Abstract
Extracellular vesicles are released by the majority of cell types and circulate in body fluids. They function as a long-distance cell-to-cell communication mechanism that modulates the gene expression profile and fate of target cells. Increasing evidence has established a central role of extracellular vesicles in kidney physiology and pathology. Urinary extracellular vesicles mediate crosstalk between glomerular and tubular cells and between different segments of the tubule, whereas circulating extracellular vesicles mediate organ crosstalk and are involved in the amplification of kidney damage and inflammation. The molecular profile of extracellular vesicles reflects the type and pathophysiological status of the originating cell so could potentially be exploited for diagnostic and prognostic purposes. In addition, robust preclinical data suggest that administration of exogenous extracellular vesicles could promote kidney regeneration and reduce inflammation and fibrosis in acute and chronic kidney diseases. Stem cells are thought to be the most promising source of extracellular vesicles with regenerative activity. Extracellular vesicles are also attractive candidates for drug delivery and various engineering strategies are being investigated to alter their cargo and increase their efficacy. However, rigorous standardization and scalable production strategies will be necessary to enable the clinical application of extracellular vesicles as potential therapeutics.
Subject terms: Chronic kidney disease, Kidney, Biomarkers, Acute kidney injury, Renal cancer
In this Review, the authors discuss the roles of extracellular vesicles in kidney physiology and disease as well as the beneficial effects of stem cell-derived extracellular vesicles in preclinical models of acute kidney injury and chronic kidney disease. They also highlight current and future clinical applications of extracellular vesicles in kidney diseases.
Key points
Urinary extracellular vesicles have roles in intra-glomerular, glomerulo-tubular and intra-tubular crosstalk, whereas circulating extracellular vesicles might mediate organ crosstalk; these mechanisms could amplify kidney damage and contribute to disease progression.
Urinary extracellular vesicles could potentially be analysed using multiplex diagnostic platforms to identify pathological processes and the originating cell types; technological advances including single extracellular vesicle analysis might increase the specificity of bulk analysis of extracellular vesicle preparations.
Robust standardization and validation in large patient cohorts are required to enable clinical application of extracellular vesicle-based biomarkers.
Stem cell-derived extracellular vesicles have been shown to improve renal recovery, limit progression of injury and reduce fibrosis in animal models of acute kidney injury and chronic kidney disease.
Various engineering approaches can be used to load extracellular vesicles with therapeutic molecules and increase their delivery to the kidney.
A small clinical trial that tested the efficacy of mesenchymal stem cell extracellular vesicle administration in patients with chronic kidney disease reported promising results; however, therapeutic application of extracellular vesicles is limited by a lack of scalable manufacturing protocols and clear criteria for standardization.
Introduction
Extracellular vesicles are a heterogeneous population of membrane-bound vesicles that are considered to be central messengers for intercellular communication1. They are released by the majority of cell types and can be classified into two main categories depending on their origin: exosomes, which are released by the endosomal compartment and have an average size of 100 nm, and ectosomes, which are released by budding of the plasma membrane and range in size from microvesicles to large vesicles (50 nm to 1 µm)2,3. Apoptotic bodies that are released by cells undergoing apoptosis4, large tumour-derived vesicles (oncosomes)5 and mitochondrial-derived vesicles (mitovesicles)6, are also classed as extracellular vesicles.
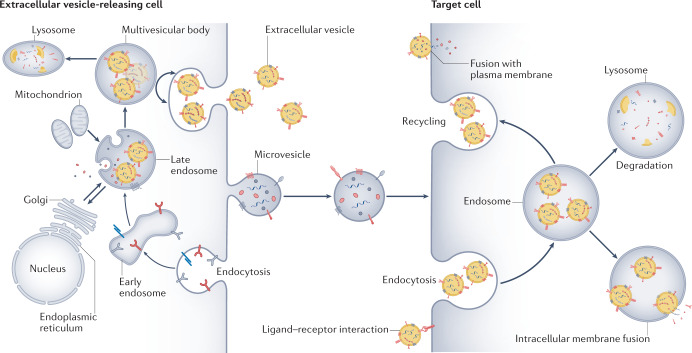
Exosomes originate from a sequential process that begins with invagination of the plasma membrane and the de novo formation of early-sorting endosomes that contain a mixture of cell-surface proteins and extracellular components. The early-sorting endosomes mature into late-sorting endosomes and the trans-Golgi network and the endoplasmic reticulum participate in sorting and defining the content of the endosomes7. The late sorting endosomes give rise to multivesicular bodies that contain intraluminal vesicles, the future exosomes (Fig. 1). Multivesicular bodies can fuse with lysosomes to be degraded or with the cellular membrane to shed mature exosomes2.
Fig. 1. Extracellular vesicle biogenesis and uptake by target cells.
Sequential invagination of the plasma membrane generates early endosomes that contain a mixture of cell-surface proteins and extracellular components. The endosome maturation process involves the formation of late-sorting endosomes and then intracellular multivesicular bodies that contain intra-luminal vesicles, the future exosomes. The trans-Golgi network and the endoplasmic reticulum participate in sorting and defining the content of the endosomes. Multivesicular bodies can fuse with lysosomes to be degraded or with the cellular membrane to release mature exosomes (extracellular vesicles) into the extracellular space. Microvesicles originate from shedding of the plasma membrane and contain cell-surface proteins and cytoplasmic components. Following their release, extracellular vesicles and microvesicles can be taken up by target cells via endocytosis or fusion with the plasma membrane, releasing their contents. They can also activate target cells via ligand binding to cell-surface receptors.
The biogenesis of exosomes is regulated by the endosomal sorting complexes required for transport (ESCRT) pathway. Some ESCRT proteins, including tumour susceptibility gene 101 protein (TSG101), ALG2-interacting protein X (ALIX), soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and heat shock protein 90 (HSP90), are present in the released exosomes8. The tetraspanins CD9, CD63 and CD81 are commonly used as biomarkers for exosome identification4. However, given the uncertain boundary between exosomes and ectosomes in terms of size and markers, some studies simply subdivide extracellular vesicles into small (<100 nm), medium (100–200 nm) and large extracellular vesicles (>200 nm)4.
Despite the heterogeneous and diverse nature of extracellular vesicles, their central role in numerous physiological and pathological conditions has been clearly defined. Extracellular vesicles are present at high levels in biological fluids and can be easily isolated from these fluids. Their complex cargo reflects the type and pathophysiological status of the originating cell and so represents an important source of information. Extracellular vesicles can be internalized by target cells in an archaic form of long-distance cell-to-cell communication that has been retained throughout evolution9. Differences in their size and surface molecules may affect their ability to be internalized. Several mechanisms of extracellular vesicle internalization have been observed, including endocytosis, micropinocytosis, phagocytosis, plasma or endosomal fusion of membranes and endocytosis mediated by clathrin or caveolin10. Following cellular uptake, extracellular vesicles may release their full cargo into the cytoplasm (Fig. 1). This transfer of biological material, including extracellular RNAs, small non-coding RNA species, lipids and proteins, may modify the recipient cells11,12. Internalized extracellular vesicles can also be degraded by lysosomes or recycled and released into the extracellular space8. Extracellular vesicles can also activate target cells by receptor–ligand interactions without being internalized and delivering their content13.
Extracellular vesicles seem to have an important role in kidney physiology, beginning from the initial phase of nephrogenesis. Embryonic kidney stem cell-derived extracellular vesicles were shown to modulate the secondary inductive interactions between the ureteric bud and metanephric mesenchyme that control organogenesis14. Moreover, extracellular vesicles present in the urine or circulation might participate in the modulation of kidney functions and in communication between different regions of the nephron15. Extracellular vesicles could also be exploited for various preclinical and clinical applications. In this Review, we discuss the role of extracellular vesicles in kidney physiology and disease as well as the beneficial effects of stem cell-derived extracellular vesicles in preclinical models of acute kidney injury (AKI), chronic kidney disease (CKD) and kidney graft preconditioning. We also highlight current and future clinical applications of extracellular vesicles in kidney diseases.
Extracellular vesicles and the kidney
Urinary extracellular vesicles are a heterogeneous population that mainly originate from the cells of the urogenital tract; the most prevalent sources of these vesicles are glomerular, tubular, prostate and bladder cells15. A proteomic analysis demonstrated that 99.96% of the proteins present in urinary extracellular vesicles are characteristic of cells of the urogenital tract15. Urinary extracellular vesicles express typical apical membrane markers, such as transporters and channels, but do not express exclusively basolateral markers, suggesting that they mainly originate from the apical membranes of urogenital cells16. A comparison of kidney-derived urinary extracellular vesicles (obtained from patients with nephrostomy drains) with extracellular vesicles derived from the complete urinary tract showed that the vast majority of urinary extracellular vesicle proteins are present in both types of samples, suggesting that the kidney is the main source of urinary extracellular vesicles17.
A small fraction of urinary extracellular vesicle proteins (0.04%) are not characteristic of urogenital cells16, raising the possibility that some of these vesicles originate from kidney-infiltrating cells or from serum-circulating extracellular vesicles. However, the latter possibility seems unlikely in the presence of an intact glomerular filtration barrier as the slit diaphragm may only allow passage of 4-nm structures18. Serum extracellular vesicles could potentially cross the podocyte layer via a process of transcytosis. A study that used a microfluidic model to reproduce the glomerular barrier demonstrated that serum extracellular vesicles that were engineered to express cel-miR-39 from Caenorhabditis elegans were able to pass through this barrier and transfer the exogenous microRNA (miRNA) to human podocytes19. Urinary excretion of intravenously injected labelled extracellular vesicles has been shown in rats20 but, to date, urinary excretion of endogenous non-urinary extracellular vesicles has not been clearly demonstrated.
Passage of extracellular vesicles through the filtration barrier can be expected in pathological conditions in which basal membrane rupture allows the passage of large structures such as erythrocytes. For example, in rodent models, AKI increased the renal biodistribution of intravenously injected labelled extracellular vesicles21. These extracellular vesicles were rapidly (5 min after injection) taken up by proximal tubule cells22,23.
Role in intra-nephron communication
Several studies have suggested a potential role of urinary extracellular vesicles in intra-nephron communication across the glomerular and tubular regions. Extracellular vesicles released by cells in the upper segments of tubules can be internalized by downstream cells, transferring active molecules and modulating the behaviour of the recipient cells. One of the first studies of intra-tubular extracellular vesicle communication reported that cultured murine kidney collecting duct cells could transfer functional aquaporin 2 (AQP2) via the release and uptake of extracellular vesicles24. A subsequent study showed that labelled extracellular vesicles isolated from proximal tubular cells could be taken up in vitro by distal tubule and collecting duct cells25. A study published in preprint form provided evidence of the physiological transfer of extracellular RNAs, likely carried by extracellular vesicles, along the nephron in mice26. In this study, podocyte RNA was labelled using a sequence-based metabolic approach (SH-linked alkylation for the metabolic sequencing of RNA) and subsequently detected in tubular cells, suggesting transfer from the glomerular to the tubular region.
Role in amplification of kidney disease
Extracellular vesicle-mediated long-distance cell-to-cell communication between different regions of the kidney and between different organs could amplify kidney damage. In vitro studies suggest that altered extracellular vesicle-mediated communication between glomerular endothelial cells and podocytes might impair podocyte functions27. Moreover, podocyte extracellular vesicles might mediate crosstalk between the glomeruli and the tubules and thereby promote tubular injury28. This mechanism could contribute to the amplification of damage, the development of tubulointerstitial fibrosis and the progression of CKD.
Cultured human podocytes that were exposed to damaging stimuli (cyclical stretch or high glucose) released an elevated number of extracellular vesicles compared with untreated podocytes29. These extracellular vesicles were taken up by proximal tubule epithelial cells in vitro and induced a profibrotic phenotype with expression of fibronectin and collagen IV and activation of p38 and mothers against decapentaplegic homologue 3 (SMAD3)30. Moreover, extracellular vesicles from puromycin-treated podocytes induced apoptosis of tubule cells via the transfer of their miRNA cargo28. Among the miRNAs that were upregulated in the injured podocyte extracellular vesicles, miR-149 and miR-424 were suggested to be responsible for the induction of apoptosis.
Extracellular vesicles might also have a role in tubulointerstitial inflammation, which promotes kidney disease progression31. In various murine models, high tubular expression of hypoxia-inducible factor 1α (HIF1α) in combination with an inflammatory milieu promoted the release of miR-23a-enriched extracellular vesicles by tubular epithelial cells32. When taken up by macrophages, these extracellular vesicles induced reprogramming of the cells into a pro-inflammatory state, amplifying the inflammatory cascade. These studies clearly indicate a role of extracellular vesicles released by injured cells along the nephron in the progression of kidney injury (Fig. 2). Whether extracellular vesicles might also be released by regenerating renal cells and support a positive paracrine effect is unknown.
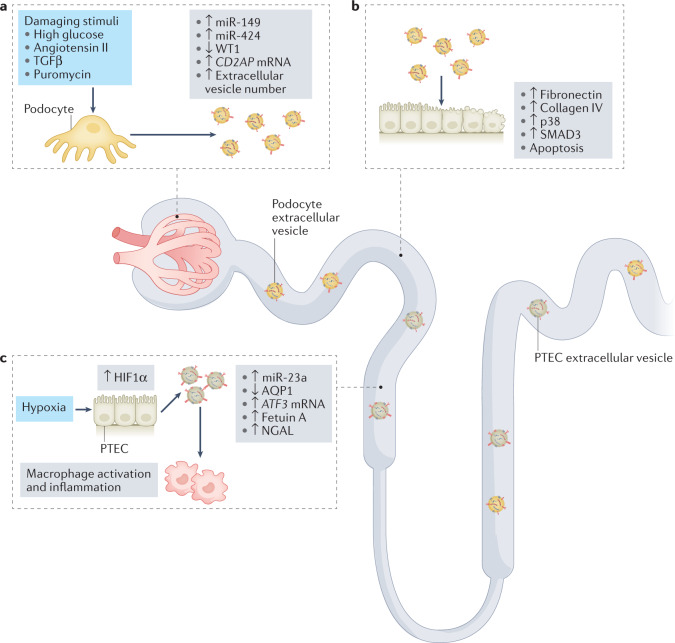
Fig. 2. The roles of extracellular vesicles in the amplification of kidney damage.
Urinary extracellular vesicles can mediate crosstalk between nephron compartments. a | Damaging stimuli induce an increase in the release of podocyte extracellular vesicles29 and modulate their content28. Upregulation of CD2AP mRNA and downregulation of Wilms tumour protein 1 (WT1) in extracellular vesicles are potential biomarkers of podocyte damage. b | Extracellular vesicles released from damaged podocytes can be taken up by proximal tubule epithelial cells (PTECs) and induce apoptosis (potentially via transfer of miR-149 and miR-424)28 and activation of pro-fibrotic pathways, resulting in phosphorylation of p38 and mothers against decapentaplegic homologue 3 (SMAD3) and upregulation of fibronectin and collagen IV30. c | In response to hypoxia, PTECs upregulate hypoxia-inducible factor 1α (HIF1α) and shed extracellular vesicles that express miR-23a. These exosomes promote macrophage activation and inflammation32. Downregulation of aquaporin 1 (AQP1) and upregulation of ATF3 mRNA, fetuin A and neutrophil gelatinase-associated lipocalin (NGAL) in extracellular vesicles are potential biomarkers of tubular damage. TGFβ, transforming growth factor-β.
Extracellular vesicles that are released into the circulation by distant tissues might have a role in intra-organ crosstalk and in the amplification of pathological conditions characterized by multi-organ dysfunction. Such crosstalk was suggested by a proteomic analysis of extracellular vesicles isolated from the murine heart that identified proteins that seemed to originate from various other organs, including the kidney33. Circulating extracellular vesicles from other organs might also affect the kidney. For example, in pre-eclampsia, placenta-derived extracellular vesicles carrying anti-angiogenic factors that are released into the maternal circulation might lead to glomerular endothelial dysfunction and proteinuria34. Circulating extracellular vesicles in autoimmune diseases, such as systemic lupus erythematosus, antiphospholipid syndrome and ANCA-associated vasculitis, might promote coagulation, thrombosis and immune-mediated renal pathological conditions35.
CKD-related cardiovascular dysfunction has been associated with high levels of circulating endothelial-derived microparticles36 that may mediate vascular remodelling, vascular wall injury and inflammation37. These microparticles are also associated with increased risk of vascular calcification, which contributes to cardiovascular disease in CKD, as well as with kidney failure38 and anaemia39. Extracellular vesicles might also contribute to the worsening of kidney damage during liver or heart disease and vice versa, thereby participating in the development of hepatorenal or cardiorenal syndrome. However, further research is needed to investigate this possibility. The development of single extracellular vesicle analysis and in vivo tracking approaches will help to elucidate the roles of extracellular vesicles in inter-organ and intra-organ communication.
Extracellular vesicle biomarkers
Urinary extracellular vesicles are promising candidates for the identification of non-invasive biomarkers to predict and monitor the progression of pathological conditions of the kidney (Table 1). An advantage of urinary extracellular vesicle biomarkers is that they could potentially be analysed using a multiplex diagnostic platform to identify pathological processes and the originating cell types. Changes in the extracellular vesicle cargo might occur before alterations of classical serum markers of kidney function, such as urea and creatinine levels, enabling early diagnosis. However, several factors currently limit the clinical application of urinary extracellular vesicle biomarkers.
Table 1.
Potential biomarkers of kidney diseases detected in urinary extracellular vesicles
| Disease | Marker | Level compared with healthy individuals | Type of damage or regeneration | Detection method | Refs |
|---|---|---|---|---|---|
| Acute kidney injury | AQP1 | Decreaseda | Tubular cell injury | Western blot | 44 |
| ATF3 mRNA | Increaseda | qRT-PCR | 46 | ||
| Fetuin A | Increaseda | MALDI-TOF, LC–MS/MS, western blot | 45 | ||
| Focal segmental glomerulosclerosis | WT1 | Increaseda | Podocyte damage | Western blot | 47 |
| Type 1 diabetes mellitus | WT1 | Increased | Podocyte damage | Western blot | 48 |
| miR-130a and miR-145 | Increaseda | Glomerular mesangial damage | qRT-PCR | 56 | |
| miR-155 and miR-424 | Decreaseda | ||||
| Chronic kidney disease | CD2AP mRNA | Decreased | Podocyte damage | qRT-PCR | 50 |
| miR-29 | Decreased | Fibrosis | qRT-PCR | 57 | |
| miR-200b | Increased | ||||
| miR-181a-5p and miR-451 | Increased | Not known | qRT-PCR | 58,59 | |
| IgA nephropathy | miR-215-5p and miR-378i | Increased | Not known | qRT-PCR | 60 |
| miR-29c and miR-205-5p | Decreased | ||||
| Lupus nephritis | miR-146a, miR-150 and miR-21 | Increased | Fibrosis | qRT-PCR | 61 |
| Kidney transplantation | NGAL | Increased | Tubular cell injury | Western blot | 51 |
| CD133 | Decreased | Tubular cell regeneration | FACS, western blot | 52 |
AQP1, aquaporin 1; FACS, fluorescence-activated cell sorting; LC, liquid chromatography; MALDI-TOF, matrix-assisted laser desorption/ionization time of flight; MS, mass spectrometry; NGAL, neutrophil gelatinase-associated lipocalin; qRT-PCR, quantitative real-time polymerase chain reaction; WT1, Wilms tumour protein 1. aAlso demonstrated in rodent models.
Standardized methods for urine collection and preservation and protocols for extracellular vesicle isolation and removal of contaminants are critical to guarantee reproducibility of results and limit artefacts15,40,41. Moreover, the lack of a standardized methodology for urinary extracellular vesicle quantification and, more importantly, normalization, might affect results and reproducibility between different centres. Urinary creatinine concentration has been proposed as a normalizer for urinary extracellular vesicle concentration because it correlates with particle number and its excretion is not affected by water intake42,43. In addition, tetraspanin levels could be used as normalizers for urinary extracellular vesicle quantity. Nephron mass directly correlates with urinary extracellular vesicle excretion and so should also be considered when comparing biomarker levels17.
Biomarkers of kidney injury and disease
Studies in rodent models of AKI identified decreased levels of aquaporin 1 (AQP1) and increased levels of fetuin A and activating transcription factor 3 (ATF3) in urinary extracellular vesicles as potential biomarkers of tubular damage44–46. However, these data have only been validated in a small number of patients. A slightly more robust body of literature supports the detection of urinary extracellular vesicle markers as an early sign of podocyte injury. For example, the number of podoplanin-expressing large extracellular vesicles in urine was reported to be a marker of diabetic glomerular injury in a mouse model29. The urinary extracellular vesicle expression of Wilms tumour protein (WT1) is another potential biomarker of podocyte injury47. The level of urinary exosomal WT1 was significantly higher in 7 patients with focal segmental glomerulosclerosis than in 5 healthy volunteers47. Similarly, in a cohort of 48 patients with type 1 diabetes mellitus (T1DM), increased urinary extracellular vesicle levels of WT1 were associated with proteinuria and a decline in estimated glomerular filtration rate48. By contrast, a study that included 40 children with idiopathic nephrotic syndrome, reported no significant differences in exosomal WT1 expression between those with different levels of proteinuria, renal pathological conditions or steroid responsiveness49. A study that included 32 patients with kidney disease reported that reduced levels of the podocyte-specific CD2AP mRNA in urinary extracellular vesicles were associated with kidney fibrosis and correlated with serum creatinine levels and glomerular filtration rate50.
Another potential application of urinary extracellular vesicles is in the monitoring of kidney graft function. The levels of urinary extracellular vesicles expressing the kidney injury marker neutrophil gelatinase-associated lipocalin (NGAL) were increased and the levels of those expressing the renal progenitor cell marker CD133 were decreased in transplant recipients with delayed graft function, reflecting tubular cell injury and regeneration, respectively51,52.
Modulation of miRNAs may also characterize renal diseases or their evolution. The presence of RNA species in extracellular vesicle cargos was first reported in 2007 (ref.53). Exosomal miRNAs are more stable than free miRNAs as they are protected from degradation owing to RNase activity in biofluids54. Numerous miRNAs within urinary extracellular vesicles are dysregulated during CKD, kidney fibrosis and diabetic kidney disease (DKD)55. A study of urinary extracellular vesicles from patients with T1DM showed enrichment of miR-130a and miR-145 and a reduction of miR-155 and miR-424 in those from patients with microalbuminuria56.
miR-29c seems to be one of the most consistently altered miRNAs in kidney disease. A study that included 32 patients with CKD reported that the levels of miR-29c were decreased and that levels of miR-200b were increased in urinary extracellular vesicles from these patients compared with healthy individuals57. Moreover, the level of miR-29c in urinary extracellular vesicles correlated with estimated glomerular filtration rate and the severity of tubulointerstitial fibrosis. Other miRNAs that are overexpressed in urinary extracellular vesicles from patients with CKD include miR-181a-5p and miR-451 (refs58,59). In urinary extracellular vesicles from 18 patients with IgA nephropathy, miR-29c and miR-205-5p were downregulated, whereas miR-215-5p and miR-378i were upregulated compared with those from healthy individuals60. Decreased levels of miR-29c and increased levels of miR-150 and miR-21 in urinary extracellular vesicles from patients with lupus nephritis were associated with kidney fibrosis. These miRNAs promote fibrosis by acting on the TGFβ–SMAD3 pathway and regulating collagen production61. Other classes of molecules in extracellular vesicles, such as small RNA species, should also be investigated as candidate biomarkers. For example, circular RNAs were reported to be upregulated in blood and urinary extracellular vesicles from patients with membranous nephropathy62.
Although a number of promising candidate biomarkers for kidney diseases have been identified, the heterogeneity of urinary extracellular vesicle analyses and the small number of patients included in published studies preclude their readiness for clinical translation. Robust standardization, studies with large numbers of patients and reproduction of findings in independent cohorts is needed to enable clinical translation. In addition, technological advances, including single extracellular vesicle analysis using flow cytometry or arrays based on extracellular vesicle-capturing chips40, might increase the specificity of results in comparison with bulk analyses of urinary extracellular vesicle preparations.
Biomarkers of renal cell carcinoma
Cancer-derived extracellular vesicles influence the tumour microenvironment and sustain and promote cancer progression63. As the levels of these extracellular vesicles increase in blood and urine as cancer develops64, they are promising candidates for the identification of non-invasive diagnostic biomarkers. One of the first studies that compared the proteomic profiles of urinary extracellular vesicles from patients with renal cell carcinoma (RCC) and healthy individuals identified several specific markers of RCC, including increased expression of metalloproteinase 9 (MMP9), ceruloplasmin, podocalyxin, carbonic anhydrase 9 (CAIX) and dickkopf-related protein 4 (DKK4), and decreased expression of neprilysin (also known as CD10), extracellular matrix metalloproteinase inducer (EMMPRIN; also known as basigin), dipeptidase 1, syntenin 1 and AQP1 (ref.65). An ongoing clinical trial is aimed at using electron microscopy to detect urinary extracellular vesicles that express CAIX in combination with the exosomal marker CD9 in the urine of patients with clear-cell renal cell carcinoma (ccRCC)66.
Urinary extracellular vesicle miRNAs have also been investigated as biomarkers of RCC. The presence of increased levels of miR-126-3p combined with miR-449a or miR-34b-5p in urinary extracellular vesicle cargo was reported to distinguish between patients with ccRCC and healthy individuals67. Another study reported upregulation of miR-204-5p in urinary exosomes from patients with Xp11 translocation RCC (a rare sporadic paediatric kidney cancer), suggesting that this upregulation could be a useful biomarker for early diagnosis68. Increased serum levels of miR-224 have been proposed as a biomarker of poor prognosis in patients with ccRCC69, whereas upregulation of serum exosomal miR-210 might distinguish patients with ccRCC from healthy individuals70. Altered miRNA expression in plasma extracellular vesicles from patients with RCC has also been described, with upregulation of miR-149-3p and miR-424-3p and downregulation of miR-92a-1-5p71.
The RNA profile64 and long non-coding RNAs72 (lncRNAs) have also been proposed as new classes of urinary extracellular vesicle RCC biomarkers. LncRNAs can be selectively compartmentalized into extracellular vesicles and can modulate cancer progression and drug resistance72. For example, sunitinib-resistant RCC cells express a high level of lncARSR compared with sunitinib-sensitive RCC cells73. This lnRNA can be transferred via extracellular vesicles to recipient cells, potentially transmitting drug resistance73.
Therapeutic use of extracellular vesicles
Evidence from preclinical models of AKI, CKD and kidney transplant preconditioning suggests that extracellular vesicles from various sources, particularly those released by stem cells, have therapeutic properties and can accelerate kidney recovery74 (Fig. 3). Mesenchymal stromal cells (MSCs) are currently the most utilized cell source for regenerative medicine owing to their well-established immunomodulatory, pro-regenerative and anti-inflammatory properties74. MSC extracellular vesicles are active components of the MSC secretome that are responsible for many of these beneficial effects74,75. However, extracellular vesicles from other sources, including kidney cells, might also have therapeutic activity.
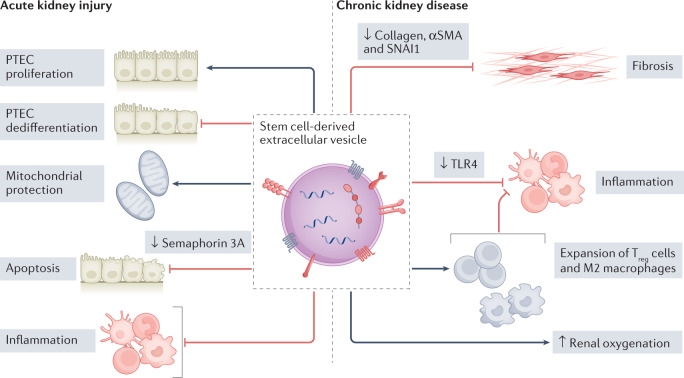
Fig. 3. Application of stem cell-derived extracellular vesicles for kidney regeneration.
Data from studies in animal models suggest that the treatment with stem cell-derived extracellular vesicles can accelerate renal repair in acute kidney injury and chronic kidney disease, promote proximal tubular epithelial cell (PTEC) proliferation, improve renal oxygenation and inhibit PTEC dedifferentiation, inflammation, apoptosis and fibrosis. Various mechanisms have been reported to underlie these beneficial effects. For example, extracellular vesicle miRNAs have been shown to induce semaphorin 3 A downregulation, resulting in a reduction in apoptosis128, to target genes involved in fibrosis progression such as Col1a1, Acta2 and Snai1 and thereby inhibit fibrosis104,105 and to downregulate Toll-like receptor 4 (TLR4) in podocytes, resulting in a reduction in inflammation and podocyte damage108. Mesenchymal stromal cell extracellular vesicles have also been reported to attenuate inflammation by promoting the expansion of regulatory T (Treg) cells and M2 macrophages99. αSMA, α-smooth muscle actin; SNAI1, zinc-finger protein SNAI1.
Stem cell-derived extracellular vesicles
Several key characteristics could potentially make stem cell-derived extracellular vesicles more effective therapeutic agents than the cells from which they are secreted. Extracellular vesicles may be less immunogenic, less toxic and more able to cross biological barriers than stem cells. Moreover, extracellular vesicles are intrinsically stable in the circulation owing to their negatively charged surface76,77 and they may have targeting properties that are dictated by their lipid composition and surface molecules78,79. Further studies are required to assess whether delivery of extracellular vesicles could confer more effective renoprotection than delivery of their parental MSCs80.
Acute kidney injury
The therapeutic effects of MSC extracellular vesicles have been well established since 2009, when a pioneering study demonstrated that intravenously injected bone marrow (BM)-MSC extracellular vesicles promoted tissue repair in a murine model of rhabdomyolysis-induced AKI22 (Supplementary Table 1). In models of toxic and ischaemic AKI, BM-MSC extracellular vesicles promoted tubular cell proliferation and limited apoptosis, resulting in amelioration of loss of kidney function and reductions in plasma urea nitrogen and creatinine levels81–83. Similarly, administration of adipose MSC extracellular vesicles reduced inflammation and ameliorated kidney damage in models of cisplatin-induced and sepsis-induced AKI84,85. The protective mechanism in septic AKI involved an increase in sirtuin 1 (SIRT1) and a reduction in NF-κB levels in renal tissues85; whereas in cisplatin-induced AKI, the mechanism involved modulation of miRNAs (miR-880, miR-141, miR-377 and miR-21) that are associated with WNT–TGFβ, fibrosis and epithelial–mesenchymal transition signalling pathways84. Wharton jelly MSC extracellular vesicles also protected against oxidative stress and apoptosis via activation of the extracellular-signal-regulated kinase (ERK) pathway in a cisplatin AKI model87.
In rat ischaemia–reperfusion injury (IRI) models, umbilical cord MSC extracellular vesicles have been reported to promote tubular cell dedifferentiation and regeneration by stimulating the production of hepatocyte growth factor86 and to mitigate apoptosis and enhance proliferation of kidney cells as well as alleviate inflammation via suppression of CX3C-chemokine ligand 1 (CX3CL1, also known as fractalkine) expression89. Furthermore, in a mouse sepsis-associated AKI model, umbilical cord MSC extracellular vesicles reduced inflammation and improved survival via upregulation of miR-146b and inhibition of NF-κB88. Similar nephroprotective effects of extracellular vesicles derived from placenta, induced pluripotent stem cell (iPSC)-derived MSCs and iPSCs have been shown in IRI models90–92. The mechanism of action of iPSC-derived MSC extracellular vesicles included delivery of the transcription factor Sp1 into target cells, which led to increased expression of sphingosine kinase 1 (SK1) and generation of sphingosine-1-phosphate (S1P), resulting in inhibition of necroptosis91. Extracellular vesicles derived from iPSCs promoted kidney regeneration by protecting mitochondrial function and reducing oxidative stress and inflammation92.
Studies using cisplatin-induced AKI and kidney IRI models have also reported that extracellular vesicles isolated from BM-MSCs and endothelial progenitor cells can limit AKI to CKD progression23,81. BM-MSC extracellular vesicles stimulated the expression of anti-apoptotic genes, such as Bcl2l1, Bcl2 and Birc8, in damaged tubular cells81, whereas endothelial progenitor cell-derived extracellular vesicles induced kidney regeneration via the transfer of angiogenic miRNAs such as miR-126 and miR-296 (ref.23). These findings are important because even a single episode of AKI can increase the risk of developing CKD or accelerate the progression of existing CKD93. Extracellular vesicles isolated from embryonic stem cells have also been shown to promote kidney repair following AKI94. An advantage of these cells is that they can proliferate indefinitely in vitro, enabling continuous isolation of extracellular vesicles94. Studies using extracellular vesicles derived from endothelial progenitor cells and endothelial colony-forming cells have also reported protective effects in various models of kidney injury23,95–97. Currently, there is no evidence to suggest that extracellular vesicles from any particular stem cell source are superior to other stem cell-derived extracellular vesicles for amelioration of kidney damage.
Chronic kidney disease
An increasing number of studies have investigated the therapeutic potential of stem cell-derived extracellular vesicles in models of CKD, using various doses and administration approaches (Supplementary Table 1). Similar to AKI studies, the most frequently utilized cell source for CKD studies is MSCs. However, unlike AKI studies, the majority of preclinical CKD studies use multiple rather than single injections of extracellular vesicles.
In a porcine model of renal artery stenosis, adipose-derived-MSC extracellular vesicles were shown to localize in tubular cells and macrophages within the stenotic kidney, attenuate renal inflammation and improve renal oxygenation and function via delivery of the anti-inflammatory cytokine IL-10 (ref.98). A subsequent study in a porcine model of metabolic syndrome and renal artery stenosis reported that intrarenal injection of MSC extracellular vesicles promoted the expansion of regulatory T cells, upregulation of anti-inflammatory M2 macrophages and a reduction in pro-inflammatory M1 macrophages99. These effects attenuate inflammation and consequently favour kidney regeneration.
Inhibiting the fibrotic process to limit or revert the progression of CKD is a major challenge in nephrology. Multiple injections of BM-MSC extracellular vesicles were shown to prevent kidney failure in a mouse five-sixth nephrectomy model, in rodent models of kidney fibrosis induced by unilateral ureteral obstruction (UUO) and in a mouse model of aristolochic acid-induced CKD100–104. Administration of BM-MSC extracellular vesicles also resulted in reversion of renal fibrosis via the delivery of anti-fibrotic miRNAs in a mouse model of streptozotocin-induced diabetic nephropathy105.
Robust data support beneficial effects of MSC extracellular vesicles in models of DKD via various mechanisms, potentially including the induction of autophagy in kidney cells106,107 and the transfer of miR-26a-5p108. This miRNA was shown to target and inhibit Toll-like receptor 4 (TLR4), resulting in inactivation of the NF-κB pathway and limiting podocyte damage108. In a rat UUO model, infusion of human umbilical cord MSC extracellular vesicles reduced kidney fibrosis through the delivery of casein kinase 1δ and E3 ubiquitin ligase β-TRCP, which promote ubiquitination and degradation of the transcriptional coactivator YAP1 (ref.109). Beneficial effects of stem cell-derived extracellular vesicles have also been shown in other CKD models, including ciclosporin, remnant kidney and DOCA salt hypertension models110–112.
Renal cell-derived extracellular vesicles
Renal cell-derived extracellular vesicles might have advantages for kidney regeneration compared with those from other sources owing to their renal commitment. Rat kidney tubular cell extracellular vesicles that were intravenously injected into a rat IRI model improved kidney function, reduced neutrophil infiltration and limited fibrosis113. In addition, urinary extracellular vesicles from healthy people improved recovery of kidney morphology and function in a mouse AKI model114. These extracellular vesicles carried the renoprotective factor klotho, which might have a central role in the observed effects.
Stem cells of renal origin are also a potential source of extracellular vesicles for therapeutic application. For example, extracellular vesicles isolated from human glomerular MSCs or from murine renal MSCs improved kidney function in mouse models of AKI115,116. In a rat model of T1DM, extracellular vesicles isolated from urinary MSCs prevented CKD progression and limited podocyte and tubular epithelial cell apoptosis through the delivery of TGFβ1, angiogenin and bone morphogenetic protein 7 (BMP7)117.
The application of renal cell-derived extracellular vesicles in preclinical studies and, more importantly, in future clinical practice, is limited owing to issues of poor availability, lack of sterility and immunogenicity of the cells of origin. However, detailed studies of their mechanisms of action might improve understanding of intra-nephron communication and kidney physiology. In addition, future approaches might involve engineering autologous non-renal extracellular vesicles to recapitulate the therapeutic properties of renal cell-derived extracellular vesicles.
Graft preconditioning
Machine perfusion of donor kidneys before transplantation could improve organ preservation and reduce ischaemic injury, potentially resulting in improved graft function. In addition, this strategy might enable organ quality and viability to be improved before transplantation, potentially increasing the donor pool118. Addition of extracellular vesicles to the perfusion solution could be an innovative strategy for delivering pro-regenerative molecules to the graft before transplantation (Fig. 4). To date, only a few studies have investigated the feasibility and efficacy of this strategy in lung, liver and kidney transplantation119. The delivery of MSC extracellular vesicles to rat deceased donor kidneys during 4 h of cold perfusion protected against ischaemic injury120. Histological and molecular analyses of the extracellular vesicle-perfused kidneys showed upregulation of enzymes involved in energy metabolism. This result was corroborated by the presence of lactate, lactate dehydrogenase and glucose in the effluent fluid, which confirmed that the extracellular vesicle-perfused kidneys had higher energy substrate consumption than the control perfused kidneys.
Fig. 4. Application of stem cell-derived extracellular vesicles for kidney graft preconditioning.
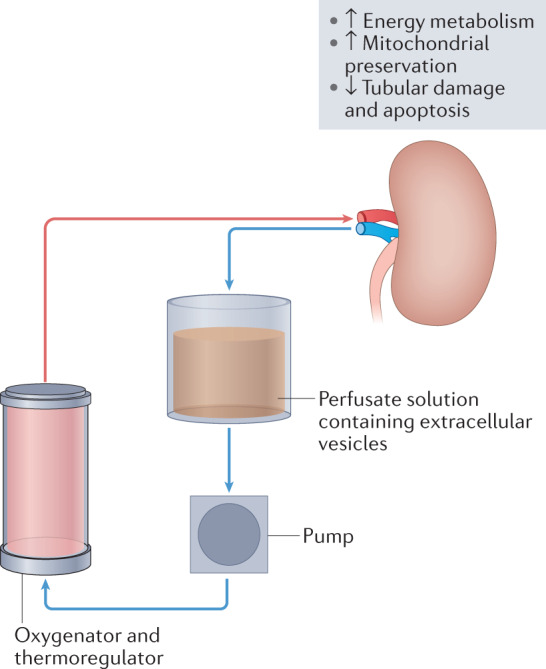
The addition of stem cell-derived extracellular vesicles to the perfusion solution during machine perfusion of donor kidneys before transplantation might protect the kidney graft from ischaemic damage, stimulate pathways involved in energy metabolism and protect mitochondria and tubular cells from damage and apoptosis121.
Similar positive results were obtained in a single-centre, prospective, pilot, randomized clinical study that evaluated the effects of 4 h of hypothermic oxygenated machine perfusion with and without the delivery of BM-MSC extracellular vesicles on marginal kidney pairs that were considered unsuitable for transplantation121. The BM-MSC extracellular vesicle-treated kidneys (n = 5) showed improved preservation of the renal architecture and mitochondrial morphology, less tubular damage and lower caspase 3 expression (indicating a protective effect against apoptosis) compared with the control perfused kidneys. Although these data are promising, further studies evaluating graft function over time are needed to investigate the long-time efficacy of the intervention.
Nephroprotective cargo molecules
The nephroprotective effects of extracellular vesicles have been attributed to the activity of numerous molecules within their cargo that reprogram the gene expression profile of recipient cells. Extracellular vesicles have anti-apoptotic, anti-inflammatory and anti-fibrotic activities. In-depth studies have dissected the roles of extracellular RNAs and, in particular, miRNAs, present within extracellular vesicles in their beneficial functions. A central role of miRNAs was supported by a study that used extracellular vesicles isolated from MSCs with knockdown for Drosha, which is a crucial nuclear enzyme involved in miRNA maturation122. These extracellular vesicles showed downregulation of miRNAs and, unlike wild type MSC extracellular vesicles, did not improve recovery of kidney function in a mouse model of AKI122. RNA sequencing analysis showed that the gene expression profiles of AKI kidneys that were treated with wild type MSC extracellular vesicles, but not those that were treated with Drosha-knockdown MSC extracellular vesicles, correlated with those of healthy kidney tissue. This finding underlines the broad effects of extracellular vesicles on injured tissues, possibly acting on multiple cells and targets through a synergy of factors. Such broad effects could be highly relevant in the context of fibrotic damage, in which overlapping pathways and mechanisms are dysregulated and therapeutic strategies that target a single factor have generally been ineffective104,105.
The beneficial effects of individual extracellular vesicle miRNAs, mainly those carried by MSC extracellular vesicles, have also been investigated. miR-133b-3p and miR-294 were reported to limit tubular TGFβ-induced epithelial-to-mesenchymal transition in vitro123. The let-7 family miRNAs might also have a role in the anti-fibrotic activity of extracellular vesicles124. Let-7c and let-7b were shown to target TGFβR1 and inhibit the TGFβ pathway, resulting in reductions in kidney fibrosis in UUO and DKD models125,126. Other miRNAs, such as miRNA-30a, miRNA-24 and miRNA-21, which are enriched in MSC extracellular vesicles derived from BM or liver tissue, target genes involved in fibrosis progression such as Col1a1, Acta2 and Snai1104,105. miR-30 carried by Wharton jelly MSC extracellular vesicles was reported to inhibit mitochondrial fission in a model of IRI127, whereas miR-26a present in adipose MSC extracellular vesicles was shown to protect against DKD by targeting TLR4 (ref.108). In a kidney IRI model, miR-199a-3p carried by MSC extracellular vesicles inhibited apoptosis by downregulating semaphorin 3A128.
Proteomic analyses of MSC extracellular vesicles have identified several growth factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin-like growth factor I (IGFI) and hepatocyte growth factor (HGF), that are involved in multiple mechanisms of tissue regeneration and repair, including regulation of inflammation, extracellular matrix remodelling, the cell cycle, cell migration, morphogenesis and angiogenesis22,116,129. These data highlight the complexity and synergistic activity of the extracellular vesicle cargo.
Biodistribution of extracellular vesicles
As extracellular vesicles are rapidly cleared by the immune system and specific organs, such as liver and spleen, their biodistribution must be considered when investigating their therapeutic potential130. Use of live and high-resolution microscopy techniques in combination with labelling strategies using lipid or luminal dyes or genetic approaches using fluorescent or bioluminescent reporters has improved understanding of the behaviour and fate of extracellular vesicles in vivo131. The most commonly used lipophilic dyes, such as PKH67, DiR and DiD, can aggregate and form particles, limiting the specificity of their application. Luminal dyes such as carboxyfluorescein diacetate succinimidyl ester (CFDA-SE), depend on the presence of luminal esterases for their conversion into a fluorescent probe. Use of these dyes reduces the fluorescent background compared with that of lipophilic dyes, but the signal is limited to extracellular vesicles that express esterases132.
Genetically engineered reporters including green or red fluorescent proteins can be linked to specific extracellular vesicle proteins, such as tetraspanins131. Crisp-Cas9 technology has also been exploited to generate functional reporters for tissue localization of extracellular vesicles. A transgenic reporter mouse that used Cre-dependent extracellular vesicle labelling under the renal promoter Pax8 enabled the detection of tubular epithelial cell-derived extracellular vesicles in urine133. This strategy provides a new approach to studying cell-specific extracellular vesicles in vivo.
Although the kidney does not seem to be a main target for extracellular vesicles, increased vascular permeability during kidney damage may favour their intrarenal localization. Extracellular vesicles preferentially localize within injured kidneys compared with healthy kidneys21. In AKI models, stem cell-derived extracellular vesicles labelled with DiD or red fluorescent dye localized within peritubular capillaries and tubules as early as 1 h after intravenous injection, with a peak after 6 h and a decrease after 24 h (refs21,23). Extracellular vesicle surface molecules might have a role in their tissue retention; for example, endothelial colony-forming cell-derived extracellular vesicles have been reported to selectively target ischaemic kidneys via the exosomal CXC-chemokine receptor 4 (CXCR4)134. In this study, the extracellular vesicles, which were labelled with lipophilic dye and detected 30 min after kidney reperfusion, rapidly transferred miR-486-5p to endothelial cells, glomeruli and proximal tubules.
Tracking of human placenta-derived MSCs extracellular vesicles expressing Gaussia luciferase using a high-resolution intravital in situ imaging technique in a transgenic mouse AKI model, confirmed their accumulation in injured kidneys and showed that they promoted the expansion of transcription factor SOX9+ cells, which are known to be involved in kidney regeneration90. Accumulation of MSC extracellular vesicles in injured kidneys was also demonstrated in an IRI mouse model using real-time in vivo imaging and labelling with an aggregation-induced emission luminogen that enabled high-resolution tracking of the extracellular vesicles135. This study reported that the extracellular vesicles were taken up by proximal tubule epithelial cells and stimulated mitochondrial antioxidant defence and ATP production, resulting in a reduction in mitochondrial fragmentation. The use of genetically engineered extracellular vesicles in combination with innovative detection technologies will enable further elucidation of the fate of extracellular vesicles in vivo following infusion and their effects at the single-cell level.
Extracellular vesicle engineering
Extracellular vesicles are an efficient, naturally targeted delivery system. They are able to protect their cargo, such as RNA species, against degradation and to evade phagocytosis more efficiently than liposomes. They also have reduced immunogenicity compared with liposomes and express surface ligands for damage-induced molecules that enable their specific uptake by damaged cells136. Extracellular vesicles are therefore attractive candidates for delivering renoprotective agents. Although the majority of applications of engineered extracellular vesicles as drug delivery systems investigated to date are in the oncology field, a few studies have shown efficacy of this approach in kidney disease models136 (Table 2). In a mouse AKI model, loading IL-10 into extracellular vesicles enhanced the stability of this cytokine and its targeting to the injured kidney137. Similarly, extracellular vesicles that were engineered to express human recombinant klotho had greater renoprotective effects than the equivalent dose of soluble klotho in a mouse model of AKI114.
Table 2.
Effects of engineered extracellular vesicles in kidney disease models
| Extracellular vesicle origin | Engineering approach | Model | Type of injury | Effects | Ref. |
|---|---|---|---|---|---|
| Urine | Klotho expression | Rhabdomyolysis | AKI | Increased endogenous klotho levels and reduced αSMA and CTGF levels in kidney cells | 114 |
| RAW 264.7 macrophages | Loading with IL-10 | IRI | AKI | Suppressed mammalian target of rapamycin signalling and promoted mitophagy and M2 macrophage polarization, which can suppress inflammation | 137 |
| Bone marrow MSCs | Transfection with specific miRNA mimics (miR-10a, miR-127 and miR-486) | Rhabdomyolysis | AKI | Reduced tubular cell injury at a lower therapeutic dose than naïve extracellular vesicles | 138 |
| Transfection with let-7i-5p antagomir | UUO | CKD | Reduced let-7i-5p level in kidney tubular epithelial cells, ECM deposition and EMT and increased activation of TSC1–mTOR signalling | 144 | |
| Kidney MSCs | Lentiviral transfection of human EPO mRNA | Renal anaemia | CKD | Induced EPO expression in kidney cells, increased haemoglobin levels and decreased serum creatinine and BUN | 143 |
| Adipose MSCs | Lentiviral transfection of GDNF mRNA | UUO | CKD | Activated the SIRT1–eNOS signalling pathway in proximal tubular cells and reduced fibrosis | 145 |
| Umbilical cord MSCs | Lentiviral transfection of OCT4 mRNA | IRI | AKI | Reduced apoptosis and increased proliferation of renal tubular epithelial cells | 146 |
| Human placental MSCs | Encapsulation of extracellular vesicles within a hydrogel containing Arg-Gly-Asp peptides | IRI | AKI and CKD | Increased efficacy of encapsulated extracellular vesicles compared with free extracellular vesicles | 149 |
| Encapsulation of extracellular vesicles within a collagen matrix | IRI | AKI | Increased efficacy of encapsulated extracellular vesicles compared with free extracellular vesicles | 150 | |
| Red blood cells | Surface expression of KIM1-binding peptide and cargo expression of P65 and Snai1 siRNAs | IRI and UUO | AKI and CKD | Reduced P-p65 and SNAI1 expression, renal inflammation and fibrosis | 152 |
αSMA, α-smooth muscle actin; AKI, acute kidney injury; CKD, chronic kidney disease; CTGF, connective tissue growth factor; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; eNOS, endothelial nitric oxide synthase; EPO, erythropoietin; IRI, ischaemia–reperfusion injury; KIM1, kidney injury molecule 1; miRNA, microRNA; MSC, mesenchymal stromal cell; mTOR, mammalian target of rapamycin; SIRT1, sirtuin 1; SNAI1, zinc-finger protein SNAI1; TSC1, tuberous sclerosis complex subunit 1; UUO, unilateral ureteral obstruction.
Engineering strategies could also be used to enhance the natural therapeutic activity of extracellular vesicles. However, direct miRNA or mRNA extracellular vesicle engineering strategies using electroporation have had limited success, possibly because of the alteration of the therapeutic cargo during this manipulation. For example, in a mouse AKI model, MSC extracellular vesicles that were loaded with selected miRNAs only improved renal regeneration compared with non-engineered MSC extracellular vesicles when the administered dose was low138. The number of miRNAs that can be packaged in extracellular vesicles is still debated; this issue is important because the absolute number of miRNA present in extracellular vesicles may correlate with their activity139. Transfection protocols should be carefully optimized by modulating voltage and pulse to minimize vesicle damage and to maximize cargo loading140. However, loading of extracellular vesicles with RNAs by electroporation is very prone to artefacts owing to the presence of residual RNA aggregates and to extracellular vesicle disruption141. For these reasons, alternative strategies to load extracellular vesicles with miRNAs such as co-incubation have also been investigated. A study that used a co-incubation strategy to load human liver stem cell extracellular vesicles with antitumour miR-145 reported that the loaded vesicles were more effective at inhibiting the invasive properties of cancer stem cells than non-loaded vesicles142.
Even more encouraging results have been obtained using extracellular vesicles derived from engineered cells. For example, extracellular vesicles from MSCs that were engineered to express human erythropoietin (EPO) were able to transfer EPO mRNA to target cells and alleviate anaemia in CKD mice143. Moreover, transfecting MSCs with a let-7i-5p antagomir increased the anti-fibrotic activity of the secreted extracellular vesicles, resulting in a reduction in extracellular matrix deposition and attenuation of epithelial-to-mesenchymal transition in vitro and in vivo in a UUO model144. In the same model, extracellular vesicles from MSCs that were engineered to express glial cell line-derived neurotrophic factor decreased peritubular capillary rarefaction and tubulointerstitial fibrosis by targeting the SIRT1 signalling pathway145. Compared with normal MSC extracellular vesicles, those that were generated from MSCs that overexpressed the transcription factor octamer-binding protein 4 (OCT4) had a greater inhibitory effect on Snai1 expression and increased anti-apoptotic and proliferative effects on renal cells in a mouse kidney IRI model146.
Preconditioning of extracellular vesicle-releasing cells might also influence their content and potentially increase their regenerative properties. For example, hypoxia preconditioning of MSCs increased the angiogenic potential of the released extracellular vesicles147. By contrast, MSCs that were cultured with pro-inflammatory cytokines released extracellular vesicles that were enriched in metalloproteinase inhibitor 1 (TIMP1), CD39 and CD73 and had greater anti-angiogenic effects than extracellular vesicles from MSCs that were cultured under normal conditions148.
An alternative strategy to increase the therapeutic efficacy of extracellular vesicles while preserving their integrity is modulation of their stability and biodistribution. The route of administration is an important factor that influences extracellular vesicle accumulation at the site of interest. The majority of preclinical studies have used tail vein injection, which enables extracellular vesicle tissue localization at the injured site via the circulation; few studies have used sub-renal capsule or direct intrarenal parenchyma administration79,83,98. Local delivery approaches may enable the use of lower doses of extracellular vesicles but are more invasive than intravenous injection.
Hydrogels containing RGD (Arg-Gly-Asp) peptides have been shown to enhance the binding of extracellular vesicles to injured kidney cells that expressed RGD binding integrins on their surface149. In a mouse AKI model, extracellular vesicles that were entrapped within hydrogels and injected under the renal capsule were more effective than free extracellular vesicles at limiting tubular injury in the early stages of AKI and limiting fibrosis in the later stages149. Similar benefits were obtained by encapsulating extracellular vesicles within a collagen matrix and injecting them intrarenally into three different sites to favour their controlled release150.
Pulsed focused ultrasound, consisting of a high-intensity pulse of sound waves, can be externally applied to control and modulate the local cargo release of circulating extracellular vesicle. This ultrasound technique increased the efficacy of MSC extracellular vesicles without altering their homing to the kidney in a mouse model of cisplatin-induced AKI151.
Finally, engineering of biologically inactive extracellular vesicles from easily available and potentially autologous sources could be a very promising approach for targeted delivery of therapeutics. For example, blood cell-derived extracellular vesicles that were engineered to express a kim1-targeting peptide and loaded with short interfering RNAs for the transcription factors p65 and snai1, efficiently delivered these short interfering RNAs to injured kidney tubules and thereby reduced tubulointerstitial inflammation and fibrosis in mouse models of AKI152.
Clinical application
A number of clinical trials are testing the safety and efficacy of extracellular vesicles from various stem cell sources for organ repair. The first in-human study reported the compassionate use of MSC extracellular vesicles in a patient with steroid-resistant graft versus host disease and showed the safety of this approach153. Administration of the extracellular vesicles quickly improved the patient’s symptoms, including diarrhoea and cutaneous and mucosal graft versus host disease153.
To date, only one trial has tested the efficacy of MSC extracellular vesicles in kidney disease. This single-centre, randomized, placebo-controlled, phase II/III clinical trial enrolled 40 patients with CKD stage III or IV154. Two doses of umbilical cord MSC extracellular vesicles were administered 1 week apart (the first dose was injected intravenously and the second dose was injected into the intrarenal arteries) and patients were followed for 12 months. The treated patients showed a significant improvement in kidney function compared with those in the placebo group, as evaluated by glomerular filtration rate, albuminuria and plasma levels of creatinine and urea. In addition, kidney biopsy samples taken from responding patients 3 months after treatment showed increases in the numbers of proliferating Ki67 cells and renal progenitor CD133+ cells, providing evidence of kidney regeneration. The treatment also induced downregulation of pro-inflammatory TNFα and upregulation of anti-inflammatory TGFβ1 and IL-10 in patient plasma. These data support the safety and efficacy of MSC extracellular vesicle administration in patients with CKD. Unfortunately, the researchers do not provide information on the development of anti-HLA antibodies in recipients of the MSC extracellular vesicles, which is of clinical relevance for patients with CKD who might require future kidney transplantation, as the presence of these antibodies can lead to hyperacute rejection and graft loss.
Since the beginning of the COVID-19 pandemic, a number of clinical trials have investigated the efficacy of MSC extracellular vesicles in patients with COVID-19 (ref.155). These trials have highlighted the feasibility of extracellular vesicle production and administration in a clinical setting, supporting their use as a novel therapeutic tool. For example, a prospective, non-randomized open-label cohort study in 24 patients with severe COVID-19 and moderate-to-severe acute respiratory distress syndrome reported that intravenous infusion of allogeneic BM-MSC extracellular vesicles was safe, improved hypoxia and immune reconstitution and downregulated the cytokine storm156. However, accurate and extensive characterization of the injected extracellular vesicle base product is necessary to guarantee reproducible, scalable, and well-controlled studies157.
The clinical application of MSC extracellular vesicles is limited by the lack of clear criteria for standardization of extracellular vesicle preparation158. Consensus has been achieved on the definition of therapeutically active MSC extracellular vesicles, which is restricted to small extracellular vesicles (<200 nm in size) expressing mesenchymal and extracellular vesicle markers coupled with a tested functional activity of relevance for the desired therapeutic use159,160. The generation of master and/or working cell banks to provide a stable pool of producer cells for extracellular vesicles is fundamental for their clinical translation. Techniques required for large-scale extracellular vesicle isolation, such as tangential flow filtration to enable their extraction from large volumes, are under development. Extracellular vesicle integrity and purity from contaminants, such as growth factors and miRNAs present in cell-culture media, as well as endotoxins, are critical factors that must be considered161. In addition, scalable, reproducible and good manufacturing practice-compliant protocols for the manufacture of extracellular vesicle products must be developed. Nevertheless, various products are under development, including cell secretome products, native extracellular vesicles and engineered extracellular vesicles, with the aim of treating a large range of conditions such as cancer and orthopaedic, neurological, ophthalmological and cardiological diseases159.
Conclusions
Extracellular vesicles have roles in in cell-to-cell and organ-to-organ crosstalk that may amplify kidney damage and contribute to the progression of kidney disease. Moreover, the complex cargo of extracellular vesicles reflects the type and pathophysiological status of the originating cells. The functions of extracellular vesicles and their applications as biomarkers and therapeutic agents constitute an expanding field of great interest for clinical translation. Researchers are now leveraging advances in nanotechnologies that enable evaluation of single extracellular vesicles, such as super resolution microscopy, nanoflow cytometry and single extracellular vesicle capture arrays, in mechanistic and biomarker studies162. Ideally, the development of approaches for single-vesicle analysis will enable correlation of the type of extracellular vesicle-releasing cell with its cargo, representing a new type of liquid biopsy.
The application of urinary extracellular vesicles as biomarkers is limited by the heterogeneity of the urine and rigorous protocols for extracellular vesicle analysis and normalization are required15. Whether organ-derived and/or serum extracellular vesicles can be identified within the urine and exploited as biomarkers is currently unclear. Although preclinical data suggest that extracellular vesicles could have beneficial effects in AKI and CKD, human studies are limited and clinical trials are needed to validate these findings. New frontiers in this field include the use of natural or autologous sources of extracellular vesicles and extracellular vesicle engineering. Increased knowledge of extracellular vesicles could also inspire the engineering of biomimetic synthetic particles, for example, by loading synthetic cargo within extracellular vesicle membrane-coated particles or by building artificial extracellular vesicle mimics.
To enable clinical translation of extracellular vesicle products, tests of their identity (that is, precise characterization relative to their mechanism of action) and their potency (that is, their effects in relation to the desired therapeutic use) need to be defined. To date, no extracellular vesicle product has been approved by regulatory agencies. The extracellular vesicle community is committed to the development of standardized protocols for the scalable production, characterization and storage of extracellular vesicles, as well as preclinical and clinical approaches to assessing their safety and efficacy.
Supplementary information
Glossary
- Transcytosis
A type of transcellular transport in which macromolecules are captured in vesicles on one side of the cell and then transported across and released on the other side of the cell.
- Flow cytometry
A technique used to detect and quantify physical and chemical characteristics of a population of cells or extracellular vesicles, which are often labelled with fluorescent antibodies.
- Extracellular vesicle-capturing chips
A technology platform in which extracellular vesicles are captured on a chip that is functionalized with antibodies.
- Mesenchymal stromal cells
(MSCs). Also known as mesenchymal stem cells. A subset of heterogeneous non-haematopoietic fibroblast-like cells that can differentiate into cells of multiple lineages including osteoblasts, chondrocytes, myocytes and adipocytes.
- Secretome
All the bioactive substances, including proteins and vesicles, that are secreted by cells into the extracellular space.
- Wharton jelly
Mucous connective tissue of the umbilical cord located between the amniotic epithelium and the umbilical vessels.
- Endothelial colony-forming cells
Adult endothelial progenitor cells that reside in the vasculature and can differentiate into endothelial cells to regenerate endothelial cell populations.
Author contributions
Both authors researched the data for the article, made substantial contributions to discussions of the content and wrote the text. B.B. reviewed and edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Nephrology thanks Alfonso Eirin, Derrick Gibbings and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41581-022-00586-9.
References
- 1.Quesenberry PJ, Aliotta J, Deregibus MC, Camussi G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res. Ther. 2015;6:153. doi: 10.1186/s13287-015-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Devel. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 4.Théry C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minciacchi VR, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327–11341. doi: 10.18632/oncotarget.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Acunzo P, et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021;7:eabe5085. doi: 10.1126/sciadv.abe5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 9.György B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratajczak J, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 12.Aliotta JM, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp. Hematol. 2010;38:233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause M, et al. Exosomes as secondary inductive signals involved in kidney organogenesis. J. Extracell. Vesicles. 2018;7:1422675. doi: 10.1080/20013078.2017.1422675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdbrügger U, et al. Urinary extracellular vesicles: a position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2021;10:e12093. doi: 10.1002/jev2.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc. Natl Acad. Sci. USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blijdorp CJ, et al. Nephron mass determines the excretion rate of urinary extracellular vesicles. J. Extracell. Vesicles. 2022;11:e12181. doi: 10.1002/jev2.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, et al. A cationic near infrared fluorescent agent and ethyl-cinnamate tissue clearing protocol for vascular staining and imaging. Sci. Rep. 2019;9:521. doi: 10.1038/s41598-018-36741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellucci L, Montini G, Collino F, Bussolati B. Mesenchymal stromal cell-derived extracellular vesicles pass through the filtration barrier and protect podocytes in a 3D glomerular model under continuous perfusion. Tissue Eng. Regen. Med. 2021;18:549–560. doi: 10.1007/s13770-021-00374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, et al. A translational study of urine miRNAs in acute myocardial infarction. J. Mol. Cell. Cardiol. 2012;53:668–676. doi: 10.1016/j.yjmcc.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grange C, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 2014;33:1055–1063. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruno S, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantaluppi V, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 24.Street JM, et al. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 2011;589:6119–6127. doi: 10.1113/jphysiol.2011.220277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gildea JJ, et al. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin. Biochem. 2014;47:89–94. doi: 10.1016/j.clinbiochem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter RW, Kumar S, Coward RJM, Buck AH, Dear JW. Extracellular RNA moves from the glomerulus to the renal tubule. bioRxiv. 2021 doi: 10.1101/2021.06.15.448584. [DOI] [Google Scholar]
- 27.Hill N, et al. Glomerular endothelial derived vesicles mediate podocyte dysfunction: a potential role for miRNA. PLoS ONE. 2020;15:e0224852. doi: 10.1371/journal.pone.0224852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon JS, et al. microRNA in extracellular vesicles released by damaged podocytes promote apoptosis of renal tubular epithelial cells. Cells. 2020;9:1409. doi: 10.3390/cells9061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger D, et al. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J. Am. Soc. Nephrol. 2014;25:1401–1407. doi: 10.1681/ASN.2013070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munkonda MN, et al. Podocyte-derived microparticles promote proximal tubule fibrotic signaling via p38 MAPK and CD36. J. Extracell. Vesicles. 2018;7:1432206. doi: 10.1080/20013078.2018.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 32.Li ZL, et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388–404. doi: 10.1016/j.kint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Claridge B, et al. Proteome characterisation of extracellular vesicles isolated from heart. Proteomics. 2021;16:e2100026. doi: 10.1002/pmic.202100026. [DOI] [PubMed] [Google Scholar]
- 34.Salomon C, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS ONE. 2014;9:e98667. doi: 10.1371/journal.pone.0098667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzariol M, Camussi G, Brizzi MF. Extracellular vesicles tune the immune system in renal disease: a focus on systemic lupus erythematosus, antiphospholipid syndrome, thrombotic microangiopathy and ANCA-Vasculitis. Int. J. Mol. Sci. 2021;22:4194. doi: 10.3390/ijms22084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amabile N, Guérin AP, Tedgui A, Boulanger CM, London GM. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrol. Dial. Transpl. 2012;27:1873–1880. doi: 10.1093/ndt/gfr573. [DOI] [PubMed] [Google Scholar]
- 37.Berezin A, Zulli A, Kerrigan S, Petrovic D, Kruzliak P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin. Biochem. 2015;48:562–568. doi: 10.1016/j.clinbiochem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Soriano S, et al. Endothelial damage and vascular calcification in patients with chronic kidney disease. Am. J. Physiol. Renal Physiol. 2014;307:F1302–F1311. doi: 10.1152/ajprenal.00114.2014. [DOI] [PubMed] [Google Scholar]
- 39.Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J. Am. Soc. Nephrol. 2016;27:12–26. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Droste M, et al. Single extracellular vesicle analysis performed by imaging flow cytometry and nanoparticle tracking analysis evaluate the accuracy of urinary extracellular vesicle preparation techniques differently. Int. J. Mol. Sci. 2021;22:12436. doi: 10.3390/ijms222212436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soekmadji C, et al. The future of extracellular vesicles as theranostics — an ISEV meeting report. J. Extracell. Vesicles. 2020;9:1809766. doi: 10.1080/20013078.2020.1809766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blijdorp CJ, et al. Comparing approaches to normalize, quantify, and characterize urinary extracellular vesicles. J. Am. Soc. Nephrol. 2021;32:1210–1226. doi: 10.1681/ASN.2020081142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter RW, Dear JW. Urinary vesicles: are they ready for real-world use? J. Am. Soc. Nephrol. 2021;32:1013–1015. doi: 10.1681/ASN.2021030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonoda H, et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemic-reperfusion injury. Am. J. Physiol. Renal Physiol. 2009;297:F1006–F1016. doi: 10.1152/ajprenal.00200.2009. [DOI] [PubMed] [Google Scholar]
- 45.Zhou H, et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou H, et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008;74:613–621. doi: 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H, et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Renal Physiol. 2013;305:F553–F559. doi: 10.1152/ajprenal.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalani A, et al. Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS ONE. 2013;8:e60177. doi: 10.1371/journal.pone.0060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HK, et al. Il Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr. Nephrol. 2012;27:317–320. doi: 10.1007/s00467-011-2035-2. [DOI] [PubMed] [Google Scholar]
- 50.Lv LL, et al. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin. Chim. Acta. 2014;428:26–31. doi: 10.1016/j.cca.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Alvarz S, et al. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant. Proc. 2013;45:3719–3723. doi: 10.1016/j.transproceed.2013.08.079. [DOI] [PubMed] [Google Scholar]
- 52.Dimuccio V, et al. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS One. 2014;9:e104490. doi: 10.1371/journal.pone.0104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masaoutis C, Al Besher S, Koutroulis I, Theocharis S. Exosomes in nephropathies: a rich source of novel biomarkers. Dis. Markers. 2020;12:8897833. doi: 10.1155/2020/8897833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barutta F, et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS ONE. 2013;8:e73798. doi: 10.1371/journal.pone.0073798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lv LL, et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Renal Physiol. 2013;305:F1220–F1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 58.Khurana R, et al. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA. 2017;23:142–152. doi: 10.1261/rna.058834.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumari M, et al. miR-451 loaded exosomes are released by the renal cells in response to injury and associated with reduced kidney function in human. Front. Physiol. 2020;11:234. doi: 10.3389/fphys.2020.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Min Q-H, et al. Differential expression of urinary exosomal microRNAs in IgA nephropathy. J. Clin. Lab. Anal. 2018;32:e22226. doi: 10.1002/jcla.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solé C, Moliné T, Vidal M, Ordi-Ros J, Cortés-Hernández J. An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells. 2019;8:773. doi: 10.3390/cells8080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma H, et al. Differential expression study of circular RNAs in exosomes from serum and urine in patients with idiopathic membranous nephropathy. Arch. Med. Sci. 2019;15:738–753. doi: 10.5114/aoms.2019.84690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grange C, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 64.De Palma G, et al. The three-gene signature in urinary extracellular vesicles from patients with clear cell renal cell carcinoma. J. Cancer. 2016;7:1960–1967. doi: 10.7150/jca.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raimondo F, et al. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol. Bio. Syst. 2013;9:1220–1233. doi: 10.1039/c3mb25582d. [DOI] [PubMed] [Google Scholar]
- 66.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04053855 (2021).
- 67.Butz H, et al. Exosomal MicroRNAs are diagnostic biomarkers and can mediate cell-cell communication in renal cell carcinoma. Eur. Urol. Focus. 2016;2:210–218. doi: 10.1016/j.euf.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Kurahashi R. MicroRNA-204-5p: a novel candidate urinary biomarker of Xp11.2 translocation renal cell carcinoma. Cancer Sci. 2019;110:1897–1908. doi: 10.1111/cas.14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujii N, et al. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget. 2017;8:109877–109888. doi: 10.18632/oncotarget.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, et al. Serum exosomal miR-210 as a potential biomarker for clear cell renal cell carcinoma. J. Cell Biochem. 2019;120:1492–1502. doi: 10.1002/jcb.27347. [DOI] [PubMed] [Google Scholar]
- 71.Xiao CT, Lai WJ, Zhu WA, Wang H. MicroRNA derived from circulating exosomes as noninvasive biomarkers for diagnosing renal cell carcinoma. Onco Targets Ther. 2020;13:10765–10774. doi: 10.2147/OTT.S271606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Z, et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol. Cancer. 2018;17:82. doi: 10.1186/s12943-018-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qu L, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Maqsood M, et al. Adult mesenchymal stem cells and their exosomes: sources, characteristics, and application in regenerative medicine. Life Sci. 2020;256:118002. doi: 10.1016/j.lfs.2020.118002. [DOI] [PubMed] [Google Scholar]
- 75.Tomasoni S, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cell Dev. 2013;22:772–780. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Börger V, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 2017;18:E1450. doi: 10.3390/ijms18071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy DE, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019;51:32. doi: 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: new nanotools for cancer treatment. Pharmacol. Res. 2016;111:487–500. doi: 10.1016/j.phrs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 79.Grange C, Skovronova R, Marabese F, Bussolati B. Stem cell-derived extracellular vesicles and kidney regeneration. Cells. 2019;8:1240. doi: 10.3390/cells8101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eirin A, Lerman LO. Mesenchymal stem/stromal cell-derived extracellular vesicles for chronic kidney disease: are we there yet? Hypertension. 2021;78:261–269. doi: 10.1161/HYPERTENSIONAHA.121.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruno S, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reis LA, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS ONE. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen B, et al. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016;2016:1240301. doi: 10.1155/2016/1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Almeida DC, et al. A regulatory miRNA-mRNA network is associated with tissue repair induced by mesenchymal stromal cells in acute kidney injury. Front. Immunol. 2017;7:645. doi: 10.3389/fimmu.2016.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao F, et al. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. doi: 10.1016/j.lfs.2020.117719. [DOI] [PubMed] [Google Scholar]
- 86.Ju GQ, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE. 2015;10:e0121534. doi: 10.1371/journal.pone.0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang R, et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol. Lett. 2020;42:669–679. doi: 10.1007/s10529-020-02831-2. [DOI] [PubMed] [Google Scholar]
- 89.Zou X, et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014;5:40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang K, et al. In vivo two-photon microscopy reveals the contribution of Sox9+ cell to kidney regeneration in a mouse model with extracellular vesicle treatment. J. Biol. Chem. 2020;295:12203–12213. doi: 10.1074/jbc.RA120.012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yuan X, et al. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017;8:3200–3218. doi: 10.1038/s41419-017-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Collino F, et al. Extracellular vesicles derived from induced pluripotent stem cells promote renoprotection in acute kidney injury model. Cells. 2020;9:453. doi: 10.3390/cells9020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsu RK, Hsu CY. The role of acute kidney injury in chronic kidney disease. Semin. Nephrol. 2016;36:283–292. doi: 10.1016/j.semnephrol.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu L, et al. Embryonic stem cell-derived extracellular vesicles promote the recovery of kidney injury. Stem Cell Res. Ther. 2021;12:379. doi: 10.1186/s13287-021-02460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cantaluppi V, et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol. Dial. Transpl. 2015;30:410–422. doi: 10.1093/ndt/gfu364. [DOI] [PubMed] [Google Scholar]
- 96.Burger D, et al. Human endothelial colony-forming cells protect against acute kidney injury role of exosomes. Am. J. Pathol. 2015;185:2309–2323. doi: 10.1016/j.ajpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Viñas JL, et al. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 2016;90:1238–1250. doi: 10.1016/j.kint.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 98.Eirin A, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114–124. doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song TR, et al. Mesenchymal stem cell-derived extracellular vesicles induce regulatory T cells to ameliorate chronic kidney injury. Hypertension. 2020;75:1223–1232. doi: 10.1161/HYPERTENSIONAHA.119.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He J, et al. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology. 2012;17:493–500. doi: 10.1111/j.1440-1797.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- 101.He J, et al. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology. 2015;20:591–600. doi: 10.1111/nep.12490. [DOI] [PubMed] [Google Scholar]
- 102.Shi Z, Wang Q, Zhang Y, Jiang D. Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem Cell Res. Ther. 2020;11:253. doi: 10.1186/s13287-020-01767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kholia S, et al. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front. Cell Dev. Biol. 2020;8:188. doi: 10.3389/fcell.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kholia S, et al. Human liver stem cell-derived extracellular vesicles prevent aristolochic acid-induced kidney fibrosis. Front. Immunol. 2018;9:1639. doi: 10.3389/fimmu.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grange C, et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019;9:4468. doi: 10.1038/s41598-019-41100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ebrahim N, et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. 2018;7:226. doi: 10.3390/cells7120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin J, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. 2019;10:95. doi: 10.1186/s13287-019-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duan Y, et al. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem. 2020;295:12868–12884. doi: 10.1074/jbc.RA120.012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ji C, et al. Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death Dis. 2020;11:327. doi: 10.1038/s41419-020-2510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang GY, et al. Extracellular-vesicles derived from human Wharton-Jelly mesenchymal stromal cells ameliorated cyclosporin A-induced renal fibrosis in rats. Int. J. Clin. Exp. Med. 2019;12:8943–8949. [Google Scholar]
- 111.Van Koppen A, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS ONE. 2012;7:e38746. doi: 10.1371/journal.pone.0038746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindoso RS, et al. Adipose mesenchymal cells-derived EVs alleviate DOCA-salt-induced hypertension by promoting cardio-renal protection. Mol. Ther. Methods Clin. Dev. 2019;16:63–77. doi: 10.1016/j.omtm.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dominguez JH, et al. Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. J. Am. Soc. Nephrol. 2017;28:3533–3544. doi: 10.1681/ASN.2016121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grange C, et al. Urinary extracellular vesicles carrying klotho improve the recovery of renal function in an acute tubular injury model. Mol. Ther. 2020;28:490–502. doi: 10.1016/j.ymthe.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ranghino A, et al. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res. Ther. 2017;8:24. doi: 10.1186/s13287-017-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi HY, et al. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS ONE. 2014;9:e87853. doi: 10.1371/journal.pone.0087853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang ZZ, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016;7:24–37. doi: 10.1186/s13287-016-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verstraeten L, Jochmans I. Sense and sensibilities of organ perfusion as a kidney and liver viability assessment platform. Transpl. Inter. 2022;35:10312. doi: 10.3389/ti.2022.10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grange C, Bellucci L, Bussolati B, Ranghino A. Potential applications of extracellular vesicles in solid organ transplantation. Cells. 2020;9:369. doi: 10.3390/cells9020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gregorini M, et al. Perfusion of isolated rat kidney with mesenchymal stromal cells/extracellular vesicles prevents ischaemic injury. J. Cell. Mol. Med. 2017;21:3381–3393. doi: 10.1111/jcmm.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rampino T, et al. Extracellular vesicles derived from mesenchymal stromal cells delivered during hypothermic oxygenated machine perfusion repair ischemic/reperfusion damage of kidneys from extended criteria donors. Biology. 2022;11:350. doi: 10.3390/biology11030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Collino F, et al. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNA. J. Am. Soc. Nephrol. 2015;26:2349–2360. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y, et al. Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-beta1-mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Res. Ther. 2015;6:185. doi: 10.1186/s13287-015-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park JT, et al. Repression of let-7 by transforming growth factor-β1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. Am. J. Physiol. Physiol. 2014;307:F1390–F1403. doi: 10.1152/ajprenal.00458.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brennan EP, et al. Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFβR1. J. Am. Soc. Nephrol. 2013;24:627–637. doi: 10.1681/ASN.2012060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang B, et al. Transforming growth factor-β1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b. Kidney Int. 2014;85:352–361. doi: 10.1038/ki.2013.372. [DOI] [PubMed] [Google Scholar]
- 127.Gu D, et al. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016;2016:2093940. doi: 10.1155/2016/2093940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu G, et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell. Physiol. 2019;234:23736–23749. doi: 10.1002/jcp.28941. [DOI] [PubMed] [Google Scholar]
- 129.Anderson JD, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappab signaling. Stem Cell. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takahashi Y, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 131.Verweij FJ, et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat. Methods. 2021;18:1013–1026. doi: 10.1038/s41592-021-01206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dehghani M, Gulvin SM, Flax J, Gaborski TR. Systematic evaluation of PKH labelling on extracellular vesicle size by nanoparticle tracking analysis. Sci. Rep. 2020;10:9533. doi: 10.1038/s41598-020-66434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nørgård MØ, et al. A new transgene mouse model using an extravesicular EGFP tag enables affinity isolation of cell-specific extracellular vesicles. Sci. Rep. 2022;12:496. doi: 10.1038/s41598-021-04512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Viñas JL, et al. Receptor-ligand interaction mediates targeting of endothelial colony forming cell-derived exosomes to the kidney after ischemic injury. Sci. Rep. 2018;8:16320. doi: 10.1038/s41598-018-34557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cao H, et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano. 2020;14:4014–4026. doi: 10.1021/acsnano.9b08207. [DOI] [PubMed] [Google Scholar]
- 136.Butreddy A, Kommineni N, Dudhipala N. Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomaterials. 2021;11:1481. doi: 10.3390/nano11061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang TT, et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci. Adv. 2020;6:eaaz0748. doi: 10.1126/sciadv.aaz0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tapparo M, et al. Renal regenerative potential of extracellular vesicles derived from miRNA-engineered mesenchymal stromal cells. Int. J. Mol. Sci. 2019;20:2381. doi: 10.3390/ijms20102381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chevillet JR, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl Acad. Sci. USA. 2014;111:14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pomatto M, et al. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019;13:133–144. doi: 10.1016/j.omtm.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kooijmans S, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release. 2013;172:229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 142.Brossa A, et al. Coincubation as miR-loading strategy to improve the anti-tumor effect of stem cell-derived EVs. Pharmaceutics. 2021;13:76. doi: 10.3390/pharmaceutics13010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi HY, et al. Kidney mesenchymal stem cell-derived extracellular vesicles engineered to express erythropoietin improve renal anemia in mice with chronic kidney disease. Stem Cell Rev. Rep. 2022;18:980–992. doi: 10.1007/s12015-021-10141-x. [DOI] [PubMed] [Google Scholar]
- 144.Jin J, et al. Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microrna Let-7i-5p antagomir. Int. J. Nanomed. 2021;16:3565–3578. doi: 10.2147/IJN.S299969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen L, et al. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signalling pathway. Theranostics. 2020;10:9425–9442. doi: 10.7150/thno.43315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang ZY, et al. Oct-4 enhanced the therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Kidney Blood Press. Res. 2020;45:95–108. doi: 10.1159/000504368. [DOI] [PubMed] [Google Scholar]
- 147.Ge L, et al. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J. Nanobiotechnol. 2021;19:380. doi: 10.1186/s12951-021-01126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Angioni R, et al. CD73+ extracellular vesicles inhibit angiogenesis through adenosine A2B receptor signalling. J. Extracell. Vesicles. 2020;9:1757900. doi: 10.1080/20013078.2020.1757900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang C, et al. Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano. 2020;14:12133–12147. doi: 10.1021/acsnano.0c05681. [DOI] [PubMed] [Google Scholar]
- 150.Liu Y, et al. Enhanced therapeutic effects of MSC-derived extracellular vesicles with an injectable collagen matrix for experimental acute kidney injury treatment. Stem Cell Res. Ther. 2020;11:161. doi: 10.1186/s13287-020-01668-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ullah M, et al. Pulsed focused ultrasound enhances the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles in acute kidney injury. Stem Cell Res. Ther. 2020;11:398. doi: 10.1186/s13287-020-01922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang TT, et al. Kim-1 targeted extracellular vesicles: a new therapeutic platform for RNAi to treat AKI. J. Am. Soc. Nephrol. 2021;32:2467–2483. doi: 10.1681/ASN.2020111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kordelas L, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 154.Nassar W, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O’Driscoll L. Extracellular vesicles from mesenchymal stem cells as a Covid-19 treatment. Drug Discov. Today. 2020;25:1124–1125. doi: 10.1016/j.drudis.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sengupta V, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lim SK, Giebel B, Weiss DJ, Witwer KW, Rohde E. Re: “Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19” by Sengupta et al. Stem Cell Dev. 2020;29:877–878. doi: 10.1089/scd.2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Börger V, et al. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy. 2020;22:482–485. doi: 10.1016/j.jcyt.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gimona M, et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy. 2021;23:373–380. doi: 10.1016/j.jcyt.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 160.Witwer KW, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles. 2019;8:1609206. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nordin JZ, Bostancioglu RB, Corso G, El Andaloussi S. Tangential flow filtration with or without subsequent bind-elute size exclusion chromatography for purification of extracellular vesicles. Methods Mol. Biol. 2019;1953:287–299. doi: 10.1007/978-1-4939-9145-7_18. [DOI] [PubMed] [Google Scholar]
- 162.Arab T, et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J. Extracell. Vesicles. 2021;10:e12079. doi: 10.1002/jev2.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.