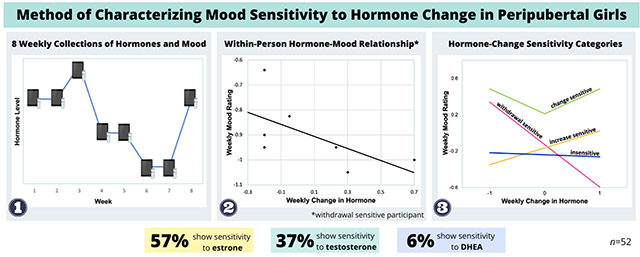
Abstract
Peripubertal females are at elevated risk for developing affective illness compared to males, yet biological mechanisms underlying this sex disparity are poorly understood. Female risk for depression remains elevated across a woman’s reproductive lifespan, implicating reproductive hormones. A sensitivity to normal hormone variability during reproductive transition events (e.g., perimenopause) precipitates affective disturbances in susceptible women; however, the extent of hormone variability during the female pubertal transition and whether vulnerability to peripubertal hormone flux impacts affective state change in peripubertal females has not been studied. 52 healthy peripubertal females (ages 11-14) provided 8 weekly salivary samples and mood ratings. 10 salivary ovarian and adrenal hormones (e.g., estrone, testosterone, dehydroepiandrosterone (DHEA)) were analyzed weekly for 8 weeks using an ultrasensitive assay to characterize the female peripubertal hormone environment and its association with affective state. Hormone variability indices, including standard deviation, mean squared and absolute successive differences of the 8 weekly measurements were analyzed by menarche status. Within-person partial correlations were computed to determine the strength of the relationship between weekly change in hormone level and corresponding mood rating for each participant. As expected, results indicated that hormone variability was greater for post- relative to pre-menarchal females and with advancing pubertal development, yet pregnenolone-sulfate and aldosterone did not differ by menarche status. Mood sensitivity to changes in estrone was exhibited by 57% of participants, whereas 37% were sensitive to testosterone and 6% were sensitive to DHEA changes. The present results offer novel evidence that a substantial proportion of peripubertal females appear to be mood-sensitive to hormone changes and may inform future investigations on the biological mechanisms underlying hormone-induced affect dysregulation in peripubertal females.
Keywords: pubertal transition, peripuberty, hormone variability, estrone, testosterone, mood
Graphical Abstract
1. Introduction
The pubertal transition (peripuberty, PT) is characterized by dramatic physical maturation, refinement of brain morphology, and a rapidly changing reproductive hormone environment. Peripuberty also marks the beginning of a sex divergence in rates of depression, with females experiencing over three times more depressive symptoms compared with males (Angold, 1993; SAMHSA, 2017). Although controlled studies in other reproductive phases, such as peripartum (Bloch et al., 2000), adult premenstrual phases (Schmidt et al., 2017, 1998) and depression during the menopause transition (perimenopause)(Gordon et al., 2016, 2015) indicate that hormonal flux precipitates affective illness in vulnerable women, the association of hormone flux and mood has not been studied in the PT. This is in part because individual differences in the dynamic nature of the hormonal milieu during the PT is yet to be well characterized.
The PT involves a cascade of hormonal events, beginning with adrenarche (increases in hypothalamic-pituitary adrenal activity) and production of adrenal androgens (e.g., dehydroepiandrosterone (DHEA) and androstenedione (A4)) followed by gonadarche (reactivation of the hypothalamic-pituitary gonadal axis), which stimulates a rapid increase in sex steroids (e.g., estradiol (E2) and estrone (E1)) in females and the subsequent maturation of secondary sex characteristics (Biro et al., 2014). Considerable increases in average levels of ovarian and adrenal hormones are observed as females progress from early to mid-puberty and to post-PT, which is ultimately characterized by regular ovulatory cycling and adult reproductive capacity (Apter, 1980). While adult hormone patterns are usually evident one year after menarche in Tanner stage 5, post-menarchal females may have luteal phase insufficiency and lower progesterone than adult women (Elmlinger et al., 2002; Sun et al., 2018; Zhang et al., 2008). That said, a direct relationship between hormone levels and pubertal stage is elusive, as individual differences in diurnal fluctuation and levels necessary to advance pubertal stage make it impossible to identify stage from hormone levels alone (Fassler et al., 2019). Further, the extent to which these hormones fluctuate during the PT, and whether peripubertal hormone flux is comparable to what is observed during other reproductive transitions, has not been elucidated.
Reproductive steroids and their metabolites interact with neurotransmitter systems to influence the activational and organizational construction of affective neural networks, thus contributing to emotion and behavior regulation (Schiller et al., 2016; Walker et al., 2004). The sex disparity in affective illness, first emerging mid-puberty and continuing throughout the female reproductive lifespan, implicates steroid hormones in vulnerability to psychopathology (Angold et al., 1999; Schiller et al., 2016). Ovarian hormones (i.e., E2, E1) have significant regulatory effects on mood, making women more likely to experience affective symptoms during reproductive events characterized by volatile hormone changes (Balzer et al., 2015; Schiller et al., 2016).
Previous studies have found associations between estrogen (E1, E2) (Balzer et al., 2015), and, more consistently, testosterone (T) and depression in adolescent females (Copeland et al., 2019). Further, along with stimulating the development of pubic and armpit hair and skin changes, DHEA has been shown to associate with anxiety symptoms and the probability of developing an anxiety disorder in adolescent females (Mulligan et al., 2020). Despite proposed relationships between mood and peripubertal hormones, these previous reports are limited by infrequent hormone collections and cross-sectional study designs that prevent the examination of mood sensitivity to endogenous peripubertal hormone changes. Thus, characterizing and differentiating ovarian and adrenal hormone variability during the PT will have significant implications for the design of future research investigating the influence of the pubertal hormonal milieu in the manifestation of female-dominated affective illness.
The objectives of this study were to 1) for the first time, characterize the extent to which ovarian and adrenal hormones fluctuate weekly during the pubertal transition, and 2) explore the association between weekly changes in E1, T and DHEA and mood symptoms in peripubertal females. It was hypothesized that the degree of ovarian and adrenal fluctuations would increase with advancing pubertal status. Our secondary hypothesis was that, in a proportion of adolescent females, there would be evidence of mood sensitivity to hormone flux, similar to what has been observed in other female reproductive phases (Gordon et al., 2020).
2. Methods
2.1. Participants
Adolescents ages 11-14 who were assigned female at birth and were undergoing a healthy PT were recruited from the local community using flyers, mass email and middle school online parent communication. Participants were self and parent-reported Tanner Stage 3 or 4, reflecting breast development and pubic hair growth underway but not complete, and pre- or within one-year post-menarche. Non-English speakers or participants with psychotic or bipolar disorders, or active suicide ideation were excluded from participation. Parental and self-report did not indicate any use of herbal and hormonal supplements or contraceptives, or medications that are known to alter mood and neurological function (e.g., antidepressants, stimulants).
2.2. Procedure
Following parental screening to evaluate eligibility, an enrollment session was completed to assess demographics and pubertal development. An abbreviated Structured Clinical Interview for DSM-V (SCID) was administered to screen for psychosis or bipolar disorder, along with self-report questionnaires on mood and stress. Height and weight measurements were collected to calculate age-corrected BMI (per Centers for Disease Control and Prevention guidelines), as BMI consistently tracks with pubertal maturation (Bini et al., 2000). Instructions were provided on best practice for self-collecting saliva samples and participants were given 8 clearly labeled collection vials. Participants and parents gave written assent and consent to participate, and participants received a $150 Visa gift card for compliance. The study protocol was approved by the local institutional review board and strictly adhered to ethical research standards consistent with the Declaration of Helsinki. After the first 18 participants, the protocol was modified to reduce participant burden, including implementing weekly rather than daily mood reports, and early morning hormone collections to capture the expected peak hormone levels (Hucklebridge et al., 2005; Janfaza et al., 2006; Kuzawa et al., 2016), rather than at-home visits.
2.3. Salivary hormone assessment and analysis.
Participants used unstimulated passive drool technique to provide 3mL of saliva into cryovials at the same time each week for 8 weeks. While the timing of saliva collections was consistent within participant, 18 participants completed saliva collection visits in the afternoon (approximately 3 PM), whereas the remaining 35 participants collected immediately upon awakening. Participants were instructed to refrain from drinking (water included) and brushing teeth at least 30 minutes prior, eating one hour prior, and to not visit the dentist within 2 days prior to the sample collection to prevent contamination and dilution. Saliva samples were immediately placed in the participant’s home freezer, before being transferred to a −80°C laboratory freezer every 1-3 weeks and later shipped in batches to ZRT Laboratory (Beaverton, OR) for analysis. Liquid chromatography- tandem mass spectrometry (LC-MS/MS) was utilized to ensure the most sensitive and accurate quantification of salivary hormones. Average inter-assay precision was 10.87% for E2, 7.27% for E1, 9.77% for T, 5.53% for androstenedione (A4), 7.03% for DHEA, 4.83% for DHEA-sulfate (DHEA-S), 12.10% for 7-keto DHEA, 11.20% for 17-hydroxyprogesterone (17-OHP4), 10.70% for pregnenolone sulfate (pregs), and 12.77% for aldosterone (aldo), with the average intra-assay coefficitents of variance as follows: 5.03% for E2, 9.47% for E1, 4.20% for T, 4.70% for A4, 7.27% for DHEA, 2.63% for DHEA-S, 7.47% for 7-keto DHEA, 6.40% for 17-OHP4, 4.57% for pregs, and 4.57% for aldo. Minimum detection limits were 0.24 pg/mL for E2, 0.4 pg/mL for E1, 3.2 pg/mL for T, 1.1 pg/mL for A4, 17.1 pg/mL for DHEA, 0.04 ng/mL for DHEA-S, 26 pg/mL for 7-keto DHEA, 2.3 pg/mL for 17-OHP4, 1.3 pg/mL for pregs and 3.3 pg/mL for aldo. Measurements that fell below assay sensitivity were assigned a value that was one-half the limit of detection. One sample of E2 from a pre-menarchal participant was identified as an outlier (20.4 pg/mL - 4 times greater and 32 standard deviations removed from the second highest value (5 pg/mL)) and excluded. 5 samples were missing from 3 post-menarchal participants. 18% (76/419) of E2 samples, 8% (34/419) of E1, 10% (43/419) of T, 3% (13/419) of DHEA, and 5% (22/419) of aldosterone samples were below the detection limit. One pre-menarchal participant was diagnosed with Swyer syndrome three years after enrollment and was removed from the analyses (n=52).
2.4. Hormone variability indices.
Variability can be defined by the amplitude (i.e., magnitude of change), the frequency (i.e., how often changes occur), and the temporal dependency of measurements (i.e., the sequence or order of collections over time) (Ebner-Priemer et al., 2007). To characterize within-person week-to-week hormone fluctuation across the 8 weeks of hormone collections, the following variability estimates were calculated: 1) within-person variance or standard deviation (SD), 2) the mean absolute successive difference (MASD), and 3) the mean squared successive difference (MSSD). While the SD captures the overall dispersion of values and is a common indicator of perimenopausal E2 variability (Gordon et al., 2016), it does not represent the temporal instability or the amount of successive change overtime (Jahng et al., 2008). Alternatively, the MASD and MSSD can account for the magnitude, frequency and temporal dependency of hormone fluctuations (Ebner-Priemer et al., 2007).
2.5. Self-Report Measures at Enrollment
Pubertal Development Scale (PDS; Petersen et al., 1988). Participants self-reported menarche status, breast development, pubic hair growth, height or growth spurt, and appearance of acne based on Tanner Stage criteria using a 4-point scale, ranging from 1) no development/ no menses, 2) barely begun, 3) definitely underway, to 4) completed/ menses, with good reliability (Cronbach’s alpha for PDS in the present sample was 0.71). The self-reported PDS has excellent agreement with a physical examination (85% within 1 Tanner stage) for female breast and pubic hair development (Schmitz et al., 2004). The category PDS score corresponds to the sum of the 5 items, as described previously (Carskadon and Acebo, 1993). Additionally, participants selected line-drawings of models at each Tanner stage of breast development and pubic hair growth that best matched their own to calculate an averaged self-rating and pictorial score for breast and pubic hair development (Taylor et al., 2001).
Mood and Feelings Questionnaire (MFQ; Eg et al., 2018) is a 33-item assessment of depression that is validated for use with children and adolescents. Participants indicate whether each statement is not true (0), somewhat true (1) and mostly true (2) for them over the past 2 weeks, and the total score reflects the sum of the 33 items and ranges from 0 to 68. A larger score reflects greater depressive symptoms, and scores 29 or above are considered clinically significant (Burleson Daviss et al., 2006). Cronbach’s alpha for MFQ in the present sample was 0.94.
Child Chronic Strain Questionnaire (CCSQ; Rudolph et al., 2001) assesses seven domains of adolescent stress on a 5-point scale (1=not at all stressful/irrelevant, to 5=very stressful), including academic (4 items; e.g., “I got bad grades on tests or report cards;” Cronbach’s α=.79), peer (11 items, e.g., “It was hard for me to make friends,” “I needed someone to talk to and didn’t have a friend to listen;” α=.92) , developmental (2 items, e.g., “I started my period,” “My body is changing and I am growing up;” α=.76), family (6 items, e.g., “I got into arguments or fights with my parent(s),” “my parents were too busy to spend time with me;” α=.92), economic (6 items, e.g., “My family didn’t have enough money to pay the bills;” α=.69), environment (5 items, e.g., “I felt unsafe walking alone in my neighborhood;” α=.71) and ethnic (6 items, e.g., “I was treated like I wasn’t smart because of my race or ethnicity,” “I was treated unfairly because of my race or ethnicity;” α=.59), with higher scores representing higher levels of chronic strain.
2.6. Weekly Mood Assessments
To examine the relationship between weekly hormone variability and mood change, an abbreviated Daily Record Severity of Problems (DRSP; Endicott et al., 2006) for the first 18 participants and Center for Epidemiologic Studies Depression Scale for Children (CESD-DC, Radloff, 1977) for the remaining 35 participants were collected at the time of saliva collection to assess mood during the previous week. DRSP and CES-DC are widely used and well-validated questionnaires for the assessment of general dysphoric mood, with items from both measures mapping onto depression, low positive affect and interpersonal symptom constructs. Weekly averaged ratings for six items (Depressed, Anxious, Rejection, Interpersonal Conflict, Anhedonia, and Anger, scored from 1 (not at all) to 6 (extreme)) were summed to assess weekly changes in mood symptoms. The CES-DC is a well-validated, highly reliable 20-item self-report inventory to assess child and adolescent depression. Each item is scored 0 (not at all) to 3 (a lot) with 4 items reverse coded for a range between 0-60. Standardized z-scores were computed for DRSP and CES-DC to use in analyses. Because the objective of the analysis was to determine the relationship between affect and weekly change in hormone level, rather than symptom severity, CES-DC and DRSP z-scores were used interchangeably to permit the evaluation of within-person hormone-induced mood change in a larger sample.
2.6. Analytic plan
Hormone variability descriptive analyses.
Analyses were conducted in SAS University Edition. Hormone fluctuation was indexed using variability indices, including standard deviation (SD), mean absolute successive difference (MASD) and mean squared successive difference (MSSD). Full descriptive profiles, including mean, range, variability indices (SD, MSSD, MASD) were computed for each hormone by menarche status (pre- vs. post-menarche). Analyses of variance (ANOVAs) were performed to determine differences in MFQ and CCSQ subscales by menarche status.
Hormone sensitivity profiles.
The strength of each participant’s mood sensitivity to week-to-week changes in E1, T, and DHEA levels was computed using methods adapted from Gordon et al. (2020). E1 was selected over E2 because E1 rises before E2 during the pubertal transition (Biro et al., 2014), E1 was easier to detect, and was highly correlated with E2 in the current sample (r=0.80, p<.0001). For each participant, a mixed model with week specified as a repeated statement using a compound symmetry correlation structure was fitted to quantify weekly mood (CES-DC or DRSP z-score) as a function of week-to-week change in hormone level (Lipsitz et al., 2001) and the absolute value of week-to-week hormone change. The solution of fixed effects with estimates and variances for each model was used to calculate the partial correlation coefficient using the following formula:
where Z is the maximum likelihood Wald statistic, and m (subject) = 1. The resulting partial correlation coefficients, ranging from −1.0 to +1.0, reflected the degree to which an individual participant exhibited mood sensitivity to increases or decreases in the hormone (e.g., E1, T, DHEA), or mood sensitivity to both weekly increases and decreases in hormone level, as indicated by the absolute hormone change correlation. Sensitivity strength was estimated as the absolute value of the largest partial correlation coefficient (i.e., hormone change or absolute hormone change) for a given participant, ranging from 0.0 to +1.0. This method of assessing the within-person correlation between hormone change and mood was chosen over Pearson correlations, which were used by Gordon et al., 2020, as it accounts for the correlation of outcomes from repeated collections within each participant (Shan et al., 2020). Following the definitions used by Gordon et al., 2020 and in line with standard definitions of moderate-to-large repeated correlations (Cohen, 1992), a participant was deemed withdrawal sensitive if the partial correlation was ≤ −0.3, and increase sensitive if the partial correlation ≥ +0.3. Using the absolute value of hormone change, a partial correlation of ≥ +0.3 indicated that the participant was hormone-change sensitive and any previous sensitivity categorization (i.e., withdrawal or increase sensitive) was overridden. Finally, participants who did not fall in any of the three hormone-change sensitive categories were considered hormone-change insensitive. Two participants did not have acceptable variance in E1 and T (≥ 6 identical hormone measurements), and one participant missed 3 mood ratings and was not included in the analyses. All remaining participants had 5 or more hormone-mood pairs.
Power Calculation.
G-power was used for power calculations. With a sample size of n=53, the current study had 90% power with α=0.05 to detect small effects (f=0.15) of within-person weekly hormone and mood associations (8 repeated measures with 0.50 correlation). This sample size was sufficient considering the much larger effects found in our previous studies of natural ovarian hormone changes on daily mood symptoms (average f=0.69; d = 1.38) (Eisenlohr-Moul et al., 2016).
3. Results
3.1. Demographic and mood characteristics.
Demographic and mood characteristics are presented in Table 1. 28 peripubertal females who were pre-menarche and 24 females who were within 1-year post-menarche were included in the analysis. The sample was composed of 73% (38/52) White, 2% Black or African American (1/52), 12% (6/52) Hispanic or Latino, and 13% (7/52) identified with more than one race. Post-menarchal females were older (F(1,50)=6.05, p=.017, ηp2=0.11), having experienced menarche at an average age of 11.79 ±1.02, had a greater BMI (F(1,49)=6.37, p=0.015, ηp2=0.12), and had more advanced pubertal developmental status than pre-menarchal participants, indicated by the category PDS score (F(1,50)=257.19, p<.0001, ηp2=0.84). MFQ symptoms were marginally higher for post-menarchal (range=1 to 53) compared with pre-menarchal (range=0 to 35) participants (F(1,50)=3.25, p=.078, ηp2=0.06). Additionally, post-menarchal participants exhibited greater peer (F(1,50)=5.46, p=.024, ηp2=0.10), developmental (F(1,50)=50.47, p<.0001, ηp2=0.50), family (F(1,50)=5.73, p=.020, ηp2=0.10) and ethnic (F(1,50)=6.84, p=.012, ηp2=0.12) stress.
Table 1.
Demographic and Mood Characteristics
| Pre-menarche (n=28) |
Post-menarche (n=24) |
F Statistic | |
|---|---|---|---|
| Age (years) | 12.16 ± 0.95 | 12.80 ± 0.89 | 6.05* |
| BMI (z-score) | −0.26 ± 0.81 | 0.30 ± 0.76 | 6.37* |
| Category PDS score | 6.30 ± 0.93 | 10.42 ± 0.92 | 257.19*** |
| Breast Dev. | 2.73 ± 0.54 | 3.17 ± 0.48 | 9.33** |
| Pubic Hair Dev. | 2.57 ± 0.57 | 3.25 ± 0.57 | 18.19*** |
| Mood & Feelings Questionnaire | 11.04 ± 8.22 | 16.79 ± 14.40 | 3.25 |
| Child Chronic Strain | |||
| Academic | 1.84 ± 0.79 | 2.16 ± 1.03 | 1.57 |
| Peer | 1.38 ± 0.57 | 1.88 ± 0.97 | 5.46* |
| Development | 1.43 ± 0.33 | 2.75 ± 0.92 | 50.47*** |
| Family | 1.27 ± 0.39 | 1.82 ± 1.13 | 5.73* |
| Economical | 1.08 ± 0.21 | 1.19 ± 0.44 | 1.45 |
| Environmental | 1.34 ± 0.60 | 1.33 ± 0.59 | 0.01 |
| Ethnic | 1.03 ± 0.11 | 1.22 ± 0.36 | 6.84* |
Note. Means are presented ± standard deviation.
p<0.05
p<.01
p<.0001.
3.2. Hormone variability and mood characteristics by menarche status
Mean levels and variability indices (SD, MSSD, MASD) for E1, A4, DHEA, DHEA-S, and 17-OHP4 were greater for participants who were post-menarche compared with pre-menarche (Table 2). Further, E2 mean and MASD, T mean, SD, and MASD, and 7-keto DHEA MSSD and MASD were significantly greater for post-menarchal participants. Pregs and aldo did not differ between pre-and post-menarchal participants (ps>0.05). E1, T and DHEA have been previously tied to mood changes during female reproductive events, (Copeland et al., 2019; Rubinow and Schmidt, 2018; Schweizer-Schubert et al., 2021) and were therefore chosen to illustrate weekly hormone variability by menarche status in Figure 1.
Table 2.
Ovarian and Adrenal Hormone Milieu Pre- and Post-Menarche
| Statistic | ||||||
|---|---|---|---|---|---|---|
| Hormone | Mean | Std.Dev | MASD | MSSD | MIN | MAX |
| E2 pg/mL(n=52) | ||||||
| pre-menarchal | 0.37 | 0.35 | 0.22 | 2.03 | 0.15 | 1.10 |
| post-menarchal | 0.63 | 0.48 | 0.54 | 0.84 | 0.15 | 5.00 |
| F statistics | 4.77* | 0.22 | 5.12* | 0.30 | ||
| E1 pg/mL (n=52) | ||||||
| pre-menarchal | 0.84 | 0.26 | 0.27 | 0.14 | 0.20 | 2.50 |
| post-menarchal | 1.48 | 0.80 | 0.92 | 2.07 | 0.20 | 7.50 |
| F statistics | 38.57*** | 28.63*** | 32.53*** | 11.31** | ||
| T pg/mL (n=52) | ||||||
| pre-menarchal | 5.21 | 1.44 | 1.50 | 7.54 | 1.50 | 23.00 |
| post-menarchal | 7.78 | 2.76 | 2.95 | 19.30 | 1.50 | 30.00 |
| F statistics | 8.20** | 10.14** | 12.78** | 3.85 | ||
| A4 pg/mL (n=52) | ||||||
| pre-menarchal | 65.32 | 14.28 | 15.07 | 390.65 | 6.00 | 167.00 |
| post-menarchal | 94.91 | 26.79 | 29.49 | 1708.49 | 17.00 | 287.00 |
| F statistics | 7.37** | 18.80*** | 19.10*** | 15.50*** | ||
| DHEA pg/mL (n=52) | ||||||
| pre-menarchal | 56.66 | 15.16 | 15.97 | 437.30 | 8.50 | 152.00 |
| post-menarchal | 92.98 | 30.01 | 33.06 | 2439.23 | 8.50 | 319.00 |
| F statistics | 15.60*** | 9.95** | 16.01*** | 9.20** | ||
| DHEA-S ng/mL (n=35) | ||||||
| pre-menarchal | 1.63 | 0.54 | 0.65 | 1.07 | 0.20 | 6.80 |
| post-menarchal | 3.67 | 1.43 | 1.63 | 7.03 | 0.30 | 19.40 |
| F statistics | 6.32* | 7.33* | 6.57* | 4.25* | ||
| 7-keto DHEA pg/mL (n=52) | ||||||
| pre-menarchal | 74.95 | 14.76 | 14.95 | 449.50 | 13.00 | 181.00 |
| post-menarchal | 91.58 | 19.23 | 22.33 | 841.84 | 13.00 | 262.00 |
| F statistics | 2.78 | 3.69 | 7.92** | 4.13* | ||
| 17-OHP4 pg/mL (n=52) | ||||||
| pre-menarchal | 12.02 | 4.70 | 5.27 | 66.15 | 1.00 | 52.00 |
| post-menarchal | 22.62 | 9.31 | 10.05 | 268.14 | 1.00 | 133.00 |
| F statistics | 7.74** | 8.69** | 6.66* | 5.43* | ||
| Pregs pg/mL (n=35) | ||||||
| pre-menarchal | 50.46 | 19.35 | 20.80 | 1582.33 | 8.00 | 366.00 |
| post-menarchal | 73.24 | 30.95 | 35.97 | 5309.13 | 14.00 | 592.00 |
| F statistics | 1.98 | 1.04 | 1.32 | 0.94 | ||
| Aldo pg/mL (n=52) | ||||||
| pre-menarchal | 29.28 | 21.84 | 23.93 | 1643.24 | 1.50 | 271.00 |
| post-menarchal | 27.54 | 21.51 | 24.19 | 1298.81 | 1.50 | 158.00 |
| F statistics | 0.19 | 0.01 | 0.00 | 0.18 | ||
Note. Estradiol (E2; limit of detection (LoD)=0.24 pg/mL), Estrone (E1; LoD=0.4 pg/mL), Testosterone (T; LoD=3.2 pg/mL), Androstenedione (A4; 1.1 pg/mL), Dehydroepiandrosterone (DHEA; LoD=17.1 pg/mL), Dehydroepiandrosterone-Sulfate (DHEA-S; LoD=0.04 ng/mL),7-keto Dehydroepiandrosterone (7k-DHEA; LoD=26 pg/mL) 17-hydroxyprogesterone (17-OHP4; LoD=2.3 pg/mL), Pregnenlone-Sulfate (pregs; LoD=1.3 pg/mL), Aldosterone (aldo; LoD=3.3 pg/mL). Standard deviation (sd), mean absolute successive difference (MASD), mean squared successive difference (MSSD).
p<0.05
p<.01
p<.0001.
Figure 1. Pre-and post-menarchal hormone variability.
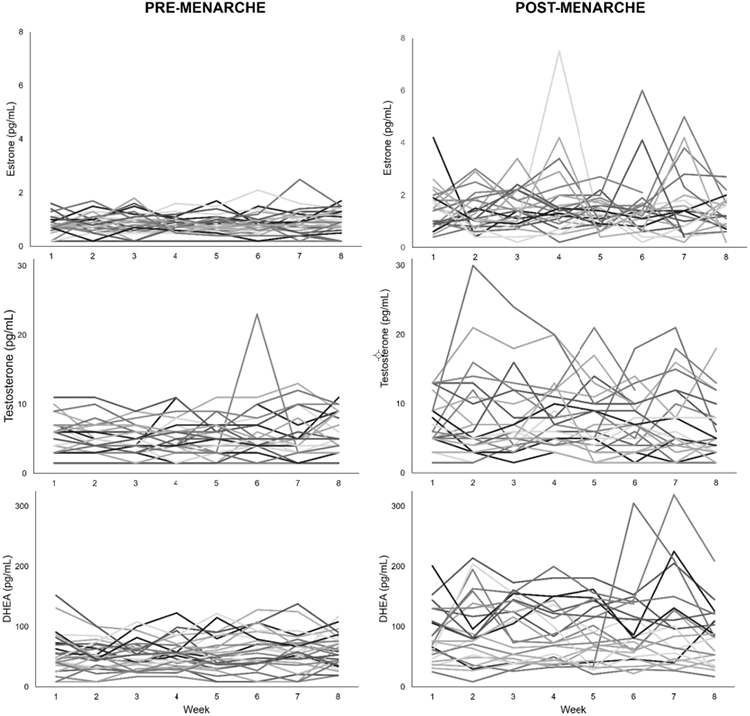
Weekly measurements of estrone (E1, pg/mL), testosterone (T, pg/mL) and dehydroepiandrosterone (DHEA, pg/mL) are presented for pre- (n=29) and post-menarchal (n=24) participants.
3.3. Distribution of hormone sensitivity profiles
Individual E1 and mood partial correlations (PCC) ranged from −0.83 to +0.85; absolute change in E1 and mood PCCs ranged from −0.92 to +0.95, and E1 sensitivity strength ranged from 0.01 to +0.95. 57% (28/49) of participants exhibited an increase in mood symptoms with changes in E1, with 8% (4/49) exhibiting increased sensitivity to E1 withdrawal, 22% (11/49) exhibiting increased sensitivity to E1-increase, 27% (13/49) exhibiting increased sensitivity to E1-change and 43% (21/49) were insensitive to E1 change. Figure 2a illustrates the relationship between changes in weekly E1 and changes in dysphoric mood by sensitivity profile (i.e., sensitive to E1 withdrawal, E1 increase, E1 change in either direction, or insensitive). Pre-menarchal females demonstrated greater E1 sensitivity strength compared with post-menarchal females (F(1,47)=7.240, p=.010, ηp2=0.13). Additionally, the distribution of hormone sensitivity profiles differed by menarche status, with pre-menarchal participants being more likely (18%) to be sensitive to increases in E1 compared with post-menarchal participants (4%) (X2(3) = 9.37, p=0.025).
Figure 2. Weekly hormone change and mood relationships by sensitivity profile.
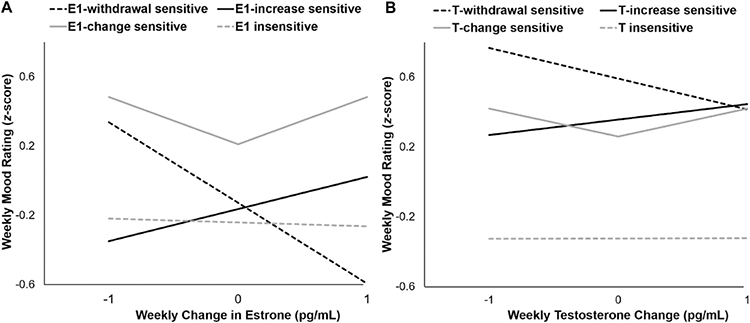
Model-based estimates of the relationships between weekly mood rating (z-score) and change in A) estrone (pg/mL) and B) testosterone (pg/mL) for each hormone-sensitivity group.
Figure 2b depicts the relationship between the weekly change in T and subsequent mood score by sensitivity profile. T and mood PCCs ranged from −0.66 to +0.72; absolute change in T and mood PCCs ranged from −0.86 to +0.90, and T sensitivity strength ranged from 0.01 to +0.90. 37% (18/49) were sensitive to changes in T, with 4% (2/49) showing T-withdrawal sensitivity, 16% (8/49) showing T-increase sensitivity, 16% (8/49) showing T-change sensitivity, and 63% (31/49) were insensitive to T change. T sensitivity strength did not differ by menarche status (p>.20).
DHEA and mood PCCs ranged from −0.21 to +0.30; absolute change in DHEA and mood PCCs ranged from −0.40 to +0.37, and DHEA sensitivity strength ranged from 0.00 to +0.37. 6% (3/53) of participants were sensitive to changes in DHEA. DHEA sensitivity strength did not differ by menarche status (p>.20).
4. Discussion
For the first time, the present study employed weekly measurements of ovarian and adrenal hormones to characterize the hormone milieu (particularly hormone variability) in females during this critical window of vulnerability for the emergence of mood and behavioral disturbances. We found that relative to pre-menarche, post-menarche was associated with an abrupt enhancement of ovarian and adrenal hormone variability for all hormones studied except aldosterone and pregnenolone-sulfate. Additionally, there were significant individual differences in hormone variability post-menarche, with some participants exhibiting minimal or extreme week-to-week changes in hormone levels. In accordance with previous reports, aldosterone levels (and variability) were independent of menarche or pubertal status (Mahler et al., 2015). However, contrary to previous examinations (Granger et al., 2003), T was highly correlated with pubertal development. Differences in the frequency of collection and method of analysis may account for this discrepancy.
The narrow window of puberty assessed in the current study (Tanner stage 3-4) is associated with increased risk for mood and behavioral disturbances, especially for adolescent females (Angold et al., 1999). The profound post-menarchal endogenous hormone variability observed in the present study is comparable to perimenopausal hormone flux (O’Connor et al., 2001), which has been associated with affective illness (Gordon et al., 2020, 2016). As such, results from the present study may provide a foundation for understanding fluctuations in hormones as potential biological mechanisms relevant to the development of affective vulnerability that emerges during the PT.
Our results offer novel evidence of a relationship between hormone change and affect state change in peripubertal females, with a significant proportion of participants showing mood sensitivity to endogenous changes in estrone, an even greater proportion than the 39% of perimenopausal women who reported mood sensitivity to changes in estrone-3-glucuronide, a urinary metabolite of estradiol (Gordon et al., 2020). While speculative, it is possible that peripubertal females are more sensitive to changing hormones than perimenopausal women because the abrupt exposure to hormone flux occurs during a sensitive developmental window of dramatic neuromaturation, and dynamic expression and regulation of estrogen receptors (McEwen, 1992; Spear, 2000). Interestingly, though, the proportion of hormone-sensitive participants falling within the withdrawal versus increase versus change sensitive profiles are remarkably similar to those observed by Gordon et al., 2020. While the majority of participants were sensitive to substantial E1 changes in either direction, participants also exhibited mood sensitivity to weekly increases or decreases in hormone level. A smaller proportion of participants were also sensitive to weekly increases and high-magnitude changes in T. Yet only 3 participants showed increased mood symptoms with changes in DHEA. As such, the distribution of hormone sensitivity profiles identified in the present study appears to reflect robust individual differences in mood sensitivity to hormone flux during a vulnerable developmental period when menstrual cycles are still irregular, and is consistent with the affective disturbances triggered by reproductive events characterized by both dramatic hormone withdrawal (i.e., peripartum symptoms) (Bloch et al., 2000) and hormone increases (i.e., lutealphase exaggeration of affective symptoms in menstrually-related mood disorders) (Schmidt et al., 2017). Further, our findings may provide partial explanation for the inconsistent reports in adolescent research linking ovarian and adrenal hormone mean levels and affective symptoms, or the failure to identify mood-hormone relationships, as the erratic variability in hormones may be more etiologically relevant to depression than are absolute levels in peripubertal females.
Despite the current evidence that peripubertal females may experience at least a moderate degree of mood sensitivity to endogenous hormone flux, the neurophysiological mechanisms underlying the differential sensitivity to ovarian and adrenal hormone flux and its role in affective disturbances is poorly understood and has not been studied in the PT. The PT is a critical developmental window for frontal brain development, making the frontal cortex and associated processes (e.g., social cognition, emotion regulation) particularly vulnerable to the deleterious effects of stress exposure during the PT (Blakemore, 2012; Crone and Konijn, 2018; Crone and Steinbeis, 2017; Zhang et al., 2016, Giedd et al., 2006; Lupien et al., 2009). Given the essential influence of ovarian and adrenal hormones in regulating the HPA axis response to stress, and frontal and limbic affect networks that are implicated in the susceptibility to stress in affective illness, (Goel et al., 2014; Kamin and Kertes, 2017; Vamvakopoulos and Chrousos, 1993;Parker et al., 2003; Spielberg et al., 2019) hormone variability may serve as a diathesis for affective dysregulation during the PT. This is particularly noteworthy given the increase in most measures of stress experienced by the post-menarchal participants in the present study. Moreover, hormone derived organizational effects have been shown to reemerge with subsequent hormone flux, supporting the importance of the PT for establishing neurodevelopmental trajectories (Blakemore, 2008; Schulz and Sisk, 2016).
While there are significant strengths of the present study, including frequent sampling of 10 hormones during a narrow pubertal window in female adolescents, there are several limitations that should be considered. First, self-reported pubertal stage simply assesses external secondary sex characteristics, and further, has not consistently shown more than moderate agreement with physical examination (Shirtcliff et al., 2009). However, self-reported puberty indices permit a less-invasive assessment of pubertal development, and allow for the integration of pubertal assessment in nonclinical settings (Morris and Udry, 1980; Shirtcliff et al., 2009). Advancing our understanding of peripubertal hormone fluctuations may inform more sensitive and comprehensive methods of defining pubertal timing and maturation. Additionally, despite the use of an ultrasensitive assay method (LC-MS/MS) in the current study, analyses may have been limited by the number of samples that were below the limit of detection (i.e., 18% of E2 samples). Moreover, saliva was chosen over other collection mediums (particularly plasma/serum) because it offers a non-invasive, less stressful method than venous blood samples, and was therefore more feasible for frequent sampling in adolescents. Although plasma is considered the “gold-standard” for hormone measurement, steroid hormones in saliva represent free plasma levels in blood, and therefore may be more relevant to understanding the effect of biologically available hormone activity on behavioral outcomes. As such, previous research identified a strong correlation between salivary hormones and behavior indices, more so than blood spot analyses (Edler et al., 2007). Nonetheless, salivary measures of some hormones are complicated by intraglandular metabolism (e.g., progesterone, testosterone), and the rapid fluctuations of salivary measures (e.g., estradiol, testosterone) suggest that multiple samples over a short period are required for optimal hormonal assessment (Chatterton et al., 2005; Wood, 2009).
Certainly, the precision of the present study’s results is limited by the reliance on only 8 weekly hormone and mood measurements. However, the degree of bias that can be expected from such a small sample is relatively modest (correlation coefficient inflated ≤ 0.14)(Bishara and Hittner, 2015). Further, because of the substantial irregularity of menstrual cycles within 1-year post-menarche and the 8-week study duration, cycle phase was not accounted for in the current analyses. Results should be interpreted in light of the fact that “mood” was not assessed with the same self-report measure for all participants; however, the standardized scores for the measures were used in the present analyses, which focused on the relationship between week-to-week changes in hormone and subsequent dysphoric mood, rather than the severity or clinical significance of mood symptoms. Nevertheless, the current method of quantifying an individual’s mood sensitivity to hormone change could be applied to predict the emergence of clinically significant depressive symptoms over an extended assessment period, consistent with what has been found in the menopause transition (Gordon et al., 2020). Accordingly, this method of characterizing mood sensitivity to hormone flux has considerable clinical implications. Additional examination of hormone-induced changes in distinct symptom domains (anxiety, irritability etc.) is warranted, particularly to examine the previously proposed relationship between anxiety symptoms and DHEA (Mulligan et al., 2020).
5. Conclusion
In conclusion, our study provides novel evidence that a substantial proportion of peripubertal females appear to be mood-sensitive to hormone changes. The present results represent a first step in establishing a relationship between hormone change and affective symptoms and provides a foundation for moving forward with future investigations of mechanisms underlying differential hormone sensitivity and affective dysregulation during the PT. Our results also suggest that an alternative, within-person methodological approach may be sensitive to capturing individual differences in the coupling of hormone and affective state changes.
Highlights.
Weekly salivary hormone levels used to capture variability in peripubertal females
Ovarian and adrenal hormone variability increase with advancing pubertal status
Peripubertal females show weekly mood changes tied to estrone and testosterone flux
Acknowledgments.
This study was supported by the National Institute of Mental Health Grant K01MH121575 (to EA), the National Institute of Health T32 postdoctoral fellowship MH093315 (to EA), the NIH Clinical Translational Science Award pilot grant UL1TR002489 (to EA), and a Foundation of Hope for Research and Treatment of Mental Illness grant (to EA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest. The authors have no conflicts of interest to disclose.
References.
- Angold A, 1993. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders 29, 145–158. 10.1016/0165-0327(93)90029-J [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM, 1999. Pubertal changes in hormone levels and depression in girls. Psychological medicine 29, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Apter D, 1980. Serum Steroids and Pituitary Hormones in Female Puberty: A Partly Longitudinal Study. Clinical Endocrinology 12, 107–120. 10.1111/j.1365-2265.1980.tb02125.x [DOI] [PubMed] [Google Scholar]
- Balzer BWR, Duke SA, Hawke CI, Steinbeck KS, 2015. The effects of estradiol on mood and behavior in human female adolescents: a systematic review. Eur J Pediatr 174, 289–298. 10.1007/s00431-014-2475-3 [DOI] [PubMed] [Google Scholar]
- Bini V, Celi F, Berioli MG, Bacosi ML, Stella P, Giglio P, Tosti L, Falorni A, 2000. Body mass index in children and adolescents according to age and pubertal stage. European Journal of Clinical Nutrition 54, 214–218. 10.1038/sj.ejcn.1600922 [DOI] [PubMed] [Google Scholar]
- Biro FM, Pinney SM, Huang B, Baker ER, Walt Chandler D, Dorn LD, 2014. Hormone Changes in Peripubertal Girls. The Journal of Clinical Endocrinology & Metabolism 99, 3829–3835. 10.1210/jc.2013-4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishara AJ, Hittner JB, 2015. Reducing Bias and Error in the Correlation Coefficient Due to Nonnormality. Educational and Psychological Measurement 75, 785–804. 10.1177/0013164414557639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, 2012. Development of the social brain in adolescence. J R Soc Med 105, 111–116. 10.1258/jrsm.2011.110221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, 2008. The social brain in adolescence. Nature Reviews Neuroscience 9, 267–277. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau MA, Murphy J, Nieman LK, Rubinow DR, 2000. Effects of Gonadal Steroids in Women With a History of Postpartum Depression. The American Journal of Psychiatry 157, 924–930. [DOI] [PubMed] [Google Scholar]
- Burleson Daviss W, Birmaher B, Melhem NA, Axelson DA, Michaels SM, Brent DA, 2006. Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects: Criterion validity of Mood and Feelings Questionnaire. Journal of Child Psychology and Psychiatry 47, 927–934. 10.1111/j.1469-7610.2006.01646.x [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, 1993. A self-administered rating scale for pubertal development. Journal of Adolescent Health 14, 190–195. 10.1016/1054-139X(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Mateo ET, Hou N, Rademaker AW, Acharya S, Jordan VC, Morrow M, 2005. Characteristics of salivary profiles of oestradiol and progesterone in premenopausal women. Journal of Endocrinology 186, 77–84. 10.1677/joe.1.06025 [DOI] [PubMed] [Google Scholar]
- Cohen J, 1992. A power primer. Psychological Bulletin 112, 155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Copeland WE, Worthman C, Shanahan L, Costello EJ, Angold A, 2019. Early Pubertal Timing and Testosterone Associated With Higher Levels of Adolescent Depression in Girls. Journal of the American Academy of Child & Adolescent Psychiatry 58, 1197–1206. 10.1016/j.jaac.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Konijn EA, 2018. Media use and brain development during adolescence. Nature communications 9, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Steinbeis N, 2017. Neural Perspectives on Cognitive Control Development during Childhood and Adolescence. Trends in Cognitive Sciences 21, 205–215. 10.1016/j.tics.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Kuo J, Kleindienst N, Welch SS, Reisch T, Reinhard I, Lieb K, Linehan MM, Bohus M, 2007. State affective instability in borderline personality disorder assessed by ambulatory monitoring. Psychol Med 37, 961–970. 10.1017/S0033291706009706 [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK, 2007. Ovarian hormones and binge eating in bulimia nervosa. Psychological medicine 37, 131. [DOI] [PubMed] [Google Scholar]
- Eg J, Bilenberg N, Costello EJ, Wesselhoeft R, 2018. Self- and parent-reported depressive symptoms rated by the mood and feelings questionnaire. Psychiatry Research 268, 419–425. 10.1016/j.psychres.2018.07.016 [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, Girdler SS, 2016. Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology 67, 142–152. 10.1016/j.psyneuen.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmlinger MW, Kühnel W, Ranke MB, 2002. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clinical Chemistry and Laboratory Medicine 40, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, 2006. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health 9, 41–49. 10.1007/s00737-005-0103-y [DOI] [PubMed] [Google Scholar]
- Fassler CS, Gutmark-Little I, Xie C, Giannini CM, Chandler DW, Biro FM, Pinney SM, 2019. Sex Hormone Phenotypes in Young Girls and the Age at Pubertal Milestones. The Journal of Clinical Endocrinology & Metabolism 104, 6079–6089. 10.1210/jc.2019-00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP, 2006. Puberty-related influences on brain development. Molecular and Cellular Endocrinology 254–255, 154–162. 10.1016/j.mce.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V, 2014. Sex Differences in the HPA Axis. Comprehensive Physiology 4, 36. [DOI] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, Wisner KL, 2015. Ovarian Hormone Fluctuation, Neurosteroids, and HPA Axis Dysregulation in Perimenopausal Depression: A Novel Heuristic Model. American Journal of Psychiatry 172, 227–236. 10.1176/appi.ajp.2014.14070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS, 2016. Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition: Menopause 23, 257–266. 10.1097/GME.0000000000000528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Sander B, Eisenlohr-Moul TA, Sykes Tottenham L, 2020. Mood sensitivity to estradiol predicts depressive symptoms in the menopause transition. Psychol. Med 1–9. 10.1017/S0033291720000483 [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn–Waxler C, Usher B, Klimes-Dougan B, Hastings P, 2003. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: Individual differences and developmental effects. Development and Psychopathology 15, 431–449. [PubMed] [Google Scholar]
- Hucklebridge F, Hussain T, Evans P, Clow A, 2005. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology 30, 51–57. 10.1016/j.psyneuen.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Jahng S, Wood PK, Trull TJ, 2008. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychological Methods 13, 354–375. 10.1037/a0014173 [DOI] [PubMed] [Google Scholar]
- Janfaza M, Sherman TI, Larmore KA, Brown-Dawson J, Klein KO, 2006. Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: a 10-year experience. Journal of Pediatric Endocrinology and Metabolism 19, 901–910. [DOI] [PubMed] [Google Scholar]
- Kamin HS, Kertes DA, 2017. Cortisol and DHEA in development and psychopathology. Hormones and Behavior 89, 69–85. 10.1016/j.yhbeh.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Georgiev AV, McDade TW, Bechayda SA, Gettler LT, 2016. Is There a Testosterone Awakening Response in Humans? Adaptive Human Behavior and Physiology 2, 166–183. 10.1007/s40750-015-0038-0 [DOI] [Google Scholar]
- Lipsitz SR, Leong T, Ibrahim J, Lipshultz S, 2001. A Partial Correlation Coefficient and Coefficient of Determination for Multivariate Normal Repeated Measures Data. Journal of the Royal Statistical Society: Series D (The Statistician) 50, 87–95. 10.1111/1467-9884.00263 [DOI] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews neuroscience 10, 434. [DOI] [PubMed] [Google Scholar]
- Mahler B, Kamperis K, Ankarberg-Lindgren C, Djurhuus JC, Rittig S, 2015. The effect of puberty on diurnal sodium regulation. American Journal of Physiology-Renal Physiology 309, F873–F879. 10.1152/ajprenal.00319.2014 [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1992. Steroid hormones: effect on brain development and function. Horm Res 37 Suppl 3, 1–10. 10.1159/000182393 [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR, 1980. Validation of a self-administered instrument to assess stage of adolescent development. Journal of youth and adolescence 9, 271–280. [DOI] [PubMed] [Google Scholar]
- Mulligan EM, Hajcak G, Crisler S, Meyer A, 2020. Increased dehydroepiandrosterone (DHEA) is associated with anxiety in adolescent girls. Psychoneuroendocrinology 119, 104751. 10.1016/j.psyneuen.2020.104751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KA, Holman DJ, Wood JW, 2001. Menstrual cycle variability and the perimenopause. American Journal of Human Biology 13, 465–478. 10.1002/ajhb.1078 [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM, 2003. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and behavior 43, 60–66. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence 17, 117–133. [DOI] [PubMed] [Google Scholar]
- SAMHSA. 2017. National Survey on Drug Use and Health: Detailed Tables. [Google Scholar]
- Rubinow DR, Schmidt PJ, 2018. Is there a role for reproductive steroids in the etiology and treatment of affective disorders? Dialogues Clin Neurosci 20, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Kurlakowsky KD, Conley CS, 2001. Developmental and social–contextual origins of depressive control-related beliefs and behavior. Cognitive Therapy and Research 25, 447–475. [Google Scholar]
- Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR, 2016. Reproductive Steroid Regulation of Mood and Behavior, in: Terjung R (Ed.), Comprehensive Physiology. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 1135–1160. 10.1002/cphy.c150014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, Wakim PG, Rubinow DR, 2017. Premenstrual Dysphoric Disorder Symptoms Following Ovarian Suppression: Triggered by Change in Ovarian Steroid Levels But Not Continuous Stable Levels. The American Journal of Psychiatry. 10.1176/appi.ajp.2017.16101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR, 1998. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New England Journal of Medicine 338, 209–216. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL, 2016. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience & Biobehavioral Reviews 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer-Schubert S, Gordon JL, Eisenlohr-Moul TA, Meltzer-Brody S, Schmalenberger KM, Slopien R, Zietlow A-L, Ehlert U, Ditzen B, 2021. Steroid Hormone Sensitivity in Reproductive Mood Disorders: On the Role of the GABAA Receptor Complex and Stress During Hormonal Transitions. Front Med (Lausanne) 7. 10.3389/fmed.2020.479646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan G, Zhang H, Jiang T, 2020. Correlation Coefficients for a Study with Repeated Measures. Computational and Mathematical Methods in Medicine 2020, e7398324. 10.1155/2020/7398324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD, 2009. Pubertal development: correspondence between hormonal and physical development. Child development 80, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews 24, 417–463. 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Schwarz JM, Matyi MA, 2019. Anxiety in transition: Neuroendocrine mechanisms supporting the development of anxiety pathology in adolescence and young adulthood. Frontiers in Neuroendocrinology 55, 100791. 10.1016/j.yfrne.2019.100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BZ, Kangarloo T, Adams JM, Sluss PM, Welt CK, Chandler DW, Zava DT, McGrath JA, Umbach DM, Hall JE, Shaw ND, 2018. Healthy Post-Menarchal Adolescent Girls Demonstrate Multi-Level Reproductive Axis Immaturity. J Clin Endocrinol Metab 104, 613–623. 10.1210/jc.2018-00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG, 2001. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric and perinatal epidemiology 15, 88–94. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP, 1993. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. Journal of Clinical Investigation 92, 1896–1902. 10.1172/JCI116782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huot R, 2004. Pubertal neuromaturation, stress sensitivity, and psychopathology. Development and Psychopathology 16. 10.1017/S0954579404040027 [DOI] [PubMed] [Google Scholar]
- Wood P, 2009. Salivary steroid assays – research or routine? Ann Clin Biochem 46, 183–196. 10.1258/acb.2008.008208 [DOI] [PubMed] [Google Scholar]
- Zhang K, Pollack S, Ghods A, Dicken C, Isaac B, Adel G, Zeitlian G, Santoro N, 2008. Onset of Ovulation after Menarche in Girls: A Longitudinal Study. None 93, 1186–1194. 10.1210/jc.2007-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ding Q, Chen N, Wei Q, Zhao C, Zhang P, Li X, Liu Q, Li H, 2016. The development of automatic emotion regulation in an implicit emotional Go/NoGo paradigm and the association with depressive symptoms and anhedonia during adolescence. NeuroImage: Clinical 11, 116–123. 10.1016/j.nicl.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]


