Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a chronic and progressive disease, often punctuated by recurrent flare‐ups or exacerbations. Magnesium sulfate, having a bronchodilatory effect, may have a potential role as an adjunct treatment in COPD exacerbations. However, comprehensive evidence of its effects is required to facilitate clinical decision‐making.
Objectives
To assess the effects of magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease in adults.
Search methods
We searched the Cochrane Airways Trials Register, CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, the World Health Organization (WHO) trials portal, EU Clinical Trials Register and Iranian Registry of Clinical Trials. We also searched the proceedings of major respiratory conferences and reference lists of included studies up to 2 August 2021.
Selection criteria
We included single‐ or double‐blind parallel‐group randomised controlled trials (RCTs) assessing magnesium sulfate in adults with COPD exacerbations. We excluded cross‐over trials.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently selected trials for inclusion, extracted data and assessed risk of bias. The primary outcomes were: hospital admissions (from the emergency room); need for non‐invasive ventilation (NIV), assisted ventilation or admission to intensive‐care unit (ICU); and serious adverse events. Secondary outcomes were: length of hospital stay, mortality, adverse events, dyspnoea score, lung function and blood gas measurements. We assessed confidence in the evidence using GRADE methodology. For missing data, we contacted the study investigators.
Main results
We identified 11 RCTs (10 double‐blind and 1 single‐blind) with a total 762 participants. The mean age of participants ranged from 62 to 76 years. Trials were single‐ or two‐centre trials conducted in Iran, New Zealand, Nepal, Turkey, the UK, Tunisia and the USA between 2004 and 2018. We judged studies to be at low or unclear risk of bias for most of the domains. Three studies were at high risk for blinding and other biases.
Intravenous magnesium sulfate versus placebo
Seven studies (24 to 77 participants) were included. Fewer people may require hospital admission with magnesium infusion compared to placebo (odds ratio (OR) 0.45, 95% CI 0.23 to 0.88; number needed to treat for an additional beneficial outcome (NNTB) = 7; 3 studies, 170 participants; low‐certainty evidence). Intravenous magnesium may result in little to no difference in the requirement for non‐invasive ventilation (OR 0.74, 95% CI 0.31 to 1.75; very low‐certainty evidence). There were no reported cases of endotracheal intubation (2 studies, 107 participants) or serious adverse events (1 study, 77 participants) in either group. Included studies did not report intensive care unit (ICU) admission or deaths. Magnesium infusion may reduce the length of hospital stay by a mean difference (MD) of 2.7 days (95% CI 4.73 days to 0.66 days; 2 studies, 54 participants; low‐certainty evidence) and improve dyspnoea score by a standardised mean difference of ‐1.40 (95% CI ‐1.83 to ‐0.96; 2 studies, 101 participants; low‐certainty evidence). We were uncertain about the effect of magnesium infusion on improving lung function or oxygen saturation. For all adverse events, the Peto OR was 0.14 (95% CI 0.02 to 1.00; 102 participants); however, the event rate was too low to reach a robust conclusion.
Nebulised magnesium sulfate versus placebo
Three studies (20 to 172 participants) were included. Magnesium inhalation may have little to no impact on hospital admission (OR 0.77, 95% CI 0.21 to 2.82; very low‐certainty evidence) or need for ventilatory support (NIV or mechanical ventilation) (OR 0.33, 95% CI 0.01 to 8.20; very low‐certainty evidence). It may result in fewer ICU admissions compared to placebo (OR 0.39, 95% CI 0.15 to 1.00; very low‐certainty evidence) and improvement in dyspnoea (MD ‐14.37, 95% CI ‐26.00 to ‐2.74; 1 study, 20 participants; very low‐certainty evidence). There were no serious adverse events reported in either group. There was one reported death in the placebo arm in one trial, but the number of participants was too small for a conclusion. There was limited evidence about the effect of magnesium inhalation on length of hospital stay, lung function outcomes or oxygen saturation. Included studies did not report adverse events.
Magnesium sulfate versus ipratropium bromide
A single study with 124 participants assessed nebulised magnesium sulfate plus intravenous magnesium infusion versus nebulised ipratropium plus intravenous normal saline. There was little to no difference between these groups in terms of hospital admission (OR 1.62, 95% CI 0.78 to 3.37), endotracheal intubation (OR 1.69, 95% CI 0.61 to 4.71) and length of hospital stay (MD 1.10 days, 95% CI ‐0.22 to 2.42), all with very low‐certainty evidence. There were no data available for non‐invasive ventilation, ICU admission and serious adverse events. Adverse events were not reported.
Authors' conclusions
Intravenous magnesium sulfate may be associated with fewer hospital admissions, reduced length of hospital stay and improved dyspnoea scores compared to placebo. There is no evidence of a difference between magnesium infusion and placebo for NIV, lung function, oxygen saturation or adverse events. We found no evidence for ICU admission, endotracheal intubation, serious adverse events or mortality.
For nebulised magnesium sulfate, we are unable to draw conclusions about its effects in COPD exacerbations for most of the outcomes. Studies reported possibly lower ICU admissions and a lesser degree of dyspnoea with magnesium inhalation compared to placebo; however, larger studies are required to yield a more precise estimate for these outcomes. Similarly, we could not identify any robust evidence for magnesium sulfate compared to ipratropium bromide. Future well‐designed multicentre trials with larger samples are required, including subgroups according to severity of exacerbations and COPD phenotypes.
Plain language summary
Is magnesium sulfate effective for chronic obstructive pulmonary disease (COPD) flare‐ups?
Background
COPD is a long‐standing disease of the lungs that causes airway narrowing. COPD flare‐ups are episodes of worsening symptoms in people diagnosed with COPD, described as exacerbations in this review. Magnesium sulfate is reported to be able to widen the airways to help breathing. Magnesium sulfate can be given as an infusion into the veins or as an inhalation via a device called a nebuliser. Some studies have shown it to be helpful as an add‐on to usual care in people with COPD flare‐ups. Therefore, we wanted to discover whether using magnesium sulfate was better or worse than other alternatives, such as usual care alone or placebo. Placebo is an infusion or inhalation of normal saline (salt water) through a nebuliser.
Study characteristics
We included 11 studies involving 762 people with COPD flare‐ups. These studies were funded by local health authorities, researchers or universities where researchers work. Usually, neither the participants nor the people doing the research knew which treatment the participants were getting; although in one study, treatment was known to the people who were doing the research. Studies were done in one or two centres in many countries between 2004 and 2018. The average age of participants ranged from 62 to 76 years. Seven studies tested magnesium infusion, three studies assessed magnesium inhalation, and one study examined both. The evidence in this review is current to 2 August 2021.
Key results
People who received magnesium infusion may have fewer admissions to hospital from the emergency room. Seven people with COPD flare‐ups would need to be treated with magnesium infusion to prevent one additional person being admitted to hospital. There was little to no difference in terms of breathing support without intubation (putting a tube into the windpipe to help a person to breathe). None of the participants required breathing support with intubation. Included studies did not report ICU admission or deaths. Only one trial reported on serious adverse events, but no‐one in the study experienced any. Magnesium infusion may shorten the duration of hospital stay and reduce breathlessness. However, we were not clear about its effect on lung function, oxygen concentration in blood or adverse events.
Magnesium inhalation (nebuliser) had little or no effect on hospital admission or the need for breathing support (with or without intubation) compared to placebo. Nebulised magnesium may reduce ICU admission and improve breathlessness. However, we are not confident of these findings due to small number of participants and study limitations. There is no evidence of a difference for duration of hospital stay, lung function or oxygen saturation in blood. Serious adverse events were not reported. There is no available data for adverse events. One trial reported one death in the placebo group, but we are not confident to draw any conclusion as the trial had very few participants.
Only one study compared magnesium sulfate inhalation and infusion versus inhaled ipratropium bromide and placebo infusion. We could not identify any differences between the effects of these treatments.
Limitations of the evidence
Magnesium infusion may reduce hospital admissions, shorten length of hospital stay and improve breathlessness compared to placebo. We are very uncertain about its effect on the need for breathing support, lung function or blood oxygen concentration because the studies were small. We do not have enough information to assess any effects on serious adverse events or deaths. The effects of magnesium inhalation compared to placebo or magnesium sulfate versus ipratropium bromide are unclear.
Magnesium sulfate infusion may be useful as an add‐on treatment for COPD flare‐ups. However, we cannot draw conclusions about whether magnesium sulfate inhalation is helpful for use in people with COPD flare‐ups.
Summary of findings
Summary of findings 1. Intravenous magnesium sulfate + standard care compared to placebo + standard care for acute exacerbations of chronic obstructive pulmonary disease.
| Intravenous magnesium sulfate + standard care compared to placebo + standard care for acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: acute exacerbations of chronic obstructive pulmonary disease Setting: emergency department Intervention: intravenous magnesium sulfate + standard care Comparison: placebo + standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with intravenous magnesium sulfate | |||||
| Proportion of people with hospital admissions (from the emergency room) | 593 per 1000 | 396 per 1000 (251 to 562) | OR 0.45 (0.23 to 0.88) | 170 (3 RCTs) | ⊕⊕⊝⊝ LOW a,b | |
| Proportion of people requiring non‐invasive ventilation | 382 per 1000 | 314 per 1000 (161 to 519) | OR 0.74 (0.31 to 1.75) | 107 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW a,c | There were no people who need endotracheal intubation in either magnesium or placebo groups. No trial assessed ICU admission. |
| Proportion of people with serious adverse events | There were no reported serious adverse events for magnesium and placebo groups. | ‐ | 77 (1 RCT) | ‐ | ||
| Length of hospital stay | The mean length of hospital stay ranged from 5.47 to 7.33 days | MD 2.7 days shorter (4.73 shorter to 0.66 shorter) | ‐ | 54 (2 RCTs) | ⊕⊕⊝⊝ LOW a,b | |
| Change in oxygen saturation (SpO2) | The mean change in SpO2 was 8.42% | MD 0.32% higher (1.53 lower to 2.17 higher) | ‐ | 77 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,c | |
| Change in dyspnoea score | The mean dyspnoea score ranged from 1.08 to 2.05 | SMD 1.4 lower (1.83 lower to 0.96 lower) | ‐ | 101 (2 RCTs) | ⊕⊕⊝⊝ LOW a,b | DSS score and Borg dyspnoea score were used. The lower the score, the lesser the severity of dyspnoea. |
| Lung function: FEV1 change from baseline at 60 min | The mean FEV1 at 60 min was 0.043 L | MD 0.0 L (0.04 lower to 0.05 higher) | ‐ | 30 (1 RCT) | ⊕⊕⊝⊝ LOW c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSS: dyspnoea severity score; FEV1: forced expiratory volume in 1 second; RCT: randomised controlled trial; OR: odds ratio; MD: mean difference; SpO2: peripheral oxygen saturation | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations (risk of selection, performance, detection and reporting bias) bDowngraded one level for serious imprecision (few events or wide confidence interval) cDowngraded two levels for very serious imprecision (few events and CI includes both appreciable benefit and harm)
Summary of findings 2. Nebulised magnesium sulfate + standard care compared to placebo + standard care for acute exacerbations of chronic obstructive pulmonary disease.
| Nebulised magnesium sulfate + standard care compared to placebo + standard care for acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: acute exacerbations of chronic obstructive pulmonary disease Setting: emergency department Intervention: nebulised magnesium sulfate + standard care Comparison: placebo + standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with nebulised magnesium sulfate | |||||
| Proportion of people with hospital admissions (from the emergency room) | 918 per 1000 | 896 per 1000 (702 to 969) | OR 0.77 (0.21 to 2.82) | 109 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,c | |
| Proportion of people requiring ICU admission | 109 per 1000 | 45 per 1000 (18 to 109) | OR 0.39 (0.15 to 1.00) | 281 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWd,e | |
| Proportion of people requiring ventilatory support (NIV or assisted ventilation) | 12 per 1000 | 4 per 1000 (0 to 88) | OR 0.33 (0.01 to 8.2) | 172 (1 RCT) | ⊕⊝⊝⊝ VERY LOW c,e | |
| Proportion of people with serious adverse events | No trial assessed this outcome. | |||||
| Length of hospital stay | The mean length of hospital stay was 10.2 days | MD 0.8 lower (4.63 lower to 3.03 higher) | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,c | |
| Change in oxygen saturation (SaO2) | The mean change in SaO2 was 4% | MD 1.1% lower (4.6 lower to 2.4 higher) | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,c | |
| Change in dyspnoea score | The mean dyspnoea score was 9.4 | MD 14.37 lower (26 lower to 2.74 lower) | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,d | VAS dyspnoea score was used. The higher the score, the greater the severity of dyspnoea. |
| Lung function: FEV1 at 60 min | The mean FEV1 at 60 min was 0.81 L | MD 0.05 L lower (0.17 lower to 0.07 higher) | ‐ | 109 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; ICU: intensive care unit ; RCT: randomised controlled trial; SaO2: arterial oxygen saturation; OR: odds ratio; MD: mean difference; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations (risk of selection and reporting bias) bDowngraded two levels for study limitations (risk of selection, reporting bias and very small participant numbers) cDowngraded two levels for very serious imprecision (few events and CI includes both appreciable benefit and harm) dDowngraded one level for serious imprecision (few events or CI includes non‐appreciable benefit and potential harm) eDowngraded two levels for study limitations (single blinded study with high risk of performance bias)
Summary of findings 3. Magnesium sulfate compared to standard care (ipratropium bromide) for acute exacerbations of chronic obstructive pulmonary disease.
| Magnesium sulfate compared to (standard care) ipratropium bromide for acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: acute exacerbations of chronic obstructive pulmonary disease Setting: emergency department Intervention: magnesium sulfate Comparison: ipratropium bromide | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ipratropium bromide | Risk with magnesium sulfate | |||||
| Proportion of people with hospital admissions (from the emergency room) | 323 per 1000 | 435 per 1000 (271 to 616) | OR 1.62 (0.78 to 3.37) | 124 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | |
| Proportion of people requiring endotracheal intubation | 113 per 1000 | 177 per 1000 (72 to 375) | OR 1.69 (0.61 to 4.71) | 124 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ICU admission or non‐invasive ventilation not reported |
| Proportion of people with serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Length of hospital stay | The mean length of hospital stay was 6.6 days | MD 1.1 higher (0.22 lower to 2.42 higher) | ‐ | 124 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | |
| Change in oxygen saturation (SaO2) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Change in dyspnoea score | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Lung function: FEV1 at 60 min | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; ICU: intensive care unit ; RCT: randomised controlled trial; SaO2: arterial oxygen saturation; OR: odds ratio; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for very serious imprecision (few events and CI includes both appreciable benefit and harm) bDowngraded one level for study limitations (risk of detection and other bias)
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) refers to a group of lung diseases characterised by airflow obstruction that interferes with normal breathing (American Lung Association 2013). Clinical diagnosis of COPD is considered in people who experience breathlessness, chronic cough or sputum production, with a history of exposure to known risk factors (WHO 2021). Smoking and ambient particulate matter are the main risk factors for COPD (GBD 2017). Confirmation of COPD requires spirometry to demonstrate persistent airflow limitation according to the criterion of a postbronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of less than 0.7 (GOLD 2021).
There were an estimated 3.17 million COPD‐related deaths, accounting for 5% of total deaths globally, in 2015 (WHO 2021). According to the Global Burden of Disease (GBD) study, 251 million people had COPD worldwide in 2016, and it caused 2.6% of disability‐adjusted life years in 2015 alone (GBD 2017). In 2016, chronic respiratory diseases contributed to 8.96% of worldwide non‐communicable disease deaths, of which 2.93 million deaths were due to COPD (Ngahavi 2017). In the 1990s, COPD was the sixth leading cause of death; it has become the fourth leading cause since 2000 and is the third leading cause of death worldwide, causing 3.23 million deaths in 2019 (GOLD 2021; Lopez‐Campos 2016; WHO 2020). The principal causes of death in people with mild to moderate COPD are lung cancer (26.5%) and cardiovascular disease (21.6%), while acute respiratory failure (25.8%) is the main cause of death in people with very severe COPD, based on the analysis of 2826 deaths in 13 Spanish centres (Soto‐Campos 2013). Morbidity due to COPD is also high worldwide, with 29.4 million years lost due to disability in 2015 (Lopez‐Campos 2016).
The chronic and progressive course of COPD is often punctuated by episodes of exacerbations. Exacerbations are defined as "an acute worsening of respiratory symptoms that result in additional therapy" (GOLD 2021; O'Donnell 2006; Wedzicha 2017). COPD exacerbations are more frequent in the winter months for people living in temperate climates (Jenkins 2012), and are mainly triggered by respiratory infections (Wedzicha 2007). Other non‐infective causes, such as air pollution and pulmonary embolus, can also trigger the exacerbations (Celli 2007). People experience worsening symptoms, including breathlessness or cough with increased sputum volume or purulence, and require increased use of maintenance medications. Mild exacerbations can be treated with short‐acting bronchodilators only, whereas more severe exacerbations require the addition of a course of systemic steroids or antibiotics, hospitalisation or an emergency room visit (GOLD 2021). These exacerbations, especially when frequent, can compromise quality of life (Connors 1996; David 2012; Miravitlles 2004; Seemungal 1998; Spencer 2001), accelerate lung function decline (Anzueto 2009; Celli 2008; Donaldson 2002), reduce physical capacity (Donaldson 2005; Pitta 2006), result in hospital admissions (Mullerova 2015), and increase mortality (Almagro 2002; Groenewegen 2003; Soler‐Cataluna 2005). In addition, severe COPD exacerbations that require hospital admission exert a direct and independent effect on survival, with a reported mortality rate of 50% within five years, similar to an oncologic mortality rate (Garcia‐Aymerich 2011; Nannini 2012).
Acute COPD exacerbations are reported to be more frequent in people with severe disease, with an annual exacerbation frequency of 3.43, compared with 2.68 for those with moderate disease (Anzueto 2010). Similarly, in the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End‐points) study, exacerbation rates in the first year of follow‐up were 0.85, 1.34 and 2.00 per person for people with GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage 2, 3 and 4, respectively, while 22%, 33% and 47% were reported to have two or more exacerbations over the same period (Hurst 2010). However, most of the COPD exacerbation data have been estimated in populations with moderate to severe COPD requiring hospital care, thus leading to the possibility of a higher number of less severe forms being under‐diagnosed (Borrell 2009).
Description of the intervention
Magnesium is the second most common intracellular cation in the body, found principally in bone (53%), muscle (27%) and soft tissues (19%); less than 1% of total body magnesium is present in the blood (Elin 1988; Fawcett 1999). It is involved in many biological actions, such as energy production, glycolysis (breakdown of glucose), synthesis of nucleic acids and proteins, transmembrane ion flux, regulation of adenylate cyclase, muscle contraction and neuronal activity (Costello 2016; Grober 2015; Romani 2013). It acts as a physiological calcium channel antagonist, stimulates prostacyclin and nitric oxide production, and diminishes vascular reactivity to a variety of pressor agents (drugs to increase blood pressure) (Fawcett 1999; Laires 2004). Magnesium prevents calcium ion movement into vascular and bronchial smooth muscle cells via voltage‐dependent calcium channels, so it is believed to play a major role in vasodilatation and bronchodilatation (Gourgoulianis 2001; Kew 2014; Spivey 1990). Magnesium also inhibits the release of acetylcholine from cholinergic nerve endings and histamine from mast cells, leading to possible anticholinergic and antihistamine effects (Del‐Castillo 1954). Furthermore, some evidence suggests that magnesium may reduce the neutrophilic burst of inflammatory response with a possible beneficial anti‐inflammatory effect (Cairns 1996).
Recent clinical guidelines advise that a single dose of intravenous magnesium sulfate can be considered for adults with severe life‐threatening asthma exacerbations, adults and children who fail to respond to initial treatment with persistent hypoxaemia, and children who fail to achieve 60% of predicted FEV1 value after one hour of care. The recommended dosage of intravenous magnesium sulfate for adults is 1.2 g to 2 g, delivered by infusion over 20 minutes (BTS/SIGN 2019; GINA 2018). However, routine use of magnesium sulfate in acute exacerbations of asthma is not recommended (GINA 2018). Similarly, nebulised magnesium sulfate is not routinely recommended for adults with acute asthma or children with mild to moderate asthma attacks, although 150 mg of nebulised magnesium sulfate can be considered as an adjunct to nebulised salbutamol and ipratropium in the first hour for children with severe asthma exacerbations (BTS/SIGN 2019).
How the intervention might work
The characteristic response in COPD exacerbations is increased airway inflammation, hyperinflation and gas trapping, with reduced expiratory flow accounting for increased breathlessness. Treatment of acute exacerbation of COPD aims to minimise the negative impact of the episode and prevent subsequent events. The current guidelines recommend the use of short‐acting beta2‐agonists (SABA), muscarinic antagonists, systemic corticosteroids, antibiotics and non‐invasive ventilation for COPD exacerbations (GOLD 2021).
Magnesium sulfate may have potential benefits as an adjunct therapy in acute exacerbations of COPD. This is because low serum magnesium levels are reported to be associated with an increased risk of exacerbation in people with COPD, according to a retrospective study (Aziz 2005), and a small prospective study (Gumus 2014). Moreover, studies have reported that hypomagnesaemia (low serum magnesium level) is an independent predictor of frequent readmission for acute exacerbations of COPD (Bhatt 2008), or exacerbation frequency in people with COPD (Gumus 2014).
Intravenous magnesium sulfate, in addition to bronchodilators, reduces hospital admissions and improves lung function when the response to bronchodilators during acute asthma exacerbations is inadequate (Kew 2014; Rowe 2000). However, evidence for the use of inhaled magnesium sulfate during acute exacerbations of asthma, either alone or in addition to bronchodilators, does not demonstrate clinically important benefits, and further trials are needed to establish its usefulness (Knightly 2017).
Over the past few years, there has been a marked interest in a subset of people with airways disease who have features of both asthma and COPD, known as asthma‐COPD overlap (ACO) (Cosio 2018). People with asthma who smoke are reported to have more symptoms than people with asthma who do not smoke (Leung 2017). In the absence of a standard definition for ACO diagnosis, the prevalence estimates vary from 3.2% in the USA (Kumbhare 2016), to 11.1% in Italy (Sorino 2016). The prevalence of ACO ranges from 6% to 55% in cohorts of people with COPD, and from 10% to 31% in cohorts of people with asthma (Leung 2017). People with ACO have more severe and frequent exacerbations, and have thicker airway walls than people with COPD alone (Hardin 2014), leading to more hospitalisations and emergency department visits (Kumbhare 2016). Furthermore, they have a significantly lower quality of life (Kauppi 2011), a more rapid decline in lung function (Lange 2016), higher disease burden (including respiratory symptoms and activity limitation) (Hines 2017), and a higher mortality rate compared to people with asthma or COPD alone (Gibson 2009; Sorino 2016). As some people with COPD may also have asthmatic features, it is reasonable to assume there may be some benefits of magnesium sulfate for acute exacerbations of COPD, as well as for acute asthma. Moreover, bronchodilatation (Spivey 1990), anticholinergic (Del‐Castillo 1954) and anti‐inflammatory properties of magnesium (Cairns 1996) could lead to potential therapeutic effects for acute exacerbations of COPD.
Why it is important to do this review
Exacerbations play a major role in the morbidity and mortality of people with COPD, resulting in a significant health burden. Therefore, a potentially effective add‐on treatment would be useful for people with COPD and healthcare providers. The potential clinical benefits of intravenous or nebulised magnesium sulfate for acute exacerbations of COPD have been studied; however, published studies have found conflicting and inconclusive results for its effectiveness. A non‐Cochrane systematic review on magnesium sulfate reported that it appeared to potentiate the bronchodilatory effect of inhaled beta2‐agonists, but did not find differences in dyspnoea scores, hospital admission rates, or emergency department readmission rates, compared to placebo (Shivanthan 2014). Another recent review demonstrated a reduction in hospital admissions in people with COPD exacerbations receiving magnesium sulfate infusion (Jahangir 2022). Currently, standard guidelines do not recommend magnesium sulfate as a treatment for acute exacerbation of COPD, but it is nonetheless used by some clinicians in practice. Therefore, we would like to establish evidence regarding its usage as an adjunct treatment for acute exacerbations of COPD in people not responding to conventional measures, based on current available data from randomised controlled trials.
Objectives
To assess the effects of magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with a parallel‐group design, regardless of the language in which they were published. We included studies reported in full text, those published as an abstract only and unpublished data. We excluded studies with a cross‐over design, due to the carry‐over effects of the intervention.
Types of participants
We included adults aged 35 years and over with acute exacerbations of COPD (defined as a worsening of a previously stable condition with increasing respiratory symptoms, particularly dyspnoea, cough, sputum production and increased sputum purulence). We included studies where diagnosis of COPD was physician‐diagnosed or guideline‐based, according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (GOLD 2021), the American Thoracic Society (ATS) and European Respiratory Society (ERS) (ATS/ERS 2011), the Thoracic Society of Australia and New Zealand (TSANZ) (Yang 2019), or the UK National Institute for Health and Care Excellence (NICE) (NICE 2019).
We planned to include trials that assessed participants with mixed COPD and asthma features (asthma‐COPD overlap, ACO), based on the consensus published by the Global Initiative for Asthma (GINA) and GOLD (GOLD ACO 2015), provided that the trials reported outcomes separately for the different participant groups. We excluded participants with the following comorbidities or characteristics: pneumothorax, bronchiectasis, cystic fibrosis, other chronic lung diseases or heart failure.
If we found trials in which only a subset of participants had a diagnosis of COPD, we had planned to include these participants if we could obtain disaggregated data from the trial authors. We also planned to include studies that recruited participants with other pulmonary diseases, provided the results of a subset of participants with COPD were available to extract separately. However, we did not encounter such studies.
Types of interventions
We included studies that compared magnesium sulfate (irrespective of dose and route of administration, such as intravenous or inhalation), as an adjunct to standard therapy for acute exacerbation of COPD.
We compared magnesium sulfate with standard therapy (ipratropium bromide), or with a placebo. We allowed standard therapy as co‐interventions, provided that they were not part of the randomised treatment: e.g. systemic corticosteroids; antibiotics; short‐acting bronchodilators, such as salbutamol or ipratropium bromide; mucolytics; intravenous aminophylline or oxygen therapy.
For intravenous magnesium sulfate, we studied the following comparisons.
Intravenous magnesium sulfate + standard care versus placebo + standard care
Intravenous magnesium sulfate + standard care versus standard care
For inhaled/nebulised magnesium sulfate, we studied the following comparisons.
Inhaled magnesium sulfate + standard care versus placebo + standard care
Inhaled magnesium sulfate + standard care versus standard care
Types of outcome measures
Primary and secondary outcomes of this review are as follows.
Primary outcomes
Proportion of people with hospital admissions (from the emergency room)
Proportion of people requiring non‐invasive ventilation (NIV), assisted ventilation or admission to intensive‐care unit (ICU)
Proportion of people with serious adverse events
Secondary outcomes
Length of hospital stay (inpatients) or time to emergency room discharge (outpatients)
Proportion of people with all‐cause mortality
Proportion of people with adverse events/side effects
Arterial‐blood gas measurements: arterial partial pressure of carbon dioxide (PaCO2), arterial partial pressure of oxygen (PaO2) and pH
Lung function measurements: forced expiratory volume in the first second (FEV1), if available, or peak expiratory flow rate (PEFR) if the trial did not report FEV1
Symptom scores measuring breathlessness, cough and sputum production using validated scales; e.g. Exacerbations of Chronic Pulmonary Disease Tool (EXACT) total score
If the trial measured arterial‐blood gas, lung function and symptom scores at multiple time points, we used the data at (or as close as possible to) 60 minutes postbaseline for meta‐analysis. We chose this time point as we expected that most participants will have a response to treatment within an hour, and to maximise the homogeneity of pooled results. Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from searches of the following databases and trial registries up to 2 August 2021, with no restriction on language or type of publication:
Cochrane Airways Trials Register (Cochrane Airways 2019), via the Cochrane Register of Studies, all years to date;
Cochrane Central Register of Controlled Trials (CENTRAL), via the Cochrane Register of Studies, all years to date;
MEDLINE OvidSP, 1946 to date;
Embase OvidSP, 1974 to date;
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch);
EU Clinical Trials Register;
Iranian Registry of Clinical Trials.
The search strategies for each database are in Appendix 1. The Cochrane Airways Information Specialist developed and conducted the searches, in collaboration with the authors. We followed the Cochrane guidance for developing search strategies and applying study design filters (Lefebvre 2021).
Searches of the Cochrane Airways Trials Register and the CENTRAL database incorporated handsearched conference abstracts and grey literature.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We also searched on PubMed for errata or retractions from included studies published in full text, on 8 November 2021.
Data collection and analysis
Selection of studies
Two review authors (HN and SZA) screened the titles and abstracts of the search results independently, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports of all potentially eligible studies, and two review authors (HN and SZA) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third review author (CN). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we used as a pilot on one study in the review. Two review authors (HN and SZA) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of trial authors.
Two review authors (HN and SZA) independently extracted outcome data from included studies. We added notes in the characteristics of included studies table if a trial did not report outcome data in a usable way. We resolved disagreements by consensus as well as by involving a third review author (CN). One review author (HN) transferred data into the Review Manager file (Review Manager 2020) or RevMan Web 2022. We double‐checked that we entered the data correctly by comparing the data presented in the systematic review with the study reports. A second review author (SZA) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (HN and SZA) assessed risk of bias independently for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We resolved any disagreements by discussion as well as by involving another author (CN). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We judged each potential source of bias as high, low or unclear, and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we included a note in the risk of bias table.
When considering treatment effects, we took into account the risk of bias for the studies which contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and justified any deviations from it in the Differences between protocol and review section of this systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR) and continuous data as the mean difference (MD) or standardised mean difference (SMD). For rare events, we used Peto ORs. When we combined data from rating scales in a meta‐analysis, we ensured that we entered these with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only where this was meaningful; that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We described skewed data narratively (for example, as medians and interquartile ranges for each group).
If a trial reported both change‐from‐baseline and endpoint scores for continuous data, we used change‐from‐baseline data. If a study reported outcomes at multiple time points, we used the data collected at or as close as possible to 60 minutes postbaseline.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses where trials reported these (i.e. those where trialists had imputed data for participants who were randomly assigned, but did not complete the study), instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. the number of participants with a hospital admission rather than the number of admissions per participant). We planned to analyse on the basis of events rather than participants if a trial reported rate ratios; however, none of the included studies in this review used rate ratios.
Where a single study reported multiple trial arms, we included only the relevant arms. If we combined two comparisons in the same meta‐analysis (e.g. intravenous magnesium sulfate versus placebo and inhaled magnesium sulfate versus placebo), we halved the control group to avoid double‐counting.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when we only identified a study as an abstract). Where this was not possible, and we thought the missing data could introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis, and interpreted this following Higgins 2021, as:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When we identified substantial heterogeneity (I² > 50%), we reported it and explored the possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We planned to create and examine a funnel plot to explore possible small study and publication biases if we were able to pool more than 10 studies (Higgins 2021). Though we identified 11 included studies, most analyses in this review included only one or two studies.
Data synthesis
We used a random‐effects model, and performed a sensitivity analysis with a fixed‐effect model. As we gathered data from a series of studies performed by different researchers operating independently, the studies were not all functionally equivalent with a common effect estimate. Therefore, the random‐effects model was more justified than the fixed‐effect model. We used a fixed‐effect model for analyses using the Peto OR method as it required this type of model.
Subgroup analysis and investigation of heterogeneity
We had planned to carry out the following subgroup analyses:
concomitant treatment with systemic corticosteroids (yes versus no);
blood eosinophil count (≥ 300/µL versus < 300/µL);
COPD versus asthma‐COPD overlap
In each subgroup analysis, we had planned to use the following outcomes:
need for admission to hospital (from the emergency department);
need for NIV, assisted ventilation or admission to ICU;
length of hospital stay (inpatients) or time to emergency room discharge (outpatients).
However, we were unable to carry out subgroup analyses as none of the included trials reported data separately for the planned subgroups.
Sensitivity analysis
We included all trials, irrespective of risk of bias, in the primary analysis.
We had planned to carry out the following sensitivity analyses for the primary outcomes:
removing studies with unclear or high risk of performance or detection bias due to lack of appropriate blinding;
comparing the results from inclusion and exclusion of imputed data values;
comparing the results from a fixed‐effect model with those from a random‐effects model.
However, we did not carry out these sensitivity analyses as they were not applicable for this review.
Summary of findings and assessment of the certainty of the evidence
We created three summary of findings tables, one for each comparison, using the following outcomes:
proportion of people with hospital admissions (from the emergency room);
proportion of people requiring non‐invasive ventilation, endotracheal intubation, ventilatory support (NIV or assisted ventilation) or ICU admission;
proportion of people with serious adverse events;
length of hospital stay;
change in oxygen saturation (SaO2/SpO2);
change in dyspnoea score;
lung function: FEV1 at 60 min.
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence in relation to the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021), using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Refer to Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies for details.
Results of the search
We performed an initial search of the databases (Cochrane Airways Group Register of trials, CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, WHO ICTRP) in December 2019, an updated search in April 2021, and authors updated the search in August 2021. We also searched the reference lists of all primary studies and review articles. From the search, we identified a total of 376 records, of which 127 were duplicates. We screened the titles and abstracts of the remaining 249 records, out of which we excluded 207 reports. We assessed 42 full‐text articles for eligibility and excluded 18 studies (23 references). One trial was ongoing, and 11 studies (18 references) met the inclusion criteria of our review. For details of the search results, see Figure 1.
1.
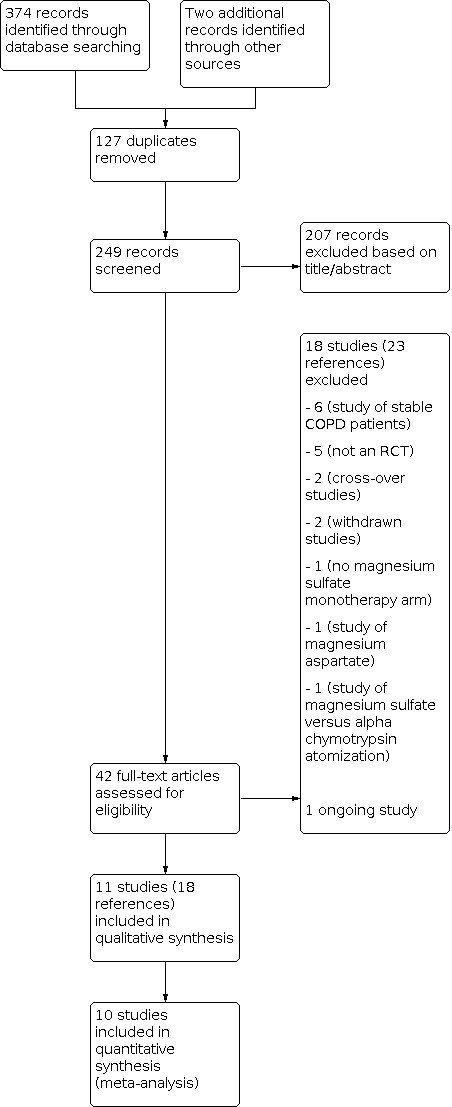
Study flow diagram
Included studies
For details, see Characteristics of included studies and Table 4.
1. Overview of included studies.
| Study ID | Study centres | Study location | Number randomised | Intervention | Comparison | Concomitant treatment | Hospitalisations in the last 12 months, n (%) | ICU admission in the last 12 months | Serum magnesium level: mean (SD) |
| Bajracharya 2021 | Single | Nepal | 172 | Nebulised MgSO4 + Nebulised salbutamol | Placebo + nebulised salbutamol | Oxygen, ipratropium, hydrocortisone | NR | NR | NR |
| Comert 2016 | Single | Turkey | 20 | Nebulised MgSO4 + standard care | Placebo + standard care | Oxygen, steroids, antibiotics and ipratropium | MgSO4: 3 (30) Placebo: 4 (40) |
MgSO4: 0 Placebo: 0 |
NR |
| Edwards 2013 | Two | New Zealand | 116 | Nebulised MgSO4 + Nebulised salbutamol | Placebo + nebulised salbutamol | Oxygen | MgSO4: 1 Placebo: 1.3 |
NR | mmol/L MgSO4: 0.81 (0.08) Placebo: 0.78 (0.10) |
| Hogg 2004 | Single | UK | 24 | IV MgSO4 infusion | Placebo | NR | NR | NR | NR |
| Jahanian 2021 | Single | Iran | 60 | IV MgSO4 infusion | Placebo | Oxygen, inhaled salbutamol and ipratropium, intravenous hydrocortisone | NR | NR | NR |
| Moradi 2021 | Single | Iran | 77 | IV MgSO4 infusion | Placebo | Oxygen, nebulised salbutamol, nebulised ipratropium, IV hydrocortisone, IV antibiotics | NR | NR | NR |
| Mukerji 2015 | Single | New Zealand | 33 | IV MgSO4 infusion | Placebo | Nebulised salbutamol | NR | NR | mmol/L MgSO4: 0.79 (0.1) Placebo: 0.78 (0.1) |
| Nouira 2014 | Two | Tunisia | 124 | Nebulised MgSO4 + IV MgSO4 infusion | Nebulised ipratropium + IV normal saline | IV methyl prednisolone, antibiotics, and nebulised terbutaline | MgSO4: 3.6 (2.4) Ipratropium: 3.1 (1.7) |
NR | NR |
| Pishbin 2018 | Single | Iran | 34 | IV MgSO4 infusion + standard care | Placebo + standard care | NR | NR | NR | NR |
| Skorodin 1995 | Two | USA | 72 | IV MgSO4 infusion | Placebo | Nebulised albuterol | NR | NR | mmol/L MgSO4: 0.94 (0.16) Placebo: 0.98 (0.16) |
| Solooki 2014 | Single | Iran | 30 | IV MgSO4 infusion + standard treatment | Placebo + standard treatment | Bronchodilators, oxygen, corticosteroid and antibiotics | MgSO4: 9 (60) Placebo: 10 (67) | NR | mEq/L MgSO4: 2.11 (0.28) Placebo: 2.05 (0.40) |
Abbreviations: MgSO4: magnesium sulfate; NR: not reported
Study Design
Ten included studies were double‐blind randomised controlled trials that assessed magnesium sulfate in COPD exacerbations, while one study was a single‐blind RCT (Bajracharya 2021). Eight studies were single‐centre trials (Bajracharya 2021; Comert 2016; Hogg 2004; Jahanian 2021; Moradi 2021; Mukerji 2015; Pishbin 2018; Solooki 2014). These were conducted in Iran (Jahanian 2021; Moradi 2021; Pishbin 2018; Solooki 2014), New Zealand (Mukerji 2015), Nepal (Bajracharya 2021), Turkey (Comert 2016) and the UK (Hogg 2004). The other three trials were conducted at two centres in New Zealand (Edwards 2013), Tunisia (Nouira 2014), and the USA (Skorodin 1995). The dates of study were not available for four trials (Hogg 2004; Pishbin 2018; Skorodin 1995; Solooki 2014), while the other studies were performed between 2004 and 2018.
Participants
The studies randomised a total of 762 participants; Bajracharya 2021 was the largest trial with 172 participants, while Comert 2016 was the smallest with only 20 participants. The studies included male and female adult participants over 35 years old, with a mean age range from 62 to 76 years. Participants had clinically diagnosed COPD and presented with an exacerbation, precipitated by either infection or non‐infective causes. Definitions for acute exacerbations were mentioned in four studies (Comert 2016; Edwards 2013; Jahanian 2021; Nouira 2014), while other trials did not specify these (refer to Characteristics of included studies for details). Participants presenting to the emergency department were included in all trials except Hogg 2004, which included hospital inpatients. None of the trials classified the severity of COPD exacerbations.
Interventions
Seven trials assessed intravenous magnesium sulfate infusion plus standard care versus placebo plus standard care (Hogg 2004; Jahanian 2021; Moradi 2021; Mukerji 2015; Pishbin 2018; Skorodin 1995; Solooki 2014). Three trials studied nebulised magnesium sulfate plus standard care versus placebo plus standard care (Bajracharya 2021; Comert 2016; Edwards 2013). Nouira 2014 studied nebulised magnesium sulfate plus intravenous magnesium sulfate versus nebulised ipratropium bromide plus intravenous normal saline. Participants in all trials received standard initial treatment before allocation to intervention arms. The standard care included supplemental oxygen via nasal cannula, nebulised salbutamol or ipratropium bromide, or both. In eight trials they also received systemic corticosteroids (Bajracharya 2021; Comert 2016; Edwards 2013; Jahanian 2021; Moradi 2021; Mukerji 2015; Nouira 2014; Solooki 2014). Participants in four studies received antibiotics (Comert 2016; Moradi 2021; Nouira 2014; Solooki 2014).
The dose of magnesium sulfate was 1.2 to 2.0 g infused over 20 minutes, except in Moradi 2021 where 2.5 g was infused over 15 minutes. For inhaled magnesium sulfate, the dose administered was 150 mg per dose. Magnesium was administered 20 minutes after initial standard treatment with no improvement in lung function in four trials (Bajracharya 2021; Edwards 2013; Moradi 2021; Skorodin 1995). It was administered concurrently or immediately after the standard therapy in three trials (Mukerji 2015; Nouira 2014; Solooki 2014). Jahanian 2021 reported that magnesium was given within the first 60 minutes of standard therapy. There was no information on timing of magnesium administration in the remaining three studies (Comert 2016; Hogg 2004; Pishbin 2018).
Comparison
With the exception of Nouira 2014, all trials compared intervention to placebo, with both arms receiving standard care. Nouira 2014 compared magnesium sulfate (both nebulised and IV) with nebulised ipratropium bromide and IV normal saline.
Primary outcomes
Four trials reported the proportion of participants who needed hospital admission (Edwards 2013; Mukerji 2015; Nouira 2014; Skorodin 1995), and five reported the proportion of participants who need NIV, mechanical ventilation or ICU admission as one of their outcomes (Bajracharya 2021; Edwards 2013; Moradi 2021; Mukerji 2015; Nouira 2014).
Secondary outcomes
Length of hospital stay was reported in four trials (Hogg 2004; Mukerji 2015; Nouira 2014; Solooki 2014), while only two studies reported hospital death rate (Bajracharya 2021; Nouira 2014). Arterial blood gas was one of the outcomes in four trials (Comert 2016; Jahanian 2021; Nouira 2014; Solooki 2014). Most trials measured lung function as a primary outcome; five trials reported FEV1 (Comert 2016; Edwards 2013; Jahanian 2021; Mukerji 2015; Solooki 2014); and six reported PEFR (Bajracharya 2021; Comert 2016; Moradi 2021; Nouira 2014; Skorodin 1995; Solooki 2014. The trials measured dyspnoea score in various ways: a visual analogue scale (VAS) dyspnoea score (Comert 2016); Borg dyspnoea score (Hogg 2004; Jahanian 2021); dyspnoea severity score (DSS) score (Moradi 2021) and unspecified (Nouira 2014; Skorodin 1995).
Funding
Included studies were funded by the researcher (Comert 2016), affiliated universities (Jahanian 2021; Moradi 2021; Nouira 2014), or local health councils (Edwards 2013; Mukerji 2015; Skorodin 1995). There was no information on funding for the other four trials (Bajracharya 2021; Hogg 2004; Pishbin 2018; Solooki 2014).
Excluded studies
We excluded a total of 18 trials during the full‐text review; six trials assessed the effect of magnesium in people with stable COPD (ACTRN12608000502336; Ahmed 2020; Amaral 2012; NCT01118936 2010; NCT02680769 2016; Tagaya 2004), five studies were not RCTs (CTRI/2018/01/011354 2018; CTRI/2018/04/013309 2018; Jenner 2004; Schenk 2001; Sternfeld 1994), two trials were withdrawn (ISRCTN65174202 2006; NCT02498496 2015), another two were of cross‐over design (Abreu 2006; Marino 1999), one studied magnesium aspartate (Friemann 1991), one trial had no magnesium monotherapy arm (Skorodin 1998), and another study assessed inhaled magnesium sulfate versus alpha chymotrypsin atomisation inhalation (Fan 2015). See Characteristics of excluded studies for details.
Risk of bias in included studies
Refer to Figure 2 for an overview of risk of bias assessment for the included studies. Support for judgements for individual domains are presented in Characteristics of included studies tables.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Of the 11 included studies, we judged five to be of low risk of bias for random sequence generation (Edwards 2013; Jahanian 2021; Moradi 2021; Mukerji 2015; Nouira 2014); the remaining six studies had an unclear risk for this domain due to limited information about how the randomisation process was performed. Six studies reported allocation concealment methods (Bajracharya 2021; Edwards 2013; Jahanian 2021; Moradi 2021; Mukerji 2015; Skorodin 1995). The other trials gave no further information, so we judged them to have an unclear risk for this domain.
Blinding
Ten included studies were double‐blind RCTs, where participants and investigators were unaware of the intervention arm they were involved in (performance bias). However, only seven trials reported detailed process of blinding with low risk of performance bias. Solooki 2014 did not provide details, while Hogg 2004 and Pishbin 2018 were available as abstracts only, and we judged these to be at unclear risk. Bajracharya 2021 was a single‐blind study where the investigator was aware of the assignment; we judged this to be of high risk. Blinding of outcome assessors (detection bias) was at low risk of bias for four studies (Comert 2016; Moradi 2021; Mukerji 2015; Nouira 2014), and unclear for seven studies due to limited information (Bajracharya 2021; Edwards 2013; Hogg 2004; Jahanian 2021; Pishbin 2018; Skorodin 1995; Solooki 2014).
Incomplete outcome data
We rated eight studies to have a low risk of attrition bias: six studies had no dropouts (Bajracharya 2021; Comert 2016; Jahanian 2021; Nouira 2014; Skorodin 1995; Solooki 2014), and two had a low attrition rate (Edwards 2013; Mukerji 2015). We considered the remaining three studies to be at unclear risk due to limited information (Hogg 2004; Pishbin 2018), or no details about the breakdown of dropouts across the intervention arms though the total number lost to follow‐up was reported (Moradi 2021).
Selective reporting
Three studies had a low risk of bias for selective reporting because the trial protocol was available on registries and all outcomes were reported as planned (Jahanian 2021; Moradi 2021; Mukerji 2015). We considered the other studies to be at unclear risk for reporting bias for various reasons: trial could not be identified on registry websites (Bajracharya 2021; Comert 2016; Skorodin 1995; Solooki 2014); only available as abstract (Hogg 2004; Pishbin 2018); registered on website with no outcomes provided in the protocol (Nouira 2014); and failed to report one of the outcomes stated in the trial protocol on the registry website (Edwards 2013).
Other potential sources of bias
We assumed that the risk of other bias was likely to be high for Hogg 2004 and Pishbin 2018, as there were no full‐text publications available and the reason for not publishing was not clear. We did not identify any other potential sources of bias for the remaining studies
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1; Table 2 and Table 3.
1. Intravenous magnesium sulfate + standard care compared to placebo + standard care
Proportion of people with hospital admissions (from the emergency room)
Three studies (170 participants) reported the number of participants who needed hospital admission from the emergency room. The result of the analysis suggests a reduction in hospitalisation with intravenous magnesium sulfate compared to placebo (OR 0.45, 95% CI 0.23 to 0.88; I2 = 0%; Analysis 1.1). In absolute terms, there was a reduction of 197 participants per 1000 with magnesium sulfate infusion compared to placebo for hospital admissions (95% CI 31 lower to 342 lower); the number needed to treat for an additional beneficial outcome (NNTB) is seven. Using GRADE, we judged the certainty of the evidence to be low due to serious concerns about study limitations and imprecision due to few events.
1.1. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 1: Proportion of people with hospital admissions (from the emergency room)
Proportion of people needing non‐invasive ventilation, assisted ventilation or admission to intensive care unit
The need for NIV and endotracheal intubation was reported by Moradi 2021and Mukerji 2015, but no trial assessed ICU admission. The pooled analysis for NIV indicated no evidence of a difference between magnesium and placebo arms (OR 0.74, 95% CI 0.31 to 1.75; I2 = 0%; Analysis 1.2). We graded the level of certainty as very low due to concerns over study limitations and imprecision. There were no people who needed endotracheal intubation in either the magnesium or placebo groups in either of the studies (107 participants).
1.2. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 2: Proportion of people with need for non‐invasive ventilation (NIV)
Proportion of people with serious adverse events
There were no reported serious adverse events for magnesium and placebo groups in Moradi 2021 (77 participants). Other trials did not assess this outcome.
Length of hospital stay (inpatients) or time to emergency room discharge (outpatients)
Two trials (54 participants) reported on the length of hospital stay for inpatients (Hogg 2004; Mukerji 2015), but no study assessed time to discharge from the emergency room. There was a mean difference in favour of magnesium infusion of ‐2.70 days (95% CI ‐4.73 to ‐0.66; I2 = 0%; Analysis 1.3). Using GRADE, we downgraded the certainty of evidence by two levels to low due to concerns over study limitations (Hogg 2004 is available as abstract only) and imprecision (few events).
1.3. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 3: Length of hospital stay (days)
Proportion of people with all‐cause mortality
No study reported this outcome.
Proportion of people with adverse events/side effects
Mukerji 2015 and Skorodin 1995 reported on adverse events, with no adverse events in the magnesium group in either of the trials. However, four participants in the placebo arm reported adverse events; one had a flushing feeling in the hands and face (Mukerji 2015), and three experienced nausea and weakness, dizziness and increased secretions (Skorodin 1995). Though the Peto odds ratio was 0.14 (95% CI 0.02 to 1.00; I2 = 0%; 102 participants; Analysis 1.4), the small number of participants and low event rate limit our confidence in conclusions.
1.4. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 4: Proportion of people with adverse events
Arterial‐blood gas measurements
No study measured arterial‐blood gas (ABG) values. However, Moradi 2021 (77 participants) reported the change in oxygen saturation (SaO2). Jahanian 2021 also assessed oxygen saturation before and 45 minutes after the intervention, but change from baseline data values were not available to be included in the analysis. The mean difference was 0.32% (95% CI ‐1.53 to 2.17; Analysis 1.5), indicating little to no difference between the groups. We graded the certainty of evidence as very low due to concerns over study limitations and very serious imprecision (few events and the confidence interval includes both appreciable benefit and appreciable harm).
1.5. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 5: Change in SpO 2 (%)
Lung function measurements
Mukerji 2015 assessed change from baseline in FEV1 at 60 minutes, whereas Jahanian 2021 reported post‐intervention FEV1 at 45 minutes. Two studies measured PEFR in L/min (Skorodin 1995; Solooki 2014), and Moradi 2021 reported change in PEFR as percentage of predicted value.
There was a mean difference of 0.00 L (95% CI ‐0.04 to 0.05) for change in FEV1 at 60 minutes, indicating no evidence of a difference between the interventions (Analysis 1.6). Using GRADE, we downgraded the certainty of evidence two levels to low due to very serious imprecision as the confidence interval includes both appreciable benefit and appreciable harm and few events.
1.6. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 6: FEV 1 change from baseline at 60 min (L)
Post‐intervention FEV1 was not different between intravenous magnesium and placebo at 45 minutes (MD 2.10 mL, 95% CI ‐0.89 to 5.09; Analysis 1.7).
1.7. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 7: FEV 1 at 45 min (mL)
Similarly, no evidence of a difference was noted between magnesium and placebo for the change in PEFR (MD 9.12 L/min, 95% CI ‐6.20 to 24.44; I2 = 56%; 102 participants; Analysis 1.8). However, for the change in PEFR % predicted, there was a mean difference of 10.64 % predicted (95% CI 8.38 to 12.90), in favour of magnesium sulfate infusion (Analysis 1.9).
1.8. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 8: Change in PEFR (L/min)
1.9. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 9: Change in PEFR (% predicted)
Symptom scores measuring breathlessness, cough and sputum production using validated scales
Symptom score was measured in three trials using different validated scales; DSS score in Moradi 2021, and Borg dyspnoea score in Hogg 2004; Jahanian 2021. Data for change in dyspnoea score from baseline for individual intervention arms was not available for Jahanian 2021 so we could not include it in the analysis. There was a reduction in dyspnoea score, with a standardised mean difference of ‐1.40 (95% CI ‐1.83 to ‐0.96; I2 = 0%; 101 participants; Analysis 1.10), indicating a difference in favour of magnesium infusion. We graded the level of certainty for this as low due to concerns over study limitations and imprecision (few events).
1.10. Analysis.

Comparison 1: Intravenous magnesium sulfate + standard care versus placebo + standard care, Outcome 10: Change in dyspnoea score
2. Nebulised magnesium sulfate + standard care compared to placebo + standard care
Proportion of people with hospital admissions (from the emergency room)
Edwards 2013 reported hospital admissions in 109 participants. There was little to no difference between the groups (OR 0.77, 95% CI 0.21 to 2.82). We graded the certainty of evidence as very low due to concerns over study limitations and very serious imprecision as the confidence interval includes both appreciable benefit and appreciable harm (Analysis 2.1).
2.1. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 1: Proportion of people with hospital admissions (from the emergency room)
Proportion of people needing non‐invasive ventilation, assisted ventilation or admission to intensive care unit
Two studies reported ICU admissions (Bajracharya 2021; Edwards 2013), with no events in the study by Edwards 2013. With 281 participants, magnesium inhalation may be associated with fewer ICU admissions compared to placebo (OR 0.39, 95% CI 0.15 to 1.00; Analysis 2.2). However, the certainty of evidence was very low as the single‐blinded study Bajracharya 2021 had a high risk of performance bias, and there were few events.
2.2. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 2: Proportion of people who need ICU admission
There was little to no difference between nebulised magnesium and placebo in requirement for ventilatory support (either NIV or assisted ventilation) in Bajracharya 2021 (OR 0.33, 95% CI 0.01 to 8.20; 172 participants; Analysis 2.3; very low‐certainty evidence).
2.3. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 3: Proportion of people who need ventilatory support (non‐invasive ventilation or assisted ventilation)
There were no reported cases of NIV requirement (Comert 2016; Edwards 2013; 129 participants) or endotracheal intubation (Comert 2016, 20 participants) for magnesium and placebo groups.
Proportion of people with serious adverse events
There were no serious adverse events for magnesium and placebo groups (Comert 2016, 20 participants).
Length of hospital stay (inpatients) or time to emergency room discharge (outpatients)
Comert 2016 reported length of hospital stay for inpatients, but no studies reported time to emergency room discharge for outpatients. There was little to no difference between magnesium and placebo groups for the length of hospital stay, with a mean difference of ‐0.80 days (95% CI ‐4.63 to 3.03; Analysis 2.4). The certainty of evidence for this outcome was very low due to concerns over study limitations and very serious imprecision (few events and the confidence interval includes both appreciable benefit and appreciable harm).
2.4. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 4: Length of hospital stay (days)
Proportion of people with all‐cause mortality
Bajracharya 2021 reported one death in the placebo arm and no deaths in the magnesium arm. However, the number of participants and the event rate are too small to reach a conclusion (OR 0.33, 95% CI 0.01 to 8.20; Analysis 2.5). There was no report on this outcome in Comert 2016 and Edwards 2013.
2.5. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 5: All‐cause mortality
Proportion of people with adverse events/side effects
There were no reported adverse events for either magnesium or placebo arms (Comert 2016; Edwards 2013; 129 participants).
Lung function measurements:
Edwards 2013 reported FEV1 at 60 minutes for 109 participants. There was little to no difference between the groups, with a mean difference of ‐0.05 L (95% CI ‐0.17 to 0.07; Analysis 2.6). We graded the evidence as very low certainty due to study limitations and very serious imprecision (few events and the confidence interval includes both appreciable benefit and appreciable harm).
2.6. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 6: FEV 1 at 60 min (L)
Comert 2016 reported change from baseline in PEFR at 60 minutes in 20 participants, with a mean difference of 7.60 L/min (95% CI ‐4.38 to 19.58) between magnesium and placebo groups, indicating little to no difference between the groups (Analysis 2.7).
2.7. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 7: PEFR change from baseline at 60 min (L/min)
Bajracharya 2021 assessed post‐intervention PEFR at 60 minutes in 172 participants. Magnesium inhalation was associated with a higher PEFR compared to the placebo group, with a mean difference of 7.40 L/min (95% CI 1.81 to 12.99; Analysis 2.8). However, data were too limited for us to be able to reach a conclusion.
2.8. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 8: PEFR at 60 min (L/min)
Symptom scores measuring breathlessness, cough and sputum production using validated scales
Comert 2016 assessed improvement in symptoms using the VAS dyspnoea score in 20 participants, and revealed a mean difference in favour of nebulised magnesium of ‐14.37 points (95% CI ‐26.00 to ‐2.74; Analysis 2.9); the evidence had a very low level of certainty due to concerns over study limitations and small participant numbers. The effect estimate of 14.37 points lower is more than the minimal clinically important difference (MCID) of 10 units for the VAS dyspnoea score (Ries 2005). However, the numbers of participants and events were too small to reach a robust conclusion.
2.9. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 9: Change in dyspnoea score
Arterial‐blood gas measurements:
Comert 2016 reported change in SaO2 in 20 participants, with a mean difference of ‐1.10 (95% CI ‐4.60 to 2.40), indicating little to no difference between magnesium and placebo (Analysis 2.10). We graded the certainty of evidence for this outcome as very low due to concerns over study limitations and very serious imprecision (few events and the confidence interval includes both appreciable benefit and appreciable harm).
2.10. Analysis.

Comparison 2: Nebulised magnesium sulfate + standard care versus placebo + standard care, Outcome 10: Change in SaO 2 (%)
3. Magnesium sulfate compared to standard care (ipratropium bromide)
Proportion of people with hospital admissions (from the emergency room)
There was little to no difference between magnesium sulfate and ipratropium bromide for hospital admission (OR 1.62, 95% CI 0.78 to 3.37; Nouira 2014; 124 participants; Analysis 3.1). The certainty of evidence for this outcome was very low due to very serious imprecision (the confidence interval includes both appreciable benefit and appreciable harm, and there were few events), as well as concerns over study limitations.
3.1. Analysis.

Comparison 3: Magnesium sulfate versus standard care (ipratropium bromide), Outcome 1: Proportion of people with hospital admissions (from the emergency room)
Proportion of people needing non‐invasive ventilation, assisted ventilation or admission to intensive care unit
Nouira 2014 reported the need for endotracheal intubation in 124 participants. Analysis indicated little to no difference between the groups (OR 1.69, 95% CI 0.61 to 4.71; Analysis 3.2). We graded the certainty of evidence for this outcome as very low due to very serious imprecision (the confidence interval includes both appreciable benefit and appreciable harm, and there were few events), as well as concerns over study limitations.
3.2. Analysis.

Comparison 3: Magnesium sulfate versus standard care (ipratropium bromide), Outcome 2: Proportion of people with the need for endotracheal intubation
There was no report on non‐invasive ventilation or admission to ICU.
Proportion of people with serious adverse events
There was no report on this outcome.
Length of hospital stay (inpatients) or time to emergency room discharge (outpatients)
There was little to no difference in the length of hospital stay between the groups, with a mean difference of 1.10 days (95% CI ‐0.22 to 2.42; Nouira 2014; 124 participants; Analysis 3.3). The certainty of evidence for this outcome was very low due to very serious imprecision (the confidence interval includes both appreciable benefit and appreciable harm, and there were few events), as well as concerns over study limitations.
3.3. Analysis.

Comparison 3: Magnesium sulfate versus standard care (ipratropium bromide), Outcome 3: Length of hospital stay (days)
Proportion of people with all‐cause mortality
There was little to no difference between magnesium and ipratropium in all‐cause mortality (OR 0.51, 95% CI 0.05 to 4.97; Nouira 2014; 124 participants; Analysis 3.4), but events were rare (1/62 in the magnesium arm and 2/62 in the control arm).
3.4. Analysis.

Comparison 3: Magnesium sulfate versus standard care (ipratropium bromide), Outcome 4: All cause mortality
Proportion of people with adverse events/side effects
There was no report on this outcome.
Arterial‐blood gas measurements
There was no report on this outcome.
Lung function measurements
Improvement in PEFR was reported, favouring ipratropium bromide with a mean difference of 32.00 L/min (95% CI 19.00 to 45.00; Nouira 2014; 124 participants; Analysis 3.5). However, data were too limited for us to be able to reach a conclusion.
3.5. Analysis.

Comparison 3: Magnesium sulfate versus standard care (ipratropium bromide), Outcome 5: Change in PEFR (L/min)
Symptom scores measuring breathlessness, cough and sputum production using validated scales
There was no report on this outcome.
Discussion
This systematic review evaluated RCTs that assessed the effects of magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease in adults. We included a total of 11 RCTs (10 double‐blind and one single‐blind) conducted in Iran, New Zealand, Nepal, Turkey, the UK, Tunisia and the USA between 2004 and 2018. We judged these studies to be at low or unclear risk of bias for most of the domains. We considered three studies to be at high risk for blinding and other biases. There were a total of 762 participants, whose mean ages ranged from 62 to 76 years.
Summary of main results
Intravenous magnesium sulfate versus placebo
We identified seven studies that assessed intravenous magnesium sulfate infusion versus placebo, with both arms receiving usual care. Low‐certainty evidence from three trials indicates that a smaller proportion of people required hospital admissions from the emergency room in the magnesium infusion group compared to the placebo group (170 participants, NNTB = 7). There was no clear difference in the need for non‐invasive ventilation. None of the participants in either group required endotracheal intubation or ICU admission or experienced serious adverse events. From a pooled analysis of two trials, length of hospital stay was shorter with magnesium infusion than placebo in 54 participants; we graded this as low‐certainty evidence. However, we could not be certain that the same benefit of reducing hospital admissions and the length of hospital stay would be observed in the larger population with COPD exacerbations.There was a lack of data on all‐cause mortality. Two studies reported no adverse events in the magnesium group; however, four participants in the placebo arm reported adverse events, with no clinically important difference between the groups. No clear difference was observed for lung function between magnesium infusion and placebo, as measured by change from baseline in FEV1 at 60 minutes, post‐intervention FEV1 and change from baseline PEFR at 45 minutes. In one trial, which reported change in PEFR as percentage predicted, magnesium infusion demonstrated a possible improvement compared to placebo. There was low‐certainty evidence indicating that magnesium infusion reduced dyspnoea score in two trials that used different tools (Borg score and DSS score). One other study assessed the Borg score, but the change from baseline score was not available for meta‐analysis, which reduced our confidence in this finding. Magnesium infusion was no better than placebo in improving oxygen saturation.
Nebulised magnesium sulfate versus placebo
Three trials studied nebulised magnesium compared to placebo in addition to standard care for acute COPD exacerbations. Magnesium inhalation was no better than placebo in reducing hospitalisation. ICU admission was lower in people who received magnesium inhalation than those in the placebo arm; however, the certainty of evidence was very low. The proportion of people who needed ventilatory support (NIV or assisted ventilation) and length of hospital stay were not different between nebulised magnesium and placebo groups. There were no reported serious adverse events in either of the groups. There was one reported death in the placebo arm; however, we were not confident to reach a conclusion due to the small number of participants. Little to no difference was observed in lung function in terms of post‐intervention FEV1 and change from baseline PEFR at 60 minutes. Post‐intervention PEFR at 60 minutes was observed to be higher with nebulised magnesium compared to placebo in one trial, but data were too limited for us to be able to reach a conclusion. Likewise, very low‐certainty evidence demonstrated a reduction in dyspnoea with magnesium inhalation in a small trial with 20 participants. There was no clear difference in oxygen saturation between these two arms.
Magnesium sulfate versus ipratropium bromide
One trial assessed inhaled magnesium sulfate plus intravenous magnesium sulfate compared to inhaled ipratropium bromide plus intravenous normal saline. We could not determine a clear difference between these groups in terms of hospital admission, endotracheal intubation and length of hospital stay (very low‐certainty evidence). Similarly, all‐cause mortality was not different for these two arms. However, change in PEFR was observed to be greater for the ipratropium and placebo arm compared to the magnesium sulfate arm. Due to the small number of participants, few events and study limitations, we could not draw a robust conclusion.
Overall completeness and applicability of evidence
We identified a total of 11 RCTs eligible for this review: seven trials that investigated intravenous magnesium sulfate infusion compared to placebo; three trials on nebulised magnesium sulfate versus placebo; and one trial that assessed combined intravenous and nebulised magnesium sulfate versus nebulised ipratropium bromide plus placebo. All of these trials determined the efficacy of magnesium in COPD exacerbations as an adjunctive measure to the usual standard care including oxygen, antibiotics, bronchodilators and systemic corticosteroids.
The findings for magnesium infusion were based on analyses that involved one or two small studies with few participants, which is a limitation of the evidence. We could not determine any benefit of magnesium sulfate compared to ipratropium bromide, due to limited studies and relatively unreliable data. Based on a single trial, there was little to no difference between magnesium and ipratropium in hospital admission, length of hospital stay, all‐cause mortality or the need for endotracheal intubation. The trial did not report on ICU admission, non‐invasive ventilation, adverse events, serious adverse events, dyspnoea score or oxygen saturation.
Most studies were conducted in single‐ or two‐centre settings with a small number of participants, which is the major limitation that impacts our ability to apply the evidence of this review to clinical practice in managing people with COPD exacerbations. The trials were based in middle‐ to high‐income countries, which may limit the applicability of the findings to low‐income countries. Current guidelines recommend the use of a single dose of magnesium sulfate infusion in acute exacerbation of bronchial asthma not responding to standard care. The trials in this review included adults over the age of 35 who were clinically diagnosed with COPD exacerbations. There were no specific criteria described for exclusion of people with overlap asthma features (asthma COPD overlap), except in the study by Mukerji 2015 which excluded participants having asthma‐type COPD. Thus, we could not rule out the possibility that the findings in this review are partly contributed by participants who had asthma COPD overlap features. We had planned to do subgroup analyses by blood eosinophil count to find out which group of people with COPD exacerbations would benefit from magnesium sulfate, but could not do so due to limited data. Similarly, none of the included studies reported the disease severity of participants, so we could not investigate the effects of magnesium sulfate in particular groups of people with COPD exacerbations.
Quality of the evidence
Studies that contributed to the outcomes of this review were single‐ or two‐centre double‐blind RCTs with small number of participants, and one single‐blind trial. The majority of studies were at unclear risk of bias in several domains due to lack of information, with the exception of Mukerji 2015, in which reporting was sufficiently clear for us to make low risk judgements for every domain. Comparisons for the outcomes were direct and most of the outcomes were based on one or two trials without any significant heterogeneity. Reasons for downgrading were mainly due to study limitations and imprecision of the pooled effect estimates (including wide or very wide confidence intervals, and small participant numbers).
We rated the certainty of evidence for intravenous magnesium sulfate infusion to be low for hospital admission, length of hospital stay, symptomatic improvement and lung function (FEV1); and very low for non‐invasive ventilation and oxygen saturation. As for inhaled magnesium, the level of certainty was very low for all outcomes. Therefore, we have very little confidence in the effect estimates for magnesium inhalation compared to placebo. Similarly, the level of certainty was very low for the key outcomes (hospital admission, endotracheal intubation and length of hospital stay) for comparison between magnesium sulfate and ipratropium bromide.
Pooled effects for nebulised magnesium were based on data from three trials, which we judged to be of poor quality with unclear risk for selection, detection and reporting biases. One trial was a single‐blinded study where investigators were unblinded, and another trial included only 20 participants (10 in each arm). The effects of nebulised magnesium sulfate on hospital admission, need for ventilatory support (NIV or assisted ventilation), length of hospital stay, lung function and oxygen saturation were of very low‐certainty evidence. No clear benefit could be demonstrated in improving these outcomes with the use of nebulised magnesium sulfate as an adjunctive therapy to usual standard care in COPD exacerbations compared to placebo. ICU admission was reportedly lower in participants who received magnesium inhalation compared to those in the placebo group. Likewise, dyspnoea measured by VAS score was observed to improve with nebulised magnesium compared to placebo in 20 participants. However, we are not confident of these findings, and data from larger multicentre trials are required to provide more robust evidence. We could not identify enough evidence to say that magnesium inhalation was not associated with important harms such as all‐cause mortality, adverse events or serious adverse events. Similarly, there is very limited evidence to support the efficacy of nebulised magnesium sulfate as an adjunctive therapy for COPD exacerbations.
We planned to perform subgroup analyses if we found high levels of heterogeneity; however, this was not necessary as there was low heterogeneity. We could not investigate publication bias because of the small number of studies in analyses. Overall, we are not confident in the pooled effect estimates of this review, since the majority were calculated using data from one or two single/double centre trials that determined the effect of magnesium sulfate on a relatively small number of participants with COPD exacerbations. Additional information from further multicentre trials in a larger population is very likely to alter our confidence in these results and provide a better evidence for clinical practice.
Potential biases in the review process
This review was based on a published protocol (Ni 2020), and any deviations from the published protocol were noted in Differences between protocol and review. Incomplete identification of studies for this review is unlikely as we performed a comprehensive search of databases, websites, clinical trial registries and reference lists. However, there are areas which may have introduced bias into the review. We identified one trial registered in the Iranian Registry of Clinical Trials (IRCT) that planned to start in 2016. We contacted the trial investigators for its current status but received no reply. We also contacted the authors of included trials for details of study characteristics and clarification on data. Only Moradi 2021 replied and provided the necessary information about the trial. Jahanian 2021 assessed some outcomes relevant to this review, but only lung function data were available; dyspnoea score and oxygen saturation could not be included in the analyses. Bajracharya 2021 was a single‐blinded study, unregistered on a trial registry, with limited information on the conduct of the trial, which downgrades our confidence in its data. Moreover, two included trials were only available as abstracts with no full text publication (Hogg 2004; Pishbin 2018), resulting in high risk of bias. In addition, assessment of publication bias through examination of funnel plots was not possible because only one or two trials were included in the analyses.
Recently, the European Respiratory Society issued a statement on core outcomes for clinical trials assessing AECOPD (acute exacerbations of COPD) management, which included mortality (any cause or due to COPD exacerbations), treatment success, need for higher level of care (hospital or ICU admission), arterial blood gases, patient‐reported outcomes (breathlessness, quality of life, activities of daily living), disease progression, future exacerbations and hospital admissions, treatment safety (adverse events) and adherence (Mathioudakis 2022). The intended outcomes of this review are included in this recommended outcome set, except for length of hospital stay and lung function improvement. However, we were not able to synthesise evidence for all the planned outcomes since most of the COPD exacerbation trials assessed lung function as the primary outcome and clinically relevant outcomes were usually not included. Therefore, it is likely that this review is lacking in evidence for patient‐centred, clinically important outcomes.
Agreements and disagreements with other studies or reviews
There is limited evidence for the role of magnesium sulfate in acute COPD exacerbations, with very few published reviews to date. We could only identify three similar published systematic reviews focusing on the effects of magnesium in COPD exacerbations (Alzaid 2021; Jahangir 2022; Shivanthan 2014). Intravenous magnesium reduced hospital admission in our analysis with a NNTB of seven. Two of the earlier published reviews failed to detect this (Alzaid 2021; Shivanthan 2014), possibly due to their searches predating the publication of the latest trial included in our analysis (Moradi 2021, which had a relatively large number of participants. However, the recent review by Jahangir 2022 identified a reduction in hospital admissions with intravenous magnesium, reporting the same summary statistics as our review. Shortening of hospital stay and improvement in dyspnoea score with magnesium infusion were not detected in these reviews. Variable severity of COPD exacerbations in the included trials could affect our findings on hospital admissions and length of hospital stay; however, the available baseline characteristics are comparable across the intervention arms.
Low serum magnesium level is an important predictor of frequency of acute COPD exacerbations (Aziz 2005; Gumus 2014; Kshirsagar 2021). Furthermore, low serum magnesium level at the time of admission was reported to be an independent predictor of readmission for AECOPD (Bhatt 2008). Since the participants in our analyses had normal serum magnesium levels, which were similar for both groups, we are quite confident that serum magnesium level at the time of presentation to the emergency room had no significant impact on our finding of hospital admission.
Our review failed to yield a positive effect of magnesium infusion on lung function in terms of FEV1 or oxygen saturation. This finding was shared by Jahangir 2022, which also showed no significant change in FEV1 using a random‐effects model, though the fixed‐effect analysis demonstrated a significant effect. In our review, there was a possible improvement in PEFR, calculated as change from baseline percentage of predicted value. A similar finding was reported by Shivanthan 2014 as an increase in PEFR, with a mean percentage change of 24%. Due to considerable variability between FEV1 and PEFR, especially when expressed as percentage of predicted value (Llewellin 2002), FEV1 is the preferred measure of airway obstruction in COPD exacerbations (Emerman 1996). Thus, this finding is not strong enough for clinical use and requires further data for assessing the role of magnesium for lung function improvement.
None of the previous reviews supported the efficacy of magnesium sulfate inhalation in COPD exacerbations compared to placebo, which is not altered by the results of our review. Similarly, evidence for nebulised magnesium versus ipratropium bromide is limited, with only one available trial. This consolidates the need for further well‐designed trials that extensively investigate the effects of magnesium sulfate, to yield better certainty evidence for its clinical use in COPD exacerbations.
Authors' conclusions
Implications for practice.
We have limited confidence to conclude a possible beneficial effect of intravenous magnesium sulfate infusion in reducing the number of people admitted to hospital from the emergency room, shortening length of hospital stay and improving dyspnoea. We could not identify evidence for intensive care unit (ICU) admission or endotracheal intubation. We had very little confidence in the effect of magnesium infusion on the need for non‐invasive ventilation (NIV), or for improving lung function and oxygen saturation. Since the data on adverse events, serious adverse events and mortality were limited, further safety data are required before using intravenous magnesium infusion as a routine addition to standard care in people with chronic obstructive pulmonary disease (COPD) exacerbations.
Current evidence for inhaled magnesium sulfate is very uncertain, and could not demonstrate that it is beneficial in reducing hospital admissions, the need for ventilatory support, length of hospital stay or all‐cause mortality, or in improving lung function and oxygen saturation. Fewer ICU admissions were noted with nebulised magnesium, and a small trial favoured inhaled magnesium over placebo in improving dyspnoea, though we graded both these outcomes as very uncertain evidence.
Similarly, evidence from a single trial was very uncertain and could not demonstrate a clear benefit of magnesium over ipratropium bromide on the need for hospital admission or endotracheal intubation, length of hospital stay or all‐cause mortality.
Overall, the evidence in this review should be interpreted with caution as larger studies are required to demonstrate the effects of magnesium sulfate in COPD exacerbations.
Implications for research.
Further research is indicated, including multicentre trials in larger populations with COPD exacerbations (better‐powered trials) using robust methods, which should be reported transparently. Trialists should define and categorise participants according to severity of COPD exacerbations and, if possible, provide disaggregated data for different groups. It is also recommended to include COPD phenotypes of participants to delineate the possible impact of asthma/COPD overlap on the evidence. Further trials assessing the effects of magnesium sulfate with participant‐related outcomes (e.g. ICU admission, need for NIV or mechanical ventilation, length of hospital stay and dyspnoea score) as primary endpoints rather than lung function improvement would be more appropriate for applicability to clinical practice in people with COPD exacerbations. Follow‐up data would be beneficial, especially to identify any impact of magnesium sulfate on recurrent exacerbations and readmissions. Since currently available evidence did not identify any study on cost‐effectiveness, future research on this outcome is also required.
History
Protocol first published: Issue 12, 2019
Acknowledgements
We would like to thank the editors and staff of Cochrane Airways for their utmost help and support, especially the Deputy Co‐ordinating Editor, Dr Emma Dennett, for advice, and Information Specialist, Ms Elizabeth Stovold, for her input in writing the search strategy.
We thank Haldun Akoglu and Anja Leider for their expertise in providing us with translations of studies.
We are also grateful to our respective institutions for their support and encouragement to conduct this review.
We based the Background and Methods sections of this review on a standard template used by Cochrane Airways.
The authors and Cochrane Airways Editorial Team are grateful to the following peer reviewers for their time and comments: Juan Abreu González (Spain) and Richard Dekhuijzen (the Netherlands).
Appendices
Appendix 1. Database search strategies
Cochrane Airways Register & CENTRAL (searched via Cochrane Register of Studies)
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All #2 MeSH DESCRIPTOR Bronchitis, Chronic #3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) #4 COPD:MISC1 #5 (COPD OR AECOPD):TI,AB,KW #6 #1 OR #2 OR #3 OR #4 OR #5 #7 MESH DESCRIPTOR Magnesium #8 MESH DESCRIPTOR Magnesium Sulfate #9 magnesium*:ti,ab,kw #10 (MgSO4 or MG SO4):ti,ab,kw #11 #7 OR #8 OR #9 OR #10 #12 #11 AND #6
MEDLINE (Ovid) ALL
1 Lung Diseases, Obstructive/ 2 exp Pulmonary Disease, Chronic Obstructive/ 3 emphysema$.tw. 4 (chronic$ adj3 bronchiti$).tw. 5 (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 6 (COPD or AECOPD or AECB).ti,ab. 7 or/1‐6 8 Magnesium/ 9 Magnesium Sulfate/ 10 magnesium$.tw. 11 (MgSO4 or MG SO4).tw. 12 or/8‐11 13 7 and 12 14 (controlled clinical trial or randomized controlled trial).pt. 15 (randomized or randomised).ab,ti. 16 placebo.ab,ti. 17 dt.fs. 18 randomly.ab,ti. 19 trial.ab,ti. 20 groups.ab,ti. 21 or/14‐20 22 Animals/ 23 Humans/ 24 22 not (22 and 23) 25 21 not 24 26 13 and 25
Embase (Ovid)
1 exp chronic obstructive lung disease/ 2 obstructive airway disease/ 3 (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 4 (chronic$ adj3 bronchiti$).tw. 5 emphysema$.tw. 6 (COPD or AECOPD or AECB).ti,ab. 7 or/1‐6 8 magnesium/ 9 magnesium sulfate/ 10 magnesium$.tw. 11 (MgSO4 or MG SO4).tw. 12 or/8‐11 13 7 and 12 14 Randomized Controlled Trial/ 15 randomization/ 16 controlled clinical trial/ 17 Double Blind Procedure/ 18 Single Blind Procedure/ 19 Crossover Procedure/ 20 (clinica$ adj3 trial$).tw. 21 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 22 exp Placebo/ 23 placebo$.ti,ab. 24 random$.ti,ab. 25 ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 26 (crossover$ or cross‐over$).ti,ab. 27 or/14‐26 28 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 29 human/ or normal human/ or human cell/ 30 28 and 29 31 28 not 30 32 27 not 31 33 13 and 32
ClinicalTrials.gov
| Search field | Search terms |
| Study type | Interventional |
| Condition | COPD |
| Intervention | magnesium |
WHO ICTRP
| Search field | Search terms |
| Condition | COPD |
| Intervention | magnesium |
Data and analyses
Comparison 1. Intravenous magnesium sulfate + standard care versus placebo + standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of people with hospital admissions (from the emergency room) | 3 | 170 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.23, 0.88] |
| 1.2 Proportion of people with need for non‐invasive ventilation (NIV) | 2 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.31, 1.75] |
| 1.3 Length of hospital stay (days) | 2 | 54 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐4.73, ‐0.66] |
| 1.4 Proportion of people with adverse events | 2 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.02, 1.00] |
| 1.5 Change in SpO 2 (%) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 FEV 1 change from baseline at 60 min (L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7 FEV 1 at 45 min (mL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8 Change in PEFR (L/min) | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 9.12 [‐6.20, 24.44] |
| 1.9 Change in PEFR (% predicted) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10 Change in dyspnoea score | 2 | 101 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.83, ‐0.96] |
Comparison 2. Nebulised magnesium sulfate + standard care versus placebo + standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of people with hospital admissions (from the emergency room) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Proportion of people who need ICU admission | 2 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.15, 1.00] |
| 2.3 Proportion of people who need ventilatory support (non‐invasive ventilation or assisted ventilation) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Length of hospital stay (days) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 All‐cause mortality | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6 FEV 1 at 60 min (L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.7 PEFR change from baseline at 60 min (L/min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.8 PEFR at 60 min (L/min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.9 Change in dyspnoea score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.10 Change in SaO 2 (%) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Magnesium sulfate versus standard care (ipratropium bromide).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion of people with hospital admissions (from the emergency room) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 Proportion of people with the need for endotracheal intubation | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Length of hospital stay (days) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4 All cause mortality | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3.5 Change in PEFR (L/min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bajracharya 2021.
| Study characteristics | ||
| Methods | Study design: single‐blinded randomised control trial of parallel design Number of study centres: single centre Study location: Tribhuvan University Teaching Hospital, Kathmandu, Nepal Study setting: emergency room Date of study: December 2016 to March 2017 Withdrawals: none |
|
| Participants | Number screened: 200 Number randomised: 172 Numbers in treatment group: 86 Number in placebo group: 86 Numbers of withdrawals: 0 Numbers completing trial: 172 Mean age (years): 70.01 (SD 10.39) (magnesium); 68.58 (SD 14.71) (placebo) Gender (male/female): 43/43 (magnesium); 37/49 (placebo) Diagnostic criteria: clinical diagnosis Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention: 3 ml isotonic magnesium sulfate (50% solution, 8.30 mosm/mL, 500 mg/mL) by nebuliser + 5 mg nebulised salbutamol Comparator: 3 mL normal saline by nebuliser + 5 mg nebulised salbutamol Concomitant medications
|
|
| Outcomes |
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: only mentioned as a randomised control trial without further details on random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomised by drawing lots to receive one of two sequences of treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: single‐blinded trial; only participants were blinded; medications were prepared by the investigator. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details provided for outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants completed the study with no withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol was unavailable, unclear if outcomes were reported as planned. |
| Other bias | Low risk | Comment: no other apparent biases identified. |
Comert 2016.
| Study characteristics | ||
| Methods | Study design: randomised placebo‐controlled trial of parallel design Number of study centres: single centre Study location: Turkey University hospital Study setting: ambulatory care unit, Department of Pulmonology, School of Medicine, Istanbul University Date of study: December 2004 to September 2005 Withdrawals: none |
|
| Participants | Number randomised: 20
Numbers in treatment group: 10
Number in placebo group: 10
Numbers of withdrawals: 0
Numbers completing trial: 20 Mean age (years): 62.1 (SD 12) (magnesium); 71.3 (SD 5) (placebo) Gender (male/female): 8/2 (magnesium); 10/0 (placebo) Diagnostic criteria: clinical diagnosis Mean duration of COPD (years): 11.4 (magnesium); 13.5 (placebo) History of hospitalisation in recent year, n (%): 3(30) (magnesium); 4(40) (placebo) History of ICU in recent year, n (%): 0 (magnesium); 0 (placebo) Long‐term oxygen use, n (%): 2 (20) (magnesium); 6 (60) (placebo) Corticosteroid use in last 6 months, n (%): 3 (30) (magnesium); 4(40) (placebo) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention: 2.5 mL isotonic magnesium sulfate (250 mmol/L, 151 mg/dose) by nebuliser + standard care Comparator: 2.5 mL isotonic saline solution by nebuliser + standard care Concomitant medications
|
|
| Outcomes | At 24 and 48 hours: FEV1, ABG (PaO2, PaCO2, pH, SaO2), VAS dyspnoea score (0 ‐ 100), serum magnesium level 10, 30, 60 and 120 mins after each nebule treatment: PEFR 10, 30, and 120 mins after each nebule treatment: BP and HR |
|
| Notes | Funding: researcher Full text article in Turkish; translation done by Cochrane Airways. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: "1:1 randomisation in two groups" with no further information. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: authors stated that all measurements were made by a single researcher; participants and the researcher who made the measurements did not know in which group the participant was. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of participants and researcher who made the measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants completed the study with no withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol was not available, unclear if outcomes were reported as planned. |
| Other bias | Low risk | Comment: no other apparent biases identified. |
Edwards 2013.
| Study characteristics | ||
| Methods | Study design: randomised double‐blind placebo‐controlled trial Number of study centres and location: two university hospitals in New Zealand (Wellington Regional Hospital and Hutt Hospital) Study setting: emergency departments Withdrawals: stated Date of study: June 2008 to November 2011 |
|
| Participants | Number screened: 161
Number randomised: 116
Number allocated in treatment group: 52
Number allocated in placebo group: 64
Numbers of withdrawals: 0
Numbers excluded from analysis: 4 (magnesium); 3 (placebo) Number included in analysis: 109 Number included in analysis: 48 (magnesium): 61 (placebo) Mean age/SD (years): 73.2/9.8 (magnesium); 69.5/11.9 (placebo) Gender (male/female): 27/21 (magnesium); 31/30 (placebo) Diagnostic criteria: clinical diagnosis Baseline lung function: Mean FEV1 at presentation (% of predicted): 28.2 (magnesium); 29.7 (placebo) Mean FEV1 at presentation: 0.69 L (magnesium); 0.72 L (placebo) Smoking history: Mean amount of smoking (pack‐years): 41.3 (magnesium); 45 (placebo) Current smokers: 18 (magnesium); 22 (placebo) Never smokers: 1 (magnesium); 2 (placebo) Average number of hospital admission in last year: 1 (magnesium); 1.3 (placebo) Serum magnesium level (mmol/L) mean/SD: 0.81 (SD 0.08) (magnesium); 0.78 (SD 0.1) (placebo) Inclusion criteria: age ≥ 35 years with a doctor diagnosis of COPD, FEV1/FVC < 70% and an FEV1 ≤ 50% predicted 20 min after initial treatment with 2.5 mg salbutamol and 500 mg ipratropium bromide by nebulisation. Exclusion criteria: those who required intubation or NIV, were unable to perform spirometry or had evidence of pneumothorax, hypotension, any other serious medical condition that would prevent their participation in the trial or were pregnant. |
|
| Interventions | Intervention: jet nebulisation 2.5 mg salbutamol (GlaxoSmithKline, London, UK) mixed with 2.5 ml isotonic magnesium sulfate (250 mmol/L, tonicity 289 mosmol; 151 mg per dose) on 3 occasions at 30 min intervals Comparator: jet nebulisation 2.5 mg salbutamol (GlaxoSmithKline, London, UK) mixed with 2.5 mL isotonic saline (placebo) on 3 occasions at 30 min intervals Concomitant medications:
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Funding for studies: The Health Research Council of New Zealand. Conflicts of interest of trial authors: Declared "None"; trial registry: ACTRN12608000167369 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study statistician performed block randomisation with a block size of eight using a computer‐generated random sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly allocated in a double‐blind fashion to receive one of the treatment regimens. This was administered by a third‐party process" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants and investigators were unaware of treatment allocation through provision by the hospital pharmacy of pre‐prepared identical syringes containing the study drug or placebo according to random allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3 (4.9%) from magnesium group and 4 (8.3%) from placebo group were excluded from the analyses, with reasons provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trial was registered on website and results for most of the outcomes were reported, except for mortality at 30 days. |
| Other bias | Low risk | Comment: no other apparent biases identified. |
Hogg 2004.
| Study characteristics | ||
| Methods | Study design: double‐blind placebo‐controlled pilot study Number of study centres and location: single centre, UK Study setting: hospital inpatients Withdrawals: not stated Date of study: not stated |
|
| Participants | Number randomised: 24
Numbers in treatment group: not stated
Number in placebo group: not stated Mean age: not stated Gender: not stated Diagnostic criteria: not stated Baseline lung function: not stated Inclusion criteria: known COPD patients presenting with an exacerbation Exclusion criteria: not stated |
|
| Interventions | Intervention: intravenous infusion of 1.2 g magnesium sulfate over 20 minutes Comparator: intravenous infusion of normal saline as placebo Concomitant medications: standard treatment |
|
| Outcomes | Modified Borg dyspnoea score, length of inpatient stay | |
| Notes | Abstract only; full text not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: only described as a randomised study, without details |
| Allocation concealment (selection bias) | Unclear risk | Comment: only described as a randomised study, without details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: only described as a double‐blind study, without details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: only described as a double‐blind study, without details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no details provided, no full‐text publication |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol was not accessible; unclear if all the outcomes were reported as planned. |
| Other bias | High risk | Comment: as there is no full‐text publication of this study, publication bias cannot be excluded |
Jahanian 2021.
| Study characteristics | ||
| Methods | Study design: phase 3 randomised double‐blind placebo‐controlled trial with parallel assignment Number of study centres and location: single centre; Imam Khomeini Hospital in Sari, Mazandaran Province, Iran Study setting: emergency department (ED) Withdrawals: stated Date of study: September 2016 to February 2018 |
|
| Participants | Number screened: 72
Number randomised: 60
Number in treatment group: 30
Number in placebo group: 30
Mean age (years): 62.9 (SD 7.3) (magnesium); 65.8 (SD 3.1) (placebo) Gender (male/female): 5/25 (magnesium); 9/21 (placebo) Diagnostic criteria: physician diagnosed Disease duration of less than 5 years (n): 20 (magnesium); 18 (placebo) Smoking history Current smokers: 16 (magnesium); 17 (placebo) Inclusion criteria: adults with known clinically diagnosed moderate COPD (FEV1/FVC < 70%, 30 < FEV1 < 50, often symptomatic with shortness of breath) with the acute attack symptoms including changes in the severity of shortness of breath, cough, and sputum volume Exclusion criteria
|
|
| Interventions | Intervention: intravenous infusion of magnesium sulfate (2 g in 100 mL of normal saline) over 30 minutes Placebo: intravenous infusion of 0.9% normal saline (100 mL) over 30 minutes After receiving standard treatment for COPD exacerbation:
|
|
| Outcomes | FEV1, RR, PR, SpO2 and 0 to 10 Borg dyspnoea scale at 45 minutes and 6 hours after the commencement of intervention | |
| Notes | Funding for studies: Clinical Research Development Unit of Imam Khomeini Hospital, Mazandaran University of Medical Sciences, Sari, Iran Conflicts of interest of trial authors: declared none Clinical Trials Registry: IRCT20150315021480N8 (Iranian Registry of Clinical Trials Database) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated random numbering system with the help of a nurse who was blinded to the study groups. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done using a sealed envelope technique with the help of a nurse who was blinded to the study groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All drugs were prepared in syringes in the same size, colour, volume, and shape. The syringes were labelled as A or B by a pharmacist and were administered by a nurse. Particpants were unaware of the type of medication they were receiving. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided for outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts from either arm. |
| Selective reporting (reporting bias) | Low risk | Trial was registered on website, all outcome measures were reported as planned. Moreover, the authors stated their willingness to share the data upon request. |
| Other bias | Low risk | None identified. |
Moradi 2021.
| Study characteristics | ||
| Methods | Study design: randomised double‐blind placebo‐controlled trial Number of study centres and location: single centre; Emam Reza hospital in north‐east of Iran, affiliated with Mashhad University of Medical Sciences Study setting: emergency department (ED) Withdrawals: stated Date of study: October 2017 to March 2018 |
|
| Participants | Number screened: 167
Number randomised: 77
Number in treatment group: 39
Number in placebo group: 38 Mean age (years): 71.9 (SD 7) (magnesium); 69.8 (SD 8.2) (placebo) Gender (male/female): 20/19 (magnesium); 22/16 (placebo) Diagnostic criteria: physician diagnosed Baseline lung function: Mean PEFR at presentation (% of predicted): 27.3 (magnesium); 28.1 (placebo) Smoking history Current smokers: 5 (magnesium); 7 (placebo) Ex smokers: 19 (magnesium); 20 (placebo) Non‐smokers: 15 (magnesium); 11 (placebo) Inclusion criteria: adults with the initial diagnosis of AECOPD Exclusion criteria: people who:
|
|
| Interventions | Intervention: intravenous infusion of 2.5 g of MgSO4 (5 mL of 50% solution) in 50 mL of normal saline over 15 minutes Comparator: intravenous infusion of 5 mL sterile water in 50 mL of normal saline over 15 minutes as placebo Concomitant medications: standard treatment with supplemental oxygen to maintain SpO2 > 90%, nebulized salbutamol, nebulized ipratropium, IV hydrocortisone, IV ceftriaxone and oral azithromycin |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Funding for studies: Mashhad University of Medical Sciences Conflicts of interest of trial authors: declared none Clinical Trials Registry: IRCT2014111519962N1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by block randomisation (blocks of 4) by a third party." |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly allocated to either group A (MgSO4) or group B (placebo) by a third party process so that patients and researchers were unaware of allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, treating physician, and investigators were blinded to the treatment. A nurse who was not involved in patients’ care prepared the MgSO4/placebo vials by filling similarly appearing vials with 5 mL of MgSO4 (50% solution) or sterile water daily and kept the codes for content of each bottle in a log not accessible by the investigators until the completion of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: "Participant, care provider, investigator and outcome assessor" were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comments: 4 participants lost to follow‐up (5.2 %), reasons not provided. |
| Selective reporting (reporting bias) | Low risk | Comment: trial was registered on website, all outcomes were reported as planned. |
| Other bias | Low risk | Comment: no other apparent biases identified. |
Mukerji 2015.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, parallel‐group, placebo‐controlled trial Number of study centres and location: single centre; Palmerston North Hospital, New Zealand Study setting: emergency department (ED) Withdrawals: stated Date of study: July and October 2013 |
|
| Participants | Number screened: 37
Number randomised: 33 Numbers in treatment group: 14 Number in placebo group: 19 Numbers of withdrawals: 1 (magnesium), 2 (placebo) Numbers completing trial: 13 (magnesium), 17 (placebo) Number included in analysis: 30 Mean age SD (years): 76.1 (SD 12.47) (magnesium), 72.9 (SD 9.39) (placebo) Gender (male/female): 11/2 (magnesium), 10/7 (placebo) Diagnostic criteria: clinically by the attending physician who was not one of the investigators Baseline lung function Mean FEV1 at presentation: 0.637 L (magnesium); 0.691 L (placebo) Number of participants with FEV1 at presentation < 50% of predicted: 13 (magnesium); 17 (placebo) Smoking history Mean amount of smoking (pack‐years): 40.0 (magnesium); 38.8 (placebo) Current smokers: 4 (magnesium); 5 (placebo) Never smokers: 1 (magnesium); 1 (placebo) Serum magnesium level (mmol/L) mean/SD: 0.79/0.1 (magnesium); 0.78/0.1 (placebo) Inclusion criteria: non‐infective and infective cases of AECOPD in people above the age of 35 years, who had a previously documented diagnosis of COPD by either their general practitioner or in‐hospital respiratory specialists Exclusion criteria:
|
|
| Interventions | Intervention: 2 g IV magnesium sulphate made up to 20 mL in 0.9% sodium chloride solution (saline) + standard therapy Comparator: 20 ml of IV saline as placebo + standard therapy Concomitant medications 5 mg of nebulised salbutamol Standard therapy 5 mg salbutamol and 500 mcg ipratropium Bromide by jet nebulisation • 60 mg of oral prednisone or 100 mg of IV hydrocortisone • Oxygen: 2 L per min via nasal prongs if the patient’s pulse oximetry revealed saturations of < 90% |
|
| Outcomes | Primary outcomes percentage change in FEV1 and FVC at 0, 60 and 120 minutes Secondary outcomes:
|
|
| Notes | Funding for studies: Midcentral District Health Board, Palmerston North, New Zealand. Conflicts of interest of trial authors: declared "nil" The trial was registered with Australian New Zealand Clinical Trials Registry: (ANZCTR) (ACTRN12613000837729). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The senior ED pharmacist performed block randomisation. A block size of 20 was used with a 1:1 allocation ratio" |
| Allocation concealment (selection bias) | Low risk | Quote: "The senior ED pharmacist provided identical numbered pre‐made syringes with either trial drug or placebo" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The senior ED pharmacist provided identical pre‐made syringes with either trial drug or placebo, as per randomisation, to maintain investigator and patient masking" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: participants, investigators, outcome assessors were all masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rates were similar in both groups (7.1% vs 5.2%). |
| Selective reporting (reporting bias) | Low risk | Comment: trial was registered on website, all outcome measures were reported as planned. |
| Other bias | Low risk | Comments: no other apparent biases identified. |
Nouira 2014.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, controlled trial Number of study centres and location: 2; Fattouma Bourguiba University Hospital (Monastir, Tunisia) and Tahar Sfar University Hospital (Mahdia, Tunisia) Study setting: emergency department (ED) Withdrawals: stated Date of study: January 2005 and June 2007 |
|
| Participants | Number screened: 208
Number randomised: 124
Numbers in treatment group: 62
Number in placebo group: 62
Numbers of withdrawals: 2 (magnesium); 2 (ipratropium)
Numbers completing trial: 60 (magnesium); 60 (ipratropium) Number included in analysis: 62 (magnesium); 62 (ipratropium) Mean age (years): 69.2 (SD 8.6) (magnesium), 68.9 (SD 7.8) (ipratropium) Gender (male/female): 48/14 (magnesium), 47/15 (ipratropium) Diagnostic criteria: clinical Smoking history: current smokers: 58 (magnesium); 52 (ipratropium) Hospitalisation for COPD within the last year, n (%): 3.6 (2.4) (magnesium); 3.1 (1.7) (ipratropium) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: magnesium sulfate 150 mg in 4 mL of normal saline delivered via aerosol mask at 10 L/min driven by pressurised air plus IV magnesium sulfate 1.5 g in 10 mL, followed by 4 doses of magnesium sulfate with terbutaline at 30 min apart Comparator: ipratropium bromide 0.5 mg in 3 mL of normal saline delivered via aerosol mask at 10 L/min driven by pressurised air plus IV 10 mL of normal saline as placebo, followed by 4 doses of nebulized ipratropium bromide with terbutaline at 30 min apart Concomitant medications: standard treatment consisting of intravenous methylprednisolone, parenteral fluid therapy, antibiotics, and nebulised terbutaline 5 mg in 4 mL of normal saline |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Funding for studies: University of Monastir Conflicts of interest of trial authors: not described; proposal was approved by the Ethic committee Clinical Trials Registry: NCT01136421 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment preparation and allocation were performed by the hospital pharmacy in random sequence using a random table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients were assigned in a randomised double‐blind fashion; treatment preparation performed by the hospital pharmacy were kept identical in their appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: masking includes participant, care provider, investigator and outcomes assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no dropouts from the study arms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial was registered on website; however, outcomes were not provided on the website so unclear if all outcomes were reported as planned. |
| Other bias | Low risk | Comment: no other apparent biases identified. |
Pishbin 2018.
| Study characteristics | ||
| Methods | Study design:randomised controlled trial Number of study centres and location: Iran Study setting: emergency department (ED) Withdrawals: not stated Date of study: not stated |
|
| Participants | Number randomised: 34 Numbers in treatment group: 17 Number in placebo group: 17 Mean age: not stated Gender: 16 male; 18 female Diagnostic criteria: not stated Baseline lung function: not stated Smoking history: not stated Inclusion criteria: acute exacerbation of COPD Exclusion criteria: not stated |
|
| Interventions | Intervention: standard treatment plus 2 g of IV magnesium sulfate Comparator: standard treatment plus placebo |
|
| Outcomes | Admission rate, intubation rate, changes in PEFR, SpO2 and dyspnoea severity score | |
| Notes | Funding for studies: not provided Conflicts of interest of trial authors: not declared Abstract only; full text not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated as "randomly allocated into two groups" without further information. |
| Allocation concealment (selection bias) | Unclear risk | Comment: stated as "randomly allocated into two groups" without further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no details provided for blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details provided for blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information on withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Comment: abstract only, protocol not available, so unclear if outcomes were reported as planned. |
| Other bias | High risk | Comment: there is no full‐text publication of this study; publication bias cannot be excluded |
Skorodin 1995.
| Study characteristics | ||
| Methods | Study design: double‐blind randomised controlled trial Number of study centres and location: 2; Edward Hines Jr. Veterans Affairs Hospital, Hines, Illinois and Augusta (GA) Veterans Affairs Medical Center Study setting: emergency department (ED) Withdrawals: not stated Date of study: not stated |
|
| Participants | Number randomised: 72
Numbers in treatment group: 36
Number in placebo group: 36 Mean age (years): 62.8 (SD 9.0) (magnesium); 66.5 (SD 7.3) (placebo) Gender (male/female): 35/1 (magnesium); 35/1 (placebo) Diagnostic criteria: ATS Mean duration of COPD (years): 11 (SD 10.6) (magnesium); 11 (SD 13) (placebo) Baseline lung function: Mean PEFR at presentation (L/min): 122.8 (magnesium); 119.7 (placebo) Smoking history: Mean duration of smoking (years): 39.7 (magnesium); 39.5 (placebo) Serum magnesium level (mmol/L) mean: 0.94 (SD 0.16) (magnesium); 0.98 (SD 0.16) (placebo) Inclusion criteria: people aged 35 years or older who presented with acute exacerbation of COPD Exclusion criteria:
|
|
| Interventions | Intervention: IV 1.2 g of magnesium sulfate in 150 mL of normal saline over 20 minutes Comparator: IV 2.5 mL of saline in 150 mL of normal saline over 20 minutes as placebo Concomitant medications:
|
|
| Outcomes | Dyspnoea score, PEFR, hospital admission, emergency department visit in next two weeks, MIP and MEP | |
| Notes | Funding for studies: Veterans Affair Health Services Research & Development grant 90‐091; magnesium sulfate was donated by Abbot Lab Conflicts of interest of trial authors: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated as "coded from a randomised list", with no elaboration on how sequence generation was performed. |
| Allocation concealment (selection bias) | Low risk | Quote: "placebo and magnesium sulfate solutions were prepackaged and coded from a randomised list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomised in a double‐blind fashion" Quote: "placebo and magnesium sulfate solutions were prepackaged in identical vials in the pharmacy and added to the normal saline in the pharmacy before delivery to the emergency department" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details provided for outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no dropouts from the study arms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear if all outcomes were reported as planned since trial protocol was not available. |
| Other bias | Low risk | Comment: no other apparent biases identified. |
Solooki 2014.
| Study characteristics | ||
| Methods | Study design: double‐blind randomised controlled trial Number of study centres and location: single centre; Imam Hussein Hospital affiliated to Shahid Beheshti University of Medical Sciences in Eastern Tehran, Iran Study setting: emergency department (ED) Withdrawals: not stated Date of study: not stated |
|
| Participants | Number randomised: 30
Numbers in treatment group: 15
Number in placebo group: 15
Mean age (years): 67 (SD 10) (magnesium), 70 (SD 8) (placebo) Gender (male/female): 11/4 (magnesium), 10/5 (placebo) Diagnostic criteria: not stated Baseline lung function: Baseline mean FEV1 (% of predicted): 26 (magnesium); 35 (placebo) Baseline mean PEFR (L/min): 126 (magnesium); 142 (placebo) Smoking history: Mean amount of smoking (pack‐years): 23 (magnesium); 22 (placebo) History of admission, n (%): 9 (60) (magnesium); 10 (67) (placebo) Serum magnesium level (mEq/L) mean/SD: 2.11/0.28 (magnesium); 2.05/0.40 (placebo) Inclusion criteria: 40 years or older with COPD exacerbation Exclusion criteria:
|
|
| Interventions | Intervention: 2 g of magnesium sulfate diluted in 100 ml normal saline, infused over 20 min, concurrent with standard treatment Comparator: placebo of 100 mL normal saline with standard treatment Concomitant medications:
|
|
| Outcomes | PEFR, FEV1, SaO2, duration of hospital stay | |
| Notes | Funding: not mentioned Conflicts of interest of trial authors: not explicitly described, but the proposal was approved by the research ethic board |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated as "patients were randomly divided" with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Comment: stated as "randomised‐control double blind study" with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: stated to be a double‐blind study, but no further details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: stated to be a double‐blind study, but no further details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no dropouts form the study arms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol was not available; unclear if all outcomes were reported as planned. |
| Other bias | Low risk | Comment: no other apparent biases identified. |
ABG: arterial blood gas; AECOPD: acute exacerbations of COPD; ATS: American Thoracic Society; BP: blood pressure; bpm: beats per minute; COPD: chronic obstructive pulmonary disease; CXR: chest X‐ray; DSS: Dyspnea Severity Score; ED: emergency department; ECG: electrocardiogram; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HR: heart rate; ICU: intensive care unit; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; mmHg: millimetre of mercury; NIMV: noninvasive mechanical ventilation; NIV: non‐invasive ventilation; PaO2: partial pressure of arterial oxygen; PaCO2: partial pressure of arterial carbon dioxide; PEFR: peak expiratory flow rate; PO: per oral; PR: pulse rate; RR: respiratory rate; SaO2: arterial oxygen saturation; SD: standard deviation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abreu 2006 | cross‐over design |
| ACTRN12608000502336 | study of people with stable COPD |
| Ahmed 2020 | study of intraoperative magnesium sulfate infusion in people with stable COPD |
| Amaral 2012 | study in people with stable COPD |
| CTRI/2018/01/011354 2018 | not an RCT |
| CTRI/2018/04/013309 2018 | not an RCT |
| Fan 2015 | study of magnesium sulfate inhalation versus alpha chymotrypsin atomisation inhalation |
| Friemann 1991 | study of magnesium aspartate hydrochloride |
| ISRCTN65174202 2006 | study was stopped due to participant recruitment issues |
| Jenner 2004 | not an RCT |
| Marino 1999 | cross‐over trial |
| NCT01118936 2010 | study in people with stable COPD |
| NCT02498496 2015 | study was withdrawn due to financing delays |
| NCT02680769 2016 | study in people with stable COPD |
| Schenk 2001 | not an RCT |
| Skorodin 1998 | study of magnesium sulfate and ipratropium bromide combination versus salbutamol and normal saline without magnesium sulfate monotherapy arm |
| Sternfeld 1994 | not an RCT |
| Tagaya 2004 | study in people with stable COPD |
Characteristics of ongoing studies [ordered by study ID]
IRCT2016012420024N3 2016.
| Study name | Nebulised magnesium sulfate treatment in acute exacerbation of chronic obstructive pulmonary disease |
| Methods | Study design: phase 2 to 3 randomised double‐blind placebo‐controlled trial of parallel assignment |
| Participants | Inclusion criteria
Exclusion criteria
Age minimum: 40 years Age maximum: no limit Gender: both |
| Interventions | Intervention: 2.5 mLmagnesium sulfate Vial 20% by nebulizer mask Placebo: 2.5 mLsodium chloride 0.9% |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 22 January 2016 |
| Contact information | Dr. Besharat Rahimi Advanced Thoracic Research Cente, Imamkhomeini medical complex, Keshavarz blvd.Tehran Iran (Islamic Republic of) besharatrahimi@yahoo.com |
| Notes | Sponsor: Vice chancellor for research, Tehran University of Medical Sciences |
Differences between protocol and review
In the protocol, we had defined adults as ≥ 40 years; however, three of the included studies in this review defined adults as ≥ 35 years (Edwards 2013; Mukerji 2015; Skorodin 1995).
We could not compare between intravenous magnesium sulfate + standard care versus standard care as we identified no studies reporting this comparison. We added the comparison of magnesium sulfate versus ipratropium bromide, which is the standard therapy of COPD exacerbations. In data analysis, we used Peto OR for rare events. We could not perform subgroup and sensitivity analyses as planned in the protocol as the trials did not report data on prespecified groups of people. We analysed oxygen saturation as both arterial (SaO2) and peripheral (SpO2).
We edited two of the outcomes in the Methods section 'Summary of findings and assessment of the certainty of the evidence' to be more specific: ‘arterial blood gas measurements (e.g. PaCO2)’ became oxygen saturation; and 'symptom scores, as measured by validated scales; e.g. EXACT total score’ became dyspnoea.
Contributions of authors
Han Ni (HN): designed the work, conducted the literature search, screened articles, assessed risk of bias, extracted data from included studies, performed data analyses, wrote the review and revised the manuscript critically for important intellectual content.
Swe Zin Aye (SZA): conducted the literature search, screened titles, assessed risk of bias, extracted data from included studies, checked data entry and drafted the review.
Cho Naing (CN): commented on the review, checked data analyses and revised the manuscript critically for important intellectual and statistical content.
All authors reviewed and agreed on the review prior to submission for editorial review.
Contributions of editorial team
Emma Dennett (Deputy Co‐ordinating Editor): co‐ordinated the editorial process; advised on content; edited the review; signed off the review for publication with assistance from the contact editor. Alexander Mathioudakis (contact Editor): edited the review, assisted with sign‐off of the review. Rebecca Fortescue (Co‐ordinating Editor): edited the review; advised on methodology. Chris Cates (Co‐ordinating Editor): checked the data analyses. Emma Jackson (Managing Editor): conducted peer review; edited the references and other sections of the review. Elizabeth Stovold (Information Specialist): designed the search strategy, conducted the searches.
Sources of support
Internal sources
-
Newcastle University Medicine, Malaysia
Allowed Han Ni to work on this systematic review during office hours
-
QUEST International University Perak, Malaysia
Permitted Swe Zin Aye to work on this review during office hours
-
International Medical University (IMU), Malaysia and The James Cook University, Queensland, Australia, Malaysia
Allowed CN to work on this systematic review during office hours.
CN was at the IMU during the data synthesis phase of study.
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure funding to Cochrane Airways. [The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.]
Declarations of interest
Han Ni: none known. Cho Naing: none known. Swe Zin Aye: none known.
New
References
References to studies included in this review
Bajracharya 2021 {published data only (unpublished sought but not used)}
- Bajracharya M, Acharya RP, Neupane RP, Sthapit R, Tamrakar AR.Nebulized magnesium sulphate versus saline as an adjuvant in acute exacerbation of chronic obstructive pulmonary disease in a tertiary centre of Nepal: a randomised control study. Journal of Institute of Medicine Nepal 2021;43(1):5-10. [DOI: 10.3126/jiom.v43i1.37461] [DOI] [Google Scholar]
Comert 2016 {published and unpublished data}
- Comert S, Kiyan E, Okumus G, Arseven O, Ece T, Issever H.Efficiency of nebulised magnesium sulphate in infective exacerbations of chronic obstructive pulmonary disease. Tuberkuloz ve Toraks 2016;64(1):17-26. [DOI] [PubMed] [Google Scholar]
- Sunmez S, Kiyan E, Okumus G, Arseven O, Ece T, Issever H.Efficiency of nebulised magnesium sulphate in exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2006;28(Suppl 50):1275. [Google Scholar]
Edwards 2013 {published data only (unpublished sought but not used)}
- ACTRN12608000167369.A study to investigate the effects of nebulised magnesium in severe exacerbations of chronic obstructive pulmonary disease (COPD). www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82699 (first received 27 March 2008).
- Edwards L, Shirtcliffe P, Wadsworth K, Healy B, Jefferies S, Weatherall M, et al.Use of nebulised magnesium sulphate as an adjuvant in the treatment of acute exacerbations of COPD in adults: a randomised double-blind placebo-controlled trial. Thorax 2013;68(4):338-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hogg 2004 {published data only}
- Hogg JS, Mulrenan SA, Everett C, Morice AH.A single centre double blind placebo controlled pilot study in the use of intravenous magnesium sulphate for the treatment of acute exacerbation of chronic obstructive airways disease (COPD). European Respiratory Journal 2004;24:343s. [Google Scholar]
Jahanian 2021 {published data only (unpublished sought but not used)}
- IRCT20150315021480N8.The effect of intravenous magnesium sulfate as a complementary therapy to COPD [Study of the effect of intravenous magnesium sulfate on clinical performance as a complementary therapy of the patients with COPD in the emergency department]. en.irct.ir/trial/18822 (first received 9 May 2018).
- Jahanian F, Khatir IG, Ahidashti HA, Amirifard S.The effect of intravenous magnesium sulphate as an adjuvant in the treatment of acute exacerbations of COPD in the emergency department: a double-blind randomised clinical trial. Ethiopian Journal of Health Sciences 2021;31(2):267-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moradi 2021 {published and unpublished data}
- IRCT2014111519962N1.The effect of IV magnesium sulfate on dyspnoea improving of patients with chronic obstructive pulmonary disease. en.irct.ir/trial/17716 (first received 4 February 2015).
- Vafadar Moradi E, Pishbin E, Habibzadeh SR, Talebi Doluee M, Soltanifar A.The adjunctive effect of intravenous magnesium sulfate in acute exacerbation of chronic obstructive pulmonary disease: a randomised controlled clinical trial. Academic Emergency Medicine 2021;28(3):359-62. [DOI] [PubMed] [Google Scholar]
Mukerji 2015 {published data only}
- ACTRN12613000837729.Intravenous magnesium as an adjunct treatment in acute exacerbations of chronic obstructive pulmonary disease [Intravenous magnesium sulphate will improve bronchodilation in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) compared to placebo]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364675 (first received 25 July 2013).
- Mukerji S, Shahpuri B, Clayton-Smith B, Smith N, Armstrong P, Hardy M, et al.Intravenous magnesium sulphate as an adjuvant therapy in acute exacerbations of chronic obstructive pulmonary disease: a single centre, randomised, double-blinded, parallel group, placebo-controlled trial: a pilot study. New Zealand Medical Journal 2015;128(1425):34-42. [PMID: ] [PubMed] [Google Scholar]
Nouira 2014 {published data only (unpublished sought but not used)}
- NCT01136421.Magnesium sulfate versus ipratropium bromide in acute exacerbation of chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01136421 (first received 3 June 2010).
- Nouira S, Bouida W, Grissa MH, Beltaief K, Trimech MN, Boubaker H, et al.Magnesium sulfate versus ipratropium bromide in chronic obstructive pulmonary disease exacerbation: a randomised trial. American Journal of Therapeutics 2014;21(3):152-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pishbin 2018 {published data only (unpublished sought but not used)}
- Pishbin E, Moradi EV.Intravenous magnesium sulfate in the treatment of acute exacerbations of COPD: a randomised controlled trial. Critical Care 2018;22(Suppl 1):P228. [Google Scholar]
- Pishbin E.Intravenous magnesium sulfate in the treatment of acute exacerbations of COPD. Journal of Emergency Medicine 2017;53(3):442-3. [Google Scholar]
Skorodin 1995 {published data only}
- Skorodin MS, Tenholder MF, Yetter B, Owen KA, Waller RF, Khandelwahl S, et al.Magnesium sulfate in exacerbations of chronic obstructive pulmonary disease. Archives of Internal Medicine 1995;155(5):496-500. [PMID: ] [PubMed] [Google Scholar]
Solooki 2014 {published data only (unpublished sought but not used)}
- Solooki M, Miri M, Mokhtari M, Valai M, Sistanizad M, Kouchek M.Magnesium sulfate in exacerbations of COPD in patients admitted to internal medicine ward. Iranian Journal of Pharmaceutical Research 2014;13(4):1235-9. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abreu 2006 {published data only}
- Abreu Gonzalez J, Hernandez Garcia C, Abreu Gonzalez P, Martin Garcia C, Jimenez A.Effect of intravenous magnesium sulfate on chronic obstructive pulmonary disease exacerbations requiring hospitalisation: a randomised placebo-controlled trial. Archivos de Bronconeumologia 2006;42(8):384-7. [DOI] [PubMed] [Google Scholar]
ACTRN12608000502336 {unpublished data only}
- ACTRN12608000502336.Effects of inhaled magnesium on pulmonary function and walking distance of patients with stable chronic obstructive pulmonary disease (COPD). www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83127 (first received 3 September 2008).
Ahmed 2020 {published data only}
- Ahmed A, Sayed AH, Elkholy J, Elshal S, Badwy A, Abdelhamid B, et al.Intraoperative MgSO4 infusion protects oxygenation and lung mechanics in COPD patients during general anaesthesia. A randomised clinical trial. Acta Anaesthesiologica Scandinavica 2020;64(10):1460-8. [DOI] [PubMed] [Google Scholar]
Amaral 2012 {published data only}
- Amaral AF, Gallo L Jr, Vannucchi H, Crescencio JC, Vianna EO, Martinez JA.The effect of acute magnesium loading on the maximal exercise performance of stable chronic obstructive pulmonary disease patients. Clinics 2012;67(6):615-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral AF, Rodrigues-Junior AL, Terra Filho J, Vannucchi H, Martinez JA.Effects of acute magnesium loading on pulmonary function of stable COPD patients. Medical Science Monitor 2008;14(10):CR524-9. [PubMed] [Google Scholar]
- Baddini MJ, do Amaral AF, Crescencio JC, Vannucchi H, Vianna EO, Gallo L.Effects of acute magnesium loading on maximal exercise performance of stable COPD patients. ATS Journals 2012;185:A1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JA, Amaral AF, Teixeira CA, Gallo L, Terra Filho J, Kazuki RT.The effects of intravenous magnesium loading on pulmonary function of COPD patients in stable clinical conditions. In: American Thoracic Society International Conference; 2007 May 18-23; San Francisco. 2007.
- NCT00500864.Magnesium loading in chronic obstructive pulmonary disease [Effects of acute intravenous magnesium loading on pulmonary function parameters and maximal exercise capacity of patients with chronic obstructive pulmonary disease in stable clinical conditions]. clinicaltrials.gov/ct2/show/NCT00500864 (first received 13 July 2007).
CTRI/2018/01/011354 2018 {unpublished data only}
- CTRI/2018/01/011354.A study to compare and know the beneficial role of intravenous magnesium sulfate vs placebo as an add on therapy in the treatment of acute exacerbations of chronic obstructive pulmonary disease [A prospective study to compare the efficacy of I.V Magnesium sulfate infusion vs. placebo, as an adjuvant to nebulised salbutamol in acute exacerbations of chronic obstructive pulmonary disease]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/01/011354 (first received 16 January 2018).
CTRI/2018/04/013309 2018 {unpublished data only}
- CTRI/2018/04/013309.A study to compare the costs between intravenous Magnesium Sulfate and placebo in acute exacerbations of chronic obstructive pulmonary disease [A prospective study to compare the cost effectiveness of i.v. magnesium sulfate infusion vs. Placebo, as adjutant to nebulised salbutamol in acute exacerbations of chronic obstructive pulmonary disease.]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/04/013309 (first received 17 April 2018).
Fan 2015 {published data only}
- Fan MS, Wu WH, Chen JY.Effect of magnesium sulfate and oxygen inhalation on pulmonary function in elderly chronic obstructive pulmonary disease during acute attack. Journal of Tropical Medicine 2015;15:959-62. [Google Scholar]
Friemann 1991 {published data only}
- Friemann EF, Lasch HG, Friemann S, Golf S, Enzinger D, Temme H, et al.Effect of a magnesium therapy on chronic obstructive lung diseases. Die Medizinische Welt 1991;42:311-5. [Google Scholar]
ISRCTN65174202 2006 {unpublished data only}
- EUCTR2006-002484-99-GB.The use of nebulised magnesium sulphate in exacerbations of chronic obstructive pulmonary disease (COPD) - nebulised magnesium in COPD. www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2006-002484-99-GB (first received 10 January 2007).
- ISRCTN65174202.The use of nebulised magnesium sulphate in exacerbations of chronic obstructive pulmonary disease. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN65174202 (first received 4 December 2006).
Jenner 2004 {published data only}
- Jenner R, Body R.Best evidence topic report. Intravenous magnesium in chronic obstructive pulmonary disease. Emergency Medicine Journal 2004;21(2):203. [PMC free article] [PubMed] [Google Scholar]
Marino 1999 {published data only}
- Marino P, Belloli E, Invernizzi F.Magnesium sulfate in chronic obstructive pulmonary disease. In: European Respiratory Society 9th Annual Congress; 1999 Oct 9-13; Madrid. 1999.
NCT01118936 2010 {unpublished data only}
- NCT01118936.Daily magnesium-treatment of patients with chronic obstructive pulmonary disease [Daily mablet-treatment of patients with COPD]. clinicaltrials.gov/ct2/show/NCT01118936 (first received 7 May 2010).
NCT02498496 2015 {unpublished data only}
- NCT02498496.Magnesium sulfate to prevent hospitalisation of acute exacerbations of chronic obstructive pulmonary disease [MASTER-ED: magnesium sulfate to prevent hospitalisation of acute exacerbations of chronic obstructive pulmonary disease seen in the emergency department]. clinicaltrials.gov/ct2/show/NCT02498496 (first received 15 July 2015).
NCT02680769 2016 {unpublished data only}
- NCT02680769.Effect of magnesium supplementation in COPD [Daily supplementation of magnesium citrate in moderate-severe chronic obstructive pulmonary disease: a randomised, controlled, double-blind trial]. clinicaltrials.gov/ct2/show/NCT02680769 (first received 12 February 2016).
Schenk 2001 {published data only}
- Schenk P, Vonbank K, Schnack B, Haber P, Lehr St, Smetana R.Magnesium in obstructive respiratory tract disease. Journal fur Mineralstoffwechsel 2001;8(3):38-41. [Google Scholar]
Skorodin 1998 {published data only}
- Skorodin M, Shorten J, Lyon K, Smallwood J, Owen W, Emery L, et al.Magnesium sulfate and ipratropium bromide combination in the treatment of COPD exacerbations. Archives of Internal Medicine 1998;155(5):496-500. [PubMed] [Google Scholar]
Sternfeld 1994 {published data only}
- Sternfeld M, Eliraz A.Magnesium and obstructive lung diseases. Harefuah 1994;126(11):681-3. [PubMed] [Google Scholar]
Tagaya 2004 {published data only}
- Tagaya E, Tamaoki J, Kawatani K, Nagai A.The effect of isotonic nebulized magnesium sulfate on pulmonary function in moderate to severe COPD. In: American Thoracic Society 100th international conference; 2004 May 21-26; Orlando. 2004.
References to ongoing studies
IRCT2016012420024N3 2016 {unpublished data only}
- IRCT2016012420024N3.Nebulized magnesium sulfate treatment in acute exacerbation of chronic obstructive pulmonary disease. irct.ir/trial/17769 (first received 6 February 2016).
Additional references
Almagro 2002
- Almagro P, Calbo E, Ochoa de Echaguen A, Barreiro B, Quintana S, Heredia JL, et al.Mortality after hospitalisation for COPD. Chest 2002;121(5):1441-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Alzaid 2021
- Alzaid H, Aldossari F, Bin Nafisah S.Magnesium administration for COPD exacerbation: a systematic review and meta-analysis. Journal of Medicine, Law and Public Health 2021;1(3):38-46. [DOI: 10.52609/jmlph.v1i3.22] [DOI] [Google Scholar]
American Lung Association 2013
- American Lung Association.Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality; 2013. www.lung.org/getmedia/4f74781e-481f-4f10-9255-8dfc9dc56974/copd-trend-report.pdf.pdf.
Anzueto 2009
- Anzueto A, Leimer I, Kesten S.Impact of frequency of COPD exacerbations on pulmonary function, health status and clinical outcomes. International Journal of Chronic Obstructive Pulmonary Disease 2009;4:245-51. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anzueto 2010
- Anzueto A.Impact of exacerbations on COPD. European Respiratory Review 2010;19(116):113-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ATS/ERS 2011
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al.Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179–91. [DOI] [PubMed] [Google Scholar]
Aziz 2005
- Aziz HS, Blamoun AI, Shubair MK, Ismail MM, DeBari VA, Khan MA.Serum magnesium levels and acute exacerbation of chronic obstructive pulmonary disease: a retrospective study. Annals of Clinical and Laboratory Science 2005;35(4):423-7. [PMID: ] [PubMed] [Google Scholar]
Bhatt 2008
- Bhatt SP, Khandelwal P, Nanda S, Stoltzfus JC, Fioravanti GT.Serum magnesium is an independent predictor of frequent readmissions due to acute exacerbation of chronic obstructive pulmonary disease. Respiratory Medicine 2008;102(7):999-1003. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borrell 2009
- Borrell E, Rodriguez M, Toran P, Munoz L, Pera G, Montella N, et al.Incidence and risk factors of exacerbations among COPD patients in primary health care: APMPOC study. BMC Public Health 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS/SIGN 2019
- Scottish Intercollegiate Guidelines Network, British Thoracic Society.British guideline on the management of asthma: a national clinical guideline: revised edition published July 2019. www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma.html.
Cairns 1996
- Cairns CB, Kraft M.Magnesium attenuates the neutrophil respiratory burst in adult asthmatic patients. Academic Emergency Medicine 1996;3(12):1093-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Celli 2007
- Celli BR, Barnes PJ.Exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2007;29:1224-38. [DOI: 10.1183/09031936.00109906] [DOI] [PubMed] [Google Scholar]
Celli 2008
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al.Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. American Journal of Respiratory and Critical Care Medicine 2008;178(4):332-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochrane Airways 2019
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 7 May 2019).
Connors 1996
- Connors AF Jr, Dawson NV, Thomas C, Harrell FE Jr, Desbiens N, Fulkerson WJ, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). American Journal of Respiratory and Critical Care Medicine 1996;154(4 Pt 1):959-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cosio 2018
- Cosio BG, Dacal D, Perez de Llano L.Asthma-COPD overlap: identification and optimal treatment. Therapeutic Advances in Respiratory Disease 2018;12:1753466618805662. [DOI: 10.1177/1753466618805662] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costello 2016
- Costello R, Wallace TC, Rosanoff A.Magnesium. Advances in Nutrition 2016;7(1):199-201. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2012
- David A, Corlateanu A.Influence of COPD exacerbations on health related quality of life. European Respiratory Journal 2012;40:P4821. [Google Scholar]
Del‐Castillo 1954
- Del-Castillo J, Engbaek L.The nature of the neuromuscular block produced by magnesium. Journal of Physiology 1954;124(2):370-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 2002
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA.Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57(10):847-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 2005
- Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA.Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171(5):446-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Elin 1988
- Elin RJ.Magnesium metabolism in health and disease. Disease-a-month 1988;34(4):161-218. [PMID: ] [DOI] [PubMed] [Google Scholar]
Emerman 1996
- Emerman CL, Cydulka RK.Use of peak expiratory flow rate in emergency department evaluation of acute exacerbation of chronic obstructive pulmonary disease. Annals of Emergency Medicine 1996;27(2):159-63. [DOI: 10.1016/s0196-0644(96)70317-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fawcett 1999
- Fawcett WJ, Haxby EJ, Male DA.Magnesium: physiology and pharmacology. British Journal of Anaesthesia 1999;83(2):302-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Aymerich 2011
- Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ.Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax 2011;66(7):585-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
GBD 2017
- Chronic Respiratory Disease Collaborators.Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma,1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respiratory Medicine 2017;5:691-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2009
- Gibson PG, Simpson JL.The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009;64(8):728-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
GINA 2018
- Global Initiative for Asthma: Global strategy for asthma management and prevention (2018 update). ginasthma.org/gina-ebooks (accessed 30 March 2019).
GOLD 2021
- 2021 Global Strategy for Prevention, Diagnosis and Management of COPD. goldcopd.org/2021-gold-reports/ (accessed 30 September 2021).
GOLD ACO 2015
- Global Initiative for Asthma, Global Initiative for Chronic Obstructive Lung Disease.Diagnosis of diseases of chronic airflow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS); 2015. goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome.
Gourgoulianis 2001
- Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA.Magnesium as a relaxing factor of airway smooth muscles. Journal of Aerosol Medicine 2001;14(3):301-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed 30 March 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grober 2015
- Grober U, Schmidt J, Kisters K.Magnesium in prevention and therapy. Nutrients 2015;7(9):8199-226. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Groenewegen 2003
- Groenewegen KH, Schols AM, Wouters EF.Mortality and mortality-related factors after hospitalisation for acute exacerbation of COPD. Chest 2003;124(2):459-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gumus 2014
- Gumus A, Haziroglu M, Gunes Y.Association of serum magnesium levels with frequency of acute exacerbations in chronic obstructive pulmonary disease: a prospective study. Pulmonary Medicine 2014;2014:329476. [DOI: 10.1155/2014/329476] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardin 2014
- Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, et al.The clinical and genetic features of COPD-asthma overlap syndrome. European Respiratory Journal 2014;44(2):341-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hines 2017
- Hines KL, Peebles RS Jr.Management of the asthma-COPD overlap syndrome (ACOS): a review of the evidence. Current Allergy and Asthma Reports 2017;17(3):15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hurst 2010
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al.Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128-38. [DOI: 10.1056/NEJMoa0909883] [DOI] [PubMed] [Google Scholar]
Jahangir 2022
- Jahangir A, Zia Z, Niazi MR, Sahra S, Jahangir A, Sharif MA, et al.Efficacy of magnesium sulfate in the chronic obstructive pulmonary disease population: a systematic review and meta-analysis. Advances in Respiratory Medicine 2022 Jan 31 [Epub ahead of print]. [DOI: 10.5603/ARM.a2022.0012] [PMID: ] [DOI] [PubMed]
Jenkins 2012
- Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, Vestbo J, et al.Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. European Respiratory Journal 2012;39(1):38-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kauppi 2011
- Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpelainen M, Kinnula VL, et al.Overlap syndrome of asthma and COPD predicts low quality of life. Journal of Asthma 2011;48(3):279-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kew 2014
- Kew KM, Kirtchuk L, Michell CI.Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD010909. [DOI: 10.1002/14651858.CD010909.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knightly 2017
- Knightly R, Milan SJ, Hughes R, Knopp-Sihota JA, Rowe BH, Normansell R, et al.Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD003898. [DOI: 10.1002/14651858.CD003898.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kshirsagar 2021
- Kshirsagar K, Patil VC.Chronic obstructive pulmonary disease: Is serum magnesium level risk factor for its acute exacerbation? Caspian Journal of Internal Medicine 2021;12(2):223-7. [DOI: 10.22088/cjim.12.2.223] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumbhare 2016
- Kumbhare S, Pleasants R, Ohar JA, Strange C.Characteristics and prevalence of asthma/chronic obstructive pulmonary disease overlap in the United States. Annals of the American Thoracic Society 2016;13(6):803-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laires 2004
- Laires MJ, Monteiro CP, Bicho M.Role of cellular magnesium in health and human disease. Frontiers in Bioscience 2004;9:262-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lange 2016
- Lange P, Colak Y, Ingebrigtsen TS, Vestbo J, Marott JL.Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respiratory Medicine 2016;4(6):454-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Leung 2017
- Leung JM, Sin DD.Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ 2017;358:j3772. [PMID: ] [DOI] [PubMed] [Google Scholar]
Llewellin 2002
- Llewellin P, Sawyer G, Lewis S, Cheng S, Weatherall M, Fitzharris P, et al.The relationship between FEV1 and PEF in the assessment of the severity of airways obstruction. Respirology 2002;7(4):333-7. [DOI: 10.1046/j.1440-1843.2002.00417.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lopez‐Campos 2016
- Lopez-Campos JL, Tan W, Soriano JB.Global burden of COPD. Respirology 2016;21(1):14-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mathioudakis 2022
- Mathioudakis AG, Abroug F, Agusti A, Ananth S, Bakke P, Bartziokas K, et al.DECODE-NET. ERS Statement: A core outcome set for clinical trials evaluating the management of chronic obstructive pulmonary disease (COPD) exacerbations. European Respiratory Journal 2022;59:2102006. [DOI: 10.1183/13993003.02006-2021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miravitlles 2004
- Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, et al.Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004;59(5):387-95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullerova 2015
- Mullerova H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha JA, et al.Hospitalised exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest 2015;147(4):999-1007. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nannini 2012
Ngahavi 2017
- Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence.Chronic obstructive pulmonary disease in over 16s: diagnosis and management. www.guidelines.co.uk/respiratory/nice-copd-guideline/454912.article (accessed 8 December 2019). [PubMed]
O'Donnell 2006
- O'Donnell DE, Parker CM.COPD exacerbations: pathophysiology. Thorax 2006;61(4):354-61. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitta 2006
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R.Physical activity and hospitalisation for exacerbation of COPD. Chest 2006;129(3):536-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web).Version 4.6.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Ries 2005
- Ries AL.Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD 2005;2(1):105-10. [DOI: 10.1081/copd-200050655] [PMID: ] [DOI] [PubMed] [Google Scholar]
Romani 2013
- Romani AM.Magnesium in health and disease. Metal Ions in Life Sciences 2013;13:49-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rowe 2000
- Rowe BH, Bretzlaff J, Bourdon C, Bota G, Blitz S, Camargo Jr CA.Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No: CD001490. [DOI: 10.1002/14651858.CD001490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Seemungal 1998
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA.Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(5 Pt 1):1418-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shivanthan 2014
- Shivanthan MC, Rajapakse S.Magnesium for acute exacerbation of chronic obstructive pulmonary disease: a systematic review of randomised trials. Annals of Thoracic Medicine 2014;9(2):77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soler‐Cataluna 2005
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R.Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60(11):925-31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorino 2016
- Sorino C, Pedone C, Scichilone N.Fifteen-year mortality of patients with asthma-COPD overlap syndrome. European Journal of Internal Medicine 2016;34:72-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soto‐Campos 2013
- Soto-Campos JG, Plaza V, Soriano JB, Cabrera-Lopez C, Almonacid-Sanchez C, Vazquez-Oliva R, et al.Causes of death in asthma, COPD and non-respiratory hospitalised patients: a multicentric study. BMC Pulmonary Medicine 2013;13:73. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2001
- Spencer S, Calverley PM, Sherwood Burge P, Jones PW.Health status deterioration in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(1):122-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spivey 1990
- Spivey WH, Skobeloff EM, Levin RM.Effect of magnesium chloride on rabbit bronchial smooth muscle. Annals of Emergency Medicine 1990;19(10):1107-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wedzicha 2007
- Wedzicha JA, Seemungal TA.COPD exacerbations: defining their cause and prevention. Lancet 2007;370(9589):786-96. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wedzicha 2017
- Wedzicha JA, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al.Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. European Respiratory Journal 2017;49(3):1600791. [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2020
- WHO Global Health Estimates: The top 10 causes of death; 2020. www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
WHO 2021
- World Health Organization.Chronic obstructive pulmonary disease (COPD) Fact sheets; 21 June 2021. www.who.int/en/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed prior to 4 May 2022).
Yang 2019
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald V, et al.The COPD-X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2019. Version 2.59, August 2019. copdx.org.au/copd-x-plan/.
References to other published versions of this review
Ni 2020
- Ni H, Naing C, Aye SZ.Magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD013506. [DOI: 10.1002/14651858.CD013506] [DOI] [PMC free article] [PubMed] [Google Scholar]


