Summary
Pregnancy stimulates an intricately coordinated assortment of physiological changes to accommodate growth of the developing fetus, while simultaneously averting rejection of genetically foreign fetal cells and tissues. Despite increasing evidence that expansion of immune-suppressive maternal regulatory T cells enforces fetal tolerance and protects against pregnancy complications, the pregnancy-associated signals driving this essential adaptation remain poorly understood. Here we show that the female reproductive hormone, progesterone, coordinates immune tolerance by stimulating expansion of FOXP3+ regulatory T cells. Conditional loss of the canonical nuclear progesterone receptor in maternal FOXP3+ regulatory T cells blunts their proliferation and accumulation, which is associated with fetal wastage and decidual infiltration of activated CD8+ T cells. Reciprocally, the synthetic progestin 17α-hydroxyprogesterone caproate (17-OHPC) administered to pregnant mice reinforces fetal tolerance and protects against fetal wastage. These immune modulatory effects of progesterone that promote fetal tolerance establish a molecular link between immunological and other physiological adaptions during pregnancy.
Subject areas: Immunology, Immune response, Endocrinology, Female reproductive endocrinology
Graphical abstract
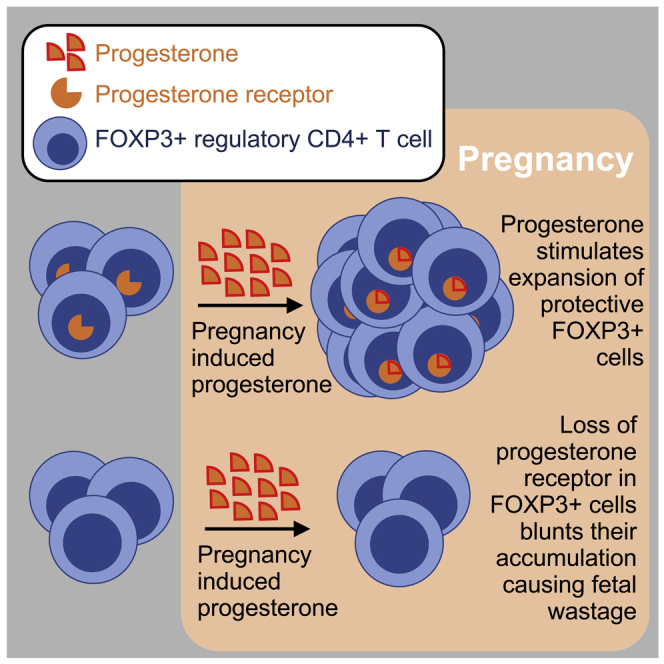
Highlights
-
•
Progesterone receptor in maternal FOXP3+ Tregs protects against fetal wastage
-
•
Progesterone receptor stimulation drives maternal Treg proliferation and accumulation
-
•
Loss of progesterone receptor unleashes decidual CD8+ T cell infiltration
-
•
Exogenous 17-OHPC progesterone reinforces fetal tolerance
Immunology; Immune response; Endocrinology; Female reproductive endocrinology
Introduction
Pregnancy-associated expansion of maternal FOXP3+ regulatory CD4+ T cells (Tregs) is widely conserved across eutherian mammalian species consistent with a shared requirement for expanded tolerance to foreign paternal-fetal alloantigens (Erlebacher, 2013; Ruocco et al., 2014; Salvany-Celades et al., 2019; Samstein et al., 2012; Tilburgs et al., 2008). Decreased expansion of maternal Tregs is associated with a variety of pregnancy complications, including spontaneous abortion and preeclampsia (Gomez-Lopez et al., 2020; Inada et al., 2013; Jiang et al., 2014; Sasaki et al., 2004; Somerset et al., 2004). Complete or partial depletion of maternal FOXP3+ cells to prepregnancy levels in mice impairs fetal tolerance, leading to fetal wastage (Aluvihare et al., 2004; Rowe et al., 2011). Maternal Treg expansion is initiated as early as conception through paternal antigen stimulation and cytokines such as TGF-β in the seminal fluid (Moldenhauer et al., 2009). Maternal Tregs with fetal specificity further accumulate during pregnancy in mice (Rowe et al., 2012) and humans (Tilburgs et al., 2008, 2009). This necessity for maternal Treg expansion raises important questions regarding the pregnancy-associated factors that promote their expansion in coordination with other pregnancy-induced physiological changes.
Progesterone is a highly conserved female reproductive hormone essential for initiating and maintaining pregnancy across viviparous species (Ramathal et al., 2010; Wu et al., 2018). Rising progesterone levels during the luteal phase of the menstrual cycle prepare the uterine lining for implantation (Graham and Clarke, 1997). Significantly more elevated progesterone levels maintain relaxation of the uterine smooth muscle and closure of the uterine cervix during pregnancy. These actions are mediated by the canonical nuclear progesterone receptor (PR), as female PR-deficient mice are infertile (Lydon et al., 1995), and in all species treatment with the PR antagonist mifepristone (RU486) terminates pregnancy (el-Refaey et al., 1995; Mao et al., 2010; Renthal et al., 2010).
Recent evidence suggests immune-modulatory roles for progesterone that contribute to expanded tolerance during pregnancy. Conditional PR deficiency in CD11c+ antigen-presenting cells causes intrauterine growth restriction that parallels modestly reduced accumulation of uterine Tregs (Thiele et al., 2019). Progesterone can also directly stimulate CD4+ T cells to undergo Treg differentiation in vitro, which requires PR expression by T cells (Lee et al., 2012; Mao et al., 2010). However, whether progesterone directly stimulates maternal Tregs to promote their accumulation required for sustaining fetal tolerance during pregnancy remains uncertain. This knowledge gap was addressed by evaluating pregnancy outcomes in mice with conditional loss of PR in maternal Treg cells.
Results and discussion
To investigate the importance of PR in maternal Tregs, FOXP3Cre and PRflox/flox mice were intercrossed (Fernandez-Valdivia et al., 2010; Rubtsov et al., 2008) to generate FOXP3Cre/CrePRflox/flox (FOXP3-PRKO) mice with selective PR deficiency only in FOXP3+ cells. We found allogeneic pregnancies sired by male BALB/c mice consistently showed increased rates of fetal wastage with reciprocal loss of live pups in female FOXP3-PRKO compared with FOXP3-wild-type (WT) mice (Figure 1A). Similar fertility and numbers of concepti per litter indicate defects in maintaining pregnancy are responsible for negative pregnancy outcomes in FOXP3-PRKO mice (Figure 1A). Normal allogeneic pregnancy in FOXP3Cre/Cre control mice indicate fetal wastage in FOXP3-PRKO mice is not explained by potentially reduced FOXP3 expression in FOXP3Cre knock-in mice (Franckaert et al., 2015; Rubtsov et al., 2008) (Figure 1A).
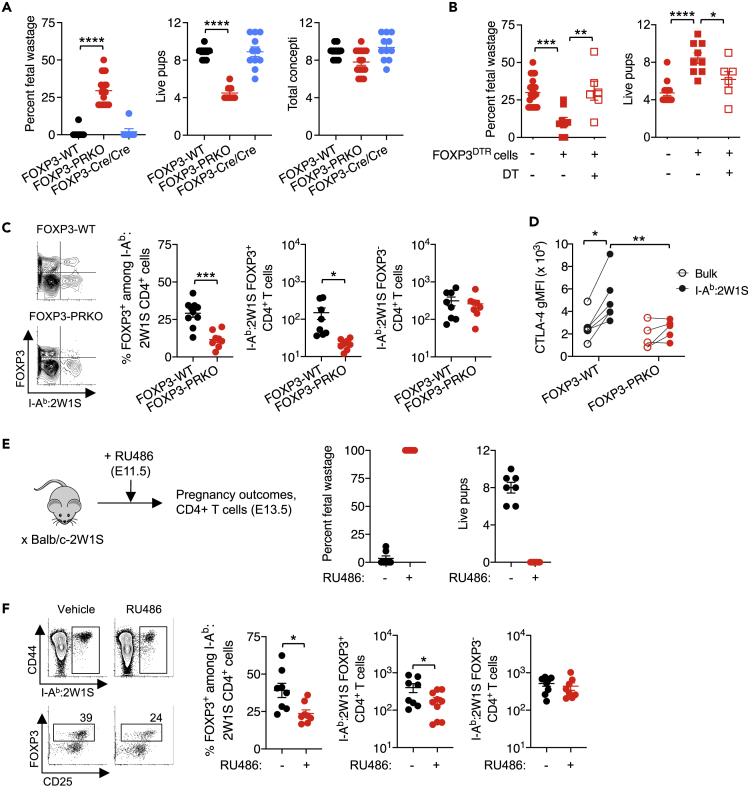
Figure 1.
Progesterone receptor signaling in maternal FOXP3+ cells protects against fetal wastage
(A) Percent fetal wastage, number of live pups in utero, and concepti in FOXP3-WT (black), FOXP3-PRKO [FOXP3Cre/CrePRflox/flox] (red), and FOXP3-Cre/Cre (blue) female mice at mid-gestation (E11.5) during allogeneic pregnancies sired by Balb/c-2W1S/OVA (H-2d) male mice.
(B) Percent fetal wastage and number of live pups for FOXP3-PRKO female mice at mid-gestation (E11.5) during allogeneic pregnancies sired by Balb/c-2W1S/OVA (H-2d) male mice, including those adoptively transferred PR-sufficient Treg from FOXP3DTR donor mice prior to mating (squares) and whether or not DT was administered to selectively eliminate donor FOXP3+ Tregs (open).
(C) Frequency of FOXP3+ and number of FOXP3+ and FOXP3− CD4+ T cells with I-Ab:2W1S55-68 specificity in the spleen and pooled peripheral lymph nodes for mice described in panel A.
(D) CTLA-4 expression (gMFI) after cell-surface + intracellular staining among I-Ab:2W1S55-68-specific (filled) or bulk (open) CD4+ T cells in the spleen and pooled peripheral lymph nodes for mice described in panel A.
(E) Percent fetal wastage and number of live pups 2 days following administration of RU486 (red) or vehicle (black) in allogeneic pregnant female mice.
(F) Representative FACS plots and summary data showing number of I-Ab:2W1S55-68 CD4+ T cells and frequency of FOXP3+ Treg cells in the spleen and pooled peripheral lymph nodes for mice described in panel E. Data are from at least three independent experiments each with similar results, with each point representing data from an individual mouse. Bar, mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001.
To more definitively investigate the cause of fetal wastage in FOXP3-PRKO mice, complementation studies evaluated how adoptively transferred PR-sufficient WT cells impact pregnancy outcomes in FOXP3-PRKO recipient mice. Donor splenocytes from FOXP3DTR mice with co-expression of human diphtheria toxin receptor (DTR) with FOXP3 were used for complementation, allowing selective depletion of PR-sufficient FOXP3 cells in recipient FOXP3-PRKO mice after low-dose diphtheria toxin (DT) administration (Kim et al., 2007). These experiments showed fetal wastage, and loss of live pups in FOXP3-PRKO mice is reversed by adoptive transfer of whole splenocytes containing PR-sufficient FOXP3+ cells, whereas pregnancy complications persisted when PR-sufficient donor FOXP3+ cells were selectively eliminated (Figure 1B). Thus, fetal wastage in FOXP3-PRKO mice reflects the selective loss of PR in maternal FOXP3+ cells and is also not explained by potential low-level promiscuous Cre-activity (Bittner-Eddy et al., 2019; Franckaert et al., 2015).
To examine the role of paternal antigens on maternal Treg expansion, BALB/c male mice with constitutive expression of the 2W1S55-68 peptide as a recombinant protein in all cells (Balb/c-2W1S/OVA) (Moon et al., 2011) were used to sire allogeneic pregnancy. This approach transforms the MHC class II I-Ab restricted 2W1S55-68 peptide into a surrogate fetal antigen, allowing precise tracking of maternal CD4+ T cells responsive to fetal I-Ab:2W1S55-68 stimulation (Figure S1) (Rowe et al., 2012). These experiments showed fetal wastage in FOXP3-PRKO mice was associated with sharply reduced percentage and total number of FOXP3+ Tregs with I-Ab:2W1S fetal specificity, whereas accumulation of I-Ab:2W1S FOXP3− CD4+ T cells was similar compared with FOXP3-WT control mice (Figure 1C).
Pregnancy also stimulates functional changes in maternal FOXP3+ cells. A prominent example is expression of cytotoxic T cell Lymphocyte antigen 4 (CTLA-4), which mediates many aspects of immune suppression by FOXP3+ Tregs and is increased in maternal Tregs during healthy pregnancy (Heikkinen et al., 2004; Tilburgs et al., 2008) but reduced with pregnancy complications including preterm birth and spontaneous abortion (Jin et al., 2009; Sasaki et al., 2004). We found increased CTLA-4 expression by maternal FOXP3+ Tregs with I-Ab:2W1S fetal specificity compared with tetramer negative bulk FOXP3+ cells during healthy pregnancies in FOXP3-WT mice but not in FOXP3-PRKO mice (Figure 1D), indicating PR is essential for activation as well as numerical expansion of FOXP3+ cells during pregnancy.
The immune modulatory effects of progesterone were further investigated using RU486, which blocks PR signaling and as a consequence induces parturition in mice within 48 h after administration (Figure 1E). RU486-induced pregnancy termination was accompanied by reductions in both the percentage and number of FOXP3+ Tregs with fetal-I-Ab:2W1S55-68 specificity but did not affect accumulation of I-Ab:2W1S FOXP3− CD4+ T cells (Figure 1F). This necessity for PR by maternal FOXP3+ cells, and in particular maternal Tregs with fetal specificity, parallels significantly increased levels of Pgr mRNA among splenocytes in pregnant compared with virgin control mice, albeit at sharply reduced levels compared with uterine cells (Figure S2), and is in agreement with prior studies showing only low-level Pgr mRNA levels in bulk maternal CD4+ T cells during pregnancy (Engler et al., 2017; Lissauer et al., 2015). Thus, despite RU486 functionally antagonizing progesterone/PR across a variety of maternal cells and tissues, and likely through a variety of receptors including the canonical nuclear PR plus glucocorticoid receptor (Beck et al., 1993; Zhang et al., 2006), these results together with reduced expansion of fetal-specific Tregs in FOXP3-PRKO mice highlight the importance of progesterone/PR signaling in maintaining expanded accumulation of maternal FOXP3+ Tregs during pregnancy.
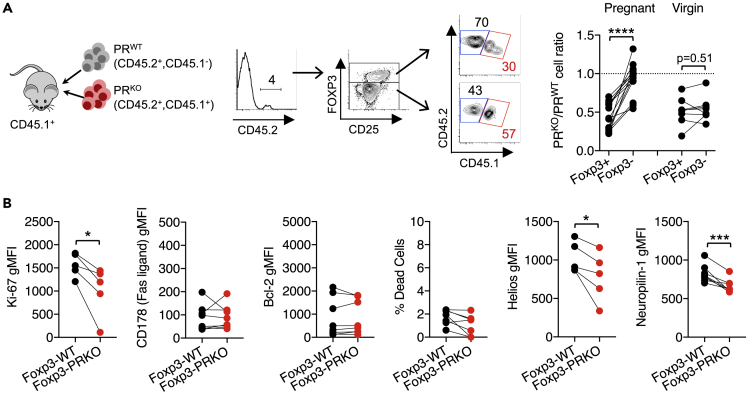
To establish the causal relationship between reduced maternal FOXP3+ Treg expansion and fetal wastage in FOXP3-PRKO mice, pregnancy-induced accumulation of donor PR-deficient and PR-sufficient CD4+ T cells was compared after co-transfer into WT recipient mice (Figure 2A). Donor CD4+ T cells were identified based on discordant expression of the CD45.1/CD45.2 congenic markers from endogenous WT cells in recipient mice. We reasoned that if loss of PR-mediated expansion of maternal Tregs drives fetal wastage in FOXP3-PRKO mice, healthy pregnancies in WT recipient mice would exhibit diminished accumulation of donor PRKO compared with PRWT FOXP3+ cells. In agreement, the ratio of PR-deficient to PR-sufficient donor cells was significantly reduced among FOXP3+ compared with FOXP3− cells in pregnant recipient but not virgin control mice (Figure 2A). Reduced expansion of donor PR-deficient FOXP3+ cells reflects diminished proliferation given reduced Ki-67 expression, as opposed to differences in cell death shown by similar levels of the apoptosis markers CD178 (Fas ligand), B-cell lymphoma 2 (Bcl-2), and membrane integrity cell viability staining (Figure 2B) (Allison et al., 2004; Ramaswamy et al., 2009). This platform allowing CD4+ T cell PR stimulation to be evaluated during healthy pregnancy also revealed reduced Helios and Neuropilin-1 (Nrp-1) expression by PR-deficient compared with PR-sufficient FOXP3+ cells, suggesting progesterone selectively promotes accumulation of natural, as opposed to induced, Tregs during pregnancy (Figure 2B) (Curotto de Lafaille and Lafaille, 2009; Rowe et al., 2012). Thus, PR signaling in FOXP3+ cells promotes the proliferation and accumulation of this protective maternal CD4+ T cell subset during pregnancy.
Figure 2.
Progesterone receptor stimulation drives proliferation and accumulation of maternal FOXP3+ cells
(A) FACS plots and summary graphs showing ratio of adoptively transferred PRKO [CD45.2+, CD45.1+] (red) to PRWT [CD45.2+, CD45.1−] (gray) FOXP3+ and FOXP3− maternal CD4+ splenocytes during allogeneic pregnancy (E11.5) sired by BALB/c males compared with virgin control mice.
(B) Expression of Ki-67, CD178 (Fas ligand), Bcl-2, Helios, Neuropilin, and percent dead cells identified by staining with a membrane integrity dye by donor PR-sufficient (FOXP3-WT) compared with PR-deficient (FOXP3-PRKO) FOXP3+ cells for mice described in panel A. Data are from at least three independent experiments, each with similar results, with each point representing data from an individual mouse. Bar, mean ± SEM. ∗p < 0.05, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001.
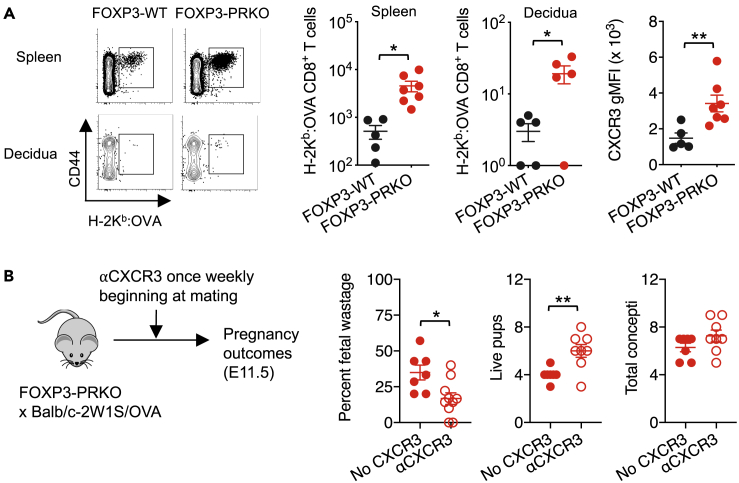
One mechanism whereby Tregs may protect against fetal wastage is through suppressed activation and decidual accumulation of cytolytic CD8+ T cells (Chaturvedi et al., 2015; Samstein et al., 2012). To test this possibility, fetal-specific CD8+ T cells were evaluated in FOXP3-PRKO mice. Co-expression of OVA with 2W1S in Balb/c-2W1S-OVA transgenic mice used to sire allogeneic pregnancy allows endogenous CD8+ T cells with surrogate fetal-OVA specificity to be identified by H-2Kb:OVA257-264 tetramer staining (Moon et al., 2011). These experiments showed fetal wastage in FOXP3-PRKO mice paralleled sharply increased systemic and decidual accumulation of CD8+ T cells with fetal-OVA specificity in comparison with healthy pregnancy in FOXP3-WT mice (Figures 3A and S3). Expanded fetal-OVA-specific CD8+ T cells in FOXP3-PRKO mice also showed increased expression of the chemokine receptor, CXCR3 (Figure 3A), required for decidual infiltration (Chaturvedi et al., 2015). In turn, CXCR3 blockade efficiently overturned fetal wastage in FOXP3-PRKO mice (Figure 3B), similar to fetal wastage triggered by prenatal infection or partial transient depletion of maternal FOXP3+ cells (Chaturvedi et al., 2015). Thus, natural progesterone produced during pregnancy stimulates expansion of maternal Treg through PR that protects against fetal wastage and decidual accumulation of CXCR3+ fetal-specific CD8+ T cells.
Figure 3.
Selective loss of progesterone receptor by maternal FOXP3+ cells unleashes activation and decidual infiltration of CD8+ T cells with fetal specificity
(A) Number of H-2Kb:OVA257-264 CD8+ T cells in the spleen and peripheral lymph nodes or decidua and CXCR3 gMFI by H-2Kb:OVA257-264 CD8+ T cells in the spleen and lymph nodes of FOXP3-WT (black) or FOXP3-PRKO (red) female during allogeneic pregnancies (E11.5) sired by Balb/c-2W1S/OVA (H-2d) male mice.
(B) Percent fetal wastage, number of live pups in utero, and concepti at mid-gestation following once weekly administration of anti-CXCR3 (open) or isotype (filled) antibodies in FOXP3-PRKO females initiated at time of mating with Balb/c-2W1S/OVA (H-2d) male mice. Data are from at least three independent experiments, each with similar results, with each point representing data from an individual mouse. Bar, mean ± SEM. ∗p < 0.05, ∗∗p < 0.01.
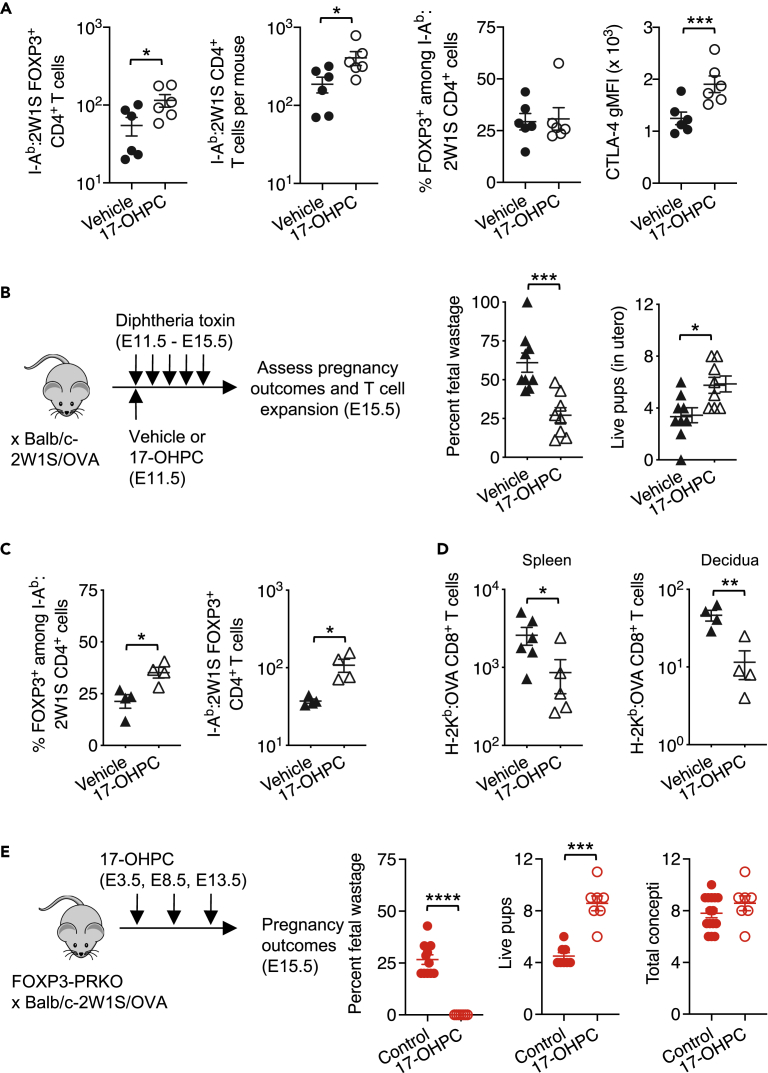
A variety of synthetic progestins with unique pharmacodynamic and biological properties have been developed with the goal of therapeutically preventing preterm birth and other pregnancy complications (Coomarasamy et al., 2019; Palacio et al., 2016; Saccone et al., 2017; Stephenson et al., 2017). A widely used synthetic compound is the long-lasting progestin, 17α-hydroxyprogesterone caproate (17-OHPC) (Romero and Stanczyk, 2013). Supplementation with 17-OHPC, but not natural progesterone, reduces the incidence of miscarriage in women with high-risk pregnancies (Saccone et al., 2017). However, how 17-OHPC works remains uncertain. Given the importance of PR in maternal Tregs for sustaining fetal tolerance, the potential for reinforced fetal tolerance by exogenous 17-OHPC was evaluated. Significantly increased accumulation of maternal FOXP3+ Tregs and CD4+ T cells with I-Ab:2W1S fetal specificity was found 48 h after a single dose of 17-OHPC at mid-gestation (E11.5) (Figure 4A). Interestingly, although the frequency of I-Ab:2W1S fetal-specific Tregs did not further increase from expanded pregnancy levels, CTLA-4 expression was significantly increased among FOXP3+ Tregs with fetal-2W1S specificity in 17-OHPC-treated mice (Figure 4A). Thus, exogenous 17-OHPC exerts distinct immune-modulatory properties even in the presence of high endogenous natural progesterone levels during pregnancy.
Figure 4.
17-OHPC progesterone protects against fetal wastage
(A) Number of FOXP3+ and CD4+ T cells with I-Ab:2W1S55-64 specificity, percent FOXP3+ among CD4+ T cells with I-Ab:2W1S55-64 specificity, and CTLA-4 gMFI among 2W1S-specific FOXP3+ cells in the spleen and pooled peripheral lymph nodes 48 h following administration of 17-OHPC (open) or vehicle (filled) in C57BL/6 female mice mid-gestation (E11.5) during allogeneic pregnancy sired by Balb/c-2W1S/OVA (H-2d) male mice.
(B) Percent fetal wastage, number of live pups in utero, and total concepti for 17-OHPC (open black) or vehicle-treated (closed black) Foxp3DTR/WT female mice five days after initiating daily diphtheria toxin (DT) treatment during allogeneic pregnancy sired by Balb/c-2W1S/OVA (H-2d) male mice.
(C) Percent and number of FOXP3+ Tregs with I-Ab:2W1S55-68 specificity one day after initiating DT treatment for groups described in panel b.
(D) Number of H-2Kb:OVA257-264-specific CD8+ T cells in the spleen and pooled peripheral lymph nodes or decidua five days after initiation of DT for groups described in panel B.
(E) Percent fetal wastage, number of live pups in utero, and total concepti for 17-OHPC-treated FOXP3-PRKO (open) or control FOXP3-PRKO (closed) mice. Data are from at least three independent experiments, each with similar results, with each point representing data from an individual mouse. Bar, mean ± SEM. ∗p < 0.05, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001.
Protective impacts of 17-OHPC were further investigated using an established model of fetal wastage triggered by partial depletion of maternal FOXP3+ Tregs. FOXP3 is encoded on the X chromosome, and random inactivation of this chromosome in female mice heterozygous for co-expression of diphtheria toxin receptor with FOXP3 (FOXP3DTR/WT mice) allows partial transient depletion of maternal FOXP3+ cells that nadir within the first 24 h after initiating low-dose DT treatment during pregnancy (Rowe et al., 2011, 2012). Remarkably, 17-OHPC reduced fetal wastage and loss of live pups that normally occur with DT administration in FOXP3DTR/WT pregnant mice (Figure 4B). Protection against fetal wastage paralleled significantly increased percentage and numbers of FOXP3+ Treg cells with I-Ab:2W1S fetal specificity (Figure 4C), with reciprocally reduced systemic and decidual expansion of activated maternal CD8+ T cells with fetal-OVA specificity in 17-OHPC-treated mice (Figure 4D). Thus, exogenous 17-OHPC reinforces fetal tolerance and protects against fetal wastage when maternal Tregs are depleted to prepregnancy levels, to mimic their blunted expansion in human pregnancy complications such as preeclampsia and spontaneous abortion (Jiang et al., 2014; Sasaki et al., 2004, 2007; Somerset et al., 2004).
Along with the canonical nuclear PR, progesterone can stimulate cells through other receptors including membrane bound PRs and glucocorticoid receptor (GR) (Vrachnis et al., 2012), each with considerably higher expression by maternal T cells during pregnancy (Engler et al., 2017; Lissauer et al., 2015). Because 17-OHPC and natural progesterone are molecular distinct with discordant relative affinity to PR and these noncanonical receptors (Attardi et al., 2007; Boucher et al., 2014; Courtin et al., 2012; Romero and Stanczyk, 2013), we evaluated whether exogenous 17-OHPC can bypass the necessity for PR in maternal FOXP3+ Tregs during pregnancy by treating FOXP3-PRKO mice with 17-OHPC. Consistent with this hypothesis, 17-OHPC administered 3 days after mating reversed fetal wastage and loss of live pups in FOXP3-PRKO mice (Figure 4E). Thus, 17-OHPC exerts distinct immune-modulatory properties, even in the presence of endogenous natural progesterone, to reinforce tolerance and protect against fetal wastage.
Taken together, these results indicate that although PR in maternal FOXP3+ is essential for protection against fetal wastage in response to physiologically increased natural progesterone levels during pregnancy, this necessity can be overcome by exogenous progestins. The expanded protective effects of 17-OHPC may reflect stimulation of other cell types through PR and/or noncanonical PR receptors. For example, 17-OHPC, but not micronized progesterone, has been reported to reduce CXCL9 in the placenta and IL-1β in the brain of fetal mice after LPS-induced intrauterine inflammation (Novak et al., 2018), which may explain increased protection by 17-OHPC compared with natural progesterone against miscarriage (Saccone et al., 2017). The importance of nuclear PR expression by CD11c+ antigen-presenting cells for optimal fetal growth (Thiele et al., 2019) suggests these cells are likely candidates to respond to increased progestin levels after 17-OHPC treatment. With regard to noncanonical receptors, GR is a likely candidate given its necessity in maternal T cells for protection against autoimmunity (self-tolerance) during pregnancy (Engler et al., 2017). Thus, although protection against fetal wastage in 17-OHPC-treated mice parallels significantly increased expansion of FOXP3+ Treg cells with I-Ab:2W1S fetal specificity (Figures 4A and 4C), stimulation of other cell types and through noncanonical receptors cannot be excluded and is in fact likely.
Based on this complex division of labor between progesterone responsive cells, and the receptors they utilize for optimal pregnancy outcomes, more comprehensive analyses of how progesterone works temporally throughout pregnancy, and across multiple maternal leukocyte subsets, represent important areas for future investigation. In turn, differences in how natural progesterone and synthetic progestins work may explain the variable success of 17-OHPC for the prevention of preterm birth (Blackwell et al., 2020; Meis et al., 2003). From a broader translational perspective, the immune-modulatory effects of progesterone are likely not restricted only to pregnancy, and further establishing the mechanism for how natural progesterone and synthetic progestins work will likely unveil improved therapeutic approaches for autoimmunity, transplantation, and other physiological contexts where expanded immunological tolerance is desired.
Limitations of the study
Progesterone is known to induce differentiation of CD4+ T cells into FOXP3+ Tregs after stimulation in vitro, and PR is essential for CD4+ T cell responsiveness to progesterone stimulation in this context (Lee et al., 2012; Mao et al., 2010). However, maternal T and other leukocyte subsets show only low-level Pgr mRNA levels during pregnancy (Engler et al., 2017; Lissauer et al., 2015). One explanation for this discordance is that PR expression in maternal T cells has been evaluated and purified without consideration of fetal-antigen specificity. We found sharply reduced expansion of maternal Tregs when PR is selectively eliminated in maternal FOXP3+ cells using tools that track maternal CD4+ T cells with fetal specificity. Despite the current lack of antibodies required for evaluating PR expression among individual cells by flow cytometry, and the relative scarcity of these cells that precludes their purification by FACS sorting, selective PR expression by maternal Tregs with fetal specificity is consistent with significantly increased but low-level PR expression among unfractionated maternal splenocytes (Figure S2). More definitive identification of the maternal leukocyte subset(s) that express PR during pregnancy will require the development tools for detecting PR protein, even among potentially rare cells (e.g. maternal T cells with fetal specificity). Another consideration is that although the necessity for PR expression by maternal FOXP3+ cells for averting fetal wastage in response to endogenous natural progesterone physiologically produced during pregnancy is clearly demonstrated (Figures 1, 2 and 3), we also find that exogenous progestins likely work by stimulating unique immune cell types and/or through noncanonical PR receptors. Although this complexity was not evaluated beyond PR expression in maternal FOXP3+ cells, the scientific basis for analysis of bioactive progesterone levels after administration of 17-OHPC compared with natural progesterone during pregnancy and systematic analysis of PR compared with noncanonical receptors among individual maternal leukocyte subsets systemically and at the maternal-fetal interface is nonetheless established.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE/Cy5 anti-mouse/human CD11b Antibody, Clone M1/70 | BioLegend | Cat# 101210; RRID:AB_312793 |
| PE/Cy5 anti-mouse/human CD45R/B220 Antibody, Clone RA3-B62 | BioLegend | Cat# 103210; RRID:AB_312995 |
| PE/Cy7 anti-mouse CD62L Antibody, Clone MEL-14 | BioLegend | Cat# 104418; RRID:AB_313103 |
| APC anti-mouse CD152 (CTLA-4) Antibody, Clone UC10-4B9 | BioLegend | Cat# 106310; RRID:AB_2087653 |
| Alexa Fluor 700 anti-mouse/human CD44 Antibody, Clone IM7 | BioLegend | Cat# 103026; RRID:AB_493713 |
| PE/Cy7 anti-mouse CD45.1 Antibody, Clone A20 | BioLegend | Cat# 110730; RRID:AB_1134168 |
| Brilliant Violet 421 anti-mouse CD45.2 Antibody, Clone 104 | BioLegend | Cat# 109832; RRID:AB_2565511 |
| eFluor 450 anti-mouse CD4 Antibody, Clone GK1.5 | Invitrogen | Cat# 48-0041-82; RRID:AB_10718983 |
| APC-eFluor 780 anti-mouse CD8alpha Antibody, Clone 53-6.7 | Invitrogen | Cat# 47-0081-82; RRID:AB_1272185 |
| PE/Cy5 anti-mouse CD11c Antibody, Clone N418 | Invitrogen | Cat# 15-0114-82; RRID:AB_468717 |
| PE/Cy5 anti-mouse F4/80 Antibody, Clone BM8 | Invitrogen | Cat# 15-4801-82; RRID:AB_468798 |
| FITC anti-mouse CD4 Antibody, Clone GK1.5 | Invitrogen | Cat# 11-0041-82; RRID:AB_464892 |
| PE/Cy7 anti-mouse CD8alpha Antibody, Clone 53-6.7 | Invitrogen | Cat# 25-0081-82; RRID:AB_469584 |
| PE/Cy7 anti-mouse CD3epsilon Antibody, Clone 145-2C11 | Invitrogen | Cat# 25-0031-82; RRID:AB_469572 |
| FITC anti-mouse FOXP3 Clone FJK-16S |
Invitrogen | Cat# 11-5773-82; RRID:AB_465243 |
| eFluor 450 anti-mouse Ki-67 Clone SolA15 |
Invitrogen | Cat# 48-5698-82; RRID:AB_11149124 |
| APC anti-mouse/human Helios Clone 22F6 |
BioLegend | Cat# 137222; RRID:AB_10662900 |
| eFluor anti-mouse Neuropilin-1 Clone 3DS304M |
Invitrogen | Cat# 48-3041-82; RRID:AB_2574051 |
| PE anti-mouse CD178 (Fas Ligand) Clone MFL3 |
BioLegend | Cat# 106606; RRID:AB_313279 |
| AF647 anti-mouse Bcl-2 Clone 10C4 |
BioLegend | Cat# 633510; RRID:AB_2274702 |
| APC anti-CD183 (CXCR3) | Invitrogen | Cat# 17-1831-82; RRID:AB_1210791 |
| InVivoPlus anti-mouse CXCR3 Clone CXCR3-173 |
BioXCell | Cat# BE0249; RRID:AB_2687730 |
| Chemicals, peptides, and recombinant proteins | ||
| H-2Kb:OVA257-64 MHC Class I Monomer | NIH Tetramer Core | N/A |
| Streptavidin, R-Phycoerythrin Conjugate (SAPE) | Invitrogen | S866 |
| I-Ab:2W1S55-64 MHC Class II Tetramer | NIH Tetramer Core | N/A |
| Mifepristone, RU486 | Sigma Aldrich | M8046 |
| 17alpha-hydroxyprogesterone caproate | Sigma Aldrich | H5752 |
| LIVE/DEAD Near-IR staining kit | ThermoFisher | L34976 |
| Critical commercial assays | ||
| Fixation/Permeabilization Solution Kit | BD Biosciences | 554714 |
| Anti-PE Microbeads | Miltenyi | 130-048-801 |
| Experimental models: Organisms/strains | ||
| Balb/c-Act-2W1S/OVA | Marc Jenkins (University of Minnesota) | Published in Moon et al. (2011) |
| Backcrossed 10 generations to BALB/c background | ||
| C57BL/6NCr | Charles River Laboratories (NCI) | #556 |
| Balb/cAnNCr | Charles River Laboratories (NCI) | #555 |
| B6-Ly5.1/Cr | Charles River Laboratories (NCI) | #564 |
| B6.129(Cg)-Foxp3tm4(YFP/icre)/Ayr/J | Jackson Laboratory | #016959 |
| PRflox/flox | Laboratory of John Lydon and Francisco De-Mayo | Published in Lydon et al. (1995) |
| Software and algorithms | ||
| FlowJo | BD Biosciences | N/A |
| GraphPad Prism | GraphPad Software | N/A |
Resource availability
Lead contact
Further information and requests for resources or reagents should be directed to and will be fulfilled by the lead contact, Dr. Sing Sing Way (singsing.way@cchmc.org).
Materials availability
Mouse strains and other reagent describe in this paper are available from the lead contact upon request with a completed Material Transfer Agreement.
Experimental model and subject details
Mice
C57BL/6 (B6, H-2b; CD45.2), BALB/c (H-2d), and B6-Ly5.1/Cr (H-2b; CD45.1) mice were purchased from the National Cancer Institute colony at Charles River Laboratories. Balb/c-2W1S/OVA transgenic mice that constitutively express recombinant 2W1S55-68 and OVA protein behind the β-actin promoter, and Foxp3DTR/DTR and FOXP3DTR/WT mice where all or half of FOXP3+ cells are susceptible to diphtheria toxin induced ablation have been described (Kim et al., 2007; Rowe et al., 2011, 2012). PRflox/flox mice and FOXP3Cre mice with IRES-YFP-Cre cDNA knocked into the 3′ UTR of the Foxp3 gene have been described (Fernandez-Valdivia et al., 2010; Rubtsov et al., 2008). FOXP3CRE mice were backcrossed >10 times onto the C57BL/6 background prior to intercrossing with PRflox/flox mice to generate FOXP3Cre/Cre PRflox/flox mice (FOXP3-PRKO). For mating, Balb/c-2W1S/OVA male mice backcrossed on the BALB/c background were combined with virgin females for 24 h and visualization of the copulation plug was determined as embryonic day (E) 0.5. Fetal wastage was assessed as in utero resorption or fetal death together with placental friability. All mice were housed under specific pathogen-free conditions and used in accordance with the Animal Care and Use Committee of Cincinnati Children’s Hospital.
Method details
qPCR analysis
Spleen and uteri were harvested during mid-gestation (E11.5) during allogeneic pregnancy or virgin control mice on the C57BL/6 background. Tissues were cut into several small pieces, immediately submerged in RNAlater solution (Invitrogen), lysed using the RNAaqueous-4PCR kit (Invitrogen), and passed through a homogenizer tube before precipitation. At least 1 μg of isolated RNA was added to each cDNA reaction. cDNA synthesis was performed using both Oligo-d(T)16 nucleotide and a PR-specific reverse transcription primers (5′TTGAAATAATGGGTGAAATATA-3′) using the TaqMan Reverse Transcription platform (Applied Biosystems). qPCR reactions were set up using the TaqMan Fast Advanced Master Mix (Applied Biosystems). Four different exon-spanning PR TaqMan probes were used: Mm00435625 (Exon 4–5), Mm00435626 (Exon 5–6), Mm01176082 (Exon 6–7), and Mm00435628 (Exon 7–8). Samples were run on a 7500 Fast Real-Time PCR system, and normalized to ActB (Mm04394036) within each sample (DCT).
In vivo treatments
For progesterone receptor blockade, pregnant mice were subcutaneously administered 250ug mifepristone [RU486] (Sigma Aldrich, USA) in 100uL sesame oil at E12.5. Treg complementation experiments were performed by adoptively transferring whole splenocytes from FOXP3DTR/DTR donor mice into FOXP3-PRKO recipient mice on the day when pulse mating with allogeneic male mice was initiated. For depletion of PR-sufficient FOXP3DTR/DTR donor Treg cells, FOXP3-PRKO recipient mice were administered DT (0.5 μg) immediately after adoptive cell transfer, and twice weekly (0.5 μg/dose) until pregnancy outcomes evaluated at mid-gestation. For adoptive cell transfer, CD4+ splenocytes from donor PR-sufficient WT CD45.2+ and FOXP3-PRKO CD45.2 + CD45.1 + mice were purified by negative selection (Miltenyi Biotec), mixed at a 1:1 ratio, and intravenously transferred into CD45.1+, CD45.2- recipient mice immediately prior to mating. To neutralize CD8+ T cell decidual infiltration, FOXP3-PRKO females were injected intraperitoneally with 500ug anti-CXCR3 (CXCR3-173, BioXcell) or Hamster IgG once weekly beginning at the time of mating through mid-gestation (E11.5). For exogenous progestin, mice were injected subcutaneously with 2 mg of 17α-hydroxyprogesterone caproate [17-OHPC] (Sigma Aldrich, USA) in 200 μL sesame oil or vehicle control. For partial transient ablation of maternal Treg cells, FOXP3DTR/WT females were administered diphtheria toxin (Sigma Aldrich, USA) [0.5ug first dose, followed by 0.1ug/dose) beginning mid-gestation (E11.5) for five consecutive days with fetal wastage being assessed at E15.5. Where indicated, mice were injected with 17-OHPC (2 mg/200uL) or vehicle 12 h prior to initiation of DT treatment.
Tetramer enrichment and flow cytometry
Single cell suspensions were prepared from the spleen and peripheral (axillary, brachial, cervical, inguinal, mesenteric, pancreatic, para-aortic/uterine) lymph nodes by grinding with glass slides. Decidua tissue was isolated and processed as previously described (Chaturvedi et al., 2015). Specifically, placentas were removed from fetuses and separated at the labyrinth and junctional zone interface. Tissue was placed in RBC lysis buffer and single-cell suspension was generated by grinding between frosted glass slides. Complete DMEM was used to quench the lysis reaction and samples were then filtered through 70 micron cell strainers and pelleted by centrifugation (530 g for 5 min). For tracking, CD4+ I-Ab:2W1S55-68 or CD8+ H-2Kb:OVA257-264 cells, single cell suspensions were incubated with PE-conjugated or APC-conjugated tetramers and enriched with anti-fluorochrome microbeads (Miltenyi Biotec) as previously described. Bound fractions were eluted and stained with fluorochrome-labeled cell surface antibodies for CD4 (GK1.5), CD8a (53-7.3), CD25 (PC61), CD45.1 (A20), CD45.2 (104), CD44 (IM7), CD11b (M1/70), CD11c (N418), B220 (RA3-B62), F4/80 (BM8), CXCR3 (CXCR3-173), CTLA-4 (UC10-4B9). For Treg cell analysis, splenocytes were stained for Ki-67 (SolA15), Helios (22F6), Neuropilin-1 (3DS304M), CD178 (MFL3), Bcl-2 (10C4), and a membrane integrity dye that identified dead cells (Thermo Fisher). For intranuclear staining, cells were fixed and permeabilized (eBioscience) and stained with antibodies against FOXP3 (FJK-16S). Cells were analyzed on a FACSCanto cytometer (BD Biosciences) and analyzed using FlowJo (TreeStar) software.
Quantification and statistical analysis
For all experiments, female mice were randomly assigned to experimental groups. Distribution of data points were first evaluated for having a Gaussian normal distribution (Prism, GraphPad). Differences between normally distributed datasets were analyzed using either the Student’s paired or unpaired t-test, while non-normally distributed datasets were evaluated using non-parametric Mann-Whitney analysis. A one-way ANOVA was used to analyze mean difference between three or more groups with a single variable and two-way ANOVA was used to analyze multiple groups with more one independent variable (Prism, GraphPad). For each analysis, a p value of <0.05 was taken as statistical significance.
Acknowledgments
We are indebted to Drs. John Lydon and Francesco De-Mayo for providing PRflox/flox mice, the staff of Research Animal Resources at Cincinnati Children’s Hospital led by Dr. Saigiridhar Tummala for the conscientious care of experimental mice, and the NIH Tetramer Core Facility (supported by NIH-NIAID contract 75N93020D00005) for providing MHC monomer and tetramer reagents. This work was supported by NIH-NIAID through grants R01AI45840, R01AI124657, and DP1AI131080 and the March of Dimes Ohio Collaborative on Prematurity Research. A.L.S is supported by T32DK007727 (PI, Dr. Lee Armistead Denson). S.S.W. is supported by the HHMI Faculty Scholar’s program (grant #55108587) and Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease Award (grant #1011031).
Author contributions
A.L.S, J.M.K., L.X., T.T., W.A.G., S.M., and S.S.W. designed the project and experiments; A.L.S, J.M.K., L.X., A.R.B, T-Y.S., and G.P. performed the experiments; A.L.S, J.M.K., T.T., W.A.G., S.M., and S.S.W. organized the data and wrote the paper with editorial input from all the authors.
Declaration of interests
The authors declare no competing financial interests.
Published: June 17, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104400.
Supplemental information
Data and code availability
-
•
All data reported will be shared by the lead contact upon request.
-
•
This paper does not report original code. Additional information required to analyze the data reported in this paper is available from the lead contact upon request.
References
- Allison D.D., Drazba J.A., Vesely I., Kader K.N., Grande-Allen K.J. Cell viability mapping within long-term heart valve organ cultures. J. Heart Valve Dis. 2004;13:290–296. [PubMed] [Google Scholar]
- Aluvihare V.R., Kallikourdis M., Betz A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Attardi B.J., Zeleznik A., Simhan H., Chiao J.P., Mattison D.R., Caritis S.N., Obstetric-Fetal Pharmacology Research Unit, N Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins. Am. J. Obstet. Gynecol. 2007;197:599.e1–599.e7. doi: 10.1016/j.ajog.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C.A., Estes P.A., Bona B.J., Muro-Cacho C.A., Nordeen S.K., Edwards D.P. The steroid antagonist RU486 exerts different effects on the glucocorticoid and progesterone receptors. Endocrinology. 1993;133:728–740. doi: 10.1210/endo.133.2.8344212. [DOI] [PubMed] [Google Scholar]
- Bittner-Eddy P.D., Fischer L.A., Costalonga M. Cre-loxP reporter mouse reveals stochastic activity of the Foxp3 promoter. Front. Immunol. 2019;10:2228. doi: 10.3389/fimmu.2019.02228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell S.C., Gyamfi-Bannerman C., Biggio J.R., Jr., Chauhan S.P., Hughes B.L., Louis J.M., Manuck T.A., Miller H.S., Das A.F., Saade G.R., et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am. J. Perinatol. 2020;37:127–136. doi: 10.1055/s-0039-3400227. [DOI] [PubMed] [Google Scholar]
- Boucher E., Provost P.R., Tremblay Y. Ontogeny of adrenal-like glucocorticoid synthesis pathway and of 20alpha-hydroxysteroid dehydrogenase in the mouse lung. BMC Res. Notes. 2014;7:119. doi: 10.1186/1756-0500-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V., Ertelt J.M., Jiang T.T., Kinder J.M., Xin L., Owens K.J., Jones H.N., Way S.S. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J. Clin. Invest. 2015;125:1713–1725. doi: 10.1172/JCI78578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomarasamy A., Devall A.J., Cheed V., Harb H., Middleton L.J., Gallos I.D., Williams H., Eapen A.K., Roberts T., Ogwulu C.C., et al. A randomized trial of progesterone in women with bleeding in early pregnancy. N. Engl. J. Med. 2019;380:1815–1824. doi: 10.1056/NEJMoa1813730. [DOI] [PubMed] [Google Scholar]
- Courtin A., Communal L., Vilasco M., Cimino D., Mourra N., de Bortoli M., Taverna D., Faussat A.M., Chaouat M., Forgez P., Gompel A. Glucocorticoid receptor activity discriminates between progesterone and medroxyprogesterone acetate effects in breast cells. Breast Cancer Res. Treat. 2012;131:49–63. doi: 10.1007/s10549-011-1394-5. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille M.A., Lafaille J.J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- el-Refaey H., Rajasekar D., Abdalla M., Calder L., Templeton A. Induction of abortion with mifepristone (RU 486) and oral or vaginal misoprostol. N. Engl. J. Med. 1995;332:983–987. doi: 10.1056/NEJM199504133321502. [DOI] [PubMed] [Google Scholar]
- Engler J.B., Kursawe N., Solano M.E., Patas K., Wehrmann S., Heckmann N., Luhder F., Reichardt H.M., Arck P.C., Gold S.M., Friese M.A. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc. Natl. Acad. Sci. U S A. 2017;114:E181–E190. doi: 10.1073/pnas.1617115114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R., Jeong J., Mukherjee A., Soyal S.M., Li J., Ying Y., Demayo F.J., Lydon J.P. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis. 2010;48:106–113. doi: 10.1002/dvg.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franckaert D., Dooley J., Roos E., Floess S., Huehn J., Luche H., Fehling H.J., Liston A., Linterman M.A., Schlenner S.M. Promiscuous Foxp3-cre activity reveals a differential requirement for CD28 in Foxp3(+) and Foxp3(-) T cells. Immunol. Cell Biol. 2015;93:417–423. doi: 10.1038/icb.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N., Arenas-Hernandez M., Romero R., Miller D., Garcia-Flores V., Leng Y., Xu Y., Galaz J., Hassan S.S., Hsu C.D., et al. Regulatory T cells play a role in a subset of idiopathic preterm labor/birth and adverse neonatal outcomes. Cell Rep. 2020;32:107874. doi: 10.1016/j.celrep.2020.107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J.D., Clarke C.L. Physiological action of progesterone in target tissues. Endocr. Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- Heikkinen J., Mottonen M., Alanen A., Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol. 2004;136:373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada K., Shima T., Nakashima A., Aoki K., Ito M., Saito S. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J. Reprod. Immunol. 2013;97:104–111. doi: 10.1016/j.jri.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Jiang T.T., Chaturvedi V., Ertelt J.M., Kinder J.M., Clark D.R., Valent A.M., Xin L., Way S.S. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J. Immunol. 2014;192:4949–4956. doi: 10.4049/jimmunol.1400498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L.P., Chen Q.Y., Zhang T., Guo P.F., Li D.J. The CD4+CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin. Immunol. 2009;133:402–410. doi: 10.1016/j.clim.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Lydon J.P., Kim C.H. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur. J. Immunol. 2012;42:2683–2696. doi: 10.1002/eji.201142317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissauer D., Eldershaw S.A., Inman C.F., Coomarasamy A., Moss P.A., Kilby M.D. Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur. J. Immunol. 2015;45:2858–2872. doi: 10.1002/eji.201445404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon J.P., DeMayo F.J., Funk C.R., Mani S.K., Hughes A.R., Montgomery C.A., Jr., Shyamala G., Conneely O.M., O'Malley B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mao G., Wang J., Kang Y., Tai P., Wen J., Zou Q., Li G., Ouyang H., Xia G., Wang B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- Meis P.J., Klebanoff M., Thom E., Dombrowski M.P., Sibai B., Moawad A.H., Spong C.Y., Hauth J.C., Miodovnik M., Varner M.W., et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N. Engl. J. Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- Moldenhauer L.M., Diener K.R., Thring D.M., Brown M.P., Hayball J.D., Robertson S.A. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J. Immunol. 2009;182:8080–8093. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- Moon J.J., Dash P., Oguin T.H., 3rd, McClaren J.L., Chu H.H., Thomas P.G., Jenkins M.K. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc. Natl. Acad. Sci. U S A. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak C.M., Ozen M., McLane M., Alqutub S., Lee J.Y., Lei J., Burd I. Progesterone improves perinatal neuromotor outcomes in a mouse model of intrauterine inflammation via immunomodulation of the placenta. Am. J. Reprod. Immunol. 2018;79:e12842. doi: 10.1111/aji.12842. [DOI] [PubMed] [Google Scholar]
- Palacio M., Ronzoni S., Sanchez-Ramos L., Murphy K.E. Progestogens as maintenance treatment in arrested preterm labor: a systematic review and meta-analysis. Obstet. Gynecol. 2016;128:989–1000. doi: 10.1097/AOG.0000000000001676. [DOI] [PubMed] [Google Scholar]
- Ramaswamy M., Cleland S.Y., Cruz A.C., Siegel R.M. Many checkpoints on the road to cell death: regulation of Fas-FasL interactions and Fas signaling in peripheral immune responses. Results Probl. Cell Differ. 2009;49:17–47. doi: 10.1007/400_2008_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal C.Y., Bagchi I.C., Taylor R.N., Bagchi M.K. Endometrial decidualization: of mice and men. Semin. Reprod. Med. 2010;28:17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal N.E., Chen C.C., Williams K.C., Gerard R.D., Prange-Kiel J., Mendelson C.R. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc. Natl. Acad. Sci. U S A. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Stanczyk F.Z. Progesterone is not the same as 17alpha-hydroxyprogesterone caproate: implications for obstetrical practice. Am. J. Obstet. Gynecol. 2013;208:421–426. doi: 10.1016/j.ajog.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J.H., Ertelt J.M., Aguilera M.N., Farrar M.A., Way S.S. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J.H., Ertelt J.M., Xin L., Way S.S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov Y.P., Rasmussen J.P., Chi E.Y., Fontenot J., Castelli L., Ye X., Treuting P., Siewe L., Roers A., Henderson W.R., Jr., et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Ruocco M.G., Chaouat G., Florez L., Bensussan A., Klatzmann D. Regulatory T-cells in pregnancy: historical perspective, state of the art, and burning questions. Front. Immunol. 2014;5:389. doi: 10.3389/fimmu.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone G., Schoen C., Franasiak J.M., Scott R.T., Jr., Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta-analysis of randomized, controlled trials. Fertil. Steril. 2017;107:430–438.e3. doi: 10.1016/j.fertnstert.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Salvany-Celades M., van der Zwan A., Benner M., Setrajcic-Dragos V., Bougleux Gomes H.A., Iyer V., Norwitz E.R., Strominger J.L., Tilburgs T. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019;27:2537–2547.e5. doi: 10.1016/j.celrep.2019.04.109. [DOI] [PubMed] [Google Scholar]
- Samstein R.M., Josefowicz S.Z., Arvey A., Treuting P.M., Rudensky A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Darmochwal-Kolarz D., Suzuki D., Sakai M., Ito M., Shima T., Shiozaki A., Rolinski J., Saito S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Sakai M., Miyazaki S., Higuma S., Shiozaki A., Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Somerset D.A., Zheng Y., Kilby M.D., Sansom D.M., Drayson M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M.D., McQueen D., Winter M., Kliman H.J. Luteal start vaginal micronized progesterone improves pregnancy success in women with recurrent pregnancy loss. Fertil. Steril. 2017;107:684–690.e2. doi: 10.1016/j.fertnstert.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Thiele K., Hierweger A.M., Riquelme J.I.A., Solano M.E., Lydon J.P., Arck P.C. Impaired progesterone-responsiveness of CD11c(+) dendritic cells affects the generation of CD4(+) regulatory T cells and is associated with intrauterine growth restriction in mice. Front. Endocrinol. (Lausanne) 2019;10:96. doi: 10.3389/fendo.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburgs T., Roelen D.L., van der Mast B.J., de Groot-Swings G.M., Kleijburg C., Scherjon S.A., Claas F.H. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- Tilburgs T., Scherjon S.A., van der Mast B.J., Haasnoot G.W., Versteeg V.D.V.-M.M., Roelen D.L., van Rood J.J., Claas F.H. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 2009;82:148–157. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Vrachnis N., Malamas F.M., Sifakis S., Tsikouras P., Iliodromiti Z. Immune aspects and myometrial actions of progesterone and CRH in labor. Clin. Dev. Immunol. 2012;2012:937618. doi: 10.1155/2012/937618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.P., Li R., DeMayo F.J. Progesterone receptor regulation of uterine adaptation for pregnancy. Trends Endocrinol. Metab. 2018;29:481–491. doi: 10.1016/j.tem.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tsai F.T., Geller D.S. Differential interaction of RU486 with the progesterone and glucocorticoid receptors. J. Mol. Endocrinol. 2006;37:163–173. doi: 10.1677/jme.1.02089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported will be shared by the lead contact upon request.
-
•
This paper does not report original code. Additional information required to analyze the data reported in this paper is available from the lead contact upon request.