Abstract
The pathophysiology associated with sickle cell disease (SCD) includes hemolytic anemia, vaso-occlusive events, and ultimately end organ damage set off by the polymerization of deoxygenated hemoglobin S (HbS) into long fibers and sickling of red blood cells (RBCs). One approach toward mitigating HbS polymerization is to pharmacologically stabilize the oxygenated (R) conformation of HbS and thereby reduce sickling frequency and SCD pathology. GBT440 is an α-subunit-specific modifying agent that has recently been reported to increase HbS oxygen binding affinity and consequently delay in vitro polymerization. In addition, animal model studies have demonstrated the potential for GBT440 to be a suitable therapeutic for daily oral dosing in humans. Here, we report an optimized method for detecting GBT440 intermediates in human patient hemolysate using a combination of HPLC and mass spectrometry analysis. First, oxygen dissociation curves (ODCs) analyzed from patient blood showed that oxygen affinity increased in a dose dependent manner. Second, HPLC and integrated mass spectrometric analysis collectively confirmed that GBT440 labeling was specific to the α N-terminus thereby ruling out other potential ligand binding sites. Finally, the results from this optimized analytical approach allowed us to detect a stable α-specific GBT440 adduct in the patient’s hemolysate in a dose dependent manner. The results and methods presented in this report could therefore potentially help therapeutic monitoring of GBT440 induced oxygen affinity and reveal critical insight into the biophysical properties of GBT440 Hb complexes.
Graphical Abstract
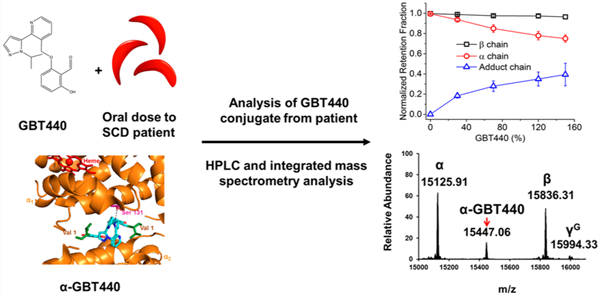
Sickle Cell Disease (SCD) is a genetic disorder resulting from a single point mutation at the sixth position of the hemoglobin β subunit (β6Glu → Val). This valine amino acid replacement results in a hydrophobic sticky patch on the β subunit surface of HbS.1 Under hypoxic conditions, the resulting HbS molecules polymerize into long fibers which lead to formation of sickled red blood cells (SS RBC), also referred to as “sickling”.1 SCD is characterized by hemolytic anemia, inflammation, vaso-occlusion, and chronic vasculopathy that ultimately lead to end organ damage and progressive organ failure.2 After several cycles of sickling and unsickling, SS RBCs rupture, releasing a mixture of fibrils and Hb molecules into circulation.3 Therapeutic interventions have been designed to control SCD related hemolytic symptoms by targeting HbS polymerization and subsequent sickling (for review, see Eaton and Bunn4). To date, SCD treatment is primarily limited to the use of hydroxyurea (HU), blood transfusions,5 and supportive therapy during a vaso-occlusive crisis. HU treatment (an FDA approved drug for SCD) induces HbF expression and, as a result, reduces HbS polymerization. An alternative to HbF induction could be the use of allosteric modulators to reduce polymerization by preferentially binding to the R (oxygenated) quaternary conformation of Hb, which does not polymerize.6 Because of the high affinity of the R conformation, the potential downside of this approach is that the oxygen delivery capacity of the blood may be reduced.5,7 A variety of allosteric modulators have been identified, including most recently GBT440 (also known as Voxelotor).8,9
GBT440 (2-hydroxyl-6 ((2-(1-isopropyl-1H-pyrazol-5-yl) pyridine-3-yl) methoxy) benzaldehyde) is a small molecule drug developed for the treatment of SCD, based on its unique Hb-oxygen modifying properties.8 While other ligand binding sites are possible, recent studies have indicated that GBT440 forms a reversible covalent bond (via a Schiff base) with the HbS α chain N-terminal valine (in a 1:1 stoichiometry to the HbS tetramer) which ultimately results in allosteric modification and stabilization of the desired oxygenated Hb conformation.9,10 Recent X-ray analysis has also shown that the N-terminal valine residue of one α globin chain exhibits a hydrogen bond to Ser 131 of the second α-chain via the pyrazole substituent (see Figure 1). This additional hydrogen bond interaction has been suggested to prevent binding of a second GBT440 molecule to the remaining α chain N-terminus and consequently induces changes in in Hb structure, including changes to the dimer:dimer interface of Hb as well as changes to the heme pocket.8
Figure 1.
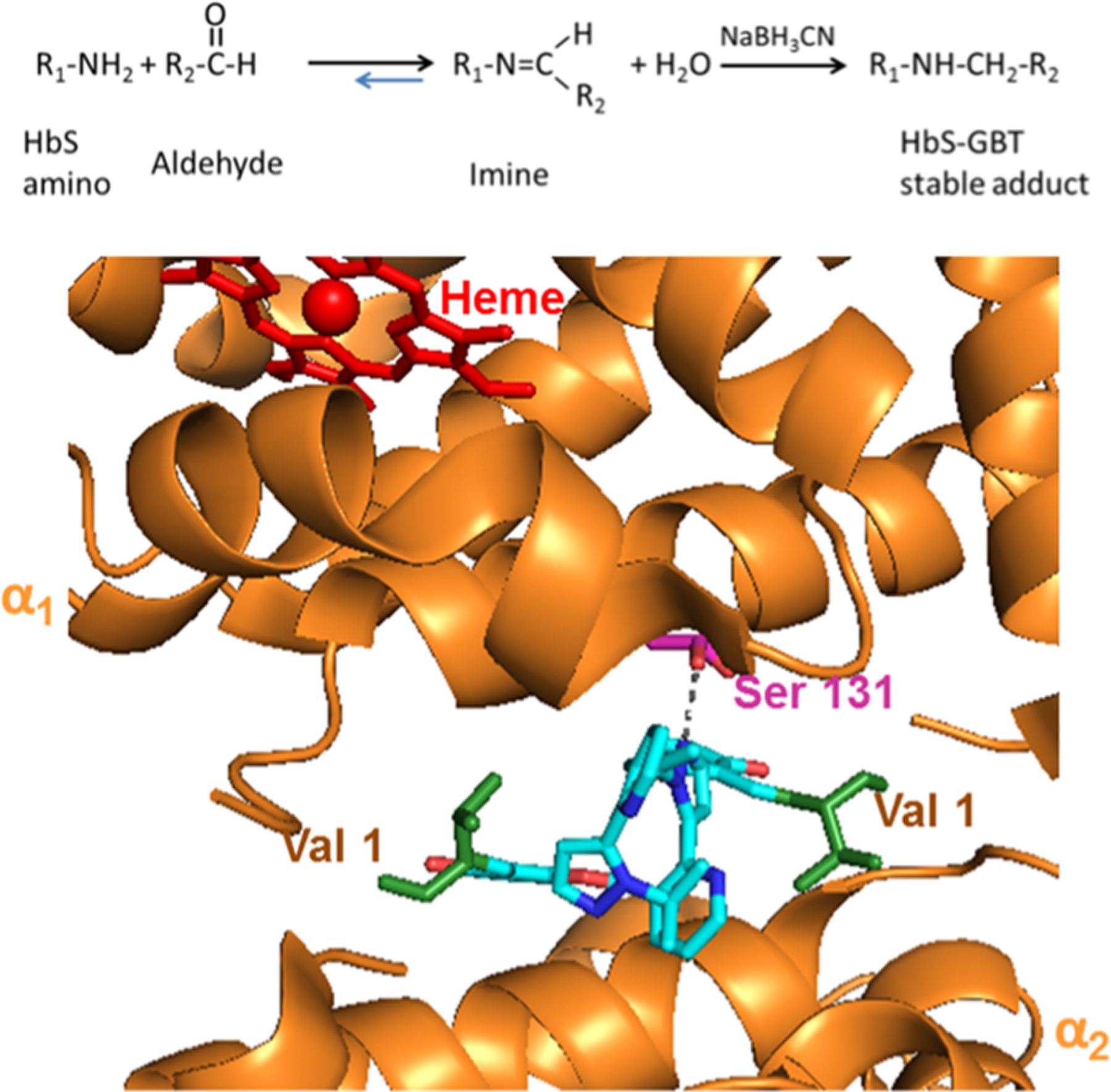
Scheme (top panel) represents the chemical reaction showing how GBT440 modifies HbS primary amines to form a reversible Schiff base imine. Na BH3CN converts the reversible Schiff base imine into a covalent HbS-GBT440 stable adduct that can easily be detected and characterized using reverse phase HPLC and mass spectrometry. The panel below represents the structure of GBT440 (cyan) complexed with HbS (PDB 5E83). The α subunits are represented by orange, the heme by red, and corresponding N-terminal valines by green. The image was made using PyMOL software.
A recent study performed using a murine SCD model has shown that oral dosing of GBT440 prevented ex vivo RBC sickling, reducing reticulocyte counts and prolonging RBC half-life.8 Taken together with the above structural studies, these SCD murine data demonstrated the potential for GBT440 to be a suitable therapeutic for daily oral dosing in humans. As a result, the NIH recently obtained an FDA approved emergency investigational new drug (eIND) application to treat one patient, for whom blood transfusion proved very challenging due to the presence of multiple alloantibodies, on a compassionate basis with GBT440 for life-saving anemia. Upon monitoring this patient during treatment, an electrophoretic and HPLC adduct formation was noted which prompted the need to perform a more in-depth analysis of Hb modifications during exposure to this drug.11 In-depth characterization studies could potentially help monitor therapeutic response and potential toxicity. Furthermore, biophysical studies of in vitro oxygen dissociation curves from GBT440 could help predict effective in vivo dosing.
The data presented in this report is from an investigation designed to elucidate the structure and functional relationship of GBT440 complexes with Hb. Figure 2 shows the oxygen dissociation curve data for red cells from an SS patient incubated with incrementally increasing concentrations of GBT440. As seen in a previous animal model study, incrementally increasing GBT440 concentrations in whole SS blood resulted in a dose dependent increase in oxygen affinity as demonstrated by a left shifting of the oxygen dissociation curves (ODCs). However, ODCs shown in Figure 2 are not sigmoidal but biphasic in nature, because there are two species in the solution—a high affinity species with the drug bound and a species with no drug bound having a sigmoidal binding curve.13
Figure 2.
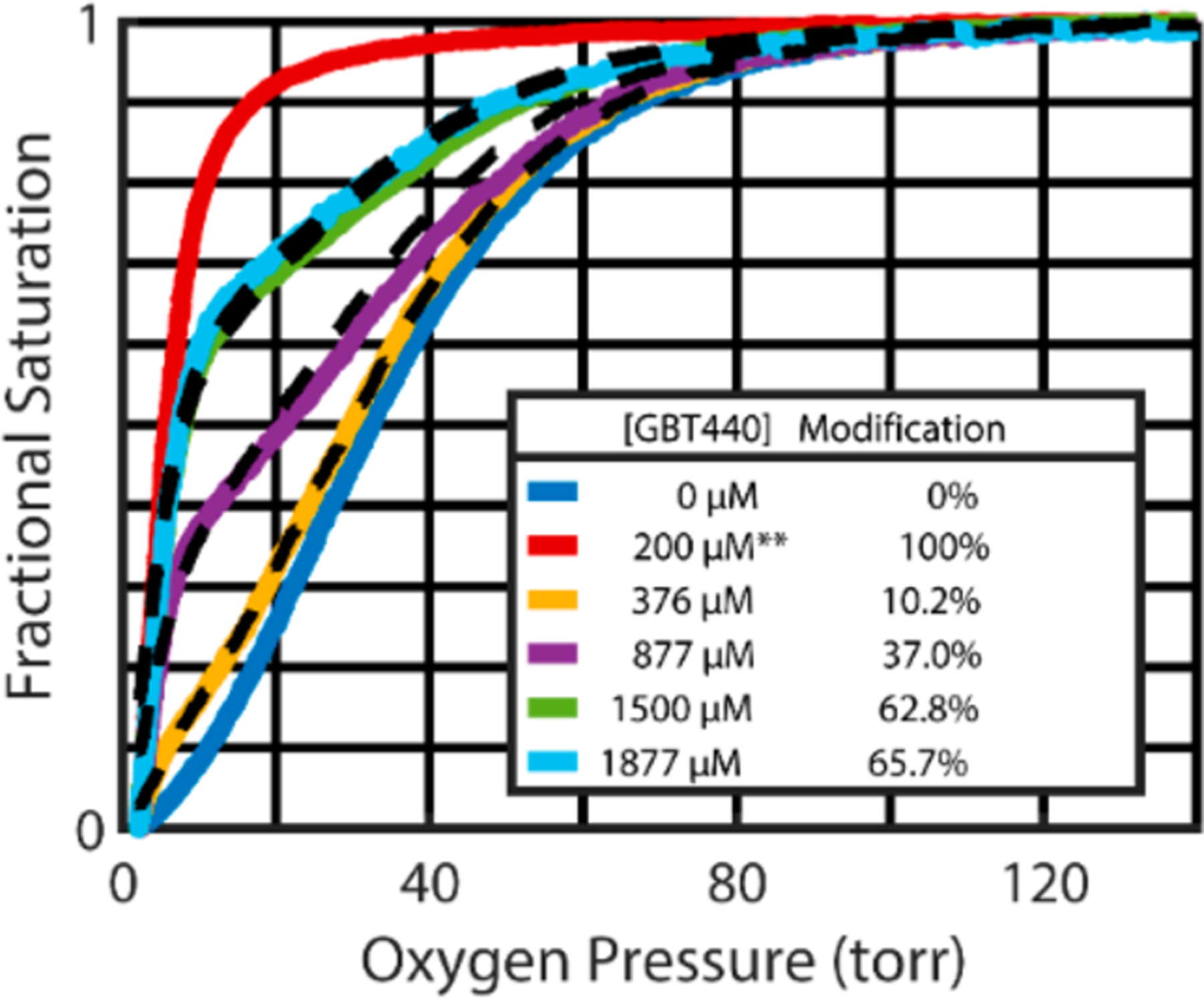
Representative red cell oxygen dissociation curves (ODC) analyzed after incubating SS patient blood for 1 h at incrementally increasing GBT440 concentrations. These ODC curves, measured after a 50-fold dilution of whole blood into physiological buffer, show that the oxygen affinity increases in a dose dependent manner. Because dissociation of the drug from hemoglobin is so slow (hours), the suspending buffer for the ODC measurements contained no drug except for the red curve**, which contained sufficient GBT440 (200 μM) to saturate 100% of the Hb with the drug.12 At saturating concentrations, the R-T quaternary conformational equilibrium shifts completely to the noncooperative, high affinity oxygen dissociation curve of the R conformation. The black dashed curves show the optimal linear combination of the blue curve (absence of drug) and the red curve (saturating concentrations of drug) to yield the percent modified Hb. Additional data on the percent hemoglobin modified as a function of drug concentration is in Figure S1 of the Supporting Information.
To determine if GBT440 binding involved additional Hb sites for covalent attachment (in addition to the α subunit) and/or resulted in a heterogeneous population of GBT440-HbS species, we utilized an analytical approach involving HPLC and mass spectrometry to analyze GBT440-HbS taken from a single patient hemolysate. GBT440 is an aromatic aldehyde that reacts with primary amines to form a reversible Schiff base imine. As shown in the scheme listed in Figure 1, an HbS primary amine reacts with the aldehyde, which has previously been shown to be the α N-terminal amino group but could also include the β N-terminal amino group and/or Hb lysine side chains. The resulting Schiff base imine is reversible and labile under conditions used for RP HPLC and mass spectrometry analysis. To improve detection sensitivity, sodium borohydride (Na BH3CN) was added to GBT440-incubated HbS-containing samples (see Figure 1). Na BH3CN converts the reversible Schiff base imine into a covalent GBT440-HbS stable adduct that can easily be detected and characterized using reverse phase HPLC and mass spectrometry.
HPLC data, presented in Figure 3 (Panel A), showed an increase in the formation of GBT440-HbS stable adducts (from an SS patient sample) in a dose dependent manner.
Figure 3.
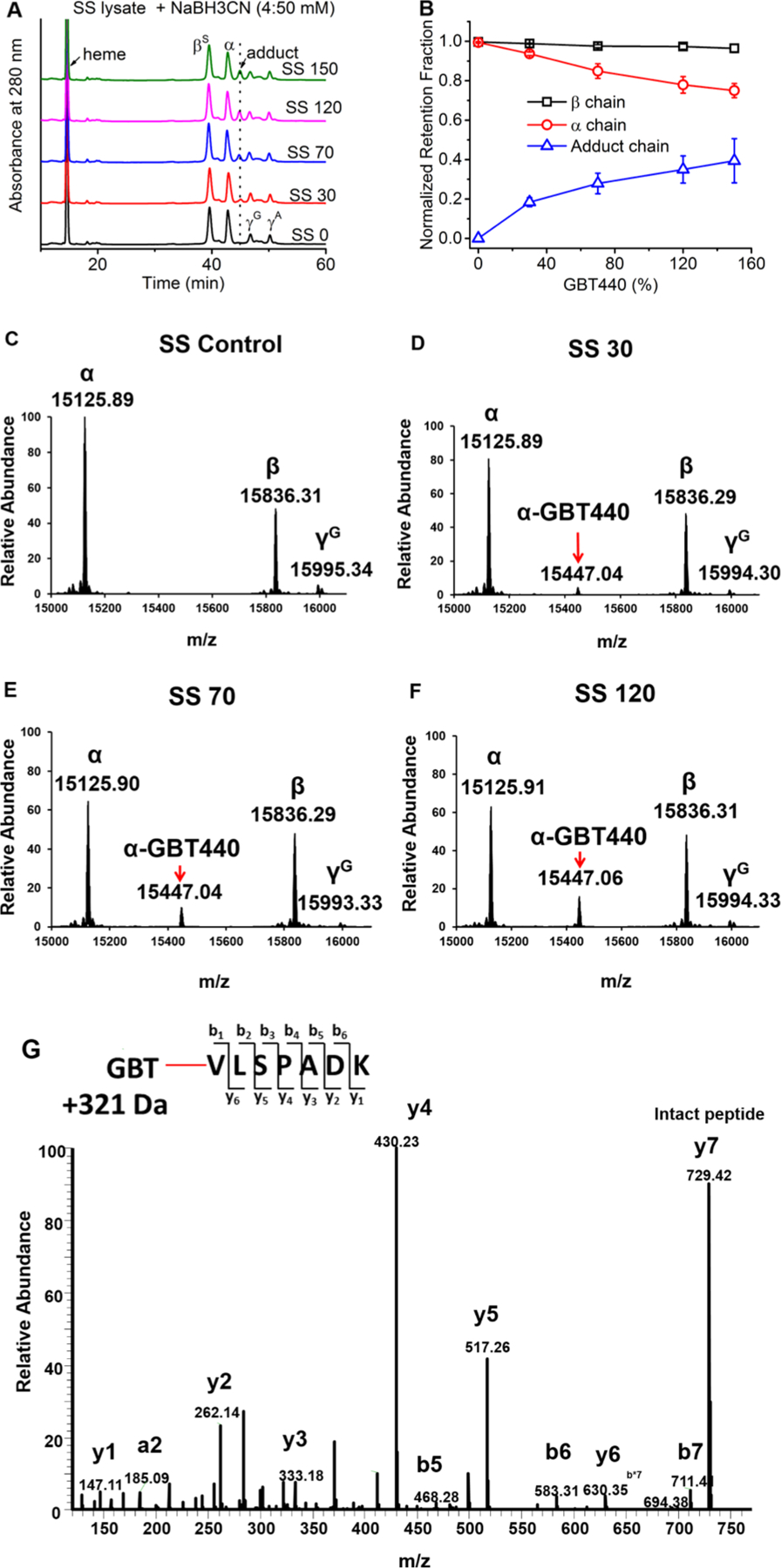
(A) RP-HPLC analysis of SS lysate was performed after incubation with NaBH3CN. (B) The relationship of GBT levels and the retention fraction of subunit globins. The growing fractions of GBT-Hb adducts were also plotted in the figure (n = 3). The fractions of all the species were normalized by the globin chain area compared to the heme area (calculated from the Figure 3A). (C−F) Intact mass spectrometric analysis of Hb subunits from patient hemolysate (including control) treated with increasing dosages of GBT440. (G) LC/MS/MS fragmentation spectrum representing y and b fragment ions for the GBT440 labeled N-terminal α peptide. The GBT440 concentrations 30, 70, 120, and 150 correspond to % values of the 1 mM HbS tetramer (i.e., SS 30 contains 0.3 mM GBT440). These mass spectrometry data confirm that GBT440 labeling is specific to the α N-terminus.
Furthermore, the normalized retention data in Panel B indicated that as GBT440 concentration increases the amount of GBT440 adducts increases simultaneously with a reduction in detected free α subunits. During the course of these HPLC assays, β subunit levels remained constant. Taken together, these HPLC data indicate that GBT440 labeling in the human patient lysate was specific to the α subunit and increased in a dose dependent manner (α chains come from both HbS and HbF).
To follow up on these HPLC results we utilized a comprehensive mass spectrometry approach that included both intact mass (Top-down) and digested peptide (Bottom-up) LC/MS/MS analysis to further characterize the HbS adducts (see Materials and Methods in SI) seen from the HPLC data. In preliminary pilot experiments aimed to optimize GBT440 HbS labeling (using a 10:1 dose of GBT440 with free HbS), intact and LC/MS/MS mass spectrometry data indicated that both α and β N-termini were labeled with a preference for the α subunit (data not shown).
When we performed intact mass analysis on human patient lysate samples incubated with GBT440, we identified a deconvoluted peak that corresponds to the α subunit intact mass plus a single covalently attached GBT440 molecule (Panel D). Panels D−F showed a dose dependent increase in peak intensity for this GBT440-HbS species that correlates with Panel B and indicates that GBT440 labeling of the α subunit is dose dependent. Another important observation from these results is that we did not detect any other GBT440 adducts in the patient hemolysate under the doses used.
The LC/MS/MS data shown in panel G represents a fragmentation spectrum of the N-terminal peptide showing y and b ions that are mass shifted by the MW of GBT440. Interestingly, our LC/MS/MS analysis only detected modification at N-terminal peptides; we did not see any evidence of GBT440 labeling of Hb lysines. Taken together, the intact mass and LC/MS/MS data collectively confirm that GBT440 labeling is highly specific to the α N-terminus and that GBT440 labeling involves 1:1 stoichiometry. These MS data are orthogonal to the HPLC results that indicate the same result.13
CONCLUSION
The results from these studies provide an in-depth characterization of GBT440-HbS adducts identified in an SS patient hemolysate and show unambiguously that GBT440 labeling based on the doses used in this study are specific to the α subunit and occur in a 1:1 stoichiometry. Our analysis did not detect additional GBT440-HbS adducts which support previous animal model studies. The results and methods presented in this report could therefore potentially help therapeutic monitoring of GBT440, aid in determining optimal dosing and accessing patient compliance, and may also reveal critical insight into the biophysical properties of GBT440-HbS complexes.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the U.S. Food and Drug Administration (MODSCI) and by the National Institutes of Health (NIH/NHLBI) under grant HL110900 to AIA. We acknowledge Global Blood Therapeutics, GBT, for the kind gift of material GBT440 used in this study. The authors thank Tigist Kassa for creating the Pymol image shown in Figure 1.
ABBREVIATIONS
- ODC
oxygen dissociation curve
- SS
patient Homozygous sickle Cell patient
- HPLC
high performance liquid chromatography
- LC/MS/MS
liquid chromatography tandem mass spectrometry
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Hebbel RP (2011) Reconstructing sickle cell disease: a data-based analysis of the ″hyperhemolysis paradigm″ for pulmonary hypertension from the perspective of evidence-based medicine. Am. J. Hematol 86, 123–154. [DOI] [PubMed] [Google Scholar]
- (2).Bunn HF (1997) Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med 337, 762–769. [DOI] [PubMed] [Google Scholar]
- (3).Hebbel RP (2014) Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol Oncol Clin North Am 28, 181–198. [DOI] [PubMed] [Google Scholar]
- (4).Eaton WA, and Bunn HF (2017) Treating sickle cell disease by targeting HbS polymerization. Blood 129, 2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wahl S, and Quirolo KC (2009) Current issues in blood transfusion for sickle cell disease. Curr. Opin. Pediatr 21, 15–21. [DOI] [PubMed] [Google Scholar]
- (6).Sunshine HR, Hofrichter J, Ferrone FA, and Eaton WA (1982) Oxygen binding by sickle cell hemoglobin polymers. J. Mol. Biol 158, 251–273. [DOI] [PubMed] [Google Scholar]
- (7).Safo MK, and Kato GJ (2014) Therapeutic strategies to alter the oxygen affinity of sickle hemoglobin. Hematol Oncol Clin North Am 28, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Oksenberg D, Dufu K, Patel MP, Chuang C, Li Z, Xu Q, Silva-Garcia A, Zhou C, Hutchaleelaha A, Patskovska L, et al. (2016) GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br. J. Haematol 175, 141–153. [DOI] [PubMed] [Google Scholar]
- (9).Metcalf B, Chuang C, Dufu K, Patel MP, Silva-Garcia A, Johnson C, Lu Q, Partridge JR, Patskovska L, Patskovsky Y, et al. (2017) Discovery of GBT440, an Orally Bioavailable R-State Stabilizer of Sickle Cell Hemoglobin. ACS Med. Chem. Lett 8, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Torres L, and Conran N (2019) Emerging pharmacotherapeutic approaches for the management of sickle cell disease. Expert Opin. Pharmacother 20, 173–186. [DOI] [PubMed] [Google Scholar]
- (11).Thein SL, and Shet A, NHLBI. Personal communication
- (12).Li Q, Henry ER, Hofrichter J, Smith JF, Cellmer T, Dunkelberger EB, Metaferia BB, Jones-Straehle S, Boutom S, Christoph GW, et al. (2017) Kinetic assay shows that increasing red cell volume could be a treatment for sickle cell disease. Proc. Natl. Acad. Sci. U. S. A 114, E689–E696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Rutherford NJ, Thoren KL, Shajani-Yi Z, and Colby JM (2018) Voxelotor (GBT440) produces interference in measurements of hemoglobin S. Clin. Chim. Acta 482, 57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


