Summary
Background
In animal models, immunity to mosquito salivary proteins protects animals against mosquito-borne disease. These findings provide a rationale to vaccinate against mosquito saliva instead of the pathogen itself. To our knowledge, no vector salivary protein-based vaccine has been tested for safety and immunogenicity in humans. We aimed to assess the safety and immunogenicity of Anopheles gambiae saliva vaccine (AGS-v), a peptide-based vaccine derived from four A gambiae salivary proteins, in humans.
Methods
In this randomised, placebo-controlled, double-blind, phase 1 trial, participants were enrolled at the National Institutes of Health Clinical Center in Bethesda, MD, USA. Participants were eligible if they were healthy adults, aged 18–50 years with no history of severe allergic reactions to mosquito bites. Participants were randomly assigned (1:1:1), using block randomisation and a computer-generated randomisation sequence, to treatment with either 200 nmol of AGS-v vaccine alone, 200 nmol of AGS-v with adjuvant (Montanide ISA 51), or sterile water as placebo. Participants and clinicians were masked to treatment assignment. Participants were given a subcutaneous injection of their allocated treatment at day 0 and day 21, followed by exposure to feeding by an uninfected Aedes aegypti mosquito at day 42 to assess subsequent risk to mosquito bites in a controlled setting. The primary endpoints were safety and immunogenicity at day 42 after the first immunisation. Participants who were given at least one dose of assigned treatment were assessed for the primary endpoints and analysis was by intention to treat. The trial was registered with ClinicalTrials.gov, NCT03055000, and is closed for accrual.
Findings
Between Feb 15 and Sept 10, 2017, we enrolled and randomly assigned 49 healthy adult participants to the adjuvanted vaccine (n=17), vaccine alone (n=16), or placebo group (n=16). Five participants did not complete the two-injection regimen with mosquito feeding at day 42, but were included in the safety analyses. No systemic safety concerns were identified; however, one participant in the adjuvanted vaccine group developed a grade 3 erythematous rash at the injection site. Pain, swelling, erythema, and itching were the most commonly reported local symptoms and were significantly increased in the adjuvanted vaccine group compared with both other treatment groups (nine [53%] of 17 participants in the adjuvanted vaccine group, two [13%] of 16 in the vaccine only group, and one [6%] of 16 in the placebo group; p=0·004). By day 42, participants who were given the adjuvanted vaccine had a significant increase in vaccine-specific total IgG antibodies compared with at baseline than did participants who were give vaccine only (absolute difference of log10-fold change of 0·64 [95% CI 0·39 to 0·89]; p=0·0002) and who were given placebo (0·62 [0·34 to 0·91]; p=0·0001). We saw a significant increase in IFN-γ production by peripheral blood mononuclear cells at day 42 in the adjuvanted vaccine group compared with in the placebo group (absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control 0·17 [95% CI 0·061 to 0·27]; p=0·009) but we saw no difference between the IFN-γ production in the vaccine only group compared with the placebo group (0·022 [−0·072 to 0·116]; p=0·63).
Interpretation
AGS-v was well tolerated, and, when adjuvanted, immunogenic. These findings suggest that vector-targeted vaccine administration in humans is safe and could be a viable option for the increasing burden of vector-borne disease.
Funding
Office of the Director and the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, and National Institutes of Health.
Introduction
Epidemics from mosquito-borne disease are often difficult to control and are occurring with increasing frequency each year.1-3 Promising pathogen-targeted vaccines are in the pipeline for known mosquito-borne pathogens, but diverse tools are needed to combat the increase in both expected and unexpected mosquito-borne diseases. The innovative concept of vector-targeted vaccine development builds on clinical observations extending back to 1940 when physicians first hypothesised that mosquito saliva contained immunomodulatory molecules.4 Pathogens transmitted via vector saliva seem to initiate or enhance severity of host infection by taking advantage of interactions between the vertebrate host and the saliva itself.5-8 This interaction leads to alteration of the cutaneous environment and modulation of the host’s innate and adaptive immune responses,9,10 thereby providing a rationale for creating vaccines against vector salivary proteins rather than the pathogens contained in the saliva.11
Novel salivary proteins across a variety of vectors have now been shown to enhance infectivity—eg, with sand flies and leishmaniasis, ticks and Borrelia, tsetse flies and trypanosomiasis, and mosquitoes and arboviruses.6,12-14 Within the salivary repertoire of all three clinically relevant mosquito vectors (Anopheles spp, Aedes spp, and Culex spp), multiple immunomodulatory proteins exist and often facilitate infection in the host.5,6,15-18 Passive immunisation of mice with Aedes aegypti salivary proteins NeST and AgBR1 improved survival against Zika-virus-infected mosquito challenge by preventing early changes in the inflammatory milieu.16,17 Immunisation with the Anopheles gambiae salivary protein AgTRIO provided protection in mice against malaria by reducing movement of Plasmodium sporozoites in the dermis.15 In a translational research study of macaques, vaccination with the sandfly salivary protein PdSP15 reduced lesion size and parasite load in cutaneous leishmaniasis.19 Despite compelling evidence in a variety of animal models, to our knowledge, no salivary components from arthropod vectors have been tested in humans to date.
A gambiae saliva vaccine (AGS-v) is a synthetic vaccine composed of four salivary peptides derived from A gambiae salivary glands. We aimed to assess the safety and immunogenicity of AGS-v delivered by subcutaneous injection in humans.
Method
Study design and participants
In this randomised, placebo-controlled, double-blind, phase 1 trial, we enrolled participants at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD, USA. Eligible participants were healthy adults aged 18–50 years who did not have a history of severe allergic reaction to mosquito bites, grade 2 or higher laboratory or electrocardiogram or imaging abnormalities, refusal to use contraceptives, positive drug screens, or current use of antihistamines.
All participants provided written informed consent before enrolment, and the study was done in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was reviewed and approved by the National Institute of Allergy and Infectious Diseases (NIAID) Institutional Review Board. NIAID investigators ran the clinical trial and carried out all safety and immunogenicity testing and analyses.
Randomisation and masking
Participants were randomly assigned (1:1:1) to either adjuvanted vaccine, vaccine only, or placebo, with a minimum of 15 individuals per group. A staggered enrolment scheme was created by the study statistician (SHu) using block randomisation with computer-generated randomisation codes. The study pharmacist was unmasked to study group assignment and maintained the randomisation codes that were assigned to participants. The study nurse who administering the study drugs was masked to the formulation (adjuvanted or non-adjuvanted vaccine, or placebo) by use of an opaque label placed over the content of the syringe by the pharmacist. Participants and clinicians were masked to treatment assignment.
An interim safety analysis was done after the first six participants were randomly assigned, vaccinated, and monitored for 28 days, at which point accrual was paused. No pausing or halting criteria were met and so nine more patients were enrolled and underwent study procedures until day 28 without safety concerns, again halting accrual during this period. The remaining participants were then enrolled and followed study procedures.
Procedures
The vaccine was developed by SEEK (London, UK) and produced according to current Good Manufacturing Practices by CordenPharma (Plankstadt, Germany). AGS-v is a vaccine that contains a combination of four synthetic salivary peptides, derived from A gambiae salivary proteins and ranging from 32 to 44 amino acids in length, that were identified by a proprietary T-cell epitope algorithm and then manufactured via acetate peptides and Fmoc-based peptide synthesis (appendix p 1). The vaccine peptides were reconstituted individually in 50% acetic acid and mixed to create a bulk solution of each peptide at 100 nmol/mL. Sterile glass vials were each filled with 0·5 mL of sterile water containing 200 nmol total peptide, 824 μg, and lyophilised to remove the water and acetic acid. For non-adjuvanted AGS-v vaccination, vials of AGS-v were reconstituted with 0·5 mL of sterile water for injection. For adjuvanted AGS-v vaccination, AGS-v vials were reconstituted in 0·25 mL of water for injection and emulsified with 0·25 mL of Montanide ISA 51 (Seppic, France), a sterile, manufactured adjuvant composed of a mineral oil and mannide monooleate, allowing generation of a water-in-oil emulsion to enhance immune cell recruitment to the injection site and slow antigen clearance. The placebo was 0·5 mL of sterile water.
On day 0 and day 21, the randomly allocated treatment was administered by subcutaneous injection into the fatty tissue of the triceps. The study team observed participants for 2 h after injection. Blood samples for immunogenicity measures were collected on days 0 (before vaccination), 42 (before mosquito feeding), 102, and 332. Blood samples for safety analysis were collected on approximately days 0, 21, 42, 102, 162, 222, 282, and 332 (before vaccination or feeding if during same visit). On day 42, ten starved, uninfected female A aegypti mosquitoes in a feeding device made of disposable mesh were placed on participants’ ventral forearm skin for 15 min. Redness and swelling, measured in mm, was assessed immediately and 30 min after feeding. 48 h after the mosquito feed, the study nurse called the participant on the telephone to verbally complete a safety checklist of symptoms and to self-report redness and swelling, measured in mm.
We collected serum samples to measure vaccine-specific immune responses at days 0, 42, 102, and 332 (before vaccination or feeding if during same visit). We used ELISA to measure binding antibody responses in 96-well Immulon plates (ThermoFisher, Waltham, MA, USA) in triplicate to AGS-v peptides with 100 μL per well at 2 μM concentration diluted in carbonate-bicarbonate buffer, and we report these data in arbitrary ELISA units.19 All four timepoints per participant were run on the same plate. Three controls were included on each plate: a control blank, which was three wells coated with AGS-v peptides and blocked, but without any sample from the participant, to act as the control for non-specific induction of colour for any of the reagents used in the test; a negative control, which was three wells with AGS-v and a human serum sample known to be non-reactive to AGS-v peptides; and a positive control, which was three wells with the same serum sample known to be positive to AGS-v peptides per plate to test plate variation and to normalise optical density (OD) values. IgG antibody concentrations were normalised as ΔOD=(average patient OD value [triplicate] – blank OD value) / positive control OD value. The negative and positive controls were chosen from a stored bank of human serum samples that were well characterised by reactivity to the saliva of various vectors from the Laboratory of Malaria and Vector Research (Rockville, MD, USA).
To observe cellular immunogenicity, whole blood was collected at day 42 (before feeding) and day 102 to obtain peripheral blood mononuclear cells, which were frozen (−196°C) for subsequent cytokine profiling on a Luminex platform (Luminex Corporation, Austin, TX, USA). To assess vaccine-specific T-cell responses, cryopreserved peripheral blood mononuclear cells obtained at day 42 and day 102 were stimulated with 4 μM concentrations of vaccine antigens in duplicate.20 Cytokine production was measured using the Luminex Cytokine Human Magnetic 10-Plex Panel (catalogue number LHC001M, Luminex Corporation) designed for quantifying human granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, IL-β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and TNF-α. Stimulation with media served as negative control and concanavalin-A (ConA) for positive control.
Starved female A aegypti mosquitoes, aged approximately 5–7 days, were selected from a mosquito colony approved for human feeding studies at the insectary of the Laboratory of Malaria and Vector Research at NIAID (Rockville, MD, USA) to test the safety of exposure to an abundant mosquito genus in the state of Maryland, USA. After the day 42 feeding as described, mosquitoes remained in the feeding devices and were returned to the laboratory for further study. Mosquitoes that took a blood meal were followed up to assess changes in life span, number of eggs laid, number of surviving larvae, and number of pupae that then developed in adults.
Outcomes
The primary endpoints were safety and immunogenicity at day 42 after completion of the vaccination regime on day 21 and before mosquito feeding. For safety, participants kept a daily symptom diary for 7 days after each injection that was reviewed by a study physician (JEM, MJM) and nurse (HAB) at day 7. Investigators also solicited information on local and systemic reactogenicity at each scheduled visit. From day 0 to day 42, study physicians followed up participants weekly during a visit to the clinic at the study site and did safety laboratory evaluations including complete blood counts, comprehensive metabolic panels, creatinine kinase, and urinalysis. After day 42, participants were followed up in clinic once every 60 days until 12 months after the initial vaccination. If necessary, a participant was seen between scheduled visits if they had any symptoms that could be attributed to vaccination or mosquito feeding. Adverse events were graded according to Common Terminology Criteria for Adverse Events. For the primary endpoint of immunogenicity at day 42, vaccine-specific total IgG antibody levels were assessed for humoral immunogenicity and T-cell responses were assessed for cellular immunogenicity.
Secondary endpoints were safety and immunogenicity at 102 days after initial vaccination (ie, 60 days after mosquito feeding) in addition to mosquito mortality and fecundity after feeding on participants at day 42. Another secondary outcome was also planned to develop an in-vitro assay to assess the infectivity of Zika virus after incubation with participants’ T cells, but we were unable to develop a working assay.
Statistical analysis
We aimed to have at least 15 people completing day 42 follow-up in each treatment group. We based the sample size on having a 90% power to detect a between-group difference in the immunoglobulin endpoint of 0·95 SDs using a one-sided Student’s t test with a significance level of 0·1. Safety and immunogenicity analyses were intention-to-treat and included any participant who received at least one dose of assigned treatment. We tabulated adverse events by treatment group and compared them using a one-sided Fisher’s exact test with each treatment group being compared with placebo. We considered p values of less than 0·1 to be significant for safety analyses. We analysed antibody and cytokine responses by taking the average of the replicates and doing a log10 transformation. For antibody responses, we compared each measurement from a participant with their own baseline sample (eg, day 42 ΔOD / baseline ΔOD). For cytokine responses, we compared each participant response to AGS-v stimulation with their own negative control (eg, day 42 AGS-v stimulation / media stimulation). We used Student’s t test to compare the immunological, cytokine, and mosquito rearing data. For these antibody, cytokine, and mosquito rearing tests, we compared all three treatment groups to each other. For these analyses, we used the Holm’s procedure at the two-sided 0·05 level to control for multiple comparisons. In the Holm’s procedure, the vaccine-specific antibody concentrations measured on day 42 was a measure of the primary outcome (ie, primary analysis; controlled for three comparisons). We considered the day 102 and 332 analyses to be secondary analyses (controlled for six comparisons) while IFN-γ at day 42 was considered a measure of the primary outcome and the Holm’s procedure was used to control for three comparisons (similarly to the analysis of the sample from day 102). The other cytokine data (seven cytokines and 2 days and three treatment groups) totalled 42 comparisons that were also controlled for using the Holm’s procedure. For our secondary outcome, we observed five secondary endpoints from the mosquito rearing data: mosquito fecundity, overall mosquito mortality, larvae mortality, pupae mortality, and adult mortality (controlled for 15 comparisons).
We did all analyses using R (version 3.6.1). This study is registered on ClinicalTrials.gov, NCT03055000.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Feb 15 and Sept 10, 2017, of 73 individuals screened for eligibility, 49 participants were enrolled and randomly assigned to the adjuvanted vaccine group (n=17), the vaccine only group (n=16) or the placebo group (n=16; figure 1). Investigators were unmasked to treatment assignment on Oct 25, 2018, after the 12-month follow-up visit of the final participant was completed. The study population comprised 30 women and 19 men, of whom 21 (43%) were white, 18 (37%) were black, and ten (20%) were multiracial or of unknown race, and the median age was 30·5 years (IQR 24·5–35·0; table). Geometric mean IgE concentrations were similar across the treatment groups.
Figure 1: Trial profile.
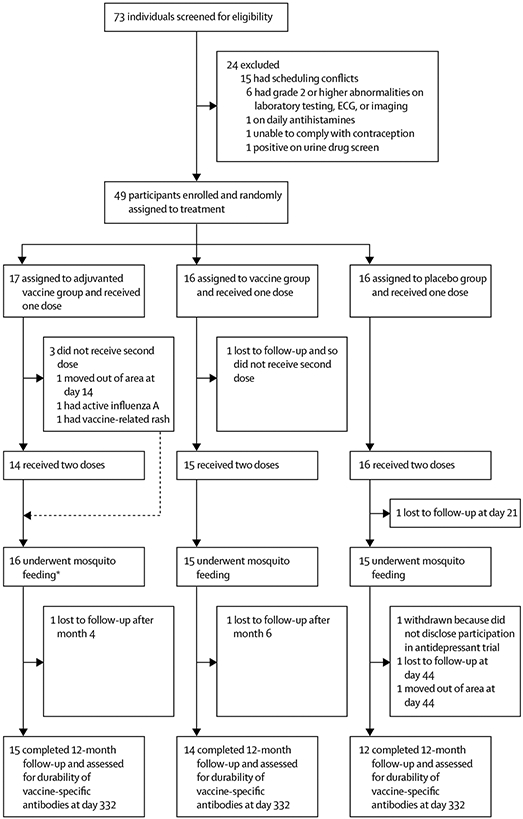
Three treatment groups underwent staggered enrolment and block randomisation. All available study data and samples were used for intention-to-treat analyses. ECG=electrocardiogram. *The two participants who did not receive second doses of the vaccine still participated in the day 42 mosquito feeding and completed 12-month follow-up.
Table:
Baseline demographic and clinical characteristics
| Adjuvanted vaccine group (n=17) |
Vaccine only group (n=16) |
Placebo group (n=16) |
|
|---|---|---|---|
| Sex | |||
| Male | 9 (53%) | 7 (44%) | 3 (19%) |
| Female | 8 (47%) | 9 (56%) | 13 (81%) |
| Age, years | 27 (22–33) | 32 (27–37) | 31 (27–34) |
| Race* | |||
| American Indian or Alaskan Native | 0 | 0 | 0 |
| Asian | 0 | 0 | 0 |
| Black or African American | 4 (23%) | 6 (38%) | 8 (50%) |
| Native Hawaiian or Pacific Islander | 0 | 0 | 0 |
| White | 7 (41%) | 9 (56%) | 5 (31%) |
| Multiracial | 3 (18%) | 1 (6%) | 3 (19%) |
| Unknown | 3 (18%) | 0 | 0 |
| Ethnicity* | |||
| Hispanic or Latino | 6 (35%) | 3 (19%) | 1 (6%) |
| Not Hispanic or Latino | 11 (65%) | 13 (18%) | 14 (88%) |
| Unknown | 0 | 0 | 1 (6%) |
| Body-mass index, kg/m2 | 25·2 (4·3) | 28·6 (4·5) | 25·5 (2·4) |
| IgE concentration, IU/mL†‡ | 29·6 (106·9) | 19·8 (151·1) | 28·3 (36·0) |
| Baseline AGS-v-specific total IgG antibody, ΔOD‡ | 0·554 (0·650) | 0·489 (0·566) | 0·378 (0·547) |
Data are n (%), median (IQR), or mean (SD) unless otherwise stated. AGS-v=Anopheles gambiae saliva vaccine.
Self-reported.
The validated range of normal values is 0–91 IU/mL.
Geometric mean.
44 participants completed the two-injection series and mosquito feeding. Of the five participants who did not complete the study per protocol until the day 42 primary endpoint, one in the placebo group was lost to follow-up given lack of transportation after a car accident on day 21; one in the vaccine only group was lost to follow-up at day 14; one in the adjuvanted vaccine group had active influenza A infection at the time of the second dose but continued in the study until completion, including the mosquito feeding; one in the adjuvanted vaccine group moved out of the area on day 14; and one in the adjuvanted vaccine group developed a vaccine-attributable non-tender erythema at the injection site with mild, localised urticaria after the first dose. This participant was otherwise asymptomatic, but the erythema was classified as grade 3 given its 8 cm size extending along the triceps. Oral antihistamines and steroids were administered at day 4 after the erythema did not spontaneously regress. The participant’s condition resolved within 2 weeks, but the second dose of study drug at day 21 was not given as a precautionary measure. No unanticipated adverse events occurred during or after the participants’ day 42 mosquito feeding. All 49 participants, including the two who missed the second dose of the vaccine but otherwise participated in mosquito feeding and completed day 42 follow-up, were included in the primary intention-to-treat analyses for safety and immunogenicity.
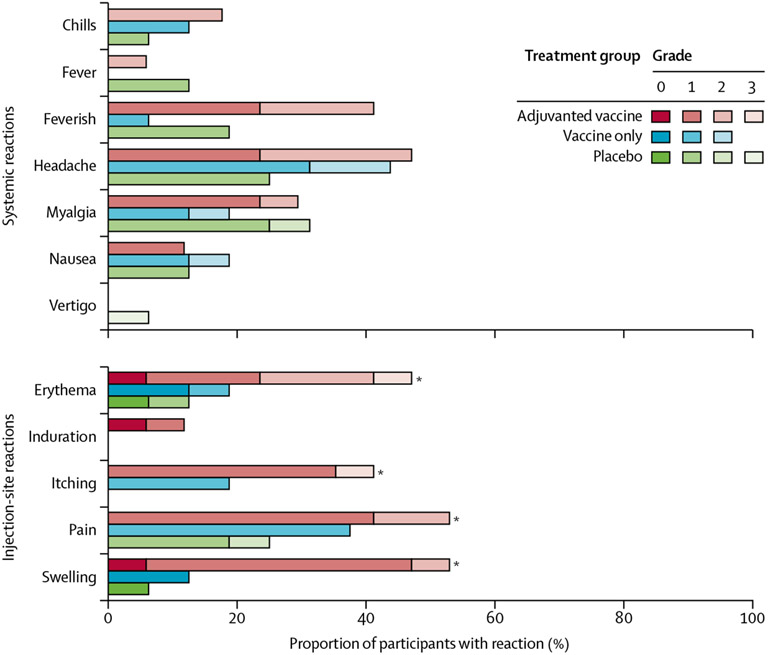
Over the 12-month follow-up period, the most frequently reported events were pain, redness, swelling, and itching at the injection site, which occurred more frequently in participants who were given adjuvanted vaccine (more pronounced with the first dose) than in those given placebo (figure 2). A complete list of all adverse events is in the appendix (pp 7–25) Injection-site swelling was the most notable adverse event difference in participants who received at least one dose of study drug (nine [53%] of 17 participants in the adjuvanted vaccine group, two [13%] of 16 in the vaccine only group [adjuvanted vaccine vs vaccine only p=0·004], and one [6%] of 16 in the placebo group; p=0·50). No significant difference was found in adverse events reported by participants in the vaccine only group compared with those in the placebo group (figure 2; appendix pp 49–53). Systemic adverse events were uncommon and included myalgia, headache, fever, and fatigue. Excluding the injection-site reactions, the investigators considered that, of all 512 systemic adverse events, 385 (75%) were unrelated or unlikely to be related to vaccine administration (appendix pp 2–53). Transient abnormalities included decreased absolute neutrophil count, increased liver enzymes, and increased creatine kinase, which comprised 168 events in 41 patients, although none were deemed definitely related to study drug administration by investigators. Itching, redness, and swelling occurred as expected in participants during the controlled A aegypti mosquito feeding or during the 48-h follow-up period and resolved appropriately (appendix pp 118–31).
Figure 2: Adverse events after vaccine administration until 12-months of follow-up as a proportion of total study population.
Listed are all injection-site adverse events and the systemic adverse events that were deemed clinically relevant, reported by study participants or noted by study clinicians, during the vaccine follow-up period of 12 months. *p<0·1 comparing adjuvanted vaccine group with placebo group using one-sided Fisher’s exact test.
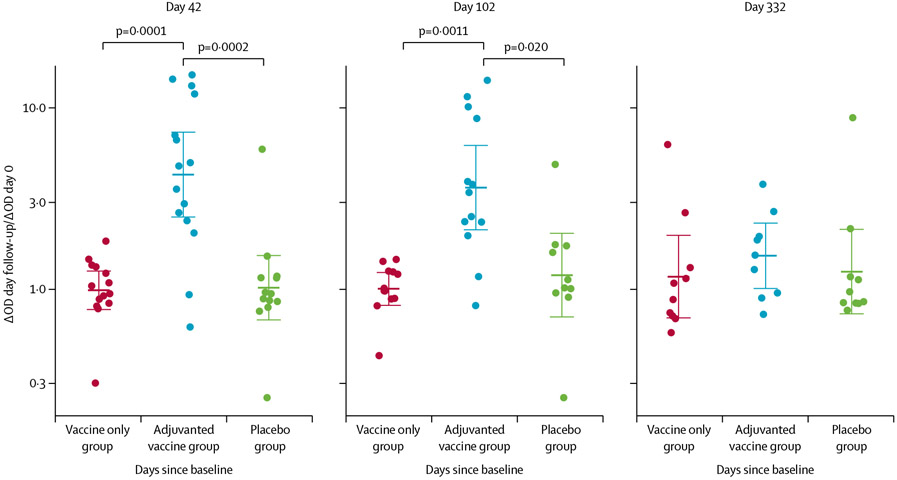
Baseline AGS-v-specific total IgG antibody concentrations were similar across the three groups (table). Participants in the adjuvanted vaccine group had significantly higher vaccine-specific total IgG antibody responses than those in the vaccine only or placebo groups at day 42 (4·31 in the adjuvanted vaccine group vs 0·99 in the vaccine only group [Student’s t test p=0·0001] and 1·02 in the placebo group [p=0·0002]; absolute difference of log10-fold change of 0·64 [95% CI 0·39 to 0·89] for adjuvanted vaccine vs vaccine only, and 0·62 [0·34–0·91] for adjuvanted vaccine vs placebo) and day 102 (3·65 in the adjuvanted vaccine group vs 1·00 in the vaccine only group [Student’s t test p=0·0011] and 1·20 in the placebo group [p=0·020]; absolute difference of log10-fold change of 0·56 [95% CI 0·32–0·80] for adjuvanted vaccine vs vaccine only and 0·48 [0·18–0·78] for adjuvanted vaccine vs placebo; figure 3; appendix p 112). These IgG antibodies were primarily of the IgG3 subtype (appendix p 115). We saw no noticeable difference between antibody levels by day 332 (figure 3).
Figure 3: Vaccine-specific total IgG antibody responses in three treatment groups.
Each datapoint is the ratio of ΔOD values at the day of follow-up compared with at baseline and bold bars are geometric means of the ratios and the whiskers are 95% CIs. Each treatment group included at least 15 participants in the intention-to-treat analysis. Holm’s-corrected p values for treatment group differences that are significant are shown at the top of the figure. OD=optical density.
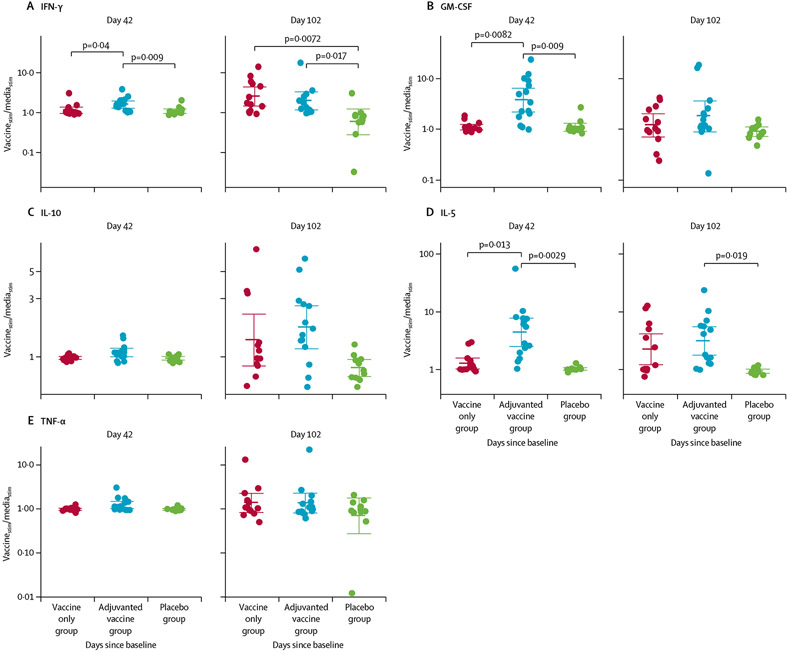
We assessed vaccine-induced T-cell responses, as determined by expression of cytokines (GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and TNF-α) in pg/mL in response to stimulation of participants’ peripheral blood mononuclear cells with AGS-v peptides versus media alone, at day 42 and day 102. IL-6 and IL-8 were excluded from this analysis because all values were outside of the range of detection of the assay. By day 42, participants in the adjuvanted vaccine group had higher production of IFN-γ, GM-CSF, and IL-5 than did those in the placebo group (IFN-γ Student’s t test p=0·009, absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control 0·17 [95% CI 0·061 to 0·27]; GM-CSF p=0·009, absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control 0·53 [95% CI 0·29 to 0·78]; and IL-5 p=0·0029, absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control 0·63 [95% CI 0·39–0·88]; figure 4; appendix pp 112–13, 116). We saw no difference between in IFN-γ, GM-CSF, or IL-5 production between the vaccine only group and the placebo group (absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control for IFN-γ 0·022 [95% CI −0·072 to 0·12; p=0·63]; GM-CSF 0·002 [−0·089 to 0·094; p=1·00]; and IL-5 0·094 [−0·0027 to 0·19; p=1·00]). By day 102, the mean levels of IL-10 and IL-5 were significantly increased in the adjuvanted vaccine group compared with in the placebo group (IL-10 Student’s t test p=0·05, absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control of 0·33 [95% CI 0·15 to 0·52]; and IL-5 p=0·015, absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control of 0·52 [95% CI 0·28 to 0·77]) and no significant difference was seen in the levels of IL-10 and IL-5 between the vaccine only and placebo groups at day 42 or day 102 (appendix p 113), whereas IFN-γ was significantly increased in both the adjuvanted vaccine and vaccine only groups compared with placebo (adjuvanted vaccine group Student’s t test p=0·017, absolute difference of log10 ratio of vaccine peptide-stimulated vs negative control 0·53 [95% CI 0·15 to 0·90]; and vaccine-only group Student’s t test p=0·0072, absolute difference of log10 ratio of vaccine peptide-stimulated versus negative control of 0·64 [95% CI 0·26–1·02]).
Figure 4: Cellular response to vaccine peptides.
Each datapoint is each participant’s ratio of cytokine response when stimulated with vaccine peptides (vaccinestim) compared with media (mediastim), and bold bars are geometric means of the ratios and the whiskers are 95% CIs. Peripheral blood mononuclear cells collected on day 42 and day 102 were stimulated with: vaccine peptides at 4 μM, media as a negative control, and Concanavalin A as a positive control. Holm’s-corrected p values for treatment group differences that are significant are shown at the top of the figure.
Nearly all mosquitoes took successful bloodmeals, laid eggs, and survived to day 7 regardless of treatment group from which they fed (data not shown). Mosquitoes produced similar numbers of eggs and pupae in each treatment group (appendix p 116). The number of pupae that developed into viable adults did not significantly differ between the treatment groups.
Discussion
In this, to our knowledge, first-in-human vector saliva-based vaccine trial, we found that AGS-v was well tolerated and adjuvanted AGS-v produced an increase in both saliva vaccine peptide-specific antibodies and IFN-γ release compared with placebo at the primary endpoint of day 42. These findings suggest that vector-targeted vaccine administration in humans is safe and could be a viable option for the increasing burden of vector-borne disease, particularly for unexpected epidemics for which no pathogen-directed vaccines are available.
Historically, safety concerns specific to a vector saliva-based vaccine have included induction of a hypersensitivity or an exaggerated Th1-skewed response to naturally occurring mosquito bites.21 No unexpected adverse events occurred in participants during the controlled A aegypti mosquito feeding or during the 48-h follow-up period.
In this study, A aegypti mosquitoes were selected for the controlled mosquito feeding for two reasons. First, previous entomological surveys of the greater Washington, DC, and Baltimore, MD, areas of the USA, where most participants of this study reside, showed Aedes spp and Culex spp to be the most abundant species of mosquito, so a controlled Aedes feeding allowed for the assessment of potential subsequent risk in the natural environment of the greater Washington, DC, area.22 Second, if any boosting of the AGS-v-specific response was observed after the controlled A aegypti mosquito feeding, it would provide preliminary rationale for broader cross-reactivity across mosquito genera. To increase generalisability of our results, we did not assess participants for mosquito saliva exposure before enrolment nor did we exclude participants if they had high baseline IgE concentrations given lack of specificity of the cause. During the extended follow-up period of 12 months, participants were asked to anecdotally note mosquito bites sustained in their communities. Since the study was extended over the summer period, most volunteers self-reported mosquito bites at follow-up visits without any appreciable difference in characteristics compared with previous mosquito bites in their lifetime, although this was not a formal analysis.
One limitation of this study might be that AGS-v is made up of peptides derived from A gambiae salivary proteins, such that salivary proteins injected during feeding by other mosquito genus, such as Aedes, do not induce an immune response. This possibility might have resulted in a more favourable safety profile than if the trial had been done in an area with abundant Anopheles spp mosquitoes.
The rates and severity of local reactogenicity in participants who were given the AGS-v peptide vaccine adjuvanted with Montanide ISA 51 were higher than among those in the placebo and vaccine only groups, but were similar to previous reports of this adjuvant in over 200 clinical trials with Montanide ISA 51 as adjuvant.23-25 Analysis of Montanide ISA 51 as an adjuvant in 6000 patients in cancer and HIV vaccination trials has shown mild-to-moderate adverse events involving mostly transient local pain or redness at the injection site.24 One previous study of an HIV peptide vaccine adjuvanted with Incomplete Freund’s Adjuvant resulted in premature study termination because of extended duration of pain, formation of sterile abscess, and clinically unacceptable systemic reactions after the second dose of vaccine was given.23 In our study, the more severe episodes of local reactogenicity, based on the size of redness, induration, and swelling, occurred predominantly after the first dose of vaccine. Participants in both the adjuvanted vaccine and vaccine only groups reported transient pain with injection, and then a notable lingering and intense pruritis, as one would expect with high doses of synthetic salivary antigen.
The saliva vaccine-specific antibody duration observed here to at least 102 days supports studies done in France and Colombia suggesting that the anti-mosquito salivary IgG response is genus-specific and short-lived, whereas IgM responses are less specific and likely to last longer due to boosting from other mosquito genera.26,27 Indeed, we saw a noticeable AGS-v vaccine-specific antibody increase at day 102 after the day 42 A aegypti mosquito feeding occurred. Whether this observed increase is boosted due to the controlled exposure to mosquito saliva or due to the follow-up occurring mostly in the late spring to late summer of 2017 when exposure to mosquito bites might be increased (despite directions to participants to use personal protective measures), is unclear. Changes in adjuvant selection or dosing schedule in future studies might be needed to produce more durable antibody responses.
Determination of appropriate correlates of protection for cellular immunogenicity in a non-pathogen-targeted vaccine is a challenge. Stimulation of participants’ peripheral blood mononuclear cells with AGS-v peptides produced a significant difference in cytokine profiles, an increase in IL-5, GM-CSF, and IFN-γ in the adjuvanted vaccine group compared with the placebo group, suggesting a mixed Th1-Th2 environment. However, these findings cannot be extrapolated to the vaccine’s effects on a mosquito-borne pathogen without a controlled human infection study, such as a malaria sporozoite challenge or a large field trial in an endemic area. Despite a large body of animal-based evidence showing disease reduction after immunisation with saliva,6,12,15,18 the exact mechanism by which a mosquito saliva-based vaccine might protect a human host is not known. As such, in this study we considered both antibody-mediated and cell-mediated protection as possible contributors to the so-called bystander phenomenon in which the pathogen’s effects are mitigated despite the vaccine targeting the vector saliva11,28 Another challenge to determining a protective mechanism is to consider that cellular populations in the epidermal–dermal microenvironment that rapidly release cytokines, such as tissue-resident CD4 or CD8 T cells, activated macrophages, or plasmacytoid dendritic cells, are responding to local signals, and thus such responses are not reproducible in vitro. A follow-up study of this phase 1 trial (NCT04009824) is ongoing with an updated version of AGS-v and includes a skin biopsy of the inoculation site at 48 h in response to these questions. Although further clinical studies are needed to understand the dermal immune response to vector saliva in general, vector-targeted vaccine development will likely require an in-vitro pathogen-specific correlate of protection as proof of principle before moving into larger, more expensive field studies in areas endemic for mosquito-borne diseases.
The implications for a safe, effective, vector-based vaccine are broad and clinically significant in the world of emerging vector-borne diseases. Even if universal protection across the Culicidae family of mosquitoes cannot be achieved with a single vaccine, the use of an Anopheles genus-targeted vaccine or adjuvant could potentially protect against the approximately 70 species of Anopheles mosquitoes that are competent to transmit Plasmodium parasites that cause malaria in humans.29 Similarly, a single species-targeted vaccine against A aegypti vectors would encompass a range of clinically significant pathogens including dengue virus, chikungunya, Zika virus, yellow fever, Mayaro viruses, and other emerging pathogens.11,30 Given unexpected mosquito-borne outbreaks of Zika virus disease in 2015 and of Eastern Equine Encephalitis in 2019,2,31 consideration of vector-targeted vaccines that might potentially reduce pathogenesis or the spread of emerging arboviruses is needed.
In this first-in-human phase 1 trial, we have shown the initial safety and immunogenicity of a mosquito saliva-based vaccine. This preliminary evidence expands the scope for innovative development of vector-borne disease vaccines because of the possibilities to target a vector, pathogen, or both. In conjunction with promising synthetic DNA and mRNA vaccine platforms that allow rapid pathogen antigen design, vector-targeted vaccines or adjuvants are an additional tool to combat emerging diseases that are spread by mosquitoes, ticks, or other arthropod vectors. Further studies of AGS-v and other vector-targeting vaccines are warranted to investigate the ability of these vaccines to provide meaningful protection against vector-borne diseases.
Supplementary Material
Research in context.
Evidence before this study
Emerging mosquito-borne diseases have led to unprecedented outbreaks in the past several years. Often, no vaccines are immediately available while pathogen-specific vaccines are developed. A vector-targeted vaccine is an innovative concept that takes advantage of the fact that vector saliva potentiates pathogen transmission in the mammal host. For nearly 80 years, scientists have observed the immunomodulatory effects of mosquito saliva. Recent advances have allowed further characterisation of vector saliva’s effects in animal models of disease including flaviviruses, bunyaviruses, alphaviruses, leishmaniasis, malaria, and tick-borne bacteria. We searched PubMed for publications from database inception to Feb 15, 2020, with no language restrictions, using the terms “vector saliva” OR “mosquito saliva” AND (“pathogenesis” OR “immunization” OR “vaccine”). We excluded papers related to use of human saliva or viral vectors for vaccine deployment. We identified 151 papers since 1984 describing vector-saliva mediated pathogenesis or immunisation in a variety of animal models. We had no results when we restricted our search to clinical trials and species to human. We found no studies describing human immunisation with saliva or synthetic saliva derivatives from any arthropod vector. We assessed Anopheles gambiae saliva vaccine (AGS-v), a vaccine cocktail of synthetic saliva peptides from A gambiae mosquitoes but conserved across Aedes spp and Culex spp mosquitoes, for safety and immunogenicity in a double-blinded, placebo-controlled, phase 1 clinical trial in healthy volunteers. Montanide ISA 51 was selected as an adjuvant given its availability and extensive use in previous vaccine trials for both cancer and infectious diseases.
Added value of this study
To our knowledge, this is the first study in which humans have been vaccinated with vector saliva of any kind. We found AGS-v to be systemically safe and well tolerated after subcutaneous injection, although 16 (94%) of 17 participants in the adjuvanted group had an injection site-related complaint rated grade 1 or 2, with the exception of one participant (2%) who had a grade 3 vaccine-related rash at the injection site. The vaccine produced vaccine-specific antibodies of at least 3 months’ duration in the adjuvanted vaccine group. Participants had no unexpected adverse events after being challenged with Aedes aegypti mosquitoes at day 42.
Implications of all the available evidence
The results of this study lay the foundation for future development of vector-targeted vaccine administration in humans. With emerging infectious diseases in a variety of arthropod vectors, improved understanding of the immunomodulatory effects of vector saliva in potentiating infection is crucial so that more therapeutic and preventive vector-derived tools can be developed to combat disease in the human population.
Acknowledgments
We thank the study participants for their time and dedication and the staff of the Special Clinical Studies Unit at the National Institutes of Health (NIH) Clinical Center. This work was supported by the Office of the Director and the Intramural Research Program at the National Institute of Allergy and Infectious Diseases. PepTcell (trading as SEEK) provided the vaccine product and assistance with Investigational New Drug submission. The statistical analysis was funded in part with federal funds from the National Cancer Institute, NIH (contract numbers HHSN261200800001E and 75N9101D00024, task order number 75N91019F00130). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organisations imply endorsement by the US Government. The vaccine manufacturers were not involved in the conduct of the study, analysis of the clinical data, nor did they contribute to the preparation or writing of the manuscript although they were allowed to comment on factual inaccuracies related to vaccine development in the manuscript prior to publication.
Footnotes
Declaration of interests
GS declares holdings in PepTcell (SEEK). All other authors declare no competing interests.
Contributor Information
Jessica E Manning, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Fabiano Oliveira, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Iliano V Coutinho-Abreu, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Samantha Herbert, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Claudio Meneses, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Shaden Kamhawi, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Holly Ann Baus, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Alison Han, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Lindsay Czajkowski, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Luz Angela Rosas, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Adriana Cervantes-Medina, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Rani Athota, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Susan Reed, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Allyson Mateja, Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research, sponsored by the National Cancer Institute, National Institutes of Health, Frederick, MD, USA.
Sally Hunsberger, Biostatistics Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Emma James, SEEK, PepTcell, London, UK.
Olga Pleguezuelos, SEEK, PepTcell, London, UK.
Gregory Stoloff, SEEK, PepTcell, London, UK.
Jesus G Valenzuela, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Matthew J Memoli, LID Clinical Studies Unit, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Data sharing
The clinical protocol including the statistical analysis plan and de-identified participant data in aggregate for primary and secondary endpoints are available online.
References
- 1.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015; 372: 1231–39. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016; 374: 601–04. [DOI] [PubMed] [Google Scholar]
- 3.Dorigatti I, Hamlet A, Aguas R, et al. International risk of yellow fever spread from the ongoing outbreak in Brazil, December 2016 to May 2017. Euro Surveill 2017; 22: 30572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellanby K Man’s reaction to mosquito bites. Nature 1946; 158: 554. [DOI] [PubMed] [Google Scholar]
- 5.Conway MJ, Watson AM, Colpitts TM, et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol 2014; 88: 164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCracken MK, Christofferson RC, Grasperge BJ, Calvo E, Chisenhall DM, Mores CN. Aedes aegypti salivary protein “aegyptin” co-inoculation modulates dengue virus infection in the vertebrate host. Virologgy 2014; 468-70: 133–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pingen M, Bryden SR, Pondeville E, et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 2016; 44: 1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Styer LM, Lim P-Y, Louie KL, Albright RG, Kramer LD, Bernard KA. Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol 2011; 85: 1517–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt MB, Lahon A, Arya RP, et al. Mosquito saliva alone has profound effects on the human immune system. PLoS Negl Trop Dis 2018; 12: e0006439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bizzarro B, Barros MS, Maciel C, et al. Effects of Aedes aegypti salivary components on dendritic cell and lymphocyte biology. Parasit Vectors 2013; 6: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning JE, Morens DM, Kamhawi S, Valenzuela JG, Memoli M. Mosquito saliva: the hope for a universal arbovirus vaccine? J Infect Dis 2018; 218: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science 2000; 290: 1351–54. [DOI] [PubMed] [Google Scholar]
- 13.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun 1997; 65: 335–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caljon G, Van Den Abbeele J, Sternberg JM, Coosemans M, De Baetselier P, Magez S. Tsetse fly saliva biases the immune response to Th2 and induces anti-vector antibodies that are a useful tool for exposure assessment. Int J Parasitol 2006; 36: 1025–35. [DOI] [PubMed] [Google Scholar]
- 15.Dragovic SM, Agunbiade TA, Freudzon M, et al. Immunization with AgTRIO, a protein in Anopheles saliva, contributes to protection against Plasmodium infection in mice. Cell Host Microbe 2018; 23: 523–35.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastings AK, Uraki R, Gaitsch H, et al. Aedes aegypti NeSt1 protein enhances Zika virus pathogenesis by activating neutrophils. J Virol 2019; 93: e00395–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uraki R, Hastings AK, Brackney DE, Armstrong PM, Fikrig E. AgBR1 antibodies delay lethal Aedes aegypti-borne West Nile virus infection in mice. NPJ Vaccines 2019; 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schleicher TR, Yang J, Freudzon M, et al. A mosquito salivary gland protein partially inhibits Plasmodium sporozoite cell traversal and transmission. Nat Commun 2018; 9: 2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira F, Rowton E, Aslan H, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med 2015; 7: 290ra90. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira F, Traoré B, Gomes R, et al. Delayed-type hypersensitivity to sand fly saliva in humans from a leishmaniasis-endemic area of Mali is Th1-mediated and persists to midlife. J Invest Dermatol 2013; 133: 452–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider DS. Battling the bite: tradeoffs in immunity to insect-borne pathogens. Immunity 2016; 44: 1251–52. [DOI] [PubMed] [Google Scholar]
- 22.LaDeau SL, Leisnham PT, Biehler D, Bodner D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int J Environ Res Public Health 2013; 10: 1505–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham BS, McElrath MJ, Keefer MC, et al. Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS One 2010; 5: e11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ascarateil S, Puget A, Koziol M-E. Safety data of montanide ISA 51 VG and montanide ISA 720 VG, two adjuvants dedicated to human therapeutic vaccines. J Immunother Cancer 2015; 3: 428. [Google Scholar]
- 25.Wu Y, Ellis RD, Shaffer D, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 2008; 3: e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontaine A, Pascual A, Orlandi-Pradines E, et al. Relationship between exposure to vector bites and antibody responses to mosquito salivary gland extracts. PLoS One 2011; 6: e29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Londono-Renteria B, Cardenas JC, Cardenas LD, et al. Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS One 2013; 8: e81211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning JE, Cantaert T. Time to micromanage the pathogen-host-vector interface: considerations for vaccine development. Vaccines (Basel) 2019; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinka ME, Bangs MJ, Manguin S, et al. A global map of dominant malaria vectors. Parasit Vectors 2012; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira TN, Rocha MN, Sucupira PHF, Carvalho FD, Moreira LA. Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci Rep 2018; 8: 6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morens DM, Folkers GK, Fauci AS. Eastern equine encephalitis virus - another emergent arbovirus in the United States. N Engl J Med 2019; 381: 1989–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical protocol including the statistical analysis plan and de-identified participant data in aggregate for primary and secondary endpoints are available online.