Purpose and Scope of This Practice Guidance
This is the first American Association for the Study of Liver Diseases (AASLD) practice guidance on the management of malnutrition, frailty, and sarcopenia in patients with cirrhosis. This guidance represents the consensus of a panel of experts after a thorough review and vigorous debate of the literature published to date, incorporating clinical experience and common sense to fill in the gaps when appropriate. Our goal was to offer clinicians pragmatic recommendations that could be implemented immediately in clinical practice to target malnutrition, frailty, and sarcopenia in this population.
This AASLD guidance document differs from AASLD guidelines, which are supported by systematic reviews of the literature, formal rating of the quality of the evidence and strength of the recommendations, and, if appropriate, meta-analysis of results using the Grading of Recommendations Assessment Development and Evaluation system. In contrast, this guidance was developed by consensus of an expert panel and provides guidance statements based on formal review and analysis of the literature on the topics, with oversight provided by the AASLD Practice Guidelines Committee at all stages of guidance development. The AASLD Practice Guidelines Committee chose to perform a guidance on this topic because a sufficient number of randomized controlled trials (RCTs) were not available to support the development of a guideline.
Definitions of Malnutrition, Frailty, and Sarcopenia and Their Relationship in Patients With Cirrhosis
Cirrhosis is a major predisposing condition for the development of malnutrition, frailty, and sarcopenia. Multiple, yet complementary, definitions of these conditions exist in the published domain outside of the field of hepatology; but consensus definitions have not yet been established by the AASLD for patients with cirrhosis. Furthermore, there has been ambiguity related to operationalization of these constructs in clinical practice. To address this, we offer definitions of the theoretical constructs of malnutrition, frailty, and sarcopenia as commonly represented in all populations, partnered with operational definitions, developed by consensus, to facilitate pragmatic implementation of these constructs in clinical practice as applied to patients with cirrhosis (Table 1).
TABLE 1.
Definitions for the Theoretical Constructs of Malnutrition, Frailty, and Sarcopenia and Consensus-Derived Operational Definitions Applied to Patients with Cirrhosis
| Construct | Theoretical Definitions | Operational Definitions |
|---|---|---|
| Malnutrition | A clinical syndrome that results from deficiencies or excesses of nutrient intake, imbalance of essential nutrients, or impaired nutrient use(4) | An imbalance (deficiency or excess) of nutrients that causes measurable adverse effects on tissue/body form (body shape, size, composition) or function and/or clinical outcome(1) |
| Frailty | A clinical state of decreased physiologic reserve and increased vulnerability to health stressors(2) | The phenotypic representation of impaired muscle contractile function |
| Sarcopenia | A progressive and generalized skeletal muscle disorder associated with an increased likelihood of adverse outcomes including falls, fractures, disability, and mortality(3) | The phenotypic representation of loss of muscle mass |
Malnutrition is a clinical syndrome that results from “an imbalance (deficiency or excess) of nutrients that causes measurable adverse effects on tissue/body form (body shape, size, composition) or function, and/or clinical outcome.”(1) Key to this definition is the recognition that malnutrition represents a spectrum of nutritional disorders across the entire range of body mass index (BMI)—from underweight to obese. By this definition, malnutrition leads to adverse physical effects, which, in patients with cirrhosis, are commonly manifested phenotypically as frailty or sarcopenia.
Frailty has most commonly been defined as a clinical state of decreased physiologic reserve and increased vulnerability to health stressors, a definition that has its roots in the field of geriatrics.(2) However, the weight of evidence available to date in patients with cirrhosis has focused predominantly on one component of frailty: physical frailty. Although this representation deviates somewhat from the classic “geriatric” definition of frailty as a global construct, physical frailty represents clinical manifestations of impaired muscle contractile function that are commonly reported by patients with cirrhosis such as decreased physical function, decreased functional performance, and disability.
Sarcopenia has been defined by the European Working Group on Sarcopenia as “a progressive and generalized skeletal muscle disorder associated with an increased likelihood of adverse outcomes including falls, fractures, disability, and mortality,” combining both muscle mass and muscle strength or muscle performance in its definition.(3) However, the majority of studies in patients with cirrhosis have investigated sarcopenia using measures of muscle mass alone. Therefore, based on the evidence available to date on patients with cirrhosis, we have developed a consensus definition for operationalization of sarcopenia in patients with cirrhosis as the phenotypic manifestation of loss of muscle mass.
Although we have, for the purposes of this guidance, developed separate operational definitions for malnutrition, frailty, and sarcopenia, we acknowledge that these three constructs are interrelated and in practice are often recognized simultaneously in an individual patient. For example, a patient with cirrhosis who presents to clinic with severe muscle wasting might be described as “malnourished,” “frail,” and “sarcopenic,” each descriptor conveying similar information about the patient’s poor clinical condition and prognosis. Despite the overlap of these three constructs in clinical practice, there is value in understanding each as a separate entity as well as the relationship between the three in order to develop tailored behavioral interventions and targeted pharmacotherapies for these conditions.
Herein, we propose a conceptual framework for this relationship (Fig. 1). There are a number of factors that lead to malnutrition in patients with cirrhosis, which is challenging to identify at the bedside unless it manifests phenotypically as frailty and/or sarcopenia. Malnutrition is not the only factor that contributes to frailty and sarcopenia; other factors such as cirrhosis complications, other systems-related factors (e.g., systemic inflammation, metabolic dysregulation), physical inactivity, and environmental/organizational factors can contribute to frailty and/or sarcopenia within or independent of the malnutrition pathway. In addition, frailty and sarcopenia can contribute to each other—impaired muscle contractile function can accelerate loss of muscle mass and vice versa. It is these clinical phenotypes—frailty and sarcopenia—that ultimately lead to adverse health outcomes including hepatic decompensation, increased health care use, worse health-related quality of life, adverse posttransplant outcomes, and increased overall risk of death.
FIG. 1.
Factors contributing to malnutrition, frailty, and sarcopenia and the relationship between these three constructs. Cirrhosis-related and other systems-related factors, along with physical inactivity and environmental/organizational factors, contribute to malnutrition—which then leads to frailty and sarcopenia. These factors can also contribute directly to frailty and sarcopenia independently of malnutrition.
Factors That Contribute to Frailty and Sarcopenia in Patients With Cirrhosis
Here, we describe the factors that have been shown to contribute to frailty and sarcopenia in patients with cirrhosis. We acknowledge that these factors are, in some cases, interrelated; but for the purposes of ease of clinical implementation, we have categorized these factors broadly as (1) malnutrition, (2) cirrhosis-related, (3) other systems–related, (4) physical inactivity, and (5) environmental/organizational factors.
MALNUTRITION
Impaired Intake of Macronutrients
Reduced oral intake results from many factors including early satiety, anorexia, nausea and vomiting, dysgeusia, diet unpalatability (e.g., low sodium or low potassium), impaired level of consciousness, free water restriction, and frequent fasting due to procedures and hospitalizations.(5) Excess oral intake is a root cause of obesity and is influenced by a variety of biological, sociocultural, and psychological factors.(6) Many patients with cirrhosis have limited knowledge about disease self-management, including nutrition therapy.(7,8) Inadequate food knowledge/preparation skills and food insecurity can impact dietary intake—through either reduced or excess intake—across the spectrum of nutritional disorders from undernutrition to obesity.(7-9)
Impaired Intake of Micronutrients
Malabsorption leads to high rates of micronutrient deficiency in patients with cirrhosis. Factors leading to impaired macronutrient intake and absorption also contribute to deficiency of many micronutrients. In particular, folate, thiamine, zinc, selenium, vitamin D, and vitamin E deficiencies have been reported in patients with alcohol-associated liver disease; and fat-soluble vitamin deficiencies have been well documented in patients with cholestatic liver disease.(10-14) Several of these micronutrients have a strong link with frailty or sarcopenia. Vitamin D deficiency is associated with impaired muscle contractile function in the general population.(15) Although studies evaluating the role of vitamin D deficiency on frailty and sarcopenia in patients with cirrhosis are lacking, vitamin D deficiency is prevalent in patients with cirrhosis(16-18) and may contribute to the development and progression of frailty in this population. Deficiency of zinc, a cofactor in the urea cycle that metabolizes ammonium, is associated with HE, frailty, and sarcopenia in patients with cirrhosis.(19-21) Magnesium deficiency occurs because of malabsorption of magnesium in the small intestine and is exacerbated by diuretic use. Magnesium deficiency is associated with reduced cognitive performance as well as reduced muscle strength in adults with cirrhosis(22-24) and with increased bone resorption in children with cholestatic liver disease.(25)
Impaired Nutrient Uptake
Impaired nutrient uptake is multifactorial, resulting from malabsorption, maldigestion, and altered macronutrient metabolism. Cholestasis leads to alterations in the enterohepatic circulation of bile salts and maladaptation of bile salt regulation. This may result in elevated serum and tissue levels of potentially toxic bile salts as well as impaired metabolism and malabsorption of long-chain fatty acids and fat-soluble vitamin deficiency in both adults and children.(26-28) Other contributors to malabsorption and maldigestion in patients with cirrhosis include portosystemic shunting, pancreatic enzyme deficiency, bacterial overgrowth, altered intestinal flora, and enteropathy.(5) Altered macronutrient metabolism or “accelerated starvation” occurs as a result of reduced hepatic glycogen synthesis and storage during the postprandial state, an early shift from glycogenolysis to gluconeogenesis, fatty acid oxidation, and increased rates of whole-body protein breakdown.(29,30) Hypermetabolism has been variably defined in the literature (e.g., resting energy expenditure [REE] + 1 SD or REE:REE predicted + 2SD).(31,32) With its associated catabolic state, hypermetabolism also contributes to the imbalance between intake and requirements, occurring in at least 15% of patients with cirrhosis without a clear correlation of hypermetabolism with disease severity or other predictors.(32,33)
CIRRHOSIS-RELATED
Cirrhosis itself leads to frailty and sarcopenia through a number of pathways. At the pathophysiological level, the altered catabolic state in cirrhosis leads to an imbalance between energy needs and intake. Altered protein metabolism, particularly of branched-chain amino acids (BCAAs) that are essential for supporting glutamine synthesis and extrahepatic ammonia detoxification, results in reduced levels of circulating BCAAs, which leads to accelerated muscle breakdown.(34-36) Impaired hepatic ammonia clearance from loss of metabolic capacity, in combination with increased portosystemic shunting, increases systemic ammonia concentration with pathologic effects on the muscle.(37-39) Ammonia is myotoxic through mechanisms that include decreased protein synthesis, increased autophagy, proteolysis, and mitochondrial oxidative dysfunction in the skeletal muscle. Posttranslational modifications of contractile proteins with bioenergetic dysfunction result in muscle contractile dysfunction and loss of muscle mass.(40-42)
The etiology of liver disease has been associated with differences in the prevalence of sarcopenia.(43,44) For example, alcohol-associated liver disease has been associated with a particularly high prevalence of sarcopenia, affecting 80% of patients with decompensated cirrhosis—although sarcopenia was reported in approximately 60% of patients with cirrhosis from NASH, chronic HCV, and autoimmune hepatitis.(45) Patients with alcohol-associated cirrhosis display the most rapid rate of reduction in muscle areas compared with other etiologies.(43) Alcohol exposure increases muscle autophagy, inhibits proteasome activity, and decreases the anabolic hormone insulin-like growth factor 1.(46-48) Patients with cirrhosis secondary to NASH may be at increased risk of sarcopenia due to the additive effects of insulin resistance and chronic systemic inflammation.(49) Finally, cholestasis-predominant liver diseases, such as primary sclerosing cholangitis, lead to elevated serum bile acid levels that may induce skeletal muscle atrophy through the bile acid receptor G protein–coupled bile acid receptor 1 (or TGR5) that is expressed in healthy muscles.(50)
Complications of portal hypertension also contribute to malnutrition and muscle dysfunction. HE is associated with anorexia, reduced physical activity, and frequent hospitalizations.(37,51) Ascites contributes to anorexia, early satiety, increased REE, and limited physical activity.(52,53) Both HE and ascites are strongly associated with frailty.(54)
OTHER SYSTEMS
Systemic Inflammation, Endocrine Factors, Metabolic Dysregulation, and Other Aging-Related Conditions
Circulating levels of inflammatory markers such as IL-1, IL-6, IL-10, C-reactive protein, and TNF-α are elevated in patients with cirrhosis.(55,56) Low-grade endotoxemia may result from increased gut permeability, from impaired hepatic clearance of lipopolysaccharide and portosystemic shunting, and potentially from cirrhosis-related changes in the gut microbiome.(57) This chronic systemic inflammation may promote the development of frailty, sarcopenia, and their subsequent complications through reduced muscle protein synthesis and increased protein degradation.(58-61)
Even in the absence of cirrhosis, chronic liver disease may lead to systemic inflammation and vulnerability to developing frailty and sarcopenia. Inflammatory cytokines are elevated in chronic HCV; eradication of HCV with antiviral agents results in a decrease of these markers.(62,63) Both alcohol-associated liver diseases and NAFLDs are also characterized by elevated systemic inflammatory markers.(64)
Further disruption of mediators of the “liver–muscle axis” may result from cirrhosis-related reduction in circulating levels of testosterone and changes in growth hormone secretion and sensitivity.(65) Low testosterone levels have been observed in male patients with cirrhosis and sarcopenia compared with patients who are nonsarcopenic.(66) Testosterone replacement resulted in improvements in total lean body mass,(67) further supporting the role of low testosterone in the development and progression of sarcopenia.
Obesity has been associated with frailty and sarcopenia in patients with cirrhosis and is of increasing relevance given the rapidly rising prevalence of obesity-related liver diseases.(6,68-71) Obesity is associated with metabolic dysregulation, visceral fat accumulation, insulin resistance, and anabolic resistance. A strong link has been demonstrated between obesity and muscle loss in patients with cirrhosis, with nearly one third of patients with obesity and cirrhosis meeting criteria for sarcopenia by skeletal muscle index (SMI).(70) With regard to muscle function, obesity has not been associated with an increased rate of frailty, although one multicenter study of patients with cirrhosis awaiting liver transplantation did demonstrate a significant interaction between obesity and frailty on clinical outcomes: patients with a BMI ≥ 35 kg/m2 who were frail experienced a 3-fold increased risk of waitlist mortality compared with similar-weight patients who were nonfrail.(68)
Consistent with the general population, there has been a rapid rise in the prevalence of cirrhosis in older adults.(72) In older adults with cirrhosis, a combination of primary (aging-related) and secondary (chronic disease–related) sarcopenia occurs simultaneously and has been referred to as “compound sarcopenia.”(73) In hospitalized patients, compound sarcopenia was associated with higher odds of death (OR, 1.06; 95% CI, 1.04-1.08) and greater resource use (OR, 1.10; 95% CI, 1.04-1.08) than patients with cirrhosis but without compound sarcopenia.(73)
Physical Inactivity
Physical inactivity and sedentary behavior are common in patients with cirrhosis and are associated with frailty and sarcopenia as well as mortality.(74-76) In one small study of 53 liver transplant candidates, participants spent 76% of their waking hours in sedentary time and completed a mean of only 3,000 steps per day.(76) Physical inactivity was significantly higher among liver transplant candidates who experienced waitlist mortality than in those who experienced other outcomes on the waitlist (e.g., transplant, removed for social reasons, or still waiting).(75) In a survey of liver transplant candidates and their caregivers, only 60% of patients and caregivers reported feeling that their clinicians “encouraged exercise,”(77) suggesting that one possible barrier to engaging in physical activity is the patient–provider communication around the benefits of physical activity.
There are no prospective longitudinal studies evaluating the direct role of physical inactivity on progressive frailty and/or sarcopenia. However, a number of trials have demonstrated a benefit of interventions to increase physical activity (in combination with nutritional counseling) on muscle function, muscle mass, and functional capacity.(78-82) These studies suggest that physical inactivity may, in part, contribute to decline in muscle function and/or muscle mass.
Social Determinants of Health
Social determinants of health—that is, where we live, learn, work, and play(83)—also play a role in the development of malnutrition, frailty, and sarcopenia. Health literacy is primarily governed by socioeconomic factors and is associated with physical frailty among liver transplant candidates.(84) Food insecurity owing to social factors such as poverty, isolation, or limited access to nutritious food is associated with advanced liver disease in patients with NAFLD.(85) Financial strain may limit caregiver presence in the home, resulting in limited monitoring, limited supervision for physical activity, and less attentive management of cirrhosis complications (e.g., timely lactulose therapy for HE). Conversely, increased patient needs impact caregiver productivity and earning potential. Some caregivers of patients with cirrhosis lose employment,(86) potentially worsening financial strain and thus the ability to provide adequate nutrition and management of cirrhosis complications that contribute to malnutrition.
Organizational Factors
Factors at the local, community, and national levels can exacerbate the development of malnutrition, frailty, and sarcopenia in this population. Community-level barriers to access to nutritious food may accelerate the development of all of these factors, including obesity, in some populations and drive adverse outcomes. In pediatric liver transplant recipients, neighborhood deprivation, an administrative metric of socioeconomic status, has been shown to be independently associated with mortality.(87) Given the complexity of managing patients with cirrhosis, there may be insufficient time during clinical visits to devote to identifying factors and developing strategies to target the contributing causes. Although a referral to, or comanagement with, a registered dietician with expertise in managing patients with advanced liver disease is ideal, some health care systems may not offer this resource or allow for longitudinal follow-up to assess for response to treatment recommendations. Furthermore, there may be confusion about which provider is responsible for management (e.g., primary care physician, hepatologist, registered dietician), despite the importance of a multimodal, multidisciplinary approach.
Clinical Manifestations of Muscle Dysfunction: Frailty and Sarcopenia
FRAILTY
Assessment of Frailty in Adults and Children
Tools to assess frailty as a multidimensional construct (e.g., global frailty) or its individual components (e.g., physical frailty, disability, functional status) that have been studied in adults or children with cirrhosis are listed in Table 2.(115-128) The tools are organized in the table from subjective, survey-based tools assessed by the patient, caregiver, or clinician to objective, performance-based assessments. The majority of these tools have been studied in the ambulatory setting only, underscoring the original “geriatric” construct of frailty as a chronic state of decreased physiologic reserve. However, the strong prognostic value of the two tools that have been studied in the acute care setting—activities of daily living (ADLs) and Karnofsky Performance Status (KPS)—highlights the pragmatic need for tools to measure the effects of frailty and sarcopenia in patients with acute cirrhosis complications.
TABLE 2.
Tools to Assess Frailty or Individual Frailty Components that have been Studied in Patients with Cirrhosis
| Tool | Setting Studied |
Administration Time |
Equipment Needed | Component(s) of Frailty Measured |
Details Regarding Administration | |
|---|---|---|---|---|---|---|
| Objective→-----------------------------------------------------------←subjective | Clinical Frailty Scale(96,115) | Ambulatory inpatient | <1 minute | None | Global frailty | Rapid survey-based instrument using clinician assessment on a scale of 1-9 where 1 = very fit, 5 = mildly frail, and 9 = terminally ill |
| ADLs (97,100,113) | Ambulatory inpatient | 2-3 minutes | None | Ability to conduct basic tasks to function within one’s home | Patient or caregiver assesses difficulty or dependence with six activities that are essential to function within one’s home (e.g., basic hygiene, eating, ambulation) | |
| KPS(88,89,116,117) | Ambulatory inpatient | <1 minute | None | Ability to carry out normal ADLs | Patient, caregiver, or clinician assesses functional limitations ranging from 100 (normal, no complaints, no evidence of disease) to 50 (requires considerable assistance and frequent medical care) to 10 (moribund, fatal processes progressing rapidly). | |
| Lansky Play-Performance Scale(94) | Ambulatory inpatient | <1 minute | None | Usual play activity in children | Studied in children aged 1-17 years listed for liver transplant. Similar to KPS scale ranging from 100 (fully active, normal) to 50 (lying around much of the day, no active playing but participates in all quiet play and activities) to 10 (does not play) | |
| Eastern Cooperative Oncology Group(103,104,118-121) | Ambulatory | <1 minute | None | Ability to carry out normal ADLs | Patient, caregiver, or clinician assesses functional limitations ranging 0-5, where 0 = asymptomatic, 2 = < 50% in bed during the day, and 4 = bedbound. | |
| Fried Frailty Instrument(75,106) | Ambulatory | 5-10 minutes | Hand dynamometer,stopwatch,tape measure | Physical frailty | Consists of five domains: (1) weight loss (question), (2) exhaustion (question), (3) slowness (short gait speed), (4) weakness (hand grip strength), and (5) low physical activity level (questionnaire) | |
| Modified Fried Frailty Instrument(93) | Ambulatory | 60 minutes | Hand dynamometer,stopwatch,tape measure | Physical frailty | Developed for children with chronic liver disease aged 5-17 years. Consists of five domains: (1) weight loss (triceps skinfold thickness), (2) exhaustion (questionnaire), (3) slowness (gait speed), (4) weakness (grip strength), and (5) low physical activity (questionnaire) | |
| Hand grip strength(122,123) | Ambulatory | 2-3 minutes | Hand dynamometer | Physical frailty | The patient is asked to grip a dynamometer using the dominant hand with their best effort. The test is repeated 3 times, and the values are averaged. | |
| Short gait speed(105) | Ambulatory | ~1 minute | stopwatch, tape measure | Functional mobility | One of the components of the Short Physical Performance Battery | |
| 6-minute walk test(107) | Ambulatory | 6 minutes | Stopwatch, tape measure | Submaximal aerobic capacity and endurance | Distance walked on a flat surface at usual walking speed within 6 minutes | |
| Short Physical Performance Battery(75) | Ambulatory | ~3 minutes | Stopwatch,tape measure, chair | Lower extremity physical function | Consists of three components: (1) 8-foot gait speed, (2) timed chair stands (5 times), and (3) balance testing three positions (feet together, semitandem, tandem) for 10 seconds each | |
| Liver Frailty Index(54,90-92,124-127) | Ambulatory inpatient | ~3 minutes | Stopwatch, hand dynamometer, chair | Physical frailty | Cirrhosis-specific tool consisting of grip strength, chair stands, and balance testing. Changes in Liver Frailty Index are associated with outcomes. | |
| Cardiopulmonary exercise test(102,108,128) | Ambulatory | 60 minutes | Cardiopulmonary stress diagnostic system | Maximal aerobic capacity | Noninvasive test of functional capacity through measurement of gas exchange at rest and during exercise to evaluate both submaximal and peak exercise responses |
Some scales have validated thresholds to grade the severity of frailty. Specifically, patients can be categorized as having high, moderate, or low performance status using KPS thresholds of 80-100, 50-70, or 10-40, respectively.(88,89) The Liver Frailty Index also has established cut-points to define robust (Liver Frailty Index < 3.2), prefrail (Liver Frailty Index 3.2-4.3), and frail (Liver Frailty Index ≥ 4.4).(90,91) Poor performance according to some scales (e.g., ADLs), however, suggests a greater burden of functional deficits than others (e.g., walk speed). The only tools that have also evaluated the associations between longitudinal assessments and outcomes in patients with cirrhosis are the KPS scale and the Liver Frailty Index.(88,92)
When it comes to assessing frailty in children, the well-established tools for assessment of frailty in adults are challenging to administer given the need for participation in the tests (either by survey or by performance) and consideration of age-related and sex-related norms. However, a few studies have demonstrated that the concept of frailty has clear applicability to children with chronic liver disease. The traditional Fried frailty phenotype, developed in older adults and validated in patients with cirrhosis of all ages, has been modified for children.(93) Although assessment of frailty was feasible in this cohort of children 5-17 years of age, the majority of children undergoing liver transplantation are too young to use the Modified Fried Frailty Instrument (median age 18 years), highlighting the need to derive an objective pediatric frailty assessment tool for children < 2 years of age. One promising metric is the Lansky Play-Performance Scale, a measure of global functional status developed for children with cancer aged 1-16 years, which can be assessed by the patient, caregiver, or clinical provider.(94) Gaps remain in the measurement of muscle contractile function among those < 1 year of age.
Prevalence and Natural History
Frailty is common among patients with cirrhosis; its prevalence increases with liver disease severity. Estimates of frailty prevalence in this population have varied because of the use of a number of different tools to capture impaired muscle contractile function. Among patients with cirrhosis in the ambulatory setting, the reported prevalence of frailty has ranged from 17% to 43%.(54,75,95,96) Among hospitalized patients with cirrhosis, the prevalence of frailty is as high as 38% for inpatients with HE (and 18% for those without HE) when measured as disability using the ADL tool.(97,98) Rates of frailty have been reported to be as high as 68% when measured as impaired performance status using the KPS scale.(89) Using the Modified Fried Frailty Instrument, 24% of children with chronic liver disease met the criteria for frailty, with rates as high as 46% among children with more advanced/end-stage liver disease.(93)
Frailty worsens in the majority of patients with cirrhosis over time.(88,92) Among patients awaiting liver transplantation in the United States, < 20% displayed improved or stable KPS scores.(88) After liver transplantation, at least 90% experience some improvement in their KPS scores, with a median improvement of 20% by 1 year posttransplant.(88) Frailty, as measured by the Liver Frailty Index, improved in only 16% of 1,093 patients with cirrhosis awaiting liver transplantation during a median follow-up time of 10.6 months on the waitlist.(92) At 3, 6, and 12 months after liver transplantation, Liver Frailty Index scores worsened from pretransplant values in 59%, 41%, and 32% of patients, respectively. Only 20% of patients achieved functional “robustness” as defined by a Liver Frailty Index score of ≤ 3.2 by 1 year after liver transplantation.(99)
Association With Outcomes
Frailty has been strongly linked with mortality in both the ambulatory and acute care settings as well as the posttransplant setting.(54,75,88,89,92-94,96,97,100-108) For example, frailty, by the Liver Frailty Index, was associated with a nearly 2-fold increased adjusted risk of death in a study of > 1,000 ambulatory patients with cirrhosis awaiting liver transplantation at 9 US centers (sub-HR, 1.82; 95% CI, 1.31-2.52).(54) In another study including 734 hospitalized patients with cirrhosis, disability, as assessed by the need for some assistance with three or more ADLs, was associated with a nearly 2-fold increased adjusted odds of 90-day mortality (OR, 1.83; 95% CI, 1.05-3.20).(97) In the posttransplant setting, compromised functional performance, by the KPS score, was associated with higher HRs for death after liver transplantation (for KPS 50%-70%: HR, 1.18; 95% CI, 1.13-1.24; for KPS 10%-40%: HR, 1.43; 95% CI, 1.35-1.52).(88)
Importantly, changes in frailty over time—both worsening and improvement—are informative of mortality risk.(88,92) Among 1,093 patients with cirrhosis at eight US sites, each 0.1 unit change in the Liver Frailty Index over 3 months was associated with a 2-fold increased hazard of waitlist mortality (HR, 2.04; 95% CI, 1.35-3.09), independent of baseline frailty and Model for End-Stage Liver Disease-Sodium (MELD-Na) score. Cumulative rates of waitlist mortality at 6 months were 12.1% among those who experienced severe worsening compared with 7% among those who remained stable. Although this was a purely observational study, it is worth noting that those who displayed improved frailty scores demonstrated a 6-month cumulative incidence of waitlist mortality of only 0.6%, suggesting the potential benefit of interventions targeting frailty to reduce mortality in this population.(92)
Baseline frailty measures have been linked with outcomes other than mortality. These outcomes include metrics of health care use in both ambulatory patients (e.g., unplanned hospitalizations, health care costs, recovery of physical function after liver transplantation) and hospitalized patients (e.g., readmissions, prolonged length of stay, discharge to a rehabilitation facility).(89,97,99,105,109) Furthermore, frailty is strongly associated with patient-reported outcomes, including development of falls, depression, disability, and global health-related quality of life.(109-114)
SARCOPENIA
Assessment of Sarcopenia in Adults and Children
Methods to assess muscle mass in patients with cirrhosis are detailed in Table 3.(171-178)
TABLE 3.
Tools to Assess Muscle Mass that have been Studied in Patients with End-Stage Liver Disease
| Method | Equipment Needed |
Advantages | Disadvantages | Outcomes Studied | Summary Notes |
|---|---|---|---|---|---|
| Anthropometrics(142,171) (MAMC, triceps skinfold thickness) | Tape measure, skinfold thickness, calipers | Safe, rapid, bedside tool, accessible, minimal training, repeatable | Low reproducibility; affected by fluid overload, adipose tissue loss; weak correlation with cross-sectional imaging | Concordance between DEXA and CT, post–liver transplant morbidity and mortality | Practical for large patient populations but poor accuracy and precision; interpret with caution |
| Anthropometrics (pediatric)(150) | Comparison between MAMC and CT | ||||
| BIA(135-139) | BIA device | Safe, rapid, accessible, minimal to moderate training, repeatable | Strict parameters around nutritional intake and exercise before the test, positioning challenging in patients with obesity | Hepatic decompensation, pretransplant mortality | Fluid retention may impact the reliability of lean body mass estimates; data using phase angle show good reliability even in patients with fluid retention |
| Ultrasound(165,172,173) | Ultrasound device |
Safe, rapid, accessible, repeatable | Operator-dependent, challenging in patients with obesity, lack of normative data | Ultrasound of psoas compared with CT-based SMI, hospitalizations and mortality, severity of liver disease | More data are needed to standardize technique; able to provide echogenicity data for tissue integrity |
| MRI(134,174) | MRI machine, image analysis software | Accurate, no radiation, measures muscle quantity and quality | Costly, limited availability | Validated against CT imaging, acute-on-chronic liver failure and mortality | Muscle mass has been defined by fat-free muscle area |
| DEXA(142,144,145,158,175) | DEXA scanner | Safe, rapid | Radiation exposure (low),edema can limit accuracy | Mortality | Low concordance between DEXA and CT in patients with cirrhosis DEXA appendicular mass improves accuracy compared with CT |
| CT(131,154,157,159,160,166,169,176,177) | CT scanner, image analysis software | Accurate, rapid, measures muscle quantity and quality, requires a high level of training to interpret | Radiation exposure, not available at bedside, varying cut-points/sites of measurement, not easily repeatable | Waitlist mortality, posttransplant mortality, decompensation, acute care use, quality of life | Has the most evidence to support its use but has challenges with radiation exposure and repeatability Muscle mass measures that have been studied:
|
| CT (pediatric)(150-152,155,156,178) | Comparison between MAMC and CT, comparison with healthy children, motor delay, infections, hospitalizations |
CT imaging is currently the gold standard for assessment of muscle mass in cirrhosis, but cost and exposure to ionizing radiation make routine use of CT solely for the purpose of detecting sarcopenia impractical in many clinical settings.(129) However, when abdominal CT imaging is performed for clinical reasons—such as in patients with HCC or for surgical planning (e.g., transplant, hepatectomy)—muscle mass measurement can be obtained from clinical scans using readily available quantitative morphomics software.(130) Muscle mass is conventionally reported as the SMI, calculated as the total skeletal muscle area at L3 normalized to height.(131) Total psoas muscle area has also been studied in patients with cirrhosis (along with psoas muscle index, calculated as total psoas muscle area normalized to height) but has been shown to be less strongly correlated with total body protein as determined by dual-energy X-ray absorptiometry (DEXA) than skeletal muscle area.(132) Furthermore, psoas muscle index led to greater misclassification of mortality risk in adult patients with cirrhosis when compared with SMI.(133) Quantitative morphomics by MRI is less well studied in patients with cirrhosis but offers the same theoretical advantages as CT-based measures of muscle mass (and is often more costly and less readily available in resource-limited settings).(134)
Measures of muscle mass other than cross-sectional imaging have been studied in patients with cirrhosis. Assessment of fat-free mass by bioelectrical impedance analysis (BIA), including segmental BIA, has been shown to modestly correlate with muscle mass and is associated with mortality in patients with cirrhosis.(71,135-140) Fluid retention impacts the reliability of lean body mass estimates by BIA.(141) Phase angle measurements have good reliability in patients with cirrhosis, even among those with ascites.(136) Availability of BIA devices for routine clinical practice is currently limited, although availability of portable BIA devices may increase the acceptability of BIA measures of body composition. Other methods to assess muscle mass, such as DEXA scanning or anthropometrics, may be more available in some practice settings worldwide but have limitations in patients with cirrhosis due to fluid retention in certain body compartments.(138,142-145) Anthropometrics, although valuable in pediatric populations,(146) are vulnerable to high interobserver variability and the inability to distinguish different body compartments (lean versus fat mass), which is of particular relevance given increasing rates of obesity in populations with cirrhosis.(142)
Sarcopenia assessment is particularly useful in the pediatric population because muscle contractile function can be difficult to assess in young children. Measures of muscle mass can provide an objective measure of growth because anthropometric measures such as weight, BMI, midarm circumference, triceps skin fold thickness, and serum markers such as albumin are often confounded by concurrent ascites, peripheral edema, and organomegaly.(147-149) This is particularly relevant in infants, for whom ascites limits the value of standard anthropometric measurements. Similar to adults, CT imaging with quantitative morphomics provides the most accurate assessment of muscle mass, with more data supporting the use of total psoas muscle versus total skeletal muscle mass, including reference values for children aged 1-16 years.(147,150-152) Longitudinal measurements, including rate of change, are even more relevant given the dynamic changes with development in children.
Prevalence
Sarcopenia is common in adults with cirrhosis, affecting 30%-70% of patients with end-stage liver disease.(153) Similar to the general population, there are strong sex-based differences in the prevalence of sarcopenia, with 21% of women and 54% of men with cirrhosis awaiting liver transplantation meeting criteria for sarcopenia by SMI in one large multicenter study.(131) The degree of muscle loss correlates with severity of liver disease in men but not women.(154) In children, sarcopenia has been reported in 17%-40% of those with end-stage liver disease.(151,155,156)
Association With Outcomes
Studies investigating sarcopenia in patients with cirrhosis have largely focused on muscle mass assessments in the ambulatory setting. Sarcopenia has been shown to be a robust predictor of a wide spectrum of outcomes in adults with cirrhosis both with and without HCC.(70,73,131,142,147,151,154,157-165) These outcomes have included not only mortality both before and after liver transplantation(131,160,161) but also hepatic decompensation,(166) reduced quality of life, (167) increased risk of infection,(157) and prolonged hospitalization.(44,73,168) In a meta-analysis of 3,803 liver transplant candidates across 19 studies in partly overlapping cohorts published between 2000 and 2015, “sarcopenia,” as defined by a wide range of CT-assessed skeletal muscle mass cut-points, was associated with a pooled HR of 1.72 (95% CI, 0.99-3.00) for waitlist mortality and 1.84 (95% CI, 1.11-3.05) for posttransplant mortality.(159) A separate North American multicenter cohort of nearly 400 patients with cirrhosis listed for liver transplantation identified SMI cut-points to predict waitlist mortality: < 39 cm2/m2 in women and < 50 cm2/m2 in men.(131,169) These SMI cut-points were further validated in a separate cohort of all White patients.(131,169) Although the original derivation cohort consisted of patients with liver transplants in the ambulatory setting at five centers in North America, it predominantly consisted of non-Hispanic and Hispanic White patients, so additional validation in more diverse cohorts is warranted to evaluate the prognostic value of SMI across all populations. Most studies to date have used a static measure of sarcopenia, but recent data suggest that sarcopenia is progressive and that dynamic measures of rate of muscle loss from serial/longitudinal measures are predictors of clinical outcomes.(43)
In children with end-stage liver disease, sarcopenia has been associated with adverse outcomes including growth failure, hospitalizations, infections, and motor delay.(151,155,156)
Sarcopenic Obesity
“Sarcopenic obesity” refers to the state of decreased muscle mass in the setting of increased fat mass. This phenotype presents a unique clinical challenge in that it can be difficult to detect without dedicated testing because fat mass can mask underlying muscle wasting.(6) The prevalence of sarcopenic obesity in patients with cirrhosis ranges from 20% to 35%.(70,71,139) NAFLD has been shown to be a strong risk factor for sarcopenic obesity, even after adjustment for metabolic comorbidities.(69,170) Sarcopenic obesity, defined as low sex-adjusted SMI and BMI ≥ 25 kg/m2, is an independent risk factor for mortality in patients with cirrhosis.(70,139) Rates of sarcopenic obesity are likely to increase as cirrhosis related to NAFLD increases.
Practical Considerations for Assessing Frailty and Sarcopenia in Clinical and Research Settings
Measures of muscle mass, particularly by cross-sectional imaging, have the advantage of being objective, reliable, and more easily reproducible than measures of muscle function. However, despite the prognostic importance of sarcopenia in patients with cirrhosis, its clinical use is currently hampered by the lack of inexpensive, safe, and readily available tests for assessment. On the other hand, many tools to assess frailty can be administered quickly in the ambulatory setting and at low cost and, perhaps most importantly, can be repeated at follow-up intervals. Furthermore, measures of muscle contractile function may be more closely associated than measures of muscle mass, with additional patient-reported outcomes including depression and the ability to complete basic life activities.(98,111,113,179,180)
For the purposes of clinical practice, one tool does not fit all. The choice of whether to measure frailty or sarcopenia (or both) depends on the specific clinical scenario and resources available. Given the ease, low cost, and repeatability of frailty metrics, we recommend routine assessment of frailty using a standardized tool—from which there are many to choose (Table 2)—in all ambulatory patients with cirrhosis. Assessment of muscle mass, on the other hand, may be useful in select groups, especially those in whom measures of frailty are unobtainable or unreliable. Such groups may include hospitalized patients, who often cannot perform performance-based tests of muscle contractile function. In this setting, preserved muscle mass may be an indicator of underlying physiologic reserve and suggest high potential for reversal of the patient’s acute presentation. Children with end-stage liver disease represent another subgroup in whom assessment of muscle mass may be more useful than measures of muscle contractile function given the limitations of performance-based testing in very young individuals (including infants).
For the purposes of research, frailty and sarcopenia represent important and complementary endpoints because they are robust and consistent predictors of outcomes in patients with cirrhosis. Given that the pathophysiology of sarcopenia in patients with cirrhosis is better elucidated than frailty,(57) sarcopenia may offer more precise mechanistic targets for drug development. In addition, assessment of muscle mass does not require active patient participation and therefore may be more appropriate as a research tool in patients who are critically ill and immobilized (e.g., on mechanical ventilation). However, frailty has the advantage of directly measuring how an individual functions and correlating strongly with how the individual feels, so frailty may be a more direct measure of a patient’s quality of life than sarcopenia. For these reasons, we recommend the inclusion of both frailty and sarcopenia as complementary endpoints in research studies.
Interventions
ALGORITHM FOR THE MANAGEMENT OF MALNUTRITION, FRAILTY, AND SARCOPENIA IN CLINICAL PRACTICE
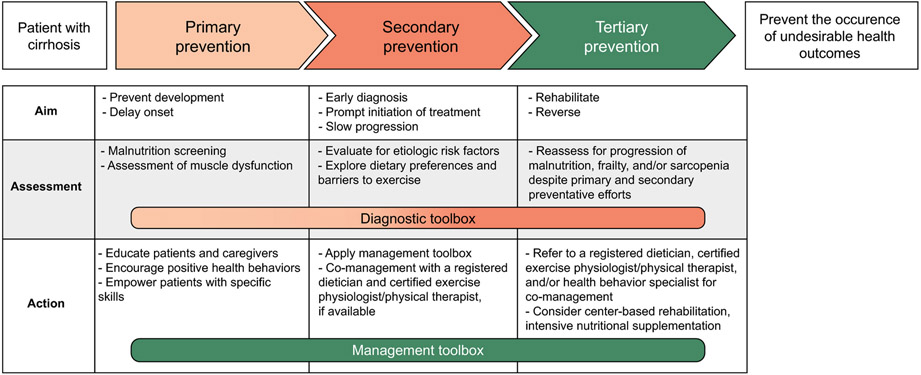
Ideally, all patients with cirrhosis would receive intensive efforts to preserve muscle mass and contractile function on diagnosis of cirrhosis, but we recognize that this is not practical in most clinical settings given resource limitations. In an effort to guide the greatest resource allocation to those with the greatest need, we have grounded our recommendations within a classic three-level framework for disease prevention and health promotion, with each level representing different aims at different stages of disease requiring increasing intensities of assessment and action (Fig. 2). “Primary prevention” refers to routine screening to identify patients with sarcopenia or frailty. “Secondary prevention” refers to the initiation of therapy in patients diagnosed with sarcopenia or frailty. “Tertiary prevention” refers to the intensification of therapy in patients with sarcopenia or frailty not responding to first-line therapy. The ultimate goal is to prevent the occurrence of adverse health outcomes attributable to malnutrition, frailty, and sarcopenia.
FIG. 2.
The three levels of disease prevention and health promotion as applied to management of malnutrition, frailty, and sarcopenia in patients with cirrhosis.
Using this framework, we have developed a clinical practice algorithm for screening, assessment, and management of malnutrition, frailty, and sarcopenia in patients with cirrhosis (Fig. 3). Key to this algorithm is the importance of reassessment of malnutrition risk, frailty, and sarcopenia—whether it be rescreening for the development or evaluating for worsening of these conditions. Although definitive intervals for reassessment have not been established in the literature, there are three points that have informed our recommendations for reassessment intervals. First, rates of frailty and sarcopenia increase with worsening liver disease severity, so patients with decompensated cirrhosis should be assessed more frequently than those with compensated cirrhosis. Second, clinical trials of interventions that target muscle dysfunction, such as testosterone,(67) nocturnal nutritional supplementation(181) or exercise,(79,80) evaluated outcomes at intervals no shorter than 8 weeks but as long as 3, 6, and 12 months. Third, the recent International Conference of Frailty and Sarcopenia Research consensus guidelines on frailty screening and management in primary care recommended screening for frailty (in the general geriatric population) on an annual basis.(182) Based on these points, we recommend that reassessment of malnutrition risk (refer to the “Screening for Malnutrition Risk” section), frailty, and sarcopenia occurs at least annually for patients with well-compensated disease but as frequently as every 8-12 weeks among those with decompensated cirrhosis and/or those undergoing active management for malnutrition, frailty, and sarcopenia.
FIG. 3.
Algorithm for screening, assessment, and management of malnutrition, frailty, and sarcopenia in patients with cirrhosis.
Ideally, a multidisciplinary team, consisting of the patient’s primary care provider, gastroenterologist/hepatologist, registered dietician, certified exercise physiologist/physical therapist, and health behavior specialist—especially ones with expertise in managing patients with serious medical conditions, including advanced liver disease—would be involved with each level of management; but this may not be feasible in many practice settings. At a minimum, a patient should be referred to a registered dietician and a certified exercise physiologist/physical therapist if malnutrition, frailty, and/or sarcopenia are progressive despite primary and secondary preventive efforts.
Given the interdependence of malnutrition, frailty, and sarcopenia in patients with cirrhosis, interventions that target one condition likely impact the other two conditions as well. Here, we provide pragmatic guidance for the management of malnutrition, frailty, and sarcopenia in patients with cirrhosis (Fig. 4). This information was intended for medical providers who are not specialists in nutrition or exercise to engage in primary and secondary prevention efforts (Fig. 2).
FIG. 4.
Diagnostic and management toolboxes with specific tools to facilitate diagnosis and management of malnutrition, frailty, and sarcopenia in patients with cirrhosis.
SCREENING FOR MALNUTRITION RISK
Multiple tools to screen for malnutrition have been evaluated in patients with cirrhosis.(183-187) Of these, the Royal Free Hospital Nutrition Prioritizing Tool (RFH-NPT) has been the most consistently associated with a diagnosis of malnutrition.(185-187) Patients are classified into three nutritional risk categories (low, moderate, and high) based on a combination of (1) presence of acute hepatitis or need for enteral nutritional support; (2) low BMI, unexplained weight loss, or maintenance of volitional nutritional intake; and (3) whether fluid overload interferes with ability to eat. Patients at high risk for malnutrition based on the RFH-NPT classification system have been shown to experience worse clinical outcomes including reduced survival, worsened liver function, and reduced quality of life.(187) Improvement in the RFH-NPT has been associated with improved survival.(187)
CIRRHOSIS-RELATED INTERVENTIONS
Disease-Specific
When possible, the cause of underlying chronic liver disease should be addressed. Eradication of chronic HCV is associated with a reduction in systemic inflammation, although levels of inflammatory biomarkers among individuals with advanced fibrosis remained elevated above levels measured in individuals who are not infected with HCV. Alcohol-associated skeletal myopathy may be partially reversible with alcohol cessation.(188) Although the exact mechanisms linking NAFLD with sarcopenia and sarcopenic obesity are not well understood, the shared pathophysiologic processes of chronic inflammation and insulin resistance (that lead to both NAFLD and sarcopenia) suggest that interventions targeting NAFLD have the potential to prevent muscle loss.
Management of HE
A strong theoretical basis exists for the management of frailty and sarcopenia with agents that lower circulating blood ammonia concentration or reduce its production. In an animal model, combined use of rifaximin and L-ornithine L-aspartate lowered plasma and muscle ammonia concentrations and improved muscle mass and function.(189) These data raise the possibility that agents used to manage HE may have a role in prevention and treatment of sarcopenia as well. However, data specifically evaluating the benefit of HE management strategies on muscle contractile function or muscle mass in patients with cirrhosis are lacking. Carnitine plays a key role in mitochondrial fatty acid oxidation, a process impaired by ammonia and central to mitochondrial function and energy metabolism. In small studies, administration of L-carnitine was associated with dose-related lowering of blood ammonia levels, a lower rate of muscle loss, reversal of existing sarcopenia, and increased levels of physical activity(190-192) However, a recent systematic review did not show benefit of acetyl-L-carnitine for the treatment of HE,(193) so its availability for the management of frailty and/or sarcopenia in clinical practice may be limited.
Management of Ascites
Medical therapy of fluid retention should be optimized as ascites and edema lead to early satiety, limit exercise capacity, and compromise mobility. In some patients, therapeutic paracentesis may improve anorexia, satiety, caloric intake, and exercise tolerance as well as reduce REE.(52,53,194) Use of loop diuretics in patients with cirrhosis and ascites has been associated with loss of muscle mass, although this finding is limited to one study and has not been confirmed in other cohorts (and its use must be balanced against the risk of poorly controlled fluid retention).(195)
Transjugular Intrahepatic Portosystemic Shunt for Management of Portal Hypertension
Placement of a transjugular intrahepatic portosystemic shunt (TIPS) in patients with portal hypertensive complications has been associated with marked improvement in body composition with gain of lean body mass, lower visceral fat, and an increase in total and fat-free muscle mass.(134,196-199) However, failure to increase muscle mass after TIPS is seen in up to one third of patients and is associated with increased mortality; baseline sarcopenia is a strong risk factor for failure to improve muscle mass after TIPS.(134,197) There is currently no evidence supporting the use of TIPS explicitly for the management of frailty and/or sarcopenia, although TIPS placement for standard indications (e.g., ascites, variceal bleed) may offer indirect benefits to the patient in the form of improvement in muscle mass.
Liver Transplantation for Management of Portal Hypertension
Liver transplantation is associated with improvement of frailty and sarcopenia in some, but not all, liver transplant recipients and often not to levels of age-matched and sex-matched norms.(99,101,168,200-203) In a prospective study that included 118 liver transplant recipients without HCC, the proportion of patients who were frail (Liver Frailty Index score ≥ 4.5) decreased from 29% pretransplant to 9% at 12 months after transplant, but only 30% met criteria for “robust” by a Liver Frailty Index <3.2.(99) Rates of improvement were related to the severity of pretransplant frailty,(99) highlighting a need both for pretransplant interventions to prevent or minimize frailty and for mechanisms to identify and transplant patients before they become severely frail. With respect to sarcopenia, two separate studies including 53 and 40 liver transplant recipients demonstrated rates of improvement in muscle mass between 25% and 34% after liver transplantation; however, one of the studies demonstrated that 26% developed new-onset sarcopenia after liver transplant but did not identify any specific predictors of posttransplant muscle loss.(201,203) Similar to our recommendations regarding TIPS, liver transplantation may offer indirect benefits to improving frailty and/or sarcopenia in recipients but cannot be recommended specifically for the treatment of these two conditions.
Although frailty and/or sarcopenia may improve after liver transplantation in some patients, both pretransplant frailty and sarcopenia are associated with adverse outcomes, including mortality after liver transplantation.(88,99,160-163,204) When considering the presence of frailty or sarcopenia in the assessment of a patient’s candidacy for liver transplantation, we recommend the use of objective, standardized metrics for frailty and/or sarcopenia for transplant decision-making. However, in the absence of data demonstrating specific thresholds of objective metrics of frailty or sarcopenia that balance risk of waitlist with posttransplant mortality, we do not recommend using frailty or sarcopenia as absolute contraindications against liver transplantation.
INTAKE-RELATED INTERVENTIONS
A personalized nutrition “prescription” should be provided to all patients with cirrhosis that is tailored to current nutritional status (i.e., a patient who meets criteria for frailty or sarcopenia should receive more intensive nutritional support to reach their targets than a patient who does not meet these criteria for malnutrition). Reassessment of nutritional intake should be repeated at regular intervals, with more frequent intervals reserved for those meeting criteria for frailty or sarcopenia at baseline and/or displaying worsening impairment of muscle contractile function or mass. If clinical deterioration or lack of improvement occurs despite target calorie and protein intake, additional causes should be considered, barriers addressed, and the nutrition prescription refined.
Energy Intake
One of the most important elements of developing a personalized intake prescription is to calculate the patient’s REE. Indirect calorimetry using a metabolic cart is the gold standard for measuring actual REE but is not widely available in all practice settings. Use of handheld calorimeters, a relatively inexpensive option to measure REE, has been validated in patients with cirrhosis to quantify REE and can be used at the bedside with high reliability in measuring REE (based on the gold standard of metabolic cart indirect calorimetry).(205,206) In the absence of indirect calorimetry, predictive equations (e.g., Harris-Benedict, Mifflin-St. Jeor) can be used to estimate an individual’s daily energy expenditure; but there is considerable interindividual variation in measured versus predicted values of REE.(33)
Studies evaluating energy expenditure in patients with cirrhosis have demonstrated that total energy expenditure ranges from 28 to 38 kcal/kg/day.(207-210) Based on these data, current nutrition guidelines for patients with chronic liver diseases and/or cirrhosis recommend a weight-based daily caloric intake of at least 35 kcal/kg/day.(211-213) In patients with fluid retention, dry weight can be estimated using subjective assessments based on either (1) postparacentesis weight or (2) subtracting a percentage of weight based on the amount of fluid retention (mild, 5%; moderate, 10%; severe, 15%; additional 5% taken off with bilateral pedal edema to the knees).(211) Although data are lacking on actual energy use among patients with cirrhosis across the spectrum of BMI, there is increasing acceptance of the need for BMI-adjusted energy intake goals. In light of this, weight-based energy intake recommendations may be modified to 25-35 kcal/kg/day for individuals with BMI 30-40 kg/m2 and 20-25 kcal/kg/day for individuals with BMI ≥ 40 kg/m2.(213) Further research is required to evaluate the accuracy of weight-based equations across BMI strata. Until that time, given their ease of use in a busy clinical practice for patients who are nonhospitalized and patients who are clinically stable, we support using BMI-adjusted, weight-based energy intake calculations to develop personalized daily caloric targets when indirect calorimetry is not available and use of predictive equations (e.g., Harris-Benedict) is not practical.
Sodium restriction may reduce the palatability of food, representing a barrier to adequate nutrition intake. In a study of 120 outpatients with cirrhosis and ascites, only 31% were adherent to a 2-g-sodium diet, and adherent patients had a 20% lower daily caloric intake.(9) When patients are prescribed a sodium-restricted diet, it should be balanced with educational resources that offer suggestions to improve diet palatability. Liberalization of sodium restriction should be considered if the patient is unable to maintain nutritional targets because of diet unpalatability.
Protein Intake
Studies dating as far back as the 1980s have established that patients with cirrhosis have increased protein needs.(210,214-216) In these studies, a positive protein balance was achieved above a protein intake of 1.2 g/kg/day(214,216); another study in patients with cirrhosis demonstrated the ability to use up to 1.8 g/kg/day of protein(210) A small randomized clinical trial of 30 hospitalized patients with cirrhosis and HE who received either protein restriction (0 g/day for the first 3 days, then gradual increase to 1.2 g/kg/day for the next 2 days) versus a normal-protein diet of 1.2 g/kg/day demonstrated accelerated protein catabolism in the protein-restricted group with no difference in evolution of HE between the two groups.(217) Based on these data, we recommend a protein intake of 1.2-1.5 g/kg/day for adults with cirrhosis because it is safe, does not worsen HE, and minimizes protein loss compared with lower protein doses.(217,218) For children with cirrhosis, protein intake of up to 4 g/kg/day has been shown to be safe and effective at improving anthropometrics (based on a single study with 10 children).(219) Although the existing literature lacks consistency on whether the weight on which to calculate protein targets should be measured, dry, or ideal body weight, we recommend using ideal body weight (based on height) for pragmatic reasons.
Data evaluating the effect of the type of protein on nutritional status in patients with cirrhosis are limited. Several studies have demonstrated the benefits of vegetable and casein-based protein diets over meat protein diets to reduce HE.(220-222) In light of the limited evidence on malnutrition and the fact that meat may be a staple protein source for many patients, we currently do not recommend limiting the intake of meat-based protein sources. However, patients should be encouraged to consume protein from a diverse range of sources, including vegetable and dairy products when possible.
Some studies support the use of BCAA supplementation (e.g., leucine, isoleucine, and valine) in the management of cirrhosis-related complications, primarily HE, some of which evaluated the effect of BCAAs on nutritional status.(223-226) Two studies have demonstrated a reduction in clinical events and an improvement in quality of life with longer-term use of BCAAs.(223,225) However, in a meta-analysis of 16 RCTs evaluating BCAA supplementation (either orally or i.v.) in patients with HE, BCAAs had no effect on mortality, quality of life, or nutritional parameters(227) Children with chronic cholestatic liver disease have significantly higher BCAA requirements than healthy children.(228) One RCT of children with end-stage liver disease demonstrated benefit of BCAA-enriched nutritional support over a standard formula with respect to midarm muscular circumference (MAMC) and triceps skinfold thickness, but this study included only 12 children(229) Given that BCAAs are naturally present in protein-containing foods, we do not recommend long-term BCAA supplementation beyond recommended protein intake targets from a diverse range of protein sources.
Several other amino acid–based treatments have been studied in patients with cirrhosis, but there is currently insufficient patient-level evidence to definitively support their use for management of malnutrition, frailty, or sarcopenia in this population. These include ß-hydroxy-ß-methylbutyrate (a metabolite of leucine),(230,231) acetyl-L-carnitine (an amino acid that has been shown to reduce blood ammonia levels),(193) and L-ornithine L-aspartate (a combination of two endogenous amino acids that reduces blood ammonia levels).(232)
Timing of Nutritional Intake
Timing of nutritional intake is essential to manage nutritional status in patients with cirrhosis. Prolonged periods of fasting should be avoided in cirrhosis, with evidence supporting the benefits on muscle mass of an early morning breakfast, late evening snack, and intake of small, frequent meals and snacks every 3-4 hours while awake.(181,233,234) A landmark study randomized 103 patients to daytime or nighttime supplemental nutrition of 710 kcal/day who otherwise had isocaloric, isonitrogenous diets.(181) Although most sustained in the Child-Turcotte-Pugh A patients, significant improvement in total body protein and fat-free mass was demonstrated in patients receiving nocturnal supplementation across all Child-Turcotte-Pugh classes. A diverse range of late-night snack options have been evaluated in the literature, with snacks varying from 149 to 710 kcal with varying carbohydrate and protein composition.(233) Given the range of personal habits regarding timing of regular food intake and preferences for types of snacks, we suggest a personalized approach to providing patients with recommendations on the timing of additional snacks (e.g., early breakfast versus late-evening snack) as well as snack content (e.g., protein bar, rice ball, yogurt).
Method of Nutritional Intake
A retrospective study of 75 patients with cirrhosis and known esophageal varices who underwent enteric tube placement demonstrated that 15% of patients experienced a gastrointestinal bleed within 48 hours of placement.(235) Higher MELD-Na score was a strong predictor of gastrointestinal bleeding. On the other hand, in a study of 14 outpatients with cirrhosis, continuous feeding through an enteric tube was associated with significant improvement of ascites, need for paracenteses, and handgrip strength without any reported complications.(236) Percutaneous gastrostomy placement is associated with a high risk of complications and mortality in patients with cirrhosis.(237) Based on these data, we recommend considering an enteric tube only in patients who have failed a trial of oral supplementation; we strongly advise against placement of percutaneous feeding devices in patients with cirrhosis and ascites.
Weight Loss With Obesity
In patients who are overweight/obese with compensated cirrhosis, weight loss of 5%-10% has been associated with reduced disease progression and reduction of portal hypertension(238) but the effects of intentional weight loss on nutritional parameters, muscle contractile function, and muscle mass are less well studied. In a study of 160 dieting older adults without liver disease, intentional weight loss was associated with decreases in lean mass and bone mineral density; but this was mitigated by resistance training.(239) Given the evidence supporting the role of adequate protein intake in the preservation of overall nitrogen balance (see the “Protein Intake” section), we advise caution when recommending weight loss in patients with decompensated cirrhosis or known sarcopenic obesity.(6) If weight loss through caloric restriction must be prescribed in patients with cirrhosis for clinical reasons (e.g., to reduce NASH progression, for transplant listing), we recommend (1) ensuring adequate protein intake (1.2-1.5 g/kg/day) and (2) combination with an exercise program.
Nutritional Intake in the Hospitalized Setting
Existing meta-analyses of nutritional supplementation in hospitalized patients with cirrhosis have not been able to demonstrate an impact on mortality.(240,241) However, a subgroup analysis of three studies evaluating oral nutritional supplementation alone demonstrated a benefit in mortality in hospitalized patients with cirrhosis (risk ratio, 0.40; 95% CI, 0.18-0.90).(241) The benefit of oral supplementation is further supported by an RCT of patients hospitalized with severe acute alcohol-associated hepatitis in which enteral versus oral supplementation did not offer a benefit in mortality but adequate oral intake (defined as ≥ 22 kcal/kg/day)—regardless of mode of administration—reduced mortality by 67% (0.19-0.57) compared with patients who consumed < 22 kcal/kg/day. One study conducted at a single Veterans Affairs medical center demonstrated that a cirrhosis-specific nutrition education intervention targeted to physicians and dieticians involved in caring for inpatients with cirrhosis resulted in increased nutritional intake and reduction in 90-day hospital readmissions.(243) We recommend that all hospitalized patients with cirrhosis receive formal consultation by a registered dietician within 24 hours of admission or, if not possible, with the RFH-NPT.(213) Barriers to oral intake (e.g., fasting time, HE, nausea) should be promptly identified and addressed. In patients who screen positive for malnutrition in whom barriers have been addressed and who are unable to meet their nutrition targets through oral intake alone, enteral nutrition should be considered within 48-72 hours of hospital admission.(244,245) One common barrier is prolonged periods of fasting that result from frequent nil per os (NPO) orders for procedures; strategies to minimize this fasting period or frequency of NPO orders (e.g., prebedtime snack, early-morning snack if the procedure will be in the late afternoon, consider advancing diet rapidly when there is no indication for NPO status) should be implemented.
In the intensive care unit (ICU) population, limited cirrhosis-specific data are available to guide energy targets. Indirect calorimetry is the gold standard for determining total energy requirements in this setting. However, given the limited availability of indirect calorimetry in many hospital settings, predictive or weight-based estimations of energy needs may be used, recognizing the potential underestimation of energy needs in light of the dynamic metabolic requirements and fluid overload in patients with cirrhosis who are acutely ill.(244) In patients who are critically ill, it is generally recommended to use higher protein goals, targeting 1.2-2.0 g/kg/day.(244,246,247) Again, the literature is not clear whether these recommendations are based on dry or ideal body weight; therefore, we recommend using ideal body weight for pragmatic purposes.
For hospitalized patients with cirrhosis who are unable to meet energy needs through oral intake alone, enteral feeding should be considered (time course has not been established in the literature); for those who are critically ill and unable to maintain volitional intake, enteral feeding should be initiated within 24-48 hours of ICU admission. A meta-analysis of 21 RCTs comparing early versus delayed enteral nutrition in all patients who are critically ill (including those with cirrhosis) demonstrated a significant reduction in mortality (relative risk, 0.70; 9% CI, 0.49-1.00) and infectious morbidity (relative risk, 0.74; 95% CI, 0.58-0.93) among those receiving early enteral nutrition versus delayed enteral nutrition or standard of care.(244) In one RCT of 136 patients with severe alcohol-associated hepatitis randomized (1:1) to intensive enteral nutritional support versus oral supplementation, enteral feeding had to be discontinued early in 49% of patients and was associated with three (4%) serious adverse events that were determined to be related to the intervention (one aspiration pneumonia, one decompensated diabetes, one severe worsening of HE); mortality benefit of supplemental nutrition was demonstrated only in patients ≥ 21.5 kcal/kg body weight/day, regardless of intervention arm. In two studies of early enteral feeding in patients with cirrhosis admitted with an esophageal variceal hemorrhage, placement of a nasogastric feeding tube was associated with a 10%-33% rate of rebleeding.(235,248)
Data around the use of parenteral nutrition in cirrhosis are limited, but meta-analyses in the general critically ill population have reported a higher incidence of hyperglycemia and sepsis (but an improvement in mortality when compared with patients receiving enteral nutrition).(249,250) Parenteral nutrition should be considered as a second-line option to enteral nutrition in patients who are unable to meet their nutritional requirements by oral intake alone and is strongly preferable to no nutritional supplementation in patients who are hospitalized and meet criteria for frailty or sarcopenia.
Micronutrients
Vitamin and mineral deficiencies are common in cirrhosis regardless of etiology of liver disease and are particularly prevalent in patients with advanced disease, cholestasis, or acute illness.(251,252) Routine assessment for micronutrient deficiencies and appropriate repletion are recommended in patients with cirrhosis.(253) Recommendations for repletion of certain micronutrients that have been better studied in patients with cirrhosis are detailed in Table 4.(255-261) There is little evidence to guide longer-term maintenance dosing once deficiency has been corrected. Decisions for use of longer-term maintenance dosing will depend on assessment of whether the patient remains at nutritional risk (e.g., still consuming alcohol or with low oral intake).
TABLE 4.
Micronutrient Supplementation in Cirrhosis
| Symptoms of Deficiency | Repletion | Comments/Monitoring | ||
|---|---|---|---|---|
| Fat-soluble vitamins | Vitamin A |
|
Vitamin A 2,000-200,000 IU/day PO according to deficiency syndrome and severity for 4-8 weeks(255,256) | |
| Vitamin D |
|
Repletion: 50,000 IU/week vitamin D2 or D3 for 8 weeks followed by maintenance of 1,500-2,000 IU/day(259) |
|
|
| Vitamin E |
|
α-Tocopherol acetate 400-800 IU/day PO |
|
|
| Vitamin K |
|
Phytonadione 1-10 mg PO, s.c.,or i.v. |
|
|
| Water-soluble vitamins | Thiamine, vitamin B1 |
|
Asymptomatic: thiamine 100 mg/day Suspected Wernicke encephalopathy: thiamine 500 mg i.v. 3×/day on days 1 and 2, then 250 mg i.v. 3×/day on days 3-5 |
|
| Niacin, vitamin B3 |
|
300-1,000 mg/day PO for deficiency states |
|
|
| Pyridoxine, vitamin B6 |
|
Vitamin B6 100 mg PO per day |
|
|
| Folic acid, vitamin B9 |
|
Folate 1-5 mg PO per day |
|
|
| Cobalamin, vitamin B12 |
|
Vitamin B12 1,000 μg i.m. monthly or 1,000-2,000 μg PO per day |
|
|
| Ascorbic acid, vitamin C |
|
Vitamin C 500-1,000 mg PO per day |
|
|
| Trace elements | Zinc |
|
30-50 mg elemental zinc PO per day(258) |
|
| Selenium |
|
50-100 μg/day PO |
|
|
| Copper |
|
2-4 mg i.v. for 6 days, followed by 3-8 mg per day PO until level normalization or symptom resolution(261) |
|
Abbreviation: PO, per os.
However, because vitamin and mineral status may not be regularly assessed in clinical practice because of competing demands from other cirrhosis complications (e.g., management of protein-calorie malnutrition, ascites, HE, etc.)—and multivitamin supplementation is inexpensive and essentially free of side effects—we support a pragmatic approach of an empiric course of oral multivitamin supplementation in patients with cirrhosis who display any evidence of frailty or sarcopenia, as has been proposed in the general population.(254)
PHYSICAL ACTIVITY–RELATED INTERVENTIONS
Physical activity–based interventions have been shown to improve muscle contractile function and muscle mass as well as cardiopulmonary function and quality of life in patients with cirrhosis.(78-81,238,262-265) The caveat to interpretation of these studies in this population is that they have been limited by small sample size and inclusion of primarily well-compensated patients.
There are three general principles to consider when recommending activity-based interventions for patients with cirrhosis: (1) assess frailty and/or sarcopenia with a standardized tool, (2) recommend a combination of aerobic and resistance exercises, and (3) tailor recommendations based on the physical assessment and reassessments.
Assess frailty and/or sarcopenia with a standardized tool. This should occur at baseline and longitudinally to assess response to the intervention. Improvement in frailty has been associated with lower rates of mortality compared with worsening of frailty in patients with decompensated cirrhosis.(92) Data are lacking on the potential benefits of changes in sarcopenia.
Recommend a combination of aerobic and resistance exercises. Activity-based interventions in patients with cirrhosis have ranged in duration from 8 to 64 weeks.(266) Exercise prescriptions can be guided by principles of frequency, intensity, time, and type, as detailed in the management toolbox (Fig. 4) and adapted from the American College of Sports Medicine.(267) Although the type of exercise (e.g., aerobic only or combination aerobic and resistance training) has varied in these studies, the general consensus is that the optimal activity-based intervention should include a combination of aerobic and resistance training. The theoretical framework for this recommendation is that aerobic training may address impaired muscular endurance and cardiopulmonary fitness, whereas resistance training specifically addresses skeletal muscle strength and mass.(263) Data on what constitutes an adequate level of aerobic activity in patients with cirrhosis are limited, although it is reasonable to follow Centers for Disease Control guidelines to achieve 150-300 minutes of moderate to vigorous intensity exercise per week and muscle-strengthening exercises at least 2 days per week.(268) The use of technology, including fitness trackers, can provide more accurate and objective data on an individual’s actual physical activity better than self-report alone.(76) Smartphone-based fitness apps designed for persons with cirrhosis may have a role in facilitating increased exercise and activity in patients with cirrhosis.(269)
Tailor recommendations based on baseline assessment and reassessments. This includes modifying the intensity of the physical activity intervention based on the presence of frailty and/or sarcopenia.(270) It also includes adapting recommendations based on risk for adverse events related to increased mobility, such as musculoskeletal injury and falls.(271) Studies evaluating activity-based interventions in patients with cirrhosis to date have demonstrated a good safety profile of exercise,(238,272,273) but most studies to date have primarily included patients with well-compensated cirrhosis. A number of unanswered questions regarding an exercise program in cirrhosis remain, including the duration, time of day, and impact of concurrent exercise on responses.(274) Notwithstanding these, it is prudent for patients to optimize their portal hypertensive complications (e.g., ascites control, variceal prophylaxis, optimal HE therapies) before initiating an activity-based program.
INTERVENTIONS TARGETING OTHER SYSTEMS
Hormone-Associated Interventions
In one study of 101 male patients with cirrhosis and low testosterone (defined as total testosterone < 12 nmol/L or free testosterone < 230 pmol/L), testosterone replacement increased muscle mass, decreased fat mass, and improved glucose metabolism.(67) The anabolic effect of testosterone on muscle may be related to suppression of muscle cell apoptosis and myostatin production.(275) However, exogenous testosterone is also associated with increased risk for HCC and thrombophilia. Testosterone therapy may be indicated for some men with cirrhosis, but the risk/benefit profile must be individualized. For men with a personal or family history of HCC, prostate cancer, or thrombophilia, the risk of testosterone replacement may outweigh potential benefits.
Suggestions for Future Research
In patients with cirrhosis, frailty and sarcopenia are prevalent and lethal. The last decade has welcomed a large body of literature solidifying the importance of frailty and sarcopenia on outcomes in patients with cirrhosis and has brought with it exciting opportunities for future research. Although the possibilities are endless, we highlight three that we believe are the most urgently needed:
Standardized, feasible assessment of frailty and sarcopenia in diverse populations of patients with cirrhosis with respect to sex/gender, race/ethnicity, and clinical acuity. The literature has been lacking on detailed comparisons of frailty and sarcopenia by not only biological sex but also self-identified gender. Cohorts should be enriched with patients of diverse racial/ethnic backgrounds to better understand variation in racial/ethnic differences in manifestations of malnutrition, frailty, and sarcopenia and the implications on clinical outcomes. Lastly, more studies are needed in patients with decompensated cirrhosis, particularly those who are acutely ill.
Longitudinal assessment of frailty and sarcopenia. This includes evaluation of the natural progression as well as predictors of accelerated decline. Studies evaluating any treatments in this population (e.g., antiviral agents, alcohol abstinence, management of ascites, TIPS, liver transplantation) should also investigate the impact of those interventions on muscle function and muscle mass.
Development of therapeutics and multimodal strategies targeting frailty and sarcopenia. Frailty and sarcopenia are measurable and clinically significant. The objective measurement of these conditions enables a field of research focused on reversing or slowing their consequences. This will require collaboration across many disciplines, including hepatology, surgery, nutrition, and physiotherapy; but it will also require collaboration across industries, including academia and pharmaceutical and biotechnology firms. One essential step for the development of therapeutics is certification by the Food and Drug Administration of clinically meaningful endpoints of frailty and sarcopenia.
Guidance Statement:
- All patients with cirrhosis should be assessed for frailty with a standardized tool both at baseline and longitudinally.
- 1a. There are insufficient data to recommend the use of one frailty tool over another. Instead, we recommend that selection of the standardized frailty tool in clinical practice should depend upon the relative need for efficiency versus objectivity of assessment within that clinical scenario. In patients with compensated cirrhosis, annual frailty assessment may be sufficient, whereas patients with decompensated cirrhosis may benefit from more frequent (every 3-6 months) assessment.
All patients with cirrhosis should be counseled on the risks and adverse clinical consequences of frailty regardless of their baseline frailty status.
Given the strong and consistent association between muscle mass and outcomes in both adults and children with cirrhosis, objective measures of muscle loss should be considered to assess risk for poor outcomes in patients with cirrhosis.
- SMI as assessed by CT image analysis is recommended as the most consistent and reproducible method to quantify muscle mass in patients with cirrhosis.
- 4a There are currently insufficient data to support a bedside tool to assess muscle mass in patients with cirrhosis.
- 4b MRI measurement of skeletal muscle mass has not been validated in patients with cirrhosis but theoretically provides the same information on muscle mass as CT imaging.
Because of the risk of exposure to radiation, use of abdominal CT solely for the purpose of muscle mass measurement is not recommended for routine use; but quantification of skeletal muscle mass should be considered when an abdominal CT is obtained as part of clinical care or in patients in whom assessment of muscle contractile function is not practical or feasible (e.g., acutely ill patients, very young children).
- In clinical settings, we recommend systematic assessment of frailty and/or sarcopenia in all patients with cirrhosis using a standardized instrument.
- 6a Frailty testing may be particularly useful in the ambulatory setting and when intermediate-term and long-term longitudinal assessments are needed to assess natural progression or response to treatment.
- 6b Sarcopenia testing may be particularly useful for patients in whom administration of tests of frailty is not feasible or is impractical (e.g., because of acute severe illness or inability to participate in testing such as in very young children).
In research studies of patients with cirrhosis, including clinical trials evaluating interventions related to malnutrition and/or muscle dysfunction, we recommend assessment of both frailty and sarcopenia, when possible, to more comprehensively capture the impact of interventions on these complementary endpoints.
All patients with cirrhosis should receive education, motivation, and behavioral skills support to reduce their risk of developing these conditions (primary prevention).
A positive frailty or sarcopenia screen should prompt evaluation for underlying etiologic risk factors and the development of an ambulatory personalized management plan (secondary prevention).
Reassessment of frailty or sarcopenia using the same standardized tool as baseline assessment should occur at least annually for patients with well-compensated disease but as frequently as every 8-12 weeks for those with decompensated cirrhosis and/or those undergoing active management for these conditions.
Patients with progressive frailty or sarcopenia despite initiation of secondary prevention efforts should undergo more intensive nutrition and exercise rehabilitation under the direct supervision of a registered dietician and certified exercise physiologist/physical therapist (tertiary prevention).
Management should involve a multidisciplinary team consisting of the patient’s primary care provider, gastroenterologist/hepatologist, registered dietician, certified exercise physiologist/physical therapist, and health behavior specialist (if there is a concurrent mental health condition) when possible. However, if not available at all levels of prevention/health promotion, then at a minimum, referral to a registered dietician and certified exercise physiologist/physical therapist is recommended at the tertiary prevention level.
Treatment of inflammatory conditions that lead to cirrhosis, such as HCV, insulin resistance, obesity, and alcohol use disorder, is recommended to manage malnutrition, frailty, and sarcopenia.
Identification and management of cirrhosis-specific complications (e.g., HE, ascites) is recommended in all patients with cirrhosis to manage malnutrition, frailty, and sarcopenia.
TIPS placement for standard indications (e.g., ascites, acute variceal bleeding) may offer an indirect benefit of improving muscle mass.
In the absence of specific data on which patients will experience improvement in frailty and sarcopenia posttransplant, liver transplantation cannot be recommended specifically for the treatment of frailty or sarcopenia.
We do not recommend using frailty or sarcopenia as an absolute contraindication against liver transplantation.
All patients with cirrhosis (regardless of a diagnosis of malnutrition) should receive educational resources and counseling regarding the association between nutritional status and outcomes and to optimize nutritional status.
Patients with cirrhosis who screen positive for malnutrition risk, frailty, or sarcopenia should receive a personalized intake “prescription” that is tailored to actual needs and incorporates individual habits around nutrition.
- Calorie needs should be personalized to the patient.
- 20a When possible, indirect calorimetry should be used to measure the patient’s REE in order to provide a personalized intake prescription.
- 20b In the absence of indirect calorimetry, data, although limited, support the use of the following:
- Traditional predictive equations, such as the Harris-Benedict equation
- Weight-based equations (using ideal body weight)
- Nonobese—target of at least 35 kcal/kg body weight/day
- Obese (nonhospitalized, clinically stable)—use of caloric targets stratified by BMI: 25-35 kcal/kg/day for individuals with BMI 30-40 kg/m2 and 20-25 kcal/kg/day for individuals with BMI ≥ 40 kg/m2
- 20c In patients who screen positive for frailty or sarcopenia and cannot meet nutritional targets on a sodium-restricted diet, liberalization of sodium restriction should be considered to facilitate adequate oral intake.
- Recommended protein intake for adults with cirrhosis is 1.2-1.5 g/kg ideal body weight per day.
- 21a For adults with cirrhosis who are critically ill, a target of 1.2-2.0 g/kg ideal body weight per day is recommended.
- 21b A diverse range of protein sources, including vegetable and dairy products, should be encouraged.
- 21c BCAA supplementation is not recommended beyond emphasizing the importance of meeting daily overall protein targets from a diverse range of protein sources.
For children with chronic liver disease, recommended protein intake should be up to 4 g/kg ideal body weight per day.
Protein intake should not be restricted in patients with HE.
Fasting time should be minimized, with a maximum interval of 3-4 hours between nutritional intake while awake.
To minimize nocturnal fasting time, an early breakfast and/or late-evening snack should be recommended.
In ambulatory patients with cirrhosis and children with cirrhosis/end-stage liver disease who do not meet dietary intake requirements with oral intake, enteral nutritional supplementation may be considered to achieve targets.
Percutaneous gastrostomy tubes should not be placed in patients with cirrhosis and ascites.
- If medically required, weight loss should be undertaken under the supervision of a multidisciplinary team.
- 28a Particular caution should be applied to prescribing weight loss in a patient with decompensated cirrhosis.
- 28b Intake of target protein and physical activity are required to reduce the loss of muscle contractile function and muscle mass that can occur with weight loss.
All hospitalized patients with cirrhosis should receive formal consultation with a registered dietician within 24 hours of admission or, if not available, then assessment for malnutrition using the RFH-NPT.
Strategies to minimize this fasting period or frequency of NPO orders (e.g., prebedtime snack, early-morning snack if the procedure will be in the late afternoon, consider advancing diet rapidly when there is no indication for NPO status) should be implemented.
Oral nutritional supplementation is the first-line therapy for hospitalized patients with cirrhosis who are unable to meet energy needs through volitional intake alone.
- In hospitalized patients with cirrhosis who are unable to meet energy needs with volitional intake and oral nutritional supplementation, enteral nutritional supplementation should be considered to achieve targets.
- 32a Precautions should be taken to reduce risk of aspiration and development of hyperglycemia.
- 32b The presence of esophageal varices is not an absolute contraindication to placement of an enteric feeding tube, but close monitoring is warranted for signs of rebleeding if an enteric tube is required after recent banding of esophageal varices.
- 32c Parenteral nutritional should be reserved for patients with cirrhosis who are intolerant of enteral nutrition and unable meet dietary intake requirements through oral intake alone.
In patients who are critically ill with cirrhosis, a higher protein target of 1.2-2.0 g/kg ideal body weight/day is recommended.
In hospitalized patients with decompensated cirrhosis, parenteral nutritional support should be considered in those who are unable to meet nutritional requirements through oral intake alone and are unable to receive enteral nutritional support.
Micronutrient deficiencies should be assessed at least annually, repleted if deficient, and reassessed after repletion.
Physical activity–based interventions are recommended to improve muscle contractile function and muscle mass in patients with cirrhosis.
The three components to activity-based interventions in patients with cirrhosis should include (1) assessing and reassessing frailty and/or sarcopenia using standardized tools, (2) recommending a combination of aerobic and resistance exercises, and (3) tailoring recommendations based on assessments.
In men with cirrhosis who may be candidates for testosterone therapy, testosterone levels should be checked at baseline.
- Testosterone replacement may be considered in select men with low testosterone to improve muscle mass.
- 39a Relative contraindications to use of testosterone include a history of HCC, other malignancy, or thrombosis.
Acknowledgment:
The authors are grateful for the valuable contributions of the AASLD Practice Guideline Committee (PGC), particularly Barry Schlansky. Members of the PGC include George Ioannou (chair), Rabab Ali, Scott W. Biggins, Roniel Cabrera, Henry Chang, Michael F. Chang, David S. Goldberg, Ruben Hernaez, Binu John, Patricia D. Jones, W. Ray Kim (board liaison), Cynthia Levy, Jeff McIntyre, Jessica L. Mellinger, Mindie H. Nguyen, Nadia Ovchinsky, Mary E.M. Rinella (board liaison), Anjana A. Pillai, Daniel S. Pratt, Hugo R. Rosen, Barry Schlansky, Matthew J. Stotts, Christopher J. Sonnenday, Lisa B. VanWagner, and Elizabeth C. Verna.
Supported by the American Association for the Study of Liver Diseases. Dr. Lai is partially supported by R01 AG059183 and R21 AG067554. Srinivasan Dasarathy is partially supported by NIH RO1 GM119174; RO1 DK113196; P50 AA024333; RO1 AA021890; 3U01AA026976 - 03S1; UO1 AA 026976; R56HL141744;UO1 DK061732; 5U01 DK062470-17S2.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- ADLs
activities of daily living
- BCAA
branched-chain amino acid
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- DEXA
dual-energy X-ray absorptiometry
- KPS
Karnofsky Performance Status
- MAMC
midarm muscular circumference
- MELD-Na
Model for End-Stage Liver Disease-Sodium
- NPO
nil per os
- RCT
randomized controlled trial
- REE
resting energy expenditure
- RFH-NPT
Royal Free Hospital Nutrition Prioritizing Tool
- SMI
skeletal muscle index
- TIPS
transjugular intrahepatic portosystemic shunt.
Footnotes
Potential conflict of interest: Dr. Lai received grants from Axcella and Lipocine. Dr. Bernal advises Versantis.
REFERENCES
- 1).Lochs H, Allison SP, Meier R, Pirlich M, Kondrup J, Schneider ST, et al. Introductory to the ESPEN Guidelines on Enteral Nutrition: terminology, definitions and general topics. Clin Nutr 2006;25:180–186. 10.1016/j.clnu.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 2).Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).World Health Organization. Malnutrition. https://www.who.int/health-topics/malnutrition#tab=tab_1. Accessed June 15, 2020.
- 5).Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther 2020;51:64–77. 10.1111/apt.15571. [DOI] [PubMed] [Google Scholar]
- 6).Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis—the confluence of 2 prognostic titans. Liver Int 2018;38:1706–1717. 10.1111/liv.13876. [DOI] [PubMed] [Google Scholar]
- 7).Volk ML, Fisher N, Fontana RJ. Patient knowledge about disease self-management in cirrhosis. Am J Gastroenterol 2013;108:302–305. 10.1038/ajg.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Beg S, Curtis S, Shariff M. Patient education and its effect on self-management in cirrhosis: a pilot study. Eur J Gastroenterol Hepatol 2016;28:582–587. 10.1097/MEG.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 9).Morando F, Rosi S, Gola E, Nardi M, Piano S, Fasolato S, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int 2015;35:1508–1515. 10.1111/liv.12583. [DOI] [PubMed] [Google Scholar]
- 10).Wu J, Meng QH. Current understanding of the metabolism of micronutrients in chronic alcoholic liver disease. World J Gastroenterol 2020;26:4567–4578. 10.3748/wjg.v26.i31.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Sanvisens A, Zuluaga P, Pineda M, Fuster D, Bolao F, Juncà J, et al. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend 2017;180:417–422. 10.1016/j.drugalcdep.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 12).Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care 2020;23:138–144. 10.1097/MCO.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 13).Phillips JR, Angulo P, Petterson T, Lindor KD. Fat-soluble vitamin levels in patients with primary biliary cirrhosis. Am J Gastroenterol 2001;96:2745–2750. [DOI] [PubMed] [Google Scholar]
- 14).Jorgensen RA, Lindor KD, Sartin JS, LaRusso NF, Wiesner RH. Serum lipid and fat-soluble vitamin levels in primary sclerosing cholangitis. J Clin Gastroenterol 1995;20:215–219. 10.1097/00004836-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 15).Holick MF Vitamin D deficiency. N Engl J Med 2007;357:266–281. 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 16).Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci 2009;55:2624–2628. 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 17).Kubesch A, Quenstedt L, Saleh M, Rüschenbaum S, Schwarzkopf K, Martinez Y, et al. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: a prospective cohort study. PLoS One 2018;13:e0207162. 10.1371/journal.pone.0207162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Guo G-Y, Shi Y-Q, Wang L, Ren X, Han Z-Y, Guo C-C, et al. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment Pharmacol Ther 2015;42:221–230. 10.1111/apt.13244. [DOI] [PubMed] [Google Scholar]
- 19).Riggio O, Merli M, Capocaccia L, Caschera M, Zullo A, Pinto G, et al. Zinc supplementation reduces blood ammonia and increases liver ornithine transcarbamylase activity in experimental cirrhosis. Hepatology 1992;16:785–789. 10.1002/hep.1840160326. [DOI] [PubMed] [Google Scholar]
- 20).Nishikawa H, Yoh K, Enomoto H, Iwata Y, Sakai Y, Kishino K, et al. Serum zinc level is associated with frailty in chronic liver diseases. J Clin Med 2020;9:1570. 10.3390/jcm9051570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, et al. Serum zinc concentration and sarcopenia: a close linkage in chronic liver diseases. J Clin Med 2019;8:336. 10.3390/jcm8030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Rocchi E, Borella P, Borghi A, Paolillo F, Pradelli M, Farina F, et al. Zinc and magnesium in liver cirrhosis. Eur J Clin Invest 1994;24:149–155. 10.1111/j.1365-2362.1994.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 23).Aagaard NK, Andersen H, Vilstrup H, Clausen T, Jakobsen J, Dørup I. Muscle strength, Na, K-pumps, magnesium and potassium in patients with alcoholic liver cirrhosis—relation to spironolactone. J Intern Med 2002;252:56–63. 10.1046/j.1365-2796.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- 24).Aagaard NK, Andersen H, Vilstrup H, Clausen T, Jakobsen J, Dørup I. Decreased muscle strength and contents of Mg and Na, K-pumps in chronic alcoholics occur independently of liver cirrhosis. J Intern Med 2003;253:359–366. 10.1046/j.1365-2796.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 25).Heubi JE, Higgins JV, Argao EA, Sierra RI, Specker BL. The role of magnesium in the pathogenesis of bone disease in childhood cholestatic liver disease: a preliminary report. J Pediatr Gastroenterol Nutr 1997;25:301–306. 10.1097/00005176-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 26).Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis 2010;42:409–418. 10.1016/j.dld.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 27).Yu R, Wang Y, Xiao Y, Mo L, Liu A, Li D, et al. Prevalence of malnutrition and risk of undernutrition in hospitalised children with liver disease. J Nutr Sci 2017;6:e55. 10.1017/jns.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Venu M, Martin E, Saeian K, Gawrieh S. High prevalence of vitamin A deficiency and vitamin D deficiency in patients evaluated for liver transplantation. Liver Transpl 2013;19:627–633. 10.1002/lt.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Owen OE, Reichle FA, Mozzoli MA, Kreulen T, Patel MS, Elfenbein IB, et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest 1981;68:240–252. 10.1172/JCI110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Owen OE, Trapp VE, Reichard GA, Mozzoli MA, Moctezuma J, Paul P, et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest 1983;72:1821–1832. 10.1172/JCI111142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Madden AM, Morgan MY. Resting energy expenditure should be measured in patients with cirrhosis, not predicted. Hepatology 1999;30:655–664. 10.1002/hep.510300326. [DOI] [PubMed] [Google Scholar]
- 32).Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr 2007;85:1257–1266. [DOI] [PubMed] [Google Scholar]
- 33).Eslamparast T, Vandermeer B, Raman M, Gramlich L, Den Heyer V, Belland D, et al. Are predictive energy expenditure equations accurate in cirrhosis? Nutrients 2019;11:334. 10.3390/nu11020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Mullen KD, Denne SC, McCullough AJ, Savin SM, Bruno D, Tavill AS, et al. Leucine metabolism in stable cirrhosis. Hepatology 1986;6:622–630. 10.1002/hep.1840060412. [DOI] [PubMed] [Google Scholar]
- 35).Yamato M, Muto Y, Yoshida T, Kato M, Moriwaki H. Clearance rate of plasma branched-chain amino acids correlates significantly with blood ammonia level in patients with liver cirrhosis. Int Hepatol Commun 1995;3:91–96. 10.1016/0928-4346(94)00159-3. [DOI] [Google Scholar]
- 36).Montanari A, Simoni I, Vallisa D, Trifirò A, Colla R, Abbiati R, et al. Free amino acids in plasma and skeletal muscle of patients with liver cirrhosis. Hepatology 1988;8:1034–1039. 10.1002/hep.1840080509. [DOI] [PubMed] [Google Scholar]
- 37).Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 38).Shawcross DL, Wright GAK, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, et al. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology 2008;48:1202–1212. 10.1002/hep.22474. [DOI] [PubMed] [Google Scholar]
- 39).Shalimar Sheikh MF, Mookerjee RP, Agarwal B, Acharya SK, Jalan R. Prognostic role of ammonia in patients with cirrhosis. Hepatology 2019;70:982–994. 10.1002/hep.30534. [DOI] [PubMed] [Google Scholar]
- 40).Chen HW, Dunn MA. Muscle at risk: the multiple impacts of ammonia on sarcopenia and frailty in cirrhosis. Clin Transl Gastroenterol 2016;7:e170. 10.1038/ctg.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Dasarathy S, Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care 2018;21:30–36. 10.1097/MCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Davuluri G, Allawy A, Thapaliya S, Rennison JH, Singh D, Kumar A, et al. Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J Physiol 2016;594:7341–7360. 10.1113/JP272796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Welch N, Dasarathy J, Runkana A, Penumatsa R, Bellar A, Reen J, et al. Continued muscle loss increases mortality in cirrhosis: impact of aetiology of liver disease. Liver Int 2020;40:1178–1188. 10.1111/liv.14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Vural A, Attaway A, Welch N, Zein J, Dasarathy S. Skeletal muscle loss phenotype in cirrhosis: a nationwide analysis of hospitalized patients. Clin Nutr 2020;39:3711–3720. 10.1016/j.clnu.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).DiMartini A, Cruz RJ, Dew MA, Myaskovsky L, Goodpaster B, Fox K, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl 2013;19:1172–1180. 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Prasad SVN, et al. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 2014;10:677–690. 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Bousquet-Dubouch M-P, Nguen S, Bouyssié D, Burlet-Schiltz O, French SW, Monsarrat B, et al. Chronic ethanol feeding affects proteasome-interacting proteins. Proteomics 2009;9:3609–3622. 10.1002/pmic.200800959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab 2004;286:E916–E926. 10.1152/ajpendo.00554.2003. [DOI] [PubMed] [Google Scholar]
- 49).Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055–2065. 10.1002/hep.29420. [DOI] [PubMed] [Google Scholar]
- 50).Abrigo J, Gonzalez F, Aguirre F, Tacchi F, Gonzalez A, Meza MP, et al. Cholic acid and deoxycholic acid induce skeletal muscle atrophy through a mechanism dependent on TGR5 receptor. J Cell Physiol 2021;236:260–272. 10.1002/jcp.29839. [DOI] [PubMed] [Google Scholar]
- 51).Tapper EB, Aberasturi D, Zhao Z, Hsu CY, Parikh ND. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Aliment Pharmacol Ther 2020;51:1397–1405. 10.1111/apt.15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Aqel BA, Scolapio JS, Dickson RC, Burton DD, Bouras EP. Contribution of ascites to impaired gastric function and nutritional intake in patients with cirrhosis and ascites. Clin Gastroenterol Hepatol 2005;3:1095–1100. 10.1016/S1542-3565(05)00531-8. [DOI] [PubMed] [Google Scholar]
- 53).Dolz C, Raurich JM, Ibáñez J, Obrador A, Marsé P, Gayá J. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterology 1991;100:738–744. 10.1016/0016-5085(91)80019-6. [DOI] [PubMed] [Google Scholar]
- 54).Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology 2019;156:1675–1682. 10.1053/j.gastro.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol 2019;54:845–859. 10.1007/s00535-019-01605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Santetti D, de Albuquerque Wilasco MI, Dornelles CTL, Werlang ICR, Fontella FU, Kieling CO, et al. Serum proinflammatory cytokines and nutritional status in pediatric chronic liver disease. World J Gastroenterol 2015;21:8927–8934. 10.3748/wjg.v21.i29.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–1244. 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol 2014;61:396–407. 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 59).Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385–1396. 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 60).Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol 2017;8:1045. 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8. 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 62).Nishitsuji H, Funami K, Shimizu Y, Ujino S, Sugiyama K, Seya T, et al. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J Virol 2013;87:8169–8178. 10.1128/JVI.00974-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Saraiva GN, Rosário NFd, Medeiros T, Leite PEC, Lacerda GdS, Andrade TGd, et al. Restoring inflammatory mediator balance after sofosbuvir-induced viral clearance in patients with chronic hepatitis C. Mediators Inflamm 2018;2018:8578051. 10.1155/2018/8578051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Gao B, Tsukamoto H. Inflammation in alcoholic and nonalcoholic fatty liver disease: friend or foe? Gastroenterology 2016;150:1704–1709. 10.1053/j.gastro.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Dasarathy S The pathogenesis of physical frailty and sarcopenia. In: Tandon P, Montano-Loza AJ, eds. Frailty and Sarcopenia in Cirrhosis. Cham, Switzerland: Springer International Publishing; 2020:33–53. 10.1007/978-3-030-26226-6_4. [DOI] [Google Scholar]
- 66).Moctezuma-Velázquez C, Low G, Mourtzakis M, Ma M, Burak KW, Tandon P, et al. Association between low testosterone levels and sarcopenia in cirrhosis: a cross-sectional study. Ann Hepatol 2018;17:615–623. 10.5604/01.3001.0012.0930. [DOI] [PubMed] [Google Scholar]
- 67).Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol 2016;65:906–913. 10.1016/j.jhep.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 68).Haugen CE, McAdams-DeMarco M, Verna EC, Rahimi RS, Kappus MR, Dunn MA, et al. Association between liver transplant wait-list mortality and frailty based on body mass index. JAMA Surg 2019;154:1103–1109. 10.1001/jamasurg.2019.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Kashiwagi K, Takayama M, Fukuhara K, Shimizu-Hirota R, Chu P-S, Nakamoto N, et al. A significant association of non-obese non-alcoholic fatty liver disease with sarcopenic obesity. Clin Nutr ESPEN 2020;38:86–93. 10.1016/j.clnesp.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 70).Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CMM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2015;7:126–135. 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Nishikawa H, Nishiguchi S. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med 2016;55:855–856. 10.2169/internalmedicine.55.6298. [DOI] [PubMed] [Google Scholar]
- 72).Orman ES, Roberts A, Ghabril M, Nephew L, Desai AP, Patidar K, et al. Trends in characteristics, mortality, and other out-comes of patients with newly diagnosed cirrhosis. JAMA Netw Open 2019;2:e196412. 10.1001/jamanetworkopen.2019.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Welch N, Attaway A, Bellar A, Alkhafaji H, Vural A, Dasarathy S. Compound sarcopenia in hospitalized patients with cirrhosis worsens outcomes with increasing age. Nutrients 2021;13:659. 10.3390/nu13020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Hayashi F, Matsumoto Y, Momoki C, Yuikawa M, Okada G, Hamakawa E, et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol Res 2013;43:1264–1275. 10.1111/hepr.12085. [DOI] [PubMed] [Google Scholar]
- 75).Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–1879. 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, et al. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 2016;22:1–22. 10.1002/lt.24506. [DOI] [PubMed] [Google Scholar]
- 77).Chascsa DM, Lai JC, Dunn MA, Montano-Loza AJ, Kappus MR, Dasarathy S, et al. Patient and caregiver attitudes and practices of exercise in candidates listed for liver transplantation. Dig Dis Sci 2018;63:3290–3296. 10.1007/s10620-018-5271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Román E, Torrades MT, Nadal MJ, Cárdenas G, Nieto JC, Vidal S, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014;59:1966–1975. 10.1007/s10620-014-3086-6. [DOI] [PubMed] [Google Scholar]
- 79).Román E, García-Galcerán C, Torrades T, Herrera S, Marín A, Doñate M, et al. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One 2016;11:e0151652. 10.1371/journal.pone.0151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920–1926. e2. 10.1016/j.cgh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 81).Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, et al. Home exercise training improves exercise capacity in cirrhosis patients: role of exercise adherence. Sci Rep 2018;8:99. 10.1038/s41598-017-18320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Hiraoka A, Michitaka K, Kiguchi D, Izumoto H, Ueki H, Kaneto M, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2017;29:1416–1423. 10.1097/MEG.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 83).Centers for Disease Control and Prevention. Social determinants of health: Know what affects health. https://www.cdc.gov/socialdeterminants/index.htm. Accessed August 16, 2020.
- 84).Bittermann T, Dwinnells K, Chadha S, Wolf MS, Olthoff KM, Serper M. Low health literacy is associated with frailty and reduced likelihood of liver transplant listing: a prospective cohort study. Liver Transpl 2020;26:1409–1421. 10.1002/lt.25830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Golovaty I, Tien PC, Price JC, Sheira L, Seligman H, Weiser SD. Food insecurity may be an independent risk factor associated with nonalcoholic fatty liver disease among low-income adults in the United States. J Nutr 2020;150:91–98. 10.1093/jn/nxz212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011;106:1646–1653. 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant 2020;20:1597–1605. 10.1111/ajt.15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky performance status before and after liver transplantation predicts graft and patient survival. J Hepatol 2018;69:818–825. 10.1016/j.jhep.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 89).Tandon P, Reddy KR, O’Leary JG, Garcia-Tsao G, Abraldes JG, Wong F, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology 2017;65:217–224. 10.1002/hep.28900. [DOI] [PubMed] [Google Scholar]
- 90).Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–574. 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Kardashian A, Ge J, McCulloch CE, Kappus MR, Dunn MA, Duarte-Rojo A, et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology 2020;73:1132–1139. 10.1002/hep.31406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Lai JC, Dodge JL, Kappus MR, Dunn MA, Volk ML, Duarte-Rojo A, et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol 2020;73:575–581. 10.1016/j.jhep.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Lurz E, Quammie C, Englesbe M, Alonso EM, Lin HC, Hsu EK, et al. Frailty in children with liver disease: a prospective multicenter study. J Pediatr 2018;194:109–115.e4. 10.1016/j.jpeds.2017.10.066. [DOI] [PubMed] [Google Scholar]
- 94).Perito ER, Bucuvalas J, Lai JC. Functional status at listing predicts waitlist and posttransplant mortality in pediatric liver transplant candidates. Am J Transplant 2018;19:1–21. 10.1111/ajt.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am J Transplant 2018;18:2566–2570. 10.1111/ajt.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Tandon P, Tangri N, Thomas L, Zenith L, Shaikh T, Carbonneau M, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the Clinical Frailty Scale. Am J Gastroenterol 2016;111:1759–1767. 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 97).Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology 2015;62:584–590. 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98).Tapper EB, Baki J, Parikh ND, Lok AS. Frailty, psychoactive medications, and cognitive dysfunction are associated with poor patient-reported outcomes in cirrhosis. Hepatology 2019;69:1676–1685. 10.1002/hep.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Lai JC, Segev DL, McCulloch CE, Covinsky KE, Dodge JL, Feng S. Physical frailty after liver transplantation. Am J Transplant 2018;18:1986–1994. 10.1111/ajt.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Samoylova ML, Covinsky KE, Haftek M, Kuo S, Roberts JP, Lai JC. Disability in patients with end-stage liver disease: results from the functional assessment in liver transplantation study. Liver Transpl 2017;23:292–298. 10.1002/lt.24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Thuluvath PJ, Thuluvath AJ, Savva Y, Zhang T. Karnofsky performance status following liver transplantation in patients with multiple organ failures and probable acute-on-chronic liver failure. Clin Gastroenterol Hepatol 2020;18:234–241. 10.1016/j.cgh.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 102).Bernal W, Martin-Mateos R, Lipcsey M, Tallis C, Woodsford K, Mcphail MJ, et al. Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transpl 2013;20:54–62. 10.1002/lt.23766. [DOI] [PubMed] [Google Scholar]
- 103).Wallace D, Cowling TE, Walker K, Suddle A, Gimson A, Rowe I, et al. The impact of performance status on length of hospital stay and clinical complications following liver transplantation. Transplantation 2020. Oct 8. 10.1097/TP.0000000000003484. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 104).Wallace D, Cowling T, McPhail MJ, Brown SE, Aluvihare V, Suddle A, et al. Assessing the time-dependent impact of performance status on outcomes after liver transplantation. Hepatology 2020;72:1341–1352. 10.1002/hep.31124. [DOI] [PubMed] [Google Scholar]
- 105).Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol 2016;111:1768–1775. 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 106).Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol 2017;23:899–905. 10.3748/wjg.v23.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107).Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl 2010;16:1373–1378. 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 108).Prentis JM, Manas DMD, Trenell MI, Hudson M, Jones DJ, Snowden CP. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl 2012;18:152–159. 10.1002/lt.22426. [DOI] [PubMed] [Google Scholar]
- 109).Tapper EB, Zhao L, Nikirk S, Baki J, Parikh ND, Lok AS, et al. Incidence and bedside predictors of the first episode of overt hepatic encephalopathy in patients with cirrhosis. Am J Gastroenterol 2020;115:2017–2025. 10.14309/ajg.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Tapper EB, Derstine B, Baki J, Su GL. Bedside measures of frailty and cognitive function correlate with sarcopenia in patients with cirrhosis. Dig Dis Sci 2019;64:3652–3659. 10.1007/s10620-019-05713-4. [DOI] [PubMed] [Google Scholar]
- 111).Cron DC, Friedman JF, Winder GS, Thelen AE, Derck JE, Fakhoury JW, et al. Depression and frailty in patients with end-stage liver disease referred for transplant evaluation. Am J Transplant 2016;16:1805–1811. 10.1111/ajt.13639. [DOI] [PubMed] [Google Scholar]
- 112).Derck JE, Thelen AE, Cron DC, Friedman JF, Gerebics AD, Englesbe MJ, et al. Quality of life in liver transplant candidates. Transplantation 2015;99:340–344. 10.1097/TP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 113).Lai JC, Dodge JL, McCulloch CE, Covinsky KE, Singer JP. Frailty and the burden of concurrent and incident disability in patients with cirrhosis: a prospective cohort study. Hepatol Commun 2020;4:126–133. 10.1002/hep4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114).Tapper EB, Nikirk S, Parikh N, Zhao L. Falls are common, morbid, and predictable in patients with cirrhosis. J Hepatol 2021. Apr 19. 10.1016/j.jhep.2021.04.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Kremer WM, Nagel M, Reuter M, Hilscher M, Michel M, Kaps L, et al. Validation of the Clinical Frailty Scale for the prediction of mortality in patients with liver cirrhosis. Clin Transl Gastroenterol 2020;11:e00211. 10.14309/ctg.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Orman ES, Ghabril M, Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:1189–1195.e1. 10.1016/j.cgh.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117).Dolgin NH, Martins PNA, Movahedi B, Lapane KL, Anderson FA, Bozorgzadeh A. Functional status predicts postoperative mortality after liver transplantation. Clin Transplant 2016;30:1403–1410. 10.1111/ctr.12808. [DOI] [PubMed] [Google Scholar]
- 118).Hsu C-Y, Lee Y-H, Hsia C-Y, Huang Y-H, Su C-W, Lin H-C, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 2013;57:112–119. 10.1002/hep.25950. [DOI] [PubMed] [Google Scholar]
- 119).Jacob M, Copley LP, Lewsey JD, Gimson A, Rela M, van der Meulen JHP. Functional status of patients before liver transplantation as a predictor of posttransplant mortality. Transplantation 2005;80:52–57. 10.1097/01.TP.0000163292.03640.5C. [DOI] [PubMed] [Google Scholar]
- 120).Jo HB, Lee JK, Kang HW, Kim JH, Lim YJ, Koh M-S, et al. Safety and effectiveness of midazolam for cirrhotic patients undergoing endoscopic variceal ligation. Turk J Gastroenterol 2018;29:448–455. 10.5152/tjg.2018.17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121).Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 2014;60:192–201. 10.1002/hep.27057. [DOI] [PubMed] [Google Scholar]
- 122).Álvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005;21:113–117. 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 123).Wang CW, Feng S, Covinsky KE, Hayssen H, Zhou L-Q, Yeh BM, et al. A comparison of muscle function, mass, and quality in liver transplant candidates. Transplantation 2016;100:1692–1698. 10.1097/TP.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124).Haugen CE, McAdams-DeMarco MA, Verna EC, et al. Association between liver transplant wait-list mortality and frailty based on body mass index. JAMA Surg. 2019;154 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Haugen CE, McAdams-DeMarco M, Holscher CM, Ying H, Gurakar AO, Garonzik-Wang J, et al. Multicenter study of age, frailty, and waitlist mortality among liver transplant candidates. Ann Surg 2020;271:1132–1136. 10.1097/SLA.0000000000003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126).Lai JC, Ganger DR, Volk ML, Dodge JL, Dunn MA, Duarte-Rojo A, et al. Association of frailty and sex with wait list mortality in liver transplant candidates in the Multicenter Functional Assessment in Liver Transplantation (FrAILT) Study. JAMA Surg 2021;156:256–262. 10.1001/jamasurg.2020.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127).Skladany L, Drotarova Z, Vnencakova J, Jancekova D, Molcan P, Koller T. Applicability and prognostic value of frailty assessment tools among hospitalized patients with advanced chronic liver disease. Croat Med J 2021;62:8–16. 10.3325/cmj.2021.62.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128).Ney M, Haykowsky MJ, Vandermeer B, Shah A, Ow M, Tandon P. Systematic review: pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment Pharmacol Ther 2016;44:796–806. 10.1111/apt.13771. [DOI] [PubMed] [Google Scholar]
- 129).Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology 2019;70:1816–1829. 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Paris MT, Tandon P, Heyland DK, Furberg H, Premji T, Low G, et al. Automated body composition analysis of clinically acquired computed tomography scans using neural networks. Clin Nutr 2020;39:3049–3055. 10.1016/j.clnu.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131).Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625–633. 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132).Wells CI, McCall JL, Plank LD. Relationship between total body protein and cross-sectional skeletal muscle area in liver cirrhosis is influenced by overhydration. Liver Transpl 2019;25:45–55. 10.1002/lt.25314. [DOI] [PubMed] [Google Scholar]
- 133).Ebadi M, Wang CW, Lai JC, Dasarathy S, Kappus MR, Dunn MA, et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle 2018;9:1053–1062. 10.1002/jcsm.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Praktiknjo M, Book M, Luetkens J, Pohlmann A, Meyer C, Thomas D, et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology 2018;67:1014–1026. 10.1002/hep.29602. [DOI] [PubMed] [Google Scholar]
- 135).Pirlich M, Schutz T, Spachos T, Ertl S, Weis M, Lochs H, et al. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology 2000;32:1208–1215. 10.1053/jhep.2000.20524. [DOI] [PubMed] [Google Scholar]
- 136).Ruiz-Margáin A, Xie JJ, Román-Calleja BM, Pauly M, White MG, Chapa-Ibargüengoitia M, et al. Phase angle from bioelectrical impedance for the assessment of sarcopenia in cirrhosis with or without ascites. Clin Gastroenterol Hepatol 2020. Sep 2. 10.1016/j.cgh.2020.08.066. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 137).Fernando Gomes Romeiro L, Augusti S. Nutritional assessment in cirrhotic patients with hepatic encephalopathy. World J Hepatol 2015;7:2940–2954. 10.4254/wjh.v7.i30.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Hara N, Iwasa M, Iwata K, Miyachi H, Tanaka H, Takeo M, et al. Value of the extracellular water ratio for assessment of cirrhotic patients with and without ascites. Hepatol Res 2009;39:1072–1079. 10.1111/j.1872-034X.2009.00546.x. [DOI] [PubMed] [Google Scholar]
- 139).Hara N, Iwasa M, Sugimoto R, Mifuji-Moroka R, Yoshikawa K, Terasaka E, et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med 2016;55:863–870. 10.2169/internalmedicine.55.5676. [DOI] [PubMed] [Google Scholar]
- 140).Viertel M, Bock C, Reich M, Löser S, Plauth M. Performance of CT-based low skeletal muscle index, low mean muscle attenuation, and bioelectric impedance derived low phase angle in the detection of an increased risk of nutrition related mortality. Clin Nutr 2019;38:2375–2380. 10.1016/j.clnu.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 141).Bellafronte NT, Diani LM, Vega-Piris L, Cuadrado GB, Chiarello PG. Comparison between dual-energy X-ray absorptiometry and bioelectrical impedance for body composition measurements in adults with chronic kidney disease: a cross-sectional, longitudinal, multi-treatment analysis. Nutrition 2021;82:111059. 10.1016/j.nut.2020.111059. [DOI] [PubMed] [Google Scholar]
- 142).Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, et al. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-fay absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 2015;27:328–334. 10.1097/MEG.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 143).Labio ED, Del Rosario DB, Strasser SI, McCaughan GW, Crawford BA. Effect of ascites on bone density measurement in cirrhosis. J Clin Densitom 2007;10:391–394. 10.1016/j.jocd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 144).Lindqvist C, Brismar TB, Majeed A, Wahlin S. Assessment of muscle mass depletion in chronic liver disease: dual-energy X-ray absorptiometry compared with computed tomography. Nutrition 2019;61:93–98. 10.1016/j.nut.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 145).Sinclair M, Hoermann R, Peterson A, Testro A, Angus PW, Hey P, et al. Use of dual X-ray absorptiometry in men with advanced cirrhosis to predict sarcopenia-associated mortality risk. Liver Int 2019;39:1089–1097. 10.1111/liv.14071. [DOI] [PubMed] [Google Scholar]
- 146).WHO Multicentre Growth Reference Study Group. Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl 2007;450:38–46. 10.1111/j.1651-2227.2006.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 147).Mangus RS, Bush WJ, Miller C, Kubal CA. Severe sarcopenia and increased fat stores in pediatric patients with liver, kidney, or intestine failure. J Pediatr Gastroenterol Nutr 2017;65:579–583. 10.1097/MPG.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 148).Squires RH, Ng V, Romero R, Ekong U, Hardikar W, Emre S, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology 2014;60:362–398. 10.1002/hep.27191. [DOI] [PubMed] [Google Scholar]
- 149).Young S, Kwarta E, Azzam R, Sentongo T. Nutrition assessment and support in children with end-stage liver disease. Nutr Clin Pract 2013;28:317–329. 10.1177/0884533612474043. [DOI] [PubMed] [Google Scholar]
- 150).Grutters LA, Pennings JP, Bruggink JLM, Viddeleer AR, Verkade HJ, de Kleine RHJ, et al. Body composition of infants with biliary atresia: anthropometric measurements and computed tomography-based body metrics. J Pediatr Gastroenterol Nutr 2020;71:440–445. 10.1097/MPG.0000000000002859. [DOI] [PubMed] [Google Scholar]
- 151).Ooi PH, Thompson-Hodgetts S, Pritchard-Wiart L, Gilmour SM, Mager DR. Pediatric sarcopenia: a paradigm in the overall definition of malnutrition in children? JPEN 2020;44:407–418. 10.1002/jpen.1681. [DOI] [PubMed] [Google Scholar]
- 152).Lurz E, Patel H, Lebovic G, Quammie C, Woolfson JP, Perez M, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle 2020;11:405–414. 10.1002/jcsm.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153).Mazurak VC, Tandon P, Montano-Loza AJ. Nutrition and the transplant candidate. Liver Transpl 2017;23:1451–1464. 10.1002/lt.24848. [DOI] [PubMed] [Google Scholar]
- 154).Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl 2012;18:1209–1216. 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 155).Ooi PH, Mazurak VC, Bhargava R, Dunichand-Hoedl A, Romero RA, Gilmour SM, et al. Myopenia and reduced subcutaneous adiposity in children with liver disease are associated with adverse outcomes. JPEN 2020. July 25. 10.1002/jpen.1963. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 156).Woolfson JP, Perez M, Chavhan GB, Johara FT, Lurz E, Kamath BM, et al. Sarcopenia in children with end-stage liver disease on the transplant waiting list. Liver Transpl 2021;27:641–651. 10.1002/lt.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157).Montano–Loza AJ, Meza–Junco J, Prado CMM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166–173.e1. 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 158).Belarmino G, Gonzalez MC, Sala P, Torrinhas RS, Andraus W, D’Albuquerque LAC, et al. Diagnosing sarcopenia in male patients with cirrhosis by dual-energy X-ray absorptiometry estimates of appendicular skeletal muscle mass. JPEN 2018;42:24–36. 10.1177/0148607117701400. [DOI] [PubMed] [Google Scholar]
- 159).van Vugt JLA, Levolger S, de Bruin RWF, van Rosmalen J, Metselaar HJ, IJzermans JNM. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant 2016;16:2277–2292. 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 160).Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010;211:271–278. 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161).Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 2013;13:1549–1556. 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- 162).Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 2014;20:1413–1419. 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 163).Kuo SZ, Ahmad M, Dunn MA, Montano-Loza AJ, Carey EJ, Lin S, et al. Sarcopenia predicts posttransplant mortality in acutely ill men undergoing urgent evaluation and liver transplantation. Transplantation 2019;103:2312–2317. 10.1097/TP.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164).Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol 2020;55:927–943. 10.1007/s00535-020-01711-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165).Hari A, Berzigotti A, Štabuc B, Cagleviǐ N. Muscle psoas indices measured by ultrasound in cirrhosis—preliminary evaluation of sarcopenia assessment and prediction of liver decompensation and mortality. Dig Liver Dis 2019;51:1502–1507. 10.1016/j.dld.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 166).Tapper EB, Zhang P, Garg R, Nault T, Leary K, Krishnamurthy V, et al. Body composition predicts mortality and decompensation in compensated cirrhosis patients: a prospective cohort study. JHEP Rep 2020;2:100061. 10.1016/j.jhepr.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167).Ando Y, Ishigami M, Ito T, Ishizu Y, Kuzuya T, Honda T, et al. Sarcopenia impairs health-related quality of life in cirrhotic patients. Eur J Gastroenterol Hepatol 2019;31:1550–1556. 10.1097/MEG.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 168).Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CMM, Ma M, Meeberg G, et al. Severe muscle depletion predicts post-operative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014;20:640–648. 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 169).Georgiou A, Papatheodoridis GV, Alexopoulou A, Deutsch M, Vlachogiannakos I, Ioannidou P, et al. Validation of cutoffs for skeletal muscle mass index based on computed tomography analysis against dual energy X-ray absorptiometry in patients with cirrhosis: the KIRRHOS study. Ann Gastroenterol 2020;33:80–86. 10.20524/aog.2019.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170).Carias S, Castellanos AL, Vilchez V, Nair R, Dela Cruz AC, Watkins J, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31:628–633. 10.1111/jgh.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171).Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-Stage Liver Disease score. J Cachexia Sarcopenia Muscle 2016;8:113–121. 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172).Kobayashi K, Maruyama H, Kiyono S, Ogasawara S, Suzuki E, Ooka Y, et al. Application of transcutaneous ultrasonography for the diagnosis of muscle mass loss in patients with liver cirrhosis. J Gastroenterol 2018;53:652–659. 10.1007/s00535-017-1378-2. [DOI] [PubMed] [Google Scholar]
- 173).Tandon M, Singh H, Singla N, Jain P, Pandey CK. Tongue thickness in health vs cirrhosis of the liver: prospective observational study. World J Gastrointest Pharmacol Ther 2020;11:59–68. 10.4292/wjgpt.v11.i3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174).Tandon P, Mourtzakis M, Low G, Zenith L, Ney M, Carbonneau M, et al. Comparing the variability between measurements for sarcopenia using magnetic resonance imaging and computed tomography imaging. Am J Transplant 2016;16:2766–2767. 10.1111/ajt.13832. [DOI] [PubMed] [Google Scholar]
- 175).Strauss BJ, Gibson PR, Stroud DB, Borovnicar DJ, Xiong DW, Keogh J. Total body dual X-ray absorptiometry is a good measure of both fat mass and fat-free mass in liver cirrhosis compared to “gold-standard” techniques. Ann N Y Acad Sci 2000;904:55–62. [DOI] [PubMed] [Google Scholar]
- 176).Wang NC, Zhang P, Tapper EB, Saini S, Wang SC, Su GL. Automated measurements of muscle mass using deep learning can predict clinical outcomes in patients with liver disease. Am J Gastroenterol 2020;115:1210–1216. 10.14309/ajg.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177).Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One 2017;12:e0186990. 10.1371/journal.pone.0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178).Lurz E, Patel H, Frimpong RG, Ricciuto A, Kehar M, Wales PW, et al. Sarcopenia in children with end-stage liver disease. J Pediatr Gastroenterol Nutr 2018;66:222–226. 10.1097/MPG.0000000000001792. [DOI] [PubMed] [Google Scholar]
- 179).Foster C, Baki J, Nikirk S, Williams S, Parikh ND, Tapper EB. Comprehensive health-state utilities in contemporary patients with cirrhosis. Hepatol Commun 2020;4:852–858. 10.1002/hep4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180).Derck JE, Thelen AE, Cron DC, Friedman JF, Gerebics AD, Englesbe MJ, et al. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation 2015;99:340–344. 10.1097/TP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 181).Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology 2008;48:557–566. 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- 182).Ruiz JG, Dent E, Morley JE, Merchant RA, Beilby J, Beard J, et al. Screening for and managing the person with frailty in primary care: ICFSR Consensus Guidelines. J Nutr Health Aging 2020;24:920–927. 10.1007/s12603-020-1492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183).Boulhosa RSSB, Lourenço RP, Côrtes DM, Oliveira LPM, Lyra AC, de Jesus RP. Comparison between criteria for diagnosing malnutrition in patients with advanced chronic liver disease: GLIM group proposal versus different nutritional screening tools. J Hum Nutr Diet 2020;33:862–868. 10.1111/jhn.12759. [DOI] [PubMed] [Google Scholar]
- 184).Traub J, Bergheim I, Horvath A, Stadlbauer V. Validation of malnutrition screening tools in liver cirrhosis. Nutrients 2020;12:1306. 10.3390/nu12051306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185).Wu Y, Zhu Y, Feng Y, Wang R, Yao N, Zhang M, et al. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br J Nutr 2020;124:1293–1302. 10.1017/S0007114520002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186).Georgiou A, Papatheodoridis GV, Alexopoulou A, Deutsch M, Vlachogiannakos I, Ioannidou P, et al. Evaluation of the effectiveness of eight screening tools in detecting risk of malnutrition in cirrhotic patients: the KIRRHOS study. Br J Nutr 2019;122:1368–1376. 10.1017/S0007114519002277. [DOI] [PubMed] [Google Scholar]
- 187).Borhofen SM, Gerner C, Lehmann J, Fimmers R, Görtzen J, Hey B, et al. The Royal Free Hospital-Nutritional Prioritizing Tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci 2015;61:1735–1743. 10.1007/s10620-015-4015-z. [DOI] [PubMed] [Google Scholar]
- 188).Peters TJ, Martin F, Ward K. Chronic alcoholic skeletal myopathy—common and reversible. Alcohol 1985;2:485–489. 10.1016/0741-8329(85)90120-X. [DOI] [PubMed] [Google Scholar]
- 189).Kumar A, Davuluri G, Silva RNE, Engelen MPKJ, Ten Have GAM, Prayson R, et al. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology 2017;65:2045–2058. 10.1002/hep.29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190).Hiramatsu A, Aikata H, Uchikawa S, Ohya K, Kodama K, Nishida Y, et al. Levocarnitine use is associated with improvement in sarcopenia in patients with liver cirrhosis. Hepatol Commun 2019;3:348–355. 10.1002/hep4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191).Ohara M, Ogawa K, Suda G, Kimura M, Maehara O, Shimazaki T, et al. L-Carnitine suppresses loss of skeletal muscle mass in patients with liver cirrhosis. Hepatol Commun 2018;2:910–922. 10.1002/hep4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192).Malaguarnera M, Vacante M, Giordano M, Pennisi G, Bella R, Rampello L, et al. Oral acetyl-l-carnitine therapy reduces fatigue in overt hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr 2011;93:799–808. 10.3945/ajcn.110.007393. [DOI] [PubMed] [Google Scholar]
- 193).Martí-Carvajal AJ, Gluud C, Arevalo-Rodriguez I, Martí-Amarista CE. Acetyl-L-carnitine for patients with hepatic encephalopathy. Cochrane Database Syst Rev 2019;2019:CD011451. 10.1002/14651858.CD011451.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194).Makhlouf NA, Mahran ZG, Sadek SH, Magdy DM, Makhlouf HA. Six-minute walk test before and after large-volume paracentesis in cirrhotic patients with refractory ascites: a pilot study. Arab J Gastroenterol 2019;20:81–85. 10.1016/j.ajg.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 195).Hanai T, Shiraki M, Miwa T, Watanabe S, Imai K, Suetsugu A, et al. Effect of loop diuretics on skeletal muscle depletion in patients with liver cirrhosis: loop diuretics and muscle in cirrhosis. Hepatol Res 2019;49:82–95. 10.1111/hepr.13244. [DOI] [PubMed] [Google Scholar]
- 196).Allard JP, Chau J, Sandokji K, Blendis LM, Wong F. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol 2001;96:2442–2447. 10.1111/j.1572-0241.2001.04051.x. [DOI] [PubMed] [Google Scholar]
- 197).Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol 2013;25:85–93. [DOI] [PubMed] [Google Scholar]
- 198).Artru F, Miquet X, Azahaf M, Labreuche J, Ntandja Wandji LC, Sergent G, et al. Consequences of TIPSS placement on the body composition of patients with cirrhosis and severe portal hypertension: a large retrospective CT-based surveillance. Aliment Pharmacol Ther 2020;52:1516–1526. 10.1111/apt.16080. [DOI] [PubMed] [Google Scholar]
- 199).Benmassaoud A, Roccarina D, Arico F, Leandro G, Yu B, Cheng F, et al. Sarcopenia does not worsen survival in patients with cirrhosis undergoing transjugular intrahepatic portosystemic shunt for refractory ascites. Am J Gastroenterol 2020;115:1911–1914. 10.14309/ajg.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 200).Kaido T, Tamai Y, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017;33:195–198. 10.1016/j.nut.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 201).Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, et al. Post–liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol 2014;29:1250–1257. 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202).Dasarathy S Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci 2013;58:3103–3111. 10.1007/s10620-013-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203).Bergerson JT, Lee J-G, Furlan A, Sourianarayanane A, Fetzer DT, Tevar AD, et al. Liver transplantation arrests and reverses muscle wasting. Clin Transplant 2015;29:216–221. 10.1111/ctr.12506. [DOI] [PubMed] [Google Scholar]
- 204).Bhanji RA, Takahashi N, Moynagh MR, Narayanan P, Angirekula M, Mara KC, et al. The evolution and impact of sarcopenia pre– and post–liver transplantation. Aliment Pharmacol Ther 2019;49:807–813. 10.1111/apt.15161. [DOI] [PubMed] [Google Scholar]
- 205).Glass C, Hipskind P, Tsien C, Malin SK, Kasumov T, Shah SN, et al. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol 2013;114:559–565. 10.1152/japplphysiol.01042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206).Glass C, Hipskind P, Cole D, Lopez R, Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract 2012;27:677–688. 10.1177/0884533612446195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207).Greco AV, Mingrone G, Benedetti G, Capristo E, Tataranni PA, Gasbarrini G. Daily energy and substrate metabolism in patients with cirrhosis. Hepatology 1998;27:346–350. 10.1002/hep.510270205. [DOI] [PubMed] [Google Scholar]
- 208).Guglielmi F, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism: multicentre prospective study by the ‘Nutritional Problems in Gastroenterology’ Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis 2005;37:681–688. 10.1016/j.dld.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 209).Riggio O, Angeloni S, Ciuffa L, Nicolini G, Attili AF, Albanese C, et al. Malnutrition is not related to alterations in energy balance in patients with stable liver cirrhosis. Clin Nutr 2003;22:553–559. 10.1016/s0261-5614(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 210).Nielsen K, Kondrup J, Martinsen L, Døssing H, Larsson B, Stilling B, et al. Long-term oral refeeding of patients with cirrhosis of the liver. Br J Nutr 1995;74:557–567. 10.1079/BJN19950158. [DOI] [PubMed] [Google Scholar]
- 211).European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172–193. 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212).Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38:485–521. 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213).Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013;58:325–336. 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 214).Swart GR, van den Berg JWO, van Vuure JK, Rietveld T, Wattimena DL, Frenkel M. Minimum protein requirements in liver cirrhosis determined by nitrogen balance measurements at three levels of protein intake. Clin Nutr 1989;8:329–336. 10.1016/0261-5614(89)90008-3. [DOI] [PubMed] [Google Scholar]
- 215).Swart GR, van den Berg JWO, Wattimena JLD, Rietveld T, Van Vuure JK, Frenkel M. Elevated protein requirements in cirrhosis of the liver investigated by whole body protein turnover studies. Clin Sci 1988;75:101–107. 10.1042/cs0750101. [DOI] [PubMed] [Google Scholar]
- 216).Nielsen K, Kondrup J, Martinsen L, Stilling B, Wikman B. Nutritional assessment and adequacy of dietary intake in hospitalized patients with alcoholic liver cirrhosis. Br J Nutr 1993;69:665–679. 10.1079/BJN19930068. [DOI] [PubMed] [Google Scholar]
- 217).Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol 2004;41:38–43. 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 218).Gheorghe L, Iacob R, Vădan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005;14:231–238. [PubMed] [Google Scholar]
- 219).Charlton CP, Buchanan E, Holden CE, Preece MA, Green A, Booth IW, et al. Intensive enteral feeding in advanced cirrhosis: reversal of malnutrition without precipitation of hepatic encephalopathy. Arch Dis Child 1992;67:603–607. 10.1136/adc.67.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220).Uribe M, Marquez MA, Ramos GG, Ramos-Uribe MH, Vargas F, Villalobos A, et al. Treatment of chronic portal–systemic encephalopathy with vegetable and animal protein diets: a controlled crossover study. Dig Dis Sci 1982;27:1109–1116. 10.1007/BF01391449. [DOI] [PubMed] [Google Scholar]
- 221).Bianchi GP, Marchesini G, Fabbri A, Rondelli A, Bugianesi E, Zoli M, et al. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized crossover comparison. J Intern Med 1993;233:385–392. 10.1111/j.1365-2796.1993.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 222).Jonung T, Jeppsson B, Äslund U, Nair BM. A comparison between meat and vegan protein diet in patients with mild chronic hepatic encephalopathy. Clin Nutr 1987;6:169–174. 10.1016/0261-5614(87)90052-5. [DOI] [Google Scholar]
- 223).Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 2003;124:1792–1801. 10.1016/S0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 224).Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, et al. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. J Hepatol 1990;11:92–101. 10.1016/0168-8278(90)90278-y. [DOI] [PubMed] [Google Scholar]
- 225).Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol 2005;3:705–713. 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 226).Plauth M, Egberts E-H, Hamster W, Török M, Müller PH, Brand O, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. J Hepatol 1993;17:308–314. 10.1016/S0168-8278(05)80210-7. [DOI] [PubMed] [Google Scholar]
- 227).Gluud LL, Dam G, Les I, Córdoba J, Marchesini G, Borre M, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev 2015:CD001939. 10.1002/14651858.CD001939.pub2. [DOI] [PubMed] [Google Scholar]
- 228).Mager DR, Wykes LJ, Roberts EA, Ball RO, Pencharz PB. Branched-chain amino acid needs in children with mild-to-moderate chronic cholestatic liver disease. J Nutr 2006;136:133–139. 10.1093/jn/136.1.133. [DOI] [PubMed] [Google Scholar]
- 229).Chin SE, Shepherd RW, Thomas BJ, Cleghorn GJ, Patrick MK, Wilcox JA, et al. Nutritional support in children with end-stage liver disease: a randomized crossover trial of a branched-chain amino acid supplement. Am J Clin Nutr 1992;56:158–163. 10.1093/ajcn/56.1.158. [DOI] [PubMed] [Google Scholar]
- 230).Gielen E, Beckwée D, Delaere A, De Breucker S, Vandewoude M, Bautmans I, et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev 2021;79:121–147. 10.1093/nutrit/nuaa011. [DOI] [PubMed] [Google Scholar]
- 231).Singh SS, Kumar A, Welch N, Sekar J, Mishra S, Bellar A, et al. Multiomics-identified intervention to restore ethanol-induced dysregulated proteostasis and secondary sarcopenia in alcoholic liver disease. Cell Physiol Biochem 2021;55:91–116. 10.33594/000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232).Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. L-Ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev 2018:CD012410. 10.1002/14651858.CD012410.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233).Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol 2012;27:430–441. 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 234).Vaisman N, Katzman H, Carmiel-Haggai M, Lusthaus M, Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr 2010;92:137–140. 10.3945/ajcn.2010.29211. [DOI] [PubMed] [Google Scholar]
- 235).Al-Obaid LN, Bazarbashi AN, Cohen ME, Kim J, Lei Y, Axelrad JE, et al. Enteric tube placement in patients with esophageal varices: risks and predictors of postinsertion gastrointestinal bleeding. JGH Open 2020;4:256–259. 10.1002/jgh3.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236).Vidot H, Bowen DG, Carey S, McCaughan GW, Allman-Farinelli M, Shackel NA. Aggressive nutrition intervention reduces ascites and frequency of paracentesis in malnourished patients with cirrhosis and ascites: ascites and frequency of paracentesis. JGH Open 2017;1:92–97. 10.1002/jgh3.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237).Baltz JG, Argo CK, Al-Osaimi AMS, Northup PG. Mortality after percutaneous endoscopic gastrostomy in patients with cirrhosis: a case series. Gastrointest Endosc 2010;72:1072–1075. 10.1016/j.gie.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 238).Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology 2017;65:1293–1305. 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 239).Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med 2017;376:1943–1955. 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240).Koretz RL, Avenell A, Lipman TO. Nutritional support for liver disease. Cochrane Database Syst Rev 2012:CD008344. 10.1002/14651858.CD008344.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241).Ney M, Vandermeer B, van Zanten SJV, Ma MM, Gramlich L, Tandon P. Meta-analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment Pharmacol Ther 2013;37:672–679. 10.1111/apt.12252. [DOI] [PubMed] [Google Scholar]
- 242).Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology 2016;150:903–910.e8. 10.1053/j.gastro.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 243).Reuter B, Shaw J, Hanson J, Tate V, Acharya C, Bajaj JS. Nutritional assessment in inpatients with cirrhosis can be improved after training and is associated with lower readmissions. Liver Transpl 2019;25:1790–1799. 10.1002/lt.25602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244).McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enter Nutr 2016;40:159–211. 10.1177/0148607115621863 [DOI] [PubMed] [Google Scholar]
- 245).McClave SA, DiBaise JK, Mullin GE, Martindale RG. ACG clinical guideline: nutrition therapy in the adult hospitalized patient. Am J Gastroenterol 2016;111:315–334. 10.1038/ajg.2016.28. [DOI] [PubMed] [Google Scholar]
- 246).Patel JJ, McClain CJ, Sarav M, Hamilton-Reeves J, Hurt RT. Protein requirements for critically ill patients with renal and liver failure. Nutr Clin Pract 2017;32(1 Suppl):101S–111S. 10.1177/0884533616687501. [DOI] [PubMed] [Google Scholar]
- 247).Nanchal R, Subramanian R, Karvellas CJ, Hollenberg SM, Peppard WJ, Singbartl K, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med 2020;48:e173–e191. 10.1097/CCM.0000000000004192. [DOI] [PubMed] [Google Scholar]
- 248).de Lédinghen V, Beau P, Mannant PR, Borderie C, Ripault MP, Silvain C, et al. Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig Dis Sci 1997;42:536–541. 10.1023/a:1018838808396. [DOI] [PubMed] [Google Scholar]
- 249).Peter JV, Moran JL, Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med 2005;33:213–220; discussion 260-261. 10.1097/01.ccm.0000150960.36228.c0. [DOI] [PubMed] [Google Scholar]
- 250).Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med 2005;31:12–23. 10.1007/s00134-004-2511-2. [DOI] [PubMed] [Google Scholar]
- 251).Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol 2012;10:117–125. 10.1016/j.cgh.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 252).Himoto T, Masaki T. Current trends of essential trace elements in patients with chronic liver diseases. Nutrients 2020;12:2084. 10.3390/nu12072084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253).Kozeniecki M, Ludke R, Kerner J, Patterson B. Micronutrients in liver disease: roles, risk factors for deficiency, and recommendations for supplementation. Nutr Clin Pract 2020;35:50–62. 10.1002/ncp.10451. [DOI] [PubMed] [Google Scholar]
- 254).Sriram K, Lonchyna VA. Micronutrient supplementation in adult nutrition therapy: practical considerations. J Parenter Enteral Nutr 2009;33:548–562. 10.1177/0148607108328470. [DOI] [PubMed] [Google Scholar]
- 255).Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract 2013;28:15–29. 10.1177/0884533612469027. [DOI] [PubMed] [Google Scholar]
- 256).Newsome PN, Beldon I, Moussa Y, Delahooke TE, Poulopoulos G, Hayes PC, et al. Low serum retinol levels are associated with hepatocellular carcinoma in patients with chronic liver disease. Aliment Pharmacol Ther 2000;14:1295–1301. 10.1046/j.1365-2036.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 257).Schölmerich J, Löhle E, Köttgen E, Gerok W. Zinc and vitamin A deficiency in liver cirrhosis. Hepatogastroenterology 1983;30:119–125. [PubMed] [Google Scholar]
- 258).Koop AH, Mousa OY, Pham LE, Corral-Hurtado JE, Pungpapong S, Keaveny AP. An argument for vitamin D, A, and zinc monitoring in cirrhosis. Ann Hepatol 2018;17:920–932. 10.5604/01.3001.0012.7192. [DOI] [PubMed] [Google Scholar]
- 259).Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930. 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 260).Heubi JE, Hollis BW, Specker B, Tsang RC. Bone disease in chronic childhood cholestasis: vitamin D absorption and metabolism. Hepatology 1989;9:258–264. 10.1002/hep.1840090216. [DOI] [PubMed] [Google Scholar]
- 261).Altarelli M, Ben-Hamouda N, Schneider A, Berger MM. Copper deficiency: causes, manifestations, and treatment. Nutr Clin Pract 2019;34:504–513. 10.1002/ncp.10328. [DOI] [PubMed] [Google Scholar]
- 262).Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, Ponce-de-León-Rosales S, Vargas-Vorácková F, García-Flores O, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol 2016;7:e180. 10.1038/ctg.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263).Aamann L, Dam G, Borre M, Drljevic-Nielsen A, Overgaard K, Andersen H, et al. Resistance training increases muscle strength and muscle size in patients with liver cirrhosis. Clin Gastroenterol Hepatol 2020;18:1179–1187.e6. 10.1016/j.cgh.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 264).Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci 2020;65:3350–3359. 10.1007/s10620-019-06034-2. [DOI] [PubMed] [Google Scholar]
- 265).Lai JC, Dodge JL, Kappus MR, Wong R, Mohamad Y, Segev DL, et al. A multicenter pilot randomized clinical trial of a home-based exercise program for patients with cirrhosis: the Strength Training Intervention (STRIVE). Am J Gastroenterol 2021;116:717–722. 10.14309/ajg.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266).Williams FR, Berzigotti A, Lord JM, Lai JC, Armstrong MJ. Review article: impact of exercise on physical frailty in patients with chronic liver disease. Aliment Pharmacol Ther 2019;50:988–1000. 10.1111/apt.15491. [DOI] [PubMed] [Google Scholar]
- 267).Moore GE, Durstine JL, Painter PL, eds. ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities. 4th ed. Champaign, IL: Human Kinetics; 2016. [Google Scholar]
- 268).US Deparment of Health & Human Services. Executive summary: physical activity guidelines for Americans. 2nd ed. https://health.gov/sites/default/files/2019-10/PAG_ExecutiveSummary.pdf. Published 2019. Accessed April 23, 2021. [Google Scholar]
- 269).Duarte-Rojo A, Bloomer PM, Rogers RJ, Hassan MA, Dunn MA, Tevar AD, et al. Introducing EL-FIT (Exercise and Liver FITness): a smartphone app to prehabilitate and monitor liver transplant candidates. Liver Transpl 2021;27:502–512. 10.1002/lt.25950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270).Lai JC, Sonnenday CJ, Tapper EB, Duarte-Rojo A, Dunn MA, Bernal W, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant 2019;19:1896–1906. 10.1111/ajt.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271).Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol 2018;69:1164–1177. 10.1016/j.jhep.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 272).Lin FP, Ferrando AA, Dennis RA, Dunn MA, Kim WR, Duarte-Rojo A. Exercise-induced hyperammonemia does not precipitate overt hepatic encephalopathy. Hepatology 2020;72:778–780. 10.1002/hep.31148. [DOI] [PubMed] [Google Scholar]
- 273).Bandi J-C, García-Pagán JC, Escorsell A, François E, Moitinho E, Rodes J, et al. Effects of propranolol on the hepatic hemodynamic response to physical exercise in patients with cirrhosis. Hepatology 1998;28:677–682. 10.1002/hep.510280312. [DOI] [PubMed] [Google Scholar]
- 274).Bellar A, Welch N, Dasarathy S. Exercise and physical activity in cirrhosis: opportunities or perils. J Appl Physiol 2020;128:1547–1567. 10.1152/japplphysiol.00798.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275).Kovacheva EL, Hikim APS, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 2010;151:628–638. 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]