Abstract
Rationale & Objective
Some US hemodialysis (HD) facilities switched from oral cinacalcet to intravenous etelcalcetide as the primary calcimimetic therapy to control parathyroid hormone (PTH) levels after the introduction of etelcalcetide in 2017. Although clinical trials have demonstrated the superior efficacy of etelcalcetide versus cinacalcet, evidence comparing real-world effectiveness is lacking.
Study Design
Prospective cohort.
Setting & Participants
Patients receiving HD enrolled in US Dialysis Outcomes and Practice Patterns Study facilities.
Exposure
We classified HD facilities on the basis of whether >75% of calcimimetic users were prescribed etelcalcetide (“etelcalcetide-first”) or cinacalcet (“cinacalcet-first”) from March-August 2019.
Outcomes
PTH, calcium, and phosphorus levels among calcimimetic users, all averaged in the 6 months after the exposure assessment period.
Analytical Approach
We used adjusted linear regression to compare outcomes using 2 approaches: (1) cross-sectional comparison of etelcalcetide-first and cinacalcet-first HD facilities; (2) pre-post comparison of HD facilities that switched from cinacalcet-first to etelcalcetide-first using facilities that remained cinacalcet-first as a comparison group.
Results
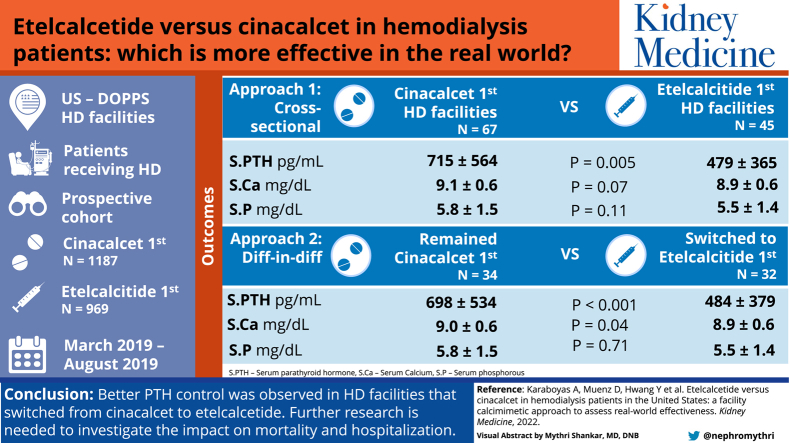
We identified 45 etelcalcetide-first and 67 cinacalcet-first HD facilities; etelcalcetide-first (vs cinacalcet-first) facilities were more likely to be from small or independent dialysis organizations (86% vs 22%) and had higher total calcimimetic use (43% vs 29%) and lower active vitamin D use (66% vs 82%). In the cross-sectional analysis comparing etelcalcetide-first and cinacalcet-first HD facilities, the adjusted mean difference in PTH levels was −115 pg/mL (95% CI, −196 to −34) and the prevalence of a PTH level of >600 pg/mL was lower (prevalence difference, −11.4%; 95% CI, −19.3% to −3.5%). Among facilities that switched to etelcalcetide-first, the mean PTH level decreased from 671 to 484 pg/mL and the prevalence of a PTH level of >600 pg/mL decreased from 39% to 21%. Among facilities that remained cinacalcet-first, the mean PTH level increased from 632 to 698 pg/mL and the prevalence of a PTH level of >600 pg/mL increased from 37% to 43%. The adjusted difference-in-difference between the switch to etelcalcetide-first and the continuation of cinacalcet-first was −169 pg/mL (−249 to −90 pg/mL) for the mean PTH and −14.4% (−22.0% to −6.8%) for a PTH level of >600 pg/mL. We also observed slightly lower serum calcium levels and minimal differences in serum phosphorus levels between the etelcalcetide-first and the cinacalcet-first facilities.
Limitations
Residual confounding.
Conclusions
We observed better PTH control in HD facilities that switched from using cinacalcet to etelcalcetide as the primary calcimimetic therapy. Further research is needed to investigate how the greater real-world effectiveness of intravenous etelcalcetide (vs oral cinacalcet) may affect clinical outcomes.
Index Words: Calcimimetics, calcium, cinacalcet, etelcalcetide, phosphorus, PTH
Graphical abstract
Plain-Language Summary.
Elevated parathyroid hormone levels are associated with higher mortality risk and can be treated with 2 types of calcimimetic medications: oral cinacalcet, limited by patient nonadherence, and intravenous etelcalcetide, a newer but more expensive drug shown to be superior to cinacalcet in a randomized trial setting. To gain real-world data insights, we performed a natural experiment by leveraging variation in US hemodialysis facility calcimimetic preference and found that facilities that switched from cinacalcet to etelcalcetide as the primary calcimimetic therapy had lower parathyroid hormone levels than facilities that continued to use cinacalcet as the primary calcimimetic therapy following the introduction of etelcalcetide in 2017. Despite the demonstrated comparative effectiveness, etelcalcetide use remains limited because of cost incentives in the US dialysis setting.
Secondary hyperparathyroidism is common among patients receiving hemodialysis (HD),1 particularly in the United States where parathyroid hormone (PTH) levels of >600 pg/mL have become increasingly common in the past decade.2 PTH levels of >600 pg/mL are beyond the upper limit of the KDIGO (Kidney Disease Improving Global Outcomes) clinical practice guidelines (2-9 times the normal range, equating to ∼130-585 pg/mL)3 and have been associated with elevated mortality risk in multiple large observational studies of patients receiving HD.4
Calcimimetic therapy is often used to control PTH levels in patients receiving HD; oral cinacalcet therapy was approved for US commercial use in 2004, whereas intravenous (IV) etelcalcetide was introduced in 2017. Etelcalcetide was shown to be safe and effective in randomized trials,5, 6, 7 demonstrating superior efficacy compared with placebo8 and cinacalcet.9 Daily oral cinacalcet is susceptible to challenges of self-management and adherence.10, 11, 12, 13 IV etelcalcetide has a longer half-life14,15 and is administered thrice weekly at the end of the HD session, thus improving adherence and decreasing pill burden.
Etelcalcetide use in the US HD setting must be placed into the context of payment reimbursement. Under the Medicare end-stage renal disease prospective payment system introduced in 2011, oral renal medications without an injectable equivalent, such as cinacalcet, were reimbursed outside the bundled payment via Medicare Part D.16 The introduction of IV etelcalcetide triggered a transitional drug add-on payment adjustment period during which calcimimetics were reimbursed under Medicare Part B for 3 years (January 1, 2018-January 1, 2021) before the fixed bundled payment was adjusted to include both drugs.16 As a result of these incentives, etelcalcetide use increased dramatically throughout 2018, particularly in small and independent dialysis organizations that may have had greater flexibility to evaluate etelcalcetide during the transitional drug add-on payment adjustment period.17 Etelcalcetide use remained high (20%-25%) in small and independent dialysis organizations in 2019 despite the new availability of generic cinacalcet.17 With etelcalcetide use expected to be disincentivized in favor of cinacalcet following the expiration of the transitional drug add-on payment adjustment period on January 1, 2021, evidence comparing outcomes between the 2 calcimimetics is needed.16
In this study, we investigated the comparative effectiveness of etelcalcetide versus cinacalcet in terms of PTH control and other mineral and bone disorder (MBD) markers, including serum calcium and phosphorus. A prior study of US patients receiving HD showed that PTH levels are very high among etelcalcetide initiators (median 762 [interquartile range: 510-1158 pg/mL]),16 likely because etelcalcetide may, in some facilities, be reserved for only those with the highest PTH levels. We performed a natural experiment to limit the impact of this confounding by indication by leveraging the variation in HD facility calcimimetic preference. We identified and compared US HD facilities that transitioned most or all of their calcimimetic users to etelcalcetide (“etelcalcetide-first”) with facilities that continued to use cinacalcet as the primary calcimimetic therapy (“cinacalcet-first”) to address the hypothesis that etelcalcetide is more effective at reducing PTH levels than cinacalcet in the real-world setting.
Methods
Data Source
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is an international prospective cohort study of adult in-center patients receiving HD, ongoing since 1996.18, 19, 20, 21 Study approval and patient consent were obtained as required by Ethical and Independent (E&I) Review Services. In this analysis, the source population of potentially eligible patients included those receiving HD in US DOPPS facilities between October 2016 and February 2020 (most updated DOPPS data when beginning analysis). This US DOPPS sample consisted of data from 3 electronic health record sources, including 2 large dialysis organizations and a separate data extract from medium/small dialysis organizations and independent units.
Study Design
To evaluate the comparative effectiveness of etelcalcetide versus cinacalcet in terms of PTH control, we used 2 complementary approaches: (1) Between-facility cross-sectional comparison—do HD facilities that use etelcalcetide as the primary calcimimetic therapy have better PTH control than HD facilities that use cinacalcet as the primary calcimimetic therapy?; (2) Within-facility pre-post comparison—do HD facilities that transitioned from cinacalcet to etelcalcetide as the primary calcimimetic therapy have subsequently better PTH control than HD facilities that continued to use cinacalcet as the primary calcimimetic therapy?
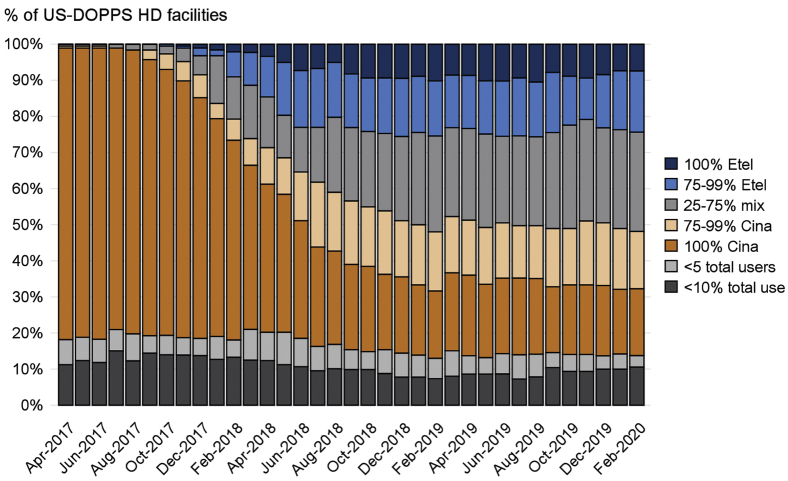
Etelcalcetide use was first observed in US DOPPS facilities in April 2017, with prevalence steadily increasing through early 2019. Figure 1 illustrates that many facilities transitioned all, or nearly all, of their calcimimetic users from cinacalcet to etelcalcetide by early 2019 (“etelcalcetide-first”). In contrast, other facilities continued to prescribe cinacalcet as the primary calcimimetic therapy (“cinacalcet-first”), providing us the opportunity for a natural experiment. On the basis of these empirical data (Fig 1), we chose to utilize the most contemporary data for approach 1 (2019+, after etelcalcetide use had plateaued). For approach 2, we defined a period 1 (pre-April 2017, to coincide with the pre-etelcalcetide era) and period 2 (2019+, as the post period) to evaluate changes in PTH control among HD facilities that switched to etelcalcetide as the primary calcimimetic therapy using facilities that continued to use cinacalcet as the comparison group.
Figure 1.
Facility calcimimetic type in US hemodialysis (HD) facilities from April 2017 to February 2020. The denominator is the total number of enrolled US Dialysis Outcomes and Practice Patterns Study (DOPPS) HD facilities each month. Proportions represent the percentage of calcimimetic users within each facility prescribed etelcalcetide (Etel) or cinacalcet (Cina), which will always sum to 100% (e.g., 90% Cina implies 10% Etel). We did not calculate % Etel/Cina for HD facilities with <5 total users or <10% total calcimimetic use because facility calcimimetic preference could not be reliably defined.
Exposure
For both approaches, the exposure of interest was HD facility calcimimetic preference. We defined “etelcalcetide-first” HD facilities as >75% of calcimimetic users prescribed etelcalcetide and “cinacalcet-first” HD facilities as >75% of calcimimetic users prescribed cinacalcet. We used 75% as the cut-off to flag HD facilities where a large majority of patients were treated with 1 type of calcimimetic, indicating a clear facility preference. This 75% cut-off point was chosen as a compromise between 50% (larger sample, but no clear preference) and 100% (very clear preference, but limited sample size). We excluded facilities with little to no calcimimetic use (<10% use or <5 total users) because calcimimetic preference could not be reliably defined and facilities with no clear calcimimetic preference (25%-75% etelcalcetide use among calcimimetic users). Calcimimetic type was assessed during a single 6-month run-in period for approach 1 (March-August 2019) and during 2 distinct 6-month run-in periods for approach 2 corresponding to before (period 1: May-October 2016) and after (period 2: March-August 2019) the introduction of etelcalcetide in the United States. Facility percent etelcalcetide use during each run-in period was based on the total number of patient-months with a prescription for etelcalcetide divided by the total number of patient-months with a prescription for any calcimimetic.
Outcomes
The primary outcome was serum intact PTH level, and secondary outcomes included albumin-corrected serum calcium and serum phosphorus. All MBD marker outcomes were averaged across all available measurements over the 6 months after each exposure run-in period; patients were not required to survive or remain in the study during the entire 6-month outcome ascertainment period, and all patients were weighted equally regardless of the number of PTH measurements during the outcome ascertainment period. These patient-level outcomes were assessed as the difference in mean values and as the difference in proportion above the target range, corresponding to KDIGO guideline recommendations for the upper limit of PTH level (approximately >600 pg/mL), lower limit of serum calcium (<8.4 mg/dL), and upper limit of serum phosphorus (>5.5 mg/dL).3,22
Inclusion/Exclusion Criteria
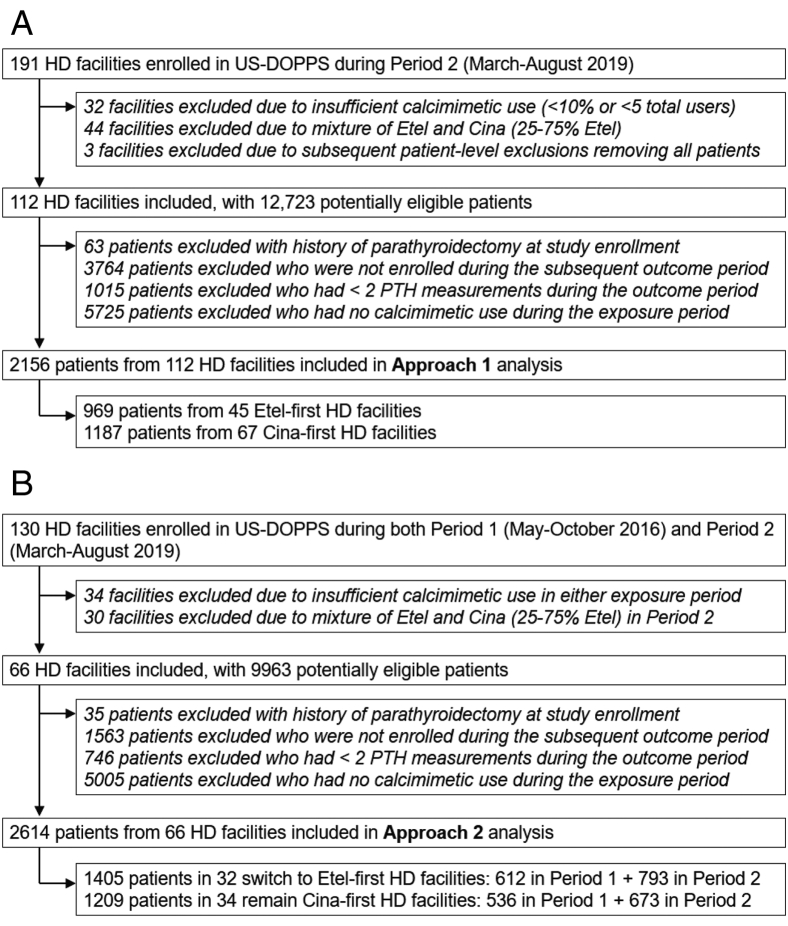
Patients aged 18 years and older undergoing maintenance HD in a US DOPPS facility during the study period were eligible for this analysis. To address our research question in an appropriate target population, patients were required to have been prescribed a calcimimetic at any time during the 6-month exposure run-in period and to have at least 2 PTH measurements (to provide a more stable estimate of PTH control) during the subsequent outcome ascertainment period. Patients with a prior history of parathyroidectomy at study enrollment were excluded. All the inclusion/exclusion criteria were implemented separately for approach 1 and approach 2 and separately for period 1 and period 2 within approach 2 (Fig 2).
Figure 2.
Flow chart illustrating the inclusion/exclusion criteria for HD facilities and patients in (A) approach 1 and (B) approach 2. Exclusions were first made at the HD facility level to identify eligible facilities, and then at the patient-level to identify eligible patients within these facilities. Approach 1 was a cross-sectional analysis focused on Period 2 (March-August 2019). Approach 2 was a pre-post analysis where HD facilities were required to be eligible in both periods whereas individual patients could contribute to one or both periods depending on their period-specific eligibility.
Statistical Analysis
When treating the outcome (PTH, calcium, phosphorus) as a continuous or binary variable, we used linear mixed models with a random facility intercept; for binary outcomes, these linear models are often referred to as “linear probability models.” Models were adjusted for both facility-level confounders (dialysis organization size, facility size, facility percent Black race, hospital-based, and facility percent total calcimimetic use) and patient-level confounders (age, sex, Black race, dialysis vintage, body mass index, serum albumin, hemoglobin, serum potassium, 13 summary comorbid conditions, and catheter use). As a secondary analysis, we also present models that include potential mediators (facility percent vitamin D use, facility percent calcium-based and non–calcium-based phosphate binder use, and facility mean dialysate calcium concentration) that may be potentially influenced by facility calcimimetic preference. Additionally, we performed a sensitivity analysis stratifying the results by dialysis organization size to confirm that the results were robust to any imbalance of facility calcimimetic preference by dialysis organization size.
For approach 1, the parameter of interest was the coefficient of an indicator variable for the etelcalcetide-first (reference group: cinacalcet-first) HD facility. For approach 2, we used a difference-in-differences approach to compare the pre-post (period 1 to period 2) difference in MBD marker outcomes in HD facilities that switched from cinacalcet-first to etelcalcetide-first with that in facilities that remained cinacalcet-first in period 2. The difference-in-differences approach implicitly accounts for differences in period 1 PTH levels by estimating the between-group difference between their differences in period 2 versus period 1 PTH levels. The parameter of interest for approach 2 was the interaction effect between period and facility calcimimetic preference, which reflects whether the within-facility change in PTH levels from period 1 to period 2 differed for facilities that switched from cinacalcet-first to etelcalcetide-first versus facilities that remained cinacalcet-first in both periods.
Results
Study Sample
For approach 1, our study sample included 2,156 calcimimetic users: 969 patients from 45 etelcalcetide-first facilities and 1,187 patients from 67 cinacalcet-first facilities. We excluded 38 HD facilities with little to no calcimimetic use (<10% use or <5 total users) because calcimimetic preference could not be reliably defined and 44 HD facilities with no clear calcimimetic preference (25%-75% etelcalcetide use among calcimimetic users). For approach 2, our study sample included 34 HD facilities that were cinacalcet-first in both period 1 and period 2 and 32 facilities that were cinacalcet-first in period 1 but switched to etelcalcetide-first in period 2. The number of calcimimetic users eligible for the approach 2 analysis ranged from 500-800 per period in each group (Fig 2).
Patient and Facility Characteristics
The mean (standard deviation) dose among users was 15.6 (9.2) mg/week for etelcalcetide and 45.6 (32.9) mg/day for cinacalcet. The proportion of calcimimetic users who initiated treatment during the 6-month exposure period was 16% in etelcalcetide-first facilities and 15% in cinacalcet-first facilities. Other patient and facility characteristics for etelcalcetide-first and cinacalcet-first HD facilities are shown in Table 1. Etelcalcetide-first (vs cinacalcet-first) HD facilities were much less likely to be affiliated with a large or medium dialysis organization (10+ affiliated HD facilities) (14% vs 78%) and thus also had a higher proportion that was hospital-based (28% vs 4%). Etelcalcetide-first (vs cinacalcet-first) facilities also had a higher proportion of patients prescribed any calcimimetic (43% vs 29%) and a lower proportion of patients prescribed active vitamin D (66% vs 82%). Patients in etelcalcetide-first (vs cinacalcet-first) facilities tended to be older (64 vs 59 years) and less likely Black (36% vs 45%), with a shorter median dialysis vintage (3.6 vs 4.7 years). Similar patterns were observed when comparing HD facilities that switched to etelcalcetide-first with those that remained cinacalcet-first (Table S1).
Table 1.
Patient and Facility Characteristics in Etelcalcetide-First Versus Cinacalcet-First HD Facilities
| Characteristics | Cinacalcet-First (n = 1,187) | Etelcalcetide-First (n = 969) |
|---|---|---|
| N facilities | 67 | 45 |
| Demographics | ||
| Age (y) | 59 ± 14 | 64 ± 14 |
| Sex (% male) | 56% | 56% |
| Race (% Black) | 45% | 36% |
| Dialysis vintage (y) | 4.7 (2.6-7.8) | 3.6 (1.8-7.0) |
| Body mass index (kg/m2) | 29.6 ± 7.7 | 30.6 ± 7.9 |
| Comorbid history (%) | ||
| Coronary artery disease | 27% | 23% |
| Cerebrovascular disease | 7% | 9% |
| Heart failure | 20% | 24% |
| Peripheral vascular disease | 15% | 11% |
| Hypertension | 92% | 81% |
| Other cardiovascular disease | 14% | 25% |
| Cancer (nonskin) | 5% | 5% |
| Diabetes | 63% | 62% |
| Gastrointestinal bleeding | 6% | 7% |
| Lung disease | 5% | 8% |
| Neurologic disease | 9% | 4% |
| Psychiatric disorder | 26% | 27% |
| Recurrent cellulitis, gangrene | 9% | 6% |
| Markers of nutrition and inflammation | ||
| Serum albumin (g/dL) | 3.9 ± 0.3 | 3.8 ± 0.3 |
| Hemoglobin (g/dL) | 10.8 ± 1.0 | 10.7 ± 1.1 |
| Serum potassium (mEq/L) | 4.8 ± 0.5 | 4.7 ± 0.5 |
| Dialysis treatments | ||
| Catheter use (%) | 10% | 17% |
| Facility-level characteristics | ||
| Dialysis organization size (% LDO/MDO vs SDO/Ind) | 78% | 14% |
| Facility size (N patients) | 87 ± 40 | 73 ± 31 |
| Facility % Black race | 38 ± 30 | 32 ± 30 |
| Facility % calcimimetic use | 29 ± 10 | 43 ± 17 |
| Facility % vitamin D use | 82 ± 9 | 66 ± 14 |
| Facility % phosphate binder (calcium-based) use | 47 ± 28 | 52 ± 23 |
| Facility % phosphate binder (non–calcium-based) use | 47 ± 22 | 49 ± 21 |
| Facility mean dialysate calcium (mEq/L) | 2.5 ± 0.1 | 2.6 ± 0.2 |
| Facility type (% hospital-based) | 4% | 28% |
| Facility location (% rural) | 19% | 8% |
Note: Results are shown as mean ± standard deviation, median (interquartile range), or percentage. The proportion with missing data was <2% for all variables, except for Black race (14%) and dialysis vintage (7%).
Abbreviations: HD, hemodialysis; Ind, independent HD units; LDO/MDO, large/medium dialysis organizations with 10+ affiliated HD units; SDO, small dialysis organizations (<10 affiliated units).
Approach 1: Between-Facility Cross-Sectional Comparison
MBD markers in etelcalcetide-first versus cinacalcet-first HD facilities are summarized in Table 2. During the outcome ascertainment period (September 2019-February 2020), the mean PTH was 479 pg/mL in etelcalcetide-first facilities compared with 715 pg/mL in cinacalcet-first facilities. The proportion of calcimimetic users with a PTH level of >600 pg/mL was 21% in etelcalcetide-first facilities and 44% in cinacalcet-first facilities. Comparing etelcalcetide-first with cinacalcet-first facilities in models adjusted for confounders, the mean PTH difference was −115 (95% confidence interval [CI], −196 to −34) pg/mL and the prevalence difference of PTH >600 pg/mL was −11.4% (95% CI, −19.3% to −3.5%). Adjusted mean levels of serum calcium and serum phosphorus were slightly lower in etelcalcetide-first versus cinacalcet-first facilities (Table 2). Because of the substantial differences between the crude and adjusted estimates, we summarized the impact of stepwise adjustment for different sets of covariates; the large crude association with PTH was somewhat attenuated by adjustment for facility characteristics (eg, dialysis organization size, total calcimimetic use) rather than patient factors (Table S2). The adjusted mean PTH difference comparing etelcalcetide-first and cinacalcet-first facilities was similar among large and medium dialysis organizations (−121; 95% CI, −250 to 8) and among small and independent dialysis organizations (−142; 95% CI, −266 to −17) (Table S3).
Table 2.
MBD Marker Outcomes in Etelcalcetide-First Versus Cinacalcet-First US HD Facilities: Approach 1 Results.
| Etelcalcetide-First HD Facilities | Cinacalcet-First HD Facilities | Adjusted Difference (95% CI) | P | |
|---|---|---|---|---|
| N facilities | 45 | 67 | -- | -- |
| N calcimimetic users | 969 | 1,187 | -- | -- |
| Continuous outcomes | ||||
| PTH (pg/mL) | 479 ± 365 | 715 ± 564 | −115 (−196, −34) | 0.005 |
| Serum Ca (mg/dL) | 8.9 ± 0.6 | 9.1 ± 0.6 | −0.12 (−0.25, 0.01) | 0.07 |
| Serum P (mg/dL) | 5.5 ± 1.4 | 5.8 ± 1.5 | −0.18 (−0.40, 0.04) | 0.11 |
| Binary outcomes | ||||
| PTH >600 pg/mL | 21% | 44% | −11.4% (−19.3, −3.5) | 0.005 |
| Ca <8.4 mg/dL | 21% | 13% | 5.0% (−3.0, 13.0) | 0.22 |
| P >5.5 mg/dL | 45% | 53% | −1.1% (−8.0, 5.8) | 0.76 |
Note: Results are shown as crude mean ± standard deviation and prevalence (%). Linear mixed models with random facility intercept adjusted for HD facility characteristics (dialysis organization size, facility size, facility % Black race, hospital-based, facility % total calcimimetic use) and patient characteristics (age, sex, Black race, dialysis vintage, body mass index, serum albumin, hemoglobin, serum potassium, 13 summary comorbid conditions, catheter use). After implementing inclusion criteria requiring PTH data, >99% of patients also had data on serum calcium and serum phosphorus.
Abbreviations: Ca, calcium; CI, confidence interval; HD, hemodialysis; P, phosphorus; PTH, parathyroid hormone.
Approach 2: Within-Facility Pre-Post Comparison
MBD markers in HD facilities that switched to etelcalcetide-first versus those that remained cinacalcet-first are summarized in Table 3. From period 1 to period 2, the mean PTH level decreased from 671 to 484 pg/mL in facilities that switched to etelcalcetide-first and increased from 632 to 698 pg/mL in facilities that remained cinacalcet-first. The adjusted difference-in-difference between facilities that switched versus those that continued was −169 (95% CI, −249 to −90) pg/mL. From period 1 to period 2, the percentage of calcimimetic users with PTH level >600 pg/mL decreased from 39% to 21% in facilities that switched to etelcalcetide-first and increased from 37% to 43% in facilities that remained cinacalcet-first. The adjusted difference-in-difference for percent PTH >600 pg/mL was −14.5% (95% CI, −22.4% to −6.7%). Serum calcium levels were slightly lower in facilities that switched to etelcalcetide-first versus those that remained cinacalcet-first, whereas serum phosphorus was not associated with facility calcimimetic preference (Table 3). As in approach 1, the large crude association with PTH was attenuated more so by adjustment for facility characteristics than patient factors (Table S4).
Table 3.
MBD Marker Outcomes in US HD Facilities That Switched to Etelcalcetide-First Versus Remained Cinacalcet-First: Approach 2 Results.
| Switched to Etelcalcetide-first | Remained Cinacalcet-first | Adjusted Diff-in-diff (95% CI) | P | |||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | |||
| N facilities | 32 | 32 | 34 | 34 | -- | -- |
| N calcimimetic users | 612 | 793 | 536 | 673 | -- | -- |
| Continuous outcomes | ||||||
| PTH (pg/mL) | 671 ± 580 | 484 ± 379 | 632 ± 463 | 698 ± 534 | −169 (−249, −90) | <0.001 |
| Serum Ca (mg/dL) | 9.1 ± 0.6 | 8.9 ± 0.6 | 9.1 ± 0.5 | 9.0 ± 0.6 | −0.10 (−0.20, −0.01) | 0.04 |
| Serum P (mg/dL) | 5.5 ± 1.3 | 5.5 ± 1.4 | 5.6 ± 1.4 | 5.8 ± 1.5 | 0.04 (−0.17, 0.25) | 0.71 |
| Binary outcomes | ||||||
| PTH >600 pg/mL | 39% | 21% | 37% | 43% | −14.4% (−22.0, −6.8) | <0.001 |
| Ca <8.4 mg/dL | 12% | 19% | 10% | 13% | 5.5% (−0.2, 11.3) | 0.06 |
| P >5.5 mg/dL | 48% | 46% | 48% | 53% | −1.9% (−9.6, 5.8) | 0.62 |
Note: Results shown as crude mean ± standard deviation and prevalence (%) in period 1 and period 2 columns; Linear mixed models with random facility intercept adjusted for HD facility characteristics (dialysis organization size, facility size, facility % Black race, hospital-based, facility % total calcimimetic use) and patient characteristics (age, sex, Black race, dialysis vintage, body mass index, serum albumin, hemoglobin, serum potassium, 13 summary comorbid conditions, catheter use); Adjusted diff-in-diff (95% CI) parameter derived from the interaction effect between period and facility calcimimetic preference. Abbreviations: Ca, calcium; CI, confidence interval; HD, hemodialysis; P, phosphorus; PTH, parathyroid hormone; Outcomes in period 1 assessed November 2016-April 2017; outcomes in period 2 assessed September 2019-February 2020. After implementing inclusion criteria requiring PTH data, >99% of patients also had data on serum calcium and serum phosphorus.
Discussion
In this large cohort of US patients receiving HD, we identified HD facilities that transitioned from cinacalcet to etelcalcetide as the primary calcimimetic therapy (etelcalcetide-first), and found that PTH levels in these facilities were considerably lower than those in HD facilities that continued to use cinacalcet as the primary calcimimetic therapy (cinacalcet-first). Results were consistent with our hypothesis that IV etelcalcetide would have greater effectiveness, with respect to PTH control, than oral cinacalcet in the real-world setting, likely because of a combination of greater efficacy9 and improved adherence to IV compared with oral administration. When comparing etelcalcetide-first to cinacalcet-first HD facilities, we also observed slightly lower serum calcium levels and a minimal difference in phosphorus levels.
We leveraged variation in US HD facility practice to conduct a natural experiment following the introduction of etelcalcetide therapy in 2017. Etelcalcetide is often reserved for patients with severe secondary hyperparathyroidism16; many patients are prescribed etelcalcetide as a last resort because alternative therapies (potentially including cinacalcet) have not effectively reduced their PTH levels. Thus, a standard approach directly comparing initiators of etelcalcetide to cinacalcet would likely be severely confounded by indication. By isolating HD facilities with a clear preference for one calcimimetic, the prescribed calcimimetic type for patients included in our study was more likely determined by facility preference rather than biased by patient indication.
Effect estimates for the difference in PTH levels—∼115 pg/mL in approach 1 and ∼170 pg/mL in approach 2—were even larger before covariate adjustment. Given the HD facility-based design, it was important to account for differences in not only patient characteristics but also facility characteristics that may have influenced both facility calcimimetic preference and PTH levels, such as dialysis organization size and overall percent calcimimetic use. While imbalance between etelcalcetide-first and cinacalcet-first HD facilities was observed for other facility-level characteristics (eg, percent active vitamin D use), we considered these factors to be possible mediators rather than confounders (eg, greater etelcalcetide use may effectively reduce PTH levels such that less vitamin D is required); thus, in the primary analyses, we did not adjust for these variables thought to be in the causal pathway. Nonetheless, adjustment for these potential mediators had minimal impact on our results (Tables S2, S4).
While Block et al9 demonstrated greater efficacy of etelcalcetide than cinacalcet in reducing PTH levels, real-world evidence has been limited to a handful of small studies evaluating the impact of individual patients switching from cinacalcet to etelcalcetide.23,24 To our knowledge, this is the first large-scale study to formally evaluate the comparative effectiveness of etelcalcetide versus cinacalcet in a real-world setting. These prior small studies,23,24 as well as a meta-analysis of randomized trials25 all demonstrated better PTH control—with a potentially higher risk of hypocalcemia—with etelcalcetide than with cinacalcet. Our results were consistent with these studies with respect to PTH control although we found only modestly lower (∼0.1 mg/dL) levels of albumin-adjusted serum calcium levels in etelcalcetide-first versus cinacalcet-first facilities, perhaps because of an increase in medications to control calcium levels.17
The greater effectiveness of etelcalcetide compared to cinacalcet is likely driven by a combination of superior efficacy9 and increased adherence to a medication administered intravenously (vs orally). Indeed, prior work by Arenas et al24 showed that patients switching from cinacalcet to etelcalcetide had improved PTH control—dramatically better among patients who were not adherent to cinacalcet, but also better among patients who were adherent to cinacalcet. In our facility calcimimetic preference study, decisions to “switch” from cinacalcet to etelcalcetide were made largely at the HD facility level, and thus unlikely due predominantly to tolerability or adherence among individual patients.
Transitioning from cinacalcet to etelcalcetide as the primary calcimimetic therapy may improve the achievement of KDIGO guideline recommendations,3 which has been relatively poor in the United States where >20% of patients have PTH >600 pg/mL.2 Elevated mortality risk at PTH >600 pg/mL has been demonstrated in large observational studies of patients receiving HD,4,26 though treatment with cinacalcet (vs placebo) did not result in a statistically significant reduction in the composite outcome of mortality + cardiovascular events in the EVOLVE trial (hazard ratio, 0.93; 95% CI, 0.85-1.02), despite effectively reducing PTH levels (EVOLVE 2012).27 One may speculate that initiating etelcalcetide as the primary calcimimetic therapy, rather than in patients “failing” cinacalcet, may help to control PTH levels before they become more difficult to lower into the target range, potentially by limiting the development of parathyroid gland hyperplasia. Our results showing slightly lower serum calcium levels in etelcalcetide-first than in cinacalcet-first HD facilities also underscore the importance of monitoring calcium levels following etelcalcetide initiation, though trials have shown a low frequency of symptomatic hypocalcemia.5,6
Our study had some general limitations. First, residual confounding may have biased results, given the observational study design. We implemented 2 distinct approaches, with complementary strengths and weaknesses, and accounted for many potential confounders to mitigate this potential bias. Second, we do not have access to provider protocols to understand motivations for specific treatment strategies. We thus chose to define preference (etelcalcetide-first vs cinacalcet-first) empirically, based on actual usage patterns. Third, the PTH assay was not standardized across US DOPPS facilities, so interassay variability may have resulted in measurement error, which would artificially increase variability in the outcome but would be unlikely to bias results in either direction. Fourth, while the mean PTH models could be biased due to the skewed distribution of PTH, we also identified strong associations between facility calcimimetic preference and the binary outcome (PTH >600 pg/mL), confirming that the associations estimated in the mean PTH models were not driven by outlier values. Our study also had some approach-specific limitations. For approach 1, we observed differences between etelcalcetide-first and cinacalcet-first facilities—many of which were likely driven by small and independent dialysis organizations being far more likely than large/medium dialysis organizations to transition to etelcalcetide as the primary calcimimetic therapy. Reasons for this discrepancy in treatment protocols were not studied; we adjusted for many confounders, including facility percentage total calcimimetic use, but may not have been able to fully account for differences in the patient populations (including socioeconomic status) and other facility protocols/practices. However, we demonstrated the robustness of results within strata of dialysis organization size (Table S3). For approach 2 (evaluating change within facilities), differences between facilities were mitigated, but differences in PTH levels between period 1 and period 2 may have been influenced by other factors changing within the same facility that may not have been fully accounted for in our models.
Our study also had some strengths. First, we took advantage of the introduction and adoption of etelcalcetide in some, but not all, US HD facilities to conduct this natural experiment. Second, the large US DOPPS sample allowed us to compare calcimimetic users across >100 US HD facilities. We excluded many facilities where calcimimetic preference could not be reliably defined, either because of little to no calcimimetic use or a balanced mix (25%-75%) of etelcalcetide and cinacalcet use among calcimimetic users. Although it may appear that results are no longer fully generalizable to the US HD population once all of these facilities were excluded, our approach required the comparison of facilities with a clear calcimimetic preference, and so these excluded facilities would have biased results. Third, the detailed collection of demographics, comorbidities, labs, and treatments in the DOPPS allowed us to account for many potential confounders. Finally, we utilized 2 distinct approaches that complemented one another, as each approach addressed limitations inherent to the other approach: approach 1 used a cross-sectional between-facility design to compare etelcalcetide-first versus cinacalcet-first HD facilities, while approach 2 used a difference-in-differences design to assess within-facility changes in PTH levels among HD facilities that transitioned from cinacalcet-first to etelcalcetide-first, using those that remained cinacalcet-first as the comparison group. Obtaining qualitatively similar results when using different methods with unique strengths and weaknesses strengthens our findings.28
Our results demonstrate superior real-world effectiveness of etelcalcetide versus cinacalcet as the primary calcimimetic therapy to control PTH levels, using an innovative HD facility calcimimetic preference approach. Although superior efficacy of etelcalcetide compared with that of cinacalcet has been shown in a randomized trial,9 we extended these results to a real-world setting, where the advantages of etelcalcetide versus cinacalcet could be expected to be even more pronounced because of better adherence of IV versus oral medications. Despite advantages in efficacy and effectiveness, etelcalcetide use remains limited, largely because of the cost environment in the United States.29 With the transitional drug add-on payment adjustment period expiring on January 1, 2021, continued monitoring and analysis of trends in MBD markers and treatments will be needed to assess the impact of incentivizing the use of generic oral cinacalcet versus a more expensive, but more effective, medication.16 Further research is also needed to investigate the degree to which the greater real-world effectiveness of IV etelcalcetide (vs oral cinacalcet) may be driven by therapy adherence and the impact on clinical outcomes.
Article Information
Authors’ Full Names and Academic Degrees
Angelo Karaboyas, PhD, Daniel Muenz, PhD, Yunji Hwang, PhD, William Goodman, MD, Sunfa Cheng, MD, Pooja Desai, PhD, Kathleen M. Fox, PhD, Bruce M. Robinson, MD, and Ronald L. Pisoni, PhD.
Authors’ Contributions
Research idea and study design: AK, BMR, RLP; data acquisition: AK, DM, BMR, RLP; data analysis/interpretation: AK, DM, YH, WG, SC, PD, KMF, BMR, RLP; statistical analysis: DM; supervision or mentorship: AK, BMR, RLP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study was funded by Amgen. Arbor Research authors developed the study design, carried out analyses, and wrote the manuscript; Amgen authors provided feedback on the study design and manuscript drafts without restrictions on publishing this work. Global support for the ongoing Dialysis Outcomes and Practice Patterns Study (DOPPS) Program is provided without restriction on publications by a consortium of industry and public funders. See https://www.dopps.org/AboutUs/Support.aspx for a full list of up-to-date funders. As of May 29, 2021, these include the following: Amgen Inc, United States (since 1996, founding sponsor); Astellas Pharma Inc, Japan; AstraZeneca Pharmaceuticals LP, United Kingdom; Baxter Healthcare Corp, United States; Bayer Yakuhin, Ltd, Japan; Cara Therapeutics, Inc; Chugai Pharmaceutical Co, Ltd, Japan; GlaxoSmithKline LLC, United Kingdom; Horizon Therapeutics USA, Inc; Italian Society of Nephrology; Japanese Society for Peritoneal Dialysis; JMS Co, Ltd; Kidney Research UK; Kidney Foundation Japan; Kissei Pharmaceutical Co, Ltd; Kyowa Kirin Co, Ltd (since 1999 for Japan DOPPS); Merck Sharp & Dohme Corp; Nikkiso Co, Ltd; ONO Pharmaceutical Co, Ltd; Terumo Corporation; Torii Pharmaceutical Co, Ltd; and Vifor-Fresenius Medical Care Renal Pharma Ltd. Public funding and support has been provided for specific DOPPS projects, ancillary studies, or affiliated research projects by the following: France: French National Institute of Health and Medical Research (Inserm); Thailand: Thailand Research Foundation, Chulalongkorn University Matching Fund, King Chulalongkorn Memorial Hospital Matching Fund, and National Research Council of Thailand; United Kingdom: National Institute for Health Research via the Comprehensive Clinical Research Network; United States: Agency for Healthcare Research and Quality and National Institutes of Health.
Financial Disclosure
Drs Karaboyas, Muenz, Robinson, and Pisoni are employees of Arbor Research Collaborative for Health, which administers the DOPPS. All funds are made to Arbor Research Collaborative for Health and not directly to the authors. Drs Hwang, Goodman, Cheng, Desai, and Fox are employees and stockholders of Amgen.
Acknowledgement
Shauna Leighton, BA, an employee of Arbor Research Collaborative for Health, provided editorial support.
Peer Review
Received December 16, 2021 as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form March 7, 2022.
Footnotes
Complete author and article information provided before references.
Table S1: Patient and facility characteristics in US HD facilities that switched to Etelcalcetide-first vs. remained Cinacalcet-first.
Table S2: MBD marker outcomes in Etelcalcetide-first vs. Cinacalcet-first US HD facilities: Approach 1 results by level of adjustment.
Table S3: MBD marker outcomes in Etelcalcetide-first vs. Cinacalcet-first US HD facilities: Approach 1 results stratified by dialysis organization size.
Table S4: MBD marker outcomes in US HD facilities that switched to Etelcalcetide-first vs. remained Cinacalcet-first: Approach 2 results by level of adjustment.
Supplementary Material
Tables S1-S4.
References
- 1.Hedgeman E., Lipworth L., Lowe K., Saran R., Do T., Fryzek J. International burden of chronic kidney disease and secondary hyperparathyroidism: a systematic review of the literature and available data. Int J Nephrol. 2015;2015:184321. doi: 10.1155/2015/184321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbor Research Collaborative for Health Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor. https://www.dopps.org/DPM
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl (2011) 2017;7(1):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K., Kuwae N., Regidor D.L., et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 5.Block G.A., Chertow G.M., Sullivan J.T., et al. An integrated analysis of safety and tolerability of etelcalcetide in patients receiving hemodialysis with secondary hyperparathyroidism. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushinsky D.A., Chertow G.M., Cheng S., et al. One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism. Nephrol Dial Transplant. 2020;35(10):1769–1778. doi: 10.1093/ndt/gfz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham J., Block G.A., Chertow G.M., et al. Etelcalcetide is effective at all levels of severity of secondary hyperparathyroidism in hemodialysis patients. Kidney Int Rep. 2019;4(7):987–994. doi: 10.1016/j.ekir.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block G.A., Bushinsky D.A., Cunningham J., et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317(2):146–155. doi: 10.1001/jama.2016.19456. [DOI] [PubMed] [Google Scholar]
- 9.Block G.A., Bushinsky D.A., Cheng S., et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317(2):156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 10.Lee A., Song X., Khan I., et al. Association of cinacalcet adherence and costs in patients on dialysis. J Med Econ. 2011;14(6):798–804. doi: 10.3111/13696998.2011.627404. [DOI] [PubMed] [Google Scholar]
- 11.Fuller D.S., Hallett D., Dluzniewski P.J., et al. Predictors of cinacalcet discontinuation and reinitiation in hemodialysis patients: results from 7 European countries. BMC Nephrol. 2019;20(1):169. doi: 10.1186/s12882-019-1355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilpatrick R.D., Newsome B.B., Zaun D., et al. Evaluating real-world use of cinacalcet and biochemical response to therapy in US hemodialysis patients. Am J Nephrol. 2013;37(4):389–398. doi: 10.1159/000350213. [DOI] [PubMed] [Google Scholar]
- 13.Reams B.D., Dluzniewski P.J., Do T.P., et al. Dynamics of cinacalcet use and biochemical control in hemodialysis patients: a retrospective new-user cohort design. BMC Nephrol. 2015;16:175. doi: 10.1186/s12882-015-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsabiv. Package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208325Orig1s000Lbledt.pdf
- 15.Sensipar. Package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021688s017lbl.pdf
- 16.Lin E., Watnick S. Calcimimetics and bundled reimbursement. Am J Kidney Dis. 2019;73(3):385–390. doi: 10.1053/j.ajkd.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaboyas A., Muenz D., Fuller D.S., et al. Etelcalcetide utilization, dosing titration, and chronic kidney disease–mineral and bone disease (CKD-MBD) marker responses in US hemodialysis patients. Am J Kidney Dis. 2022;79(3):362–373. doi: 10.1053/j.ajkd.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Young E.W., Goodkin D.A., Mapes D.L., et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57(suppl 74):S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 19.Pisoni R.L., Gillespie B.W., Dickinson D.M., Chen K., Kutner M.H., Wolfe R.A. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(5 suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Robinson B., Fuller D., Zinsser D., et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: rationale and methods for an initiative to monitor the new US bundled dialysis payment system. Am J Kidney Dis. 2011;57(6):822–831. doi: 10.1053/j.ajkd.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DPM sampling, study design, and calculation methods Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor Methods. https://www.dopps.org/DPM/Data_Sources_Methods.pdf
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;(113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 23.Xipell M., Montagud-Marrahi E., Rubio M.V., et al. Improved control of secondary hyperparathyroidism in hemodialysis patients switching from oral cinacalcet to intravenous etelcalcetide, especially in nonadherent patients. Blood Purif. 2019;48(2):106–114. doi: 10.1159/000496562. [DOI] [PubMed] [Google Scholar]
- 24.Arenas M.D., Rodelo-Haad C., Pendón-Ruiz de Mier M.V., Rodriguez M. Control of hyperparathyroidism with the intravenous calcimimetic etelcalcetide in dialysis patients adherent and non-adherent to oral calcimimetics. Clin Kidney J. 2020;14(3):840–846. doi: 10.1093/ckj/sfaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer S.C., Mavridis D., Johnson D.W., Tonelli M., Ruospo M., Strippoli G.F.M. Comparative effectiveness of calcimimetic agents for secondary hyperparathyroidism in adults: a systematic review and network meta-analysis. Am J Kidney Dis. 2020;76(3):321–330. doi: 10.1053/j.ajkd.2020.02.439. [DOI] [PubMed] [Google Scholar]
- 26.Tentori F., Wang M., Bieber B.A., et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10(1):98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EVOLVE Trial Investigators. Chertow G.M., Block G.A., et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 28.Lawlor D.A., Tilling K., Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–1886. doi: 10.1093/ije/dyw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wetmore J.B. Calcimimetics: a promise unfulfilled. Am J Kidney Dis. 2020;76(3):308–310. doi: 10.1053/j.ajkd.2020.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4.