Abstract
It remains undetermined whether burden of diabetes newly detected during acute COVID-19 persist in post-acute COVID phase. This meta-analysis was conducted to summarize the available literature and provide a pooled estimate of the risk of developing incident diabetes following hospital discharge or at least 28 days after the COVID-19 diagnosis compared to matched controls or severity matched influenza/ non-COVID-19 acute upper respiratory tract infections (AURI). Pooled analysis of 5787,027 subjects from four observational studies showed 59 % higher risk of developing incident diabetes in post-acute COVID-19 phase versus healthy controls (HR:1.59; 95 % CI:1.40–1.81, p < 0.001, I2=94 %, random-effects model). The high degree of heterogeneity in pooled estimate can be attributed to difference in demographic characteristics, hospitalization rates or disease severity between study subjects. Pooling data from three studies, higher risk of incident diabetes was also observed following COVID-19 versus severity matched non-COVID-19 respiratory tract infections (moderate-severe/hospitalized cases, HR 1.52; 95 % CI: 1.36–1.70, p < 0.01, I2=0 %, fixed-effects model; mild cases, HR 1.22; 95 % CI: 1.14–1.31, p < 0.001; I2=0 %, fixed-effects model). Majority of studies had median follow-up period of around 4 months. In view of several limitations due to retrospective design of these studies, prospective studies with long term follow-up are warranted.
1. Introduction
Presence of newly diagnosed diabetes in a significant proportion of patients during acute COVID-19 phase with or without hospitalization has been established in previous systematic reviews [1], [2]. However, it is unclear whether such metabolic abnormalities are persistent in long COVID. Emerging literature points towards an increasing burden of incident diabetes during post-COVID-19 period as well [3], [4], [5], [6], [7], [8], [9]. The CDC defines post–COVID-19 conditions as new, returning, or ongoing health problems occurring ≥ 4 weeks after being infected with COVID-19 [10]. However, this cut-off remains a matter of debate with some researchers suggesting COVID diagnosis plus 21 days as a reasonable start to the post-acute phase [5]. In this context, we aimed to provide a pooled estimate of the risk of developing incident diabetes following hospital discharge or at least 28 days after the COVID-19 diagnosis compared to matched controls. In addition, we also planned to collate the effect of COVID-19 versus severity matched non-COVID-19 acute upper respiratory infections (AURI) on the risk of incident diabetes.
2. Methods
The meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement [11]. We (MB and RP) independently performed a systematic search of the literature across the PubMed, Embase and Web of Science databases from inception till April 2, 2022, using relevant Mesh terms, Emtree terms and keywords interposed with appropriate Boolean operators (Supplementary Appendix).
Observational studies (prospective/retrospective, case-control/cohort design) were included wherever they reported the adjusted rate ratio (RR)/hazard ratio (HR) of incident diabetes in patients after at least 28 days of COVID diagnosis or following discharge after first hospital admission for COVID-19 as compared to a non-COVID-19 population (either healthy controls or non-COVID-19 AURI). Reviews, commentaries, articles in non-English language, pre-prints and incomplete/duplicate data were excluded. Any discrepancy was solved via discussion with third reviewer (SD). Study quality was assessed using the Newcastle-Ottawa scale (NOS) [12].
Statistical analysis was performed using the RevMan 5.4 software following data extraction. Adjusted estimates (expressed as HR/RR) from each study were pooled together with the generic inverse variance method using the fixed-effects/random-effects model. Heterogeneity was measured using I 2 statistics and quantified as low, moderate, and high with upper limits of 25 %, 50 %, and 75 % for I 2, respectively. A two-tailed p < 0.05 was considered to be statistically significant.
3. Results
After a scrupulous literature search, we included 7 observational studies in our meta-analysis (Supplementary Figure 1). Out of these 7 studies, 4 studies (5 datasets) reported the risk of incident diabetes post-COVID-19 versus matched controls pooling data from 5787,027 subjects [3], [4], [5], [6]. The rest of the 3 studies reported the risk of incident diabetes in COVID-19 versus severity matched influenza, pooling data from both mild (n = 308613) and moderate-severe/hospitalized (n = 24090) COVID-19 patients [7], [8], [9]. One preprint study was excluded as per inclusion criteria [13]. Two studies reported rate ratios [6], [8], while others reported hazard ratios for incident diabetes. Incidence RR can be roughly interpreted as HR, especially when a single HR averaged over the duration of the study’s follow-up has been reported and the follow-up period is comparable among studies [14]. Since we planned to pool reported HR/RR from studies, the study by Wander et al. was excluded that reported significantly higher odds ratio (OR) for incident DM in men following COVID-19 adjusting for greater surveillance post-hospitalization [15]. Characteristics of all studies and quality assessment have been demonstrated (Supplementary Table 1–2).
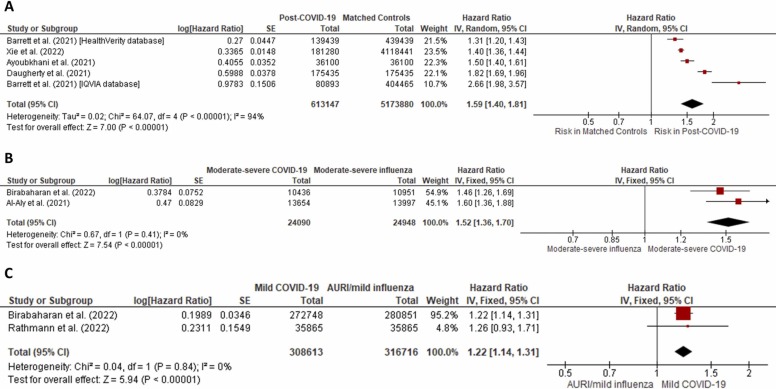
The pooled analysis showed that post-COVID-19 subjects were at 59 % higher risk of developing incident diabetes when compared to controls (HR 1.59; 95 %CI: 1.40–1.81, p < 0.001, I 2=94%, random-effects model) ( Fig. 1A). Similar estimates were observed on analysis including only those studies that reported post-COVID diabetes at least 28 days after COVID diagnosis (HR 1.64; 95 %CI: 1.37–1.96, p < 0.001, I 2=95 %, random-effects model) (Supplementary Figure 2 ). Sensitivity analysis revealed that results derived from one dataset [4] and two studies [5], [6] contributed to the high heterogeneity (Supplementary Figure 3 ). Compared to patients with moderate-to-severe/hospitalized influenza, patients with moderate-severe COVID-19 showed significantly higher risk of developing incident diabetes (HR 1.52; 95% CI: 1.36–1.70, p < 0.01, I 2=0%, fixed-effects model) (Fig. 1B). Risk of diabetes was also significantly higher in patients in mild COVID-19 versus subjects with mild flu/AURI (HR 1.22; 95 % CI: 1.14–1.31, p < 0.001, I 2=0 %, fixed-effects model) (Fig. 1C).
Fig. 1.
(1A) Forest plot depicting the risk of incident diabetes in post-COVID-19 subjects as compared to controls (HR 1.59; 95 % CI: 1.40–1.81, p < 0.001, I2=94 %, random-effects model). (1B) Forest plot depicting the risk of incident diabetes in moderate-to-severe post-COVID-19 subjects as compared to patients with moderate-to-severe/hospitalized influenza (HR 1.52; 95 % CI: 1.36–1.70, p < 0.01, I2=0 %, fixed-effects model). (1C) Forest plot depicting the risk of incident diabetes in mild post-COVID-19 subjects as compared to patients with mild flu or acute upper respiratory infection (HR 1.22; 95 % CI: 1.14–1.31, p < 0.001, I2=0 %, fixed-effects model).
4. Discussion
This systematic review and meta-analysis showed higher risk of incident diabetes post-acute COVID-19 compared to healthy controls. Difference in age, ethnicity, male population, hospitalization rates or disease severity among population of included studies might have resulted in the high heterogeneity in our pooled estimates.
Despite reports of a twofold excess of incident type 1 diabetes in patients following pandemic influenza A (H1N1) in Norway [16], majority of epidemiological studies do not support temporal association of seasonal influenza with diabetes [17]. Acute influenza, being a common disease event for hospitalized patients, was taken as control group in few studies in present review. In our study, a consistent increase in risk of incident DM following COVID-19 was found with no heterogeneity when compared to severity matched flu-like illness. Furthermore, subgroup analysis based on steroid use amongst moderate/severe cases did not reveal any difference in the strength of association between new-onset T2DM and COVID-19 [9]. Therefore, clinicians need to screen for adverse metabolic consequences frequently during post-acute COVID-19 phase regardless of the disease severity or history of steroid use during acute phase [18].
Given the high risk of DM even in pediatric population, we underscore the importance of vaccinating all persons irrespective of age. Notably, two studies did not find significantly higher adjusted HR for T1DM or other forms of diabetes in post-COVID-19 versus controls, thereby suggesting major contribution of T2DM on excess burden of incident diabetes in post-acute COVID-19 [7], [8]. To explain the observed association of diabetes risk after COVID-19 in children/adolescents, Barrett et al. focused on disproportionately higher proportion of ethnic minorities contracting COVID-19 and the rising prevalence of T2DM from very early age among them [4]. Higher incidence of diabetic ketoacidosis in the pediatric cohort compared to pre-pandemic data could be attributed to delayed care seeking and less accessibility to healthcare [4]. Lack of significantly higher anti-SARS-CoV-2 antibodies in children with incident T1DM during pandemic have argued against the temporal association between COVID-19 and T1DM [19], [20]. Moreover, recent reports indicate that infection of human pancreatic islets by COVID-19 remains largely non-cytopathic and unlikely to precipitate new-onset diabetes [21]. Nonetheless, insulin resistance and disordered insulin release consequent to up-regulated cytokines during post-acute COVID-19 phase may indeed contribute to excess risk of incident T2DM in these subjects [22], [23].
We do acknowledge several limitations of this study. None of the studies accounted for greater surveillance following hospitalization like the one that was excluded from analysis [15]. However, increased risk of diabetes in patients following hospitalized COVID-19 versus influenza adds value to interpretation of our findings. All of the studies included had retrospective design. ICD-10 codes were solely used to define DM in most studies with the exception of two studies that additionally used HbA1c levels [3], [7]. This technique might be less sensitive to identify actual burden of DM. Moreover, chances of misclassification bias, information bias and residual confounding could not be ruled out from all studies. Many studies, including the study by Barrett et al. conducted in persons < 18 years age [4], did not report HR separately for type 1 and 2 DM. Owing to lack of information, subgroup analysis could not be done on important covariates, such as pre-diabetes and obesity status.
In conclusion, subjects post-acute COVID-19 have significantly higher risk of incident diabetes, most likely due to new-onset T2DM. However, long-term prospective studies are required to establish whether this burden truly reflects a permanent burden on the metabolic health.
Funding
None.
Acknowledgement
None.
Conflicts of Interest
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pcd.2022.05.009.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID ‐19 patients: a systematic review and meta‐analysis. Diabetes Obes. Metab. 2021;23:870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khunti K., Del Prato S., Mathieu C., Kahn S.E., Gabbay R.A., Buse J.B. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care. 2021;44:2645–2655. doi: 10.2337/dc21-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Y., Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022 doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett C.E., Koyama A.K., Alvarez P., Chow W., Lundeen E.A., Perrine C.G., et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 Years — United States, March 1, 2020–June 28, 2021. MMWR Morb. Mortal. Wkly Rep. 2022;71:59–65. doi: 10.15585/mmwr.mm7102e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daugherty S.E., Guo Y., Heath K., Dasmariñas M.C., Jubilo K.G., Samranvedhya J., et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayoubkhani D., Khunti K., Nafilyan V., Maddox T., Humberstone B., Diamond I., et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 8.Rathmann W., Kuss O., Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022 doi: 10.1007/s00125-022-05670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birabaharan M., Kaelber D.C., Pettus J.H., Smith D.M. Risk of new‐onset type 2 diabetes in 600 055 people after COVID ‐19: A cohort study. Diabetes Obes. Metab. 2022 doi: 10.1111/dom.14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. n.d.
- 11.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luchini C., Stubbs B., Solmi M., Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017;5:80. doi: 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 13.The OpenSAFELY Collaborative, Tazare J., Walker A.J., Tomlinson L., Hickman G., Rentsch C.T., et al. Rates of serious clinical outcomes in survivors of hospitalisation with COVID-19: a descriptive cohort study within the OpenSAFELY platform. Epidemiology. 2021 doi: 10.1101/2021.01.22.21250304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernán M.A. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wander P.L., Lowy E., Beste L.A., Tulloch-Palomino L., Korpak A., Peterson A.C., et al. The incidence of diabetes among 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care. 2022;45:782–788. doi: 10.2337/dc21-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz P.L.D., Tapia G., Bakken I.J., Håberg S.E., Hungnes O., Gulseth H.L., et al. Pandemic influenza and subsequent risk of type 1 diabetes: a nationwide cohort study. Diabetologia. 2018;61:1996–2004. doi: 10.1007/s00125-018-4662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M., Kedzierska K., Doherty P.C., et al. Influenza. Nat. Rev. Dis. Prim. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal R., Joshi A., Bhadada S.K., Banerjee M., Vaikkakara S., Mukhopadhyay S. Endocrine follow-up during post-acute COVID-19: practical recommendations based on available clinical evidence. Endocr. Pr. 2022 doi: 10.1016/j.eprac.2022.02.003. S1530891X22000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmi H., Heinonen S., Hästbacka J., Lääperi M., Rautiainen P., Miettinen P.J., et al. New-onset type 1 diabetes in Finnish children during the COVID-19 pandemic. Arch. Dis. Child. 2022;107:180–185. doi: 10.1136/archdischild-2020-321220. [DOI] [PubMed] [Google Scholar]
- 20.Messaaoui A., Hajselova L., Tenoutasse S. Anti-SARS-CoV-2 antibodies in new-onset type 1 diabetes in children during pandemic in Belgium. J. Pedia Endocrinol. Metab. 2021;34:1319–1322. doi: 10.1515/jpem-2021-0289. [DOI] [PubMed] [Google Scholar]
- 21.van der Heide V., Jangra S., Cohen P., Rathnasinghe R., Aslam S., Aydillo T., et al. Limited extent and consequences of pancreatic SARS-CoV-2 infection. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montefusco L., Ben Nasr M., D’Addio F., Loretelli C., Rossi A., Pastore I., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021;3:774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal R., Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J. Endocrinol. Investig. 2020;43:1027–1031. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material