Abstract
Objective
The present study explored whether low-intensity pulsed ultrasound (LIPUS) enhances the therapeutic efficacy of mesenchymal stem cells (MSCs) in osteoarthritis (OA) cartilage repair by regulating autophagy-mediated exosome release.
Design
MSCs were isolated from the rat bone marrow and treated with rapamycin, 3-methyladenine, or LIPUS. The mechanism of the LIPUS-stimulated exosome release by MSCs was analyzed by inhibiting autophagy. In addition, the MSCs were co-cultured with OA chondrocytes and stimulated by LIPUS, with or without exosome release inhibitor intervention. The exosome release was detected through transmission electron microscopy (TEM), nanoparticle tracking analysis, and biomarker expression analysis. Autophagy was analyzed through TEM, autophagy-related gene expression analysis, and immunofluorescence analysis in vitro. Furthermore, a rat knee OA model was constructed and treated with MSCs, GW4869, and LIPUS. The cartilage repair was assessed through histopathological analysis and extracellular matrix protein expression analysis.
Results
The in vitro results indicated that LIPUS promoted MSC exosome release by activating autophagy. The in vivo results demonstrated that LIPUS significantly enhanced the positive effects of MSCs on OA cartilage. These effects were significantly blocked by GW4869, an inhibitor of exosome release.
Conclusions
LIPUS can enhance the therapeutic efficacy of MSCs in OA cartilage repair, and the underlying mechanism is related to the increase in autophagy-mediated exosome release.
Keywords: low-intensity pulsed ultrasound, mesenchymal stem cell, osteoarthritis, autophagy, exosome
Introduction
Osteoarthritis (OA) is one of the most common clinical chronic joint diseases, and its main symptoms are joint pain, stiffness, and dysfunction. OA was listed as one of the three major threats to human health, in addition to cardiovascular disease and cancer, by the World Health Organization in 1999. In 2020, OA was reported to be the fourth leading cause of disability worldwide. 1 With the combined effects of aging, obesity, and increasing incidences of joint injury, OA is becoming increasingly common and has affected approximately 250 million people worldwide. 2
The treatment of OA has always been a challenge for clinicians, and an ideal treatment for OA is lacking to date. Drugs and physiotherapy commonly used in clinical practice are the symptomatic treatments for stiffness, swelling, and pain, whereas joint replacement is the only option to improve the quality of life of patients with late-stage OA.3,4 Mesenchymal stem cell (MSC) therapy is the focus in the field of cell transplantation and has a potential clinical application value in OA. MSCs are isolated and expanded from the bone marrow, umbilical cord blood, fat, skin, tendons, muscles, and dental pulp, and these cells have the potential for multilineage differentiation. 5 Studies have demonstrated that MSCs can efficiently promote OA cartilage repair.6,7
The mechanism of OA cartilage repair by MSCs is poorly understood. Some studies have suggested that the main medium for MSCs to play a role in cartilage repair may be exosomes. 8 Exosomes are subcellular bilayer membranous vesicles, with a diameter of 40 to 100 nm, that are secreted by cells and contain various active substances such as mRNA, miRNA, DNA, and protein. Exosomes can specifically act on target cells, regulate the microenvironment and inflammation, and promote the regeneration of injured tissue. 9 Studies have found that exosomes derived from MSCs can increase the expression of extracellular matrix protein (including type II collagen [COL2] and aggrecan [AGG]) and promote cartilage regeneration in rats.10,11
Increasing number of studies have shown that autophagy can regulate the biogenesis and release of exosomes by cells. 12 Autophagy, also known as cell self-digestion, is a highly conserved life phenomenon in eukaryotes. A study reported that the autophagy activator rapamycin can promote exosome release, suggesting that autophagy may play a key role in the regulation of exosome release. 13 Our previous studies have demonstrated that autophagy is closely related to MSC homing and chondrogenic differentiation; however, the role of autophagy in MSC exosome release remains unclear.14,15
Although MSCs have been proved to be suitable for OA cartilage repair, the biological effects of MSCs and the release of MSC exosomes are affected by the local microenvironment, which limits the therapeutic effect of MSCs. Low-intensity pulsed ultrasound (LIPUS) is a physiotherapeutic factor and can lead to biochemical changes in MSCs. In our previous study, we found that LIPUS could improve the chondrogenic differentiation of MSCs by regulating autophagy. 15 In addition, in animal experiments, we found that LIPUS could improve the cartilage repair effect of MSCs in OA. 14
In the present study, we explored the role of autophagy in MSC exosome release and investigated the direct influence of LIPUS on MSC exosome release regulated by autophagy. In addition, we demonstrated that the cartilage repair effects of MSCs on OA are augmented by LIPUS through the exosome release pathway.
Materials and Methods
The experimental protocol related to rats was in accordance with the US National Institutes of Health’s Guidelines of Laboratory Animal Use and approved by the Ethics Committee of Nanjing First Hospital.
Isolation and Culture of MSCs
Bone marrow–derived MSCs (BMSCs) were obtained from the bone marrow of 49 eight-week-old male Sprague-Dawley (SD) rats (45 rats in vitro experiment and 4 rats in vivo experiment), which were acquired from the Qinglongshan Experimental Animal Center of Jiangsu Province, China. The MSCs were identified based on their surface phenotypes and multipotency, as described previously.14,15 After the SD rats were euthanized, the bone marrow from the femur was washed out in a Petri dish with low-glucose Dulbecco’s modified Eagle’s medium (DMEM; KeyGEN, Nanjing, Jiangsu, China), containing 10% fetal bovine serum (FBS; KeyGEN), without exosomes. Then, the cells were cultured in culture dishes with 5% CO2/95% air at 37°C. After 72 hours, the medium was changed, and the cells were resuspended with 0.25% trypsin until the cells reached 80% to 90% confluence. Thereafter, the cells were re-seeded at the density of approximately 2×106 cells per dish. The follow-up experiment was performed using passage 3.
Activation and Inhibition of Autophagy
To determine the role of autophagy in MSC exosome release, the cells were treated with the autophagy inhibitor 3-methyladenine (3-MA; Selleck, Houston, TX, USA) (10 μM) or the agonist rapamycin (Selleck) (10 μM) for 24 h. The cells of the control group were treated only with the solvent dimethylsulfoxide (DMSO).
Analysis of Autophagy in MSCs
Transmission electron microscopy (TEM) (JEM-1011, JEOL, Akishima, Tokyo, Japan) was used to observe the autophagosomes of MSCs, as described previously. 14 The morphological characteristics of autophagosomes include a crescent or cup-shaped bilayer or multilayer membrane, with a tendency to enclose cytoplasmic components. Immunofluorescence analysis was performed to detect a specific autophagy marker LC3, as described previously. 14 Briefly, the cells were washed with phosphate-buffered saline (PBS) (KeyGEN) and fixed with 4% paraformaldehyde. Then, the cells were again washed with PBS and incubated with 0.5% Triton X-100 (KeyGEN). Subsequently, the cells were incubated with a blocking solution (10% goat serum in PBS) and then treated with the anti-LC3B antibody (Novus Biological, Littleton, CO, USA) overnight at 4 °C. Finally, the cells were incubated with secondary antibodies after washing with the blocking solution. The MSCs were stained with diamidine phenylindole (DAPI) (Molecular Probes, Waltham, MA, USA) and observed under a confocal microscope (Dmi 6000-B, Leica, Brunswick, Germany). Western blot analysis was used to detect the autophagy makers Beclin1 and LC3, as described previously. 14
Isolation and Analysis of Exosomes
The culture medium of MSCs was collected and centrifuged in a 50-mL centrifuge tube at 4 °C for 10 minutes, and the dead cell precipitate was removed. The supernatant was collected and placed in another 50-mL centrifugation tube. The cell debris and macromolecular proteins were further removed through centrifugation at 4 °C and 2,000 ×g for 20 minutes. The supernatant was collected again in a 50-mL centrifuge tube and then centrifuged at 4 °C and 10,000 ×g for 30 minutes; the supernatant was collected again and passed through the 0.22-μm cell filter. The supernatant was collected in an ultracentrifugation tube with a diameter greater than 0.22 μm and then subjected to ultracentrifugation for 70 minutes at 4 °C and 12,000 ×g. The supernatant was discarded, and the precipitate was resuspended with PBS and centrifuged again at 4 °C and 12,000 ×g for 70 minutes. Finally, the exosome precipitate was obtained, resuspended with 50 μL PBS, and stored at −80 °C. The exosome morphology was observed through TEM, and then, the exosomes were quantified through nanoparticle tracking analysis (NTA) (NanoSight NS300, Malvern Instruments Ltd, UK). Proteins were extracted from MSCs and exosomes by using a total protein extraction kit (KeyGEN), and the exosome surface marker proteins, namely CD63, ALIX, and TSG101, were analyzed through western blotting.
OA Chondrocyte Isolation and Culture
The OA chondrocytes were isolated from the OA rat model. The rat OA model was generated through anterior cruciate ligament transection (ACLT), as described previously. 16 The cartilage was obtained from the femoral condyle of 15 OA rats right knee joint and placed in a Petri dish filled with PBS. The cartilage was cut into small fragments and digested with 2 mL of 0.25% trypsin for half an hour, followed by incubation with 0.2% type II collagenase at 37 °C for 4 hours. After the cartilage pieces were digested, they were washed with DMEM and collected through centrifugation at 1,000 rpm for 10 minutes. The isolated chondrocytes were cultured in culture dishes with complete DMEM in 5% CO2/95% air at 37 °C. When the chondrocytes reached 80% to 90% confluence, they were split and cultured to approximately 2 × 106 cells per culture dish.
OA Chondrocyte Co-Culture With MSCs
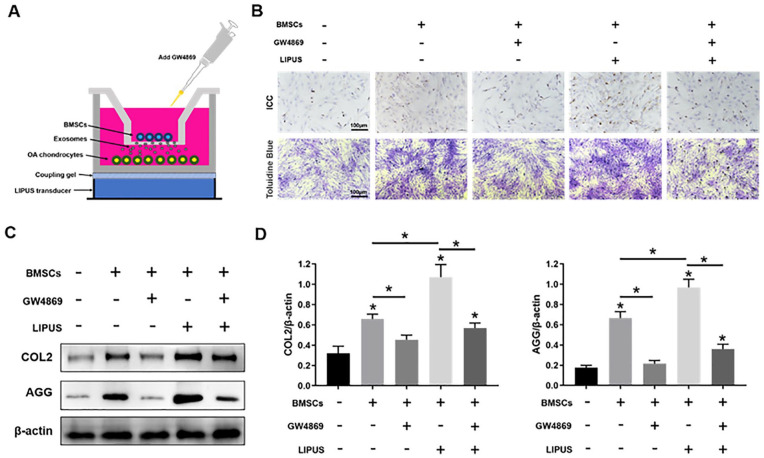
The OA chondrocyte and MSC co-culture was established in a 0.4-μm diameter co-culture chamber (Millipore, Massachusetts, USA), as shown in Figure 4A . In the co-culture chamber, MSCs (1 × 106 cells/well) were placed into the apical chamber, whereas OA chondrocytes (1 × 106 cells/well) were placed into the basolateral chamber, and the co-culture chamber was placed in a 6-well plate. 17 The culture medium was changed every 4 days, and the morphological changes in the cells were observed under a microscope.
Figure 4.
LIPUS enhances the anti-degeneration effects of MSCs on OA chondrocytes by promoting exosome release. (A) The OA chondrocyte and BMSC co-culture system was stimulated by LIPUS. (B) Immunohistochemical staining (ICC) and toluidine blue staining in OA chondrocytes. (C, D) Western blotting analysis of COL2, AGG, and β-actin expressions in OA chondrocytes. LIPUS = low-intensity pulsed ultrasound; MSCs = mesenchymal stem cells; OA = osteoarthritis; BMSC = bone marrow-derived MSC; COL2 = type II collagen; AGG = aggrecan. The values are the mean ± SD; n = 3, *P < .05.
Immunocytochemistry
COL2 expression in the OA chondrocytes was detected through immunocytochemistry, as described in our previous study. 18 The OA chondrocytes in the transwell co-culture system were fixed with 4% paraformaldehyde for 30 minutes, washed with PBS, and incubated with a 3% H2O2–methanol solution at room temperature for 10 minutes. Subsequently, the chondrocytes were washed thrice with PBS and blocked and incubated with goat serum (50–100 µL) at room temperature for 20 minutes. The cells were then incubated with COL2 antibodies (Abcam, Cambridge, UK) at 37 °C for 2 hours and washed thrice with PBS. Thereafter, 50 µL of an intensifier was added, and the cells were incubated at room temperature for 30 minutes. Then, the cells were washed with PBS and incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit-(Fab)2 antibodies (Santa Cruz, Dallas, TX, USA) at 37 °C for 30 minutes. Finally, the cells were washed with PBS again and subjected to diaminobenzidine (DAB) staining for color development. COL2 expression was observed under a microscope, and the images were captured.
Toluidine Blue Staining
To observe the proteoglycan side chains in mature AGG, the OA chondrocytes in the transwell co-culture system were washed with PBS and fixed with 4% paraformaldehyde at room temperature for 20 minutes. Then, the cells were again washed with PBS and stained with toluidine blue for 30 minutes. Finally, the cells were observed under a microscope, and the images were photographed.
GW4869 Treatment
In vitro study: to inhibit exosome release by MSCs, the secretory-specific inhibitor of exosomes, GW4869 (Selleck, Houston, TX, USA) (10 μM), was added to the co-culture system in the transwell chamber. The cells of the control group were treated with only the solvent DMSO.
In vivo study: GW4869 (10 μM) was added to the MSCs suspension and injected into the right knee joint of the OA rat.
LIPUS Stimulation
In vitro study: the LIPUS transducer (HT2009-1, Ito Corporation, Tokyo, Japan) was placed under a Petri dish and coated with a coupling agent. Then, the LIPUS (50 mW/cm2, on-off ratio of 20%, frequency of 3 MHz) waves were transferred through the bottom of the Petri dish, as described previously. 14 The control group was subjected to sham LIPUS stimulation with no ultrasound irradiation. The cells were stimulated for 20 minutes once a day for 0 (as control), 3, 7, and 10 days in 5% CO2/95% air at 37 °C.
In vivo study: the knee joint of the rats was exposed to LIPUS after MSCs intra-articular injection. According to the results in vitro experiments, we applied LIPUS stimulation for 7 days after MSCs intra-articular injection as a course of treatment. Based on our previous study, 4 times intra-articular injections of MSCs have therapeutic effects on OA cartilage. 14 Therefore, the MSCs were also injected for 4 times (once every 7 days) in this study, and the LIPUS stimulation were performed 20 minutes/day for 28 days (4 weeks). The LIPUS parameters were similar to those in the in vitro study.
Western Blotting
The extracted total protein was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. Primary anti-Beclin1 and anti-β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA); anti-LC3 and anti-AGG antibodies were purchased from Novus Biological; anti-CD63, ALIX, and TSG101 antibodies were purchased from Santa Cruz; anti-COL2 antibody was purchased from Abcam. Following treatment with the goat anti-mouse secondary antibody (Santa Cruz), blot signals were observed using the electrochemiluminescence (ECL) western blotting substrate (KeyGEN).
Animal Experiments
A total of 30 eight-week-old male SD rats (weighing 250–300 g) were used to establish OA models, and the OA rats were divided into the following 5 groups (6 rats in each group): control group, MSCs group, MSCs + GW4869 group, MSCs + LIPUS group, and MSCs + GW4869 + LIPUS group. The OA rat model was generated through ACLT, as described in our previous study. 16 The control group OA rats received vehicle injections of 0.9% normal saline and a sham LIPUS stimulation, whereas the MSCs group OA rats were administered an intra-articular injection of MSCs (1×106 MSCs were resuspended with 50 μL normal saline) through their right knee joint and a sham LIPUS stimulation. In the MSCs + GW4869 group, the OA rats received an intra-articular injection of MSCs, with addition of GW4869 and a sham LIPUS stimulation. In the MSCs + LIPUS group, the OA rats received LIPUS stimulation after an intra-articular injection of MSCs. In the MSCs + GW4869 + LIPUS group, the OA rats received an intra-articular injection of MSCs, with addition of GW4869 and LIPUS stimulation.
All the rats were euthanized 4 weeks after treatment and then subjected to histopathological examination for observation of the femoral condylar cartilage. Safranin-O/fast green staining was used to detect pathological changes in the cartilage, including surface irregularities and crack formation. In addition, we used the Mankin scores to evaluate the extent of fibrosis, matrix distribution, cartilage loss, and chondrocyte colonization (Table 1). Finally, we extracted the protein from the tibial plateau articular cartilage and determined the expression levels of COL2 and AGG through western blotting.
Table 1.
Mankin Scoring Scale.
|
Subgroup 1: fibrillation
1. Even surface 2. Uneven surface 3. Fibrillated and fissured within superficial zone only 4. Fissures and erosions extending below the surface zone, without extending beyond the radial zone 5. Fissures and erosions extending into the deeper zone Subgroup 2: matrix distribution 1. Normal staining 2. Moderate loss in staining 3. Severe loss in staining 4. No staining Subgroup 3: chondrocyte loss 1. Loss extending into superficial zone 2. Loss extending into midzone 3. Loss extending into radial zone Subgroup 4: chondrocyte cloning 1. No clusters 2. Chondrocyte clusters in superficial zone 3. Chondrocyte clusters in superficial to midzone(less than 4 cells) 4. Chondrocyte clusters of more than 4 cells located in superficial to midzone, or chondrocyte clusters in deeper zone |
Grading was performed for the femoral condyle. The minimum total score was 4, and the maximum total score is 16.
Statistical Analysis
All the experiments were performed in triplicate, and all data are reported as the mean ± standard deviation (SD) and analyzed using SPSS 23.0 software (IBM, Armonk, NY, USA). Statistical comparisons between the groups were performed using single-factor analysis of variance. The Mankin scores were analyzed using the Wilcoxon signed rank test. A P value < .05 was considered to indicate statistical significance.
Results
Autophagy Regulates Exosome Release by MSCs
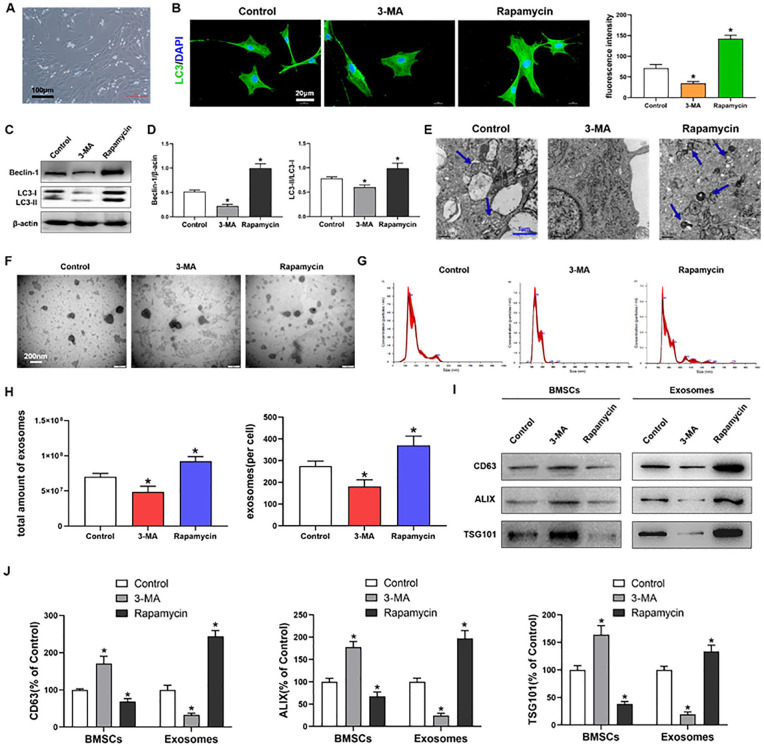
The autophagy-related genes such as Beclin1 and LC3 are essential for autophagosome formation in the MSCs. During autophagy, LC3I was gradually transformed into LC3II. Rapamycin is the specific activator, and 3-MA is the specific inhibitor in autophagy. Figure 1A shows the morphology of MSCs. Immunofluorescence staining indicated that LC3-positive cells were significantly increased (P < .05) in the rapamycin group and significantly decreased (P < .05) in the 3-MA group compared with those in the control group ( Fig. 1B ). We used western blotting to detect protein expression of the autophagy markers Beclin1 and LC3 after rapamycin or 3-MA treatment. The expression of Beclin1 and the ratio of LC3II/LC3I in MSCs were significantly increased (P < .05) after rapamycin treatment and significantly decreased (P < .05) after 3-MA treatment compared with those in the control (Fig. 1C and 1D). Autophagosome formation was observed through TEM, which showed that the number of autophagosomes was increased after rapamycin treatment and decreased after 3-MA treatment compared with that in the control ( Fig. 1E ).
Figure 1.
Autophagy regulates exosome release by MSCs. (A) BMSCs cultured in medium; scale bars = 100 μm. (B) Immunofluorescence staining depicting LC3+ cells (green) and quantitative analysis of intensity; scale bars = 20 μm. (C, D) Western blot analysis of Beclin1, LC3I, LC3II, and β-actin expressions in BMSCs. (E) TEM depicting autophagosomes (arrows); scale bars = 1 μm. (F) TEM observation of exosomes from BMSCs; scale bars = 200 nm. (G, H) NTA of the size distribution and amount of exosomes from BMSCs. (I, J) Western blot analysis of CD63, ALIX and TSG101 expressions in BMSCs and exosomes. MSCs = mesenchymal stem cells; BMSCs = bone marrow-derived MSCs; TEM = transmission electron microscopy; NTA = nanoparticle tracking analysis; DAPI: diamidine phenylindole; 3-MA = 3-methyladenine. The values are the mean ± SD; n = 3, *P < .05.
The MSC exosomes observed through TEM are shown in Figure 1F . The size distribution and amount of exosomes were detected by NTA. The NTA showed that the rapamycin and 3-MA treatments had no effect on the size distribution of MSC exosomes ( Fig. 1G ). However, the amount of exosomes was significantly increased (P < .05) after rapamycin treatment and significantly decreased (P < .05) after 3-MA treatment compared with that in the control ( Fig. 1H ). The specific markers of exosomes include CD63, ALIX, and TSG101, and they mainly involved in the formation and secretion of exosomes. The western blot results showed that the protein expression of CD63, ALIX, and TSG101 were significantly increased (P < .05) in the exosomes isolated from MSCs culture medium after rapamycin treatment and significantly decreased (P < .05) after 3-MA treatment compared with those in the control. Simultaneously, concomitant significant decrease and increase (both P < .05) in CD63, ALIX, and TSG101 expression were observed in MSCs after treatments with rapamycin and 3-MA, respectively, compared with those in the control (Fig. 1I and 1J).
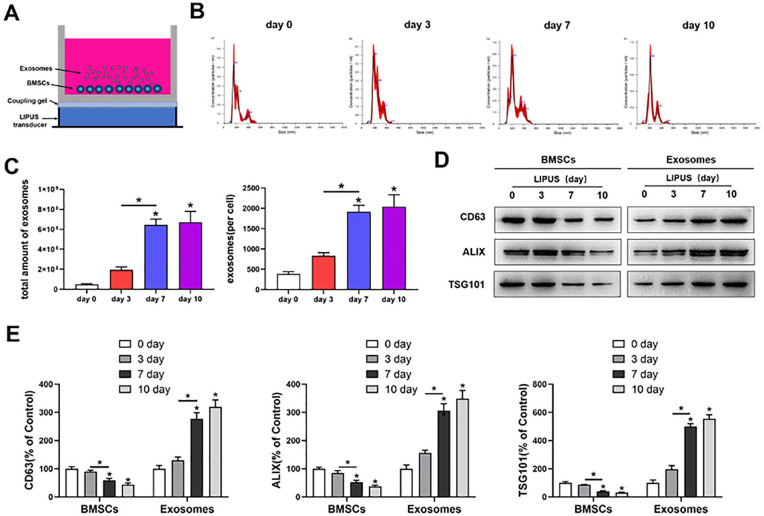
LIPUS Promotes MSC Exosome Release
The MSCs were stimulated by LIPUS, as shown in Figure 2A . The NTA showed that LIPUS has no effect on the size distribution of MSC exosomes after 3, 7, and 10 days of stimulation ( Fig. 2B ). The amount of exosomes was significantly increased (P < .05) after LIPUS stimulation for 7 and 10 days compared with that after stimulation for 3 days; however, no significant difference (P > .05) was observed in the amount of exosomes between 7- and 10-day stimulation ( Fig. 2C ). Western blot analysis showed that the protein expression of CD63, ALIX, and TSG101 was significantly increased (P < .05) in the exosomes isolated from MSCs culture medium after LIPUS stimulation for 7 and 10 days compared with those after stimulation for 3 days. Simultaneously, a concomitant significant decrease (P < .05) in CD63, ALIX, and TSG101 expression was noted in MSCs after LIPUS treatment for 7 and 10 days compared with those after stimulation for 3 days (Fig. 2D and 2E).
Figure 2.
LIPUS promotes MSC exosome release. (A) BMSCs were stimulated by LIPUS. (B, C) NTA of the size distribution and amount of exosomes from BMSCs. (D, E) Western blot analysis of CD63, ALIX, and TSG101 expressions in BMSCs and exosomes. LIPUS = low-intensity pulsed ultrasound; MSC = mesenchymal stem cell; BMSCs = Bone marrow-derived MSCs. The values are the mean ± SD; n = 3, * P < .05.
Autophagy Inhibition Decreases the Promoting Effect of LIPUS on MSC Exosome Release
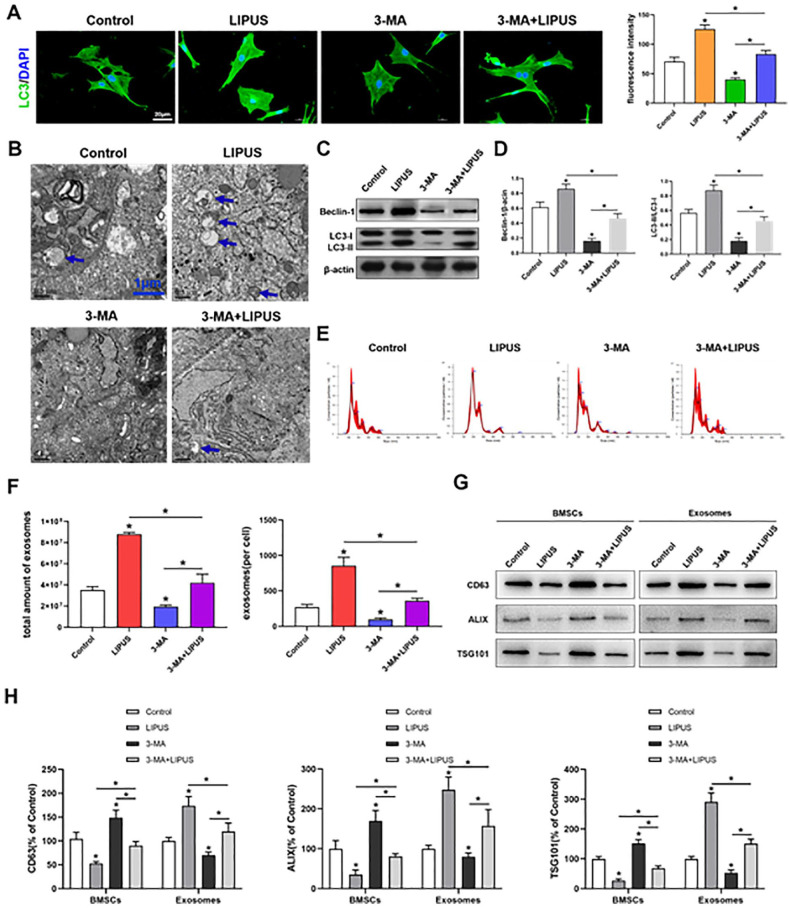
To determine whether the effect of LIPUS on MSC exosome release was regulated by autophagy, we used 3-MA to inhibit autophagy. According to the aforementioned results, autophagy was significantly increased (P < .05) after LIPUS stimulation and significantly decreased (P < .05) after 3-MA treatment compared with that in the unstimulated MSCs. In addition, compared with the LIPUS-stimulated MSCs, the 3-MA-treated MSCs exhibited a significant decrease (P < .05) in autophagy after LIPUS stimulation. However, LIPUS stimulation significantly increased (P < .05) autophagy in the 3-MA-treated MSCs compared with that in the unstimulated MSCs in the presence of 3-MA ( Fig. 3A-3D ).
Figure 3.
Inhibition of autophagy decreases the promoting effect of LIPUS on MSC exosome release. (A) Immunofluorescence staining depicting LC3+ cells (green) and quantitative analysis of intensity; scale bars = 20 μm. (B) Electron microscopy depicting autophagosomes (arrows); scale bars = 1 μm. (C, D) Western blot analysis of Beclin1, LC3I, LC3II, and β-actin expressions in BMSCs. (E, F) NTA of the size distribution and amount of exosomes from BMSCs. (G, H) Western blotting analysis of CD63, ALIX, and TSG101 expressions in BMSCs and exosomes. LIPUS = low-intensity pulsed ultrasound; MSC = mesenchymal stem cell; BMSCs = bone marrow-derived MSCs; NTA = nanoparticle tracking analysis; DAPI: diamidine phenylindole; 3-MA = 3-methyladenine. The values are the mean ± SD; n = 3, *P < .05.
Furthermore, exosome release was analyzed through NTA and western blotting. The NTA showed that no difference was observed on the size distribution of MSC exosomes in 4 groups ( Fig. 3E ). The results also showed that the exosome release was significantly increased (P < .05) in LIPUS-stimulated MSCs and significantly decreased (P < .05) in 3-MA-treated MSCs compared with that in the unstimulated MSCs. In addition, 3-MA treatment significantly decreased exosome release in the LIPUS-stimulated MSCs (P < .05), whereas LIPUS stimulation significantly increased (P < .05) exosome release in the 3-MA-treated MSCs compared with that in the unstimulated MSCs in the presence of 3-MA (Fig. 3F-3H).
LIPUS Enhances the Anti-Degeneration Effects of MSCs on OA Chondrocytes by Promoting Exosome Release
The OA chondrocytes and MSCs co-culture system was stimulated by LIPUS, as depicted in Figure 4A . Immunohistochemical staining of COL2 and toluidine blue staining in OA chondrocytes treated with MSCs and LIPUS were much stronger than those in the control, MSCs, MSCs + GW4869, and MSCs + GW4689 + LIPUS groups. Staining in the MSCs + GW4869 and MSCs + GW4689 + LIPUS groups was weaker than that in the MSCs and MSCs + LIPUS groups ( Fig. 4B ).
The western blot analysis showed that the protein expression of COL2 and AGG was significantly increased (P < .05) in the MSCs + LIPUS group compared with those in the control, MSCs, MSCs + GW4869, and MSCs + GW4689 + LIPUS groups, and the expression of COL2 and AGG was significantly decreased (P < .05) in the MSCs + GW4869 and MSCs + GW4689 + LIPUS groups compared with that in the MSCs and MSCs + LIPUS groups (Fig. 4C and 4D).
LIPUS Enhances the Repair Effects of MSCs on OA Cartilage through the Exosome Release Pathway
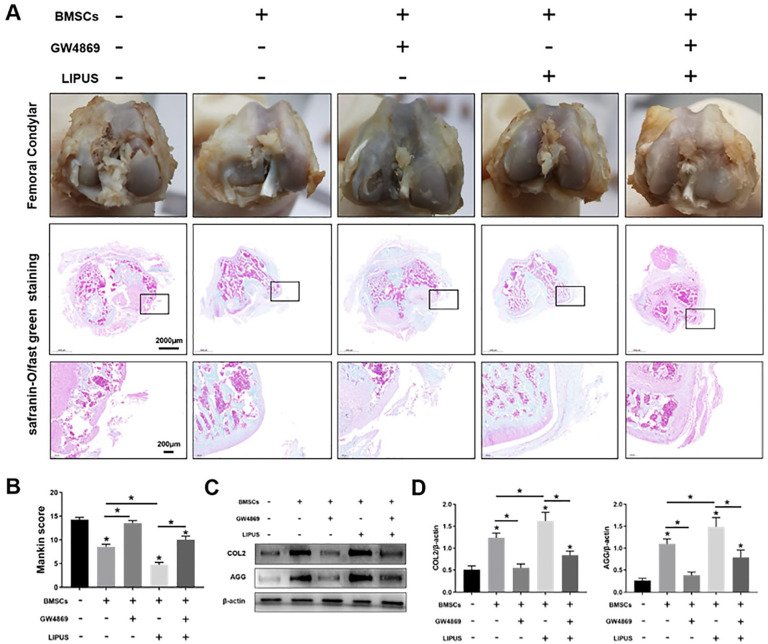
We examined the role of exosome release in LIPUS in promoting the effect of MSCs on OA cartilage repair. The femoral condylar cartilage was observed and assessed through safranin-O/fast green staining, which showed that in the control group, the cartilage was surface defected and thinned, with increased fibrosis of the surface and irregular distribution of the chondrocytes. Compared with the control group, the MSCs group and the MSCs + GW4869 + LIPUS group exhibited thicker cartilage and regular chondrocyte distribution. In addition, the surface of cartilage was more smooth and regular in the MSCs + LIPUS group than in the MSC group and the MSCs + GW4869 + LIPUS group. No difference was observed in terms of cartilage morphology and chondrocyte distribution between the control group and the MSCs + GW4869 group ( Fig. 5A ).
Figure 5.
LIPUS enhances the repair effects of MSCs on OA cartilage through the exosome release pathway. (A) The femoral condylar cartilage was observed and detected through safranin-O/fast green staining; scale bars = 2,000 μm, 200 μm. (B) Bar graph comparing the Mankin scores. (C, D) Western blotting analysis of COL2, AGG, and β-actin expressions in the cartilage. LIPUS = low-intensity pulsed ultrasound; MSCs = mesenchymal stem cells; OA = osteoarthritis; COL2 = type II collagen; AGG = aggrecan. The values are the mean ± SD; n = 6, *P < .05.
The quantitative analysis of cartilage pathological changes performed on the basis of Mankin scores showed that the scores were significantly decreased (P < .05) in the MSCs group, MSCs + GW4689 + LIPUS group, and MSCs + LIPUS group, particularly in the MSCs + LIPUS group, compared with those in the control group. Moreover, the scores were significantly increased (P < .05) in the MSCs+ GW4869+ LIPUS group compared with those in the MSCs+LIPUS group. No significant difference (P > .05) was found in the scores between the control group and the MSCs + GW4869 group ( Fig. 5B ).
Protein expression of COL2 and AGG determined through western blot analysis showed that the COL2 and AGG expression were significantly increased (P < .05) in the MSCs group, MSCs + GW4689 + LIPUS group, and MSCs + LIPUS group, particularly in the MSCs + LIPUS group, compared with those in the control group. Moreover, compared with the MSCs + LIPUS group, the MSCs + GW4869 + LIPUS group demonstrated significantly decreased COL2 and AGG expression (P < .05). No significant difference (P > .05) was found in the COL2 and AGG expression between the control group and the MSCs + GW4869 group (Fig. 5C).
Discussion
In this study, we attempted to investigate the role of autophagy in MSC exosome release and explored whether LIPUS promotes the therapeutic effects of MSCs on OA cartilage through autophagy-regulated exosome release. We found that autophagy promotes MSC exosome release, and LIPUS promotes MSC exosome release by activating autophagy. In addition, our in vitro and in vivo experiments suggested that LIPUS enhances the repair effects of MSCs on OA cartilage through the exosome release pathway.
OA is one of the most common chronic joint diseases in clinic; however, its pathogenesis is still unclear. The articular cartilage defect is the most obvious pathologic change in OA, which is caused by extracellular matrix degeneration. 19 Current OA treatment strategies, such as drug therapy, physical therapy and joint replacement, are aimed at relieving pain and improving the joint function, although these strategies cannot repair the damaged cartilage and restore the characteristics of cartilage.3,4,20
Cell therapy based on tissue engineering has become a research hotspot in the field of OA treatment in recent years. Cartilage stem/progenitor cells (CSPCs) are cells with self-renewal and multi-directional differentiation ability that exist on the surface of articular cartilage. 21 Studies have shown that CSPCs have chondrogenic differentiation capacity and cartilage repair effect.22,23 However, because CSPCs need to be extracted from cartilage tissue, there are limitations such as large trauma, high cost, few cell sources, and insufficient quantity.
MSCs mainly exist in the connective tissue and organ stroma of the whole body, and most of these cells are present in the bone marrow. These cells have strong proliferation ability and can differentiate into chondrocytes under certain conditions. 24 Some studies have shown that MSCs migrate into cartilage tissues and first become osteochondral cell populations, and then differentiate into CSPCs. 25 CSPCs are regulated by some cytokines and then differentiated into chondrocytes and secreted COL2 to form normal cartilage. 26 Accumulating preclinical studies have suggested that the use of MSCs might be a novel therapeutic strategy to protect the articular cartilage.6,27,28 Some clinical studies and our meta-analysis also confirmed that intra-articular injection of MSCs produces a strong therapeutic effect on OA.29-32
Although MSCs have been confirmed to be useful in the treatment of OA, their mechanism of action remains unclear. The traditional view is that MSCs can differentiate into specific cells to replace damaged tissues to produce repair effects. 33 However, the current point of view suggests that MSCs can secrete biologic active substances including cytokines and extracellular vesicles (EVs) to regulate the injured tissue environment and the regeneration processes, such as cell migration, proliferation, differentiation, and matrix synthesis. 34 Exosomes are the main EVs secreted by MSCs, and they support the regeneration ability of MSCs in tissue repair.35,36
Exosomes were first discovered in 1987 by Johnstone et al. 37 in sheep reticulocytes; they are a special form of extracellular vesicles, with a diameter of 40 to 100 nm, and contain various proteins, nucleic acids, lipids, and other substances. Studies have indicated that the exosomes released by MSCs can maintain chondrocyte homeostasis and inhibit chondrocyte apoptosis, thereby mediating cartilage tissue regeneration and repair.10,11,38 Although these lines of evidence indicate that exosomes are the main therapeutic factor for cartilage repair by MSCs, the release of exosomes is affected by many factors and the mechanism is still unknown.
Studies have found that mammalian target of rapamycin (mTOR), an autophagy inhibitory signal, is involved in the regulation of exosome release. The mTOR inhibitor rapamycin (which is also an autophagy activator) can promote exosome release, suggesting that autophagy plays a role in the regulation of exosome release.13,39 Autophagy is a form of cell death that maintains cell metabolism and energy balance through degradation and recycling of intracellular proteins and organelles. In our previous studies, we found that autophagy regulates the chondrogenesis and migration of MSCs.14,15 The results of the present study suggested that autophagy also participates in the exosome release by MSCs. Activation of autophagy increased the exosome release from MSCs, whereas the effect was reversed upon autophagy inhibition.
The current research focuses on improving the therapeutic effect of MSCs in OA. LIPUS is a physical therapy factor, and the mechanical stimulation produced by LIPUS can alter the biological effects of MSCs. In vivo experiments in a study indicated that LIPUS can promote BMSC homing to the fracture site to accelerate fracture healing. 40 In vitro experiments have also proved that LIPUS can affect the multilineage differentiation ability of BMSCs, which can promote transforming growth factor (TGF)-1β-induced chondrogenic differentiation.41,42 Our previous studies found that LIPUS can promote chondrogenic differentiation and migration of MSCs by regulating autophagy and thus enhance the therapeutic effect of MSCs on OA catilage.14,15 In the present study, we found that although LIPUS has no effect on the shape and size of exosomes, stimulation for 7 and 10 days can increase the number of exosomes released by MSCs. Moreover, we found that LIPUS can activate autophagy and promote exosome release by MSCs, and the inhibition of autophagy with 3-MA reduced the effect of LIPUS on the exosome release by MSCs.
Although studies have shown that MSCs have a certain effect on OA cartilage regeneration,24,27,28,43 and our previous study also demonstrated that LIPUS can enhance the therapeutic effect of MSCs in OA cartilage repair, 14 it is unknown whether exosomes play a role in the MSC-mediated repair process. The present in vitro and in vivo studies confirmed that MSCs can increase the synthesis of extracellular matrix proteins of OA chondrocytes and protect cartilage. LIPUS was found to significantly enhance the therapeutic effect of MSCs in OA cartilage repair; however, inhibition of the release of exosomes blocked the therapeutic effect of MSCs and decreased the enhancement effect of LIPUS. These results indicated that MSCs mediate the repair effect on OA cartilage mainly through exosome release, and LIPUS enhances the therapeutic effect of MSCs on OA cartilage by promoting exosome release.
In conclusion, autophagy participates in exosome release by MSCs. Activation of autophagy promoted exosome release by MSCs, and inhibition of autophagy displayed the opposite effects. In addition, the study demonstrated that LIPUS promotes exosome release by activating autophagy and enhances the therapeutic effect of MSCs on OA cartilage through the exosome release pathway. Our results revealed the effects of LIPUS on MSC exosome release, as well as the mechanism of the effect of LIPUS on MSCs in OA cartilage repair. These findings will provide a theoretical basis for the combined application of LIPUS and MSCs.
Footnotes
Authors’ Note: All the authors are researchers at Department of Rehabilitation Medicine, Nanjing First Hospital, Nanjing Medical University, China. Peng Xia and Xueping Li are the leaders of our research group.
Author Contributions: Peng Xia and Xueping Li conceived and designed the study. Peng Xia, Qi Wang, and Jiulong Song performed the experiments. Peng Xia wrote the manuscript. Xiaoju Wang, Qiang Lin, Kai Cheng, Anliang Chen, and Xueping Li reviewed and edited the manuscript. All authors read and approved the manuscript.
Acknowledgments and Funding: We thank TopEdit (www.topeditsci.com) for English language editing of this manuscript and the National Natural Science Foundation of China (No.81772437, 82072541) for financial support.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The experimental protocol relating to rats was approved by the Nanjing Medical University Ethics Committee of Nanjing Hospital.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Animal Welfare: The experimental protocol relating to rats was in accordance with the US National Institutes of Health’s Guidelines of Laboratory Animal Use and approved by the Nanjing Medical University Ethics Committee of Nanjing Hospital.
ORCID iD: Xueping Li  https://orcid.org/0000-0003-0481-5162
https://orcid.org/0000-0003-0481-5162
Availability of Data and Materials: All data generated and/or analyzed during this study are included in this published article.
References
- 1. Bortoluzzi A, Furini F, Scirè CA. Osteoarthritis and its management—epidemiology, nutritional aspects and environmental factors. Autoimmun Rev. 2018;17(11):1097-104. doi: 10.1016/j.autrev.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 2. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745-59. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43(6):701-12. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4. Block JA. Osteoarthritis: OA guidelines: improving care or merely codifying practice? Nat Rev Rheumatol. 2014;10(6):324-6. doi: 10.1038/nrrheum.2014.61. [DOI] [PubMed] [Google Scholar]
- 5. Chen FH, Rousche KT, Tuan RS. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2(7):373-82. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Yuan M, Guo QY, Lu SB, Peng J. Mesenchymal stem cells for treating articular cartilage defects and osteoarthritis. Cell Transplant. 2015;24(9):1661-78. doi: 10.3727/096368914X683485. [DOI] [PubMed] [Google Scholar]
- 7. Kilmer CE, Battistoni CM, Cox A, Breur GJ, Panitch A, Liu JC. Collagen type I and II blend hydrogel with autologous mesenchymal stem cells as a scaffold for articular cartilage defect repair. ACS Biomater Sci Eng. 2020;6(6):3464-76. doi: 10.1021/acsbiomaterials.9b01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56-64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 9. Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838-48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 10. Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-40. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 11. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xing H, Tan J, Miao Y, Lv Y, Zhang Q. Crosstalk between exosomes and autophagy: a review of molecular mechanisms and therapies. J Cell Mol Med. 2021;25(5):2297-308. doi: 10.1111/jcmm.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zou W, Lai M, Zhang Y, Zheng L, Xing Z, Li T, et al. Exosome release is regulated by mTORC1. Adv Sci. 2019;6(3):1801313. doi: 10.1002/advs.201801313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia P, Wang X, Wang Q, Wang X, Lin Q, Cheng K, et al. Low-intensity pulsed ultrasound promotes autophagy-mediated migration of mesenchymal stem cells and cartilage repair. Cell Transplant. 2021;30:963689720986142. doi: 10.1177/0963689720986142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Lin Q, Zhang T, Wang X, Cheng K, Gao M, et al. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res Ther. 2019;10(1):41. doi: 10.1186/s13287-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia P, Shen S, Lin Q, Cheng K, Ren S, Gao M. et al. Low-intensity pulsed ultrasound treatment at an early osteoarthritis stage protects rabbit cartilage from damage via the integrin/focal adhesion kinase/mitogen-activated protein kinase signaling pathway. J Ultrasound Med. 2015;34(11):1991-9. doi: 10.7863/ultra.14.10016. [DOI] [PubMed] [Google Scholar]
- 17. Zuo Q, Cui W, Liu F, Wang Q, Chen Z, Fan W. Co-cultivated mesenchymal stem cells support chondrocytic differentiation of articular chondrocytes. Int Orthop. 2013;37(4):747-52. doi: 10.1007/s00264-013-1782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia P, Ren S, Lin Q, Cheng K, Shen S, Gao M, et al. Low-intensity pulsed ultrasound affects chondrocyte extracellular matrix production via an integrin-mediated p38 MAPK signaling pathway. Ultrasound Med Biol. 2015;41(6):1690-700. doi: 10.1016/j.ultrasmedbio.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 19. Zhang XX, He SH, Liang X, Li W, Li TF, Li DF. Aging, cell senescence, the pathogenesis and targeted therapies of osteoarthritis. Front Pharmacol. 2021;12:728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higashi H, Barendregt JJ. Cost-effectiveness of total hip and knee replacements for the Australian population with osteoarthritis: discrete-event simulation model. PLoS ONE. 2011;6(9):e25403. doi: 10.1371/journal.pone.0025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJR, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889-97. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 22. Yu Y, Brouillette MJ, Seol D, Zheng H, Buckwalter JA, Martin JA. Use of recombinant human stromal cell-derived factor 1alpha-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol. 2015;67(5):1274-85. doi: 10.1002/art.39049. [DOI] [PubMed] [Google Scholar]
- 23. Levato R, Webb WR, Otto IA, Mensinga A, Zhang Y, van Rijen M, et al. The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017;61:41-53. doi: 10.1016/j.actbio.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: a review. Am J Transl Res. 2021;13(2):448-61. [PMC free article] [PubMed] [Google Scholar]
- 25. Schminke B, Miosge N. Cartilage repair in vivo: the role of migratory progenitor cells. Curr Rheumatol Rep. 2014;16(11):461. doi: 10.1007/s11926-014-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang QO, Shakib K, Heliotis M, Tsiridis E, Mantalaris A, Ripamonti U, et al. TGF-beta3: a potential biological therapy for enhancing chondrogenesis. Expert Opin Biol Ther. 2009;9(6):689-701. doi: 10.1517/14712590902936823. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Q, Xiang E, Rao W, Zhang YQ, Xiao CH, Li CY. et al. Intra-articular injection of human umbilical cord mesenchymal stem cells ameliorates monosodium iodoacetate-induced osteoarthritis in rats by inhibiting cartilage degradation and inflammation. Bone Joint Res. 2021;10(3):226-36. doi: 10.1302/2046-3758.103.BJR-2020-0206.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang R, Meng F, Zhang Q, Zou Z, Xiao K, Zhu T, et al. Allogeneic adipose-derived mesenchymal stem cells promote the expression of chondrocyte redifferentiation markers and retard the progression of knee osteoarthritis in rabbits. Am J Transl Res. 2021;13(2):632-45. [PMC free article] [PubMed] [Google Scholar]
- 29. Kim SH, Ha CW, Park YB, Nam E, Lee JE, Lee HJ. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139(7):971-80. doi: 10.1007/s00402-019-03140-8. [DOI] [PubMed] [Google Scholar]
- 30. Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504-11. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Molto F, Nunez-Cordoba JM, Lopez-Elio S, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2018;16(1):213. doi: 10.1186/s12967-018-1591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363-72. doi: 10.1007/s00264-015-2785-8. [DOI] [PubMed] [Google Scholar]
- 33. Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5-6):419-27. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 34. Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10(8):1959. doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bao C, He C. The role and therapeutic potential of MSC-derived exosomes in osteoarthritis. Arch Biochem Biophys. 2021;710:109002. doi: 10.1016/j.abb.2021.109002. [DOI] [PubMed] [Google Scholar]
- 36. Tang Y, Zhou Y, Li HJ. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12(1):71. doi: 10.1186/s13287-021-02138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412-20. [PubMed] [Google Scholar]
- 38. Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960-76. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei FY, Leung KS, Li G, Qin J, Chow SK, Huang S. et al. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS ONE. 2014;9(9):e106722. doi: 10.1371/journal.pone.0106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebisawa K, Hata K, Okada K, Kimata K, Ueda M, Torii S, et al. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10(5-6):921-9. doi: 10.1089/1076327041348437. [DOI] [PubMed] [Google Scholar]
- 42. Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289(15):10330-44. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ding N, Li E, Ouyang X, Guo J, Wei B. The therapeutic potential of bone marrow mesenchymal stem cells for articular cartilage regeneration in osteoarthritis. Curr Stem Cell Res Ther. 2021;16:840-7. [DOI] [PubMed] [Google Scholar]