Abstract
Background
Respiratory viruses pose an important public health threat to most communities. Nonpharmaceutical interventions (NPIs) such as masks, hand hygiene or physical distancing, among others, are believed to play an important role in reducing transmission of respiratory viruses. In this umbrella review, we summarise the evidence of the effectiveness of NPIs for the prevention of respiratory virus transmission in the community setting.
Observations
A systematic search of PubMed, Embase, Medline and Cochrane reviews resulted in a total of 24 studies consisting of 11 systematic reviews and meta-analyses, 12 systematic reviews without meta-analyses and one standalone meta-analysis. The current evidence from these data suggests that hand hygiene is protective against respiratory viral infection. The use of hand hygiene and facemasks, facemasks alone and physical distancing were interventions with inconsistent evidence. Interventions such as school closures, oral hygiene or nasal saline rinses were shown to be effective in reducing the risk of influenza; however, the evidence is sparse and mostly of low and critically low quality.
Conclusions
Studies on the effectiveness of NPIs for the prevention of respiratory viral transmission in the community vary in study design, quality and reported effectiveness. Evidence for the use of hand hygiene or facemasks is the strongest; therefore, the most reasonable suggestion is to use hand hygiene and facemasks in the community setting.
Short abstract
Evidence from this umbrella review point to hand hygiene and facemasks as effective nonpharmaceutical methods to prevent respiratory viral transmission in the community setting https://bit.ly/3x6rGu8
Introduction
Since the start of the coronavirus disease 2019 (COVID-19) pandemic, recommendations for nonpharmaceutical interventions (NPIs) such as facemask wearing, physical distancing and quarantining were advocated. Numerous reports of transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from mildly symptomatic or asymptomatic individuals suggest a critical period during which NPIs may have an impact [1–4]. When implemented appropriately, there is potential to mitigate the transmission of respiratory viruses such as SARS-CoV-2 and reduce the burden on healthcare systems. Understanding the role of NPIs during pandemics is essential to guide public health strategies both for affording time for vaccine development and rollout and as an adjunct to vaccinations to provide improved infection control.
A growing number of studies have been conducted on hand hygiene, mask efficacy and physical distancing in preventing respiratory viral transmission and this has been paralleled by the growing pool of narrative and systematic reviews [5, 6]. This unprecedented pace of research output on COVID-19, while highly productive, hinders the interpretation of systematic reviews and meta-analyses, which are conventionally few in number and provide high-quality summaries of data. The purpose of our study is to perform a systematic review of systematic reviews and meta-analyses, otherwise known as an umbrella review, to condense this rapidly expanding pool of data and provide context for the interpretation and comparison of the current state of the literature. In this umbrella review, we synthesise systematic review and meta-analysis data regarding the effectiveness of NPIs for reducing viral transmission in the community setting and assess the quality of evidence.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used as a framework to guide and present the workflow of this umbrella review [7]. Findings are reported at the individual level of each reference and as the aggregate of multiple references. This study is an umbrella review of systematic reviews and meta-analyses of NPIs in the community setting, conducted with reference to standard Cochrane methods [8]. The purpose of this study is to provide an overview of the systematic review and meta-analysis evidence addressing the effectiveness of NPIs in decreasing respiratory virus transmission and infection in community settings, and assess the quality of evidence.
Search strategy
We conducted a comprehensive search of the following databases: PubMed, Embase, Medline and Cochrane reviews. The search strategy was developed with assistance from the university librarian. All systematic reviews published between 1 January 2000 and 31 December 2020 were included in the search, as most studies of mask efficacy appeared in the aftermath of the SARS-CoV pandemic of 2003 [9]. Only articles published in the English language were reviewed. Our search syntax was adapted from the Cochrane review by Jefferson et al. [10], and updated with a systematic review search hedge for the purposes of this study [11]. The screening strategy is presented in table 1 and the complete search strategy with database-specific variations can be found in the supplementary material.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion | Exclusion | |
| Population | Community setting (e.g. school, household, workplace, childcare centre, assisted living facility) | Healthcare workers (including dentists, mainly taking place in clinics) |
| Intervention | Any nonpharmaceutical intervention (e.g. facemask and variations, hand hygiene with soap, sanitiser or education, nasal or oral hygiene with over-the-counter products, school closure) | Pharmaceutical interventions (e.g. prescription medications, vaccines, professional oral hygiene, antiviral agents), surface or object disinfection Population-scale interventions (e.g. mass quarantine, airport screening, lockdowns, travel restrictions) |
| Comparison | No intervention or control | |
| Outcome | Self-reported or clinically diagnosed ARI, ILI, CRI Laboratory-confirmed influenza |
Bacterial infection, fungal infection, parasitic infection |
| Language | English | Language other than English |
| Study type | Systematic reviews of interventional, observational, epidemiological studies | Nonsystematic reviews, systematic reviews containing modelling or simulation studies, umbrella reviews, meta-reviews |
| Time period | 1 January 2020 to 31 December 2020 | Before 1 January 2020 or after 31 December 2020 |
| Other | Must have at least two contributing authors | Single author, full text unretrievable |
ARI: acute respiratory illness; ILI: influenza-like illness; CRI: clinical respiratory infection.
Eligibility and inclusion criteria
Systematic reviews looking at the impact of NPIs used in community settings were included. Community settings included, but were not limited to, mass gathering settings (such as the Hajj), household settings, workplaces, schools (residence halls, classrooms), childcare centres (including daycare), assisted-living facilities (senior homes, long-term care centres), military settings (navy recruits, military trainees) and general community settings. Healthcare settings such as clinics, hospitals or dental offices, or populations including healthcare workers were excluded from our study, because transmission patterns, preventative measures and exposure risks differ. Eligible systematic reviews and meta-analyses addressed at least one NPI. Eligible NPIs included mask use, hand hygiene, social distancing, nasal rinses, mouthwashes (with nonpharmaceutical ingredients), school closure or change in government policy acting on the aforementioned interventions. Included studies reported on at least one of reverse transcriptase (RT)-PCR-confirmed respiratory viral illness (including influenza and coronaviruses), influenza-like illness (ILI), clinical respiratory infection (CRI), acute respiratory illness (ARI), viral attack rate or work- or school-related sick leave secondary to respiratory illness. Detailed criteria can be found in table 1.
We included systematic reviews and/or meta-analyses containing more than one relevant randomised controlled trial and/or observational study (cohort, case–control, cross-sectional, case series, case reports) whose conclusions were separated from healthcare population data and modelling study data. Combined results that included modelling studies were excluded, as they premise their conclusions on a series of assumptions, which may not accurately represent the complexity of interactions in the community. Combined community and healthcare setting results were excluded, as such settings vary significantly from community settings and results may be skewed. Studies with only one author were excluded because the rigorous methodology of systematic reviews and meta-analyses may not necessarily be ascertained without multiple authors (e.g. lack of data extraction in duplicate). Eligible studies were published in peer-reviewed journals between 1 January 2000 and 31 December 2020. Nonoriginal publications (narrative reviews, commentaries, letters) and conference abstracts were excluded (table 1).
Data extraction and analysis
Screening phase
We catalogued the results of the search in Endnote Reference Manager, then uploaded records into the systematic review software Covidence (Veritas Health Innovation, Australia). After removal of duplicates, titles and abstracts, followed by full texts, were screened independently and in duplicate (H. Zhao, S. Jatana) for possible inclusion. Any conflicts were first deliberated between H. Zhao and S. Jatana and any that remained unresolved were discussed in the presence of a third reviewer.
Data extraction phase
The following data were extracted independently and in duplicate from each systematic review and meta-analysis by H. Zhao and S. Jatana: bibliographic information of the study (author, publication year), databases searched, number and types of studies included (randomised trials, cohort, case–control, case series, case reports) (where obtainable), total number of participants, types of NPIs, pertinent outcomes (RT-PCR-confirmed respiratory virus including influenza and coronaviruses, patient-reported or clinically diagnosed ILI, ARI or CRI, and work or school absenteeism (herein referred to as sick leave) secondary to respiratory illness; sensitivity or other post hoc analysis and adherence to outcome, if observed, were also included), key findings and knowledge gaps. Key findings were identified from reading through each reference's abstract, results, tables and figures and conclusion to identify conclusions according to our inclusion criteria (e.g. results pertinent to viral respiratory infections that were not combined with those of modelling studies or studies involving healthcare workers).
Quality assessment
The methodological quality of included systematic reviews and meta-analyses was assessed using of AMSTAR2 (A Measurement Tool to Assess Systematic Reviews) [12]. AMSTAR2 is a critical appraisal tool for systematic reviews and meta-analyses that include randomised or nonrandomised studies of healthcare interventions. It grades studies according to a 16-point questionnaire and overall confidence in the results were categorised as high, moderate, low or critically low. A questionnaire provided by Shea et al. [12] from the AMSTAR team was used to assess the quality of the studies. As per Shea et al., high-quality studies had zero or one noncritical weakness, moderate-quality studies had more than one noncritical weakness, low-quality studies had one critical flaw with or without noncritical weaknesses and critically low studies had more than one critical flaw with or without noncritical weaknesses. AMSTAR2 was chosen over the Risk of Bias in Systematic Reviews tool because of slightly superior inter-rater reliability [13]. This stage undertaken done in duplicate by H. Zhao and S. Jatana, and if necessary, a third reviewer was present to resolve disagreements.
Data synthesis
We narratively synthesised and tabulated information regarding the study design and quality of the included systematic reviews and meta-analyses, along with information regarding relevant interventions, comparators, settings or populations and outcomes. Where available, quantitative data were included to provide support for or refute the utility of various NPIs. Given significant qualitative heterogeneity in the published literature, we opted to provide narrative synthesis only and as we did not conduct a meta-analysis, we did not exclude systematic reviews that referenced a similar set of studies.
Ethics and dissemination
We did not require ethics approval as this study was conducted in a setting that did not interact directly with human or animal subjects.
Results
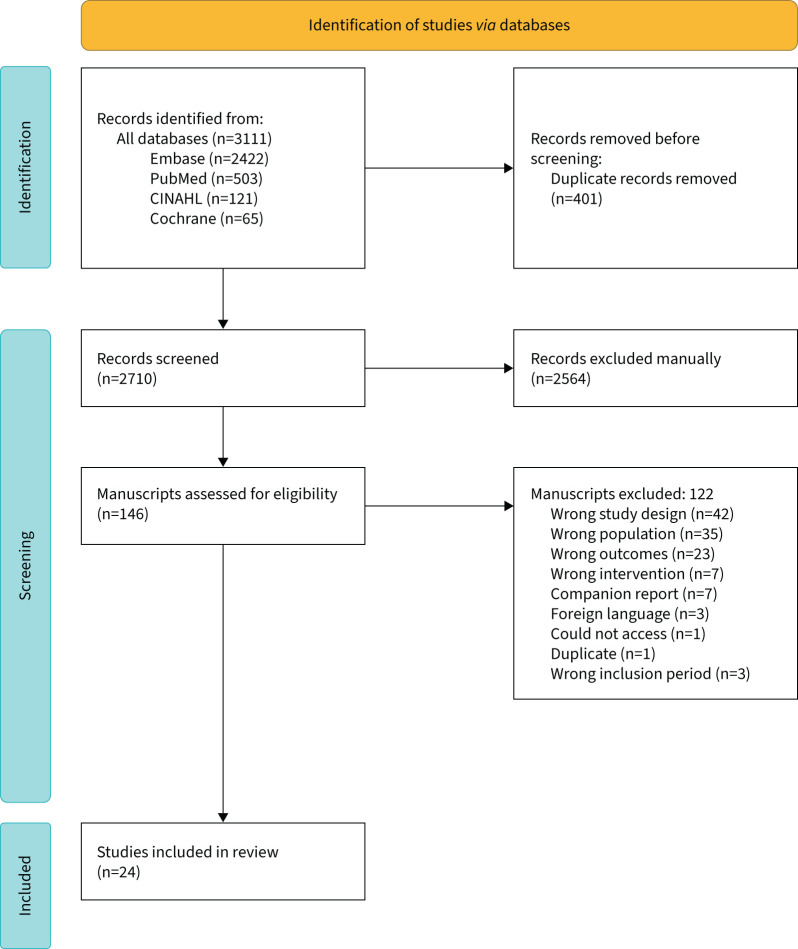
Between the years 2000 and 2020, we identified a total of 2710 records, of which 146 were selected for full-text review, and 24 met inclusion criteria for this study. The study flow diagram is shown in figure 1. The studies were graded according to AMSTAR 2, which resulted in seven low-quality studies and 17 critically low quality studies (table 2). Inter-rater agreement for AMSTAR 2 was 83.3% (95% CI 61.8–94.5%) or κ 0.84 (95% CI 0.68–1.0), signalling near-perfect agreement, and disagreements differed by one quality grade level at most.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for the identification of systematic reviews and meta-analyses. CINAHL: Cumulative Index to Nursing and Allied Health database.
TABLE 2.
Systematic reviews and meta-analyses
| First author, year [ref.] | Total studies (n) | Relevant studies/total studies in meta-analysis (n/n) | Population | Interventions | Outcome | Findings |
| Low quality | ||||||
| Chu, 2020 [14] | 172 | 3/44 Observational studies |
General community# Household |
Facemask | Probable or confirmed SARS-CoV-1 infection | Facemask use by those exposed to infected contacts decreases the risk of infection (relative risk 0.56, 95% CI 0.40–0.79; low–moderate credibility) |
| Jefferson, 2020 [10] | 67 | 16/35 Hand hygiene RCTs |
School Childcare centre Household Workplace Military (navy) Assisted-living facility Mass gatherings General community# |
Hand hygiene | ARI Laboratory-confirmed influenza Laboratory-confirmed other virus Sick leave |
Hand hygiene decreases the composite outcome of ARI, ILI or laboratory-confirmed influenza relative to control (risk ratio 0.89, 95% CI 0.84–0.95; low-certainty evidence) with high heterogeneity Hand hygiene reduces the risk of ARI relative to control (risk ratio 0.84, 95% CI 0.82–0.86; moderate-certainty evidence) Hand hygiene does not reduce the risk of ILI and laboratory-confirmed influenza (risk ratio 0.91, 95% CI 0.63–1.30; low-certainty evidence) Hand hygiene reduces the rate of sick leave compared to control (risk ratio 0.64; 95% CI 0.58–0.71) |
| Facemask | Mask results were not differentiated between community and healthcare settings | |||||
| Facemask and hand hygiene | Hand hygiene with facemasks does not reduce the risk of ILI (risk ratio 1.03, 95% C1 0.77–1.37) or laboratory-confirmed influenza (risk ratio 0.99, 95% CI 0.69–1.36) compared to control | |||||
| Gargling | Gargling does not reduce the risk of viral illness compared to control (risk ratio 0.91, 95% CI 0.63–1.31) | |||||
| Wong, 2014 [15] | 18 | 10/10 RCTs | Household School Workplace |
Hand hygiene | ILI Laboratory-confirmed influenza |
Hand hygiene alone compared to control does not demonstrate a significant benefit for ILI (risk ratio 0.86, 95% CI 0.71–1.04) and laboratory-confirmed influenza (risk ratio 0.90, 95% CI 0.67–1.20) |
| Hand hygiene and facemask | Hand hygiene with facemask use compared to control is associated with significantly decreased ILI (risk ratio 0.73, 95% CI 0.6–0.89) and laboratory-confirmed influenza (risk ratio 0.73, 95% CI 0.53–0.99) | |||||
| Hand hygiene ± facemask | Hand hygiene with or without facemask compared to control is associated with a significant decrease in ILI (risk ratio 0.78, 95% CI 0.68–0.9), but a nonsignificant effect on laboratory-confirmed influenza (risk ratio 0.82, 95% CI 0.66–1.02) Subgroup analysis of less-developed countries for the same interventions and outcomes does not demonstrate statistically significant results |
|||||
| Critically low quality | ||||||
| Abdullahi, 2020 [16] | 17 | 7/7 RCTs and observational studies | Low- to middle-income countries (China, Bangladesh, Thailand) Household School General community# |
Facemask | SARS and influenza incidence | Facemask use demonstrates no significant benefit to the composite of influenza and SARS spread versus control (risk ratio 0.78, 95% CI 0.36–1.67) |
| Hand hygiene | Hand hygiene demonstrates no significant benefit to SARS and influenza spread versus control (risk ratio 0.95, 95% CI 0.83–1.08) | |||||
| Facemask and hand hygiene | Facemasks with hand hygiene demonstrates no significant benefit to influenza spread versus control (risk ratio 0.94, 95% CI 0.58–1.54) | |||||
| Social distancing | Social distancing interventions may slow down the spread of influenza (low-certainty evidence, 9 studies not pooled) | |||||
| Aggarwal, 2020 [17] | 9 | 8/9 RCTs |
Household School |
Facemask | Clinically diagnosed influenza or ILI | Facemasks show no significant reduction of ILI compared to control (effect size −0.17, 95% CI −0.43–0.10)¶ |
| Facemask and hand hygiene | Mask and hand hygiene show no significant reduction of ILI compared to control (effect size −0.09, 95% CI −0.58–0.4)¶ | |||||
| Gera, 2018 [18] | 41 | 8/34 RCTs and non-RCTs |
Low- to middle-income countries Individuals, families or communities Children aged <18 years |
Hand hygiene | ARI Laboratory-confirmed influenza School sick leave |
Hand hygiene compared to control decreases the risk of ARI (risk ratio 0.76, 95% CI 0.59–0.98), 6 studies, moderate-quality evidence Hand hygiene compared to control decreases laboratory-confirmed influenza (risk ratio 0.5, 95% CI 0.41–0.62), 1 study, very low quality evidence Hand hygiene compared to control decreases school sick leave (risk ratio 0.78, 95% CI 0.76–0.8), 4 studies, moderate-quality evidence |
| Liang, 2020 [19] | 21 | 8/8 RCTs and observational studies | School Mass-gathering (Hajj) Workplace Household General community# |
Facemask | Laboratory confirmed respiratory virus Clinically diagnosed ARI |
Facemask use compared to control significantly reduces laboratory-confirmed viral infection by 47% (OR 0.53, 95% CI 0.36–0.79) |
| Rabie, 2006 [20] | 8 | 8/8 RCTs and interventional studies |
School Childcare centre Military (navy) |
Hand hygiene | ARI Duration of respiratory illness |
Hand hygiene measures lower risk of respiratory infection by 24% (relative risk 0.76, 95% CI 0.6–0.96) Sensitivity analysis excluding one uncontrolled study of hand hygiene measures (n=7) demonstrate decreased risk of respiratory infection by 16% (relative risk 0.84, 95% CI 0.79–0.89); note, studies were of poor quality Sensitivity analysis excluding crossover or poor-quality studies had no significant effect |
| Rainwater-Lovett, 2014 [21] | 37 | 10 personal protective equipment |
Assisted-living facility | Personal protective equipment (hand hygiene, mask, droplet precautions) |
ILI with minor variations Laboratory-confirmed influenza All studies required laboratory testing to establish influenza as the cause of the outbreak |
Personal protective equipment is not associated with decreased influenza A or B attack rate (OR 0.63, 95% CI 0.33–1.19) |
| 18 social distancing | Social distancing (no new admissions, visitor restriction, ward transfer restrictions, isolation or cohorting) | Social distancing is not associated with decreased influenza A or B attack rate (OR 1.31, 95% CI 0.78–2.18) | ||||
| Wang, 2020 [22] | 15 | 10/10 observational studies | School Household Mass gathering (Hajj) In-flight setting |
Facemask ± hand hygiene | ARI Laboratory-confirmed influenza |
Facemask use is not associated with reduced ARI incidence (OR 0.96, 95% CI 0.8–1.15) Subgroup analysis of laboratory-confirmed viral infection (OR 0.82, 95% CI 0.63–1.07) does not demonstrate any benefit Subgroup analysis of self-reported/clinically diagnosed ARI (OR 1.1, 95% CI 0.84–1.45) does not demonstrate any benefit |
| Xiao, 2020 [23] | 18 | 12/12 hand-hygiene studies 10/10 facemask studies |
School Household Mass gathering |
Facemask and hand hygiene | Laboratory-confirmed influenza | Facemask use with hand hygiene does not significantly decrease laboratory-confirmed influenza (risk ratio 0.91, 95% CI 0.73–1.13; 6 studies) |
| Facemask | Facemask use alone does not significantly decrease laboratory-confirmed influenza (risk ratio 0.78, 95% CI 0.51–1.20; 7 studies) | |||||
| Facemask ± hand hygiene | Facemask use with or without hand hygiene does not decrease laboratory-confirmed influenza (risk ratio 0.92, 95% CI 0.75–1.12; 10 studies) | |||||
| Hand hygiene | No pooled estimate for hand hygiene alone or with optional facemask use due to high heterogeneity |
SARS-CoV: severe acute respiratory syndrome coronavirus; ARI: acute respiratory illness; ILI: influenza-like illness; RCT: randomised controlled trial. #: general community settings refer to all other community-based settings not fitting into any of the major categories such as school, household, assisted living facility, childcare centre or workplace. ¶: the effect size was calculated as log(OR). A negative number represents a protective effect.
Systematic reviews and meta-analyses
The search identified 11 systematic reviews with accompanying meta-analyses, which were subsequently classified according to their AMSTAR 2 quality grading (table 2). No studies were categorised as high or moderate quality. Three studies were categorised as low quality and demonstrated effectiveness of NPIs such as hand hygiene and mask use [10, 14, 15]. Jefferson et al. [10] exclusively examined randomised trials and concluded that hand hygiene decreased the composite outcome of ARI, ILI and laboratory-confirmed influenza by 11% relative to no intervention (risk ratio 0.89, 95% CI 0.84–0.95) or decreased the risk of ARI alone by 16% relative to no intervention (risk ratio 0.84, 95% CI 0.82–0.86). However, there was no effectiveness of hand hygiene against ILI or laboratory-confirmed influenza alone, and gargling did not modify the risk of viral illness compared to control. The efficacy of masks in the community setting could not be delineated due to the admixture of healthcare studies [10]. In the second study, Chu et al. [14] conducted a large systematic review and meta-analysis of 44 studies, of which only three observational studies were separately synthesised for the effectiveness of NPIs in the community [14]. These studies demonstrated that facemask use by those exposed to infected contacts decreased the risk of infection by 44% (relative risk 0.56, 95% CI 0.40–0.79) [14]. Wong et al. [15] also found a significant protective effect against ILI and laboratory-confirmed influenza when hand hygiene was used in combination with facemasks (risk ratio 0.73, 95% CI 0.60–0.89 and risk ratio 0.73, 95% CI 0.53–0.99, respectively), but not individually.
The remaining eight studies were of critically low quality [16–23]. Hand hygiene as a standalone intervention was evaluated in four studies [15, 18, 20, 23] and was found to be effective in reducing the risk of respiratory virus infection by 24% by both Gera et al. [18] (risk ratio 0.76, 95% CI 0.59–0.98) and Rabie and Curtis [20] (relative risk 0.76, 95% CI 0.6–0.96). Conversely, Abdullahi et al. [16] found no benefit for severe acute respiratory syndrome or influenza incidence, while Xiao et al. [23] omitted a pooled estimate, citing high heterogeneity. Of the four studies assessing facemask use alone [19, 16, 22, 23], only Liang et al. [19] found a significant reduction in respiratory viral spread (OR 0.53, 95% CI 0.36–0.79). No studies found a significant benefit for facemasks and hand hygiene in combination [16, 17, 23] and social distancing was only assessed in one study, where no impact on influenza A or B attack rate was reported (OR 1.31, 95% CI 0.78–2.18) [21].
We further delineated this cluster of 11 systematic reviews and meta-analyses assessing the effectiveness of hand hygiene or facemask use by illness outcomes, as both these NPIs are hypothesised to prevent transmission via respiratory droplets, aerosols and surface contamination and have the most data available in nonhealthcare settings. The most common outcomes studied were laboratory-confirmed influenza or other respiratory virus, ARI, ILI and sick leave. Most systematic reviews and meta-analyses did not report a decrease in the risk of laboratory-confirmed viral infection, the most standardised measure, with regard to facemasks (none out of three reporting significant effect) [10, 22, 23], hand hygiene (one out of three) [10, 15, 18] or a combination of the two interventions (none out of two) [10, 23]; thus, Gera et al. [18] was unique in demonstrating a protective effect of hand hygiene for laboratory-confirmed influenza. For ILI, only Wong et al. [15] showed a benefit for a combination of hand hygiene and facemasks, whereas no benefit was found with this combination nor hand hygiene alone by Jefferson et al. [10]. Systematic reviews and meta-analyses evaluating ARI demonstrated a benefit of hand hygiene interventions [10, 18], but not mask interventions [22]. For the rate of sick leave secondary to respiratory illness, hand hygiene interventions appear to be effective in two systematic reviews and meta-analyses, but lose significance when combined with facemask interventions [10, 18].
Systematic reviews without meta-analysis
There were 12 systematic reviews without meta-analyses [24–35] (table 3). Of these, four were of low quality [24–27]. Most of these studies demonstrated a protective effect of hand hygiene, although there was considerable heterogeneity in setting and outcomes across these systematic reviews. McGuinness et al. [24] suggested a protective effect of hand hygiene interventions with variability in effectiveness across childcare, schools, workplaces and domestic settings. Willmott et al. [27] and Smith et al. [26] reached similar conclusions regarding a positive effect of hand hygiene in reducing the incidence of respiratory tract infection, including both ILI and laboratory-confirmed influenza, and sick leave in children. Furthermore, a comprehensive layered NPI approach with an educational component may contribute to improved intervention effects [26]. For reasons related to the heterogeneity and poor quality of available evidence, Moncion et al. [25] were unable to provide a reasonable conclusion of the effectiveness of hand hygiene on preventing influenza transmission in the community.
TABLE 3.
Systematic review only
| First author, year [ref.] | Relevant studies/total studies (n/n) | Population | Intervention | Outcome | Findings |
| Low quality | |||||
| McGuinness, 2018 [24] | 14/14 | Low- to middle-income countries School Childcare centre Household General community# |
Hand hygiene (education, promotion and infrastructure) | ARI ILI Laboratory-confirmed viral infection ARI-related sick leave and deaths |
Hand-hygiene interventions can reduce ARI morbidity in childcare, school and domestic settings, but depend on setting, intervention target and compliance In childcare settings, there is a reduction in ARI-related sick leave and illness (low–moderate-quality evidence) In school settings, there is a reduction in ARI-related sick leave and laboratory-confirmed influenza (moderate–high-quality evidence) but no reduction in ARI illness (low-quality evidence) In domestic settings, there is reduction in ARI and pneumonia in urban settings (high-quality evidence), no reduction in ARI and pneumonia in rural settings (low-quality evidence) and no reduction in secondary influenza transmission in household settings (moderate-quality evidence) |
| Moncion, 2019 [25] | 16/16 | Household Mass gathering (Hajj) General community# |
Hand hygiene | Laboratory-confirmed influenza Possible influenza infection (e.g. ARI, ILI) |
Effectiveness of hand hygiene against influenza virus infection and transmission in community settings is difficult to determine due to heterogeneity and poor quality of evidence 6 out of 9 studies (1 out of 2 RCTs, 5 out of 7 observational studies) suggest that hand hygiene reduces laboratory-confirmed or possible influenza infection 2 out of 7 studies find hand hygiene to be effective in preventing laboratory-confirmed or possible influenza infection transmission |
| Smith, 2015 [26] | 6/7 | School Household Assisted-living facility General community# |
Hand hygiene | ILI Laboratory-confirmed influenza Evidence of decreased transmission (influenza/ILI attack rates, secondary infections ratios, viral illness severity, mortality rates, healthcare utilisation) |
Handwashing appears to be helpful in decreasing viral transmission |
| Facemask | Not able to fully assess, secondary to significant design flaws | ||||
| Combination NPIs (one or more of hand hygiene, facemask, education) | Not able to fully assess, secondary to significant design flaws | ||||
| Education as component of other NPI interventions | An NPI approach with an educational component (education, guidance or advice) appears to be effective in decreasing viral transmission | ||||
| Gargling/oral hygiene | Oral hygiene appears to be helpful in decreasing viral transmission | ||||
| Willmott, 2016 [27] | 13/18 | School (ages 3–11 years) Childcare centre |
Hand hygiene | Incidence of respiratory tract infections (composite) Laboratory-confirmed respiratory tract infections School sick leave |
Hand hygiene may reduce respiratory tract infection incidence, laboratory-confirmed respiratory tract infection and sick leave |
| Critically low quality | |||||
| Chou, 2020 [28] | 15/39 | School Household Mass gathering (Hajj) General community# |
Facemasks | SARS-CoV-1 infection SARS-CoV-2 infection MERS-CoV infection ILI CRI Laboratory-confirmed viral infection/influenza |
No studies of mask effectiveness for prevention of SARS-CoV-2 in the community Facemask use compared to control may decrease risk of SARS-CoV-1 infection based on 3 observational studies Facemask use may have no effect on risk of ILI, CRI or laboratory-confirmed virus/influenza for both the index case or contacts, based on 12 RCTs |
| Cowling, 2010 [29] | 5/12 | School General community# |
Facemask | ILI Laboratory-confirmed influenza |
Some evidence to support that wearing masks or respirators is beneficial in preventing influenza transmission if worn during illness Less evidence to support that wearing masks or respirators has benefit in preventing influenza transmission if worn to prevent infection Note, many studies included in the systematic review had masks as part of a combined hand hygiene and facemask group only |
| Fong, 2020 [30] | 57/101 | School Workplace General community# |
School closure in Asia, Europe, America, Africa, and Australia Due to outbreak report or teacher's strike, planned holiday, reactive closure, pre-emptive closure |
Effectiveness of school closure (poorly defined) | Planned school closure (holiday) may decrease influenza transmission during closure, but leads to increase after opening Pre-emptive school closure may have a moderate impact in reducing influenza transmission by delaying epidemic peak, affecting mean peak and reducing overall attack rate Reactive school closure effectiveness varies |
| MacIntyre, 2020 [31] | 11/19 | School General community# |
Facemasks ± hand hygiene | ILI Laboratory-confirmed influenza Influenza infection (poorly defined) |
In community settings, masks appear to be effective with and without hand hygiene, and both together are more protective; interventions appear to be more likely to be more effective if used within 36 h of exposure |
| Mbakaya, 2017 [32] | 8/8 | Developing countries School (ages 6–12 years) |
Hand hygiene (education, promotion and infrastructure) | ARI School sick leave |
Hand hygiene compared to control decreases ARI (risk ratio 0.77, 95% CI 0.62–0.95) Reduction in school sick leave secondary to ILI is 40% (p<0.0001) |
| Singh, 2020 [33] | 7/8 | School Assisted-living facility Adults and children diagnosed with acute URTI General community# |
Nasal wash with isotonic/hypertonic solutions Gargling saline/tea Kunjal/stomach saline wash Steam inhalation |
CRI Time to resolution of symptomatic illness Viral shedding Transmission to household contacts Adverse events from treatment Sick leave Antibiotic and URTI medication use |
Hypertonic saline gargles and nasal wash may help prevent or improve symptoms of respiratory illness, reduce transmission, reduce need for medication and reduce viral loads in patients with common cold |
| Wang, 2017 [34] | 9/19 | School (ages 4–15 years) | Hand hygiene | Sick leave secondary to respiratory illness | Inadequate evidence to show that hand-hygiene interventions have an effect on ARI-associated sick leave; note, 5 out of 9 studies show hand-hygiene intervention has significant reduction in ARI-associated sick leave compared to control (30.9–52.6% reduction) |
| Warren-Gash, 2013 [35] | 16/16 | School Childcare centre Assisted-living facility Workplace Household General community# |
Hand hygiene | ILI ARI Laboratory-confirmed influenza |
Hand hygiene interventions have the potential to reduce influenza and ARI, but their effectiveness depends on setting, context and compliance Hand hygiene is associated with a large decrease in influenza and ARI in institutional settings (school) and domestic settings (squatter settlement) (moderate–high-quality evidence) Hand hygiene is associated with a small reduction in ARI in daycare centres (high-quality evidence) and in school and workplaces (lower-quality evidence) Hand hygiene did not prevent secondary influenza transmission in households with index cases (moderate–high-quality evidence) |
ARI: acute respiratory illness; ILI: influenza-like illness; RCT: randomised controlled trial; NPI: nonpharmaceutical intervention; SARS-CoV: severe acute respiratory syndrome coronavirus; MERS-CoV: Middle East respiratory syndrome coronavirus; CRI: clinical respiratory infection; URTI: upper respiratory tract infection. #: general community settings refer to all other community-based settings not fitting into any of the major categories such as school, household, assisted living facility, childcare centre or workplace.
Eight systematic reviews without meta-analyses were graded critically low quality [26–31, 33, 34] and there is consensus among these studies, reporting that facemasks, either with or without hand hygiene, and the use of respirators may be effective in preventing influenza transmission or SARS-CoV-2 transmission [28, 29, 31, 32, 34, 35]. This benefit is thought to be most pronounced if used promptly and by sick patients as a form of source control [29, 31]. Individuals with the common cold may find benefit with hypertonic saline gargles and nasal washes in reducing viral transmission [26]. Finally, pre-emptive and planned school closures may help in curtailing influenza transmission, but the evidence was less clear-cut with regard to reactive school closures [30].
Meta-analysis without systematic review
There was only one study that conducted a meta-analysis without a systematic review and was graded as critically low quality [36] (table 4). Aiello et al. [36] examined the use of various hand hygiene interventions such as hand washing with antibacterial or normal soaps, hand sanitisers and education in school or daycare settings. The sum of all hand hygiene interventions was effective in reducing the risk of respiratory illness by 21% (95% CI 5–34%). Either antibacterial soap (rate ratio 0.5, 95% CI 0.4–0.61) or non-antibacterial soap (rate ratio 0.49, 95% CI 0.4–0.61) in conjunction with hygiene education appeared to be the most effective method of preventing respiratory illness. Among solutions not requiring running water, benzalkonium chloride-based hand sanitiser was most effective (rate ratio 0.6, 95% CI 0.45–0.81) [36].
TABLE 4.
Meta-analyses only
| First author, year [ref.] | Relevant/total studies (n/n) | Population | Intervention | Outcome | Findings |
| Critically low quality | |||||
| Aiello, 2008 [36] | 13/30 | School Childcare centre General community# |
Hand hygiene | Reported or diagnosed respiratory illness | All hand hygiene interventions versus no intervention leads to 21% reduction in respiratory illness (95% CI 5–34%) Benzalkonium chloride-based hand sanitiser is 40% effective versus no intervention Antimicrobial soap with education is the best method |
#: general community settings refer to all other community-based settings not fitting into any of the major categories such as school, household, assisted living facility, childcare centre or workplace.
The composite of all the studies, interventions and their direction of effect can be found in table 5.
TABLE 5.
Summary of study findings for reducing respiratory viral transmission
| First author, year [ref.] | Study type | AMSTAR2 rating | Facemask | Facemask+hand hygiene | Hand hygiene | Social distancing | School closure | Oral hygiene, gargle, or nasal wash |
| Chu, 2020 [14] | SR+MA | L | Probable or confirmed SARS-CoV-1 relative risk 0.56 (0.40–0.79) | n/a | n/a | n/a | n/a | n/a |
| Jefferson, 2020 [10] | SR+MA | L | n/a | ILI risk ratio 1.03 (0.77–1.37) Laboratory-confirmed influenza risk ratio 0.99 (0.69–1.36) |
Composite risk ratio 0.89 (0.84–0.95) ARI risk ratio 0.84 (0.82–0.86) ILI and laboratory-confirmed influenza risk ratio 0.91 (0.63–1.30) Rate of sick leave risk ratio 0.64 (0.58–0.71) |
n/a | n/a | Risk of viral illness risk ratio 0.91 (0.63–1.31) |
| Wong, 2014 [15] | SR+MA | L | n/a | ILI risk ratio 0.73 (0.6–0.89) | ILI risk ratio 0.86 (0.71–1.04) Laboratory-confirmed influenza risk ratio 0.90 (0.67–1.20) |
n/a | n/a | n/a |
| Abdullahi, 2020 [16] | SR+MA | CL | SARS and influenza spread risk ratio 0.78 (0.36–1.67) | Influenza spread risk ratio 0.94 (0.58–1.54) | SARS and influenza spread risk ratio 0.95 (0.83–1.05) | + | n/a | n/a |
| Aggarwal, 2020 [17] | SR+MA | CL | n/a | ILI effect size# −0.09 (−0.58–0.4) | ILI effect size# −0.17 (−0.43–0.10) | n/a | n/a | n/a |
| Gera, 2018 [18] | SR+MA | CL | n/a | n/a | ARI risk ratio 0.76 (0.59–0.98) Laboratory-confirmed influenza risk ratio 0.5 (0.41–0.62) School sick leave risk ratio 0.78 (0.76–0.8) |
n/a | n/a | n/a |
| Liang, 2020 [19] | SR+MA | CL | Viral infection OR 0.53 (0.36–0.79) | n/a | n/a | n/a | n/a | n/a |
| Rabie, 2006 [20] | SR+MA | CL | n/a | n/a | Respiratory infection relative risk 0.76 (0.6–0.96) | n/a | n/a | n/a |
| Rainwater-Lovett, 2014 [21] | SR+MA | CL | n/a | Influenza A or B attack rate OR 0.63 (0.33–1.19)¶ | n/a | Influenza A or B attack rate OR 1.31 (0.78–2.18) | n/a | n/a |
| Wang, 2020 [22] | SR+MA | CL | ARI OR 0.96 (0.8–1.15) Laboratory-confirmed viral infection OR 0.82 (0.63–1.07) Self-reported and clinically diagnosed ARI OR 1.1 (0.84–1.45) |
n/a | n/a | n/a | n/a | n/a |
| Xiao, 2020 [23] | SR+MA | CL | Laboratory-confirmed influenza risk ratio 0.78 (0.51–1.20) | Laboratory-confirmed influenza risk ratio 0.91 (0.73–1.13) | n/a | n/a | n/a | n/a |
| McGuinness, 2018 [24] | SR | L | n/a | n/a | ++ | n/a | n/a | n/a |
| Moncion, 2019 [25] | SR | L | n/a | n/a | + | n/a | n/a | n/a |
| Smith, 2015 [26] | SR | L | n/a§ | n/a§ | ++ | n/a | n/a | ++ |
| Willmott, 2016 [27] | SR | L | n/a | n/a | ++ | n/a | n/a | n/a |
| Chou, 2020 [28] | SR | CL | − | n/a | n/a | n/a | n/a | n/a |
| Cowling, 2010 [29] | SR | CL | + | n/a | n/a | n/a | n/a | n/a |
| Fong, 2020 [30] | SR | CL | n/a | n/a | n/a | n/a | ++ | n/a |
| MacIntyre, 2020 [31] | SR | CL | ++ | ++ | n/a | n/a | n/a | n/a |
| Mbakaya, 2017 [32] | SR | CL | n/a | n/a | ++ | n/a | n/a | n/a |
| Singh, 2020 [33] | SR | CL | n/a | n/a | n/a | n/a | n/a | + |
| Wang, 2017 [34] | SR | CL | n/a | n/a | + | n/a | n/a | n/a |
| Warren-Gash, 2013 [35] | SR | CL | n/a | n/a | ++ | n/a | n/a | n/a |
| Aeillo, 2008 [36] | MA | CL | n/a | n/a | Reduction in respiratory illness 21% (5–34%) | n/a | n/a | n/a |
Includes study type and AMSTAR (A Measurement Tool to Assess Systematic Reviews) 2 rating. Data are presented as ratio value (95% CI), unless otherwise stated. SR: systematic review; MA: meta-analysis; SARS-CoV: severe acute respiratory syndrome coronavirus; n/a: not applicable; ILI: influenza-like illness; ARI: acute respiratory illness; L: low quality; CL: critically low quality; ++: findings suggest positive effect; +: findings suggest potential positive effect; −: findings suggest no effect/neutral effect. #: effect size=log(OR); ¶: personal protective equipment as an intervention was categorised as masks+hand hygiene; §: no clear summary statement and not able to fully assess, because of methodological flaws in studies included in systematic review.
Discussion
Our data represent a broad overview of the systematic review and meta-analysis evidence on NPIs for the prevention of respiratory illnesses. We included studies of various community settings, qualities and study questions to explore large quantities of evidence evaluating the effectiveness of various NPIs for the prevention of respiratory viral illness. Most importantly, the strength of this study is derived from the inclusion and synthesis of results from a large number of systematic reviews, with or without meta-analyses, offering pertinent quantitative and qualitative evidence.
The paucity of rigorous studies in the community setting makes it difficult to draw definitive conclusions on the effectiveness of various NPIs. The systematic review and meta-analysis data offer the strongest support for hand hygiene. Although overall the data are in support of an effect of facemask use to reduce respiratory viral transmission in the community, data are inconsistent. Taken in context with high heterogeneity in methodology, respiratory viruses, mask type and outcomes, it is unreasonable to estimate an overall effect. For instance, there are several less standardised outcomes reported by systematic review and meta-analyses, including “reduction in respiratory illness” [36], “influenza A or B attack rate” [21], “viral infection” [19], “SARS and influenza spread” [16], “respiratory infection” [20], “effect size” [17], “probable/confirmed SARS-CoV-1” [14] or a composite outcome of the aforementioned outcomes [10]. Current evidence supports the use of hand hygiene preferably with facemask use in the community setting to reduce the risk of contracting or spreading respiratory viral illness. Facemask practices should be implemented as early as possible as to reduce the risk of asymptomatic transmission for both wearers and their contacts and should be worn with good technique and compliance. Less frequently studied measures such as pre-emptive school closures and nasopharyngeal saline rinses may also play a role in the reduction of influenza transmission.
Recently, a cluster-randomised study comparing the impact of mask use on COVID-19 seroprevalence in a true community setting offered support for the use of facemasks. Abaluck et al. [37] compared an intervention arm where either cloth or surgical masks were used along with education on proper use, to a control group of no intervention. The intervention acheived a relative reduction of symptomatic seroprevalence by 9.5% from 0.76% to 0.68% (p=0.03). More specifically, groups randomised to surgical masks had an 11.1% relative reduction in symptomatic seroprevalence (0.81% to 0.72%), whereas cloth masks did not significantly reduce symptomatic seroprevalence (0.67% to 0.61%). This further adds to the evidence base that suggests a protective effect of masks in the community and is consistent with conclusions derived from our research. Where vaccination rates have yet to reach a satisfactory level, such as those in low to middle income countries, mask wearing continues to play an instrumental role in preventing the spread of COVID-19 [38].
Respiratory droplets and aerosols are the major routes of transmission for respiratory viruses such as influenza and SARS-CoV-2 [39]. Studies have reported presence of viral particles on surfaces in the vicinity of COVID-19-positive patients, which can remain viable for several days depending on the surface material [40]. Hence, the efficacy of hand hygiene is directly related to its ability to eliminate pathogens present on surfaces, bodily fluids or secretions that one comes into contact with prior to inoculation via mucous membranes in the nose and eyes [41]. The efficacy of masks is from their ability to filter droplets (>5–10 μm diameter), which are created by forceful expiratory manoeuvres such as sneezing, coughing and talking, and aerosols (<5–10 μm) [42, 43]. Surgical masks undergo a series of tests regulated by the American Society for Testing and Materials including two measuring filtration efficiencies. The bacterial filtration efficiency test uses aerosols that are 3.0 μm in diameter, which requires a minimum of 95% filtration efficiency, and particulate filtration efficiency test uses 0.1 μm charged latex sphere aerosols [44]. Cloth facemasks are not subject to the same rigorous testing standards, leaving greater tolerance for filtration efficacy, which may range between 10% and 26% when evaluated with National Institute for Occupational Safety and Health testing standards [45]. Given that the modality of transmission of SARS-CoV-2 is primarily through respiratory droplets and aerosols, it is reasonable to expect that facemasks would provide a protective effect [46]. The inconsistent evidence for mask interventions could be attributed to heterogeneity in virus studied, poor compliance with the intervention, crossover of interventions, improper technique and imprecise estimates, which was suggested by several systematic reviews and meta-analyses [16, 24, 28, 35].
Mouthwashes and nasal rinses have been recommended less frequently for the prevention of COVID-19. They function to reduce viral load following contact with mucous membranes and may demonstrate some efficacy through secondary prevention [47]. Social distancing and school closures are upstream measures to avoid exposure to contagious contacts and thus prevent viral transmission. Consequently, they are both expected to decrease transmission, although the evidence appears to suggest a benefit of school closures as opposed to physical distancing [16, 21]. However, for both interventions, there were limited, poor-quality systematic review and meta-analysis data available and we encourage further research to elucidate the efficacy of these measures.
The limitations of our study include the difficulty of drawing conclusions on a small number of systematic reviews and meta-analyses on individual NPIs such as nasal rinses, hypertonic saline gargles, social distancing and school closures. Furthermore, due to the substantial heterogeneity and low-quality evidence in the literature in terms of the implementation of NPIs, the viral agents in question and the definition of outcomes, we did not feel that we could provide a reasonable, rigorous pooled estimate of the systematic reviews. Such estimates may be inappropriate, due to the presence of 25 studies [48–72] that have appeared more than once among the systematic reviews and meta-analyses and the sole meta-analysis. Although the aforementioned limitations make it difficult to draw conclusions about effectiveness of NPIs, a key aim of our article is to address and present this heterogeneity in the literature through a narrative synthesis, in order to guide future studies about NPIs.
At the time of writing, there was limited evidence addressing the effectiveness of NPIs in the prevention of SARS-CoV-2 transmission. However, with additional consideration of experimental data and evidence of behaviour of other respiratory viruses, such as SARS-CoV, influenza and Middle East respiratory syndrome-related coronavirus, we believe that these data are useful in the current pandemic and in support of NPIs for the community setting. As the emerging omicron variant continues to spread rapidly and COVID-19 cases worldwide exceed 400 million [73], the use of NPIs in the community setting should take on greater importance.
Conclusion
Systematic reviews and meta-analyses assessing NPIs are of mostly low and critically low quality. Hand hygiene appears to be effective in decreasing viral transmission according to most systematic reviews and meta-analyses (10 studies with positive or potentially positive findings [18, 20, 22, 24–27, 32, 35, 36]; three studies without positive findings [15–17]; one study with findings dependent on outcome [10]), while evidence supporting the effectiveness of facemasks appears to be more heterogeneous (four studies with positive or potentially positive findings [14, 19, 29, 31] and three studies without positive findings [16, 22, 23, 28]). Additionally, the combination of facemasks and hand hygiene together appears to provide no additional benefit, which could be explained by fewer systematic reviews and meta-analyses evaluating this specific NPI (two studies with positive findings [15, 31] and five studies without positive findings [10, 165, 17, 21, 23]). Other NPIs studied included school closures, which may be beneficial, and social distancing, for which there is uncertainty of its benefit based on the current literature.
In summary, umbrella review data point to hand hygiene and facemasks each as individual methods to prevent respiratory viral transmission. Future studies should focus on assessing the effectiveness of NPIs in true community settings and use standardised illness outcomes, which improves interpretation and provides greater generalisability to guide public health recommendations.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00650-2021.SUPPLEMENT (645.2KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Data availability: Source data extracted from reviews are available upon request.
Conflict of interest: There is no conflict of interest to report.
References
- 1.Li Q, Guan X, Wu P, et al. . Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 2020; 382: 1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauer SA, Grantz KH, Bi Q, et al. . The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172: 577–582. doi: 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X, Lau EHY, Wu P, et al. . Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26: 672–675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . How to Protect Yourself & Others. 2021. www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html/ Date last accessed: 24 July 2021. Date last updated: 11 June 2021.
- 6.World Health Organization . Advice for the Public. Coronavirus Disease (COVID-19). 2021. www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/ Date last accessed: 24 July 2021. Date last updated: 1 July 2021.
- 7.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane, 2021. www.training.cochrane.org/handbook/ [Google Scholar]
- 9.Jefferson T, Foxlee R, Del Mar C, et al. . Interventions for the interruption or reduction of the spread of respiratory viruses. Cochrane Database Syst Rev 2007; 4: CD006207. doi: 10.1002/14651858.CD006207.pub2 [DOI] [PubMed] [Google Scholar]
- 10.Jefferson T, Del Mar CB, Dooley L, et al. . Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev 2020; 11: CD006207. doi: 10.1002/14651858.CD006207.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American University of Beirut . Systematic Reviews: Health Sciences. 2021. https://aub.edu.lb.libguides.com/SML-Systematic_Review/ Date last accessed: 24 July 2021.
- 12.Shea BJ, Reeves BC, Wells G, et al. . AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieper D, Puljak L, González-Lorenzo M, et al. . Minor differences were found between AMSTAR 2 and ROBIS in the assessment of systematic reviews including both randomized and nonrandomized studies. J Clin Epidemiol 2019; 108: 26–33. doi: 10.1016/j.jclinepi.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 14.Chu DK, Akl EA, Duda S, et al. . Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020; 395: 1973–1987. doi: 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong VWY, Cowling BJ, Aiello AE. Hand hygiene and risk of influenza virus infections in the community: a systematic review and meta-analysis. Epidemiol Infect 2014; 142: 922–932. doi: 10.1017/S095026881400003X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdullahi L, Onyango JJ, Mukiira C, et al. . Community interventions in low-and middle-income countries to inform COVID-19 control implementation decisions in Kenya: a rapid systematic review. PLoS One 2020; 15: e0242403. doi: 10.1371/journal.pone.0242403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal N, Dwarakanathan V, Gautam N, et al. . Facemasks for prevention of viral respiratory infections in community settings: a systematic review and meta-analysis. Indian J Public Health 2020; 64: S192–S200. doi: 10.4103/ijph.IJPH_470_20 [DOI] [PubMed] [Google Scholar]
- 18.Gera T, Shah D, Sachdev HS. Impact of water, sanitation and hygiene interventions on growth, non-diarrheal morbidity and mortality in children residing in low- and middle-income countries: a systematic review. Indian Pediatr 2018; 55: 381–393. doi: 10.1007/s13312-018-1279-3 [DOI] [PubMed] [Google Scholar]
- 19.Liang M, Gao L, Cheng C, et al. . Efficacy of face mask in preventing respiratory virus transmission: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 36: 101751. doi: 10.1016/j.tmaid.2020.101751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabie T, Curtis V. Handwashing and risk of respiratory infections: a quantitative systematic review. Trop Med Int Health 2006; 11: 258–267. doi: 10.1111/j.1365-3156.2006.01568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainwater-Lovett K, Chun K, Lessler J. Influenza outbreak control practices and the effectiveness of interventions in long-term care facilities: a systematic review. Influenza Other Respir Viruses 2014; 8: 74–82. doi: 10.1111/irv.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang MX, Gwee SXW, Chua PEY, et al. . Effectiveness of surgical face masks in reducing acute respiratory infections in non-healthcare settings: a systematic review and meta-analysis. Front Med 2020; 7: 564280. doi: 10.3389/fmed.2020.564280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao J, Shiu EYC, Gao H, et al. . Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings – personal protective and environmental measures. Emerg Infect Dis 2020; 26: 967–975. doi: 10.3201/eid2605.190994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuinness SL, Barker SF, O'Toole J, et al. . Effect of hygiene interventions on acute respiratory infections in childcare, school and domestic settings in low- and middle-income countries: a systematic review. Trop Med Int Health 2018; 23: 816–833. doi: 10.1111/tmi.13080 [DOI] [PubMed] [Google Scholar]
- 25.Moncion K, Young K, Tunis M, et al. . Effectiveness of hand hygiene practices in preventing influenza virus infection in the community setting: a systematic review. Can Commun Dis Rep 2019; 45: 12–23. doi: 10.14745/ccdr.v45i01a02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SMS, Sonego S, Wallen GR, et al. . Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology 2015; 20: 896–903. doi: 10.1111/resp.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willmott M, Nicholson A, Busse H, et al. . Effectiveness of hand hygiene interventions in reducing illness absence among children in educational settings: a systematic review and meta-analysis. Arch Dis Child 2016; 101: 42–50. doi: 10.1136/archdischild-2015-308875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou R, Dana T, Jungbauer R, et al. . Masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings: a living rapid review. Ann Intern Med 2020; 173: 542–555. doi: 10.7326/M20-3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowling BJ, Zhou Y, Ip DK, et al. . Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect 2010; 138: 449–456. doi: 10.1017/S0950268809991658 [DOI] [PubMed] [Google Scholar]
- 30.Fong MW, Gao H, Wong JY, et al. . Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings – social distancing measures. Emerg Infect Dis 2020; 26: 976–984. doi: 10.3201/eid2605.190995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud 2020; 108: 103629. doi: 10.1016/j.ijnurstu.2020.103629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mbakaya BC, Lee PH, Lee RLT. Hand hygiene intervention strategies to reduce diarrhoea and respiratory infections among schoolchildren in developing countries: a systematic review. Int J Environ Res Public Health 2017; 14: 371. doi: 10.3390/ijerph14040371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S, Sharma N, Singh U, et al. . Nasopharyngeal wash in preventing and treating upper respiratory tract infections: could it prevent COVID-19? Lung India 2020; 37: 246–251. doi: 10.4103/lungindia.lungindia_241_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Lapinski M, Quilliam E, et al. . The effect of hand-hygiene interventions on infectious disease-associated absenteeism in elementary schools: a systematic literature review. Am J Infect Control 2017; 45: 682–689. doi: 10.1016/j.ajic.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 35.Warren-Gash C, Fragaszy E, Hayward AC. Hand hygiene to reduce community transmission of influenza and acute respiratory tract infection: a systematic review. Influenza Other Respir Viruses 2013; 7: 738–749. doi: 10.1111/irv.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aiello AE, Coulborn RM, Perez V, et al. . Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health 2008; 98: 1372–1381. doi: 10.2105/AJPH.2007.124610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abaluck J, Kwong LH, Styczynski A, et al. . The impact of community masking on COVID-19: a cluster-randomized trial in Bangladesh. Science 2022; 375: eabi9069. doi: 10.1126/science.abi9069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathieu E, Ritchie H, Ortiz-Ospina E, et al. . A global database of COVID-19 vaccinations. Nat Hum Behav 2021; 5: 947–953. doi: 10.1038/s41562-021-01122-8 [DOI] [PubMed] [Google Scholar]
- 39.Leung NHL, Chu DKW, Shiu EYC, et al. . Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26: 676–680. doi: 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Doremalen N, Bushmaker T, Morris DH, et al. . Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020; 382: 1564–1567. doi: 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention . Show Me the Science – Why Wash Your Hands? 2020. www.cdc.gov/handwashing/why-handwashing.html/ Date last accessed: 6 January 2022. Date last updated: 10 September 2020.
- 42.Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med 2020; 8: 914–924. doi: 10.1016/S2213-2600(20)30323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stadnytskyi V, Bax CE, Bax A, et al. . The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci USA 2020; 117: 11875–11877. doi: 10.1073/pnas.2006874117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.PRI·MED Medical Products . Mask Protection Standards & Medical Face Mask Information for Use. www.primed.com/resources/astm-mask-protection-standards/ Date last accessed: 14 February 2022.
- 45.Rengasamy S, Eimer B, Shaffer RE. Simple respiratory protection – evaluation of the filtration performance of cloth masks and common fabric materials against 20–1000 nm size particles. Ann Occup Hyg 2010; 54: 789–798. doi: 10.1093/annhyg/meq044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Government of Canada . Update with Consideration of Omicron – Interim COVID-19 Infection Prevention and Control in the Health Care Setting When COVID-19 is Suspected or Confirmed – December 23, 2021. www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/omicron-infection-prevention-control-health-care-settings-covid-19-suspected-confirmed.html/ Date last accessed: 6 January 2022. Date last updated: 29 December 2021.
- 47.Carrouel F, Gonçalves LS, Conte MP, et al. . Antiviral activity of reagents in mouth rinses against SARS-CoV-2. J Dent Res 2021; 100: 124–132. doi: 10.1177/0022034520967933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aiello AE, Murray GF, Perez V, et al. . Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. J Infect Dis 2010; 201: 491–498. doi: 10.1086/650396 [DOI] [PubMed] [Google Scholar]
- 49.Aiello AE, Perez V, Coulborn RM, et al. . Facemasks, hand hygiene, and influenza among young adults: a randomized intervention trial. PLoS One 2012; 7: e29744. doi: 10.1371/journal.pone.0029744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barasheed O, Almasri N, Badahdah A-M, et al. . Pilot randomised controlled trial to test effectiveness of facemasks in preventing influenza-like illness transmission among Australian Hajj pilgrims in 2011. Infect Disord Drug Targets 2014; 14: 110–116. doi: 10.2174/1871526514666141021112855 [DOI] [PubMed] [Google Scholar]
- 51.Correa JC, Pinto D, Salas LA, et al. . A cluster-randomized controlled trial of handrubs for prevention of infectious diseases among children in Colombia. Rev Panam Salud Publica 2012; 31: 476–484. doi: 10.1590/S1020-49892012000600005 [DOI] [PubMed] [Google Scholar]
- 52.Cowling BJ, Chan K-H, Fang VJ, et al. . Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med 2009; 151: 437–446. doi: 10.7326/0003-4819-151-7-200910060-00142 [DOI] [PubMed] [Google Scholar]
- 53.Cowling BJ, Fung ROP, Cheng CKY, et al. . Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS One 2008; 3: e2101. doi: 10.1371/journal.pone.0002101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyer DL, Shinder A, Shinder F. Alcohol-free instant hand sanitizer reduces elementary school illness absenteeism. Fam Med 2000; 32: 633–638. [PubMed] [Google Scholar]
- 55.Hübner N-O, Hübner C, Wodny M, et al. . Effectiveness of alcohol-based hand disinfectants in a public administration: impact on health and work performance related to acute respiratory symptoms and diarrhoea. BMC Infect Dis 2010; 10: 250. doi: 10.1186/1471-2334-10-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larson EL, Ferng Y-H, Wong-McLoughlin J, et al. . Impact of non-pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep 2010; 125: 178–191. doi: 10.1177/003335491012500206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lau JTF, Lau M, Kim JH, et al. . Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis 2004; 10: 235–243. doi: 10.3201/eid1002.030626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luby SP, Agboatwalla M, Feikin DR, et al. . Effect of handwashing on child health: a randomised controlled trial. Lancet 2005; 366: 225–233. doi: 10.1016/S0140-6736(05)66912-7 [DOI] [PubMed] [Google Scholar]
- 59.MacIntyre CR, Cauchemez S, Dwyer DE, et al. . Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis 2009; 15: 233–241. doi: 10.3201/eid1502.081166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicholson JA, Naeeni M, Hoptroff M, et al. . An investigation of the effects of a hand washing intervention on health outcomes and school absence using a randomised trial in Indian urban communities. Trop Med Int Health 2014; 19: 284–292. doi: 10.1111/tmi.12254 [DOI] [PubMed] [Google Scholar]
- 61.Niffenegger JP. Proper handwashing promotes wellness in child care. J Pediatr Health Care 1997; 11: 26–31. doi: 10.1016/S0891-5245(97)90141-3 [DOI] [PubMed] [Google Scholar]
- 62.Ram PK, DiVita MA, Khatun-e-Jannat K, et al. . Impact of intensive handwashing promotion on secondary household influenza-like illness in rural Bangladesh: findings from a randomized controlled trial. PLoS One 2015; 10: e0125200. doi: 10.1371/journal.pone.0125200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts L, Smith W, Jorm L, et al. . Effect of infection control measures on the frequency of upper respiratory infection in child care: a randomized, controlled trial. Pediatrics 2000; 105: 738–742. doi: 10.1542/peds.105.4.738 [DOI] [PubMed] [Google Scholar]
- 64.Sandora TJ, Taveras EM, Shih M-C, et al. . A randomized, controlled trial of a multifaceted intervention including alcohol-based hand sanitizer and hand-hygiene education to reduce illness transmission in the home. Pediatrics 2005; 116: 587–594. doi: 10.1542/peds.2005-0199 [DOI] [PubMed] [Google Scholar]
- 65.Simmerman JM, Suntarattiwong P, Levy J, et al. . Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. Influenza Other Respir Viruses 2011; 5: 256–267. doi: 10.1111/j.1750-2659.2011.00205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stebbins S, Cummings DAT, Stark JH, et al. . Reduction in the incidence of influenza A but not influenza B associated with use of hand sanitizer and cough hygiene in schools: a randomized controlled trial. Pediatr Infect Dis J 2011; 30: 921–926. doi: 10.1097/INF.0b013e3182218656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suess T, Remschmidt C, Schink SB, et al. . The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009–2011. BMC Infect Dis 2012; 12: 26. doi: 10.1186/1471-2334-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talaat M, Afifi S, Dueger E, et al. . Effects of hand hygiene campaigns on incidence of laboratory-confirmed influenza and absenteeism in schoolchildren, Cairo, Egypt. Emerg Infect Dis 2011; 17: 619–625. doi: 10.3201/eid1704.101353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White C, Kolble R, Carlson R, et al. . The effect of hand hygiene on illness rate among students in university residence halls. Am J Infect Control 2003; 31: 364–370. doi: 10.1016/S0196-6553(03)00041-5 [DOI] [PubMed] [Google Scholar]
- 70.White CG, Shinder FS, Shinder AL, et al. . Reduction of illness absenteeism in elementary schools using an alcohol-free instant hand sanitizer. J Sch Nurs 2001; 17: 258–265. [PubMed] [Google Scholar]
- 71.Wu J, Xu F, Zhou W, et al. . Risk factors for SARS among persons without known contact with SARS patients, Beijing, China. Emerg Infect Dis 2004; 10: 210–216. doi: 10.3201/eid1002.030730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L, Peng Z, Ou J, et al. . Protection by face masks against influenza A(H1N1)pdm09 virus on trans-Pacific passenger aircraft, 2009. Emerg Infect Dis 2013; 19: 1403–1410. doi: 10.3201/eid1909.121765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization (WHO) . WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ Date last accessed: 14 February 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00650-2021.SUPPLEMENT (645.2KB, pdf)