Abstract
There is now considerable evidence that in Europe, babesiosis is an emerging infectious disease, with some of the causative species spreading as a consequence of the increasing range of their tick vector hosts. In this review, we summarize both the historic records and recent findings on the occurrence and incidence of babesiosis in 20 European countries located in southeastern Europe (Bosnia and Herzegovina, Croatia, and Serbia), central Europe (Austria, the Czech Republic, Germany, Hungary, Luxembourg, Poland, Slovakia, Slovenia, and Switzerland), and northern and northeastern Europe (Lithuania, Latvia, Estonia, Iceland, Denmark, Finland, Sweden, and Norway), identified in humans and selected species of domesticated animals (cats, dogs, horses, and cattle). Recorded cases of human babesiosis are still rare, but their number is expected to rise in the coming years. This is because of the widespread and longer seasonal activity of Ixodes ricinus as a result of climate change and because of the more extensive use of better molecular diagnostic methods. Bovine babesiosis has a re-emerging potential because of the likely loss of herd immunity, while canine babesiosis is rapidly expanding in central and northeastern Europe, its occurrence correlating with the rapid, successful expansion of the ornate dog tick (Dermacentor reticulatus) populations in Europe. Taken together, our analysis of the available reports shows clear evidence of an increasing annual incidence of babesiosis across Europe in both humans and animals that is changing in line with similar increases in the incidence of other tick-borne diseases. This situation is of major concern, and we recommend more extensive and frequent, standardized monitoring using a “One Health” approach.
Keywords: Babesia, emerging, One Health, tick, vector
1. Introduction
Babesiosis, also known as piroplasmosis, is a multisystem disease caused by the protozoan parasites of the genus Babesia [1,2,3,4]. Babesiosis is an important emerging infectious disease of global significance, affecting both humans and domesticated animals [5,6]. The main route of transmission of babesiae to mammalian hosts is through a tick bite, although other routes of transmission (e.g., vertical transmission, transmission through blood transfusion or organ transplantation) have been documented among both humans and animals, including also in wildlife reservoir hosts [7]. Hard ticks (Ixodidae) act as vectors of babesiae, with evidence of specificity in Babesia-tick vector interactions [8]. The course of babesiosis can differ considerably, from entirely subclinical infections, through mild non-specific manifestations, to fatal, multisystem disease. Additionally, there is growing evidence that the co-infection of Babesia and other tick-borne diseases may contribute to a more severe course of disease in patients [5]. Notably, co-infection of Babesia microti and Borrelia burgdorferi s.l. has been documented in the United States of America (USA) and Europe, with Lyme disease patients experiencing more symptoms and for a longer duration if coinfected with B. microti [9,10].
From a historical perspective, babesiosis has been well-described as a dangerous, potentially lethal, tick-borne disease of dogs [11,12] and cattle [13,14], the latter contributing to marked financial losses in the cattle industry. In current times, with tick control and efficient treatments for babesiosis being readily available, the disease appears to have lesser importance than in earlier periods. However, the number of human cases has been growing recently, especially in the US, with about 2000 new cases being reported annually, and in Canada and China as well [5,15,16].
The situation in Europe appears to be less acute and the disease is less recognized in the medical profession, although there are some recent reports of human cases, in particular from France, Germany, and the United Kingdom [3]. Moreover, the number of cases attributed to other important tick-borne diseases (e.g., borreliosis, rickettsiosis, tick-borne encephalitis) has been growing also and the One Health approach has been applied in investigating all of these diseases (e.g., [17,18,19,20]). Taken together, current observations suggest that there is increasing exposure of humans and animals to ticks in Europe (as reviewed for Ixodes ricinus in [21]).
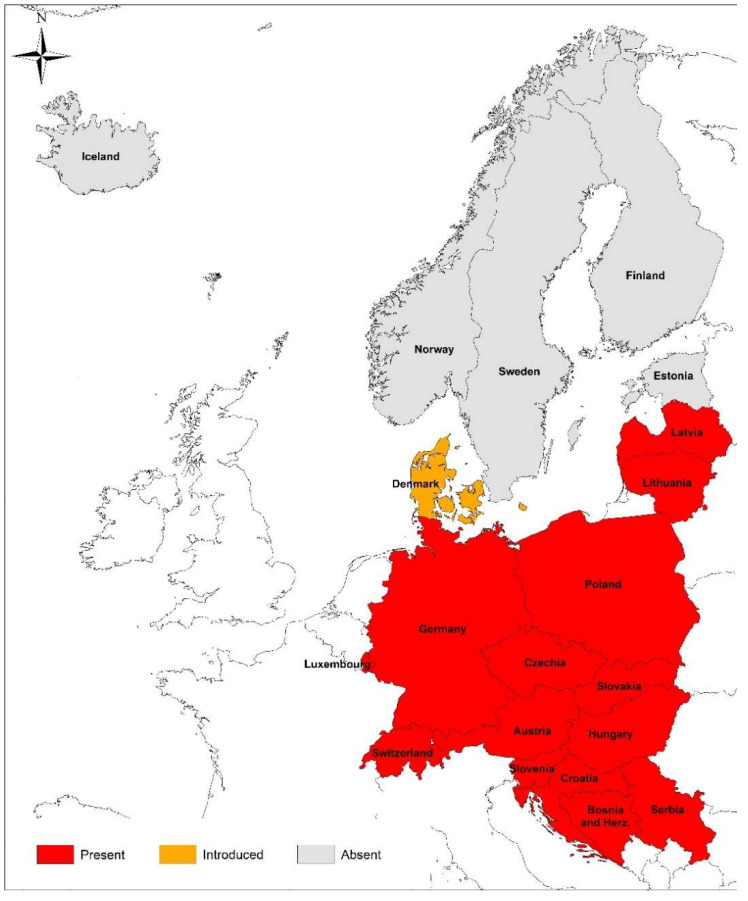
The ornate dog tick, Dermacentor reticulatus, is one of the fastest-spreading tick species in central and northeastern Europe, with increasing significance as a vector of tick-borne pathogens for domesticated animals [22,23,24,25]. Dermacentor reticulatus is the main, if not the only, vector of Babesia canis and its spread is accompanied by the expansion of canine babesiosis and the appearance of new foci of the disease [8,26]. Additionally, this tick species may act as a vector of B. caballi and Theileria equi [25,27,28]. Despite focusing on Babesia spp. in this review, we have also included Theileria equi, which was originally referred to as B. equi, and is one of the two common piroplasm species of horses. In addition, the two piroplasms are closely related and some molecular diagnostic assays fail to distinguish between isolates of T. equi and Babesia spp. While other Theileria species are known to exist in wild animals, such as deer and boars, these do not pose a threat to human populations or our domestic animals in the region of Europe that is covered in our review [29]. Therefore, in the current review, we summarize recent findings on the occurrence and incidence of babesiosis in southeastern, central, and northeastern Europe (Bosnia and Herzegovina, Croatia, Serbia, Austria, Luxembourg, Switzerland, Germany, Hungary, the Czech Republic, Slovakia, Slovenia, Poland, Lithuania, Latvia, Estonia, Iceland, Denmark, Finland, Sweden, and Norway), in humans and selected species of domesticated animals (cats, dogs, horses, and cattle), and T. equi in horses.
2. South-Eastern Europe
2.1. Bosnia and Herzegovina (BiH)
2.1.1. Babesiosis in Humans
There are no published reports to date of human babesiosis in BiH.
2.1.2. Babesiosis in Animals
In June 2017 and July 2018, two cases of bovine babesiosis occurring in two small farms in central BiH were attributed to Babesia divergens, after confirmation by molecular methods (Polymerase chain reaction [PCR] and sequencing) [30]. In a recent molecular screening of samples from 142 horses from the Central Balkan region, including Serbia, Montenegro, and BiH, the overall prevalence was reported to be 22.5% and 2.1% for T. equi and B. caballi, respectively [31]. This study indicated for the first time that T. equi is actually present in BiH.
Dermacentor reticulatus is a tick species that is currently expanding its range in BiH. In a recent (2017–2019) monitoring study, this tick species was found in regions of the country where it had not been recorded earlier and was identified in new host species for the country [32]. Between 2014 and 2016, the DNA of B. canis was identified by PCR and sequencing in 85% of eighty symptomatic dogs that had never left the country [33]. Accordingly, the parasite is considered autochthonous and canine babesiosis is treated as an expanding disease in BiH. Additionally, the DNA of B. canis has been recorded in one red fox (Vulpes vulpes) (prevalence 0.8%). Babesia vulpes (formerly known as B. microti-like) (31.9%) and Hepatozoon canis (38.6%) have also been detected in red foxes (n = 119) in BiH, identified in animals hunted between October 2013 and April 2014 [34].
There are no published records of Babesia in domestic cats; however, Babesia sp. badger-type A was identified in one of 18 examined European wildcats (Felis silvestris) in BiH [35].
2.2. Croatia
2.2.1. Babesiosis in Humans
The first reported case of human babesiosis in Europe, and indeed in the world, occurred in 1956 in former Yugoslavia, now Croatia. The patient was a 33-year-old tailor and part-time farmer who had been splenectomized following a traffic accident 11 years earlier [36]. Based on morphological characteristics, and the fact that the patient was a farmer, the authors concluded that the infection was caused by Babesia bovis. The patient presented with fever and severe hemoglobinuria eight days after first feeling unwell and died two days later. Because B. bovis is not known to be zoonotic, and the photomicrographs in the published case study show divergent piroplasms that are characteristic of B. divergens, this is currently regarded as the first human case of B. divergens. To date, this case remains the only confirmed case of babesiosis in Croatia, although, in 2003, a low titer of antibodies to B. microti was detected in a single serum from 102 patients with a history of tick bites [37].
2.2.2. Babesiosis in Animals
The first records of piroplasmosis in Croatia date back to 1912, when studies on babesiosis in sheep were conducted in Dalmatia. Further studies performed by the same author in 1921 confirmed piroplasmosis in cattle, horses, and dogs from the same Croatian littoral region [38].
The results of the first molecular study were published in 2002 and in these, B. canis infection was confirmed in eight dogs from the Zagreb region of Croatia showing the clinical signs of babesiosis, including apathy, fever, and anemia [39]. In the second molecular study, which included 81 microscopically positive dogs with babesiosis, six piroplasm species were detected. Babesia canis was the dominant species, identified in 78 of the symptomatic dogs (96%), followed by single infections with B. vogeli, B. caballi, and T. equi [40]. In a group of randomly selected, apparently healthy dogs in the same study, the prevalence was 3.4% (29 of 848). Besides the confirmation of B. canis in 20 dogs (69%), B. gibsoni was detected for the first time in six dogs (21%), B. vogeli in two dogs (7%), and B. vulpes in one dog (3%). Babesia canis was the only species detected in dogs with lethargy, anorexia, fever, dark urine, and thrombocytopenia [41]. More recently, B. gibsoni has been confirmed in three dogs and B. vulpes in one dog showing anemia and thrombocytopenia [42]. Antibodies to B. canis were detected in 20% (95% CI 16.3–24.1%) of 435 randomly selected, apparently healthy dogs from 13 different locations in Croatia using indirect immunofluorescence [43].
In retrospective post-mortem studies on archived, formalin-fixed, paraffin-embedded tissue blocks (FFPEB) from dogs that had died due to a hemolytic crisis, B. canis was confirmed in all cases except one, Theileria capreoli being identified in the heart tissue of the latter case [44]. Septic shock was the cause of death in the T. capreoli-infected dog, based on gross and histological criteria. Babesia canis was confirmed by sequencing from archived Romanowsky stained cytological slides, on which positivity for canine piroplasmosis had been previously identified after microscopic examination [45]. The slides consisted of five clinical blood smears and nine different organ imprints; these were shown to be suitable for the molecular typing of the archival samples to enable species confirmation. Moreover, B. canis was amplified from the different tissues of 15 dogs that had shown gross findings consistent with hemolytic disease, despite clearance of the diagnostic life stages soon after treatment [45]. Among wildlife, B. canis has been found in grey wolves [46], B. vulpes in red foxes [47], and T. capreoli (previously Theileria sp. 3182/05) in grey wolves and foxes [46,47].
In 2015, Gotic [48] identified the DNA of B. caballi and T. equi in 3.6% (13/362) and 13.2% (48/362) of 362 horses, respectively. Two genotypes were detected, T. equi genotype E in 10.8% (39/362) of the horses and T. equi genotype A in 2.5% (9/362) of the horses. Babesia caballi and T. equi genotype A were found in the continental part of the country, while T. equi genotype E was found exclusively in coastal areas [48].
In 2020, three cows that had died, with icterus, splenomegaly, and dark urine, were found to be co-infected with A. bovis and T. orientalis [49]. In 2001, Theileria ovis and Theileria sp. OT3 were identified in both healthy and sick sheep in southern Croatia [50]. A third ovine species, Babesia ovis, was responsible for several cases of babesiosis in the Dubrovnik region in 2018 (unpublished data). In a single study on wild ungulates, sequence analysis of piroplasms from the spleens of red deer, fallow deer, and roe deer revealed the presence of B. crassa, B. divergens/capreoli, B. venatorum (previously Babesia sp. EU1), T. ovis and T. capreoli (Theileria sp. 3185/02) [51].
The DNA of Babesia sp. was detected in 7.1% of I. ricinus ticks from Zagreb, while in contrast, B. canis was found in 77% of D. reticulatus ticks from the same location [52]. In southern Croatia, the DNA of T. ovis was detected in the Rhipicephalus turanicus, while the DNA of B. ovis and T. ovis was detected in the tick, R. bursa [53].
2.3. Serbia
2.3.1. Babesiosis in Humans
Both the microscopic and molecular presence of Babesia in human blood samples still remains unconfirmed among Serbian citizens, despite a broad “One Health” approach study on tick-borne diseases in Serbia [54].
2.3.2. Babesiosis in Animals
The first records of seasonal hemoglobinuria associated with tick infestations in grazing cattle, sheep, goats, and horses on Serbian territory were recorded in the 19th century. Although piroplasms were not established as the etiological cause of disease in this case, the authors recommended prevention measures aimed at ticks in order to decrease exposure to infection. The first microscopic evidence of piroplasms in dogs from Serbia was published in 1953 [38]. Awareness of the importance of canine piroplasmosis, based on clinical and microscopic diagnoses by veterinarians from Belgrade and Novi Sad working in the domain of “small animal practice”, was raised during the early 1980s [55]. Available data indicated that the Belgrade district was not a highly endemic region for canine babesiosis at that time. Sporadic cases of babesiosis were recorded in hunting and companion dogs that had returned from vacations. In their study of 1997–2001, Pavlović et al. [56] examined a total of 3945 dogs with febrile disease accompanied by anemia, hemoglobinuria, general pallor, and icterus or tick infestation. In this study, B. canis was found in 74% of blood smears, confirming its endemic status in the region of the Serbian capital. The prevalence of infection was observed to increase between March and April and then to decrease by July. A second peak occurred in September and additional cases continued to appear until December [56]. In a similar study conducted in 2015–2017 on 1085 dogs with overt signs of babesiosis, prevalence by microscopy was 35%, half that recorded in the earlier study [57]. Morphological appearance allowed for the identification of B. canis in 95% of infected animals. Another study in 2013–2016 confirmed the endemic status and seasonal appearance of canine babesiosis due to B. canis in Belgrade [58].
In 2012–2013, in the region of Vojvodina (northern Serbia), Babesia spp. were diagnosed by microscopy in 12.2% of 41 dogs suspected of having babesiosis [59].
The first molecular study on Babesia species in Serbia was published in 2015; this was based on 60 dogs with clinical signs of babesiosis [60]. For the first time, B. canis and B. gibsoni were identified in Serbian dogs by PCR-RFLP and PCR and sequencing. In a more recent study encompassing 111 dogs, including 46 from shelters, 31 free-roaming, and 34 hunting dogs, B. canis and B. gibsoni were detected in 16.2% of the dogs through species-specific real-time PCR. None of the dogs tested positive for B. vogeli [61]. Furthermore, B. canis was confirmed by PCR (tick/vector comprehensive real PCR panel—canine) to be the only species found in 29 dogs microscopically diagnosed with babesiosis in Belgrade [62].
During 2012 to 2014, an overall Babesia prevalence of 21.5% was found in 158 healthy, asymptomatic, outdoor dogs originating from Pančevo and Ðurđevo (northern Serbia) and Niš and Prokuplje (southern Serbia). Five species of piroplasms were confirmed by sequencing: B. vulpes (10.1%), B. gibsoni (5.7%), B. vogeli (1.9%), B. caballi (1.9%), and B. microti (1.9%). Dogs from Prokuplje were more frequently infected (59.1%) than dogs from Panćevo (11.9%) or Niš (4.5%). No infected dogs were found in Ðurđevo [63].
So far, only a single study has been conducted on equine piroplasmosis in Serbia [31]. In 2014, 94 apparently healthy horses from four locations were examined with multiplex PCR and sequencing. The overall prevalence was 28.7% and the predominant species was T. equi (27.7%), with a single sample testing positive for B. caballi (1.1%). The prevalence of EP varied within a range of 0–7.7% between collection sites in three NW counties and 92.3% in the single SE county included in this survey. The prevalence was associated with the exposure of farming horses to ticks when grazing throughout most of the year [31]. The similar high prevalence of T. equi (50%) was found in 70 blood samples from apparently healthy donkeys [64]. Hematological alterations were detected in 54% of the tested donkeys, while the DNA of T. equi was detected in 92% of donkeys with hematological abnormalities. Interestingly, B. caballi was not detected in these donkeys.
PCR testing to investigate bovine piroplasmosis was conducted by Vasic and co-workers [65], who detected Theileria spp. in 5 out of 135 bovine blood samples from northern and central Serbia. Sequences were 100% identical with GenBank entries from Italy (Theileria sergenti), China (Theileria spp.), and Korea (Theileria buffeli isolate HS252). The blood samples had been collected during May and June of 2013 from six geographically different locations in the northern to southern regions of Serbia. All the infected animals were found to have originated from Banatski Karlovac, northeastern Serbia, and were free of any clinical signs that could be related to theileriosis.
Babesia canis was confirmed by PCR and sequencing in 4.2% (9/216) of golden jackals [66]. In red foxes, two Babesia species were identified by molecular methods: B. vulpes (28.7%) and B. canis in a single fox (0.8%) [67]. Co-infection with B. vulpes and H. canis was present in 20.2% of foxes.
In several studies, both zoonotic (B. microti, B. venatorum) and non-zoonotic (B. canis) Babesia species have been detected in ticks collected from vegetation and animals in Serbia [54,68,69].
3. Central Europe
3.1. Austria
3.1.1. Babesiosis in Humans
To date, three cases of human babesiosis have been described in Austria. Two of these cases were attributed to B. venatorum, including one in a 56-year-old splenectomized huntsman who remembered a tick-bite two weeks before the onset of symptoms [70], and one in a splenectomized 68-year-old male patient with acute renal failure, whose serum revealed an anti-Babesia antibody titer of 1:1024 in an immunofluorescence assay [71]. The third case occurred in a non-splenectomized 63-year-old male patient who had spent four weeks in Massachusetts, USA, occupied in mainly outdoor activities shortly before the onset of symptoms. In this case, the causative agent was identified as B. microti by PCR, and sequencing [72].
In a recent unpublished study, 1253 hunters and 414 other individuals, each of the latter with a history of tick bites, were screened for antibodies against Babesia spp. with an in-house immunofluorescent antibody test (IFAT) using Fluoline H conjugates (Biomerieux, Vienna, Austria). Of the hunters and individuals with a history of tick bites, 101 (8.1%) and 35 (8.4%), respectively, tested positive for Babesia spp. The age range of positive humans was 17–73 years (mean 54.02) and titers ranged from 1:16 to 1:256. Additional blood samples were obtained from all serologically positive individuals who were available for follow-up and were investigated by a commercial PCR. Altogether, seven individuals were found to be positive for Babesia spp. by PCR, and in one of these individuals, the intra-erythrocytic stages of Babesia sp. were also detected microscopically. Five of the PCR-positive individuals were symptomatic, while three were co-infected with Borrelia spp. The sequencing of PCR products revealed B. venatorum and B. microti. One sequence could not be identified because of poor sequence data.
3.1.2. Babesiosis in Animals
Several Babesia species, including B. canis, B. capreoli, B. divergens, B. microti, B. venatorum, and B. vulpes have been detected in Austrian pets, livestock, and wildlife animals, but also in ticks [73,74,75,76,77,78,79,80]. In a large study of Babesia in ticks, the most prevalent Babesia species were from the B. divergens/B. capreoli cluster [73].
Autochthonous babesiosis has become a common disease in dogs in Austria but is absent in cats. The presence of B. canis is thought to be focused in the eastern Austrian regions where D. reticulatus is endemic [81,82]. In some of these endemic areas, up to 25% of D. reticulatus ticks are infected with B. canis [76]. Canine babesiosis became endemic within the last 30 years, possibly introduced by hunting dogs that frequently crossed the border from western Hungary. Nowadays, autochthonous cases are reported especially from areas of eastern Austria at low altitude levels (below 800 m), thus representing a suitable habitat for these ticks [83]. In a recent study, the DNA of B. canis was detected in six out of 94 (6.4%) clinically healthy military dogs kept in kennels in Burgenland (Eastern Austria) [84].
Another study reported several cases from alpine valleys in Tyrol and Carinthia in western and southern Austria [85]. Strobl et al. [86] determined the proportion of autochthonous canine cases with clinical signs in Austria to be 59.6%. A study from Eastern Austria calculated an annual risk for infection of 6.9% for dogs, leading to clinical signs in 50% of the infected animals [87]. A fatality rate of 10%, independent of the therapeutic measures applied, has been recorded in canine babesiosis within the last decades in Eastern Austria. Infection has also been documented in a red fox (V. vulpes) (1/351 blood samples; 0.3%) but it was concluded that red foxes are unlikely to have a significant impact as a reservoir or as spreader hosts [34,82].
Babesia gibsoni was found in 2015 in a dog with a history of tick infestation from the “Mauererwald” forest, close to Vienna (Prof. Walter G. Url, personal communication), and also more recently [88]. The last case attracted some interest from researchers because it was a co-infection of B. canis with B. gibsoni in a dog imported from Serbia [88]. However, it is still unclear whether B. gibsoni is currently endemic in Austria.
In Austria, foxes are acknowledged as the main hosts of B. vulpes (formerly known as B. microti-like or “Babesia sp. from Spanish dog”), a parasite known to rarely infect dogs [89]. Various studies have revealed a high prevalence of B. vulpes in foxes in western and eastern Austria [82,90].
Cattle are mainly affected by B. divergens, a species that is widespread in the Austrian alpine regions [74]. Between 1998 and 2016, a total of 1257 fatal cases of babesiosis were reported in the federal province of Styria (southeastern Austria), with high-risk clusters in the central, northern, and western regions of Styria [91]. Babesia divergens has also been documented in red deer populations in Styria (1/37; 2.9%) and Tyrol (10/196; 5.1%) [79,92].
Autochthonous cases of B. caballi have not as yet been recorded in Austria. However, an autochthonous case of T. equi was reported recently in its easternmost province (Burgenland) in a horse and in D. reticulatus [27].
3.2. Czech Republic
3.2.1. Babesiosis in Humans
To date, only two cases have been reported from the Czech Republic (CR). The first case of human babesiosis was recognized in 2000 [93], being also the first case of a symptomatic B. microti infection imported from the USA to Europe. Babesia microti infection was diagnosed on the basis of a positive blood smear and antibody detection by IFAT [93].
Recently, babesiosis was diagnosed and successfully treated in a patient initially diagnosed with Reiter’s syndrome because of symptoms corresponding to the classic triad of arthritis, conjunctivitis, and non-specific urethritis [94]. This was also the first suspected case of post-transfusion babesiosis, as the patient, a 36-year-old male who had experienced a motorcycle accident with consequent severe polytrauma, received repeated blood transfusions. Six months following the transfusions, the patient suffered from non-specific symptoms, including dysuria and periurethral itch, mild edema and itching of the eyelids, and joint pains. The abdominal ultrasonographic examination revealed mild splenomegaly and slight hepatomegaly, with normal echogenicity and without focal changes in the parenchyma. The diagnosis of babesiosis due to B. microti was made by an immunoassay, the lymphocyte transformation test (LTT, ELISPOT) [94].
3.2.2. Babesiosis in Animals
Despite the widespread occurrence of B. divergens in red deer and, to a lesser extent, in sika deer, no bovine babesiosis cases have been reported from the CR for many decades now [95]. Moreover, no Babesia DNA was detected among the 100 blood samples collected in 2014–2015 from cattle [95].
In a recent study performed in 2014–2018, blood and serum samples were collected from 711 healthy horses [96]. Antibodies to T. equi were detected by cELISA in eight (1.1%) horses and antibodies to B. caballi in three (0.4%) horses that were also positive for T. equi. Seropositivity to T. equi and B. caballi was confirmed by IFAT in five and three of the horses, respectively, that had shown positivity via cELISA. Samples that were seropositive for T. equi (n = 8) and B. caballi (n = 3) were then tested by PCR; the DNA of T. equi was identified in five horses, but no B. caballi DNA was detected in any of the serologically positive horses.
Canine babesiosis is considered to be an emerging disease in the CR, following the ongoing expansion of the range of D. reticulatus ticks in Europe [97]. Until recently, babesiosis caused by B. canis was reported frequently in the CR but only as an imported disease [98,99]. Then, in a group of 41 non-traveling dogs from the South Moravian region, 12% of the dogs tested seropositive, although the DNA of B. canis was not detected either in the examined dogs or in 340 questing D. reticulatus ticks collected in the region [100]. South Moravia, in the southeastern region of the CR, has been known for over 50 years to be an endemic area for D. reticulatus [101,102], and ticks of this species were found in 2009–2010 in numerous localities in South Moravia [103]. However, the DNA of B. canis was identified in shelter dogs from this region of the country only in 2017, and the first clinical autochthonous case of B. canis was diagnosed in a non-traveling dog from this area one year later [104,105]. The most recent study of the distribution of D. reticulatus in the CR, based on a citizen science campaign of 2018–2021, revealed that D. reticulatus is actually present in all regions of the CR. This work suggested a real risk of emergence of canine babesiosis in at least two regions with well-established tick populations, in the southeastern and northwestern CR [97].
In 2017, B. gibsoni was confirmed by PCR-sequencing in an American pit bull terrier with clinical signs of acute babesiosis [104]. The dog originated from Slovakia, where B. gibsoni was reported for the first time in two pit bull terriers in 2013 [106]. No cases of babesiosis in cats have been reported to date from the CR [12].
3.3. Germany
3.3.1. Babesiosis in Humans
Among the zoonotic Babesia spp., B. divergens, B. microti, B. venatorum and B. motasi have been reported in Germany. Babesia microti was first identified in European field voles in Germany in the 1970s, but autochthonous human infections were not yet known at the time [107]. The first confirmed autochthonous clinical case due to B. microti in Europe was reported in a German patient with myeloid leukemia in 2007, and the source of infection was attributed to a blood transfusion [108]. In one of the donors from whom the patient had received blood products, a borderline B. microti IgG titer was demonstrated but active infection could not be confirmed. Neither the patient nor the blood donor had traveled to North America or Asia. The obtained 284-bp 18S rDNA sequence from the leukemia patient showed 100% identity with the Gray strain [108]. A second clinical case involving B. microti in Germany has been published, but the infection was acquired in North America [109]. One further autochthonous babesiosis case was reported in 2007 in a splenectomized and immunocompromised patient, and the causative agent was identified as B. venatorum (known as Babesia sp. EU1 at the time) [110]. Human B. divergens infections have not as yet been reported in Germany [3].
The first serological study on Babesia exposure was conducted by Krampitz et al. [111], who detected a B. microti seroprevalence of 0.25% among 798 healthy forestry workers from the federal state of Bavaria in southern Germany. In the same study, four sera (0.5%) were positive for B. divergens antibodies by ELISA but not by IFAT. Further studies in the following years indicated seroprevalence values of 1.7–13.9% for B. microti [112,113,114] and 0.8–4.9% for B. divergens [112]. In general, higher values were found in risk groups (forestry workers, Lyme borreliosis patients, and humans exposed to ticks) rather than in non-risk groups (Table 1). Exposure to B. venatorum or B. motasi has not been studied. Babesia motasi has been implicated in human babesiosis cases in Asia [5]. This ovine piroplasm was detected in herds of sheep in northwestern and central parts of Germany in the 1950s [115]. However, to our knowledge, there are no recent reports of B. motasi in Germany, neither in sheep nor in humans.
Table 1.
Summary of studies reporting the (sero-)prevalence of Babesia spp. in Germany.
| Reference | Host Species/Group (No. of Individuals Examined) | Babesia Species (No. of Cases) | Babesia Species (No. of Seropositive) | Prevalence/Seroprevalence |
|---|---|---|---|---|
| Krampitz et al. 1986 [111] | Humans, healthy forestry workers (798) | n.a. | B. microti (2) | 0.25% |
| n.a. | B. divergens (4) | 0.5% | ||
| Hunfeld et al. 1998 [114] | Humans, Lyme borreliosis patients (76) | n.a. | B. microti (9) | 11.8% |
| Humans, seropositive but asymptomatic Lyme patients (44) | n.a. | B. microti (4) | 9.1% | |
| Humans, syphilis patients (50) | n.a. | B. microti (2) | 4.0% | |
| Humans, healthy blood donors (100) | n.a. | B. microti (8) | 8.0% | |
| Hunfeld et al. 2002 [112] | Humans exposed to ticks (225) | n.a. |
B. microti (21), B. divergens (11) |
B. microti: 9.3%, B. divergens: 4.9% |
| Humans with various infectious diseases (122) | n.a. |
B. microti (2), B. divergens (5) |
B. microti: 1.6%, B. divergens: 4.1% |
|
| Humans, healthy blood donors (120) | n.a. |
B. microti (2), B. divergens (1) |
B. microti: 1.7%, B. divergens: 0.8% |
|
| Scheller 2004 [113] | Humans, forestry workers with fever (490) | n.a. | B. microti (68) | 13.9% |
| Weiland et al. 1980 [117] | Cattle (1220) | n.a. | B. divergens (256) | 21.0% |
| Ullmann et al. 1984 [118] | Cattle (1616) | n.a. | B. divergens (211) | 13.1% |
| Ganse-Dumrath 1986 [119] | Cattle from farms with history of babesiosis (251) | B. divergens (29) | B. divergens (108) | 43.0% |
| Niepold 1990 [120] | Cattle, Borrelia-positive animals (212) | n.a. | B. divergens (0) | 0.0% |
| Cattle, farms with suspected babesiosis (354) | n.a. | B. divergens (0) | 0.0% | |
| Cattle, farms with history of babesiosis (200) | n.a. | B. divergens (8) | 4.0% | |
| Huwer et al. 1994 [121] | Cattle, farms with babesiosis history (187) | B. divergens (14) | B. divergens (88) | 47.1% |
| Lengauer et al. 2006 [122] | Cattle (287) | n.a. | B. divergens (1) | 0.3% |
| Springer et al. 2020 [123] | Cattle, one farm with history of babesiosis (95) | B. divergens (30) | B. divergens (36) | 37.9% |
| Pikalo et al. 2016 [124] | Horses (314) | n.a. |
T. equi (19), B. caballi (1) |
T. equi: 6.1%, B. caballi: 0.3% |
| Boch 1985 [125] | Horses (321) | n.a. |
T. equi (18), B. caballi (4) |
T. equi: 5.6%, B. caballi: 1.2% |
| Dogs with suspected babesiosis (116) | n.a. | B. canis complex * (46) | 39.7% | |
| Hirsch and Pantchev 2008 [126] | Dogs, imported or traveling (5142) | Babesia spp. (n.a.) | n.a. | 2.1–2.7% |
| Menn et al. 2010 [127] | Dogs, imported or traveling (4681) | n.a. | B. canis complex * (1138) | 24,3% |
| Hamel et al. 2011 [128] | Dogs, traveling (648) | n.a. | B. canis complex * (32) | 4.9% |
| Dogs, traveling (508) | B. canis complex * (19) | n.a. | 3.7% | |
| Röhrig et al. 2011 [129] | Dogs, imported (2819) | n.a. | B. canis complex * (251) | 8.9% |
| Dogs, imported (2288) | B. canis complex * (5) | n.a. | 0.5% | |
| Pantchev [130] | Dogs, imported or traveling (4579) | n.a. | B. canis complex * (319) | 7,0% |
| Dogs with suspected babesiosis (937) | n.a. | B. canis complex * (119) | 12.7% | |
| Liesner et al. 2016 [131] | Dogs (1023) | B. canis (1) | n.a. | 0.1% |
| Vrhovec et al. 2017 [132] | Dogs (9966) | B. canis complex * (170) | n.a. | 1.7% |
| Dogs (15,555) | Babesia spp. (502) | n.a. | 3.3% | |
| Dogs (2653) | n.a. | Babesia spp. | 11.5% | |
| Schäfer et al. 2019 [133] | Dogs, imported (98) | Babesia spp. (3) | n.a. | 3.1% |
| Dogs, imported (214) | n.a. | B. canis/B. vogeli (22) | 10.3% | |
| Schäfer et al. 2019 [134] | Dogs, traveling (127) | Babesia spp. (3) | n.a. | 2,4% |
| Dogs, traveling (160) | n.a. | B. canis/B. vogeli (8) | 0.5% | |
| Schäfer et al. 2021 [135] | Dogs (20,914) | Babesia spp. (659) | n.a. | 3.2% |
| Dogs, never been abroad (692) | Babesia spp. (54) | n.a. | 7.8% |
n.a.—not applicable. * Note that the B. canis complex was previously classified as a complex of B. canis, B. vogeli and B. rossi as subspecies.
In summary, only three human babesiosis cases have been reported in Germany, two being autochthonous and one imported, and all three patients were splenectomized and/or immunocompromised. In contrast, high seroprevalence values of anti-Babesia antibodies have been found, particularly in high-risk groups such as forestry workers. This may indicate that Babesia strains circulating in Germany show low pathogenicity, or that the disease is underreported.
3.3.2. Babesiosis in Animals
Two causative agents of bovine babesiosis occur in Germany, B. divergens and B. major. However, the latter is only present on certain islands where the tick vector Haemaphysalis punctata occurs [116]. Reports of babesiosis in German cattle are rare, and most studies date from the 1980s. At the time, seroepidemiological investigations were conducted in the federal state of Bavaria in southern Germany, resulting in B. divergens seroprevalence rates of 13.0–21.0%, with considerable differences between districts [117,118] (Table 1). Furthermore, serological investigations have been conducted on farms with the suspicion or a history of bovine babesiosis that are located in the federal state of Lower Saxony in northern Germany, where seroprevalences of 4.0–43.0% were recorded [119,120] (Table 1).
As B. divergens antibody titers show strong seasonal variation [121,136], the timing of sample collection with respect to the grazing season may explain the large variation in these results. Furthermore, B. divergens seems to occur in a focal pattern. This is exemplified by the study of Huwer, Schwarzmaier, Hamel and Will [121], who located three valleys in the federal state of Baden-Wuerttemberg (near Freiburg i. Br.), where bovine babesiosis occurred. Since 2000, reports of bovine babesiosis in Germany are rare. A decline in bovine babesiosis seroprevalence or incidence has been reported in other European countries [137,138], but comparable data from Germany are lacking. However, a recent report illustrates that the introduction of infected ticks or animals may lead to B. divergens (re-)emergence, with considerable economic losses for farmers. In this case, 25 animals died of bovine babesiosis on an affected farm between 2018 and 2019 [123]. Seroepidemiological investigations revealed that only one herd, which had grazed on a particular pasture, was affected during the first year of the outbreak, whereas events during the second year suggested that infected ticks had spread further afield on the farm [123].
Currently, equine piroplasmosis (EP) is considered to be non-endemic in Germany, although competent vectors, such as D. reticulatus and D. marginatus, are present [22]. Therefore, the importing of infected horses or ticks from endemic countries poses a risk of becoming endemic. As no import restrictions exist with regard to EP, seropositive horses frequently enter Germany. Between 1997 and 1999, 19 of 42 B. caballi- and/or T. equi-seropositive horses that were diagnosed at the Ludwig-Maximilians-University, Munich, were imported or had a travel history, whereas information was lacking for the remaining animals [139]. A more recent study detected 6.1% of T. equi- and 0.3% B. caballi-seropositive animals among 314 German horses where the travel history was not reported [124].
Among the published clinical cases, two involved B. caballi and eight, T. equi [27,30,140,141]. Five infections were imported and four were considered to be autochthonous, while the situation was unclear in a further case. The first, presumably autochthonous, case involved T. equi and was reported in 2003 in a mare that had only left Germany once to visit the Netherlands, several years prior to the onset of illness [140]. The authors discussed the introduction of infected ticks, such as Hyalomma spp., as a possible source of infection. Indeed, Hyalomma spp. are increasingly found on horses in Germany during particularly warm and dry summers [142]. A further case involved a gelding that originated from France and had traveled to various EP-endemic areas earlier in life [143]. Therefore, this case may represent a reactivated B. caballi infection. Furthermore, two T. equi infections imported from France were reported in 2020 [141]. Dirks et al. [27] summarized six EP cases diagnosed in Germany between 2014 and 2019: three were presumably autochthonous, while two infections were subclinical and had probably been acquired before the importation of the horses from Russia. For the remaining animal, no travel history was provided [27]. In summary, the number of autochthonous EP cases currently seems to be increasing in Germany.
The first reported case of canine babesiosis in Germany dates back to 1909, when four dogs in a fox-terrier kennel were affected within a few weeks [144]. Further reports followed from the mid-1970s onward, and by the end of this decade, 32 cases had been diagnosed in traveling or imported dogs, 31 of which were caused by B. canis and one by B. gibsoni (summarized in [145]). In the 1980s, the number of reported Babesia infections in dogs increased [125,146,147], and in 1989, the first autochthonous infections and the existence of an endemic focus were postulated in the literature, after 70 dogs from the area of Offenburg, a federal state of Baden-Wurttemberg, southern Germany, were diagnosed with B. canis without having been abroad. Up until the early 21st century, further endemic B. canis foci were discovered in the southern German federal states of Baden-Wurttemberg, Bavaria, Rhineland-Palatinate, and Saarland [146,148,149,150,151,152], while only a few sporadic autochthonous B. canis cases were reported from dogs in the northern part of Germany [151,153,154].
At the same time, two cases of B. gibsoni (Asian genotype) infection were reported in American pit bull terriers that had been presented independently to the same veterinarian. Both dogs were born and lived in the county of Ravensburg, southern Germany, and had never been abroad [155]. The dogs had no known contact with each other, nor was there any history of dog-fighting or blood transfusions. The authors also considered transplacental transmission in both dogs to be unlikely and, therefore, described the cases as the first two autochthonous B. gibsoni infections in Germany [155]. Regarding new imported pathogens, a newly detected B. microti-like species, for which the name B. vulpes has since been proposed [156], was described in a German dog that had acquired the infection in 1994 during a stay in the Pyrenean region of Spain [150].
The first major epidemiological datasets on canine babesiosis include data from the first decade of the 21st century. These retrospective studies (listed in Table 1) included several thousand diagnostic samples from imported or traveling dogs, between 2004 and 2008, and reported 0.5–3.7% blood-smear-positive [128,129,132] and 2.1–3.3% Babesia spp. PCR-positive dogs [126,132]. The indirect detection of antibodies against the B. canis complex (previously classified as a complex of B. canis, B. vogeli, and B. rossi as subspecies) resulted in 4.9–24.3% of seropositive dogs [127,128,129,132]. Similar values have been found in recent studies covering the period from 2007 to 2018. Babesia canis/B. vogeli seroprevalences were 0.5–10.3% [130,133,134] in imported and traveling dogs and 12.7% in suspected cases of babesiosis [130]. Additionally, two out of 35 dogs tested positive for antibodies against B. gibsoni [133,134]. Babesia spp. DNA was found in 0.1–3.1% of the dogs [52,53,54]. Sequencing was reported from five samples only, three of which were identified as B. canis [131,133] and two as B. gibsoni [134].
Currently, D. reticulatus shows a continuing range expansion in Germany and has now colonized the entire national territory [22]. The authors warned that this spreading is of major importance for veterinarians and dog owners in terms of canine babesiosis outbreaks or endemic status in what were hitherto B. canis-free areas [22]. Shortly thereafter, an increase in autochthonous cases of canine babesiosis in the federal states of Berlin/Brandenburg, northern Germany, was reported [157]. While only five autochthonous B. canis infections were diagnosed in 2015–2016, there were 20 cases in 2019–2021. Similarly, 77 autochthonous canine babesiosis cases were reported between 2018 and 2020 in the Rhine-Main area of the federal state of Hesse, which was not previously known as a B. canis-endemic focus [158]. Increasing numbers of autochthonous B. canis infections are also evident from a recent study analyzing the PCR results of diagnostic dog samples from 2007 to 2020 [135]. Of the total of 20,914 samples screened, 3.2% (659 samples) were positive for Babesia spp., mostly B. canis (199/205 identified samples). This percentage is comparable to previous results [126,131,132,133,134]; however, a somewhat high percentage of 7.8% (54/692) of dogs that had not stayed abroad tested PCR-positive, and high incidence correlated with areas of high levels of activity of D. reticulatus in Germany [22], indicating that autochthonous B. canis infections occur in considerable numbers in dogs in Germany [135].
The only case of feline babesiosis so far in Germany was reported in 1997 [159]. A 10-month-old Norwegian Forest cat, presenting with fever, anemia, and icterus, was imported from Sweden 7 months prior and, ever since, had lived exclusively in its new home area. The morphology of the Babesia in the blood smear roughly corresponded to those described in a Siamese cat in Zimbabwe [160].
3.4. Hungary
3.4.1. Babesiosis in Humans
To date, no reports of human babesiosis in Hungary have been published so far. The first likely autochthonous case of babesiosis due to B. microti was diagnosed by PCR in 2021 in a 64-year-old man from the southern part of Hungary (Farkas, personal communication).
3.4.2. Babesiosis in Animals
The presence of equine piroplasmosis in Hungary, caused by B. caballi, was first described by Buza et al. in the 1950s [161,162]. Half a century later, a serological survey by cELISA of 371 horses, kept in 23 different locations, confirmed the stable endemic focus of this piroplasm in the same area of the country, in Hortobágy, where it was first detected [163]. These authors also reported the first serological evidence of horses naturally infected with B. canis in seven regions of Hungary. No information had been available about the other species until 2004, when the first autochthonous case caused by T. equi was identified in a horse with clinical signs of piroplasmosis [164]. A few years later, the first serological and molecular study on T. equi infection in horses was carried out [165]. The results indicated that the parasite was present subclinically in the western as well as in the eastern parts of Hungary. Based on these findings, the prevalence of T. equi was much higher than expected and the species was present in many regions of the country, unlike B. caballi.
According to unpublished data from the period between 1958 and 1967, there were several endemic foci of bovine babesiosis caused by B. divergens in northeastern Hungary [166]. At that time, the number of clinical cases was fluctuating, with intervals of 4–5 years and monophasic seasonality, peaking in June. Although Kotlán et al. [167] reported that some cases were attributed to B. bigemina, its main vector, Rhipicephalus spp., had not been identified by that time in Hungary. Therefore, these infections were most likely caused by B. major, transmitted by H. punctata. In a more recent study of 654 cattle kept outdoors, grazing in potential tick habitats in or near the endemic area, only two individuals had antibodies to B. divergens. The results of this first report on the prevalence of B. divergens infection in cattle suggest that this Babesia species was becoming extinct in northeastern Hungary [138]. This dramatic change could have been triggered by the massively reduced population of cattle and possibly also because fewer susceptible animals had been imported into the region. The last case of known clinical B. divergens infection was diagnosed in May 2008 (unpublished result). A few years later, however, the first evidence for the occurrence of B. major and T. buffeli in local cattle was reported [168]. In a herd of 88 beef cattle from northeastern Hungary, B. major was identified in five animals, two of which died, while four cattle harbored T. buffeli, of which one was anemic. In another study, T. orientalis was identified in 20 blood samples from 90 dairy cattle in northern Hungary, the first report of this piroplasm species in Europe [169]. This report also provided the first molecular evidence of the presence of piroplasms of sheep and goats in Hungary (B. motasi in central–eastern Europe, and a B. crassa-like strain in Europe), which were detected in the tick species Haemaphysalis concinna and H. inermis, respectively.
Based on the reported data, B. canis is endemic in Hungary. Autochthonous canine babesiosis in Hungary was first described more than a century ago [170]. A few decades later, Miklósi [171] wrote about piroplasms in the blood samples of three hunting dogs, identified by microscopy. Further autochthonous cases have been reported since then [172,173], based on the clinical picture of babesiosis in dogs. Microscopic evaluation of the blood smears has demonstrated the presence of the large (3–5 µm), pyriform, frequently paired parasites. Clinical and hematological findings thus indicated that B. canis is endemic in some parts of Western Hungary and Budapest. Csikós et al. [174] observed the well-documented seasonality of clinical babesiosis in dogs, with peaks around March–April and October–November, in an eight-year-long study of the occurrence/outbreaks of the disease. Two studies [175,176] have reported that the main vector species, D. reticulatus, occurs in a greater geographical range than Horváth and Papp [172] had previously described.
Until 2005, the identification of piroplasms had been based merely on the size and morphology of the intraerythrocytic parasites. The first molecular survey on natural Babesia infections in dogs in Hungary, using PCR and sequence analysis, was attempted subsequently to clarify the species (genotype) and to obtain information on the occurrence of B. canis. A piroplasm-specific PCR amplifying the partial 18S rRNA gene yielded an approximately 450 bp PCR product in 39 (88.6%) of 44 blood samples from dogs with acute babesiosis. This was the first molecular confirmation of B. canis in Hungary [177]. Thirteen positive samples originated from Budapest and 26 came from 21 other locations. The geographical origin of the PCR-positive samples indicated the presence of piroplasms in many districts of Budapest and in several other parts of the country, including northeastern and southeastern regions, from which no babesiosis cases had been reported previously.
Hornok et al. [178] reported first on the seroprevalence of canine babesiosis in Hungary. In total, 651 blood samples were collected from urban and rural dogs in various parts of Hungary to measure antibody levels for B. canis by IFAT. Thirty-seven (5.7%) sera were positive, with titers of between 1:80 and 1:10,240. Seroconverted dogs were found in 13 locations in the country, indicating that canine babesiosis was becoming more prevalent in Eastern Hungary.
The first reports on the occurrence of small Babesia spp. in dogs in Hungary were based on blood and splenic impression smears [179,180]. However, their species status remains uncertain, due to the lack of molecular identification, especially since B. canis can also exhibit small Babesia spp.-like morphology, depending on the sampling conditions [181]. Recently, a badger-associated Babesia sp. DNA was detected for the first time in dogs used for hunting, one of which showed the relevant clinical signs [182].
The most recent data on small Babesia spp. in dogs were reported by Tuska et al. [183]. In this study, blood samples from 79 American Staffordshire Terrier dogs, confiscated from their owners for participation in illegal dog fights, were molecularly analyzed for tick-borne pathogens. Babesia gibsoni was detected in 32 dogs (prevalence of 40.5%). Based on a partial fragment of the 18S rRNA gene, B. gibsoni from Hungary exhibited complete sequence identity with conspecific strains reported from Europe and Asia. There are only sporadic cases of B. gibsoni infections in the country, partly due to the absence of indigenous status of the main vector, Rhipicephalus sanguineus sensu stricto (s.s.). However, recently, the emergence and establishment of this tick species have been reported in the country [184]. Babesia vulpes has also been found in dogs in Hungary (n = 8; prevalence of 10.1%), where previously this piroplasm had only been reported in red foxes (V. vulpes) [185].
So far there have not been any published reports of autochthonous or imported babesiosis in cats from Hungary.
3.5. Luxembourg
3.5.1. Babesiosis in Humans
There are no reports of human babesiosis from Luxembourg in the literature [186]. To the best of the authors’ knowledge, no specific studies on babesiosis in humans have been conducted to date in Luxembourg. The only study on all relevant tick-borne pathogens was performed in 2007 by PCR on ticks of the species I. Ricinus collected from all regions of Luxembourg [187]. According to this study, Babesia spp. were detected in 2.7% (n = 37) of I. ricinus ticks, with B. venatorum being predominant (59.5%), and B. microti being the second most common species (35.1%). Babesia divergens and H. canis were each detected in only one tick (2.7%).
3.5.2. Babesiosis in Animals
Similarly, thus far, there is no report in the literature on babesiosis in animals from Luxembourg. To date, only one unpublished case of babesiosis in a stray cat (Heddergott, personal communication) is available from Luxembourg. According to this, a road-killed 7-year-old male cat from the administrative district of Echternach in Northern Luxembourg was found to have B. canis-like piroplasm in a blood smear. However, PCR-sequencing of a fragment of the 18S rDNA showed only a 96% similarity with B. canis. A study on the prevalence and incidence of canine babesiosis in Western European countries, by means of questionnaires in veterinary clinics in 2010, did not yield any cases of canine babesiosis for Luxembourg [188]. However, a recent study reported the occurrence of the ornate dog tick, D. reticulatus, in the southern parts of Luxembourg ([189]; Figure 1).
Figure 1.
Occurrence of the ornate dog tick, Dermacentor reticulatus, in the reviewed countries according to https://www.ecdc.europa.eu/sites/default/files/images/Dermacentor_reticulatus_2021_09.png (accessed on 20 March 2022).
3.6. Poland
3.6.1. Babesiosis in Humans
In Poland, the first case of babesiosis, likely due to B. microti, was described as an imported infection in a 37-year-old immunocompetent sailor following his return from Brazil [190]. The first case of babesiosis in an immunosuppressed patient (a patient with ulcerative colitis, treated with immunosuppressive drugs) was reported in 2004 [191]. Although infection was confirmed by the successful cross-infection of mice, molecular identification of the Babesia species involved was unsuccessful [191]. No new cases were reported until 2010, when an asymptomatic infection with B. venatorum/B. divergens was identified by PCR in an immunocompetent forester from southeastern Poland [192]. In 2015, an asymptomatic infection with B. microti, Jena strain, was identified in two immunocompetent foresters from Białowieża, northeastern Poland (2 were positive out of 58 tested, 3.3% [193]). In 2016, another B. microti (identical to the pathogenic US Jena strains) infection was diagnosed in a hospitalized immunocompetent woman, following her return from the US and Canada (likely an imported case). The woman was co-infected with Lyme borreliae [194].
In addition to these sporadic cases, four epidemiological studies have been conducted in northeastern Poland, a region that is well known as (hyper-)endemic for other tick-borne diseases, including borreliosis (Lyme disease), anaplasmosis, and tick-borne encephalitis (TBE) [195,196,197,198]. In the first study, anti-B. microti IgG were found in five healthy foresters (5/114 = 4.4%) from Białowieża (local seroprevalence: 9.2%) [199]. In a second, larger study, which focused on Babesia infections in patients hospitalized/treated because of non-specific symptoms (fever, muscle pain, joint pain, headache, vertigo, nausea, and vomiting) following a tick bite, 548 patients were tested by a range of methods, including molecular methods (PCR and sequencing) and serology [200]. Babesia infection was diagnosed by PCR (6) and serology (3) in six patients (about 1%). Analyses of the obtained sequences revealed infection with a B. microti variant identical to the Munich strain (or Omsk-vole110 or UR2), a grouping that is considered to be non-pathogenic in humans, comprising isolates derived from rodents, mainly voles (Microtus spp.) [200]. In a study of 110 patients diagnosed initially with TBE, one patient (0.9%) tested positive for B. microti (by PCR and sequencing [201]. In another study performed by the same group, one of 118 patients (0.8%) with non-specific symptoms following a tick bite was diagnosed with co-infection with Anaplasma phagocytophilum and a Babesia sp. (undetermined species and strain) [202].
In a recent serological study focusing on the detection of tick-borne pathogens in HIV-positive patients and blood donors, anti-B. microti IgM was detected in 9.3% of HIV-infected patients (21/227) and in 1.0% of blood donors (2/199) [203]. Anti-B. microti IgG was detected in 2.2% of HIV-infected patients (5/227) and in 1.5% of blood donors (3/199) [203].
To summarize, both imported and autochthonous, asymptomatic, and symptomatic infections with B. microti have been reported in humans in Poland. Most of the positive cases have been observed in immunocompetent subjects. Interestingly, infections were caused both by well-known zoonotic B. microti strains (US or Jena or Gray) or by strains considered to be non-zoonotic, such as the Munich strain (or UR2 or Omsk-vole110) [193,194,200]. Additionally, an asymptomatic human infection with B. venatorum/B. divergens has also been identified [192]. Serological studies have revealed a relatively high seroprevalence of anti-B. microti antibodies in groups at risk (about 9% in foresters and HIV-infected patients).
3.6.2. Babesiosis in Animals
Cases of babesiosis in animals other than dogs are rarely reported in Poland. There is a single published case of feline babesiosis reported to date: a 10-year-old cat, showing weakness, anemia, fever, and hematuria, which recovered fully after the administration of imidocarb [204,205]. Piroplasms in the blood smear of this animal resembled B. canis, but the sequencing of a fragment of the 18S rDNA showed only 95% homology with B. canis. However, in central Poland, a region that is hyperendemic for B. canis, cases of babesiosis in cats are suspected to occur sporadically: 2–3 cases per year, in comparison to an incidence of 240/1000 dogs at the same veterinary clinic [206].
There are few reports of Babesia spp. infections in cattle and horses, although several cases of bovine piroplasmosis have been diagnosed, including fatal cases, likely due to B. divergens in northwestern Poland (Choszczno) in 2017 and 2018 [207]. In eastern Poland (Lubelskie province) Babesia spp. DNA has been detected in 10.4% of apparently healthy dairy cows (20/192), with no effect on the hematological and biochemical parameters or milk productivity in the positive animals [208,209]. The exact identification of the piroplasm species involved was not possible because the sequencing of 18S rDNA revealed only a low similarity (max 93% identity) to B. occultans.
There are few cases of piroplasmosis in horses reported from Poland [210,211]. The first case was described in 2008, in a 2-year-old stallion with fever, anemia, loss of appetite, and muscle weakness. PCR sequencing revealed that infection was caused by T. equi [210]. Similarly, in the most recent study, the presence of T. equi DNA (identity above > 99.5%) was detected in 37 out of 512 (7.2%) horses with clinical signs following tick bites [211]. No Babesia infections were noted among these horses, which presented with lethargy, anemia, and thrombocytopenia [211].
Babesiosis in dogs is an increasing veterinary problem in large areas of Poland and is strictly associated with the occurrence of D. reticulatus ticks [212]. Before 2000, canine babesiosis was limited to the eastern region of the country [213]. However, with the expansion of the range of D. reticulatus toward west, central and eastern Poland, these regions currently constitute a large (hyper-)endemic area for canine Babesia infections [212,214,215], with thousands of canine babesiosis cases treated annually [210,212,216,217,218]. Interestingly, a new population of ornate dog ticks has been found in western Poland [219,220], but no B. canis DNA was detected to date in about 2100 examined ticks from this area [212,220]. In a recent country-wide epidemiological study, the incidence of canine babesiosis was calculated for different regions, based on the number of dogs treated for babesiosis by veterinary practitioners in 2018 [212]. The overall annual incidence of clinical babesiosis among the Polish dog population was 20/1000 dogs (2%), with marked differences between three regions of the country. The study revealed few cases and low incidence in western Poland (a total of 19 cases/year and 0.4/1000 dogs) and in the “gap” region where no D. reticulatus has been found to date (only 7 cases/year and 0.9/1000 dogs). Many more cases (1532) and a much higher incidence (53/1000 dogs), accompanied by 2.5% fatality, have been reported in the areas of central and eastern Poland. In an earlier study [217], the number of canine babesiosis cases was six times higher in dogs in the eastern regions of Poland compared to the western regions. Canine babesiosis is currently the most common cause for renal replacement therapy in dogs [221]. Babesia canis is reported almost exclusively as the etiological agent of canine babesiosis in Poland [214,215,218,222,223], with only four cases of B. gibsoni identified recently [224,225].
3.7. Slovakia
3.7.1. Babesiosis in Humans
In a recent review [226] on ticks and tick-borne disease occurrences in Slovakia, three unpublished cases of human babesiosis are mentioned. No more data (i.e., species involved, imported vs. autochthonous, or the region of the country) has been provided to date.
3.7.2. Babesiosis in Animals
No reports of babesiosis in cattle or cats have been published over the past 20 years, and no horses were found to be positive among 39 from southern and southwestern Slovakia, as examined recently by PCR for Babesia and Theileria [227].
Canine babesiosis is an emerging disease in Slovakia. Two studies describing the first recognized cases of babesiosis were published in 2001 [228] and 2002 [229], respectively. Swan et al. [228] described three cases of clinical babesiosis diagnosed in southwestern Slovakia (near Bratislava) in early autumn 2000. The infection was confirmed by the presence of Babesia sp. in erythrocytes, observed by microscopic examination of the blood of the dogs (one crossbreed, two Irish Setters). The outcome of the infection was fatal in the case of a female Irish Setter. Chandoga et al. [229] reported the first case of canine babesiosis in southeastern Slovakia, near Kosice, in a 1.5-year-old male Siberian husky in May 2000. In autumn 2000 and February 2001, two additional cases were diagnosed in this region [229]. Then, B. canis was found in 1% of D. reticulatus ticks in southwestern Slovakia in 2002 [230]. During an outbreak of babesiosis in the period from 2004 to 2005 and until 2010, veterinary practitioners from areas with the endemic occurrence of canine babesiosis in southern Slovakia collected 87 samples from dogs with clinical signs of babesiosis, and molecular techniques (PCR and sequencing) confirmed B. canis infection in 80/87 dogs [231]. In the same area, 326 (204 females and 122 males) questing adult D. reticulatus ticks were collected by flagging. The DNA of B. canis was identified in 35.6% of the ticks. Furthermore, D. reticulatus ticks were observed across the entire territory of Slovakia but were seen less frequently in northern areas of the country [232].
Marked west-to-east differences have been observed in the prevalence of B. canis in D. reticulatus ticks collected in Slovakia [233]. The highest prevalence of B. canis has been observed in D. reticulatus from eastern Slovakia (14.7%), whereas the prevalence in the southeastern region was significantly lower (2.3%). Notably, all 874 D. reticulatus ticks collected in western Slovakia (Záhorská Nízina lowland) tested B. canis-negative. In a more recent study (2010–2011), a group of 217 dogs, encompassing both healthy individuals and those treated for babesiosis, was tested both by molecular techniques and serology [234]. The dogs originated from western (near Bratislava) and southern Slovakia (near Nové Zámky and Komarno). Interestingly, no dogs from the western region tested positive for B. canis (including dogs with suspected babesiosis), in comparison to 31–35% of (sero-)positive dogs from southern Slovakia [234]. Thus, the authors concluded that canine babesiosis is surely endemic in southeastern and southern Slovakia but may not yet be endemic in western regions of the country, despite the recent expansion of the parasite in a northwestern direction ([234], Siroky, unpublished).
In 2013, two pit bull terriers from one household, both with clinical signs of babesiosis, were treated with imidocarb without success. Molecular testing revealed B. gibsoni infection in both dogs [106], despite the absence of the main tick vector, R. sanguineus, in Slovakia.
3.8. Slovenia
3.8.1. Babesiosis in Humans
Testing of sera from seven febrile patients with a history of tick bites by an “in-house” IFAT [230] resulted in the detection of anti-B. divergens antibodies in five patients. However, molecular testing revealed negative results for these patients and they also tested negative in an anti-B. microti IFAT.
In 2014, a B. crassa–like infection was diagnosed by a range of methods, including molecular testing (PCR and sequencing) in a 55-year-old asplenic woman [235]. The patient sought medical treatment after a 6-day history of intermittent fever up to 39 °C, myalgia, headache, poor appetite concomitant with weight loss, fatigue, sweating, and dark urine. She reported no history of travel, tick bites, animal contact, or blood transfusions.
3.8.2. Babesiosis in Animals
There are only very limited data on babesiosis in domestic animals from Slovenia. In the period from 2000 to 2002, based on clinical, microscopic, and molecular investigations, 14 (5.9%) of 238 dogs admitted to the animal clinic in Ljubljana were infected with Babesia spp. Clinical signs relating to acute hemolysis, fever, anorexia, depression, and hematological abnormalities such as anemia and thrombocytopenia were noticed in the majority of the 14 infected dogs. Babesia canis was detected in 11 dogs (4.6%) and B. vogeli in 3 dogs (1.3%) via PCR and sequencing [236].
Spleen samples from 51 roe deer (Capreolus capreolus) and 30 red deer (Cervus elaphus) were tested for piroplasms between 1996 and 2000. Both B. divergens and B. venatorum were detected in roe deer (76.5%); however, more animals were infected with B. divergens (54.9%) than B. venatorum (21.6%). Only 16.7% of red deer tested positive for B. divergens alone [237]. Additionally, B. venatorum DNA was detected in I. ricinus ticks from Slovenia [238].
3.9. Switzerland
3.9.1. Babesiosis in Humans
Overall, reports on human babesiosis in Switzerland are rare, despite a high incidence of other tick-borne infections [239]. There are only three clinical human case studies in Switzerland in the last 30 years: one was due to an autochthonous infection with B. microti, one was a B. microti infection in a tourist from the eastern United States, and one was an imported case tested positive for B. divergens, in a patient returning from Wales [240,241,242]. All patients had no history of immunosuppression and showed only a mild course of the infection with fever. Interestingly, in a region with 4% B. microti PCR-positive questing I. ricinus ticks, a seroprevalence of 1.5% has been found by a B. microti IFAT in 396 residents [243].
3.9.2. Babesiosis in Animals
Local outbreaks of babesiosis in cattle have been described in the past in different regions throughout Switzerland, including the western, central, alpine, and southern parts of the country [244]. These cases were due to small Babesia sp., most probably B. divergens, as this species could be identified in the tick vectors [245,246]. Sporadic Babesia outbreaks in farms are known to occur in the region of the Jura mountains (close to the Swiss-French border) up to the geographical tripoint where the borders of Switzerland, France, and Germany meet, and enzootic stability in the affected farms is suspected. Accordingly, a study indicated 90% seropositivity with antibodies against B. divergens in cattle in this region, but only a few (<1%) clinical cases were observed [247].
The presence of large Babesia species in cattle was identified in the southern part of Switzerland, and this was assumed to be B. major, based on the presence of the tick vector H. punctata [244]. More recently, the presence of B. major has been confirmed by PCR in ticks from the region [244].
Furthermore, a severe outbreak was reported on a trading farm for dairy cows in eastern Switzerland, with 3% (n = 8) of all examined animals testing positive for large Babesia sp. [248]. Species-specific Babesia sequencing for the positive samples was achieved at a later stage, with 99.7% sequence identity to B. bigemina [246]. Interestingly, 90% of the animals in this farm had up to five other tick-borne infections, presenting also with Theileria sp., Anaplasma marginale, A. phagocytophilum and Mycoplasma wenyonii. The Theileria sp. belonged to the complex of T. buffeli/orientalis/sergenti, which are considered to be less pathogenic and are disseminated throughout southern Europe [249,250].
Equine piroplasmosis is considered a sporadic disease in Switzerland but is estimated to be highly underdiagnosed [251]. The first description of EP in Switzerland was from 1985, when a stable with sports horses had an extensive outbreak [252]. Although some of the infected horses had traveled to sports events in other parts of Europe (mainly to France), most of the infected animals had no history of travel. Furthermore, autochthonous cases of tick-transmitted or iatrogenic infections in horses have been described in Switzerland [253]. An overall seroprevalence of 7.3% for EP was demonstrated in 689 horses, with a significantly higher seroprevalence of T. equi infections (5.8%) compared to B. caballi (2.9%), among which 10 horses (1.5%) were seropositive for both parasites. In imported horses, the seroprevalence of T. equi was significantly higher than in indigenous horses [254]. Between 2006 and 2016, 29 horses were presented at clinics for equine medicine at the Vetsuisse Faculty of the University of Zurich, with a diagnosis of EP [255]. The majority (62.1%) had a confirmed infection with T. equi, 34.5% with B. caballi, and one horse showed a co-infection with both species. Most of these animals (22 of 29) had a travel history to another country prior to the onset of symptoms. Most affected stables with autochthonous infections were located in the Jura mountains region, where tick vectors are present. Altogether, EP is prevalent in Switzerland, with sporadic outbreaks in regions where the tick vector is present, and constant monitoring is recommended [254].
The regional distribution of canine babesiosis in Switzerland is similar to the epidemiological pattern of EP. The first cases of B. canis, as the only large Babesia species prevalent in Switzerland, were described in 1974 in the region of Geneva [256]. Since then, a stable endemic focus has been present in the western part of Lake Geneva and along the region of the Jura mountains [257,258]. Sporadic outbreaks with severe infections in dogs without any travel history have been identified in eastern areas of the Swiss midlands and in northeastern Switzerland, where tick vectors (D. reticulatus) were found by flagging for questing ticks [26,259,260,261]. In these studies, 10 of the 50 dogs that had been admitted to veterinary practices between 2003 and 2015 died despite the initiation of treatment. After a few years, no further infections were observed and no endemic regions have been established so far, other than the one bordering France.
To our knowledge, there are no reports of autochthonous infections with small Babesia species (e.g., B. gibsoni and B. felis) in dogs and cats in Switzerland. In cats, infections with another apicomplexan tick-borne hemoprotozoan, Cytauxzoon spp., emerged, with a report in 2018 of five domestic kittens from the northwest and west of Switzerland presenting with severe anemia [262]. Furthermore, six infected domestic cats were identified in 2019 in the central part of Switzerland, triggering a nationwide epidemiological study, in which one seropositive (1 of 881 randomly selected cats), and one PCR-positive cat (1 of 501 anemic cats), both originating from the region of the Jura mountains, were identified [263]. In summary, these cases in companion animals indicate a higher risk of hemoprotozoan infections in the western part of Switzerland, where also tick vectors such as D. reticulatus can frequently be encountered [25]. Furthermore, babesiosis still remains an important imported and travel disease for companion animals in Switzerland, as supported, for example, by a report of several vector-borne diseases in rescued dogs imported from Hungary, which included two cases (15.4%) of B. canis [264].
Three Babesia species involved in human infections, B. divergens, B. venatorum, and B. microti, have been described in ticks from urban and suburban areas throughout Switzerland, representing a potential risk of infection for local inhabitants [243,245,246,247,265,266]. In individual studies, species identification has been carried out, and this has indicated a low prevalence of questing ticks (mainly I. ricinus), with 0.2–0.8% testing positive for B. divergens, 0.4–2.0% for B. venatorum, and 0.2–0.7% for B. microti [245,246,266]. In urban areas, B. venatorum has been detected only in areas with a known presence of wild ungulates [267].
4. Northern and Northeastern Europe
4.1. Denmark
4.1.1. Babesiosis in Humans
The first case of human babesiosis in Denmark was an imported case of B. microti infection from the US, reported in 2013 [268]. No locally acquired cases have been reported to date.
4.1.2. Babesiosis in Animals
Bovine babesiosis is endemic in Denmark but has received relatively little attention [269]. In 2018, the first autochthonous case of canine babesiosis was diagnosed in a Golden Retriever with no history of travel or import [270]. Earlier, a fatal case was reported in a Miniature Schnauzer that had traveled to Hungary [271]. In February 2017, 21 adult male D. reticulatus ticks were found on a migrating golden jackal (Canis aureus) that had been hunted in Western Denmark, about 200 km north of the Denmark–Germany border [272].
Babesia microti and B. venatorum were reported in ticks collected from domestic dogs in 2011 in Denmark [273], and B. venatorum in ticks from raccoon dogs (Nyctereutes procyonoides) [274].
4.2. Estonia
4.2.1. Babesiosis in Humans
No published data are available from Estonia on babesiosis in humans.
4.2.2. Babesiosis in Animals
There is still a paucity of information on the current situation regarding babesiosis in animals in Estonia because, thus far, no large studies have been undertaken among different animal species. According to old records from the period 1960–1968, bovine babesiosis was then estimated to affect 0.36% of cattle in the Estonian Soviet Socialistic Republic [275].
According to the Estonian Veterinary and Food Laboratory’s yearly report on animal diseases in 2020, 42 blood samples from horses were tested for EP and 14 (33.3%) were positive for piroplasms (Estonian Veterinary and Food Laboratory, 2020).
There have been two case studies published on canine babesiosis relating to Estonia. In 2010, six dogs attended a race meeting in Estonia. After their return to Poland, two of the dogs developed clinical signs of babesiosis [276]. Babesia DNA was detected in their blood samples and sequenced. Based on a phylogenetic tree, no regional specificity of the parasites was observed; therefore, the origin of the infection in the two cases could not be clearly determined. High homology to genotype 2 and an isolate from D. reticulatus from Kury, Poland, suggested that the dogs had been infected in Poland [276].
In 2015, a previously splenectomized dog was admitted to a small animal clinic of the Estonian University of Life Sciences in Tartu, Estonia, showing signs of lethargy, a change in urine color, and lack of appetite, was diagnosed with babesiosis by blood smear microscopy [277]. The clinical status of the dog worsened within 8 h, with the onset of unresponsive seizures; therefore, with the owner’s consent, the dog was euthanized. Blood samples from the dog and from two other dogs from the same household tested positive for Babesia using molecular methods, and the sequencing of the 18S rRNA gene fragment confirmed the causative species as B. canis. All the dogs had attended a dog show in the United Kingdom and clinical signs developed 13 days after their participation in the show; thus, it was considered likely that the infection had been acquired abroad. This case study is a useful reminder of the possible rapid progression of the disease in splenectomized dogs [277].
Various zoonotic Babesia species, for example, B. microti, B. divergens and B. venatorum, have been identified in ticks from Estonia [278].
4.3. Finland
4.3.1. Babesiosis in Humans
There is one published case study of fatal human babesiosis caused by B. divergens, in a 53-year-old man with a rudimentary spleen and comorbidities [279]. The man had no travel history in the previous five years and the infection was considered to have been acquired from a local cattle reservoir through a tick bite [279].
4.3.2. Babesiosis in Animals
Bovine babesiosis is endemic in Finland [279,280] although, currently, cases are reported to be far less common than in the 1960s, when thousands of cases were reported annually [281]. The most recent case of EP was reported in 2020 in an imported horse [280]. Canine babesiosis due to B. canis has been diagnosed in imported dogs [282,283]. There are no published reports of the endemic occurrence of D. reticulatus in Finland (ECDC website, Figure 1).
4.4. Iceland
Neither imported nor autochthonous cases of babesiosis in humans or animals have been reported from Iceland. No endemic tick populations have been detected in the country (ECDC website, Figure 1).
4.5. Latvia
4.5.1. Babesiosis in Humans
There are no published reports of human babesiosis in Latvia to date.
4.5.2. Babesiosis in Animals
No cases in cats or horses in Latvia have been reported. The first documented cases of babesiosis in dogs without a travel history occurred in Latvia between 2009 and 2011 [284]. Since then, canine babesiosis has become an endemic disease in the southern and western regions of Latvia and is caused solely by the large species, B. canis [285]. A seasonal pattern has been observed in Latvia for disease outbreaks, as the majority of canine babesiosis cases have occurred between April and June [285]. The distribution of B. canis in Latvia is most likely linked primarily to the expansion of the range of the main vector—D. reticulatus. A recent study has shown that D. reticulatus ticks are present in the southern, central, and western regions of the country; moreover, this tick has been detected in geographically separate small localities in the Riga region, outside of the major region of endemicity [286]. In accordance with this finding, many canine babesiosis cases have been reported from Riga, the capital of Latvia [285]. In recent assessments, the reported overall prevalence of B. canis in field-collected D. reticulatus ticks in Latvia was found to be relatively low (0.34%); in contrast, 14.8% of D. reticulatus ticks removed from Latvian dogs were B. canis-positive [286,287]. Additionally, B. canis DNA was detected in 0.91% of the field-collected I. ricinus ticks; however, B. canis-positive samples were found only in areas with the sympatric occurrence of I. ricinus and D. reticulatus [286,288]. Several Babesia species have been identified in field-collected ticks, including the human pathogens B. microti and B. venatorum [286,288].
4.6. Lithuania
4.6.1. Babesiosis in Humans
In Lithuania, babesiosis is not a nationally notifiable disease and no human cases have been reported.
4.6.2. Babesiosis in Animals
Confirmed babesiosis cases among domestic animals have been reported only in dogs. There are no reports of Babesia infection in cats, cattle, or horses in Lithuania. However, during observations of the health status of European bison (Bison bonasus) in Lithuania, B. divergens was detected in two out of 37 (5.4%) spleen samples from these animals and in an engorged I. ricinus feeding on the bison. These findings were based only on molecular evidence (PCR and sequence analysis). However, visual examination of the internal organs of the animals revealed pathological changes that were compatible with those expected of Babesia infection [289]. The detection of B. divergens in European bison and I. ricinus ticks in Lithuania implies that domestic livestock coexisting with European bison are at high risk of contracting this pathogen.
The first cases of canine babesiosis were recorded and confirmed by microscopic analysis in central Lithuania in the early 2000s. Although previously a rare infection in the country, canine babesiosis has been reported more frequently in recent years in Lithuania. According to the observations of veterinary practitioners in the period from 2003 to 2010, the highest incidence of canine babesiosis was observed at the time in the central and southwestern parts of Lithuania [290]. However, over the last decade, canine babesiosis has been spreading continuously in Lithuania and an increasing number of cases, with a wide variety of clinical signs, have been recorded throughout the country. Currently, the disease occurs almost across the entire country. Both complicated and uncomplicated forms of canine babesiosis have been recorded [291,292].
Babesia canis is reported as the main etiological agent of the disease in dogs in Lithuania [290,291,292]. The expansion of the range of B. canis in Lithuania is directly related to the expanding range of the main vector—D. reticulatus. Evidence for the changing distribution of D. reticulatus ticks in the Baltic countries has been provided by the occurrence of canine babesiosis in new locations in Lithuania and Latvia since 2013 [284]. In Lithuania, the presence of D. reticulatus (2013–2015) has been confirmed in 38 localities in which this species had not been previously reported [293]. Babesia canis DNA was detected in 1.2% (26 of 2255) of D. reticulatus ticks collected from 40 locations across Lithuania. The highest prevalence of infection was observed in the central (7%) and southern (11%) parts of the country, where populations of D. reticulatus ticks have been observed since the last century and dogs are frequently diagnosed with canine babesiosis [294].
The State Food and Veterinary Service of Lithuania does not hold statistics on the overall annual incidence of clinical babesiosis cases, nor does it record mortality among the Lithuanian dog population. From 2011 to 2018, veterinary practitioners from different small animal clinics in central (Kaunas), eastern (Vilnius), and western (Klaipeda) parts of Lithuania reported between 20 and 150 babesiosis cases per year, with the highest number of cases detected in central regions of the country. According to information provided by the major veterinary clinic in Central Lithuania (Dr. L Kriaučeliūnas Small Animal Clinic; Faculty of Veterinary Medicine, Lithuanian University of Health Sciences, Kaunas), the incidence of canine babesiosis (based on the number of treated babesiosis cases) in 2019 was 59 cases/year and 8.6/1000 dogs; in 2020, the figure was 74 cases/year and 10.5/1000 dogs; and in 2021, it was 55 cases/year and 7.1/1000 dogs (Radzijevskaja et al., unpublished). During the past few years, some veterinary practitioners have noticed a trend of decreasing canine babesiosis cases, especially of the complicated forms of the disease [291], most likely attributable to the increasing public availability of information and the implementation of measures for the prevention of canine babesiosis.
The causative agents of human babesiosis B. divergens, B. venatorum and B. microti have been detected in I. ricinus and D. reticulatus tick populations in the country [289,294,295].
4.7. Norway
4.7.1. Babesiosis in Humans
The first case of severe autochthonous babesiosis in Norway was reported to have occurred in 2007 [296]. The patient was a splenectomized 58-year-old male veterinarian who worked with cattle. He was frequently exposed to tick bites in an area endemic for bovine babesiosis in western Norway [297,298]. The patient presented with severe hemolysis and multi-organ failure. Microscopic observation revealed a 30% parasitemia with a small Babesia, and IFAT confirmed a B. divergens infection [296].
In a study of 188 patients with erythema migrans, no Babesia DNA was detected in skin biopsies, nor were anti-B. microti antibodies detected in the serum samples by IFAT [299]. No Babesia DNA was detected in blood samples from 163 immunosuppressed patients with systemic rheumatic, gastrointestinal, and neurological autoimmune diseases from southern Norway [300]. In contrast to these findings, among the adult general population in Søgne in the southernmost part of Norway, IgG antibodies to B. microti were found in 2.1% (33/1537) of serum samples [301].
4.7.2. Babesiosis in Animals
Norway is endemic for bovine babesiosis, with a regular occurrence of cases [297,298,302]. Records of the tick-borne disease, bovine babesiosis, are available from the Norwegian Cattle Health Recording system (NCHRS) for the time period from 2006 to 2015 [297,298,302]. The registration is based on clinical symptoms, rather than confirmed cases based on laboratory diagnostics. Records of babesiosis in cattle are rare in eastern Norway but are regularly reported in the western regions of the country, with incidence values ranging from 5 to 30/1000 [297,298].
Until recently, canine babesiosis caused by B. canis was considered to be an imported disease; imported cases of acute babesiosis were diagnosed sporadically in dogs returning from Central Europe (Brun-Hansen and Øines, unpublished). The first likely autochthonous case was diagnosed in early May 2009, in an Irish Setter from the Oslo area [303]. The 6-year-old male dog presented at a veterinary clinic with lethargy, anorexia, and polydipsia. The owner had noticed the dog becoming sick one day after a large (about 8–10 mm) gray tick had been removed from its neck. Unfortunately, the tick was not available for species identification or further investigation. Babesiosis due to B. canis was diagnosed, based on microscopy and molecular methods (PCR and sequencing). There are no data on the occurrence/distribution of D. reticulatus in Norway (Figure 1).
4.8. Sweden
4.8.1. Babesiosis in Humans
The literature records two human cases of babesiosis in Sweden to date. The first report was a 34-year-old splenectomized man who presented in October 1989 with fever, myalgia, and dysuria [304]. His condition rapidly deteriorated as he became anuric and developed severe hemolytic anemia, thrombocytopenia, and fibrinolysis. Peripheral blood smears revealed 40% parasitemia with B. divergens [304]. The second case was of a 52-year-old splenectomized man, who presented with recurrent fever between March and May 2015, muscle pain, and dark urine [305]. Babesia sp. infection was diagnosed, based on microscopy, and B. venatorum infection was identified by PCR and sequencing [305]. An epidemiological study from southern Sweden estimated the seroprevalence of IgG antibodies to B. microti/B. divergens to be 2.5% (5/197) among healthy individuals and 16.3% (14/86) among individuals seropositive for Borrelia burgdorferi s.l. antibodies [306]. In a recent study, the seroprevalence of antibodies to B. microti was 4.4% (7/159) among individuals bitten by ticks [307].
4.8.2. Babesiosis in Animals
Bovine and canine babesiosis are notifiable animal diseases in Sweden [308]. Bovine babesiosis is endemic in Sweden [309,310] although no cases were recorded in 2019 [308]. Canine babesiosis is sporadically diagnosed as an imported disease. Four cases of babesiosis due to B. canis infection and one case due to B. gibsoni infection were reported in 2019 [308]. No cases of feline or equine babesiosis were published or recorded.
5. Discussion
A thorough review of the epidemiological situation in the range of countries covered in this paper revealed an interesting picture. First, cases of human babesiosis have occurred in almost all regions of Europe (in 13/20 of the reviewed countries) and their number is slowly but steadily growing. In addition to the well-known cause of human babesiosis in Europe, B. divergens, there are increasing numbers of cases/infections attributable to other species, i.e., B. venatorum, B. crassa-like, and B. microti. Furthermore, infections due to the last species seem to be more prevalent than generally believed, as is indicated by the frequent detection of antibodies to B. microti in different European populations and the detection of B. microti DNA in patients from different countries (Austria, Germany, Hungary, the Czech Republic, Switzerland, and Poland) [3]. A recent study has reported that the molecular detection of B. microti enabled convincing identification of this parasite as the etiological cause of non-specific symptoms leading to patients’ hospitalization in northeastern Poland [200]. The study also provided evidence that a strain of B. microti, common in voles (B. microti Munich) [7] and regarded as non-zoonotic, can lead to the clinical manifestation of the infection in humans [200]. Moreover, the study carries an important message, i.e., that babesiosis due to B. microti may be easily overlooked and may not be recognized in immunocompetent patients due to the non-specific symptoms and to the generally low awareness of general practitioners regarding this tick-borne zoonosis. There is a clear need not only to standardize diagnostic tests and to employ antigens from European B. microti strains in tests but also to develop tests for the detection of antibodies against B. divergens and B. crassa- like organisms.
In Western Europe, human babesiosis is rare but cases due to B. divergens and B. microti (autochthonous or imported) have been regularly reported [3]. In France, 21 cases have been described to date, including 19 cases due to B. divergens and two cases due to B. microti (imported from the US) [3]. In the British Isles, nine cases have been reported to date, including eight cases due to B. divergens (7 in the UK and one in Ireland) and one imported case due to B. microti [3]. In southern Europe, ten cases have been reported in Spain (5 × B. divergens, 5 × B. microti, including four likely imported cases of the latter) [3]. The number of described human babesiosis cases from eastern Europe is much lower, despite the fact that TBD (mainly Lyme disease, TBE, or rickettsioses) constitute a serious health problem in this region [311,312]. There have been at least two cases from Russia [3,313,314], both due to B. divergens. No human cases have been reported from Belarus to date. No human cases have been reported from Ukraine; however, in a recent serological study from Kharkiv, antibodies to B. divergens (6.9%) and B. microti (3.4%) were detected, with higher frequency in HIV-infected individuals (26.7%) and in Lyme disease patients (16.7%) than in blood donors (1.7%) [315].
Co-infection of Babesia spp. and Borrelia burgdorferi s.l., as well as Babesia spp. and A. phagocytophilum, or TBE have been reported in patients from the reviewed countries.
In view of the present emergence of human babesiosis in the US, Canada, China, and other countries outside Europe [5,16], and the emergence of other tick-borne diseases in Europe, a very likely scenario for the next decade is a more rapid increase in the cases of babesiosis, especially in the central and eastern regions of Europe, as reviewed herein. Among animal babesiosis, canine babesiosis is a disease of significant impact, both in well-known endemic (Central Europe) or newly identified (Northeastern Europe, including the Baltic states) regions. Interestingly, the occurrence of (hyper)endemic foci/regions of babesiosis due to B. canis is rather patchy/mosaic and depends on the occurrence of tick vector populations, which are not evenly distributed across the terrain of most countries [23,24,25,316]. Although the ECDC recognized the great majority of the reviewed countries as being endemic for D. reticulatus (Figure 1), the exact distribution rarely covers the whole territory of a specific country. Nevertheless, B. canis is well established in the endemic areas/spots of Austria, BiH, Croatia, Serbia, Hungary, Poland, Slovenia, Slovakia, Switzerland, Latvia, and Lithuania, and newly endemic foci have appeared in many other countries, for example, Germany and the Czech Republic. Overall, autochthonous B. canis infections have been reported from 15 out of 20 reviewed countries. Although in some countries (e.g., in Switzerland and Austria) the number of cases appears sporadic but stable, in other Central European countries, babesiosis due to B. canis is an emerging and fast-spreading disease (Supplementary Table S1). Nordic countries are an example, a region where the spread to new areas is currently being observed, with the first autochthonous published cases of B. canis infections in dogs from Norway and Denmark [270,303]. The occurrence of sporadic cases, together with reports on D. reticulatus ticks, for example, near Stockholm or in other southern parts of Scandinavia, constitute the first steps for the development of local endemicity [272,303]. Furthermore, marked differences in terms of protective practices against ticks have been identified in the Nordic countries [317].
A questionnaire study reported that 23% of veterinarians working in the Baltic and Nordic countries saw at least one canine babesiosis case in 2016 [318]. A veterinarian working in the Baltic countries had 12.2 times higher odds of seeing a canine babesiosis case than a veterinarian working in the Nordic countries. Almost half (48%) of the veterinarians responding to the survey answered correctly that canine babesiosis is not considered a zoonosis [318].
The emergence of canine babesiosis due to B. canis is closely connected to the expansion of the range of D. reticulatus [22,97,212,319]. Countries in which the occurrence of D. reticulatus has been confirmed are shown in Figure 1. However, there are marked differences in the prevalence of B. canis in D. reticulatus from different parts of central Europe, with generally higher prevalence in the eastern European tick populations [220,294,316,320,321]. There are a few reports of very low prevalence, at below 1% and even zero [97,322], among the western European tick populations, although sporadically very high prevalence has also been observed, notably in novel local foci, such as those reported in the UK and Switzerland [26,319]. The prevalence of B. canis in ornate tick populations from the different areas of a single country may differ markedly, as observed in the Czech Republic, Slovakia, and Poland [97,233,316]. Accordingly, the highest incidence of canine babesiosis (from 5 to 250 cases/1000 dogs) is reported from areas with a high percentage of infected ticks—i.e., in eastern Poland, western Ukraine or southwestern Lithuania [212,294,320,323]. In the future, it will be important to monitor carefully whether these differences in the prevalence of B. canis between tick foci/populations are maintained, or if prevalence changes with further increases in tick density/population/range.
Besides B. canis, several other species of piroplasms, such as B. vogeli, B. gibsoni, B. vulpes, B. caballi, and T. equi [40], have been identified in dogs and, recently, T. capreoli was confirmed by PCR and sequencing as the cause of the septic shock and death of an infected dog [44]. In some regions, the fatality rate in dogs from Babesia/Theileria infection could be higher than suspected, since post-mortem molecular detection has not been performed on a regular basis and infections may have been misdiagnosed, possibly being confused with A. phagocytophilum, Borrelia burgdorferi s.l., Leptospira infection [44] or intoxication. Furthermore, due to the rapid clearance of merozoites after treatment in cases with a history of anti-babesial treatment, PCR diagnostic testing should be used rather than the microscopical examination of blood smears, since it is virtually impossible to detect any merozoites on slides within 24 h after treatment [45].
Wild carnivores, such as grey wolves and red foxes, may play an important role as subclinical carriers of babesiosis, as well as vector tick species, and spread both to non-endemic regions [46,47,324,325]. In a recent study, a young collared and telemetrically followed grey wolf infected with B. canis roamed over a large area, covering 563 km2 during a 12-month period, and, throughout, may have constituted a source of Babesia infection for D. reticulatus ticks that it picked up during its travels in the region [46].
Feline babesiosis is still very rarely reported globally [12], including among the reviewed countries. Although there are several species of Babesia that can infect cats [12], these piroplasms have not been identified in questing I. ricinus or D. reticulatus (reviewed in [8]). Cats are less frequently infested with D. reticulatus than dogs [22,326], and their susceptibility to B. canis is still to be confirmed. Interestingly, in a hyper-endemic region for canine babesiosis in central Poland, sporadic feline cases are treated based on clinical symptoms that are indicative of babesiosis (Olszak, personal communication). Unfortunately, there have been no attempts to reach a species-level diagnosis in these cases.
Piroplasmosis in horses is reported regularly in several of the countries reviewed herein, and it is caused most frequently by T. equi. Again, the tick vectors in central and northeastern Europe occur focally (i.e., H. punctata in Germany) [116], or are not reported (there are no reports of T. equi in D. reticulatus; [8]). Therefore, many cases are likely to have been imported (i.e., to the Czech Republic, Germany or Finland) [123]. Moreover, because Theileria spp. are transmitted only transstadially (no transovarial transmission) in ticks, this precludes the establishment of endemic foci within tick populations in the absence of infected animals (source of infection for ticks). Theileria equi occurs commonly in southern Europe, where the tick species that act as vectors are mainly R. sanguineus s.s. or Haemaphysalis spp., in contrast to central and northern Europe, where D. reticulatus or other Dermacentor sp. or Hyalomma ticks may be involved. With climate change and the global movement of equids, it is likely that Theileria spp. will become more common in central Europe, as has recently been observed in Germany [27] and Hungary. In contrast, B. caballi infections, transmitted by Dermacentor and Hyalomma spp., seem still to be rare in the reviewed countries [27,211]. However, due to the transovarial transmission of Babesia spp. in ticks, the establishment of new endemic foci may increase in the future.
Nearly all the reviewed countries (16/20; Supplementary Table S1) have endemic areas for bovine babesiosis due to B. divergens. Babesia divergens is a zoonotic species and infections can be fatal in both cattle and humans [3,13]; thus, the occurrence of babesiosis due to B. divergens in cattle is of special relevance. Interestingly, in the last twenty years, the number of cases has decreased in parallel in both humans [3] and cattle [123,141], a trend that has been observed across several of the countries reviewed herein, this despite the repeated recent reports of the occurrence of this piroplasm in I. ricinus ticks over much of Europe [8,327]. The trend is usually explained by the less extensive use of pastures, anti-tick prophylaxis, and by the maintenance of immunity in parasite-exposed herds, leading to enzootic stability [123,141,328]. However, new outbreaks of bovine babesiosis have been observed recently, e.g., between 2017 and 2019, in northern Germany [123], BiH, and likely northwestern Poland (Supplementary Table S1). These outbreaks draw attention to bovine babesiosis as a potential re-emerging disease.
Despite variations in the methods applied in the studies covered by this review, precluding direct comparisons in many cases, the extensive epidemiological and molecular data collated suggest the expansion (and emergence) of new piroplasm species in central, northern, and northeastern Europe. For instance, sporadic cases of canine babesiosis due to B. gibsoni have been reported in Austria, the Czech Republic, Slovakia, Germany, Hungary, Poland, and Sweden (Supplementary Table S1), despite the lack of the main vector (R. sanguineus) in most of these countries. Babesia gibsoni infections could have been imported or transmitted by alternative routes, through dog fights/biting, or transplacentally [329,330]. Interestingly, almost all reported cases have been described in American pit bull terriers or American Staffordshire terriers, suggesting either the higher susceptibility of these breeds to piroplasms or specific but as yet unidentified transmission routes within these breeds. Furthermore, B. vogeli was identified in dogs from Croatia, Serbia, and Slovenia. In Hungary, B. major, T. buffeli, and T. orientalis were recently identified in cattle [168].
Considering the geographical occurrence of babesiosis in animals, the greatest diversity of piroplasms has been observed in southern Europe (Supplementary Table S1). Central and northeastern Europe (the Baltic states) are the main areas of expansion and the emergence of new foci for Babesia/Theileria species. Fennoscandia is endemic for B. divergens, enabled by the occurrence of I. ricinus ticks [21], but it seems to be largely free of the endemic occurrence of canine babesiosis and other Babesia spp. due to the lack of established tick vector populations. However, with the expected further, future, successful expansion of D. reticulatus in central and northeastern Europe ([22,212,293,294,331], Figure 1), and due to the transovarial and transstadial transmission of Babesia spp. within tick species, it is likely that areas of Finland and southern Scandinavia will become endemic for piroplasms vectored by this tick species in the relatively near future.
Undoubtedly, the situation in Europe is changing, but a clear picture of future trends is not easy to generate, given the data currently in the public domain and the lack of attention to human and animal babesiosis in some countries. Different sampling approaches and different methods have been applied, complicating a direct comparison between studies and the synthesis of a global picture. Increasing awareness about babesiosis in both the medical and veterinary professions, as well as among the general public, is urgently required. However, in order to enable the identification of convincing temporal changes and trends in the prevalence and associated disease status of babesiosis, we firmly recommend the standardization of data collection and the better coordination of studies across the countries, sectors, and disciplines involved.
6. Conclusions
This review of the occurrence of piroplasms in humans, animals, and ticks from 20 countries, covering southeastern, central, northern, and northeastern Europe, has revealed the widespread occurrence of these pathogens. Recorded cases of human babesiosis are still rare but their number is expected to rise in the coming years, due to the widespread and longer seasonal activity of I. ricinus, with more cases attributable to B. microti and because of better diagnostic methods. Bovine babesiosis has re-emerging potential because of the likely loss of herd immunity and the return to more extensive cattle management. Canine babesiosis due to B. canis is a rapidly expanding and emerging tick-borne disease in central and northeastern Europe, the prevalence of which correlates with the rapid and successful expansion of the ornate dog tick population. There is clear potential for the local establishment of new “hotspots” and, on the basis of currently identified trends, the focal emergence of disease in areas of Fennoscandia in the near future. In horses, T. equi infections appear to be spreading in central Europe, mainly due to the mobility of horses. Feline babesiosis is still reported only rarely in Europe but the susceptibility of cats to B. canis infection requires reassessment and their risk of infection needs re-evaluation, especially in those regions of Europe that are endemic for babesiosis.
Finally, in this review, we have drawn attention to the still largely incomplete datasets currently available about the extent of babesiosis in Europe. Despite this, there is clear evidence of an increasing annual incidence of piroplasmosis across Europe, which is changing in line with similar increases in the incidence of other tick-borne diseases that have been documented elsewhere (ECDC). This situation is of concern, and we recommend careful, standardized monitoring using a “One Health” approach.
Acknowledgments
Not applicable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10050945/s1, Table S1: Babesiosis cases in humans and animals in the reviewed countries.
Author Contributions
A.B. (Anna Bajer) conceived and planned the manuscript; A.B. (Ana Beck), R.B., J.M.B., D.D.-S., R.M.E., R.F., H.-P.F., M.H., P.J., M.L., V.O., A.P., J.R., R.R., M.S., A.S., C.S., K.T. and J.W. reviewed the available literature and prepared reports for the countries included. All authors participated in preparing the final version of the article and have approved its content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uilenberg G. Babesia—A historical overview. Vet. Parasitol. 2006;138:3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Krause P.J. Human babesiosis. Int. J. Parasitol. 2019;49:165–174. doi: 10.1016/j.ijpara.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Hildebrandt A., Zintl A., Montero E., Hunfseld K.P., Gray J. Human babesiosis in Europe. Pathogens. 2021;10:1165. doi: 10.3390/pathogens10091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vannier E.G., Diuk-Wasser M.A., Ben Mamoun C., Krause P.J. Babesiosis. Infect. Dis. Clin. N. Am. 2015;29:357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A., O’Bryan J., Krause P.J. The global emergence of human babesiosis. Pathogens. 2021;10:1447. doi: 10.3390/pathogens10111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centre for Disease Control and Prevention [CDC] [(accessed on 3 March 2022)]; Available online: https://www.cdc.gov/
- 7.Tolkacz K., Bednarska M., Alsarraf M., Dwuznik D., Grzybek M., Welc-Faleciak R., Behnke J.M., Bajer A. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae) Parasit. Vectors. 2017;10:66. doi: 10.1186/s13071-017-2007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajer A., Dwuznik-Szarek D. The specificity of Babesia-tick vector interactions: Recent advances and pitfalls in molecular and field studies. Parasit. Vectors. 2021;14:507. doi: 10.1186/s13071-021-05019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause P.J., Telford S.R., Spielman A., Sikand V., Ryan R., Christianson D., Burke G., Brassard P., Pollack R., Peck J. Concurrent Lyme disease and babesiosis: Evidence for increased severity and duration of illness. Jama. 1996;275:1657–1660. doi: 10.1001/jama.1996.03530450047031. [DOI] [PubMed] [Google Scholar]
- 10.Diuk-Wasser M.A., Vannier E., Krause P.J. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32:30–42. doi: 10.1016/j.pt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solano-Gallego L., Sainz Á., Roura X., Estrada-Peña A., Miró G. A review of canine babesiosis: The European perspective. Parasit. Vectors. 2016;9:336. doi: 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penzhorn B.L. Don’t let sleeping dogs lie: Unravelling the identity and taxonomy of Babesia canis, Babesia rossi and Babesia vogeli. Parasit. Vectors. 2020;13:184. doi: 10.1186/s13071-020-04062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zintl A., Mulcahy G., Skerrett H.E., Taylor S.M., Gray J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 2003;16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock R., Jackson L., De Vos A., Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:S247–S269. doi: 10.1017/S0031182004005190. [DOI] [PubMed] [Google Scholar]
- 15.Gray E.B., Herwaldt B.L. Babesiosis surveillance—United States, 2011–2015. MMWR Surveill. Summ. 2019;68:1–11. doi: 10.15585/mmwr.ss6806a1. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y., Christie J., Köster L., Du A., Yao C. Emerging human babesiosis with “ground zero” in North America. Microorganisms. 2021;9:440. doi: 10.3390/microorganisms9020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonteri E., Jokelainen P., Matala J., Pusenius J., Vapalahti O. Serological evidence of tick-borne encephalitis virus infection in moose and deer in Finland: Sentinels for virus circulation. Parasit. Vectors. 2016;9:54. doi: 10.1186/s13071-016-1335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markowicz M., Schötta A.-M., Höss D., Kundi M., Schray C., Stockinger H., Stanek G. Infections with tickborne pathogens after tick bite, Austria, 2015–2018. Emerg. Infect. Dis. 2021;27:1048–1056. doi: 10.3201/eid2704.203366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millins C., Leo W., MacInnes I., Ferguson J., Charlesworth G., Nayar D., Davison R., Yardley J., Kilbride E., Huntley S., et al. Emergence of Lyme disease on treeless islands, Scotland, United Kingdom. Emerg. Infect. Dis. 2021;27:538–546. doi: 10.3201/eid2702.203862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevestre J., Diarra A.Z., Oumarou H.A., Durant J., Delaunay P., Parola P. Detection of emerging tick-borne disease agents in the Alpes-Maritimes region, southeastern France. Ticks Tick Borne Dis. 2021;12:101800. doi: 10.1016/j.ttbdis.2021.101800. [DOI] [PubMed] [Google Scholar]
- 21.Medlock J.M., Hansford K.M., Bormane A., Derdakova M., Estrada-Peña A., George J.-C., Golovljova I., Jaenson T.G.T., Jensen J.-K., Jensen P.M., et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drehmann M., Springer A., Lindau A., Fachet K., Mai S., Thoma D., Schneider C.R., Chitimia-Dobler L., Bröker M., Dobler G., et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—Evidence of a continuing spread of Dermacentor reticulatus. Front. Vet. Sci. 2020;7:578220. doi: 10.3389/fvets.2020.578220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubel F., Brugger K., Belova O.A., Kholodilov I.S., Didyk Y.M., Kurzrock L., García-Pérez A.L., Kahl O. Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp. Appl. Acarol. 2020;82:95–123. doi: 10.1007/s10493-020-00533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubel F., Brugger K., Pfeffer M., Chitimia-Dobler L., Didyk Y.M., Leverenz S., Dautel H., Kahl O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224–233. doi: 10.1016/j.ttbdis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Földvári G., Široký P., Szekeres S., Majoros G., Sprong H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors. 2016;9:314. doi: 10.1186/s13071-016-1599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaarschmidt D., Gilli U., Gottstein B., Marreros N., Kuhnert P., Daeppen J.A., Rosenberg G., Hirt D., Frey C.F. Questing Dermacentor reticulatus harbouring Babesia canis DNA associated with outbreaks of canine babesiosis in the Swiss Midlands. Ticks Tick Borne Dis. 2013;4:334–340. doi: 10.1016/j.ttbdis.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Dirks E., Werner G., Schwarz B., Trübenbach L., Schwendenwein I., Joachim A., Cavalleri J. Die equine piroplasmose im deutschsprachigen raumeine unterdiagnostizierte Krankheit? Prakt. Tierarzt. 2021;102:1078–1088. doi: 10.2376/0032-681X-2150. [DOI] [Google Scholar]
- 28.Jongejan F., Ringenier M., Putting M., Berger L., Burgers S., Kortekaas R., Lenssen J., van Roessel M., Wijnveld M., Madder M. Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasit. Vectors. 2015;8:232. doi: 10.1186/s13071-015-0841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnittger L., Ganzinelli S., Bhoora R., Omondi D., Nijhof A.M., Florin-Christensen M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 2022;121:1207–1245. doi: 10.1007/s00436-022-07424-8. [DOI] [PubMed] [Google Scholar]
- 30.Stevanović O., Jurković D., Polkinghorne A., Ćeleš A., Ilić T., Dimitrijević S., Nedić D., Beck R. Molecular detection of Babesia divergens and Mycoplasma wenyonii infection in cattle from Bosnia and Herzegovina. Parasitol. Res. 2020;119:1423–1427. doi: 10.1007/s00436-020-06630-6. [DOI] [PubMed] [Google Scholar]
- 31.Davitkov D., Vucicevic M., Stevanovic J., Krstic V., Slijepcevic D., Glavinic U., Stanimirovic Z. Molecular detection and prevalence of Theileria equi and Babesia caballi in horses of central Balkan. Acta Parasitol. 2016;61:337–342. doi: 10.1515/ap-2016-0044. [DOI] [PubMed] [Google Scholar]
- 32.Omeragić J., Šerić-Haračić S., Klarić Soldo D., Kapo N., Fejzić N., Škapur V., Medlock J. Distribution of ticks in Bosnia and Herzegovina. Ticks Tick Borne Dis. 2022;13:101870. doi: 10.1016/j.ttbdis.2021.101870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ćoralić A., Gabrielli S., Zahirović A., Stojanović N.M., Milardi G.L., Jažić A., Zuko A., Čamo D., Otašević S. First molecular detection of Babesia canis in dogs from Bosnia and Herzegovina. Ticks Tick Borne Dis. 2018;9:363–368. doi: 10.1016/j.ttbdis.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Hodžić A., Alić A., Fuehrer H.-P., Harl J., Wille-Piazzai W., Duscher G.G. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit. Vectors. 2015;8:88. doi: 10.1186/s13071-015-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodžić A., Alić A., Duscher G.G. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: A molecular study. Ticks Tick Borne Dis. 2018;9:589–593. doi: 10.1016/j.ttbdis.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Skrabalo Z., Deanovic Z. Piroplasmosis in man; report of a case. Doc. Med. Geogr. Trop. 1957;9:11–16. [PubMed] [Google Scholar]
- 37.Topolovec J., Puntarić D., Antolović-Pozgain A., Vuković D., Topolovec Z., Milas J., Drusko-Barisić V., Venus M. Serologically detected “new” tick-borne zoonoses in Eastern Croatia. Croat. Med. J. 2003;44:626–629. [PubMed] [Google Scholar]
- 38.Babić I. An overview of the development of Yugoslav medical (human and veterinary) parasitology until 1960 and its further basic tasks. Rev. Yugosl. Acad. Sci. Arts. 1965:160–174. [Google Scholar]
- 39.Cacciò S.M., Antunovic B., Moretti A., Mangili V., Marinculic A., Baric R.R., Slemenda S.B., Pieniazek N.J. Molecular characterisation of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet. Parasitol. 2002;106:285–292. doi: 10.1016/S0304-4017(02)00112-7. [DOI] [PubMed] [Google Scholar]
- 40.Beck R., Vojta L., Mrljak V., Marinculić A., Beck A., Živičnjak T., Cacciò S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009;39:843–848. doi: 10.1016/j.ijpara.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Brkljacić M., Matijatko V., Kis I., Kucer N., Forsek J., Rafaj R.B., Grden D., Torti M., Mayer I., Mrljak V. Molecular evidence of natural infection with Babesia canis canis in Croatia. Acta Vet. Hung. 2010;58:39–46. doi: 10.1556/avet.58.2010.1.4. [DOI] [PubMed] [Google Scholar]
- 42.Jurković D., Csik V.-A., Rebselj B., Petak A., Stublić M., Beck R. First report of Babesia gibsoni and Babesia vulpes in symptomatic dogs from Croatia: From clinics to therapy; Proceedings of the 8th International Congress “Veterinary Science and Profession”; Zagreb, Croatia. 10–12 October 2019; p. 87. [Google Scholar]
- 43.Mrljak V., Kuleš J., Mihaljević Ž., Torti M., Gotić J., Crnogaj M., Živičnjak T., Mayer I., Šmit I., Bhide M., et al. Prevalence and geographic distribution of vector-borne pathogens in apparently healthy dogs in Croatia. Vector Borne Zoonotic Dis. 2017;17:398–408. doi: 10.1089/vbz.2016.1990. [DOI] [PubMed] [Google Scholar]
- 44.Beck A., Huber D., Antolić M., Anzulović Ž., Reil I., Polkinghorne A., Baneth G., Beck R. Retrospective study of canine infectious haemolytic anaemia cases reveals the importance of molecular investigation in accurate postmortal diagnostic protocols. Comp. Immunol. Microbiol. Infect. Dis. 2019;65:81–87. doi: 10.1016/j.cimid.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Huber D., Beck A., Anzulović Ž., Jurković D., Polkinghorne A., Baneth G., Beck R. Microscopic and molecular analysis of Babesia canis in archived and diagnostic specimens reveal the impact of anti-parasitic treatment and postmortem changes on pathogen detection. Parasit. Vectors. 2017;10:495. doi: 10.1186/s13071-017-2412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck A., Huber D., Polkinghorne A., Kurilj A.G., Benko V., Mrljak V., Reljić S., Kusak J., Reil I., Beck R. The prevalence and impact of Babesia canis and Theileria sp. in free-ranging grey wolf (Canis lupus) populations in Croatia. Parasit. Vectors. 2017;10:168. doi: 10.1186/s13071-017-2106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dezdek D., Vojta L., Curković S., Lipej Z., Mihaljević Z., Cvetnić Z., Beck R. Molecular detection of Theileria annae and Hepatozoon canis in foxes (Vulpes vulpes) in Croatia. Vet. Parasitol. 2010;172:333–336. doi: 10.1016/j.vetpar.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Gotić J. Ph.D. Thesis. University of Zagreb; Zagreb, Croatia: 2015. Clinical and serological Diagnostics and Molecular Typing of Equine Piroplasmosis Aetiological Agents in the Republic of Croatia. [Google Scholar]
- 49.Jurković D., Mihaljević Ž., Duvnjak S., Silaghi C., Beck R. First reports of indigenous lethal infection with Anaplasma marginale, Anaplasma bovis and Theileria orientalis in Croatian cattle. Ticks Tick Borne Dis. 2020;11:101469. doi: 10.1016/j.ttbdis.2020.101469. [DOI] [PubMed] [Google Scholar]
- 50.Duh D., Punda-Polic V., Trilar T., Avšič-Županc T. Molecular detection of Theileria sp. in ticks and naturally infected sheep. Vet. Parasitol. 2008;151:327–331. doi: 10.1016/j.vetpar.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Pintur K. Ph.D. Thesis. University of Zagreb; Zagreb, Croatia: 2012. Genetic Characterization of Piroplasms Infecting Cervids in Croatia. [Google Scholar]
- 52.Beck R., Habrun B., Bosnić S., Benić M., Nemeth-Blažić T., Barišin A.D.S. Identification of pathogens in Ixodes ricinus and Dermacentor reticulatus from public gardens in Zagreb, Croatia; Proceedings of the 12th International Conference on Lyme Borreliosis and Other Tick-Borne Diseases; Ljubljana, Slovenia. 26–29 November 2010; p. 95. [Google Scholar]
- 53.Beck R., Jurković D., Krčmar S., Šarić T., Brezak R., Bosnić S., Stublić M. Diversity of piroplasms detected in ticks: Individual sequencing approach; Proceedings of the 7th International Congress “Veterinary Science and Profession”; Zagreb, Croatia. 5–7 October 2017; p. 89. [Google Scholar]
- 54.Banović P., Díaz-Sánchez A.A., Simin V., Foucault-Simonin A., Galon C., Wu-Chuang A., Mijatović D., Obregón D., Moutailler S., Cabezas-Cruz A. Clinical aspects and detection of emerging rickettsial pathogens: A “One Health” approach study in Serbia, 2020. Front. Microbiol. 2021;12:797399. doi: 10.3389/fmicb.2021.797399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krstić V., Trailović D., Andrić N., Čalić M., Jovanović M. Contribution to epizootiology of dog babesiosis in Belgrade area; Proceedings of the 7th Conference of Veterinarians of Serbia; Zlatibor, Yugoslavia. 13–16 September 1994; pp. 25–26. [Google Scholar]
- 56.Pavlovic I., Milutinović M., Petković D., Terzin D., Terzin V. Epizootiological research of canine babesiosis in the Belgrade district. J. Protozool. Res. 2003;12:10–15. [Google Scholar]
- 57.Pavlovic I., Antić V., Terzin V., Petković D., Terzin D., Ćurčin L., Pesut B., Ćurčin K., Vasić A., Zdravković N. Canine babesiosis in Belgrade area in period 2015-2017; Proceedings of the Workshop on Arthropod-Borne Diseases Transmitted by Ticks, Mites, Fleas, and Lice; Greifswald, Germany. 15–16th November 2018. [Google Scholar]
- 58.Janjić F., Sarvan D., Tomanović S., Ćuk J., Krstić V., Radonjić V., Kovačević Filipović M., Ajtić J. A short-term and long-term relationship between occurrence of acute canine babesiosis and meteorological parameters in Belgrade, Serbia. Ticks Tick Borne Dis. 2019;10:101273. doi: 10.1016/j.ttbdis.2019.101273. [DOI] [PubMed] [Google Scholar]
- 59.Savić S., Vidić B., Grgić Z., Potkonjak A., Spasojevic L. Emerging vector-borne diseases—Incidence through vectors. Front. Public Health. 2014;2:267. doi: 10.3389/fpubh.2014.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davitkov D., Vucicevic M., Stevanovic J., Krstic V., Tomanovic S., Glavinic U., Stanimirovic Z. Clinical babesiosis and molecular identification of Babesia canis and Babesia gibsoni infections in dogs from Serbia. Acta Vet. Hung. 2015;63:199–208. doi: 10.1556/avet.2015.017. [DOI] [PubMed] [Google Scholar]
- 61.Kovačević Filipović M.M., Beletić A.D., Ilić Božović A.V., Milanović Z., Tyrrell P., Buch J., Breitschwerdt E.B., Birkenheuer A.J., Chandrashekar R. Molecular and serological prevalence of Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeenses, E. ewingii, Borrelia burgdorferi, Babesia canis, B. gibsoni and B. vogeli among clinically healthy outdoor dogs in Serbia. Vet. Parasitol. Reg. Stud. Rep. 2018;14:117–122. doi: 10.1016/j.vprsr.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Milanović Z., Beletić A., Vekić J., Zeljković A., Andrić N., Božović A.I., Spariosu K., Radaković M., Ajtić J., Filipović M.K. Evidence of acute phase reaction in asymptomatic dogs naturally infected with Babesia canis. Vet. Parasitol. 2020;282:109140. doi: 10.1016/j.vetpar.2020.109140. [DOI] [PubMed] [Google Scholar]
- 63.Gabrielli S., Otašević S., Ignjatović A., Savić S., Fraulo M., Arsić-Arsenijević V., Momčilović S., Cancrini G. Canine babesioses in noninvestigated areas of Serbia. Vector Borne Zoonotic Dis. 2015;15:535–538. doi: 10.1089/vbz.2015.1797. [DOI] [PubMed] [Google Scholar]
- 64.Davitkov D., Davitkov D., Vucicevic M., Stanisic L., Radakovic M., Glavinic U., Stanimirovic Z. A molecular and haematological study of Theileria equi in Balkan donkeys. Acta Vet. Hung. 2017;65:234–241. doi: 10.1556/004.2017.023. [DOI] [PubMed] [Google Scholar]
- 65.Vasić A., Nieder M., Zdravković N., Bojkovski J., Bugarski D., Pavlović I., Silaghi C. Tick infestation and occurrence of Anaplasma phagocytophilum and piroplasms in cattle in the Republic of Serbia. Parasitol. Res. 2018;117:1813–1818. doi: 10.1007/s00436-018-5867-x. [DOI] [PubMed] [Google Scholar]
- 66.Sukara R., Chochlakis D., Ćirović D., Penezić A., Mihaljica D., Ćakić S., Valčić M., Tselentis Y., Psaroulaki A., Tomanović S. Golden jackals (Canis aureus) as hosts for ticks and tick-borne pathogens in Serbia. Ticks Tick Borne Dis. 2018;9:1090–1097. doi: 10.1016/j.ttbdis.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Juwaid S., Sukara R., Penezić A., Mihaljica D., Veinović G., Kavallieratos N.G., Ćirović D., Tomanović S. First evidence of tick-borne protozoan pathogens, Babesia sp. and Hepatozoon canis, in red foxes (Vulpes vulpes) in Serbia. Acta Vet. Hung. 2019;67:70–80. doi: 10.1556/004.2019.008. [DOI] [PubMed] [Google Scholar]
- 68.Tomanović S., Chochlakis D., Radulović Z., Milutinović M., Cakić S., Mihaljica D., Tselentis Y., Psaroulaki A. Analysis of pathogen co-occurrence in host-seeking adult hard ticks from Serbia. Exp. Appl. Acarol. 2013;59:367–376. doi: 10.1007/s10493-012-9597-y. [DOI] [PubMed] [Google Scholar]
- 69.Potkonjak A., Gutiérrez R., Savić S., Vračar V., Nachum-Biala Y., Jurišić A., Kleinerman G., Rojas A., Petrović A., Baneth G., et al. Molecular detection of emerging tick-borne pathogens in Vojvodina, Serbia. Ticks Tick Borne Dis. 2016;7:199–203. doi: 10.1016/j.ttbdis.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Herwaldt B.L., Caccio S., Gherlinzoni F., Aspock H., Slemenda S.B., Piccaluga P., Martinelli G., Edelhofer R., Hollenstein U., Poletti G., et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg. Infect. Dis. 2003;9:942–948. doi: 10.3201/eid0908.020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blum S., Gattringer R., Haschke E., Walochnik J., Tschurtschenthaler G., Lang F., Oberbauer R. The case: Hemolysis and acute renal failure. Babesiosis. Kidney Int. 2011;80:681–683. doi: 10.1038/ki.2011.184. [DOI] [PubMed] [Google Scholar]
- 72.Ramharter M., Walochnik J., Lagler H., Winkler S., Wernsdorfer W.H., Stoiser B., Graninger W. Clinical and molecular characterization of a near fatal case of human babesiosis in Austria. J. Travel. Med. 2010;17:416–418. doi: 10.1111/j.1708-8305.2010.00446.x. [DOI] [PubMed] [Google Scholar]
- 73.Blaschitz M., Narodoslavsky-Gfoller M., Kanzler M., Stanek G., Walochnik J. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl. Environ. Microbiol. 2008;74:4841–4846. doi: 10.1128/AEM.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edelhofer R., Baumgartner W. Seroepidemiological studies of bovine Anaplasma marginale and Babesia divergens in Austria, involving autochthonous infections; Proceedings of the World Association for Buiatrics Congress; Edinburgh, Scotland. 8–12 July 1996; pp. 472–476. [Google Scholar]
- 75.Edelhofer R., Muller A., Schuh M., Obritzhauser W., Kanout A. Differentiation of Babesia bigemina, B. bovis, B. divergens and B. major by Western blotting—First report of B. bovis in Austrian cattle. Parasitol. Res. 2004;92:433–435. doi: 10.1007/s00436-003-1060-x. [DOI] [PubMed] [Google Scholar]
- 76.Leschnik M.W., Khanakah G., Duscher G., Wille-Piazzai W., Hörweg C., Joachim A., Stanek G. Species, developmental stage and infection with microbial pathogens of engorged ticks removed from dogs and questing ticks. Med. Vet. Entomol. 2012;26:440–446. doi: 10.1111/j.1365-2915.2012.01036.x. [DOI] [PubMed] [Google Scholar]
- 77.Silaghi C., Hamel D., Pfister K., Rehbein S. Babesia species and co-infection with Anaplasma phagocytophilum in free-ranging ungulates from Tyrol (Austria) Wien. Tierarztl. Mon. 2011;98:268–274. [Google Scholar]
- 78.Rehbein S., Visser M., Jekel I., Silaghi C. Endoparasites of the fallow deer (Dama dama) of the Antheringer Au in Salzburg, Austria. Wien. Klin. Wochenschr. 2014;126:37–41. doi: 10.1007/s00508-014-0506-8. [DOI] [PubMed] [Google Scholar]
- 79.Kogler S., Gotthalmseder E., Shahi-Barogh B., Harl J., Fuehrer H.-P. Babesia spp. and Anaplasma phagocytophilum in free-ranging wild ungulates in central Austria. Ticks Tick Borne Dis. 2021;12:101719. doi: 10.1016/j.ttbdis.2021.101719. [DOI] [PubMed] [Google Scholar]
- 80.Wijnveld M., Schötta A.-M., Stelzer T., Duscher G., Leschnik M., Stockinger H., Lindgren P.-E., Stanek G. Novel protozoans in Austria revealed through the use of dogs as sentinels for ticks and tick-borne pathogens. Microorganisms. 2021;9:1392. doi: 10.3390/microorganisms9071392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duscher G.G., Feiler A., Leschnik M., Joachim A. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from Eastern Austria and the influence of acaricides/repellents on these parameters. Parasit. Vectors. 2013;6:76. doi: 10.1186/1756-3305-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hodžić A., Zörer J., Duscher G.G. Dermacentor reticulatus, a putative vector of Babesia cf. microti (syn. Theileria annae) piroplasm. Parasitol. Res. 2017;116:1075–1077. doi: 10.1007/s00436-017-5379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leschnik M., Kirtz G., Tichy A., Leidinger E. Seasonal occurrence of canine babesiosis is influenced by local climate conditions. Int. J. Med. 2008;298:243–248. doi: 10.1016/j.ijmm.2008.03.008. [DOI] [Google Scholar]
- 84.Sonnberger B.W., Graf B., Straubinger R.K., Rackl D., Obwaller A.G., Peschke R., Shahi Barogh B., Joachim A., Fuehrer H.-P. Vector-borne pathogens in clinically healthy military working dogs in eastern Austria. Parasitol. Int. 2021;84:102410. doi: 10.1016/j.parint.2021.102410. [DOI] [PubMed] [Google Scholar]
- 85.Kirtz G., Leschnik M., Hooijberg E., Tichy A., Leidinger E. In-clinic laboratory diagnosis of canine babesiosis (Babesia canis canis) for veterinary practitioners in Central Europe. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere. 2012;40:87–94. [PubMed] [Google Scholar]
- 86.Strobl A., Künzel F., Tichy A., Leschnik M. Complications and risk factors regarding the outcomes of canine babesiosis in Central Europe—A retrospective analysis of 240 cases. Acta Vet. Hung. 2020;68:160–168. doi: 10.1556/004.2020.00031. [DOI] [PubMed] [Google Scholar]
- 87.Leschnik M., Feiler A., Duscher G.G., Joachim A. Effect of owner-controlled acaricidal treatment on tick infestation and immune response to tick-borne pathogens in naturally infested dogs from Eastern Austria. Parasit. Vectors. 2013;6:62. doi: 10.1186/1756-3305-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strobl A., Pantchev N., Martin L., Guija-De-Arespacochaga A., Hinney B., Fuehrer H.-P., Leschnik M. Co-infection with Babesia canis and Babesia gibsoni in a dog. Acta Vet. Hung. 2021;69:347–353. doi: 10.1556/004.2021.00048. [DOI] [PubMed] [Google Scholar]
- 89.Duscher G.G., Leschnik M., Fuehrer H.-P., Joachim A. Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int. J. Parasitol. Parasites Wildl. 2015;4:88–96. doi: 10.1016/j.ijppaw.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duscher G.G., Fuehrer H.-P., Kübber-Heiss A. Fox on the run—Molecular surveillance of fox blood and tissue for the occurrence of tick-borne pathogens in Austria. Parasit. Vectors. 2014;7:521. doi: 10.1186/s13071-014-0521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schlögl K.S., Hiesel J.A., Wolf R., Kopacka I., Wagner P., Kastelic J., Deutz A. Spatiotemporal cluster and incidence analysis of cattle mortality caused by bovine babesiosis in Styria, Austria, between 1998 and 2016. Parasitol. Res. 2020;119:1117–1123. doi: 10.1007/s00436-020-06604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cézanne R., Mrowietz N., Eigner B., Duscher G.G., Glawischnig W., Fuehrer H.-P. Molecular analysis of Anaplasma phagocytophilum and Babesia divergens in red deer (Cervus elaphus) in Western Austria. Molec. Cell. Probes. 2017;31:55–58. doi: 10.1016/j.mcp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Nohýnková E., Kubek J., Mĕst’ánková O., Chalupa P., Hubálek Z. A case of Babesia microti imported into the Czech Republic from the USA. Cas. Lek. Cesk. 2003;142:377–381. [PubMed] [Google Scholar]
- 94.Strizova Z., Havlova K., Patek O., Smrz D., Bartunkova J. The first human case of babesiosis mimicking Reiter’s syndrome. Folia Parasitol. 2020;67:1–5. doi: 10.14411/fp.2020.031. [DOI] [PubMed] [Google Scholar]
- 95.Hrazdilova K., Rybarova M., Siroky P., Votypka J., Zintl A., Burgess H., Steinbauer V., Zakovcik V., Modry D. Diversity of Babesia spp. in cervid ungulates based on the 18S rDNA and cytochrome c oxidase subunit I phylogenies. Infect. Genet. Evol. 2020;77:104060. doi: 10.1016/j.meegid.2019.104060. [DOI] [PubMed] [Google Scholar]
- 96.Belkova T., Bartova E., Ricarova D., Jahn P., Jandova V., Modry D., Hrazdilova K., Sedlak K. Theileria equi and Babesia caballi in horses in the Czech Republic. Acta Trop. 2021;221:105993. doi: 10.1016/j.actatropica.2021.105993. [DOI] [PubMed] [Google Scholar]
- 97.Danek O., Hrazdilová K., Kozderková D., Jirků D., Modrý D. The distribution of Dermacentor reticulatus in the Czech Republic re-assessed: Citizen Science approach to understanding current distribution of vector of Babesia canis. Parasit. Vectors. 2022 doi: 10.1186/s13071-022-05242-6. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kucera J. Babesiosis in a dog. Description of an imported case and literature review (in Czech) Veterinarstvi. 1992;42:371–373. [Google Scholar]
- 99.Svobodova Z., Svobodova V. Babesiosis in dogs in the Czech Republic (In Czech) Veterinarstvi. 2004;54:76–79. [Google Scholar]
- 100.Konvalinová J., Rudolf I., Šikutová S., Hubálek Z., Svobodová V., Svoboda M. Contribution to canine babesiosis in the Czech Republic. Acta Vet. Brno. 2012;81:91–95. doi: 10.2754/avb201281020091. [DOI] [Google Scholar]
- 101.Cerny V. The tick fauna of Czechoslovakia. Folia Parasitol. 1972;19:87–92. [PubMed] [Google Scholar]
- 102.Nosek J. The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in Central Europe. Folia Parasitol. 1972;19:93–102. [PubMed] [Google Scholar]
- 103.Široký P., Kubelová M., Bednář M., Modrý D., Hubálek Z., Tkadlec E. The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic—How much is range of this vector expanding? Vet. Parasitol. 2011;183:130–135. doi: 10.1016/j.vetpar.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Mitkova B., Hrazdilova K., Novotna M., Jurankova J., Hofmannova L., Forejtek P., Modry D. Autochthonous Babesia canis, Hepatozoon canis and imported Babesia gibsoni infection in dogs in the Czech Republic. Vet. Med. 2017;62:138–146. doi: 10.17221/152/2016-VETMED. [DOI] [Google Scholar]
- 105.Křivánková J., Lásková K., Sitařová B., Hrazdilová K., Modrý D., Hanzlíček D. Autochtonní babezióza u psa, popis klinického případu. Autochthonous babesiosis in a dog—A description of clinical case. Veterinářství. 2018;68:763–766. [Google Scholar]
- 106.Víchová B., Horská M., Blaňarová L., Švihran M., Andersson M., Peťko B. First molecular identification of Babesia gibsoni in dogs from Slovakia, central Europe. Ticks Tick Borne Dis. 2016;7:54–59. doi: 10.1016/j.ttbdis.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Krampitz H.E. Babesia microti: Morphology, distribution and host relationship in Germany. Zentralbl. Bakteriol. Orig. A. 1979;244:411–415. [PubMed] [Google Scholar]
- 108.Hildebrandt A., Hunfeld K.P., Baier M., Krumbholz A., Sachse S., Lorenzen T., Kiehntopf M., Fricke H.J., Straube E. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26:595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- 109.Berens-Riha N., Zechmeister M., Hirzmann J., Draenert R., Bogner J., Löscher T. Babesiose bei einem splenektomierten Reisenden aus den USA–Nach Deutschland importierte Infektion durch Zecken. Flugmed. Tropenmed. Reisemed. 2012;19:113–115. doi: 10.1055/s-0032-1321513. [DOI] [Google Scholar]
- 110.Häselbarth K., Tenter A.M., Brade V., Krieger G., Hunfeld K.-P. First case of human babesiosis in Germany—Clinical presentation and molecular characterisation of the pathogen. Int. J. Med. 2007;297:197–204. doi: 10.1016/j.ijmm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Krampitz H., Buschmann H., Münchhoff P. Gibt es latente Babesien-infektionen beim Menschen in Süddeutschland? Mitt. Österr. Ges. Tropenmed. Parasitol. 1986;8:233–243. [Google Scholar]
- 112.Hunfeld K.-P., Lambert A., Kampen H., Albert S., Epe C., Brade V., Tenter A.M. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J. Clin. Microbiol. 2002;40:2431–2436. doi: 10.1128/JCM.40.7.2431-2436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scheller M. Epidemiological Studies of Human Granulocytic Ehrlichiosis and Babesiosis in Forestry Workers in Southern Germany. Julius-Maximilians-University of Würzburg; Würzburg, Germany: 2004. [Google Scholar]
- 114.Hunfeld K.P., Allwinn R., Peters S., Kraiczy P., Brade V. Serologic evidence for tick-borne pathogens other than Borrelia burgdorferi (TOBB) in Lyme borreliosis patients from midwestern Germany. Wien. Klin. Wochenschr. 1998;110:901–908. [PubMed] [Google Scholar]
- 115.Enigk K. Die Schafpiroplasmose in Deutschland. Dtsch. Tierarztl. Wochenschr. 1956;63:161–162. [Google Scholar]
- 116.Liebisch A., Melfsen J., Rahman M.S. Occurrence of the tick Haemaphysalis punctata (Can. et Fanz., 1877) and of Babesia major in cattle in North Germany. Berl. Munch. Tierarztl. Wochenschr. 1976;89:477–480. [PubMed] [Google Scholar]
- 117.Weiland G., Reif L., Schmidt M., Boch J. Serological investigations on Babesia divergens infections in cattle. Berl. Munch. Tierarztl. Wochenschr. 1980;93:261–264. [PubMed] [Google Scholar]
- 118.Ullmann B., Centurier C., Weiland G., Boch J. The occurrence of Babesia divergens in cattle in the Western Allgaeu (Germany, FR) Berl. Munch. Tierarztl. Wochenschr. 1984;97:265–269. [PubMed] [Google Scholar]
- 119.Ganse-Dumrath D. Ph.D. Thesis. University of Veterinary Medicine Hannover; Hannover, Germany: 1986. Epidemiology of Babesia divergens Infection in Cattle in Northern Germany. [Google Scholar]
- 120.Niepold J. Ph.D. Thesis. University of Veterinary Medicine Hannover; Hannover, Germany: 1990. Serologic Investigations into Simultaneous Infections with Borrelia burgdorferi and Babesia divergens. [Google Scholar]
- 121.Huwer M., Schwarzmaier A., Hamel H., Will R. The occurrence of Babesia divergens in the Freiburg i. Br. district and piroplasmosis prevention trials in cattle. Berl. Munch. Tierarztl. Wochenschr. 1994;107:198–202. [PubMed] [Google Scholar]
- 122.Lengauer H., Just F.T., Edelhofer R., Pfister K. Tick infestation and the prevalence of Borrelia burgdorferi and Babesia divergens in cattle in Bavaria. Berl. Munch. Tierarztl. Wochenschr. 2006;119:335–341. [PubMed] [Google Scholar]
- 123.Springer A., Höltershinken M., Lienhart F., Ermel S., Rehage J., Hülskötter K., Lehmbecker A., Wohlsein P., Barutzki D., Gietl C., et al. Emergence and epidemiology of bovine babesiosis due to Babesia divergens on a northern German beef production farm. Front. Vet. Sci. 2020;7:649. doi: 10.3389/fvets.2020.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pikalo J., Sattler T., Eichinger M., Loitsch A., Schmoll F., Schusser G.F. Seroprevalence of Babesia caballi and Theileria equi in horses in Central Germany. Pferdeheilkunde. 2016;32:254–259. doi: 10.21836/PEM20160309. [DOI] [Google Scholar]
- 125.Boch J. Babesia infections in horses, cattle and dogs in southern Germany. Tierarztl. Prax. Suppl. 1985;1:3–7. [PubMed] [Google Scholar]
- 126.Hirsch M., Pantchev N. Vorkommenshäufigkeit der Reisekrankheiten Leishmaniose, Ehrlichiose, Babesiose und Dirofilariose bei in Deutschland lebenden Hunden. Kleintierpraxis. 2008;53:154–165. [Google Scholar]
- 127.Menn B., Lorentz S., Naucke T.J. Imported and travelling dogs as carriers of canine vector-borne pathogens in Germany. Parasit. Vectors. 2010;3:34. doi: 10.1186/1756-3305-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamel D., Röhrig E., Pfister K. Canine vector-borne disease in travelled dogs in Germany—A retrospective evaluation of laboratory data from the years 2004–2008. Vet. Parasitol. 2011;181:31–36. doi: 10.1016/j.vetpar.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 129.Röhrig E., Hamel D., Pfister K. Retrospective evaluation of laboratory data on canine vector-borne infections from the years 2004–2008. Berl. Munch. Tierarztl. Wochenschr. 2011;124:411–418. [PubMed] [Google Scholar]
- 130.Pantchev N. Zeckenübertragene reiseinfektionen beim hund: Ehrlichiose und babesiose. Vet. Spieg. 2013;22:162–170. doi: 10.1055/s-0032-1327972. [DOI] [Google Scholar]
- 131.Liesner J.M., Krücken J., Schaper R., Pachnicke S., Kohn B., Müller E., Schulze C., von Samson-Himmelstjerna G. Vector-borne pathogens in dogs and red foxes from the federal state of Brandenburg, Germany. Vet. Parasitol. 2016;224:44–51. doi: 10.1016/j.vetpar.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 132.Vrhovec M.G., Pantchev N., Failing K., Bauer C., Travers-Martin N., Zahner H. Retrospective analysis of canine vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of leishmaniosis. Parasitol. Res. 2017;116:131–144. doi: 10.1007/s00436-017-5499-6. [DOI] [PubMed] [Google Scholar]
- 133.Schäfer I., Volkmann M., Beelitz P., Merle R., Müller E., Kohn B. Retrospective evaluation of vector-borne infections in dogs imported from the Mediterranean region and southeastern Europe (2007–2015) Parasit. Vectors. 2019;12:30. doi: 10.1186/s13071-018-3284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schäfer I., Volkmann M., Beelitz P., Merle R., Müller E., Kohn B. Retrospective analysis of vector-borne infections in dogs after travelling to endemic areas (2007–2018) Vet. Parasitol. 2019;276:100015. doi: 10.1016/j.vpoa.2019.100015. [DOI] [PubMed] [Google Scholar]
- 135.Schäfer I., Helm C., Marsboom C., Hendrickx G., Kohn B., Krücken J., Samson-Himmelstjerna G., Müller E. Infections with Babesia spp. in dogs living in Germany (2007–2020) J. Vet. Intern. Med. 2021;35:3199. [Google Scholar]
- 136.L’Hostis M., Chauvin A., Valentin A., Precigout E., Gorenflot A. Survey of Babesia divergens antibody kinetics in cattle in western France. Vet. Res. 1997;28:481–488. [PubMed] [Google Scholar]
- 137.Zintl A., McGrath G., O’Grady L., Fanning J., Downing K., Roche D., Casey M., Gray J.S. Changing incidence of bovine babesiosis in Ireland. Ir. Vet. J. 2014;67:19. doi: 10.1186/2046-0481-67-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hornok S., Edelhofer R., Szotáczky I., Hajtós I. Babesia divergens becoming extinct in cattle of Northeast Hungary: New data on the past and present situation. Acta Vet. Hung. 2006;54:493–501. doi: 10.1556/AVet.54.2006.4.7. [DOI] [PubMed] [Google Scholar]
- 139.Zahler M., Gothe R. Infections of horses with Babesia caballi and Theileria equi in Germany-an epidemiological analysis. Tierarztl Umsch. 2000;55:655–657. [Google Scholar]
- 140.Scheidemann W., Liebisch G., Liebisch A., Budde K. Equine Piroplasmose-Fallbericht einer akuten Infektion mit Theileria equi (syn. Babesia equi) in Deutschland. Pferdeheilkunde. 2003;19:16–20. doi: 10.21836/PEM20030102. [DOI] [Google Scholar]
- 141.Springer A., Ehrmann C., Lembcke M., Roscher K., Strube C. Theileria equi-infection in 2 German horses returning from a trail ride in southern France. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere. 2020;48:124–129. doi: 10.1055/a-1110-2998. [DOI] [PubMed] [Google Scholar]
- 142.Chitimia-Dobler L., Schaper S., Rieß R., Bitterwolf K., Frangoulidis D., Bestehorn M., Springer A., Oehme R., Drehmann M., Lindau A. Imported Hyalomma ticks in Germany in 2018. Parasit. Vectors. 2019;12:134. doi: 10.1186/s13071-019-3380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Adam M., Pikalo J., Snyder A., Steinrigl A., Koeller G., Schusser G.F. Equine piroplasmosis–a case of severe Babesia caballi infection associated with acute renal failure. Berl. Munch. Tierarztl. Wochenschr. 2017;130:113–118. doi: 10.2376/0005-9366-16064. [DOI] [Google Scholar]
- 144.Stahn Piroplasmose bei Hunden. Z. Veterinaerkd. 1910;22:35–36. [Google Scholar]
- 145.Dennig H.K., Centurier C., Göbel E., Weiland G. Canine babesiasis and its importance in the Federal Republic of Germany and West Berlin. Berl. Munch. Tierarztl. Wochenschr. 1980;93:373–379. [PubMed] [Google Scholar]
- 146.Gothe R., Wegerdt S. Babesiosis of dogs in Germany: Epidemiologic case analysis. Tierarztl. Prax. 1991;19:170–173. [PubMed] [Google Scholar]
- 147.Gothe R., Wegerdt S., Walden R., Walden A. Zur Epidemiologie von Babesia canis-und Babesia gibsoni-Infektionen bei Hunden in Deutschland. Kleintierpraxis. 1989;34:309–320. [Google Scholar]
- 148.Zahler M., Loster F., Merkle C., Rinder H., Gothe R. Risk of infection for dogs in Regensburg–A new endemic focus for Babesia canis and Dermacentor reticulatus in Germany. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. 2000;28:395. [Google Scholar]
- 149.Zahler M., Steffen T., Lutz S., Hähnel W., Rinder H., Gothe R. Babesia canis and Dermacentor reticulatus in Munich: A new endemic focus in Germany. Tierärztliche Praxis. Ausgabe K Kleintiere/Heimtiere. 2000;28:116–120. [Google Scholar]
- 150.Zahler M., Rinder H., Schein E., Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet. Parasitol. 2000;89:241–248. doi: 10.1016/S0304-4017(00)00202-8. [DOI] [PubMed] [Google Scholar]
- 151.Heile C., Heydorn A.-O., Schein E. Dermacentor reticulatus (Fabricius, 1794)—Distribution, biology and vector for Babesia canis in Germany. Berl. Munch. Tierarztl. Wochenschr. 2006;119:330–334. [PubMed] [Google Scholar]
- 152.Beelitz P., Schumacher S., Marholdt F., Pfister K., Silaghi C. Studies on the prevalence of Babesia canis canis in marsh ticks (Dermacentor reticulatus) in the Saarland. Berl. Munch. Tierarztl. Wochenschr. 2012;125:168–171. [PubMed] [Google Scholar]
- 153.Jensen J., Nolte I. Autochthone Babesia canis-Infektion bei einem Hund aus Norddeutschland. Tierärztl. Prax. Ausg. K. Kleintiere Heimtiere. 2005;33:408–412. [Google Scholar]
- 154.Gothe R., Schmid I. Epidemiologische Fallanalyse Babesiose-erkrankter Hunde in Deutschland. Kleintierpraxis. 1995;40:169–179. [Google Scholar]
- 155.Hartelt K., Rieker T., Oehme R., Brockmann S., Müller W., Dorn N. First evidence of Babesia gibsoni (Asian genotype) in dogs in Western Europe. Vector Borne Zoonotic Dis. 2007;7:163–166. doi: 10.1089/vbz.2006.0580. [DOI] [PubMed] [Google Scholar]
- 156.Baneth G., Florin-Christensen M., Cardoso L., Schnittger L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit. Vectors. 2015;8:207. doi: 10.1186/s13071-015-0830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Helm C., Weingart C., Schäfer I., Pachnicke S., Müller E., von Samson-Himmelstjerna G., Krücken J., Kohn B. Kanine babesiose—15 autochthone fälle in Berlin/Brandenburg. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. 2021;49:P11. [Google Scholar]
- 158.Hof S., Rohrberg A., Stockinger A., Schaalo S., März I. Occurrence of canine babesiosis in dogs in the Rhine-Main area of Hessen, Germany—A case study of 81 dogs. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. in press . [DOI] [PubMed]
- 159.Moik K., Gothe R. Babesia infections of felids and a report on a case in a cat in Germany. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. 1997;25:532–535. [Google Scholar]
- 160.Stewart C.G., Hackett K.J.W., Collett M.G. An unidentified Babesia of the domestic cat (Felis domesticus) J. S. Afr. Vet. Assoc. 1980;51:219–221. [PubMed] [Google Scholar]
- 161.Buza L., Hajdú G., Temesi Z. Occurrence of horse babesiosis in Hungary (in Hungarian) Magy. Allatorv. Lap. 1953;8:265–268. [Google Scholar]
- 162.Buza L., Hajdú G., Temesi Z. New cases of horse babesiosis in Hungary (in Hungarian) Magy. Allatorv. Lap. 1955;10:427–429. [Google Scholar]
- 163.Hornok S., Edelhofer R., Földvári G., Joachim A., Farkas R. Serological evidence for Babesia canis infection of horses and an endemic focus of B. caballi in Hungary. Acta Vet. Hung. 2007;55:491–500. doi: 10.1556/avet.55.2007.4.8. [DOI] [PubMed] [Google Scholar]
- 164.Hevesi A., Ütő D., Kis J., Balogh N., Bakos Z. Theileria equi infection in Hungary. Literature review and case report. (in Hungarian) Magy. Allatorv. Lap. 2006;128:195–199. [Google Scholar]
- 165.Farkas R., Tánczos B., Gyurkovszky M., Földvári G., Solymosi N., Edelhofer R., Hornok S. Serological and molecular detection of Theileria equi infection in horses in Hungary. Vet. Parasitol. 2013;192:143–148. doi: 10.1016/j.vetpar.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 166.Bernard A. Data on the treatment of cattle piroplasmoses. (In Hungarian) Magy. Alltorv. Lap. 1963;16:373–374. [Google Scholar]
- 167.Kotlán A., Versényi L., Janisch M. Über das Vorkommen von Piroplasma bigeminum in Ungarn. Acta Vet. Hung. 1959;9:131–133. [Google Scholar]
- 168.Hornok S., Mester A., Takács N., Fernández de Mera I.G., de la Fuente J., Farkas R. Re-emergence of bovine piroplasmosis in Hungary: Has the etiological role of Babesia divergens been taken over by B. major and Theileria buffeli? Parasit. Vectors. 2014;7:434. doi: 10.1186/1756-3305-7-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hornok S., Takács N., Kontschán J., György Z., Micsutka A., Iceton S., Flaisz B., Farkas R., Hofmann-Lehmann R. Diversity of Haemaphysalis-associated piroplasms of ruminants in Central-Eastern Europe, Hungary. Parasit. Vectors. 2015;8:627. doi: 10.1186/s13071-015-1236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wetzl J. Piroplasmosis of dogs. (in Hungarian) Állatorvos Lapok. 1905;28:505–517. [Google Scholar]
- 171.Miklósi M. Dog piroplasmosis (in Hungarian) Állatorvos Lapok. 1932;5:75–77. [Google Scholar]
- 172.Horváth L., Papp L. Incidence, symptomatology and treatment of canine babesiosis. Magy. Allatorv. Lap. 1996;51:180–187. [Google Scholar]
- 173.Máthé A., Vörös K., Papp L., Reiczigel J. Clinical manifestations of canine babesiosis in Hungary (63 cases) Acta Vet. Hung. 2006;54:367–385. doi: 10.1556/avet.54.2006.3.7. [DOI] [PubMed] [Google Scholar]
- 174.Csikós K., Varga J., Hadházy Á., Bándy P. Babesiosis of dogs: Changes of epidemiology and clinical pattern in Szekszárd between 1992 and 1999 (in Hungarian) Magy. Allatorv. Lap. 2001;123:259–264. [Google Scholar]
- 175.Földvári G., Farkas R. Review of literature relating to Dermacentor reticulatus (Acari: Ixodidae) and newer data on the occurrence in Hungary. Magy. Allatorv. Lap. 2005;127:289–298. [Google Scholar]
- 176.Farkas R., Földvári G. Examination of dog’s and cat’s tick infestation in Hungary (in Hungarian) Magy. Allatorv. Lap. 2001;123:534–539. [Google Scholar]
- 177.Földvári G., Hell E., Farkas R. Babesia canis canis in dogs from Hungary: Detection by PCR and sequencing. Vet. Parasitol. 2005;127:221–226. doi: 10.1016/j.vetpar.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 178.Hornok S., Edelhofer R., Farkas R. Seroprevalence of canine babesiosis in Hungary suggesting breed predisposition. Parasitol. Res. 2006;99:638–642. doi: 10.1007/s00436-006-0218-8. [DOI] [PubMed] [Google Scholar]
- 179.Farkas R., Földvári G., Fenyves B., Kótai I., Szilágyi A., Hegedüs G.T. First detection of small babesiae in two dogs in Hungary. Vet. Rec. 2004;154:176–178. doi: 10.1136/vr.154.6.176. [DOI] [PubMed] [Google Scholar]
- 180.Dékay V. Babesia gibsoni infection in a pit bull terrier (in Hungarian) Kisállatpraxis. 2013;14:214–218. [Google Scholar]
- 181.Demeter Z., Palade E.A., Balogh E., Jakab C., Farkas R., Tánczos B., Hornok S. Postmortem small babesia-like morphology of Babesia canis—Short communication. Acta Vet. Hung. 2011;59:427–432. doi: 10.1556/avet.2011.029. [DOI] [PubMed] [Google Scholar]
- 182.Hornok S., Horváth G., Takács N., Kontschán J., Szőke K., Farkas R. Molecular identification of badger-associated Babesia sp. DNA in dogs: Updated phylogeny of piroplasms infecting Caniformia. Parasit. Vectors. 2018;11:235. doi: 10.1186/s13071-018-2794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tuska-Szalay B., Vizi Z., Hofmann-Lehmann R., Vajdovich P., Takács N., Meli M.L., Farkas R., Stummer-Knyihár V., Jerzsele Á., Kontschán J., et al. Babesia gibsoni emerging with high prevalence and co-infections in “fighting dogs” in Hungary. CRPVBD. 2021;1:100048. doi: 10.1016/j.crpvbd.2021.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hornok S., Kováts D., Horváth G., Kontschán J., Farkas R. Checklist of the hard tick (Acari: Ixodidae) fauna of Hungary with emphasis on host-associations and the emergence of Rhipicephalus sanguineus. Exp. Appl. Acarol. 2020;80:311–328. doi: 10.1007/s10493-019-00461-6. [DOI] [PubMed] [Google Scholar]
- 185.Farkas R., Takács N., Hornyák Á., Nachum-Biala Y., Hornok S., Baneth G. First report on Babesia cf. microti infection of red foxes (Vulpes vulpes) from Hungary. Parasit. Vectors. 2015;8:55. doi: 10.1186/s13071-015-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Berger S. Infectious Diseases of Luxembourg: 2021 Edition. GIDEON Informatics, Inc.; Los Angeles, CA, USA: 2021. [Google Scholar]
- 187.Reye A.L., Hübschen J.M., Sausy A., Muller C.P. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl. Environ. Microbiol. 2010;76:2923–2931. doi: 10.1128/AEM.03061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Halos L., Lebert I., Abrial D., Danlois F., Garzik K., Rodes D., Schillmeier M., Ducrot C., Guillot J. Questionnaire-based survey on the distribution and incidence of canine babesiosis in countries of Western Europe. Parasite. 2014;21:13. doi: 10.1051/parasite/2014015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weigand A., Teixeira J., Christian S. First record of Hyalomma marginatum sensu stricto C.L. Koch, 1844 and distribution of Dermacentor reticulatus (Fabricius, 1794) (Acari, Ixodidae) in Luxembourg. Bull. Société Nat. Luxemb. 2020;122:253–263. [Google Scholar]
- 190.Humiczewska M., Kuźna-Grygiel W. A case of imported human babesiosis in Poland. Wiad. Parazytol. 1997;43:227–229. [PubMed] [Google Scholar]
- 191.Przybylińska A., Bielicki D., Kuźna-Grygiel W. The case of babesiosis in a patient with ulcerative colitis treated with immunosuppressive drugs. Gastroenterol. Pol. 2004;11:607–609. [Google Scholar]
- 192.Welc-Falęciak R., Hildebrandt A., Siński E. Co-infection with Borrelia species and other tick-borne pathogens in humans: Two cases from Poland. Ann. Agric. Environ. Med. 2010;17:309–313. [PubMed] [Google Scholar]
- 193.Welc-Falęciak R., Pawełczyk A., Radkowski M., Pancewicz S.A., Zajkowska J., Siński E. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann. Agric. Environ. Med. 2015;22:51–54. doi: 10.5604/12321966.1141394. [DOI] [PubMed] [Google Scholar]
- 194.Jabłońska J., Żarnowska-Prymek H., Stańczak J., Kozłowska J., Wiercińska-Drapało A. Symptomatic co-infection with Babesia microti and Borrelia burgdorferi in patient after international exposure; a challenging case in Poland. Ann. Agric. Environ. Med. 2016;23:387–389. doi: 10.5604/12321966.1203914. [DOI] [PubMed] [Google Scholar]
- 195.Moniuszko-Malinowska A., Guziejko K., Czarnowska A., Kułakowska A., Zajkowska O., Pancewicz S., Świerzbińska R., Dunaj J., Czupryna P., Róg-Makal M., et al. Assessment of anti-HSV antibodies in patients with facial palsy in the course of neuroborreliosis. Int. J. Clin. Pract. 2021;75:e13749. doi: 10.1111/ijcp.13749. [DOI] [PubMed] [Google Scholar]
- 196.Moniuszko-Malinowska A., Dunaj J., Andersson M.O., Czupryna P., Zajkowska J., Guziejko K., Garkowski A., Grygorczuk S., Kondrusik M., Pancewicz S. Assessment of Anaplasma phagocytophilum presence in early Lyme borreliosis manifested by erythema migrans skin lesions. Travel. Med. Infect. Dis. 2020;36:101648. doi: 10.1016/j.tmaid.2020.101648. [DOI] [PubMed] [Google Scholar]
- 197.Moniuszko-Malinowska A., Dunaj J., Andersson M.O., Chmielewski T., Czupryna P., Groth M., Grygorczuk S., Zajkowska J., Kondrusik M., Kruszewska E., et al. Anaplasmosis in Poland—Analysis of 120 patients. Ticks Tick Borne Dis. 2021;12:101763. doi: 10.1016/j.ttbdis.2021.101763. [DOI] [PubMed] [Google Scholar]
- 198.Krawczuk K., Czupryna P., Pancewicz S., Ołdak E., Moniuszko-Malinowska A. Comparison of tick-borne encephalitis between children and adults—Analysis of 669 patients. J. Neurovirol. 2020;26:565–571. doi: 10.1007/s13365-020-00856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pancewicz S., Moniuszko A., Bieniarz E., Puciło K., Grygorczuk S., Zajkowska J., Czupryna P., Kondrusik M., Swierzbińska-Pijanowska R. Anti-Babesia microti antibodies in foresters highly exposed to tick bites in Poland. Scand. J. Infect. Dis. 2011;43:197–201. doi: 10.3109/00365548.2010.538930. [DOI] [PubMed] [Google Scholar]
- 200.Moniuszko-Malinowska A., Swiecicka I., Dunaj J., Zajkowska J., Czupryna P., Zambrowski G., Chmielewska-Badora J., Żukiewicz-Sobczak W., Swierzbinska R., Rutkowski K., et al. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect. Dis. 2016;48:537–543. doi: 10.3109/23744235.2016.1164339. [DOI] [PubMed] [Google Scholar]
- 201.Moniuszko A., Dunaj J., Swięcicka I., Zambrowski G., Chmielewska-Badora J., Zukiewicz-Sobczak W., Zajkowska J., Czupryna P., Kondrusik M., Grygorczuk S., et al. Co-infections with Borrelia species, Anaplasma phagocytophilum and Babesia spp. in patients with tick-borne encephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1835–1841. doi: 10.1007/s10096-014-2134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dunaj J., Moniuszko-Malinowska A., Swiecicka I., Andersson M., Czupryna P., Rutkowski K., Zambrowski G., Zajkowska J., Grygorczuk S., Kondrusik M., et al. Tick-borne infections and co-infections in patients with non-specific symptoms in Poland. Adv. Med. Sci. 2018;63:167–172. doi: 10.1016/j.advms.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 203.Pawełczyk A., Bednarska M., Kowalska J.D., Uszyńska-Kałuża B., Radkowski M., Welc-Falęciak R. Seroprevalence of six pathogens transmitted by the Ixodes ricinus ticks in asymptomatic individuals with HIV infection and in blood donors. Sci. Rep. 2019;9:2117. doi: 10.1038/s41598-019-38755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Adaszek Ł., Winiarczyk S., Łukaszewska J. Babesiose bei einer Katze. Kleintierpraxis. 2010;55:624–634. [Google Scholar]
- 205.Adaszek L., Ukaszewska J., Winiarczyk S., Kunkel M. The first case of feline babesiosis in Poland. Zycie Wet. 2008;83:668–670. [Google Scholar]
- 206.Olszak A. Personal communication. 2022.
- 207.Kretschmer M. (Vetlab sp. z o. o., Veterinary Diagnostic Laboratory, Wrocław, Poland). Personal communication. 2022.
- 208.Staniec M., Buczek K., Milczak A., Adaszek L., Winiarczyk S. Epidemiological studies towards cattle babesiosis-tick-borne disease. Scient. Messeng. LNU Vet. Med. Biotech. 2016;18:228–239. doi: 10.15421/nvlvet7153. [DOI] [Google Scholar]
- 209.Staniec M., Adaszek Ł., Buczek K., Winiarczyk S. Molecular identification of Babesia spp. isolated from Polish cattle with asymptomatic protozoa infections. Pol. J. Vet. Sci. 2018;21:287–291. doi: 10.24425/119050. [DOI] [PubMed] [Google Scholar]
- 210.Adaszek L., Winiarczyk S. Molecular characterization of Babesia canis canis isolates from naturally infected dogs in Poland. Vet. Parasitol. 2008;152:235–241. doi: 10.1016/j.vetpar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 211.Teodorowski O., Kalinowski M., Winiarczyk D., Janecki R., Winiarczyk S., Adaszek Ł. Molecular surveillance of tick-borne diseases affecting horses in Poland—Own observations. Vet. Med. Sci. 2021;7:1159–1165. doi: 10.1002/vms3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dwużnik-Szarek D., Mierzejewska E.J., Rodo A., Goździk K., Behnke-Borowczyk J., Kiewra D., Kartawik N., Bajer A. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasit.Vectors. 2021;14:267. doi: 10.1186/s13071-021-04758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Szymanski S. Distribution of the tick Dermacentor reticulatus (Fabricus, 1794) (Ixodidae) in Poland. Acta Parasitol. Pol. 1986;31:143–154. [Google Scholar]
- 214.Bajer A., Mierzejewska E.J., Rodo A., Welc-Falęciak R. The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland. Part 2: Occurrence and control of babesiosis in a sled dog kennel during a 13-year-long period. Vet. Parasitol. 2014;202:234–240. doi: 10.1016/j.vetpar.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 215.Bajer A., Mierzejewska E.J., Rodo A., Bednarska M., Kowalec M., Welc-Falęciak R. The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland: Part 1: A population study on sled dogs during the racing season. Vet. Parasitol. 2014;202:276–286. doi: 10.1016/j.vetpar.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 216.Adaszek L., Winiarczyk S. Application of the SYBR Green real-time HRM PCR technique in the differentiation of the Babesia canis canis protozoa isolated in the areas of eastern Poland. Parasitol. Res. 2010;106:1253–1256. doi: 10.1007/s00436-010-1784-3. [DOI] [PubMed] [Google Scholar]
- 217.Adaszek Ł., Martinez A.C., Winiarczyk S. The factors affecting the distribution of babesiosis in dogs in Poland. Vet. Parasitol. 2011;181:160–165. doi: 10.1016/j.vetpar.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 218.Zygner W., Górski P., Wędrychowicz H. Detection of the DNA of Borrelia afzelii, Anaplasma phagocytophilum and Babesia canis in blood samples from dogs in Warsaw. Vet. Rec. 2009;164:465–467. doi: 10.1136/vr.164.15.465. [DOI] [PubMed] [Google Scholar]
- 219.Nowak M. Discovery of Dermacentor reticulatus (Acari: Amblyommidae) populations in the Lubuskie Province (Western Poland) Exp. Appl. Acarol. 2011;54:191–197. doi: 10.1007/s10493-010-9422-4. [DOI] [PubMed] [Google Scholar]
- 220.Mierzejewska E.J., Pawełczyk A., Radkowski M., Welc-Falęciak R., Bajer A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit. Vectors. 2015;8:490. doi: 10.1186/s13071-015-1099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Szymula Z., Stepkowski J., Kosieradzki M., Stepkowska-Kwasiuk N., Krainska-Losek M. Babesia canis infection as the most common cause of renal replacement therapy in a dog—Hemodialysis (in Polish) Mag. Wet. 2017;26:10–17. [Google Scholar]
- 222.Bajer A., Rodo A., Mierzejewska E.J., Tołkacz K., Welc-Faleciak R. The prevalence of Dirofilaria repens in cats, healthy dogs and dogs with concurrent babesiosis in an expansion zone in central Europe. BMC Vet. Res. 2016;12:183. doi: 10.1186/s12917-016-0816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Welc-Falęciak R., Rodo A., Siński E., Bajer A. Babesia canis and other tick-borne infections in dogs in Central Poland. Vet. Parasitol. 2009;166:191–198. doi: 10.1016/j.vetpar.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 224.Adaszek L., Lyp P., Poblocki P., Skrzypczak M., Mazurek L., Winiarczyk S. The first case of Babesia gibsoni infection in a dog in Poland. Vet. Med. 2018;63:225–228. doi: 10.17221/155/2017-VETMED. [DOI] [Google Scholar]
- 225.Teodorowski O., Kalinowski M., Skrzypczak M., Witt K., Madany J., Winiarczyk S., Adaszek L. Babesia gibsoni infection in dogs in Poland. Pol. J. Vet. Sci. 2020;23:469–471. doi: 10.24425/pjvs.2020.134694. [DOI] [PubMed] [Google Scholar]
- 226.Stanko M., Derdáková M., Špitalská E., Kazimírová M. Ticks and their epidemiological role in Slovakia: From the past till present. Biologia. 2021:1–36. doi: 10.1007/s11756-021-00845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Slivinska K., Víchová B., Werszko J., Szewczyk T., Wróblewski Z., Peťko B., Ragač O., Demeshkant V., Karbowiak G. Molecular surveillance of Theileria equi and Anaplasma phagocytophilum infections in horses from Ukraine, Poland and Slovakia. Vet. Parasitol. 2016;215:35–37. doi: 10.1016/j.vetpar.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 228.Swan M., Jurasik D., Kovacicova H. Occurrence of babesiosis in dogs in West Slovakia. Veterinarstvi. 2001;51:60–62. [Google Scholar]
- 229.Chandoga P., Goldová M., Baranová D., Kozák M. First cases of canine babesiosis in the Slovak Republic. Vet. Rec. 2002;150:82–84. doi: 10.1136/vr.150.3.82. [DOI] [PubMed] [Google Scholar]
- 230.Duh D., Slovák M., Saksida A., Strašek K., Petrovec M., Avšič-Županc T. Molecular detection of Babesia canis in Dermacentor reticulatus ticks collected in Slovakia. Biologia. 2006;61:231–233. doi: 10.2478/s11756-006-0035-7. [DOI] [Google Scholar]
- 231.Majláthová V., Majláth I., Víchová B., Gul’ová I., Derdáková M., Sesztáková E., Pet’ko B. Polymerase chain reaction confirmation of Babesia canis canis and Anaplasma phagocytophilum in dogs suspected of babesiosis in Slovakia. Vector Borne Zoonotic Dis. 2011;11:1447–1451. doi: 10.1089/vbz.2010.0276. [DOI] [PubMed] [Google Scholar]
- 232.Bullová E., Lukáň M., Stanko M., Peťko B. Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet. Parasitol. 2009;165:357–360. doi: 10.1016/j.vetpar.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 233.Kubelová M., Tkadlec E., Bednář M., Roubalová E., Široký P. West-to-east differences of Babesia canis canis prevalence in Dermacentor reticulatus ticks in Slovakia. Vet. Parasitol. 2011;180:191–196. doi: 10.1016/j.vetpar.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 234.Kubelová M., Sedlák K., Panev A., Široký P. Conflicting results of serological, PCR and microscopic methods clarify the various risk levels of canine babesiosis in Slovakia: A complex approach to Babesia canis diagnostics. Vet. Parasitol. 2013;191:353–357. doi: 10.1016/j.vetpar.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 235.Strasek-Smrdel K., Korva M., Pal E., Rajter M., Skvarc M., Avsic-Zupanc T. Case of Babesia crassa-like infection, Slovenia, 2014. Emerg. Infect. Dis. 2020;26:1038–1040. doi: 10.3201/eid2605.191201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Duh D., Tozon N., Petrovec M., Strasek K., Avsic-Zupanc T. Canine babesiosis in Slovenia: Molecular evidence of Babesia canis canis and Babesia canis vogeli. Vet. Res. 2004;35:363–368. doi: 10.1051/vetres:2004018. [DOI] [PubMed] [Google Scholar]
- 237.Duh D., Petrovec M., Bidovec A., Avsic-Zupanc T. Cervids as babesiae hosts, Slovenia. Emerg. Infect. Dis. 2005;11:1121–1123. doi: 10.3201/eid1107.040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Duh D., Petrovec M., Avsic-Zupanc T. Molecular characterization of human pathogen Babesia EU1 in Ixodes ricinus ticks from Slovenia. Int. J. Parasitol. 2005;91:463–465. doi: 10.1645/GE-394R. [DOI] [PubMed] [Google Scholar]
- 239.(BAG), B.f.G. Zeckenübertragene Krankheiten—Lagebericht Schweiz. [(accessed on 14 February 2022)]. Available online: https://www.bag.admin.ch/bag/de/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/zeckenuebertragene-krankheiten.html.
- 240.Loutan L., Rossier J., Zufferey G., Cuenod D., Hatz C., Marti H.P., Gern L. Imported babeslosis diagnosed as malaria. Lancet. 1993;342:749. doi: 10.1016/0140-6736(93)91744-7. [DOI] [PubMed] [Google Scholar]
- 241.Baumann D., Pusterla N., Péter O., Grimm F., Fournier P., Schär G., Bossart W., Lutz H., Weber R. Fieber nach Zeckenstich: Klinik und Diagnostik von akuten Zeckenstich-assoziierten Infektionskrankheiten in der Nordostschweiz. DMW-Dtsch. Med. Wochenschr. 2003;128:1042–1047. doi: 10.1055/s-2003-39103. [DOI] [PubMed] [Google Scholar]
- 242.Meer-Scherrer L., Adelson M., Mordechai E., Lottaz B., Tilton R. Babesia microti infection in Europe. Curr. Microbiol. 2004;48:435–437. doi: 10.1007/s00284-003-4238-7. [DOI] [PubMed] [Google Scholar]
- 243.Foppa I.M., Krause P.J., Spielman A., Goethert H., Gern L., Brand B., Telford III S.R. Entomologic and serologic evidence of zoonotic transmission of Babesia microti, eastern Switzerland. Emerg. Infect. Dis. 2002;8:722. doi: 10.3201/eid0807.010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Aeschlimann A., Brossard M., Quenet G. Contribution to the knowledge of Swiss piroplasmas. Acta Trop. 1975;32:281–289. [PubMed] [Google Scholar]
- 245.Casati S., Sager H., Gern L., Piffaretti J.C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006;13:65–70. [PubMed] [Google Scholar]
- 246.Hilpertshauser H., Deplazes P., Schnyder M., Gern L., Mathis A. Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in southern Switzerland. Appl. Environ. Microbiol. 2006;72:6503–6507. doi: 10.1128/AEM.00823-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gern L., Brossard M. Evolution annuelle de l’infestation de bovins par la tique Ixodes ricinus L. et de l’infection de ces ectoparasites par Babesia divergens dans le Clos du Doubs Jura, Suisse. Schweiz. Archiv. Tierheilkd. 1986;128:361–363. [PubMed] [Google Scholar]
- 248.Hofmann-Lehmann R., Meli M.L., Dreher U.M., Gönczi E., Deplazes P., Braun U., Engels M., Schüpbach J., Jörger K., Thoma R. Concurrent infections with vector-borne pathogens associated with fatal hemolytic anemia in a cattle herd in Switzerland. J. Clin. Microbiol. 2004;42:3775–3780. doi: 10.1128/JCM.42.8.3775-3780.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Almería S., Castella J., Ferrer D., Ortuno A., Estrada-Peña A., Gutierrez J. Bovine piroplasms in Minorca (Balearic Islands, Spain): A comparison of PCR-based and light microscopy detection. Vet. Parasitol. 2001;99:249–259. doi: 10.1016/S0304-4017(01)00464-2. [DOI] [PubMed] [Google Scholar]
- 250.Georges K., Loria G.R., Riili S., Greco A., Caracappa S., Jongejan F., Sparagano O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001;99:273–286. doi: 10.1016/S0304-4017(01)00488-5. [DOI] [PubMed] [Google Scholar]
- 251.Joachim A., Cavalleri J.V., Berger S. Equine anaplasmosis and equine piroplasmosis in Germany, Austria and Switzerland -previously anecdotal, now relevant? Schweiz. Arch. Tierheilkd. 2022;164:35–50. doi: 10.17236/sat00335. [DOI] [PubMed] [Google Scholar]
- 252.Hermann M., Baumann D., Weiland G., von Salis B. Erstmalige feststellung von equiner babesiose als bestandsproblem in der Schweiz. Pferdeheilkunde. 1987;3:17–24. doi: 10.21836/PEM19870103. [DOI] [Google Scholar]
- 253.Gottstein B. Babesia equi-Infektion bei einem Pferd ohne Auslandaufenthalt. Swiss. Vet. 1995;12:14–19. [Google Scholar]
- 254.Sigg L., Gerber V., Gottstein B., Doherr M.G., Frey C.F. Seroprevalence of Babesia caballi and Theileria equi in the Swiss horse population. Parasitol. Int. 2010;59:313–317. doi: 10.1016/j.parint.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 255.Buchs B. Master’s Thesis. Unviersity of Zurich; Zurich, Switzerland: 2018. Untersuchungen zur Piroplasmose Beim Pferd. [Google Scholar]
- 256.Jacquier C. Canine piroplasmosis, first case in Geneva. Schweiz. Arch. Tierheilkd. 1974;116:307–308. [PubMed] [Google Scholar]
- 257.Pfister K., Schwalbach B., Chuit P., Liz J., Aeschlimann A. Präliminare Untersuchungen zur endemischen Ausbreitung von Babesia canis und der Zecke Dermacentor reticulatus in der Schweiz. Mitt. Österr. Ges. Tropenmed. Parasitol. 1993;15:1–5. [Google Scholar]
- 258.Porchet M.J., Sager H., Muggli L., Oppliger A., Müller N., Frey O.F., Gottstein B. Etude épidémiologique descriptive de la Babésiose canine dans la Région Lémanique. Schweiz. Arch. Tierheilk. 2007;149:457–465. doi: 10.1024/0036-7281.149.10.457. [DOI] [PubMed] [Google Scholar]
- 259.Sager H., Casati S., Hartmeier G., Sommer B. Autochthone Fälle von caniner Babesiose im Kanton Solothurn. Schweiz. Arch. Tierheilk. 2005;147:259–265. doi: 10.1024/0036-7281.147.6.259. [DOI] [PubMed] [Google Scholar]
- 260.Schaarschmidt D., Trächsel M., Achermann R., Hartelt K., Oehme R., Müller W. Bedeutung der PCR in der diagnostik der caninen Babesiose. Schweiz. Arch. Tierheilk. 2006;148:633–640. doi: 10.1024/0036-7281.148.12.633. [DOI] [PubMed] [Google Scholar]
- 261.Eichenberger R.M., Deplazes P., Mathis A. Ticks on dogs and cats: A pet owner-based survey in a rural town in northeastern Switzerland. Ticks Tick Borne Dis. 2015;6:267–271. doi: 10.1016/j.ttbdis.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 262.Nentwig A., Meli M.L., Schrack J., Reichler I.M., Riond B., Gloor C., Howard J., Hofmann-Lehmann R., Willi B. First report of Cytauxzoon sp. infection in domestic cats in Switzerland: Natural and transfusion-transmitted infections. Parasit. Vectors. 2018;11:292. doi: 10.1186/s13071-018-2728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Willi B., Meli M.L., Cafarelli C., Gilli U.O., Kipar A., Hubbuch A., Riond B., Howard J., Schaarschmidt D., Regli W. Cytauxzoon europaeus infections in domestic cats in Switzerland and in European wildcats in France: A tale that started more than two decades ago. Parasit. Vectors. 2022;15:19. doi: 10.1186/s13071-021-05111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Willi B., Spiri A.M., Meli M.L., Grimm F., Beatrice L., Riond B., Bley T., Jordi R., Dennler M., Hofmann-Lehmann R. Clinical and molecular investigation of a canine distemper outbreak and vector-borne infections in a group of rescue dogs imported from Hungary to Switzerland. BMC Vet. Res. 2015;11:154. doi: 10.1186/s12917-015-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gern L., Brossard M., Aeschlimann A., Broquet C.A., Quenet G., Stucki J.P., Ackermann J. Bovine piroplasmosis in the Clos-du-Doubs (Jura, Switzerland): Preliminary observations. Schweiz. Arch. Tierheilkd. 1982;124:549–556. [PubMed] [Google Scholar]
- 266.Gigandet L., Stauffer E., Douet V., Rais O., Moret J., Gern L. Prevalence of three zoonotic Babesia species in Ixodes ricinus (Linné, 1758) nymphs in a suburban forest in Switzerland. Vector Borne Zoonotic Dis. 2011;11:363–366. doi: 10.1089/vbz.2010.0195. [DOI] [PubMed] [Google Scholar]
- 267.Oechslin C.P., Heutschi D., Lenz N., Tischhauser W., Péter O., Rais O., Beuret C.M., Leib S.L., Bankoul S., Ackermann-Gäumann R. Prevalence of tick-borne pathogens in questing Ixodes ricinus ticks in urban and suburban areas of Switzerland. Parasit. Vectors. 2017;10:558. doi: 10.1186/s13071-017-2500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Holler J., Röser D., Nielsen H., Eickhardt S., Chen M., Lester A., Bang D., Frandsen C., David K. A case of human babesiosis in Denmark. Travel Med. Infect. Dis. 2013;11:324–328. doi: 10.1016/j.tmaid.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 269.Petersen H., Hansen M., Larsen G., Chriél M., Roug N., Christiansen L. Bovine babesiosis: A persistent vector-borne parasitic disease. Dan. Veterinærtidsskrift. 2018;101:20–23. [Google Scholar]
- 270.Hørlück A., Øines Ø., Friislund H., Thamsborg S., Enemark H. Babesiosis in dogs—A new diagnosis in Denmark. Dan. Veterinærtidsskrift. 2019;102:12–15. [Google Scholar]
- 271.Kappelgaard L., Steensborg K. Ferie med dødelig udgang. DVT. 2012;12:14–15. [Google Scholar]
- 272.Klitgaard K., Chriél M., Isbrand A., Jensen T.K., Bødker R. Identification of Dermacentor reticulatus ticks carrying Rickettsia raoultii on migrating jackal, Denmark. Emerg. Infect. Dis. 2017;23:2072–2074. doi: 10.3201/eid2312.170919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Stensvold C.R., Al Marai D., Andersen L.O.B., Krogfelt K.A., Jensen J.S., Larsen K.S., Nielsen H.V. Babesia spp. and other pathogens in ticks recovered from domestic dogs in Denmark. Parasit. Vectors. 2015;8:262. doi: 10.1186/s13071-015-0843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kjær L.J., Jensen L.M., Chriél M., Bødker R., Petersen H.H. The raccoon dog (Nyctereutes procyonoides) as a reservoir of zoonotic diseases in Denmark. Int. J. Parasitol. Parasites Wildl. 2021;16:175–182. doi: 10.1016/j.ijppaw.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Praks J.O. Voprosõ Etiologii I Rasprostranenija Babezioza Krupnogo Rogatogo Skota v Estonskoi SSR I Merõ Borbõ s Etim Zabolevanijem. Estonian Agricultural Academy of the Ministry of Agriculture of the USSR; Tartu, Finland: 1969. [Google Scholar]
- 276.Bajer A., Rodo A., Bednarska M., Mierzejewska E., Welc-Falęciak R. Babesia canis and tick-borne encephalitis virus (TBEV) co-infection in a sled dog. Ann. Agric. Environ. Med. 2013;20:426–430. [PubMed] [Google Scholar]
- 277.Tiškina V., Capligina V., Must K., Berzina I., Ranka R., Jokelainen P. Fatal Babesia canis canis infection in a splenectomized Estonian dog. Acta Vet. Scan. 2016;58:7. doi: 10.1186/s13028-016-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Katargina O., Geller J., Vasilenko V., Kuznetsova T., Järvekülg L., Vene S., Lundkvist Å., Golovljova I. Detection and characterization of Babesia species in Ixodes ticks in Estonia. Vector Borne Zoonotic Dis. 2011;11:923–928. doi: 10.1089/vbz.2010.0199. [DOI] [PubMed] [Google Scholar]
- 279.Haapasalo K., Suomalainen P., Sukura A., Siikamaki H., Jokiranta T.S. Fatal babesiosis in man, Finland, 2004. Emerg. Infect. Dis. 2010;16:1116–1118. doi: 10.3201/eid1607.091905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Finnish Food Authority . Animal Diseases in Finland 2020. Volume 4. Finnish Food Authority Publications; Helsinki, Finland: 2021. p. 66. (In Finnish, with summary in English) [Google Scholar]
- 281.Finnish Food Authority . Tutkimus Ilmastonmuutoksen Vaikutuksesta Tauteihin Hyötyy eri Alojen Asiantuntemuksesta. Finnish Food Authority Publications; Helsinki, Finland: 2020. (In Finnish) [Google Scholar]
- 282.Louhelainen M., Spillmann T. Case report: Babesia canis infection in a dog. Suom. Eläinlääkärilehti. 2009;115:143–148. (In Finnish, with summary in English) [Google Scholar]
- 283.Rossow H., Joutsen S., Tuominen P. Zoonoottiset Taudinaiheuttajat Tuontikoirissa. Ruokavirasto; Helsinki, Finland: 2019. [Google Scholar]
- 284.Berzina I., Capligina V., Baumanis V., Ranka R., Cirule D., Matise I. Autochthonous canine babesiosis caused by Babesia canis canis in Latvia. Vet. Parasitol. 2013;196:515–518. doi: 10.1016/j.vetpar.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 285.Seleznova M., Kivrane A., Namina A., Krumins R., Aleinikova D., Lazovska M., Akopjana S., Capligina V., Ranka R. Babesiosis in Latvian domestic dogs, 2016–2019. Ticks Tick Borne Dis. 2020;11:101459. doi: 10.1016/j.ttbdis.2020.101459. [DOI] [PubMed] [Google Scholar]
- 286.Capligina V., Seleznova M., Akopjana S., Freimane L., Lazovska M., Krumins R., Kivrane A., Namina A., Aleinikova D., Kimsis J., et al. Large-scale countrywide screening for tick-borne pathogens in field-collected ticks in Latvia during 2017–2019. Parasit. Vectors. 2020;13:351. doi: 10.1186/s13071-020-04219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Namina A., Capligina V., Seleznova M., Krumins R., Aleinikova D., Kivrane A., Akopjana S., Lazovska M., Berzina I., Ranka R. Tick-borne pathogens in ticks collected from dogs, Latvia, 2011–2016. BMC Vet. Res. 2019;15:398. doi: 10.1186/s12917-019-2149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Capligina V., Berzina I., Bormane A., Salmane I., Vilks K., Kazarina A., Bandere D., Baumanis V., Ranka R. Prevalence and phylogenetic analysis of Babesia spp. in Ixodes ricinus and Ixodes persulcatus ticks in Latvia. Exp. Appl. Acarol. 2016;68:325–336. doi: 10.1007/s10493-015-9978-0. [DOI] [PubMed] [Google Scholar]
- 289.Paulauskas A., Aleksandravičienė A., Lipatova I., Griciuvienė L., Kibiša A., Žukauskienė J., Radzijevskaja J. Molecular detection of Babesia spp. in European bison (Bison bonasus) and their ticks. Ticks Tick Borne Dis. 2021;12:101807. doi: 10.1016/j.ttbdis.2021.101807. [DOI] [PubMed] [Google Scholar]
- 290.Paulauskas A., Radzijevskaja J., Karveliene B., Grigonis A., Aleksandravičienė A., Zamokas G., Babickaitė L., Sabunas V., Petkevicius S. Detection and molecular characterization of canine babesiosis causative agent Babesia canis in the naturally infected dog in Lithuania. Vet. Parasitol. 2014;205:702–706. doi: 10.1016/j.vetpar.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 291.Sabūnas V. Ph.D. Thesis. Vytautas Magnus University; Kaunas, Lithuania: 2019. Prevalence of Vector-Borne Pathogens in Dogs. [Google Scholar]
- 292.Radzijevskaja J., Tamoliūnaitė D., Sabunas V., Aleksandravičienė A., Paulauskas A. Prevalence and co-infection of mosquito- and tick-borne pathogens in domestic dogs suspected for canine babesiosis in Lithuania. Biologija. 2020;66:2. doi: 10.6001/biologija.v66i2.4256. [DOI] [Google Scholar]
- 293.Paulauskas A., Radzijevskaja J., Mardosaitė-Busaitienė D., Aleksandravičienė A., Galdikas M., Krikštolaitis R. New localities of Dermacentor reticulatus ticks in the Baltic countries. Ticks Tick Borne Dis. 2015;6:630–635. doi: 10.1016/j.ttbdis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 294.Radzijevskaja J., Mardosaitė-Busaitienė D., Aleksandravičienė A., Paulauskas A. Investigation of Babesia spp. in sympatric populations of Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania and Latvia. Ticks Tick Borne Dis. 2018;9:270–274. doi: 10.1016/j.ttbdis.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 295.Radzijevskaja J., Paulauskas A., Rosef O. Prevalence of Anaplasma phagocytophilum and Babesia divergens in Ixodes ricinus ticks from Lithuania and Norway. Int. J. Med. Microbiol. 2008;298:218–221. doi: 10.1016/j.ijmm.2008.01.008. [DOI] [Google Scholar]
- 296.Mørch K., Holmaas G., Frolander P.S., Kristoffersen E.K. Severe human Babesia divergens infection in Norway. Int. J. Infect. Dis. 2015;33:37–38. doi: 10.1016/j.ijid.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 297.Mysterud A., Stigum V.M., Seland I.V., Herland A., Easterday W.R., Jore S., Østerås O., Viljugrein H. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasit. Vectors. 2018;11:309. doi: 10.1186/s13071-018-2890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mysterud A., Jore S., Østerås O., Viljugrein H. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Sci. Rep. 2017;7:16316. doi: 10.1038/s41598-017-15742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Eliassen K.E., Ocias L.F., Krogfelt K.A., Wilhelmsson P., Dudman S.G., Andreassen Å., Lindbak M., Lindgren P.-E. Tick-transmitted co-infections among erythema migrans patients in a general practice setting in Norway: A clinical and laboratory follow-up study. BMC Inf. Dis. 2021;21:1044. doi: 10.1186/s12879-021-06755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Quarsten H., Salte T., Lorentzen Å.R., Hansen I.J.W., Hamre R., Forselv K.J.N., Øines Ø., Wennerås C., Noraas S. Tick-borne pathogens detected in the blood of immunosuppressed Norwegian patients living in a tick-endemic area. Clin. Infect. Dis. 2021;73:e2364–e2371. doi: 10.1093/cid/ciaa971. [DOI] [PubMed] [Google Scholar]
- 301.Thortveit E.T., Aase A., Petersen L.B., Lorentzen Å.R., Mygland Å., Ljøstad U. Human seroprevalence of antibodies to tick-borne microbes in southern Norway. Ticks Tick Borne Dis. 2020;11:101410. doi: 10.1016/j.ttbdis.2020.101410. [DOI] [PubMed] [Google Scholar]
- 302.Østerås O., Solbu H., Refsdal A.O., Roalkvam T., Filseth O., Minsaas A. Results and evaluation of thirty years of health recordings in the Norwegian dairy cattle population. J. Dairy Sci. 2007;90:4483–4497. doi: 10.3168/jds.2007-0030. [DOI] [PubMed] [Google Scholar]
- 303.Øines Ø., Storli K., Brun-Hansen H. First case of babesiosis caused by Babesia canis canis in a dog from Norway. Vet. Parasitol. 2010;171:350–353. doi: 10.1016/j.vetpar.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 304.Uhnoo I., Cars O., Christensson D., Nyström-rosander C. First documented case of human babesiosis in Sweden. Scand. J. Infect. Dis. 1992;24:541–547. doi: 10.3109/00365549209052642. [DOI] [PubMed] [Google Scholar]
- 305.Bläckberg J., Lazarevic V.L., Hunfeld K.-P., Persson K.E. Low-virulent Babesia venatorum infection masquerading as hemophagocytic syndrome. Ann. Hematol. 2018;97:731–733. doi: 10.1007/s00277-017-3220-6. [DOI] [PubMed] [Google Scholar]
- 306.Svensson J., Hunfeld K.P., Persson K.E.M. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks Tick Borne Dis. 2019;10:186–190. doi: 10.1016/j.ttbdis.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 307.Wilhelmsson P., Lövmar M., Krogfelt K.A., Nielsen H.V., Forsberg P., Lindgren P.E. Clinical/serological outcome in humans bitten by Babesia species positive Ixodes ricinus ticks in Sweden and on the Åland Islands. Ticks Tick Borne Dis. 2020;11:101455. doi: 10.1016/j.ttbdis.2020.101455. [DOI] [PubMed] [Google Scholar]
- 308.Årsstatistik. 2019. [(accessed on 18 March 2022)]. Available online: https://Jordbruksverket.se.
- 309.Christensson D.A. Inverse age resistance to experimental Babesia divergens infection in cattle. Acta Vet. Scand. 1989;30:453–464. doi: 10.1186/BF03548023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lundström S. Babesios: En Fallbeskrivning Samt Diskussion av Fästingburna Sjukdomar Hos Nöt i SVERIGE. SLU, Dept. of Ruminant Medicine and Veterinary Epidemiology; Uppsala, Sweden: 2004. [Google Scholar]
- 311.Dedkov V., Simonova E., Beshlebova O., Safonova M., Stukolova O., Verigina E., Savinov G., Karaseva I., Blinova E., Granitov V. The burden of tick-borne diseases in the Altai region of Russia. Ticks Tick Borne Dis. 2017;8:787–794. doi: 10.1016/j.ttbdis.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 312.Karan L., Makenov M., Kolyasnikova N., Stukolova O., Toporkova M., Olenkova O. Dynamics of spirochetemia and early PCR detection of Borrelia miyamotoi. Emerg. Infect. Dis. 2018;24:860–867. doi: 10.3201/eid2405.170829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kukina I.V., Zelya O.P., Guzeeva T.M., Karan L.S., Perkovskaya I.A., Tymoshenko N.I., Guzeeva M.V. Severe babesiosis caused by Babesia divergens in a host with intact spleen, Russia, 2018. Ticks Tick Borne Dis. 2019;10:101262. doi: 10.1016/j.ttbdis.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 314.Kukina I.V., Guzeeva T.M., Zelya O.P., Ganushkina L.A. Fatal human babesiosis caused by Babesia divergens in an asplenic host. IDCases. 2018;13:e00414. doi: 10.1016/j.idcr.2018.e00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bondarenko A.V., Torianyk I.I., Pokhil S.I., Katsapov D.V., Lytvynenko M.V., Lantukh I.V., Bocharova T.V., Gargin V.V. Seroprevalence of babesiosis in immunocompetent and immunocompromised individuals. Pol. Med. J. 2021;49:193–197. [PubMed] [Google Scholar]
- 316.Dwuznik-Szarek D., Mierzejewska E.J., Kiewra D., Czułowska A., Robak A., Bajer A. Update on prevalence of Babesia canis and Rickettsia spp. in adult and juvenile Dermacentor reticulatus ticks in the area of Poland (2016–2018) Sci. Rep. 2022;12:5755. doi: 10.1038/s41598-022-09419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jepsen M.T., Jokelainen P., Jore S., Boman A., Slunge D., Krogfelt K.A. Protective practices against tick bites in Denmark, Norway and Sweden: A questionnaire-based study. BMC Public Health. 2019;19:1344. doi: 10.1186/s12889-019-7613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tiškina V., Jokelainen P. Vector-borne parasitic infections in dogs in the Baltic and Nordic countries: A questionnaire study to veterinarians on canine babesiosis and infections with Dirofilaria immitis and Dirofilaria repens. Vet. Parasitol. 2017;244:7–11. doi: 10.1016/j.vetpar.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 319.Wright I. Babesiosis in Essex, UK: Monitoring and learning lessons from a novel disease outbreak. Parasit. Vectors. 2018;11:132. doi: 10.1186/s13071-018-2718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Levytska V.A., Mushinsky A.B., Zubrikova D., Blanarova L., Długosz E., Vichova B., Slivinska K.A., Gajewski Z., Gizinski S., Liu S., et al. Detection of pathogens in ixodid ticks collected from animals and vegetation in five regions of Ukraine. Ticks Tick Borne Dis. 2021;12:101586. doi: 10.1016/j.ttbdis.2020.101586. [DOI] [PubMed] [Google Scholar]
- 321.Hamel D., Silaghi C., Zapadynska S., Kudrin A., Pfister K. Vector-borne pathogens in ticks and EDTA-blood samples collected from client-owned dogs, Kiev, Ukraine. Ticks Tick Borne Dis. 2013;4:152–155. doi: 10.1016/j.ttbdis.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 322.Silaghi C., Weis L., Pfister K. Dermacentor reticulatus and Babesia canis in Bavaria (Germany)—A georeferenced field study with digital habitat characterization. Pathogens. 2020;9:541. doi: 10.3390/pathogens9070541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Bajer A., Kowalec M., Levytska V., Mierzejewska E.J., Alsarraf M., Poliukhovych V., Rodo A., Wężyk D., Dwużnik-Szarek D. Tick-borne pathogens, Babesia canis and Borrelia burgdorferi s.l., in sled and companion dogs from Central and North-Eastern Europe. Pathogens. 2022 doi: 10.3390/pathogens11050499. in review . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mierzejewska E.J., Dwużnik D., Koczwarska J., Stańczak Ł., Opalińska P., Krokowska-Paluszak M., Wierzbicka A., Górecki G., Bajer A. The red fox (Vulpes vulpes), a possible reservoir of Babesia vulpes, B. canis and Hepatozoon canis and its association with the tick Dermacentor reticulatus occurrence. Ticks Tick Borne Dis. 2021;12:101551. doi: 10.1016/j.ttbdis.2020.101551. [DOI] [PubMed] [Google Scholar]
- 325.Dwużnik D., Mierzejewska E.J., Kowalec M., Alsarraf M., Stańczak Ł., Opalińska P., Krokowska-Paluszak M., Górecki G., Bajer A. Ectoparasites of red foxes (Vulpes vulpes) with a particular focus on ticks in subcutaneous tissues. Parasitology. 2020;147:1359–1368. doi: 10.1017/S003118202000116X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mierzejewska E.J., Welc-Faleciak R., Karbowiak G., Kowalec M., Behnke J.M., Bajer A. Dominance of Dermacentor reticulatus over Ixodes ricinus (Ixodidae) on livestock, companion animals and wild ruminants in eastern and central Poland. Exp. Appl. Acarol. 2015;66:83–101. doi: 10.1007/s10493-015-9889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Onyiche T.E., Răileanu C., Fischer S., Silaghi C. Global distribution of Babesia species in questing ticks: A systematic review and meta-analysis based on published literature. Pathogens. 2021;10:230. doi: 10.3390/pathogens10020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Gern L., Kindler A., Brossard M. Annual evolution of cattle immunity against Babesia divergens in Northern Switzerland. Prev. Vet. Med. 1988;6:9–16. doi: 10.1016/0167-5877(88)90021-9. [DOI] [Google Scholar]
- 329.Cannon S., Levy J., Kirk S., Crawford P., Leutenegger C., Shuster J., Liu J., Chandrashekar R. Infectious diseases in dogs rescued during dogfighting investigations. Vet. J. 2016;211:64–69. doi: 10.1016/j.tvjl.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Fukumoto S., Suzuki H., Igarashi I., Xuan X. Fatal experimental transplacental Babesia gibsoni infections in dogs. Int. J. Parasitol. 2005;35:1031–1035. doi: 10.1016/j.ijpara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 331.Deksne G., Davidson R.K., Buchmann K., Kärssin A., Kirjušina M., Gavarāne I., Miller A.L., Pálsdóttir G.R., Robertson L.J., Mørk T. Parasites in the changing world–Ten timely examples from the Nordic-Baltic region. Parasite Epidemiol. Control. 2020;10:e00150. doi: 10.1016/j.parepi.2020.e00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.