Abstract
Background
While meta-analyses confirm treatment for chronic post-stroke aphasia is effective, a lack of comparative evidence for different interventions limits prescription accuracy. We investigated whether Constraint-Induced Aphasia Therapy Plus (CIAT-plus) and/or Multimodality Aphasia Therapy (M-MAT) provided greater therapeutic benefit compared with usual community care and were differentially effective according to baseline aphasia severity.
Methods
We conducted a three-arm, multicentre, parallel group, open-label, blinded endpoint, phase III, randomised-controlled trial. We stratified eligible participants by baseline aphasia on the Western Aphasia Battery-Revised Aphasia Quotient (WAB-R-AQ). Groups of three participants were randomly assigned (1:1:1) to 30 hours of CIAT-Plus or M-MAT or to usual care (UC). Primary outcome was change in aphasia severity (WAB-R-AQ) from baseline to therapy completion analysed in the intention-to-treat population. Secondary outcomes included word retrieval, connected speech, functional communication, multimodal communication, quality of life and costs.
Results
We analysed 201 participants (70 in CIAT-Plus, 70 in M-MAT and 61 in UC). Aphasia severity was not significantly different between groups at postintervention: 1.05 points (95% CI −0.78 to 2.88; p=0.36) UC group vs CIAT-Plus; 1.06 points (95% CI −0.78 to 2.89; p=0.36) UC group vs M-MAT; 0.004 points (95% CI −1.76 to 1.77; p=1.00) CIAT-Plus vs M-MAT. Word retrieval, functional communication and communication-related quality of life were significantly improved following CIAT-Plus and M-MAT. Word retrieval benefits were maintained at 12-week follow-up.
Conclusions
CIAT-Plus and M-MAT were effective for word retrieval, functional communication, and quality of life, while UC was not. Future studies should explore predictive characteristics of responders and impacts of maintenance doses.
Trial registration number
ACTRN 2615000618550.
Keywords: aphasia, rehabilitation, randomised trials, stroke
Key messages.
What is already known on this topic
Previous evidence for constraint-induced aphasia therapy (CIAT-Plus) and Multimodality Aphasia Therapy (M-MAT) is limited by small sample sizes, inadequate comparator groups, and recruitment and detection bias.
What this study adds
This large-scale, phase III trial confirmed the efficacy of an intensive dose of CIAT Plus and M-MAT with clinically meaningful effects on word retrieval, functional communication and quality of life.
How this study might affect research, practice or policy
CIAT Plus and M-MAT are efficacious interventions in the chronic phase of aphasia recovery. Research is required to explore methods to enhance maintenance of effects and potential impacts of aphasia severity on treatment prescription.
Introduction
Aphasia is an acquired language disability, impacting all aspects of communication underpinned by language: understanding speech, reading, writing and speaking. Aphasia persists into the chronic phase in approximately 20% of stroke survivors,1 with significant negative impacts on mental health2 and quality of life.3 In the first year after stroke, aphasia is responsible for 8.5% of stroke related healthcare costs.4 The 2016 Cochrane review of speech and language therapy for aphasia after stroke showed statistically significant treatment effects immediately after intervention for functional communication (SMD 0.28, 95% CI 0.06 to 0.49, p=0.01) when treatment was provided with sufficient intensity (5–10 hours per week), but not at 6 month follow-up.5 The review lacked evidence for comparative treatment effects,5 limiting the prescription of effective treatment. Additional evidence for the effectiveness of aphasia therapy in the chronic phase (>6 months) comes from two high quality randomised controlled trials (RCTs): the FCET2EC trial6 and the Big CACTUS trial.7 FCET2EC6 utilised a mean of 46 hours of linguistic and pragmatically focused, clinician delivered, individual therapy showing significant effects (moderate ESs) on functional communication immediately post-intervention and at 6 month follow-up. Big CACTUS7 used computer-based naming therapy and demonstrated significant effects (moderate ESs) on word finding for trained items but not functional communication. Both trials6 7 confirmed significant variability in participant treatment response, with stroke6 and aphasia severity7 as probable moderator variables. There is a need for properly powered trials comparing the effectiveness of different aphasia treatments in the chronic phase.
Constraint-induced Aphasia Therapy Plus (CIAT-Plus)8 and Multimodality Aphasia Therapy (M-MAT)9 are intensive, high-dose interventions delivered in a small group setting of 2–4 participants, aimed at improving verbal communication. They involve different therapeutic strategies: CIAT-plus preferences speech production and verbal therapist cueing; M-MAT includes multimodal tasks and cues (drawing, gesturing and writing). CIAT-Plus and M-MAT are hypothesised to rely on different underlying neural recovery mechanisms and may be differentially effective based on aphasia severity.9 10 Systematic reviews of trials of CIAT-Plus and M-MAT reveal moderate-high effect sizes11–13 but studies are limited by small sample sizes (n<15), inadequate comparator groups, and recruitment and detection bias. Determining the most effective intervention for severity-based and other sub-groups of people with aphasia may lead to improved patient outcomes and reduced healthcare costs.
The aim of our multicentre RCT of constraint-induced or multimodality personalised aphasia rehabilitation (COMPARE) was to assess the comparative effectiveness of a 2-week intensive dose (30 hours) of treatment (CIAT-Plus or M-MAT) in chronic aphasia after stroke compared with low dose usual community care (usual care, UC). We aimed to investigate potential differential impacts according to pre-treatment aphasia severity. Our primary hypothesis was that, compared with UC, both CIAT-Plus and M-MAT would lead to significantly reduced aphasia severity immediately post-intervention, with M-MAT superior for mild and severe aphasia, and CIAT-Plus superior for moderate aphasia.9 10 We further hypothesised that, compared with UC, both treatments would lead to improved word retrieval, functional communication, multimodal communication and quality of life. A tertiary objective (not reported here) was to report on the potential incremental cost-effectiveness of these interventions.
Methods
Study design
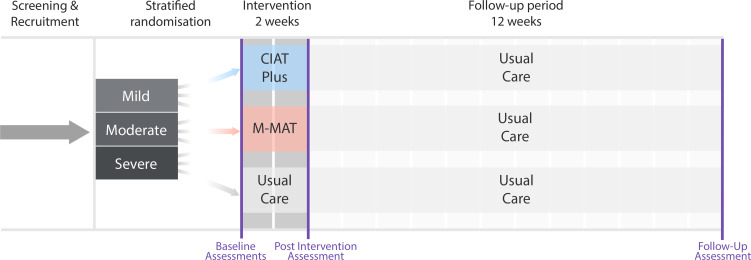
COMPARE was a three-arm, multicentre, parallel group, open-label, blinded endpoint, phase III RCT (figure 1). The trial protocol has been published.14 CIAT-Plus and M-MAT were provided by 30 trial trained therapists in community settings in ten cities across Australia and New Zealand. We used usual community care as the control condition, which in Australia and New Zealand comprises either no therapy or limited, non-intensive therapy of less than 1 hour per week.15 The trial was coordinated from the Aphasia Rehabilitation Research Centre at La Trobe University in Melbourne. A data safety and management committee comprising staff independent of the principal investigators (a biostatistician, an experienced stroke rehabilitation trialist, and a speech pathologist with additional expertise in bioethics) monitored study progress and safety.
Figure 1.
Trial timeline. CIAT, constraint-induced aphasia therapy; M-MAT, Multimodality Aphasia Therapy.
Participants
Eligible participants were: aged 18 years or older; living in the community; had chronic aphasia resulting from stroke of any kind (>6 months duration) confirmed by an aphasia quotient <93.8 on the Western Aphasia Battery-Revised Aphasia Quotient (WAB-R-AQ)16 at the time of screening; fluent in English prior to stroke; independent in toileting or had a caregiver who could assist with toileting during therapy. Participants provided their own written informed consent. Modified consent processes were provided including the use of supported communication strategies and aphasia accessible consent documents. Participants were excluded if they had a neurological condition other than stroke, severe apraxia of speech or dysarthria (Apraxia Severity Rating Scale)17 uncorrected sensory loss preventing participation in group communication, or a diagnosis of a self-reported untreated mental health condition preventing adherence to the study protocol. Participants were recruited through 19 hospital sites and via direct community advertising.
Randomisation and masking
Therapy was provided face to face, in groups of three participants, requiring the members of a treatment group to be in the same geographic location. Once enrolled in the trial, participants were allocated to treatment groups based on their aphasia severity (WAB-R-AQ mild: 93.7–62.6; moderate: 62.5–31.3; severe:≤31.3). Groups of three participants were randomised to one of three arms (M-MAT, CIAT-Plus or UC) in a 1:1:1 ratio via a central allocation system using blocked randomisation within each severity stratum. Only the independent randomisation statistician knew the block sizes, and these were not disclosed until trial completion. COMPARE involved complex behavioural interventions in which the treating therapists were aware of the treatment they provided. Participants assigned to UC were aware they were not receiving intensive treatment. Therefore, neither the participants nor the treating therapists were blinded. All assessments were conducted by independent assessors who were blinded to treatment allocation.
Procedures
Full details of the intervention are provided in the published COMPARE Trial protocol14 and online trial (https://cloudstor.aarnet.edu.au/plus/s/dkST1BSrG6r8ooT) and intervention protocols (https://cloudstor.aarnet.edu.au/plus/s/F74O828J71IDYKa; https://cloudstor.aarnet.edu.au/plus/s/crCAZe09ggGCosV). We used the Template for Intervention Description and Replication reporting template18 to guide reporting of this complex behavioural intervention (online supplemental material p.4).
jnnp-2021-328422supp001.pdf (506.9KB, pdf)
CIAT-Plus and M-MAT treatment sessions ran 3 hours a day, 5 days per week for 2 weeks (30 hours). Rest breaks of 15–30 min were provided between every 1-hour session. A 15 min daily home practice communication task was prescribed, checked for completion the following day, and logged in the COMPARE Research Electronic Data Capture (REDCap) database. CIAT-Plus and M-MAT were provided in community settings by qualified and study-trained speech pathologists. UC comprised no direct intervention for some, and non-intense, individual, computerised or social/support group sessions for others. UC was documented in a participant-logged diary, collected by blinded assessors at the baseline and 12-week follow-up time points. All participants could undertake UC throughout the trial allowing for the effects of UC alone to be compared with the addition of 2 weeks of CIAT-Plus or M-MAT.
CIAT-Plus and M-MAT involved six different structured, protocolised communication activities including requesting items, clarifying requests, recalling items from memory and naming items. A prescribed set (easy, moderate, hard) of 80 coloured picture cards (48 nouns; 32 verbs) was utilised in therapy according to participant pretreatment picture naming accuracy. Therapists prescribed production targets ranging from single nouns/verbs to complex sentences. Therapist decisions to select targets and picture sets were guided by a detailed protocol. All session details and individual participant performance were logged in the trial REDCap database.
In CIAT-Plus, participants were not permitted to use paper and pencil or augmentative communication devices, focusing all activity on spoken communication. Visual barriers (23 cm high) placed between participants blocked sight of therapy stimulus cards, limiting nonverbal communication attempts. When participants could not spontaneously produce specific targets (word, phrase, sentence) within 10 s, the therapist commenced a strict cueing hierarchy: (1) provision of initial sound of target (eg, starts with ‘/k/’); (2) provision of written form of target to read aloud (eg, cup); (3) spoken form of target provided for the participant to repeat aloud three times. In M-MAT, multimodal communication and cues were utilised and there were no visual barriers. When participants could not spontaneously produce targets, therapists implemented a strict cueing hierarchy: (1) participant asked to produce a gesture of, and attempt to name, the target; (2) therapist provided gesture and spoken target word for participant to copy; (3) participant asked to draw target and name it; (4) participant provided with written target to read aloud and repeat three times. All therapy sessions were videorecorded and monitored by an independent therapy fidelity monitor. All videos from day 1 of intervention and 25% of day 6 were reviewed for therapy adherence, with feedback to therapists within 24 hours of receipt (fidelity monitoring protocol online https://cloudstor.aarnet.edu.au/plus/s/k37xblJGkSsxLYR).
Demographic, medical/health, mood, speech and language characteristics were collected at screening prior to study inclusion (table 1; online supplemental material p.5). All primary and secondary outcomes were collected within 2 weeks prior to the intervention period commencing (baseline), within 7 days of ending the intervention (immediate postintervention), and within 7 days of the 12-week follow-up time point. All assessment data were collected in participants’ homes except for 15 participants (30 assessments) due to COVID-19 pandemic hospital and university policies requiring completion via video teleconference. Validity of video teleconference administration of the outcome measures have been shown to be equivalent to face to face.19 20
Table 1.
Baseline characteristics of intention to treat population and therapy characteristics
| CIAT Plus (n=71) | M-MAT (n=75) | UC (n=70) | |
| Recruitment region | |||
| Australia | 71 (100%) | 60 (80%) | 64 (91.43%) |
| New Zealand | 0 (0%) | 15 (20%) | 6 (8.57%) |
| Sex | |||
| Male | 46 (64.79%) | 53 (70.67%) | 48 (68.57%) |
| Female | 25 (35.21%) | 22 (29.33%) | 22 (31.43%) |
| Age at time of consent (median (IQR) | 63.93 (19.79) | 63.77 (21.02) | 63.16 (14.10) |
| <55 | 21 (29.58%) | 25 (33.33%) | 14 (20%) |
| 55–70 | 26 (36.62%) | 30 (40%) | 35 (50%) |
| >70 | 24 (33.80%) | 20 (26.67%) | 21 (30%) |
| Education (median (IQR) | 14 (5) | 13 (6) | 14 (5) |
| Time post most recent stroke onset (years) (median (IQR) | 2.41 (4.22) | 2.97 (3.81) | 2.58 (2.87) |
| Baseline mRS | |||
| Low (0–2) | 29 (41.43%) | 30 (41.67%) | 31 (48.44%) |
| High (3–6) | 41 (58.57%) | 42 (58.33%) | 33 (51.56%) |
| Stroke type | |||
| Haemorrhage | 19 (26.76%) | 16 (21.33%) | 13 (18.57%) |
| Infarct | 44 (61.97%) | 48 (64%) | 48 (68.57%) |
| Infarct and haemorrhagic | 1 (1.41%) | 4 (5.33%) | 3 (4.29%) |
| Not known | 7 (9.86%) | 7 (9.33%) | 6 (8.57%) |
| Aphasia type | |||
| Anomic | 32 (45) | 29 (39) | 30 (43) |
| Broca’s | 20 (28) | 18 (24) | 15 (21) |
| Conduction | 13 (18) | 16 (21) | 11 (16) |
| Wernicke’s | 4 (6) | 6 (8) | 9 (13) |
| Global | 0 (0) | 2 (3) | 0 (0) |
| Transcortical motor | 0 (0) | 1 (1) | 2 (3) |
| Transcortical sensory | 0 (0) | 1 (1) | 0 (0) |
| Unclassifiable | 2 (3) | 2 (3) | 3 (4) |
| WAB-R-AQ | 71.33 (16.98) | 68.68 (19.57) | 72.69 (18.13) |
| Above cut-off (93.7–100) | 1 (1.21%) | 1 (1.33%) | 3 (4.29%) |
| Mild (62.6–93.6) | 51 (71.83%) | 49 (65.33%) | 49 (70%) |
| Moderate (31.3–62.5) | 17 (23.94%) | 21 (28%) | 18 (25.71%) |
| Severe (0–31.2) | 2 (2.82%) | 4 (5.33%) | 0 (0%) |
| WAB-R-AQ subtests | |||
| Spontaneous speech (mean, SD) /20 | 14.03 (4.24) | 13.43 (4.35) | 14.41 (4.32) |
| Auditory Verbal Comprehension (mean, SD) /10 |
8.23 (1.29) | 7.89 (1.72) | 8.08 (1.61) |
| Repetition (Mean, SD) /10 | 6.65 (2.20) | 6.28 (2.52) | 6.57 (2.41) |
| Naming and Wording Finding (mean, SD) /10 |
6.75 (2.24) | 6.74 (2.49) | 7.28 (1.93) |
| COMPARE Naming Battery (mean, SD) /80 (treated items) |
40.46 (18.38) | 40.91 (18.85) | 47.33 (17.38) |
| COMPARE Naming Battery (mean, SD) /100 (untreated items) |
61.41 (28.06) | 60.63 (31.06) | 67.74 (25.73) |
| Communication accuracy and efficiency | |||
| No of CIUs (mean, SD) | 217.04 (183.13) | 178.4 (146.1) | 235.72 (176.31) |
| CIUs per minute (mean, SD) | 22.82 (17.02) | 20.30 (16.29) | 27.86 (19.1) |
| Communicative Effectiveness Index (mean, SD) /100 | 56.49 (17.43) | 52.28 (17.64) | 59.28 (16.82) |
| Scenario test (mean, SD) /54 | 45.33 (11.16) | 44.03 (10.85) | 46.55 (8.64) |
| Stroke and Aphasia Quality of Life Scale-39g | |||
| Composite (mean, SD) /5 | 3.72 (0.62) | 3.67 (0.74) | 3.69 (0.56) |
| Physical (mean, SD) /5 | 4.08 (0.76) | 4.1 (0.89) | 4.1 (0.74) |
| Communication (mean, SD) /5 | 3.08 (0.86) | 2.89 (0.88) | 3.05 (0.72) |
| Psychosocial (mean, SD) /5 | 3.63 (0.81) | 3.59 (0.92) | 3.56 (0.76) |
| Trial provided intervention | |||
| No of therapy hours (median (IQR) | 29.20 (3.02) | 29.91 (2.69) | NA |
| Length of sessions (mean minutes (SD)) | 59.35 (1.67) | 59.80 (1.42) | NA |
| Intervention compliant (>24 hours) | 65 | 68 | NA |
| Non trial provided intervention | |||
| No | 40 (56.3%) | 52 (69.3%) | 47 (67.1%) |
| Yes | 31 (43.7%) | 23 (30.7%) | 23 (32.9%) |
| No of speech therapy sessions (median (IQR) | 10 (5, 28) | 9 (3, 24) | 10 (5, 20) |
| Stimulus set | |||
| Easy | 12 (16.9%) | 18 (24%) | NA |
| Moderate | 8 (11.27%) | 2 (2.67%) | NA |
| Hard | 51 (71.83%) | 55 (73.33%) | NA |
CIAT-Plus, Constraint-Induced Aphasia Therapy Plus; CIUs, correct information units; COMPARE, Constraint-Induced or Multi-Modality Personalised Aphasia Rehabilitation; M-MAT, Multimodality Aphasia Therapy; mRS, Modified Rankin Scale; N/A, not available; UC, usual care; WAB-R-AQ, Western Aphasia Battery-Revised Aphasia Quotient.
Outcomes
Outcomes included four of the recommended measures from the core outcome set for aphasia studies.21 The primary outcome measure was change in aphasia severity from baseline to immediately post-intervention as assessed by the WAB-R-AQ. Secondary outcomes included change in word retrieval (COMPARE naming battery: treated 80-item set; untreated 100-item set13 (online supplemental material p.1)), functional communication (Communication Effectiveness Index (CETI)22), multimodal communication (Scenario Test,23 quality of life (Stroke and Aphasia Quality of Life Scale-39g (SAQOL-39g),24 and efficiency of connected speech (correct information units (CIUs)/min.25 Additional secondary outcomes included maintenance of treatment effects as measured by repeating all outcome measures at a 12-week follow-up visit. All adverse events were reported to the study coordination team within 24 hours of occurrence. All serious adverse events were reported to the trial steering committee and the lead Human Research Ethics Committee.
Statistical analysis
The published statistical analysis plan provides full details of the analyses and was submitted for publication ahead of database lock (September 2020).26 At the time of study design, a 5-point difference on the WAB-R-AQ was considered clinically meaningful.27 Therefore, the study was powered to detect a 5-point difference on the WAB-R-AQ at therapy completion. Given previous reports of moderate effect sizes following CIAT-Plus and M-MAT, and without considering a possible clustering effect for group intervention, a sample size of 198 was required to achieve 80% power at the 5% significance level. Adjusting for the clustering effect, we anticipated a relatively small intraclass correlation coefficient of 0.04 and, with a group size of three, we calculated a maximum design effect of 1.08. We multiplied this design effect to the naïve (unclustered) sample size of 198, and rounded off to a multiple of three, to obtain balanced allocation across the three treatment groups, yielding the required sample size of 216.
All statistical analyses were performed by a statistician not employed at the trial coordination centre (TR). Analyses for all outcome measures were conducted on an intention to treat basis. Imputation for missing data was not required as <10% of primary outcome data were missing. The planned per-protocol analysis was also not required as only five participants in CIAT-Plus and two in M-MAT did not receive the minimum 24 hours of intervention.
We used linear mixed effects regression models for the primary outcome analysis with WAB-R-AQ as the outcome measure, adjusted for baseline aphasia severity (WAB-R-AQ) and baseline stroke severity (mRS) as fixed effects; treatment group and individual/participant were included as random effects. 95% CIs were calculated and reported. For all secondary outcomes, we used linear mixed effects regression models with CIUs/min, CETI, Scenario Test, SAQOL-39g or COMPARE Naming Battery as the outcome measure, adjusted for baseline aphasia severity (WAB-R-AQ), baseline stroke severity (mRS) and baseline score of the relevant outcome measure as fixed effects, with treatment group and participant as random effects. The potential impacts of aphasia severity on outcomes were analysed with linear mixed effects regression models with group allocation and baseline severity included as an interaction term, while between group differences were assessed with group allocation and timepoint as an interaction term. Unadjusted results for both primary and secondary analyses are reported (online supplemental material p.7). All analyses were conducted using the R Statistical Programming Language (V.4.0.5).28
Results
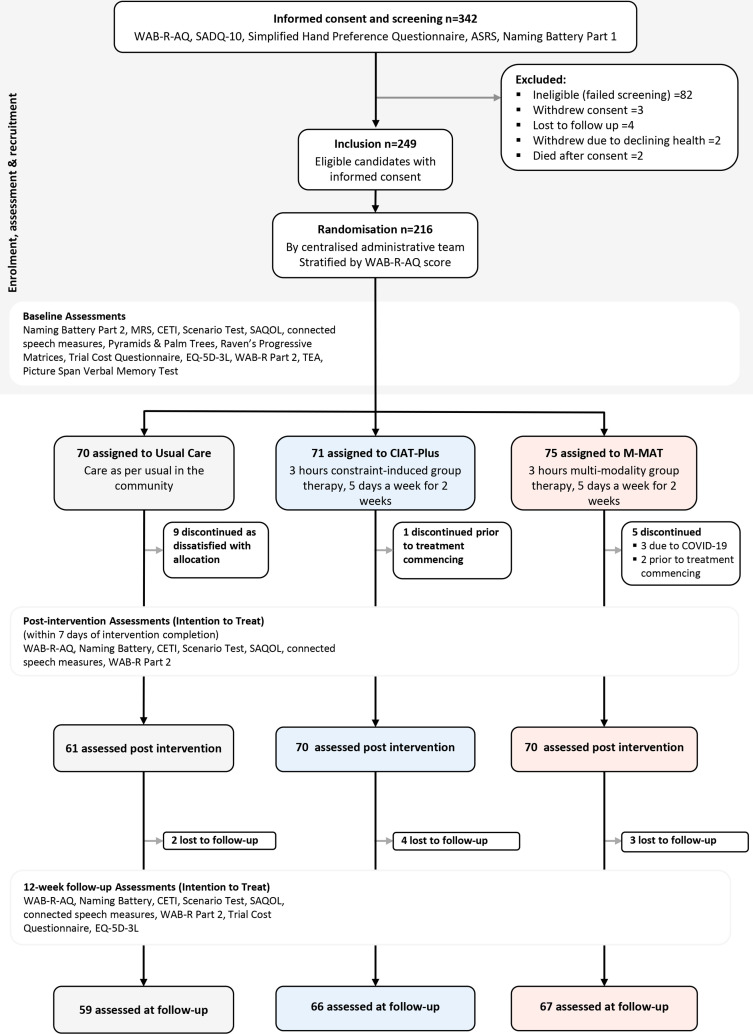
We screened 342 potential participants for eligibility, and 249 eligible participants consented to the trial (figure 2). We randomised 216 from the 249 eligible participants in groups of three according to their severity strata and geographical location between 15 July 2016 and 30 March 2021: 71 (33%) participants were randomised to CIAT-Plus, 75 (35%) to M-MAT and 70 (32%) to UC. Three participants elected to drop out before commencing treatment, and three were not provided with M-MAT due to COVID-19 restrictions forcing cancellation of their therapy group. Nine participants from UC discontinued due to dissatisfaction with group allocation. In total, 201 (93.1%) participants completed postintervention assessment and were included in the primary analysis (70 CIAT-Plus, 70 M-MAT, 61 UC). COVID-19 data collection restrictions led to 15 participants undertaking postintervention and follow-up assessments via video teleconference (figure 2).
Figure 2.
CONSORT diagram. CETI, Communication Effectiveness Index; CIAT, Constraint-Induced Aphasia Therapy; CONSORT, Consolidated Standards of Reporting Trials; SAQOL, Stroke and Aphasia Quality of Life; WAB-R-AQ, Western Aphasia Battery-Revised Aphasia Quotient.
Baseline demographic and clinical characteristics are reported in table 1 (online supplemental material p.5). The median age of all participants was 63.6 years (IQR 18.7) with a range of 18–92 years and 147 (68%) were male. At baseline, 154 (71.3%) participants had mild aphasia, 56 (25.9%) were moderate and 6 (2.8%) were severe. Participants were a median of 2.55 years (IQR 3.75 years) post-stroke (range 6.4 months to 28 years). Intervention groups were broadly similar at baseline although UC participants were more independent (lower mRS score), were younger, had no participants with severe aphasia, and had higher discourse and naming scores.
A median of 29.2 hours (IQR 3.02) of intervention was provided in CIAT-Plus and 29.9 hours (IQR 2.69) in M-MAT (table 1). Seventy-seven participants undertook non-trial related UC speech therapy during the study period. As expected, this comprised low dose and low intensity intervention, with a median of ten therapy sessions (IQR 15) or approximately 7.5 hours across the 15 week trial period (table 1). Trial therapy integrity was high: 124 (97.7%) of reviewed sessions were adherent at day 1; 121 (100%) at day 6 (online supplemental material p.4).
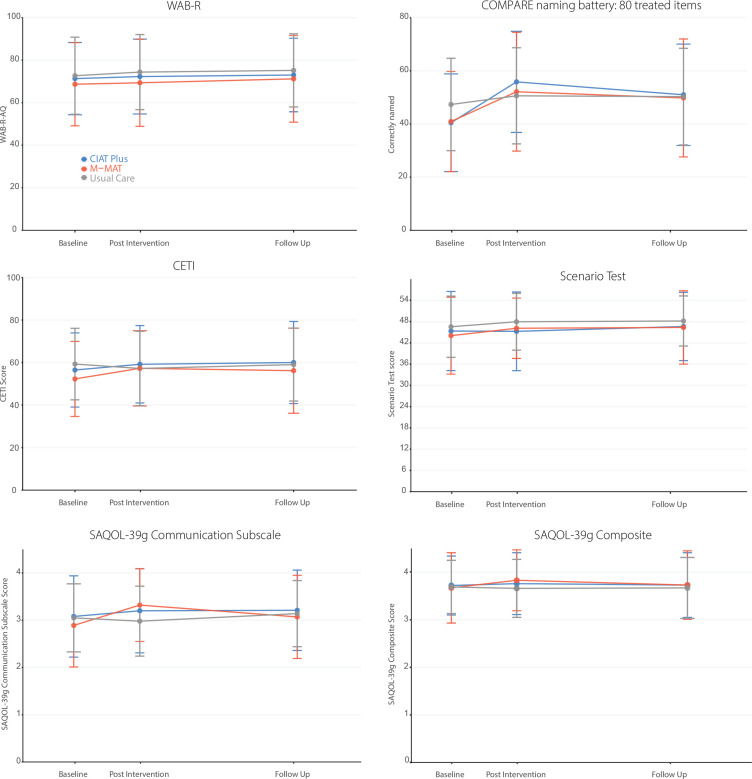
For the primary outcome measure (WAB-R-AQ), on average there were no significant differences between the three arms immediately post-intervention and no within group baseline to post-intervention average change that exceeded the prespecified 5-point clinically meaningful difference (table 2, figure 3). Participant sex, age and stroke severity did not significantly moderate the primary outcomes (online supplemental material p.9–10). At post-intervention there was a significant difference between CIAT-Plus and M-MAT for participants with severe aphasia favouring M-MAT (difference of 7.83 points (95% CI 0.87 to 14.8); p=0.03) and for moderate aphasia favouring CIAT-Plus (difference of 3.71 points (95% CI 1.06 to 6.36); p=0.006), but no difference for mild aphasia (difference 0.93 (95% CI −0.71 to 2.56); p=0.26). At 3 months follow-up, there was a significant difference between CIAT Plus and UC favouring UC (difference of 2.39 points (95% CI 0.53 to 4.24); p=0.008) and there was a significant difference (two points, (95% CI 0.38 to 3.67), p=0.02) favouring M-MAT for people with mild aphasia, but no other significant differences (table 2).
Table 2.
Treatment effects at immediately postintervention and 12-week follow-up in the intention-to-treat population
| CIAT Plus | M-MAT | Usual care | CIAT Plus versus usual care | M-MAT versus usual care | M-MAT versus CIAT Plus | ||||
| Unadjusted mean change score PIV-Baseline (SD) | Unadjusted mean change score PIV-Baseline (SD) | Unadjusted mean change score PIV-Baseline (SD) | Adjusted mean difference (95% CI) | P value | Adjusted mean difference (95% CI) | P value | Adjusted mean difference (95% CI) | P value | |
| Primary outcome | |||||||||
| Aphasia severity, WAB-R-AQ | 0.96 (4.81) | 1.04 (5.17) | 2.06 (6.56) | −1.05 (−2.88 to 0.78) | 0.36 | −1.06 (−2.89 to 0.78) | 0.36 | −0.004 (−1.77 to 1.76) | 1.00 |
| Key secondary outcomes at post-intervention | |||||||||
| Aphasia severity, WAB-R-AQ by severity stratum | |||||||||
| Mild | 0.51 (4.56) | 1.43 (3.57) | 1.11 (5.25) | + | + | 0.93 (−0.71 to 2.56) | 0.26 | ||
| Moderate | 2.35 (5.60) | −1.33 (6.54) | 4.74 (9.00) | + | + | −3.71 (−6.36 to 1.06) | 0.006* | ||
| Severe | 0.40 (3.68) | 8.35 (7.05) | NA (n=0) | + | + | 7.83 (0.87 to 14.80) | 0.03* | ||
| Functional communication, CETI | 2.96 (11.85) | 4.29 (10.47) | −1.83 (10.15) | 4.32 (0.03 to 8.61) | 0.048* | 4.64 (0.32 to 8.95) | 0.032* | 0.31 (−3.81 to 4.44) | 0.98 |
| Multimodal communication, Scenario Test | −0.30 (4.62) | 1.83 (5.78) | 1.20 (4.39) | −1.57 (−3.29 to 0.15) | 0.08 | 0.31 (−1.40 to 2.02) | 0.90 | 1.88 (0.195 to 3.57) | 0.0245* |
| Overall quality of life, SAQOL-39g: Mean Score | 0.06 (0.35) | 0.17 (0.38) | −.02 (0.37) | 0.09 (−0.03 to 0.21) | 0.17 | 0.20 (0.08 to 0.31) | 0.0004* | 0.10 (−0.01 to 0.22) | 0.085 |
| Communication-related quality of life, SAQOL-39g: Communication Scale | 0.15 (0.64) | 0.41 (0.49) | −.03 (0.57) | 0.20 (0.01 to 0.39) | 0.039* | 0.43 (0.24 to 0.62) | <0.0001* | 0.24 (0.05 to 0.42) | 0.008* |
| COMPARE naming battery: 80 treated items | 15.61 (11.38) | 11.30 (10.95) | 3.90 (7.73) | 10.73 (7.83 to 13.63) | <0.0001* | 7.49 (4.58 to 10.41) | <0.0001* | −3.24 (−6.05 to 0.43) | 0.019* |
| COMPARE naming battery: 100 untreated items | 4.09 (9.86) | 2.32 (8.95) | 0.51 (10.09) | 3.64 (0.79 to 6.50) | 0.0081* | 1.71 (−1.16 to 4.58) | 0.3402 | −1.93 (−4.71 to 0.84) | 0.2281 |
| Key secondary outcomes at 12 week follow-up | |||||||||
| Aphasia severity, WAB-R-AQ | 0.65 (5.85) | 2.0 (5.02) | 3.07 (6.58) | −2.39 (−4.24 to 0.53) | 0.008* | −1.15 (−3.01 to 0.71) | 0.32 | 1.24 (−0.56 to 3.04) | 0.24 |
| Aphasia severity, WAB-R-AQ by severity stratum | |||||||||
| Mild | 0.37 (5.52) | 2.38 (3.89) | 1.78 (5.84) | + | + | 2.03 (0.38 to 3.67) | 0.02* | ||
| Moderate | 1.40 (7.03) | 0.74 (7.56) | 6.54 (7.39) | + | + | −0.64 (−3.42 to 2.13) | 0.65 | ||
| Severe | 2.60 (NA; n=1) | 3.00 (3.08) | NA (n=0) | + | + | 1.33 (−7.37 to 10.03) | 0.76 | ||
| Functional communication, CETI | 2.64 (15.05) | 3.83 (16.58) | 0.20 (13.31) | 2.14 (−2.31 to 6.59) | 0.49 | 2.68 (−1.77 to 7.13) | 0.33 | 0.54 (−3.73, 4.81) | 0.95 |
| Multimodal communication, Scenario Test | 0.57 (6.10) | 1.76 (5.90) | 1.45 (6.17) | −0.91 (−2.67 to 0.85) | 0.45 | 0.30 (−1.43 to 2.03) | 0.91 | 1.21 (−0.5 to 2.92) | 0.22 |
| Overall quality of life, SAQOL-39g: Mean Score | −0.02 (0.38) | 0.08 (0.35) | 0.01 (0.36) | −0.03 (−0.15 to 0.10) | 0.87 | 0.07 (−0.05 to 0.19) | 0.38 | 0.09 (−0.25 to 0.211) | 0.15 |
| Communication-related quality of life, SAQOL-39g: Communication Scale | 0.11 (0.60) | 0.17 (0.64) | 0.11 (0.67) | −0.001 (−0.194 to 0.193) | 0.9999 | 0.036 (−0.157 to 0.229) | 0.898 | 0.037 (−0.151 to 0.225) | 0.887 |
| COMPARE naming battery: 80 treated items | 9.35 (7.76) | 8.61 (8.55) | 4.00 (7.80) | 4.23 (1.30 to 7.16) | 0.002* | 4.42 (1.49 to 7.35) | 0.001* | 0.186 (−2.67 to 3.04) | 0.987 |
| COMPARE naming battery: 100 untreated items | 3.24 (8.24) | 3.46 (9.52) | 2.97 (6.76) | 0.06 (-2.83 to 2.96) | 0.9985 | 0.26 (-2.64 to 3.16) | 0.9753 | 0.20 9 (2.63 to 3.02) | 0.953 |
NB: Bold and *: Statistically significant difference; +These comparisons were not planned for in our statistical analysis plan.23
CETI, Communication Effectiveness Index; CIAT-Plus, Constraint-Induced Aphasia Therapy; COMPARE, Constraint-Induced or Multi-Modality Personalised Aphasia Rehabilitation; M-MAT, Multimodality Aphasia Therapy; N/A, not available; PIV, Post-intervention; SAQOL-39g, Stroke and Aphasia Quality of Life Scale-39g; WAB-R-AQ, Western Aphasia Battery-Revised Aphasia Quotient.
Figure 3.
Comparative mean outcomes measures at baseline, post-intervention and follow-up. CIAT, Constraint-Induced Aphasia Therapy-Plus; CETI, Communication Effectiveness Index; COMPARE, Constraint-Induced or Multi-Modality Personalised Aphasia Rehabilitation; M-MAT, Multimodality Aphasia Therapy; SAQOL, Stroke and Aphasia Quality of Life; WAB-R, Western Aphasia Battery-Revised. Error bars represent standard deviations.
Significant differences were found on secondary outcomes immediately post-intervention (table 2, figure 3): Functional communication (CETI) was significantly better for M-MAT compared with UC (4.64 (95% CI 0.32 to 8.95); p=0.032) and CIAT-Plus compared with UC (4.32 (95% CI 0.03 to 8.61); p=0.048) but there was no difference between CIAT-Plus and M-MAT (0.31 (95% CI −3.81 to 4.44); p=0.98). For communication-related quality of life (SAQOL-39 g Communication Scale), there were significant differences favouring CIAT-Plus over UC (0.20 (95% CI 0.01 to 0.39); p=0.039), M-MAT over UC (0.43 (95% CI 0.24 to 0.62); p=0.0001) and M-MAT over CIAT-Plus (0.24 (95% CI 0.05 to 0.42); p=0.008). Compared with UC, naming of treated items was significantly better following M-MAT (7.49 (95% CI 4.58 to 10.41); p<0.0001) and CIAT-Plus (10.73 (95% CI 7.83 to 13.63); p<0.0001), and for CIAT-Plus compared with M-MAT (3.24 (95% CI 0.43 to 6.05); p=0.019). Multimodal communication (Scenario test) was significantly better for M-MAT compared with CIAT-Plus (1.88 (95% CI 0.195 to 3.57); p=0.025) but there were no differences between CIAT-Plus and UC (−1.57 (95% CI −0.15 to 3.29); p=0.08) or M-MAT and UC (0.31 (95% CI −1.40 to 2.02); p=0.90. There were minimal impacts of the intervention on discourse measures with high levels of baseline variability (online supplemental material p.8).
Thirty-six serious adverse events occurred during the trial for 31 participants; 18 (3.6 per year) in CIAT-Plus, 10 (1.4 per year) in M-MAT and 8 (1.8 per year) in UC; all were deemed unrelated to study participation (table 3).
Table 3.
Adverse events and serious adverse events
| CIAT Plus | M-MAT | UC | |
| Adverse events | 39 | 36 | 34 |
| Deaths | 0 | 0 | 0 |
| Serious adverse events | 18 | 10 | 8 |
| Serious adverse events linked to the trial | 0 | 0 | 0 |
| Participants with serious adverse events | 15 (21.13%) | 7 (9.33%) | 9 (12.86%) |
| 0 | 24 (33.80%) | 29 (38.67%) | 25 (35.71%) |
| 1 | 13 (18.31%) | 6 (8%) | 8 (11.43%) |
| 2 | 1 (1.41%) | 1 (1.33%) | 1 (1.43%) |
| >2 | 1 (1.41%) | 0 (0%) | 0 (0%) |
CIAT-Plus, Constraint-Induced Aphasia Therapy Plus; M-MAT, Multimodality Aphasia Therapy; UC, usual care.
Discussion
We present data from the first phase III, multisite, trial comparing an intensive dose of CIAT-Plus or M-MAT to UC in a large, inclusive sample of people with chronic post-stroke aphasia. Overall, on average, 30 hours of CIAT-Plus or M-MAT delivered over 2 weeks did not significantly reduce global aphasia severity compared with limited community UC. However, baseline aphasia severity may impact outcome, with differences between WAB-R-AQ at post-intervention favouring CIAT-Plus for moderate aphasia, M-MAT for severe aphasia, and M-MAT for mild aphasia at follow-up. Compared with UC, both treatments significantly improved word retrieval, functional communication and communication-related quality of life with M-MAT superior for communication-related quality of life and CIAT-Plus superior for word retrieval. These are important differential findings rejecting the ‘one size fits all’ approach to aphasia therapy prescription in this highly heterogenous population. The results are a major step forward towards more targeted allocation of aphasia treatment and personalised rehabilitation.
Most previous studies of Constraint and Multimodal aphasia therapies for chronic aphasia utilised non-randomised designs or were small phase I or II pilot trials subject to participant selection and other biases.8 9 11–13 These study limitations may account for the differences in findings between COMPARE and those of the preliminary trials that showed significant change in global aphasia severity. We utilised liberal inclusion criteria without limitations on age or time after stroke onset beyond the minimum of 6 months, whereas the previous studies had narrower selection criteria which may have impacted outcomes. Given recent trial evidence it is perhaps unsurprising that global aphasia severity (WAB-R-AQ) was not reduced across the entire treated population in COMPARE. For example, in FCT2EC6 only people <70 years were recruited while in COMPARE there was no age restriction, and participants in FCET2EC were provided a higher dose (median of 46 hours including self-practice) of therapy. In FCET2EC, significant improvements were observed in functional communication which were maintained at 6 month follow-up, but a global aphasia severity outcome measure was not used. Similarly, in the Big CACTUS trial7 of computer-based word retrieval treatment, effects were found on picture naming but not functional communication, and there was no measure of change in global aphasia severity.
We demonstrated an unambiguous outcome of significantly improved word retreival for treated items immediately following CIAT-Plus and M-MAT compared with UC, with gains exceeding a previously reported clinically meaningful difference of 10%,7 and maintenance of approximately 50% of the original posttherapy gain at 12 weeks follow-up. The limited generalisation at the group level to untreated items or to discourse measures seen here is consistent with the Big CACTUS7 results. There was a large degree of variability in our discourse measures undermining confidence in their psychometric properties and future work should explore the stability of these measures and critical thresholds for meaningful change. Although maintenance of word retrieval effects were seen at 12 weeks follow-up these were not seen at the group level for functional communication, multimodal communication or quality of life outcomes. This latter finding is consistent with the Cochrane Review of SLT5 and a recent systematic review of maintenance of outcomes following intensive aphasia interventions,29 which showed on average a 50% loss of gains at follow-up. Such loss highlights the need for research into the impacts of maintenance doses of intervention on preserving therapeutic gains after intensive aphasia interventions as well as careful examination of participant factors associated with treatment response and maintenance of response.
Study limitations
One limitation of COMPARE concerns the participant numbers recruited in each severity strata: although we aimed to recruit participants evenly across the severity strata, 69% had mild aphasia while only 3% had severe aphasia. Thus, caution is required in interpreting the results for the severe stratum. A further potential limitation common to all rehabilitation trials employing complex behavioural interventions is lack of participant and therapist blinding. We minimised this risk by using blinded assessors with checks on potential assessor unblinding throughout.
Summary
In conclusion, COMPARE is the first multicentre, phase III RCT in which the effects of two different aphasia treatments, CIAT-Plus and M-MAT, were compared for people with chronic aphasia. We found significant improvement in word retrieval, functional communication, and quality of life immediately following both treatments and maintenance of word retrieval effects at follow-up, with a differential benefit of M-MAT for multimodal communication and communication-related quality of life and CIAT-Plus for word retrieval. The possible differential effects by baseline aphasia severity are clinically important for treatment prescription and require further investigation. Further research should investigate the impacts of increased dose on global aphasia severity outcomes and maintenance of effects, and further refine treatment prescription algorithms.
Footnotes
Twitter: @rose_mirandaros, @LyndseyNickels, @ErinGodecke
Contributors: MLR, LN, DC, LT, EG, MM, TR, DAC, JK, AF, MC, MH and GS devised the study protocol. Julie Bernhardt (JB), Leonid Churilov, Alison Ferguson were members of the COMPARE Data Safety Management Committee; JB was the chairperson. MLR, LN, DC, LT, EG, MM, AF, MC and MH developed the CIAT-Plus and M-MAT therapy treatment manuals. CW, JP, GS, MH, MC participated in data collection and processing. MLR, LN, DAC, LT, EG, MM, TR, DC, JK, MC, MH and JP contributed to data analysis and interpretation. MLR wrote the first draft of the report. MLR, LN, DAC, LT, EG, MM, TR, DC, JK, MH, CW and JP edited the report. All authors approved the final version of the manuscript.
Funding: This trial was funded by the Australian National Health and Medical Research Council (#1083010). Additional funding was provided by La Trobe University.
Competing interests: MLR, LN, DC, LT, EG, MM, TR, DAC, JK and MH were members of the COMPARE steering committee, chaired by MLR.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Human Research Ethics Committee of the lead recruitment site (Gold Coast University Hospital; HREC 15 QGC 291) and 25 other participating hospitals and universities in Australia and New Zealand.
References
- 1. Flowers HL, Skoretz SA, Silver FL, et al. Poststroke aphasia frequency, recovery, and outcomes: a systematic review and meta-analysis. Arch Phys Med Rehabil 2016;97:2188–201. 10.1016/j.apmr.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 2. Kauhanen ML, Korpelainen JT, Hiltunen P, et al. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis 2000;10:455–61. 10.1159/000016107 [DOI] [PubMed] [Google Scholar]
- 3. Hilari K, Byng S. Health-Related quality of life in people with severe aphasia. Int J Lang Commun Disord 2009;44:193–205. 10.1080/13682820802008820 [DOI] [PubMed] [Google Scholar]
- 4. Ellis C, Simpson AN, Bonilha H, et al. The one-year attributable cost of poststroke aphasia. Stroke 2012;43:1429–31. 10.1161/STROKEAHA.111.647339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brady MC, Kelly H, Godwin J, et al. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 2016:CD000425. 10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breitenstein C, Grewe T, Flöel A, et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet 2017;389:1528–38. 10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- 7. Palmer R, Dimairo M, Cooper C, et al. Self-managed, computerised speech and language therapy for patients with chronic aphasia post-stroke compared with usual care or attention control (big cactus): a multicentre, single-blinded, randomised controlled trial. Lancet Neurol 2019;18:821–33. 10.1016/S1474-4422(19)30192-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meinzer M, Rodriguez AD, Gonzalez Rothi LJ. First decade of research on constrained-induced treatment approaches for aphasia rehabilitation. Arch Phys Med Rehabil 2012;93:S35–45. 10.1016/j.apmr.2011.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rose ML, Attard MC, Mok Z, et al. Multi-Modality aphasia therapy is as efficacious as a constraint-induced aphasia therapy for chronic aphasia: a phase 1 study. Aphasiology 2013;27:938–71. 10.1080/02687038.2013.810329 [DOI] [Google Scholar]
- 10. Rose ML. Releasing the constraints on aphasia therapy: the positive impact of gesture and multimodality treatments. Am J Speech Lang Pathol 2013;22:S227–39 10.1044/1058-03602012/12-0091 [DOI] [PubMed] [Google Scholar]
- 11. Zhang J, Yu J, Bao Y, et al. Constraint-induced aphasia therapy in post-stroke aphasia rehabilitation: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2017;12:e0183349. 10.1371/journal.pone.0183349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang G, Ge L, Zheng Q, et al. Constraint-induced aphasia therapy for patients with aphasia: a systematic review. Int J Nurs Sci 2020;7:349–58. 10.1016/j.ijnss.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pierce JE, Menahemi-Falkov M, O'Halloran R, et al. Constraint and multimodal approaches to therapy for chronic aphasia: a systematic review and meta-analysis. Neuropsychol Rehabil 2019;29:1005–41. 10.1080/09602011.2017.1365730 [DOI] [PubMed] [Google Scholar]
- 14. Rose ML, Copland D, Nickels L, et al. Constraint-induced or multi-modal personalized aphasia rehabilitation (compare): a randomized controlled trial for stroke-related chronic aphasia. Int J Stroke 2019;14:972–6. 10.1177/1747493019870401 [DOI] [PubMed] [Google Scholar]
- 15. Rose M, Ferguson A, Power E, et al. Aphasia rehabilitation in Australia: current practices, challenges and future directions. Int J Speech Lang Pathol 2014;16:169–80. 10.3109/17549507.2013.794474 [DOI] [PubMed] [Google Scholar]
- 16. Kertesz A. Western aphasia Battery-Revised. New York: Grune & Stratton, 2007. [Google Scholar]
- 17. Strand EA, Duffy JR, Clark HM, et al. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord 2014;51:43–50. 10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 19. Dekhtyar M, Braun EJ, Billot A, et al. Videoconference administration of the Western aphasia Battery-Revised: feasibility and validity. Am J Speech Lang Pathol 2020;29:673–87. 10.1044/2019_AJSLP-19-00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caute A, Northcott S, Clarkson L, et al. Does mode of administration affect health-related quality-of-life outcomes after stroke? Int J Speech Lang Pathol 2012;14:329–37. 10.3109/17549507.2012.663789 [DOI] [PubMed] [Google Scholar]
- 21. Wallace SJ, Worrall L, Rose T, et al. A core outcome set for aphasia treatment research: the Roma consensus statement. Int J Stroke 2019;14:180–5. 10.1177/1747493018806200 [DOI] [PubMed] [Google Scholar]
- 22. Lomas J, Pickard L, Bester S, et al. The communicative effectiveness index: development and psychometric evaluation of a functional communication measure for adult aphasia. J Speech Hear Disord 1989;54:113–24. 10.1044/jshd.5401.113 [DOI] [PubMed] [Google Scholar]
- 23. van der Meulen I, van de Sandt-Koenderman WME, Duivenvoorden HJ, et al. Measuring verbal and non-verbal communication in aphasia: reliability, validity, and sensitivity to change of the scenario test. Int J Lang Commun Disord 2010;45:424–35. 10.3109/13682820903111952 [DOI] [PubMed] [Google Scholar]
- 24. Hilari K, Byng S, Lamping DL, et al. Stroke and aphasia quality of life Scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke 2003;34:1944–50. 10.1161/01.STR.0000081987.46660.ED [DOI] [PubMed] [Google Scholar]
- 25. Nicholas LE, Brookshire RH. Presence, completeness, and accuracy of main concepts in the connected speech of non-brain-damaged adults and adults with aphasia. J Speech Hear Res 1995;38:145–56. 10.1044/jshr.3801.145 [DOI] [PubMed] [Google Scholar]
- 26. Rose ML, Rai T, Copland D, et al. Statistical analysis plan for the compare trial: a 3-arm randomised controlled trial comparing the effectiveness of Constraint-induced aphasia therapy plus and multi-modality aphasia therapy to usual care in chronic post-stroke aphasia (compare). Trials 2021;22:303. 10.1186/s13063-021-05238-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gilmore N, Dwyer M, Kiran S. Benchmarks of significant change after aphasia rehabilitation. Arch Phys Med Rehabil 2019;100:1131–9. 10.1016/j.apmr.2018.08.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. www.R-project.org/ [Google Scholar]
- 29. Menahemi-Falkov M, Breitenstein C, Pierce JE, et al. A systematic review of maintenance following intensive therapy programs in chronic post-stroke aphasia: importance of individual response analysis. Disabil Rehabil 2021:1–16. 10.1080/09638288.2021.1955303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2021-328422supp001.pdf (506.9KB, pdf)
Data Availability Statement
Data are available on reasonable request.