Abstract
Spatial memory is critical for many tasks necessary for survival (i.e., locating mates and food resources). The two mammalian nonapeptides arginine vasopressin (AVP) and oxytocin (OT) are mechanistically important in modulating memory ability, albeit in contrasting ways. In general, AVP facilitates memory consolidation and retrieval while OT is an amnesic. Although AVP and OT are known to have these memory effects, past work has focused on their impact in social memory with little research on their effects on spatial memory. In this experiment, we tested the impact of AVP and OT on spatial memory as determined by performance in the Morris water maze (MWM). We administered doses of AVP, OT, or saline (a control) intranasally to male prairie voles (Microtus ochrogaster), a species whose spatial memory is hypothesized to impact their mating tactics. We also investigated if acute doses (given immediately prior to the memory trial in the MWM) and chronic doses (given daily during adolescence) had differing impacts on spatial cognition. We found that chronic intranasal administration of AVP during post-wean development improved spatial memory performance. In contrast, both chronic and acute administration of OT and acute administration of AVP had no impact on spatial memory. These results together suggest that 1) chronic exposure to AVP has organizational effects on spatial memory in the prairie vole, and 2) acute administration of nonapeptides does not impact the retrieval of spatial memories.
Keywords: Vasopressin, oxytocin, intranasal, post-weaning development, spatial memory
1. INTRODUCTION
Spatial memory is essential for locating resources important for survival and reproduction (Schwagmeyer, 1994; Smulders et al., 1995). The mammalian nonapeptides, arginine vasopressin (AVP) and oxytocin (OT), have important, yet contrasting, roles in regulating learning and memory. Work in this area has ranged, including the impact of AVP and OT in navigation and hippocampal-dependent cognition (e.g., Engelmann et al., 1992), passive or active avoidance learning (e.g., de Wied, 1991), and social recognition and social memory (e.g., Albers, 2012). Increasing attention has aimed to understand the roles of nonapeptides in the hippocampus and hippocampal-dependent memory. For instance, Egashira et al. (2004) showed that mice insensitive to AVP due to a lack of the AVP 1a receptor (V1aR) demonstrate impairments in a hippocampal-dependent spatial memory task. Additionally, Rice et al. (2017) demonstrated that male prairie voles perform better in a hippocampal-dependent water task than females, and that this sex difference was not related to size of or cell number in the hippocampus, but instead to the density of oxytocin receptors (OTR) within it. These results indicate that lower sensitivity to the effects of OT could explain better performance in this classic test of spatial memory. In general, AVP and OT appear to affect behavioral change by modulating information transfer through alterations in the ‘gain,’ or magnitude of a neuronal response, of conventional synapses (Grinevich & Ludwig, 2021). More specifically, AVP and OT can modulate synaptic activity by modulating the potency of GABA and/or glutamate synapses (Grinevich & Ludwig, 2021).
Nonapeptides have broad effects on learning and memory, but it is unclear to what extent their effects are ubiquitous, or consistent across forms of learning and memory or methods of manipulation. Generally, AVP facilitates memory consolidation and retrieval, whereas OT can serve as an amnestic, a memory enhancer, or have no effect (McEwen, 2004; Ophir, 2017). For example, central and peripheral injections of AVP facilitate social memory, whereas OT injections have no effect or interfere with it (Dantzer et al., 1987; Popik & Vetulani, 1991; Benelli et al., 1995). Moreover, OT appears to have a dose-dependent effect on memory (Popik et al., 1992; Benelli et al., 1995). These inconsistent impacts of OT could be explained by cross reactivity between OT, AVP, and their receptors (de Wied, 1991; Manning et al., 2008; Song et al., 2014; Song et al., 2016). Importantly, few studies that have compared the impacts of chronic or acute administration of nonapeptides on memory.
The behavioral variability observed resulting from the actions of AVP and OT and the differences between long-term or short-term exposure raise important questions about their roles in memory function. Importantly, the timing and degree of exposure to nonapeptides could have different impacts on spatial cognition, suggesting activational and organizational roles. More specifically, hormones may affect behavior by activating existing neural pathways or by organizing neural systems through exposure during critical periods of development. Work in rodents suggests that nonapeptides have both activational and organizational roles, and differences in the timing and length of administration results in varying behavioral effects. For instance, Huang et al. (2014) found that male mice given chronic doses of OT reduced sniffing behavior towards females, whereas males receiving acute doses of OT increased their investigation of females. This suggests that although acute administration activates existing receptors, chronic administration and the sustained increase in AVP or OT concentrations may lead to a desensitization of nonapeptide receptors (Huang et al., 2014) or changes in brain-wide connectivity (Pagani et al., 2020). Indeed, chronic intranasal delivery of AVP or OT produces varying behavioral results in many other contexts as well (Bales et al., 2013; Simmons et al., 2017; Pagani et al., 2020). Moreover, chronic intranasal manipulations during the juvenile and adolescent period can influence future adult behavior through organizational effects (Simmons et al., 2017; Prounis & Ophir 2019). Peripheral manipulations of nonapeptides given early in postnatal development can also lead to lasting changes in adult behavior, presumably through organizational effects (i.e., Stribley & Carter, 1999; Carter, 2003; for a review of the impacts of developmental AVP and OT, see Hammock, 2015). These studies, however, have focused on the activational and organizational roles of nonapeptides in social behavior; none to our knowledge have examined their role in cognition.
Here, we address whether chronic or acute intranasal administration of nonapeptides impact spatial memory. Intranasal administration of AVP and OT provides a non-invasive method of increasing nonapeptide levels in the extracellular fluid in the brain to study their proximate effects on behavior (Neumann et al., 2013). The non-invasiveness of intranasal delivery easily accommodates both chronic and acute administration. We chose to answer this question using male prairie voles (Microtus ochrogaster) for several reasons. Firstly, intranasal delivery of nonapeptides has been validated in this species (Bales et al., 2013; Simmons et al., 2017; Prounis & Ophir, 2019) enabling reproducible behavioral and physiological effects using predetermined concentrations of AVP and OT. The nonapeptide system and its effect in prairie voles is well described for their roles in social behavior (Walum & Young, 2018) and cognition (Prounis et al., 2015; Rice et al. 2017; Ophir, 2017). Finally, male prairie voles are believed to rely on spatial cognition to inform their mating decisions (Ophir, 2017). Indeed, male prairie voles have better spatial memory ability than females (Rice et al., 2017), and some have argued that male socio-spatial memory underlies the male-male interactions shaping social monogamy in this species (Phelps & Ophir, 2009; Rice et al., 2019; Ophir, 2017). Therefore, manipulations of spatial memory via nonapeptides in males specifically could reveal important insights into the mechanisms that contribute to reproductive decision-making in this model for monogamous social attachment.
We ran a series of experiments investigating how manipulating AVP or OT through acute or chronic intranasal administration impacts spatial memory in adult male prairie voles. Specifically, we investigated the effects of acute administration of AVP or OT delivered immediately before a memory task (i.e., activational role), and chronic administration of AVP or OT delivered throughout adolescence (i.e., organizational role). We tested spatial memory using the Morris water maze, a spatial memory task in which subjects must remember the learned location of a hidden platform using visual cues (Morris, 1984). We predicted that OT would impair spatial memory and that AVP would improve it. We also predicted that acute doses of AVP or OT should impact spatial memory due to the immediate bolus of peptide received, but that chronic intranasal administration would be more likely to alter spatial memory because of the long-term developmental or compensatory effects described above.
2. MATERIALS AND METHODS
2.1. Animals
All animals in this study were the laboratory-bred male offspring from unrelated pairs of F1 or wild-caught prairie voles in our breeding colony. These wild-caught breeders were originally trapped in Urbana-Champaign, IL, USA. Pups were weaned at 21 days old and then housed with same-sex siblings in standard polycarbonate cages (29 cm x 18 cm x 13 cm) containing Sani-chip bedding and nesting material. Animals were kept on a 14:10 light:dark cycle and were provided rodent chow (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO, USA) and water ad libitum. Ambient temperature was maintained at 20°C (± 2°C). All procedures were approved by, and in compliance with, the Institutional Animal Care and Use Committee of Cornell University (Protocol 2013-0102).
Randomly selected males from the breeding colony served as subjects in treatment or control groups for each experiment. Individuals received either saline, AVP, or OT intranasally. Doses for AVP and OT were based on published reports and calculated based on the prairie vole weight-adjusted dose used in human studies (Bales et al., 2013; Simmons et al., 2017; Prounis & Ophir, 2019, see supplementary material, table 1).
2.2. Acute intranasal administration
Subjects were chosen and ran through the Morris water maze (MWM) beginning at PND 60 (Figure 1a; see section 2.4 for MWM procedure). All animals progressed through the 9 learning trials without any experimental manipulation. We then administered saline (N = 10), AVP (N = 10; 1 μg/μl [or 600 IU/ml] in 0.9% saline; Sigma-Aldrich, St. Louis, MO), or OT (N = 10; 1 μg/μl [or 600 IU/ml] in 0.9% saline; Bachem Americas, Inc., Torrance, CA) intranasally 30 min prior to the MWM memory trial. To be consistent with the existing literature, our doses were comparable to those used in other studies that administered acute intranasal OT or AVP (e.g., Huang et al., 2014; Pagani et al., 2020; see Supplementary Table 1 for dose comparisons between studies). Furthermore, these doses are known to raise nonapeptide levels in the extracellular fluid and throughout brain plasma, with levels peaking 30 minutes after administration (Neumann et al., 2013). Intranasal administrations took place between 14:00h and 16:00h. Following the acute intranasal administration protocol outlined in Neumann et al. (2013), we held subjects in a supine position and applied 12 μl of solution to the nostrils, alternating between sides so that each nostril received 6 μl. All subjects inhaled the solution successfully.
Figure 1.
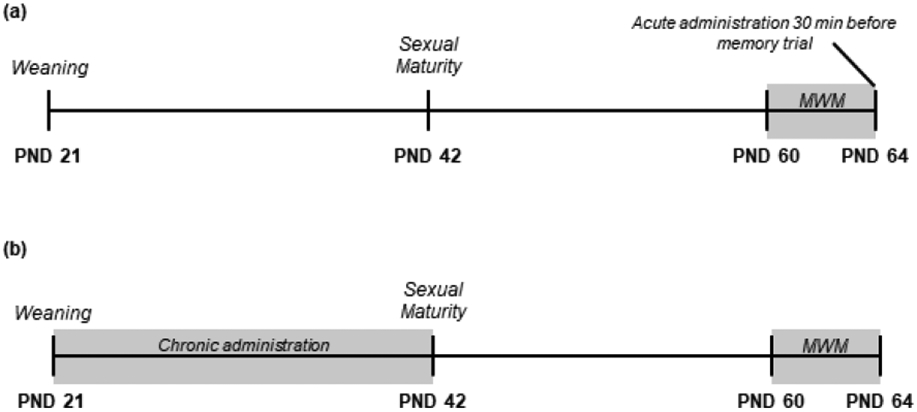
Timeline of experimental procedures for (a) acute intranasal administration and (b) chronic intranasal administration.
2.3. Chronic intranasal administration
Subjects received daily intranasal treatments of saline (N = 10), AVP (N = 9), or OT (N = 10) between PND 21 (i.e., weaning age) and PND 42 (i.e., subadult age; Figure 1b). Intranasal administrations took place between 08:00h and 12:00h. Studies administering intranasal nonapeptides chronically have used significantly smaller doses to achieve behavioral or physiological effects (e.g., Bales et al., 2013; Prounis & Ophir, 2019). In this experiment, we used doses of AVP (0.0005 IU/ml in 0.9% saline; Sigma-Aldrich, St. Louis, MO) and OT (0.0008 IU/ml in 0.9% saline; Bachem Americas, Inc., Torrance, CA) that are consistent with other chronic intranasal studies in prairie voles (Bales et al., 2013; Prounis & Ophir, 2019; see Supplementary Table 1 for dose comparisons between studies) and that are comparable to a weight-adjusted dose commonly used in human studies (reviewed in Grace et al., 2018). We adjusted concentrations of solution given intranasally to maintain the 0.0005 IU/ml (AVP) and 0.0008 IU/ml (OT) doses as the animals grew and gained weight. We administered intranasal solution to subjects held in a supine position as described above. To be consistent with other chronic intranasal studies in prairie voles (Bales et al., 2013; Prounis & Ophir, 2019), we applied doses in larger volumes of vehicle, such that 25 μl of saline, AVP, or OT were administered with a pipette tip around the nasal cavity. We alternated administration between nostrils so that 12.5 μl was applied to each nostril. We confirmed that solution was successfully inhaled. Like the acute intranasal experiment, the MWM learning trials began when the subjects reached PND 60. No intranasal administration of any substance occurred after PND 42 or at any point during the MWM procedure.
2.4. Morris water maze
Spatial memory was assessed in all experiments at PND 60 using the Morris water maze (Morris, 1984), following the procedure outlined in Rice et al. (2017). The apparatus consisted of a 1000 L tank (measuring 140 cm in diameter and 59 cm tall), a submerged removable platform (diameter: 11.5 cm, area: 103.87 cm2, depth below surface: 3 cm), and room dividers (171 cm tall) surrounding the tank. We maintained the water temperature at 30°C ±2°C with a baptismal heater (120V Baptistry Immersion Heater, product number 6HI-HL-120, Little Giant Manufacturing Company, Inc., Orange, TX). We concealed the location of the platform from the voles by making the water opaque with non-toxic white paint powder (Fresco Tempera Paint, Rich Art, Northvale, NJ). Visual cues were placed on room dividers that surrounded the tank to assist the subjects in finding the platform.
Testing in the MWM paradigm took place over 5 consecutive days and consisted of ten trials, with one ‘morning’ trial occurring between 09:00h and 11:00h and one ‘afternoon’ trial occurring between 14:00h and 16:00h. The first nine trials lasted until the subject located the platform or for a maximum of 2 min (Vorhees & Williams, 2006). If two minutes elapsed without the subject finding the platform, the subject was gently guided to it with a stick and their latency to locate the platform was coded as taking the total 120 seconds. We removed the platform from the testing apparatus before initiating the final ‘test’ trial. The test trial lasted 1 min, at which point the subject was removed from the apparatus with a net.
To begin a trial, subjects were placed in the apparatus at a randomized start position and allowed to freely swim until they reached the platform, or until time expired (either 2 min had elapsed in Trials 1-9, or until 1 min had elapsed in Trial 10). After each trial, the subjects were dried with a warm soft towel and placed on a heating pad for 2 min before being returned to their home cages.
A video camera (GoPro Hero3, GoPro, San Mateo, CA) was suspended above the tank and recorded all trials. We analyzed swimming performance in these videos using the Noldus EthoVision XT 14 software package (Noldus, Leesburg, VA). We divided the apparatus into four quadrants, and we outlined a zone consisting of the entire submerged platform. We then calculated the duration of time spent in each quadrant and on the platform. We interpreted a reduction in latency to find the platform across Trials 1-9 as evidence that the subjects learned to locate the platform using spatial cues and that they had formed a memory of the relative location of the platform to draw upon in the spatial memory test. Time spent swimming in the quadrant that formerly contained the platform and time spent in the specific location of the platform were used to assess spatial memory for the platform location.
2.5. Statistical Analysis
All data were analyzed using R (R Core Team, 2020). We determined normality both visually (i.e., histograms and Q-Q plots) and with the Shapiro-Wilk test. We determined outliers statistically via Grubbs’s tests (Lukasz, 2011), and found no outliers in our data. Significance threshold was set at P ≤ 0.05 for all statistical tests. Multiple helper packages were used to import and visualize the data (Wickham, 2009; Fox & Weisberg, 2011; R Core Team, 2015; Auguie, 2019). R-code for our analyses is provided in the supplementary material.
We first confirmed that subjects successfully learned the location of the platform by comparing the latency to reach it across Trials 1-9 using repeated-measures two-way ANOVAs. Each ANOVA contained treatment (saline, AVP, or OT) as a between-subjects factor and trial number (1-9) as a within-subjects factor, with animal ID as the repeated measure. These repeated-measures two-way ANOVAs were analyzed with the rstatix package (Alboukadel, 2021). We concluded that learning occurred if we found a main effect of trial.
We next evaluated memory performance (Trial 10 data) by examining the amount of time animals spent in the quadrant previously containing the platform. When data were normally distributed, we analyzed memory trial data with a one-way ANOVA using treatment as the independent variable. The Kruskal-Wallis test was used for non-normal data, followed by the Wilcoxon test for pairwise differences.
3. RESULTS
3.1. Acute intranasal administration
Subjects successfully learned the location of the hidden platform over Trials 1-9 before intranasal delivery of saline, AVP, or OT (main effect of trial: F(8,216) = 11.69, P < 0.001; Figure 2a). We found no significant effect of treatment (main effect of treatment: F(2,27) = 1.24, P = 0.31) and no interaction (F(16,216) = 0.45, P = 0.97).
Figure 2.
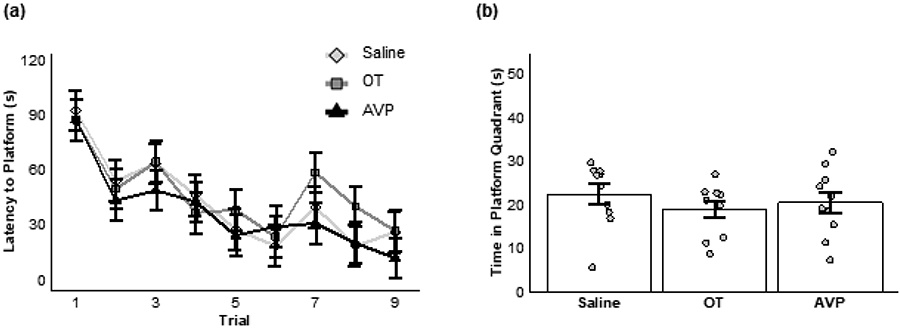
Acute nonapeptide administration. (a) Mean (±SE) latency (in seconds, s) to reach the platform across nine learning trials for animals receiving saline (diamonds), OT (squares), or AVP (triangles). (b) Mean (±SE) duration of time (in seconds) spent during the memory trial in the quadrant where a platform was previously located for animals given saline, OT, or AVP 30 minutes before the memory trial.
Spatial memory was not significantly impacted following acute intranasal pharmacological manipulation of AVP, OT, or saline. Specifically, subjects did not differ in their ability to locate the former location of the platform immediately after receiving either intranasal AVP, OT, or saline (one-way ANOVA: F(2,27) = 0.64, P = 0.53; Figure 2b).
3.3. Chronic intranasal administration
All subjects demonstrated significant improvement in locating the platform across trials as adults, after receiving chronic AVP, OT, or saline during post-wean development (main effect of trial: F(8,192) = 7.95, P < 0.001; Figure 3a). However, neither chronic AVP administration nor chronic OT administration during post-wean development impacted overall spatial learning in adulthood (main effect of treatment: F(2,24) = 1.95, P = 0.16), and no interaction effect was found (F(16,192) = 1.39, P = 0.15).
Figure 3.
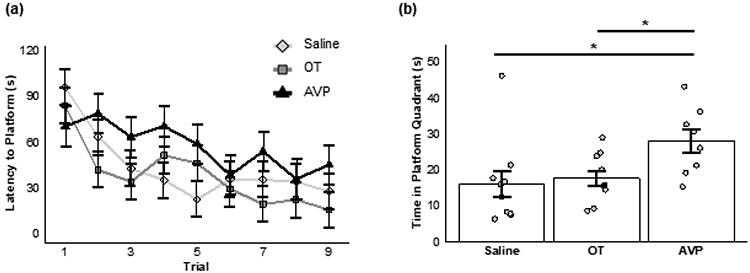
Chronic nonapeptide administration. (a) Mean (±SE) latency (in seconds, s) to reach the platform across nine learning trials for animals receiving saline (diamonds), OT (squares), or AVP (triangles). (b) Mean (±SE) duration of time (in seconds) spent during the memory trial in the quadrant where a platform was previously located for animals chronically administered saline, OT, or AVP during adolescence. Bars show significant post hoc differences; * = p ≤ 0.05.
In contrast and unlike learning, we found a significant effect of treatment on the amount of time subjects spent in the quadrant previously containing the platform during the memory trial (Kruskal-Wallis: X2 = 7.27, P = 0.03; Figure 3b). More specifically, animals that received chronic AVP administration demonstrated better spatial memory as measured by significantly greater time spent in the quadrant that previously contained the hidden platform compared to animals that received OT (P = 0.04) or saline (P = 0.04). Subjects that received chronic administration of OT did not significantly differ in the time spent in the quadrant that previously contained the hidden platform compared to saline animals (P = 0.35).
4. DISCUSSION
We examined how intranasal administration of AVP or OT given either acutely (30 min before testing) or chronically (daily during adolescence) impacted spatial memory in adult male prairie voles. Acute doses of AVP and OT had no significant effect on spatial memory. In contrast, chronic intranasal AVP, but not OT, during post-weaning development improved spatial memory performance in the Morris water maze. More specifically, animals that received chronic intranasal AVP spent more time in the quadrant that previously contained the platform than animals that received chronic intranasal OT or saline. There was also no significant difference in memory performance between animals that received chronic intranasal OT and chronic intranasal saline. Spatial learning was not significantly impacted by chronic intranasal AVP or chronic intranasal OT. These results suggest that chronic AVP during post-wean development plays an organizational role in spatial memory ability, whereas neither OT nor AVP have an activational role for this same behavior.
4.1. Chronic administration of AVP
We found that chronic administration of AVP during postweaning adolescence improved adult spatial memory in the MWM. This result corresponds with prior work showing that AVP facilitates performance in other memory paradigms (McEwen, 2004). Moreover, the fact that chronic administration of AVP during adolescence resulted in improved spatial memory suggests that AVP asserts important organizational effects on spatial cognition. Indeed, previous studies in both rats and humans have demonstrated that chronic administration of AVP during adolescence, but not acute administration, improves memory (Hamburger-Bar et al., 1985; Hamburger-Bar et al., 1987). It is unclear what the mechanism by which early-life exposure to AVP impacts spatial cognition. We suspect that chronic administration of AVP during adolescence likely increased the density of V1aR present in areas of the brain associated with memory, thereby increasing the sensitivity to endogenous AVP in adulthood (Csaba, 1986, see below). Indeed, past work indicates that exposure to nonapeptides during development increases adult sensitivity to nonapeptides in adulthood through hormonal imprinting, or amplifications of nonapeptide receptor density and sensitivity (Csaba et al., 1980; Csaba, 1986).
Our choice to use intranasal administration in this experiment may have also increased the impact of chronic AVP during post-weaning development on the brain. Intranasal administration is inherently non-specific; the delivered substance can be carried throughout the brain once it reaches the extracellular fluid (reviewed in Mittal et al., 2013). Indeed, non-localized increases in brain AVP delivered intraperitoneally 1 hour before MWM learning trials improved spatial memory in adult rats by improving synaptic plasticity in the hippocampus (Yang et al., 2017), indicating that non-specific manipulations can have far-reaching impacts on spatial memory. Our manipulation was also likely able to reach multiple regions of the brain, including many regions that are critical for spatial memory and MWM performance (e.g., the hippocampus, amygdala, prefrontal cortex, anterior thalamus, and retrosplenial cortex; Ophir, 2017). Thus, the non-specific nature of the intranasal delivery method raises the possibility that increasing AVP over post-wean development impacted many aspects of brain function that ultimately impacted one or more neural circuits associated with adult spatial memory performance.
Our study found chronic intranasal AVP did not affect spatial learning. This, together with its impact on spatial memory ability, suggests AVP likely only influences memory retrieval and not the initial formation or consolidation of memories (i.e., learning). Indeed, one study found AVP had the greatest impact on learning and memory when it was administered in the later stages of memory consolidation (Alescio-Lautier et al., 2000). Furthermore, this lack of influence over spatial learning suggests chronic AVP does not change the density of V1aR in areas of the brain that contribute to spatial learning, or if it does, it is not in a way that is behaviorally evident. Indeed, if chronic intranasal AVP during adolescence had made brain regions important for spatial learning more sensitive to AVP, we would expect animals treated with chronic AVP to learn the location of the platform faster.
The effects of chronic AVP exposure during development on spatial cognition speak to a larger question about how social behavior, cognition, and neocortical development are sensitive to environmental perturbations. It is established that neural variation in AVP-containing cells and V1aR density have the potential to directly impact behavior. For instance, V1aR knockout mice have greater numbers of parvalbumin immunoreactive cells than wild-type mice, a difference that may impact information processing (Hammock & Levitt, 2012). Moreover, V1aR variability among brain areas associated with spatial cognition is a strong predictor of reproductive success within male prairie vole mating tactics (Ophir et al. 2008 PNAS). Notably, low levels of neonatal AVP in cerebrospinal fluid accurately predicts later diagnoses of autism (Oztan et al., 2020), suggesting that developmental exposure to AVP might go beyond spatial cognition and could impact many other forms of cognition, carrying significant consequences with it.
Circulating levels of AVP during development may also be influenced by environmental experiences. For instance, smoking and exposure to second-hand smoke increase plasma AVP levels in humans (Fuxe et al., 1989). Furthermore, direct manipulation of AVP in humans can occur in clinical settings, and such treatment has the potential to shape behavior. For example, treating 6- to 12-year old autistic children for just four weeks with daily intranasal AVP improves social deficits (Parker et al., 2019). Moreover, variability in rearing conditions can impact the sensitivity to AVP; paternal deprivation during postnatal development increases prosocial behavior and V1aR mRNA in the lateral septum of male prairie voles (Kelly et al., 2020). These studies indicate that natural or experimentally/clinically induced variation in AVP has lasting impacts on cognition and behavior.
4.2. Chronic intranasal administration of OT
We found that chronic intranasal administration of OT during post-weaning developmental had no statistically significant impact on spatial memory in adulthood. Although OT is typically considered to be an amnesic, prior work has shown that the relationship between OT and memory is unclear. Indeed, OT may impair, improve, or have no effect on memory, depending on the circumstance (McEwen, 2004; Ophir, 2017). In particular, spatial memory may be less affected by perturbations in OT and OTR than other types of memory, like social cognition and social memory (Ferguson et al., 2000; Takayanagi et al., 2005). OT knockout mice, for example, show no spatial memory deficits but they do demonstrate pervasive social deficits (Takayanagi et al., 2005). Thus, despite its important role in memory in general, one reason we did not find a significant effect of chronic OT on adult spatial memory could be because OT is less involved in the neurobiological organization of this specific form of memory.
Another possible explanation for our results relates to the size of dose we used in our chronic OT treatment. The relationship between OT and memory is murky in part because OT has dose-dependent impacts on memory (Popik et al., 1992; Benelli et al., 1995). For example, different doses of chronic intranasal OT differentially impact prairie vole pair bond formation (Bales et al., 2013). We specifically chose our OT concentration (8 x 10−4 IU/ml) to match the dose Bales et al. (2013) used to influence social behavior, predicting that this concentration would also alter other behaviors, including spatial memory. Interestingly, Bales et al. (2013) found that this dose did not influence autogrooming, alloparental care, or performance in an elevated plus-maze. At least two interpretations for the impact of intranasal OT on general behavior can be inferred from these results. One possibility is that OT has a limited scope of efficacy and only alters pair bonding, but not other social and non-social behaviors. A second possibility is that non-pair bonding behaviors might be affected by exogenous exposure to OT during development, but only at other (larger) doses. We believe that the latter interpretation is unlikely for two reasons. First, Bales et al. (2013) used three different doses of OT in their experiment and found that none of them impacted behaviors other than pair bond formation. Second, as mentioned above, OT might have little influence on spatial behavior despite its effects on other forms of learning and memory (Ferguson et al., 2000; Takayanagi et al., 2005). Taken together, we believe that if developmental OT does have the potential to organize adult spatial memory, we would have seen it at the dose we used in our chronic treatment. The lack of significant effect strengthens the argument that spatial memory is not organized during development by OT, although more research is needed to fully support this conclusion.
4.3. Possible impacts of chronic administration on nonapeptide receptors
Chronic administration of AVP and OT during post-wean development may have changed the densities of OTR and V1aR present in the brain. This organizational effect would presumably then lead to changes in adult spatial memory behavior. To our knowledge, no studies have examined AVP receptor densities or other structural changes after chronic AVP administration during a developmental time period. However, chronic intranasal OT administration during development has been shown to impact nonapeptide receptor densities, with the specific effect depending on brain region. For example, chronic developmental OT increases OTR density in the nucleus accumbens of female prairie voles, and reduces V1aR and OTR densities in the forebrain of male rats (Guoynes et al., 2018; Freeman et al., 2018). The structural similarities between OT and AVP, and the fact that they can interact with each other’s receptors (Manning et al., 2008), implies that chronic AVP administration could have also shaped V1aR (or OTR) densities, and thus increased overall potential for binding.
More work has been done on the organizational effect of OT on receptor densities in the brain, and this work has shown that chronic OT can have impacts outside of just the OT system. For example, OT can bind to and interact with V1aR (de Wied, 1991; Manning et al., 2008; Song et al., 2014), and chronic intranasal OT administration leads to a decrease in AVP immunoreactive neurons (Guoynes et al., 2018). Moreover, chronic intranasal OT administration reduced V1aR density in rats (Freeman et al., 2018), indicating a reduction of AVP sensitivity. However, if chronic administration of OT had decreased the density and binding capabilities of V1aR in our adult subjects, we would expect them to demonstrate impaired spatial memory compared to control animals. Given the lack of effect post-weaning (adolescent) administration of chronic OT had on adult spatial memory in our experiment, the impact of chronic OT on V1aR in the brain is likely to be small and potentially have few, if any, behavioral consequences.
4.4. Acute intranasal administration
Our subjects demonstrated neither significant impairments nor improvements in spatial memory 30 minutes after intranasal administration of either nonapeptide when compared to saline-treated animals. This is unlikely to be the result of nonapeptides not reaching memory structures in the brain, because these relatively high doses increase nonapeptide concentration in the extracellular fluid surrounding the hippocampus, amygdala, and cerebrospinal fluid 30 minutes after intranasal administration (Neumann et al., 2013). Furthermore, multiple studies in titi monkeys (Callicebus cupreus), mice, and prairie voles have demonstrated changes in social behavior within just 5 minutes of an acute intranasal administration of either AVP or OT (Jarcho et al., 2011; Bales et al., 2013; Huang et al., 2014), suggesting that nonapeptides can quickly reach receptors and impact social behavior. Another possibility is that our acute doses were not high enough to have an effect. We believe this is an unlikely explanation for our results because the doses we used in the acute manipulations were extraordinarily high. Indeed, our acute doses were approximately 1,000,000 times more concentrated than our chronic doses and the doses used in previous intranasal studies with voles (see Bales et al., 2013; Huang et al., 2014; Simmons et al., 2017; Prounis & Ophir, 2019). We, therefore, conclude that the acute administration of AVP or OT simply did not have an activational influence on spatial memory.
As discussed above, AVP and OT affect many forms of memory and learning. It is possible that the potential for AVP and/or OT to modify adult spatial memory exists but their effects were undetected in our experiment because of the timing of the acute doses during the Morris water maze procedure. Notably, information must first be consolidated from temporary memory into a stable form of memory for long-term retrieval to occur (Squire et al., 2015). Studies have shown that the same manipulation can have different effects on memory depending on when it occurs in the memory consolidation and retrieval process (Roozendaal, 2002; Alvares et al., 2008). For example, AVP appears to be most effective at modulating later stages of memory consolidation (Alescio-Lautier et al., 2000), and OT has timepoint-dependent effects on fear extinction (Toth et al., 2012). Our acute administration effectively targeted memory retrieval because it occurred after the learning trials and presumably after memory consolidation. Thus, our data indicate that acute administration of AVP and OT does not affect the retrieval of previously consolidated spatial memory. Whether either AVP or OT has the potential to impact acquisition or other non-recall aspects of spatial cognition remains to be tested.
4.5. Organizational and activational roles of nonapeptides in spatial cognition
Past work on nonapeptides suggests that they can play both an organizational and activational role in social behavior (Jarcho et al., 2011; Bales et al., 2013; Huang et al., 2014; Simmons et al., 2017; Prounis & Ophir 2019; Pagani et al., 2020). Our results expand upon this research by adding spatial memory as another domain that they can influence. More specifically, exposure to AVP during post-wean development has an organizational role on spatial memory ability, likely through brain-wide changes to receptor densities and connectivity. Although both AVP and OT are known to impact several forms of memory (e.g., navigation and hippocampal-dependent cognition [Engelmann et al., 1992]; passive or active avoidance learning [de Wied, 1991]; and social recognition and social memory [Albers, 2012]), our results indicate that they do not have an activational role in spatial memory retrieval. Yet, unlike their impacts on social behavior, our work indicates that AVP, but not OT, organizes adult spatial memory in male prairie voles. Given the potential role that spatial memory might have in prairie vole social monogamy (Phelps & Ophir, 2009; Rice et al., 2019; Ophir 2017), this work suggests that exposure to relatively high levels of AVP during male prairie vole adolescence could alter adult male reproductive decision-making due to the improvement in spatial memory it produces.
5. CONCLUSIONS
We examined the impacts of chronic and acute intranasal administration of AVP and OT on spatial memory performance in the adult male prairie vole. Chronic exposure to AVP during post-weaning adolescent development improved adult spatial memory, whereas chronic exposure to OT had no effect. These results suggest that AVP, but not OT, has a specific role in organizing adult spatial cognition. Acute doses of both AVP and OT given after spatial learning consolidation but before recall did not alter spatial memory performance, suggesting that intranasal nonapeptides do not activate memory retrieval. Unlike social behavior, where nonapeptides can have both activational and organizational effects, AVP and OT do not appear to activationally impact spatial memory retrieval, and only AVP delivered during development organized adult spatial cognition.
Supplementary Material
Highlights.
Chronic AVP during post-wean development improves adult spatial memory
Chronic OT during post-wean development had no effect on adult spatial memory
AVP, but not OT, organizes adult spatial memory
Administration of AVP or OT before a memory task did not impact spatial memory
AVP and OT do not affect the retrieval of previously consolidated spatial memory
6. ACKNOWLEDGMENTS
We would like to thank George Prounis for assistance with intranasal administration and Angela Freeman for comments on an early version of this manuscript.
Funding:
This work was supported by the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development HD079573 to AGO) and National Science Foundation (1354760 to AGO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLATRATIONS OF INTEREST:
We attest that we have no competing or conflicts of interest.
REFRENCES
- 1.Albers HE (2012). The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Hormones and Behavior 61, 283–292. 10.1016/j.yhbeh.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 2.Alboukadel Kassambara (2021). rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.0. https://CRAN.R-project.org/package=rstatix [Google Scholar]
- 3.Alescio-Lautier B, Paban V, & Soumireu-Mourat B (2000). Neuromodulation of memory in the hippocampus by vasopressin. European Journal of Pharmacology 405, 63 – 72. 10.1016/S0014-2999(00)00542-2 [DOI] [PubMed] [Google Scholar]
- 4.Alvares LDO, Genro BP, Diehl F, & Quillfeldt JA (2008). Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiology of Learning and Memory 90, 1 – 9. 10.1016/j.nlm.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 5.Auguie B (2019). egg: Extensions for ‘ggplot2’: Custom geom, custom themes, plot alignment, labelled panels, symmetric scales, and fixed panel size. R package version 0.4.5. https://CRAN.R-project.org/package=egg [Google Scholar]
- 6.Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, et al. (2013). Chronic intranasal oxytocin causes long-term impacts in partner preference formation in male prairie voles. Biological Psychiatry 74, 180 – 188. doi: 10.1016/j.biopsych.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, & Arletti R (1995). Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides 28, 251–255. 10.1016/0143-4179(95)90029-2 [DOI] [PubMed] [Google Scholar]
- 8.Carter CS (2003). Developmental consequences of oxytocin. Physiology & Behavior 79, 383 – 397. 10.1016/S0031-9384(03)00151-3 [DOI] [PubMed] [Google Scholar]
- 9.Csaba G, Rónai A, LZszló V, Darvas Z, & Berzétei I (1980). Amplification of hormones receptors by neonatal oxytocin and vasopressin treatment. Hormone and Metabolic Research 12, 28–31. DOI: 10.1055/s-2007-996189 [DOI] [PubMed] [Google Scholar]
- 10.Csaba G (1986). Receptor ontogeny and hormonal imprinting. Experientia 42, 750 – 750. 10.1007/BF01941521 [DOI] [PubMed] [Google Scholar]
- 11.Dantzer R, Bluthe R-M, Koob GF, & Le Moal M (1987). Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology 91, 363–368. 10.1007/BF00518192 [DOI] [PubMed] [Google Scholar]
- 12.de Wied D (1991). The effects of neurohypophyseal hormones and related peptides on learning and memory processes. In Frederickson RCA, McGaugh JL, & Felten DL (Eds.), Peripheral signaling of the brain: Role in neural-immune interactions and learning and memory (pp. 335–350). Hogrefe & Huber Publishers. [Google Scholar]
- 13.Egashira N, Tanoue A, Higashihara F, Mishima K, Fukue Y, Takano Y, Tsujimoto G, Iwasaki K, & Fuijwara M (2004). V1a receptor knockout mice exhibit impairment of spatial memory in an eight-arm radial maze. Neuroscience Letters 356, 195 – 198. 10.1016/j.neulet.2003.11.050 [DOI] [PubMed] [Google Scholar]
- 14.Engelmann M, Bureš J, & Landgraf R (1992). Vasopressin administration via microdialysis into the septum interferes with the acquisition of spatial memory in rats. Neuroscience Letters 142, 69–72. 10.1016/0304-3940(92)90622-E [DOI] [PubMed] [Google Scholar]
- 15.Ferguson JN, Young LJ, Hearn EG, Matzuk MM, Insel TR, & Winslow JT (2000). Social amnesia in mice lacking the oxytocin gene. Nature Genetics 25, 284 – 288. 10.1038/77040 [DOI] [PubMed] [Google Scholar]
- 16.Fox J, & Weisberg S (2011). An {R} companion to applied regression. URL., second edition. Sage, Thousand Oaks CA. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- 17.Freeman SM, Ngo J, Singh B, Masnaghetti M, Bales KL, & Blevins JE (2018). Effects of chronic oxytocin administration and diet composition on oxytocin and vasopressin receptor binding in the rat brain. Neuroscience 392, 241 – 251. 10.1016/j.neuroscience.2018.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuxe K, Andersson K, Eneroth P, Härfstrand A, & Agnati L (1989). Neuroendocrine actions of nicotine and of exposure to cigarette smoke: Medical implications. Psychoneuroendocrinology 14, 19 – 41. 10.1016/0306-4530(89)90054-1 [DOI] [PubMed] [Google Scholar]
- 19.Grace SA, Rossell SL, Heinrichs M, Kordsachia C, & Labuschagne I (2018). Oxytocin and brain activity in humans: A systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology 96, 6 – 24. 10.1016/j.psyneuen.2018.05.031 [DOI] [PubMed] [Google Scholar]
- 20.Grinevich V, & Ludwig M (2021). The multiple faces of the oxytocin and vasopressin systems in the brain. Journal of Neuroendocrinology, e13004. 10.1111/jne.13004 [DOI] [PubMed] [Google Scholar]
- 21.Guoynes CD, Simmons TC, Downing GM, Jacob S, Solomon M, & Bales KL (2018). Chronic intranasal oxytocin has dose-dependent effects on central oxytocin and vasopressin systems in prairie voles (Microtus ochrogaster). Neuroscience 369, 292 – 302. 10.1016/j.neuroscience.2017.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamburger-Bar R, Klein A, & Belmaker RH (1985). The effect of chronic vs. acute injection of vasopressin on animal learning and memory. Peptides 6, 23 – 25. 10.1016/0196-9781(85)90070-1 [DOI] [PubMed] [Google Scholar]
- 23.Hamburger-Bar R, Eisenberg J, & Belmaker RH (1987). Animal and clinical studies of vasopressin effects on learning and memory. Israel Journal of Medical Sciences 23, 12 – 18. PMID: 2952619 [PubMed] [Google Scholar]
- 24.Hammock EAD, & Levitt P (2012). Modulation of parvalbumin interneuron number by developmentally transient neocortical vasopressin receptor 1a (V1aR). Neuroscience 222, 20 – 28. 10.1016/j.neuroscience.2012.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni BC, & Papaleo F (2014). Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 39, 1102 – 1114. 10.1038/npp.2013.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarcho MR, Mendoza SP, Mason WA, Yang X, & Bales KL (2011). Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes, Brain, and Behavior 10, 375 – 383. 10.1111/j.1601-183X.2010.00677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly AM, Ong JY, Witmer RA, & Ophir AG (2020). Paternal deprivation impairs social behavior putatively via epigenetic modification to lateral septum vasopressin receptor. Science Advances 6, eabb9116. DOI: 10.1126/sciadv.abb9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukasz K (2011). outliers: Test for outliers. R package version 0.14. https://CRAN.R-project.org/package=outliers [Google Scholar]
- 29.Manning M, Stoev S, Chini B, Durroux T, Mouillac B, & Guiillon G (2008). Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2, and OT receptors: research tools and potential therapeutic agents. Progress in Brain Research 170, 473–512. 10.1016/S0079-6123(08)00437-8 [DOI] [PubMed] [Google Scholar]
- 30.McEwen B (2004). The Roles of Vasopressin and Oxytocin in Memory Processing. San Diego, CA: Elsevier Academic Press. [DOI] [PubMed] [Google Scholar]
- 31.Mittal D, Ali A, Md. S, Baboota S, Sahni JK, & Ali J (2013). Insights into direct nose to brain delivery: current status and future perspective. Drug Delivery 21, 75–86. 10.3109/10717544.2013.838713 [DOI] [PubMed] [Google Scholar]
- 32.Morris R (1984). Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods 11, 47 – 60. doi: 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 33.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, & Landgraf R (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38, 1985 – 1993. 10.1016/j.psyneuen.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 34.Ophir AG (2017). Navigating monogamy: Nonapeptide sensitivity in a memory neural circuit may shape social behavior and mating decisions. Frontiers in Neuroscience. 10.3389/fnins.2017.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oztan O, Garner JP, Constantino JN, & Parker KJ (2020). Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proceedings of the National Academy of Sciences 117, 10609 – 10613. 10.1073/pnas.1919050117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagani M, De Felice A, Montani C, Galbusera A, Papaleo F, & Gozzi A (2020). Acute and repeated intranasal oxytocin differentially modulate brain-wide functional connectivity. Neuroscience 445, 83 – 94. 10.1016/j.neuroscience.2019.12.036 [DOI] [PubMed] [Google Scholar]
- 37.Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, … & Hardan AY (2019). A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Science translational medicine 11. DOI: 10.1126/scitranslmed.aau7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelps SM, & Ophir AG (2009). 9. Monogamous Brains and Alternative Tactics: Neuronal ViaR, Space Use, and Sexual Infidelity among Male Prairie Voles. In Dukas R & Ratcliffe J (Ed.), Cognitive Ecology II (pp. 156–176). Chicago: University of Chicago Press. 10.7208/9780226169378-010 [DOI] [Google Scholar]
- 39.Popik P, & Vetulani J (1991). Opposite action of oxytocin and its peptide antagonists on social memory in rats. Neuropeptides 18, 23–27. 10.1016/0143-4179(91)90159-G [DOI] [PubMed] [Google Scholar]
- 40.Popik P, Vetulani J, & van Ree JM (1992). Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology 106, 71–74. 10.1007/BF02253591 [DOI] [PubMed] [Google Scholar]
- 41.Prounis GS, Foley L, Rehman A, & Ophir AG (2015). Perinatal and juvenile social environments interact to shape cognitive behaviour and neural phenotype in prairie voles. Proceedings of the Royal Society B: Biological Sciences 282, 20152236. 10.1098/rspb.2015.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prounis GS, & Ophir AG (2019). The impact of early postnatal and juvenile social environments on the effects of chronic intranasal oxytocin in the prairie vole. Frontiers in Behavioral Neuroscience 13, 206. 10.3389/fnbeh.2019.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team (2015). Foreign: Read data stored by Minitab, S, SAS, SPSS, Stata, Systat, Weka, dBase, … R package version 0.8-66. https://CRAN.R-project.org/package=foreign. [Google Scholar]
- 44.R Core Team (2020). R: A language and environment for statistical computing. URL. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- 45.Rice MA, Hobbs LE, Wallace KJ, & Ophir AG (2017). Cryptic sexual dimorphism in spatial memory and hippocampal oxytocin receptors in prairie voles (Microtus ochrogaster). Hormones and Behavior 95, 94 – 102. 10.1016/j.yhbeh.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice MA, Sanín G, & Ophir AG (2019). Social context alters spatial memory performance in free-living male prairie voles. Royal Society Open Science 6, 190743. 10.1098/rsos.190743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roozendaal B (2002). Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory 78, 578 – 595. doi: 10.1006/nlme.2002.4080 [DOI] [PubMed] [Google Scholar]
- 48.Schwagmeyer PL (1994). Competitive mate searching in thirteen-lined ground squirrels (Mammalia, Sciuridae): potential roles of spatial memory. Ethology 98, 265 – 276. doi: 10.1111/j.1439-0310.1994.tb01075.x [DOI] [Google Scholar]
- 49.Simmons TC, Balland JF, Dhauna J, Yang SY, Traina JL, Vazquez J, & Bales KL (2017). Early intranasal vasopressin administration impairs partner preference in adult male prairie voles (Microtus ochrogaster). Frontiers in Endocrinology 8, 145. 10.3389/fendo.2017.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smulders TV, Sasson AD, & DeVoogd TJ (1995). Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee. Journal of Neurobiology 27, 15 – 25. doi: 10.1002/neu.480270103 [DOI] [PubMed] [Google Scholar]
- 51.Song Z, McCann KE, McNeill IV JK, Larkin II TE, Huhman KL, & Albers HE (2014). Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 50, 14–19. 10.1016/j.psyneuen.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Z, Larkin TE, O’Malley M, & Albers HE (2016). Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP 1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus). Hormones and Behavior 81, 20–27. 10.1016/j.yhbeh.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 53.Squire LR, Genzel L, Wixted JT, & Morris RG (2015). Memory consolidation. Cold Spring Harbor Perspectives in Biololgy 7, a021766. 10.1101/cshperspect.a021766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stribley JM, & Carter CS (1999). Developmental exposure to vasopressin increases aggression in adult prairie voles. Proceedings of the National Academy of Sciences of the United States of America 96, 12601 – 12604. 10.1073/pnas.96.22.12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, & Nishimori K (2005). Pervasive social deficits, but normal parturition, in oxytocin-receptor deficient mice. Proceedings of the National Academy of Sciences 102, 16096 – 16101. 10.1073/pnas.0505312102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toth I, Neumann ID, & Slattery DA (2012). Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharamachology 223, 149 – 158. 10.1007/s00213-012-2702-4 [DOI] [PubMed] [Google Scholar]
- 57.Vorhees CV, & Williams MT (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols 1, 848 – 858. 10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walum H, & Young LJ (2018). The neural mechanisms and circuitry of the pair bond. Nature Reviews Neuroscience 19, 643–654. 10.1038/s41583-018-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickham H (2009). ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York. [Google Scholar]
- 60.Yang C, Zhang X, Gao J, Wang M, & Yang Z (2017). Arginine vasopressin ameliorates spatial learning impairments in chronic cerebral hypoperfusion via V1a receptor and autophagy signaling partially. Translational Psychiatry 7, e1174. 10.1038/tp.2017.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


