Abstract
Objectives
To evaluate the ocular surface, meibomian glands and corneal structural changes using in vivo confocal microscopy (IVCM) in patients receiving aromatase inhibitor (AI) therapy due to the breast cancer.
Methods
This prospective observational study included 13 patients undergoing AI therapy. The patients were evaluated before the treatment, at 3- and 6-month timepoints of AI therapy. To examine the ocular surface and tear film, corneal sensitivity (CS) measurement with Cochet–Bonnet Aesthesiometer, tear film break-up time (TBUT), lissamine green (LG) staining, Schirmer I test with anaesthesia (ST) and the ocular-surface disease index (OSDI) questionnaire were performed consecutively. Corneal cell densities and sub-basal nerve plexus were evaluated with IVCM (ConfoScan 4, Nidek, Japan). Finally, quantitative MG drop-out assessment was made using infrared meibography. Shapiro Wilk, Friedman’s and Post-hoc Dunn tests were used for the statistical analysis.
Results
TBUT, ST scores, basal epithelium, anterior and posterior keratocytes and endothelial cell densities, long and total sub-basal nerve densities were found to be decreased (p < 0.001, p = 0.023, p < 0.001, p = 0.01, p = 0.002, p = 0.004, p < 0.001, p < 0.001), and meiboscore, CS, OSDI scores and sub-basal nerve tortuosity values were increased (p < 0.001, p = 0.015, p = 0.001, p = 0.004) during the treatment. Endothelial pleomorphism rates were lower at the 3- and 6-month timepoints compared to before the treatment (p = 0.04).
Conclusion
This study showed that aromatase inhibitor therapy causes deteriorations in many of the ocular-surface parameters and corneal structural changes in relation with the duration of treatment. These patients should be observed during the therapy in terms of the ocular-surface side effects.
Subject terms: Eye manifestations, Chemotherapy
Introduction
Aromatase inhibitors (AIs) are standard adjuvant treatment regimens for hormone-positive breast cancer in postmenopausal women. These drugs inhibit aromatase, which converts androgen to oestrogen in peripheral tissues [1]. A significant reduction of oestrogen in peripheral tissue is the basis of the mechanism of action of these drugs [1]. Nowadays, the decision of adjuvant therapy is based on the clinical and biological criteria for each patient and the therapeutic strategy should be adapted accordingly. AIs have many systemic side effects on the neurological, urogenital and musculoskeletal system, such as cognitive dysfunction, arthralgia, stiffness, urinary urgency and frequency [2].
AIs are also known to be associated with various ophthalmological disorders [3]. Alpha and beta receptors of oestrogen are basically found in human retinal pigment epithelium, neurosensorial retina and the choroid in the eye and these receptors provide neuroprotection to the retinal cells [4–6]. The posterior segment of the eye may be affected by changes in oestrogen level and it has been reported in the literature that AIs cause visual disturbance, retinal haemorrhage, macular oedema, uveitis, hemi-central retinal artery occlusion and vitreoretinal traction [7–10].
In addition to the posterior segment effects of these drugs, their anterior segment side effects have also become an important research topic nowadays. Sex steroids have a critical role in maintaining ocular-surface stabilization and tear film continuity [11, 12]. The tear film consists of mucous, aqueous and lipid layers [13]. While these layers provide lubrication of the ocular surface, androgen levels affect the lacrimal and meibomian glands (MGs), which are responsible for the production of the aqueous and lipid layers of the tear film.
The circulating form and amount of sex steroids can vary depending on age and gender. The main circulating androgen in men is testosterone, and oestrogen accounts for a small amount. On the other hand, the main sex steroid in circulation in women is oestrogen, and 17 B-oestradiol, which shows a fluctuation in the menstrual cycle, constitutes the main form in the circulation in the reproductive period with their highest level, decreases by age, and has a horizontal course including the menopause period [14]. In the postmenopausal age group using aromatase inhibitor, the main form of oestrogen is estrone, and its production originates from peripheral adipose tissue rather than ovaries [15]. Further, the amount of circulating dehydroepiandrosterone, a precursor inactive sex steroid, decreases on average by 60% in postmenopausal women with a significant decline in androgen and oestrogen synthesis [16]. Circulating oestrogen levels in the postmenopausal age group reflect the small amount of leakage from many peripheral tissues and are not a good indicator of the effects of oestrogen on the ocular surface. Serum oestrogen level is more associated with ocular-surface changes in premenopausal women [17]. The general opinion is that androgen provides a protective effect on ocular-surface homeostasis, whereas oestrogen has a negative impact on these structures [18]. If the amount of circulating oestrogen is considered to be lowest, the increased frequency of dry eye in postmenopausal women is quite confusing and this puzzle is still not fully resolved [18]. Consequently, the changes in the androgen–oestrogen balance are mainly associated with deterioration of tear film and ocular-surface homeostasis and may cause in inflammation [19–22].
Ocular-surface diseases have been reported to be more severe and frequent in patients receiving AIs [23, 24]. However, there are no data in the literature evaluated changes in the ocular surface, corneal structure and meibomian glands using meibography and in vivo confocal microscopy (IVCM) of patients using AIs. Thus, the aim of this study was to investigate the possible changes of the ocular surface due to AIs and to determine the longitudinal effects of these drugs during the treatment.
Materials and methods
This prospective observational study was included 13 eyes of 13 patients who were receiving anastrozole/letrozole, which are non-steroidal AIs, due to the breast cancer with no history of radiotherapy or adjuvant chemotherapy. The tenets of the Declaration of Helsinki were followed throughout the study. Informed consent was obtained from all patients and the study was conducted with the approval of the Hacettepe University (KA-17135) and Turkish Medicines and Medical Devices Agency (18-AKD-03) Clinical Investigation Review Board.
All the participants underwent a detailed ophthalmic examination including best corrected visual acuity, anterior segment assessment with a slit lamp biomicroscopy, dilated fundus examination and intraocular pressure measurement using Goldmann applanation tonometer. Patients who had a history of ocular-surface surgery or ocular trauma, contact lens wear, any topical-systemic medication which may have corneal toxicity or had any systemic disease that may adversely affect the corneal sub-basal nerve plexus were excluded from the study.
Patients were evaluated pre-treatment, then after 3- and 6-months period of the treatment. The ocular-surface tests of all the subjects were performed by an observer. For all participants, the following tests were performed sequentially; corneal sensitivity (CS) measurement with Cochet–Bonnet Aesthesiometer, standard tear break-up time (TBUT) testing, lissamine green (LG) staining of the conjunctiva and cornea and Schirmer I test with topical anaesthesia. All the patients were examined at the same time of day to eliminate any possible effects of environmental factors and diurnal variability.
Corneal sensitivity was measured using a Cochet–Bonnet aesthesiometer (Luneau Ophthalmics, France), which is a nominal nylon filament, 0.12 mm in diameter. With the subject gazing directly at a target ~3 m away, the central threshold is determined at the corneal apex. The recorded nylon lengths for the thresholds were converted into pressure (mg/mm2) using a specifically calibrated curve for the filament used.
In TBUT, sterile fluorescein test strips soaked with saline solution are applied. The patient is instructed to blink several times for a few seconds and the time between the final blink and the appearance of the first corneal dry area is defined as the TBUT. For ocular-surface staining, an LG strip was applied to the inferior conjunctival sac. Staining of the cornea and the conjunctiva were assessed under white light with scores between 0 and 5 according to the Oxford Scheme [25]. The Schirmer I test was applied with the dropping of topical anaesthetic (proparacaine hydrochloride 0.5%, Alcaine; Alcon Laboratories Inc, Puurs, Belgium). With the subject looking straight ahead for 5 min, sterilized strips of filter paper were placed in the lower fornix, between the lateral third and the middle third of the eyelid. The Ocular Surface Disease Index (OSDI; Allergan Inc, Irvine, CA) questionnaire was applied to all the participants to assess the severity of subjective symptoms [26].
Meibography
Meibography was performed using a Scheimpflug camera-Placido topography device (Sirius, C.S.O, Florence, Italy; bon Optic, Lübeck, Germany). First, the participant was seated and asked to focus on the target of the placido disc system. Then, the upper and the lower eyelids were everted, respectively, by the examiner using a cotton-tipped applicator and the image of the entire area of the MG was taken. The images of the MG were taken by the same examiner three times at the same contrast setting, taking care that the entire MG area was included and well visible. The images were evaluated with the Phoenix-Meibography Imaging Module. Although the procedure was computer-assisted, manual gland boundary tracing was required. Initially, the best image was selected by the observer and then the upper or lower eyelid evaluation was selected. The lid area and afterwards the gland boundaries were manually marked. The area of loss score was automatically calculated together with a pre-established degree in the Meibomian Scale [27]. The values of the meiboscores refer to the percentage of the lost field as 0 = 0%, 1 ≤ 25%, 2 = 25–50%, 3 = 51–75%, 4 ≥ 75% [27]. The scores of the upper and lower eyelids were calculated to obtain a total score from 0 to 8 for each participant. Infrared meibography pictures of a patient receiving Ais are shown in Fig. 1.
Fig. 1. Infrared meibography images of a patient receiving AIs.
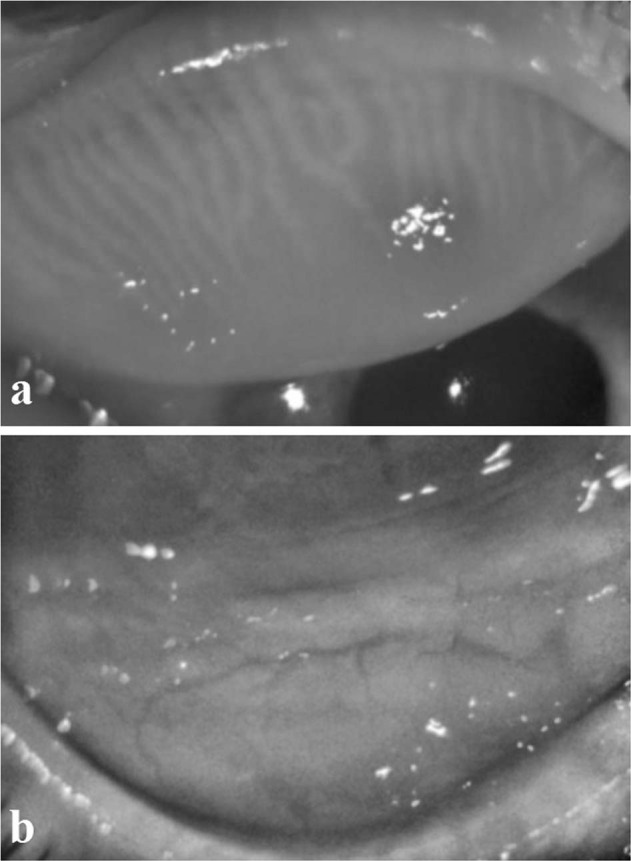
a Upper eyelid. b Lower eyelid.
In vivo confocal microscopy
A Confoscan 4.0 (Nidek, Gamagori, Japan) affixed to an immersion lens (Achroplan ×40/0.75 W; Zeiss, Oberkochen, Germany) was used to apply in vivo confocal microscopy of the corneal sections. The technique has been explained in detail elsewhere. At ×13 magnification, a working distance of 1.98 mm was set for the immersion lens, with numerical aperture of 0.75, and 16.61 mm² front surface area. Following data collection for both eyes, 1 eye was randomly selected for each patient. Anaesthetic of proparacaine hydrochloride (Alcaine 0.5%; Alcon Laboratories Inc) was applied to the cornea before the examination. Viscotears (Carbomer 0.2%; Novartis, Basel, Switzerland) was applied as a coupling agent to the applanating lens, then the full thickness of the central cornea was scanned. A total of 4–6 complete full-layer corneal scans were obtained for each subject by the same observer who masked to the clinical test results (SK). Each image covered an area of 450 × 340 mm, with lateral resolution of 1 mm, and 10 mm depth resolution. On a 15-inch display (1024 × 768 pixels), ×500 mean magnification was obtained.
Image analysis
After obtaining central corneal images from all the participants, evaluation was made of the 2 best-focused images from the epithelium, sub-basal nerve plexus, anterior stroma, posterior stroma and endothelium. Using the software provided by Confoscan 4.0, keratocyte density was determined using a manual method of counting images of cell nuclei in a predefined area of confocal images. Quantification of the keratocyte, endothelial cell, and basal epithelial cell densities was made using a boundary box positioned in the centre of the image. The dimensions (200 × 300 mm) and area (0.060 mm2) of the box were kept constant for all the subjects. When cells overlapped the boundary box, only those in the superior and left half of the box were counted. Keratocyte densities were measured in the anterior (0–100 mm posterior to the basal epithelium), and posterior (0–100 mm anterior to the endothelium) stromal layers. The Confoscan 4.0 software automatically calculated the percentages of endothelial polymegethism and pleomorphism. The evaluation of sub-basal nerves was made on the images showing the greatest number of nerve fibres. Sub-basal nerve densities were evaluated in respect of the number of long nerve fibres (LNFs) per frame, the total number of nerve branches (NBs) per frame, and the sub-basal nerve fibre length (mm) per frame. For sub-basal nerve analysis, three non-overlapping images were selected for each eye. After exporting the images from the Confoscan 4.0 device, density measurements were taken using ImageJ software (https://imagej.nih.gov/ij/). Sub-basal nerve tortuosity was classified in four groups; grade 1 represents perfectly straight nerves and grade 4 represents grossly tortuous nerves with significant convolutions throughout their course.
Statistical analysis
Data were evaluated in collaboration with the Department of Biostatistics. Data analysis was performed using SPSS 23.0 software (Statistical Package for Social Sciences; SPSS Inc IBM, Armonk, NY, USA). Quantitative variables were evaluated for normality of data distribution by using Shapiro Wilk test. Descriptive statistics were expressed as mean ± standard deviation and median (min-max). Friedman’s test was used to evaluate the significance of differences over time for non-normal quantitative variables. After Friedman’s test, post-hoc Dunn test was used to examine for multiple comparisons. A post-hoc power calculation was applied to determine the resultant power of the study. The power was calculated by using G*Power 3.1 software. A p < 0.05 was accepted as statistically significant.
Results
The mean age of the patients was 65.8 ± 7.12 y (range, 53–78 years; 13 female). Ocular surface and meiboscores of the patients receiving AIs are highlighted in Table 1. OSDI scores, CS and meiboscores were increased (p = 0.001, p = 0.015, p < 0.001; respectively), and Schirmer I test values and TBUT scores were decreased during the treatment period (p < 0.023, p < 0.001; respectively). The LG staining scores was found to be similar at all examination timepoints (p = 0.47) (Table 2). Basal epithelial cells, anterior and posterior stromal keratocytes and endothelium cell densities were found to be decreased during the treatment. (p < 0.001, p = 0.01, p = 0.002, p = 0.004; respectively). The results of corneal cell densities are outlined in Table 2. The number of corneal sub-basal LNFs, the total number of nerve fibres and total nerve densities were found to be significantly lower (p < 0.001 for all parameters), and the tortuosity of the sub-basal nerve plexus was found to be significantly higher (p = 0.004) at the last examination compared to the first examination (Table 3). The results of post-hoc Dunn test for the variables that were statistically significant are highlighted in Table 4. With a probability of error set to 0.5 and a total sample size of 13 patients, the post-hoc power of this study was calculated to be ~90% using the Friedman’s test. The comparative IVCM sections of sub-basal nerve plexus are shown in Fig. 2.
Table 1.
Comparison of ocular-surface parameters and meiboscore of patients during aromatase inhibitor therapy.
| Pre-treatment (n = 13 eyes) Median (min-max) | At 3. month (n = 13 eyes) Median (min-max) | At 6. month (n = 13 eyes) Median (min-max) | P | |
|---|---|---|---|---|
| TBUT, s | 14 (4–20) | 7 (3–15) | 7 (2–14) | <0.001* |
| LG staining, grade | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.47 |
| Schirmer I test, mm | 10 (4–22) | 8 (5–25) | 6 (4–23) | 0.023* |
| OSDI score, points | 10.4 (0–42.5) | 20.8 (0–35.4) | 31.2 (0–77) | 0.001* |
| CS test, mg/mm2 | 11 (11–11) | 11 (11–13) | 11 (11–13) | 0.015* |
| Meiboscore, points | 3 (2–6) | 5 (4–6) | 5 (4–8) | <0.001* |
TBUT tear break-up time, LG lissamine green, OSDI ocular-surface disease index, CS Corneal Mechanical Sensitivity with Cochet–Bonnet Aesthesiometer.
*Statistically significant.
Table 2.
Comparison of cell densities at different corneal layers of patients during aromatase inhibitor therapy.
| Pre-treatment (n = 13 eyes) Median (min-max) | At 3. month (n = 13 eyes) Median (min-max) | At 6. month (n = 13 eyes) Median (min-max) | P | |
|---|---|---|---|---|
|
Basal epithelium, cells/mm² |
3692 (2377–5771) | 3184 (1892–4700) | 3045 (1465–3950) | <0.001* |
|
Anterior stroma, cells/mm² |
382 (203–486) | 340 (270–400) | 311 (255–451) | 0.01* |
|
Posterior stroma, cells/mm² |
395 (201–565) | 370 (217–543) | 322 (217–434) | 0.002* |
|
Endothelium, cells/mm² |
2281 (1276–3011) | 2119 (1095–2850) | 1987 (1166–2826) | 0.004* |
| Endothelial polymegethism rate/% | 41.6 (32–66.1) | 47.2 (28.9–79.1) | 46.7 (27–70.5) | 0.39 |
| Endothelial pleomorphism rate/% | 50 (25–65) | 44.4 (15.5–60) | 42.8 (14.7–72) | 0.04* |
*Statistically significant.
Table 3.
Comparison of sub-basal nerve plexus densities of patients during the aromatase inhibitor therapy.
| Pre-treatment (n = 13 eyes) Median (min-max) | At 3. month (n = 13 eyes) Median (min-max) | At 6. month (n = 13 eyes) Median (min-max) | P | |
|---|---|---|---|---|
| Number of long nerves fibres, nerves/frame | 3 (2–6) | 2 (1–6) | 2 (1–4) | <0.001* |
| Total number of nerves, nerves/frame | 5 (2–8) | 4 (2–7) | 4 (2–5) | <0.001* |
| Total nerve density, mm/frame | 1148.87 (955.32–3184.74) | 1065 (629.52–2543.45) | 995.56 (543.49–2245.56) | <0.001* |
| Tortuosity | 1 (1–1) | 1 (1–2) | 2 (1–2) | 0.004* |
*Statistically significant.
Table 4.
The results of post-hoc Dunn test for the variables that were statistically significant.
| Between first and third examinations (p-value) | Between second and third examinations (p-value) | Between first and second examinations (p-value) | |
|---|---|---|---|
| TBUT | 0.002* | 1.00 | 0.003* |
| Schirmer I test | 0.28 | 0.04* | 1.00 |
| OSDI score | 0.002* | 0.18 | 0.35 |
| Meiboscore | <0.001* | 0.05 | 0.23 |
| CS test | 0.14 | 0.23 | 0.76 |
| Basal epithelium cells | <0.001* | 0.07 | 0.03* |
| Anterior stroma cells | 0.01* | 0.71 | 0.23 |
| Posterior stroma cells | 0.001* | 0.23 | 0.23 |
| Endothelium cells | 0.003* | 0.50 | 0.15 |
| Endothelium pleomorphism rate | 0.04* | 1.00 | 0.28 |
| Number of long nerve fibres | 0.004* | 0.42 | 0.23 |
| Total number of nerves | 0.001* | 0.18 | 0.18 |
| Total nerve density | <0.001* | 0.15 | 0.03* |
| Tortuosity | 0.03* | 0.55 | 0.14 |
TBUT tear break-up time, OSDI ocular-surface disease index, CS Corneal Mechanical Sensitivity with Cochet–Bonnet Aesthesiometer.
*Statistically significant.
Fig. 2. IVCM sections of sub-basal nerve plexus in a patient receiving AIs.
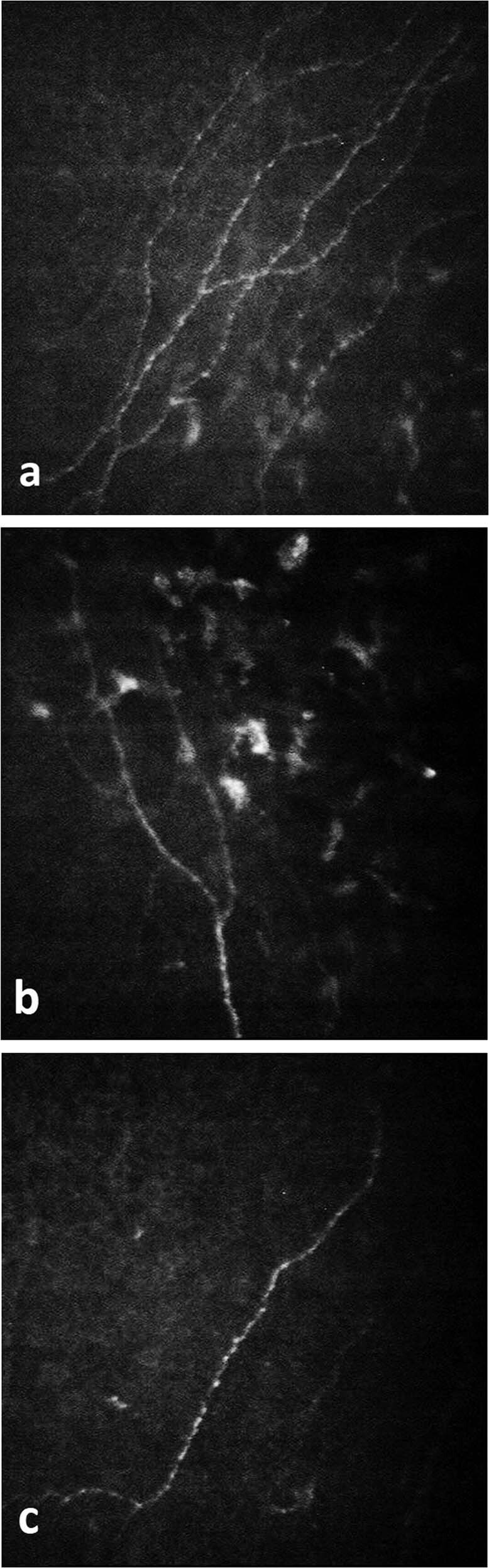
a Performed at the beginning of treatment. b Performed in the 3rd month of treatment. c Performed in the 6th month of treatment.
Discussion
Research on treatment of breast cancer—one of the most common types of cancer in women—have become an important focus with developing biotechnology [28]. By the discovery of AIs, a significant improvement has been achieved in the treatment of the patients with hormone-positive breast cancer, and they are widely used nowadays [28]. We able to show with this study the effects of AIs on ocular surface, MGs and corneal structure. The results of this study suggested that these drugs cause deterioration in both objective and subjective ocular-surface parameters, alteration in intensity and morphology of MGs, and damage to cornea at the cellular level depending on the duration of the treatment. In addition, structural changes in corneal nerves were found, which may contribute to the dry eye pathogenesis in these patient group.
Oestrogen plays a key role in the maintenance of the ocular surface and corneal hemostasis [4, 29]. Oestrogen receptors are commonly found in the MGs, lacrimal gland, ciliary body, cornea and conjunctiva. The anti-estrogenic effect of AIs is thought to result in changes in the ocular surface, cornea and MGs. Impaired oestrogen production is associated with decreased lacrimal secretion, which causes an increase in ocular-surface symptoms [30]. Androgens are also an important element in the production of the tear lipid layer and in the regulation of MG function [31]. Changes in the balance between androgens and oestrogens lead to a variety of conditions by affecting these structures [4, 29].
Limited data are available in the literature evaluating the relationship between AIs and the ocular surface, and MGs. Turaka et al. [24] and Chatziralli et al. [3] evaluated the effects of AI therapy on the ocular-surface parameters. They reported that ocular-surface signs and symptoms, such as irritation/foreign body sensation, decreased tear function and conjunctival injection were higher in patients receiving aromatase inhibitor therapy compared to control groups [3, 24]. In the study by Inglis et al. [23], which the prevalence of dry eye syndrome (DES) was evaluated, they found that the DES was more frequent in patients receiving AI therapy than normal subjects (35% vs. 18%) [23]. Similarly, Cuzick et al. [32] reported that the frequency of DES was two-fold higher in patients receiving AI therapy than in those taking placebo. However, in a recent published cross-sectional study [33], it has been reported that there was no difference between ocular-surface parameters such as conjunctival and corneal staining, BUT, PRT (phenol read threat) and OSDI scores in these patients compared to the controls. In the same study, they showed that the pain perception score increased in the patient group. In our study, we evaluated before and during the treatment process for six months prospectively, and showed that the TBUT and Schirmer I test scores decreased, while the LG staining score remained unchanged during the follow-up period compared to the pre-treatment examination. The mean OSDI score increased from 12.01 to 31.58 and was associated with more frequent occurrence of subjective symptoms during the follow-up period.
MG secretions are mediated by hormonal factors such as androgens and estrogens [19, 34]. While androgens stimulate MG production and meibum secretion, oestrogens act on this structure in the opposite direction [19, 34]. However, the effects of oestrogen on MGs are still controversial. Serum oestradiol suppresses lipid synthesis, increases lipid catabolism in MG and provides the basis for the formation of DES [34]. Furthermore, Kam et al. [35] reported that oestradiol could downregulate cyclic AMP signalling in MG epithelial cells and reduce cell proliferation. However, there is scarce in the literature about the effect of AIs on MG. High frequency of MG dysfunction and worse MG expressibility score were reported in patients receiving AIs [3, 33]. On the other hand, Darabad et al. [36] investigated the effects of aromatase absence on the histologic and gene expression traits of meibomian gland by comparing in wild-type and aromatase knockout mouse. It was determined that aromatase significantly affects gene expression in a sex-dependent manner. However, it has been found that aromatase absence does not have a significant impact on the histologic sections of the meibomian gland. As Tong et al. [37] pointed out, animal models for meibomian gland may not give optimal results with some limitations and may not provide a definite idea about the clinical effects on human meibomian gland. In addition to the studies in the literature, in the current study the meiboscore, which enables us to perform qualitative and quantitative analysis of MGs, increased during the follow-up period of the treatment. It could be thought that these changes in ocular-surface parameters and MG density are associated with oestrogen degradation and deterioration in the oestrogen-androgen balance due to AI therapy, and may contribute to the negative effects on ocular-surface parameters and DES severity.
Most important aspect of our study from the publishes in the literature is the assessment of corneal nerve plexus and cell layers using IVCM during the follow-up period. Alterations in the epithelium and sub-basal nerves may contribute to the pathogenesis of DES in these patients.
Corneal nerves have a strong role in maintaining the anatomical integrity of the ocular surface and epithelial cell morphology [38]. In diseases with neurotrophy such as diabetes, a reduction in the length of the sub-basal nerve fibre layer was associated with decreased corneal epithelial cell density [39]. In our study, it is thought that the decrease in the density and length of the sub-basal nerve fibre layer leads to decreased corneal sensitivity. It has been reported that structural and functional changes such as decreased sub-basal corneal nerves and increased tortuosity are associated with conditions included in the DES spectrum such as Sjogren’s syndrome and ocular chronic graft-versus-host disease [40–42]. However, decreased Schirmer I test score and increased BUT in relation to the duration of treatment support decreased tear production and the presence of impaired tear film stability. Zhang et al. [43] showed that the reduction in corneal epithelial cells was associated with the severity of DES. However, it is not known whether the instability on the ocular surface is due to a decrease in sub-basal nerves or apoptotic cell death by deterioration in tear film parameters or effect of hormones at the cellular level on cornea or alterations in the MG. As stated in the DEWS II report, nerve plexus plays a significant role in maintaining ocular-surface homeostasis, tear secretion and epithelial integrity [44]. In this setting, the decreased sub-basal nerve density and reduced corneal sensitivity measurements in our study may contribute to the ocular-surface deterioration and DES in these patients. In addition, increased tortuosity of corneal sub-basal nerve plexus could be an evidence of inflammation, and the decrease in keratocyte density may be the result of ocular-surface inflammation. Meanwhile, the presence of oestrogen receptor in the corneal layers remains controversial in the literature. Previous studies have shown oestrogen receptor α-β and androgen receptor in human, rat and mouse cornea [45–47]. Oestrogen receptor α was shown in epithelial and stromal layers of cornea, oestrogen β and androgen receptor was shown in epithelial, stroma and endothelial layers of cornea [48]. Changes in these hormones eventually affect the cornea structurally and functionally due to association with protein synthesis in corneal cells [49]. Consequently, the cornea can be directly affected by changes in the amount of systemic sex hormones due to menstruation, drug use or aging.
The limitation of this study is relatively limited sample size. However, only postmenopausal hormone receptor positive breast cancer patients who receiving only anastrozole/letrozole were included to prevent any confounding factor of other treatments for breast cancer including radiotherapy and chemotherapy.
In conclusion, to the best of our knowledge this is the first prospective study which evaluated of the effects of AIs on MGs both qualitatively and quantitatively, and corneal layers and sub-basal nerve plexus. Our results showed a decrease in sub-basal nerve densities with a decreased corneal sensitivity, and in all corneal cell densities in these patient group. This structural changes in corneal nerves may contribute to the dry eye pathogenesis in these patient group. In addition, we were able to show that AIs cause deterioration in the ocular-surface parameters depending on the duration of the treatment. According to these findings, these patients should be observed during the AI therapy in terms of the ocular-surface side effects.
Summary
What was known before
Aromatase inhibitors increase subjective symptoms and induce dry eye syndrome by causing deterioration in ocular-surface parameters.
Aromatase inhibitors are associated with the development of meibomian gland dysfunction.
What this study adds
Aromatase inhibitors cause alterations on the ocular surface, meibomian gland and cornea at the cellular level.
Aromatase inhibitors cause a decrease in the number of meibomian glands, corneal nerves and cells.
The deteriorational effects of aromatase inhibitors on the ocular surface, meibomian gland and cornea increase depending on the duration of treatment.
Acknowledgements
This work was presented in part at the 52nd Turkish Ophthalmological Association National Congress (November,13-18,2018, Antalya, Turkey).
Author contributions
Design of the study: SK, MI; data collection: AA, AYG, SA; analysis and interpretation: AA, SK, JK, MI; manuscript preparation: AA, SK, MI; critical appraisal of the manuscript: SK, MI
Funding
This study was funded by the authors and did not receive any grant from finance agencies in the public or commercial sectors.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lonning PE. The potency and clinical efficacy of aromatase inhibitors across the breast cancer continuum. Ann Oncol. 2011;22:503–14. doi: 10.1093/annonc/mdq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomao F, Spinelli G, Vici P, Pisanelli GC, Cascialli G, Frati L, et al. Current role and safety profile of aromatase inhibitors in early breast cancer. Expert Rev Anticancer Ther. 2011;11:1253–63. doi: 10.1586/era.11.96. [DOI] [PubMed] [Google Scholar]
- 3.Chatziralli I, Sergentanis T, Zagouri F, Chrysikos D, Ladas I, Zografos GC, et al. Ocular surface disease in breast cancer patients using aromatase inhibitors. Breast J. 2016;22:561–3. doi: 10.1111/tbj.12633. [DOI] [PubMed] [Google Scholar]
- 4.Ogueta SB, Schwartz SD, Yamashita CK, Farber DB. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest Ophthalmol Vis Sci. 1999;40:1906–11. [PubMed] [Google Scholar]
- 5.Munaut C, Lambert V, Noel A, Frankenne F, Deprez M, Foidart JM, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol. 2001;85:877–82. doi: 10.1136/bjo.85.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliot SJ, Catanuto P, Espinosa-Heidmann DG, Fernandez P, Hernandez E, Saloupis P, et al. Estrogen receptor beta protects against in vivo injury in RPE cells. Exp Eye Res. 2010;90:10–16. doi: 10.1016/j.exer.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisner A, Falardeau J, Toomey MD, Vetto JT. Retinal hemorrhages in anastrozole users. Optom Vis Sci. 2008;85:301–8. doi: 10.1097/OPX.0b013e31816bea3b. [DOI] [PubMed] [Google Scholar]
- 8.Karagoz B, Ayata A, Bilgi O, Uzun G, Unal M, Kandemir EG, et al. Hemicentral retinal artery occlusion in a breast cancer patient using anastrozole. Onkologie. 2009;32:421–3. doi: 10.1159/000218369. [DOI] [PubMed] [Google Scholar]
- 9.Eisner A, Thielman EJ, Falardeau J, Vetto JT. Vitreo-retinal traction and anastrozole use. Breast Cancer Res Treat. 2009;117:9–16. doi: 10.1007/s10549-008-0156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sathiamoorthi S, Ruddy KJ, Bakri SJ. Association of uveitis and macular edema with anastrozole therapy. JAMA Ophthalmol. 2018;136:837–9. doi: 10.1001/jamaophthalmol.2018.1700. [DOI] [PubMed] [Google Scholar]
- 11.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–9. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 12.Affinito P, Di Spiezio Sardo A, Di Carlo C, Sammartino A, Tommaselli GA, Bifulco G, et al. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause. 2003;10:482–7. doi: 10.1097/01.GME.0000063568.84134.35. [DOI] [PubMed] [Google Scholar]
- 13.Berman ER. Tears. In: Biochemistry of the Eye. Boston: Springer US; 1991. p. 63–88.
- 14.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–53. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 15.Rizner TL, Thalhammer T, Ozvegy-Laczka C. The importance of steroid uptake and intracrine action in endometrial and ovarian cancers. Front Pharmacol. 2017;8:346. doi: 10.3389/fphar.2017.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–8. doi: 10.1016/S0039-128X(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul Surf. 2017;15:284–333. doi: 10.1016/j.jtos.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Versura P, Giannaccare G, Campos EC. Sex-steroid imbalance in females and dry eye. Curr Eye Res. 2015;40:162–75. doi: 10.3109/02713683.2014.966847. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan DA, Sullivan BD, Ullman MD, Rocha EM, Krenzer KL, Cermak JM, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41:3732–42. [PubMed] [Google Scholar]
- 20.Sullivan DA, Rocha EM, Ullman MD, Kreuzer KL, Gao J, Toda I et al. Androgen Regulation of the Meibomian Gland. In: Sullivan DA, Dartt DA, Meneray MA (eds). Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2: Basic Science and Clinical Relevance. Boston: Springer US; 1998. p. 327–31. [DOI] [PubMed]
- 21.Liu M, Richards SM, Schirra F, Yamagami H, Sullivan BD, Sullivan DA. Identification of androgen-regulated genes in the lacrimal gland. Adv Exp Med Biol. 2002;506:129–35. doi: 10.1007/978-1-4615-0717-8_17. [DOI] [PubMed] [Google Scholar]
- 22.Smith RE. The tear film complex: pathogenesis and emerging therapies for dry eyes. Cornea. 2005;24:1–7. doi: 10.1097/01.ico.0000141486.56931.9b. [DOI] [PubMed] [Google Scholar]
- 23.Inglis H, Boyle FM, Friedlander ML, Watson SL. Dry eyes and AIs: If you don’t ask you won’t find out. Breast. 2015;24:694–8. doi: 10.1016/j.breast.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Turaka K, Nottage JM, Hammersmith KM, Nagra PK, Rapuano CJ. Dry eye syndrome in aromatase inhibitor users. Clin Exp Ophthalmol. 2013;41:239–43. doi: 10.1111/j.1442-9071.2012.02865.x. [DOI] [PubMed] [Google Scholar]
- 25.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:108–52. [DOI] [PubMed]
- 26.Irkec MT, Group TOS. Reliability and validity of Turkish translation of the ocular surface disease index (OSDI) in dry eye syndrome. Invest Ophthalmol Vis Sci. 2007;48:408–408. [Google Scholar]
- 27.Pult H, Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye. 2013;36:22–27. doi: 10.1016/j.clae.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 28.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spelsberg H, Klueppel M, Reinhard T, Glaeser M, Niederacher D, Beckmann MW, et al. Detection of oestrogen receptors (ER) alpha and beta in conjunctiva, lacrimal gland, and tarsal plates. Eye (Lond) 2004;18:729–33. doi: 10.1038/sj.eye.6701314. [DOI] [PubMed] [Google Scholar]
- 30.Song X, Zhao P, Wang G, Zhao X. The effects of estrogen and androgen on tear secretion and matrix metalloproteinase-2 expression in lacrimal glands of ovariectomized rats. Invest Ophthalmol Vis Sci. 2014;55:745–51. doi: 10.1167/iovs.12-10457. [DOI] [PubMed] [Google Scholar]
- 31.Krenzer KL, Dana MR, Ullman MD, Cermak JM, Tolls DB, Evans JE, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab. 2000;85:4874–82. doi: 10.1210/jcem.85.12.7072. [DOI] [PubMed] [Google Scholar]
- 32.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–8. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 33.Gibson E, Stapleton F, Dear R, Wolffsohn JS, Golebiowski B. Dry eye signs and symptoms in aromatase inhibitor treatment and the relationship with pain. Ocul Surf. 2020;18:108–13. doi: 10.1016/j.jtos.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Schirra F, Richards SM, Jensen RV, Sullivan DA. Estrogen and progesterone control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2008;49:1797–808. doi: 10.1167/iovs.07-1458. [DOI] [PubMed] [Google Scholar]
- 35.Kam W, Sullivan D. Suppressive effects of 17β-estradiol on immortalized human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2013;54:4316–4316. [Google Scholar]
- 36.Darabad RR, Suzuki T, Richards SM, Jensen RV, Jakobiec FA, Zakka FR, et al. Influence of aromatase absence on the gene expression and histology of the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2013;54:987–98. doi: 10.1167/iovs.12-10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong L, Gupta PK. Need for animal models of meibomian gland dysfunction. Ophthalmol Ther. 2016;5:129–34. doi: 10.1007/s40123-016-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–9. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai D, Zhu M, Petroll WM, Koppaka V, Robertson DM. The impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epithelium. Am J Pathol. 2014;184:2662–70. doi: 10.1016/j.ajpath.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Diaz-Valle D, Gegundez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 41.Labbe A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–31. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 42.Steger B, Speicher L, Philipp W, Bechrakis NE. In vivo confocal microscopic characterisation of the cornea in chronic graft-versus-host disease related severe dry eye disease. Br J Ophthalmol. 2015;99:160–5. doi: 10.1136/bjophthalmol-2014-305072. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Chen Q, Chen W, Cui L, Ma H, Lu F. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118:902–7. doi: 10.1016/j.ophtha.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15:404–37. doi: 10.1016/j.jtos.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tachibana M, Kasukabe T, Kobayashi Y, Suzuki T, Kinoshita S, Matsushima Y. Expression of estrogen receptor alpha and beta in the mouse cornea. Invest Ophthalmol Vis Sci. 2000;41:668–70. [PubMed] [Google Scholar]
- 46.Tachibana M, Kobayashi Y, Kasukabe T, Kawajiri K, Matsushima Y. Expression of androgen receptor in mouse eye tissues. Invest Ophthalmol Vis Sci. 2000;41:64–66. [PubMed] [Google Scholar]
- 47.Suzuki T, Kinoshita Y, Tachibana M, Matsushima Y, Kobayashi Y, Adachi W, et al. Expression of sex steroid hormone receptors in human cornea. Curr Eye Res. 2001;22:28–33. doi: 10.1076/ceyr.22.1.28.6980. [DOI] [PubMed] [Google Scholar]
- 48.Hadeyama T, Nakayasu K, Ha NT, Nakamura S. Expression of estrogen receptors alpha and beta, androgen receptors and progesterone receptors in human cornea. Nippon Ganka Gakkai Zasshi. 2002;106:557–64. [PubMed] [Google Scholar]
- 49.Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand. 2000;78:146–53. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]


