Abstract
The cortex is a highly organized structure that develops from the caudal regions of the segmented neural tube. Its spatial organization sets the stage for future functional arealization. Here, we suggest using a developmental perspective to describe and understand the etiology of common cortical malformations and their manifestation in the human brain.
Keywords: cortical development, neuronal migration, holoprosencephaly, lissencephaly, forebrain, telencephalon, signaling factors
1. Introduction
Understanding the origins of higher cortical functions and their malfunction in developmental diseases requires delineating how brain areas are formed during development. It has been long appreciated that the human brain is composed of defined areal and laminar structures that differ in composition [1,2,3,4]. These areas are specified during embryonic development due to orchestrated evolutionary conserved processes that sculpt the brain. The limited access to human embryos, ethical considerations, and the lack of suitable in vitro human model systems make human brain organization a complex topic to study. Today’s information is an accumulation of data from different model organisms and, in part, from human embryos. This review explores major developmental events leading to the formation of regional forebrain domains and cortical layers. It examines how these processes contribute to our understanding of the pathophysiology of common human brain developmental disorders.
1.1. Forebrain Induction and Patterning
The first indication of the developing human central nervous system (CNS) appears in the third week of human embryonic development in a process termed neurulation. A plate of thickened ectoderm appears in the mid-dorsal region of the tri-laminar embryo, the neural plate. The formation of the anterior neural plate, which will give rise to the forebrain, is induced by signals from the prechordal plate (PrCP), a cell population that originates from the mesendoderm and migrates rostrally from the primitive node along the midline between the ectoderm and the endoderm layers. In a series of extensive morphogenetic movements, the neural plate bends inwards to generate a tubular structure with open neural folds, then brought together at the dorsal midline to generate the neural tube (scheme in Figure 1). While neural plate induction occurs along the entire rostral–caudal axis at the dorsal midline, the PrCP is responsible for the rostral neural plate cells to adopt an anterior fate and antagonizes the activity of caudalizing factors that are secreted at more posterior mesodermal domains [5].
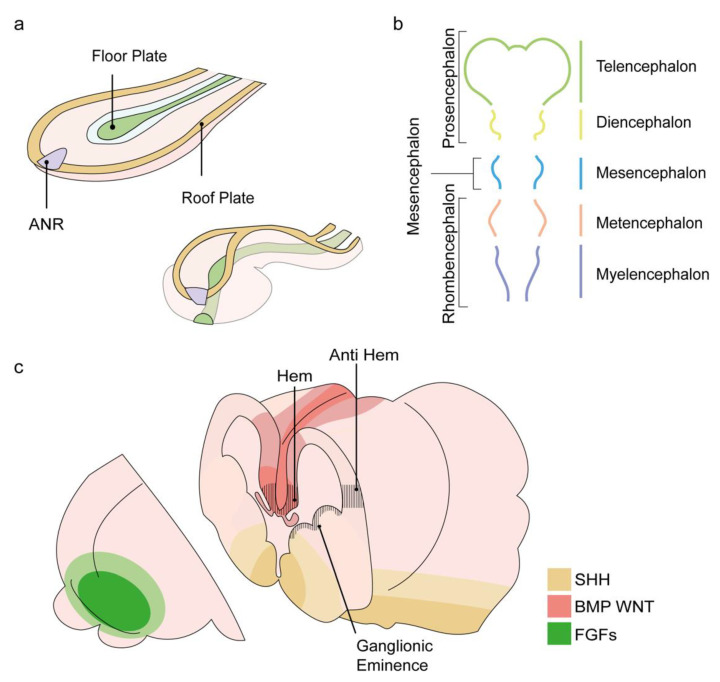
Figure 1.
Schematic presentation of the events leading to forebrain formation. (a) Rostro-lateral representation of the neural plate as it folds and fuses at the midline to form the neural tube. The anterior neural ridge (ANR), shown in purple, is an organizer of the forebrain. Positioned at the most rostral part of the neural plate, its signaling will promote the formation of the future forebrain structure, the prosencephalon. Modified from [6]. (b) The primary vesicles appear in the fourth week of development and introduce three morphological segments of the brain: the prosencephalon, the mesencephalon, and the rhombencephalon. By the following week, the prosencephalon will further subdivide into the future forebrain structures: the anterior neural tube, the two telencephalon vesicles, and the diencephalon. (c) Sources of key morphogens are indicated; FGFs in green, BMPs and WNTs in red, SHH in yellow. The positions of the hem, anti-hem, and ganglionic eminences are indicated with black lines. Modified from [7].
By the end of the fourth week of development, the rostrally positioned primary vesicles become noticeable and form the morphological segmentation that will become the distinct functional units of the brain [8]. The primary vesicles include three vesicles: prosencephalon, mesencephalon, and rhombencephalon (which will later be divided into the metencephalon and myelencephalon(. A week later, the primary prosencephalus becomes subdivided into three secondary vesicles: at the anterior end of the neural tube, the left and right telencephalic vesicles, and a more caudal diencephalon vesicle (Figure 1b). The telencephalon will eventually give rise to the left and right cerebral hemispheres, the olfactory bulbs, and the hippocampus.
The diencephalon will generate the optic vesicles that become the retina, thalamus, and hypothalamus, which develop from dorsal or ventral domains. Notably, the current view supports the hypothesis that the segmented neuroaxis evolved from a series of many more repeated segments than those morphologically evident, termed metameric units. This organization paradigm has early evolutionary origins, is already apparent in the embryonic insect, and is dissected by homologous segment identity genes in vertebrates. The prosomeric model depicts a segmental subdivision of the body axis, based on gene expression patterns, to the so-called “prosomere,” separated by nonidentical boundaries [9]. Metamerism was suggested to extend to the nervous system’s anterior parts, including the caudal forebrain, but is missing in the prosencephalon (telencephalon and hypothalamus) [9].
1.2. Signaling Factors That Control Forebrain Development
The organization along the anterior–posterior (AP) and dorsal–ventral (DV) axes of both the spinal cord and the anterior structures of the CNS is governed by the production of instructive molecules—morphogens—that are expressed by small cell populations, often referred to as patterning centers or organizers. A fundamental question is how positional information is conveyed and interpreted by the cells to express subsets of transcription factors that will execute the developmental fate. A model for the interpretation of morphogens areal information into distinct cell fates was proposed by Wolpert (the French flag model) [10]. According to this model, effective patterning driven by morphogen gradients will obey three required elements: (i) polarity, (ii) differential response of cells, and (iii) at least one spontaneous self-limiting reaction. The robustness of morphogen patterning requires a buffer mechanism that ensures a reproducible response [11].
The anterior neural ridge (ANR) is an example of an organizing center that governs forebrain patterning via positional information. The ANR is a temporary structure located at the most-rostral edge of the neural plate (Figure 1a). The ANR is initially necessary for prosencephalon induction. Moreover, it also sets a “protomap” upon which the prosencephalon is programmed to subdivide into the telencephalon, optic vesicles, and diencephalon domains, based on the regionalized expression of regulatory genes [12,13,14,15,16]. The ANR, which later becomes the anterior telencephalon, is the source of soluble morphogens that belong to the fibroblast growth factor (FGF) family, such as FGF8 and FGF17 [17,18,19]. The instructive role of FGF8 signaling in shaping the anterior–posterior neocortical map and its particular role in the shaping of the most anterior part of the telencephalon were highlighted by a detailed analysis of the arealization of the forebrain in mutant mice embryos with reduced FGF8 levels (Fgf8 neo/neo and Fgf8 neo/null hypomorphic mutants [20,21,22,23]).
Similarly, ablation of the ANR or loss of Fgf8 in the anterior neural plate cells of zebrafish embryos resulted in a distorted telencephalon with abnormal morphology, altered gene expression, and axonal misprojection [24]. Furthermore, in utero electroporation of a soluble FGF8 receptor that competed with the endogenous FGF8 receptors resulted in shifts in telencephalic boundaries [14]. Conversely, forced expression of Fgf8 in E11.5–E12.5 mouse embryos in the anterior cortical primordium resulted in enlarged cortical areas with anterior identity and a reciprocal reduction of posterior fates [25]. Notably, Fgf17 function was found to be similar to that of Fgf8, with a more selective role in regulating the properties of the dorsal frontal cortex [26].
Following the formation of the left and right telencephalic vesicles, the mediolateral axis of their dorsal cortical primordium develops into two signaling centers: the hem and the anti-hem. Both centers flank the cortical neuroepithelium and express different types of signaling molecules. The cortical hem expresses Wingless (Wnt) genes (Wnt2b, 3a, 5a, 7b, 8b) and bone morphogenetic protein (BMP) genes (Bmp2, 4, 5, 6, 7) and serves as the organizer of the hippocampus. Indeed, the induction of ectopic hem structures adjacent to Lhx2 null cells can produce multiple hippocampal fields [27,28]. The anti-hem is located at the pallial/subpallial boundary and expresses secreted signaling molecules, including FGF7 and three epidermal growth factor (EGF) family molecules, yet its organizing functions are not yet fully understood [28].
In addition to FGF, BMP, and Wnt signals governing the AP and mediolateral patterning of the dorsal telencephalon, additional signaling factors participate in the patterning of the ventral and the lateral telencephalon. Sonic Hedgehog (Shh), a morphogen known for its graded function in the ventral spinal cord, has been shown to work in two phases during forebrain development. In the early stage, Shh is expressed in the PrCP and mediates the initiation of forebrain development from the anterior neural plate as well as the subdivision of the anterior prosencephalon into left and right vesicles. Next, the early Shh signal triggers a secondary induction activity in the forebrain, where it will sequentially specify the progenitors of the medial ganglionic eminence (MGE) and, later, of the lateral ganglionic eminence (LGE) (Figure 1c) [29].
Retinoic acid (RA), a metabolite of retinol (vitamin A), functions as a ligand for nuclear retinoic acid receptors (RARs), which regulate the expression of numerous downstream target genes during both early embryogenesis and adulthood. Early in development, RA appears in a morphogenic gradient (high caudally, low rostrally) established by an interplay between diffusion gradients and localized RA metabolism, thereby allowing RA to control the patterning of various structures [30]. RA is locally synthesized in the telencephalon neuroepithelium in the early stages of forebrain development. Prevention of RA signal transduction will significantly impair forebrain development by reducing neurogenesis and cell proliferation and increasing cell death [31,32]. Later, RA is required to properly differentiate GABAergic and dopaminergic neurons in the striatum and neocortical lamination and for radial migration during mouse corticogenesis [33,34].
An essential principle in forebrain induction and patterning is that signaling molecules, such as those mentioned, do not work independently but rather display an intricate regulatory network. Different secreted morphogenetic molecules in and around the patterning centers govern direct or indirect interactions between multiple signaling pathways and their downstream transcription factors. For instance, Fgfs, expressed at the ANR, are regulators of mid-line patterning and antagonize Wnt and BMP of the cortical hem [35]. At later stages, the proliferative activity of RA involves a crosstalk of Shh and FGF signaling [36].
Finally, while the local AP planar induction sets up a transverse regionalization of the major forebrain parts, DV patterning along the neural tube is also fundamental for defining longitudinal zones. The best-studied DV patterning of cellular and molecular events in the CNS is that that shapes the hindbrain and the spinal cord DV axis, in which distinct neuronal subtypes emerge from pre-patterned progenitor domains at a defined location along the DV axis [37,38,39,40] to govern sensory and motor neural activities, respectively. Although less knowledge exists on the anterior neural tube, recent data indicate that the dorsal or ventral fates of neural progenitors are similarly patterned in the human forebrain [41,42].
1.3. Neuronal Migration and Cortical Layers
The cerebral cortex has an intrinsic functional architecture critical for its proper performance. It is derived from the dorsal and ventral telencephalon. The dorsal telencephalic domain gives rise to the significant neuronal population that will occupy the brain, the excitatory neurons, while the ventral telencephalon includes the ganglionic eminences (GE) from which the minor population of GABAergic interneurons originates. The emergence of different functional areas results from orchestrated events discussed elsewhere [43,44].
Cortical development involves several modes of migration during which cells move away from their place of birth. Migratory events are generally categorized based on their general movement pattern (radial or tangential) or by the characteristic morphological or dynamic behavior of the migrating cells (multipolar migration, somal translocation). An important example of how cell migration shapes the brain is that of the early-born neurons, the Cajal–Retzius cells (CRs). CRs are one of several transient cell populations in the developing brain [45] and spread along the superficial preplate and marginal zone throughout corticogenesis. They are well known for their role as regulators of radial migration by the secretion of an instructive extracellular matrix protein, Reelin, and their direct interaction with the end-feet of the radially migrating cells [46,47]. Additionally, CRs from different origins spread in a complementary manner throughout the cortical surface and set boundaries of functional areas within the cortex [45].
The primordial progenitors of the cortex are the neuroepithelial cells (NECs). NECs are elongated cells that span the entire thickness of the neuroepithelium from the ventricular (apical) surface to the laminal (basal) side [48,49] (Figure 2). During proliferation, the nuclei move within the cells’ cytoplasm, traveling large distances in synchronization with the cell cycle phase [50,51]. Nuclei undergoing mitosis occupy the apical surface. This is followed by an apical-to-basal motion of the daughters’ nuclei and progression of the G1 phase. The S phase occurs as the nuclei reach the basal side of the cells, and G2 occurs during basal-to-apical motion.
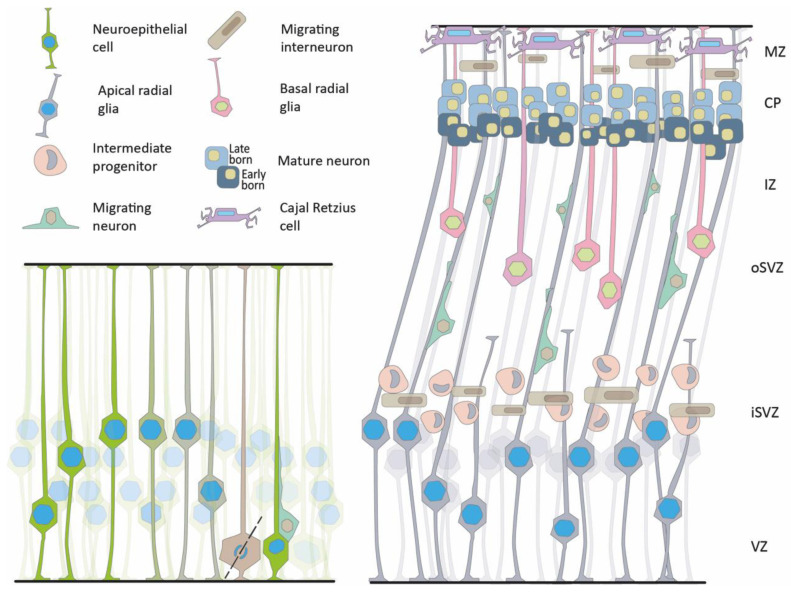
Figure 2.
The neuroepithelium and neuronal migration. Left, schematic presentation of the early neuroepithelium, showing interkinetic nuclear motility and asymmetric cell division leading to the formation of two daughter cells, a progenitor and a migrating neuron. Right, Later stages of brain development show three proliferative areas, the ventricular zone (VZ), the inner subventricular zone (iSVZ), and the outer subventricular zone (oSVZ). Different types of progenitors can be detected in the proliferative zones, radial glia, intermediate progenitors, and basal radial glia. Migrating neurons can be seen in the intermediate zone (IZ). The pyramidal neurons are organized in layers in the forming cortical plate (CP) based on their birth date. Cajal–Retzius cells that are born the earliest are detected in the marginal zone (MZ). Migrating interneurons are visible in two migratory streams.
This cyclic motion, termed interkinetic nuclear migration or motion, was first discovered by Schaper in 1897 and re-discovered by Sauer in 1935 [52,53,54]. Later, the neuroepithelium gives rise to and is eventually replaced by apical radial glia (aRG) that divide symmetrically to increase the pool of progenitors. Alternatively, asymmetrical divisions result in new types of progenitors, including intermediate progenitors (IPs), basal radial glial progenitors (bRGs), or postmitotic neurons. Postmitotic neurons migrate along the radial glial fibers or the bRG to the cortical plate [55]. It has been suggested that during cortical expansion, the processes of the aRG are not reaching the whole width of the cortex; therefore, the migrating neurons possibly migrate along non-continuous radial fibers generated from the bRG [56]. The expanded proliferative area of the oSVZ is considered a new niche of progenitors. It is defined by the expression of specific extracellular matrix proteins that can be involved in forming folds in the human brain [55,57]. The considerable expansion of the outer subventricular zone (oSVZ) is of evolutionary significance in the increase in brain volume as well as in the appearance of cortical gyrification [56,58,59,60,61,62,63,64].
Notch is a central signaling pathway that acts at several key time points during corticogenesis, ensuring progenitor pool expansion and maintenance, differentiation and neurogenesis, as well as folding of the cortex. The Notch pathway was first identified in the fruit fly and is highly conserved [65]. Decades of studies have expanded our understanding of the canonical and noncanonical Notch signaling events, its downstream target genes, regulation, and interplay with other signaling pathways (for recent reviews, see [66,67,68]). In a simplified overview, the canonical Notch involves juxtapositioned signal-sending cells that express the bound ligands of the Delta/Serrate/Lag2 family and a receiving cell that expresses the membrane-bound Notch receptors (Notch1-4). The canonical pathway is direct, namely, following ligand binding, the receptor itself undergoes three cleavages, and its intracellular domain (NICD) directly translocates into the nucleus, releasing corepressor complexes (CSL, CBF-1/suppressor of hairless/Lag1), recruits transcription coactivators (MAMLs, Mastermind-like proteins), and promotes target genes expression. The NICD can remain in the cytoplasm, where it will crosstalk with other signaling pathways [69].
Early studies showed that forced activation of Notch signaling (NICD expression) in the mouse forebrain promoted radial glia identity [70], pointing at Notch’s role during the NEC transition to RG. More recently, in human studies, developmental trajectories based on scRNA seq analysis at early neurogenic timepoints recorded the transcriptional transition from early to mature progenitor populations linking it to the disappearance of the Notch1 inhibitor DLK1 [71].
Notch activity suppresses neurogenesis and promotes the maintenance of neural precursors by activating canonical Notch target genes, Hairy/Enhancer of Split (HES), and Hairy/Enhancer of Split related to the YRPW motif (HEY) [68]. This well-establish notion holds for the significant proliferative zone in the human cortex, the osVZ. oRGs express the Notch effector HES1 and undergo neuronal differentiation following pharmacological inhibition of Notch signaling [71]. Interestingly, Notch signaling in progenitors displays an oscillatory behavior that is critical for its ability to sustain them in the proliferative state [72,73]. Being at the crossroad of fundamental developmental events requires the Notch regulatory network to be intrinsicaly robust and coordinated with other processes. We found that Shootin1 plays a dual role as a Notch activator and that the polarity regulator allows radial migrating cells to transit from a multipolar to a bipolar morphology. The enhancing activity of Shootin involves the promotion of the removal of the Notch inhibitor Numb (via interaction with LNX1) and the protection of NICD from degradation (via interaction with Itch) [74].
Finally, Notch activity was linked to the evolutionary expansion and folding of the human cortex. Disturption of neurogenic symmetry, regardless of bRG expansion, is suggested to allow cortical folding. This is achieved by local disruption of Notch activity while maintaining niches of sustain Notch activity [75]. Three paralogs of human-specific NOTCH2NL were found to be highly expressed in RG and were able to sustain their proliferative state, suggesting a possible contribution to human brain evolution [76,77,78,79].
As radial migration proceeds, early-born neurons will generally contribute to the deep layers, whereas late neurons will migrate through the earlier neurons to form the more superficial layers [80]. This organization is known as an “inside-out” organization, wherein newly generated neurons display a multipolar morphology regulated by subplate neurons. These multipolar neurons migrate slowly in the subventricular and intermediate zones [81]. Approximately 80% of the neurons in the cerebral cortex that compose the population of the excitatory or pyramidal neurons follow this radial migration path [55,56,82,83,84]. The inhibitory or GABAergic neurons are born in the GE and travel longer, tangential, migratory routes. Once the GABAergic neurons reach the cortex, they adopt a radial migration pattern. Their position in the cortical layer matches that of the pyramidal neurons born at a particular birthdate [85]. In humans, there is an expanded oSVZ also in the GE, which is particularly marked by the increased size of the caudal ganglionic eminence. These features allow for a higher proportion of interneurons that populate the human brain [84,86]. Another human-specific phenomenon is the continuation of an extensive migration of interneurons during the early postnatal period, which has not been observed in rodents [87].
1.4. Human Diseases Associated with Forebrain Development
1.4.1. Holoprosencephaly
One group of human genetic diseases that exhibits aberrant signaling related to brain patterning is holoprosencephaly (HPE) [88,89,90,91,92,93]. HPE is the most common malformation of the forebrain in humans, with an embryonic prevalence of 1 to 250 and a live births prevalence of 1 in 10,000–20,000. HPE is manifested by an incomplete cleavage of the forebrain (prosencephalon) into the right and left hemispheres [93]. The disease is very heterogeneous in appearance and severity, and multiple genes and environmental factors are implicated. This condition can be detected usually at low frequency in many domestic animals such as sheep, horses, and cattle, and in extreme cases can be manifested as cyclopia (single-eye). During the 1950s, there were episodic cases of cycloptic lambs that, in some cases, appeared in up to 25% of a flock’s lambs [94,95]. Due to the economic consequences of these malformations, a lengthy investigative process excluded genetic causes and eventually attributed these cases to the first identified Hedgehog-signaling inhibitor, cyclopamine, found in Veratrum californicum plants in the area [96,97]. Over the years, additional environmental factors that can lead to HPE were identified [98]. These included pesticides such as piperonyl butoxide [99], cannabis-derived phytocannabinoids [100], and exposure to retinoid compounds [101]. Alcohol can also be considered an important contributor to HPE and other congenital disabilities [102].
The genetic etiology of HPE is highly heterogenous and may exhibit a highly variable phenotype. Different cytogenic abnormalities are a leading cause of HPE among live-born patients, and the most prominent ones are trisomy 13 and trisomy 18 [93]. Mutations in members of at least four families of morphogens, SHH, FGF, NODAL, and BMP, are involved in the etiology of HPE [103,104,105] (Figure 3).
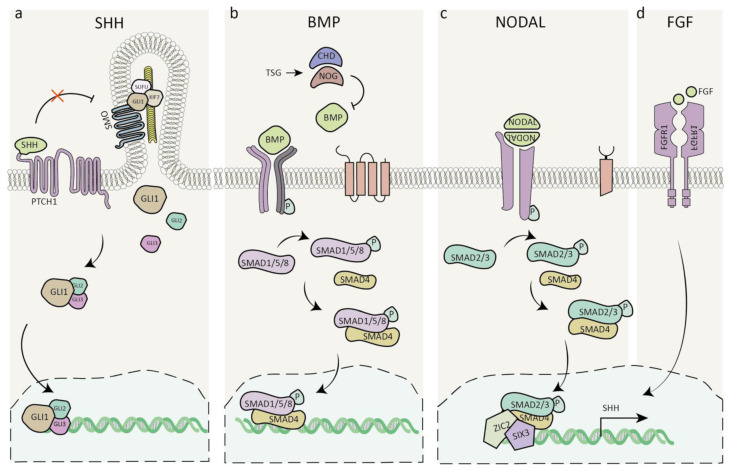
Figure 3.
Signalling pathways involved in holoprosencephaly. (a) SHH signaling pathway. (b) BMP antagonists TSG/CHD/NOG suppress the activation of BMP downstream effectors. (c) Nodal signaling leads to the phosphorylation of smad2/3, which translocates to the nucleus, where it is thought to interact with ZIC2 and regulate SHH expression. (d) FGF signaling maintains SHH expression. Abbreviations: SHH, Sonic hedgehog; GLI1-3, glioma-associated oncogene homolog; PTCH1, Patched1; SMO, Smoothened; SUFU, Suppressor of fused homolog; KIF7, Kinesin family member 7; TSG, Twisted gastrulation; CHD, Chordin; NOG, Noggin; BMP, Bone morphogenic protein; P, Phosphate; SMAD, Mothers against decapentaplegic homolog; NODAL, Nodal growth differentiation factor; ZIC2, Zinc finger protein 2; SIX3, SIX homeobox 3; FGF/FGFR1, Fibroblast growth factor/receptor 1.
The four major mutated genes in HPE are SHH, ZIC2, SIX3, and FGFR1 [93]. SHH was the first HPE gene identified and is the most common one to be mutated [106,107]. Some carriers can be asymptomatic even within the same pedigree, while others exhibit a mild or severe phenotype [93]. It has been hypothesized that mutant proteins can act as modifiers in some cases, and an “autosomal dominant with modifier” model has been suggested [108]. A recent study highlighted two such modifier genes that are positive regulators of SHH signaling. These two genes are highly expressed in LRP2-deficient FVB/N mice, preventing HPE. Both modulators, ULK4 and PTTG1, are microtubule-associated protein components of primary cilia in the neuroepithelium [109]. As another example, among the SHH coreceptors, mutations in CDON and GAS1 were identified to play a role in HPE [110,111,112,113,114,115]; however, mutations in the BOC gene, an SHH gene coreceptor, can modify the observed phenotype [116,117,118,119].
Mutations in the transcription factor ZIC2 are common in HPE. Mice with hypomorphic alleles exhibit middle interhemispheric variant HPE [120,121].
ZIC2 has been suggested to act downstream of NODAL, a secreted member of the transforming growth factor ß (TGF-ß) family, as it is essential for stabilizing the epiblast state and the specification of mesoderm and endoderm, including the PrCP mesoderm, whose instructive functions were discussed. Nodal signals as a heterodimer with TGF-ß family members GDF1/3 and the activated receptor complex phosphorylates the signal transducers Smad2/3 at their C-terminal. ZIC2 possibly acts by direct interaction with SMAD2 and SMAD3 [122]. In addition to its early roles, ZIC2 is working at later stages of brain development and may be involved in limiting Hedgehog signaling [123].
Mutations in SIX3 are another leading cause of HPE [124]. SIX3 is involved in the expression of SHH in the rostral diencephalon ventral midline (in zebrafish) and does so by direct binding to a remote SHH enhancer element (in mice) [125,126]. Mutations in FGFR1 can result in multiple diseases, including HPE [127,128]. FGFR1 belongs to the tyrosine kinase receptor superfamily and contains an extracellular ligand-binding domain and a cytoplasmic domain bearing tyrosine kinase activity. As mentioned above, FGF signaling maintains Shh expression in the prechordal tissue, where it plays a crucial role in the induction of the ventral forebrain [129,130]. Examples of the complex genetics of HPE include documented cases where double mutations in two different genes were reported [131,132]. One of the cases involved a deleterious FGFR1 allele transmitted from one parent and a loss-of-function allele in FGF8 from the other parent [132]. Several such cases with two mutations included the following combinations: FGF8/FGFR1, FGF8/DLL1, DLL1/SHH, DISP1/DISP1, and DISP1/SUFU [128].
Microdeletions and missense mutations in 5′-TG-3′-interacting factor (TGIF) also result in HPE. TGIF interacts with several pathways that are important in the developing brain. TGIF regulates the TGFb/Nodal signaling pathway and SHH signaling independently [133]. In addition, TGIF can function as a repressor to regulate RA-responsive genes [134,135].
BMP signaling has a clear role in brain patterning. Nevertheless, so far, there are no specific mutations in HPE patients related to this pathway. However, several mouse mutants are exhibiting HPE-like phenotypes with mutations in genes associated with this signaling pathway. These mutations include BMP receptors [136], chordin and noggin, antagonists of BMPs in the developing mammalian head [137,138], and mutations in Twisted gastrulation (Tsg) that regulates the pathway through interactions with BMP and chordin [139,140]. In addition, HPE-like phenotypes were observed in mice lacking megalin, which resulted in enhanced Bmp4 expression [141,142].
Mutations in additional genes causing HPE include proteins with several known functions that are likely to regulate the signaling pathways, as mentioned earlier. However, direct connections are yet to be identified. Mutations in CNOT1 that encode for a subunit of the CCR4–NOT Transcription Complex Subunit 1 result in HPE [143,144]. Mutations in members of the cohesin complex of proteins (cohesinopathy), including mutations in STAG2, SMC1A, RAD21, and SMC3, all result in HPE [145,146,147,148]. Loss of function, de novo mutations in protein phosphatase 1 and regulatory subunit 12a (PPP1R12A), an important developmental gene involved in cell migration, adhesion, and morphogenesis, were also associated with HPE [149]. In addition, mutations in the histone lysine methyltransferase 2D (KMT2D) have also been detected in HPE patients [150,151]. In summary, holoprosencephaly can be manifested as an extreme condition. The pathophysiology of the disease process converges on multiple signaling pathways, most of which directly participate in brain patterning.
1.4.2. Retinoic Acid Signaling and Neurodevelopmental Disorders
Consistent with the known functions of RA, loss of RA signaling in the developing fetus has detrimental effects on early arealization and later neurodevelopmental processes. Mutations in genes involved in RA signaling have been implicated in a wide range of diseases, including cancer, metabolic disorders, eye development, retinal function, and neurodegenerative diseases [34,152]. Mutations and gene dosage variation in downstream effectors of RA signaling are implicated in severe developmental syndromes. RAI1 encodes for the transcription factor retinoic acid-induced 1 protein (RAI1). It is involved in both Smith–Magenis syndrome (SMS) and Potocki–Lupski syndrome (PTLS), in which deletions or duplications encompassing the gene were identified, respectively. SMS is a complex disorder characterized by developmental delay, cognitive impairment, and atypical behavioral phenotype [153]. Individuals that suffer from PTLS syndrome have delayed development, mild-to-moderate intellectual disability, behavioral problems, and a high incidence of autism spectrum disorders [154].
Interestingly, abnormal RA signaling is not exclusively the result of a genetic mutation. Exposure of developing fetuses to alcohol is known to cause congenital disabilities and intellectual and neurodevelopmental disabilities. Fetal alcohol syndrome (FASD) was first described in the early 1970s as a specific cluster of congenital disabilities resulting from chronic prenatal alcohol exposure. In the case of the severe form of FASD, the patients have craniofacial malformations (“sentinel facial features”), a high prevalence of microcephaly, prenatal and postnatal growth restriction, and central nervous system neurodevelopmental abnormalities [155]. FASD is considered the leading preventable cause of congenital disabilities and intellectual and neurodevelopmental disabilities, with an estimated global prevalence rate of 0.77%, much higher rates in Europe and North America (1–3%), and lowest rates (0.2%) in countries where religious customs of alcohol abstinence are common. Despite this alarming data, the numbers are considered an underestimation, as the drinking habits of pregnant mothers are often not disclosed [155]. Evidence linking reduced RA signaling during embryogenesis to in utero exposure to alcohol has provided one of the prominent etiological models for the syndrome. Other suggested disease mechanisms consider the maleffects of ethanol on cellular and subcellular levels. As discussed earlier, ethanol itself, rather than its metabolites, is regarded as an HPE-inducing teratogen and can act as a modifier that synergizes with loss-of-function mutations in the SHH signaling pathway [116,156]. An alternative, or rather a complementary model, namely, the reduction of RA during early development, can be linked to multiple disease phenotypes and explain the severe teratogenic outcome of prenatal alcohol exposure [157,158].
The connection between ethanol metabolism and retinol arises from the biochemical similarity between ethanol clearance from the body and RA biosynthesis [158,159]. Ethanol competes for the enzymes with alcohol dehydrogenase activity, suggesting that ethanol or its oxidation metabolite, retinaldehyde, can competitively inhibit the production of retinoic acid [159]. In fact, acetaldehyde is the preferred substrate of RALDH2, one of two retinaldehyde dehydrogenases that oxidize retinaldehyde to form retinoic acid.
1.4.3. Lissencephaly with Regional Gradients of Severity
In the mature brain, the areal organization can be manifested in differences in the cytoarchitecture, cell number, cell density, and lamination in different brain areas. Histological differences define anatomical subdivisions rooted in the developmental phases of forebrain development. They may result in differential sensitivity to pathological mutations or variations in gene dosage that dictate the disease phenotype.
The lissencephaly–pachygyria spectrum of diseases defines a variety of brain malformations that cause relative smoothness of the brain surface and includes lissencephaly (smooth brain surface), agyria (no gyri), and pachygyria (broad gyri) (Figure 4). These brain malformations are partially due to the impairment of neuron migration in the developing brain. Only four layers can be observed instead of the normal six layers in the cortex [160,161].
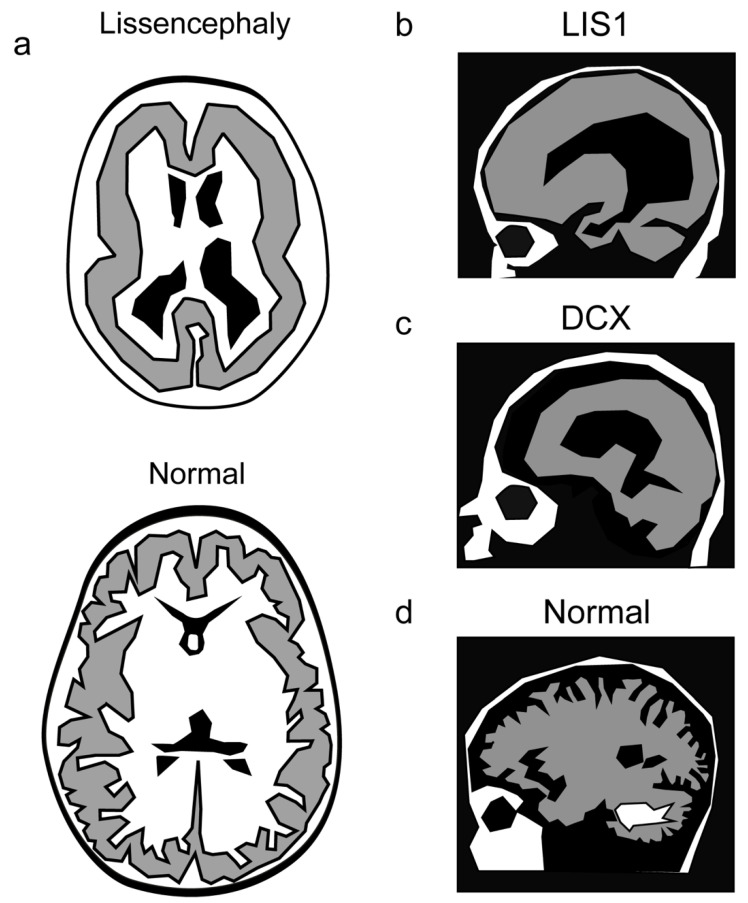
Figure 4.
Lissencephaly manifests with a reduction in the normal brain folds. (a) A schematic of a lissencephalic and a normal brain. (b–d) Mutations in LIS1 result in a more severe phenotype in the caudal part of the brain (b), whereas mutations in DCX affect more the rostral part of the brain (c), as shown in comparison with the normal brain (d).
Several of the lissencephalies exhibit area-specific severity of the phenotype [4,162]. In a few cases, the preferential brain region exhibiting a more pronounced phenotype has been attributed to the expression pattern of the mutated gene or its protein interactor [163]. However, in most cases, the regional preference is not understood and requires additional research.
Regional severity has been noted in lissencephaly caused by mutations in LIS1, Lissencephaly 1 [164], or DCX, Doublecortin [165,166]. LIS1 mutations result in a more severe phenotype in the parietal and occipital cortex, whereas in the case of mutations in DCX, the malformation is more pronounced in the frontal cortex [167,168]. A smooth cerebral surface with a posterior-to-anterior gradient of severity was noted in cases of mutations in ARX [169,170], KIF2A [171], MACF1 [172], DYNC1H1 [173,174,175]. The same trend was noted for mutations in several tubulin genes; TUBA1A [176,177,178], TUBG1 [179], TUBB2B, TUBB3 [180], TUBB5 [181], TUBA8 [182]. Mutations in the centrosomal-associated protein CEP85L result in posterior predominant lissencephaly [183]. A different study demonstrated that the severity gradient reflected the expression pattern and that CEP85L is required to localize and activate the phosphorylation of CDK5 at the centrosome [184].
Anterior-to-posterior increased severity also involves genes of the actin superfamily of proteins: ACTG1 [185], ACTB [185,186], CRADD [187], and RTTN [188].
2. Concluding Remarks
Understanding and identifying developmental principles and signaling pathways that shape the anterior CNS have progressed considerably. The areal organization of the human brain is tightly linked to brain function. Thus, the proper progression of the developmental plan is critical for brain functions. A deeper understanding of the etiology of neurodevelopmental conditions is rooted in uncovering the essential steps during brain patterning. This may be true not only when gross brain abnormalities are seen but also when abnormalities are manifested in subtle arealization defects that are not always appreciated but can profoundly influence an individual’s cognitive and social skills.
Author Contributions
Conceptualization, T.S., D.S.-D. and O.R.; writing—original draft preparation, T.S., D.S.-D. and O.R.; writing—review and editing, T.S., D.S.-D., M.K. and O.R.; visualization, M.K.; funding acquisition, O.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by research grants from the William and Joan Brodsky Foundation and the Edward F. Anixter Family Foundation, the Helen and Martin Kimmel Institute for Stem Cell Research, the Nella and Leon Benoziyo Center for Neurological Diseases, the David and Fela Shapell Family Center for Genetic Disorders Research, the Brenden-Mann Women’s Innovation Impact Fund, The Irving B. Harris Fund for New Directions in Brain Research, the Irving Bieber, M.D. and Toby Bieber, M.D. Memorial Research Fund, The Leff Family, Barbara & Roberto Kaminitz, Sergio & Sônia Lozinsky, Debbie Koren, Jack and Lenore Lowenthal, and the Dears Foundation, a research grant from the Weizmann SABRA—Yeda-Sela—WRC Program, the Estate of Emile Mimran, and The Maurice and Vivienne Wohl Biology Endowment Canadian Institutes of Health Research (CIHR), the International Development Research Centre (IDRC), the Israel Science Foundation (ISF) and the Azrieli Foundation (2397/18), United States-Israel Binational Science Foundation (BSF; Grant No. 2017006).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brodmann K. Brodmann’s Localisation in the Cerebral Cortex. Springer; Lausanne, Switzerland: 1909. [Google Scholar]
- 2.Cadwell C.R., Bhaduri A., Mostajo-Radji M.A., Keefe M.G., Nowakowski T.J. Development and Arealization of the Cerebral Cortex. Neuron. 2019;103:980–1004. doi: 10.1016/j.neuron.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Leary D.D., Chou S.J., Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Klingler E., Francis F., Jabaudon D., Cappello S. Mapping the molecular and cellular complexity of cortical malformations. Science. 2021;371:eaba4517. doi: 10.1126/science.aba4517. [DOI] [PubMed] [Google Scholar]
- 5.Wilson S.W., Houart C. Early steps in the development of the forebrain. Dev. Cell. 2004;6:167–181. doi: 10.1016/S1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puelles L., Martínez S., Martínez-De-La-Torre M., Rubenstein J.L.R. Chapter 1—Gene maps and related histogenetic domains in the forebrain and midbrain. In: Paxinos G., editor. The Rat Nervous System (Fourth Edition) Academic Press; San Diego, CA, USA: 2015. pp. 3–24. [Google Scholar]
- 7.Moore S.A., Iulianella A. Development of the mammalian cortical hem and its derivatives: The choroid plexus, Cajal-Retzius cells and hippocampus. Open Biol. 2021;11:210042. doi: 10.1098/rsob.210042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami Y. The origin of vertebrate brain centers. In: Shigeno S., Murakami Y., Nomura T., editors. Brain Evolution by Design: From Neural Origin to Cognitive Architecture. Springer; Tokyo, Japan: 2017. pp. 215–252. [Google Scholar]
- 9.Puelles L., Rubenstein J.L. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 10.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969;25:1–47. doi: 10.1016/S0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 11.Shilo B.Z., Barkai N. Buffering Global Variability of Morphogen Gradients. Dev. Cell. 2017;40:429–438. doi: 10.1016/j.devcel.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein J.L. Intrinsic and extrinsic control of cortical development. Novartis Found. Symp. 2000;228:67–75, 109–113. doi: 10.1002/0470846631.ch6. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Arrones L., Stern C.D., Bovolenta P., Puelles L. Sharpening of the anterior neural border in the chick by rostral endoderm signalling. Development. 2012;139:1034–1044. doi: 10.1242/dev.067934. [DOI] [PubMed] [Google Scholar]
- 14.Grove E.A., Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu. Rev. Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- 15.O’Leary D.D., Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr. Opin. Neurobiol. 2002;12:14–25. doi: 10.1016/S0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- 16.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 17.Bachler M., Neubuser A. Expression of members of the Fgf family and their receptors during midfacial development. Mech. Dev. 2001;100:313–316. doi: 10.1016/S0925-4773(00)00518-9. [DOI] [PubMed] [Google Scholar]
- 18.Crossley P.H., Martin G.R. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 19.Maruoka Y., Ohbayashi N., Hoshikawa M., Itoh N., Hogan B.L., Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech. Dev. 1998;74:175–177. doi: 10.1016/S0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 20.Meyers E.N., Lewandoski M., Martin G.R. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 21.Garel S., Huffman K.J., Rubenstein J.L. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 22.Storm E.E., Rubenstein J.L., Martin G.R. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proc. Natl. Acad. Sci. USA. 2003;100:1757–1762. doi: 10.1073/pnas.0337736100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storm E.E., Garel S., Borello U., Hebert J.M., Martinez S., McConnell S.K., Martin G.R., Rubenstein J.L. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 24.Shanmugalingam S., Houart C., Picker A., Reifers F., Macdonald R., Barth A., Griffin K., Brand M., Wilson S.W. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development. 2000;127:2549–2561. doi: 10.1242/dev.127.12.2549. [DOI] [PubMed] [Google Scholar]
- 25.Sato T., Kikkawa T., Saito T., Itoi K., Osumi N. Organizing activity of Fgf8 on the anterior telencephalon. Dev. Growth Differ. 2017;59:701–712. doi: 10.1111/dgd.12411. [DOI] [PubMed] [Google Scholar]
- 26.Cholfin J.A., Rubenstein J.L. Patterning of frontal cortex subdivisions by Fgf17. Proc. Natl. Acad. Sci. USA. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangale V.S., Hirokawa K.E., Satyaki P.R., Gokulchandran N., Chikbire S., Subramanian L., Shetty A.S., Martynoga B., Paul J., Mai M.V., et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian L., Remedios R., Shetty A., Tole S. Signals from the edges: The cortical hem and antihem in telencephalic development. Semin. Cell Dev. Biol. 2009;20:712–718. doi: 10.1016/j.semcdb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Q., Guo L., Moore H., Waclaw R.R., Campbell K., Anderson S.A. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith D., Wagner E., Koul O., McCaffery P., Drager U.C. Retinoic acid synthesis for the developing telencephalon. Cereb. Cortex. 2001;11:894–905. doi: 10.1093/cercor/11.10.894. [DOI] [PubMed] [Google Scholar]
- 31.Schneider R.A., Hu D., Rubenstein J.L., Maden M., Helms J.A. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- 32.Haushalter C., Asselin L., Fraulob V., Dolle P., Rhinn M. Retinoic acid controls early neurogenesis in the developing mouse cerebral cortex. Dev. Biol. 2017;430:129–141. doi: 10.1016/j.ydbio.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Shibata M., Pattabiraman K., Lorente-Galdos B., Andrijevic D., Kim S.K., Kaur N., Muchnik S.K., Xing X., Santpere G., Sousa A.M.M., et al. Regulation of prefrontal patterning and connectivity by retinoic acid. Nature. 2021;598:483–488. doi: 10.1038/s41586-021-03953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghyselinck N.B., Duester G. Retinoic acid signaling pathways. Development. 2019;146:dev167502. doi: 10.1242/dev.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimogori T., Banuchi V., Ng H.Y., Strauss J.B., Grove E.A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- 36.Ribes V., Le Roux I., Rhinn M., Schuhbaur B., Dolle P. Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development. 2009;136:665–676. doi: 10.1242/dev.016204. [DOI] [PubMed] [Google Scholar]
- 37.Ulloa F., Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- 38.Dessaud E., McMahon A.P., Briscoe J. Pattern formation in the vertebrate neural tube: A sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch D., Kohl A., Wang Y., Sela-Donenfeld D. Axonal Projection Patterns of the Dorsal Interneuron Populations in the Embryonic Hindbrain. Front. Neuroanat. 2021;15:793161. doi: 10.3389/fnana.2021.793161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Miranda L.R., Muller T., Birchmeier C. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev. Biol. 2017;432:34–42. doi: 10.1016/j.ydbio.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Chi L., Fan B., Feng D., Chen Z., Liu Z., Hui Y., Xu X., Ma L., Fang Y., Zhang Q., et al. The Dorsoventral Patterning of Human Forebrain Follows an Activation/Transformation Model. Cereb. Cortex. 2017;27:2941–2954. doi: 10.1093/cercor/bhw152. [DOI] [PubMed] [Google Scholar]
- 42.De Santis R., Etoc F., Rosado-Olivieri E.A., Brivanlou A.H. Self-organization of human dorsal-ventral forebrain structures by light induced SHH. Nat. Commun. 2021;12:6768. doi: 10.1038/s41467-021-26881-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tole S., Hébert J. Chapter 1—Telencephalon patterning. In: Rubenstein J.L.R., Rakic P., editors. Patterning and Cell Type Specification in the Developing CNS and PNS. Academic Press; Oxford, UK: 2013. pp. 3–24. [Google Scholar]
- 44.Agirman G., Broix L., Nguyen L. Cerebral cortex development: An outside-in perspective. FEBS Lett. 2017;591:3978–3992. doi: 10.1002/1873-3468.12924. [DOI] [PubMed] [Google Scholar]
- 45.Barber M., Pierani A. Tangential migration of glutamatergic neurons and cortical patterning during development: Lessons from Cajal-Retzius cells. Dev. Neurobiol. 2016;76:847–881. doi: 10.1002/dneu.22363. [DOI] [PubMed] [Google Scholar]
- 46.Gil-Sanz C., Franco S.J., Martinez-Garay I., Espinosa A., Harkins-Perry S., Muller U. Cajal-Retzius cells instruct neuronal migration by coincidence signaling between secreted and contact-dependent guidance cues. Neuron. 2013;79:461–477. doi: 10.1016/j.neuron.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Causeret F., Moreau M.X., Pierani A., Blanquie O. The multiple facets of Cajal-Retzius neurons. Development. 2021;148:dev199409. doi: 10.1242/dev.199409. [DOI] [PubMed] [Google Scholar]
- 48.Noctor S.C., Flint A.C., Weissman T.A., Dammerman R.S., Kriegstein A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 49.Taverna E., Götz M., Huttner W.B. The cell biology of neurogenesis: Toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- 50.Frade J.M. Interkinetic nuclear movement in the vertebrate neuroepithelium: Encounters with an old acquaintance. Prog. Brain Res. 2002;136:67–71. doi: 10.1016/s0079-6123(02)36007-2. [DOI] [PubMed] [Google Scholar]
- 51.Baye L.M., Link B.A. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:10143–10152. doi: 10.1523/JNEUROSCI.2754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaper A. The Earliest Differentiation in the Central Nervous System of Vertebrates. Science. 1897;5:430–431. [Google Scholar]
- 53.Sauer F. The interkinetic migration of embryonic epithelial nuclei. J. Morphol. 1936;60:1–11. doi: 10.1002/jmor.1050600102. [DOI] [Google Scholar]
- 54.Sauer F. Mitosis in the neural tube. J. Comp. Neurol. 1935;62:377–405. doi: 10.1002/cne.900620207. [DOI] [Google Scholar]
- 55.Wilsch-Brauninger M., Florio M., Huttner W.B. Neocortex expansion in development and evolution—From cell biology to single genes. Curr. Opin. Neurobiol. 2016;39:122–132. doi: 10.1016/j.conb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Lui J.H., Hansen D.V., Kriegstein A.R. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long K.R., Newland B., Florio M., Kalebic N., Langen B., Kolterer A., Wimberger P., Huttner W.B. Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron. 2018;99:702–719.e6. doi: 10.1016/j.neuron.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Dehay C., Kennedy H., Kosik K.S. The outer subventricular zone and primate-specific cortical complexification. Neuron. 2015;85:683–694. doi: 10.1016/j.neuron.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 59.Li Y., Muffat J., Omer A., Bosch I., Lancaster M.A., Sur M., Gehrke L., Knoblich J.A., Jaenisch R. Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell. 2017;20:385–396.e3. doi: 10.1016/j.stem.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollen A.A., Nowakowski T.J., Chen J., Retallack H., Sandoval-Espinosa C., Nicholas C.R., Shuga J., Liu S.J., Oldham M.C., Diaz A., et al. Molecular identity of human outer radial glia during cortical development. Cell. 2015;163:55–67. doi: 10.1016/j.cell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reillo I., Borrell V. Germinal zones in the developing cerebral cortex of ferret: Ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb. Cortex. 2012;22:2039–2054. doi: 10.1093/cercor/bhr284. [DOI] [PubMed] [Google Scholar]
- 62.Fietz S.A., Kelava I., Vogt J., Wilsch-Brauninger M., Stenzel D., Fish J.L., Corbeil D., Riehn A., Distler W., Nitsch R., et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 63.Fietz S.A., Lachmann R., Brandl H., Kircher M., Samusik N., Schroder R., Lakshmanaperumal N., Henry I., Vogt J., Riehn A., et al. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc. Natl. Acad. Sci. USA. 2012;109:11836–11841. doi: 10.1073/pnas.1209647109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansen D.V., Rubenstein J.L., Kriegstein A.R. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 2011;70:645–660. doi: 10.1016/j.neuron.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guruharsha K.G., Kankel M.W., Artavanis-Tsakonas S. The Notch signalling system: Recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falo-Sanjuan J., Bray S.J. Decoding the Notch signal. Dev. Growth Differ. 2020;62:4–14. doi: 10.1111/dgd.12644. [DOI] [PubMed] [Google Scholar]
- 67.Nian F.S., Hou P.S. Evolving Roles of Notch Signaling in Cortical Development. Front. Neurosci. 2022;16:844410. doi: 10.3389/fnins.2022.844410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou B., Lin W., Long Y., Yang Y., Zhang H., Wu K., Chu Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target Ther. 2022;7:95. doi: 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borggrefe T., Lauth M., Zwijsen A., Huylebroeck D., Oswald F., Giaimo B.D. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFbeta/BMP and hypoxia pathways. Biochim. Biophys. Acta. 2016;1863:303–313. doi: 10.1016/j.bbamcr.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 70.Gaiano N., Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu. Rev. Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 71.Eze U.C., Bhaduri A., Haeussler M., Nowakowski T.J., Kriegstein A.R. Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat. Neurosci. 2021;24:584–594. doi: 10.1038/s41593-020-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kageyama R., Shimojo H., Isomura A. Oscillatory Control of Notch Signaling in Development. Adv. Exp. Med. Biol. 2018;1066:265–277. doi: 10.1007/978-3-319-89512-3_13. [DOI] [PubMed] [Google Scholar]
- 73.Kageyama R., Shimojo H., Ohtsuka T. Dynamic control of neural stem cells by bHLH factors. Neurosci. Res. 2019;138:12–18. doi: 10.1016/j.neures.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Sapir T., Levy T., Kozer N., Shin I., Zamor V., Haffner-Krausz R., McGlade J.C., Reiner O. Notch Activation by Shootin1 Opposing Activities on 2 Ubiquitin Ligases. Cereb. Cortex. 2018;28:3115–3128. doi: 10.1093/cercor/bhx180. [DOI] [PubMed] [Google Scholar]
- 75.Han S., Okawa S., Wilkinson G.A., Ghazale H., Adnani L., Dixit R., Tavares L., Faisal I., Brooks M.J., Cortay V., et al. Proneural genes define ground-state rules to regulate neurogenic patterning and cortical folding. Neuron. 2021;109:2847–2863.e11. doi: 10.1016/j.neuron.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Lodewijk G.A., Fernandes D.P., Vretzakis I., Savage J.E., Jacobs F.M.J. Evolution of Human Brain Size-Associated NOTCH2NL Genes Proceeds toward Reduced Protein Levels. Mol. Biol. Evol. 2020;37:2531–2548. doi: 10.1093/molbev/msaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiddes I.T., Lodewijk G.A., Mooring M., Bosworth C.M., Ewing A.D., Mantalas G.L., Novak A.M., van den Bout A., Bishara A., Rosenkrantz J.L., et al. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell. 2018;173:1356–1369.e1322. doi: 10.1016/j.cell.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Florio M., Heide M., Pinson A., Brandl H., Albert M., Winkler S., Wimberger P., Huttner W.B., Hiller M. Evolution and cell-type specificity of human-specific genes preferentially expressed in progenitors of fetal neocortex. eLife. 2018;7:e32332. doi: 10.7554/eLife.32332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki I.K., Gacquer D., Van Heurck R., Kumar D., Wojno M., Bilheu A., Herpoel A., Lambert N., Cheron J., Polleux F., et al. Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. Cell. 2018;173:1370–1384.e1316. doi: 10.1016/j.cell.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rakic P. Neurons in rhesus monkey visual cortex: Systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 81.Ohtaka-Maruyama C., Okado H. Molecular Pathways Underlying Projection Neuron Production and Migration during Cerebral Cortical Development. Front. Neurosci. 2015;9:447. doi: 10.3389/fnins.2015.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reiner O., Sapir T. Polarity Regulation in Migrating Neurons in the Cortex. Mol. Neurobiol. 2009;40:1–14. doi: 10.1007/s12035-009-8065-0. [DOI] [PubMed] [Google Scholar]
- 83.Reiner O., Karzbrun E., Kshirsagar A., Kaibuchi K. Regulation of neuronal migration, an emerging topic in autism spectrum disorders. J. Neurochem. 2016;136:440–456. doi: 10.1111/jnc.13403. [DOI] [PubMed] [Google Scholar]
- 84.Molnar Z., Clowry G.J., Sestan N., Alzu’bi A., Bakken T., Hevner R.F., Huppi P.S., Kostovic I., Rakic P., Anton E.S., et al. New insights into the development of the human cerebral cortex. J. Anat. 2019;235:432–451. doi: 10.1111/joa.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silva C.G., Peyre E., Adhikari M.H., Tielens S., Tanco S., Van Damme P., Magno L., Krusy N., Agirman G., Magiera M.M., et al. Cell-Intrinsic Control of Interneuron Migration Drives Cortical Morphogenesis. Cell. 2018;172:1063–1078.e1019. doi: 10.1016/j.cell.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dzaja D., Hladnik A., Bicanic I., Bakovic M., Petanjek Z. Neocortical calretinin neurons in primates: Increase in proportion and microcircuitry structure. Front. Neuroanat. 2014;8:103. doi: 10.3389/fnana.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paredes M.F., James D., Gil-Perotin S., Kim H., Cotter J.A., Ng C., Sandoval K., Rowitch D.H., Xu D., McQuillen P.S., et al. Extensive migration of young neurons into the infant human frontal lobe. Science. 2016;354:81–89. doi: 10.1126/science.aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muenke M., Beachy P.A. Genetics of ventral forebrain development and holoprosencephaly. Curr. Opin. Genet Dev. 2000;10:262–269. doi: 10.1016/S0959-437X(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 89.Roessler E., Muenke M. The molecular genetics of holoprosencephaly. Am. J. Med. Genet. C Semin. Med. Genet. 2010;154C:52–61. doi: 10.1002/ajmg.c.30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monuki E.S. The morphogen signaling network in forebrain development and holoprosencephaly. J. Neuropathol. Exp. Neurol. 2007;66:566–575. doi: 10.1097/nen.0b013e3180986e1b. [DOI] [PubMed] [Google Scholar]
- 91.Walsh C.A. Genetic malformations of the human cerebral cortex. Neuron. 1999;23:19–29. doi: 10.1016/S0896-6273(00)80749-7. [DOI] [PubMed] [Google Scholar]
- 92.Golden J.A. Holoprosencephaly: A defect in brain patterning. J. Neuropathol. Exp. Neurol. 1998;57:991–999. doi: 10.1097/00005072-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 93.Kruszka P., Gropman A.L., Muenke M. Cassidy and Allanson’s Management of Genetic Syndromes. Wiley & Sons; Hoboken, NJ, USA: 2021. Holoprosencephaly; pp. 487–503. [Google Scholar]
- 94.Binns W., Thacker E.J., James L.F., Huffman W.T. A congenital cyclopiantype malformation in lambs. J. Am. Vet. Med. Assoc. 1959;134:180–183. [PubMed] [Google Scholar]
- 95.Binns W., James L.F., Shupe J.L., Thacker E.J. Cyclopian-type malformation in lambs. Arch. Environ. Health. 1962;5:106–108. doi: 10.1080/00039896.1962.10663251. [DOI] [PubMed] [Google Scholar]
- 96.Keeler R.F. Cyclopamine and related steroidal alkaloid teratogens: Their occurrence, structural relationship, and biologic effects. Lipids. 1978;13:708–715. doi: 10.1007/BF02533750. [DOI] [PubMed] [Google Scholar]
- 97.Chen J.K. I only have eye for ewe: The discovery of cyclopamine and development of Hedgehog pathway-targeting drugs. Nat. Prod. Rep. 2016;33:595–601. doi: 10.1039/C5NP00153F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beames T.G., Lipinski R.J. Gene-environment interactions: Aligning birth defects research with complex etiology. Development. 2020;147:dev191064. doi: 10.1242/dev.191064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Everson J.L., Sun M.R., Fink D.M., Heyne G.W., Melberg C.G., Nelson K.F., Doroodchi P., Colopy L.J., Ulschmid C.M., Martin A.A., et al. Developmental Toxicity Assessment of Piperonyl Butoxide Exposure Targeting Sonic Hedgehog Signaling and Forebrain and Face Morphogenesis in the Mouse: An in Vitro and in Vivo Study. Environ. Health Perspect. 2019;127:107006. doi: 10.1289/EHP5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khaliullina H., Bilgin M., Sampaio J.L., Shevchenko A., Eaton S. Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc. Natl. Acad. Sci. USA. 2015;112:3415–3420. doi: 10.1073/pnas.1416463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohen M.M., Jr., Sulik K.K. Perspectives on holoprosencephaly: Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J. Craniofac. Genet. Dev. Biol. 1992;12:196–244. [PubMed] [Google Scholar]
- 102.Sulik K.K. Genesis of alcohol-induced craniofacial dysmorphism. Exp. Biol. Med. 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- 103.Grove E.A., Monuki E.S. Chapter 1—Morphogens, patterning centers, and their mechanisms of action. In: Rubenstein J., Rakic P., Chen B., Kwan K.Y., editors. Patterning and Cell Type Specification in the Developing CNS and PNS. 2nd ed. Academic Press; Cambridge, MA, USA: 2020. pp. 3–21. [Google Scholar]
- 104.Barratt K.S., Drover K.A., Thomas Z.M., Arkell R.M. Patterning of the antero-ventral mammalian brain: Lessons from holoprosencephaly comparative biology in man and mouse. WIREs Mech. Dis. 2022:e1552. doi: 10.1002/wsbm.1552. [DOI] [PubMed] [Google Scholar]
- 105.Geng X., Oliver G. Pathogenesis of holoprosencephaly. J. Clin. Investig. 2009;119:1403–1413. doi: 10.1172/JCI38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roessler E., Belloni E., Gaudenz K., Jay P., Berta P., Scherer S.W., Tsui L.C., Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat. Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 107.Belloni E., Muenke M., Roessler E., Traverso G., Siegel-Bartelt J., Frumkin A., Mitchell H.F., Donis-Keller H., Helms C., Hing A.V., et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat. Genet. 1996;14:353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- 108.Roessler E., Velez J.I., Zhou N., Muenke M. Utilizing prospective sequence analysis of SHH, ZIC2, SIX3 and TGIF in holoprosencephaly probands to describe the parameters limiting the observed frequency of mutant genexgene interactions. Mol. Genet. Metab. 2012;105:658–664. doi: 10.1016/j.ymgme.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mecklenburg N., Kowalczyk I., Witte F., Gorne J., Laier A., Mamo T.M., Gonschior H., Lehmann M., Richter M., Sporbert A., et al. Identification of disease-relevant modulators of the SHH pathway in the developing brain. Development. 2021;148:dev199307. doi: 10.1242/dev.199307. [DOI] [PubMed] [Google Scholar]
- 110.Bae G.U., Domene S., Roessler E., Schachter K., Kang J.S., Muenke M., Krauss R.S. Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am. J. Hum. Genet. 2011;89:231–240. doi: 10.1016/j.ajhg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cole F., Krauss R.S. Microform holoprosencephaly in mice that lack the Ig superfamily member Cdon. Curr. Biol. 2003;13:411–415. doi: 10.1016/S0960-9822(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 112.Pineda-Alvarez D.E., Roessler E., Hu P., Srivastava K., Solomon B.D., Siple C.E., Fan C.M., Muenke M. Missense substitutions in the GAS1 protein present in holoprosencephaly patients reduce the affinity for its ligand, SHH. Hum. Genet. 2012;131:301–310. doi: 10.1007/s00439-011-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ribeiro L.A., Quiezi R.G., Nascimento A., Bertolacini C.P., Richieri-Costa A. Holoprosencephaly and holoprosencephaly-like phenotype and GAS1 DNA sequence changes: Report of four Brazilian patients. Am. J. Med. Genet. A. 2010;152A:1688–1694. doi: 10.1002/ajmg.a.33466. [DOI] [PubMed] [Google Scholar]
- 114.Martinelli D.C., Fan C.M. A sonic hedgehog missense mutation associated with holoprosencephaly causes defective binding to GAS1. J. Biol. Chem. 2009;284:19169–19172. doi: 10.1074/jbc.C109.011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seppala M., Depew M.J., Martinelli D.C., Fan C.M., Sharpe P.T., Cobourne M.T. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J. Clin. Investig. 2007;117:1575–1584. doi: 10.1172/JCI32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hong M., Srivastava K., Kim S., Allen B.L., Leahy D.J., Hu P., Roessler E., Krauss R.S., Muenke M. BOC is a modifier gene in holoprosencephaly. Hum. Mutat. 2017;38:1464–1470. doi: 10.1002/humu.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seppala M., Xavier G.M., Fan C.M., Cobourne M.T. Boc modifies the spectrum of holoprosencephaly in the absence of Gas1 function. Biol. Open. 2014;3:728–740. doi: 10.1242/bio.20147989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang W., Hong M., Bae G.U., Kang J.S., Krauss R.S. Boc modifies the holoprosencephaly spectrum of Cdo mutant mice. Dis. Model. Mech. 2011;4:368–380. doi: 10.1242/dmm.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Echevarria-Andino M.L., Allen B.L. The hedgehog co-receptor BOC differentially regulates SHH signaling during craniofacial development. Development. 2020;147:dev189076. doi: 10.1242/dev.189076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nagai T., Aruga J., Minowa O., Sugimoto T., Ohno Y., Noda T., Mikoshiba K. Zic2 regulates the kinetics of neurulation. Proc. Natl. Acad. Sci. USA. 2000;97:1618–1623. doi: 10.1073/pnas.97.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elms P., Siggers P., Napper D., Greenfield A., Arkell R. Zic2 is required for neural crest formation and hindbrain patterning during mouse development. Dev. Biol. 2003;264:391–406. doi: 10.1016/j.ydbio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 122.Houtmeyers R., Tchouate Gainkam O., Glanville-Jones H.A., Van den Bosch B., Chappell A., Barratt K.S., Souopgui J., Tejpar S., Arkell R.M. Zic2 mutation causes holoprosencephaly via disruption of NODAL signalling. Hum. Mol. Genet. 2016;25:3946–3959. doi: 10.1093/hmg/ddw235. [DOI] [PubMed] [Google Scholar]
- 123.Sedykh I., Yoon B., Roberson L., Moskvin O., Dewey C.N., Grinblat Y. Zebrafish zic2 controls formation of periocular neural crest and choroid fissure morphogenesis. Dev. Biol. 2017;429:92–104. doi: 10.1016/j.ydbio.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wallis D.E., Roessler E., Hehr U., Nanni L., Wiltshire T., Richieri-Costa A., Gillessen-Kaesbach G., Zackai E.H., Rommens J., Muenke M. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat. Genet. 1999;22:196–198. doi: 10.1038/9718. [DOI] [PubMed] [Google Scholar]
- 125.Geng X., Speirs C., Lagutin O., Inbal A., Liu W., Solnica-Krezel L., Jeong Y., Epstein D.J., Oliver G. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev. Cell. 2008;15:236–247. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jeong Y., Leskow F.C., El-Jaick K., Roessler E., Muenke M., Yocum A., Dubourg C., Li X., Geng X., Oliver G., et al. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat. Genet. 2008;40:1348–1353. doi: 10.1038/ng.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Courage C., Jackson C.B., Owczarek-Lipska M., Jamsheer A., Sowinska-Seidler A., Piotrowicz M., Jakubowski L., Dalleves F., Riesch E., Neidhardt J., et al. Novel synonymous and missense variants in FGFR1 causing Hartsfield syndrome. Am. J. Med. Genet. A. 2019;179:2447–2453. doi: 10.1002/ajmg.a.61354. [DOI] [PubMed] [Google Scholar]
- 128.Dubourg C., Carre W., Hamdi-Roze H., Mouden C., Roume J., Abdelmajid B., Amram D., Baumann C., Chassaing N., Coubes C., et al. Mutational Spectrum in Holoprosencephaly Shows That FGF is a New Major Signaling Pathway. Hum. Mutat. 2016;37:1329–1339. doi: 10.1002/humu.23038. [DOI] [PubMed] [Google Scholar]
- 129.Ellis P.S., Burbridge S., Soubes S., Ohyama K., Ben-Haim N., Chen C., Dale K., Shen M.M., Constam D., Placzek M. ProNodal acts via FGFR3 to govern duration of Shh expression in the prechordal mesoderm. Development. 2015;142:3821–3832. doi: 10.1242/dev.119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gutin G., Fernandes M., Palazzolo L., Paek H., Yu K., Ornitz D.M., McConnell S.K., Hebert J.M. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- 131.Mouden C., de Tayrac M., Dubourg C., Rose S., Carre W., Hamdi-Roze H., Babron M.C., Akloul L., Heron-Longe B., Odent S., et al. Homozygous STIL mutation causes holoprosencephaly and microcephaly in two siblings. PLoS ONE. 2015;10:e0117418. doi: 10.1371/journal.pone.0117418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hong S., Hu P., Marino J., Hufnagel S.B., Hopkin R.J., Toromanovic A., Richieri-Costa A., Ribeiro-Bicudo L.A., Kruszka P., Roessler E., et al. Dominant-negative kinase domain mutations in FGFR1 can explain the clinical severity of Hartsfield syndrome. Hum. Mol. Genet. 2016;25:1912–1922. doi: 10.1093/hmg/ddw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wotton D., Taniguchi K. Functions of TGIF homeodomain proteins and their roles in normal brain development and holoprosencephaly. Am. J. Med. Genet. C Semin. Med. Genet. 2018;178:128–139. doi: 10.1002/ajmg.c.31612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bartholin L., Powers S.E., Melhuish T.A., Lasse S., Weinstein M., Wotton D. TGIF inhibits retinoid signaling. Mol. Cell Biol. 2006;26:990–1001. doi: 10.1128/MCB.26.3.990-1001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gongal P.A., Waskiewicz A.J. Zebrafish model of holoprosencephaly demonstrates a key role for TGIF in regulating retinoic acid metabolism. Hum. Mol. Genet. 2008;17:525–538. doi: 10.1093/hmg/ddm328. [DOI] [PubMed] [Google Scholar]
- 136.Fernandes M., Gutin G., Alcorn H., McConnell S.K., Hebert J.M. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- 137.Anderson R.M., Lawrence A.R., Stottmann R.W., Bachiller D., Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- 138.Bachiller D., Klingensmith J., Kemp C., Belo J.A., Anderson R.M., May S.R., McMahon J.A., McMahon A.P., Harland R.M., Rossant J., et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- 139.Zakin L., De Robertis E.M. Inactivation of mouse Twisted gastrulation reveals its role in promoting Bmp4 activity during forebrain development. Development. 2004;131:413–424. doi: 10.1242/dev.00946. [DOI] [PubMed] [Google Scholar]
- 140.Petryk A., Anderson R.M., Jarcho M.P., Leaf I., Carlson C.S., Klingensmith J., Shawlot W., O’Connor M.B. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev. Biol. 2004;267:374–386. doi: 10.1016/j.ydbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 141.Willnow T.E., Hilpert J., Armstrong S.A., Rohlmann A., Hammer R.E., Burns D.K., Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc. Natl. Acad. Sci. USA. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spoelgen R., Hammes A., Anzenberger U., Zechner D., Andersen O.M., Jerchow B., Willnow T.E. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–414. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- 143.De Franco E., Watson R.A., Weninger W.J., Wong C.C., Flanagan S.E., Caswell R., Green A., Tudor C., Lelliott C.J., Geyer S.H., et al. A Specific CNOT1 Mutation Results in a Novel Syndrome of Pancreatic Agenesis and Holoprosencephaly through Impaired Pancreatic and Neurological Development. Am. J. Hum. Genet. 2019;104:985–989. doi: 10.1016/j.ajhg.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kruszka P., Berger S.I., Weiss K., Everson J.L., Martinez A.F., Hong S., Anyane-Yeboa K., Lipinski R.J., Muenke M. A CCR4-NOT Transcription Complex, Subunit 1, CNOT1, Variant Associated with Holoprosencephaly. Am. J. Hum. Genet. 2019;104:990–993. doi: 10.1016/j.ajhg.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cratsenberg D.M., Winningham P.J., Starr L.J. Second reported individual with a partial STAG2 deletion: Middle interhemispheric variant holoprosencephaly in STAG2-related cohesinopathy. Clin. Dysmorphol. 2021;30:159–163. doi: 10.1097/MCD.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 146.Kruszka P., Berger S.I., Casa V., Dekker M.R., Gaesser J., Weiss K., Martinez A.F., Murdock D.R., Louie R.J., Prijoles E.J., et al. Cohesin complex-associated holoprosencephaly. Brain. 2019;142:2631–2643. doi: 10.1093/brain/awz210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krab L.C., Marcos-Alcalde I., Assaf M., Balasubramanian M., Andersen J.B., Bisgaard A.M., Fitzpatrick D.R., Gudmundsson S., Huisman S.A., Kalayci T., et al. Delineation of phenotypes and genotypes related to cohesin structural protein RAD21. Hum. Genet. 2020;139:575–592. doi: 10.1007/s00439-020-02138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kruszka P. Reply: Another case of holoprosencephaly associated with RAD21 loss-of-function variant. Brain. 2020;143:e65. doi: 10.1093/brain/awaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hughes J.J., Alkhunaizi E., Kruszka P., Pyle L.C., Grange D.K., Berger S.I., Payne K.K., Masser-Frye D., Hu T., Christie M.R., et al. Loss-of-Function Variants in PPP1R12A: From Isolated Sex Reversal to Holoprosencephaly Spectrum and Urogenital Malformations. Am J Hum Genet. 2020;106:121–128. doi: 10.1016/j.ajhg.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tekendo-Ngongang C., Kruszka P., Martinez A.F., Muenke M. Novel heterozygous variants in KMT2D associated with holoprosencephaly. Clin. Genet. 2019;96:266–270. doi: 10.1111/cge.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tan Y.C., Chow V.T. Novel human HALR (MLL3) gene encodes a protein homologous to ALR and to ALL-1 involved in leukemia, and maps to chromosome 7q36 associated with leukemia and developmental defects. Cancer Detect. Prev. 2001;25:454–469. [PubMed] [Google Scholar]
- 152.Das B.C., Thapa P., Karki R., Das S., Mahapatra S., Liu T.C., Torregroza I., Wallace D.P., Kambhampati S., Van Veldhuizen P., et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014;22:673–683. doi: 10.1016/j.bmc.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Falco M., Amabile S., Acquaviva F. RAI1 gene mutations: Mechanisms of Smith-Magenis syndrome. Appl. Clin. Genet. 2017;10:85–94. doi: 10.2147/TACG.S128455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Potocki L., Neira-Fresneda J., Yuan B. Potocki-Lupski syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Mirzaa G., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. [PubMed] [Google Scholar]
- 155.Wozniak J.R., Riley E.P., Charness M.E. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 2019;18:760–770. doi: 10.1016/S1474-4422(19)30150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hong M., Krauss R.S. Ethanol itself is a holoprosencephaly-inducing teratogen. PLoS ONE. 2017;12:e0176440. doi: 10.1371/journal.pone.0176440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Petrelli B., Bendelac L., Hicks G.G., Fainsod A. Insights into retinoic acid deficiency and the induction of craniofacial malformations and microcephaly in fetal alcohol spectrum disorder. Genesis. 2019;57:e23278. doi: 10.1002/dvg.23278. [DOI] [PubMed] [Google Scholar]
- 158.Fainsod A., Bendelac-Kapon L., Shabtai Y. Fetal Alcohol Spectrum Disorder: Embryogenesis Under Reduced Retinoic Acid Signaling Conditions. Subcell. Biochem. 2020;95:197–225. doi: 10.1007/978-3-030-42282-0_8. [DOI] [PubMed] [Google Scholar]
- 159.Shabtai Y., Fainsod A. Competition between ethanol clearance and retinoic acid biosynthesis in the induction of fetal alcohol syndrome. Biochem. Cell Biol. 2018;96:148–160. doi: 10.1139/bcb-2017-0132. [DOI] [PubMed] [Google Scholar]
- 160.Reiner O., Sapir T. LIS1 functions in normal development and disease. Curr. Opin. Neurobiol. 2013;23:951–956. doi: 10.1016/j.conb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 161.Di Donato N., Chiari S., Mirzaa G.M., Aldinger K., Parrini E., Olds C., Barkovich A.J., Guerrini R., Dobyns W.B. Lissencephaly: Expanded imaging and clinical classification. Am. J. Med. Genet. A. 2017;173:1473–1488. doi: 10.1002/ajmg.a.38245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Francis F., Cappello S. Neuronal migration and disorders—An update. Curr. Opin. Neurobiol. 2021;66:57–68. doi: 10.1016/j.conb.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 163.Yeo N.C., Chavez A., Lance-Byrne A., Chan Y., Menn D., Milanova D., Kuo C.C., Guo X., Sharma S., Tung A., et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods. 2018;15:611–616. doi: 10.1038/s41592-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W.B., Caskey C.T., Ledbetter D.H. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 165.Des Portes V., Francis F., Pinard J.M., Desguerre I., Moutard M.L., Snoeck I., Meiners L.C., Capron F., Cusmai R., Ricci S., et al. Doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH) Hum. Mol. Genet. 1998;7:1063–1070. doi: 10.1093/hmg/7.7.1063. [DOI] [PubMed] [Google Scholar]
- 166.Gleeson J.G., Allen K.M., Fox J.W., Lamperti E.D., Berkovic S., Scheffer I., Cooper E.C., Dobyns W.B., Minnerath S.R., Ross M.E., et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/S0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 167.Dobyns W.B., Truwit C.L., Ross M.E., Matsumoto N., Pilz D.T., Ledbetter D.H., Gleeson J.G., Walsh C.A., Barkovich A.J. Differences in the gyral pattern distinguish chromosome 17-linked and X-linked lissencephaly. Neurology. 1999;53:270–277. doi: 10.1212/WNL.53.2.270. [DOI] [PubMed] [Google Scholar]
- 168.Pilz D.T., Matsumoto N., Minnerath S., Mills P., Gleeson J.G., Allen K.M., Walsh C.A., Barkovich A.J., Dobyns W.B., Ledbetter D.H., et al. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum. Mol. Genet. 1998;7:2029–2037. doi: 10.1093/hmg/7.13.2029. [DOI] [PubMed] [Google Scholar]
- 169.Bonneau D., Toutain A., Laquerriere A., Marret S., Saugier-Veber P., Barthez M.A., Radi S., Biran-Mucignat V., Rodriguez D., Gelot A. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): Clinical, magnetic resonance imaging, and neuropathological findings. Ann. Neurol. 2002;51:340–349. doi: 10.1002/ana.10119. [DOI] [PubMed] [Google Scholar]
- 170.Dobyns W.B., Berry-Kravis E., Havernick N.J., Holden K.R., Viskochil D. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia. Am. J. Med. Genet. 1999;86:331–337. doi: 10.1002/(SICI)1096-8628(19991008)86:4<331::AID-AJMG7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 171.Cavallin M., Bijlsma E.K., El Morjani A., Moutton S., Peeters E.A., Maillard C., Pedespan J.M., Guerrot A.M., Drouin-Garaud V., Coubes C., et al. Recurrent KIF2A mutations are responsible for classic lissencephaly. Neurogenetics. 2017;18:73–79. doi: 10.1007/s10048-016-0499-8. [DOI] [PubMed] [Google Scholar]
- 172.Dobyns W.B., Aldinger K.A., Ishak G.E., Mirzaa G.M., Timms A.E., Grout M.E., Dremmen M.H.G., Schot R., Vandervore L., van Slegtenhorst M.A., et al. MACF1 Mutations Encoding Highly Conserved Zinc-Binding Residues of the GAR Domain Cause Defects in Neuronal Migration and Axon Guidance. Am. J. Hum. Genet. 2018;103:1009–1021. doi: 10.1016/j.ajhg.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jamuar S.S., Lam A.T., Kircher M., D’Gama A.M., Wang J., Barry B.J., Zhang X., Hill R.S., Partlow J.N., Rozzo A., et al. Somatic mutations in cerebral cortical malformations. N. Engl. J. Med. 2014;371:733–743. doi: 10.1056/NEJMoa1314432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Poirier K., Lebrun N., Broix L., Tian G., Saillour Y., Boscheron C., Parrini E., Valence S., Pierre B.S., Oger M., et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat. Genet. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Willemsen M.H., Vissers L.E., Willemsen M.A., van Bon B.W., Kroes T., de Ligt J., de Vries B.B., Schoots J., Lugtenberg D., Hamel B.C., et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J. Med. Genet. 2012;49:179–183. doi: 10.1136/jmedgenet-2011-100542. [DOI] [PubMed] [Google Scholar]
- 176.Sohal A.P., Montgomery T., Mitra D., Ramesh V. TUBA1A mutation-associated lissencephaly: Case report and review of the literature. Pediatr. Neurol. 2012;46:127–131. doi: 10.1016/j.pediatrneurol.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 177.Morris-Rosendahl D.J., Najm J., Lachmeijer A.M., Sztriha L., Martins M., Kuechler A., Haug V., Zeschnigk C., Martin P., Santos M., et al. Refining the phenotype of alpha-1a Tubulin (TUBA1A) mutation in patients with classical lissencephaly. Clin. Genet. 2008;74:425–433. doi: 10.1111/j.1399-0004.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 178.Lecourtois M., Poirier K., Friocourt G., Jaglin X., Goldenberg A., Saugier-Veber P., Chelly J., Laquerriere A. Human lissencephaly with cerebellar hypoplasia due to mutations in TUBA1A: Expansion of the foetal neuropathological phenotype. Acta Neuropathol. 2010;119:779–789. doi: 10.1007/s00401-010-0684-z. [DOI] [PubMed] [Google Scholar]
- 179.Brock S., Stouffs K., Scalais E., D’Hooghe M., Keymolen K., Guerrini R., Dobyns W.B., Di Donato N., Jansen A.C. Tubulinopathies continued: Refining the phenotypic spectrum associated with variants in TUBG1. Eur. J. Hum. Genet. 2018;26:1132–1142. doi: 10.1038/s41431-018-0146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fallet-Bianco C., Laquerriere A., Poirier K., Razavi F., Guimiot F., Dias P., Loeuillet L., Lascelles K., Beldjord C., Carion N., et al. Mutations in tubulin genes are frequent causes of various foetal malformations of cortical development including microlissencephaly. Acta Neuropathol. Commun. 2014;2:69. doi: 10.1186/2051-5960-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Breuss M., Heng J.I., Poirier K., Tian G., Jaglin X.H., Qu Z., Braun A., Gstrein T., Ngo L., Haas M., et al. Mutations in the beta-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep. 2012;2:1554–1562. doi: 10.1016/j.celrep.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abdollahi M.R., Morrison E., Sirey T., Molnar Z., Hayward B.E., Carr I.M., Springell K., Woods C.G., Ahmed M., Hattingh L., et al. Mutation of the variant alpha-tubulin TUBA8 results in polymicrogyria with optic nerve hypoplasia. Am. J. Hum. Genet. 2009;85:737–744. doi: 10.1016/j.ajhg.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tsai M.H., Muir A.M., Wang W.J., Kang Y.N., Yang K.C., Chao N.H., Wu M.F., Chang Y.C., Porter B.E., Jansen L.A., et al. Pathogenic Variants in CEP85L Cause Sporadic and Familial Posterior Predominant Lissencephaly. Neuron. 2020;106:237–245.e238. doi: 10.1016/j.neuron.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kodani A., Kenny C., Lai A., Gonzalez D.M., Stronge E., Sejourne G.M., Isacco L., Partlow J.N., O’Donnell A., McWalter K., et al. Posterior Neocortex-Specific Regulation of Neuronal Migration by CEP85L Identifies Maternal Centriole-Dependent Activation of CDK5. Neuron. 2020;106:246–255.e246. doi: 10.1016/j.neuron.2020.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Riviere J.B., van Bon B.W., Hoischen A., Kholmanskikh S.S., O’Roak B.J., Gilissen C., Gijsen S., Sullivan C.T., Christian S.L., Abdul-Rahman O.A., et al. De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat. Genet. 2012;44:440–444. doi: 10.1038/ng.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Forman M.S., Squier W., Dobyns W.B., Golden J.A. Genotypically defined lissencephalies show distinct pathologies. J. Neuropathol. Exp. Neurol. 2005;64:847–857. doi: 10.1097/01.jnen.0000182978.56612.41. [DOI] [PubMed] [Google Scholar]
- 187.Di Donato N., Jean Y.Y., Maga A.M., Krewson B.D., Shupp A.B., Avrutsky M.I., Roy A., Collins S., Olds C., Willert R.A., et al. Mutations in CRADD Result in Reduced Caspase-2-Mediated Neuronal Apoptosis and Cause Megalencephaly with a Rare Lissencephaly Variant. Am. J. Hum. Genet. 2016;99:1117–1129. doi: 10.1016/j.ajhg.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vandervore L.V., Schot R., Kasteleijn E., Oegema R., Stouffs K., Gheldof A., Grochowska M.M., van der Sterre M.L.T., van Unen L.M.A., Wilke M., et al. Heterogeneous clinical phenotypes and cerebral malformations reflected by rotatin cellular dynamics. Brain. 2019;142:867–884. doi: 10.1093/brain/awz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.