Summary
Background
The long-term prognosis of COVID-19 survivors remains poorly understood. It is evidenced that the lung is the main damaged organ in COVID-19 survivors, most notably in impairment of pulmonary diffusion function. Hence, we conducted a meta-analysis of the potential risk factors for impaired diffusing capacity for carbon monoxide (DLCO) in convalescent COVID-19 patients.
Methods
We performed a systematic search of PubMed, Web of Science, Embase, and Ovid databases for relevant studies from inception until January 7, 2022, limited to papers involving human subjects. Studies were reviewed for methodological quality. Fix-effects and random-effects models were used to pool results. Heterogeneity was assessed using I2. The publication bias was assessed using the Egger's test. PROSPERO registration: CRD42021265377.
Findings
A total of eighteen qualified articles were identified and included in the systematic review, and twelve studies were included in the meta-analysis. Our results showed that female (OR: 4.011; 95% CI: 2.928–5.495), altered chest computerized tomography (CT) (OR: 3.002; 95% CI: 1.319–6.835), age (OR: 1.018; 95% CI: 1.007–1.030), higher D-dimer levels (OR: 1.012; 95% CI: 1.001–1.023) and urea nitrogen (OR: 1.004;95% CI: 1.002–1.007) were identified as risk factors for impaired DLCO.
Interpretation
Pulmonary diffusion capacity was the most common impaired lung function in recovered patients with COVID-19. Several risk factors, such as female, altered chest CT, older age, higher D-dimer levels and urea nitrogen are associated with impairment of DLCO. Raising awareness and implementing interventions for possible modifiable risk factors may be valuable for pulmonary rehabilitation.
Funding
This work was financially supported by Emergency Key Program of Guangzhou Laboratory (EKPG21-29, EKPG21-31), Incubation Program of National Science Foundation for Distinguished Young Scholars by Guangzhou Medical University (GMU2020-207).
Keywords: COVID-19, DLCO, Meta-analysis, Pulmonary diffusion function, Risk factors
Abbreviations: ARDS, acute respiratory distress syndrome; ACE2, angiotensin-converting enzyme 2; COVID-19, Coronavirus Disease 2019; CIs, confidence intervals; CT, computerized tomography; DLCO, diffusing capacity for carbon monoxide; DLNO, diffusion capacity for nitric oxide; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HR, hazard ratio; MERS, Middle East Respiratory Syndrome; NOS, Newcastle-Ottawa scale; OR, odds ratio; PFTs, pulmonary function tests; RT-PCR, reverse transcription-polymerase chain reaction; RR, relative risk; RASI, renin-angiotensin system inhibitors; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; TSS, total severity score; WHO, World Health Organization
Research in context.
Evidence before this study
Increasing evidence suggests that the lung is the main damaged organ in COVID-19 survivors, most notably by impairment of pulmonary diffusion function. We performed a systematic search in PubMed, Web of Science, Embase and Ovid for relevant studies from inception until 7 January 2022, using search terms (“COVID-19” OR “SARS-CoV-2”) AND (“Respiratory Function Tests” OR “Lung Function Tests” OR “diffusing capacity for carbon monoxide”). We found some studies reporting risk factors for the diffusing capacity of carbon monoxide (DLCO) in convalescent COVID-19 patients. However, a comprehensive review of risk factors for impaired DLCO in COVID-19 survivors is still lacking.
Added value of this study
This study is the first meta-analysis that summarizes the potential risk factors for impaired DLCO in convalescent COVID-19 patients. Our results showed that 1) the percentage of abnormal DLCO among recovered COVID-19 subjects who underwent follow-up ranged from 14% to 67%; 2) female, altered chest computerized tomography (CT), older age, high levels of D-dimer and urea nitrogen were identified as risk factors for impaired DLCO in these subjects.
Implications of all the available evidence
Female, older patients, patients with specific characteristics (altered chest CT, high levels of D-dimer or urea nitrogen) are at high risk of impaired DLCO among COVID-19 survivors. Raising awareness and implementing interventions for possible modifiable risk factors may be worthy of consideration for pulmonary rehabilitation.
Alt-text: Unlabelled box
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) emerged in late 2019 and has caused a global pandemic of acute respiratory diseases, named "Coronavirus Disease 2019" (COVID-19), threatening human health and public safety. According to the World Health Organization (WHO), more than 298 million cases of COVID-19 have been confirmed worldwide, and the global death toll has risen to 5.4 million (Last update: January 9, 2022).1 Since the outbreak, clinical and epidemiological data related to COVID-19 have updated rapidly,2,3 but the long-term prognosis still remains poorly understood.
The lung is the primary target organ for COVID-19. Infected lung tissue has multiple forms of pathophysiological changes, including diffuse alveolar epithelial destruction, hyaline membrane formation, capillary damage and hemorrhage, alveolar septal fibroplasia and pulmonary solidification.4 It has been reported that 5-10% of patients with COVID-19 may develop critical illness, including acute respiratory distress syndrome (ARDS),5 which itself causes residual respiratory physiological impairment. Similar to classic ARDS, 49% of patients with COVID-19-associated ARDS (C-ARDS) still had reduced lung diffusion capacity for carbon monoxide (DLCO) at 3 months after discharge.6 Previous studies have shown that pulmonary impairment caused by SARS coronavirus can last for months or even years,7 and impaired diffusion function (defined as diffusing capacity for carbon monoxide (DLCO) < 80% predicted value) is the most common abnormality.8, 9, 10 A team of researchers performed pulmonary function tests (PFTs) on convalescent COVID-19 patients and showed that >80% of critically ill patients presented impaired pulmonary function.11 The impaired diffusing capacity was the main feature.12,13 As with Severe Acute Respiratory Syndrome (SARS) or Middle East Respiratory Syndrome (MERS) survivors, DLCO appears to be a relevant functional parameter for assessing respiratory function in recovered patients with COVID-19.7
Impaired lung function is significantly associated with a decrease in health-related quality of life.14 There have been many studies on subsequent lung function in COVID-19 patients discharged from hospitals,11,15, 16, 17, 18 For instance, a study conducted by Nicla Orzes, et al showed that leaving more than one-third of convalescent COVID-19 patients still had abnormal DLCO at six months.19 Despite this understanding of the long-term effects of COVID-19 on lung function, particularly on diffusion function, remains limited. At present, there's still a lack of description of the prevalence of impaired DLCO and comprehensive analysis of its risk factors in patients with COVID-19. Therefore, this systematic review and meta-analysis aims to provide a review of risk factors for impaired DLCO in recovered patients with COVID-19, and to conduct a meta-analysis of factors in homogeneous studies. It may provide preliminary evidence for estimating the prognosis of respiratory function in convalescent COVID-19 patients.
Methods
This systematic review was completed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement20 and was registered with PROSPERO prior to completion of the initial search (registration No: CRD42021265377).
Study strategy
We performed a systematic search of PubMed, Web of Science, Embase, and Ovid databases for relevant studies from inception until 7 January 2022, limited to papers involving human subjects. The following search terms were used: 'COVID-19′, 'SARS-CoV-2′, 'Respiratory Function Tests', 'Lung Function', 'Pulmonary Function', 'DLCO' and 'diffusing capacity for carbon monoxide'. In addition, we undertook manual searches according to cited references of retrieved articles. The full search strategy is available in the e-Appendix 1 in Supplement 1.
Study selection
Studies were eligible for the meta-analysis if they met the following criteria: (1) The population of the study was patients who have been diagnosed with COVID-19 by reverse transcription-polymerase chain reaction (RT-PCR) analysis or serological methods; (2) Patients who recovered from acute infection and were followed up for at least 12 weeks or three months after discharge from hospital; (3) Patients who were followed up were tested and assessed by lung function tests during the follow-up; (4) Studies had complete risk estimate data and clear outcomes; (5) Studies reported the estimates of odds ratio (OR), hazard ratio (HR) or relative risk (RR) with corresponding 95% confidence intervals (CIs); (6) Study design was a cohort study, case-control study, cross-sectional study or randomized controlled trial; (7) Study with a longer follow-up period was included when there were studies with the same outcomes in duplicated samples.
The following types of publications were excluded: (1) Patients in the studies were not infected with COVID-19. (2) Patients infected with COVID-19 died or had lost to follow-up; (3) Comments, case reports, conference papers, animal experiments, letters, and review articles; (4) Full text was not accessible; (5) Studies with the same outcomes in a duplicated samples; (6) Studies with the uncertain definition of impaired pulmonary diffusion function. (7) Literatures published in a non-English language.
Data extraction
Extracted data consisted of demographic characteristics (First author, publication year, country, sex, et.al), study design, diagnostic criteria for DLCO impairment, risk factors, risk estimate (univariate OR or multivariate OR), and a measure of the precision of the point estimate (95% CIs). When data were missing, the corresponding authors of the concerned articles were contacted. Data extraction was initially conducted by one author (ZMY) and subsequently reviewed by a second author (LYT). Disagreements were resolved by discussion between the two authors. If the risk factor data from the different stratifications (such as populations, period, etc.) was found in the same study, the relevant data was extracted separately according to the stratifications.
Quality and risk of bias assessment
We used the Newcastle-Ottawa scale (NOS) to assess the methodological quality of observational studies with its design.21 NOS score was categorized into three levels: low, moderate, and high quality with the NOS scores of 0-5, 6-7, and 8-9.22 Two reviewers (LYT; ZZF) independently assessed the quality of each study. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used for rating the quality of evidence.23 According to the GRADE approach, the quality of evidence for each outcome was graded for risk of bias, imprecision, inconsistency, indirectness, and publication bias, which can fell into one of four categories from high to very low. Any conflicts of the assessment were solved through discussion.
Statistical methods
The meta-analysis was conducted in accordance with the Cochrane handbook. The ORs and their 95% confidence intervals (95%CI) were calculated for each potential risk factor for impaired pulmonary diffusing function in convalescent COVID-19 patients. We extracted ORs from the multivariate analysis if both univariate and multivariate analyses were provided. Otherwise, the univariate ORs were chosen. The meta-analysis was conducted for outcomes reported in at least two studies. To pool study results, the random-effects model or fix-effect model were fitted in Stata 15.1 (Stata Corp, College Station, TX, USA).24 Based on the expectation about whether or not the studies share a common effect size and on our goals in performing the analysis, the random-effect model or fixed-effect model were chosen to pool study results. Heterogeneity was evaluated using Cochran's Q and I2 statistics, in which p-value of < 0.1 and I2 > 50% were defined as statistically significant heterogeneity. The subgroup analysis was used to explore the heterogeneity sources. The subgroup analysis was performed for follow-up time. The detection of potential publication bias was performed on the factors mentioned in more than 7 studies. The Egger's test and Begg's test were provided with quantitative evidence. Studies of high quality (NOS scores ≥ 8) were selected for sensitivity analysis.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Literature search
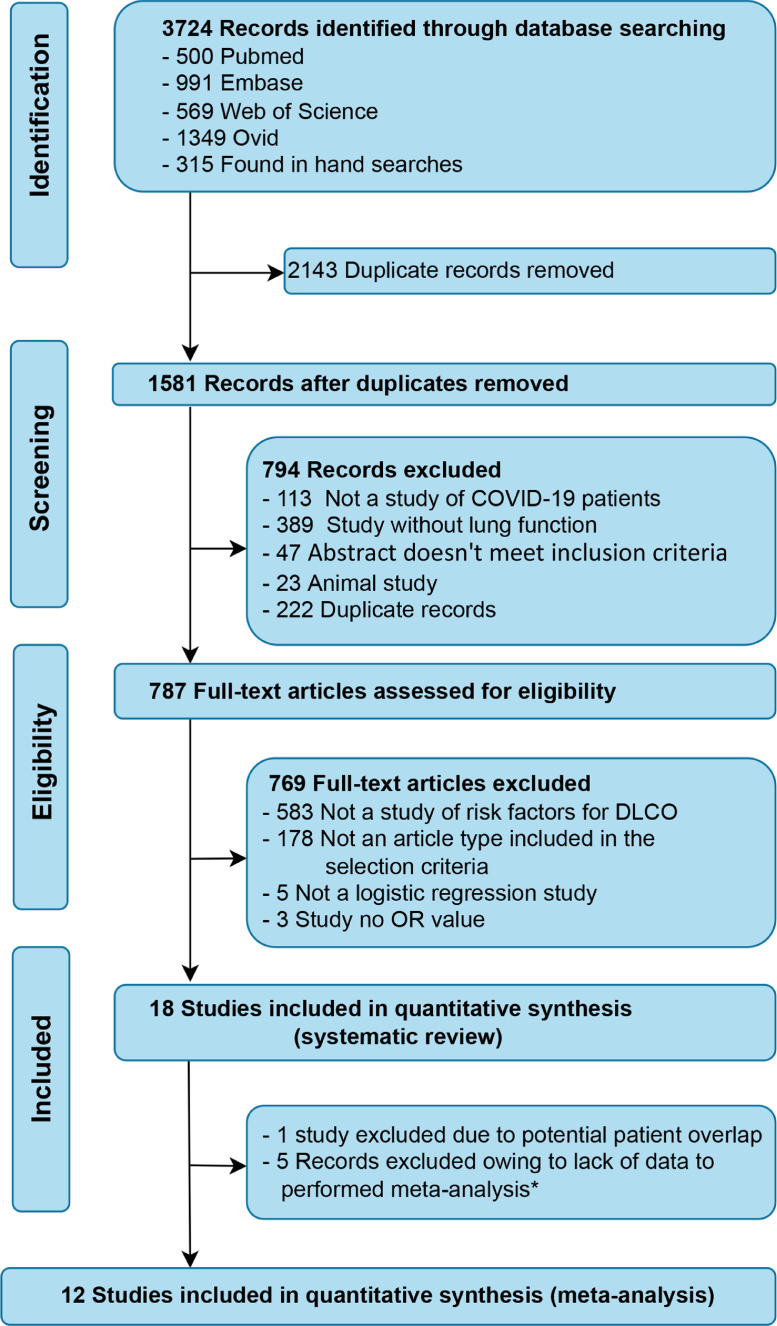
The initial search yielded a total of 3724 potential studies (3409 from selected databases and 315 from manual searches). In total, 2143 duplicate records were deleted. After the initial screening of titles and abstracts and exclusion of duplicates, a total of 787 articles were retained for full-text review. Ultimately, 18 studies met the criteria for eligibility and 12 studies were included in the meta-analysis. The study selection process is shown in Figure 1.
Figure 1.
Flow chart for study inclusion and exclusion.
*: The meta-analysis could not be performed because there was only one study in every outcome reported.
DLCO, diffusing capacity for carbon monoxide.
Study characteristics
All of the 18 included studies are observational studies, of which 16 are cohort studies,17,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 one is a prospective, cross-sectional study40 and one is a cross-sectional case-control study.41 The included studies were published between 2020 and 2021 and were conducted in seven countries including China, Germany, Italy, Spain, Chile, Norway and Saudi Arabia. A total of 4112 patients were included, including 2127 males and 1985 females, of whom 2414 (58.7%) patients completed the carbon monoxide diffusing test. The mean/median age of the subjects of the 11 studies25,26,28, 29, 30, 31, 32,34,35,37,38 on DLCO was over 50 years.
17 papers17,25,26,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 reported risk factors associated with DLCO impairment and 12 studies were included in the meta-analysis.17,25,26,28,34, 35, 36, 37, 38, 39, 40, 41 One mentioned risk factor for diffusion capacity for nitric oxide (DLNO) impairment.27 Due to insufficient data and uncertain definitions of DLNO and Restrictive Pattern, this review focuses on a meta-analysis of risk factors for impaired DLCO.
In most studies, the diagnostic criterion for the impairment of DLCO was DLCO < 80% of predicted.17,25,26,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 Except for the study performed by Ayad Mohammed Salem,41 the diagnostic criterion was DLCO < 75% of predicted. There were 100% follow-up rates in 14 studies,17,26, 27, 28, 29, 30,33, 34, 35, 36, 37, 38,40,41 90% in two studies31,32, 51% in one study25 and 87.3% in one study39 Seventeen studies17,25,26,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 have reported 14.3% to 67% COVID-19 survivors with impaired diffusing capacity for carbon monoxide. Table 1 shows the characteristics of the included studies in detail.
Table 1.
Study characteristics of convalescent COVID-19 patients with pulmonary diffusion function in the included studies.
| Study | Country | Study design | Total | Sex (M/F) | Age, Mean (±SD) /Median (IQR) | Follow-up rate (%) | Follow-up Duration | Diagnostic criteria | DLCO impairment proportion (%) | NOS | Risk factors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Steinbeis F et al (2021) | Germany | prospective observational study | 180 | 112/68 | 56.50(43.25-65.75) | 100.0 | 12 months | DLCO <80%, of predicted or <LLN | 60.6 | 9 | Age; Altered CT; Others |
| Blanco JR et al (2021) | Spain | prospective cohort study | 100 | 64 /36 | 54.98±10.72 | 92.6 | 104 days (IQR 89.25, 126.75) | DLCO <80%, of predicted | 52.0 | 9 | Others |
| Huang LX et al (2021) | China | ambidirectional cohort study | 1276 | 681/595 | 59 (49-67) | 51.7 | 12 months | DLCO <80%, of predicted | 34.5 | 8 | Female; Age; Others |
| Bellan M et al (2021) | Italy | prospective cohort study | 238 | 142/96 | 61 (50-71) | 92.0 | 3 to 4 months | DLCO <80%, of predicted | 67.1 | 8 | Female;Age; Others1a |
| Wu XJ et al (2021) | China | prospective cohort study | 83 | 47/36 | 60 (52-66) | 100.0 | 12-month | DLCO <80%, of predicted | 33.0 | 8 | Female;Age; Others |
| Qin W et al (2021) | China | prospective cohort study | 647 | 287/360 | 58±15 | 100.0 | 3 months | DLCO <80%, of predicted | 54.3 | 8 | Age; Altered CT; D-dimer; Others |
| Salem AM et al (2021) | Saudi Arabia | cross-sectional case-control study | 50 | 34/16 | post covid-19: 47.05 ± 11.57 control: 41.93 ± 11.27 |
100.0 | 166.52 days (102–283 days) | Restrictive Pattern a DLCO <75% |
35.0 | 8 | Female; Age; D-dimer; Others |
| Liao TT et al (2021) | China |
prospective cohort study |
303 | 59/244 | 39 (33-48) | 100.0 | 12 months | DLCO <80%, of predicted |
43.5 |
7 | Female; Age; Others |
| Bellan M et al (2021) | Italy | prospective cohort study | 200 | 122/78 | 62 (51-71) | 100.0 | 12 months | DLCO <80%, of predicted |
49.0 |
7 | Female; Age; Altered CT; Others1b |
| Labarca G et al (2021) | Chile | prospective,cross-sectional study | 60 | 32/28 | mild 39.2 (±14.3) moderate 47.4 (±11) severe 50.0 (±10.3) |
100.0 | 4 months | DLCO <80%, of predicted | 25.0 | 7 | Age; Others2a |
| Labarca G et al (2021) | Chile | prospective cohort study | 60 | 32/28 | Control:40.4 (±23.6) ARDS:51 (±11.6) |
100.0 | 3-6months | DLCO <80%, of predicted | 25.0 | 7 | Others2b |
| Zhang SD et al (2021) | China | retrospective cohort study | 40 | 19/21 | 57 (40-68) | 100.0 | 249 ± 15 days | DLCO <80%, of predicted | 32.5 | 7 | Others |
| Safont B et al(2021) | Spain | prospective cohort study | 313 | 184/129 | 61.12 ± 12.26 | 100.0 | 6 months | DLCO <80%, of predicted | 54.7(2 months) 47(6 months) |
7 | Female; Age; Others |
| Zhao YM et al (2021) | China | prospective cohort study | 94 | 54/40 | 61(50-71) | 100.0 | 12 months | DLCO <80%, of predicted |
14.3 |
7 | Urea nitrogen; Others |
| Marta NF et al (2021) | Spain | prospective cohort study | 200 | 119/81 | 62 (50-71) | 100.0 | 3 months | reduced DLNO | 58.0 | 7 | Others |
| Zhao YM et al (2020) | China | retrospective cohort study | 55 | 32/23 | 47.74±15.49 | 100.0 | 3 months | DLCO <80%, of predicted | 16.4 | 8 | Urea nitrogen; D-dimer |
| Lerum TV et al (2020) | Norway | prospective cohort study | 103 | 54/49 | 59 (49-72) | 100.0 | 3 months | DLCO <80%, of predicted | Not mentioned | 7 | Others |
| Chen M et al (2021) |
China | prospective cohort study | 110 | 53/57 | 45.0 (33.8-56.3) | 87.3 | 6 months | DLCO <80%, of predicted | 32.6% | 7 | Female; Age; Altered CT; Others |
1. Data are Mean±SD/median (IQR), or N (%). M/F: Ratio of males to females.
2.Others: Severe pneumonia; Chronic respiratory disease; Tumor; Length of hospital stay; Chronic obstructive pulmonary disease (COPD); lower serum lactate dehydrogenase (LDH) levels; etc.
3.a Restrictive Pattern:FEV1/FVC%≥70%of predicted with TLC < 80% or FVC < 80%of predicted.
4.1a:COPD, Atrial fibrillation, etc; 1b: CAD, CIRS, etc; 2a: ARDS, steroids, etc; 2b: HFNC.
5. Abbreviations: ARDS, acute respiratory distress syndrome; DLCO, diffusing capacity of the lung for carbon monoxide; LLN, lower limit of normal; DLNO, diffusion capacity of nitric oxide; CT, computerized tomography; ICU, intensive care unit; BMI, body mass index; WBCs, white blood cells; NOS, Newcastle-Ottawa scale; CAD,coronary artery disease; CIRS, cumulative illness rating scale; HFNC, high-flow nasal canula.
Study quality
All selected studies were assessed for methodological quality with NOS. The NOS score of 12 studies included in the meta-analysis was 7.6 (IQR: 7.0–9.0), indicating that all included studies were of high (50%)17,25,26,28,34,41 or moderate (50%)35, 36, 37, 38, 39, 40 quality literature. The quality of evidence (GRADE) in the analyzed studies was “low” or “very low”. These results are shown in Table 1 and e-Table 1-2.
Risk factors for impaired DLCO
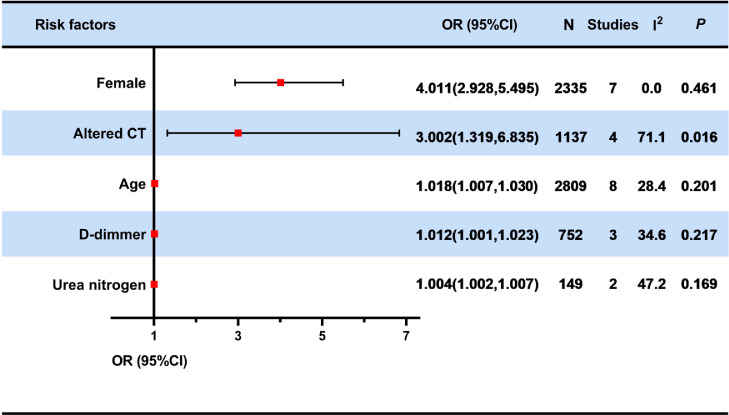
The meta-analysis was performed on five risk factors, including sex (female), altered chest CT, older age, high levels of D-dimer and urea nitrogen. ORs with 95% CIs are summarized in Figure 2.
Figure 2.
Forest plot of meta-analyses on the potential risk factors for impaired diffusing capacity of the lung for carbon monoxide.
Red squares and their corresponding lines are the point estimates and 95% CIs. No statistical difference was observed with the red squares and their corresponding line crossing the line of effect. The red squares and their corresponding line in the risk factors of female, altered chest computerized tomography (CT), age, higher D-dimer levels and urea nitrogen were not crossing the line of effect. Therefore, the above factors were identified as risk factors for impaired DLCO. Heterogeneity was evaluated using Cochran's Q and I2 statistics, in which P-values of < 0.1 and I2 > 50% were defined as statistically significant heterogeneity.
CI, confidence intervals; N, sample size; OR, Odds ratio; CT, computerized tomography.
Sex (female)
A total of seven studies25,26,35, 36, 37,39,41 have shown that being female is associated with a higher risk for impaired DLCO. Female recovered patients showed a 4-fold greater risk of impaired DLCO than male recovered patients (OR: 4.011; 95% CI: 2.928–5.495). Four researches25,26,35,36 performed pulmonary diffusion function tests one year after the patient's discharge and the remaining studies37,39,41 performed pulmonary diffusion function tests between three and six months. All of the studies25,26,35, 36, 37,39,41 conducted multivariate logistic regression analysis. Heterogeneity was considered insignificant (I2=0.0%, p=0.461). Subgroup analysis was carried out to explore the source of heterogeneity and the risk estimate in each subgroup. For female, the subgroup analysis based on follow-up time showed that the OR was 3.473 (95% CI: 2.193 - 5.502, I2=0.0%, p = 0.521) at 3-6 months after discharge and 4.553 (95% CI: 2.958 - 7.010, I2=17.9%, p = 0.301) at 1 year after discharge. (e-Figure 1-2)
Altered CT
Four articles28,34,35,39 described the relation between altered chest CT [altered chest CT is represented by the abnormal score of the TSS (total severity score)] and the impairment of DLCO (OR: 3.002; 95% CI: 1.319–6.835). Two studies34,35 performed pulmonary diffusion function tests one year after the patient's discharge and two study28,39 performed pulmonary diffusion function tests after three months. All studies were subjected to multiple logistic regression analyses. The heterogeneity was significant (I2=71.1%, p = 0.016). (e-Figure 3)
Age
A total of ten literature data reported the association between older age and impaired DLCO.25,26,28,34, 35, 36, 37,39, 40, 41 One study was excluded because it was discussed in two age groups (age < 60 and age≥60).39 Finally, eight articles were included for meta-analysis after excluding one study36 by performing sensitivity analysis (e-Figures 4-5). Four studies28,34,40,41 performed pulmonary diffusion function tests between three and six months after the patient's discharge, and the remaining four studies25,26,35,37 performed pulmonary function tests one year after discharge. All of eight articles25,26,28,34,35,37,40,41 were all multivariate logistic regression analyses. Our results show that age is a risk factor for impaired DLCO (OR: 1.018; 95% CI: 1.007–1.030). Heterogeneity was considered insignificant (I2=28.4%, p = 0.201). The subgroup analysis was carried out to explore the source of heterogeneity and the risk estimate in each subgroup. For age, the subgroup analysis by the follow-up time showed that there was no significant difference in ORs between 3-6 months after discharge and 1 year after discharge (OR:1.022, 95% CI: 1.005 - 1.039 VS OR: 1.015, 95% CI: 0.999 - 1.031). (e-Figures 6-7)
D-dimer
Three studies17,28,41 have pointed out the association between D-dimer and DLCO impairment. All COVID-19 survivors were examined for pulmonary diffusion function between three to six months after discharge from the hospital. Data from multivariate logistic regression analyses are provided by studies performed by Salem et al. and Zhao et al.17,41 Qin et al. reported the results of univariate logistic regression.28 The results showed that as the patient's D-dimer increased during the follow-up period, the DLCO was more likely to be impaired (OR: 1.012; 95% CI:1.001–1.023). Heterogeneity was considered insignificant (I2=34.6%, p = 0.217). (e-Figure 8)
Urea nitrogen
Two studies reported the relationship between high levels of Urea nitrogen and impaired DLCO.17,38 Convalescent COVID-19 patients with higher level of urea nitrogen are more likely to present damaged DLCO (OR: 1.004; 95% CI:1.002–1.007, I2= 47.2%, p = 0.169). One study38 performed pulmonary diffusion function tests at one year after discharge and the remaining one17 performed pulmonary diffusion function tests after three months. All of data were extracted from multivariate logistic regression analyses. (e-Figure 9).
Bias and sensitivity analyses
As mentioned in the previous statistical analysis, potential publication bias was assessed on the factors mentioned in more than 7 studies. Therefore, we performed the tests for bias for the age risk factor. And it was showed that both Begg's test and Egger's test showed a low likelihood of bias (p = 0.386; p = 0.338). (e-Figures 10-12) Sensitivity analyses were performed for those studies with high NOS. The sensitivity analyses showed that our results were generally robust, regardless of small differences between the high NOS and overall results. (e-Table 3)
Discussion
Eighteen studies that investigated risk factors for impaired pulmonary diffusion function in recovered COVID-19 patients were identified in our systematic review. Our results showed that 1) the percentage of abnormal DLCO among recovered COVID-19 patients who underwent follow-up ranged from 14% to 67%; 2) Female, altered chest CT, older age, higher levels of D-dimer, and urea nitrogen were identified as risk factors for impaired DLCO in these patients. This is the first meta-analysis to summarize the potential risk factors for impaired DLCO in convalescent COVID-19 patients. And it may provide some suggestions for COVID-19 patients' individual treatment during hospitalization and after discharge. Half of the reports included in this review were of high quality according to the NOS score. And the results of the studies were considered objective and reliable. The studies included in this review were prospective and had relatively uniform diagnostic criteria, which allowed for better control of bias and confounding factors arising in the review.
The risk of impaired DLCO in female recovered COVID-19 patients is about four times higher than male recovered COVID-19 patients after discharge. It is suggested that the damage of alveolar capillaries and microvasculature may be more severe in female COVID-19 survivors than in males. Firstly, there are sex differences in the immune response of patients infected with COVID-19. During SARS-CoV-2 infection, male patients had higher plasma levels of innate immune cytokines and stronger induction of non-classical monocytes, while T cell activation in female patients was stronger than that in male patients.42 The existence of different immune response mechanisms between men and women may lead to more serious damage to alveolar capillaries and micro-vessels in women, which in turn affects the ability of pulmonary diffusion. Secondly, greater expression of ACE2 in women leads to a worse prognosis for SARS-CoV-2.43 This may be related to why women are more likely to develop DLCO damage during follow-up. However, the specific mechanisms are not clear, and more researches are needed to confirm them further.
Our meta-analysis found that the higher levels of D-dimer during follow-up were also one risk factor for impaired DLCO in COVID-19 survivors. Measurement of D-dimer levels on admission may help predict the impairment of pulmonary diffusion function.17 As previous studies reported, patients with elevated D-dimer can lead to pulmonary vascular damage, thrombosis, and microangiopathy, which may affect pulmonary diffusion capacity.44,45
D-dimer has now been identified as a prognostic indicator of disease severity and poor outcome.46 It has been revealed that COVID-19 patients with a high risk of venous thromboembolism had poorer outcomes than patients with a low risk.47 A retrospective study found that anticoagulant therapy appears to be associated with a better prognosis in severe COVID-19 patients with markedly elevated D-dimer.48 Therefore, dynamically detecting the D-dimer level may be a potential way for estimating the recovery of diffusion capacity in COVID-19 survivors. The effect of early thromboprophylaxis on improving the prognosis of COVID-19 patients may deserve further studies.
Four studies28,34,35,39 showed that CT alteration was a risk factor for DLCO damage. Chest CT total severity score was related to the severity of inflammation in the lung.49 The higher the score, the more severe the lung damage.50 There was considerable evidence showing that the percentage of DLCO prediction was negatively correlated to persistent CT abnormalities.51, 52, 53 Abnormal TSS was significantly associated with impaired DLCO, indicating that the severity of lung inflammation may be one aspect of impaired DLCO. DLCO mainly correlates to two factors, including the rate constant for carbon monoxide uptake from alveolar gas (KCO) and the accessible alveolar volume (VA). In the early stage of the disease, one study using both lung ultrasound and chest CT has identified signs of interstitial-alveolar damage in patients with mild to severe COVID-19 pneumonia.54 Alveolar-capillary damage, microvascular pathology or anemia can lead to the decline of KCO. Decreased VA occurs in reduced alveolar expansion, alveolar damage or loss, or maldistribution of inspired gases with airflow obstruction.55 These suggested that the impaired lung diffusion capacity might correlate with severe pulmonary pathological changes, such as capillary component damage and reduced accessible alveolar volume. But more investigation is still needed to explore the comprehensive pathological changes. Observing dynamic changes of lung function should be considered for COVID-19 survivors with abnormal chest CT.
Our results show that age is also a risk factor for impaired DLCO. A growing number of studies have shown that elderly patients are more likely to develop DLCO impairment. A recent study found that older age was linearly associated with poorer predictive values of DLCO at follow-up in COVID-19 patients.56 Lewis et al. also showed that older age was independently associated with a decrease in DLCO following SARS-CoV-2 infection.57 In an editorial on the pathogenesis of COVID-19, Prof. Mason commented that immune response disorders in elderly patients may make it easier for the virus to spread to the gas exchange units in the lungs.58 The specific mechanism of elderly patients being more likely to have DLCO damage is uncertain. Some studies proposed that it may be related to the higher expression of Angiotensin-Converting Enzyme 2 (ACE2) in elderly patients. SARS-CoV-2 interacts with ACE2 receptors and thereby leads to COVID-19.59,60 Previous studies have reported that elevated levels of ACE2 expression were associated with older age.61 Elderly patients with previous Renin-Angiotensin System Inhibitors (RASI) use were less likely to have abnormal DLCO at follow-up period compared to non-RASI users.56 In addition, the decrease of DLCO in elderly patients may be related to the severity of the disease.62,63 A recent study found that requiring invasive mechanical ventilation (IMV) or developing ARDS were associated with poorer pulmonary diffusion function in COVID-19 patients.64 DLCO damage is more common in critically COVID-19 patients.11,31 And the older COVID-19 patients may tend to be more serious.62,63 Additionally, patients with COVID-19, especially the elderly, have various degrees of respiratory, physical, and psychological impairment. Therefore, for this group, in addition to active treatment to improve their condition during hospitalization, respiratory rehabilitation during convalescence for improving respiratory function and the quality of life may be worthy of further studies.65
Several limitations should be noted in our systematic review. Firstly, as we only included the English literatures for analysis, the scope of our search was not necessarily comprehensive. Secondly, there are few studies addressing risk factors for impaired DLCO during recovery in COVID-19 patients. The amount of literatures included and data available for analysis were limited. Therefore the subgroup analysis on certain confounders (such as follow-up time, disease severity, virus variants, etc) could not be performed currently. Thirdly, there are two literatures about urea nitrogen were from the same author,17,39 and the potential overlap of the data may not be completely ruled out. Finally, there may be additional features that contribute to impaired DLCO, and we are unable to report on them due to the limited amount of studies. We only analyzed and discussed risk factors that have been mentioned in at least two papers and above or with relatively significant ORs. Despite some limitations, this study was designed strictly in accordance with the PRISMA list and was registered in advance. To our knowledge, this study is the first to explore the risk factors for impaired DLCO in convalescent COVID-19 patients. It may provide preliminary results for estimating the recovery of respiratory function in convalescent COVID-19 patients.
Pulmonary diffusion capacity was the most common impaired lung function in recovered patients with COVID-19. Several risk factors, such as female, altered chest CT, older age, higher D-dimer levels and urea nitrogen are associated with impairment of DLCO. Raising awareness and implementing interventions for possible modifiable risk factors maybe valuable for pulmonary rehabilitation.
Contributions
Jing Li, Nanshan Zhong, Mei Jiang and Ruchong Chen designed the study. Haopeng Zhi, Xiaolong Ji, Zifan Zhao, Hanwen Liang and Shuxin Zhong conducted the literature search and searched the articles. Yiting Luo, Mingyu Zhong and Chen Zhan contributed to the data extraction process. Haopeng Zhi, Xiaolong Ji, Zifan Zhao, Hanwen Liang and Shuxin Zhong, Yi Gao, Xilong Deng and Shiyue Li analyzed the data and interpretation of data. Haopeng Zhi, Xiaolong Ji, Zifan Zhao, Hanwen Liang and Shuxin Zhong drafted the manuscript. All the authors revised the article and approved the final version.
Declaration of interests
All authors declare that they have no conflict of interest.
Acknowledgments
Funding
This work was financially supported by Emergency Key Program of Guangzhou Laboratory (EKPG21-29, EKPG21-31), Incubation Program of National Science Foundation for Distinguished Young Scholars by Guangzhou Medical University (GMU2020-207).
Data sharing statement
Review protocol has been available via PROSPERO website. All extracted and calculated data are available upon appropriate requests by emailing to co-corresponding authors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101473.
Contributor Information
Jing Li, Email: lijing@gird.cn.
Nanshan Zhong, Email: nanshan@vip.163.com.
Mei Jiang, Email: jiangmei927@163.com.
Ruchong Chen, Email: chen_rch@163.com.
Appendix. Supplementary materials
References
- 1.https://covid19.who.int/. Accessed 9 January 2022.
- 2.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. Jama. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Cevik M, Kuppalli K, Kindrachuk J, et al. Virology, transmission, and pathogenesis of SARS-CoV-2. Bmj. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 4.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latronico N, Peli E, Calza S, et al. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax. 2022;77(3):300–303. doi: 10.1136/thoraxjnl-2021-218064. [DOI] [PubMed] [Google Scholar]
- 7.Ngai JC, Ko FW, Ng SS, et al. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie L, Liu Y, Fan B, et al. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. 2005;6(1):5. doi: 10.1186/1465-9921-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie L, Liu Y, Xiao Y, et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127(6):2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27(4):328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekbom E, Frithiof R, Öi Emilsson, et al. Impaired diffusing capacity for carbon monoxide is common in critically ill Covid-19 patients at four months post-discharge. Respir Med. 2021;182 doi: 10.1016/j.rmed.2021.106394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schelling G, Stoll C, Vogelmeier C, et al. Pulmonary function and health-related quality of life in a sample of long-term survivors of the acute respiratory distress syndrome. Intensive Care Med. 2000;26(9):1304–1311. doi: 10.1007/s001340051342. [DOI] [PubMed] [Google Scholar]
- 15.Frija-Masson J, Debray MP, Gilbert M, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56(2) doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orzes N, Pini L, Levi G, et al. A prospective evaluation of lung function at three and six months in patients with previous SARS-COV-2 pneumonia. Respir Med. 2021;186 doi: 10.1016/j.rmed.2021.106541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Wells G, Shea B, O'connell D, et al. 2008. The Newcastle-Ottawa Scale (NOS) Forassessing the Quality of Nonrandomised Studies in Meta-Analyses. http. ohri. ca/programs/clinical_epidemiology/oxfordasp (accessed Jun 2014) [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Núñez-Fernández M, Ramos-Hernández C, García-Río F, et al. Alterations in respiratory function test three months after hospitalisation for COVID-19 Pneumonia: value of determining nitric oxide diffusion. J Clin Med. 2021;10(10):2119. doi: 10.3390/jcm10102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin W, Chen S, Zhang Y, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58(1) doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4) doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Bai W, Yue J, et al. Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep. 2021;11(1):13854. doi: 10.1038/s41598-021-93191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco JR, Cobos-Ceballos MJ, Navarro F, et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect. 2021;27(6):892–896. doi: 10.1016/j.cmi.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labarca G, Henriquez-Beltran M, Llerena F, et al. Undiagnosed sleep disorder breathing as a risk factor for critical COVID-19 and pulmonary consequences at the midterm follow-up. Sleep Med. 2022;91:196–204. doi: 10.1016/j.sleep.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbeis F, Thibeault C, Doellinger F, et al. Severity of respiratory failure and computed chest tomography in acute COVID-19 correlates with pulmonary function and respiratory symptoms after infection with SARS-CoV-2: An observational longitudinal study over 12 months. Respir Med. 2022;191 doi: 10.1016/j.rmed.2021.106709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellan M, Baricich A, Patrucco F, et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021;11(1):22666. doi: 10.1038/s41598-021-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao T, Meng D, Xiong L, et al. Long-term effects of COVID-19 on health care workers 1-year post-discharge in Wuhan. Infect Dis Ther. 2022;11(1):145–163. doi: 10.1007/s40121-021-00553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safont B, Tarraso J, Rodriguez-Borja E, et al. Lung function, radiological findings and biomarkers of fibrogenesis in a cohort of COVID-19 patients six months after hospital discharge. Arch Bronconeumol. 2022;58(2):142–149. doi: 10.1016/j.arbres.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Yang C, An X, et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis. 2021;112:173–182. doi: 10.1016/j.ijid.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, Liu J, Peng P, et al. Dynamic changes of pulmonary diffusion capacity in survivors of non-critical COVID-19 during the first six months. EClinicalMedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labarca G, Henríquez-Beltrán M, Lastra J, et al. Analysis of clinical symptoms, radiological changes and pulmonary function data 4 months after COVID-19. Clin Respir J. 2021;15(9):992–1002. doi: 10.1111/crj.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem AM, Al Khathlan N, Alharbi AF, et al. The long-term impact of COVID-19 pneumonia on the pulmonary function of survivors. Int J Gen Med. 2021;14:3271–3280. doi: 10.2147/IJGM.S319436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gemmati D, Bramanti B, Serino ML, et al. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int J Mol Sci. 2020;21(10):3474. doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colombo C, Garatti L, Ferrante G, et al. Cardiovascular injuries and SARS-COV-2 infection: focus on elderly people. J Geriatr Cardiol. 2021;18(7):534–548. doi: 10.11909/j.issn.1671-5411.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung M, Bernheim A, Mei X, et al. CT Imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santus P, Flor N, Saad M, et al. Trends over time of lung function and radiological abnormalities in COVID-19 Pneumonia: a prospective, observational, cohort study. J Clin Med. 2021;10(5):1021. doi: 10.3390/jcm10051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76(4):402–404. doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- 53.Barisione G, Brusasco V. Lung diffusing capacity for nitric oxide and carbon monoxide following mild-to-severe COVID-19. Physiol Rep. 2021;9(4):e14748. doi: 10.14814/phy2.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fratianni G, Malfatto G, Perger E, et al. Lung ultrasound in patients with SARS-COV-2 pneumonia: correlations with chest computed tomography, respiratory impairment, and inflammatory cascade. J Ultrasound Med. 2021 doi: 10.1002/jum.15831. [published online ahead of print, 2021 Sep 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes JM, Pride NB. Examination of the carbon monoxide diffusing capacity (DL(CO)) in relation to its KCO and VA components. Am J Respir Crit Care Med. 2012;186(2):132–139. doi: 10.1164/rccm.201112-2160CI. [DOI] [PubMed] [Google Scholar]
- 56.Gori M, Ghirardi A, D'Elia E, et al. Association between inhibitors of the renin-angiotensin system and lung function in elderly patients recovered from severe COVID-19. Eur J Prev Cardiol. 2021:zwab143. doi: 10.1093/eurjpc/zwab143. [published online ahead of print, 2021 Sep 18] [DOI] [PubMed] [Google Scholar]
- 57.Lewis KL, Helgeson SA, Tatari MM, et al. COVID-19 and the effects on pulmonary function following infection: a retrospective analysis. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4) doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallentin L, Lindbäck J, Eriksson N, et al. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur Heart J. 2020;41(41):4037–4046. doi: 10.1093/eurheartj/ehaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu K, Chen Y, Lin R, et al. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80(6):e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anastasio F, Barbuto S, Scarnecchia E, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;58(3) doi: 10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu K, Zhang W, Yang Y, et al. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39 doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.