Abstract
Toxic ingredients in food can lead to serious food-related diseases. Such compounds are bacterial toxins (Shiga-toxin, listeriolysin, Botulinum toxin), mycotoxins (aflatoxin, ochratoxin, zearalenone, fumonisin), pesticides of different classes (organochlorine, organophosphate, synthetic pyrethroids), heavy metals, and natural antinutrients such as phytates, oxalates, and cyanide-generating glycosides. The generally regarded safe (GRAS) status and long history of lactic acid bacteria (LAB) as essential ingredients of fermented foods and probiotics make them a major biological tool against a great variety of food-related toxins. This state-of-the-art review aims to summarize and discuss the data revealing the involvement of LAB in the detoxification of foods from hazardous agents of microbial and chemical nature. It is focused on the specific properties that allow LAB to counteract toxins and destroy them, as well as on the mechanisms of microbial antagonism toward toxigenic producers. Toxins of microbial origin are either adsorbed or degraded, toxic chemicals are hydrolyzed and then used as a carbon source, while heavy metals are bound and accumulated. Based on these comprehensive data, the prospects for developing new combinations of probiotic starters for food detoxification are considered.
Keywords: food, lactic acid bacteria, toxins, mycotoxins, pesticides, heavy metals, antinutrients
1. Introduction
In addition to nutrients, human food sometimes contains components and ingredients of a toxic nature. Food poisoning and foodborne illness outbreaks have been a problem for human communities since the dawn of civilization. Such data go back to antiquity when the population of ancient Rome used lead pipes to build aqueducts and sweetened the wine with lead acetate (Pb(C2H3O2)2·3H2O), known as lead sugar. The Middle Ages in Europe were marked by numerous incidents of human poisoning after eating rye-flour bread infected with ergot fungi. The types, severity, and consequences of food-related diseases have changed over the centuries and remain diverse in different regions and communities. In the last two decades, we have witnessed the deadliest outbreaks caused by toxigenic microorganisms. Listeria monocytogenes struck South Africa in 2017, poisoning 1060 people and killing 216; new toxigenic strain E. coli O104:H4 caused 53 deaths and serious illness of more than 3950 people in Europe in 2011; aflatoxin contamination of maize in Kenya resulted in 317 cases of hepatic failure and 125 deaths in 2004 [1,2]. In terms of chemical contamination, a significant incident occurred in China in 2008, when infant milk formula was contaminated with melamine, resulting in 294,000 affected babies, 6 of whom died [3].
Today, more than 200 diseases are caused by eating food contaminated with bacteria, viruses, parasites, toxins, or chemicals. This contributes significantly to the global increase in morbidity and mortality. Worldwide, about 600 million people get sick each year from eating contaminated food, which leads to 420,000 deaths annually, mostly of children and vulnerable people [4]. That is why the WHO established the Foodborne Disease Burden Epidemiology Reference Group (FERG). According to the report, food poisoning has exerted a significant socio-economic impact and emerged as a growing public health problem in the last decade. The Secretariat of the International Food Safety Authorities Network (INFOSAN) reported that only during the fourth quarter of 2021, 64 food safety incidents of great importance, involving 86 countries, occurred. Thirty-three of them posed a serious biological hazard to society and were caused by toxigenic Salmonella spp., Lis. monocytogenes, E. coli, Bacillus cereus, Vibrio spp., Clostridium botulinum, Staphylococcus aureus, and Shigella sonnei [4].
However, food containing chemical agents does not pose a lesser risk to the health of consumers. Notably, 140,000 tons of pesticides are sprayed on crops in the European Union every year [5]. The toxicological evaluation of pesticide residues in food performed by the Food and Agriculture Organization (FAO) showed that the residual concentration of 13 toxic pesticides must be continuously monitored because they leave significant traces in food commodities [5].
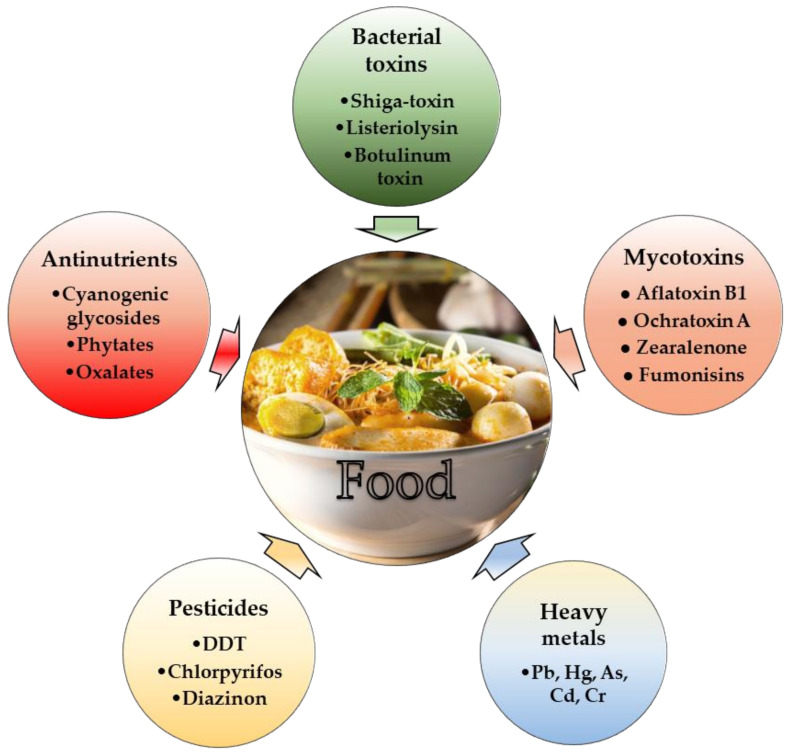
According to the World Health Organization (WHO), toxic ingredients in food may be classified as (i) toxicants derived from microorganisms; (ii) toxic chemicals (pesticides, heavy metals); and (iii) naturally occurring toxicants and antinutrients derived from plant material before processing. All these agents cause gastrointestinal tract (GIT) disorders and inflict considerable neurological, cardiovascular, immunological, and psychological damage. A schematic overview of toxic food ingredients is shown in Figure 1.
Figure 1.
Scheme of the toxic compounds that could be found in food products.
Food spoilage can occur at various stages of food production, supply, and consumption. That is why food safety receives a lot of attention in wealthier societies, but it is a much more pressing concern in developing countries. One reason for food contamination is the polluted water used for washing and processing; others include primitive ways of production and improper use of agricultural chemicals, poor storage, and lack of regulations. Many agents that cause diseases are transmitted from domestic animals to humans through food products; in addition, the warm climate further contributes to the spread of natural toxigenic producers in tropical countries.
Lactic acid fermentation is the oldest and most widely used method to improve the safety and nutritional value of foods. It has been employed from the very beginning of agriculture and animal husbandry to preserve cereal, milk, fish, and meat products from bacterial contamination, prolong their shelf life, and enrich them with probiotic bacterial strains [6,7]. Lactic acid bacteria (LAB) are routinely used to produce traditional functional foods such as yogurt, cheese, sauerkraut, pickles, and fermented cereal meals and beverages [6,7,8]. Dozens of LAB strains have been evaluated as probiotics due to the production of metabolites with health benefits that are scientifically confirmed and well-documented [9,10,11,12]. However, Markowiak and Śliżewska have underlined that one of the requirements for a particular strain to be evaluated as a probiotic should be its ability to inhibit the production of bacterial toxins, inactivate them, or facilitate their removal from the human body [13]. The probiotics exhibiting detoxifying properties contain unique, strain-related characteristics, and their selection deserves special attention. On the other hand, over the past decade, hundreds of scientific studies have highlighted the role of LAB in food detoxification [14,15,16]. Large-scale food production and increasing environmental pollution make the topic of natural food purification via microbial fermentation extremely important and relevant. Biological detoxification of food can be achieved with various LAB degrading, metabolizing, or adsorbing toxins and thus effectively neutralizing them. The present state-of-the-art review aims to summarize the available data and elucidate the current role of LAB in food detoxification. Due to their wide substrate spectrum and diverse enzyme pool [17,18,19], LAB can ferment almost any food of dubious quality and potentially detoxify it. The unique properties of LAB that make them the “panacea” for food detoxification are described below.
2. Lactic Acid Bacteria as Probiotics
Although the original concept of probiotics was first proposed more than a hundred years ago by Élie Metchnikoff, the term was introduced in 1965 by Lilly and Stillwell to describe the consumption of a living microorganism with a positive effect on the resident microflora [20]. In 2010, Fujiya and Kohgo widened the definition by including other positive effects on human health, such as “maintaining intestinal development, nutrition and treatment of intestinal inflammation, functional disorders and other extraintestinal diseases” [21]. Indeed, besides the ability to maintain the proper balance between pathogens and the beneficial bacteria in order to prevent gastrointestinal infections and disorders [22], probiotics also possess immunomodulatory action on the host [23], alleviate allergies and atopic diseases [24], and help in cholesterol removal [25].
The most significant share of probiotic microorganisms is occupied by LAB species of the genus Lactobacillus as well as the species Enterococcus faecalis and Ent. faecium, Lactococcus lactis, Leuconostoc mesenteroides, Pediococcus acidilactici, Sporolactobacillus inulinus, and Streptococcus thermophilus [26]. Among lactobacilli, the most popular pharmaceutical probiotics contain Lactobacillus acidophilus, Lacticaseibacillus casei, Lactobacillus gasseri, Limosilactobacillus reuteri, and Lactobacillus helveticus, while most often used in the production of functional foods are the species L. amylovorus, Lactiplantibacillus plantarum, Lacticaseibacillus paracasei, Lactobacillus johnsonii, Lactiplantibacillus pentosus, and Lactiplantibacillus rhamnosus [13].
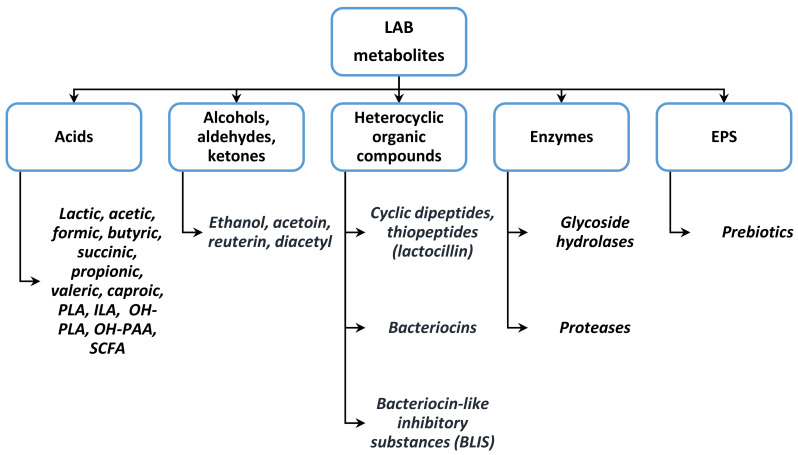
The main LAB metabolites that may be used against toxigenic producers are shown in Figure 2. Lactic acid (LA) has a well-established antimicrobial activity. According to Arena et al. [27], LA acts in its protonated form by impairing the pH gradient between the cytosol (alkaline) and the external environment (acidic), thus dissipating the membrane potential and destroying the pathogenic cells. Birt et al. [28] showed that other organic acids with antimicrobial effects are branched short-chain fatty acids (SCFA) such as isobutyrate and isovalerate. Fazeli et al. [29] reported the same effect in the production of acetate, butyrate, formate, succinate, propionate, valerate, and caproic acid.
Figure 2.
LAB metabolites involved in their activity against toxigenic producers. Designations: PLA, phenyllactic acid; ILA, indolelactic acid; OH-PAA, hydroxyphenylacetic acid; OH-PLA, 4-hydroxyphenyllactic acid; SCFA, branched short-chain fatty acids; EPS, exopolysaccharides.
Van der Meulen et al. [30] showed that LAB also form phenyllactic, indolelactic, 4-hydroxyphenyllactic, and hydroxyphenylacetic acids, all possessing antimicrobial activity in sourdough, while Negatu et al. [31] noted the fungus-inhibitory activity of benzoic acid, methylhydantoin, and mevalonolactone. Indole-3-propionic prevents endotoxins leakage through the intestinal epithelial barrier [32]. Other antimicrobial compounds are carbonyl derivatives such as diacetyl, acetaldehyde, acetoin, and 2,3-butanediol, as they act against toxigenic E. coli, Lis. monocytogenes, and S. aureus [33]. Carbon dioxide works by inhibiting enzymatic decarboxylation and increasing the membrane permeability, while hydrogen peroxide damages cellular structures through its oxidative effect and disrupts the membrane redox potential [34].
Bacteriocins are ribosomally produced, heat- and acid-resistant LAB oligopeptides with antimicrobial activity against foodborne pathogenic bacteria and fungi. According to the nucleotide sequence of the responsible genes and amino acid composition, bacteriocins are classified as (1) smaller than 5 kDa, heat-stable, and lanthionine-containing; (2) bacteriocins below 10 kDa, heat-stable, and non-lanthionine-containing; (3) proteins with Mw higher than 30 kDa, heat-sensitive; and (4) bacteriolysins. Due to their efficiency, class II (pediocin-like bacteriocins) are considered an alternative to chemical preservatives because they are highly active against Lis. monocytogenes. The specific structure of these molecules includes conserved Tyrosine-Glycine-Asparagine-Glycine-Valine (YGNGV) motif and disulfide bonds in the N-terminal region [35]. Bacteriocins with a pronounced antitoxigenic activity belong to the third class: helveticin M, helveticin J, and enterolysin A, produced by Lactobacillus crispatus, L. helveticus, and Ent. faecalis, while in the fourth class are affiliated with leuconocin S and lactocin 27, which comprise complexes of protein, lipids, and carbohydrates. One of the most active antibacterial compounds, plantaricin A, is produced by many food-derived LAB: Lp. plantarum, Furfurilactobacillus rossiae, Levilactobacillus brevis, Companibacillus paralimentarius, Leuc. mesenteroides, Leuc. pseudomesenteroides, and Leuc. citreum; Weissella paramesenteroides, W. cibaria, Lactiplantibacillus paraplantarum, and Latilactobacillus curvatus [36]. Other bacteriocins secreted by food LAB are sakacin (Leuc. citreum and Latilactobacillus graminis), bavaricin (Latilactobacillus sakei), and pentocin. L. gasseri produces gassericin A and the thiopeptide antibiotic lactocillin, which prevents the growth of Staph. aureus, Corynebacterium aurimucosum, and Str. sobrinus [37].
Other LAB metabolites with strong antifungal activity are cyclic dipeptides. They are often produced by sourdough lactobacilli species such as Fur. rossiae, L. harbinensis, L. amylovorus, Limosilactobacillus reuteri, Lev. brevis, and Levilactobacillus spicheri [38]. Different Lp. plantarum strains generate the fungistatic peptides cyclo(Gly-L-Leu), cyclo(L-Phe–L-Pro), cyclo(L-Phe–trans-4-OH-L-Pro), cyclo(L-Leu-L-Pro), and cyclo(L-Phe-L-Pro) [31,39,40]. Other antifungal cyclopeptides produced by sourdough LAB are cyclo(L-Pro-L-Pro) [41], cyclo(L-Tyr-L-Pro) [42], cyclo(L-Met-L-Pro) [43], cyclo(L-His-L-Pro) [44], and cyclo(Leu-Leu) [45].
3. LAB against Bacterial Toxins and Their Producers
There are three types of bacterial foodborne diseases: intoxications, infections, and toxico-infections. Intoxication occurs by ingesting food containing a pre-formed bacterial toxin (for example, produced by S. aureus or C. botulinum), which causes intoxication. The second type of foodborne infection is a result of the consumption of food containing viable toxigenic bacteria (such as serotypes of E. coli, Salmonella or Listeria), which multiply in the host and cause disease. The third variant (toxico-infection) is caused by species such as C. perfringens: When food containing viable vegetative cells is consumed, bacterial cells undergo sporulation in the small intestine and produce an enterotoxin, which is released with the spores during cell lysis. The enterotoxin has a cytotoxic effect on GIT epithelial cells by damaging the cell membrane structure.
3.1. LAB against Toxigenic Escherichia coli
Many authors studying spontaneously fermented ethnic foods believe that the presence of probiotics in the diet can serve as a preventive measure against infectious diseases associated with the consumption of contaminated foods [7]. However, besides enteritis and diarrhea, the toxigenic E. coli strains can cause urinary tract infections, septicemia, neonatal meningitis, and cardiovascular and central nervous system diseases by cytotoxin production and are the most clinically significant pathogen in European countries [46]. Shiga toxin-producing E. coli (STEC) are enterohemorrhagic E. coli (EHEC) strains that cause either enteric disease (bloody diarrhea, hemorrhagic colitis) or hemolytic uremic syndrome (HUS). EHEC colonizes the host’s large intestine, causing the so-called attaching-and-effacing (AE) lesions. The adherence to epithelial cells with localized destruction occurs with the aid of Shiga toxins 1 and 2 (Stx1, Stx2). These two cytotoxins are immunologically different, as Stx1 is identical to the Shiga toxin produced by Shigella dysenteriae type I [47]. Both toxins are encoded on chromosomal lysogenic bacteriophages. Although many variations are found in the Stx family, all Shiga toxins have an A-B subunit structure. Subunit A has N-glycosidase activity, while subunit B binds to a membrane glycolipid. Subunit A cleaves a single adenine residue from the 28S rRNA component of eukaryotic ribosomes, resulting in inhibition of protein synthesis in the cells of the renal glomeruli [48].
STEC infections can lead to death, especially in young children and elderly people. Various foods contain such strains: ground beef, fresh milk, apple cider [49,50,51,52,53,54,55], or fermented hard salami [56]. Although the most dangerous EHEC, E. coli O157:H7, has been associated with foods of bovine origin in Michigan and Oregon, USA, in 1982 [57], it was also found in goat’s milk, lettuce, and alfalfa sprouts [58,59,60,61]. Investigating the presence of STEC in 4330 Korean food samples, Ryu et al. [62] determined the highest prevalence of the bacterium in yukhoe (forged raw meat), cold bean soup, gimbal (meat broth for cold noodles), and sprouts, as well as that 17.7% of the obtained E. coli strains, were resistant to antibiotics. A study was also conducted in central Egypt to determine whether E. coli O157:H7 was present in 175 samples of raw ground beef, chicken, lamb, and unpasteurized milk obtained from slaughterhouses, supermarkets, and farms [63]. In Greece, 1–2% of samples of ewes’ milk, sausages, and swine intestines contained E. coli O157:H7; similar values were obtained in the Czech Republic and Spain [64,65,66]. STEC/enteroaggregative E. coli O104:H4 was the causative agent of the outbreak that occurred in Germany in 2011 and took at least 40 lives from more than 4000 cases of diarrhea; almost one-fourth of the cases (908) and more than 75 percent of the deaths (34) were accompanied by hemolytic-uremic syndrome [67].
LAB can inhibit the growth of STEC/EHEC E. coli serotypes by direct or indirect interaction with the pathogen. For instance, Orihuel et al. [68] tested LAB antagonistic activity against STEC strains in co-cultures. Bacteriocin producer L. curvatus CRL705 showed only a slight decrease in the E. coli population, whereas the bacteriocinogenic strain Ent. mundtii CRL 35 and Lp. plantarum CRL 681 (non-bacteriocinogenic) significantly reduced E. coli viability and put its growth into the death phase after 8 h. In order to assess the antagonistic mechanisms, a proteomics approach was applied. Differences in the proteome were connected with carbohydrate and amino acid metabolism, energy production, transcription, and translation; cell division was also involved, suggesting that Ent. mundtii CRL35 used the competition strategy.
The inhibitory characteristics of probiotic strains Lc. casei Shirota and L. acidophilus YIT 0070 were investigated toward three clinical isolates of E. coli O157:H7. During batch co-fermentation, both probiotic lactobacilli exerted growth inhibitory and bactericidal activity on EHEC [69]. The same authors, Ogawa et al. [70], used a newborn rabbit model of experimental infection to investigate the protective effects of oral administration of the probiotic Lc. casei strain Shirota against EHEC infection. Daily consumption of the probiotic from birth prevented colonization in the GIT and reduced the concentrations of both Stx1 and Stx2 toxins. The reason for the protective effect of Lc. casei Shirota was due to local immune response enhancement and STEC cell elimination, which consequently reduced toxin levels in the gut. Byakika et al. [71] revealed the antimicrobial effect of Lp. plantarum, Lactococcus lactis, W. confusa, and Lc. rhamnosus GG against acid- and antibiotic-resistant E. coli producing Stx2 toxin and isolated from Obushera, a Ugandan cereal drink. The data concerning the molecular mechanisms involved in the antimicrobial activity of LAB against STEC and EHEC are summarized in Table 1.
Table 1.
Established mechanisms of antibacterial activity of lactic acid bacteria (LAB) against toxigenic E. coli strains.
| E. coli Strain | LAB Species, Strain | Source/Model System | Agent/Bioactive Molecule | Mode of Action | References |
|---|---|---|---|---|---|
| O157:H7 | Lc. casei strain Shirota, L. acidophilus YIT 0070 | Yakult, Japan | Low pH, undissociated lactic acid | Growth inhibitory and bactericidal activities | [69] |
| O157:H7 | L. lactis | Raw chicken meat | H2O2 | Growth inhibition | [72] |
| O127:H6 | Li. reuteri ATCC PTA 6475, ATCC 53608 | Human, pig | Adhesins MUB, CmbA, MapA | Mucus layer binding and E. coli adherence decrease | [73] |
| O157:H7 | Li. reuteri ATCC PTA 6475 | Germfree mice | Reuterin | Decreased E. coli colonization, amended necrosis of the kidneys | [74] |
| O157:H7 | L. acidophilus NP51 | Cattle | Reuterin | Effective reduction of E. coli in cattle feces | [75] |
| EDL933 | Lc. casei LC wt, LC CLA | Batch fermentation | Conjugated linoleic acid | Downregulation of EHEC virulence genes | [76] |
Several different studies claimed that the popular probiotic L. acidophilus strain La-5 is effective against infection with toxigenic E. coli O157:H7. Zeinhom et al. [77] observed an antivirulence effect of an “active fraction” extracted from La-5 cell-free spent medium incorporated in yogurt and tested using a mice model. Strain-derived metabolites prevented the epithelium attachment and GIT colonization by STEC, along with crucial downregulation of the stxB2 gene encoding Shiga toxin. This study confirmed earlier works with the same probiotic, which reported the ability of L. acidophilus strain La-5 to prevent EHEC from adhering to epithelial cells and to concentrate F-actin at adhesion sites [78]. The surface-layer protein (SLP) extracts of L. helveticus and Lc. rhamnosus metabolites decrease the AE lesions of E. coli O157:H7. Both species preserve the barrier function of Hep-2 and T84 cells monolayers by metabolites produced in the culture medium [79]. Hirano et al. [80] also found that Lc. rhamnosus prevents EHEC adhesion to human colon epithelial cell line C2Bbe1, but only when living probiotic cells are used.
Lc. rhamnosus GR-1 and Li. reuteri RC-14 were studied for their effects on growth and virulence expression factors in uropathogenic E. coli C1212. LA and other metabolites secreted by lactobacilli downregulate genes for proteins critical for the pathogen’s attachment [81]. Caridi et al. [82] evaluated Lc. paracasei subsp. paracasei isolated from Italian cheese as E. coli antagonist due to bacteriocin production. A recent study by Fijan et al. [83] revealed the high potential of Li. reuteri DSM 17,938 to diminish EHEC growth; however, the authors admitted that the most effective antagonism against EHEC was displayed by multi-strain culture containing lactobacilli, bifidobacteria, and enterococci.
3.2. LAB against Listeria Monocytogenes
Lis. monocytogenes is a non-spore-forming opportunistic pathogen, an intracellular parasite expressing β–hemolysin [84,85]. In nature, it grows in soil, water, and plant material. This pathogen causes listeriosis, characterized by central nervous system disorders, mainly meningitis and encephalitis, pneumonia, respiratory problems, and hematologic deviations [86]. Susceptible to listeriosis are immunocompromised individuals, pregnant women, newborns, and the elderly, as 20–30% of the infected people reach a lethal end [87]. If untreated during pregnancy, the illness could lead to amnionitis and fetus infection, premature birth, or abortion [88]. The responsible factor for this severe infection is a listerial toxin, listeriolysin O (LLO), accompanied by transcriptional activator (PrfA), actin (ActA), and surface proteins internalins InlA and InlB. The presence of responsible genes in food is evidence of Listeria infection [89]. LLO is a cytolysin that is activated by reducing agents (thiol groups), with maximal cytolytic activity at pH 5.5 and 37 °C. The toxin is activated in the phagosomes and lyses them, thus allowing Lis. monocytogenes to escape into the cytosol and persist intracellularly, protected from the immune system [90].
The basic way of introducing foodborne pathogens into the human organism is via food products—most often the so-called ready-to-eat food. Lis. monocytogenes can be found in fruits, vegetables, meat, poultry, raw milk and dairy products, and seafood [91]. Among fresh products, Lis. monocytogenes is found to grow on cabbage, potatoes, asparagus, green beans, broccoli, radishes, corn, cauliflower, lettuce [90], in refrigerated and cooked eggs [92], and cantaloupe [93]. Besides food, strains of Lis. monocytogenes can also contaminate non-food contact surfaces, such as sinks and grounds, persisting for a long period even without growth [94]. It can survive in critical conditions—temperatures between −0.5 °C and 45 °C, high osmotic pressure up to 10% NaCl, and low pH values such as 3.3–4.2 [95,96,97]. The pathogen adapts to stress conditions by altering its membrane fluidity [98], synthesizing σ-factors and osmoprotectant molecules—proline, glycine, betaine, acylcarnitine, and carnitine [99].
Traditional approaches against foodborne infections with Lis. monocytogenes include heating, salting, acid treatment, and drying [100]. Modern technologies include the application of high hydrostatic pressure, pulsed electric field, new packaging methods, and biocontrol. With the latter, the environmental method, LAB metabolites are involved [101]. LAB counteract toxigenic strains of Listeria with all available antimicrobial agents, the most effective against this pathogen being organic acids and bacteriocins. The biocontrol is accomplished by two methods which introduce bacteriocins into the food. In the direct approach, the bacteriocin is added in the form of concentrated dried powder. In the indirect method, bacteriocin-producing LAB strains are incorporated into the food and secrete bacteriocins in situ. In order to prevent the decrease in activity over time because of enzymatic degradation, interference with food components, or food processing, methods for the inclusion of bacteriocin in structures consisting of alginate, gelatin, starch, guar gum, xanthan gum, or liposomes are developed [102]. The most promising LAB strains in the fight against Lis. monocytogenes and the main tools they use are listed in Table 2.
Table 2.
Antibacterial activity of lactic acid bacteria (LAB) against Listeria monocytogenes toxigenic producers.
| Mechanism | LAB Species/Strain | Source | Agent/Action | References |
|---|---|---|---|---|
| Organic acids production | Lactococcus lactis LM0230, Lp. plantarum, La. sakei | Calabrian cheeses | Intracellular pH acidification for unfavorable microenvironment for non-acidophiles | [103,104] |
| CO2 | Heterofermentative LAB | Foods | Anaerobic environment support; inhibition of enzyme decarboxylation; cell membrane disruption | [103] |
| H2O2 | Heterofermentative LAB | Foods | Inactivation of essential biomolecules by superoxide anion chain reaction; activation of the lactoperoxidase system | [105,106] |
| Diacetyl | Lactobacillus sp., Leuconostoc sp., P. aidilactici CC 8081, Streptococcus sp. | Foods | Affects the arginine-binding proteins | [105,106,107] |
| Bacteriocins production |
Lactococcus lactis subsp. lactis, P. acidilactici, Ent. faecium, La. sakei, Li. reuteri INIA P572, Leu. gelidum UAL 187, Lc. rhamnosus CJNU 0519 | Drinks, Foods, Meats, Salads, Antimicrobial packaging | Bacteriocin synthesis: nisin, pediocin PA-1, enterocin A, sakacin A, reuterin, leucocin, rhamnocin 519 | [89,105,108] |
| Nutrients competition | Carnobacterium piscicola, Lactococcus piscium | Ready-to-eat meat products | Quick uptake of nutrients by LAB; bacteriocin synthesis | [89,109,110] |
| Niche competition | Li. reuteri, Li. fermentum, Lc. rhamnosus GC mutant | Foods, probiotics |
Prevent the attachment on host cells through colonization and saturation of Lis. monocytogenes attachment receptor | [111,112] |
| Reduction of L. monocytogenes virulence | Li. reuteri, Li. fermentum, Lp. plantarum, Lactococcus lactis, Leu. mesenteroides, La. sakei | Human intestinal epithelial cells (Caco-2) | Competition for adhesion receptors expressed on host cells through downregulation of virulence gens (prfA, plcA, plcB, hly, actA, inlA, inlB, iap, luxS) | [113,114,115] |
| Protection of Gastrointestinal Tract from L. monocytogenes Invasion | Lc. casei, Li. reuteri, Lc. rhamnosus, Str. thermophilus | Human | MUC2 and TFF3 overexpression; mucus layer integrity conservation; serum cholesterol decrease | [89,116] |
| Host immune response modulation | L. bulgaricus, L. acidophilus, Lc. casei, L. salivarius, Lp. plantarum, Li. reuteri, Lc. rhamnosus, Lev. brevis, Str. thermophilus | Human | Reduction of the pro-inflammatory cytokines (IL-8) and anti-inflammatory cytokines (IL-10) increase | [89,117] |
| Vaccine vector | Lactococcus lactis | Human | Delivery and expression of listerial antigens | [118] |
LAB, which produce bacteriocins with anti-listerial activity, belong to the genera Lactococcus, Lactobacillus, Leuconostoc, Enterococcus, Pediococcus, and Carnobacterium [119]. The most studied bacteriocins with bacteriostatic activity are nisin produced by some Lactococcus lactis spp. lactis; pediocin—by Pediococcus spp. [108]; and plantaricin—by Lp. plantarum. Bavaricin A has a bactericidal mode of action on 90% of the tested Lis. monocytogenes strains [36]. It is produced by the sourdough strain L. bavaricus MI401. Similar to nisin, it is synthesized at temperatures from 4 °C to 30 °C. Another sourdough isolate, Fructilactobacillus sanfranciscensis strain C57, produces a chromosomally-encoded bacteriocin-like inhibitory substance (BLIS) active against the same pathogen. Nisin is approved as a legal food additive in many countries [89]. Some successful encapsulations of nisin in soy-lecithin are available in the literature [120,121]; however, the non-encapsulated one demonstrates stronger anti-listerial activity [122]. According to Thomas and Wimpenny [123], nisin activity increases with the decrease in temperature and pH. For achieving enhanced nisin activity against foodborne pathogens, combinations of nisin with other compounds have been applied. For example, the product Nisaplin® consists of nisin (2.5% w/w), NaCl (77.5% w/w), protein (12% w/w), and carbohydrates (6% w/w) [89]. Notably, LAB inhibit Lis. monocytogenes in food products under refrigerating temperatures. Amezquita and Brashears [109] registered strong anti-listerial activity of P. acidilactici, Lc. casei, and Lc. paracasei at 5 °C isolated from ready-to-eat foods. Even higher activity is observed in cases of co-cultivation of different LAB strains in combination with ProH (whey protein hydrolyzed with pepsin) in traditional Spanish cheese [124]. Morandi et al. [125] achieved total inhibition of the pathogen throughout the co-cultivation of Lactococcus lactis FT27 and Carnobacterium divergens SCA, inoculated in Gorgonzola cheese, and the addition of lactic acid/sodium lactate.
Another strategy for Lis. monocytogenes prevention is the potential of LAB to be employed as a vaccine vector. The LLO possesses important features, enhancing its potential in antitumor vaccines, such as the ability to live intracellularly in a host cell that is not infected by other toxin-producing bacteria [126] and the ability to provide cytosolic access for antigens in antigen-presenting cells via pores formation [127]. As the infection occurs through contaminated food and the pathogen succeeds in bypassing the mucosal barrier, the mucosal vaccines would offer higher effectiveness than those with a parenteral delivery route [128]. However, antigen delivered by mucosa leads to a weak immune response, most likely due to fast disruption in the mucosal secretion, low microbial adsorption, and mucosal tolerance [129]. The safe oral uptake of LAB makes them quite attractive to be employed as a live vector. In this regard, the most studied are the LAB exhibiting probiotic features [130]. Lactococcus lactis appears to be the most suitable for vaccine production as its safety is confirmed and its genome is completely sequenced [118]. Its capability to express different antigens intra- and extracellularly resulted in the development of an inducible expression system. This system should be used for listerial antigens expression delivered orally and be involved in the vaccine construction. LAB are also reported to demonstrate single-chain antibody fragments, which could be employed for generating passive immunity [131]. This is a possible strategy for Lis. monocytogenes treating, as it would exhibit a more direct and fast response. However, the questions about the horizontal transfer of plasmid carrying antibiotic resistance marker to the environmental and host microflora [118], the immune response regarding administration, and the rate of antigen production in vivo to stimulate future vaccine production based on the LAB system remain to be studied in more detail.
3.3. LAB Preventing the Growth and Toxin Production by Clostridium botulinum
C. botulinum is an obligately anaerobic, spore-forming microorganism, and first isolated from raw ham and human liver. Botulinum neurotoxins (BoNTs) are the most powerful natural toxins known to humankind [132]. They cause botulism, a rare but potentially fatal paralytic disease affecting both humans and animals. There are seven types of botulinum neurotoxins (A–G) and many subtypes (e.g., A1–A5 and several subtypes B, E, and F) with different amino acid sequences. BoNTs are initially formed as single-chain polypeptides with a molecular weight of about 150 kDa and relatively low toxicity. According to Lund and Peck [133], in the case of proteolytic C. botulinum (A, B, and F neurotoxins of Group I), the single-chain protein is cleaved by proteases to form a double-chained, highly toxic form. In non-proteolytic C. botulinum (B, E, and F type, Group II), the single-chain pre-toxin is not activated by the same proteases but by unidentified proteases in host cells. The responsible genes for the above-described groups of neurotoxins are either chromosomal or plasmid-located, while in groups III (C and D) and IV (G), neurotoxin genes are always plasmid-localized. Some strains contain genes for toxins of two different antigenic types, one synthesized in large quantities and the other in insignificant amounts. Although the vegetative cells of C. botulinum are sensitive to air, in spore form, they can retain viability for long periods. Spores of C. botulinum Group II pose the highest risk of food poisoning due to their ubiquitous presence in the environment and their ability to survive pasteurization [134], thus germinating in toxic cultures at low temperatures. Proteolytic strains can grow at temperatures below 10–12 °C (non-proteolytic—at 3–4 °C), and contaminate raw meat, fruits, vegetables, and seafood [135,136].
To prevent C. botulinum from spreading, many preservatives are used in food: 3.5% salt in the aqueous phase in chilled ready-to-eat foods, sodium or potassium nitrite and nitrate, etc. Many of these ingredients have detrimental effects on human health, mainly through the formation of carcinogenic substances such as nitrosamines [137].
Recently, the use of lactic acid bacteria was evaluated as a very effective approach to bio-control of C. botulinum [138]. The species that have been applied in solving this task until now are listed in Table 3.
Table 3.
LAB against C. botulinum growth and toxin production in foods.
There are various antimicrobials produced by LAB as part of their defense mechanisms that can improve their ability to compete with C. botulinum. Substances such as hydrogen peroxide, fatty acids, organic acids, ethanol, enzymes, and antibiotics are also involved in food defense against C. botulinum [144]. The use of bacteriocins in heat-treated foods can reduce the intensity of the heat process, minimize the cost of heat treatment and, at the same time, improve the nutritional and organoleptic properties of food [145]. Nisin effectively inhibits the growth of C. botulinum and its spores and prolongs the shelf life at room temperature [142]. To date, eight nisin types have been observed and characterized: Nisin A, Z, F, and Q are produced by Lactococcus lactis, while nisin U, U2, P, and H are produced by some strains of Streptococcus [146,147]. The concentration of 500–1000 IU/g nisin effectively inhibits C. botulinum in cheeses made from pasteurized milk [148,149]. Other LAB-derived bacteriocins, such as pediocin PA-1, mersacidin, mutacin, and lacticin, are used as preservatives in the food industry, as they are also able to prevent the growth of C. botulinum, E. coli, Lis. monocytogenes, S. aureus, and other food pathogens [150,151,152].
3.4. LAB Preventing the Growth and Toxin Production by Other Pathogenic Bacteria
Other widespread toxigenic foodborne pathogens are C. perfringens, Bacillus cereus, S. aureus, Ps. fluorescens, and Ps. putida. C. perfringens is a ubiquitous spore-forming bacterium, a contaminant of water and dust, but also foods such as meat and milk, even processed. The strains produce 18 different toxins and are classified into five toxin types (A, B, C, D, and E) according to the production of four major toxins (α, β, ε, and ι), and the sequences and localization of the toxin-encoding genes [153]. Five of the serotypes of the pathogen produce α-toxin, an enzyme of the family of bacterial zinc-metallo-phospholipases [154]. Both cells and cell-free supernatants of Chinese isolates of L. acidophilus and Li. fermentum inhibited the growth and α-toxin production by C. perfringens. In vitro experiments showed that both lactobacilli are able to degrade α-toxin [155].
B. cereus is another widespread food-spoiling and toxin-producing pathogen, the cause of many food-poisoning outbreaks. Its spores can be found in water, soil, air, cereals, rice, vegetables, milk, dairy products, and meat [156,157,158,159,160]. It is a common contaminant in raw milk, ice cream, milk powder, fermented milk, and pasteurized milk [161], as its spores are heat-resistant and survive pasteurization and chemicals. The ability of the bacterium to form biofilms makes it difficult to clean and disinfect. Once in the gastrointestinal tract, it causes two types of disease. Emetic syndrome is caused by the formation of heat-resistant emetic cereulide toxins (cyclic peptides), which the bacterium forms during its active phase of growth in food, and diarrhea syndrome, which is due to protein enterotoxin complexes, mainly hemolysin BL, non-hemolytic enterotoxin (NHE), and cytotoxin K produced during bacterial growth in the small intestine [162]. The clinical picture of ingestion of food contaminated with cereulide toxin includes nausea, vomiting and abdominal cramps appearing from the first to the fifth hour, and recovery is usually within 6–24 h [163]. The different strains of B. cereus have diverse pathogenic effects, with a dose for the diarrheal syndrome 105–108 CFU/g (colony-forming units per gram of food) of vegetative cells or spores, but there are exceptions, and food poisoning has also been reported with doses below 105 CFU/g. Often two of the three enterotoxins work together and are responsible for gastrointestinal disorders by forming pores in the membranes of epithelial cells in the small intestine [164].
LAB act against B. cereus with the production of metabolites such as organic acids, hydrogen peroxide, bacteriocins, and other antimicrobial peptides [165]. Wang et al. [166] report that the antibacterial effect of LA is most likely due to physiological and morphological changes caused in the bacterial cytoplasmic membrane, leading to leakage of cytoplasmic content. In vacuum-packed raw meats and fish that are kept chilled, LAB become the dominant population and preserve the meat through so-called “hidden” fermentation. Tirloni et al. [167] report that the addition of natural microflora rich in lactic acid bacteria to yogurt, raw milk, and Taleggio cheese has led to inhibition of spore formation and subsequent development and growth of the vegetative cells of B. cereus. L. acidophilus LF221 and Lactococcus lactis have an enormous antibacterial activity against B. cereus in skim milk and fresh cheese due to the synthesis of lactic and acetic acids, while Lacticaseibacillus paracasei also prevents biofilm formation [167,168,169,170].
Besides B. cereus, LAB isolated from fermented foods display strong antagonism toward S. aureus and Pseudomonas spp., as reported by Olaniyi et al. [171]. Ps. fluorescens has generally been considered a saprophytic rhizobacterium; however, it has been isolated from human clinical samples and is known as a common contaminant of packaged vegetables, fish, chicken, beef, fruit milk, goat’s milk [172,173,174,175,176,177], as well as the raw milk in 28 different farms in the Lombardy region of Northern Italy in 2014 [178].
Pseudomonas spp. produce a large number of harmful extracellular substances: phytotoxic compounds, pigments, hydrocyanic acid, proteolytic enzymes, phospholipase, and several enterotoxins [179]. Ps. fluorescens also produces heat-resistant lipases and proteases, indigoidin (causing blue spots on mozzarella cheese), biosurfactants (in the chilled chicken meat), methyl mercaptan, and dimethyl disulfide in fish samples [174,179,180,181]. Exotoxins produced by Pseudomonas spp. are proteinaceous substances. When consumed with the food, they cause leukopenia, acidosis, circulatory collapse, liver necrosis, pulmonary edema, hemorrhage, and tubular necrosis of the kidneys, while proteolytic enzymes are responsible for hemorrhagic and necrotic changes in the skin, as well as corneal destruction in some eye infections [182]. LAB possess significant bactericidal activity against pseudomonads, as the main antagonistic tools are LA and bacteriocins. Among fifteen LAB isolated by Okorhi et al. [183], 80% showed antagonist activity against Pseudomonas spp., including Lp. plantarum, Li. fermentum, L. acidophilus, Str. thermophilus and Lactococcus lactis. Table 4 presents a summary of the most notable examples of antibacterial activity shown by LAB, including Lc. paracasei FX-6, which is highly effective against Ps. putida [184], and Lc. rhamnosus, which inhibits the formation of biofilm by the same pathogen [170].
Table 4.
Antibacterial activity of Lactic acid bacteria against other bacterial toxigenic producers.
| Inhibited Pathogen |
LAB Species, Strain | Source | Agent | Mode of Action | References |
|---|---|---|---|---|---|
| C. perfringens | L. acidophilus CGMCC No. 1.1878, Li. fermentum CGMCC No. 1.2029 | Chicken | Lactic acid | Bacteriostatic effect on pathogen’s growth, repression of α-toxin synthesis, α-toxin degradation by lactobacilli, L. acidophilus inhibits C. perfringens adherence to GIT epithelium | [155] |
| B. cereus |
Lactococcus lactis, Lactobacillus spp. |
Skim milk, fresh cheese | Organic acids, H2O2, nisin | Bactericidal effect on pathogen’s growth by leakage of cytoplasmic content of the pathogen | [166,167,168] |
| B. cereus | L. acidophilus LF221 | Infant feces | Acidocin LF221 A and B | Bactericidal effect on pathogen’s growth | [169] |
| B. cereus | Lactococcus lactis C660, Lc. paracasei ATCC 27092 | Raw milk, human | Organic acids, H2O2, nisin | Reduced adhesion of the pathogen, prevention of biofilm formation | [170] |
| Pseudomonas spp. | Lp. plantarum, Li. fermentum, L. acidophilus, Str. thermophilus, Lactococcus lactis | Milk | Lactic, acetic, citric acids | Reduced growth | [183] |
| Ps. putida |
Lc. paracasei FX-6, Lc. rhamnosus |
Milk | Organic acids | Antibacterial activity, prevention of biofilm formation | [184] |
| S. aureus | Lactococcus lactis | Cheese | Lantibiotics | Reduced growth by cells disruption | [185] |
S. aureus is a non-spore-forming opportunistic pathogen producing cytotoxins, exotoxins, and exfoliative toxins [186]. It causes many skin infections such as boils, pimples, cellulite and osteomyelitis, impetigo, and abscesses, as well as life-threatening diseases such as endocarditis, pneumonia, meningitis, and septicemia [187]. Staphylococcal food poisoning causing gastroenteritis is accompanied by symptoms of sudden onset of nausea, vomiting, abdominal cramps, and diarrhea, caused by ingestion and absorption of enterotoxins previously formed in food [188]. Up to half of the human population carries this bacterium; in addition, it grows in a wide pH and temperature range (pH 4.2 to 9.3, T °C to 48.5 °C), and up to 15% NaCl. However, LAB can minimize its spreading in food mainly by the action of lantibiotics produced by Lactococcus lactis. Felicio et al. [185] used nisin with concentrations of 400 and 500 IU/mL against the growth of S. aureus in Minas Frescal cheese and nisin-producing strain Lactococcus lactis UL730 against the enterotoxigenic S. aureus J10 in fresh Moroccan cheese. Lp. plantarum and Lc. casei active against S. aureus were isolated from Indian traditional fermented product dosa [189,190].
4. LAB against Mycotoxins and Their Producers
4.1. Mycotoxins—Overview and Medical Relevance
Mycotoxins are low-molecular secondary metabolites produced by molds. Mycotoxicoses are examples of poisoning as a result of exposure (mostly dietary but sometimes respiratory or even dermal) to mycotoxins. They may be acute or chronic and generally affect more people in developing countries, where they can worsen the effects of vitamin deficiency and malnutrition [191]. Mycotoxins are absorbed in the upper parts of the GIT, but to a greatly different degree that varies between more than 80% (aflatoxins) to less than 10% (fumonisins) [192]. Many mycotoxins can permeate the skin, although not, it seems, in sufficient doses to cause serious health problems [193].
The number of currently known mycotoxins varies between sources, but it is probably between 400 and 500. They are extremely diverse chemically but, unlike many bacterial toxins, are not proteins. Most of them are produced by relatively few genera of fungi. Those most hazardous to human health are briefly described below in Table 5.
Table 5.
Most harmful mycotoxins that often contaminate human food.
| Type * | Genus | Foods | Clinical Picture | Molecular Mechanisms | References |
|---|---|---|---|---|---|
| Aflatoxin B1 (AFB1) | Aspergillus | Nuts, peanuts, maize | Extremely potent carcinogen, strongly linked with liver cancer; immunosuppression; stunted growth | Mutagenic and genotoxic effects: binds N7 of guanine; GC to TA transversions; (–) transcription, (+) oxidative stress | [191,194,195] |
| Ochratoxin A (OTA) | Aspergillus | Cereals, coffee, figs, raisins, pork kidneys | Nephrotoxic effects in all species tested; liver damage, immune suppression, and teratogenic effects in animals | (–) Phe metabolism; (–) mitochondrial ATP production; (–) tumor-suppressor gene dmrt-1 in mice; (+) lipid peroxidation | [195,196] |
| Zearalenone (ZEA) | Fusarium | Maize, corn, other cereals | Reduced fertility, stillbirths in females; testicular atrophy and reduced spermatogenesis in males; hemato- and hepatoxic effects | ZEA-estrogen receptor complex is translocated into the nucleus which regulates the transcription of many genes | [195,197] |
| Fumonisins | Fusarium | Maize, rice, beans, beer, soybeans | Suppression of the immune response; pulmonary edema, esophageal cancer | (–) Sphingolipid synthesis; (–) mitochondrial ETC; (+) ROS generation; (+) cytotoxicity | [191,195] |
| Trichothecenes | Fusarium, Cephalosporium, Myrothecium, Stachybotrys, Trichothecium | Grains: rice, barley, oats, maize, eggs, milk, meat | Alimentary toxic aleukia (ATA): fever, diarrhea, nausea, vomiting, agranulocytosis, necrotic angina, bleeding; reduced serum levels of WBC and Ig in mice | (–) Translation; (–) mitochondrial ETC; (+) lipid peroxidation and membrane remodeling; (+) apoptosis | [191,196,198,199] |
| Patulin | Penicillium | Apples, pears, other fruits | Neurotoxic and immunotoxic effects reported in animals | As yet unknown | [195] |
| Citrinin | Penicillium, Aspergillus, Monascus | Cereals, Italian sausages | Nephrotoxic effects in all species tested; reproductive toxicity and chromosome aberrations in mice | (–) DNA and RNA synthesis; (–) microtubules assembly; (–) HSP90 multichaperone complex; (+) ROS generation | [191] |
| Ergot alkaloids | Claviceps | Various grasses and grains | Ergotism, convulsions, ataxia, gangrene, abortion | As yet unknown | [191,195] |
* Trichothecenes mycotoxins are classified in groups A (T-2, HT-2); B (Deoxynivalenol, DON); C (Crotocin), and D (Verrucarins, Roridin, Satratoxins). Designations: (–), inhibits; (+), stimulates; WBC, white blood cells; Ig, immunoglobulins; ROS, reactive oxygen species; ETC, electron-transport chain; Phe, Phenylalanine.
Aflatoxins are the most important group of mycotoxins concerning human health. Over a dozen different aflatoxins are known, the four major ones being B1, B2, G1, and G2, classified according to their green or blue fluorescence under UV light. Aspergillus flavus and a few others from the same genus are the best-known producers of aflatoxins. Aflatoxin B1 (AFB1) is usually the major aflatoxin produced by toxigenic strains. It is one of the most potent carcinogens yet discovered, especially associated with liver cancer in chronic aflatoxicosis. Acute poisoning with aflatoxin is rare but could be fatal [191]. The death of 13 children in northwestern Malaysia in 1988 from acute hepatic encephalopathy and of at least 125 people (from 317 cases) in Kenya in 2004 were traced to Chinese noodles and homegrown maize, respectively, contaminated with aflatoxins [195].
Ochratoxin A (OTA) is comparable in importance to the aflatoxins, usually produced by many Aspergillus spp. and at least two Penicillium spp. (P. nordicum and P. verrucosum). It is often found in infected barley, oats, rye, wheat, coffee beans, and other plants of commercial value. OTA is a potent nephrotoxin to all animal species, associated with porcine nephropathy in Denmark and endemic nephropathy in Balkan countries such as Bulgaria, Romania, and the former Yugoslavia [191]. OTA half-life in humans can be as long as 35 days, considerably longer than in mice, pigs, or rats. Acute renal failure in humans has been associated with long-term exposure to ochratoxins in an agricultural setting [195,196].
Zearalenone (ZEA) is produced by Fusarium spp. and is most often found in cereals (especially maize). It is a structural analog of 17β-estradiol. Widely studied in various animal models (pigs, ruminants, mice), ZEA is best-known for its strong estrogenic and anabolic effects, to a lesser extent, hemato- and hepatotoxic effects. In pregnant women, long-term consumption of foods contaminated with ZEA presumably leads to reduced fetal weight and milk production; even changes in uterine tissue morphology have been suggested [195,197].
Among fumonisins, fumonisin B1 is the most prominent and the most toxic member of this group produced by Fusarium spp., which grows as corn endophytes. The toxins inhibit the synthesis of sphingolipids and cause various diseases depending on the species and the dose. In humans, fumonisins are strongly associated with esophageal cancer, especially in South Africa, China, and northeast Italy. Together with deoxynivalenol, fumonisins have also been implicated in the suppression of the immune response, for instance, significantly decreased levels of IL-8, IL-1β, IL-6, and macrophage inflammatory protein (MIP)-1β in piglets [200].
Trichothecenes are a large family divided into four groups. Groups A and B, produced by Fusarium spp., include all trichothecenes of major importance, namely T-2, HT-2, and deoxynivalenol (DON); groups C and D include less important members such as crotocin, verrucarins, and others. DON (aka vomitoxin or food refusal factor) may cause nausea, vomiting, and diarrhea in farm animals if ingested in high doses. Trichothecenes are commonly found in various grains (corn, barley, rye, wheat) and are strongly associated with alimentary toxic aleukia (ATA), whose acute phase is characterized by necrosis of the oral cavity, bleeding from various organs (nose, mouth, vagina), and CNS disorders. It was common in 19th-century Russia and the former Soviet Union, for instance, in the Orenburg district during the Second World War, when a large number of people got sick from eating overwintered grain infected with Fusarium [191,195,198].
Patulin was first isolated in the 1940s from Penicilium patulum (later renamed P. urticae and P. griseofulvum), tested as an antibiotic in the 1950s, and classified as a mycotoxin in the 1960s. Nowadays, patulin contamination most often comes from P. expansum, the blue mold that causes the soft rot of apples, pears, cherries, and other fruits [195].
Citrinin was originally isolated from P. citrinum, later also from a dozen of other Penicillium spp. (including P. camemberti of cheese fame) and several Aspergillus (such as A. oryzae used to make sake, miso, and soy sauce). Citrinin is a nephrotoxin in all species tested, although toxic doses vary greatly. It is found in many kinds of cereal as well as in some naturally fermented sausages in Italy [191].
Ergot alkaloids are a toxic cocktail found in the sclerotia of Claviceps spp., common pathogens on various grasses and grains, and are known, as well as the ergotism, from antiquity. It was a scourge in Europe during the Middle Ages when its two forms, gangrenous and convulsive, were responsible for high-mortality outbreaks; some 20,000 people were believed to have died from the disease only in the Aquitaine region in 944–945 AD. Though rare in humans nowadays, ergotism remains a major veterinarian problem [195].
4.2. LAB Detoxification of Mycotoxins
Mycotoxins are highly resistant to harsh conditions, including high temperatures during cooking, which makes them particularly difficult to be eliminated from contaminated foods. Crops may be contaminated with mycotoxins in the field, but this usually happens during prolonged and poor storage. No actual or precise figures about the worldwide loss due to fungal growth and mycotoxin production are available, but 25% of feed and food annually sounds like a reasonable estimation, which makes mycotoxins almost as much an economic threat as they are a health hazard [201].
Synthetic antifungal preservatives such as benzoate, sorbate, and propionate have been implicated in health issues ranging from irritability and inattentiveness to cancer and damage to the nervous system. LAB are safer and more desirable antifungal preservatives [202,203]. The antifungal properties of LAB are two major and essentially different types: (i) inhibition of fungal growth and (ii) neutralization of mycotoxins. Several studies have reported a broad spectrum of antifungal activity by many Lactobacillus spp. due to various organic acids production [204,205]. According to Lavermicocca et al. [206], PLA and OH-PLA synthesized by Lp. plantarum have anti-mold activity against Aspergillus, Penicillium, Eurotium, Endomyces, and Monilia as the minimum fungistatic concentration of PLA is 7.5 mg/mL, and the minimum fungicidal concentration is 10 mg/mL. These results are similar to the effect of caproic acid produced by Fr. sanfranciscensis CB1 against spoilage of bread by Fusarium, Monilia, Penicillium, and Aspergillus. PLA, ILA, and OH-PLA produced by Lp. plantarum and Lentilactobacillus buchneri have been shown to inhibit the growth of Penicillium nordicum and the synthesis of mycotoxins [207].
Besides antagonists to fungi, LAB are an antidote to mycotoxins. However, the exact mechanisms of this action remain elusive. The most studied is the adsorption of mycotoxins on the cell surface of LAB, where a complex network of teichoic and lipoteichoic acids, S-layer proteins, and exopolysaccharides plays a vital role in the process. The peptidoglycan layer has also been implicated. However, the binding capacity is highly variable: species- and strain-specific, greatly affected by pH and temperature, and mostly reversible [204,208]. Other mechanisms, such as the degradation of mycotoxins or their conversion to less toxic metabolites, are still waiting for proper experimental support [201]. Some remarkable feats of detoxification are summarized in Table 6.
Table 6.
Major studies of LAB-mediated mycotoxin-related detoxification.
| Target Toxin | LAB Strain | Mechanism of Action | Maximum effectiveness | References |
|---|---|---|---|---|
| Aflatoxin B1 | ||||
| L. amylovorus CSCC 5197 and CSCC 5160, Lc. rhamnosus Lc1/3 | Probable adsorption on the cell surface | >50% AFB1 bound from solution, but reversibly | [209] | |
| Lc. rhamnosus LBGG and LC-705 | None proposed | 80% removal from liquid media, very rapidly | [210] | |
| Lc. paracasei LOCK 0920, Lev. brevis LOCK 9044, Lp. plantarum LOCK 0945 | None proposed | 39–55% decrease, depending on the initial concentration of AFB1 | [211] | |
| Lactococcus lactis, Lp. plantarum | Low-molecular proteins involved, possibly bacteriocins | 81% combined, 27–46% separately | [212] | |
| L. kefiri KFLM3 | Toxin-binding on the cell surface | 80% decrease in milk, 0% in MRS | [213] | |
| Lev. brevis NM101-1, Lc. paracasei ABRIINW.F58 | Antifungal compounds caused 52–80% transcriptional inhibition of the omt-A gene, a key player in the biosynthesis of AFB1 | 90–96% reduction of the AFB1 production by A. flavus and A. parasiticus | [214] | |
| Levilactobacillus spp. 2QB383, Lp. plantarum 1QB147, 1QB314 and 3QB350 | Toxin binding is assumed for the reduced amounts; no mechanism proposed for the reduced production | >50% reduced amount by inactivated strains in PPB *; >50% reduced production in YES broth at 25 °C | [215] | |
| Ochratoxin A | ||||
| Str. thermophilus T4, L. delbrueckii subsp. bulgaricus LB-51 | None proposed | Complete elimination of 0.5 mg/L in milk; 36 and 26% drop with 1.0 and 1.5 mg/L | [216] | |
| L. bulgaricus 259/2 and 171/2 | None proposed | Up to 94% detoxification, but very much strain-dependent | [217] | |
| Lc. rhamnosus GG, L. acidophilus CH-5, L. helveticus 8, Lactococcus lactis 202 | Toxin binding on the cell surface is assumed, another mechanism hypothesized | 60–87% decrease, rapid process but partially reversible | [218] | |
| L. acidophilus VM 20 | Toxin-binding on the cell surface | 96–97% decrease for 4 h | [219] | |
| P. parvulus UTAD 473 | Degradation by putative peptidase | 100% degradation in MRS for 7 days at 30 °C | [220] | |
| Lb. kefiri KFLM3 | Toxin-binding on the cell surface | 81% decrease in milk, 15% in MRS | [213] | |
| Lc. rhamnosus CECT 749, Lp. plantarum CECT 749 and CECT 288, Lc. casei CECT 4045, Lc. casei CECT 4040, L. bulgaricus CECT 4005 | >90% degradation by proteolytic activity; very little adsorption | 97–99% in MRS at pH 6.5 | [221] | |
| Lp. plantarum 3QB361 | Toxin-binding on cell surface assumed | ~60% reduced amount by inactivated strain in PPB | [215] | |
| Patulin | ||||
| Lev. brevis 20023 | Adsorption on the cell wall | 65% adsorption | [222] | |
| Lp. plantarum ATCC 8014 | Adsorption on the cell wall, proteins mediated | 96% decrease in apple juice during 6 weeks of cold storage | [223] | |
| L. kefiranofaciens JKSP109 | Adsorption on the cell wall | 93% removal at pH 4.6 and 15° Brix | [224] | |
| Deoxynivalenol | ||||
| Lp. plantarum GT III | Adsorption assumed; metabolic degradation suggested | 67% reduction by unviable cells (sterilized) | [225] | |
| Lc. paracasei LHZ-1 | Cell wall adsorption confirmed as the major mechanism | 40.7% reduction by the cell wall fraction, only 10.5 & 8.9% by SN or cellular lysate | [226] | |
| Fumonisins | ||||
|
Lactococcus lactis, L. delbrueckii |
Toxin-binding on the cell surface | 75% recovery from spiked maize meal after 4 days | [227] | |
| Lp. paraplantarum CNRZ 1885, Str. thermophilus RAR1 | Toxin binding was assumed; the role of peptidoglycan confirmed | 19–37% bound FB1, 65–76% FB2, both after TCA treatment | [228] | |
| Zearalenone | ||||
|
Lactococcus lactis, L. delbrueckii |
Toxin binding assumed | 68% recovery from spiked maize meal after 4 days | [227] | |
| Lp. plantarum A1 | Toxin-binding on the cell surface | 99% immediately, 77% after 72 h | [229] | |
| Lb. kefiri KFLM3 | Toxin-binding on the cell surface | 100% decrease in milk, 60% in MRS | [213] | |
| Lactococcus lactis | Surface adsorption assumed, interactions with surface proteins and intracellular uptake | 90% bound in the first 20 min | [230] | |
| Lp. plantarum 3QB361 | Toxin-binding on the cell surface | 70–80% amount reduction by inactivated strain in PPB | [215] |
* Abbreviations: PPB, Potassium Phosphate Buffer; YES, Yeast Extract Sucrose; MRS, De Man, Rogosa and Sharpe medium; AFB1, aflatoxin B1; SN, supernatant; FB1 and FB2, fumonisins B1 and B2; TCA, Trichloroacetic Acid.
4.2.1. LAB against Aflatoxin B1 (AFB1)
AFB1 in cereals and cereal‐based products, to a lesser extent its less toxic but still dangerous metabolite AFM1 in milk and fermented milk products, remains a major global health problem for mycotoxins. Binding on the cell wall is the major mechanism by which LAB neutralize aflatoxins. Of 20 strains of LAB and bifidobacteria tested by Peltonen et al. [209], the most efficient were L. amylovorus CSCC 5160 and CSCC 5197, and Lc. rhamnosus Lc1/3. They were able to bind more than 50% of AFB1 from solution (5 μg/mL), 59.7, 57.8 and 54.6%, respectively, within 24 h. However, the binding was reversible. Upon incubation in toxin-free solution, various amounts of AFB1, 48.6, 30.7, and 26.5% for CSCC 5160, CSCC 5197, and Lc1/3, respectively, were dissociated from the bacteria and released back into the medium. Of the three Lactococcus strains studied, the most efficient proved to be Lactococcus lactis ssp. cremoris ARH74 with 41.1% binding of AFB1 [209]. Hence, favorable binding kinetics are necessary but, in itself, not a sufficient condition for a successful anti-mycotoxin probiotic. The cell count and the type of medium are important factors that may have a decisive influence. Lc. rhamnosus LBGG and LC-705 achieved 80% removal of AFB1 (5 μg/mL) from liquid media. The process was very rapid, reaching maximum in the very beginning and maintaining similar values for the next 72 h. Strains of L. gasseri, L. acidophilus, and Lc. casei were also tested, but their binding capacity was significantly lower and less consistent in time. Notably, however, even LBGG and LC-705 required very high cell densities, approximately 2 × 109 CFU/mL, for effective detoxification. This makes the strains somewhat unsuitable as toxin-protecting food additives [210].
Of 11 LAB strains isolated from kefir, L. kefiri KFLM3 proved to be the most potent in eliminating AFB1 (1 μg/mL). Toxin binding, the assumed mechanism, was reversible and very much dependent on the pH and the medium. The AFB1 binding capacity of L. kefiri KFLM3 improved from 0% in MRS to 80% in milk. The bacteria/mycotoxin complex was found to be more stable at pH 7–8 and more prone to dissociate at pH 3: 12 and 37%, respectively, of the bound AFB1 were recovered [213]. LAB strains isolated from Brazilian artisanal cheeses were able to reduce the AFB1 levels much more effectively in phosphate buffer (>80% for some) compared to milk (>50% for all). The binding was time- and pH-dependent as well and, on the whole, much more effective close to neutral levels (6.5) than in a highly acidic environment (pH 3.0) and slightly better for 5 than for 15 min [215].
While the probiotic design is difficult under such conditions, it has been attempted in specific settings. Lc. paracasei LOCK 0920, Lev. brevis LOCK 9044 and Lp. plantarum LOCK 0945 achieved dose-dependent detoxification of broiler feed: 55% when contaminated with a low concentration of AFB1 (1 mg/kg) and 39% when contaminated with a high concentration of AFB1 (5 mg/kg). These results were obtained after 6 h of fermentation and remained stable 12 and 24 h after adding the strains, which the authors finally evaluated as a promising probiotic supplement for broiler feed [211]. An innovative study of ten LAB strains isolated from Brazilian artisanal cheeses, most notably Levilactobacillus spp. 3QB398, Lp. plantarum 3QB350 and Lev. brevis 2QB422 were shown to inhibit the production of aflatoxins B1, B2, G1, and G2 by A. parasiticus. The authors found that the time of inoculation with the LAB strains, simultaneously with the fungus or 24/48 h later, was critical for inhibition of the AFB1 production. Curiously enough, on the whole, these LAB strains appeared to be least effective against the most important aflatoxin, AFB1. Nevertheless, there were some notable exceptions. Three Lp. plantarum strains, 1QB147, 1QB314, and 3QB350, were able to reduce AFB1 production by more than 50%. Levilactobacillus spp. 2QB383 was the only strain with something like 100% effectiveness: even when it was inoculated 48 h after the fungus, no detectable levels of AFB1 were observed [215].
At least two different mechanisms, the involvement of bacteriocins and transcriptional inhibition of aflatoxin production, have been proposed based on some experimental evidence. Mixed culture of Lp. plantarum and Lactococcus lactis achieved an 81% reduction of AFB1 (0.05 μg/mL) in MRS broth after only 6 h of cultivation, and that level remained stable for another 24 h. This was considerably better than both species separately (46% and 27%, respectively) or common food preservatives such as benzoic and propionic acids (39% and 6%, respectively). The authors speculated that bacteriocins are largely responsible for the effect because they obtained their best detoxification values (90%) with a crude protein extract filtered through a 1000-Da dialysis membrane [212]. One of the few studies to propose a more sophisticated mechanism of LAB action against mycotoxins was published by Gomaa et al. [214]. Of 38 Lactobacillus species isolated from dairy products, Lev. brevis NM101-1 and Lp. paracasei ABRIINW.F58 were selected for their conventional antifungal activity (i.e., growth inhibition). This was found to be due to an antifungal compound of protein nature which remained active within a large range of temperatures and pH but lost its inhibitory effect upon treatment with proteases. Most interestingly, these antifungal compounds caused significant inhibition on the transcriptional level of the omt-A gene, which encodes a key enzyme in the biosynthesis of AFB1. The effect was species-dependent, more pronounced with the compounds from Lev. brevis, which reached 80 and 64.5% inhibition of A. flavus and A. parasiticus, respectively. The antifungal compounds from Lc. paracasei were somewhat weaker but still reached 70 and 52% inhibition, respectively, of the omt-A gene in the same two Aspergillus spp. [214].
In regard to LAB and AFB1, it may be concluded that the suitable strains for effective detoxification are relatively few and need rigorous testing before they are approved as probiotics.
4.2.2. LAB against Ochratoxin A (OTA)
Adsorption on the cell wall of LAB appears to be the most predominant mechanism of detoxification of OTA [196]. Yogurt bacteria are capable of remarkable reduction of OTA content in milk. Str. thermophilus T4 and L. bulgaricus LB-51 achieved complete elimination of 0.5 mg/L OTA after 18 h of incubation; and 36 and 26% drop of OTA with concentrations of 1.0 and 1.5 mg/L, respectively. The strains were less effective separately, with 79% and 62% OTA removal for Str. thermophilus and L. bulgaricus, respectively. The authors reported a change in morphology in the lactobacilli (longer rods, thinner cell walls) at high OTA concentrations [216]. Another strain of L. bulgaricus, 259/2 was able to reduce OTA (with a concentration of 50 ppb) by 94% after 48 h incubation in MRS medium. However, other studies showed great variability in OTA binding by L. bulgaricus (6 to 34%), which implies great strain specificity [231]. Two strains of L. acidophilus (1A and 4A) were also able to reduce OTA by 46.5–32.7% [217]; and L. helveticus—by between 67.1 and 71.9% [217,231]. Notably, different authors used various OTA concentrations (50–1000 ppb) and media; therefore, the data comparison was difficult. For example, L. kefiri KFLM3 decreased OTA (1 μg/mL) by 81% in milk but only by 15% in MRS [213].
A very impressive degree of detoxification of OTA has been achieved by LAB in Douro wines. P. parvulus strains achieved 89–98% degradation of OTA (1 μg/mL) in MRS medium after 5 days of incubation at 30 °C with 103 CFU/mL. P. parvulus UTAD 473 reached 100% degradation of OTA under these conditions; 16 other LAB strains (mostly Lp. plantarum and Oenococcus oeni) also decreased OTA by 10–20%. The rate of the process was dependent on the inoculum size (almost five times faster with 109 CFU/mL) and the incubation temperature (~30% slower at 37 °C). The presence of ochratoxin α (a degradation product of OTA) was confirmed by LC-MS/MS, which suggested peptidase activity displayed by the strain. The study of OTA adsorption on P. parvulus cells was only 1.3%, thus suggesting that the main mechanism of detoxication by this strain is OTA degradation [220].
Another study of OTA degradation compared 27 commercial LAB strains cultivated in MRS contaminated with 0.6 μg/mL OTA for 24 h at 37 °C. The authors concluded that among the six strains that showed 97–99% total reduction of OTA at pH 6.5, hydrolysis was by far the predominant mechanism; only 2–4% were due to adsorption. Curiously, the hydrolysis was less effective in a more acidic medium (pH 3.5). Degradation products ochratoxin α and phenylalanine were confirmed by mass spectrometry [221].
Interestingly, a study of OTA reduction by L. bulgaricus also tested the ability of these lactobacilli to neutralize several different trichothecenes, such as nivalenol (1 ppm), deoxynivalenol (1 ppm), diacetoxyscirpenol (500 ppb), and T2 toxin (500 ppb), but no effect was observed [217]. The same lack of correlation between detoxifying capacities was demonstrated for OTA and patulin by Lactobacillus and Bifidobacterium [219]. On the whole, the efficiency of LAB as OTA scavengers is considerable and reinforces their role as probiotics with anti-mycotoxin action. However, as in the case of AFB1 detoxification, strains must be selected with great care regarding their capacity to neutralize OTA and their optimal conditions.
4.2.3. LAB against Patulin
In recent years, perhaps because of its easy availability on moldy fruits, patulin has attracted some notable attention in the field of LAB detoxification. An intriguing study with heat-inactivated LAB used methods such as Fourier Transform Infrared Spectroscopy (FTIR), Zeta Potential, and Contact Angle to confirm the importance of physical and chemical parameters such as specific surface area, cell wall volume, and N/C ratio for the binding capacity of patulin. Since CO-, OH-, and NH- were the main functional groups involved, probably polysaccharides and/or proteins are the crucial binding molecules. Among the studied LAB, Lev. brevis 20,023 was found to have the highest specific surface area, greatest cell wall volume, and, expectedly, highest capacity (65.02%) to adsorb patulin (4 mg/L) from aqueous solution [222]. Lp. plantarum ATCC 8014 achieved 96% patulin removal from apple juice during 6 weeks of cold storage after the juice was purposefully contaminated with 100 μg/L of the toxin. However, very high cell density was required (3.6 × 1011 CFU/mL), as well as the addition of prebiotic fructooligosaccharide (2.3%), ascorbic acid (213 mg/L), and citric acid (1.4 g/L). SDS-PAGE was used to confirm that S-layer proteins were involved in the adsorption of patulin. The electrophoresis showed a sharp decline in the amount of a 50-kDa fraction on the first day of incubation, which is in agreement with the kinetics of patulin decrease: almost 70% on the first day, a much slower but steady decrease until the 42nd day [223]. A recent study used LAB from Tibetan kefir grains for the detoxification of apple juice and went into some detail about the adsorption mechanism. FTIR was used to establish the most important functional groups, and while the result (C–O, OH, C–H, N–O) was somewhat different from the study mentioned above [222], the authors reached the same, admittedly rather general, conclusion: Proteins and polysaccharides on the cell surface must be responsible for the patulin adsorption. Of the five strains tested, L. kefiranofaciens JKSP109 was the finest patulin scavenger—93% at 100 μg/L but only 56% at 200 μg/L. The adsorption capacity was found to depend on pH and the °Brix, in which the higher, the better in both cases [224].
4.2.4. LAB against Deoxynivalenol (DON), Fumonisins, and Zearalenone (ZEA)
DON has been a somewhat unpopular research subject in the last few decades, which is surprising considering its prevalence in cereal crops. According to some studies, 65% of the maize kernels harvested in France from 2004–2006 were contaminated with DON and fumonisins; another study of corn samples from several European countries found that 52 of 67 contaminated samples (78%) contained DON and while only two of them exceeded the EU recommended values (8 mg/kg in grain and grain products), six others exceeded 1 mg/kg; concentrations from 100 to 1000 μg/kg appeared to be quite common in Europe [192,232]. A couple of recent studies have dealt with LAB as DON detractors in a somewhat illuminating way.
Altogether 16 LAB strains, eight commercially available in probiotic formulae (e.g., Lyofast LPRA, Yo-flex YC-180), and eight isolated from cereals and kefir (mostly Lp. plantarum), were tested for anti-fungal activity and DON reduction. Six of them significantly inhibited the growth (agar halos bigger than 30 mm in diameter) of Fusarium graminearum JAPAR 2218, a confirmed DON producer and an economic scourge for grain crops worldwide. DON reduction studies were conducted with 1.5 μg/mL toxin in MRS for 4 h in a volume of 2 mL with average cell densities of 1010 CFU/mL and three types of bacteria, viable and heat-inactivated. In all cases, the sterilized cells showed a better ability to reduce DON, usually 20–30% higher than that of the viable cells. However, the best in DON detoxication Lp. plantarum GT III (67% decrease), was not the most potent fungicide (27 mm halo) [225]. Lc. paracasei LHZ-1 isolated from yogurt achieved a 40.7% reduction of DON (50 μg/mL) by the cell wall fraction in PBS for 24 h at 37 °C. In contrast, only 10.5% and 8.9% were reduced by culture supernatant or cellular lysate, respectively. Laser scanning confocal microscopy was used to elucidate further the mechanism of DON detoxification. DON was labeled with AMCA-X SE to produce blue fluorescence and thus obtained visual evidence that DON does form complexes with the bacterial cell wall. As in the aforementioned study [225], pasteurized and sterilized cells removed DON more efficiently than viable cells, only in this case, the increase was only 5–6% at most.
After DON, fumonisins are the next most prominent contaminants of food and feed [232]. It was found that LAB starter culture (Lactococcus lactis, L. delbrueckii) added to a maize meal could reduce the levels of fumonisin B1 (2 μg/g meal) by almost 75% for 4 days. This fermented meal was comparatively less toxic to SNO human esophageal carcinoma cell line, but the difference was not significant. The authors perceptively note that the reduction of the toxin level may not necessarily result in reduced toxicity because the LAB fermentation does not alter the bioavailability of the toxin. Chronic complications from trace amounts of the toxin remain a potential problem [227]. Another study provided some insight into the exact components of the LAB cell wall that bind fumonisin B1 and B2 (FB1, FB2). The importance of peptidoglycan (PG) was confirmed in two different ways. Mutants with defective PG layer displayed decreased toxin binding, which affected only FB2, and only with 20–25%. Purified PG bound fumonisins (5 μg/mL each) in a similar, but somewhat lower, degree to LAB (20% for FB1, 60% for FB2, both at 2 mg/mL PG). Mutants with a defective synthesis of lipoteichoic acids showed negligible difference (5–10%) compared to the wild type, indicating that this component of the cell wall is unimportant as far as fumonisin binding is concerned. The tricarballylic acid chains of the fumonisins were confirmed to be essential for the toxin-binding, which decreased when the chains were hydrolyzed. The authors also claimed that treatment with lipases and proteases had no effect on the toxin binding, and neither did the use of mutants lacking exopolysaccharides [228].
Zearalenone (ZEA) has been reported in foods and body fluids (animal as well as human) with an alarming frequency [197]. As in the cases of AFB1 and OTA, a great deal of work has been done on LAB detoxification of ZEA, but the molecular mechanisms remain elusive. One promising probiotic of the future against ZEA is Lp. plantarum A1, a strain with a potent and rapid ability to bind ZEA (20 μg/mL). The process was partially reversible, dropping from immediate 99% to 77% after 72 h cultivation in MRS broth, but the relatively small inoculum (108 CFU/mL) was a point in the strain’s favor [229]. Similar kinetics were obtained with Lactococcus lactis isolated from milk products and 130 μg/mL ZEA, although in this case, the process appeared to be virtually irreversible [230]. LAB starter culture (Lactococcus lactis, L. delbrueckii) added to a maize meal reduced the levels of ZEA (2 μg/g meal) by 68% for 4 days; as in the case of FB1, the decreased toxicity on the SNO cell line was not significant [227]. L. kefiri KFLM3 achieved a 100% decrease of ZEA (1 μg/mL) in milk, but only 60% in MRS, yet another reminder of the importance of the medium [213]. Lp. plantarum 3QB361, isolated from Brazilian cheese and inactivated in phosphate buffer, managed to reduce ZEA (2 μg/mL) with 70–80% at pH 6.5, but five other strains (from ten tested) hardly managed 20–40%—another timely reminder, this time of species- and strain-specificity [215].
5. Lactic Acid Bacteria for Reducing Pesticide Levels in Food
The toxic effects of various pesticides in humans include neurotoxicity, skin irritation, carcinogenesis, and endocrine disruption [15]. Among the symptoms are abdominal pain, nausea, vomiting, diarrhea, headache, lethargy, tremor, muscle spasm, coma, kidney insufficiency, upper airway and mucous membrane irritation, tachycardia, weakness, acidosis, hypotension, ataxia, hypertonia, etc. [4]. For instance, the mechanism of action of organophosphate pesticides is to inhibit acetylcholinesterase, which leads to an impaired connection between acetylcholine and its receptor in nerve and muscle cells. The toxicology research also shows that pesticide exposure induces oxidative stress and DNA, protein, and lipid damage, followed by adverse health and psychological effects [16,233].
There are several types of pesticides according to their chemical structure: organochlorine, organophosphorus, neonicotinoid, benzimidazoles, carbamates, and synthetic pyrethroids. Organochlorine pesticides, also known as “contact” insecticides, can be accumulated in fatty tissues and milk. They are highly persistent in the environment, and for this reason, their application in most countries is prohibited. The oldest and best-known organochlorine is the insecticide DDT (1, 1, 1-trichloro-2,2- bis (4 -chlorophenyl) ethane); it is a usual contaminant of hen eggs and milk products. Common organophosphate pesticides are chlorethoxyfos, chlorpyrifos, and diazinon, esters of ortho-, thio-, and pyro-phosphoric acids. Organophosphate pesticides act as acaricides but primarily as insecticides. They are highly toxic to bees, wildlife, and humans. Urea Pesticides are another class of herbicides; they are inhibitors of photosynthesis in plants. The most commonly applied among them are isoproturon, chlortoluron, and fluometuron. Other herbicides are dinitroaniline pesticides (trifluralin; pendimethalin; oryzalin; prodiamine; ethalfluralin; benfluralin). The organophosphate pesticides can be found in foods such as milk and yogurt, wheat flour, cabbage, eggplants, cucumbers, maize, and tomatoes. Carbamates are selective herbicides, insecticides, acaricides, nematicides, molluscicides, or fungicides in fruits and vegetables. The most widespread carbamate pesticide, aldicarb, found in high concentrations in watermelons, caused food poisoning that affected more than 2000 people in the USA in 1985 [234]. Quaternary ammonium salts (paraquat; diquat; chlormequat) are the most toxic of all insecticides or herbicides. Nearly 25% of the global market for insecticides is occupied by the class of neuro-active insecticides neonicotinoids (chemically similar to nicotine).
Many LAB of Lactobacillus and Leuconostoc genera can metabolize a broad spectrum of synthetic insecticides and use them as carbon and energy source. The mode of action is through their esterase and phosphatase enzymes [13]. DDT degradation (1 ppm in milk and cheese) by Str. thermophilus and L. bulgaricus was shown by Abou-Arab two decades ago [235]; however, the drop was only 10.8–11.8%. Later, La. sakei pro7 isolated from soil reached 95.1% biodegradation of DDT with a concentration of 20 ppm [236]. The following LAB able to convert chlorpyrifos were isolated from kimchi: Leuc. mesenteroides WCP907, Lp. plantarum WCP931, La. sakei WCP904, and Lev. brevis WCP902 [237]. The last strain consumed 83.3% of 30 mg/l of the pesticide in 3 days and completely assimilated it after 9 days. In search of the molecular mechanisms of degradation, the responsible opdB gene was determined, cloned, and the relevant enzyme OpdB (274 amino acids) was purified. It contains the “Gly-X-Ser-X-Gly” motif typical for bacterial organophosphorus hydrolases and is a member of the esterase family [238]. Kimchi LAB strains are also known to degrade coumaphos, diazinon, methylparathion, and parathion [237]. Recently, Maden and Kumral [239] investigated the degradation of insecticides in sauerkraut samples with or without the presence of lactic acid bacteria during fermentation. Lp. plantarum 112 (previously isolated from olive brines, 109 CFU/mL) contributed for malathion (2 mg/kg) and chlorpyrifos-methyl (4 mg/kg) degradation. However, the decrease of λ-cyhalothrin was low. The same team [240] tested Lp. plantarum strains for pesticide removal in the course of black olive fermentation. At the end of fermentation (after 60 days), 61% of deltamethrin, 68% of dimethoate, and 50% of imidacloprid were removed by Lp. plantarum 123. Significant success was achieved in the detoxification from synthetic pyrethroids. Dorđević et al. [241] underlined the role of LAB in bifenthrin removal from wheat flour; then Lp. pentosus 3–27 was applied for the successful elimination of beta-cypermethrin from silage [242]. The strain degraded 96% of the pesticide with a concentration of 50 mg/L. LAB species and strains capable of removing pesticides from foods are shown in Table 7.
Table 7.
Detoxification of pesticides falling in food content by lactic acid bacteria (LAB).
| Pesticide | LAB Species/Strain | Sample/Food | Mode of Action | References |
|---|---|---|---|---|
| Organochlorine | ||||
| DDT | Lactobacillus spp. | Cereals | Phosphotriesterase | [13] |
| DDT | Streptococcus, Lactobacillus | Ras cheese | Biodegradation | [235] |
| DDT | La. sakei | Soil | Biodegradation | [236] |
| Organophosphorus | ||||
| Chlorpyrifos, coumaphos, diazinon, parathion, methyl parathion | Leuc. mesenteroides WCP907, Lev. brevis WCP902, Lp. plantarum WCP931, La. sakei WCP904 | Kimchi | Biodegradation | [237] |
| Chlorpyrifos, coumaphos, diazinon, parathion, methyl parathion | Lev. brevis WCP902 | Kimchi | Organophosphorus hydrolase OpdB | [238] |
| λ-Cyhalothrin, malathion, chlorpyrifos-methyl | Lp. plantarum 112 | Sauerkraut | Low pH | [239] |
| Deltamethrin, dimethoate, imidacloprid | Lp. plantarum 112,Lp. plantarum 123 | Black olives | Biodegradation | [240] |
| Pirimiphos-methyl | Lp. plantarum | Wheat | Organophosphorus hydrolase, low pH | [243] |
| Chlorpyrifos, dichlorvos, phorate, trichlorphon | Lp. plantarum | Wheat dough, Chinese cabbage, Tofu | Biodegradation | [244] |
| Dimethoate, parathion methyl, trichlorfon | Lp. plantarum subsp. plantarum CICC 20261 | Batch process | Phosphatase and Antioxydation | [245] |
| Phorate | Lp. plantarum | Corn silage | Enzyme hydrolysis | [246] |
| Diazinon | L. acidophilus | Apple juice | Enzyme hydrolysis | [247] |
| Diazinon, chlorpyrifos, fenitrothion, malathion | Lev. brevis 1.0209 | Milk | Enzyme hydrolysis | [248] |
| Pyrethroids | ||||
| Bifenthrin | Lp. plantarum | Wheat flour | Enzyme hydrolysis | [241] |
| Beta-cypermethrin | Lp. pentosus 3-27 | Alfalfa Silage | Enzyme hydrolysis | [242] |
As summarized by Mohammadi et al. [249], the most common mechanism of pesticide elimination by LAB is the enzymatic hydrolysis by carboxylesterases, organophosphate hydrolases, phosphotriesterases, and phosphatases. That is why LAB are potent detoxifiers of food from organochlorine, organophosphorus, and pyrethroids, but there is no evidence that they can degrade carbamate pesticides. However, some species of lactobacilli, such as the sourdough isolate Fru. sanfranciscensis DSM 20451T are highly resistant to the carbamate paraquat [250], while others (such as the probiotic Li. fermentum) have been used successfully to alleviate oxidative stress in piglets caused by diquat [251].
6. LAB against Heavy Metals Intoxication
Foodstuff can also be contaminated with other toxic and non-degradable elements such as heavy metals. Heavy metals are defined as metallic elements with a density above 5 g/L [252]. Most metals toxic to human health are considered cadmium (Cd), lead (Pb), mercury (Hg), arsenic (As), and chromium (Cr) [253,254]. However, even in low concentrations, many physiologically essential for the human body heavy metals such as iron (Fe), zinc (Zn), copper (Cu), cobalt (Co), manganese (Mn), etc., can also be hazardous [254]. Sources of heavy metal pollution are several industries [252], pesticide and veterinary drug residues [255], packaging materials [256], technological incidents, and many others, which contaminate foodstuff and drinking water directly or by distribution in the environment and slow accumulation in food chains through polluted agricultural soils or intoxicated aquatic animals [257]. In case of prolonged ingestion, heavy metals accumulate in the human body, adversely affecting the nervous, cardiovascular, and reproductive systems, causing renal and lung diseases, hepatic damage, skin problems, and bone demineralization [258,259,260,261,262,263]. Moreover, most heavy metals are defined as carcinogenic (e.g., As, Cd, Cr) or possibly carcinogenic (e.g., Pb) to humans [264]. In oral intoxication, the gastrointestinal tract is the first organ where metals are absorbed [263], but once in the bloodstream, they accumulate mainly in the kidney and liver [265,266]. In addition, long-term exposure to both Cd and Pb disrupts calcium homeostasis and causes mitochondrial damage [267] and oxidative stress leading to lipid peroxidation [268] and DNA fragmentation [269]. On the other hand, arsenic, as a proven genotoxic agent, can cause skin, lung, and kidney cancer in case of prolonged intoxication [270].
Current methods for metal detoxification are divided into physical, chemical, and biological [271]. The most employed techniques for metal removal from contaminated industrial areas, waters, and the environment are chemical precipitation, ion exchange, membrane filtration, and solvent extraction [272,273,274]. Detoxification of heavy metals in vivo has been achieved with various chelating agents such as dimercaptosuccinic acid (DMSA) and ethylenediaminetetraacetic acid (EDTA), which promote the excretion of heavy metals [252]. However, the described chemical methods for both in vitro and in vivo metal detoxification have serious drawbacks limiting their application. The chemical methods designed to detoxify the environment are extremely expensive or ineffective and generate additional toxic waste [275,276,277]. On the other hand, detoxification by chelators is effective but not suitable for prolonged treatment [278] due to safety concerns [279,280].
Biological methods based on biosorption of metals or metal-containing compounds by plants [281], algae [277,282], bacteria [283], and fungi [284] are promising options due to their high efficiency and specificity, lack of side effects, and low investment cost [285]. The biosorbents have the capacity to decrease heavy metal concentration from ppm to ppb levels in aqueous solutions [277]. Although the process of metal biosorption is initially well studied in other organisms (e.g., Aspergillus spp., Penicillium spp., Bacillus spp., Saccharomyces cerevisiae, etc.), the use of LAB as a biosorbent is of particular interest, due to their GRAS status and probiotic nature. Moreover, LAB could be easily added to the diet in an attempt to alleviate heavy metal intoxication in the human body.
As with other organisms, the capacity for metal removal by LAB is strain-specific and depends on many factors such as cell surface content, protein production, pH of the environment, temperature, type of metal element, and both cell and metal concentrations [254,286]. The overall process consists of two distinct mechanisms: binding of metals to the bacterial cell wall by electrostatic interaction (biosorption) and passage of metal ions through the cell membrane and accumulation inside the cell (bioaccumulation) [256]. The former mechanism is fast and metabolically independent; the latter is slow, requires metabolic activity, and takes place only when biosorption has reached its limit [287].
As Gram-positive bacteria, LAB possess on their cell wall surface a thick layer of peptidoglycan, teichoic acids, and S-layer proteins, which play a key role in the ability to bind and sequester metals by ion-exchange reactions [288]. LAB have a negatively charged surface and are suitable for binding cations such as Hg2+, Cd2+, Pb2+, etc. [289]. Moreover, LAB strains, which produce exopolysaccharides, have additional negatively charged groups on their surface (carboxyl, hydroxyl, phosphate) and an additional number of ligands for binding metal cations [290]. When the negatively charged group on LAB cell wall surfaces, such as carboxyl, phosphoryl, or carboxylate, are neutralized, the binding properties of the bacterium sharply decrease [291,292,293]. Environmental pH is another factor that strongly affects biosorption in LAB. Adsorption of Cd and Pb is very low at pH ≤ 3.0 and gradually increase above 3.0 to reach its maximum between pH 4.0 and pH 6.0 [252]. For example, the best rates of metal removal by Li. fermentum ME3, Lc. rhamnosus GG, and L. acidophilus X37 were observed at pH 6.0, for L. bulgaricus—at pH 5.0 [271]. Likewise, the production of specific proteins appears to be of vital importance for LAB biosorption. Kinoshita et al. [286] identified ~14 kDa mercury-binding protein from the cell surface of Weissela viridescens MYU 205. This protein contains the “CXXC” motif (as “X” is any amino acid), which is a well-known heavy metal-binding motif contained in various proteins with the confirmed binding ability of Cd2+, Co2+, Cu2+, and Zn2+ [294,295].
Since biosorption is not a metabolically connected mechanism, LAB can be used for metal removal both in viable and non-viable conditions. Several authors suggest that living cells have a higher binding capacity than dead ones [296,297,298]. Tian et al. [298] studied the binding capacities to cooper of 16 different LAB strains, and the results showed that all of them have a higher binding capacity as living cells. In another study, however, Lp. plantarum PTCC 1896 showed an increased biosorption of Cd2+ and decreased—of Pb2+ when the cells were heat-killed [299]. W. viridescens MY 205 showed decreased removal of Cd2+ and Pb2+ but increased—of Hg2+ after cells’ heat inactivation [300]. A probable explanation of these results is that the change in sorption capacity after inactivation might be dependent on both the strain and type of metal. Nevertheless, LAB could be applied successfully for heavy metal detoxification as a viable culture without losing their probiotic characteristics.
For selection as suitable biosorbents, LAB strains are tested for their metal-resistant and metal-removal abilities. To assess the metal resistance of the strain is used the term minimum inhibitory concentration (MIC), which is the lowest metal concentration that completely inhibits the growth of the strain [301]. LAB strains displayed a wide spectrum of MIC values. Bhakta et al. [302] tested 26 LAB strains for Cd- and Pb-resistance and reported that the MIC values for Cd are in the range from 50 to >1000 mg/L for the different strains, while for Pb are >2000 mg/L for all strains tested. It is considered that strains with MIC values exceeding those of the control organism E. coli K-12 (e.g., MIC >100 mg/L for Cd and MIC > 1600 for Pb) are tolerant to the respective metal [303]. This indicates that, in general, LAB strains are relatively resistant to heavy metals.
The metal-removal abilities of LAB have been confirmed in many in vitro and in vivo studies. The biosorption capacity is strain-specific, and rarely is a strain a good sorbent of many different metals. According to Kinoshita et al. [286], LAB exhibited the following order of preferential sorption in regard to the most toxic metals: Hg2+ > Cd2+ > Pb2+ = As3+. However, the same authors concluded that Hg most strongly inhibits bacterial growth due to its higher toxicity. On the other hand, LAB strains possess notably high levels of resistance to Pb, which allows the sorption performance at higher metal concentrations [255,299,304,305]. Contrariwise, the biosorption of arsenic can be implemented only at comparably low initial metal concentrations [289,306]. In addition, other potentially hazardous metals, such as Cu, Fe, and Zn, can be adsorbed very successfully by LAB strains in vitro [307,308]. The most successful metal biosorptions in vitro by living LAB strains are listed in Table 8.
Table 8.
In vitro biosorption of heavy metals by living LAB and Bifidobacterium strains.
| Heavy Metal | Biosorbent | Initial Metal Concentration (mg/L) |
Metal Removal (%) |
Metal Removal Capacity (mg/g Dry Biomass) |
References |
|---|---|---|---|---|---|
| Hg | Weissella viridescens MY 205 | 1 | 79.6 | [300] | |
| Cd | Propionibacterium freudenreichii shermanii JS | 50 | 49.1 | [305] | |
| L. acidophilus ATCC 20552 | 50 | 65.5 | [304] | ||
| Lc. rhamnosus, L. acidophilus, Bifidobacterium longum | 43 a,* | 48.0 | [310] | ||
| Bifidobacterium longum 46 | 10 | 54.7 | [314] | ||
| Ent. faecium EF031 | 10 | 97.5 | [315] | ||
| Lp. plantarum PTCC 1896 | 10 | 90.9 | 122.7 b,c | [299] | |
| Lp. plantarum CCFM8610 | 5 | 77.0 | 3.85 | [311] | |
| Li. reuteri Cd70-13 | 1 | 25.0 | [302] | ||
| P. acidilactici As105-7 | 1 | 0.13 d | [306] | ||
| W. viridescens MY 205 | 1 | 54.1 | [286] | ||
| Pb | Lp. plantarum LAB-32 | 200 | 82.25 | 57.31 b | [255] |
| Lp. plantarum PTCC 1896 | 50 | 65.4 | 34.5 b,c | [299] | |
| L. acidophilus ATCC 20552 | 50 | 72.6 | [304] | ||
| Propionibacterium freudenreichii shermanii JS | 50 | 69.9 | [305] | ||
| Li. reuteri Pb71-1 | 6 | 59.0 | [302] | ||
| P. acidilactici As105-7 | 6 | 0.76 d | [306] | ||
| As | L. acidophilus | 1 | 60.0 | [316] | |
| L. acidophilus ATCC 20552 | 0.5 | 49.8 | [304] | ||
| Lc. casei DSM20011 | 0.1 | 38.1 | 0.312 c | [289] | |
| P. acidilactici As102-4 | 0.1 | 0.006 d | [306] | ||
| Al | Lp. plantarum CCFM639 | 50 | 26.83 | [312] | |
| Cu | Ent. faecium | 250 | 106.38 c | [308] | |
| Lentilactobacillus buchneri DSM 20057 | 40 | 46.17 c | [317] | ||
| Lev. brevis | 20 | 26.5 c | [318] | ||
| Fe | L. bulgaricus Lb-12 | 100 | 99.3 | [307] | |
| Str. thermophilus STM-7 | 100 | 100.0 | [307] | ||
| Zn | L. bulgaricus Lb-12 | 100 | 90.2 | [307] | |
| Str. thermophilus STM-7 | 100 | 92.8 | [307] | ||
| Leuc. mesenteroides | 20 | 27.10 c | [319] | ||
| W. viridescens MY 205 | 1 | 20.0 | [300] |
* Designations: a, estimated from 70 ppm CdCl2; b, mg removed metal per gram wet biomass; c, maximum removal capacity, calculated from Langmuir isotherm; d, metal removal efficiency (mg removed metal per hour per g wet biomass).
Over the last decade, many in vivo studies have revealed that LAB strains (especially Lactobacillus spp.) have a remarkable influence on the heavy metal intoxicated human body. Orally taken by fermented foods consumption, they can detoxify different organs and tissues [288]. Thus, the prolonged intake of yogurt with concentrated cell culture of Lc. rhamnosus GR-1 can prevent further increment of Hg and As blood levels of pregnant women subjected to chronic exposure [278]. In experiments with mice, the addition of Lp. plantarum CCFM8610 and L. bulgaricus CCFM8004 in soymilk have a protective effect against chronic Cd exposure [309]. Jama et al. [310] used a combination of Lc. rhamnosus, L. acidophilus, and Bifidobacterim longum against Cd-induced genotoxicity in rats and succeeded in reducing it by 20%. Likewise, different Lp. plantarum strains are successfully applied for Cd sequestration in mice intestines [311], reduction of Pb levels in mice blood and tissues [296], and reduction of Al and Cu levels in mice livers, brains, and kidneys [298,312]. The authors connected the LAB antioxidative properties with the complex action on the metal intoxicated body.
In addition to metal adsorption in tissues, LAB also alleviate oxidative stress [311], protect the intestinal barrier [313], prevent losses of essential metals [298,311], and finally, remove metals from the body by defecation [286].
LAB can be successfully used for the metal detoxification of foods and drinks. For example, treatment with Lp. plantarum CCFM8610 removed up to 82% of the Cd from nine types of fruit and vegetable juices [320].
In conclusion, LAB have a high potential as a biosorbent of heavy metals both from foodstuff and from the intoxicated human body. LAB can be easily applied as a biosorbent in the form of food additive, especially in fermented foods and drinks, providing a comprehensive reduction in damage from metal intoxication.
7. LAB in Detoxication of Food from Natural Antinutrients
Leaf vegetables, legumes, and cereals food contain antinutrients—natural compounds that interfere with the absorption of nutrients. They are toxic or are a platform for toxic compound synthesis during their degradation in the human body. Examples of antinutrients include phytic acid, cyanogenic glycosides, oxalates, and protease inhibitors. Other chemicals considered to be antinutrients are toxic only in certain cases of insufficiently processed foods (lectins). Some of them have a controversial role in the human body (for example, phenolic acids have an antioxidant effect) and, so far, cannot be attributed to the group of antinutrients [321]. Phytate is one of the most studied antinutrients, as it can chelate various nutrients and reduce their bioavailability. It causes mineral deficiencies because it inhibits the absorption of zinc and iron in human GIT [322]. LAB fermentation is a good approach to diminish the adverse effect of phytate-rich cereals such as pearl millet and maize, but also other cereals and pseudocereals. These foods are a source of LAB displaying phytase activity, for instance, Lp. plantarum and Li. fermentum isolated from the fermented teff meal injera and the pearl-millet fermented gruel ben-saalga [323]. According to Sharma et al. [324], both L. amylovorus and Lp. plantarum from sourdough show high phytase activity: 125–146 U/mL and 60–74.4 U/mL, respectively. Traditional Asian sourdoughs for dosa and idli (made of rice and black gram dhal) are subjected to natural lactic acid fermentation for at least 20 h for reduction of the phytates content [324]. Castro-Alba et al. [325] fermented quinoa, amaranth, and canihua with Lp. plantarum 299v, thus reducing phytate concentrations by 47–51%, 12–14%, and 25–27%, respectively. However, the presence of some phytates and tannins in food and tea may decrease the bioaccessibility of mercury and prevent heavy metals poisoning [258].
Cyanogenic glycosides are the substrate that releases the respiratory inhibitor hydrogen cyanide after hydrolysis in the human organism. HCN is in lethal dose if consumed in an amount higher than 3.5 mg per kg body weight. Plant foods contain about 25 cyanogenic glycosides, such as linamarin (cassava, white clover, flaxseed), dhurin (in all sorghum cereals), prunazine, and amygdalin (apples, apricots, plums, almonds, cherries). Amygdalin in apricot kernels reaches 17.5 mg/g. Apple seeds can contain up to 4 mg/g of this glucoside, and that is why commercial apple juice usually contains 0.1 mg/mL of amygdalin [326]. LAB converting cyanogenic glycosides are relatively rare: among 25 strains representing 23 species of LAB screened by Menon et al. [327], only Lp. plantarum and Lp. paraplantarum grew well and degraded amygdalin, similarly to Lei et al. [328]. Linustatin, neolinustatin, and linamarin found in linseed were destroyed by L. acidophilus, reaching a 66% reduction in the total amount of cyanogenic glycosides. L. delbrueckii starter cultures were used for cyanide detoxification of Tanzanian cassava meal Mchuchume by decreasing cyanogenic glycosides from 72.72 to 5.18 mg/kg [329]. In all these cases, as during the LAB fermentation of bamboo [330] or hemp sourdough made by the use of Lp. plantarum, P. acidilactici, and Leuc. mesenteroides starter [331], a significant decrease in the concentration of phytic acid, tannins, and saponins, was observed during fermentation.
Oxalates—salts of oxalic acid occur naturally in many plants. In addition to being consumed, oxalates are also obtained in the human body as waste from the breakdown of food. Various (otherwise useful foods) are high in oxalates: leafy greens and legumes. The danger of consuming many oxalates comes from their ability to bind calcium, thus increasing the risk of kidney stones in some people. Oxalates consumption is linked to pathologic conditions such as hyperoxaluria, urolithiasis, renal failure, cardiomyopathy, and cardiac conductance disorders [332]. Several LAB species can degrade oxalates in vitro and in vivo. L. acidophilus breaks down 11.8% of 10 mM ammonium oxalate, while Str. thermophilus—2.3% [333]. Other species reducing oxalate absorption in GIT are Lev. brevis, Lc. casei, L. gasseri, L. salivarius, Li. fermentum, Weissella confusa, and W. cibaria [334]. Azcarate-Peril et al. [335] showed that frc and oxc genes encoding functional oxalate-degrading enzymes were identified in L. acidophilus NCFM and L. gasseri AM63T. However, one of the strongest oxalates destroyers is Ent. faecalis, which is “oxalotroph” and uses oxalates as a sole carbon source [336]. Hokama et al. [337] found that the oxalate-degrading Ent. faecalis produces three unique proteins involved in the oxalate degradation. Murru et al. [333] reported that Lp. rhamnosus GG diminishes the oxalates content of food in vitro. Currently, a number of probiotics are developed to prevent calcium oxalate urolithiasis [338].
Other substances classified as antinutrients are amylase/trypsin inhibitors or ATI. They are small, cysteine-rich proteins involved in the wheat defense system against insects and fungi. They are classified as antinutrients as they cause non-celiac wheat sensitivity (NCWS), an immunological disorder that shares the symptoms of celiac disease and irritable bowel syndrome [339]. LAB can hydrolyze ATI, as shown in vivo in mice [340]. Strains that showed the highest ATI-degrading activity are Ligilactobacillus salivarius H32.1, Li. mucosae D5a1, and Lc. rhamnosus LE3, as well as the sourdough, isolates Fru. sanfranciscensis, Li. reuteri, and La. sakei [341].
8. Conclusions
The 21st century is associated with food shortage, global warming, and increasing pollution of waters and soils. For instance, due to climate change, the aflatoxins that were common in hot and humid regions until now are expected to increase their deadly presence as major contaminants in European foods. The contemporary hopes of the food industry are in environmentally friendly approaches to food detoxification.
Although the action of LAB species as detoxifiers is usually limited to a specific toxicological agent, there are strains capable of significant and complex reduction in the amount of several toxic ingredients in food. Considering the wide spectrum of toxic food substances, LAB are most effective against mycotoxins and bacterial toxigenic producers. LAB’s major mechanisms are neutralizing the toxins, in one way or another, by metabolic degradation or biosorption, for example, and/or inhibiting the growth of the producers themselves. LAB are capable of enzymatic hydrolysis of several types of pesticides. The data in this direction are impressive and promising, and we may express certain hope for even better results in the future. The case of LAB against various antinutrients is likewise hopeful, although to a lesser degree. Concerning the detoxification of heavy metals, LAB act on the one hand preventively, purifying the soil and water used for food production, and on the other hand, as a means of combating the already existing poisoning of the human body. Here they can, at best, act as a probiotic remedy for more effective excretion of heavy metals from the body.
It should be noted that many toxins enter the food simultaneously and may act in combination, for example, mycotoxins and pesticides, bacterial toxins and antinutrients, mycotoxins and antinutrients, etc. In this case, LAB prove to be indispensable. During lactic acid fermentation, probiotic LAB strains achieve both toxins removal and the food’s nutritional value increase, especially with regard to foods of plant and cereal origin. In the future, the search for and application of new probiotic LAB strains for potential detoxification of mixed toxic agents is very promising and may be expected to become even more important than it is at present. The combination of different LAB strains with various detoxifying capabilities could serve as starter cultures for the production of safer and healthier functional foods.
Author Contributions
Conceptualization, P.P. and K.P.; writing—original draft preparation, P.P., A.A., F.T., T.P.-M., E.V., L.T. and K.P.; writing—review and editing, K.P. and A.A.; project administration, K.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Bulgarian Ministry of Education and Science with financial support from the “Healthy Foods for a Strong Bio-Economy and Quality of Life”, National Research Programme approved by DCM # 577/17.08.2018 and SOURDOMICS—COST Action 18101.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.E. Coli: Rapid Response in a Crisis. [(accessed on 3 March 2022)]. Available online: http://www.efsa.europa.eu/en/press/news/120711.
- 2.Investigations of Foodborne Illness Outbreaks. US Food and Drugs Administration. [(accessed on 30 March 2022)]; Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/investigations-foodborne-illness-outbreaks.
- 3.Gossner C.M., Schlundt J., Ben Embarek P., Hird S., Lo-Fo-Wong D., Beltran J.J., Teoh K.N., Tritscher A. The melamine incident: Implications for international food and feed safety. Environ. Health Perspect. 2009;117:1803–1808. doi: 10.1289/ehp.0900949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.INFOSAN Quarterly Summary, 2021 #4. [(accessed on 25 February 2022)]. Available online: https://www.who.int/news/item/04-02-2022-infosan-quarterly-summary-2021-4.
- 5.Regueiro J., López-Fernández O., Rial-Otero R., Cancho-Grande B., Simal-Gándara J. A Review on the Fermentation of Foods and the Residues of Pesticides—Biotransformation of Pesticides and Effects on Fermentation and Food Quality. Crit. Rev. Food Sci. Nutr. 2015;55:839–863. doi: 10.1080/10408398.2012.677872. [DOI] [PubMed] [Google Scholar]
- 6.Pesticide Residues in Food—2017: Toxicological Evaluations. [(accessed on 28 February 2022)]. Available online: https://www.who.int/publications/i/item/9789240006775.
- 7.Petrova P., Ivanov I., Tsigoriyna L., Valcheva N., Vasileva E., Parvanova-Mancheva T., Arsov A., Petrov K. Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits. Microorganisms. 2021;9:480. doi: 10.3390/microorganisms9030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrova P., Petrov K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients. 2020;12:1118. doi: 10.3390/nu12041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrova P., Petrov K. Prebiotic–probiotic relationship: The genetic fundamentals of polysaccharides conversion by Bifidobacterium and Lactobacillus genera. In: Grumezescu A.M., Holban A.M., editors. Handbook of Food Bioengineering. 1st ed. Volume 2. Elsevier Inc.; San Diego, CA, USA: 2017. pp. 237–278. [Google Scholar]
- 10.Ranjha M.M.A.N., Shafique B., Batool M., Kowalczewski P.Ł., Shehzad Q., Usman M., Manzoor M.F., Zahra S.M., Yaqub S., Aadil R.M. Nutritional and Health Potential of Probiotics: A Review. Appl. Sci. 2021;11:11204. doi: 10.3390/app112311204. [DOI] [Google Scholar]
- 11.Grumet L., Tromp Y., Stiegelbauer V. The Development of High-Quality Multispecies Probiotic Formulations: From Bench to Market. Nutrients. 2020;12:2453. doi: 10.3390/nu12082453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Żółkiewicz J., Marzec A., Ruszczyński M., Feleszko W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients. 2020;12:2189. doi: 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertin Y., Habouzit C., Duniere L., Laurier M., Durand A., Duchez D., Segura A., Thevenot-Sergentet D., Baruzzi F., Chaucheyras-Durand F. Lactobacillus reuteri suppresses E. coli O157:H7 in bovine ruminal fluid: Toward a pre-slaughter strategy to improve food safety? PLoS ONE. 2017;12:e0187229. doi: 10.1371/journal.pone.0187229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinder M., Bisanz J.E., Burton J.P., Reid G. Probiotic lactobacilli: A potential prophylactic treatment for reducing pesticide absorption in humans and wildlife. Benef. Microbes. 2015;6:841–847. doi: 10.3920/BM2015.0022. [DOI] [PubMed] [Google Scholar]
- 16.Grzywacz J.G., Belden J.B., Robertson A.M., Hernandez D.C., Carlos Chavez F.L., Merten M.J. Parenting, Pesticides and Adolescent Psychological Adjustment: A Brief Report. Int. J. Environ. Res. Public Health. 2022;19:540. doi: 10.3390/ijerph19010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh Y.J., Klaenhammer T.R. Genetic Mechanisms of Prebiotic Oligosaccharide Metabolism in Probiotic Microbes. Ann. Rev. Food Sci. Technol. 2015;6:137–156. doi: 10.1146/annurev-food-022814-015706. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y., Zhou T., Tang H., Li X., Chen Y., Zhang L., Zhang J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control. 2020;111:107057. doi: 10.1016/j.foodcont.2019.107057. [DOI] [Google Scholar]
- 19.Velikova P., Stoyanov A., Blagoeva G., Popova L., Petrov K., Gotcheva V., Angelov A., Petrova P. Starch utilization routes in lactic acid bacteria: New insight by gene expression assay. Starch-Stärke. 2016;68:953–960. doi: 10.1002/star.201600023. [DOI] [Google Scholar]
- 20.Gibson G.R. From probiotics to prebiotics and a healthy digestive system. J. Food Sci. 2004;69:141–143. doi: 10.1111/j.1365-2621.2004.tb10724.x. [DOI] [Google Scholar]
- 21.Fujiya M., Kohgo Y. Novel perspectives in probiotic treatment: The efficacy and unveiled mechanisms of the physiological functions; Clin. J. Gastroenterol. 2010;3:117–127. doi: 10.1007/s12328-010-0154-0. [DOI] [PubMed] [Google Scholar]
- 22.Bendali F., Madi N., Sadoun D. Beneficial effects of a strain of Lactobacillus paracasei subsp. paracasei in Staphylococcus aureus-induced intestinal and colonic injury. Int. J. Infect. Dis. 2011;15:e787–e794. doi: 10.1016/j.ijid.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Makino S., Ikegami S., Kano H., Sashihara T., Sugano H., Horiuchi H., Saito T., Oda M. Immunomodulatory Effects of Polysaccharides Produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2006;89:2873–2881. doi: 10.3168/jds.S0022-0302(06)72560-7. [DOI] [PubMed] [Google Scholar]
- 24.Kalliomaki M., Salminen S., Arvilommi H., Kero P., Koskinen P., Isolauri E. Probiotics in primary prevention of atopic disease, a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Chen C., Ai L., Zhou F., Zhou Z., Wang L., Zhang H., Chen W., Guo B. Complete genome sequence of the probiotic Lactobacillus plantarum ST-III. J. Bacteriol. 2011;193:313–314. doi: 10.1128/JB.01159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Hoorde K., Verstraete T., Vandamme P., Huys G. Diversity of lactic acid bacteria in two Flemish artisan raw milk Gouda-type cheeses. Food Microbiol. 2008;25:929–935. doi: 10.1016/j.fm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Arena M.P., Capozzi V., Russo P., Drider D., Spano G., Fiocco D. Immunobiosis and probiosis: Antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl. Microbiol. Biotechnol. 2018;102:9949–9958. doi: 10.1007/s00253-018-9403-9. [DOI] [PubMed] [Google Scholar]
- 28.Birt D.F., Boylston T., Hendrich S., Jane J.L., Hollis J., Li L., McClelland J., Moore S., Phillips G.J., Rowling M., et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013;4:587–601. doi: 10.3945/an.113.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazeli M.R., Shahverdi A.R., Sedaghat B., Jamalifar H., Samadi N. Sourdough-isolated Lactobacillus fermentum as a potent anti-mould preservative of a traditional Iranian bread. Eur. Food Res. Technol. 2004;218:554–556. doi: 10.1007/s00217-004-0898-1. [DOI] [Google Scholar]
- 30.Van der Meulen R., Scheirlinck I., Van Schoor A., Huys G., Vancanneyt M., Vandamme P., De Vuyst L. Population dynamics and metabolite target analysis of lactic acid bacteria during laboratory fermentations of wheat and spelt sourdoughs. Appl. Environ. Microbiol. 2007;73:4741–4750. doi: 10.1128/AEM.00315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negatu D.A., Gengenbacher M., Dartois V., Dick T. Indole Propionic Acid, an Unusual Antibiotic Produced by the Gut Microbiota, With Anti-inflammatory and Antioxidant Properties. Front. Microbiol. 2020;11:575586. doi: 10.3389/fmicb.2020.575586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z.H., Xin F.Z., Xue Y., Hu Z., Han Y., Ma F., Zhou D., Liu X.-L., Cui A., Liu Z., et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019;51:1–14. doi: 10.1038/s12276-019-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanciotti R., Patrignani F., Bagnolini F., Guerzoni M.E., Gardini F. Evaluation of diacetyl antimicrobial activity against Escherichia coli, Listeria monocytogenes and Staphylococcus aureus. Food Microbiol. 2003;20:537–543. doi: 10.1016/S0740-0020(02)00159-4. [DOI] [Google Scholar]
- 34.Reid G. Probiotic lactobacilli for urogenital health in women. J. Clin. Gastroenterol. 2008;42((Suppl. 3)):234–236. doi: 10.1097/MCG.0b013e31817f1298. [DOI] [PubMed] [Google Scholar]
- 35.Kumariya R., Garsa A.K., Rajput Y.S., Sood S.K., Akhtar N., Patel S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019;128:171–177. doi: 10.1016/j.micpath.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Demirbaş F., İspirli H., Kurnaz A.A., Tahsin Yilmaz M.T., Dertli E. Antimicrobial and functional properties of lactic acid bacteria isolated from sourdoughs. LWT Food Sci. Technol. 2017;79:361–366. doi: 10.1016/j.lwt.2017.01.067. [DOI] [Google Scholar]
- 37.Lo S., Thiam I., Fall B., Ba-Diallo A., Diallo O.F., Diagne R., Dia M.L., Ka R., Sarr A.M., Sow A.I. Urinary tract infection with Corynebacterium aurimucosum after urethroplasty stricture of the urethra: A case report. J. Med. Case Rep. 2015;9:156. doi: 10.1186/s13256-015-0638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Axel C., Zannini E., Arendt E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017;57:3528–3542. doi: 10.1080/10408398.2016.1147417. [DOI] [PubMed] [Google Scholar]
- 39.Strom K., Sjogren J., Broberg A., Schnurer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002;68:4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dal Bello F., Clarke C.I., Ryan L.A.M., Ulmer H., Schober T.J., Strom K., Sjogren J., van Sinderen D., Schnurer J., Arendt E.K., et al. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007;45:309–318. doi: 10.1016/j.jcs.2006.09.004. [DOI] [Google Scholar]
- 41.Axelsson L.T., Chung T.C., Dobrogosz W.J., Lindgren S.E. Production of a Broad Spectrum Antimicrobial Substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 1989;2:131–136. doi: 10.3109/08910608909140210. [DOI] [Google Scholar]
- 42.Broberg A., Jacobsson K., Strom K., Schnurer J. Metabolite Profiles of Lactic Acid Bacteria in Grass Silage. Appl. Environ. Microbiol. 2007;73:5547–5552. doi: 10.1128/AEM.02939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black B.A., Zannini E., Curtis J.M., Gänzle M.G. Antifungal Hydroxy Fatty Acids Produced during Sourdough Fermentation: Microbial and Enzymatic Pathways, and Antifungal Activity in Bread. Appl. Environ. Microbiol. 2013;79:1866–1873. doi: 10.1128/AEM.03784-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valerio F., Di Biase M., Lattanzio V.M.T., Lavermicocca P. Improvement of the antifungal activity of lactic acid bacteria by addition to the growth medium of phenylpyruvic acid, a precursor of phenyllactic acid. Int. J. Food Microbiol. 2016;222:1–7. doi: 10.1016/j.ijfoodmicro.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Mieszkin S., Hymery N., Debaets S., Coton E., Le Blay G., Valence F., Mounier J. Action mechanisms involved in the bioprotective effect of Lactobacillus harbinensis K.V9.3.1.Np against Yarrowia lipolytica in fermented milk. Int. J. Food Microbiol. 2017;248:47–55. doi: 10.1016/j.ijfoodmicro.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Allocati N., Masulli M., Alexeyev M., Di Ilio C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health. 2013;10:6235–6254. doi: 10.3390/ijerph10126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakao H., Takeda T. Escherichia coli Shiga toxin. J. Nat. Toxins. 2000;9:299–313. [PubMed] [Google Scholar]
- 48.Melton-Celsa A., Mohawk K., Teel L., O’Brien A. Pathogenesis of Shiga-Toxin Producing Escherichia coli, Ricin and Shiga. Toxins. 2011;357:67–103. doi: 10.1007/82_2011_176. [DOI] [PubMed] [Google Scholar]
- 49.Padhye N., Doyle M. Escherichia coli O157:H7: Epidemiology, pathogenesis, and methods for detection in food. J. Food Prot. 1992;55:555–565. doi: 10.4315/0362-028X-55.7.555. [DOI] [PubMed] [Google Scholar]
- 50.Griffin P., Tauxe R. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 51.Tarr P. Escherichia coli O157:H7: Clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 1995;20:1–10. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Besser R., Lett S., Weber J., Doyle M., Barrett T., Wells J., Griffin P. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. doi: 10.1001/jama.1993.03500170047032. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Update: Multistate outbreak of Escherichia coli O157:H7 infections from hamburgers—western United States 1992–1993. Morbid. Mortal. Weekly Rep. 1993;42:258–263. [PubMed] [Google Scholar]
- 54.Steele B., Murphy N., Rance C. An outbreak of hemolytic uremic syndrome associated with ingestion of fresh apple juice. J. Pediatr. 1982;101:963–965. doi: 10.1016/S0022-3476(82)80021-8. [DOI] [PubMed] [Google Scholar]
- 55.Leyer G., Wang L., Johnson E. Acid Adaptation of Escherichia coli O157:H7 Increases Survival in Acidic Foods. [(accessed on 12 March 2022)];Appl. Environ. Microbiol. 1995 61:3752–3755. doi: 10.1128/aem.61.10.3752-3755.1995. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC167674/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami—Washington and California. Morbid. Mortal. Wkly. Rep. 1995;44:157–160. [PubMed] [Google Scholar]
- 57.Riley L., Remis R., Helgerson S., McGee H., Wells J., Davis B., Herbert R., Olcott G., Johnson L., Blake N., et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype 0157:H7. N. Engl. J. Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 58.Ackers M., Mahon B., Leahy E., Goode B., Damrow T., Hayes P., Bibb W., Rice D., Barrett T., Hutwagner L., et al. An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. J. Infect. Dis. 1998;177:1588–1593. doi: 10.1086/515323. [DOI] [PubMed] [Google Scholar]
- 59.Bielaszewska M., Janda J., Blahova K., Minarikova H., Jikova E., Karmali M., Laubova J., Sikulova J., Preston M., Khakhria R., et al. Human Escherichia coli O157:H7 infection associated with the consumption of unpasteurized goat’s milk. Epidemiol. Infect. 1997;119:299–305. doi: 10.1017/S0950268897008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention (CDC) Outbreaks of Escherichia coli O157:H7 infection associated with eating alfalfa sprouts—Michigan and Virginia. Morbid. Mortal. Wkly. Rep. 1997;46:741–744. [PubMed] [Google Scholar]
- 61.Doyle M., Zhao T., Meng J., Zhao S. Escherichia coli O157:H7. In: Doyle M.P., Beuchat L.R., Montville T.J., editors. Food Microbiology, Fundamentals and Frontiers. ASM Press; Washington, DC, USA: 1997. [(accessed on 9 March 2022)]. pp. 171–191. Available online: https://www.worldcat.org/title/food-microbiology-fundamentals-and-frontiers/oclc/46642017. [Google Scholar]
- 62.Ryu S.H., Lee J.H., Park S.H., Song M.O., Park S.H., Jung H.W., Park G.Y., Choi S.M., Kim M.S., Chae Y.Z., et al. Antimicrobial resistance profiles among Escherichia coli strains isolated from commercial and cooked foods. Int. J. Food Microbiol. 2012;159:263–266. doi: 10.1016/j.ijfoodmicro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Abdul-Raouf U., Ammar M., Beuchat L. Isolation of Escherichia coli 0157:H7 from some Egyptian foods. Int. J. Food Microbiol. 1996;29:423–426. doi: 10.1016/0168-1605(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 64.Dontorou C., Papadopoulou C., Filioussis G., Economou V., Apostolou I., Zakkas G., Salamoura A., Kansouzidou A., Levidiotou S. Isolation of Escherichia coli O157:H7 from foods in Greece. Int. J. Food Microbiol. 2003;82:273–279. doi: 10.1016/S0168-1605(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 65.Lukásová J., Abraham B., Cupáková S. Occurrence of Escherichia coli O157 in raw material and food in Czech Republic. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2004;51:77–81. doi: 10.1111/j.1439-0450.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 66.Mora A., Blanco J.E., Blanco M., Alonso M.P., Dhabi G., Echeita A., González E.A., Bernárdez M.I., Blanco J. Antimicrobial resistance of Shiga toxin (verotoxin)-producing Escherichia coli O157:H7 and non-O157 strains isolated from humans, cattle, sheep and food in Spain. Res. Microbiol. 2005;156:793–806. doi: 10.1016/j.resmic.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Lee K.-S., Jeong Y.-J., Lee M.-S. Escherichia coli Shiga Toxins and Gut Microbiota Interactions. Toxins. 2021;13:416. doi: 10.3390/toxins13060416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orihuel A., Terán L., Renaut J., Vignolo G., De Almeida A., Saavedra M., Fadda S. Differential Proteomic Analysis of Lactic Acid Bacteria—Escherichia coli O157:H7 Interaction and Its Contribution to Bioprotection Strategies in Meat. Front. Microbiol. 2018;9:1083. doi: 10.3389/fmicb.2018.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa M., Shimizu K., Nomoto K., Tanaka R., Hamabata T., Yamasaki S., Takeda T., Takeda Y. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 2001;68:135–140. doi: 10.1016/S0168-1605(01)00465-2. [DOI] [PubMed] [Google Scholar]
- 70.Ogawa M., Shimizu K., Nomoto K., Takahashi M., Watanuki M., Tanaka R., Tanaka T., Hamabata T., Yamasaki S., Takeda Y. Protective Effect of Lactobacillus casei Strain Shirota on Shiga Toxin-Producing Escherichia coli O157:H7 Infection in Infant Rabbits. Infect. Immun. 2001;69:1101–1108. doi: 10.1128/IAI.69.2.1101-1108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byakika S., Mukisa I., Mugabi R., Muyanja C. Antimicrobial Activity of Lactic Acid Bacteria Starters against Acid Tolerant, Antibiotic Resistant, and Potentially Virulent E. coli Isolated from a Fermented Sorghum-Millet Beverage. Int. J. Microbiol. 2019;2019:2013539. doi: 10.1155/2019/2013539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brashears M.M., Reilly S.S., Gilliland S.E. Antagonistic action of cells of Lactobacillus lactis toward Escherichia coli O157:H7 on refrigerated raw chicken meat. J. Food Prot. 1998;61:166–170. doi: 10.4315/0362-028X-61.2.166. [DOI] [PubMed] [Google Scholar]
- 73.Walsham A.D.S., MacKenzie D.A., Cook V., Wemyss-Holden S., Hews C.L., Juge N., Schüller S. Lactobacillus reuteri Inhibition of Enteropathogenic Escherichia coli Adherence to Human Intestinal Epithelium. Front. Microbiol. 2016;7:00244. doi: 10.3389/fmicb.2016.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eaton K.A., Honkala A., Auchtung T.A., Britton R.A. Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect. Immun. 2011;79:185–191. doi: 10.1128/IAI.00880-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peterson R.E., Klopfenstein T.J., Erickson G.E., Folmer J., Hinkley S., Moxley R.A., Smith D.R. Effect of Lactobacillus acidophilus strain NP51 on Escherichia coli O157:H7 fecal shedding and finishing performance in beef feedlot cattle. J. Food Prot. 2007;70:287–291. doi: 10.4315/0362-028X-70.2.287. [DOI] [PubMed] [Google Scholar]
- 76.Aditya A., Li Y., Biswas D. Antagonistic Effects of Conjugated Linoleic Acids of Lactobacillus casei Against Foodborne Enterohemorrhagic Escherichia coli. J. Food Prot. 2022;85:712–719. doi: 10.4315/JFP-21-414. [DOI] [PubMed] [Google Scholar]
- 77.Zeinhom M., Tellez A.M., Delcenserie V., El-Kholy A.M., El-Shinawy S.H., Griffiths M.W. Yogurt Containing Bioactive Molecules Produced by Lactobacillus acidophilus La-5 Exerts a Protective Effect against Enterohemorrhagic Escherichia coli in Mice. J. Food Prot. 2012;75:1796–1805. doi: 10.4315/0362-028X.JFP-11-508. [DOI] [PubMed] [Google Scholar]
- 78.Medellin-Peña M., Griffiths M. Effect of Molecules Secreted by Lactobacillus acidophilus Strain La-5 on Escherichia coli O157:H7 Colonization. Appl. Environ. Microbiol. 2009;75:1165–1172. doi: 10.1128/AEM.01651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson-Henry K.C., Hagen K.E., Gordonpour M., Tompkins T.A., Sherman P.M. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007;9:356–367. doi: 10.1111/j.1462-5822.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 80.Hirano J., Yoshida T., Sugiyama T., Koide N., Mori I., Yokochi T. The Effect of Lactobacillus rhamnosus on Enterohemorrhagic Escherichia coli Infection of Human Intestinal Cells In Vitro. Microbiol. Immunol. 2003;47:405–409. doi: 10.1111/j.1348-0421.2003.tb03377.x. [DOI] [PubMed] [Google Scholar]
- 81.Cadieux P., Burton J., Devillard E., Reid G. Lactobacillus By-Products Inhibit The Growth And Virulence Of Uropathogenic Escherichia coli. [(accessed on 11 March 2022)];J. Phys. Pharmacol. 2009 60:13–18. Available online: https://pubmed.ncbi.nlm.nih.gov/20224146/ [PubMed] [Google Scholar]
- 82.Caridi A. Selection of Escherichia coli-inhibiting strains of Lactobacillus paracasei subsp. paracasei. J. Ind. Microbiol. Biotechnol. 2002;29:303–308. doi: 10.1038/sj.jim.7000300. [DOI] [PubMed] [Google Scholar]
- 83.Fijan S., Šulc D., Steyer A. Study of the In Vitro Antagonistic Activity of Various Single-Strain and Multi-Strain Probiotics against Escherichia coli. Int. J. Environ. Res. Public Health. 2018;15:1539. doi: 10.3390/ijerph15071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maury M.M., Tsai Y.-H., Charlier C., Touchon M., Chenal-Francisque V., Leclercq A., Criscuolo A., Gaultier C., Roussel S., Brisabois A., et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Gen. 2016;48:308–313. doi: 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farber J.M., Peterkin P.I. Listeria monocytogenes: A food borne pathogen. Microbiol. Rev. 1991;5:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kessler S.L., Dajani A.S. Listeria meningitis in infants and children. Pediatr. Infect. Dis. J. 1990;9:6–63. doi: 10.1097/00006454-199001000-00016. [DOI] [PubMed] [Google Scholar]
- 87.Buchanan R., Lindqvist R., Ross T., Smith M., Todd E., Whiting R. Risk Assessment of Listeria Monocytogenes in Ready-to-Eat Foods. Microbiol. Risk Assess. Ser. 2004, 4. Food and Agriculture Organization of the United Nations, Rome (Italy) [(accessed on 15 February 2022)]. Available online: https://www.fao.org/3/y5394e/Y5394E.pdf.
- 88.Vazquez-Boland J.-A., Kuhn M., Berche P., Chakraborty T., Domínguez-Bernal G., Goebel W., González-Zorn B., Wehland J., Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yap P.C., MatRahim N.A., AbuBakar S., Lee H.Y. Antilisterial potential of lactic acid bacteria in eliminating Listeria monocytogenes in host and ready-to-eat food application. Microbiol. Res. 2021;12:234–257. doi: 10.3390/microbiolres12010017. [DOI] [Google Scholar]
- 90.Dramsi S., Cossart P. Listeriolysin O: A genuine cytolysin optimized for an intracellular parasite. J. Cell Biol. 2002;156:943–946. doi: 10.1083/jcb.200202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glass K.A., Doyle M.P. Fate of Listeria monocytogenes in processed meat products during refrigerated storage. Appl. Environ. Microbiol. 1989;55:1565–1569. doi: 10.1128/aem.55.6.1565-1569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leasor S.B., Foegeding P.M. Listeria spp. in commercially broken raw liquid whole egg. J. Food Prot. 1989;52:777–780. doi: 10.4315/0362-028X-52.11.777. [DOI] [PubMed] [Google Scholar]
- 93.Yin H.B., Chi-Hung Chen C.H., Boomer A., Byun S., Venkitanarayanan K., Macarisin D., Patel J. Biocontrol of Listeria on cantaloupes with lactic acid bacteria. J. Food Process Preserv. 2020;44:14465. doi: 10.1111/jfpp.14465. [DOI] [Google Scholar]
- 94.Hoelzer K., Sauders B.D., Sanchez M.D., Olsen P.T., Pickett M.M., Mangione K.J., Rice D.H., Corby J., Stich S., Fortes E.D., et al. Prevalence, distribution, and diversity of Listeria monocytogenes in retail environments, focusing on small establishments and establishments with a history of failed inspections. J. Food Prot. 2011;74:1083–1095. doi: 10.4315/0362-028X.JFP-10-567. [DOI] [PubMed] [Google Scholar]
- 95.Gandhi M., Chikindas M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007;113:1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 96.McClure P.J., Roberts T.A., Otto Oguru P. Comparison of the effects of sodium chloride, pH and temperature on the growth of Listeria monocytogenes on gradient plates and in liquid medium. Lett. Appl. Microbiol. 1989;9:95–99. doi: 10.1111/j.1472-765X.1989.tb00299.x. [DOI] [Google Scholar]
- 97.Lomonaco S., Decastelli L., Nucera D., Gallina S., Bianchi D.M., Civera T. Listeria monocytogenes in Gorgonzola: Subtypes diversity and persistence over time. Int. J. Food Microbiol. 2009;128:516–520. doi: 10.1016/j.ijfoodmicro.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Miladi H., Bakhrouf A., Ammar E. Cellular lipid fatty acid profiles of reference and food isolates Listeria monocytogenes as a response to refrigeration and freezing stress. J. Food Biochem. 2013;37:136–143. doi: 10.1111/j.1745-4514.2011.00607.x. [DOI] [Google Scholar]
- 99.Bayles D., Wilkinson B. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 2000;30:23–27. doi: 10.1046/j.1472-765x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 100.Amit S.K., Uddin M.M., Rahman R., Islam S.M.R., Khan M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017;6:51. doi: 10.1186/s40066-017-0130-8. [DOI] [Google Scholar]
- 101.Scatassa M.L., Gaglio R., Cardamone C., Macaluso G., Arcuri L., Todaro M., Mancuso I. Anti-listeria activity of lactic acid bacteria in two traditional Sicilian cheeses. Ital. J. Food Safety. 2017;6:13–17. doi: 10.4081/ijfs.2017.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Linares-Morales J.R., Gutierrez-Mendez N., Rivera-Chavira B.E., Perez-Vega S.B., Nevarez-Moorillon G.V. Biocontrol processes in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front. Sustain. Food Syst. 2018;2:50. doi: 10.3389/fsufs.2018.00050. [DOI] [Google Scholar]
- 103.Kasra-Kermanshahi R., Mobarak-Qamsari E. Inhibition effect of lactic acid bacteria against food born pathogen Listeria monocytogenes. Appl. Food Biotechnol. 2015;2:11–19. doi: 10.22037/afb.v2i4.8894. [DOI] [Google Scholar]
- 104.Panebianco F., Giarratana F., Caridi A., Sidari R., De Bruno A., Giuffrida A. Lactic acid bacteria isolated from traditional Italian dairy products: Activity against Listeria monocytogenes and modelling of microbial competition in soft cheese. LWT. 2020;137:110446. doi: 10.1016/j.lwt.2020.110446. [DOI] [Google Scholar]
- 105.Mishra C., Lambert J. Production of antimicrobial substances by probiotics. [(accessed on 18 February 2022)];Asia Pac. J. Clin. Nut. 1996 5:20–24. Available online: https://apjcn.nhri.org.tw/server/APJCN/5/1/20.pdf. [PubMed] [Google Scholar]
- 106.Leroy F., Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004;15:67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- 107.Jay J. Antimicrobial properties of diacetyl. Appl. Environ. Mirobiol. 1982;44:525–532. doi: 10.1128/aem.44.3.525-532.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mulet-Powell N., Lacoste-Armynot A.M., Vicas M., De Buochberg M.S. Interactions between pairs of bacteriocins from lactic acid bacteria. J. Food Prot. 1998;61:1210–1212. doi: 10.4315/0362-028X-61.9.1210. [DOI] [PubMed] [Google Scholar]
- 109.Amezquita A., Brashears M.M. Competitive inhibition of Listeria monocytogenes in ready-to-eat meat products by lactic acid bacteria. J. Food Prot. 2001;65:316–325. doi: 10.4315/0362-028X-65.2.316. [DOI] [PubMed] [Google Scholar]
- 110.Saraoui T., Fall P.A., Leroi F., Antignac J.P., Chereau S., Pilet M.F. Inhibition mechanism of Listeria monocytogenes by a bioprotective bacteria Lactococcus piscium CNCM I-4031. Food Microbiol. 2016;53:70–78. doi: 10.1016/j.fm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Bermudez-Brito M., Plaza-Diaz J., Munoz-Quezada S., Gomez-Llorente C., Gil A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012;61:160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 112.Wang L., He Z., Tian P., Wang G. Lactic Acid Bacteria and Host Immunity. In: Chen W., editor. Lactic Acid Bacteria: Omics and Functional Evaluation. Springer; New York, NY, USA: 2019. pp. 261–296. [Google Scholar]
- 113.Upadhyay A., Upadhyaya I., Mooyottu S., Venkitanarayanan K. Eugenol in combination with lactic acid bacteria attenuates Listeria monocytogenes virulence in vitro and in invertebrate model Galleria mellonella. J. Med. Microbiol. 2016;65:443–455. doi: 10.1099/jmm.0.000251. [DOI] [PubMed] [Google Scholar]
- 114.Winkelstroter L.K., De Martinis E.C. Effect of bacteriocins and conditions that mimic food and digestive tract on biofilm formation, in vitro invasion of eukaryotic cells and internalin gene expression by Listeria monocytogenes. Probiotics Antimicrob. Proteins. 2013;5:153–164. doi: 10.1007/s12602-013-9135-1. [DOI] [PubMed] [Google Scholar]
- 115.Rios-Covian D., Nogacka A., Salazar N., Hernandez-Barranco A.M., Cuesta I., Gueimonde M., de Los Reyes Gavilan C.G. Bifidobacterium breve IPLA20005 affects in vitro the expression of hly and luxS genes, related to the virulence of Listeria monocytogenes Lm23. Can. J. Microbiol. 2018;64:215–221. doi: 10.1139/cjm-2017-0625. [DOI] [PubMed] [Google Scholar]
- 116.Fernandez N., Wrzosek L., Radziwill-Bienkowska J.M., Ringot-Destrez B., Duviau M.P., Noordine M.L., Laroute V., Robert V., Cherbuy C., Daveran-Mingot M.L., et al. Characterization of mucus-related properties of Streptococcus thermophilus: From adhesion to induction. Front. Physiol. 2018;9:980. doi: 10.3389/fphys.2018.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren C., Zhang Q., de Haan B.J., Zhang H., Faas M.M., de Vos P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci. Rep. 2016;6:34561. doi: 10.1038/srep34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Azizpour M., Hosseini S.D., Jafari P., Akbary N. Lactococcus lactis: A New Strategy for Vaccination. Avicenna J. Med. Biotechnol. 2017;9:163–168. [PMC free article] [PubMed] [Google Scholar]
- 119.Sullivan L.O., Ross R.P., Hill C. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie. 2002;84:593–604. doi: 10.1016/S0300-9084(02)01457-8. [DOI] [PubMed] [Google Scholar]
- 120.Pinilla C.M.B., Brandelli A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016;36:287–293. doi: 10.1016/j.ifset.2016.07.017. [DOI] [Google Scholar]
- 121.Malheiros S., Sant’Anna V., Barbosa S., Brandelli A., Franco B.D. Effect of liposome-encapsulated nisin and bacteriocin-like substance P34 on Listeria monocytogenes growth in Minas frescal cheese. Int. J. Food Microbiol. 2012;156:272–277. doi: 10.1016/j.ijfoodmicro.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 122.Malheiros S., Daroit D.J., Brandelli A. Inhibition of Listeria monocytogenes in minas frescal cheese by free and nanovesicle-encapsulated nisin. Braz. J. Microbiol. 2012;43:1414–1418. doi: 10.1590/S1517-83822012000400024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas L., Wimpenny J. Investigation of the effect of combined variations in temperature, pH, and NaCl concentration on nisin inhibition of Listeria monocytogenes and Staphylococcus aureus. Appl. Environ. Microbiol. 1996;62:2006–2012. doi: 10.1128/aem.62.6.2006-2012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martín I., Rodríguez A., Alía A., Martínez-Blanco M., Ojalvo D.L., Córdoba J.J. Control of Listeria monocytogenes growth and virulence in a traditional soft cheese model system based on lactic acid bacteria and a whey protein hydrolysate with antimicrobial activity. Int. J. Food Microbiol. 2022;361:109444. doi: 10.1016/j.ijfoodmicro.2021.109444. [DOI] [PubMed] [Google Scholar]
- 125.Morandi S., Silvetti T., Vezzini V., Morozzo E., Brasca M. How we can improve the antimicrobial performances of lactic acid bacteria? A new strategy to control Listeria monocytogenes in Gorgonzola cheese. Food Microbiol. 2020;90:103488. doi: 10.1016/j.fm.2020.103488. [DOI] [PubMed] [Google Scholar]
- 126.Koster S., van Pee K., Hudel M., Leustik M., Rhinow D., Kuhlbrandt W., Chakraborty T., Yildiz O. Crystal structure of listeriolysin O reveals molecular details of oligomerization and pore formation. Nat. Commun. 2014;5:3690. doi: 10.1038/ncomms4690. [DOI] [PubMed] [Google Scholar]
- 127.Hernandez-Flores K.G., Vivanco-Cid H. Biological effects of listeriolysin O: Implications for vaccination. BioMed Res. Int. 2015;2015:360741. doi: 10.1155/2015/360741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Szatraj K., Szczepankowska A.K., Chmielewska-Jeznach M. Lactic acid bacteria promising vaccine vectors: Possibilities, limitations, doubts. J. Appl. Microbiol. 2017;123:325–339. doi: 10.1111/jam.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mercenier A., Muller-Alouf H., Grangette C. Lactic acid bacteria as live vaccines. Curr. Issues Mol. Biol. 2000;2:17–25. doi: 10.21775/cimb.002.017. [DOI] [PubMed] [Google Scholar]
- 130.Wyszynska A., Kobierecka P., Bardowski J., Jagusztyn-Krynicka E.K. Lactic acid bacteria-20 years exploring their potential as live vectors for mucosal vaccination. Appl. Microbiol. Biotechnol. 2015;99:2967–2977. doi: 10.1007/s00253-015-6498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marcotte H., Koll-Klais P., Hultberg A., Zhao Y., Gmur R., Mandar R., Mikelsaar M., Hammarstrom L. Expression of single-chain antibody against RgpA protease of Porphyromonas gingivalis in Lactobacillus. J. Appl. Microbiol. 2006;100:256–263. doi: 10.1111/j.1365-2672.2005.02786.x. [DOI] [PubMed] [Google Scholar]
- 132.Dhaked R.K., Singh M.K., Singh P., Gupta P. Botulinum toxin: Bioweapon & magic drug. [(accessed on 26 March 2022)];Indian J. Med. Res. 2010 132:489–503. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3028942/ [PMC free article] [PubMed] [Google Scholar]
- 133.Lund B.M., Peck M.W. Clostridium botulinum. In: Labbé R.G., García S., editors. Guide to Foodborne Pathogens. 2nd ed. John Wiley & Sons Ltd.; Chichester, UK: 2013. pp. 91–111. [Google Scholar]
- 134.Nowakowska M.B., Selby K., Przykopanski A., Krüger M., Krez N., Dorner B.G., Dorner M.B., Jin R., Minton N.P., Rummel A., et al. Construction and validation of safe Clostridium botulinum Group II surrogate strain producing inactive botulinum neurotoxin type E toxoid. Sci. Rep. 2022;12:1790. doi: 10.1038/s41598-022-05008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alizadeh A.M., Hashempour-Baltork F., Alizadeh-Sani M., Maleki M., Azizi-Lalabad M., Khosravi-Darani K. Inhibition of Clostridium botulinum and its toxins by probiotic bacteria and their metabolites. Qual. Assur. Saf. Crops Foods. 2020;12:59–68. doi: 10.15586/qas.v12iSP1.823. [DOI] [Google Scholar]
- 136.Lynt R.K., Kautter D.A., Solomon H. Differences and Similarities among Proteolytic and Nonproteolytic Strains of Clostridium botulinum Types A, B, E and F: A Review. J. Food Prot. 1982;45:466–474. doi: 10.4315/0362-028X-45.5.466. [DOI] [PubMed] [Google Scholar]
- 137.Eskandari M.H., Hosseinpour S., Mesbahi G.R., Shekarforoush S. New composite nitrite-free and low-nitrite meat-curing systems using natural colorants. Food Sci. Nutr. 2013;1:392–401. doi: 10.1002/fsn3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lucumi-Banguero R.S., Ramírez-Toro C., Bolivar G.A. Potential Use of Lactic Acid Bacteria with Pathogen Inhibitory Capacity as a Biopreservative Agent for Chorizo. Processes. 2021;9:1582. doi: 10.3390/pr9091582. [DOI] [Google Scholar]
- 139.Okereke A., Montville T.J. Bacteriocin Inhibition of Clostridium botulinum Spores by Lactic Acid Bacteria. J. Food Prot. 1991;54:349–353. doi: 10.4315/0362-028X-54.5.349. [DOI] [PubMed] [Google Scholar]
- 140.Okereke A., Montville T.J. Bacteriocin-mediated inhibition of Clostridium botulinum spores by lactic acid bacteria at refrigeration and abuse temperatures. Appl. Environ. Microbiol. 1991;57:3423–3428. doi: 10.1128/aem.57.12.3423-3428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dobson A., Cotter P.D., Ross R.P., Hill C. Bacteriocin Production: A Probiotic Trait? Appl. Environ. Microbiol. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodgers S. Preserving non-fermented refrigerated foods with microbial cultures—A review. Trends Food Sci. Technol. 2002;12:276–284. doi: 10.1016/S0924-2244(01)00093-0. [DOI] [Google Scholar]
- 143.Lewus C.B., Kaiser A., Montville T.J. Inhibition of food-borne bacterial pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl. Environ. Microbiol. 1991;57:1683–1688. doi: 10.1128/aem.57.6.1683-1688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roces C., Rodríguez A., Martínez B. Cell Wall-active Bacteriocins and Their Applications beyond Antibiotic Activity. Probiotics Antimicrob. Proteins. 2012;4:259–272. doi: 10.1007/s12602-012-9116-9. [DOI] [PubMed] [Google Scholar]
- 145.De Arauz L.J., Jozala A.F., Mazzola P.G., Penna T.C.V. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009;20:146–154. doi: 10.1016/j.tifs.2009.01.056. [DOI] [Google Scholar]
- 146.Khan I., Oh D.H. Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. Technol. 2016;34:376–384. doi: 10.1016/j.ifset.2015.12.013. [DOI] [Google Scholar]
- 147.Wiedemann I., Breukink E., Kraaij C.v., Kuipers O.P., Bierbaum G., Kruijff B.D., Sahl H.G. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 2001;276:1772–1779. doi: 10.1074/jbc.M006770200. [DOI] [PubMed] [Google Scholar]
- 148.Hernandez-González J.C., Martinez-Tapia A., Lazcano-Hernández G., Garcia-Pérez B., Castrejon-Jiménez N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals. 2021;11:979. doi: 10.3390/ani11040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Biscola V., Todorov S.D., Capuano V.S.C., Abriouel H., Gálvez A., Franco B.D.G.M. Isolation and characterization of a nisin-like bacteriocin produced by a Lactococcus lactis strain isolated from charqui, a Brazilian fermented, salted and dried meat product. Meat. Sci. 2013;93:607–613. doi: 10.1016/j.meatsci.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 150.Field D., Daly K., O′Connor P., Cotter P.D., Hill C., Ross R. Efficacies of Nisin A and Nisin V Semipurified Preparations Alone and in Combination with Plant Essential Oils for Controlling Listeria monocytogenes. Appl. Environ. Microbiol. 2015;81:2762–2769. doi: 10.1128/AEM.00070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jabés D., Brunati C., Candiani G., Riva S., Romano G., Donadio S. Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant Gram-positive pathogens. Antimicrob. Agents Chemother. 2011;55:1671–1676. doi: 10.1128/AAC.01288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Field D., O’Conner R., Cotter P.D., Ross R.P., Hill C. In Vitro Activities of Nisin and Nisin Derivatives Alone and In Combination with Antibiotics against Staphylococcus Biofilms. Front. Microbiol. 2016;7:508. doi: 10.3389/fmicb.2016.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Petit L., Gibert M., Popoff M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/S0966-842X(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 154.Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo S., Liu D., Zhang B., Li Z., Li Y., Ding B., Guo Y. Two Lactobacillus Species Inhibit the Growth and α-Toxin Production of Clostridium perfringens and Induced Proinflammatory Factors in Chicken Intestinal Epithelial Cells in Vitro. Front Microbiol. 2017;8:2081. doi: 10.3389/fmicb.2017.02081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Banykó J., Vyletelová M. Determining the source of Bacillus cereus and Bacillus licheniformis isolated from raw milk, pasteurized milk and yoghurt. Lett. Appl. Microbiol. 2009;48:318–323. doi: 10.1111/j.1472-765X.2008.02526.x. [DOI] [PubMed] [Google Scholar]
- 157.Allende A., Bolton D., Chemaly M., Davies R., Fernández Escámez P.S., Gironés R., Wahlström H. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016;14:4524–4550. doi: 10.2903/j.efsa.2016.4524. [DOI] [Google Scholar]
- 158.Halverson L.J., Clayton M.K., Handelsman J. Variable stability of antibiotic-resistance markers in Bacillus cereus UW85 in the soybean rhizosphere in the field. Mol. Ecol. 1993;2:65–78. doi: 10.1111/j.1365-294X.1993.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 159.Jensen G.B., Hansen B.M., Eilenberg J., Mahillon J. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 2003;5:631–640. doi: 10.1046/j.1462-2920.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 160.Vilain S., Luo Y., Hildreth M.B., Brozel V.S. Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl. Environ. Microbiol. 2006;72:4970–4977. doi: 10.1128/AEM.03076-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guinebretière M.H., Thompson F.L., Sorokin A., Normand P., Dawyndt P., Ehling-Schulz M., Svensson B., Sanchis V., Nguyen-The C., Heyndrickx M., et al. Ecological diversification in the Bacillus cereus group. Environ. Microbiol. 2008;10:851–865. doi: 10.1111/j.1462-2920.2007.01495.x. [DOI] [PubMed] [Google Scholar]
- 162.Bintsis T. Foodborne pathogens. AIMS Microbiol. 2017;3:529–563. doi: 10.3934/microbiol.2017.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Senesi S., Ghelardi E. Production, secretion and biological activity of Bacillus cereus enterotoxins. Toxins. 2010;2:1690–1703. doi: 10.3390/toxins2071690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dierick K., Van Coillie E., Swiecicka I., Meyfroidt G., Devlieger H., Meulemans A., Hoedemaekers G., Fourie L., Heyndrickx M., Mahillon J. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 2005;43:4277–4279. doi: 10.1128/JCM.43.8.4277-4279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gemechu T. Review on lactic acid bacteria function in milk fermentation and preservation. Afr. J. Food Sci. 2015;9:170–175. doi: 10.5897/AJFS2015.1276. [DOI] [Google Scholar]
- 166.Wang C., Chang T., Yang H., Cui M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control. 2015;47:231–236. doi: 10.1016/j.foodcont.2014.06.034. [DOI] [Google Scholar]
- 167.Tirloni E., Ghelardi E., Celandroni F., Bernardi C., Stella S. Effect of dairy product environment on the growth of Bacillus cereus. J. Dairy Sci. 2017;100:7026–7034. doi: 10.3168/jds.2017-12978. [DOI] [PubMed] [Google Scholar]
- 168.Røssland E., Borge G.I.A., Langsrud T., Sørhaug T. Inhibition of Bacillus cereus by strains of Lactobacillus and Lactococcus in milk. Int. J. Food Microbiol. 2003;89:205–212. doi: 10.1016/S0168-1605(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 169.Bogovič-Matijašić B., Rogelj I., Nes I.F., Holo H. Isolation and characterization of two bacteriocins of Lactobacillus acidophilus LF221. J. Microbiol. Biotechnol. 1998;49:606–612. doi: 10.1007/s002530051221. [DOI] [PubMed] [Google Scholar]
- 170.Gutiérrez S., Martínez-Blanco H., Rodríguez-Aparicio L.B., Ferrero M.A. Effect of fermented broth from lactic acid bacteria on pathogenic bacteria proliferation. Int. J. Dairy Sci. 2016;99:2654–2665. doi: 10.3168/jds.2015-10439. [DOI] [PubMed] [Google Scholar]
- 171.Olaniyi O.I., Adeniran H.A., Abiose S.H. Antimicrobial characteristics of lactic acid bacteria in African yam bean-based drink. [(accessed on 18 March 2022)];Int. Food Res. J. 2019 26:1733–1740. Available online: http://www.ifrj.upm.edu.my/26%20(06)%202019/09%20-%20IFRJ16216.R1-Final.pdf. [Google Scholar]
- 172.Caldera L., Franzetti L. Effect of storage temperature on the microbial composition of ready-to-use vegetables. Curr. Microbiol. 2014;68:133–139. doi: 10.1007/s00284-013-0430-6. [DOI] [PubMed] [Google Scholar]
- 173.Miller A., III, Scanlan R.A., Lee J.S., Libbey L.M. Volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas putrefaciens, Pseudomonas fluorescens, and an Achromobacter species. Appl. Microbiol. 1973;26:18–21. doi: 10.1128/am.26.1.18-21.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mellor G.E., Bentley J.A., Dykes G.A. Evidence for a role of biosurfactants produced by Pseudomonas fluorescens in the spoilage of fresh aerobically stored chicken meat. Food Microbiol. 2011;28:1101–1104. doi: 10.1016/j.fm.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 175.Edwards R.A., Dainty R.H., Hibbard C.M. Volatile compounds produced by meat pseudomonads and related reference strains during growth on beef stored in air at chill temperatures. J. Appl. Bacteriol. 1987;62:403–412. doi: 10.1111/j.1365-2672.1987.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 176.Reichler S.J., Trmčić A., Martin N.H., Boor K.J., Wiedmann M. Pseudomonas fluorescens group bacterial strains are responsible for repeat and sporadic post pasteurization contamination and reduced fluid milk shelf life. Int. J. Dairy Sci. 2018;101:7780–7800. doi: 10.3168/jds.2018-14438. [DOI] [PubMed] [Google Scholar]
- 177.Scatamburlo T.M., Yamazi A.K., Cavicchioli V.Q., Pieri F., Nero L.A. Spoilage potential of Pseudomonas species isolated from goat milk. Int. J. Dairy Sci. 2015;98:759–764. doi: 10.3168/jds.2014-8747. [DOI] [PubMed] [Google Scholar]
- 178.Decimo M., Morandi S., Silvetti T., Brasca M. Characterization of gram-negative psychrotrophic bacteria isolated from Italian bulk tank milk. J. Food Sci. 2014;79:M2081–M2090. doi: 10.1111/1750-3841.12645. [DOI] [PubMed] [Google Scholar]
- 179.Samet-Bali O., Felfoul I., Lajnaf R., Attia H., Ayadi M.A., Route de Soukra B.P.W. Study of proteolytic and lipolytic activities of Pseudomonas spp. isolated from pasteurized milk in Tunisia. J. Agric. Sci. 2013;5:7. doi: 10.5539/jas.v5n7p46. [DOI] [Google Scholar]
- 180.Vercet A., Lopez P., Burgos J. Inactivation of heat-resistant lipase and protease from Pseudomonas fluorescens by manothermosonication. Int. J. Dairy Sci. 1997;80:29–36. doi: 10.3168/jds.S0022-0302(97)75909-5. [DOI] [Google Scholar]
- 181.Caputo L., Quintieri L., Bianchi D.M., Decastelli L., Monaci L., Visconti A., Baruzzi F. Pepsin-digested bovine lactoferrin prevents Mozzarella cheese blue discoloration caused by Pseudomonas fluorescens. Food Microbiol. 2015;46:15–24. doi: 10.1016/j.fm.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 182.Liu P.V. Extracellular toxins of Pseudomonas aeruginosa. J. Infect. Dis. 1974;130:S94–S99. doi: 10.1093/infdis/130.Supplement.S94. [DOI] [PubMed] [Google Scholar]
- 183.Okorhi B.F. Anti-pseudomonas activity of organic acids produced by lactic acid bacteria. Issues Bio. Sci. Pharma. Res. 2014;2:106–114. [Google Scholar]
- 184.Duan X., Chen S., Duan S., Lan C., Yang Z., Cao Y., Miao J. Antibiotic activities of the natural antimicrobial substance produced by Lactobacillus paracasei FX-6 against Pseudomonas putida. LWT. 2020;123:109096. doi: 10.1016/j.lwt.2020.109096. [DOI] [Google Scholar]
- 185.Felicio B.A., Pinto M.S., Oliveira F.S., Lempk M.W., Pires A.C.S., Lelis C.A. Effects of nisin on Staphylococcus aureus count and physicochemical properties of Minas Frescal cheese. J. Dairy Sci. 2015;98:4364–4369. doi: 10.3168/jds.2015-9520. [DOI] [PubMed] [Google Scholar]
- 186.Pinchuk I.V., Beswick E.J., Reyes V.E. Staphylococcal enterotoxins. Toxins. 2010;2:2177–2197. doi: 10.3390/toxins2082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sears P.M., McCarthy K.K. Management and treatment of staphylococcal mastitis. Vet. Clin. Nutr. Am. Food Anim. Pract. 2003;19:171–185. doi: 10.1016/S0749-0720(02)00079-8. [DOI] [PubMed] [Google Scholar]
- 188.Le Loir Y., Baron F., Gautier M. Staphylococcus aureus and food poisoning. [(accessed on 15 February 2022)];Genet. Mol. Res. GMR. 2003 2:63–76. Available online: https://hal.archives-ouvertes.fr/hal-01123026. [PubMed] [Google Scholar]
- 189.Amin M., Jorfi M., Khosravi A.D., Samarbafzadeh A.R., Sheikh A.F. Isolation and identification of Lactobacillus casei and Lactobacillus plantarum from Plants by PCR and detection of their antibacterial activity. Int. J. Biol. Sci. 2009;9:810–814. doi: 10.3923/jbs.2009.810.814. [DOI] [Google Scholar]
- 190.Pal V., Jamuna M., Jeevaratnam K. Isolation and characterization of bacteriocin producing lactic acid bacteria from a South Indian Special dosa (Appam) batter. [(accessed on 12 March 2022)];J. Cult. Collect. 2005 4:53–60. Available online: http://www.bioline.org.br/pdf?cc05007. [Google Scholar]
- 191.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grenier B., Applegate T.J. Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boonen J., Malysheva S.V., Taevernier L., Di Mavungu J.D., De Saeger S., De Spiegeleer B. Human skin penetration of selected model mycotoxins. Toxicology. 2012;301:21–32. doi: 10.1016/j.tox.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 194.EFSA Panel on Contaminants in the Food Chain (CONTAM) Risk assessment of aflatoxins in food. EFSA J. 2020;18:6040. doi: 10.2903/j.efsa.2020.6040. [DOI] [Google Scholar]
- 195.Awuchi C.G., Ondari E.N., Ogbonna C.U., Upadhyay A.K., Baran K., Okpala C.O.R., Korzeniowska M., Guiné R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods. 2021;10:1279. doi: 10.3390/foods10061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Abrunhosa L., Paterson R.R.M., Venâncio A. Biodegradation of Ochratoxin A for Food and Feed Decontamination. Toxins. 2010;2:1078–1099. doi: 10.3390/toxins2051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ropejko K., Twarużek M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins. 2021;13:35. doi: 10.3390/toxins13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haschek W.M., Beasley V.R. Handbook of Toxicology of Chemical Warfare Agents. Elsevier; Amsterdam, The Netherlands: 2009. pp. 353–369. [Google Scholar]
- 199.De Walle J.V., Sergent T., Piront N., Toussaint O., Schneider Y.J., Larondelle Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010;245:291–298. doi: 10.1016/j.taap.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 200.Grenier B., Loureiro-Bracarense A.P., Lucioli J., Pacheco G.D., Cossalter A.M., Moll W.D., Schatzmayr G., Oswald I.P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol. Nutr. Food Res. 2011;55:761–771. doi: 10.1002/mnfr.201000402. [DOI] [PubMed] [Google Scholar]
- 201.Muhialdin B.J., Saari N., Meor Hussin A.S. Review on the Biological Detoxification of Mycotoxins Using Lactic Acid Bacteria to Enhance the Sustainability of Foods Supply. Molecules. 2020;25:2655. doi: 10.3390/molecules25112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nasrollahzadeh A., Mokhtari S., Khomeiri M., Saris P.E.J. Antifungal Preservation of Food by Lactic Acid Bacteria. Foods. 2022;11:395. doi: 10.3390/foods11030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zoghi A., Massoud R., Todorov S.D., Chikindas M.L., Popov I., Smith S., Khosravi-Darani K. Role of the lactobacilli in food bio-decontamination: Friends with benefits. Enzyme Microb. Technol. 2021;150:109861. doi: 10.1016/j.enzmictec.2021.109861. [DOI] [PubMed] [Google Scholar]
- 204.Zhao S., Hao X., Yang F., Wang Y., Fan X., Wang Y. Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns. Foods. 2022;11:195. doi: 10.3390/foods11020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gerez C., Torino M., Rollán G., Font de Valdez G. Prevention of bread mould spoilage using lactic acid bacteria with antifungal properties. Food Control. 2009;20:144–148. doi: 10.1016/j.foodcont.2008.03.005. [DOI] [Google Scholar]
- 206.Lavermicocca P., Valerio F., Visconti A. Antifungal activity of phenyllactic acid against moulds isolated from bakery products. Appl. Environ. Microbiol. 2003;69:634–640. doi: 10.1128/AEM.69.1.634-640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guimarães A., Venancio A., Abrunhosa L. Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:1803–1818. doi: 10.1080/19440049.2018.1500718. [DOI] [PubMed] [Google Scholar]
- 208.Liu A., Zheng Y., Liu L., Chen S., He L., Ao X., Yang Y., Liu S. Decontamination of Aflatoxins by Lactic Acid Bacteria. Curr. Microbiol. 2020;77:3821–3830. doi: 10.1007/s00284-020-02220-y. [DOI] [PubMed] [Google Scholar]
- 209.Peltonen K., El-Nezami H., Haskard C., Ahokas J., Salminen S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001;84:2152–2156. doi: 10.3168/jds.S0022-0302(01)74660-7. [DOI] [PubMed] [Google Scholar]
- 210.El-Nezami H., Kankaanpaa P., Salminen S., Ahokas J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998;36:321–326. doi: 10.1016/S0278-6915(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 211.Śliżewska K., Smulikowska S. Detoxification of aflatoxin B1 and change in microflora pattern by probiotic in vitro fermentation of broiler feed. J. Anim. Feed Sci. 2011;20:300–309. doi: 10.22358/jafs/66187/2011. [DOI] [Google Scholar]
- 212.Sezer Ç., Güven A., Oral N.B., Vatansever L. Detoxification of aflatoxin B1 by bacteriocins and bacteriocinogenic lactic acid bacteria. Turk. J. Vet. Anim. Sci. 2013;37:594–601. doi: 10.3906/vet-1301-31. [DOI] [Google Scholar]
- 213.Taheur F.B., Fedhila K., Chaieb K., Kouidhi B., Bakhrouf A., Abrunhosa L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017;251:1–7. doi: 10.1016/j.ijfoodmicro.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 214.Gomaa E.Z., Abdelall M.F., El-Mahdy O.M. Detoxification of Aflatoxin B1 by Antifungal Compounds from Lactobacillus brevis and Lactobacillus paracasei, Isolated from Dairy Products. Probiotics Antimicrob. Proteins. 2018;10:201–209. doi: 10.1007/s12602-017-9350-2. [DOI] [PubMed] [Google Scholar]
- 215.Møller C.O.A., Freire L., Rosim R.E., Margalho L.P., Balthazar C.F., Franco L.T., Sant′Ana A.S., Corassin C.H., Rattray F.P., de Oliveira C.A.F. Effect of Lactic Acid Bacteria Strains on the Growth and Aflatoxin Production Potential of Aspergillus parasiticus, and Their Ability to Bind Aflatoxin B1, Ochratoxin A, and Zearalenone in vitro. Front. Microbiol. 2021;12:655386. doi: 10.3389/fmicb.2021.655386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Skrinjar M., Rasic J.L., Stojicic V. Lowering of ochratoxin A level in milk by yoghurt bacteria and bifidobacteria. Folia Microbiol. 1996;41:26–28. doi: 10.1007/BF02816335. [DOI] [PubMed] [Google Scholar]
- 217.Böhm J., Grajewski J., Asperger H., Rabus B., Razzazi E. Study on biodegradation of some trichothecenes (NIV, DON, DAS, T-2) and ochratoxin A by use of probiotic microorganisms. Mycot. Res. 2000;16:70–74. doi: 10.1007/BF02942985. [DOI] [PubMed] [Google Scholar]
- 218.Piotrowska M., Zakowska Z. The elimination of ochratoxin A by lactic acid bacteria strains. [(accessed on 23 March 2022)];Pol. J. Microbiol. 2005 54:279–286. Available online: https://pubmed.ncbi.nlm.nih.gov/16599298/ [PubMed] [Google Scholar]
- 219.Fuchs S., Sontag G., Stidl R., Ehrlich V., Kundi M., Knasmüller S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008;46:1398–1407. doi: 10.1016/j.fct.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 220.Abrunhosa L., Inês A., Rodrigues A.I., Guimarães A., Pereira V.L., Parpot P., Venâncio A. Biodegradation of ochratoxin A by Pediococcus parvulus isolated from Douro wines. Int. J. Food Microbiol. 2014;188:45–52. doi: 10.1016/j.ijfoodmicro.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 221.Luz C., Ferrer J., Mañes J., Meca G. Toxicity reduction of ochratoxin A by lactic acid bacteria. Food. Chem. Toxicol. 2018;112:60–66. doi: 10.1016/j.fct.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 222.Wang L., Yue T., Yuan Y., Wang Z., Ye M., Cai R. A new insight into the adsorption mechanism of patulin by the heat-inactive lactic acid bacteria cells. Food Control. 2015;50:104–110. doi: 10.1016/j.foodcont.2014.08.041. [DOI] [Google Scholar]
- 223.Zoghi A., Khosravi-Darani K., Sohrabvandi S., Attar H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J. Appl. Microbiol. 2019;126:1149–1160. doi: 10.1111/jam.14172. [DOI] [PubMed] [Google Scholar]
- 224.Bahati P., Zeng X., Uzizerimana F., Tsoggerel A., Awais M., Qi G., Cai R., Yue T., Yuan Y. Adsorption Mechanism of Patulin from Apple Juice by Inactivated Lactic Acid Bacteria Isolated from Kefir Grains. Toxins. 2021;13:434. doi: 10.3390/toxins13070434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Franco T.S., Garcia S., Hirooka E.Y., Ono Y.S., Dos Santos J.S. Lactic acid bacteria in the inhibition of Fusarium graminearum and deoxynivalenol detoxification. J. Appl. Microbiol. 2011;111:739–748. doi: 10.1111/j.1365-2672.2011.05074.x. [DOI] [PubMed] [Google Scholar]
- 226.Zhai Y., Hu S., Zhong L., Lu Z., Bie X., Zhao H., Zhang C., Lu F. Characterization of deoxynivalenol detoxification by Lactobacillus paracasei LHZ-1 isolated from Yogurt. J. Food Prot. 2019;82:1292–1299. doi: 10.4315/0362-028X.JFP-18-581. [DOI] [PubMed] [Google Scholar]
- 227.Mokoena M.P., Chelule P.K., Gqaleni N. Reduction of fumonisin B1 and zearalenone by lactic acid bacteria in fermented maize meal. J. Food Prot. 2005;68:2095–2099. doi: 10.4315/0362-028X-68.10.2095. [DOI] [PubMed] [Google Scholar]
- 228.Niderkorn V., Boudra H., Morgavi D.P. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol. 2006;101:849–856. doi: 10.1111/j.1365-2672.2006.02958.x. [DOI] [PubMed] [Google Scholar]
- 229.Čvek D., Markov K., Frece J., Friganović M., Duraković L., Delaš F. Adhesion of zearalenone to the surface of lactic acid bacteria cells. [(accessed on 12 March 2022)];Croat. J. Food Technol. Biotechnol. Nutr. 2012 7:49–52. Available online: https://hrcak.srce.hr/file/123168. [Google Scholar]
- 230.Król A., Pomastowski P., Rafińska K., Railean-Plugaru V., Walczak J., Buszewski B. Microbiology neutralization of zearalenone using Lactococcus lactis and Bifidobacterium sp. Anal. Bioanal. Chem. 2018;410:943–952. doi: 10.1007/s00216-017-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Piotrowska M. Microbiological Decontamination of Mycotoxins: Opportunities and Limitations. Toxins. 2021;13:819. doi: 10.3390/toxins13110819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Monbaliu S., Van Poucke C., Detavernier C., Dumoulin F., Van De Velde M., Schoeters E., Van Dyck S., Averkieva O., Van Peteghem C., De Saeger S. Occurrence of Mycotoxins in Feed as Analyzed by a Multi-Mycotoxin LC-MS/MS Method. J. Agric. Food Chem. 2010;58:66–71. doi: 10.1021/jf903859z. [DOI] [PubMed] [Google Scholar]
- 233.Allam A., Abdeen A., Devkota H.P., Ibrahim S.S., Youssef G., Soliman A., Abdel-Daim M.M., Alzahrani K.J., Shoghy K., Ibrahim S.F., et al. N-Acetylcysteine Alleviated the Deltamethrin-Induced Oxidative Cascade and Apoptosis in Liver and Kidney Tissues. Int. J. Environ. Res. Public Health. 2022;19:638. doi: 10.3390/ijerph19020638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.D′Haenens J.P., McDonald K.W., Langley R.L., Higgins S.A., Scott R., Farquhar P.N., Meggs W.J. Aldicarb: A Case Series of Watermelon-Borne Carbamate Toxicity. J. Agromed. 2013;18:174–177. doi: 10.1080/1059924X.2013.766141. [DOI] [PubMed] [Google Scholar]
- 235.Abou-Arab A.A.K. Effect of Ras cheese manufacturing on the stability of DDT and its metabolites. Food Chem. 1997;64:115–119. doi: 10.1016/S0308-8146(96)00214-2. [DOI] [Google Scholar]
- 236.Nasution L., Bakti D., Agusnar H., Harahap E.M. Role of Lactobacillus sakei strain pro7 to reduce dichlorodiphenyl trichloroethane level. J. Phys. 2018;1116:042025. doi: 10.1088/1742-6596/1116/4/042025. [DOI] [Google Scholar]
- 237.Cho K.M., Math R.K., Islam S.M., Lim W.J., Hong S.Y., Kim J.M., Yun M.G., Cho J.J., Yun H.D. Biodegradation of chlorpyrifos by lactic acid bacteria during kimchi fermentation. J. Agric. Food Chem. 2009;57:1882–1889. doi: 10.1021/jf803649z. [DOI] [PubMed] [Google Scholar]
- 238.Islam S.M.A., Math R.K., Cho K.M., Lim W.J., Hong S.Y., Kim J.M., Yun M.G., Cho J.J., Yun H.D. Organophosphorus hydrolase (OpdB) of Lactobacillus brevis WCP902 from kimchi is able to degrade organophosphorus pesticides. J. Agric. Food Chem. 2010;58:5380–5386. doi: 10.1021/jf903878e. [DOI] [PubMed] [Google Scholar]
- 239.Maden B., Kumral A.Y. Degradation Trends of Some Insecticides and Microbial Changes during Sauerkraut Fermentation under Laboratory Conditions. J. Agric. Food Chem. 2020;68:14988–14995. doi: 10.1021/acs.jafc.0c03948. [DOI] [PubMed] [Google Scholar]
- 240.Kumral Y.A., Kumral N.A., Kolcu A., Maden B., Artik B. Simulation Study for the Degradation of Some Insecticides during Different Black Table Olive Processes. ACS Omega. 2020;5:14164–14172. doi: 10.1021/acsomega.0c01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dorđević T.M., Siler-Marinkovic S.S., Durovic R.D., Dimitrijevic-Brankovic S.I., Gajic Umiljendic J.S. Stability of the pyrethroid pesticide bifenthrin in milled wheat during thermal processing, yeast and lactic acid fermentation, and storage. J. Sci. Food Agric. 2013;93:3377–3383. doi: 10.1002/jsfa.6188. [DOI] [PubMed] [Google Scholar]
- 242.Liu F., Bai J., Huang W., Li F., Ke W., Zhang Y., Xie D., Zhang B., Guo X. Characterization of a novel beta-cypermethrin-degrading strain of Lactobacillus pentosus 3-27 and its effects on bioremediation and the bacterial community of contaminated alfalfa silage. J. Hazard. Mater. 2022;423:127101. doi: 10.1016/j.jhazmat.2021.127101. [DOI] [PubMed] [Google Scholar]
- 243.Dorđević T.M., Siler-Marinković S.S., Durović-Pejčev R.D., Dimitrijević-Branković S.I., Gajić Umiljendić J.S. Dissipation of pirimiphos-methyl during wheat fermentation by Lactobacillus plantarum. Lett. Appl. Microbiol. 2013;57:412–419. doi: 10.1111/lam.12128. [DOI] [PubMed] [Google Scholar]
- 244.Zhou X.W., Liu H.F., Zhao X.H. The potencies of three microorganisms to dissipate four organophosphorus pesticides in three food materials during traditional fermentation. J. Food Sci. Technol. 2015;52:7353–7360. doi: 10.1007/s13197-015-1848-6. [DOI] [Google Scholar]
- 245.Yuan S., Li C., Yu H., Xie Y., Guo Y., Yao W. Screening of lactic acid bacteria for degrading organophosphorus pesticides and their potential protective effects against pesticide toxicity. LWT. 2021;147:111672. doi: 10.1016/j.lwt.2021.111672. [DOI] [Google Scholar]
- 246.Zhang Y.H., Xu D., Zhao X.H., Song Y., Liu Y.L., Li H.N. Biodegradation of two organophosphorus pesticides in whole corn silage as affected by the cultured Lactobacillus plantarum. 3 Biotech. 2016;6:73. doi: 10.1007/s13205-016-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rezaei F., Nejati R., Sayadi M., Nematollahi A. Diazinon reduction in apple juice using probiotic bacteria during fermentation and storage under refrigeration. Environ. Sci. Pollut. Res. 2021;28:61213–61224. doi: 10.1007/s11356-021-15007-w. [DOI] [PubMed] [Google Scholar]
- 248.Zhang Y.H., Xu D., Liu J.Q., Zhao X.H. Enhanced degradation of five organophosphorus pesticides in skimmed milk by lactic acid bacteria and its potential relationship with phosphatase production. Food Chem. 2014;164:173–178. doi: 10.1016/j.foodchem.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 249.Mohammadi M., Shadnoush M., Sohrabvandi S., Yousefi M., Khorshidian N., Mortazavian A.M. Probiotics as Potential Detoxification Tools for Mitigation of Pesticides: A Mini Review. Int. J. Food Sci. Technol. 2020;56:2078–2087. doi: 10.1111/ijfs.14880. [DOI] [Google Scholar]
- 250.Jänsch A., Korakli M., Vogel R.F., Gänzle M.G. Glutathione reductase from Lactobacillus sanfranciscensis DSM 20451T: Contribution to oxygen tolerance and thiol exchange reactions in wheat sourdoughs. Appl. Environ. Microbiol. 2007;73:4469–4476. doi: 10.1128/AEM.02322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang A.N., Cai C.J., Zeng X.F., Zhang F.R., Zhang G.L., Thacker P.A., Wang J.J., Qiao S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Microbiol. 2013;114:1582–1591. doi: 10.1111/jam.12188. [DOI] [PubMed] [Google Scholar]
- 252.Chen W., Zhai Q. Applications of Lactic Acid Bacteria in Heavy Metal Pollution Environment. In: Chen W., Narbad A., editors. Lactic Acid Bacteria in Foodborne Hazards Reduction. 1st ed. Springer; Singapore: 2018. pp. 213–248. [DOI] [Google Scholar]
- 253.Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M.R., Sadeghi M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021;12:643972. doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kumar N., Kumari V., Ram C., Thakur K., Tomar S. Bio-prospectus of cadmium bioadsorption by lactic acid bacteria to mitigate health and environmental impacts. Appl. Microbiol. Biotechnol. 2018;102:1599–1615. doi: 10.1007/s00253-018-8743-9. [DOI] [PubMed] [Google Scholar]
- 255.Lin D., Cao H., Zhong Y., Huang Y., Zou J., He Q., Ji R., Qin T., Chen Y., Wang D., et al. Screening and identification of Lactic acid bacteria from Ya’an pickle water to effectively remove Pb2. AMB Express. 2019;9:10. doi: 10.1186/s13568-018-0724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mrvčić J., Stanzer D., Šolić E., Stehlik-Tomas V. Interaction of lactic acid bacteria with metal ions: Opportunities for improving food safety and quality. World J. Microbiol. Biotechnol. 2012;28:2771–2782. doi: 10.1007/s11274-012-1094-2. [DOI] [PubMed] [Google Scholar]
- 257.Cheng W.W.L., Gobas F.A.P.C. Assessment of Human Health Risks of Consumption of Cadmium Contaminated Cultured Oysters. Hum. Ecol. Risk Assess. 2007;13:370–382. doi: 10.1080/10807030701226301. [DOI] [Google Scholar]
- 258.Witkowska D., Słowik J., Chilicka K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules. 2021;26:6060. doi: 10.3390/molecules26196060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Solon O., Riddell T.J., Quimbo S.A., Butrick E., Aylward G.P., Lou Bacate M., Peabody J.W. Associations between cognitive function, blood lead concentration, and nutrition among children in the central Philippines. J. Pediatr. 2008;152:237–243. doi: 10.1016/j.jpeds.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 260.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2017;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Orisakwe O.E. Lead and cadmium in public health in Nigeria: Physicians neglect and pitfall in patient management. N. Am. J. Med. Sci. 2014;6:61–70. doi: 10.4103/1947-2714.127740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Koyu A., Gokcimen A., Ozguner F., Bayram D.S., Kocak A. Evaluation of the effects of cadmium on rat liver. Mol. Cell Biochem. 2006;284:81–85. doi: 10.1007/s11010-005-9017-2. [DOI] [PubMed] [Google Scholar]
- 263.Nordberg G.F., Fowler B.A., Nordberg M., Friberg L.T. Introduction—General Considerations and International Perspectives. In: Nordberg G.F., Fowler B.A., Nordberg M., Friberg L.T., editors. Handbook on the Toxicology of Metals. 4th ed. Academic Press; Burlington, MA, USA: 2014. pp. 1–9. [DOI] [Google Scholar]
- 264.Cuevas-González P.F., González-Córdova A.F., Vallejo-Cordoba B., Aguilar-Toalá J.E., Hall F.G., Urbizo-Reyes U.C., Liceaga A.M., Hernandez-Mendoza A., García H.S. Protective role of lactic acid bacteria and yeasts as dietary carcinogen-binding agents—A review. Crit. Rev. Food Sci. Nutr. 2022;62:160–180. doi: 10.1080/10408398.2020.1813685. [DOI] [PubMed] [Google Scholar]
- 265.Nordberg G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009;238:192–200. doi: 10.1016/j.taap.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 266.Jihen E.H., Fatima H., Nouha A., Baati T., Imed M., Abdelhamid K. Cadmium retention increase: A probable key mechanism of the protective effect of zinc on cadmium-induced toxicity in the kidney. Toxicol. Lett. 2010;196:104–109. doi: 10.1016/j.toxlet.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 267.Ahamed M., Siddiqui M.K. Environmental lead toxicity and nutritional factors. Clin. Nutr. 2007;26:400–408. doi: 10.1016/j.clnu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 268.Farmand F., Ehdaie A., Roberts C.K., Sindhu R.K. Lead-induced dysregulation of superoxide dismutases, catalase, glutathione peroxidase, and guanylate cyclase. Environ. Res. 2005;98:33–39. doi: 10.1016/j.envres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 269.Valko M., Morris H., Cronin M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 270.Gomes-Caminero A., Howe P., Hughes M., Kenyon E., Lewis D.R., Moore M., Ng J.C., Aitio A., Becking G. Environmental Health Criteria 224 Arsenic and Arsenic Compounds. 2nd ed. World Health Organization; Geneva, Switzerland: 2001. [(accessed on 14 March 2022)]. Available online: http://apps.who.int/iris/bitstream/handle/10665/42366/WHO_EHC_224.pdf;jsessionid=BEED054DABAF62A47901D273F2A2531A?sequence=1. [Google Scholar]
- 271.Patel A., Sv A., Shah N., Verma D.K. Lactic acid bacteria as metal quenchers to improve food safety and quality. [(accessed on 14 March 2022)];AgroLife Sci. J. 2017 6:146–154. Available online: https://www.cabdirect.org/cabdirect/abstract/20183009341. [Google Scholar]
- 272.Babel S., Kurniawan T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003;97:219–243. doi: 10.1016/S0304-3894(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 273.Rahman N., Haseen U., Rashid M. Synthesis and characterization of polyacrylamide zirconium (IV) iodate ion-exchanger: Its application for selective removal of lead (II) from wastewater. Arab. J. Chem. 2007;10:S1765–S1773. doi: 10.1016/j.arabjc.2013.06.029. [DOI] [Google Scholar]
- 274.Wang J., Zhao Y., Zhang P., Yang L., Xu H., Xi G. Adsorption characteristics of a novel ceramsite for heavy metal removal from stormwater runoff. Chin. J. Chem. Eng. 2018;26:96–103. doi: 10.1016/j.cjche.2017.04.011. [DOI] [Google Scholar]
- 275.Nezamzadeh-Ejhieh A., Khorsandi S. Photocatalytic degradation of 4-nitrophenol with ZnO supported nano-clinoptilolite zeolite. J. Ind. Eng. Chem. 2014;20:937–946. doi: 10.1016/j.jiec.2013.06.026. [DOI] [Google Scholar]
- 276.Kobya M., Demirbas E., Senturk E., Ince M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005;96:1518–1521. doi: 10.1016/j.biortech.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 277.Wang J., Chen C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009;27:195–226. doi: 10.1016/j.biotechadv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 278.Bisanz J.E., Enos M.K., Mwanga J.R., Changalucha J., Burton J.P., Gloor G.B., Reid G. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio. 2014;5:e01580-14. doi: 10.1128/mBio.01580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yan H., Carter C.E., Xu C., Singh P.K., Jones M.M., Johnson J.E., Dietrich M.S. Cadmium-induced apoptosis in the urogenital organs of the male rat and its suppression by chelation. J. Toxicol. Environ. Health. 1997;52:149–168. doi: 10.1080/00984109708984058. [DOI] [PubMed] [Google Scholar]
- 280.Kojima S., Sugimura Y., Hirukawa H., Kiyozumi M., Shimada H., Funakoshi T. Effects of Dithiocarbamates on Testicular Toxicity in Rats Caused by Acute Exposure to Cadmium. Toxicol. Appl. Pharmacol. 1992;116:24–29. doi: 10.1016/0041-008X(92)90140-N. [DOI] [PubMed] [Google Scholar]
- 281.Jelenković A., Jovanović M.D., Stevanović I., Petronijević N., Bokonjić D., Zivković J., Igić R. Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother Res. 2014;28:82–87. doi: 10.1002/ptr.4962. [DOI] [PubMed] [Google Scholar]
- 282.Kratochvil D., Volesky B. Advances in the biosorption of heavy metals. Trends Biotechnol. 1998;16:291–300. doi: 10.1016/S0167-7799(98)01218-9. [DOI] [Google Scholar]
- 283.Beveridge T.J., Fyfe W.S. Metal fixation by bacterial cell walls. Can. J. Earth Sci. 1985;22:1893–1898. doi: 10.1139/e85-204. [DOI] [Google Scholar]
- 284.Iskandar I., Koike K., Sendjaja P. Identifying groundwater arsenic contamination mechanisms in relation to arsenic concentrations in water and host rocks. Environ. Earth Sci. 2012;65:2015–2026. doi: 10.1007/s12665-011-1182-x. [DOI] [Google Scholar]
- 285.Tural B., Ertaş E., Enez B., Fincan S.A., Tural S. Preparation and characterization of a novel magnetic biosorbent functionalized with biomass of Bacillus Subtilis: Kinetic and isotherm studies of biosorption processes in the removal of Methylene Blue. J. Environ. Chem. Eng. 2017;5:4795–4802. doi: 10.1016/j.jece.2017.09.019. [DOI] [Google Scholar]
- 286.Kinoshita H., Sohma Y., Ohtake F., Ishida M., Kawai Y., Kitazawa H., Saito T., Kimura K. Biosorption of heavy metals by lactic acid bacteria and identification of mercury binding protein. Res. Microbiol. 2013;164:701–709. doi: 10.1016/j.resmic.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 287.Issazadeh K., Jahanpour N., Pourghorbanali F., Raeisi G., Faekhondeh J. Heavy metals resistance by bacterial strains. Ann. Biol. Res. 2013;4:60–63. [Google Scholar]
- 288.Monachese M., Burton J.P., Reid G. Bioremediation and tolerance of humans to heavy metals through microbial processes: A potential role for probiotics? Appl. Environ. Microbiol. 2012;78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Halttunen T., Finell M., Salminen S. Arsenic removal by native and chemically modified lactic acid bacteria. Int. J. Food Microbiol. 2007;120:173–178. doi: 10.1016/j.ijfoodmicro.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 290.Landersjö C., Yang Z., Huttunen E., Widmalm G. Structural studies of the exopolysaccharide produced by Lactobacillus rhamnosus strain GG (ATCC 53103) Biomacromolecules. 2002;3:880–884. doi: 10.1021/bm020040q. [DOI] [PubMed] [Google Scholar]
- 291.Hao Z., Reiske H.R., Wilson D.B. Characterization of cadmium uptake in Lactobacillus plantarum and isolation of cadmium and manganese uptake mutants. Appl. Environ. Microbiol. 1999;65:4741–4745. doi: 10.1128/AEM.65.11.4741-4745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Teemu H., Seppo S., Jussi M., Raija T., Kalle L. Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int. J. Food Microbiol. 2008;125:170–175. doi: 10.1016/j.ijfoodmicro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 293.Gerbino E., Mobili P., Tymczyszyn E., Fausto R., Gómez-Zavaglia A. FTIR spectroscopy structural analysis of the interaction between Lactobacillus kefir S-layers and metal ions. J. Mol. Struct. 2011;987:186–192. doi: 10.1016/j.molstruc.2010.12.012. [DOI] [Google Scholar]
- 294.Boonyodying K., Watcharasupat T., Yotpanya W., Kitti T., Kawang W., Kunthalert D., Sitthisak S. Factors Affecting the Binding of a Recombinant Heavy Metal-Binding Domain (CXXC motif) Protein to Heavy Metals. Environ. Asia. 2012;5:70–75. doi: 10.14456/ea.2012.20. [DOI] [Google Scholar]
- 295.Sitthisak S., Knutsson L., Webb J.W., Jayaswal R.K. Molecular characterization of the copper transport system in Staphylococcus aureus. Microbiology. 2007;153:4274–4283. doi: 10.1099/mic.0.2007/009860-0. [DOI] [PubMed] [Google Scholar]
- 296.Tian F., Zhai Q., Zhao J., Liu X., Wang G., Zhang H., Zhang H., Chen W. Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol. Trace Elem. Res. 2012;150:264–271. doi: 10.1007/s12011-012-9462-1. [DOI] [PubMed] [Google Scholar]
- 297.Zhai Q., Wang G., Zhao J., Liu X., Tian F., Zhang H., Chen W. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl. Environ. Microbiol. 2013;79:1508–1515. doi: 10.1128/AEM.03417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tian F., Xiao Y., Li X., Zhai Q., Wang G., Zhang Q., Zhang H., Chen W. Protective Effects of Lactobacillus plantarum CCFM8246 against Copper Toxicity in Mice. PLoS ONE. 2015;10:e0143318. doi: 10.1371/journal.pone.0143318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pakdel M., Soleimanian-Zad S., Akbari-Alavijeh S. Screening of lactic acid bacteria to detect potent biosorbents of lead and cadmium. Food Control. 2019;100:144–150. doi: 10.1016/j.foodcont.2018.12.044. [DOI] [Google Scholar]
- 300.Kinoshita H., Ohtake F., Ariga Y., Kimura K. Comparison and characterization of biosorption by Weissella viridescens MYU 205 of periodic group 12 metal ions. Anim. Sci. J. 2016;87:271–276. doi: 10.1111/asj.12425. [DOI] [PubMed] [Google Scholar]
- 301.Abou-Shanab R.A., van Berkum P., Angle J.S. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere. 2007;68:360–367. doi: 10.1016/j.chemosphere.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 302.Bhakta J.N., Ohnishi K., Munekage Y., Iwasaki K., Wei M.Q. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J. Appl. Microbiol. 2012;112:1193–1206. doi: 10.1111/j.1365-2672.2012.05284.x. [DOI] [PubMed] [Google Scholar]
- 303.Akinbowale O.L., Peng H., Grant P., Barton M.D. Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int. J. Antimicrob. Agents. 2007;30:177–182. doi: 10.1016/j.ijantimicag.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 304.Elsanhoty R.M., Al-Turki I.A., Ramadan M.F. Application of lactic acid bacteria in removing heavy metals and aflatoxin B1 from contaminated water. Water Sci. Technol. 2016;74:625–638. doi: 10.2166/wst.2016.255. [DOI] [PubMed] [Google Scholar]
- 305.Halttunen T., Collado M.C., El-Nezami H., Meriluoto J., Salminen S. Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett. Appl. Microbiol. 2008;46:160–165. doi: 10.1111/j.1472-765X.2007.02276.x. [DOI] [PubMed] [Google Scholar]
- 306.Bhakta J.N., Ohnishi K., Munekage Y., Iwasaki K. Isolation and Probiotic Characterization of Arsenic-Resistant Lactic Acid Bacteria for Uptaking Arsenic. Int. J. Bioeng. Life Sci. 2010;11:831–838. doi: 10.5281/zenodo.1083022. [DOI] [Google Scholar]
- 307.Sofu A., Sayilgan E., Guney G. Experimental Design for Removal of Fe(II) and Zn(II) Ions by Different Lactic Acid Bacteria Biomasses. Int. J. Environ. Res. 2015;9:93–100. doi: 10.22059/ijer.2015.878. [DOI] [Google Scholar]
- 308.Yilmaz M., Tay T., Kivanc M., Turk H. Removal of copper ions from aqueous solution by a lactic acid bacterium. [(accessed on 22 March 2022)];Braz. J. Chem. Eng. 2010 27:309–314. doi: 10.1590/S0104-66322010000200009. Available online: https://www.scielo.br/j/bjce/a/tt74Wx9drX6S3pV4×99Nhyb/?lang=en&format=pdf. [DOI] [Google Scholar]
- 309.Zhai Q., Yue X., Fengwei T., Gang W., Jianxi Z., Xiaoming L., Yong Q., Hao Z., Wei C. Protective effects of lactic acid bacteria-fermented soymilk against chronic cadmium toxicity in mice. RSC Adv. 2015;5:4648–4658. doi: 10.1039/C4RA12865F. [DOI] [Google Scholar]
- 310.Jama A.M., Mitić-Ćulafić D., Kolarević S., Đurašević S.F., Knežević-Vukčević J. Protective effect of probiotic bacteria against cadmium-induced genotoxicity in rat hepatocytes in vivo and in vitro. Arch. Biol. Sci. 2012;64:1197–1206. doi: 10.2298/ABS1203197J. [DOI] [Google Scholar]
- 311.Zhai Q., Wang G., Zhao J., Liu X., Narbad A., Chen Y.Q., Zhang H., Tian F., Chen W. Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl. Environ. Microbiol. 2014;80:4063–4071. doi: 10.1128/AEM.00762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhai Q., Tian F., Zhao J., Zhang H., Narbad A., Chen W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl. Environ. Microbiol. 2016;82:4429–4440. doi: 10.1128/AEM.00695-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Halttunen T., Salminen S., Tahvonen R. Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int. J. Food Microbiol. 2007;114:30–35. doi: 10.1016/j.ijfoodmicro.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 314.Topcu A., Bulat T. Removal of cadmium and lead from aqueous solution by Enterococcus faecium strains. J. Food Sci. 2010;75:T13–T17. doi: 10.1111/j.1750-3841.2009.01429.x. [DOI] [PubMed] [Google Scholar]
- 315.Singh A.L., Sarma P.N. Removal of Arsenic (III) from Waste Water Using Lactobacillus acidophilus. Bioremediat. J. 2010;14:92–97. doi: 10.1080/10889861003767050. [DOI] [Google Scholar]
- 316.Yu L., Zhai Q., Liu X., Wang G., Zhang Q., Zhao J., Narbad A., Zhang H., Tian F., Chen W. Lactobacillus plantarum CCFM639 alleviates aluminium toxicity. Appl. Microbiol. Biotechnol. 2016;100:1891–1900. doi: 10.1007/s00253-015-7135-7. [DOI] [PubMed] [Google Scholar]
- 317.Schut S., Zauner S., Hampel G., König H., Claus H. Biosorption of cooper by wine-relevant lactobacilli. Int. J. Food Microbiol. 2011;145:126–131. doi: 10.1016/j.ijfoodmicro.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 318.Mrvcic J., Stanzer D., Bacun-Druzina V., Stehlik-Tomas V. Copper binding by lactic acid bacteria (LAB) Biosci. Microflora. 2009;28:1–6. doi: 10.12938/bifidus.28.1. [DOI] [Google Scholar]
- 319.Mrvčić J., Prebeg T., Barišić L., Stanzer D., Bačun-Družina V., Stehlik-Tomas V. Zinc Binding by Lactic Acid Bacteria. Food Technol. Biotechnol. 2009;47:381–388. [Google Scholar]
- 320.Zhai Q., Tian F., Wang G., Zhao J., Liu X., Cross K., Zhang H., Narbad A., Chen W. The cadmium binding characteristics of a lactic acid bacterium in aqueous solutions and its application for removal of cadmium from fruit and vegetable juices. RSC Adv. 2016;6:5990–5998. doi: 10.1039/C5RA24843D. [DOI] [Google Scholar]
- 321.Petroski W., Minich D.M. Is There Such a Thing as "Anti-Nutrients"? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020;12:2929. doi: 10.3390/nu12102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Frontela C., García-Alonso F.J., Ros G., Martinez C. Phytic acid and inositol phosphates in raw flours and infant cereals: The effect of processing. J. Food Comp. Anal. 2008;21:343–350. doi: 10.1016/j.jfca.2008.02.003. [DOI] [Google Scholar]
- 323.Songré-Ouattara L.T., Mouquet-Rivier C., Icard-Vernière C., Humblot C., Diawara B., Guyot J.P. Enzyme activities of lactic acid bacteria from a pearl millet fermented gruel (ben-saalga) of functional interest in nutrition. Int. J. Food Microbiol. 2008;128:395–400. doi: 10.1016/j.ijfoodmicro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 324.Sharma N., Angural S., Rana M., Puri N., Kondepudi K.K., Gupta N. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 2020;96:1–12. doi: 10.1016/j.tifs.2019.12.001. [DOI] [Google Scholar]
- 325.Castro-Alba V., Lazarte C.E., Perez-Rea D., Carlsson N., Almgren A., Bergenståhl B., Granfeldt Y. Fermentation of pseudocereals quinoa, canihua, and amaranth to improve mineral accessibility through degradation of phytate. J. Sci. Food Agric. 2019;99:5239–5248. doi: 10.1002/jsfa.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Soto-Blanco B. Herbal glycosides in healthcare. In: Mandal S.C., Nayak A.K., Dhara A.K., editors. Herbal Biomolecules in Healthcare Applications. 1st ed. Academic Press; Cambridge, MA, USA: 2022. pp. 239–282. [DOI] [Google Scholar]
- 327.Menon R., Munjal N., Sturino J.M. Characterization of amygdalin-degrading Lactobacillus species. J. Appl. Microbiol. 2015;118:443–453. doi: 10.1111/jam.12704. [DOI] [PubMed] [Google Scholar]
- 328.Lei V., Amoa-Awua W.K., Brimer L. Degradation of cyanogenic glycosides by Lactobacillus plantarum strains from spontaneous cassava fermentation and other microorganisms. Int. J. Food Microbiol. 1999;53:169–184. doi: 10.1016/S0168-1605(99)00156-7. [DOI] [PubMed] [Google Scholar]
- 329.Nivetha N., Suvarna V.C., Abhishek R.U. Reduction of Phenolics, Tannins and Cyanogenic Glycosides Contents in Fermented Beverage of Linseed (Linum usitatissimum) [(accessed on 27 March 2022)];Int. J. Food. Ferment. Technol. 2018 8:185–190. doi: 10.30954/2277-9396.02.2018.8. Available online: https://pdfs.semanticscholar.org/9a42/b58de8516f63e3292b071074e804679cbbbf.pdf. [DOI] [Google Scholar]
- 330.Alphonce S., Kaale L.D. Assessment of Biochemical Changes during Fermentation Process for Production of Traditional Fermented Cassava Meal “Mchuchume”. [(accessed on 27 March 2022)];Tanz. J. Sci. 2020 46:228–240. Available online: https://www.ajol.info/index.php/tjs/article/view/196280. [Google Scholar]
- 331.Chongtham N., Bisht M.S., Premlata T., Bajwa H.K., Sharma V., Santosh O. Quality improvement of bamboo shoots by removal of antinutrients using different processing techniques: A review. J. Food Sci. Technol. 2022;59:1–11. doi: 10.1007/s13197-021-04987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Nionelli L., Montemurro M., Pontonio E., Verni M., Gobbetti M., Rizzello C.G. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L.) sourdough fermentation and wheat bread fortification. Int. J. Food Microbiol. 2018;279:14–25. doi: 10.1016/j.ijfoodmicro.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 333.Murru N., Blaiotta G., Peruzy M.F., Santonicola S., Mercogliano R., Aponte M. Screening of Oxalate Degrading Lactic Acid Bacteria of Food Origin. Ital. J. Food Saf. 2017;6:6345. doi: 10.4081/ijfs.2017.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Campieri C., Campieri M., Bertuzzi V., Swennen E., Matteuzzi D., Stefoni S., Pirovano F., Centi C., Ulisse S., Famularo G., et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 2001;60:1097–1105. doi: 10.1046/j.1523-1755.2001.0600031097.x. [DOI] [PubMed] [Google Scholar]
- 335.Azcarate-Peril M.A., Bruno-Barcena J.M., Hassan H.M., Klaenhammer T.R. Transcriptional and functional analysis of oxalyl-coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes from Lactobacillus acidophilus. Appl. Environ. Microbiol. 2006;72:1891–1899. doi: 10.1128/AEM.72.3.1891-1899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Gomathi S., Sasikumar P., Anbazhagan K., Sasikumar S., Kavitha M., Selvi M.S., Selvam G.S. Screening of indigenous oxalate degrading lactic acid bacteria from human faeces and South Indian fermented foods: Assessment of probiotic potential. Sci. World J. 2014;2014:648059. doi: 10.1155/2014/648059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hokama S., Honma Y., Toma C., Ogawa Y. Oxalate-degrading Enterococcus faecalis. Microbiol. Immunol. 2000;44:235–240. doi: 10.1111/j.1348-0421.2000.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 338.Wigner P., Bijak M., Saluk-Bijak J. Probiotics in the Prevention of the Calcium Oxalate Urolithiasis. Cells. 2022;11:284. doi: 10.3390/cells11020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Schuppan D., Pickert G., Ashfaq-Khan M., Zevallos V. Non-celiac wheat sensitivity: Differential diagnosis, triggers and implications. Best Pract. Res. Clin. Gastroenterol. 2015;29:469–476. doi: 10.1016/j.bpg.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 340.Caminero A., McCarville J.L., Zevallos V.F., Pigrau M., Yu X.B., Jury J., Galipeau H.J., Clarizio A.V., Casqueiro J., Murray J.A., et al. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology. 2019;156:2266–2280. doi: 10.1053/j.gastro.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 341.Huang X., Schuppan D., Rojas Tovar L.E., Zevallos V.F., Loponen J., Gänzle M. Sourdough fermentation degrades wheat alpha-amylase/trypsin inhibitor (ATI) and reduces pro-inflammatory Activity. Foods. 2020;9:943. doi: 10.3390/foods9070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.