Abstract
Objective:
How does diet quality (DQ) moderate associations between serious childhood stress exposures and adult depression?
Methods:
We analyzed a cohort of Californian women at midlife (N=382; age 36-42). Serious childhood stress was defined as high perceived stress during childhood or adverse childhood experiences (ACEs) of physical abuse, sexual abuse, and/or household substance abuse. Women were dichotomized as having high or low current depression risk. The Healthy Eating Index (HEI)-2015 and Alternate Healthy Eating Index (AHEI)-2010 measured current DQ from 3-day food records. Interactions between childhood stress exposures and DQ indices were tested one-by-one in multivariable Poisson regression models.
Results:
Depression risks associated with endorsing all 3 ACEs differed by HEI and AHEI scores, as did risks associated with endorsing high perceived stress, physical abuse, and sexual abuse by AHEI. Where DQ moderated stress-depression associations, predicted prevalences of high depression risk did not vary with DQ among women endorsing the particular childhood stressors. However, among non-endorsing women, predicted high depression risk prevalences were significantly lower with higher DQ compared to in their stress-exposed counterparts – e.g., at the 90th AHEI percentile, depression prevalences were ~20% among “non-childhood-stressed” women versus 48.8% (high perceived stress, sexual abuse), 52.0% (physical abuse), and 73.0% (3 ACEs) in “childhood-stressed” women.
Conclusions:
Higher current diet quality, particularly as aligned with chronic disease prevention guidelines, predicts lower depression risk in women with low childhood adversity. DQ did not buffer depression risk in women with high childhood stress. Further research is warranted to examine persistent pathways of depression risk and diet’s role within.
Keywords: adverse childhood experiences, diet quality, depression, childhood stress, nutritional psychiatry
INTRODUCTION
Depression is a globally costly and pervasive mental health condition1, affecting between 102 to 20%3 of reproductive-age women in the United States. Prominent risk factors include childhood trauma and chronic or prolonged experiences of stress. In the landmark adverse childhood experiences (ACEs) study, adults endorsing even just one such experience had 50% greater odds of experiencing two or more weeks of depressed mood in the past year4. In another study, higher levels of chronic stress during emerging adulthood were positively linked to elevated depression symptoms in later years5. With 61.6% of U.S. adults endorsing at least one type of ACE exposure (e.g., 17.9% report physical abuse, 11.6% report sexual abuse, and 27.6% report having a household member with substance abuse6) and 27% of American 13-17 year olds reporting feelings of “extreme” stress during the school year7, the potential public mental health risk associated with earlier trauma and stress experiences, particularly from childhood, is considerable. This may be particularly true for women, who not only experience higher levels of ACEs (particularly sexual abuse and household substance abuse)4,6,8 and psychological stress9 than men, but also surpass men in depression rates1.
ACEs often co-occur and their psychosomatic effects on the body can be compounded4 -- both ACEs4 and chronic stress10,11 trigger biological and psychological processes that impact health and well-being. Developmental stress exposures may facilitate the development of later-life depression through various stress response pathways, including: 1) poor coping/health behaviors4 – e.g., unhealthy diets and comfort eating4,10,12,13, as well as 2) biological embedding of chronic or toxic stress (“allostatic load”)13-16 with ensuing neuroimmuno-related changes in inflammation14,15, neural plasticity15,17, and gut microbiota18. Recently, dietary approaches have been identified as possible strategies for the prevention and treatment of depression-related outcomes19-21. As the stress experience may influence eating behaviors10,12 and alter if not diminish nutrient stores in the body22, populations exposed to more serious stress exposures may also see benefits from nutritional interventions. In one study among older adults, the effect of ACEs on depressive symptoms was lower in those with higher flavonoid intakes compared to those with lower intakes23. In another trial among adult survivors of a natural disaster, both B-vitamin and broad-spectrum micronutrient supplementation decreased stress and anxiety symptoms24. Similar results have been reported for other formulations of broad-spectrum micronutrients and nutraceuticals in a related suite of trials25-27.
Considering the enormous complexity of the overall diet, however, and the myriad interactions between nutrients, foods, and other chemicals within, nutrition’s role in depression may not be completely encapsulated when focus is more singularly placed on nutrients alone28,29. Correspondingly, increased attention has been paid to prevailing dietary patterns and assessments of overall diet quality. Bolstering the evidence base for these “bigger picture”-type dietary foci in depression research30 have been systematic reviews and/or meta-analyses of observational studies31-35, as well as interventions or randomized controlled trials (RCTs)19,36. Broadly, healthy dietary patterns, which predominantly consist of whole foods such as fruits, vegetables, whole grains, fish, olive oil, and low-fat dairy with minimal animal sourcing, have been linked to better depressive outcomes30-33. Accordingly, these patterns are nutrient dense and replete with neurobiologically active vitamins, minerals, and long chain polyunsaturated fatty acids (i.e., omega-3’s). High diet quality (DQ) patterns are also aligned with nutritional recommendations, and, consequently, higher scores on dietary indices, such as the Alternate Healthy Eating Index (AHEI), which measures adherence to evidence-based recommendations for reduced chronic disease37, have also been associated with lower depression risk32. In a meta-analysis of cross-sectional and longitudinal studies of Healthy Eating Index (HEI) and AHEI scores with depressive symptoms or clinical depression, a 35% risk reduction for these depression outcomes comparing highest to lowest adherent groups was observed32.
There are notable similarities between the biological mechanisms important in depression’s developmental course post-exposure to early life stressors and those important in how diet influences depression. Components of healthy diets increase neurotrophic activity, promote glucose homeostasis, and are anti-inflammatory, anti-oxidative, and/or neurogenerative. Unhealthy diets are low in said beneficial compounds while simultaneously harboring pro-inflammatory, oxidative, and neurotoxic substances that may also increase insulin resistance. These actions are further augmented by dietary influences on intestinal permeability and/or gut microbiota30,32,38,39. Given 1) the considerable prevalence of depression as well as its risk factors of childhood trauma and chronic stress in the general population and, particularly, women, and 2) considering the demonstrated inverse relations between overall diet quality and depression, there could be great public health potential in the possibility of promoting overall diet quality to reduce public mental health risks related to preceding stress or trauma experiences. Existing work in this area has focused on nutrient or supplement-based approaches23-27 and/or had limited ability to track associations between stress and depression across life stages. Previous studies have also centered on more homogenous populations comprised of older and predominantly White adults23-27. However, recent research findings have highlighted nuances in the associations between stress and depression by demographic attributes, such as gender and race/ethnicity, and their differential contextual (e.g., social, societal, and structural) underpinnings40. Complex disparities in diet quality along similar demographic lines have also been documented41, further promoting the importance of considering diversity in forthcoming work.
This study addresses gaps in the literature by investigating the extent to which diet quality might modify associations between stress and trauma exposures in childhood and depressive outcomes in a cohort of Black and White women at midlife. We hypothesized that more healthful diets would buffer the negative associations between childhood stress exposures and adult depressive outcomes.
METHODS
This study analyzed data from the National Heart, Lung, and Blood Institute (NHLBI) Growth and Health Study (NGHS)42. Women from the original Richmond, CA site (N=883) were recruited for a follow-up study that extended the previous cardiometabolic aims to midlife. Comprehensive anthropometric, health, behavioral, psychosocial, and demographic data, collected annually from the original study period (1987-1997) when the women were age 9-10 to age 19-21, were combined with similar data collected when the women were in midlife (age 36-43). To be eligible for the midlife follow-up, women could not be pregnant, have given birth/miscarried within the last three months, or be incarcerated at recruitment. Upon re-enrollment, women completed questionnaires, including a 3-day food record (N=383). A complete case analysis approach was used and analytical sample sizes for each model were noted. This study was approved by the University of California, Berkeley Institutional Review Board.
Measures
Current Depression
At adult follow-up, women completed the 20-item Center for Epidemiologic Studies Depression (CES-D) scale, which is a tool designed to measure current depressive symptomatology in the general population with high reliability and validity for use in both Black and White women (Cronbach’s alphas were >0.80 in the Black subgroup and general population samples used in CES-D scale creation)43. The CES-D asked participants how often they felt or acted in a certain way (e.g., “I felt like everything I did was an effort.”) in the past week using a 4-point Likert scale. Response values were summed (positive items were reverse coded) and total scores could range between 0 (no disorder) and 60 (great disorder). Scores were analyzed dichotomously by applying the conventional cut-off score of 16, which is a well established point associated with high clinical depression risk commonly used and approved for depression screening43,44. This treatment of depression aimed to maximize the practical significance of our findings and has been frequently done in similar studies32.
Childhood Stress Exposure
Serious childhood stress exposure was assessed in two ways: as average chronic perceived stress, prospectively collected between ages 9/10 and 19/20, and as adverse childhood experiences before age 18, retrospectively assessed at midlife. Average psychological stress during childhood was assessed with Cohen’s Perceived Stress Scale (PSS). The scale was self-administered every other year for a total of five times during the original study. Girls completed a 14-item version of the scale (language modified to teenagers), which gauged how often they had experienced certain feelings or thoughts (e.g., “I felt nervous and like everybody was pushing me.”) in the past month using a 5-point scale corresponding to never through very often. Positive items were reverse coded and response values summed. Scores could range from 0-56 with higher total scores reflecting perceptions of higher chronic psychological stress. To ascertain chronic stress throughout childhood, an average of all available PSS scores for each girl was calculated (75.9% had all 5 scores; 13.9% had 4 of 5). To identify those with “high [perceived] stress”, a dichotomous variable was created by applying a cutoff corresponding to the 80th percentile for the mean PSS scores among all women with at least 1 days’ food record. This level was chosen from other cut-points because it proved to be most significantly and highly correlated with the other ACE experiences (yes/no) assessed, suggesting general equivalency in severity of exposure. Despite the lack of an otherwise established cut-off, our level also corresponds to similar demarcations for high/low stress in other study populations45,46. The PSS is valid and reliable for use in adolescent Black and White girls (Cronbach’s alphas were >0.80 in population samples used in scale creation)47.
At midlife, women reported exposure to the following three severe adverse experiences before age 18: physical abuse (parent/household adult often hit, grab, etc. so as to leave marks or injury), sexual abuse (parent/person 5+ years older sexually touched, forced sex, etc.), and/or household substance abuse (household member was problem drinker, used street drugs, etc.)4. Each ACE item was dichotomized (yes/no). Per prior research supporting analyses of ACEs both individually and cumulatively8,48, ACEs were examined one-by-one in models as well as by a constructed dichotomous (yes/no) variable denoting if women endorsed all three ACEs. While it is possible that retrospective assessment of ACEs may introduce recall bias as well as also potentially result in confounding of ACEs by current depressive symptoms, recent research has found recall of ACEs to be stable over time, even with changes in depression status49.
Overall Diet Quality
At follow-up, participants received comprehensive instruction on how to complete 3-day food records for three non-consecutive days (two weekdays and a weekend day) (i.e., what type of information to provide, the level of detail needed, how to estimate food amounts). Trained study staff entered the data into the University of Minnesota Nutrition Coordinating Center’s (NCC) Nutrition Data System for Research (NDSR) software. Issues identified in data entry were resolved through additional discussion with the participant. Foods not found in the NDSR database were remedied by selection of a close substitute in consultation with registered dietitians. Food record days with biologically implausible values for total kilocalorie intake (<500 kcal [n=13 days’ records] or >5000 kcal [n=21 days]) were considered invalid and excluded, resulting in a final N=382 for our analytical sample of women with at least one day’s valid and complete food record (n=376 women had 2 days and n=348 had 3 days of valid records). Women with at least one day’s valid food record at follow-up did not differ from the original cohort on race, parent education, household income, single or two parent household status, or number of siblings at baseline.
Total daily intakes of nutrients and foods from valid food records were used to calculate Healthy Eating Index (HEI)-2015 and Alternate Healthy Eating Index (AHEI)-2010 scores for overall diet quality (DQ). These indices were chosen because of their 1) clear a priori scoring definitions, 2) established associations with depression in the literature32, 3) differential, non-overlapping components, 4) ability to be universally scored, unspecific to study population, and 5) compatibility with NDSR data. The HEI-2015 measures adherence to the 2015-20 Dietary Guidelines for Americans on a 0 (no adherence) to 100 (perfect adherence) scale and is comprised of 13 adequacy (e.g., total/whole fruits and vegetables, fatty acid ratio) or moderation components (e.g., added sugars, saturated fats)50,51. The AHEI-2010 measures diet quality predictive of major chronic disease on a 0 (no adherence) −110 (perfect adherence) scale and is comprised of 11 adequacy (e.g., total/whole fruits and vegetables, long chain [omega-3] fatty acids) or moderation (e.g., sugar-sweetened beverages, red/processed meats) factors37. Publicly available syntax for the simple HEI scoring algorithm method for multiple days’ records was referenced to generate HEI-2015 component and total scores standardized to 1,000 kcal52-54. Similarly, AHEI-2010 scores were determined by assessing daily component intakes, energy adjusting via the residual approach55, and applying means across food records to calculate component and sum total scores. Scores were analyzed continuously.
Covariates and Confounders
Key variables of childhood stress-adult depression relations were identified as covariates given literature and socio-ecological theory on human development56: race, household income, highest parental education, number of parents (single or double) in household, and number of siblings. All variables were parent-reported at the original study’s baseline. Baseline values were utilized to minimize disruptions to temporality between childhood stress exposures (specifically regarding ACEs, which could have occurred at any point in time before age 18) and midlife depression given the possibility that covariates taken at other points in time between exposure and outcome might, in effect, mediate the associations of interest.
Statistical Methods
Two-sample t-tests for continuous variables and chi-squared tests for categorical variables evaluated potential differences comparing women by high (CES-D score≥16) vs. low depression risk status. Modified Poisson regression with a robust estimator of variance, which has been shown to yield more accurate standard errors for approximations of relative prevalence risk ratios when studying binary data wherein the outcome is not rare (>10%)57,58, was used to model “high depression risk” at midlife, adjusting for all baseline covariates.
For moderation analyses, interaction terms between continuous HEI and AHEI scores and each of the five childhood stress variables were created. Each term was inserted into the adjusted main effects models such that only one interaction was tested in a model at a time. Likelihood ratio testing compared null and extended models for significance of the interaction term to improve model fit to the data. Statistical significance was set to α=0.05 for all analyses except for moderation, which applied an expanded α=0.15 level considered acceptable for exploratory analyses of interaction terms59. If interaction terms (“X*Z1, …n”) were significant, post-estimation calculations (utilizing Stata’s margins command) were made for predicted prevalences of high depression risk conditional on fixed values of the moderator (“Z”) of diet quality and exposure (“X”) of childhood stressor and integrating over the covariates60. Analyses utilized Stata15 SE (College Station, TX).
RESULTS
Descriptive Results
Summary characteristics of the analytical sample are reported in Table 1. The cohort was equally (50.0% and 50.0%) Black and White with a mean age of 39.2 years. Women were well-dispersed across baseline household income and highest parental educational attainment categories. From the adult follow-up assessments, 33.8% of women had high depression risk (CES-D score≥16). The 80th percentile of mean Cohen’s Perceived Stress Scale (PSS) scores throughout ages 9/10 to 19/20 among the cohort was 27.80 (out of 56), which was set as the cut-point for identifying women with high perceived stress during childhood. Approximately 21% of women endorsed childhood physical abuse, 21% endorsed sexual abuse, and 37% endorsed household substance use. About half (49.7%) endorsed none of these ACEs and 6.3% endorsed all three ACEs. For overall diet quality, the mean (SD) HEI-2015 score was 57.0 (13.4) out of 100 and the mean (SD) AHEI-2010 score was 55.5 (14.7) out of 110 (ρ= 0.77, p<0.0001).
Table 1.
Summary Sample Characteristics -- Mean (SD) or Proportion (%)1 -- for the National Heart, Lung, and Blood Institute Growth & Health Study (NGHS) Follow-up Cohort – Overall and by Depression Risk Status
| Depression Risk Status (n=379) | ||||
|---|---|---|---|---|
| Full Analytical Sample (n=382) |
Low Depression Risk (CES-D score<16) (n=251) |
High Depression Risk (CES-D score ≥16) (n=128) |
||
| Characteristic | Mean (SD) or Proportion (%, n) |
Mean (SD) or Proportion (%, n) |
Mean (SD) or Proportion (%, n) |
p-value of difference* |
| Baseline Sociodemographic Characteristics | ||||
| Current Age (y) | 39.2 (1.1) | 39.2 (1.2) | 39.1 (1.0) | 0.48 |
| Race – Black | 50.0% (n=191) | 50.2% (n=126) | 48.4% (n=62) | 0.75 |
| Household (HH) Income | 0.56 | |||
| <$10K | 18.2% (n=66) | 16.3% (n=39) | 21.7% (n=26) | |
| $10K-$19,999 | 17.4% (n=63) | 16.7% (n=40) | 18.3% (n=22) | |
| $20K-$39,999 | 30.6% (n=111) | 31.7% (n=76) | 28.3% (n=34) | |
| $40K+ | 33.9% (n=123) | 35.4% (n=85) | 31.7% (n=38) | |
| Highest Parental Education | 0.40 | |||
| ≤High School | 22.5% (n=86) | 21.1% (n=53) | 25.0% (n=32) | |
| Some College | 46.1% (n=176) | 45.0% (n=113) | 47.7% (n=61) | |
| College Graduate+ | 31.4% (n=120) | 33.9% (n=85) | 27.3% (n=35) | |
| Single Parent Household | 33.3% (n=127) | 30.7% (n=77) | 37.5% (n=48) | 0.18 |
| Number of Siblings | 1.3 (1.1) | 1.3 (1.1) | 1.3 (1.0) | 0.65 |
| High Depression Risk (Center for Epidemiologic Studies-Depression [CES-D] score ≥16) | 33.8% (n=128) | -- | -- | -- |
| Childhood Stress Exposures | ||||
| >80th mean percentile Cohen’s Perceived Stress Scale score (age 9-19/20) | 20.0% (n=76) | 15.6% (n=39) | 28.9% (n=37) | 0.002 |
| Physical Abuse (≤18 years old) | 21.2% (n=79) | 16.3% (n=40) | 30.4% (n=38) | 0.002 |
| Sexual Abuse (≤18 years old) | 20.8% (n=75) | 17.1% (n=41) | 28.8% (n=34) | 0.010 |
| Household Substance Abuse (≤18 years old) | 37.2% (n=138) | 34.2% (n=84) | 42.9% (n=54) | 0.082 |
| Report 3 ACEs (≤18 years old) | 6.3% (n=22) | 4.7% (n=11) | 9.6% (n=11) | 0.077 |
| Diet Quality Indices | ||||
| Healthy Eating Index-2015 score | 57.0 (13.4) | 57.9 (13.3) | 55.4 (13.5) | 0.095 |
| Alternate Healthy Eating Index-2010 score | 55.5 (14.7) | 57.2 (14.4) | 52.5 (14.8) | 0.003 |
Proportions and means are calculated using only the non-missing data.
Statistical significance of difference by depression risk status was tested by two sample t-tests for continuous variables and chi-squared tests for categorical variables.
Compared to women with low depression risk, women with high risk were more likely to have had high perceived stress throughout childhood (28.9% vs. 15.6%, p=0.002) and greater exposure to physical abuse (30.4% vs. 16.3%, p=0.002) and sexual abuse (28.8% vs. 17.1%, p=0.01). Additionally, women with high depression risk had significantly lower AHEI scores (52.5 [SD=14.8] vs. 57.2 [SD=14.4], p=0.003) compared to women with low risk. Women in the analytical sample did not significantly differ on any baseline covariates by depression risk status (p>0.05).
Inferential Results
In adjusted models of estimated prevalence rates for high depression risk, likelihood ratio tests revealed five significant interactions between childhood stress and diet quality. High midlife depression risks associated with endorsing all 3 ACES (or not) varied by HEI-2015 and AHEI-2010 scores (p-interaction=0.12 for HEI, p-interaction=0.08 for AHEI). AHEI scores also moderated high midlife depression risk associated with high perceived psychological stress during childhood (p-interaction=0.14), physical abuse (p-interaction=0.11), and sexual abuse (p-interaction=0.13). No significant interactions were additionally observed by HEI scores (p-interaction=0.33, 0.27, 0.18, and 0.81, for high depression risk associated with high perceived stress, physical abuse, sexual abuse, and household substance abuse, respectively) or by AHEI for high depression risk at midlife associated with substance abuse (p-interaction=0.41)
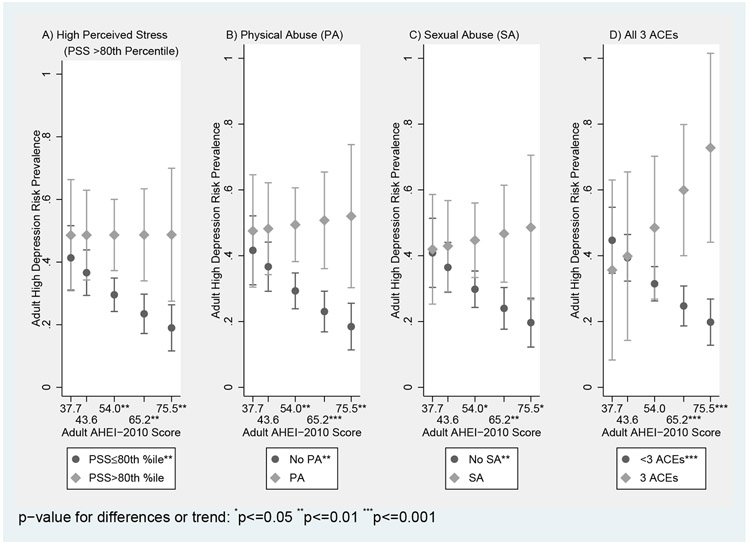
Figure 1 (with Supplemental Table 1) illustrates how predicted prevalence rates of high depression risk at midlife associated with childhood stress exposure(s) differ across the range of AHEI-2010 scores where dietary moderation was present -- specifically at select points of AHEI-2010 scores (i.e., the 10th, 25th, 50th, 75th, and 90th percentiles). Predicted prevalences of high depression risk did not vary with DQ (p-for-trends>0.05) among women exposed to childhood stressors, but did trend significantly downwards among women not exposed to the stressor (p-for-trends<0.05). For example, for women endorsing childhood physical abuse, predicted depression rates ranged between 47.5% at the 10th percentile of AHEI-2010 scores to 52.0% at the 90th percentile, but for non-endorsing women, the same rates trended downwards from 41.6% to 18.5% between the same percentiles. Moreover, significant differences were observed between predicted prevalence rates for high depression risk at the 50th, 75th, and 90th AHEI percentiles for: (A) women with high perceived stress in childhood (48.6%, 48.7%, and 48.7%, respectively) and women with lower stress (29.6%, 23.5%, and 19.0%, respectively), (B) women endorsing physical abuse (49.4%, 50.8%, and 52.0%, respectively) and those not (29.3%, 23.0%, and 18.5%, respectively), and (C) women endorsing sexual abuse (44.7%, 46.7%, and 48.6%, respectively) and those not (29.8%, 24.0%, and 19.7%, respectively). Significant differences were also observed at the 75th and 90th percentiles between (D) women endorsing all 3 ACEs (59.9% and 72.8%, respectively) and women endorsing less than 3 (24.7% and 19.8%, respectively); a similar pattern was observed with HEI comparing women endorsing all 3 ACEs to those not.
Figure 1:
Moderation of Adjusted Predicted Prevalences of High Midlife Depression Risk Associated with Specific Childhood Stress Exposures by Adult Diet Quality at Select AHEI-2010 Score Percentiles (10th, 25th, 50th, 75th, and 90th) Where Diet-Stress Interaction Was Observed
On the whole, eating a healthy diet did not change depression prevalences amongst stress exposed women, but did provide protection for women not exposed to the stressor in predicting lower depression risk. It was also notable that predicted depression prevalences at poor diet quality levels did not significantly differ between stress exposed and non-stress exposed women; at low DQ levels, depression risks in “non-exposed” women were similarly elevated as in “stress-exposed” women. For example, at the 10th percentile of AHEI scores, there was not a significant difference (p=0.56) between the predicted prevalence among women exposed to childhood physical abuse (47.5%) and that among women not-exposed (41.6%). Supplemental Table 1 provides specific p-values for differences between stress exposed and non-stress exposed women, as well as the corresponding predicted prevalences and tests for trend results graphed in Figure 1.
DISCUSSION
It is an important and novel question to understand how dietary quality might lead to protection against or vulnerability to depression in the context of high childhood stress. To our knowledge, this study is the first to explore the extent to which current overall diet quality, here assessed two ways, modifies relations between serious childhood stress exposures and adult depressive outcomes, specifically high depression risk. In our cohort of Black and White women, now at midlife, we partially confirmed our hypothesis – the association between childhood stressors and midlife depression outcomes appear to differ by adult diet quality in this study, though in an unexpected manner. Alternate Healthy Eating Index (AHEI)-2010 scores, one measure of diet quality, were consistently found to moderate associations between childhood stress exposure and high depression risk at midlife among our cohort. Adjusted depression risk related to most of the childhood stress exposures did not vary by Healthy Eating Index (HEI)-2015 scores, the other diet quality measure, and neither did risks associated with having a household member with substance abuse by AHEI. At higher DQ levels, we had expected to find associations between childhood stress and midlife depression to be dampened. However, we found this to be true only among those women not endorsing exposure to childhood stressors -- in these women without severe childhood stress, diet quality was associated with lower predicted depression risk. Moreover, at poor diet quality levels, predicted depression risks were similar between women with and without severe childhood stress.
The unexpected manner in which diet quality moderated childhood stress-midlife depression risk relations, as well as the surprising divergence in predicted prevalences of high depression risk between those exposed and unexposed to severe childhood stress at higher levels of diet quality, may underscore aspects of stress, diet, and their interaction important for appreciating their roles in the psychosomatic etiology of depression. Indeed, that women unexposed to stressors had lower depression risk at higher DQ levels, and that women regardless of stress exposure had similarly high predicted prevalences of high depression risk at low DQ levels appear testament to the strong inverse relation between diet quality and depression observed in other nutritional psychiatry studies30-33 Still there are a number of possible explanations for the nuances observed within our results. First, the strong nature of exposure(s) is likely an important consideration. We measured three ACES that reflect psychological trauma, and thus are strong predictors of depression risk. Emerging work has highlighted important variability in severity and salience of ACE events61, echoing previous studies documenting the existence of neurobiologically different subtypes of depression per the presence or absence of early life stress62. In a comparative study of ACEs and mental health, child maltreatment-type ACEs (e.g., physical and sexual abuse) but not household dysfunction-type ACEs (e.g., household substance abuse) primarily accounted for mental health symptoms61. In addition, while much remains to be discovered about the impacts of stress on nutrition, psychological stressors may influence micronutrient stores in the body -- some nutrients, such as zinc and magnesium, have been observed to become depleted in times of stress, and have also been linked to depression or depression-related stressors22. Thus, it is possible that certain, more severe childhood stressors (i.e., physical abuse) may exert such a strong influence on adult depressive outcomes that there is limited capacity for current or midlife diet to potentially effect change; usual diets of average quality may be unable to modify the relation and/or overcome pathways to high depression risk set forth in earlier years.
Second, a greater number of significant interactions were observed between childhood stressors with the chronic disease-aligned AHEI measure than with the more general HEI measure. This distinction may provide additional clues to better understand diet’s potential to interrupt stress-depression pathways. The specific differences between these two indices reflect their disparate objectives and include the more nuanced accounting of sugars and fats by the AHEI compared to the HEI. For example, some foods (e.g., fruit juices, red meats) do not count positively toward ultimate AHEI scores but do in the HEI, and sugar-sweetened beverage consumption is explicitly taxed in the AHEI but not HEI. For fats, nearly half of AHEI’s total points gauge adherence to intake recommendations for omega-3 long-chain fatty acids, PUFAs, and trans fats, as well as nuts and seeds and red/processed meats. Diets high in refined sugars and trans/saturated fats may facilitate depression’s pathology as these compounds are pro-inflammatory, increase oxidative stress, and inhibit neural plasticity30,33,63, especially in the nutrition-, stress-, and depression- pliant hippocampus14,16,30,32,33,64,65. Conversely, dietary lipids, particularly, omega-3 polyunsaturated fatty acids, are critical to brain structure, function, and plasticity38. Thus, the HEI’s sugar and fat assessments may be too blunt for depressive health, while the AHEI’s attunement to chronic disease processes, as well as the special attention it pays to these nutrients in its appraisal of overall diet quality, is a boon. Indeed, our study’s focus on overall diet quality and not micronutrient adequacy precludes conclusions from being drawn regarding micronutrient status, but significant positive relationships between overall diet quality indices and intakes and/or concentrations of vitamins and minerals do exist66,67. Overall diet quality indices that emphasize certain pro-neuronutrients while capturing the synergy between dietary components may be particularly helpful for similar studies in the future.
One final contributing reason for our observations may also be the relatively poor overall diet quality observed in the cohort, regardless of stress exposure status. While consistent with U.S. dietary studies finding poor diet quality in the general population41, both mean and median values for the two diet quality measures applied in our study were in the mid-50s, which, for the HEI (and likely the score interpretation-lacking AHEI too), are considered failing68. Additionally, the ranges of both measures were also relatively narrow at only slightly more than 35 points between the 10th and 90th percentiles (for HEI: 36.0 points, AHEI: 37.9 points). Hence, our observations may be due to floor or ceiling-type effects attributable to the limited variability observed in diet quality relative to childhood stressor exposure(s) in our sample. Moreover, as even the 90th percentile scores (76.3 for HEI and 75.6 for the AHEI) in our sample are considered mediocre68, there may simply not have been enough women with high enough diet quality to observe the full potential of diet to modify stress-depression relations, especially for very salient stressors.
Strengths of this study include its longitudinal cohort design across childhood and adolescence to midlife, its rigorous assessment of diet and perceived childhood stress, and the cohort’s racial/ethnic diversity. We controlled for important baseline measures/confounders and utilized two a priori, evidence-based measures of overall diet quality targeting general health promotion and prevention of chronic disease, whose performance as moderators we were also able to compare. Limitations of this study included the use of self-reported data for all measures and the possibility that baseline measures of covariates may not have necessarily preceded exposure to ACEs. We also did not assess adverse event frequency, and some research has suggested age or developmental stage at time of stress exposure(s) to influence outcomes69. Another limitation was also the concurrent assessment of adult diet with adult depression risk, which precludes the ability to cleanly evaluate temporality and causality in diet’s role(s) between initial childhood stress exposure(s) and midlife depression outcomes. Nonetheless, the direction of relations from our cross-sectional analyses of diet and depression risk were consistent with the literature32 and in our hypothesized direction. Some prior research has also found persons with past depression that sought treatment to consume healthier diets compared to persons with current depression70. Future work should examine diet longitudinally in the progression from childhood stress exposure to depressive outcome. It may also be informative to utilize dietary measures sensitive to specific depressive disease processes such as inflammation/oxidative health, neural plasticity, and/or the gut-brain-axis21 to highlight opportunities for nutritional or dietary intervention.
This study found high midlife depression risks associated with various childhood stress exposures to vary with overall diet quality, especially with stressors of a more serious nature. Where associations varied with diet quality, predicted prevalences of high depression risk were significantly lower for women without the childhood stress exposure(s) compared to women endorsing the same stressor(s), particularly at diet quality levels at or above the median. For women endorsing the childhood stressor(s), the prevalence of high depression risk did not significantly change with diet quality, at least not at the mediocre quality levels observed in our sample. Childhood stress exposures can deeply impact behavioral and neuroimmuno-related processes leading to depression and may also impact nutrient utilization; thus, improving diet may convey less mental health benefits or buffer for women exposed to severe childhood stressors. Additionally, the AHEI, which accounts for intakes of both a) nutrients known to be associated with depressive pathophysiology and/or chronic disease prevention as well as b) foods in its assessment, was more consistently found to modify the relationship between childhood stressors and depression risk; therefore, more targeted indices may be a more pertinent diet quality indicator than a broader measure in some instances. More research into possible nutritional influences on psychosomatic processes fostering the development of adult depression outcomes from serious childhood stress experiences is warranted.
Supplementary Material
Supplemental Table 1. Adjusted* Predicted Prevalences for High Midlife Depression Risk Conditional on Childhood Stress Exposure Status at Select Diet Quality (HEI-2015 and AHEI-2010) Score Percentiles (DQX) Where Diet-Stress Interaction was Observed
Acknowledgements:
We recognize NGHS staff past and present, especially Michael Coccia, MS, Kristy Brownell, BS, and Tashara Leak, PhD, MS, RD, for their talents and dedication without which the study and these analyses would not be possible. We also thank the Nutrition Policy Institute who provided consultation and support with historical study data. Most of all, we thank the incredible NGHS participants for their time and efforts over the years.
Disclosure Statement:
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant Race, stress and dysregulated eating: Maternal to child transmission of obesity [R01HD073568], the National Heart, Lung, and Blood Institute grant Neighborhood Environments and Intergenerational Transmission of Cardiovascular Health [R56HL141878], and the National Institute on Aging grants Early Life Adversity, Cumulative Life Stress, Race, and Cellular Aging in Midlife Women and Offspring [R56AG059677 & R01AG059677]. The authors report no conflict of interest.
Biography
DTC is a postdoctoral scholar/affiliate in the Division of Community Health Sciences at the University of California, Berkeley, and public health researcher interested in the intersection of diet and stress on mental and physical health outcomes.
EJH is a licensed clinical psychologist and postdoctoral fellow at the University of California, San Francisco. Her research focuses on the pubertal transition as a developmental period of risk for physical and mental health problems, particularly with depression.
CWL is an Assistant Professor in the Department of Nutritional Sciences at the University of Michigan, Ann Arbor, and a nutritional epidemiologist whose research focuses on diet and health disparities in vulnerable populations.
ESE is a Professor, and Vice Chair, in the Department of Psychiatry, at University of California, San Francisco as well as Associate Director of the Center for Health and Community. Her research aims to elucidate mechanisms of healthy aging, and to apply this basic science to scalable interventions that can reach vulnerable populations.
BAL is a Professor in the Division of Community Health Sciences at the University of California, Berkeley, whose research focuses on the influence of contextual level effects on dietary intake, cardiometabolic risk factors and pregnancy outcomes, especially among vulnerable populations.
Data Availability Statement:
The authors confirm that the data supporting the findings of this study are available by contacting corresponding author DTC and shareable upon reasonable request.
References:
- 1.World Health Organization. Depression. WHO Fact Sheets. https://www.who.int/news-room/fact-sheets/detail/depression. Published 2020. Accessed October 13, 2020. [Google Scholar]
- 2.Guo N, Robakis T, Miller C, Butwick A. Prevalence of Depression among Women of Reproductive Age in the United States. Obstet Gynecol. 2018;131(4):671–679. doi: 10.1097/AOG.0000000000002535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes DK, Robbins CL, Ko JY. Trends in Selected Chronic Conditions and Related Risk Factors Among Women of Reproductive Age: Behavioral Risk Factor Surveillance System, 2011–2017. J Women’s Heal. 2020;00(00):1–10. doi: 10.1089/jwh.2019.8275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE)Study. Am J Prev Med. 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 5.Goings TC, Yu T, Brody GH. Contextual risks and psychosocial outcomes among rural African American emerging adults: A latent profile analysis. Dev Psychopathol. 2020:1–13. doi: 10.1017/S0954579420001339 [DOI] [PubMed] [Google Scholar]
- 6.Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of Adverse Childhood Experiences from the 2011-2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA Pediatr. 2018;172(11):1038–1044. doi: 10.1001/jamapediatrics.2018.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychological Association. Stress in America Are Teens Adopting Adults’ Stress Habits?; 2014. https://www.apa.org/news/press/releases/stress/2013/stress-report.pdf. [Google Scholar]
- 8.Merrick MT, Ports KA, Ford DC, Afifi TO, Gershoff ET, Grogan-Kaylor A. Unpacking the impact of adverse childhood experiences on adult mental health. Child Abus Negl. 2017;69:10–19. doi: 10.1016/j.chiabu.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychological Association. Stress in America Coping With Change, Part 1.; 2017. [Google Scholar]
- 10.Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36(10):1513–1519. doi: 10.1016/j.psyneuen.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill MN, Hellemans KGC, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci Biobehav Rev. 2012;36(9):2085–2117. doi:doi: 10.1016/j.neubiorev.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 13.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: Depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075–1091. doi: 10.1176/appi.ajp.2015.15020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- 15.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. doi: 10.1542/peds.2012-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, Molecular Biology, and the Childhood Roots of Health Disparities. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Ge T, Leng Y, et al. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017;2017. doi: 10.1155/2017/6871089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rea K, Dinan TG, Cryan JF. The Brain-Gut Axis Contributes to Neuroprogression in Stress-Related Disorders. Mod Trends Pharmacopsychiatry. 2017;31:152–161. doi: 10.1159/000470813 [DOI] [PubMed] [Google Scholar]
- 19.Firth J, Marx W, Dash S, et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom Med. 2019;81(3):265–280. doi: 10.1097/PSY.0000000000000673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guu TW, Mischoulon D, Sarris J, et al. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother Psychosom. 2019;88(5):263–273. doi: 10.1159/000502652 [DOI] [PubMed] [Google Scholar]
- 21.Jacka FN. Nutritional Psychiatry: Where to Next? EBioMedicine. 2017;17:24–29. doi: 10.1016/j.ebiom.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopresti AL. The Effects of Psychological and Environmental Stress on Micronutrient Concentrations in the Body: A Review of the Evidence. Adv Nutr. 2020;11(1):103–112. doi: 10.1093/advances/nmz082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan A, Morton KR, Lee JW, Hartman R, Lee G. Adverse childhood experiences and depressive symptoms: Protective effects of dietary flavonoids. J Psychosom Res. 2020;131(February). doi: 10.1016/j.jpsychores.2020.109957 [DOI] [PubMed] [Google Scholar]
- 24.Kaplan BJ, Rucklidge JJ, Romijn AR, Dolph M. A randomised trial of nutrient supplements to minimise psychological stress after a natural disaster. Psychiatry Res. 2015;228(3):373–379. doi: 10.1016/j.psychres.2015.05.080 [DOI] [PubMed] [Google Scholar]
- 25.Rucklidge J, Johnstone J, Harrison R, Boggis A. Micronutrients reduce stress and anxiety in adults with Attention-Deficit/Hyperactivity Disorder following a 7.1 earthquake. Psychiatry Res. 2011;189(2):281–287. doi: 10.1016/j.psychres.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 26.Rucklidge JJ, Andridge R, Gorman B, Blampied N, Gordon H, Boggis A. Shaken but unstirred? Effects of micronutrients on stress and trauma after an earthquake/RCT evidence comparing formulas and doses. Hum Psychopharmacol. 2012;27:440–454. doi: 10.1002/hup.2246 [DOI] [PubMed] [Google Scholar]
- 27.Rucklidge JJ, Blampied N, Gorman B, Gordon HA, Sole E. Psychological functioning 1 year after a brief intervention using micronutrients to treat stress and anxiety related to the 2011 Christchurch earthquakes/ a naturalistic follow-up. Hum Psychopharmacol. 2014;29:230–243. doi: 10.1002/hup.2392 [DOI] [PubMed] [Google Scholar]
- 28.McNaughton SA. Dietary patterns and diet quality : approaches to assessing complex exposures in nutrition. Australas Epidemiol. 2010;17(1):35–37. [Google Scholar]
- 29.Willett W, Sampson L. Foods and Nutrients. In: Nutritional Epidemiology. Third Edit. ; 2013:17–33. [Google Scholar]
- 30.Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: The present state of the evidence. Proc Nutr Soc. 2017;76(4):427–436. doi: 10.1017/S0029665117002026 [DOI] [PubMed] [Google Scholar]
- 31.Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta - analysis of dietary patterns and depression in community - dwelling adults. Am J Clin Nutr. 2014;99:181–197. doi: 10.3945/ajcn.113.069880.Am [DOI] [PubMed] [Google Scholar]
- 32.Lassale C, Batty GD, Baghdadli A, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. 2019;24(7):965–986. doi: 10.1038/s41380-018-0237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Lv MR, Wei YJ, et al. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017;253:373–382. doi: 10.1016/j.psychres.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 34.Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. 2018;226(April 2017):346–354. doi: 10.1016/j.jad.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 35.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann Neurol. 2013;74(4):580–591. doi: 10.1002/ana.23944 [DOI] [PubMed] [Google Scholar]
- 36.Opie RS, O’Neil A, Itsiopoulos C, Jacka FN. The impact of whole-of-diet interventions on depression and anxiety: A systematic review of randomised controlled trials. Public Health Nutr. 2014;18(11):2074–2093. doi: 10.1017/S1368980014002614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiuve SE, Fung TT, Rimm EB, et al. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gómez-Pinilla F Brain foods: The effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568–578. doi: 10.1038/nrn2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godos J, Currenti W, Angelino D, et al. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants. 2020;9(346). doi: 10.3390/antiox9040346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bey GS, Waring ME, Jesdale BM, Person SD. Gendered Race Modification of the Association between Chronic Stress and Depression Among Black and White US Adults. Am J Orthopsychiatry. 2018;88(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiza HAB, Casavale KO, Guenther PM, Davis CA. Diet Quality of Americans Differs by Age, Sex, Race/Ethnicity, Income, and Education Level. J Acad Nutr Diet. 2013;113(2):297–306. doi: 10.1016/j.jand.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 42.The National Heart Lung and Blood Institute Growth and Health Study Research Group. Obesity and cardiovascular disease risk factors in Black and White girls: The NHLBI Growth and Health Study. Am J Public Health. 1992;82(12):1613–1620. doi: 10.2105/AJPH.82.12.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 44.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the center for epidemiologic studies depression (ces-d): A systematic review with meta-analysis. PLoS One. 2016;11(5):1–17. doi: 10.1371/journal.pone.0155431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amr M, El Gilany AH, El-Hawary A. Does Gender Predict Medical Students’ Stress in Mansoura, Egypt? Med Educ Online. 2008;13(1):4481. doi: 10.3402/meo.v13i.4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah M, Hasan S, Malik S, Sreeramareddy CT. Perceived stress, sources and severity of stress among medical undergraduates in a Pakistani medical school. BMC Med Educ. 2010;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 48.Campbell JA, Walker RJ, Egede LE. Associations Between Adverse Childhood Experiences, High- Risk Behaviors, and Morbidity in Adulthood. Am J Prev Med. 2016;50(3):344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frampton NMA, Poole JC, Dobson KS, Pusch D. The effects of adult depression on the recollection of adverse childhood experiences. Child Abus Negl. 2018;86:45–54. doi: 10.1016/j.chiabu.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 50.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB, Basiotis PP. Development and Evaluation of the Healthy Eating Index-2005: Technical Report.; 2007. [Google Scholar]
- 51.National Cancer Institute. Comparing the HEI-2015, HEI-2010 & HEI-2005. https://epi.grants.cancer.gov/hei/comparing.html. Published 2017. [Google Scholar]
- 52.Nutrition Coordinating Center/University of Minnesota. Healthy Eating Index (HEI). [Google Scholar]
- 53.National Cancer Institute Division of Cancer Control & Population Sciences. HEI Scoring Algorithm. https://epi.grants.cancer.gov/hei/hei-scoring-method.html. Published 2019. [Google Scholar]
- 54.Nutrition Coordinating Center/University of Minnesota. Guide for Calculating HEI-2015 Total and Component Scores Using SAS Code Developed by the Nutrition Coordinating Center (NCC) for Dietary Recall, Record, and Record-Assisted Recall Record Types. http://www.ncc.umn.edu/wp-content/uploads/2019/11/HEI-2015-sas-code-use-guide-11252019.pdf. Published 2019. Accessed October 28, 2020. [Google Scholar]
- 55.Willett W Implications of Total Energy Intake for Epidemiologic Analyses. In: Willett W, ed. Nutritional Epidemiology. Third. Oxford University Press; 2013:260–286. [Google Scholar]
- 56.Bronfenbrenner U Ecological models of human development. In: Gauvain M, Cole M, eds. Readings on the Development of Children. 2nd ed. New York: Freeman; 1993:37–43. [Google Scholar]
- 57.Zou G A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 58.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3(21). doi: 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis. 2016;8(9):E928–E931. doi: 10.21037/jtd.2016.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.www.Stata.com. Marginal means, adjusted predictions, and marginal effects. https://www.stata.com/features/overview/marginal-analysis/%0A. Accessed August 4, 2020. [Google Scholar]
- 61.Negriff S ACEs are not equal: Examining the relative impact of household dysfunction versus childhood maltreatment on mental health in adolescence. Soc Sci Med. 2020;245(July 2019):112696. doi: 10.1016/j.socscimed.2019.112696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29(4):641–648. doi: 10.1038/sj.npp.1300397 [DOI] [PubMed] [Google Scholar]
- 63.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–814. doi: 10.1016/S0306-4522(02)00123-9 [DOI] [PubMed] [Google Scholar]
- 64.Jacka FN, Cherbuin N, Anstey KJ, Sachdev P, Butterworth P. Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Med. 2015;13(1):1–8. doi: 10.1186/s12916-015-0461-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gianaros PJ, Jennings RJ, Sheua LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter. Neuroimage. 2007;35(2):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collins CE, Burrows TL, Rollo ME, et al. The comparative validity and reproducibility of a diet quality index for adults: The Australian recommended food score. Nutrients. 2015;7(2):785–798. doi: 10.3390/nu7020785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fallaize R, Livingstone KM, Celis-Morales C, et al. Association between diet-quality scores, adiposity, total cholesterol and markers of nutritional status in european adults: Findings from the Food4Me study. Nutrients. 2018;10(1). doi: 10.3390/nu10010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabrys RL, Dixon K, Anisman H. Traumatic life events in relation to cognitive flexibility: Moderating role of the BDNF Val66met gene polymorphism. Front Behav Neurosci. 2017;11(December):1–10. doi: 10.3389/fnbeh.2017.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacka FN, Cherbuin N, Anstey KJ, Butterworth P. Does reverse causality explain the relationship between diet and depression? J Affect Disord. 2015;175:248–250. doi: 10.1016/j.jad.2015.01.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Adjusted* Predicted Prevalences for High Midlife Depression Risk Conditional on Childhood Stress Exposure Status at Select Diet Quality (HEI-2015 and AHEI-2010) Score Percentiles (DQX) Where Diet-Stress Interaction was Observed
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available by contacting corresponding author DTC and shareable upon reasonable request.