Key Points
Predicting survival in patients who are critically ill with AKI-D is a patient-centered research topic that has prognostic and therapeutic implications.
A single-center, retrospective data–based prognostic model was developed using disease acuity, comorbid illness, and clinical data at RRT start.
Although robust test performance in predicting hospital survival was seen, external validation is needed to prove the generalizability of results.
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, critical care, dialysis, prognostic model, survival
Visual Abstract
Abstract
Background
Mortality of patients who are critically ill with AKI initiated on RRT is very high. Identifying modifiable and unmodifiable clinical variables at dialysis start that are associated with hospital survival can help, not only in prognostication, but also in clinical triaging.
Methods
A retrospective observational study was conducted on patients with AKI-D who were initiated on RRT in the medical and surgical intensive care units (ICUs) of a high-acuity academic medical center from January 2010 through December 2015. We excluded patients with suspected poisoning, ESKD, stage 5 CKD not on dialysis, or patients with AKI-D initiated on RRT outside of the ICU setting. The primary outcome was in-hospital mortality.
Results
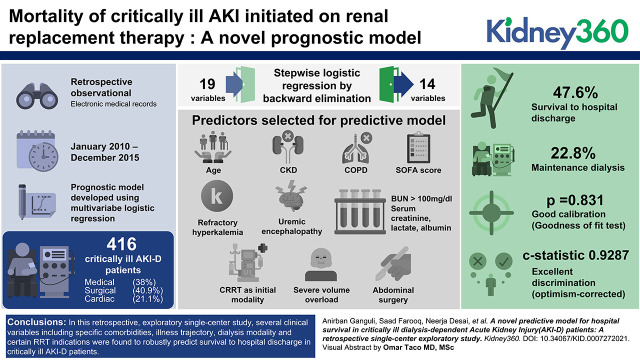
Of the 416 patients who were critically ill with AKI-D admitted to the medical (38%), surgical (41%), and cardiac (21%) ICUs, with nearly 75% on artificial organ support, the mean age 62.1±14.8 years, mean SOFA score was 11.8±4.3, dialysis was initiated using continuous RRT in 261 (63%) and intermittent hemodialysis in 155 (37%) patients. Incidence of survival to hospital discharge was 48%. Using multivariable logistic regression with stepwise backward elimination, a prognostic model was created that included the variables age, CKD, COPD, admission, and within 24 hours of the start SOFA score, refractory hyperkalemia and uremic encephalopathy as dialysis indications, BUN >100 mg/dl, serum creatinine, serum lactate, serum albumin, CRRT as initial modality, severe volume overload, and abdominal surgery. The model exhibited good calibration (goodness of fit test, P=0.83) and excellent discrimination (optimism-corrected C statistic 0.93).
Conclusions
In this single-center, diverse, critically ill AKI-D population, a novel prognostic model that combined widely used ICU scores, clinical and biochemical data at dialysis start, and dialysis indication and modality, robustly predicted short-term survival. External validation is needed to prove the generalizability of the study findings.
Introduction
Despite recent advances in critical care medicine and dialysis techniques, AKI in the intensive care unit (ICU), especially for those on dialysis, is associated with high mortality (1). Given the considerable costs and resources needed to provide critical care dialysis, strategies to optimize clinical outcomes in this population is a top-priority research topic in critical care nephrology (2). Despite the daunting ethical challenges in conducting randomized trials in critical care settings, recent multicenter randomized trials do not show incremental survival benefit, with protocolized early dialysis initiation on the basis of severity of AKI as compared with clinician-adjudicated emergent indications, underscoring the need to individualize, not generalize, the decision process (3).
Given the absence of a clear-cut, evidence-based approach, novel methods, such as prognostication tools, can be used to simplify therapeutic decision making. Other than prognostication, such tools could also help in earlier utilization of RRT if a high-risk individual is identified on the basis of dialysis indications or modality. A key factor in this is the choice of patient-centered endpoints that also provide valid measures of processes of care, so quality of services can be improved. Keeping these objectives in mind, we designed this observational study, which sought to identify clinical and biochemical data, including dialysis-specific information at RRT start in these patients, then use this information to develop a mathematical model that predicts the probability of survival to hospital discharge.
Materials and Methods
Electronic medical records were obtained for all adult patients with dialysis-dependent AKI (AKI-D) initiated on RRT in the medical and surgical ICU of Medstar Washington Hospital Center, a high-acuity academic medical center. The observation period extended from January 2010 through December 2015. Exclusion criteria included patients with ESKD, advanced CKD (stage 5, not on dialysis), patients with confirmed or suspected poisoning, patients with AKI-D transferred from other centers, initiated on RRT outside the ICU within current admission, initiated on RRT in study-center ICU but subsequently transferred to outside hospitals, or patients with missing data on clinical course during index hospitalization. Of note, we did not exclude patients with AKI-D in prior hospitalization.
Definition and staging of AKI was on the basis of serum creatinine, per the Kidney Disease Improving Global Outcomes (KDIGO) consensus criteria (4). Data collection included demographic, clinical details, and biochemical tests at time of ICU admission, at, or within 24 hours of, dialysis initiation. Biochemical panel within 24 hours of RRT was defined as test panel closest to and before the exact time of RRT start, within a 24-hour period. Acuity of illness was quantified using the Sequential Organ Failure Assessment (SOFA) score and the Acute Physiologic and Chronic Health Evaluation scoring system II (5). Comorbidity burden was quantified using the Charlson comorbidity index (6).
A semiquantitative method to grade volume overload was used as follows: 0, no O2 need, no peripheral edema; 1+, peripheral edema but no O2 need; 2+, documentation of pulmonary edema on clinical examination or chest x-ray±peripheral edema; 3+, ventilator dependent pulmonary edema.
Severity of liver disease was scored as follows: 0, no liver disease/Child Pugh class A; 1, compensated liver disease/Child Pugh class B; and 2, decompensated liver disease/Child Pugh class C.
Hyperkalemia severity was graded as follows: 0 if serum K <5.5 mmol/L; 1+ if serum K 5.5–6.5 mmol/L; 2+ if serum K 6.5–7.5 mmol/L; and 3+ if serum K >7.5 mmol/L.
Acidosis was categorized as severe if arterial pH <7.2 versus mild if >7.2, whereas BUN elevation was categorized as mild if <100 mg/dl and severe if >100 mg/dl. Details of nephrology or intensivist note were used to identify the cause of AKI, indication for RRT, and details of dialysis modality, prescription details, dialysis-related complications, and clinical outcome of hospitalization. To minimize misclassification bias, data abstractors were trained to collect data using a standard data abstraction form. Data from different abstractors were reviewed periodically to ensure accuracy and reproducibility. The principal investigator was responsible for maintaining data integrity and confidentiality. Using an anticipated probability of survival to hospital discharge of around 40% for patients admitted to the ICU with AKI-D (1,2), and keeping a margin of error ≤0.05, a minimal sample size of 369 was considered.
Statistical Methods
Analyses were performed with STATA (StataCorp 2019, Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.). Continuous variables were compared using a t test or the Wilcoxon signed-rank test, where appropriate, whereas categorical variables were compared using the chi-squared test or Fisher’s exact test as appropriate. Multiple imputation was used for missing data.
The prognostic model was constructed in the following steps:
Step 1: Identification of Candidate Variables
This was done using binary logistic regression for candidate variables using a complete dataset.
Step 2: Construction of the Regression Model
Clinically relevant covariates identified in univariate analysis with P<0.25 were included in the initial multivariable regression model. Total number of covariates were chosen using the rule of one variable per ten events (7). Stepwise logistic regression using backward elimination was done using an α-critical value of 0.15 for the exit from the previous model. All continuous covariates were assumed to have linear and additive relationships with log-odds of outcome. Wherever clinical data existed with respect to clinical outcomes, continuous variables were converted to categorical variables to simply model.
Step 3: Internal Validation
Model discrimination was assessed using area under the receiver operating characteristic curve, whereas model calibration was assessed using a calibration plot. A bootstrap validation technique using 1000 repetitions was used to generate optimism-corrected C statistics for the final model.
Step 4: Model Specification
All of the β coefficients from the final model were used to create a risk calculator on an Excel spreadsheet.
Study protocol was approved by the institutional review board. The manuscript was prepared in accordance with strengthening the reporting of observational studies in epidemiology statement for observational studies (8) and transparent reporting of a multivariable prediction model for individual prognosis or diagnosis statement for multivariable prediction model (9).
Results
Preliminary dataset obtained using International Classification of Diseases 9th revision codes (using ICU admission status CM 359.81, renal dialysis status V45.1, extracorporeal dialysis V56.0, hemodialysis PS 39.95, and procedure codes for dialysis 58001C, 58027C, 58029C for charts retrieved through September 2015) and International Classification of Diseases 10th revision codes (using ICU admission status 99291, dialysis dependence Z99.2, and dialysis procedure codes 90935, 90945, 90947, and 90999 for charts after September 2015), which yielded a general list of 968 patients, of whom 432 were excluded due to misclassification error, 97 for RRT initiated outside the ICU, and 23 because they were either transferred to other institutions or underwent organ transplantation. Final data analysis included 416 patients and included those who opted for withdrawal of care.
Baseline characteristics of patients are shown in Table 1. Mean age of patients was 62.1±14.8 years, and they were mostly male (58%) and Black (64%). The mean Charlson comorbidity index was 5.2±2.9, with nearly 48% of patients having baseline CKD. Etiology of AKI was overwhelmingly acute tubular necrosis (82%), followed by cardiorenal syndrome (12%). An even spread of patients across medical (38%), surgical (41%), and cardiac (21%) ICUs was seen, indicating a well-represented ICU population. Details of artificial organ support at RRT start (Table 1) included 71% on mechanical ventilator, 8% on a left ventricular assist device (LVAD), and 4% on extracorporeal membrane oxygenator (ECMO). Mean acuity scores at RRT start were 28.9±7.6 by Acute Physiology and Chronic Health Evaluation Score (APACHE) score and 11.8±4.3 by SOFA score. Majority of cardiovascular surgeries were emergent (67%). Dialysis modality at start was continuous RRT in 63% of patients and intermittent hemodialysis in 37% of patients. Mean time from hospitalization to ICU admission was 3.6±0.5 days. The median time lag from KDIGO stage 3 AKI to RRT start was 14 hours. The most common indication for RRT was volume overload (62%), followed by metabolic acidosis (41%), and hyperkalemia (28%). In most patients (67%), there was more than one indication for RRT initiation. The percentage of missing data varied from 0.24% to 4%.
Table 1.
Baseline characteristics of patients
| Clinical Characteristics (n=416)a | Values |
|---|---|
| Age, yr, mean±SD | 62.1±14.8 |
| Sex, n (%) | |
| M/F | 242/174 (58/42) |
| Ethnicity, n (%) | |
| Black | 266 (64) |
| Non-Hispanic White | 119 (29) |
| Others (Hispanic, Asian) | 31 (7) |
| Comorbidity profile | |
| Charlson comorbidity score, mean±SD | 5.2±2.9 |
| Pre-existing CKD, n (%) | 201 (48) |
| Pre-existing diabetes mellitus, n (%) | 188 (45) |
| Pre-existing CAD, n (%) | 147 (35) |
| Pre-existing CHF, n (%) | 165 (40) |
| Active malignancy, n (%) | 29 (7) |
| Active hematologic malignancy, n (%) | 15 (4) |
| Cause of AKI | |
| Acute tubular necrosis, n (%) | 340 (82) |
| Cardiorenal syndrome, n (%) | 48 (12) |
| Other causes (transplant rejection, obstructive uropathy, vascular events, vasculitis, AIN), mean±SD | 28 (7) |
| APACHE II score, mean±SD | 28.9±7.6 |
| SOFA score, mean±SD | 11.8±4.3 |
| Composition of ICU patients, n (%) | |
| Medical ICU | 158 (38) |
| Surgical ICU | 170 (41) |
| Cardiac ICU | 88 (21) |
| Artificial organ support, n (%) | |
| Ventilator dependent | 296 (71) |
| LVAD | 33 (8) |
| IABP | 31 (8) |
| ECMO | 15 (4) |
| Dialysis modality used in initiating RRT in ICU, n (%) | |
| Intermittent hemodialysis | 155 (37) |
| CRRT | 261 (63) |
| Median length of stay, d | 16 |
| Mean duration from hospital admission to ICU transfer, d, mean±SD | 3.6±0.5 |
| Median time-lag from KDIGO Stage 3 to RRT start, h | 14 |
| Clinical outcomes, n (%) | |
| Death | 218 (52) |
| Discharge with recovery from dialysis dependence | 97 (23) |
| Discharge on dialysis | 95 (23) |
| Discharge on hospice | 6 (2) |
M/F, male/female; CAD, coronary artery disease; CHF, congestive heart failure; AIN, acute tubulointerstitial nephritis; APACHE II, Acute Physiology and Chronic Health Evaluation Score; SOFA, Sequential Organ Failure Assessment Score; ICU, intensive care unit; LVAD, left ventricular assist device; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; CRRT, continuous RRT; KDIGO, Kidney Disease Improving Global Outcomes.
Missing data not imputed.
Overall survival to hospital discharge was 49%, with 23% recovering from a dialysis-dependent state, 23% on maintenance dialysis, and 2% receiving hospice care at time of discharge. Key clinical variables associated with primary outcome are shown in Tables 2 and 3. In the univariate analysis, positive correlation was seen with pre-existing CKD, initial ICU admission APACHE II and SOFA scores, BUN and serum creatinine and serum albumin at RRT start, time from KDIGO stage 3 to RRT start (in hours), and hyperkalemia or uremic encephalopathy as the indication for RRT start. Key factors linked with poor survival were age, ICU admission for cardiac surgery, pre-existing chronic obstructive pulmonary disease (COPD), APACHE II and SOFA score at/within 24 hours of dialysis start, serum lactate at/within 24 hours or RRT start, oliguria for >48 hours before dialysis, continuous RRT as initial RRT modality, use of either ventilatory support, ECMO, intra-aortic balloon pump or LVAD, and severity of volume overload, or severe metabolic acidosis at RRT start.
Table 2.
Comparison of clinical characteristics at dialysis initiation: Survivors and nonsurvivors
| Characteristics | Survivors (n=198) | Nonsurvivors (n=218) | Odds Ratio | 95% Confidence Interval | P Value for Odds Ratio |
|---|---|---|---|---|---|
| Age, yr, mean±SD | 60.0±14.5 | 64.0±14.4 | 0.981 | 0.967 to 0.994 | 0.006 |
| Sex, n | |||||
| M/F | 109/89 | 133/85 | 0.783 | 0.529 to 1.156 | 0.219 |
| Reason for ICU admission | 0.063 | ||||
| Medical causes, n | 122 | 117 | Reference | ||
| Cardiac surgery, n | 31 | 59 | 0.504 | 0.305 to 0.834 | 0.008 |
| Vascular surgery, n | 18 | 20 | 0.863 | 0.434 to 1.712 | 0.674 |
| Abdominal surgery, n | 19 | 11 | 1.656 | 0.756 to 3.630 | 0.207 |
| Trauma surgery, n | 3 | 4 | 0.719 | 0.158 to 3.283 | 0.671 |
| Burns, n | 2 | 5 | 0.384 | 0.073 to 2.016 | 0.258 |
| Neurosurgery, n | 3 | 2 | 1.438 | 0.787 to 8.764 | 0.693 |
| Length of stay, d, mean±SD | 26.6±18.4 | 16.1±17.1 | 1.034 | 1.024 to 1.052 | <0.001 |
| Hospital admission to ICU transfer, d, mean±SD | 2.4±5.0 | 4.6±12.5 | 0.966 | 0.937 to 0.997 | 0.030 |
| Charlson comorbidity score, mean±SD | 5.2±3.2 | 5.2±2.5 | 1.010 | 0.944 to 1.080 | 0.769 |
| No diabetes mellitus, n (%) | 111 (56) | 117 (54) | 1.057 | 0.718 to 1.556 | 0.778 |
| CAD, n (%) | 69 (35) | 78 (36) | 0.960 | 0.642 to 1.436 | 0.843 |
| CHF, n (%) | 79 (40) | 86 (40) | 1.019 | 0.688 to 1.600 | 0.925 |
| COPD, n (%) | 21 (11) | 38 (17) | 0.562 | 0.317 to 0.996 | 0.048 |
| Decompensated liver disease, n (%) | 4 (2) | 11 (5) | 0.623 | 0.122 to 1.124 | 0.110 |
| Baseline CKD, n (%) | 118 (60) | 83 (38) | 1.733 | 1.205 to 2.550 | 0.003 |
| Active malignancy (non-heme), n (%) | 14 (7) | 15 (7) | 1.029 | 0.484 to 2.191 | 0.939 |
| Hematologic malignancy, n (%) | 3 (2) | 12 (6) | 0.265 | 0.074 to 0.955 | 0.042 |
| APACHE II score, mean±SDa | |||||
| On ICU admission | 25.0±7.8 | 26.7±9.4 | 0.978 | 0.957 to 1.001 | 0.059 |
| At/within 24 hours of RRT start | 26.5±7.0 | 31.1±7.5 | 0.916 | 0.890 to 0.945 | <0.001 |
| SOFA score, mean±SDa | |||||
| On ICU admission | 9.0±3.9 | 10.8±4.6 | 0.906 | 0.866 to 0.950 | <0.001 |
| Within 24 hours of RRT start | 9.8±4.0 | 13.6±3.8 | 0.791 | 0.748 to 0.837 | <0.001 |
| BUN at start, mg/dl, mean±SD | 86.0±42.8 | 70.3±33.6 | 1.011 | 1.005 to 1.016 | <0.001 |
| S. creatinine at start, mg/dl, mean±SD | 5.77±4.95 | 3.68±2.29 | 1.334 | 1.202 to 1.480 | <0.001 |
| S. potassium at start, mmol/L, mean±SD | 5.2±1.4 | 4.8±1.2 | 1.243 | 1.068 to 1.446 | 0.005 |
| Arterial pH at start, mean±SDa | 7.29±0.12 | 7.27±0.14 | 3.755 | 0.820 to 17.191 | 0.087 |
| pCO2 at start, mm Hg, mean±SD | 38±0.9 | 39±1 | 0.991 | 0.977 to 1.006 | 0.273 |
| S. HCO3 at start, mmol/L, mean±SD | 19.3±5.9 | 19.3±6.3 | 0.999 | 0.968 to 1.031 | 0.943 |
| S. albumin, g/dL, mean±SD | 2.6±1.1 | 2.2±0.7 | 1.825 | 1.393 to 2.391 | <0.001 |
| S. lactate, mmol/L at 24 hours of starta, mean±SD | 2.0±2.0 | 4.4±4.4 | 0.756 | 0.683 to 0.837 | <0.001 |
| CRRT/IHD as initial RRT modality | 88/110 | 173/45 | 0.208 | 0.135 to 0.320 | <0.001 |
| RRT start from stage 3 AKI, h, mean±SD | 47.8±109.2 | 25.6±48.3 | 1.005 | 1.001 to 1.009 | 0.013 |
| Ventilatory support at RRT start, n (%) | 115 (58) | 181 (83) | 0.283 | 0.180 to 0.445 | <0.001 |
| ECMO at RRT start, n (%) | 0 (0) | 15 (7) | — | — | — |
| IABP at RRT start, n (%) | 22 (10) | 9 (5) | 0.422 | 0.189 to 0.940 | 0.035 |
| LVAD support at RRT start, n (%) | 10 (5) | 23 (11) | 0.448 | 0.208 to 0.968 | 0.041 |
| SCUF before RRT start, n (%) | 25 (12) | 21 (11) | 0.937 | 0.506 to 1.734 | 0.836 |
M/F, male/female; ICU, intensive care unit; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; APACHE II, Acute Physiology and Chronic Health Evaluation Score; SOFA, Sequential Organ Failure Assessment Score; S., serum; pCO2, partial pressure of CO2; HCO3, serum bicarbonate level; CRRT, continuous RRT; IHD, intermittent hemodialysis; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; SCUF, slow continuous ultrafiltration.
Only for complete patients.
Table 3.
Dialysis specific factors associated with survival outcomes
| Clinical Characteristics | Survivors (n=198) | Nonsurvivors (n=218) | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|---|---|
| Indications, n (%) | |||||
| Volume overload | 117 (60) | 139 (64) | 0.870 | 0.752 to 1.006 | 0.061 |
| Hyperkalemia | 73 (37) | 44 (20) | 2.309 | 1.489 to 3.582 | <0.001 |
| Metabolic acidosis | 79 (40) | 93 (43) | 0.892 | 0.603 to 1.319 | 0.568 |
| Uremic encephalopathy | 54 (27) | 38 (17) | 1.839 | 1.159 to 2.919 | 0.010 |
| Unclear/nonemergent | 16 (8) | 25 (12) | 0.679 | 0.351 to 1.312 | 0.249 |
| Oliguria >48 hours before starta | 84 (42) | 122 (56) | 0.610 | 0.415 to 0.896 | 0.012 |
| Degree of volume overload | 0.060 | ||||
| None, n | 68 | 64 | Ref | Ref | — |
| Mild, n | 10 | 7 | 1.344 | 0.483 to 3.745 | 0.571 |
| Moderate, n | 37 | 28 | 1.243 | 0.684 to 2.262 | 0.475 |
| Severe, n | 83 | 119 | 0.656 | 0.422 to 1.021 | 0.062 |
| Acidosis (pH <7.35) at start, n (%)a | 131 (66) | 156 (72) | 0.777 | 0.513 to 1.178 | 0.235 |
| Severe acidosis (pH <7.2) at start, n (%) | 38 (19) | 57 (26) | 0.671 | 0.421 to 1.068 | 0.093 |
| Degree of hyperkalemia mmol/L at start | 0.222 | ||||
| None (<5.5), n | 130 | 166 | Ref | Ref | |
| Mild (5.5–6.5), n | 31 | 26 | 1.502 | 0.850 to 2.654 | 0.162 |
| Moderate (6.5–7.5), n | 17 | 14 | 1.529 | 0.727 to 3.217 | 0.263 |
| Severe (>7.5), n | 18 | 13 | 1.743 | 0.824 to 3.690 | 0.146 |
| BUN >100 mg/dl at start, n (%) | 67 (34) | 45 (21) | 1.966 | 1.265 to 3.055 | 0.003 |
| Effluent/dialysate flow rate of CRRT, ml/kg per hour, mean±SD | 23.8±10.4 | 23.3±8.8 | 1.006 | 0.979 to 1.034 | 0.668 |
CRRT, continuous RRT; Ref, reference.
In the initial multivariable logistic regression model, 19 variables (Table 4) were included. Stepwise logistic regression by backward elimination was conducted using an α-critical value for an exit of 0.15. This resulted in only 14 variables being included in the final model (Table 4). Comparison for any postsurgical patient was made with patients admitted to medical ICU. Formal tests of multicollinearity for the final model using variance inflation factor method indicated that all independent variables had variance inflation factor close to 1, so multicollinearity was minimal.
Table 4.
Stepwise logistic regression using backward selection
| Models and Variables | Full Model | Final Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | Standard Error | P Value | 95% Confidence Interval | Odds Ratio | Standard Error | P Value | 95% Confidence Interval | |
| Age, yr | 0.965 | 0.009 | 0.000 | 0.948 to 0.984 | 0.965 | 0.009 | 0.000 | 0.948 to 0.983 |
| Baseline CKD | 1.384 | 0.238 | 0.058 | 0.989 to 1.935 | 1.687 | 0.302 | 0.004 | 1.187 to 2.396 |
| Baseline COPD | 0.161 | 0.058 | 0.000 | 0.080 to 0.326 | 0.146 | 0.054 | 0.000 | 0.071 to 0.300 |
| Admission SOFA score | 1.140 | 0.415 | 0.001 | 1.058 to 1.229 | 1.225 | 0.046 | 0.000 | 1.137 to 1.319 |
| SOFA score within 24 hours of RRT start | 0.689 | 0.031 | 0.000 | 0.631 to 0.753 | 0.653 | 0.030 | 0.000 | 0.597 to 0.715 |
| Refractory hyperkalemia as indication | 3.512 | 1.026 | 0.000 | 1.982 to 6.225 | 3.763 | 1.103 | 0.000 | 2.118 to 6.685 |
| Uremic encephalopathy as indication | 0.558 | 0.156 | 0.037 | 0.322 to 0.965 | 0.317 | 0.077 | 0.000 | 0.197 to 0.510 |
| BUN >100 mg/dl at start | 1.495 | 0.415 | 0.148 | 0.867 to 2.577 | 1.855 | 0.503 | 0.023 | 1.090 to 3.152 |
| S. creatinine mg/dl at start | 1.151 | 0.070 | 0.021 | 1.021 to 1.297 | 1.206 | 0.077 | 0.003 | 1.065 to 1.366 |
| S. albumin >3.5 g/dl at start | 2.284 | 1.053 | 0.073 | 0.925 to 5.639 | 3.289 | 1.472 | 0.008 | 1.368 to 7.907 |
| CRRT chosen as initial RRT modality | 0.450 | 0.136 | 0.011 | 0.293 to 0.853 | 0.514 | 0.141 | 0.015 | 0.301 to 0.879 |
| Lactate at start, mmol/L | 0.809 | 0.039 | 0.000 | 0.737 to 0.889 | 0.805 | 0.037 | 0.000 | 0.735 to 0.882 |
| Severe volume overload at start | 1.657 | 0.424 | 0.048 | 1.004 to 2.735 | 1.440 | 0.362 | 0.148 | 0.879 to 2.358 |
| ICU after abdominal surgery | 3.870 | 2.161 | 0.015 | 1.296 to 11.562 | 3.766 | 2.128 | 0.019 | 1.244 to 11.396 |
| ICU after cardiac surgery | 1.156 | 0.334 | 0.617 | 0.655 to 2.038 | — | — | — | — |
| Active hematologic malignancy | 0.434 | 0.350 | 0.301 | 0.090 to 2.107 | — | — | — | — |
| Severe acidosis at start | 1.217 | 0.481 | 0.620 | 0.561 to 2.639 | — | — | — | — |
| On LVAD at start | 1.516 | 0.588 | 0.284 | 0.708 to 3.242 | — | — | — | — |
| On IABP at start | 1.215 | 0.509 | 0.643 | 0.534 to 2.761 | — | — | — | — |
| Constant | 155.413 | 131.238 | 0.000 | 29.696 to 813.353 | 123.778 | 100.556 | 0.000 | 25.185 to 608.344 |
| LR chi2 | 439.45 | 576.54 | ||||||
| Prob >chi2 | 0.0000 | 0.0000 | ||||||
| Pseudo R2 | 0.4565 | 0.5094 | ||||||
COPD, chronic obstructive pulmonary disease; SOFA, sequential organ failure assessment score; S., serum; CRRT, continuous RRT; ICU, intensive care unit; LVAD, left ventricular assist device; IABP, intra-aortic balloon pump; LR, likelihood ratio.
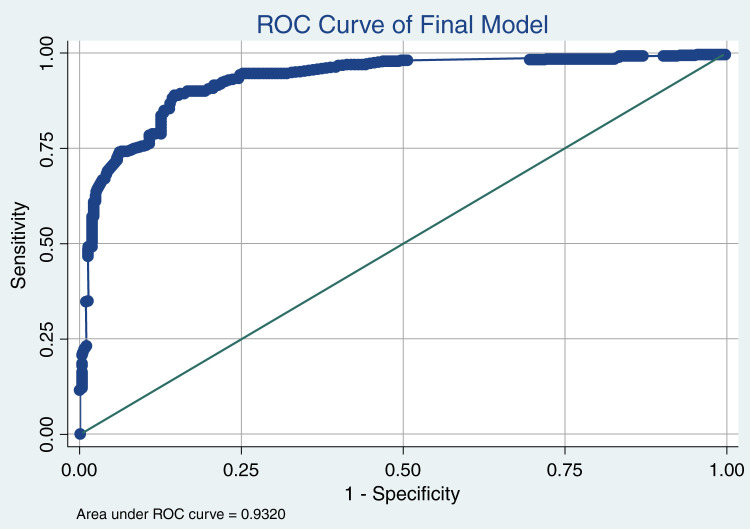
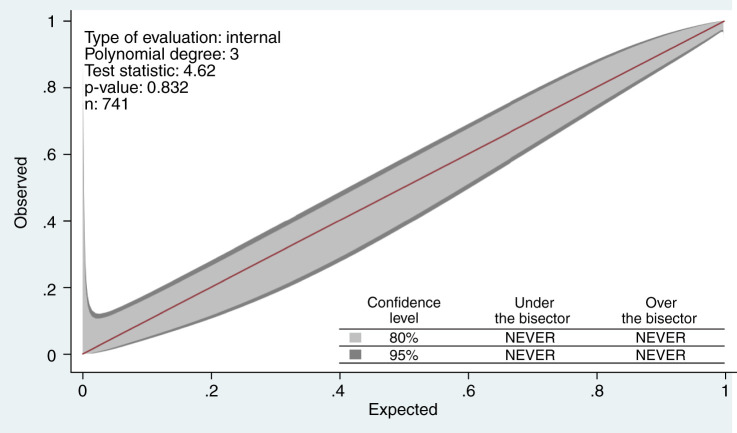
Internal validation of the final model yielded excellent discrimination (Figure 1), with a C score of 0.93 (95% confidence interval, 0.92 to 0.95). The GiViTI calibration belt shown in Figure 2 indicated good calibration (goodness of fit test, P=0.83). Additionally, the optimism corrected C statistics using bootstrapping technique for 1000 repetitions was 0.93 (95% confidence interval, 0.91 to 0.95) showing minimal optimism in the model developmental process. An Excel sheet was prepared using the final model to calculate survival probability. Given the exploratory nature of study, external validation or model updating could not be done.
Figure 1.
Receiver operating characteristic (ROC) curve for final model with the y axis representing sensitivity and x axis showing 1−specificity. The sensitivity of model is 90%, specificity 81%, positive likelihood ratio 4.86, negative likelihood ratio 0.12, positive predictive value 89%, and negative predictive value 84%.
Figure 2.
Calibration belt showing goodness of fit of final model using STATA that graphically shows significant deviation from perfect calibration that is paired to formal hypothesis testing.
Discussion
AKI affects 5%–6% of all patients in the ICU, 28%–70% of whom need dialysis at some point (1,10). Mortality in AKI sustained in the ICU is as high as 50%, with the highest incidence reported in AKI-D and varying from 45% to 79% (1,10–12). Although RRT is supposed to mitigate the detrimental effects of metabolic derangements and volume overload in AKI, their apparent lack of incremental benefit highlights the confounding effect of coexisting multiorgan failure (10,11). This paradoxical relationship between RRT and mortality in patients who are critically ill with AKI-D has recently been highlighted by the failure to demonstrate any incremental benefit of “early” or protocolized dialysis start over “usual” or “standard” initiation for emergent indications in randomized trials when patients were well matched in terms of comorbidities and acuity of illness Standard versus Accelerated Initiation of Renal-Replacement Therapy in Acute Kidney Injury (STARRT-AKI trial) (3). In view of this, strategies to optimize clinical outcomes in this high-risk cohort involve individualizing treatment thresholds on the basis of disease trajectory, while using prognostic data for informed decision making and palliative therapies.
In the last three decades, several prognostic scores dedicated to AKI and AKI-D in the critical care setting have been published, and a recent systematic review summarizing these studies is mentioned (13). Although these survival models have shown robust internal validity, utility in contemporary practice settings is limited, due to a lack of standard definition of AKI, variable inclusion-exclusion criteria, and poor performance in external validation studies (14–21). Additionally, these scores also have no utility in decision making or triaging, because prognostic variables do not include data centered around the choice of dialysis modality or clinical indication. Finally, many of the survival models use endpoints such as 7- or 60-day or ICU mortality, which may not be valid because early mortality could be confounded by the severity of extrarenal disease, whereas late mortality, extending beyond the hospitalization period, may be affected by clinical events unrelated to AKI or its therapy (19,22). To address these shortcomings, we chose a study population that was similar to major clinical trials in terms of baseline clinical acuity, comorbidities (3), and use of CRRT as the predominant dialysis modality (3,19). As a result, our clinical outcomes were similar to these studies (3,19).
Our prognostic model validated several prognostic variables for AKI-D, such as age (13,19), serum albumin (19), serum creatinine (19,22), and serum lactate (22), although identifying some novel associations. For example, baseline CKD as a good prognostic variable has previously been reported in AKI (11,23), and is speculated to be due to earlier renal consultation or ischemic preconditioning (23). Baseline COPD is another variable not previously reported, although recent observational studies have shown AKI is a risk factor for COPD exacerbation or hospitalization (24,25). Better survival after abdominal surgery is another unique observation that, we speculate, could be due to overall good prognosis for postoperative AKI (16,19,22). Although prior studies showed the highest incidence of perioperative AKI-D and mortality with cardiac and vascular surgeries, our studies showed significant association with clinical outcomes only in univariate association. This is notable, because the majority of cardiac and vascular surgeries were emergent, indicating the dominant role of multiorgan failure in determining clinical outcomes perioperatively. Our study and others (14–16,19) reiterated the negative association of mechanical ventilation on survival, although this variable was not selected in model development because ventilator dependence is a component of the SOFA score. Other artificial organ support in our study, such as ECMO, intra-aortic balloon pump, or LVAD showed similar association with the outcomes of other studies (26–28), although this was not significant in the final model, indicating they may represent surrogate markers of disease acuity. Of note, we could not test the independent association of ECMO support with outcomes, because no patients survived to hospital discharge. This leaves the question of whether ECMO use is a perfect predictor variable, or if sample representativeness was unsolved.
Our study reinforced previous data linking low serum creatinine at RRT start with poor clinical outcomes, given its association with low muscle mass and poor nutritional status (18,19,29). Serum lactate was identified as a key predictor variable in our study, underscoring the pivotal role of tissue hypoxia or circulatory failure in predicting outcomes in AKI-D (22,30–32), as in any other patient who is critically ill (33,34). In fact, recent prognostic models have demonstrated that serum lactate could potentially predict survival in patients with septic shock on CRRT (22,31). The favorable implications of elevated BUN at dialysis start in our study reflect continued uncertainty in literature about its biologic effects in uremia (34), with studies showing either no association (35) or negative association with survival (36). A possible explanation for this effect could be that BUN may be inversely related to acuity of illness, as seen previously (35).
Although general ICU scores are extensively used in critical care settings, their performance in AKI-D is very poor (13,21,37) because the developmental cohort included very few patients with AKI (38–40). Nonetheless, given their ability to predict in-hospital mortality in diverse settings such as after surgery (41), emergency room presentation (42), ICU (43), and CRRT (31,44) the SOFA score was used in our study. Another reason for using SOFA scores is that several of its component variables have shown an association with prognosis in AKI-D, such as mean arterial pressures (15,19), use of pressors (22,31), mechanical ventilation use (14–16,19,45), serum bilirubin (16,19), level of consciousness (31,45), or platelet count (16,19), thereby avoiding model instability or overfitting due to multicollinearity (46). In this regard, we used a two-point SOFA score assessment—one at ICU admission and second at RRT start was used, because delta SOFA has been shown to have greater diagnostic accuracy (43).
Among dialysis-related factors, choice of CRRT was independently associated with poor clinical outcomes. Although a protopathic bias is suspected using this variable, because CRRT is typically chosen for patients who are hemodynamically unstable and typically have poor outcomes, modality choice may be a surrogate marker for unknown covariates influencing decision making. Hyperkalemia as an indication for RRT, is a unique association and its favorable implication for survival could be due to the relative ease of correcting this metabolic complication with dialysis. As previous studies (47,48), we found severe fluid overload at RRT start to be associated with poor outcomes in univariate analysis. However, the magnitude and direction of this association changed in multivariate analysis, indicating there may be effect modifiers, such as acuity of illness at RRT start, which were not reported in previous studies (47,48) Finally, we showed presumed uremic encephalopathy at RRT start was associated with poor outcomes. Although RRT is supposed to mitigate any uremic complication, there is a possibility of misclassification of encephalopathy from nonuremic causes. This can potentially create a spurious association, especially because a lower level of consciousness, due to any reason, is associated with poor outcomes in AKI-D (12,49).
Our study has several limitations, including a small sample size, single-center data, and an absence of external validation. Some of the semiquantitative scores used in our study, such as the degree of volume overload, have not been externally validated previously, so could introduce misclassification bias. Other potential sources of error could include selection or referral bias, and indication bias. Additionally, given the absence of a contemporary dataset, applicability to the current ICU population, especially during the coronavirus disease 2019 pandemic, is questionable. Other confounders could be the inclusion of patients moving to a hospice, because this could potentially inflate mortality rates although lower the specificity of the survival model. Another potential confounder could be the dynamic nature of biochemical tests, such as serum potassium or bicarbonate before dialysis start, reflecting the confounding effects of medical treatment. Finally, given the observational nature of study, we cannot exclude the possibility of residual confounders
Despite these limitations, our retrospective data, which analyzed time elapsed from onset of AKI stage 3 to dialysis initiation as a continuous variable, showed no association with survival. This supports the conclusions of interventional data that dichotomized time to RRT start as “early” or “accelerated” versus “standard” or “usual” start strategies (3). Our data suggest that certain dialysis indications, such as hyperkalemia or volume overload, could potentially be the “low-hanging fruit” in considering early dialysis. Third, elevated serum lactate at RRT start has profound implications on outcomes in AKI-D, as for other patients who are critically ill. Fourth, specific comorbidities not overall comorbidity scores may affect survival in AKI-D. Finally, a survival model that assimilates baseline and dynamic clinical data with specific details of RRT indication and modality can potentially predict hospital survival.
To conclude, in this retrospective, exploratory single-center study, several clinical variables including specific comorbidities, illness trajectory, dialysis modality, and certain RRT indications were found to robustly predict survival to hospital discharge in patients who were critically ill with AKI-D. Future research is needed to externally validate this model for it to be an effective instrument in guiding clinical practice.
Disclosures
J. Veis reports having an advisory or leadership role with Davita as a Member of the Acute Dialysis Council; and reports other interests or relationships as a Member of the Medical Advisory Board of the National Kidney Foundation, Washington, DC, Affiliate. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The authors wish to thank the Medstar Health Research Institute, Hyattsville, in helping with obtaining a clinical database for the current research. The authors also wish to thank Dr. Shilpa Anu Krishnatry for her help in developing the prognostic score calculator for our study. The corresponding author affirms he has listed everyone who has significantly contributed to the study.
Footnotes
See related editorial, “Dissipating the Fog at the Crossroad: Predicting Survival after the Initiation of Kidney Replacement Therapy,” on pages 586–589.
Author Contributions
A. Ganguli, J. Moore, M. Sherman, and J. Veis conceptualized the study; S. Adhikari, N. Desai, S. Farooq, A. Ganguli, and V. Shah were responsible for the data curation; A. Ganguli was responsible for the formal analysis; S. Adhikari, N. Desai, S. Farooq, and V. Shah were responsible for the investigation; N. Desai was responsible for the methodology; A. Ganguli and V. Shah were responsible for the project administration; J. Moore, V. Shah, and J. Veis were responsible for the resources; A. Ganguli, J. Moore, M. Sherman, and J. Veis provided supervision; N. Desai and S. Farooq were responsible for the software; A. Ganguli and J. Veis were responsible for the validation; V. Shah and M. Sherman were responsible for the visualization; A. Ganguli wrote the original draft; A. Ganguli, M. Sherman, and J. Veis reviewed and edited the manuscript.
References
- 1.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W: Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30: 2051–2058, 2002. 10.1097/00003246-200209000-00016 [DOI] [PubMed] [Google Scholar]
- 2.Srisawat N, Lawsin L, Uchino S, Bellomo R, Kellum JA; BEST Kidney Investigators : Cost of acute renal replacement therapy in the intensive care unit: Results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care 14: R46, 2010. 10.1186/cc8933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.STARRT-AKI Investigators : Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 383: 240–251, 2020. 10.1056/NEJMoa2000741 [DOI] [PubMed] [Google Scholar]
- 4.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD: KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Acute Kidney Injury. Am J Kidney Dis 61: 649–672, 2013. 10.1053/j.ajkd.2013.02.349 [DOI] [PubMed] [Google Scholar]
- 5.Minne L, Abu-Hanna A, de Jonge E: Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 12: R161, 2008. 10.1186/cc7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 7.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR: A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49: 1373–1379, 1996. 10.1016/S0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370: 1453–1457, 2007. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 9.Collins GS, Reitsma JB, Altman DG, Moons KG: Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med 162: 55–63, 2015. 10.7326/M14-0697 [DOI] [PubMed] [Google Scholar]
- 10.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005. 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM; Program to Improve Care in Acute Renal Disease : Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 66: 1613–1621, 2004. 10.1111/j.1523-1755.2004.00927.x [DOI] [PubMed] [Google Scholar]
- 12.Liaño F, Junco E, Pascual J, Madero R, Verde E; The Madrid Acute Renal Failure Study Group : The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int Suppl 66: S16–S24, 1998 [PubMed] [Google Scholar]
- 13.Ohnuma T, Uchino S: Prediction models and their external validation studies for mortality of patients with acute kidney injury: A systematic review. PLoS One 12: e0169341, 2017. 10.1371/journal.pone.0169341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohr JW, McFarlane MJ, Grantham JJ: A clinical index to predict survival in acute renal failure patients requiring dialysis. Am J Kidney Dis 11: 254–259, 1988. 10.1016/S0272-6386(88)80158-6 [DOI] [PubMed] [Google Scholar]
- 15.Schaefer JH, Jochimsen F, Keller F, Wegscheider K, Distler A: Outcome prediction of acute renal failure in medical intensive care. Intensive Care Med 17: 19–24, 1991. 10.1007/BF01708404 [DOI] [PubMed] [Google Scholar]
- 16.Paganini EP, Halstenberg WK, Goormastic M: Risk modeling in acute renal failure requiring dialysis: the introduction of a new model. Clin Nephrol 46: 206–211, 1996 [PubMed] [Google Scholar]
- 17.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM: Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13: 1350–1357, 2002. 10.1097/01.ASN.0000014692.19351.52 [DOI] [PubMed] [Google Scholar]
- 18.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL: Mortality after acute renal failure: Models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006. 10.1038/sj.ki.5001579 [DOI] [PubMed] [Google Scholar]
- 19.Demirjian S, Chertow GM, Zhang JH, O’Connor TZ, Vitale J, Paganini EP, Palevsky PM; VA/NIH Acute Renal Failure Trial Network : Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol 6: 2114–2120, 2011. 10.2215/CJN.02900311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Doig GS, Oudemans van Straaten H, Ronco C, Kellum JA; Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators : External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med 33: 1961–1967, 2005. 10.1097/01.CCM.0000172279.66229.07 [DOI] [PubMed] [Google Scholar]
- 21.Ohnuma T, Uchino S, Toki N, Takeda K, Namba Y, Katayama S, Kawarazaki H, Yasuda H, Izawa J, Uji M, Tokuhira N, Nagata I; JSEPTIC (Japanese Society for Physicians and Trainees in Intensive Care) Clinical Trial Group : External validation for acute kidney injury severity scores: A multicenter retrospective study in 14 Japanese ICUs. Am J Nephrol 42: 57–64, 2015. 10.1159/000439118 [DOI] [PubMed] [Google Scholar]
- 22.da Hora Passos R, Ramos JG, Mendonça EJ, Miranda EA, Dutra FR, Coelho MF, Pedroza AC, Correia LC, Batista PB, Macedo E, Dutra MM: A clinical score to predict mortality in septic acute kidney injury patients requiring continuous renal replacement therapy: The HELENICC score. BMC Anesthesiol 17: 21, 2017. 10.1186/s12871-017-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khosla N, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini E, Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) : Preexisting chronic kidney disease: A potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol 4: 1914–1919, 2009. 10.2215/CJN.01690309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barakat MF, McDonald HI, Collier TJ, Smeeth L, Nitsch D, Quint JK: Acute kidney injury in stable COPD and at exacerbation. Int J Chron Obstruct Pulmon Dis 10: 2067–2077, 2015. 10.2147/COPD.S88759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, Szabo Z, Kalantar-Zadeh K, Kovesdy CP: Acute kidney injury after major surgery: A retrospective analysis of Veterans health administration data. Am J Kidney Dis 67: 872–880, 2016. 10.1053/j.ajkd.2015.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaltenmaier B, Pommer W, Kaufmann F, Hennig E, Molzahn M, Hetzer R: Outcome of patients with ventricular assist devices and acute renal failure requiring renal replacement therapy. ASAIO J 46: 330–333, 2000. 10.1097/00002480-200005000-00017 [DOI] [PubMed] [Google Scholar]
- 27.Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS, Kashani K: Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One 14: e0222894, 2019. 10.1371/journal.pone.0222894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kielstein JT, Heiden AM, Beutel G, Gottlieb J, Wiesner O, Hafer C, Hadem J, Reising A, Haverich A, Kühn C, Fischer S: Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant 28: 86–90, 2013. 10.1093/ndt/gfs398 [DOI] [PubMed] [Google Scholar]
- 29.Wilson FP, Yang W, Machado CA, Mariani LH, Borovskiy Y, Berns JS, Feldman HI: Dialysis versus nondialysis in patients with AKI: A propensity-matched cohort study. Clin J Am Soc Nephrol 9: 673–681, 2014. 10.2215/CJN.07630713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passos RDH, Ramos JGR, Gobatto A, Mendonça EJB, Miranda EA, Dutra FRD, Coelho MFR, Pedroza AC, Batista PBP, Dutra MMD: Lactate clearance is associated with mortality in septic patients with acute kidney injury requiring continuous renal replacement therapy: A cohort study. Medicine (Baltimore) 95: e5112, 2016. 10.1097/MD.0000000000005112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawarazaki H, Uchino S, Tokuhira N, Ohnuma T, Namba Y, Katayama S, Toki N, Takeda K, Yasuda H, Izawa J, Uji M, Nagata I; JSEPTIC (Japanese Society for Physicians Trainees in Intensive Care) Clinical Trial Group : Who may not benefit from continuous renal replacement therapy in acute kidney injury? Hemodial Int 17: 624–632, 2013. 10.1111/hdi.12053 [DOI] [PubMed] [Google Scholar]
- 32.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD: Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 37: 1670–1677, 2009. 10.1097/CCM.0b013e31819fcf68 [DOI] [PubMed] [Google Scholar]
- 33.Kruse O, Grunnet N, Barfod C: Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: A systematic review. Scand J Trauma Resusc Emerg Med 19: 74, 2011. 10.1186/1757-7241-19-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W; European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability [published correction appeared in Kidney Int 98: 1354, 2020]. Kidney Int 63: 1934–1943, 2003. 10.1046/j.1523-1755.2003.00924.x [DOI] [PubMed] [Google Scholar]
- 35.De Corte W, Vanholder R, Dhondt AW, De Waele JJ, Decruyenaere J, Danneels C, Claus S, Hoste EA: Serum urea concentration is probably not related to outcome in ICU patients with AKI and renal replacement therapy. Nephrol Dial Transplant 26: 3211–3218, 2011. 10.1093/ndt/gfq840 [DOI] [PubMed] [Google Scholar]
- 36.Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM: Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 1: 915–919, 2006. 10.2215/CJN.01430406 [DOI] [PubMed] [Google Scholar]
- 37.Douma CE, Redekop WK, van der Meulen JH, van Olden RW, Haeck J, Struijk DG, Krediet RT: Predicting mortality in intensive care patients with acute renal failure treated with dialysis. J Am Soc Nephrol 8: 111–117, 1997. 10.1681/ASN.V81111 [DOI] [PubMed] [Google Scholar]
- 38.Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: A severity of disease classification system. Crit Care Med 13: 818–829, 1985. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman JE, Kramer AA, McNair DS, Malila FM: Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit Care Med 34: 1297–1310, 2006. 10.1097/01.CCM.0000215112.84523.F0 [DOI] [PubMed] [Google Scholar]
- 40.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 41.Falcão ALE, Barros AGA, Bezerra AAM, Ferreira NL, Logato CM, Silva FP, do Monte ABFO, Tonella RM, de Figueiredo LC, Moreno R, Dragosavac D, Andreollo NA: The prognostic accuracy evaluation of SAPS 3, SOFA and APACHE II scores for mortality prediction in the surgical ICU: An external validation study and decision-making analysis. Ann Intensive Care 9: 18, 2019. 10.1186/s13613-019-0488-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Gigorro R, Sáez-de la Fuente I, Marín Mateos H, Andrés-Esteban EM, Sanchez-Izquierdo JA, Montejo-González JC: Utility of SOFA and Δ-SOFA scores for predicting outcome in critically ill patients from the emergency department. Eur J Emerg Med 25: 387–393, 2018. 10.1097/MEJ.0000000000000472 [DOI] [PubMed] [Google Scholar]
- 43.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL: How changes in SOFA score can predict outcome. Crit Care Med 27[Supplement]: A50, 1999. 10.1097/00003246-199912001-00102 [DOI] [Google Scholar]
- 44.Wang H, Kang X, Shi Y, Bai ZH, Lv JH, Sun JL, Pei HH: SOFA score is superior to APACHE-II score in predicting the prognosis of critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren Fail 42: 638–645, 2020. 10.1080/0886022X.2020.1788581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.d’Avila DO, Cendoroglo Neto M, dos Santos OF, Schor N, Poli de Figueiredo CE: Acute renal failure needing dialysis in the intensive care unit and prognostic scores. Ren Fail 26: 59–68, 2004. 10.1081/JDI-120028552 [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee S, Hadi AS, Price B: Regression Analysis by Example, 3rd Ed., Hoboken, NJ, John Wiley and Sons, 2000 [Google Scholar]
- 47.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) Study Group : Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76: 422–427, 2009. 10.1038/ki.2009.159 [DOI] [PubMed] [Google Scholar]
- 48.Hayes LW, Oster RA, Tofil NM, Tolwani AJ: Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24: 394–400, 2009. 10.1016/j.jcrc.2008.12.017 [DOI] [PubMed] [Google Scholar]
- 49.Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH; The Auriculin Anaritide Acute Renal Failure Study Group : Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. J Am Soc Nephrol 9: 692–698, 1998. 10.1681/ASN.V94692 [DOI] [PubMed] [Google Scholar]