SUMMARY
Following the introduction of vaccination against Haemophilus influenzae type b (Hib), cases of invasive encapsulated Hib disease have decreased markedly. This study aimed to examine subsequent epidemiological trends in invasive H. influenzae disease in Queensland, Australia and in particular, assess the clinical impact and public health implications of invasive non-typable H. influenzae (NTHi) strains. A multicentre retrospective study was conducted from July 2000 to June 2013. Databases of major laboratories in Queensland including Queensland Forensic and Scientific Services (jurisdictional referral laboratory for isolate typing) were examined to identify cases. Demographic, infection site, Indigenous status, serotype, and mortality data were collected. In total, 737 invasive isolates were identified, of which 586 (79·5%) were serotyped. Hib, NTHi and encapsulated non-b strains, respectively, constituted 12·1%, 69·1% and 18·8% of isolates. The predominant encapsulated non-b strains were f (45·5%) and a (27·3%) serotypes. Of isolates causing meningitis, 48·9% were NTHi, 14·9% Hib, 14·9% Hie, 10·6% Hif, 6·4% Hia and 4·3% were untyped. During the study period, there was an increase in the incidence of invasive NTHi disease (P = 0·007) with seasonal peaks in winter and spring (P < 0·001). The incidence of Hib disease (P = 0·295) and of encapsulated non-b disease (P = 0·122) did not change significantly. Highest overall incidence was in infants, Indigenous, and elderly patients. Australian Indigenous patients were more likely to have Hia (P > 0·001) and Hib (P = 0·039) than non-Indigenous patients. In Queensland, invasive H. influenzae disease is now predominantly encountered in adults and most commonly caused by NTHi strains with demonstrated pathogenicity extending to otherwise young or immunocompetent individuals. Routine public health notification of these strains is recommended and recent available immunization options should be considered.
Key words: Encapsulated non-b Haemophilus influenzae, Hib, invasive H. influenzae, non-Hib, non-typable H. influenzae
INTRODUCTION
Haemophilus influenzae b (Hib) is the most virulent of H. influenzae capsular strains [1] and causes life-threatening diseases such as epiglottitis, meningitis, septic arthritis and sepsis [2]. The capsular non-b strains are relatively rare but can also cause invasive disease with similar clinical manifestations [3, 4]. Non-capsulated or non-typable strains (NTHi) colonize the upper respiratory tract of up to 65% of healthy pre-school children [5] and have been implicated in mucosal infections such as acute otitis media, sinusitis and pneumonia in children [3, 6]. They can also cause opportunistic infections in patients who are aged >65 years, immunosuppressed or have a chronic respiratory disorder [3, 5, 7].
Prior to the introduction of conjugate Hib vaccines, invasive Hib disease occurred at an annual rate of 39–63 cases/100 000 Australian children aged <5 years, with much higher rates reported in the Northern Territory [8]. Hib meningitis comprised 60% of all invasive Hib cases (about 500 cases/year) and in infants aged <18 months with Hib meningitis, the case-fatality rate was 5% [9]. Aboriginal and Torres Strait Islander (ATSI) children presented earlier and had a much higher annual incidence of Hib meningitis with rates exceeding 200 cases/100 000 children. Other manifestations such as bacteraemia and septic arthritis occurred at an annual rate of 99 and 33/100 000 Indigenous children and 9 and 7/100 000 for non-Indigenous children [2].
The introduction of Hib vaccine to the national immunization programme in Australia in 1993 resulted in a marked decline in Hib disease [10]. From 1996 to 2000, the average annual incidence rate of invasive Hib disease decreased to 1·7 cases/100 000 children aged <5 years – a reduction of 87–95%, most markedly seen in ATSI children [1]. Hib epiglottitis and meningitis in children are now rare [8]. Currently, only 15–20 cases of Hib meningitis occur per year nationwide [9]. However, worldwide, rising rates of invasive NTHi infections have been reported [5] and a reversal in the ratio of Hib to non-Hib disease has been observed in a number of countries [11–13] with a shift in the ratio of adult to paediatric disease. NTHi strains now occur more commonly in patients aged >65 years [3–5] and result in higher mortality in that age group [7, 13]. While both invasive Hib and non-Hib disease including NTHi form part of the National Notifiable Diseases in other developed countries, only invasive Hib disease is reportable to the Public Health Department in Australia [14–17]. The aims of the study were to examine the epidemiological trends for invasive H. influenzae disease in Queensland for 13 years, from July 2000 to June 2013, and, in particular, to assess the implications of invasive NTHi strains for clinical practice and public health.
METHODS
Study design and study population
A multi-centre retrospective analysis of the data available from Queensland information databases of laboratory-confirmed cases of invasive H. influenzae infection was conducted. The participating Pathology sites included three of the largest pathology laboratories in the State: Pathology Queensland – the Queensland Central Laboratory, Sullivan Nicolaides Pathology and Mater Pathology. Specimens were received from 100 medical facilities across Queensland serviced by both private and public laboratories. The latest census in Queensland reported an estimated population of 4658557 including 188954 ATSI people [18].
Inclusion criteria and definitions
A laboratory-confirmed case of invasive H. influenzae infection was defined as isolation of the organism by culture from a normally sterile body site. The clinical diagnoses of bacteraemia, meningitis, septic arthritis, empyema, endophthalmitis, pericarditis, and peritonitis were assigned if H. influenzae was isolated from blood, cerebrospinal fluid, synovial fluid, pleural tissue, vitreous, pericardial, and peritoneal fluids, respectively.
Exclusion criteria
Isolates from wound swabs, ear/nose/throat specimens, sputum, bronchoalveolar lavage and chest drain fluids were considered to represent non-sterile sites. All pleural fluids were excluded as it was not possible to determine whether they were obtained by sterile technique or whether they were collected directly from a chest drain. If there was more than one positive isolate from the same site, this was counted only once.
Data collection
As Hib is a notifiable disease in Queensland, the majority of invasive H. influenzae isolates were forwarded to Queensland Health Forensic Scientific Services Reference Laboratory (QFSS) for serotyping. Documented clinical notes on laboratory databases, as provided by clinicians, were reviewed to determine the clinical presentation associated with bacteraemias.
Serotyping
At QFSS, the phenotypic identification of the isolate was confirmed with the RapidNH kit (Remel, USA). The isolate was serotyped by slide agglutination with two H. influenzae types a–f specific antisera commercial kits (Denka Seiken, Japan and Phadebact, Bactus AB, Sweden). The isolates were either untyped or classified into serotypes a–f or NT.
All isolates were screened for the capsular gene region specific to encapsulated Hib and the outer membrane protein P6 present in all H. influenzae strains using the forward primers 5’-GCG AAA GTG AAC TCT TAT CTC TC-3’ and 5’-TTG GCG GWT ACT CTG TTG CT-3’ and the reverse primers 3’-GCT TAC GCT TCT ATC TCG GTG AA-5’ and 3’-TGC AGG TTT TTC TTC ACC GT-5’, respectively [19, 20]. Previous auditing at QFSS did not justify extending the use of polymerase chain reaction (PCR) to capsular non-b serotypes as no misidentifications by slide agglutination had been detected.
Clinical syndromes
Neonatal sepsis was defined as sepsis within the first 28 days of life. Obstetric and gynaecological infections included chorioamnionitis, endometritis, pregnancy-associated sepsis, septic miscarriages, peri-partum sepsis and pelvic inflammatory disease. Mucosal disease included oropharyngeal involvement, sinusitis, conjunctivitis and otitis media. Soft tissue infections included cellulitis and wound infections. Sepsis was unspecified when the laboratory database did not have any documented source of infection.
Determination of beta-lactamase production
All laboratories used the nitrocefin or cefinase test, a colorimetric method for detecting the production of the beta-lactamase enzyme from each isolate. Beta-lactamase-negative and ampicillin-resistant (BLNAR) isolates were determined using CLSI breakpoints [21]. After 2011, Pathology Queensland and Sullivan Nicolaides Pathology also used EUCAST breakpoints [22].
Statistical analysis
Estimated annual population data for Queensland from 2000 to 2013 was obtained from the Australian Bureau of Statistics (ABS) and the Queensland population counter website [18, 23]. Estimated disease incidence was calculated from typed isolates and estimated population data as follows:
 |
Statistical analysis of data was performed using Stata/SE version 12.1 (StataCorp., USA). χ2 test, one-group/two-group proportion test and non-parametric trend test were used to evaluate differences or trends over time. A P value <0·05 was considered statistically significant. Logistic regression was used to determine factors influencing mortality.
Ethical considerations
All laboratory data was de-identified. The Royal Brisbane & Women's Hospital Human Research Ethics Committee (RBWH HREC) granted ethics approval for this multicentre study.
RESULTS
All isolates (Table 1)
Table 1.
Patients' characteristics
| Patient | Details (n, %) | ||||
|---|---|---|---|---|---|
| Gender | Females (392, 53·2%) | ||||
| Males (345, 46·8%) | |||||
| Total (737, 100·0%) | |||||
| Age range | Neonate to 100 years | ||||
| Clinical specimens | Blood (664, 90·1%) | ||||
| Cerebrospinal fluid (48, 6·5%) | |||||
| Synovial fluid (12, 1·6%) | |||||
| Other fluid* (13, 1·8%) | |||||
| Clinical syndromes | Hib | Non-b | NTHi | Untyped | Total |
| All syndromes | 71 | 110 | 405 | 151 | 737 |
| Sepsis – unspecified | 6 | 21 | 64 | 28 | 119 |
| Pneumonia ± empyema | 33 | 40 | 198 | 89 | 360 |
| Meningitis | 20 | 31 | 39 | 4 | 94 |
| Neonatal sepsis | 1 | 1 | 37 | 3 | 42 |
| O&G† infections | 0 | 0 | 24 | 8 | 32 |
| Septic arthritis | 3 | 10 | 10 | 3 | 26 |
| Mucosal infections | 0 | 4 | 15 | 5 | 24 |
| Neutropenic sepsis | 1 | 1 | 10 | 1 | 13 |
| Biliary infections | 0 | 1 | 5 | 4 | 10 |
| Urinary tract infections | 1 | 0 | 6 | 2 | 9 |
| Epiglottitis | 5 | 0 | 1 | 1 | 7 |
| Other infections‡ | 0 | 0 | 3 | 3 | 6 |
| Soft tissue infections | 1 | 1 | 3 | 0 | 5 |
Vitreous/pericardial/peritoneal/empyema fluid.
Obstetric and gynaecological.
Endophthalmitis, pericarditis, peritonitis.
A total of 737 clinically invasive H. influenzae isolates were identified. Of these, 609 (82·6%) came from Pathology Queensland, 62 (8·4%) from Mater Pathology, 58 (7·9%) from Sullivan Nicolaides Pathology and the remaining eight (1·1%) isolates from two other private laboratories. In total, 586 isolates were typed, of which 557 had been referred to QFSS and 29 had been serotyped in-house by slide agglutination only. There were 151 untyped isolates, of which 141 came from blood culture.
Clinical disease and trends
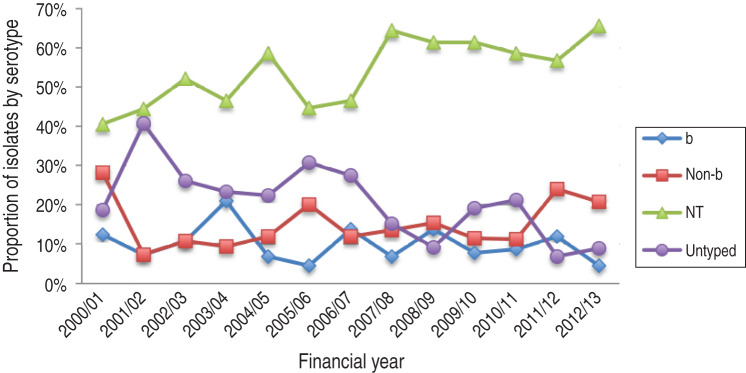
The three predominant clinical syndromes were pneumonia, meningitis and neonatal sepsis/obstetric/gynaecological disease at 48·8%, 12·8% and 8·7%, respectively. Of the meningitis cases 48·9% were attributable to NTHi, 14·9% to Hib, 14·9% to Hie, 10·6% to Hif, 6·4% to Hia and 4·3% to untyped isolates. Meningitis cases comprised of 38·3% infants, 21·3% children and 40·4% adults. Except for epiglottitis, all clinical syndromes were predominantly attributable to NTHi (Fig. 1).
Fig. 1.
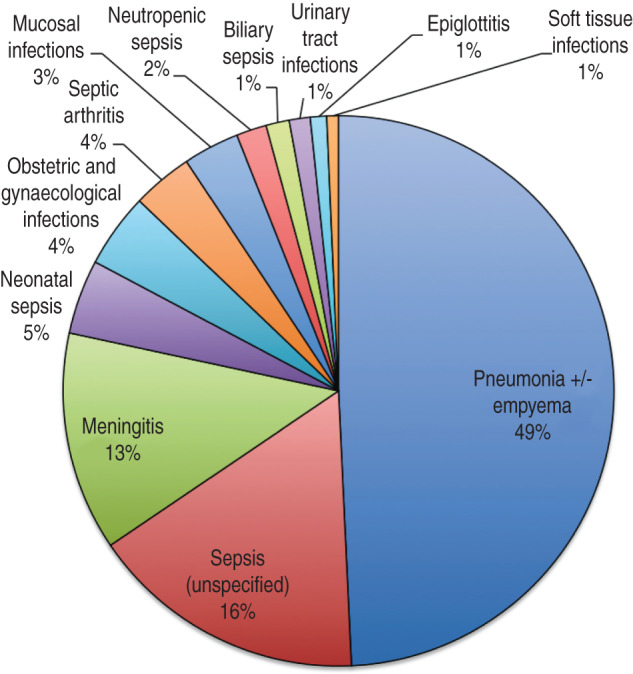
Clinical syndromes.
There was a predominance of invasive NTHi infections every year with significant increase in incidence over the study period (P = 0·007). There was a non-significant decreasing trend in invasive Hib disease (P = 0·295); the trend in encapsulated non-b disease incidence remained unchanged (P = 0·122). The overall trend of all invasive disease did not significantly increase over the study period (P = 0·757) (Fig. 2).
Fig. 2.
Overall trend of invasive disease over time.
Gender and age
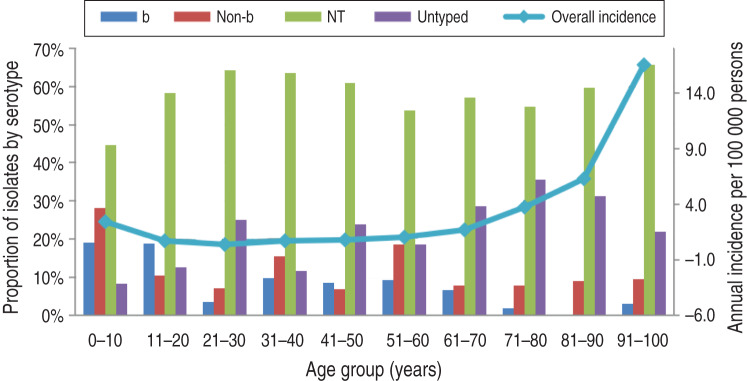
A significant increase in invasive disease incidence was observed with age, particularly in persons aged >60 years with the increase in proportion ascribed to NTHi disease (P = 0·028). Two peaks in annual disease incidence were seen: in infants and in adults aged >90 years at 14·8 and 16·5/10000 persons, respectively. In the 21–30 years age group, at least twice as many women as men had invasive disease with estimated annual incidences of 0·47/100000 for females compared to 0·26/100000 males.
Ethnicity
Ethnicity was disclosed for 572 cases; 15·9% (n = 91/572) were Indigenous.
Serotype distribution by age groups
NTHi was the predominant serotype across age groups (Fig. 3). There was a significant bias towards serotyping isolates from paediatric infections rather than adult infections (P < 0·001).
Fig. 3.
Serotypes across all age groups.
Serotype by ethnicity
Excluding untyped isolates, serotype distribution for the ATSI group was: Hia (20·9%), Hib (17·4%), Hic (4·7%), Hid (0%), Hie (2·3%), Hif (4·7%) and NTHi (50%). Corresponding serotype distribution for the non-Indigenous group was: 1·6%, 11·6%, 0%, 0·3%, 5%, 9·2%, and 72·4%, respectively. There was a significant association between Indigenous ethnicity and Hia (P < 0·001) and Hib (P = 0·039) disease compared with non-Indigenous ethnicity. ATSI and non-ATSI groups did not differ for NTHi (P = 0·089). Overall, ATSI patients were more likely to develop invasive disease than non-Indigenous patients (P< 0·001).
Beta-lactamase production
In total, 16·6% of the 737 isolates were beta-lactamase positive and there was no suggestion of an increasing trend from 2000 to 2013 (P = 0·260). Only 1·8% of the total isolates were BLNAR. Ampicillin minimum inhibitory concentrations (MICs) were reported in fewer than a quarter of the isolates. Due to the recent change in methodology from CLSI to EUCAST and related discrepancies in MIC assessment, BLNARs were not characterized further [21, 22].
Seasonality
Invasive NTHi disease was more common in winter and spring (P < 0·001) with peaks occurring in July, August and September. There was no seasonal variation with invasive encapsulated H. influenzae disease.
DISCUSSION
The overall incidence of invasive H. influenzae disease in Queensland did not increase significantly during the study period when untyped isolates were included. Of the typed isolates, there was a significant rise in NTHi disease, particularly with age, and in persons aged >60 years, as has been reported in other developed countries [7, 13, 24]. NTHi strains were associated with a wide spectrum of clinical presentations and propensity to disseminate to sites other than the central nervous system. NTHi and encapsulated non-b H. influenzae caused more meningitis and septic arthritis than Hib. Hypotheses to explain the predominance of invasive NTHi disease in developed countries include: (1) the increased opportunistic potential of NTHi strains in an ageing population, increased survival rates in premature babies, and more frequent haematological malignancies and immunosuppression due to long-term steroid use [25]; (2) the increase in nasopharyngeal carriage of NTHi strains as a result of effective vaccination programmes against Hib and Streptococcus pneumoniae [26].
In the 21–30 years age group, there were at least twice as many affected women as men. It has been suggested that disease severity and complications might be higher in women of childbearing age, with risks related to invasive gynaecological and obstetric infections caused by unusually virulent NTHi [11, 27]. The significant rate of neonatal sepsis (4·7%) in this study may also correlate with maternal sepsis/chorioamnionitis. In studies of asymptomatic pregnant females, the rate of isolation of H. influenzae from the genital tract was typically <1% [28]. In a recent study, the highest incidence of NTHi disease was observed in the first month of life [29]. A high rate of prematurity was also observed in neonates with NTHi bacteraemia without focus [30]. Another study demonstrated a 17-fold increase in invasive NTHi disease incidence in pregnant women compared to non-pregnant women, which was in turn associated with an increase in adverse fetal outcomes, including fetal loss, stillbirth and prematurity. Over 80% of the pregnant women, aged between 24 and 34 years, did not have any apparent comorbidities [31]. Resman et al. also reported that 62% of invasive NTHi cases occurred in individuals who did not have any evidence of being immunocompromised [5].
Indigenous patients were over-represented (15·6%) – only 4% of the Queensland population are of ATSI ethnicity [18]. The reasons for this over-representation are unclear. It is also unclear why Hia and Hib, in particular, are more common in the ATSI population. Hib vaccine uptake rates are comparable in both groups but it has been reported that there is persistent nasopharyngeal carriage of Hib in Indigenous infants despite vaccination [3]. This may be related to lesser vaccine responses in Indigenous children or waning antibody levels [3, 32]. A significantly higher rate of invasive Hib disease was observed in the ATSI group compared to the non-ATSI group in this study. Social factors such as overcrowding and poverty may also contribute to the persistence of Hib disease in the ATSI population [32]. Overall, Hia was the third commonest capsular serotype after Hif and Hib. Higher rates of invasive Hia disease have also been reported in specific geographical areas and populations such as Papua New Guinea, The Gambia and White Mountain Apache Indians in Arizona [33, 34].
Studies from the United States and Canada, England and Wales have shown an increasing incidence of Hif with time [4, 12, 13]. Hie has also been on the rise in England and Wales, being the third commonest capsular serotype after Hif and Hib in those countries [4]. The relative rarity of encapsulated non-b serotypes prevents the detection of any significant changes over our study period. Because comparative pre-vaccine data for the incidence of encapsulated non-b strains was unavailable, the phenomenon of strain replacement could not be adequately assessed. A recent study by Menzies et al. demonstrated no increasing trend in Hia or NTHi in the Indigenous population in South Australia and Northern Territory and overall post-vaccination rates of invasive encapsulated and non-typable disease were lower than pre-vaccination rates in that population [35]. The number of notified cases and rates of Hib Australia-wide plummeted markedly following Hib vaccine introduction [10].
This study has a number of limitations. The true incidence of empyema could not be determined as isolates from pleural fluid were excluded. The accuracy of clinical details could not be verified. It would have been interesting to assess whether episodes of neonatal sepsis correlated with maternal infections such as chorioamnionitis. This may also elucidate whether neonatal prematurity is a predisposing factor for invasive H. influenzae disease or rather a consequence of maternal sepsis. Analysis of trends and incidence in specific serotypes was limited since 20·5% of the isolates were untyped. Inclusion of the untyped isolates in the study revealed an increasing trend in laboratory serotyping over time. This could potentially have influenced the observed rise in invasive NTHi disease over the study period. Furthermore, details of untyped isolates from Sullivan Nicolaides Pathology were not available from 2000 to 2002 due to laboratory information system (LIS) constraints. Molecular serotyping is more specific and sensitive than serological typing [36]. False-positive and false-negative NTHi and capsular non-b serotypes could not be excluded in this study.
CONCLUSIONS
This study contributes to our understanding of the epidemiology and pathogenesis of NTHi and capsular non-b strains in Queensland, Australia. Twenty years after the introduction of Hib vaccination, invasive non-Hib (NTHi and encapsulated non-b) now constitutes 70% of the total H. influenzae burden in Queensland. While the pathogenicity of encapsulated non-b strains has been shown to be comparable, if not greater than Hib, the ability of NTHi to cause invasive disease in apparently immunocompetent individuals questions the traditional view that NTHi is purely opportunistic in nature. Based on the findings of this study, we recommend serotyping of all invasive H. influenzae isolates, inclusion of invasive non-Hib disease as a National Notifiable Disease and consideration of the use of recently available conjugate vaccines which include NTHi with Streptococcus pneumoniae. Vaccination against NTHi in infants and young children may decrease nasopharyngeal carriage of NTHi strains and reduce rates of otitis media, and potentially of invasive disease, as long as vaccine effects are lasting [37–39].
ACKNOWLEDGEMENTS
Dr Amy Jennison developed the multiplex PCR used in the identification of Hib isolates in QFSS. Dr Kristen Gibbons helped in the statistical data analysis. No funding was sought.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Wang H, et al. Trends in invasive Haemophilus influenzae type B disease in Australia, 1995–2005. Communicable Diseases Intelligence Quarterly Report 2008; 32: 316–325. [PubMed] [Google Scholar]
- 2.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clinical Microbiology Reviews 2000; 13: 302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal A, Murphy TF. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. Journal of Clinical Microbiology 2011; 49: 3728–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladhani SN, et al. Invasive Haemophilus influenzae serotype e and f disease, England and Wales. Emerging Infectious Diseases 2012; 18: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resman F, et al. Invasive disease caused by Haemophilus influenzae in Sweden 1997–2009; evidence of increasing incidence and clinical burden of non-type b strains. Clinical Microbiology and Infection 2011; 17: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 6.McConnell A, et al. Invasive infections caused by Haemophilus influenzae serotypes in twelve Canadian IMPACT centers, 1996–2001. Pediatric Infectious Disease Journal 2007; 26: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin MS, Park L, Borchardt SM. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons > or = 65 years old. Clinical Infectious Diseases 2007; 44: 810–816. [DOI] [PubMed] [Google Scholar]
- 8.Herceg A. The decline of Haemophilus influenzae type b disease in Australia. Communicable Diseases Intelligence 1997; 21: 173–176. [PubMed] [Google Scholar]
- 9.Australian Government DoHaA, National Health and Medical Research Council. The Australian Immunisation Handbook, 2008.
- 10.Horby P, et al. Progress towards eliminating Hib in Australia: an evaluation of Haemophilus influenzae type b prevention in Australia, 1 July 1993 to 30 June 2000. Communicable Diseases Intelligence Quarterly Report 2003; 27: 324–341. [PubMed] [Google Scholar]
- 11.Berndsen MR, Erlendsdottir H, Gottfredsson M. Evolving epidemiology of invasive Haemophilus infections in the post-vaccination era: results from a long-term population-based study. Clinical Microbiology and Infection 2012; 18: 918–923. [DOI] [PubMed] [Google Scholar]
- 12.Adam HJ, et al. Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: evidence for herd effects and strain replacement due to Hib vaccination. Vaccine 2010; 28: 4073–4078. [DOI] [PubMed] [Google Scholar]
- 13.MacNeil JR, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease – United States, 1989–2008. Clinical Infectious Diseases 2011; 53: 1230–1236. [DOI] [PubMed] [Google Scholar]
- 14.Public Health England. GOV.UK (https://www.gov.uk/notifiable-diseases-and-causative-organisms-how-to-report-list-of-notifiable-organisms-causative-agents).
- 15.CDC. (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6401md.htm?s_cid=mm6401md_w).
- 16.Public Health Agency of Canada. (http://dsol-smed.phac-aspc.gc.ca/dsol-smed/ndis/list-eng.php).
- 17.Australian Government. Department of Health (http://www.health.gov.au/internet/main/publishing.nsf/Content/cdna-casedefinitions.htm).
- 18.Australian Bureau of Statistics. (http://www.abs.gov.au).
- 19.Falla TJ, et al. PCR for capsular typing of Haemophilus influenzae. Journal of Clinical Microbiology 1994; 32: 2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stralin K, et al. Design of a multiplex PCR for Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae and Chlamydophila pneumoniae to be used on sputum samples. APMIS 2005; 113: 99–111. [DOI] [PubMed] [Google Scholar]
- 21.CLSI. M100-S24 – Performance Standards for Antimicrobial SusceptibilityTesting; Twenty-Fourth Informational Supplement.
- 22.EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 3.1, 2013 (http://www.eucast.org).
- 23.Queensland Government. (http://www.oesr.qld.gov.au).
- 24.Rubach MP, et al. Increasing incidence of invasive Haemophilus influenzae disease in adults, Utah, USA. Emerging Infectious Diseases 2011; 17: 1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gkentzi D, Slack MP, Ladhani SN. The burden of nonencapsulated Haemophilus influenzae in children and potential for prevention. Current Opinion in Infectious Diseases 2012; 25: 266–272. [DOI] [PubMed] [Google Scholar]
- 26.Wiertsema SP, et al. Predominance of nontypeable Haemophilus influenzae in children with otitis media following introduction of a 3 + 0 pneumococcal conjugate vaccine schedule. Vaccine 2011; 29: 5163–5170. [DOI] [PubMed] [Google Scholar]
- 27.Quentin R, et al. Typing of urogenital, maternal, and neonatal isolates of Haemophilus influenzae and Haemophilus parainfluenzae in correlation with clinical source of isolation and evidence for a genital specificity of H. influenzae biotype IV. Journal of Clinical Microbiology 1989; 27: 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherpes TL, Kusne S, Hillier SL. Haemophilus influenzae septic abortion. Infectious Diseases in Obstetrics and Gynecology 2002; 10: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladhani SN, Ramsay M, Slack MP. The impact of Haemophilus influenzae serotype B resurgence on the epidemiology of childhood invasive Haemophilus influenzae disease in England and Wales. Pediatric Infectious Disease Journal 2011; 30: 893–895. [DOI] [PubMed] [Google Scholar]
- 30.van Wessel K, et al. Nontypeable Haemophilus influenzae invasive disease in The Netherlands: a retrospective surveillance study 2001–2008. Clinical Infectious Diseases 2011; 53: e1–7. [DOI] [PubMed] [Google Scholar]
- 31.Collins S, et al. Risk of invasive Haemophilus influenzae infection during pregnancy and association with adverse fetal outcomes. Journal of the American Medical Association 2014; 311: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 32.Jacups SP, Morris PS, Leach AJ. Haemophilus influenzae type b carriage in Indigenous children and children attending childcare centers in the Northern Territory, Australia, spanning pre- and post-vaccine eras. Vaccine 2011; 29: 3083–3088. [DOI] [PubMed] [Google Scholar]
- 33.Ulanova M. Global epidemiology of invasive Haemophilus influenzae type a disease: do we need a new vaccine? Journal of Vaccines 2013; doi: 10.1155/2013/941461. [DOI] [Google Scholar]
- 34.Brown VM, et al. Invasive Haemophilus influenzae disease caused by non-type b strains in Northwestern Ontario, Canada, 2002–2008. Clinical Infectious Diseases 2009; 49: 1240–1243. [DOI] [PubMed] [Google Scholar]
- 35.Menzies RI, et al. No evidence of increasing Haemophilus influenzae non-b infection in Australian Aboriginal children. International Journal of Circumpolar Health 2013; doi: 10.3402/ijch.v72i0.20992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satola SW, et al. Capsule gene analysis of invasive Haemophilus influenzae: accuracy of serotyping and prevalence of IS1016 among nontypeable isolates. Journal of Clinical Microbiology 2007; 45: 3230–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prymula R, et al. Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage. Vaccine 2011; 29: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 38.Prymula R, et al. Immunological memory and nasopharyngeal carriage in 4-year-old children previously primed and boosted with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with or without concomitant prophylactic paracetamol. Vaccine 2013; 31: 2080–2088. [DOI] [PubMed] [Google Scholar]
- 39.Murphy TF. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Review of Vaccines 2005; 4: 843–853. [DOI] [PubMed] [Google Scholar]