener HC, Connolly SJ, Ezekowitz MD, et al. ; RE-LY study group . Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9(12):1157-1163. doi: 10.1016/S1474-4422(10)70274-X [DOI] [PubMed] [Google Scholar]
- 16.Rost NS, Giugliano RP, Ruff CT, et al. ; ENGAGE AF-TIMI 48 Investigators . Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: findings from ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Stroke. 2016;47(8):2075-2082. doi: 10.1161/STROKEAHA.116.013540 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Kaplan-Meier curves for ISTH major or CRNM bleeding in patients with (A) and without (B) prior stroke/TIA/TE
eFigure 2. Kaplan-Meier curves for death or hospitalization in patients with (A) and without (B) prior stroke/TIA/TE
eFigure 3. Kaplan-Meier curves for death or ischemic events in patients with (A) and without (B) prior stroke/TIA/TE
Data sharing statement