To the Editor:
The kidneys are among the most commonly affected organ systems in patients with sickle cell anemia (SCA) and an estimated 15% of deaths in patients with SCA are attributed to kidney failure.1 Albuminuria is an early manifestation of kidney disease, which is used to define stage 1 and 2 chronic kidney disease (CKD), and is present in 15 – 28% of adolescents and up to 68% of adults with SCA.1 The clinical significance of albuminuria is highlighted by its ability to predict a rapid decline in estimated glomerular filtration rate in children and adults with SCA.2
Therapies to ameliorate albuminuria in patients with SCA have been adopted from the non-SCA literature. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers lower the intra-glomerular vascular pressure and improve the urine albumin concentration in some patients, although a large proportion of patients continue to have persistent albuminuria or are intolerant to these classes of agents.3 Therapies that directly target the pathophysiology of SCA-related kidney disease may stabilize or improve kidney function. Both the rate of hemolysis and the degree of anemia are independently related to the development of CKD in patients with SCA.4,5 In this report, we evaluated whether voxelotor, a recently FDA-approved oral therapy that reduces hemoglobin polymerization and leads to an improvement in hemolytic anemia,6 would decrease albuminuria in a cohort of adult patients with SCA.
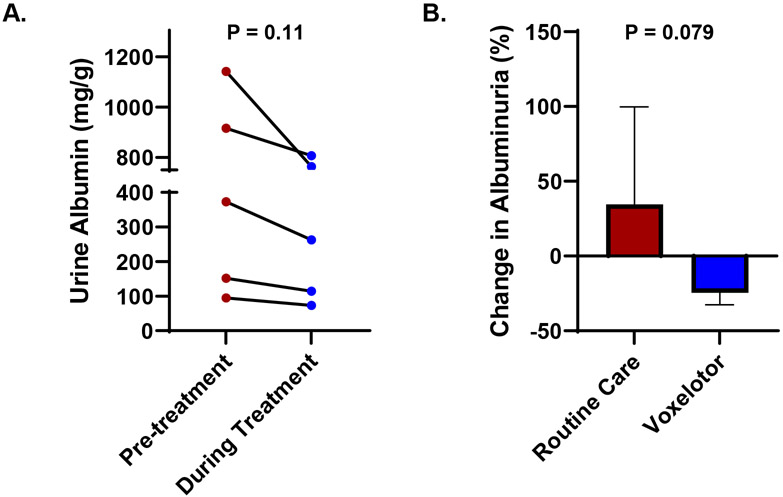
Between 8/2020 and 8/2021, ten adults with SCA (all hemoglobin SS genotype) were started on voxelotor as part of their routine medical care at the University of Illinois at Chicago (UIC) Sickle Cell Center. We focused our retrospective analysis on all five adults with CKD stage I – III. The study was approved by the UIC Institutional Review Board prior to data collection and analysis. We evaluated the change in urine albumin concentration, obtained during a routine clinic visit within six months prior to starting voxelotor and at the most recent available urine albumin concentration while on voxelotor therapy, using the paired t-test. The median age of this cohort was 47 years (range, 23–59 years), four were female, two were on hydroxyurea therapy, and three were on an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy at the time of starting voxelotor. The patients were treated with voxelotor for a median of seven months (range, 4–16 months). The doses of hydroxyurea and the angiotensin converting enzyme inhibitor or angiotensin receptor blocker remained stable during this time period. The median change in hemoglobin concentration from pre-treatment to the last time point on treatment was +0.9 g/dL (range, −0.6 to +4.2 g/dL) while the highest median change in hemoglobin concentration during treatment was +1.4 g/dL (range, +0.9 to +4.2 g/dL). The median change in the absolute reticulocyte count with voxelotor treatment was −50 x 109/L (range, −5 to −112 x 109/L). The estimated glomerular filtration rate remained stable on voxelotor treatment (median change from pre-treatment to last point on treatment: +1 ml/min/1.73m2; range, −8 to +25 mL/min/1.73m2). Urine albumin concentration declined in all five SCA patients treated with voxelotor therapy (Figure 1A). The median change in urine albumin concentration was −109 mg/g creatinine (range, −38 to −379 mg/g creatinine), which represented a 25% reduction (range, −12% to −33%) in albuminuria from baseline with voxelotor therapy.
Figure 1:
(A) Urine albumin concentration decreased during compared to values obtained within six months of starting voxelotor therapy. (B) Urine albumin concentration reduced by 25% during voxelotor therapy compared to a 34% increase in age- and sex-matched patients with SCA that did not receive voxelotor (mean and standard deviation are represented).
Next, we compared the albuminuria response with voxelotor to the changes in albuminuria in five age- and sex-matched SCA patients with a similar degree of albuminuria who did not receive voxelotor therapy. To determine the change in urine albumin concentration in this group, we used the difference between the most recent urine albumin concentration and the next available urine albumin concentration measured at least six months prior during routine clinic visits. The change in urine albumin concentration between those treated versus untreated with voxelotor was compared using the unpaired t-test. Two patients were on hydroxyurea, three patients were on angiotensin converting enzyme inhibitor or angiotensin receptor blockers, and the median interval of follow up was seven months (range, 6–9 months). In the group of SCA patients that did not receive voxelotor, three patients had an increase and two patients had a decrease in urine albumin concentration. The median urine albumin concentration change in this group was +94 mg/g creatinine (range, −179 to +288 mg/g creatinine), which represented a 34% increase (range, −34% to +101%) in albuminuria from baseline (Figure 1B).
To our knowledge, this is the first study to report a reduction in albuminuria in patients with SCA during treatment with voxelotor, an oral small molecule that prevents hemoglobin polymerization and improves hemolytic anemia. Hemolysis leads to the release of cell-free hemoglobin and heme into the circulation which may lead to kidney damage through direct oxidative damage and upregulation of inflammatory, immune response, and fibrogenic pathways.1,7 In two independent SCA cohorts, the degree of hemolysis was associated with hemoglobinuria which, in turn, predicted CKD stage and CKD progression on longitudinal follow up.4 Red blood cells are vital for oxygen delivery and anemia may exacerbate local tissue ischemia and oxidative injury. In animal models, progressive declines in hematocrit concentrations correlate with decreased cortical and medullary oxygenation.8 The tubulointerstitium may be particularly sensitive to hypoxia where anemia has been demonstrated to be independently associated with an increased risk for tubulointerstitial kidney damage in patients with early diabetic nephropathy.9 In children with SCA, a hemoglobin concentration < 8.0 g/dL was associated with a 9-fold increased risk for developing microalbuminuria compared to those with a hemoglobin concentration ≥ 8.0 g/dL.5
We report that in a cohort of SCA patients with CKD stage I to III, treatment with voxelotor was associated with both improved hemolytic anemia, as reflected by an increase in hemoglobin concentration and a reduction in absolute reticulocyte counts, and reduced urine albumin concentration. A reduction in albuminuria was observed in all five patients. In contrast, in a similar cohort of SCA patients who were not treated with voxelotor, albuminuria increased in three out of five patients. Our findings support the concept that hemolytic anemia is of clinical importance in sickle cell nephropathy; they also point to the need for future investigation of voxelotor, an FDA-approved oral therapy designed to reduce hemolytic anemia, for the treatment of sickle cell nephropathy.
Funding Statement
The project described was supported by the National Institutes of Health through grant R01 HL-153161 (S.L.S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure of Conflicts of Interest
V.G.R. and S.L.S. receive research support and provide consulting for Global Blood Therapeutics.
References
- 1.Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol. 2015;11(3):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niss O, Lane A, Asnani MR, et al. Progression of albuminuria in patients with sickle cell anemia: a multicenter, longitudinal study. Blood Adv. 2020;4(7):1501–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J, Srisuwananukorn A, Shah BN, et al. Effects of Renin-Angiotensin Blockade and APOL1 on Kidney Function in Sickle Cell Disease. EJHaem. 2021;2(3):483–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saraf SL, Zhang X, Kanias T, et al. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br J Haematol. 2014;164(5):729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aban I, Baddam S, Hilliard LM, Howard TH, Feig DI, Lebensburger JD. Severe anemia early in life as a risk factor for sickle-cell kidney disease. Blood. 2017;129(3):385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vichinsky E, Hoppe CC, Ataga KI, et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N Engl J Med. 2019;381(6):509–519. [DOI] [PubMed] [Google Scholar]
- 7.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 2012;122(4):1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johannes T, Mik EG, Nohe B, Unertl KE, Ince C. Acute decrease in renal microvascular PO2 during acute normovolemic hemodilution. Am J Physiol Renal Physiol. 2007;292(2):F796–803. [DOI] [PubMed] [Google Scholar]
- 9.von Eynatten M, Baumann M, Heemann U, et al. Urinary L-FABP and anaemia: distinct roles of urinary markers in type 2 diabetes. Eur J Clin Invest. 2010;40(2):95–102. [DOI] [PubMed] [Google Scholar]